- 1Red Blood Cell Research Group, Vetsuisse Faculty, The Zurich Center for Integrative Human Physiology (ZHIP), Institute of Veterinary Physiology, University of Zurich, Zurich, Switzerland
- 2Experimental Physics, Dynamics of Fluids, Faculty of Natural Sciences and Technology, Saarland University, Saarbrücken, Germany
- 3Theoretical Medicine and Biosciences, Medical Faculty, Saarland University, Homburg, Germany
- 4Institute for Clinical and Experimental Surgery, Saarland University, Homburg, Germany
- 5Section of Hematology/Oncology, Department of Medicine, University of Chicago, Chicago, IL, United States
Mean values of hematological parameters are currently used in the clinical laboratory settings to characterize red blood cell properties. Those include red blood cell indices, osmotic fragility test, eosin 5-maleimide (EMA) test, and deformability assessment using ektacytometry to name a few. Diagnosis of hereditary red blood cell disorders is complemented by identification of mutations in distinct genes that are recognized “molecular causes of disease.” The power of these measurements is clinically well-established. However, the evidence is growing that the available information is not enough to understand the determinants of severity of diseases and heterogeneity in manifestation of pathologies such as hereditary hemolytic anemias. This review focuses on an alternative approach to assess red blood cell properties based on heterogeneity of red blood cells and characterization of fractions of cells with similar properties such as density, hydration, membrane loss, redox state, Ca2+ levels, and morphology. Methodological approaches to detect variance of red blood cell properties will be presented. Causes of red blood cell heterogeneity include cell age, environmental stress as well as shear and metabolic stress, and multiple other factors. Heterogeneity of red blood cell properties is also promoted by pathological conditions that are not limited to the red blood cells disorders, but inflammatory state, metabolic diseases and cancer. Therapeutic interventions such as splenectomy and transfusion as well as drug administration also impact the variance in red blood cell properties. Based on the overview of the studies in this area, the possible applications of heterogeneity in red blood cell properties as prognostic and diagnostic marker commenting on the power and selectivity of such markers are discussed.
Introduction
Our understanding of red blood cells (RBCs) evolved from acknowledgment of the basic and fundamental role of these cells as key players in gas exchange to the state where we assign multiple complex functions related to sensing and signaling, maintenance of homeostasis of pH and redox state and participation in control of vascular tone, clotting (Andrews and Low, 1999; Bernhardt et al., 2019), and other processes (Helms et al., 2018; Pernow et al., 2019).
Broadening of RBC functions was accompanied with our awareness of complexity of the cellular architecture and biochemistry. Spatial compartmentalization of processes and resources in RBCs was discovered (Hoffman et al., 2009; Chu et al., 2012). Complex dynamics precise orchestration of processes occurring in the circulating RBCs in response to the changes in micro- and macro-environment (hormonal and mechanical stimulation, changes in local or ambient oxygen availability, temperature, circadian rhythm-related processes and others) is becoming evident (e.g., O’Neill and Reddy, 2011; Cahalan et al., 2015; Zhou et al., 2019).
With time it became clear that these changes and responses do not necessarily involve all the circulating cells. As our knowledge of these cells accumulates more and more reports mention the presence of “responding” and “non-responding” cells in the circulation (e.g., Kaestner et al., 2012; Makhro et al., 2013; Wang et al., 2013; Rotordam et al., 2019). As we recognize the existence of multiple fractions of RBCs that are functionally different from each other, we feel a growing need to unravel the nature of these differences, their causes and the potential information hidden in RBC heterogeneity on systemic distress and pathology. In this review we aimed to summarize the current state of knowledge in this rapidly developing research area. We focus on RBCs of healthy humans and give only a few examples of how RBC heterogeneity may be used to predict RBC disease nature and severity. Heterogeneity of stored or transfused RBCs is a broad topic also out of the scope of this review.
Inter-Individual Heterogeneity
Inter-individual variation in properties of circulating RBCs of healthy donors reflects genetic and epigenetic variance as well as the state in which the organism resides over the past 3–4 months during which the cells undergo transitions from erythroid progenitors to young, mature, and senescent state. Variance spreads to the number of copies of proteins per cell, activity of enzymes and ion transporters, shapes, differences in density, deformability, membrane stability, redox state, and the collection of Hb variants in a given cell. Most of the studies for healthy humans were performed on stored blood to assess its quality and identify a cohort of best donors (Sparrow, 2017).
The possible causes of this inter-individual variation originate at the level of erythroid precursor cells (as in case of ineffective erythropoiesis (Oikonomidou and Rivella, 2018), for more details see section Cellular Heterogeneity During Erythropoiesis) or emerge later on as the cells enter the circulation and get exposed to a variety of microenvironments (osmolarity gradients in the kidneys, shear in capillaries and spleen, changes in oxygen availability and pH within peripheral tissues, changes in redox state next to the inflammatory side or to the exercising muscle). Development of heterogeneous RBC populations may be an intrinsic property of blood (e.g., RBC aging), or be triggered by the changes in life style or environmental conditions (e.g., hypoxia, microgravity) or state of the organism (e.g., stress, inflammation, changes in dietary preferences and blood metabolites). Finally, it may result from hereditary diseases that destabilize the RBC membrane or perturb its rheological properties, redox or metabolic state. In this review we focus on the possible physiological causes of heterogeneity.
Parameters Showing Inter-Cellular Heterogeneity and Methods to Detect Them
Table 1 summarizes the information on the parameters displaying inter-individual and inter-cellular heterogeneity, as well as methodological approaches for detection of heterogeneity.
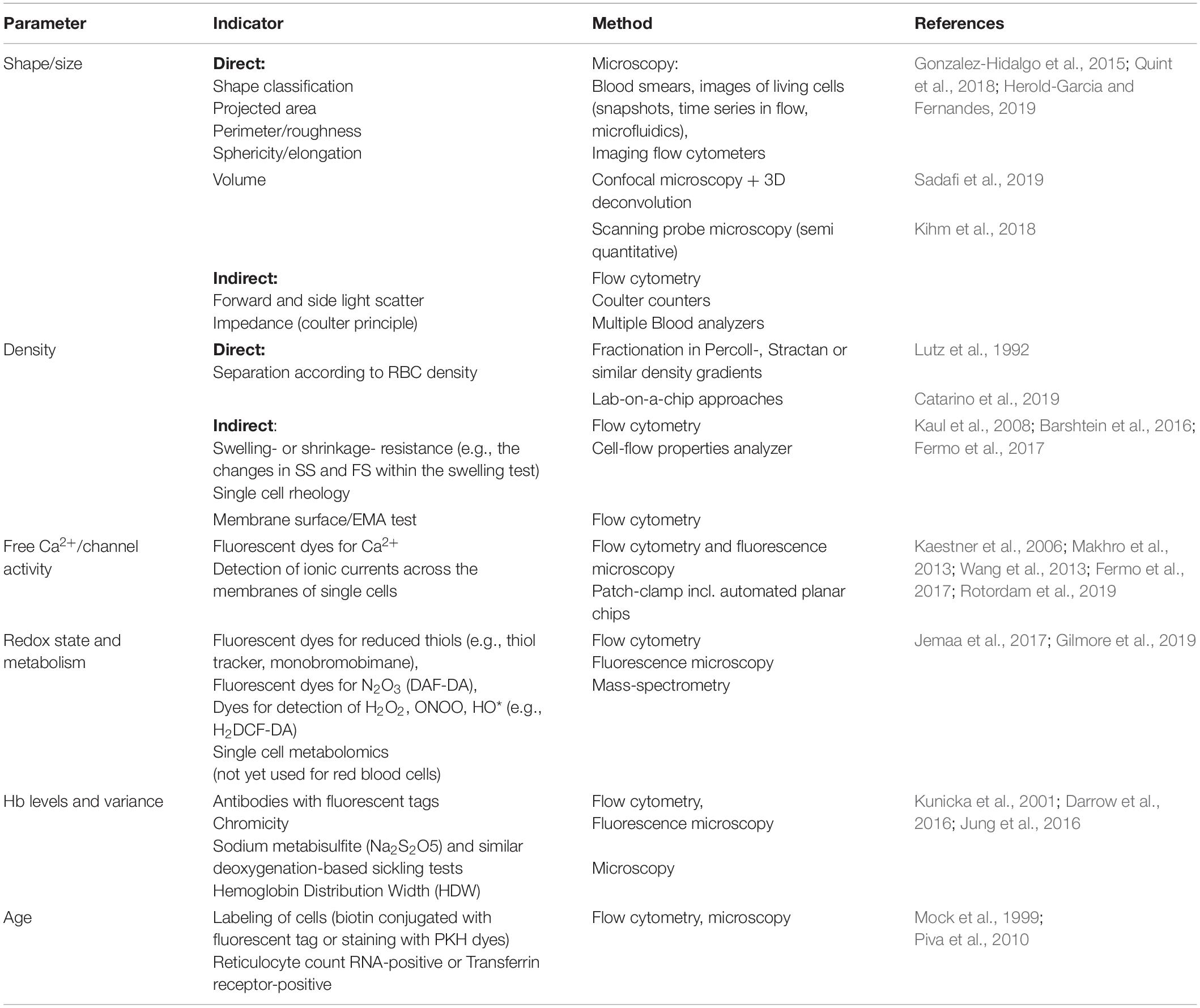
Table 1. Overview of parameters showing inter-cellular heterogeneity as well as basic principle and methodological approaches of their detection in single cells and sub-populations.
Shape and Size
First descriptions of RBCs as “red corpuscles” given by Jan Swammerdam and dates back to 1658 (Swammerdam, 1737; Bessis and Delpech, 1981; Hajdu, 2003). Since then, substantial progress was made in imaging equipment as well as in fixation and staining of RBC. Blood smears still remain a part of common diagnostic practice in most of the clinical laboratories (Bain, 2005) despite the fact that smear preparation results in distortions of RBC morphology and lysis of the most fragile of them (Wenk, 1976). This technique allows to discriminate between numerous shapes from discocytes to a broad variety of “static” shapes such as echinocytes and stomatocytes, for healthy humans. The list of shape types will extend manifolds for patients with hereditary or acute disorders.
The biggest drawback of the whole approach with smears is that it provides an immediate snapshot of the shape distribution, whereas living RBCs are very dynamic entities. So are their shapes, and, rather than discussing their “absolute shape,” it would be feasible to assign them a probability to be observed in one of the shape types. The first attempts to address RBC shapes in terms of probability density distribution are recently undertaken (Reichel et al., 2019). Each cell has its “static shape” that is preferred over the other ones if no force is applied to it. There are also several preferred shape types caused by shear stress in flow. The probability to observe one of those depends on the shear rates and flow dynamics (Abkarian et al., 2008; Dupire et al., 2015; Lanotte et al., 2016; Kihm et al., 2018; Mauer et al., 2018; Reichel et al., 2019). The restoration of the initial shape of the cells as soon as the flow stops got the name of “shape memory” (Fischer, 2004; Cordasco and Bagchi, 2017). Acute shape changes associated with the ion movements across the cell membrane (dehydration or overhydration) are often reversible (Brugnara, 1997; Cossins and Gibson, 1997; Zhu et al., 2018) whereas shape alterations related to the permanent damage of the cytoskeleton or membrane loss are irreversible (Gallagher, 2005; Perrotta et al., 2008).
Preferred shapes reflect the optimal cytoskeletal conformation, hemoglobin concentration, redox state and metabolic balance and free Ca2+ levels that, in turn, define the activity of ion transporters, hydration state and phosphorylation state of proteins. Some of these variables will be addressed below.
Parameters to describe dynamics of RBCs morphology are currently in development. Former classifications of shapes performed by eye (Bessis and Lessin, 1970; Bessis and Delpech, 1982) are non-numerical and cannot be reliably translated into the algorithms for automated segmentation and classification of smears and images of living cells. New approaches are currently developing (Tomari et al., 2014). Roundness, roughness, projected areas are among such numeric descriptors of RBC shapes. 3D volume reconstruction of, e.g., confocal recordings are more informative than 2-dimentional images. High resolution 3D-imaging was performed for fixed RBC (Abay et al., 2019). First attempts to get the 3D imaging working for RBCs in flow are undertaken but is not yet available as a high-throughput mode (Quint et al., 2018). Cell shape recognition and classification involving artificial intelligence (AI) algorithms based on artificial neural networks (Kihm et al., 2018). New optical concepts using optofluidic microlenses-like behavior of RBCs (Mugnano et al., 2018) and indirect adaptive optics as well as label-free quantitative phase imaging (Miccio et al., 2015) enables assessment of cell volume of individual cells, and monitoring of morphometric features (e.g., label-free optical markers) that make high throughput reliable quantification of cell phenotypes possible. It allows to stay unbiased, omit “human factor,” and allocate RBC shapes to a continuous scale with high throughput and precision. The challenge is that artificial neural networks need to be set up, customized and most notably trained. This type of analysis will become available routinely in the nearest future.
Hydration State and Density
The best method to visualize the variance in RBC density is fractionation on a Percoll (Figure 1), Ficoll, Stractan, or phtalate density gradient (Danon and Marikovsky, 1964; Corry et al., 1982; Salvo et al., 1982; Mosca et al., 1991; Lutz et al., 1992). Upon centrifugation in isotonic solution of any of these materials forming continuous or discontinuous gradients, RBCs distribute within them according to their densities. As RBCs of healthy human donors are fractionated on a self-forming Percoll gradient, three to five fractions may be collected. A small fraction of cells with lower density bands as the top layer, followed by one or several RBC populations with a medium density and a minor fraction of cells is presented with the highest density (Lutz et al., 1992; Makhro et al., 2013; Makhro et al., 2016b). RBCs of patients with hereditary hemolytic anemias are generally characterized with a broader variance in densities. Often this diversity may contain clinically relevant information on the severity of disease state. For sickle cell disease the abundance of dense cells was suggested to be a predictor of severity of disease manifestation due to the increased probability of irreversible aggregation of HbS (Kaul et al., 1983). For hereditary spherocytosis severity is associated with an increase in abundance of well-hydrated cells that are lost before they have time to mature and lose some of their membrane (Huisjes et al., 2019). Increase in heterogeneity is high in patients with cryohydrocytosis (Bogdanova et al., 2010), Gardos channelopathy (Fermo et al., 2017) beta-thalassemia, G6PD, and pyruvate kinase (PK) deficiency (Mosca et al., 1991).
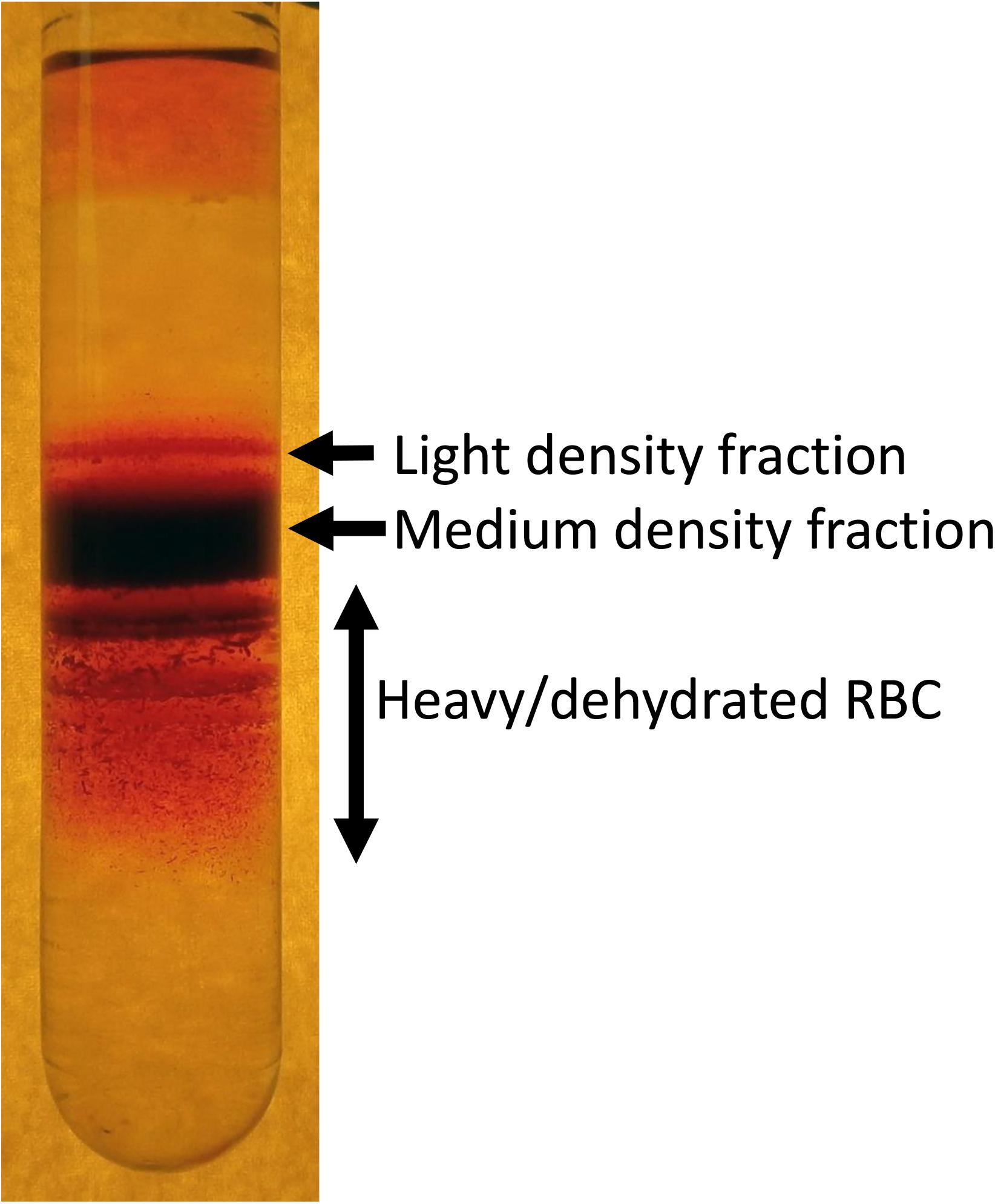
Figure 1. An example of heterogeneity of RBC density revealed by fractionation of RBCs on a self-forming Percoll density gradient. Composition of light, medium and high-density fractions vary depending on human health and environmental stress. About 15–20% of RBCs of healthy human donors forming low density are reticulocytes. However, along with young cells this fraction is “contaminated” with swollen RBCs at the terminal senescence stage (Lew and Tiffert, 2013). Medium fraction is formed by mature RBCs, and heavy dehydrated cells are those with senescent phenotype.
Factors defining RBC density include changes in water and ion content and membrane loss. During the density fractionation RBCs experience shear stress during centrifugation as they move through the isotonic Percoll solution containing micromolar concentrations of Ca2+ in the absence of EGTA. Shear forces may activate mechano-sensitive channels such as PIEZO1 channels (Cahalan et al., 2015) and NMDA receptors (Hanggi et al., 2014) that are permeable for Ca2+. Uptake of Ca2+ via these receptors triggers loss of K+ mediated by opening of Ca2+-dependent Gardos channels. Thus, fractionation of RBCs on Percoll should be viewed as a functional test in which distribution of the cells is not only driven by the steady state density, but also by their mechano-sensitivity.
Indirect methods to assess heterogeneity in RBC density include detection of hypo- and hyperchromic cells in blood smears, HDW as well as the shape of the curve in osmotic fragility test of the right arm of the osmoscan curve obtained by ektacytometry (Clark et al., 1983; Lutz et al., 1992). Hight throughput devices for evaluation of RBC density using functional tests at the single cell level are being developed.
Ca2+ Levels (Static and Dynamic Tests) and Electrophysiological Properties
Heterogeneity in basal free Ca2+ levels was recorded in RBCs of healthy humans (Kaestner et al., 2006; Makhro et al., 2013; Fermo et al., 2017). Stimulation of Ca2+ uptake by treatment of healthy human RBCs with PGE2 (Danielczok et al., 2017), lysophosphatidic acid (Steffen et al., 2011; Kaestner et al., 2012; Wang et al., 2013; Wesseling et al., 2016) or glutamate (Makhro et al., 2013; Hanggi et al., 2014; Makhro et al., 2016a; Petkova-Kirova et al., 2019) increases variance in the intracellular Ca2+. Not all cells respond to shear stress or pro-oxidative condition with an increase in Ca2+.
Molecular causes for this heterogeneity in responses to various stressors are poorly understood. It is obvious, that they relate to the differences in abundance of either Ca2+ channels (Kaestner et al., 1999; Makhro et al., 2013; Kaestner and Egee, 2018; Rotordam et al., 2019) or of the primary receptors responding to the stressor (such as LPA or prostaglandin receptors; Wang et al., 2013; Danielczok et al., 2017). In human RBCs several ion channels are known to mediate Ca2+ uptake including PIEZO1, TRPC6, NMDA receptors, CaV2.1 and several others (for a recent review see Kaestner et al., 2020). As a result of stochastic distribution and opening probability, Ca2+ entry into individual RBCs varies in response to stimulation by individual Ca2+ channels substantially giving rise to “responders” and “non-responders” cellular sup-populations. This uneven behavior may be further amplified due to the existence of feedback loops supporting Ca2+-dependent Ca2+ uptake (Kaestner et al., 2018).
Most documented is inter-cellular variance in distribution of the Ca2+-dependent K+ (Gardos) channel in RBCs. However, majority of the recordings for this best-studied channel in RBCs were performed as mean values for the unseparated populations, using radioactive tracer kinetics technique or single channel recordings. Reports based on whole-cell recordings for this channel are still sparse (Kucherenko et al., 2005; Kucherenko et al., 2013; Fermo et al., 2017). A further factor that may amplify heterogeneity of Gardos channel recordings in RBCs is its inactivation upon hypoxic exercises (Mao et al., 2011).
Redox State and Metabolism
Staining of individual cells with fluorescent probes sensitive to pro-oxidative free radicals such as dicarbofluorescein (Amer et al., 2003; Grinberg et al., 2005) and monobromobimane (Kosower and Kosower, 1995) provide a possibility to follow the changes in redox balance in individual cells. One more approach to record redox state in sub-fractions of RBCs is based on pre-fractionation of cells into low, medium and high density fractions before assessment of reduced and oxidized glutathione (GSH and GSSG) and NAD(P)H (Piccinini et al., 1995; D’Alessandro et al., 2013) in these sub-populations. Dense cells were shown to be deprived of GSH and enriched with GSSG compared to the mature RBCs of medium density. Accumulation of GSSG and reduction in GSH was not associated with any substantial changes in the intracellular ATP or NADPH (Sass et al., 1965; D’Alessandro et al., 2013). Finally, redox state of RBCs may be expressed as the ability to tolerate oxidative challenge (Lisovskaya et al., 2008; Sinha et al., 2015) which differs between individual RBCs as well.
Shifts in redox equilibrium in RBCs of healthy donors are associated with age-dependent decrease in pyruvate kinase, hexokinase, glucose-phosphate dehydrogenase, aldolase activities (Salvo et al., 1982; Suzuki and Dale, 1988). Oxidative stress is a hallmark of RBCs of patients with hereditary hemolytic anemias presented with one or two alleles of mutated glucose-6 phosphate dehydrogenase (G6PD). The resulting in acute hemolytic condition known as favism is associated with depletion in NADPH in favor of NADP+ (Mason et al., 2007; Peters and van Noorden, 2017). Furthermore, systemic oxidative stress caused by inflammatory processes, infection and other causes may result in release of reduced glutathione from RBCs and temporary increase in oxidative load and aggravate the differences in redox state between the cells of different ages (Giustarini et al., 2008).
Hb Levels and Variants
Inter-cellular heterogeneity in intracellular hemoglobin content in clinical settings is reflected by the abundance of hypochromic and hyperchromic cells in blood smears. The abundance of hypochrome RBCs for healthy humans should not exceed 2.5% of circulating RBCs (Macdougall et al., 1992; Schaefer and Schaefer, 1995; Braun et al., 1997), dropping below 1% in patients with iron overload, and increasing to 20% and more in patients with iron deficiency. Higher levels of hyperchromic cells was also reported for patients with hereditary spherocytosis (Conway et al., 2002) and sickle cell disease (Ballas and Kocher, 1988).
Even more intercellular heterogeneity is introduced by a pronounced variance in the presence of fetal hemoglobin in a small fraction of cells (F-cells) in healthy humans (Boyer et al., 1975; Thein and Craig, 1998). The abundance of F-cells increases during high altitude exposure (Narayan et al., 2005). Pregnancy has an impact on this parameter (Prus and Fibach, 2013). Moreover, the abundance of F-cells as well as the amount of HbF in them may differ from cell to cell in patients with beta-thalassemia (Narayan et al., 2005). In sickle cell disease, HbF abundance furthermore strongly depends on the haplotype (Menzel and Thein, 2019). Sickle cell trait results in uneven distribution of HbS between the cells, and the pattern for such variance seems to be hereditary (Anyaibe et al., 1985).
Causes of Heterogeneity
If we want to make extensive use of RBC heterogeneity as a diagnostic and prognostic marker, we have to understand the origin of the observed variance in RBC properties.
This may stem from the different pools of erythroid precursor cells that equip the resulting reticulocytes with various sets of proteins that may only be produced as long as the synthesis machinery is active before the enucleation.
The other cause of heterogeneity are the age-dependent differences between the young, mature and senescent RBCs. The third cause occurring at the systemic level originates from the alteration in the micro- and macro-environmental conditions (changes in hormonal and metabolic levels, inflammation, shear stress load, hyperthermia and others).
These three sources of heterogeneity will be reviewed below.
Cellular Heterogeneity During Erythropoiesis
Accumulated evidence over the last 25 years has demonstrated the existence of heterogeneity within the erythroid compartment schematically shown in Figure 2. Although it is quite expected to have a heterogenic population within the mature RBC population due to the long- life span of mature RBCs (100–120 days in human) representing cells of various ages, it is less clear and more intriguing the reasons for erythroid precursors/progenitors to be heterogeneous in multiple facets of their form and function.
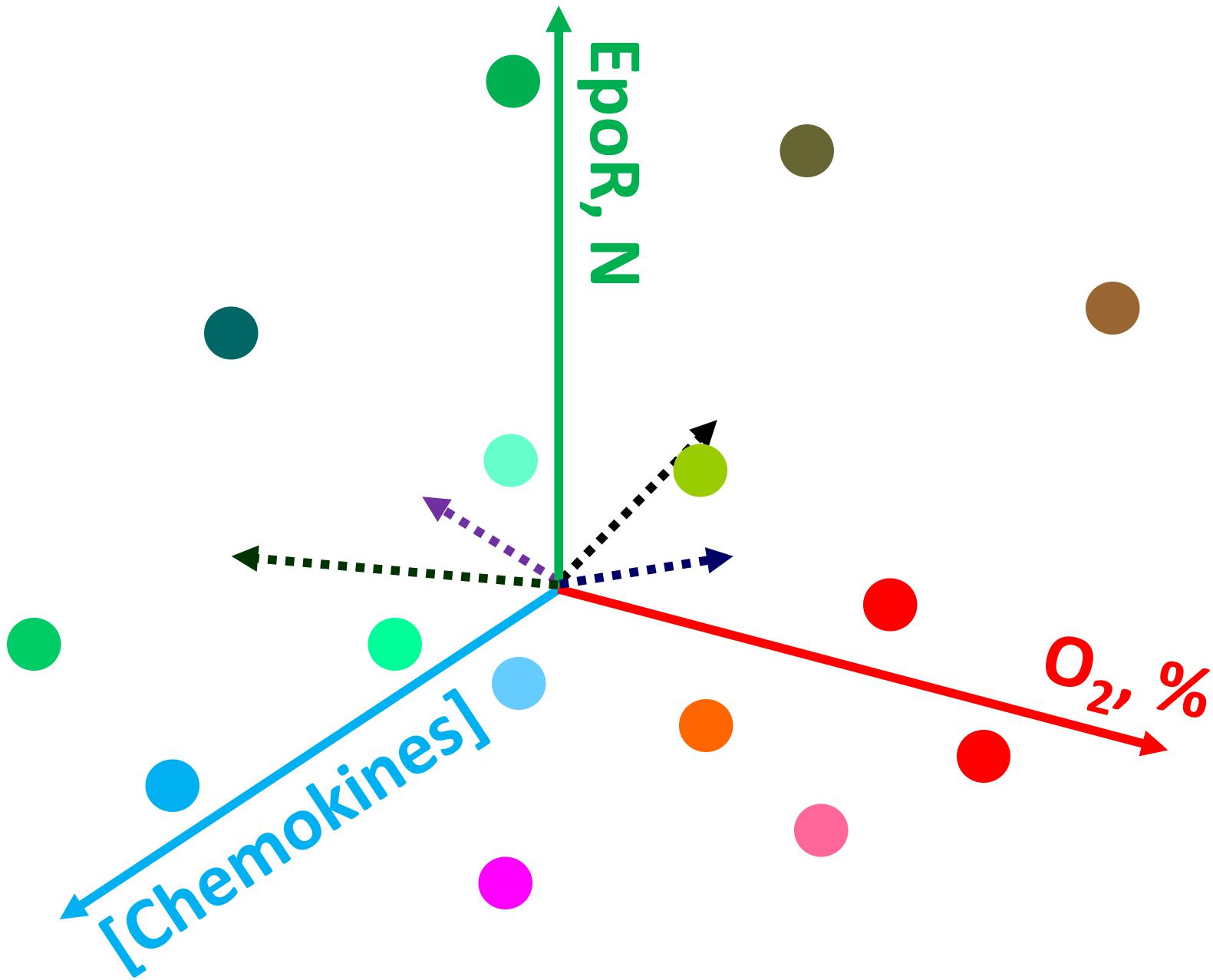
Figure 2. Schematic representation of the possible causes of heterogeneity for erythroid precursor cells within bone marrow. Gradients in oxygen availability, chemokines and other signaling messengers create a plethora of conditions in which cells find themselves during differentiation. For details see the text.
One of the most established and well-explained aspect of erythroid precursor heterogeneity pertains to erythroid precursors possessing differing sensitivities to erythropoietin (EPO). Soon after discovering the precise molecular function of EPO to be a cell survival function (Koury and Bondurant, 1988, 1990), studies revealed that even within a highly homogenous population in terms of the differentiation stage (operationally defined as colony-forming unit-erythroid; CFU-E), erythroid precursors underwent apoptosis following EPO withdrawal in an asynchronous manner (Kelley et al., 1993). These studies demonstrated a dose response effect as reflected by increasing numbers of CFU-Es undergoing apoptosis as EPO concentrations were gradually decreased. These observations clearly highlighted the built-in heterogeneity within the developing erythroid cell compartment with respect to the biochemical nature of each cell within an otherwise “homogenous” precursor pool as defined by morphological characteristics. One of the possible causes supporting heterogeneity are the gradients in various signaling messengers, growth factors, chemokines, oxygen levels and the resulting reactive oxygen species, and other factors (e.g., Thompson et al., 2010; Spencer et al., 2014; Itkin et al., 2016) making conditions in which precursor cells differentiate unique and dependent on their location within the bone marrow (Figure 2). An elegant model proposed by Koury and Bondurant (1992), explained the basis of differing EPO sensitivities as a built-in mechanism to prevent all erythroid precursors undergoing apoptosis during low EPO levels in circulation such as in patients with renal failure. The work by several other groups (Miura et al., 1991; Landschulz et al., 1992; Nakamura et al., 1992; Kelley et al., 1993) had shown that heterogeneic EPO response within the same precursor population cannot be attributed to the numbers of EPO-receptors, affinity or structure, thereby suggesting differences in signal transduction as the likely mechanism for the existence of heterogeneity in EPO response. Based on these findings one can appreciate the existence of signaling heterogeneity within the erythroid precursor compartment as a necessary component during the development process to yield mature red blood cells. Recently developed single-cell intracellular flow cytometry approaches (Liu et al., 2019) are bound to further uncover previously unrecognized levels of regulatory heterogeneity during erythroid cell development.
Besides the existence of biochemical/signaling heterogeneity within the developing erythroid precursors other aspects of erythroid precursor heterogeneity have been observed especially most recently due to the advancement of single-cell technologies at both trascriptomic and phenotypic levels (Woll et al., 2014; La Manno et al., 2018; Brierley and Mead, 2019). Within the erythroid compartment especially during the early stages of erythropoiesis a significant level of transcriptomic variability and heterogeneity seem to exist at least based on mouse bone marrow erythroid precursors (Tusi et al., 2018). The same study also found that cell cycle in erythroid precursors are continuously remodeled during the differentiation program but consistent with very early studies using bulk erythroid precursors (CFU-E), the vast majority of cells were in the S-phase of the cell cycle (Iscove, 1977). These results demonstrate that an individual cell, especially during development, has the ability to program itself to act not in concert each other with respect to signal transduction, gene transcription, cell cycle and many other aspects even though morphologically a cell population may look alike at a particular stage of differentiation.
Overall, accumulated data suggests that heterogeneity during erythroid development may not be evenly spread during the entire development cascade. Most data points to greatest level of inter-cellular heterogeneity during the early phases of development when these cells are responsive to various growth factors. Beyond the late polychromatic stage, when the cells have exited the cell cycle one observes less heterogeneity and most cells undergo dramatic reduction in cell size, chromatin condensation and enucleation. However, it is conceivable even in the bone marrow niche within the blood island not all erythroblasts undergo enucleation adding another layer of heterogeneity. It is also conceivable that due to differing levels of chemokine receptors on these cells the progenitors also exhibit varying degrees of migration within the bone marrow niche. Overall, it may seem the inter-cellular heterogeneity during erythroid precursor development. Each cell is possessing different sensitivity to EPO, and as a result a vast majority of precursors die due to apoptosis, the strategy that seems quite wasteful. However, we speculate that such heterogeneity is critical in order to respond to rapid changes in the micro and macro environment such as changes in oxygen concentration due to changes in altitude, pro-inflammatory and oxidative stress conditions as well as sudden blood loss due to trauma and onset of anemia due to renal failure.
Age of RBCs
Most of the findings for the age-related variance for RBCs of healthy humans were obtained for the fractions of cells of low, medium and high density, that were enriched with young, mature and senescent cells, respectively (Mueller et al., 1985, 1987; Lutz et al., 1992; Figure 1). Gradual changes occurring with cell aging were described in several reviews (Lutz and Bogdanova, 2013; Lew and Tiffert, 2017; Badior and Casey, 2018; Minetti et al., 2018) and article collections (Beutler, 1988; Mangani, 1991), and schematically represented in Figure 3.
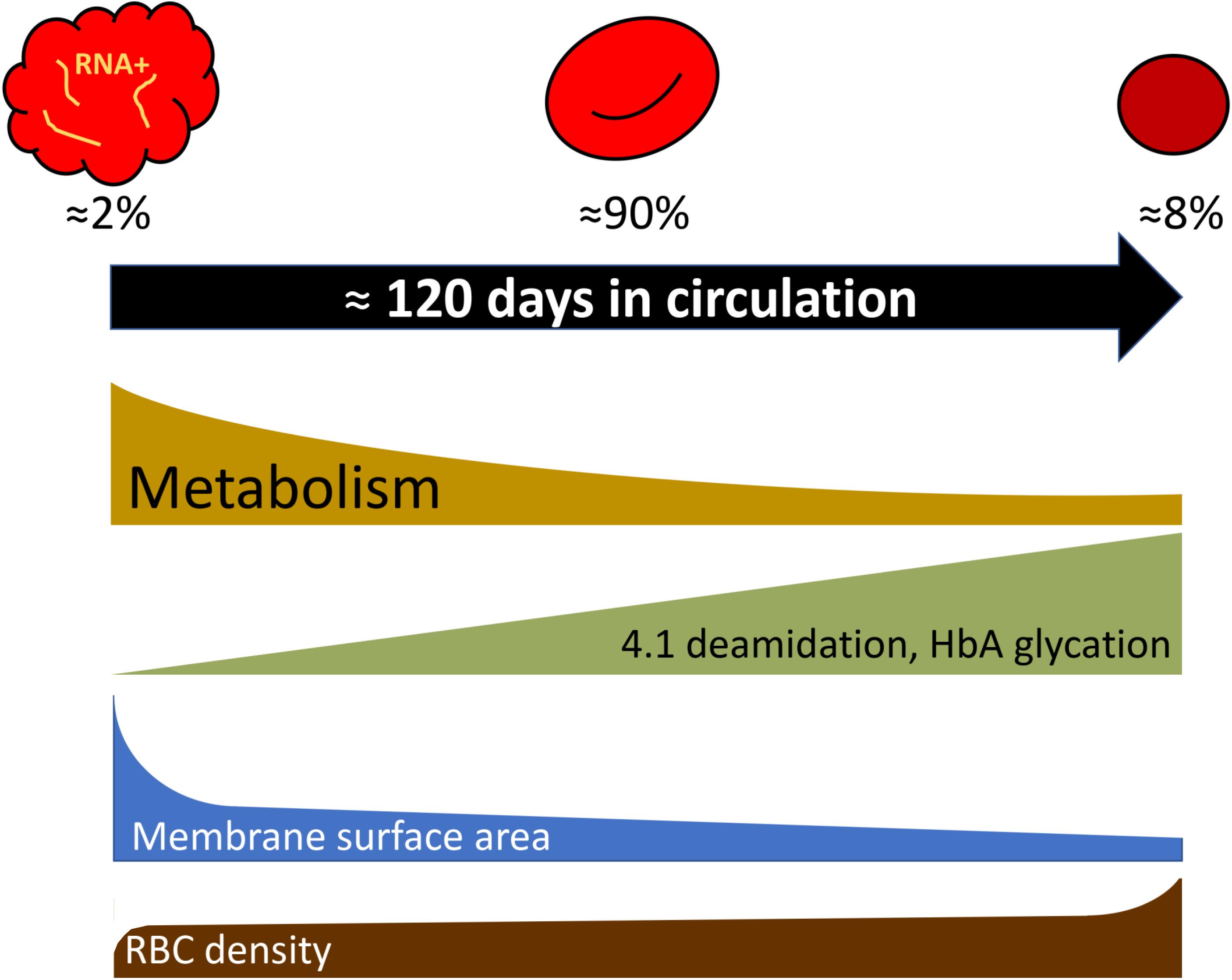
Figure 3. Selected parameters that change during RBC aging following exponential or linear kinetics as cells turn from reticulocytes to mature cells and finally enter the senescent stage. Percentage of reticulocytes, mature cells, and dense senescent cells shown in the scheme correspond to those in adult healthy donors. For more details see Lutz and Bogdanova (2013) and the text.
Recent studies of the age-dependent changes in RBCs involve single cell approaches such as flow cytometry and microscopy as well as proteomics (D’Alessandro et al., 2013; Minetti et al., 2013).
Deamidation of asparagine residue 502 of the band 4.1 protein was shown to occur gradually with RBC age as the deamidation rate is an exclusive function of temperature and time (Inaba and Maede, 1988, 1992). Deamidation is manifested as an appearance of a double band on the gels as the native and deamidated form of the protein differ in electrophoretic mobility of the protein. Fractionation of RBCs of healthy humans according to their density has shown that young cells have lower density than mature cells. Senescence is associated with further increase in RBC density and mean corpuscular hemoglobin concentration, and reduction in RBC volume. Using the changes in deamidation of band 4.1 protein or direct labeling of RBCs and monitoring of their aging (Luthra et al., 1979), increase in density were revealed as an intrinsic feature of in vivo aging of RBCs of healthy humans. Dense cells obtained by fractionation of leukodepleted RBCs on Percoll density gradient were presented with substantially lower GSH levels and GSSG levels that were doubled compared to the mature RBCs, whereas ATP and NADPH levels were only slightly reduced in the densest cell fractions (Sass et al., 1965; D’Alessandro et al., 2013). These changes were associated with the age-driven decrease in pyruvate kinase, hexokinase, glucose-6-phosphate dehydrogenase, aldolase activities (Salvo et al., 1982; Suzuki and Dale, 1988). Some of the terminally senescent RBCs, that lose control over their Na+ gradients and volume regulation due to the reduction in Na,K-ATPase activity, were reported to swell and lyse (Lew and Tiffert, 2013, 2017).
Reports on the changes in free Ca2+ levels are controversial and depend on the techniques used for assessment of these parameters (Romero and Romero, 1997, 1999; Makhro et al., 2013; Lew and Tiffert, 2017). Both Ca2+-permeable channel activity and that of plasma membrane Ca2+ pumps decreases with cellular aging (Romero et al., 2002; Makhro et al., 2013). Despite this inconsistency, changes in the intracellular free Ca2+ and the ability to maintain low levels of Ca2+ are the factors in control of RBC longevity (Bogdanova et al., 2013; Lew and Tiffert, 2017).
Further hallmarks of RBC aging include the changes in phosphorylation pattern (Fairbanks et al., 1983) and membrane loss (Mohandas and Groner, 1989).
Physical Activity, High Altitude, and Other Stress Conditions
How substantial would the change be at the level of circulating RBCs if the gene expression reprogramming occurs at the level of precursor cells? Simple calculations assuming that the RBC longevity is not affected by these changes and all cells are equally affected by this change, gives a rough estimate of ∼0.82% of RBC population changing per day for the “normal” production rate of 2.4 × 106 cells/s. If erythropoiesis is boosted to its maximum (10-fold increase, 8.2% of new cells will appear daily (Elliott and Molineux, 2009). This means that acute reversible changes at the bone marrow level will hardly be noticed if stress conditions persist for just 24 h. On the contrary, when stress conditions boosting erythropoiesis persist for a week, 5.7–57% of cells will get a new feature.
Such kinetics does not favor de novo production as an efficient strategy for acute adaptation to hypoxia or single endurance sport exercise bout, dietary changes, or to pathological conditions such as infection or sepsis, cancer, diabetes, or cardiovascular diseases (Figure 4). These changes in turn translate into the changes in shear stress, oxygen availability, pH, hormones and proinflammatory cytokines and other microenvironmental factors sensed by RBC directly. Species that undergo such acute changes from hyperoxygenation to severe hypoxia, such as Rainbow trout (Oncorhynchus mykiss) (Fago et al., 2001) or Rüppell’s griffon vulture (Gyps rueppelli). Rüppell’s griffon vulture was spotted at 37,000 feet (11277.6 m) when colliding with the plane (Laybourne, 1974) permanently possess several hemoglobin variants. Hemoglobin A and D chains are present in RBC vulture producing high and low affinity hemoglobin variants and allowing these unique birds to fly above 10,000 m with no need to engage any complex adaptive processes as they land (Weber et al., 1988; Hiebl et al., 1989).
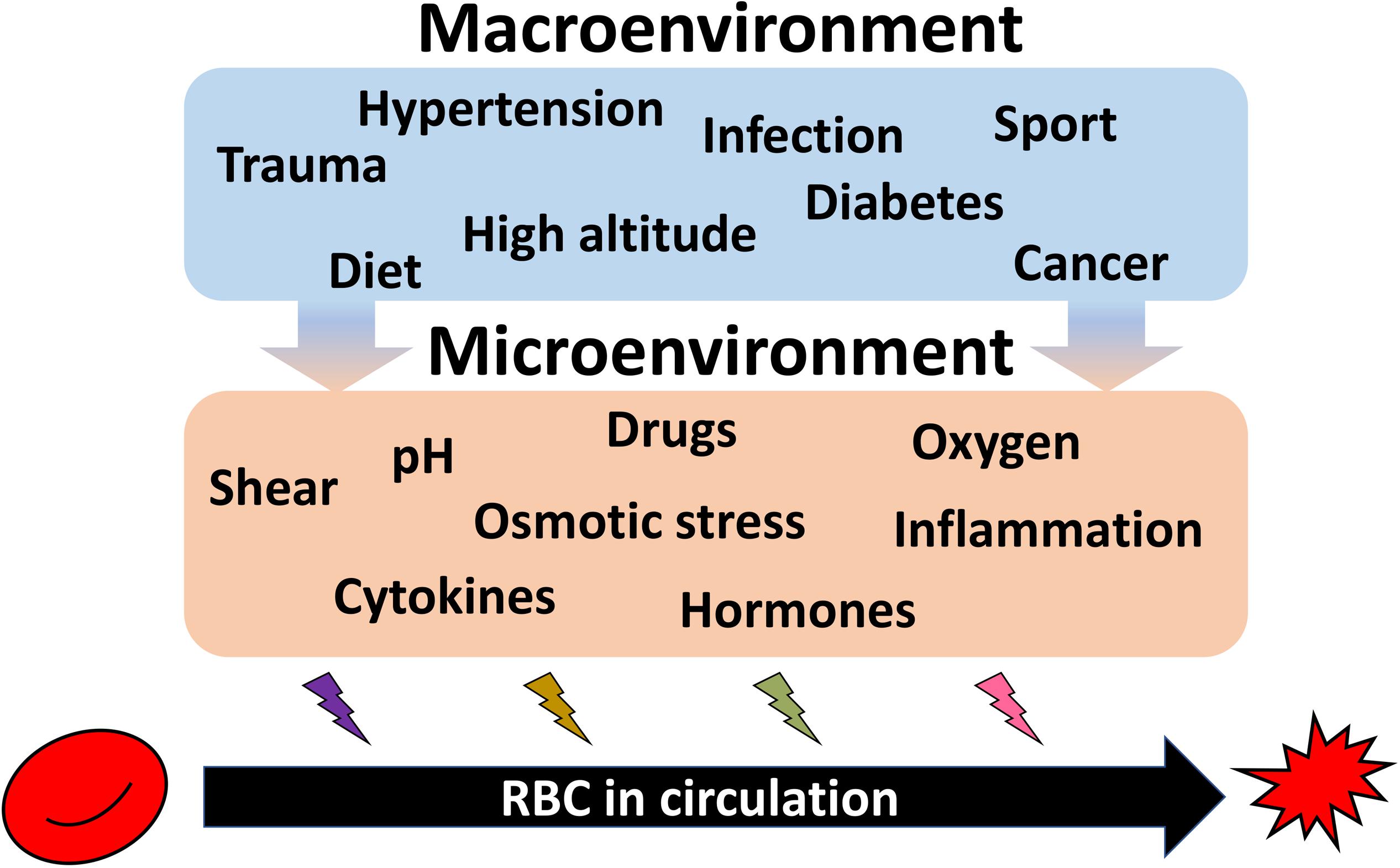
Figure 4. Summary on the environmental causes imposing heterogeneity of circulating RBCs. Exposure of the organism to high altitude or practicing endurance sport as well as dietary preferences cause durable or acute impact on the RBC properties. Along with RBC diseases (anemia, polycythemia), pathologies such as hypertension, diabetes, infection, trauma, cancer, and further systemic diseases are influencing both erythropoietic niche and the circulating cells. All these macroenvironmental stresses translated into the changes in microenvironment for erythroid precursors and circulating RBCs. Shear, alterations in pH and oxygen levels, proinflammatory cytokines, and hormones, as well as drugs work to shape the features of each individual RBC resulting in an increase in the inter-cellular heterogeneity.
Adult humans have by far lower adaptive capacity, possessing generally one Hb variant, HbA with some minor additions of HbF. However, plasticity of O2 delivery, and its fast on-demand optimization upon the changes in environmental O2 availability may be associated with other types of heterogeneity in RBC structure and function. Potential adaptive role of variance in RBC properties has to be further explored.
It is largely accepted that multiple forms of pathologies, both related to abnormal structure RBC membrane or cytosolic proteins and lipids, as well as systemic disorders such as cancer, diabetes, cardiovascular diseases, sepsis and other diseases of inflammation are associated with anemia, RBC damage and their premature removal from the circulation and increase in their heterogeneity (e.g., Salvagno et al., 2015; Feng et al., 2017; Ahmad et al., 2018; Ko et al., 2018; Yin et al., 2018; Parizadeh et al., 2019; Wang et al., 2019). The causes and consequences as well as predictive power of this increase in variability of RBC properties is out of the scope of this review but deserve special attention.
Summary and the Standing Challenges
The present collection of information on the possible causes and consequences of inter-cellular heterogeneity justifies the increasing attention of researchers to the RBC sub-populations and individual cells. It appears that vast amount of information on the near and distant (within months) past is lost when RBC properties are reduced to a set of single “mean” values. This information appears to be of substantial importance when severity of disease or efficacy of therapy are to be assessed for individual patients. At present we do not have the commercially available and standardized methodologies and machines to be able to compare the data obtained of the single cell features in different labs. These challenges are already addressed by some researchers and will drive the transformation of our understanding of red blood cell biology in the nearest future.
Author Contributions
AB and AW have composed the text. All authors contributed to editing and proofreading of the text.
Funding
AB and LK received funding from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement number 675115 – RELEVANCE – H2020-MSCA-ITN-2015/H2020-MSCA-ITN-2015 and from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement number 860436 – EVIDENCE – H2020-MSCA-ITN-2019.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abay, A., Simionato, G., Chachanidze, R., Bogdanova, A., Hertz, L., Bianchi, P., et al. (2019). Glutaraldehyde – a subtle tool in the investigation of healthy and pathologic red blood cells. Front. Physiol. 10:514. doi: 10.3389/fphys.2019.00514
Abkarian, M., Faivre, M., Horton, R., Smistrup, K., Best-Popescu, C. A., and Stone, H. A. (2008). Cellular-scale hydrodynamics. Biomed. Mater. 3:034011. doi: 10.1088/1748-6041/3/3/034011
Ahmad, H., Khan, M., Laugle, M., Jackson, D. A., Burant, C., Malemud, C. J., et al. (2018). Red cell distribution width is positively correlated with atherosclerotic cardiovascular disease 10-year risk score, age, and CRP in Spondyloarthritis with axial or peripheral disease. Int. J. Rheumatol. 2018:2476239. doi: 10.1155/2018/2476239
Amer, J., Goldfarb, A., and Fibach, E. (2003). Flow cytometric measurement of reactive oxygen species production by normal and thalassaemic red blood cells. Eur. J. Haematol. 70, 84–90. doi: 10.1034/j.1600-0609.2003.00011.x
Andrews, D. A., and Low, P. S. (1999). Role of red blood cells in thrombosis. Curr. Opin. Hematol. 6, 76–82.
Anyaibe, S., Castro, O., and Headings, V. (1985). Distributions of hemoglobins A and S among erythrocytes of heterozygotes. Hemoglobin 9, 137–155. doi: 10.3109/03630268508996996
Badior, K. E., and Casey, J. R. (2018). Molecular mechanism for the red blood cell senescence clock. IUBMB Life 70, 32–40. doi: 10.1002/iub.1703
Bain, B. J. (2005). Diagnosis from the blood smear. N. Engl. J. Med. 353, 498–507. doi: 10.1056/nejmra043442
Ballas, S. K., and Kocher, W. (1988). Erythrocytes in Hb SC disease are microcytic and hyperchromic. Am. J. Hematol. 28, 37–39. doi: 10.1002/ajh.2830280108
Barshtein, G., Pries, A. R., Goldschmidt, N., Zukerman, A., Orbach, A., Zelig, O., et al. (2016). Deformability of transfused red blood cells is a potent determinant of transfusion-induced change in recipient’s blood flow. Microcirculation 23, 479–486. doi: 10.1111/micc.12296
Bernhardt, I., Wesseling, M. C., Nguen, D. B., and Kaestner, L. (2019). “Red blood cells actively contribute to blood coagulation and thrombus formation,” in Erythrocyte, ed. A. Tombak, (London: IntechOpen).
Bessis, M., and Delpech, G. (1981). Discovery of the red blood cell with notes on priorities and credits of discoveries, past, present and future. Blood Cells 7, 447–480.
Bessis, M., and Delpech, G. (1982). Sickle cell shape and structure: images and concepts (1840-1980). Blood Cells 8, 359–435.
Bessis, M., and Lessin, L. S. (1970). The discocyte-echinocyte equilibrium of the normal and pathologic red cell. Blood 36, 399–403. doi: 10.1182/blood.v36.3.399.399
Bogdanova, A., Goede, J. S., Weiss, E., Bogdanov, N., Bennekou, P., Bernhardt, I., et al. (2010). Cryohydrocytosis: increased activity of cation carriers in red cells from a patient with a band 3 mutation. Haematologica 95, 189–198. doi: 10.3324/haematol.2009.010215
Bogdanova, A., Makhro, A., Wang, J., Lipp, P., and Kaestner, L. (2013). Calcium in red blood cells-a perilous balance. Int. J. Mol. Sci. 14, 9848–9872. doi: 10.3390/ijms14059848
Boyer, S. H., Belding, T. K., Margolet, L., and Noyes, A. N. (1975). Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science 188, 361–363. doi: 10.1126/science.804182
Braun, J., Lindner, K., Schreiber, M., Heidler, R. A., and Horl, W. H. (1997). Percentage of hypochromic red blood cells as predictor of erythropoietic and iron response after i.v. iron supplementation in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 12, 1173–1181. doi: 10.1093/ndt/12.6.1173
Brierley, C. K., and Mead, A. J. (2019). Single-cell sequencing in hematology. Curr. Opin. Oncol. 32, 139–145. doi: 10.1097/cco.0000000000000613
Brugnara, C. (1997). Erythrocyte membrane transport physiology. Curr. Opin. Hematol. 4, 122–127. doi: 10.1097/00062752-199704020-00008
Cahalan, S. M., Lukacs, V., Ranade, S. S., Chien, S., Bandell, M., and Patapoutian, A. (2015). Piezo1 links mechanical forces to red blood cell volume. eLife 4:e07370. doi: 10.7554/eLife.07370
Catarino, S. O., Rodrigues, R. O., Pinho, D., Miranda, J. M., Minas, G., and Lima, R. (2019). Blood cells separation and sorting techniques of passive microfluidic devices: from fabrication to applications. Micromachines (Basel). 10:593. doi: 10.3390/mi10090593
Chu, H., Puchulu-Campanella, E., Galan, J. A., Tao, W. A., Low, P. S., and Hoffman, J. F. (2012). Identification of cytoskeletal elements enclosing the ATP pools that fuel human red blood cell membrane cation pumps. Proc. Natl. Acad. Sci. U.S.A. 109, 12794–12799. doi: 10.1073/pnas.1209014109
Clark, M. R., Mohandas, N., and Shohet, S. B. (1983). Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood 61, 899–910. doi: 10.1182/blood.v61.5.899.bloodjournal615899
Conway, A. M., Vora, A. J., and Hinchliffe, R. F. (2002). The clinical relevance of an isolated increase in the number of circulating hyperchromic red blood cells. J. Clin. Pathol. 55, 841–844. doi: 10.1136/jcp.55.11.841
Cordasco, D., and Bagchi, P. (2017). On the shape memory of red blood cells. Phys. Fluids 29:041901. doi: 10.1063/1.4979271
Corry, W. D., Bresnahan, P. A., and Seaman, G. V. (1982). Evaluation of density gradient separation methods. J. Biochem. Biophys. Methods 7, 71–82. doi: 10.1016/0165-022x(82)90038-0
Cossins, A. R., and Gibson, J. S. (1997). Volume-sensitive transport systems and volume homeostasis in vertebrate red blood cells. J. Exp. Biol. 200, 343–352.
D’Alessandro, A., Blasi, B., D’amici, G. M., Marrocco, C., and Zolla, L. (2013). Red blood cell subpopulations in freshly drawn blood: application of proteomics and metabolomics to a decades-long biological issue. Blood Transfus. 11, 75–87. doi: 10.2450/2012.0164-11
Danielczok, J., Hertz, L., Ruppenthal, S., Kaiser, E., Petkova-Kirova, P., Bogdanova, A., et al. (2017). Does erythropoietin regulate TRPC channels in red blood cells? Cell. Physiol. Biochem. 41, 1219–1228. doi: 10.1159/000464384
Danon, D., and Marikovsky, V. (1964). Determination of density distribution of red cell population. J. Lab. Clin. Med. 64, 668–674.
Darrow, M. C., Zhang, Y., Cinquin, B. P., Smith, E. A., Boudreau, R., Rochat, R. H., et al. (2016). Visualizing red blood cell sickling and the effects of inhibition of sphingosine kinase 1 using soft X-ray tomography. J. Cell Sci. 129, 3511–3517. doi: 10.1242/jcs.189225
Dupire, J., Abkarian, M., and Viallat, A. (2015). A simple model to understand the effect of membrane shear elasticity and stress-free shape on the motion of red blood cells in shear flow. Soft Matter 11, 8372–8382. doi: 10.1039/c5sm01407g
Elliott, S. M. F., and Molineux, G. (eds.) (2009). Erythopoietins, Erythropoietic Factors, and Erythropoiesis. Molecular, Cellular, Preclinical, and Clinical Biology. Boston, MA: Birkenhäuser.
Fago, A., Forest, E., and Weber, R. E. (2001). Hemoglobin and subunit multiplicity in the rainbow trout (Oncorhynchus mykiss) hemoglobin system. Fish Physiol. Biochem. 24, 335–342.
Fairbanks, G., Palek, J., Dino, J. E., and Liu, P. A. (1983). Protein kinases and membrane protein phosphorylation in normal and abnormal human erythrocytes: variation related to mean cell age. Blood 61, 850–857. doi: 10.1182/blood.v61.5.850.850
Feng, G. H., Li, H. P., Li, Q. L., Fu, Y., and Huang, R. B. (2017). Red blood cell distribution width and ischaemic stroke. Stroke Vasc. Neurol. 2, 172–175. doi: 10.1136/svn-2017-000071
Fermo, E., Bogdanova, A., Petkova-Kirova, P., Zaninoni, A., Marcello, A. P., Makhro, A., et al. (2017). ‘Gardos Channelopathy’: a variant of hereditary Stomatocytosis with complex molecular regulation. Sci. Rep. 7:1744. doi: 10.1038/s41598-017-01591-w
Fischer, T. M. (2004). Shape memory of human red blood cells. Biophys. J. 86, 3304–3313. doi: 10.1016/s0006-3495(04)74378-7
Gallagher, P. G. (2005). Red cell membrane disorders. Hematol. Am. Soc. Hematol. Educ. Program 2005, 13–18.
Gilmore, I. S., Heiles, S., and Pieterse, C. L. (2019). Metabolic imaging at the single-cell scale: recent advances in mass spectrometry imaging. Annu. Rev. Anal. Chem. 12, 201–224. doi: 10.1146/annurev-anchem-061318-115516
Giustarini, D., Milzani, A., Dalle-Donne, I., and Rossi, R. (2008). Red blood cells as a physiological source of glutathione for extracellular fluids. Blood Cells Mol. Dis. 40, 174–179. doi: 10.1016/j.bcmd.2007.09.001
Gonzalez-Hidalgo, M., Guerrero-Pena, F. A., Herold-Garcia, S., Jaume, I. C. A., and Marrero-Fernandez, P. D. (2015). Red blood cell cluster separation from digital images for use in sickle cell disease. IEEE J. Biomed. Health Inform. 19, 1514–1525. doi: 10.1109/JBHI.2014.2356402
Grinberg, L., Fibach, E., Amer, J., and Atlas, D. (2005). N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic. Biol. Med. 38, 136–145. doi: 10.1016/j.freeradbiomed.2004.09.025
Hajdu, S. I. (2003). A note from history: the discovery of blood cells. Ann. Clin. Lab. Sci. 33, 237–238.
Hanggi, P., Makhro, A., Gassmann, M., Schmugge, M., Goede, J. S., Speer, O., et al. (2014). Red blood cells of sickle cell disease patients exhibit abnormally high abundance of N-methyl D-aspartate receptors mediating excessive calcium uptake. Br. J. Haematol. 167, 252–264. doi: 10.1111/bjh.13028
Helms, C. C., Gladwin, M. T., and Kim-Shapiro, D. B. (2018). Erythrocytes and vascular function: oxygen and nitric oxide. Front. Physiol. 9:125. doi: 10.3389/fphys.2018.00125
Herold-Garcia, S., and Fernandes, L. F. (2019). New Methods for Morphological Erythrocytes Classification. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2019, 4068–4071.
Hiebl, I., Weber, R. E., Schneeganss, D., and Braunitzer, G. (1989). High-altitude respiration of falconiformes. The primary structures and functional properties of the major and minor hemoglobin components of the adult White-Headed Vulture (Trigonoceps occipitalis, Aegypiinae). Biol. Chem. Hoppe Seyler 370, 699–706. doi: 10.1515/bchm3.1989.370.2.699
Hoffman, J. F., Dodson, A., and Proverbio, F. (2009). On the functional use of the membrane compartmentalized pool of ATP by the Na+ and Ca++ pumps in human red blood cell ghosts. J. Gen. Physiol. 134, 351–361. doi: 10.1085/jgp.200910270
Huisjes, R., Makhro, A., Llaudet-Planas, E., Hertz, L., Petkova-Kirova, P., Verhagen, L. P., et al. (2019). Density, heterogeneity and deformability of red cells as markers of clinical severity in hereditary spherocytosis. Haematologica 105, 338–347. doi: 10.3324/haematol.2018.188151
Inaba, M., and Maede, Y. (1988). Correlation between protein 4.1a/4.1b ratio and erythrocyte life span. Biochim. Biophys. Acta 944, 256–264. doi: 10.1016/0005-2736(88)90439-7
Inaba, M., and Maede, Y. (1992). The critical role of asparagine 502 in post-translational alteration of protein 4.1. Comp. Biochem. Physiol. B 103, 523–526. doi: 10.1016/0305-0491(92)90364-w
Iscove, N. N. (1977). The role of erythropoietin in regulation of population size and cell cycling of early and late erythroid precursors in mouse bone marrow. Cell Tissue Kinet. 10, 323–334. doi: 10.1111/j.1365-2184.1977.tb00300.x
Itkin, T., Gur-Cohen, S., Spencer, J. A., Schajnovitz, A., Ramasamy, S. K., Kusumbe, A. P., et al. (2016). Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328. doi: 10.1038/nature17624
Jemaa, M., Fezai, M., Bissinger, R., and Lang, F. (2017). Methods employed in cytofluorometric assessment of eryptosis, the suicidal erythrocyte death. Cell Physiol. Biochem. 43, 431–444. doi: 10.1159/000480469
Jung, J., Matemba, L. E., Lee, K., Kazyoba, P. E., Yoon, J., Massaga, J. J., et al. (2016). Optical characterization of red blood cells from individuals with sickle cell trait and disease in Tanzania using quantitative phase imaging. Sci. Rep. 6:31698. doi: 10.1038/srep31698
Kaestner, L., Bogdanova, A., and Egee, S. (2020). “Calcium channels and calcium-regulated channels in human red blood cells,” in Calcium Signalling, ed. S. Islam, (New York, NY: Springer).
Kaestner, L., Bollensdorff, C., and Bernhardt, I. (1999). Non-selective voltage-activated cation channel in the human red blood cell membrane. Biochim. Biophys. Acta 1417, 9–15. doi: 10.1016/s0005-2736(98)00240-5
Kaestner, L., and Egee, S. (2018). Commentary: voltage gating of mechanosensitive PIEZO channels. Front. Physiol. 9:1565. doi: 10.3389/fphys.2018.01565
Kaestner, L., Steffen, P., Nguyen, D. B., Wang, J., Wagner-Britz, L., Jung, A., et al. (2012). Lysophosphatidic acid induced red blood cell aggregation in vitro. Bioelectrochemistry 87, 89–95. doi: 10.1016/j.bioelechem.2011.08.004
Kaestner, L., Tabellion, W., Weiss, E., Bernhardt, I., and Lipp, P. (2006). Calcium imaging of individual erythrocytes: problems and approaches. Cell Calcium 39, 13–19. doi: 10.1016/j.ceca.2005.09.004
Kaestner, L., Wang, X., Hertz, L., and Bernhardt, I. (2018). Voltage-activated ion channels in non-excitable cells-a viewpoint regarding their physiological justification. Front. Physiol. 9:450. doi: 10.3389/fphys.2018.00450
Kaul, D. K., Fabry, M. E., Windisch, P., Baez, S., and Nagel, R. L. (1983). Erythrocytes in sickle cell anemia are heterogeneous in their rheological and hemodynamic characteristics. J. Clin. Invest. 72, 22–31. doi: 10.1172/jci110960
Kaul, D. K., Koshkaryev, A., Artmann, G., Barshtein, G., and Yedgar, S. (2008). Additive effect of red blood cell rigidity and adherence to endothelial cells in inducing vascular resistance. Am. J. Physiol. Heart Circ. Physiol. 295, H1788–H1793. doi: 10.1152/ajpheart.253.2008
Kelley, L. L., Koury, M. J., Bondurant, M. C., Koury, S. T., Sawyer, S. T., and Wickrema, A. (1993). Survival or death of individual proerythroblasts results from differing erythropoietin sensitivities: a mechanism for controlled rates of erythrocyte production. Blood 82, 2340–2352. doi: 10.1182/blood.v82.8.2340.2340
Kihm, A., Kaestner, L., Wagner, C., and Quint, S. (2018). Classification of red blood cell shapes in flow using outlier tolerant machine learning. PLoS Comput. Biol. 14:e1006278. doi: 10.1371/journal.pcbi.1006278
Ko, E., Youn, J. M., Park, H. S., Song, M., Koh, K. H., and Lim, C. H. (2018). Early red blood cell abnormalities as a clinical variable in sepsis diagnosis. Clin. Hemorheol. Microcirc. 70, 355–363. doi: 10.3233/CH-180430
Kosower, E. M., and Kosower, N. S. (1995). Bromobimane probes for thiols. Methods Enzymol. 251, 133–148. doi: 10.1016/0076-6879(95)51117-2
Koury, M. J., and Bondurant, M. C. (1988). Maintenance by erythropoietin of viability and maturation of murine erythroid precursor cells. J. Cell. Physiol. 137, 65–74. doi: 10.1002/jcp.1041370108
Koury, M. J., and Bondurant, M. C. (1990). Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science 248, 378–381. doi: 10.1126/science.2326648
Koury, M. J., and Bondurant, M. C. (1992). The molecular mechanism of erythropoietin action. Eur. J. Biochem. 210, 649–663. doi: 10.1111/j.1432-1033.1992.tb17466.x
Kucherenko, Y., Browning, J., Tattersall, A., Ellory, J. C., and Gibson, J. S. (2005). Effect of peroxynitrite on passive K+ transport in human red blood cells. Cell. Physiol. Biochem. 15, 271–280. doi: 10.1159/000087237
Kucherenko, Y. V., Wagner-Britz, L., Bernhardt, I., and Lang, F. (2013). Effect of chloride channel inhibitors on cytosolic Ca2+ levels and Ca2+-activated K+ (Gardos) channel activity in human red blood cells. J. Membr. Biol. 246, 315–326. doi: 10.1007/s00232-013-9532-0
Kunicka, J., Malin, M., Zelmanovic, D., Katzenberg, M., Canfield, W., Shapiro, P., et al. (2001). Automated quantitation of hemoglobin-based blood substitutes in whole blood samples. Am. J. Clin. Pathol. 116, 913–919.
La Manno, G., Soldatov, R., Zeisel, A., Braun, E., Hochgerner, H., Petukhov, V., et al. (2018). RNA velocity of single cells. Nature 560, 494–498. doi: 10.1038/s41586-018-0414-6
Landschulz, K. T., Boyer, S. H., Noyes, A. N., Rogers, O. C., and Frelin, L. P. (1992). Onset of erythropoietin response in murine erythroid colony-forming units: assignment to early S-phase in a specific cell generation. Blood 79, 2749–2758. doi: 10.1182/blood.v79.10.2749.bloodjournal79102749
Lanotte, L., Mauer, J., Mendez, S., Fedosov, D. A., Fromental, J. M., Claveria, V., et al. (2016). Red cells’ dynamic morphologies govern blood shear thinning under microcirculatory flow conditions. Proc. Natl. Acad. Sci. U.S.A. 113, 13289–13294. doi: 10.1073/pnas.1608074113
Laybourne, R. C. (1974). Collision between a vulture and an aircraft at an altitude of 37,000 feet. Wilson Bull. 86, 461–462.
Lew, V. L., and Tiffert, T. (2013). The terminal density reversal phenomenon of aging human red blood cells. Front. Physiol. 4:171. doi: 10.3389/fphys.2013.00171
Lew, V. L., and Tiffert, T. (2017). On the mechanism of human red blood cell longevity: roles of calcium, the sodium pump, PIEZO1, and gardos channels. Front. Physiol. 8:977. doi: 10.3389/fphys.2017.00977
Lisovskaya, I. L., Shcherbachenko, I. M., Volkova, R. I., and Tikhonov, V. P. (2008). Modulation of RBC volume distributions by oxidants (phenazine methosulfate and tert-butyl hydroperoxide): role of Gardos channel activation. Bioelectrochemistry 73, 49–54. doi: 10.1016/j.bioelechem.2008.04.008
Liu, L., Liu, C., Quintero, A., Wu, L., Yuan, Y., Wang, M., et al. (2019). Deconvolution of single-cell multi-omics layers reveals regulatory heterogeneity. Nat. Commun. 10:470. doi: 10.1038/s41467-018-08205-7
Luthra, M. G., Friedman, J. M., and Sears, D. A. (1979). Studies of density fractions of normal human erythrocytes labeled with iron-59 in vivo. J. Lab. Clin. Med. 94, 879–896.
Lutz, H. U., and Bogdanova, A. (2013). Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front. Physiol. 4:387. doi: 10.3389/fphys.2013.00387
Lutz, H. U., Stammler, P., Fasler, S., Ingold, M., and Fehr, J. (1992). Density separation of human red blood cells on self forming Percoll gradients: correlation with cell age. Biochim. Biophys. Acta 1116, 1–10. doi: 10.1016/0304-4165(92)90120-j
Macdougall, I. C., Cavill, I., Hulme, B., Bain, B., Mcgregor, E., Mckay, P., et al. (1992). Detection of functional iron deficiency during erythropoietin treatment: a new approach. BMJ 304, 225–226. doi: 10.1136/bmj.304.6821.225
Makhro, A., Haider, T., Wang, J., Bogdanov, N., Steffen, P., Wagner, C., et al. (2016a). Comparing the impact of an acute exercise bout on plasma amino acid composition, intraerythrocytic Ca(2+) handling, and red cell function in athletes and untrained subjects. Cell Calcium 60, 235–244. doi: 10.1016/j.ceca.2016.05.005
Makhro, A., Hanggi, P., Goede, J. S., Wang, J., Bruggemann, A., Gassmann, M., et al. (2013). N-methyl-D-aspartate receptors in human erythroid precursor cells and in circulating red blood cells contribute to the intracellular calcium regulation. Am. J. Physiol. Cell Physiol. 305, C1123–C1138. doi: 10.1152/ajpcell.00031.2013
Makhro, A., Huisjes, R., Verhagen, L. P., Manu-Pereira Mdel, M., Llaudet-Planas, E., Petkova-Kirova, P., et al. (2016b). Red cell properties after different modes of blood transportation. Front. Physiol. 7:288. doi: 10.3389/fphys.2016.00288
Mao, T. Y., Fu, L. L., and Wang, J. S. (2011). Hypoxic exercise training causes erythrocyte senescence and rheological dysfunction by depressed Gardos channel activity. J. Appl. Physiol. 111, 382–391. doi: 10.1152/japplphysiol.00096.2011
Mason, P. J., Bautista, J. M., and Gilsanz, F. (2007). G6PD deficiency: the genotype-phenotype association. Blood Rev. 21, 267–283. doi: 10.1016/j.blre.2007.05.002
Mauer, J., Mendez, S., Lanotte, L., Nicoud, F., Abkarian, M., Gompper, G., et al. (2018). Flow-induced transitions of red blood cell shapes under shear. Phys. Rev. Lett. 121:118103. doi: 10.1103/PhysRevLett.121.118103
Menzel, S., and Thein, S. L. (2019). Genetic modifiers of fetal haemoglobin in sickle cell disease. Mol. Diagn. Ther. 23, 235–244. doi: 10.1007/s40291-018-0370-8
Miccio, L., Memmolo, P., Merola, F., Netti, P. A., and Ferraro, P. (2015). Red blood cell as an adaptive optofluidic microlens. Nat. Commun. 6:6502. doi: 10.1038/ncomms7502
Minetti, G., Achilli, C., Perotti, C., and Ciana, A. (2018). Continuous change in membrane and membrane-skeleton organization during development from proerythroblast to senescent red blood cell. Front. Physiol. 9:286. doi: 10.3389/fphys.2018.00286
Minetti, G., Egee, S., Morsdorf, D., Steffen, P., Makhro, A., Achilli, C., et al. (2013). Red cell investigations: art and artefacts. Blood Rev. 27, 91–101. doi: 10.1016/j.blre.2013.02.002
Miura, O., D’andrea, A., Kabat, D., and Ihle, J. N. (1991). Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol. Cell. Biol. 11, 4895–4902. doi: 10.1128/mcb.11.10.4895
Mock, D. M., Lankford, G. L., Widness, J. A., Burmeister, L. F., Kahn, D., and Strauss, R. G. (1999). Measurement of red cell survival using biotin-labeled red cells: validation against 51Cr-labeled red cells. Transfusion 39, 156–162.
Mohandas, N., and Groner, W. (1989). Cell membrane and volume changes during red cell development and aging. Ann. N. Y. Acad. Sci. 554, 217–224. doi: 10.1111/j.1749-6632.1989.tb22423.x
Mosca, A., Paleari, R., Modenese, A., Rossini, S., Parma, R., Rocco, C., et al. (1991). Clinical utility of fractionating erythrocytes into “Percoll” density gradients. Adv. Exp. Med. Biol. 307, 227–238. doi: 10.1007/978-1-4684-5985-2_21
Mueller, T. J., Jackson, C. W., Dockter, M. E., and Morrison, M. (1985). Use of an in vivo enrichment procedure to study membrane skeletal protein changes during red cell aging. Prog. Clin. Biol. Res. 195, 227–236.
Mueller, T. J., Jackson, C. W., Dockter, M. E., and Morrison, M. (1987). Membrane skeletal alterations during in vivo mouse red cell aging. Increase in the band 4.1a:4.1b ratio. J. Clin. Invest. 79, 492–499. doi: 10.1172/jci112839
Mugnano, M., Memmolo, P., Miccio, L., Merola, F., Bianco, V., Bramanti, A., et al. (2018). Label-free optical marker for red-blood-cell phenotyping of inherited anemias. Anal. Chem. 90, 7495–7501. doi: 10.1021/acs.analchem.8b01076
Nakamura, Y., Komatsu, N., and Nakauchi, H. (1992). A truncated erythropoietin receptor that fails to prevent programmed cell death of erythroid cells. Science 257, 1138–1141. doi: 10.1126/science.257.5073.1138
Narayan, A. D., Ersek, A., Campbell, T. A., Colon, D. M., Pixley, J. S., and Zanjani, E. D. (2005). The effect of hypoxia and stem cell source on haemoglobin switching. Br. J. Haematol. 128, 562–570. doi: 10.1111/j.1365-2141.2004.05336.x
Oikonomidou, P. R., and Rivella, S. (2018). What can we learn from ineffective erythropoiesis in thalassemia? Blood Rev. 32, 130–143. doi: 10.1016/j.blre.2017.10.001
O’Neill, J. S., and Reddy, A. B. (2011). Circadian clocks in human red blood cells. Nature 469, 498–503. doi: 10.1038/nature09702
Parizadeh, S. M., Jafarzadeh-Esfehani, R., Bahreyni, A., Ghandehari, M., Shafiee, M., Rahmani, F., et al. (2019). The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors 45, 507–516. doi: 10.1002/biof.1518
Pernow, J., Mahdi, A., Yang, J., and Zhou, Z. (2019). Red blood cell dysfunction: a new player in cardiovascular disease. Cardiovasc. Res. 115, 1596–1605. doi: 10.1093/cvr/cvz156
Perrotta, S., Gallagher, P. G., and Mohandas, N. (2008). Hereditary spherocytosis. Lancet 372, 1411–1426. doi: 10.1016/S0140-6736(08)61588-3
Peters, A. L., and van Noorden, C. J. (2017). Single cell cytochemistry illustrated by the demonstration of Glucose-6-phosphate dehydrogenase deficiency in erythrocytes. Methods Mol. Biol. 1560, 3–13. doi: 10.1007/978-1-4939-6788-9_1
Petkova-Kirova, P., Hertz, L., Danielczok, J., Huisjes, R., Makhro, A., Bogdanova, A., et al. (2019). Red blood cell membrane conductance in hereditary haemolytic anaemias. Front. Physiol. 10:386. doi: 10.3389/fphys.2019.00386
Piccinini, G., Minetti, G., Balduini, C., and Brovelli, A. (1995). Oxidation state of glutathione and membrane proteins in human red cells of different age. Mech. Ageing Dev. 78, 15–26. doi: 10.1016/0047-6374(94)01511-j
Piva, E., Brugnara, C., Chiandetti, L., and Plebani, M. (2010). Automated reticulocyte counting: state of the art and clinical applications in the evaluation of erythropoiesis. Clin. Chem. Lab. Med. 48, 1369–1380. doi: 10.1515/CCLM.2010.292
Prus, E., and Fibach, E. (2013). Heterogeneity of F cells in beta-thalassemia. Transfusion 53, 499–504. doi: 10.1111/j.1537-2995.2012.03769.x
Quint, S., Christ, A. F., Guckenberger, A., Himbert, S., Kaestner, L., Gekle, S., et al. (2018). 3D tomography of cells in micro-channels. Appl. Phys. Lett. 111:103701. doi: 10.1063/1.4986392
Reichel, F., Mauer, J., Nawaz, A. A., Gompper, G., Guck, J., and Fedosov, D. A. (2019). High-throughput microfluidic characterization of erythrocyte shape and mechanical variability. bioRxiv [Preprint]. doi: 10.1101/488189
Romero, P. J., and Romero, E. A. (1997). Differences in Ca2+ pumping activity between sub-populations of human red cells. Cell Calcium 21, 353–358. doi: 10.1016/s0143-4160(97)90028-2
Romero, P. J., and Romero, E. A. (1999). The role of calcium metabolism in human red blood cell ageing: a proposal. Blood Cells Mol. Dis. 25, 9–19.
Romero, P. J., Salas, V., and Hernandez, C. (2002). Calcium pump phosphoenzyme from young and old human red cells. Cell Biol. Int. 26, 945–949.
Rotordam, M. G., Fermo, E., Becker, N., Barcellini, W., Bruggemann, A., Fertig, N., et al. (2019). A novel gain-of-function mutation of Piezo1 is functionally affirmed in red blood cells by high-throughput patch clamp. Haematologica 104, e179–e183.
Sadafi, A., Koehler, N., Makhro, A., Bogdanova, A., Navab, N., Marr, C. et al., (2019). “Multiclass deep active learning for detecting red blood cell subtypes in brightfield microscopy,” in Medical Image Computing and Computer Assisted Intervention – MICCAI 2019 Lecture Notes in Computer Science, ed. S. D. E. Al (Berlin: Springer).
Salvagno, G. L., Sanchis-Gomar, F., Picanza, A., and Lippi, G. (2015). Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 52, 86–105. doi: 10.3109/10408363.2014.992064
Salvo, G., Caprari, P., Samoggia, P., Mariani, G., and Salvati, A. M. (1982). Human erythrocyte separation according to age on a discontinuous “Percoll” density gradient. Clin. Chim. Acta 122, 293–300.
Sass, M. D., Caruso, C. J., and O’connell, D. J. (1965). Decreased glutathione in aging red Cells. Clin. Chim. Acta 11, 334–340.
Schaefer, R. M., and Schaefer, L. (1995). The hypochromic red cell: a new parameter for monitoring of iron supplementation during rhEPO therapy. J. Perinat. Med. 23, 83–88.
Sinha, A., Chu, T. T., Dao, M., and Chandramohanadas, R. (2015). Single-cell evaluation of red blood cell bio-mechanical and nano-structural alterations upon chemically induced oxidative stress. Sci. Rep. 5:9768. doi: 10.1038/srep09768
Sparrow, R. L. (2017). Red blood cell components: time to revisit the sources of variability. Blood Transfus. 15, 116–125. doi: 10.2450/2017.0326-16
Spencer, J. A., Ferraro, F., Roussakis, E., Klein, A., Wu, J., Runnels, J. M., et al. (2014). Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273. doi: 10.1038/nature13034
Steffen, P., Jung, A., Nguyen, D. B., Muller, T., Bernhardt, I., Kaestner, L., et al. (2011). Stimulation of human red blood cells leads to Ca2+-mediated intercellular adhesion. Cell Calcium 50, 54–61. doi: 10.1016/j.ceca.2011.05.002
Suzuki, T., and Dale, G. L. (1988). Senescent erythrocytes: isolation of in vivo aged cells and their biochemical characteristics. Proc. Natl. Acad. Sci. U.S.A. 85, 1647–1651.
Swammerdam, J. (1737). Bybel der Natuure of Historie der Insecten/Biblia Naturae sive Historia Insectorum. Utrecht: De Banier.
Thein, S. L., and Craig, J. E. (1998). Genetics of Hb F/F cell variance in adults and heterocellular hereditary persistence of fetal hemoglobin. Hemoglobin 22, 401–414.
Thompson, C. J., Schilling, T., Howard, M. R., and Genever, P. G. (2010). SNARE-dependent glutamate release in megakaryocytes. Exp. Hematol. 38, 504–515. doi: 10.1016/j.exphem.2010.03.011
Tomari, R., Zakaria, W. N. W., Jamil, M. M. A., and Fuad, N. F. N. (2014). Computer aided system for red blood cell classification in blood smear image. Proc. Comp. Sci. 42, 206–213.
Tusi, B. K., Wolock, S. L., Weinreb, C., Hwang, Y., Hidalgo, D., Zilionis, R., et al. (2018). Population snapshots predict early haematopoietic and erythroid hierarchies. Nature 555, 54–60. doi: 10.1038/nature25741
Wang, J., Wagner-Britz, L., Bogdanova, A., Ruppenthal, S., Wiesen, K., Kaiser, E., et al. (2013). Morphologically homogeneous red blood cells present a heterogeneous response to hormonal stimulation. PLoS One 8:e67697. doi: 10.1371/journal.pone.0067697
Wang, P. F., Song, S. Y., Guo, H., Wang, T. J., Liu, N., and Yan, C. X. (2019). Prognostic role of pretreatment red blood cell distribution width in patients with cancer: a meta-analysis of 49 studies. J. Cancer 10, 4305–4317. doi: 10.7150/jca.31598
Weber, R. E., Hiebl, I., and Braunitzer, G. (1988). High altitude and hemoglobin function in the vultures Gyps rueppellii and Aegypius monachus. Biol. Chem. Hoppe Seyler 369, 233–240.
Wenk, R. E. (1976). Comparison of five methods for preparing blood smears. Am. J. Med. Technol. 42, 71–78.
Wesseling, M. C., Wagner-Britz, L., Boukhdoud, F., Asanidze, S., Nguyen, D. B., Kaestner, L., et al. (2016). Measurements of intracellular Ca2+ content and phosphatidylserine exposure in human red blood cells: methodological issues. Cell. Physiol. Biochem. 38, 2414–2425. doi: 10.1159/000445593
Woll, P. S., Kjallquist, U., Chowdhury, O., Doolittle, H., Wedge, D. C., Thongjuea, S., et al. (2014). Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell 25, 794–808. doi: 10.1016/j.ccr.2014.03.036
Yin, Y., Ye, S., Wang, H., Li, B., Wang, A., Yan, W., et al. (2018). Red blood cell distribution width and the risk of being in poor glycemic control among patients with established type 2 diabetes. Ther. Clin. Risk Manag. 14, 265–273. doi: 10.2147/TCRM.S155753
Zhou, S., Giannetto, M., Decourcey, J., Kang, H., Kang, N., Li, Y., et al. (2019). Oxygen tension-mediated erythrocyte membrane interactions regulate cerebral capillary hyperemia. Sci. Adv. 5:eaaw4466. doi: 10.1126/sciadv.aaw4466
Keywords: red blood cells, heterogeneity, morphology, erythroid precursor cells, age
Citation: Bogdanova A, Kaestner L, Simionato G, Wickrema A and Makhro A (2020) Heterogeneity of Red Blood Cells: Causes and Consequences. Front. Physiol. 11:392. doi: 10.3389/fphys.2020.00392
Received: 14 January 2020; Accepted: 02 April 2020;
Published: 07 May 2020.
Edited by:
Dmitry A. Fedosov, Helmholtz Association of German Research Centers (HZ), GermanyReviewed by:
Manouk Abkarian, INSERM U1054 Centre de Biochimie Structurale de Montpellier, FranceRoberta Russo, University of Naples Federico II, Italy
Copyright © 2020 Bogdanova, Kaestner, Simionato, Wickrema and Makhro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Bogdanova, annab@access.uzh.ch
 Anna Bogdanova
Anna Bogdanova Lars Kaestner
Lars Kaestner Greta Simionato
Greta Simionato Amittha Wickrema5
Amittha Wickrema5 Asya Makhro
Asya Makhro