- 1Department of Gastroenterology, Xinyang Central Hospital, Xinyang, China
- 2National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, MD, United States
The gram-negative bacterium, Helicobacter pylori (H. pylori), infection is predominantly known for its strong association with development of gastric diseases, including gastritis, peptic ulcers, and stomach cancer. Numerous clinical reports show that ascorbic acid deficiency has been connect with gastritis. Vitamin C levels both in gastric acid and serum have constantly been affirmed to be low in subjects with H. pylori infected gastritis and peptic ulcers. Ascorbic acid supplementation likely relates to reduced incidences of bleeding from peptic ulcers and gastric cancer. H. pylori eradication is shown to increase vitamin C levels, while the benefits of ascorbic acid oral intake to increase the effectiveness of H. pylori-eradication therapy are controversial. Recent studies suggest that ascorbate intake intravenously, but not orally; pharmacologic ascorbate concentrations up to 30 mmol/L in blood, several millimolar in tissues as well as in interstitial fluid, are easily and safely achieved. Pharmacologic ascorbate can exert pro-oxidant effects locally as a drug by mediating hydrogen peroxide (H2O2) formation, which was applied to animal and clinical trials of cancer, sepsis, and severe burns etc. In this review, we summarize current understanding of the associations of vitamin C and H. pylori infection, and outline some potential strategies for H. pylori intervention from emerging advances on ascorbic acid physiology and pharmacology.
Introduction
Since Helicobacter pylori (H. pylori) was first identified in 1982 by Robin Warren and Barry Marshall, gastritis and peptic ulcer disease have been gradually approached as an infectious disease (Warren and Marshall, 1983; Suerbaum and Michetti, 2002). As one of the most common bacterial infection factors, H. pylori infects more than 50% of the world's population (Taylor and Blaser, 1991). Most infected people remain asymptomatic; however, 10 ~ 20% H. pylori infection will ultimately develop into chronic gastritis, peptic ulceration, mucosa-associated lymphoid tumors, or even gastric adenocarcinoma (Warren and Marshall, 1983; Parsonnet et al., 1991; Wündisch et al., 2005). More important, eradication of H. pylori is an effective treatment for gastritis, peptic ulcer disease, and early lymphoma of mucosal-associated lymphoid tissue (MALT); it also has the potential to reduce the risk of gastric cancer development (Parsonnet et al., 1991; Ito et al., 2002; Wong et al., 2004; Wündisch et al., 2005).
Vitamin C is one of essential micronutrients for human health. Due to the accumulation of several mutations that turned gulonolactone oxidase into a non-functional pseudogene, humans, unlike most animals, have lost the ability to perform the crucial last step of vitamin C biosynthesis (Nishikimi and Yagi, 1991; De Tullio, 2010); instead we must obtain vitamin C from diet. Two major functions of vitamin C are as antioxidants and cofactors. As a co-factor, ascorbic acid donates electrons for at least 15 mammalian enzymes, including hydroxylase and monooxygenase involved in the synthesis of carnitine, collagen, and neurotransmitters (Levine et al., 2011; Padayatty and Levine, 2016). As an antioxidant, vitamin C protects the body from various deleterious effects of free radicals and reactive oxygen species (ROS) that are produced during normal metabolic processes, via active immune cells, as well as by exposure to toxins and contaminants (Carr and Frei, 1999). Low levels of vitamin C have been associated with many conditions, including scurvy, bleeding tendency, delayed wound healing, anemia, some cancers, infections, etc. (Naidu, 2003; Grosso et al., 2013; Padayatty and Levine, 2016). Regarding peptic ulcer disease and its complications, it is well known that ascorbic acid deficiency has been related to high occurrence of gastritis and bleeding from gastric and duodenal ulcers as well (Waring et al., 1996; Zhang et al., 1998; Aditi and Graham, 2012). Lower vitamin C levels, both in gastric juice and serum, have repeatedly been linked to patients with H. pylori infected gastritis and peptic ulcers (Ruiz et al., 1994; Zhang et al., 1998; Annibale et al., 2003). Normally, gastric gland ascorbate concentrations are three to seven times higher than plasma levels, indicating that ascorbic acid is actively transported or secreted from the plasma into the gastric juice (Annibale et al., 2003; Aditi and Graham, 2012). Ascorbic acid supplementations have been shown to be inversely related to gastric cancer (Zhang et al., 2002; Wong et al., 2004; Lam et al., 2013). H. pylori eradication can reverse the negative effect and increase vitamin C levels in serum and gastric juice; however, studies of ascorbic acid oral intake on H. pylori-eradication therapy reported ambiguous results (Sobala et al., 1993; Banerjee et al., 1994; Jarosz et al., 1998; Koçkar et al., 2001; Sezikli et al., 2012; Demirci et al., 2015).
We emphasize the importance of vitamin C concentration-function relationships in human health status. Vitamin C is playing different pathological, physiological, or pharmacological functions under the recognized reference range for plasma ascorbic acid concentrations of deficiency, healthy, or therapy dosage in vivo (Levine et al., 2011; Padayatty and Levine, 2016; Robitaille and Hoffer, 2016). Even with supplementation approaching maximally tolerated oral doses at 3–4 g, plasma ascorbate concentrations will just reach a plateau of about 200 ~ 300 μmol/L. In contrast, with intravenous ascorbate intake, pharmacologic ascorbate concentration of 25 ~ 30 mmol/L has been safely attained to treat various cancers, severe burns, sepsis, and other diseases (Tanaka et al., 2000; Nathens et al., 2002; Levine et al., 2011; Parrow et al., 2013; Wilson, 2013). The purpose of this review is to update the current knowledge of pharmacological vitamin C clinical data, associations of vitamin C and H. pylori infection, and the relevance it has in clinical use since ascorbic acid treatment on H. pylori eradication is yet to be fully understood.
Current Knowledge
Vitamin C Concentration-Function Relationship
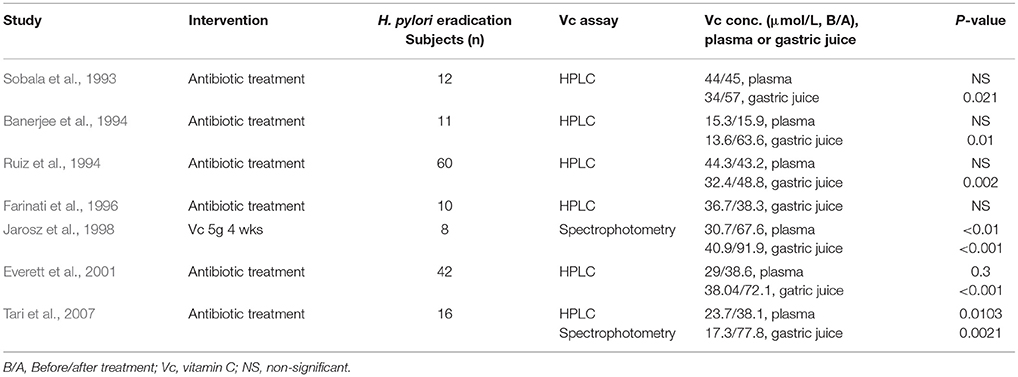
A conception was proposed more than three decades ago that ideal vitamin intake is best determined based on biochemical, functional, and/or clinical outcome in relation to vitamin concentration (Levine, 1986). The concentration-function approach applying to vitamin C may be more desirable than recommended dietary allowance (RDA) for ascorbic acid, which was based on a safety margin to prevent deficiency (Levine et al., 2011). As shown in Table 1, there is an obvious causal chain of vitamin C concentration-function relationships among vitamin C intake, plasma ascorbate concentration, and relevant functional outcomes. Scurvy usually occurs when people consumed a diet with persistent lack of sufficient amounts of vitamin C (<10 mg daily), the diagnosis is confirmed by recording the plasma vitamin C concentration <11.4 μmol/L and observing the clinical improvement after appropriate oral vitamin C administration (Table 1; Lindblad et al., 2013; Robitaille and Hoffer, 2016). Low plasma ascorbate level, or hypovitaminosis C (plasma vitamin C concentration: 11.4 ~ 27 μmol/L) associated with a variety of disease complexes including cancer, sepsis, gastric ulcer, etc, may affect ~ 10% of the general population (Lindblad et al., 2013; Robitaille and Hoffer, 2016). Clinical data of H. pylori infected gastritis showed a typical example of vitamin C concentration-function relationship in Table 2, H. pylori infection was consistently associated with low vitamin C concentrations in the gastric juice before treatment, probably due to reduced intake, increased oxidation, and impaired or absent ascorbate secretion (Sobala et al., 1993; Banerjee et al., 1994; Ruiz et al., 1994; Farinati et al., 1996; Jarosz et al., 1998; Everett et al., 2001; Woodward et al., 2001; Henry et al., 2005; Tari et al., 2007). Vitamin C concentrations in gastric juice, but not in plasma, were improved significantly after H. pylori eradication (Table 2), it implied that H. pylori eradication recovers the normal transport or secretion of ascorbic acid from plasma into gastric juice. However, a large number of population-based surveys have shown that higher serum levels of ascorbic acid were associated with a decreased seroprevalence of H. pylori and especially of the pathogenic cagA-positive strain of H. pylori (Simon et al., 2003). In normal humans, vitamin C is vigorously transported into and concentrated in gastric juice; high concentration of ascorbate in gastric juice can inactivate and denature urease secreted by H. pylori at low pH mediated by H2O2 in the presence of Fe(3+) ions, preventing H. pylori survival and colonization into acidic stomach (Krajewska and Brindell, 2011; Pal et al., 2011).
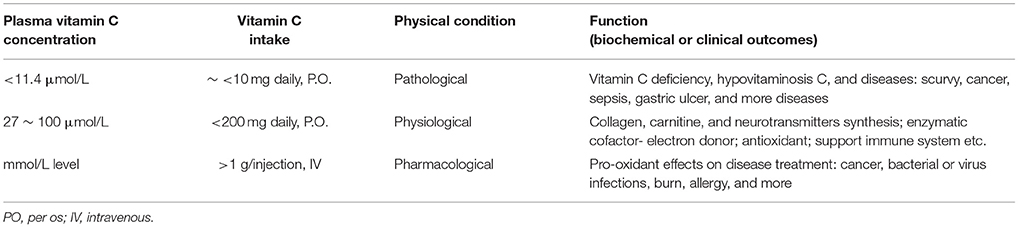
Table 1. Vitamin C concentration-function relationship: Pathology - vitamin C deficiency or low level in diseases; Physiology - normal range of plasma vitamin C level: enzymatic cofactor and antioxidant; Pharmacology - high dose intravenous vitamin C administration: pro-oxidant effects.
Normal dietary intake of ascorbic acid (≈ 40 mg per serving of fruits and vegetables, 2 ~ 5 servings daily) results in a recognized reference range for plasma ascorbic acid concentrations ranging from 27 to 100 μmol/L (Levine et al., 2001), which allows vitamin C to play its normal physiological role as enzymatic cofactors or antioxidant (Table 1). Ingestion of more vitamin C from foods will not produce higher concentrations in vivo, however, intravenous ascorbate administration produced plasma concentrations at millimolar level unachievable through oral administration (Padayatty et al., 2004; Chen et al., 2005; Parrow et al., 2013). Pharmacologic ascorbate can be used as a pro-drug for the formation of H2O2; the H2O2 concentration in extracellular fluid can reach as high as 200 μmol/L, which leads to production of large amounts of ROS inside or outside of cancer cells via iron mediated Fenton reactions and thus cause damage on macromolecules in cancer cells (Chen et al., 2007; Levine et al., 2011; Schoenfeld et al., 2017). The effects of pharmacologic ascorbate were further studied on clinical trials of cancer, bacterial or virus infections, burn, allergy, and so on (Tables 1, 4, 5).
The clinical depletion-repletion pharmacokinetic data and other studies demonstrated that the concentrations of vitamin C in plasma and tissues were strictly controlled through intestinal absorption (bioavailability), tissue transport, renal reabsorption and excretion, and probably increased utilization in diseases (Levine et al., 2011). Cellular accumulation of vitamin C is due to transport of both ascorbic acid and its oxidized form (dehydroascorbic acid / DHA) in vivo. Ascorbate is transported via at least two sodium-dependent ascorbate transporters: SVCT1/Slc23a1 and SVCT2/Slc23a2 (Sotiriou et al., 2002; Corpe et al., 2010); SVCT1, which is confined to epithelial systems including liver, intestine, and kidney; and SVCT2, which is mainly expressed in brain, skin, kidney, lung, and placenta etc. (Figure 1, upper panel). Whereas DHA is primarily transported by facilitated glucose transporters, GLUT1 ~ 4, with different affinity and tissue expression abundance, and then reduced intracellularly to ascorbate immediately (Figure 1, low panel; Rumsey et al., 1997, 2000; Corpe et al., 2013). These transporter genes with elevated expression in particular tissues are closely related to their corresponding functions involved in the tight control mechanisms over vitamin C concentrations in vivo (Figure 1; Padayatty et al., 2004; Levine et al., 2011).
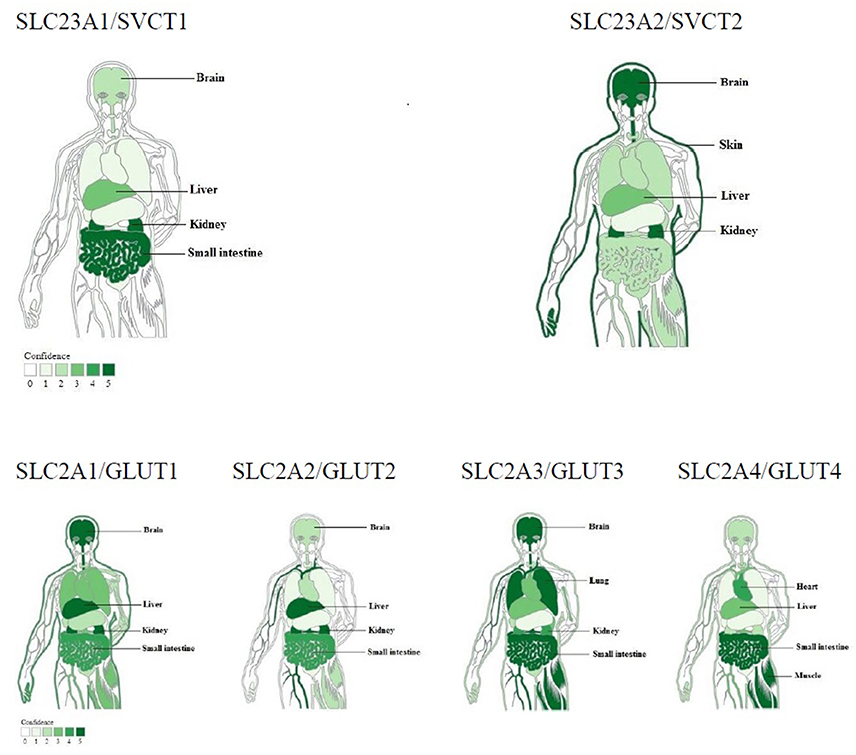
Figure 1. Tissue expression of ascorbic acid or DHA transporters responsible for tight control of vitamin C concentrations (modified from TISSUES: Tissue expression database). Ascorbic acid transporters: sodium-dependent vitamin C transporters 1 and 2. DHA transporters: Glucose transporter 1 ~ 4. Top four tissues with higher expression abundance were labeled for each transporter.
Ascorbic Acid Supplement on H. pylori Eradication: Controversial Data
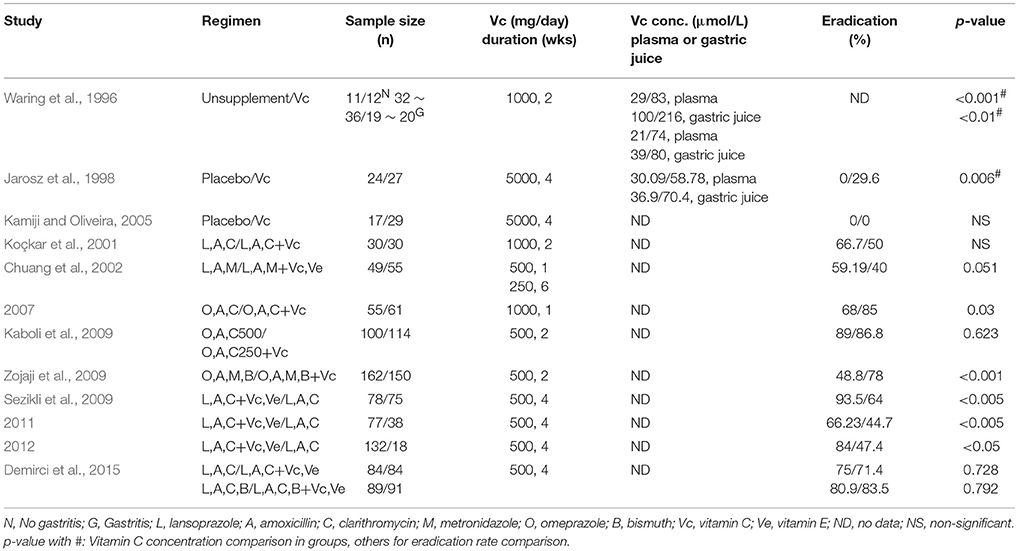
Randomized trials have produced different results in the effects of vitamin C oral supplement on H. pylori eradication (Table 3). When supplemented vitamin C alone, only Jarosz et al. reported 29.6% H. pylori eradication rate in 5 g/day over 4 weeks vitamin C group, other reports showed that vitamin C oral intake had no effects on H. pylori eradication even with significant improvement of vitamin C concentration in plasma or gastric juice (Waring et al., 1996; Jarosz et al., 1998; Kamiji and Oliveira, 2005). When supplemented vitamin C along with standard antibiotic treatment on H. pylori infection, the results of H. pylori eradication were not consistent as well (Table 3). Some trials reported significant improvement on H. pylori eradication rate in antibiotic plus vitamin C groups compared to antibiotic groups (Chuang et al., 2007; Sezikli et al., 2009, 2011, 2012; Zojaji et al., 2009), but other studies showed no benefit from vitamin C addition (Koçkar et al., 2001; Chuang et al., 2002; Kaboli et al., 2009; Demirci et al., 2015).
Although all these trials had high vitamin C dosage at 250 ~ 5,000 mg daily over 1 ~ 6 weeks with oral administration, plasma or gastric juice vitamin C concentrations were not reported in most of them (Table 3). Due to the above mentioned tight-controlled mechanisms, vitamin C daily doses for oral ingestion above 200 ~ 400 mg have no significant value for increasing vitamin C concentrations in plasma and tissues after reaching a plateau concentration of around 100 ~ 300 μmol/L (Levine et al., 1999, 2001). It thus made for a faulty experimental design for these trials without vitamin C concentration measurements (Table 3).
Several clinical attempts of intravenous injection with 500 or 1,000 mg vitamin C dosage were attempted to reverse initial low plasma or gastric juice ascorbic acid concentrations in ulcer patients. As early as in 1938, the effect of oral or intravenous administration of 1,000 mg of ascorbic acid on total body ascorbic acid stores were assessed; Portnoy and Wilkinson found that ulcer patients needed 3 ~ 4 times more amount of ascorbic acid intake to saturate body stores than normal controls (Portnoy and Wilkinson, 1938). In a case report, Sobala et al. IV injected a subject with 500 mg ascorbic acid at day 170 before H. pylori infection, day 37 and 161 after H. pylori infection respectively; it showed that the fasting gastric juice ascorbic acid rose rapidly at day170 sample, but was scarcely detectable at day 37 and remained low and rose only slightly at day 161 after the illness (Sobala et al., 1991). The effect of ethnicity, pH, and H. pylori infection on the changes of ascorbic acid concentration in gastric juice after intravenous injection of 500 mg vitamin C were examined; Correa et al. reported that 24 patients infected with H. pylori had a smaller but not statistically significant increase of ascorbic acid in gastric juice after intravenous injection (Correa et al., 1995).
Although the increased ascorbic acid in gastric juice was reported after IV injection, effects of ascorbic acid supplementation on H. pylori eradication were not mentioned in these studies (Portnoy and Wilkinson, 1938; Sobala et al., 1991; Correa et al., 1995). Notably, all these 500 or 1,000 mg vitamin C intravenous injections were administered only once, the dosage and duration of vitamin C IV injection may not be high and long enough there (Portnoy and Wilkinson, 1938; Sobala et al., 1991; Correa et al., 1995). Based on the conception of vitamin C concentration-function relationship, more clinical trials of pharmacological ascorbate on gastric ulcer and H. pylori eradication are warranted.
Recent Clinical Use of Pharmacological Ascorbate on Cancer and Other Diseases
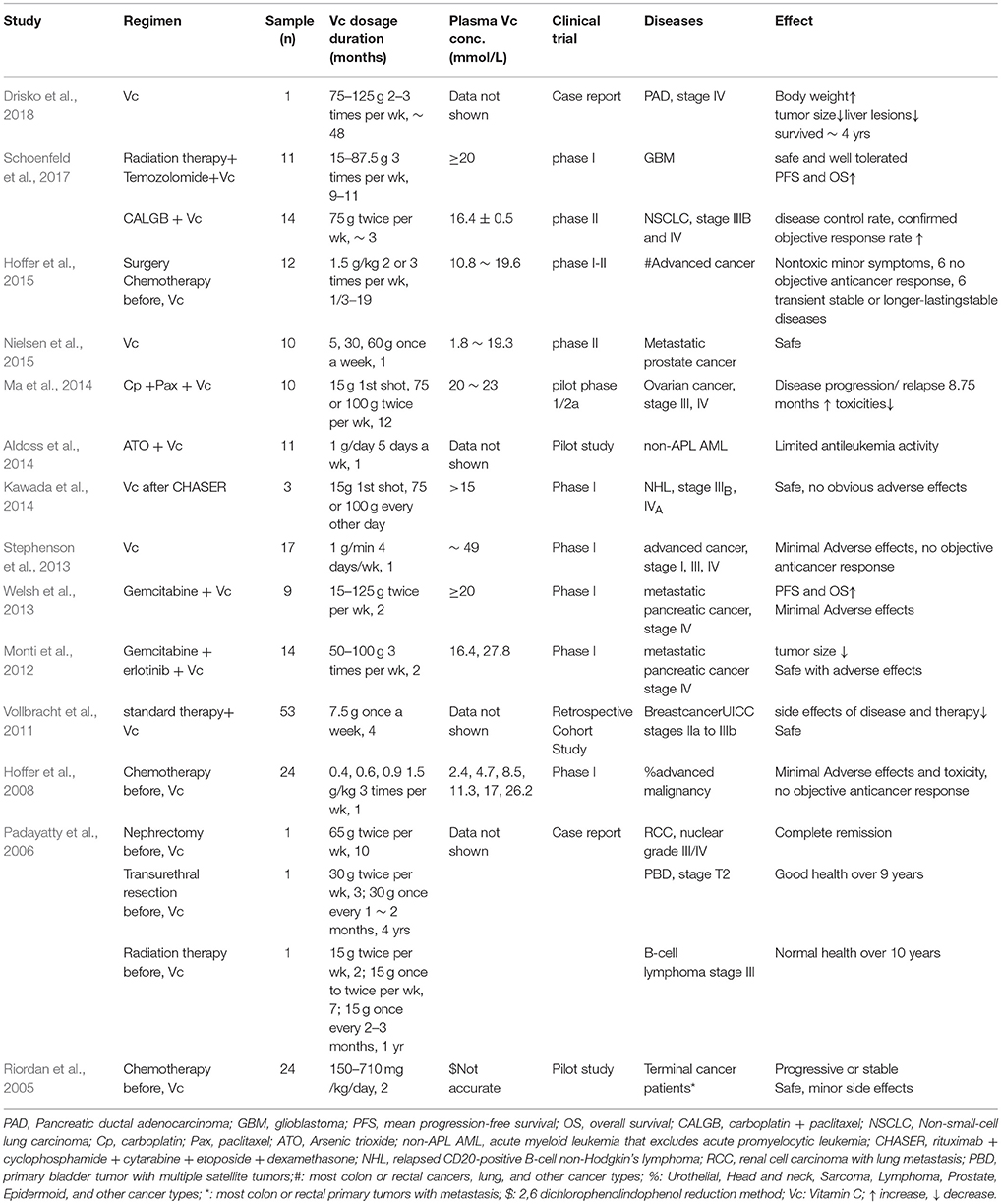
The efficacy of vitamin C in cancer treatment has a controversial history over several decades. Observational-uncontrolled trials of pharmacologic ascorbate conducted by Cameron, Campbell, and Pauling on terminal cancer patients, given in mega doses of 10 g per day intravenously for 10 days and then orally administered 10 g/day indefinitely, suggested encouraging results including decreased tumor growth, increased survival time, and improved patient well-being (Cameron and Campbell, 1974; Cameron and Pauling, 1976, 1978). However, two well designed, double-blind placebo-controlled clinical trials undertaken at the Mayo Clinic on advanced cancer patients, treated with 10 g/day of ascorbate orally, showed no survival advantage at all (Creagan et al., 1979; Moertel et al., 1985). Unfortunately, these negative data led to the suspension of ascorbic acid as a potential cancer treatment, which was almost discarded by medical and scientific communities. Both teams treated patients with 10 g/day of ascorbic acid, the different routes of vitamin C administration, orally or intravenously, were the key and brought diametrically opposed in effects of cancer treatment. Based on the clinical depletion-repletion pharmacokinetic data, it is now clear that oral vitamin C produces a strictly controlled plasma concentration of μmol/L vitamin C; and the pharmacologic concentrations of vitamin C in the plasma at a level of mmol/L can only be achieved by parenteral administration, which bypassed such tight control mechanism (Levine et al., 1996, 2001, 2011; Padayatty et al., 2004; Parrow et al., 2013). Established by seminal studies by Chen et al. and with more in vitro and animal trial data from many laboratories (Padayatty et al., 2004; Chen et al., 2005, 2007, 2008; Yun et al., 2015; Schoenfeld et al., 2017), gradually, parenteral ascorbate for cancer treatment revitalized uneasily, with recent phase I or II clinical trials on various cancer types (Table 4).
As shown in Table 4, phase I or II trials, pilot studies, case reports, and retrospective cohort study of pharmacologic ascorbic acid on cancer treatment were published, including glioblastoma, B-cell lymphoma, non-Hodgkin's lymphoma, acute myeloid leukemia, breast cancer, non-small-cell lung carcinoma, metastatic pancreatic cancer, primary bladder tumor, renal cell carcinoma, metastatic prostate cancer, ovarian cancer, and other advanced malignancy (Riordan et al., 2005; Padayatty et al., 2006; Hoffer et al., 2008, 2015; Vollbracht et al., 2011; Monti et al., 2012; Stephenson et al., 2013; Welsh et al., 2013; Aldoss et al., 2014; Kawada et al., 2014; Ma et al., 2014; Nielsen et al., 2015; Schoenfeld et al., 2017; Drisko et al., 2018). All studies reported that intravenous vitamin C at dosage from 1g/day 5 days a week over 1 month to 75–125 g 2–3 times per week over 48 months, is generally safe, no toxicities, and well tolerated with minor adverse effects (Table 4; Aldoss et al., 2014; Drisko et al., 2018). Plasma vitamin C concentrations were measured and recorded from 1.8 to 49 mmol/L (Table 4; Stephenson et al., 2013; Nielsen et al., 2015), which are 30 ~ 600-fold higher than normal physical plasma ascorbic acid level. More important, when combined with standard cancer therapy and high dosage over a long period, intravenous ascorbic acid on some cancer types showed similar clinical benefits and improvement as before (Table 4). These positive results are prompting larger, longer-duration phase II or III clinical trials to determine susceptible cancer types, proper dosage, and precise clinical efficacy; such trials of pharmacologic ascorbate on advanced colorectal, gastric cancers are currently under way (NCT02969681; NCT03015675). To determine gastric cancer incidence and cause-specific mortality of 3,365 participants, in a masked factorial placebo-controlled trial with 14.7-year follow-up, Ma et al. reported that vitamins oral supplement (250 mg vitamin C, 100 IU vitamin E, and 37.5 μg selenium from yeast twice daily for a total of 7.3 years) had no significant effect on gastric cancer incidence and mortality (Ma et al., 2012). These negative results of oral vitamin C supplement on gastric cancer made the ongoing clinical trial of pharmacologic ascorbate on gastric cancer another good example to monitor (NCT03015675).
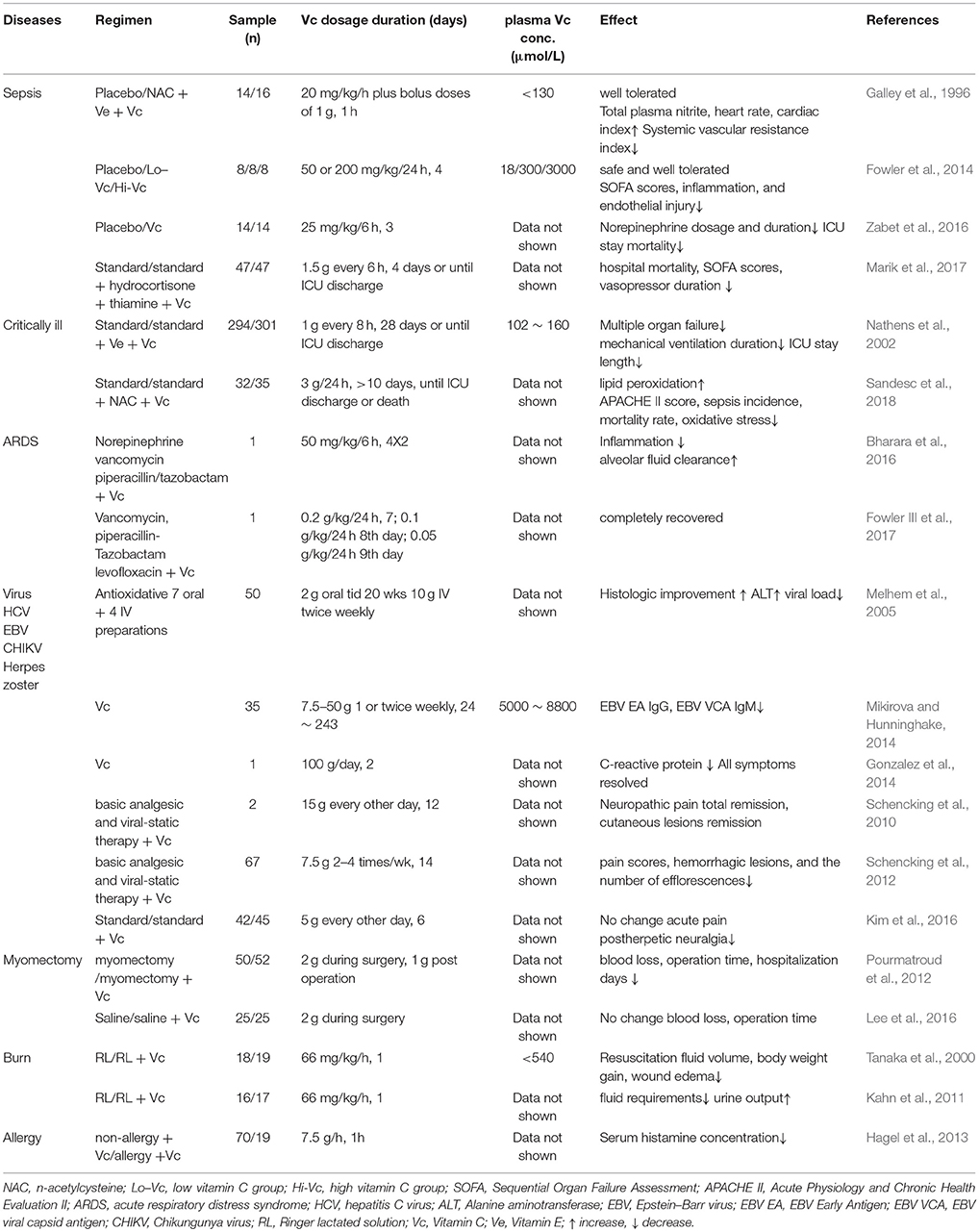
Pharmacologic ascorbate has also been widely used to treat and prevent many disorders like bacterial or virus infections, burns, allergies, and other diseases (Table 5). Intravenous vitamin C was given in doses of 1 or 2 g to 15 g per day, and plasma vitamin C concentrations could be reached 0.1 ~ 8.8 mmol/L (Table 5), which were one order of magnitude less than pharmacological ascorbate used in cancer treatments (Table 4). Clinical studies of patients with severe sepsis have found that intravenous vitamin C doses from 2.4 g over 1 h to 14 g/day over 4 days, increases total plasma nitrite, heart rate, cardiac index, and decreases the levels of pro-inflammatory biomarkers, SOFA scores, and mortality of ICU stay (Table 5; Galley et al., 1996; Fowler et al., 2014; Zabet et al., 2016; Marik et al., 2017). Two prospective trials of critically ill patients reported that standard therapy plus 3g/day intravenous ascorbic acid treatment reduced multiple organ failure, ICU stay length, and mortality rate as well (Table 5; Nathens et al., 2002; Sandesc et al., 2018). Two case reports of intravenous vitamin C injection, as adjuvant treatment for acute respiratory distress syndrome, showed reduced inflammation, increased alveolar fluid clearance, and even complete recovery (Table 5; Bharara et al., 2016; Fowler III et al., 2017). Pharmacologic ascorbate was also applied to treat virus infections like herpes zoster virus, hepatitis C virus, Epstein–Barr virus, and chikungunya virus, and most of them improved significantly (Table 5; Melhem et al., 2005; Schencking et al., 2010, 2012; Gonzalez et al., 2014; Mikirova and Hunninghake, 2014; Kim et al., 2016). Administrated 2 g ascorbic acid intravenously during myomectomy surgeries showed inconsistent effect of blood loss (Table 5; Pourmatroud et al., 2012; Lee et al., 2016). Given 66 mg/kg/h intravenous vitamin C to severe burn patients for the first day, they required less resuscitation fluid volume with more urine output (Table 5; Tanaka et al., 2000; Kahn et al., 2011). Furthermore, Hagel et al. found that intravenous infusion of 7.5 g of ascorbic acid could reduce the serum histamine concentrations in patients with infectious and allergic diseases (Table 5; Hagel et al., 2013).
Future Prospects
Low Vitamin C Levels in H. pylori Infection: Potential Mechanisms
Ascorbate concentrations are lower in H. pylori infection, probably because of insufficient vitamin C ingestion and corresponding down-regulation of vitamin C concentrations tight control mechanisms including less bioavailability, impaired stomach transport or secretion, and H. pylori-associated oxidants accelerating ascorbic acid, or DHA degradation (Woodward et al., 2001; Annibale et al., 2003; Henry et al., 2005; Levine et al., 2011; Aditi and Graham, 2012). Woodward et al. compared a large number of subjects with/without H. pylori infection and suggested that systemic bioavailability of ascorbic acid in patients with H. pylori was impaired and not related to diet (Woodward et al., 2001). Henry et al. found that proton pump inhibitor omeprazole (40 mg/day, 28 days) decreased plasma vitamin C level in both H. pylori positive and negative subjects with similar ascorbate dietary intake, and indicating a reduced bioavailability of dietary vitamin C (Henry et al., 2005). Alternatively, the lower plasma vitamin C concentrations in patients with H. pylori-infected gastritis after eradication may be the consequence of increased active transport of ascorbic acid to regain the high ratio of gastric juice to plasma ascorbic acid (Annibale et al., 2003). In addition, H. pylori infection is an inflammatory process producing great amount of ROS; therefore, it is also possible that ascorbate utilization increases in inflammation (Ellulu et al., 2015). Insufficient vitamin C ingestion might be easily avoided through more vitamin C supplementing with pills or from vegetables or fruits (Woodward et al., 2001; Henry et al., 2005). The decrease in plasma vitamin C induced by H. pylori infection and/or omeprazole depends less intestinal absorption or more renal leak (Woodward et al., 2001; Henry et al., 2005). To characterize transport or secretion of ascorbic acid from plasma into gastric juice directly (not just assuming active secretion of ascorbic acid from high gastric juice:plasma ascorbic acid ratio), It is worthwhile to further investigate how H. pylori infection (inflammatory molecules or H pylori's virulence factors) or medicine affect the function of vitamin C and DHA transporters (Figure 1).
Pharmacologic Ascorbate on H. pylori Eradication: H. pylori Antibiotic Resistance
As mentioned above, combined oral vitamin C as high as 5 g with standard antibiotic treatment on H. pylori infection, the results of H. pylori eradication were controversial; the saturated plasma ascorbic acid concentration with oral intake around 100 ~ 300 μmol/L may not be high enough for H. pylori eradication (Table 3; Levine et al., 1999, 2001). To be applied to cancer treatments or other diseases, pharmacologic ascorbate as a treatment were easily and safely reached up to 25 ~ 30 mmol/L in blood; and the concentration of H2O2 at ~ 200 μmol/L as a pro-oxidant drug induced by pharmacologic ascorbate, which was 100-fold of those concentrations that regulate normal cellular processes (Tables 4, 5; Padayatty et al., 2004; Stone and Yang, 2006; Levine et al., 2011; Parrow et al., 2013). Antibiotic treatments are still the primary methods to eradicate H. pylori; however, this strategy has been hampered by the recent emergence and spread of H. pylori antibiotic resistance in most countries worldwide with frequent treatment failures in at least 10–20% of patients (Pal et al., 2011; Megraud et al., 2013; Camargo et al., 2014; Thung et al., 2016). Taken together, pharmacologic ascorbate may be an obvious addition to existing antibiotic therapies for synergy treatment on H. pylori infection. The hundreds-fold elevated concentration of plasma vitamin C and H2O2 may be especially useful for eradication of H. pylori with multiple antibiotic resistances. If it worked as in cancer treatment, pharmacologic ascorbate would play synergic role to cope with H. pylori antibiotic resistance and reverse the low ascorbic acid concentrations in blood and gastric acid induced by H. pylori infection.
Conclusions
Current clinical data of H. pylori infected gastritis suggested a typical example of vitamin C concentration-function relationship among less vitamin C intake, low ascorbic acid concentrations in gastric juice and plasma, and relevant pathological outcomes of gastric diseases. H. pylori eradication had an inverse association with vitamin C concentrations in gastric juice and plasma. In contrast, oral ascorbic acid supplement with or without standard antibiotic treatment on H. pylori eradication yielded controversial data. The route of vitamin C administration, orally or intravenously, is critical for plasma ascorbate concentration with two orders of magnitude difference. Intravenous vitamin C, also termed pharmacological ascorbate could achieve 25 ~ 30 mmol/L and form high concentration of H2O2 as a pro-oxidant drug, which was been extensively used to treat and prevent many disorders like various cancers and other diseases. With all these knowledge and research progress, it is worthwhile to include pharmacologic ascorbate with or without standard antibiotic treatment on H. pylori eradication, especially for H. pylori with antibiotic resistances.
Author Contributions
HM and HT did literature research, wrote the manuscript, and read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Brian Brown, NIH library editing service, for reviewing the manuscript.
References
Aditi, A., and Graham, D. Y. (2012). Vitamin C, gastritis, and gastric disease: a historical review and update. Dig. Dis. Sci. 57, 2504–2515. doi: 10.1007/s10620-012-2203-7
Aldoss, I., Mark, L., Vrona, J., Ramezani, L., Weitz, I., Mohrbacher, A. M., et al. (2014). Adding ascorbic acid to arsenic trioxide produces limited benefit in patients with acute myeloid leukemia excluding acute promyelocytic leukemia. Ann. Hematol. 93, 1839–1843. doi: 10.1007/s00277-014-2124-y
Annibale, B., Capurso, G., Lahner, E., Passi, S., Ricci, R., Maggio, F., et al. (2003). Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut 52, 496–501. doi: 10.1136/gut.52.4.496
Banerjee, S., Hawksby, C., Miller, S., Dahill, S., Beattie, A. D., and McColl, K. E. (1994). Effect of Helicobacter pylori and its eradication on gastric juice ascorbic acid. Gut 35, 317–322.
Bharara, A., Grossman, C., Grinnan, D., Syed, A., Fisher, B., DeWilde, C., et al. (2016). Intravenous vitamin C administered as adjunctive therapy for recurrent acute respiratory distress syndrome. Case Rep. Crit. Care 2016:8560871. doi: 10.1155/2016/8560871
Camargo, M. C., García, A., Riquelm,e, A., Otero, W., Camargo, C. A., Hernandez-García, T., et al. (2014). The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am. J. Gastroenterol. 109, 485–495. doi: 10.1038/ajg.2014.24
Cameron, E., and Campbell, A. (1974). The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem. Biol. Interact. 9, 285–315.
Cameron, E., and Pauling, L. (1976). Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U.S.A. 73, 3685–3689.
Cameron, E., and Pauling, L. (1978). Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U.S.A. 75, 4538–4542.
Carr, A. C., and Frei, B. (1999). Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 69, 1086–1107.
Chen, Q., Espey, M. G., Krishna, M. C., Mitchell, J. B., Corpe, C. P., Buettner, G. R., et al. (2005). Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. U.S.A. 102, 13604–13609 doi: 10.1073/pnas.0506390102
Chen, Q., Espey, M. G., Sun, A. Y., Lee, J. H., Krishna, M. C., Shacter, E., et al. (2007). Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular ?uid in vivo. Proc. Natl. Acad. Sci. U.S.A. 104, 8749–8754. doi: 10.1073/pnas.0702854104
Chen, Q., Espey, M. G., Sun, A. Y., Pooput, C., Kirk, K. L., Krishna, M. C., et al. (2008). Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. U.S.A. 105, 11105–11109. doi: 10.1073/pnas.0804226105
Chuang, C. H., Sheu, B. S., Huang, A. H., Yang, H. B., and Wu, J. J. (2002). Vitamin C and E supplements to lansoprazole-amoxicillin-metronidazole triple therapy may reduce the eradication rate of metronidazole-susceptible Helicobacter pylori infection. Helicobacter 7, 310–316. doi: 10.1046/j.1523-5378.2002.00095.x
Chuang, C. H., Sheu, B. S., Kao, A. W., Cheng, H. C., Huang, A. H., Yang, H. B., et al. (2007). Adjuvant effect of vitamin C on omeprazole-amoxicillin-clarithromycin triple therapy for Helicobacter pylori eradication. Hepatogastroenterology 54, 320–324.
Corpe, C. P., Eck, P., Wang, J., Al-Hasani, H., and Levine, M. (2013). Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. J. Biol. Chem. 288, 9092–9101. doi: 10.1074/jbc.M112.436790
Corpe, C. P., Tu, H., Eck, P., Wang, J., Faulhaber-Walter, R., Schnermann, J., et al. (2010). Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J. Clin. Invest. 120, 1069–1083. doi: 10.1172/JCI39191
Correa, P., Fontham, E. T., Ruiz, B., Malcom, G. R., Hunter, F. M., and Zavala, D. E. (1995). Gastric juice ascorbic acid after intravenous injection: effect of ethnicity, pH, and Helicobacter pylori infection. J. Natl. Cancer Inst. 87, 52–53.
Creagan, E. T., Moertel, C. G., O'Fallon, J. R., Schutt, A. J., O'Connell, M. J., Rubin, J., et al. (1979). Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 301, 687–690. doi: 10.1056/NEJM197909273011303
Demirci, H., UygunIlikhan, S., Öztürk, K., Üstündag, Y., Kurt, Ö., Bilici, M., et al. (2015). Influence of vitamin C and E supplementation on the eradication rates of triple and quadruple eradication regimens for Helicobacter pylori infection. Turk. J. Gastroenterol. 26, 456–460. doi: 10.5152/tjg.2015.0233
Drisko, J. A., Serrano, O. K., Spruce, L. R., Chen, Q., and Levine, M. (2018). Treatment of pancreatic cancer with intravenous vitamin C: a case report. Anticancer Drugs 29, 373–379. doi: 10.1097/CAD.0000000000000603
Ellulu, M. S., Rahmat, A., Patimah, I., Khaza'ai, H., and Abed, Y. (2015). Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Drug Des. Devel. Ther. 9, 3405–3412. doi: 10.2147/DDDT.S83144
Everett, S. M., Singh, R., Leuratti, C., White, K. L., Neville, P., Greenwood, D., et al. (2001). Levels of malondialdehyde-deoxyguanosine in the gastric mucosa: relationship with lipid peroxidation, ascorbic acid, and Helicobacter pylori. Cancer Epidemiol. Biomarkers Prev. 10, 369–376.
Farinati, F., Della Libera, G., Cardin, R., Molari, A., Plebani, M., Rugge, M., et al. (1996). Gastric antioxidant, nitrites, and mucosal lipoperoxidation in chronic gastritis and Helicobacter pylori infection. J. Clin. Gastroenterol. 22, 275–281.
Fowler, A. A. III., Syed, A. A., Knowlson, S., Sculthorpe, R., Farthing, D., DeWilde, C., et al. (2014). Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 12:32. doi: 10.1186/1479-5876-12-32
Fowler III, A. A., Kim, C., Lepler, L., Malhotra, R., Debesa, O., Natarajan, R., et al. (2017). Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J. Crit. Care Med. 6, 85–90. doi: 10.5492/wjccm.v6.i1.85
Galley, H. F., Davies, M. J., and Webster, N. R. (1996). Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radic. Biol. Med. 20, 139–143.
Gonzalez, M. J., Miranda-Massari, J. R., Berdiel, M. J., Duconge, J., Rodríguez-López, J. L., Hunninghake, R., et al. (2014). High dose intraveneous vitamin C and chikungunya fever: a case report. J. Orthomol. Med. 29, 154–156.
Grosso, G., Bei, R., Mistretta, A., Marventano, S., Calabrese, G., Masuelli, L., et al. (2013). Effects of vitamin C on health: a review of evidence. Front. Biosci. (Landmark Ed) 18, 1017–1029. doi: 10.2741/4160.
Hagel, A. F., Layritz, C. M., Hagel, W. H., Hagel, H. J., Hagel, E., Dauth, W., et al. (2013). Intravenous infusion of ascorbic acid decreases serum histamine concentrations in patients with allergic and non-allergic diseases. Naunyn Schmiedebergs. Arch. Pharmacol. 386, 789–793. doi: 10.1007/s00210-013-0880-1
Henry, E. B., Carswell, A., Wirz, A., Fyffe, V., and McColl, K. E. (2005). Proton pump inhibitors reduce the bioavailability of dietary vitamin C. Aliment. Pharmacol. Ther. 22, 539–545. doi: 10.1111/j.1365-2036.2005.02568.x
Hoffer, L. J., Levine, M., Assouline, S., Melnychuk, D., Padayatty, S. J., Rosadiuk, K., et al. (2008). Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 19, 1969–1974. doi: 10.1093/annonc/mdn377
Hoffer, L. J., Robitaille, L., Zakarian, R., Melnychuk, D., Kavan, P., Agulnik, J., et al. (2015). High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: a Phase I-II Clinical Trial. PLoS ONE 10:e0120228. doi: 10.1371/journal.pone.0120228
Ito, M., Haruma, K., Kamada, T., Mihara, M., Kim, S., Kitadai, Y., et al. (2002). Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol. Ther. 16, 1449–1456. doi: 10.1046/j.1365-2036.2002.01311.x
Jarosz, M., Dzieniszewski, J., Dabrowska-Ufniarz, E., Wartanowicz, M., Ziemlanski, S., and Reed, P. I. (1998). Effects of high dose vitamin C treatment on Helicobacter pylori infection and total vitamin C concentration in gastric juice. Eur. J. Cancer Prev. 7, 449–454.
Kaboli, S. A., Zojaji, H., Mirsattari, D., Talaie, R., Derakhshan, F., Zali, M. R., et al. (2009). Effect of additionof vitamin C to clarithromycin-amoxicillin-omeprazol triple regimen on Helicobacter pylori eradication. Acta Gastroenterol. Belg. 72, 222–224.
Kahn, S. A., Beers, R. J., and Lentz, C. W. (2011). Resuscitation after severe burn injury using high-dose ascorbic acid: a retrospective review. J. Burn Care Res. 32, 110–117. doi: 10.1097/BCR.0b013e318204b336
Kamiji, M. M., and Oliveira, R. B. (2005). Effect of vitamin C administration on gastric colonization by Helicobacter pylori. Arq. Gastroenterol. 42, 167–172. doi: 10.1590/S0004-28032005000300008
Kawada, H., Sawanobori, M., Tsuma-Kaneko, M., Wasada, I., Miyamoto, M., Murayama, H., et al. (2014). Phase I clinical trial of intravenous L-ascorbic acid following salvage chemotherapy for relapsed B-cell non-Hodgkin's Lymphoma. Tokai J. Exp. Clin. Med. 39, 111–115.
Kim, M. S., Kim, D. J., Na, C. H., and Shin, B. S. (2016). A study of intravenous administration of vitamin C in the treatment of acute herpetic pain and postherpetic neuralgia. Ann. Dermatol. 28, 677–683. doi: 10.5021/ad.2016.28.6.677
Koçkar, C., Oztürk, M., and Bavbek, N. (2001). Helicobacter pylori eradication with beta carotene, ascorbic acid and allicin. Acta Medica (Hradec. Kralove). 44, 97–100.
Krajewska, B., and Brindell, M. (2011). Urease activity and L-ascorbic acid. J. Enzyme Inhib. Med. Chem. 26, 309–318. doi: 10.3109/14756366.2010.504675
Lam, T. K., Freedman, N. D., Fan, J. H., Qiao, Y. L., Dawsey, S. M., Taylor, P. R., et al. (2013). Prediagnostic plasma vitamin C and risk of gastric adenocarcinoma and esophageal squamous cell carcinoma in a Chinese population. Am. J. Clin. Nutr. 98, 1289–1297. doi: 10.3945/ajcn.113.061267
Lee, B., Kim, K., Cho, H. Y., Yang, E. J., Suh, D. H., No, J. H., et al. (2016). Effect of intravenous ascorbic acid infusion on blood loss during laparoscopic myomectomy: a randomized, double-blind, placebo-controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 199, 187–191. doi: 10.1016/j.ejogrb.2016.02.014
Levine, M. (1986). New concepts in the biology and biochemistry of ascorbic acid. New Engl. J. Med. 31, 892–902.
Levine, M., Conry-Cantilena, C., Wang, Y., Welch, R. W., Washko, P. W., Dhariwal, K. R., et al. (1996). Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. U.S.A. 93, 3704–3709.
Levine, M., Padayatty, S. J., and Espey, M. G. (2011). Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv. Nutr. 2, 78–88. doi: 10.3945/an.110.000109
Levine, M., Rumsey, S. C., Daruwala, R., Park, J. B., and Wang, Y. (1999). Criteria and recommendations for vitamin C intake. JAMA 281, 1415–1423.
Levine, M., Wang, Y., Padayatty, S. J., and Morrow, J. (2001). A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. U.S.A. 98, 9842–9846. doi: 10.1073/pnas.171318198
Lindblad, M., Tveden-Nyborg, P., and Lykkesfeldt, J. (2013). Regulation of vitamin C homeostasis during deficiency. Nutrients 5, 2860–2879. doi: 10.3390/nu5082860
Ma, J. L., Zhang, L., Brown, L. M., Li, J. Y., Shen, L., Pan, K. F., et al. (2012). Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J. Natl. Cancer Inst. 104, 488–492. doi: 10.1093/jnci/djs003
Marik, P. E., Khangoora, V., Rivera, R., Hooper, M. H., and Catravas, J. (2017). Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest 151, 1229–1238. doi: 10.1016/j.chest.2016.11.036
Ma, Y., Chapman, J., Levine, M., Polireddy, K., Drisko, J., and Chen, Q. (2014). High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 6:222ra18. doi: 10.1126/scitranslmed.3007154
Megraud, F., Coenen, S., Versporten, A., Kist, M., Lopez-Brea, M., Hirschl, A. M. Study Group participants., et al. (2013). Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 62, 34–42. doi: 10.1136/gutjnl-2012-302254
Melhem, A., Stern, M., Shibolet, O., Israeli, E., Ackerman, Z., Pappo, O., et al. (2005). Treatment of chronic hepatitis C virus infection via antioxidants: results of a phase I clinical trial. J. Clin. Gastroenterol. 39, 737–742. doi: 10.1097/01.mcg.0000174023.73472.29
Mikirova, N., and Hunninghake, R. (2014). Effect of high dose vitamin C on Epstein-Barr viral infection. Med. Sci. Monit. 20, 725–732. doi: 10.12659/MSM.890423
Moertel, C. G., Fleming, T. R., Creagan, E. T., Rubin, J., O'Connell, M. J., and Ames, M. M. (1985). High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N. Engl. J. Med. 312, 137–141. doi: 10.1056/NEJM198501173120301
Monti, D. A., Mitchell, E., Bazzan, A. J., Littman, S., Zabrecky, G., Yeo, C. J., et al. (2012). Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS ONE 7:e29794. doi: 10.1371/journal.pone.0029794
Naidu, K. A. (2003). Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2:7. doi: 10.1186/1475-2891-2-7
Nathens, A. B., Neff, M. J., Jurkovich, G. J., Klotz, P., Farver, K., Ruzinski, J. T., et al. (2002). Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann. Surg. 236, 814–822.
Nielsen, T. K., Højgaard, M., Andersen, J. T., Poulsen, H. E., Lykkesfeldt, J., and Mikines, K. J. (2015). Elimination of ascorbic acid after high-dose infusion in prostate cancer patients: a pharmacokinetic evaluation. Basic Clin. Pharmacol. Toxicol. 116, 343–348. doi: 10.1111/bcpt.12323
Nishikimi, M., and Yagi, K. (1991). Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am. J. Clin. Nutr. 54(Suppl. 6), 1203S−1208S. doi: 10.1093/ajcn/54.6.1203s
Padayatty, S. J., and Levine, M. (2016). Vitamin C physiology: the known and the unknown and Goldilocks. Oral Dis. 22, 463–493. doi: 10.1111/odi.12446
Padayatty, S. J., Riordan, H. D., Hewitt, S. M., Katz, A., Hoffer, L. J., and Levine, M. (2006). Intravenously administered vitamin C as cancer therapy: three cases. CMAJ 174, 937–942. doi: 10.1503/cmaj.050346
Padayatty, S. J., Sun, H., Wang, Y., Riordan, H. D., Hewitt, S. M., Katz, A., et al. (2004). Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 140:533–537.
Pal, J., Sanal, M. G., and Gopal, G. J. (2011). Vitamin-C as anti-Helicobacter pylori agent: More prophylactic than curative- critical review. Indian J. Pharmacol. 43, 624–627. doi: 10.4103/0253-7613.89814
Parrow, N. L., Leshin, J. A., and Levine, M. (2013). Parenteral ascorbate as a cancer therapeutic: a reassessment based on pharmacokinetics. Antioxid. Redox Signal. 19, 2141–2156. doi: 10.1089/ars.2013.5372
Parsonnet, J., Friedman, G. D., Vandersteen, D. P., Chang, Y., Vogelman, J. H., Orentreich, N., et al. (1991). Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325, 1127–1131.
Portnoy, B., and Wilkinson, J. F. (1938). Vitamin C deficiency in peptic ulceration and haematemesis. Br. Med. J. 1, 554–560.
Pourmatroud, E., Hormozi, L., Hemadi, M., and Golshahi, R. (2012). Intravenous ascorbic acid (vitamin C) administration in myomectomy: a prospective, randomized clinical trial. Arch. Gynecol. Obstet. 285, 111–115. doi: 10.1007/s00404-011-1897-7
Riordan, H. D., Casciari, J. J., González, M. J., Riordan, N. H., Miranda-Massari, J. R., Taylor, P., et al. (2005). A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P. R. Health Sci. J. 24, 269–276.
Robitaille, L., and Hoffer, L. J. (2016). A simple method for plasma total vitamin C analysis suitable for routine clinical laboratory use. Nutr. J. 15:40. doi: 10.1186/s12937-016-0158-9
Ruiz, B., Rood, J. C., Fontham, E. T., Malcom, G. T., Hunter, F. M., Sobhan, M., et al. (1994). Vitamin C concentration in gastric juice before and after anti-Helicobacter pylori treatment. Am. J. Gastroenterol. 89, 533–539.
Rumsey, S. C., Daruwala, R., Al-Hasani, H., Zarnowski, M. J., Simpson, I. A., and Levine, M. (2000). Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. J. Biol. Chem. 275, 28246–28253. doi: 10.1074/jbc.M000988200
Rumsey, S. C., Kwon, O., Xu, G. W., Burant, C. F., Simpson, I., and Levine, M. (1997). Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 272, 18982–18989.
Sandesc, M., Rogobete, A. F., Bedreag, O. H., Dinu, A., Papurica, M., Cradigati, C. A., et al. (2018). Analysis of oxidative stress-related markers in critically ill polytrauma patients: an observational prospective single-center study. Bosn. J. Basic Med. Sci. 18, 191–197. doi: 10.17305/bjbms.2018.2306
Schencking, M., Sandholzer, H., and Frese, T. (2010). Intravenous administration of vitamin C in the treatment of herpetic neuralgia: two case reports. Med Sci Monit. 16, CS58–CS61.
Schencking, M., Vollbracht, C., Weiss, G., Lebert, J., Biller, A., Goyvaerts, B., et al. (2012). Intravenous vitamin C in the treatment of shingles: results of a multicenter prospective cohort study. Med Sci Monit. 18, CR215–CR224. doi: 10.12659/MSM.882621
Schoenfeld, J. D., Sibenaller, Z. A., Mapuskar, K. A., Wagner, B. A., Cramer-Morales, K. L., Furqan, M., et al. (2017). O2·- and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological Ascorbate. Cancer Cell 31, 487.e8–500.e8. doi: 10.1016/j.ccell.2017.02.018
Sezikli, M., Cetinkaya, Z. A., Güzelbulut, F., Sezikli, H., Özkara, S., Coşgun, S., et al. (2011). Efficacy of vitamins supplementation to therapy on Helicobacter pylori eradication in patients with low antioxidant capacity. Clin. Res. Hepatol. Gastroenterol. 35, 745–749. doi: 10.1016/j.clinre.2011.07.001
Sezikli, M., Çetinkaya, Z. A., Güzelbulut, F., Yeşil, A., Coşgun, S., and Kurdaş, O. Ö. (2012). Supplementing vitamins C and E to standard triple therapy for the eradication of Helicobacter pylori. J. Clin. Pharm. Ther. 37, 282–285. doi: 10.1111/j.1365-2710.2011.01286.x
Sezikli, M., Cetinkaya, Z. A., Sezikli, H., Güzelbulut, F., Tiftikçi, A., Ince, A. T., et al. (2009). Oxidative stress in Helicobacter pylori infection: does supplementation with vitamins C and E increase the eradication rate? Helicobacter. 14, 280–285. doi: 10.1111/j.1523-5378.2009
Simon, J. A., Hudes, E. S., and Perez-Perez, G. I. (2003). Relation of serum ascorbic acid to Helicobacter pylori serology in US adults: the Third National Health and Nutrition Examination Survey. J. Am. Coll. Nutr. 22, 283–289. doi: 10.1080/07315724.2003.10719305
Sobala, G. M., Crabtree, J. E., Dixon, M. F., Schorah, C. J., Taylor, J. D., Rathbone, B. J., et al. (1991). Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 32, 1415–1418.
Sobala, G. M., Schorah, C. J., Shires, S., Lynch, D. A., Gallacher, B., Dixon, M. F., et al. (1993). Effect of eradication of Helicobacter pylori on gastric juice ascorbic acid concentrations. Gut 34, 1038–1041.
Sotiriou, S., Gispert, S., Cheng, J., Wang, Y., Chen, A., Hoogstraten-Miller, S., et al. (2002). Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat. Med. 8, 514–517. doi: 10.1038/nm0502-514
Stephenson, C. M., Levin, R. D., Spector, T., and Lis, C. G. (2013). Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 72, 139–146. doi: 10.1007/s00280-013-2179-9
Stone, J. R., and Yang, S. (2006). Hydrogen peroxide: a signaling messenger. Antioxid. Redox Signal. 8, 243–270. doi: 10.1089/ars.2006.8.243
Suerbaum, S., and Michetti, P. (2002). Helicobacter pylori infection. N. Engl. J. Med. 347, 1175–1186. doi: 10.1056/NEJMra020542
Tanaka, H., Matsuda, T., Miyagantani, Y., Yukioka, T., Matsuda, H., and Shimazaki, S. (2000). Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch. Surg.135, 326–331. doi: 10.1001/archsurg.135.3.326
Tari, A., Kitadai, Y., Sumii, M., Sasaki, A., Tani, H., Tanaka, S., et al. (2007). Basis of decreased risk of gastric cancer in severe atrophic gastritis with eradication of Helicobacter pylori. Dig. Dis. Sci. 52, 232–239. doi: 10.1007/s10620-006-9411-y
Taylor, D. N., and Blaser, M. J. (1991). The epidemiology of Helicobacter pylori infection. Epidemiol. Rev. 13, 42–59.
Thung, I., Aramin, H., Vavinskaya, V., Gupta, S., Park, J. Y., Crowe, S. E., et al. (2016). Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 43, 514–533. doi: 10.1111/apt.13497
Vollbracht, C., Schneider, B., Leendert, V., Weiss, G., Auerbach, L., and Beuth, J. (2011). Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo 25, 983–990.
Waring, A. J., Drake, I. M., Schorah, C. J., White, K. L., Lynch, D. A., Axon, A. T., et al. (1996). Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: effect of gastritis and oral supplementation. Gut 38, 171–176.
Warren, J. R., and Marshall, B. (1983). Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1, 1273–1275.
Welsh, J. L., Wagner, B. A., van't Erve, T. J., Zehr, P. S., Berg, D. J., Halfdanarson, T. R., et al. (2013). Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother. Pharmacol. 71, 765–775. doi: 10.1007/s00280-013-2070-8
Wilson, J. X. (2013). Evaluation of vitamin C for adjuvant sepsis therapy. Antioxid. Redox Signal. 19, 2129–2140. doi: 10.1089/ars.2013.5401
Wong, B. C., Lam, S. K., Wong, W. M., Chen, J. S., Zheng, T. T., Feng, R. E., et al. (2004). Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291, 187–194. doi: 10.1001/jama.291.2.187
Woodward, M., Tunstall-Pedoe, H., and McColl, K. E. L. (2001). Helicobacter pylori infection reduces systemic availability of dietary vitamin C. Eur J Gastroenterol. 13, 233–237.
Wündisch, T., Thiede, C., Morgner, A., Dempfle, A., Günther, A., Liu, H., et al. (2005). Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J. Clin. Oncol. 23, 8018–8024. doi: 10.1200/JCO.2005.02.3903
Yun, J., Mullarky, E., Lu, C., Bosch, K. N., Kavalier, A., Rivera, K., et al. (2015). Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350, 1391–1396. doi: 10.1126/science.aaa5004
Zabet, M. H., Mohammadi, M., Ramezani, M., and Khalili, H. (2016). Effect of high-dose Ascorbic acid on vasopressor's requirement in septic shock. J. Res. Pharm. Pract. 5, 94–100. doi: 10.4103/2279-042X.179569
Zhang, Z. W., Abdullahi, M., and Farthing, M. J. (2002). Effect of physiological concentrations of vitamin C on gastric cancer cells and Helicobacter pylori. Gut 50, 165–169. doi: 10.1136/gut.50.2.165
Zhang, Z. W., Patchett, S. E., Perrett, D., Katelaris, P. H., Domizio, P., and Farthing, M. J. (1998). The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut 43, 322–326.
Keywords: Helicobacter pylori, gastric diseases, vitamin C, concentration-function relationship, pharmacologic ascorbate, oral ingestion, I.V. administration, hydrogen peroxide (H2O2)
Citation: Mei H and Tu H (2018) Vitamin C and Helicobacter pylori Infection: Current Knowledge and Future Prospects. Front. Physiol. 9:1103. doi: 10.3389/fphys.2018.01103
Received: 30 March 2018; Accepted: 23 July 2018;
Published: 14 August 2018.
Edited by:
Gareth Davison, Ulster University, United KingdomReviewed by:
Ana Cipak Gasparovic, Rudjer Boskovic Institute, CroatiaMarcos Lopez, University of Chicago, United States
Copyright © 2018 Mei and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongbin Tu, tuhongbin@niddk.nih.gov
Haixin Mei, 652015105@qq.com
 Haixin Mei1*
Haixin Mei1* Hongbin Tu
Hongbin Tu