- 1Child Neuropsychiatric Unit, University of Bari Aldo Moro, Bari, Italy
- 2Department of Biomedical Sciences and Human Oncology, University of Bari Aldo Moro, Bari, Italy
- 3Psychiatric Emergencies in Adolescence Unit, University of Bari Aldo Moro, Bari, Italy
Autism Spectrum Disorder (ASD) has historically been studied, known, and diagnosed in males. Females tend to remain unidentified, especially those with average intelligence abilities. This sex/gender difference might be partially explained by biological risk factors, but it is probably also bound to methodological issues. The present study aims to examine phenotypic characteristics (cognitive, emotive, socio-communicative, and academic) of a group of 54 females with ASD matched to a group of 55 males with ASD (3–18 years), all without cognitive impairment. Results suggest that there are subtle, yet potentially meaningful, quantitative, and qualitative phenotypic differences between females and males that common screening tests are not always sensitive enough to recognize. Further studies to improve practice and course for the assessment of females, reducing sex/gender-based inequities in ASD care, are required.
Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental and lifelong disorder. According to the 5th edition of the Diagnostic Statistical Manual of Mental Disorders [DSM-5; (1)], specific diagnostic criteria include impairment in social skills and communication together with restrictive and repetitive behaviors, interests, or activities, as well as sensory abnormalities. In order for a diagnosis to be made, these deficits must be severe enough to significantly impair patients functioning. Although symptoms may be noticed and diagnosed in early childhood, they could also manifest later in life, when social demands and requirements exceed the limited range of functioning of an ASD individual.
In recording procedures of DSM-5, severity should be recorded in terms of the level of support needed, adding the specification of with/without accompanying intellectual and/or language impairment, which should be recorded thereafter. “High” and “low” Functioning Autism are terms, even if not used to identify nosographic categories, commonly used in scientific literature to identify patients diagnosed with ASD according to intellectual abilities (Intelligence Quotient, IQ, respectively higher or lower than 70).
The prevalence of ASD has significantly increased over the past few decades, nowadays affecting 1 in 59 children in the United States, as reported by recent findings from Centers for Disease Control data (2). ASD disproportionately affects males; the over-representation of boys with respect to girls is one of the most replicated findings in the field of ASD. The gender ratio for the spectrum. as a whole is consistently reported at around 4:1 M:F (3–6), ranging according to intelligence quotient (approximately 2:1 in ASD with intellectual impairment; up to 11:1 ASD without intellectual impairment) and age (nearly 5:1 in child and adolescents; around 2:1 in adults) (7–9). Actually, amongst epidemiological surveys screening the general population (non-referred samples), those bearing meaningful outcomes have reported a lower male to female ratio, closer to 2–3:1 (10–12). This evidence suggests that, in addition to biological factors, there could be methodological factors influencing recognition in females. Since the beginning, scientific literature has been male-based: Kanner and Asperger's samples were, respectively, prevalently (8 males in 11 children) or exclusively (4 males) composed by male subjects (13, 14); the vast majority of subsequent research was carried out on male samples; in fact, there were few detailed female-oriented studies. Additionally, they took into account only moderate sample sizes and focused especially on adults with a comorbid intellectual disability or on a limited number of domains (15–17).
Among biological factors, recent data support the existence of sex-linked genetic (e.g., X chromosome gene protective effects) and hormonal factors (e.g., prenatal hormones), which would attenuate risk in females and enhance it in males (4, 9, 18–24). Lai (21, 25) hypothesized that females have a higher genetic vulnerability threshold (higher mutational burden) than males but also that environmental and epigenetic factors can modify the vulnerability in both sexes. According to Lai's theory of higher mutational burden, Bourgeron (26) demonstrated a higher amount of copy number variation and single nucleotide polymorphism in female ASD. Concerning epigenetic factors, different studies supported the hypothesis that there may be a link between ASD or ASD traits and steroid hormones abnormalities, including elevated fetal sex steroids, pubertal hormones' imbalance, and other steroid hormones abnormalities (18, 23, 25, 27, 28). In particular, prenatal testosterone and the whole steroidogenic activity predicts, in both sexes, autistic cognitive-behavioral characteristics (29). The prenatal hormonal environment may sex-specifically cause macroglial activation in the developing brain, potentially following maternal immune activation (25, 30, 31). With regard to environmental factors, it is important to highlight how sociocultural factors have an effect on sex/gender brain differences and similarities: neuroplasticity could explain the way the experience drives and shapes brain development during childhood and adolescence. Conversely, gender may influence how individuals express their autism-related characteristics through intrapersonal processes, family, and social interactions.
Neuroimaging studies show that females and males greatly differ in some aspects of brain development, while in other ones they may be quite similar both for TD and ASD subjects. Normative sex differences have been detected both by neuroimaging studies regarding brain size, volume of defined areas (32), corpus callosum size (33), gray and white matter distribution (34, 35), both by functional imaging research regarding brain intrinsic functional organization (36), connectivity (37–39), and tasks involving empathy, emotions, language, and visuo-spatial processing (40, 41). In a paper analyzing brain volumetry, the temporal lobe was found to be larger in ASD than in TD subjects and in male than in female ASD subjects (32). Sex differences were found between ASD and TD subjects and between male and female ASD subjects also in terms of volumetric characteristics of the corpus callosum (33). Findings from a voxel-based morphometry study (35) revealed that gray and white brain patterns in females with ASD significantly resemble neural masculinization; conversely, in males with ASD, they resemble neural feminization. Consistently, connectivity studies show a pattern of hyper intra-hemispheric connectivity in female ASD subjects (more similar to male TD subjects) as well as a pattern of hypo connectivity in male ASD (more similar to female TD subjects) (42). Nevertheless, results in this field are inconclusive and heterogeneous, also considering variability over time and by age cohorts (43, 44), and the research about differences as well as similarities between sexes keeps being a topic of great scientific interest.
Among methodological factors, diagnostic criteria, tests, and professional's expertise could have a role in females' under-diagnosis, since diagnostic and screening tests of ASD are developed on the basis of the male-typical phenotype, and assessment of females is restricted to areas where they are most similar to males. Therefore, those who do not meet the male-typical behavioral descriptions are likely to be missed (9, 17, 20, 25, 45–52).
In this unclear perspective, the role of sex and gender on the phenotypic expression of ASD should be a focus of research, where the term “sex” refers to “a person's biological status,” while the term “gender” refers to “the attitudes, feelings, and behaviors that a given culture associates with a person's biological sex” (53).
This study aimed to investigate the characteristics of the female ASD phenotype in pediatric and adolescent age, recognizing ASD differences and similarities between females and males, as well as clarifying if a male bias in ASD actually exists in available screening and diagnostic tools and/or in DSM-5 diagnostic criteria.
Methods
Participants
Children and adolescents with a diagnosis of ASD were recruited between November 2017 and May 2019 at the Child Neuropsychiatric Unit and at the Adolescence Psychiatric Emergencies Unit of the University of Bari, and they were divided into two groups according to biological sex. In an attempt to create homogeneous groups, we selected the first 100 consecutive patients best matched in age. The final participants included in our analysis were 109, divided into two groups: a group of females (n = 54; age range 3–18) and a group of males (n = 55; age range 3–18). The inclusion criteria were age below 18 years, being diagnosed with level 1 and 2 ASD according to DSM-5 diagnostic criteria, and an intellectual quotient (IQ) higher than 70. All the subjects were diagnosed according to clinical judgement from the expert team and supported by standardized diagnostic tools specific for ASD such as the Autism Diagnostic Observational Schedule, Second Edition (ADOS-2) (54, 55), Autism Diagnostic Interview-Revised (ADI-R) (56), Childhood Autism Rating Scale (CARS) (57), and Michigan Autism Spectrum Questionnaire (MASQ) (58). In order to screen for other psychiatric disorders, we used medical history, clinical observation, and the children behavioral checklist (CBCL) (59).
All procedures performed were in accordance with ethical standards of the local Ethics Committee of the Azienda Ospedaliero-Universitaria Consorziale Policlinico di Bari and with the 1964 Helsinki declaration and its later amendments. Informed consent from parents and assent from children and adolescents were obtained prior to enrollment.
Procedure and Materials
The research team, composed by child and adolescent neuropsychiatrists with expertise in the autism field, collected data from anamnestic interview, clinical, and neuropsychological evaluations.
The profile of ASD-specific features was assessed with a clinical evaluation, including behavioral observation and psychiatric interviews, according to DSM-5 ASD diagnostic criteria, and with the administration of screening questionnaires and diagnostic interviews, depending on the age, to caregivers and patients.
The Autistic Spectrum Quotient Questionnaire [AQ; (60)] measures the level of autistic traits that an individual may possess. This questionnaire has three versions depending on the age of respondents: aged 4 to 11 (Child version), 12 to 15 (Adolescent version); and 16 and older (Adult version). The Adult version is a self-report questionnaire.
The Empathy Quotient Questionnaire [EQ; (61)] measures the cognitive, affective, and behavioral aspects of empathy. This questionnaire has three versions depending on the age of the respondent: aged 4 to 11 (Child version), 12 to 15 (Adolescent version); and 16 and older (Adult version). The Adult version is a self-report questionnaire.
The Systemizing Quotient Questionnaire [SQ; (61)] assesses the interest in systems across a range of different classes of systems. The questionnaire has three versions depending on the age of the respondent: aged 4 to 11 (Child version), 12 to 15 (Adolescent version); and 16 and older (Adult version). The Adult version is a self-report questionnaire.
The Australian Scale for Asperger's Syndrome [ASAS; (62)] is a questionnaire designed to identify behaviors and abilities indicative of Asperger Syndrome (AS) in children and is divided into five domains (social emotional abilities, communication skills, cognitive skills, specific interests, and movement skills). Each domain consists of different questions, having scores ranging from one (if the indicative behavior is rarely present) to six (if the indicative behavior is frequently present). Additionally, ASAS includes a section called “other characteristics” that investigates the presence/absence of atypical sensitivity, stereotypical movements, and language delay. ASAS results do not yield cut-off scores but give important information for clinical assessment. Responses to each question are in the form of “yes” (positive score) if the behavior is present (ASAS's score 4–6) or “not” (not scored) if it is not (ASAS's score 0–3).
The Autism Spectrum Diagnostic Interview [ASDI; (56)] is an investigator-based interview, built around the Gillberg and Gillberg (63) criteria for Asperger syndrome, designed for use by clinicians who want to rapidly determine whether or not an individual patient is likely to meet criteria for a diagnosis of AS or Autism. It comprises six sections: social interactions, narrow interests, routines, language, non-verbal communication, and clumsiness. Each section consists of different questions, whose scores range from one (if the indicative behavior “does not apply”) to three (if the indicative behavior “definitely applies”). The criterion is met when the score indicated for each section is obtained.
The cognitive level was assessed with the Italian version of Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV, in 59 patients) and Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III, in 29 patients); Leiter International Performance Scale Revised-Visualization and Reasoning battery (Leiter-R, in 21 patients) was administered, as an alternative to WISC-IV and WPPSI-III, to subjects with verbal disorders; academic achievement was assessed with the following batteries of tests, validated for the Italian language: MT Group Reading Tests for Primary School; MT Group Reading Tests for Middle School; MT Group Advanced Reading and Mathematics Tests for the first biennium of Secondary School; Battery for the Evaluation of Developmental Dyslexia and Dysorthography for Primary and Middle school; and Evaluation Tests of Calculation Ability for Primary School and Evaluation Tests of Calculation Ability and Problem Solving for Middle School.
Statistical Analysis
Quantitative data were summarized as mean and 95% confidence interval of the mean. To evaluate the difference by sex and level, given the adequate approach to normal distribution, the ANOVA model was applied separately for each quantitative variable (age, intelligence quotient, and AQ, EQ, and SQ scales) as dependent, sex, ASD level, and interaction as effects. Post-hoc comparisons were performed by Tukey test. Results were shown as means estimate by the linear model and its 95% confidence interval.
Qualitative variables were summarized as count and percentage. Answers to ASAS, classified in two categories, were analyzed accounting for multiplicity, therefore comparisons of percentage between the independent male and female groups, stratified by level of ASD, were all performed by Fisher exact test, and p-values were adjusted with a permutational method. The same procedure was used to analyze results and correct p-values for signs and symptoms, school abilities, and comorbidities.
Only adjusted p < 0.05 were considered for statistical significance. Analysis was performed with SAS 9.4. The PROC MULTTEST was applied to account for multiple testing.
Results
Sample Characteristics
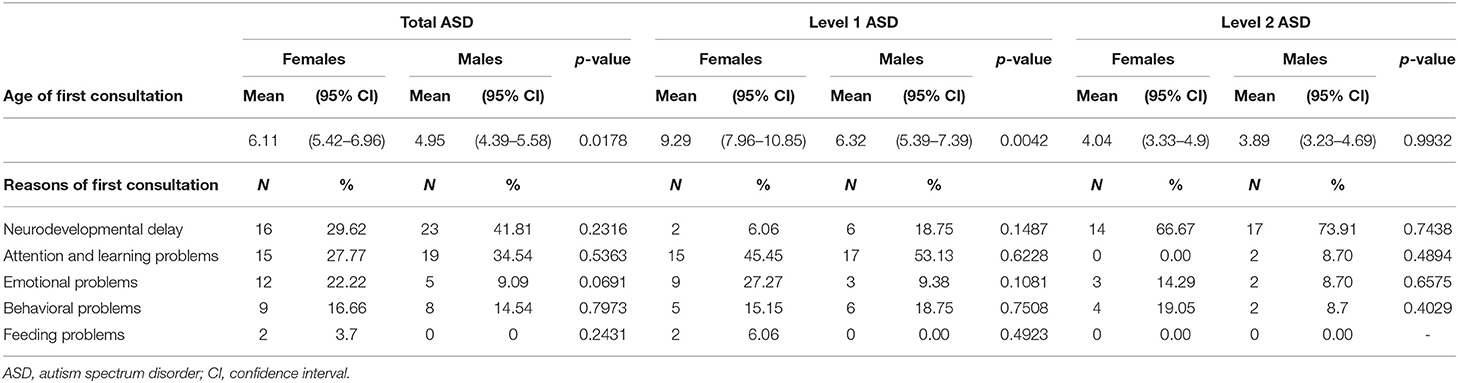
Demographic characteristics of the sample were illustrated, separately for males and females with ASD and for levels 1 and 2 of severity (Table 1).
First Medical Consultation
Age and reason for first medical consultation were collected and analyzed in relation to sex and level of severity (Table 2).
The mean (95%CI) age of the first medical consultation was statistically significantly higher in the female group than in the male group (p = 0.0178), especially in Level 1 (p = 0.0042). Females underwent their first medical consultation about 2 years later in the total sample and 3 years later than males in Level 1.
No significant differences were found in reasons for first medical consultation, even if emotional and feeding problems were higher in the female group (respectively, 22.2 and 3.7%), while neurodevelopmental and attention/learning ones were higher in the male group (respectively, 41.81 and 34.54%).
DSM-5 ASD Diagnostic Criteria
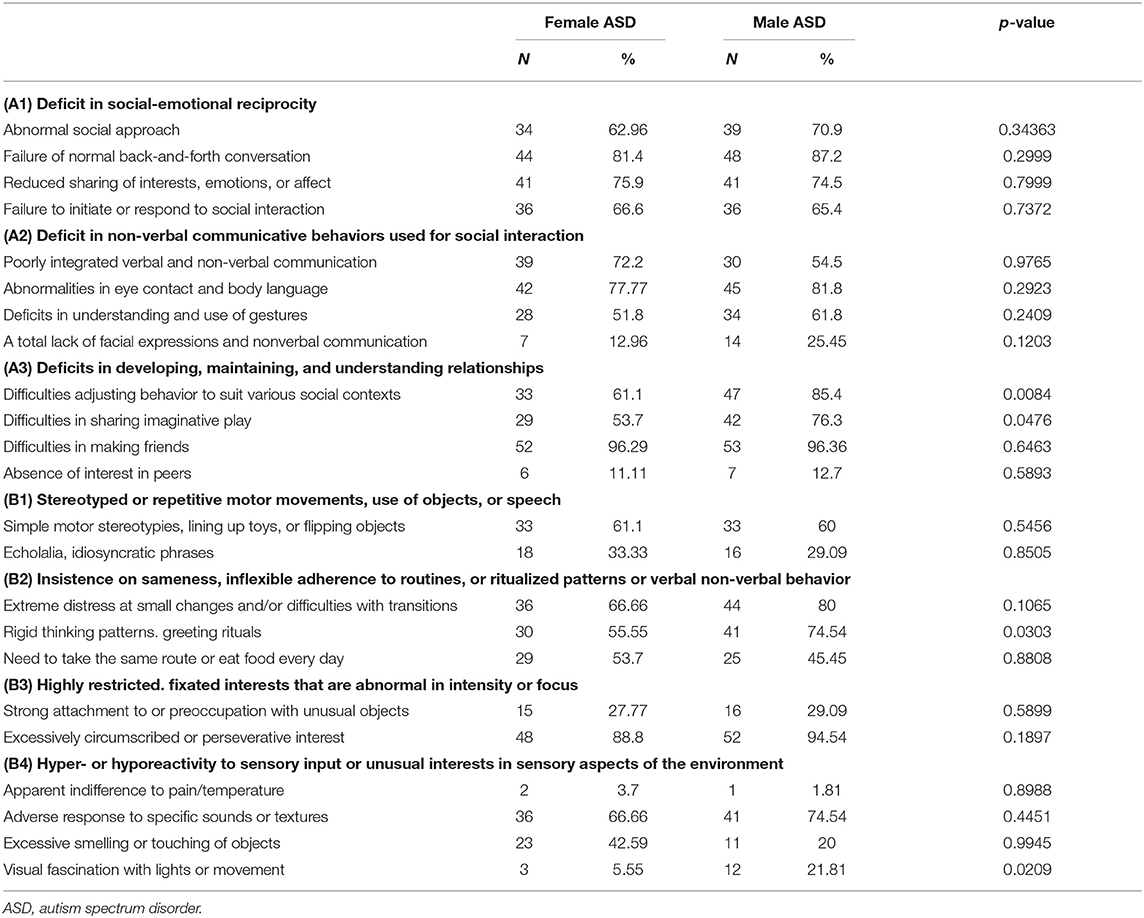
The presence or absence of persistent deficits in social communication and social interaction, as well as restricted, repetitive patterns of behavior, interests, or activities, across multiple contexts, currently or by history, listed as illustrative examples of DSM-5 ASD diagnostic criteria, was collected and analyzed in relation to sex (Table 3).
Concerning social communication and social interaction among female and male ASD, there were no statistically significant differences in social-emotional reciprocity; instead, there were some statistically significant differences in developing, maintaining, and understanding relationships. In particular, the female group was significantly less compromised than the male group in adjusting behavior to suit various social contexts (p = 0.0084) and in sharing imaginative play (p = 0.0476).
Concerning restricted, repetitive patterns of behavior, interests, or activities, the female group proved to be statistically less compromised than the male group in insistences of sameness, rigid thinking patterns, and greeting rituals (p = 0.0303). No statistically significant differences were found in stereotyped or repetitive motor movements, use of objects, speech, and highly restricted and fixated interests. The female group appeared statistically less compromised in visual fascination with light or movement (p = 0.0209).
AQ, EQ, and SQ Scores
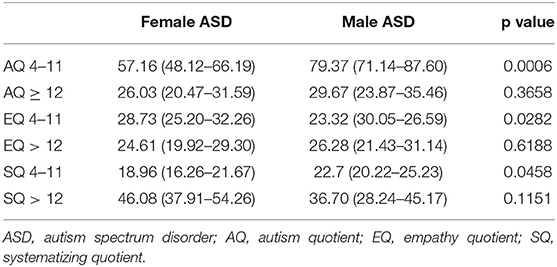
The mean (95%CI) score of AQ-child (p = 0.0006) and SQ-child (p = 0.0458) were statistically significantly higher in the male group than in the female group; on the contrary, the mean (95%CI) score of EQ-child (p = 0.0282) was significantly higher in the female group than in the male group (Table 4). Lateness in acquiring speech is 18.1% in females with ASD and 46% in males with ASD. No statistically significant differences were found in AQ, EQ, and SQ-adolescents. Females did not exceed the cut-off for either AQ-child or AQ-adolescent. The only difference, found in sub-group of the level of severity, was AQ-child in level 2, significantly lower (p = 0.0275) in females (56.6; 95% CI 44.04–69.16) than in males (81.41; 95% CI 69.61–93.21).
ASAS Score
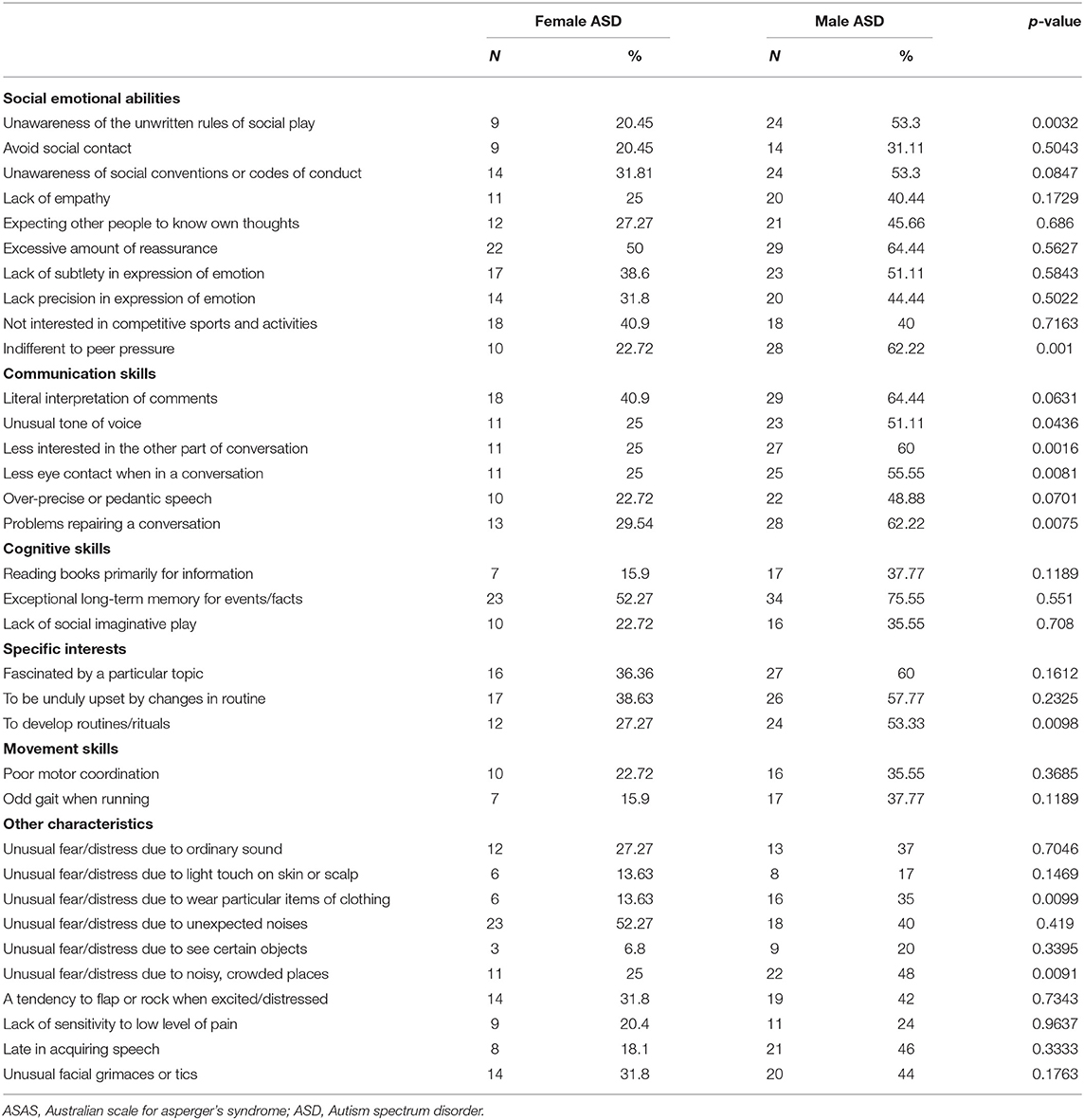
Among social emotional abilities, unawareness of social conventions or codes of conduct (p = 0.0032) and indifference to peer pressure (p = 0.001) were significantly less compromised in the female group than in the male group (Table 5).
Among communication skills, unusual tone of voice (p = 0.0436), interest in another part of the conversation (p = 0.0016), less eye contact (p = 0.0081), and problems reestablishing a conversation (p = 0.0075) were significantly less common in the female group than in the male group.
Among specific interests, developing routines/rituals was significantly lower in the female group than in the male group (p = 0.0098).
With regard to atypical sensitivity, unusual distress for wearing particular items of clothing (p = 0.0099) and too noisy and crowded places (p = 0.0091) were significantly lower in the female group than in the male group.
Considering the differences between females and males of the same level of severity, the significance was prevalently confirmed in level 1, except for rituals and distress in wearing particular clothing. Female ASD appear significantly less compromised in expecting other people to know their own thoughts (F 22.58 vs. M 55.17%, p = 0.0161) and in lack of social imaginative play (F 16.67 vs. M 44.83%, p = 0.0251) exclusively in level 1, but not in the total sample.
ASDI Scores
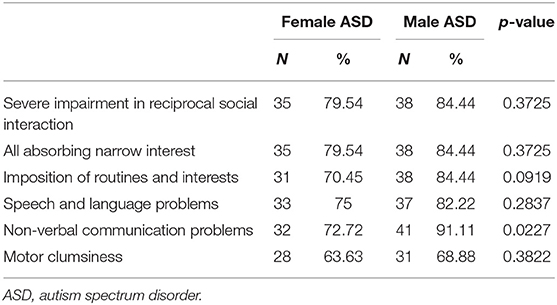
The percentage of females with ASD with “speech and language problems” is 75, whiles is 82.22 for males with ASD (Table 6). Non-verbal communication problems were statistically lower in the female group compared to the male group (p = 0.0227). Considering differences between females and males of the same level of severity, the difference in non-verbal communication was exclusively confirmed in level 1 (F 61.29 vs. M 93.10%, p = 0.023).
Intelligence Quotient
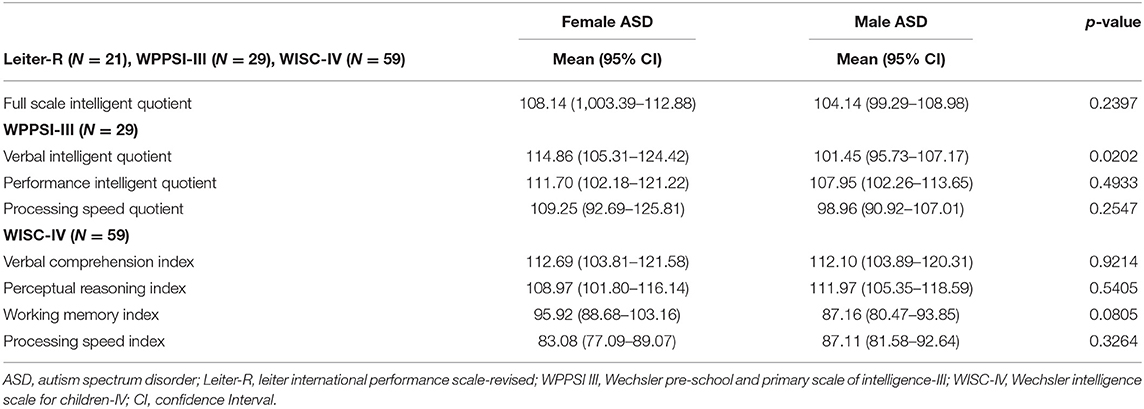
The mean IQ in females with ASD is 108.14, whiles the mean IQ in males with ASD is 104.14, and there were no statistically significant differences (Table 7). There was no significant difference between the two groups in FSIQ and in all the IQ sub-values, except to VIQ of WPPSI-III, which resulted statistically significantly higher in the female group than in the male group (p = 0.0202). Considering differences between females and males with the same level of severity, VIQ of WPPSI-III resulted statistically higher in females than males exclusively in level 2 (F 115.33 v. M 87.00; p = 0.0108).
School Learning Abilities
School learning abilities were compared and analyzed in relation to sex and level of severity (Table 8).
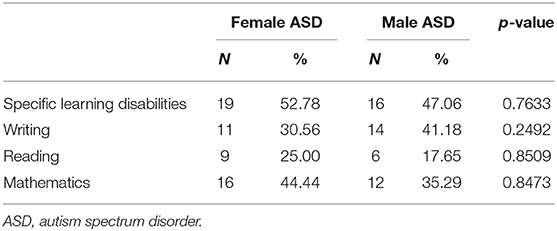
By analyzing the subsample of 70 school-aged subjects, no statistically significant differences were found in learning difficulties between females and males.
Comorbidities
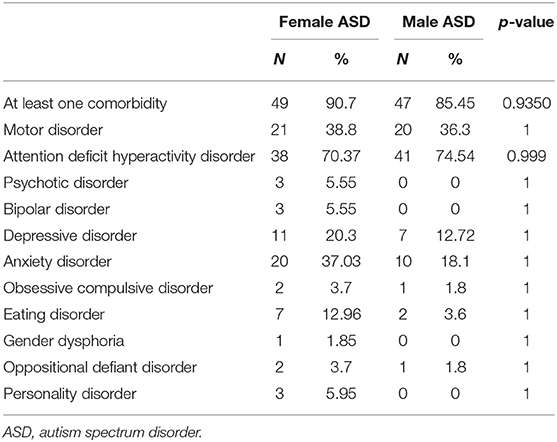
There were no significant differences in comorbidities between the female and male groups, although a non-significant trend was noticed for anxiety, depression, and eating disorders, which were higher in the female group (Table 9).
Discussion
The male bias in ASD conceptualization has led to females with ASD being under-studied.
The poor awareness of female ASD phenotype, especially when at higher functioning levels, is common even amongst professionals, and produces frequent under/misdiagnosis and consequent under-treatment, causing persistent confusion and suffering for female individuals in the autistic spectrum (15, 64–67).
Previous studies about sex/gender differences reported contrasting and not conclusive results, probably due to under-sampling and to a lack of homogeneity for age and IQ in the selected population.
This study attempts to characterize HF female ASD phenotype in pediatric and adolescent age, comparing a group of females with a group of males with a diagnosis of ASD, all without intellectual impairment.
Our aims were, first of all, to evaluate the potential sex/gender differences in clinical phenotype (core autistic domains, cognitive functioning, and co-occurring conditions) and in developmental trajectories between males and females with ASD, and secondly, to examine if a male bias exists in the available tools and in ASD DSM-5 criteria based on matching clinical findings of the expert team and scores obtained from different screening questionnaires and diagnostic interviews.
The presented results have been analyzed based on biological sex. The role of gender identity on clinical expression was not addressed in this study (even more so considering the low average age of the sample). Nevertheless, according to previous research (25), given the complexity in distinguishing the effects of sex and gender on ASD expression, especially during early pediatric age, in this study we used the term “sex/gender” to express the overlap between them.
Social-Communication Abilities
Differences in social-communication abilities between male and female children and adolescents with ASD have been reported in several previous studies, especially within ASD without intellectual impairment (25, 47, 48, 52, 68); conversely, other studies highlighted sex/gender similarities (69–72).
In this study, males and females with ASD, assessed with psychiatric interview according to DSM-5 criteria and illustrative examples, globally presented similar deficits in social communication and interaction, even if females with ASD showed greater capacity in developing, maintaining, and understanding relationships and, in particular, in adjusting behavior to suit various social contexts. This difference was also obtained from parental reports, assessed by the administration of screening questionnaires to caregivers (ASAS) in which results showed that, statistically, females are significantly better in terms of awareness of social conventions or codes of conduct.
Indeed, since Wing's hypothesis (73), until recent literature studies (15, 25, 62, 66, 68, 74–77), these relative strengths in females with ASD could reflect coping mechanism that allows them to camouflage themselves into society. “Camouflaging” is the tendency to cope (consciously or unconsciously mimicking or learning) through conventional and acceptable behaviors and strategies in social situations. Being often good observers, females with ASD could copy strategies from peers to be what the counterpart expects; nevertheless, given the artificiality of this effort, they sometimes could have difficulties understanding different types and grades of relationships, modulating behaviors, and managing conflicts. This exhausting attempt to adapt or copy other one's behaviors can bring to a sensation of losing or not developing their own identity, thus, could increase the risk of stress and unhealthy relationships, as seen from other studies (64, 78, 79).
All these social variables may be difficult for professionals to recognize (even because shyness is considered a typical pattern in cultural ideas of females), so their uncomfortable feelings are often misconstrued.
According to clinicians' reports, there were no statistical differences in “abnormalities in eye contact” between males and females with ASD. According to parents' reports, males with ASD made significantly “less eye contact when in a conversation.” This seemingly conflicting result could be explained by the difference between the wording of the illustrative example in DSM 5 criteria and the one used in questionnaires for parents (ASAS). As a matter of fact, females, rather than avoiding eye contact, often present an excessive eye-fixation (called hyper-fixation) with an abnormal, not-modulated gaze that might be mistaken to an untrained eye, according to previous literature (80, 81). Our findings converge on the idea, previously argued in the literature, that socio-cultural constructions of gender and understandings of ASD may contribute to under- or misrecognition of ASD in females.
Sameness and Sensitivity
In the present study, stereotyped or repetitive motor movements and speech, as well as extremely restricted and repetitive interests, were equally present in males and females; instead, as found from clinical findings as well as screening and diagnostic tools, the inflexible adherence to routines or ritualized patterns were statistically less common in females. This result is in accordance with previous literature which found sex/gender differences of high-functioning population prevalently in the so-called “higher-order RRBs” (that is, complex behaviors, insistence on sameness and routine and in-depth circumscribed interests) rather than in “lower-order RRBs” (that is, simple stereotyped motor movements and speech) which were often equivalent in both sexes/genders, in preschoolers and lowest functioning (70, 82, 83). In addition to the “order of RRBs,” the “type of interests and RRBs” is another point of divergence (69, 82, 84). As emerged from our clinical evaluation, even if not captured by measurement tools, the type of interests in females could appear less autistic-like (i.e., trains, numbers, and videogames), and more similar to those of their neurotypical peers (i.e., dolls, animals, horses, celebrities, fashion, make-up, arts, books, and manga).
Atypical responses to noise, texture, smell, visual stimuli were not included in diagnostic criteria from the previous edition of DSM; they appear in the last version of the Manual, within criterion B, with probable positive implications on females with ASD diagnosis. Previous research reported more sensory symptoms in females with ASD than in males with ASD (52). In the present study, females proved to be less sensitive to lights and, from parents' questionnaires, to noises and particular clothing.
Since the vast majority of available screening and diagnostic scales, including those selected in this study, do not comprise either a focus on interests and stereotypes or a focus on sensitivities (or, in any case, it does not include all the sensitivities), and considering the impact that these aspects could have in ordinary life, clinicians must carefully analyze this aspect throughout oriented questions with the aim to avoid underestimation of these features.
Cognitive Functioning
As for the domain of cognitive functioning, previous investigations have found both similar (85, 86) and divergent (87, 88) cognitive abilities between males and females with ASD. In this study, FSIQ and subitems were not statistically different, except for verbal and language abilities in preschoolers (VIQ from WPPSI-III), resulting in better scores in females. The delay in acquisition of language is more common in males, as seen in clinical experience as well as in this study; this could explain the better verbal competence of preschool females. Moreover, this finding possibly reflects the sex difference also observed in language for neurotypically developing males and females (16).
Comorbidities and Developmental Trajectories
According to recent literature (89–91), in the present study, both males and females presented a high percentage of co-occurring conditions (between 85.45 and 90.7 %). The most frequent comorbidity in both groups was ADHD, co-occurring in about 70% of cases, without significant differences between males and females. This evidence differs from previous literature in which ADHD comorbidity appeared more common in males (92, 93). In accordance with other authors, we hypothesized that the diagnosis of ADHD was underrated in females since females with ADHD have different, more hidden, symptomatology, showing inattentive rather than hyperactive manifestations (16, 94). Concerning internalizing disorders, our findings were different from previous literature reporting a statistically significant prevalence of internalizing disorders in females, in that we rather found a non-significant trend for anxiety, depressive, and eating disorders in the female group (95–100). The mean age of the sample (10 years old) could justify these findings since internalizing and feeding disorders often occur in adolescence or adulthood. In this study we found that caregivers ask for a first specialist medical consultation at different ages and for different reasons: females were referred later to clinical services, particularly for level 1 ASD (on average 3 years later). This finding fits with previous literature (7, 25, 67, 101, 102), also showing how females who received an early diagnosis of ASD often have intellectual impairment.
Diagnostic Tools and DSM-5 Criteria
Scores from screening and diagnostic tools, selected in this study, reveal some inconsistencies between the results obtained from questionnaires administered to parents (ASAS and Baron Cohen Questionnaires, i.e., the AQ, EQ, and SQ), showing greater differences between males and females with ASD, and interviews carried out by clinicians (according to DSM-5 criteria and ASDI), showing more homogeneous results. These inconsistencies could be explained by the different perceptions of clinicians in respect to parents who may underestimate or not recognize some characteristics as atypical.
In the present study, higher scores from AQ and SQ and lower ones from EQ were found in male children, with a reduction of this gap in adolescence. Population surveys on autistic traits confirmed that males have more autistic traits than females measured by Baron Cohen Questionnaires (AQ, EQ, and SQ), with attenuation in adulthood (103). Even if it is not a focus of this study, the adult attenuation of sex/gender differences could be linked with a greater insight of females growing up, as well as a decreased number of adult males that received the diagnosis. Moreover, finding specific female items or examples could be challenging, even in Baron Cohen Questionnaires.
In this study, applying examples listed in DSM-5 criteria, no sex differences were found in the broad social-communicative and sameness criteria; nonetheless, numerous differences were evident in how boys and girls come to meet each criterion. Specific sex/gender examples could be included in diagnostic criteria to detect these qualitative differences.
Conclusion
Results suggest that there are subtle, yet potentially meaningful, quantitative and qualitative ASD phenotypic differences between males and females that common screening tests are not always sensitive enough to recognize. Thus, a sex/gender disparity, in terms of identification and support, persists, above all within high functioning ASD. It might result in HFA females not receiving much-needed diagnosis and treatment, with a negative effect on their mental health and well-being. In order to prevent the risk of late- or misdiagnosis, diagnostic criteria, and tools could benefit from the addition of specifiers and female-oriented examples. In this way, all the clinicians, even with different expertise, could benefit from more sensitive measures to detect females' characteristics. This could have a positive impact on the diagnostic process, health services, and research paths.
Limitation and Future Direction
This study has two levels of limitations: the lack of a female-male neurotypical control group and the wide age range.
The absence of a control group might overrate some sex/gender differences as specific to ASD instead of normative sex differences. One of these could be females' better communicative development with respect to males, which is also common in the neurotypical population.
Having a wide age range (3–18 years), the sample does not allow consideration of sex/gender differences in infancy and adolescence. Previous findings show that sex/gender differences are less evident in toddlerhood and more evident in adolescence, both in core features and in comorbidities (16, 104). In our study, these age-related differences are just mentioned but not examined in depth. For these reasons, drawing sums on a limited sample of patients can be speculative.
Future directions should involve enrolling a larger sample, exploring sex/gender differences matching with TD subjects, and differentiating for age. Moreover, it could be useful to develop and test measures (illustrative examples and tools) that capture female as well as male manifestations of ASD.
Data Availability Statement
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to Concetta de Giambattista, concettadegiambattista@gmail.com.
Ethics Statement
All procedures performed were in accordance with ethical standards of and approved by the Ethics Committee of the Azienda Ospedaliero-Universitaria Consorziale Policlinico di Bari and with the 1964 Helsinki declaration and its later amendments. Written informed consent from parents and assent from children and adolescents were obtained prior to enrollment.
Author Contributions
CdG and PV equally contributed in designing the study. LM coordinated the study. CdG, PV, and FM participated in data collection. PT performed data analysis. All authors participated in data interpretation and helped to draft with a comorbid intellectual the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Association, DSM-5 (2013). doi: 10.1176/appi.books.9780890425596
2. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. (2018) 67:1–23. doi: 10.15585/mmwr.ss6706a1
3. Idring S, Lundberg M, Sturm H, Dalman C, Gumpert C, Rai D, et al. Changes in prevalence of autism spectrum disorders in 2001-2011: findings from the stockholm youth cohort. J Autism Dev Disord. (2014) 45:1766–73. doi: 10.1007/s10803-014-2336-y
4. Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. (2013) 26:146–53. doi: 10.1097/WCO.0b013e32835ee548
5. Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. (2011) 68:459–65. doi: 10.1001/archgenpsychiatry.2011.38
6. Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. (2009) 65:591–8. doi: 10.1203/PDR.0b013e31819e7203
7. Rutherford M, McKenzie K, Johnson T, Catchpole C, O'Hare A, McClure I, et al. Gender ratio in a clinical population sample, age of diagnosis and duration of assessment in children and adults with autism spectrum disorder. Autism. (2016) 20:628–34. doi: 10.1177/1362361315617879
8. Dworzynski K, Ronald A, Bolton P, Happ, é F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry. (2012) 51:788–97. doi: 10.1016/j.jaac.2012.05.018
9. Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the simons simplex collection. J Am Acad Child Adolesc Psychiatry. (2014) 53:329–40.e1–3. doi: 10.1016/j.jaac.2013.12.004
10. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. (2017) 56:466–74. doi: 10.1016/j.jaac.2017.03.013
11. Zwaigenbaum L, Bryson SE, Szatmari P, Brian J, Smith IM, Roberts W, et al. Sex differences in children with autism spectrum disorder identified within a high-risk infant cohort. J Autism Dev Disord. (2012) 42:2585–96. doi: 10.1007/s10803-012-1515-y
12. Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. (2011) 168:904–12. doi: 10.1176/appi.ajp.2011.10101532
14. Asperger H. Die autistisehen psychopathen im kindesalter. Archiv für Psychiatrie und Nervenkrankheit. (1944) 117:76–136. doi: 10.1007/BF01837709
15. Milner V, McIntosh H, Colvert E, Happé F. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J Autism Dev Disord. (2019) 49:2389–402. doi: 10.1007/s10803-019-03906-4
16. Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, et al. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol Autism. (2015) 6:36. doi: 10.1186/s13229-015-0019-y
17. Kirkovski M, Enticott PG, Fitzgerald PB. A review of the role of female gender in autism spectrum disorders. J Autism Dev Disord. (2013) 43:2584–603. doi: 10.1007/s10803-013-1811-1
18. Cherskov A, Pohl A, Allison C, Zhang H, Payne RA, Baron-Cohen S. Polycystic ovary syndrome and autism: a test of the prenatal sex steroid theory. Transl Psychiatry. (2018) 8:136. doi: 10.1038/s41398-018-0186-7
19. Ferri SL, Abel T, Brodkin ES. Sex differences in autism spectrum disorder: a review. Curr Psychiatry Rep. (2018) 20:9. doi: 10.1007/s11920-018-0874-2
20. Beggiato A, Peyre H, Maruani A, Scheid I, Rastam M, Amsellem F, et al. Gender differences in autism spectrum disorders: divergence among specific core symptoms. Autism Res. (2016) 10:680–9. doi: 10.1002/aur.1715
21. Lai MC, Lerch JP, Floris DL, Ruigrok AN, Pohl A, Baron-Cohen S, et al. Imaging sex/gender and autism in the brain: etiological implications. J Neurosci Res. (2017) 95:380–97. doi: 10.1002/jnr.23948
22. Singer L. Thoughts about sex and gender differences from the next generation of autism scientists. Mol Autism. (2015) 6:52. doi: 10.1186/s13229-015-0046-8
23. Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. (2011) 9:e1001081. doi: 10.1371/journal.pbio.1001081
24. Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. (2007) 176:170–86. doi: 10.1016/j.bbr.2006.08.025
25. Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry. (2015) 54:11–24. doi: 10.1016/j.jaac.2014.10.003
26. Bourgeron T. Current knowledge on the genetics of autism and propositions for future research. Comptes Rendus Biol. (2016) 339:300–7. doi: 10.1016/j.crvi.2016.05.004
27. Gillberg C, Fernell E, Kočovská E, Minnis H, Bourgeron T, Thompson L, et al. The role of cholesterol metabolism and various steroid abnormalities in autism spectrum disorders: a hypothesis paper. Autism Res. (2017) 10:1022–44. doi: 10.1002/aur.1777
28. Baron-Cohen S, Tsompanidis A, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah M, et al. Foetal oestrogens and autism. Mol Psychiatry. (2020) 25:2970–8. doi: 10.1038/s41380-019-0454-9
29. Auyeung B, Lombardo MV, Baron-Cohen S. Prenatal and postnatal hormone effects on the human brain and cognition. Pflugers Arch. (2013) 465:557–71. doi: 10.1007/s00424-013-1268-2
30. Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J. Neurosci. (2013) 33:2761–72. doi: 10.1523/JNEUROSCI.1268-12.2013
31. Suzuki K, Sugihara G, Ouchi Y. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry. (2013) 70:49–58. doi: 10.1001/jamapsychiatry.2013.272
32. Di X, Biswal BB. Similarly expanded bilateral temporal lobe volumes in female and male children with autism spectrum disorder. Biol. Psychiatry. (2016) 1:178–85. doi: 10.1016/j.bpsc.2015.11.006
33. Nordahl CW, Iosif AM, Young GS, Perry LM, Dougherty R, Lee A, et al. Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Mol Autism. (2015) 6:26. doi: 10.1186/s13229-015-0005-4
34. Kirkovski M, Fuelscher I, Hyde C, Donaldson PH, Ford TC, Rossell SL, et al. Fixel based analysis reveals atypical white matter micro- and macrostructure in adults with autism spectrum disorder: an investigation of the role of biological sex. Front Integrat Neurosci. (2020) 14:40. doi: 10.3389/fnint.2020.00040
35. Lai MC, Lombardo MV, Suckling J, Ruigrok AN, Chakrabarti B, Ecker C, et al. Biological sex affects the neurobiology of autism. Brain. (2013) 136 (Pt. 9):2799–815. doi: 10.1093/brain/awt216
36. Porcelli S, Van Der Wee N, van der Werff S, Aghajani M, Glennon JC, van Heukelum S, et al. Social brain, social dysfunction and social withdrawal. Neurosci Biobehav Rev. (2019) 97:10–33. doi: 10.1016/j.neubiorev.2018.09.012
37. Floris DL, Lai M, Nath T, Milham MP, Di Martino. A. Network-specific sex differentiation of intrinsic brain function in males with autism. Mol Autism. (2018) 9:17. doi: 10.1186/s13229-018-0192-x
38. Irimia A, Torgerson CM, Jacokes ZJ, Van Horn JD. The connectomes of males and females with autism spectrum disorder have significantly different white matter connectivity densities. Sci Rep. (2017) 7:46401. doi: 10.1038/srep46401
39. Alaerts K, Swinnen SP, Wenderoth N. Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Soc Cogn Affect Neurosci. (2016) 11:1002–16. doi: 10.1093/scan/nsw027
40. Holt RJ, Chura LR, Lai MC, Suckling J, von dem Hagen E, Calder AJ, et al. 'Reading the Mind in the Eyes': an fMRI study of adolescents with autism and their siblings. Psychol Med. (2014) 44:3215–27. doi: 10.1017/S0033291714000233
41. Kaiser A, Haller S, Schmitz S, Nitsch C. On sex/gender related similarities and differences in fMRI language research. Brain Res Rev. (2009) 61:49–59. doi: 10.1016/j.brainresrev.2009.03.005
42. Chen S, Xing Y, Kang J. Latent and abnormal functional connectivity circuits in autism spectrum disorder. Front Neurosci. (2017) 11:125. doi: 10.3389/fnins.2017.00125
43. Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. (2005) 58:1–9. doi: 10.1016/j.biopsych.2005.03.026
44. Pua EPK, Bowden SC, Seal ML. Autism spectrum disorders: neuroimaging findings from systematic reviews. Res Autism Spectr Disord. (2017) 34:28–33. doi: 10.1016/j.rasd.2016.11.005
45. Tillmann J, Ashwood K, Absoud M, Bölte S, Bonnet-Brilhault F, Buitelaar JK, et al. Evaluating sex and age differences in ADI-R and ADOS scores in a large European multi-site sample of individuals with autism spectrum disorder. J Autism Dev Disord. (2018) 48:2490–505. doi: 10.1007/s10803-018-3510-4
46. Duvekot J, van der Ende J, Verhulst FC, Slappendel G, van Daalen E, Maras A, et al. Factors influencing the probability of a diagnosis of autism spectrum disorder in girls versus boys. Autism. (2017) 21:646–58. doi: 10.1177/1362361316672178
47. Hiller RM, Young RL, Weber N. Sex differences in autism spectrum disorder based on DSM-5 criteria: evidence from clinician and teacher reporting. J Abnorm Child Psychol. (2014) 42:1381–93. doi: 10.1007/s10802-014-9881-x
48. Head AM, McGillvray JA, Stokes MA. Gender differences in emotionality and sociability in children with autism spectrum disorders. Mol Autism. (2014) 5:19. doi: 10.1186/2040-2392-5-19
49. Kreiser NL, White SW. ASD in females: are we overstating the gender difference in diagnosis? Clin Child Fam Psychol Rev. (2014) 17:67–84. doi: 10.1007/s10567-013-0148-9
50. Anderson GW, Gillberg C, Miniscalco C. Pre-school children with suspected autism spectrum disorders: do girls and boys have the same profiles? Res Dev Disabil. (2013) 34:413–22. doi: 10.1016/j.ridd.2012.08.025
51. Goldman S. Opinion: sex, gender and the diagnosis of autism — a biosocial view of the male preponderance. Res Autism Spectr Disord. (2013) 7:675–9. doi: 10.1016/j.rasd.2013.02.006
52. Lai MC, Lombardo MV, Pasco G. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS ONE. (2011) 6:e20835. doi: 10.1371/journal.pone.0020835
53. American Psychological Association. Guidelines for psychological practice with lesbian, gay, bisexual clients. Am Psychol. (2012) 67:10–42. doi: 10.1037/a0024659
54. Lord C, Luyster RJ, Gotham K, Guthrie W. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part II): Toddler Module. Torrance, CA: Western Psychological Services (2012). doi: 10.1007/978-1-4419-1698-3_2011
55. Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised Manual. Los Angeles, CA: Western Psychological Services (2003).
56. Gillberg C, Gillberg C, Råstam M, Wentz E. The asperger syndrome (and high-functioning autism) diagnostic interview (ASDI): a preliminary study of a new structured clinical interview. Autism. (2001) 5:57–66. doi: 10.1177/1362361301005001006
57. Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: childhood autism rating scale (CARS). J Autism Dev Disord. (1980) 10:91–103. doi: 10.1007/BF02408436
58. Ghaziuddin M, Welch K. The michigan autism spectrum questionnaire: a rating scale for high-functioning autism spectrum disorders. Autism Res Treat. (2013) 2013:1–5. doi: 10.1155/2013/708273
59. Achenbach TM. The child behavior checklist and related instruments. In Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Burlington: Lawrence Erlbaum Associates Publishers (1999). p. 429–466.
60. Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. (2001) 31:5–17. doi: 10.1023/a:1005653411471
61. Auyeung B, Wheelwright S, Allison C, Atkinson M, Samarawickrema N, Baron-Cohen S. The children's empathy quotient and systemizing quotient: sex differences in typical development and in autism spectrum conditions. J Autism Dev Disord. (2009) 39:1509–21. doi: 10.1007/s10803-009-0772-x
62. Attwood T. The Complete Guide to Asperger's Syndrome. Philadelphia, PA: Jessica Kingsley Publishers (2006).
63. Gillberg IC, Gillberg C. Asperger syndrome: some epidemiological considerations: a research note. J Child Psychol Psychiatry. (1989) 30:631–8. doi: 10.1111/j.1469-7610.1989.tb00275.x
64. Bargiela S, Steward R, Mandy W. The experiences of late-diagnosed women with autism spectrum conditions: an investigation of the female autism phenotype. J Autism Dev Disord. (2016) 46:3281–94. doi: 10.1007/s10803-016-2872-8
65. Haney JL. Autism, females, and the DSM-5: gender bias in autism diagnosis. Soc. Work Ment Health. (2016) 14:396–407. doi: 10.1080/15332985.2015.1031858
66. Gould J, Ashton-Smith J. Missed diagnosis or misdiagnosis? Girls and women on the autism spectrum. Good Autism Pract. (2011) 12:34–41.
67. Giarelli E, Wiggins LD, Rice CE, Levy SE, Kirby RS, Pinto-Martin J, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. (2010) 3:107–16. doi: 10.1016/j.dhjo.2009.07.001
68. Sedgewick F, Hill V, Yates R, Pickering L, Pellicano E. Gender differences in the social motivation and friendship experiences of autistic and non-autistic adolescents. J Autism Dev Disord. (2016) 46:1297–306. doi: 10.1007/s10803-015-2669-1
69. Rubenstein E, Wiggins LD, Lee LC. A review of the differences in developmental, psychiatric, and medical endophenotypes between males and females with autism spectrum disorder. J Dev Phys Disabil. (2015) 27:119–39. doi: 10.1007/s10882-014-9397-x
70. Van Wijngaarden-Cremers PJ, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, Van der Gaag RJ. Gender and age differences in the core triad of impairments in autism spectrum disorders: a systematic review and meta-analysis. J Autism Dev Disord. (2014) 44:627–35. doi: 10.1007/s10803-013-1913-9
71. Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. J Autism Dev Disord. (2012) 42:1304–13. doi: 10.1007/s10803-011-1356-0
72. Holtmann M, Bölte S, Poustka F. Autism spectrum disorders: sex differences in autistic behaviour domains and coexisting psychopathology. Dev Med Child Neurol. (2007) 49:361–6. doi: 10.1111/j.1469-8749.2007.00361.x
73. Wing L. Sex ratios in early childhood autism and related conditions. Psychiatry Research. (1981) 5:129–37. doi: 10.1016/0165-1781(81)90043-3
74. Hull L, Mandy W, Lai MC, Baron-Cohen S, Allison C, Smith P, et al. Development and validation of the camouflaging autistic traits questionnaire (CAT-Q). J Autism Dev Disord. (2019) 49:819–33. doi: 10.1007/s10803-018-3792-6
75. Dean M, Harwood R, Kasari C. The art of camouflage: gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism. (2017) 21:678–89. doi: 10.1177/1362361316671845
76. Hull L, Petrides KV, Allison C, Smith P, Baron-Cohen S, Lai MC, et al. “Putting on my best normal”: social camouflaging in adults with autism spectrum conditions. J Autism Dev Disord. (2017) 47:2519–34. doi: 10.1007/s10803-017-3166-5
77. Rynkiewicz A, Schuller B, Marchi E, Piana S, Camurri A, Baron-Cohen S, et al. An investigation of the “female camouflage effect” in autism using a computerized ADOS-2 and a test of sex/gender differences. Mol Autism. (2016) 7:10. doi: 10.1186/s13229-016-0073-0
78. Cage E, Troxell-Whitman Z. Understanding the reasons, contexts and costs of camouflaging for autistic adults. J Autism Dev Disord. (2019) 49:1899–911. doi: 10.1007/s10803-018-03878-x
79. Baldwin S, Costley D. The experiences and needs of female adults with high-functioning autism spectrum disorder. Autism. (2016) 20:483–95. doi: 10.1177/1362361315590805
80. Whyte EM, Scherf KS. Gaze following is related to the broader autism phenotype in a sex-specific way: building the case for distinct male and female autism phenotypes. Clin Psychol Sci. (2018) 6:280–7. doi: 10.1177/2167702617738380
81. Matsuyoshi D, Kuraguchi K, Tanaka Y, Uchida S, Ashida H, Watanabe K. Individual differences in autistic traits predict the perception of direct gaze for males, but not for females. Mol Autism. (2014) 5:12. doi: 10.1186/2040-2392-5-12
82. McFayden TC, Albright J, Muskett AE, Scarpa A. Brief report: sex differences in ASD diagnosis-a brief report on restricted interests and repetitive behaviors. J Autism Dev Disord. (2019) 49:1693–9. doi: 10.1007/s10803-018-3838-9
83. Reinhardt VP, Wetherby AM, Schatschneider C, Lord C. Examination of sex differences in a large sample of young children with autism spectrum disorder and typical development. J Autism Dev Disord. (2015) 45:697–706. doi: 10.1007/s10803-014-2223-6
84. Harrop C, Shire S, Gulsrud A, Chang YC, Ishijima E, Lawton K, et al. Does gender influence core deficits in ASD? An investigation into RRBs in girls and boys with ASD. J Autism Dev Disord. (2015) 45:766–77. doi: 10.1007/s10803-014-2234-3
85. Duvall SW, Huang-Storms L, Presmanes Hill A, Myers J, Fombonne E. No sex differences in cognitive ability in young children with autism spectrum disorder. J Autism Dev Disord. (2019) 27:1770–85. doi: 10.1007/s10803-019-03933-1
86. Hull L, Mandy W, Petrides KV. Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism. (2017) 21:706–27. doi: 10.1177/1362361316669087
87. Frazier TW, Hardan AY. Equivalence of symptom dimensions in females and males with autism. Autism. (2017) 21:749–59. doi: 10.1177/1362361316660066
88. Lai MC, Lombardo MV, Ruigrok AN, Chakrabarti B, Wheelwright, Baron-Cohen S, et al. Cognition in males and females with autism: similarities and differences. PLoS ONE. (2012) 7:e47198. doi: 10.1371/journal.pone.0047198
89. Margari L, Palumbi R, Peschechera A, Craig F, de Giambattista C, Ventura P, et al. Sex-Gender comparisons in comorbidities of children and adolescents with high-functioning autism spectrum disorder. Front Psychiatry. (2019) 10:159. doi: 10.3389/fpsyt.2019.00159
90. Lever AG, Geurts HM. Psychiatric co-occurring symptoms and disorders in young, middle-aged, and older adults with autism spectrum disorder. J Autism Dev Disord. (2016) 46:1916–1930. doi: 10.1007/s10803-016-2722-8
91. Solomon M, Miller M, Taylor SL, Hinshaw SP, Carter CS. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. J Autism Dev Disord. (2012) 42:48–59. doi: 10.1007/s10803-011-1215-z
92. Charman T, Loth E, Tillmann J, Crawley D, Wooldridge C, Goyard D, et al. The EU-AIMS longitudinal european autism project (LEAP): clinical characterization. Mol Autism. (2017) 23:8:27. doi: 10.1186/s13229-017-0145-9
93. May T, Cornish K, Rinehart N. Does gender matter? A one-year follow-up of autistic, attention and anxiety symptoms in high-functioning children with autism spectrum disorder. J Autism Dev Disord. (2014) 44:1077–86. doi: 10.1007/s10803-013-1964-y
94. Antshel KM, Russo N. Autism spectrum disorders and adhd: overlapping phenomenology, diagnostic issues, treatment considerations. Curr Psychiatry Rep. (2019) 22:34. doi: 10.1007/s11920-019-1020-5
95. Westwood H, Mandy W, Tchanturia K. Clinical evaluation of autistic symptoms in women with anorexia nervosa. Mol Autism. (2017) 8:12. doi: 10.1186/s13229-017-0128-x
96. Treasure JL. Getting beneath the phenotype of anorexia nervosa: the search for viable endophenotypes and genotypes. Can J Psychiatry. (2007) 52:212–9. doi: 10.1177/070674370705200402
97. Oswald TM, Winter-Messiers MA, Gibson B, Schmidt AM, Herr CM, Solomon M. Sex differences in internalizing problems during adolescence in autism spectrum disorder. J Autism Dev Disord. (2016) 46:624–36. doi: 10.1007/s10803-015-2608-1
98. Dudova I, Kocourkova J, Koutek J. Early-onset anorexia nervosa in girls with Asperger syndrome. Neuropsychiatr Dis Treats. (2015) 11:1639–43. doi: 10.2147/NDT.S83831
99. Matson JL, Cervantes PE. Commonly studied comorbid psychopathologies among persons with autism spectrum disorder. Res Dev Disabil. (2014) 35:952–62. doi: 10.1016/j.ridd.2014.02.012
100. Karjalainen L, Råstam M, Paulson-Karlsson G, Wentz E. Do autism spectrum disorder and anorexia nervosa have some eating disturbances in common? Eur Child Adolesc Psychiatry. (2019) 28:69–78. doi: 10.1007/s00787-018-1188-y
101. Begeer S, Mandell D. Wijnker-Holmes,B. Sex differences in the timing of identification among children and adults with autism spectrum disorders. J Autism Dev Disord. (2013) 43:1151–6. doi: 10.1007/s10803-012-1656-z
102. Shattuck PT, Durkin M, Maenner M. Timing of identification among children with an autism spectrum disorder: findings from a population-based surveillance study. J Am Acad Child Adolesc Psychiatry. (2009) 48:474–83. doi: 10.1097/CHI.0b013e31819b3848
103. Baron-Cohen S, Cassidy S, Auyeung B. Attenuation of typical sex differences in 800 adults with autism vs. 3900 controls. PLoS ONE. (2014) 9:e102251. doi: 10.1371/journal.pone.0102251
Keywords: autism spectrum disorder, high functioning, female, gender, sex
Citation: de Giambattista C, Ventura P, Trerotoli P, Margari F and Margari L (2021) Sex Differences in Autism Spectrum Disorder: Focus on High Functioning Children and Adolescents. Front. Psychiatry 12:539835. doi: 10.3389/fpsyt.2021.539835
Received: 02 March 2020; Accepted: 03 June 2021;
Published: 09 July 2021.
Edited by:
Kerim Munir, Boston Children's Hospital, United StatesReviewed by:
Gabriele Masi, University of Pisa, ItalyMelissa Kirkovski, Deakin University, Australia
Copyright © 2021 de Giambattista, Ventura, Trerotoli, Margari and Margari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Margari, lucia.margari@uniba.it
 Concetta de Giambattista1
Concetta de Giambattista1 Paolo Trerotoli
Paolo Trerotoli Francesco Margari
Francesco Margari Lucia Margari
Lucia Margari