- 1Sports Performance Research Institute New Zealand, Auckland University of Technology, Auckland, New Zealand
- 2AUT Roche Diagnostics Laboratory, Auckland University of Technology, Auckland, New Zealand
Keeping athletes healthy will be important for optimal athletic performance at the 2020 Tokyo Summer Olympic and Paralympic Games. Athletes will be exposed to several stressors during the preparatory and competition phases of the Summer Games that have the potential to depress immunity and increase illness risk. This mini-review provides an overview on effective and practical stressor-specific illness prevention strategies that can be implemented to maintain and protect the health of Olympic and Paralympic athletes.
Introduction
Acute illness is one of the single biggest factors that can prevent athletes from successful performance at pinnacle events (Raysmith and Drew, 2016). Upper respiratory tract symptoms (URTS) are the most common illness reported by elite athletes at the Olympic and Paralympic Games (Derman et al., 2013, 2017; Engebretsen et al., 2013; Soligard et al., 2017). Common URTS include sore throat, headache, runny nose and coughing (Walsh et al., 2011b). URTS can negatively impact training availability, reduce exercise performance, and can even result in athletes missing a major competition (Gleeson and Pyne, 2016). The importance of maintaining athlete health has been highlighted by studies demonstrating that World and Olympic winning medal athletes experience fewer URTS than less successful athletes (Raysmith and Drew, 2016; Svendsen et al., 2016). As such, keeping athletes healthy in preparatory and competition phases of the 2020 Tokyo Olympic and Paralympic Games (referred to as “Summer Games” in this mini review) will contribute toward optimal performance. The purpose of this mini review is to describe how stressors impact upon an athlete’s risk for illness and to identify which athletes may be more susceptible to illness at the Summer Games, along with outlining strategies to maintain and protect athlete health.
Preparatory Phase of the Summer Games
Keeping athletes healthy in the lead up to the Summer Games is important for optimal performance. In elite track and field athletes, having fewer illnesses (and injuries) and completing more than 80% of planned trainings in the 6-months prior to a major event increases the likelihood of achieving pre-defined performance goals (Raysmith and Drew, 2016). Nevertheless, maintaining athlete health during the preparatory phase may prove to be a challenging task, as both Northern and Southern hemisphere athletes will be exposed to different stressors that can challenge immunity and increase URTS risk, including seasonal specific stressors, limited ultraviolet B exposure (UVB), heat acclimation and travel.
Seasonal Specific Stressors
Seasonal Influenza
It is well established that the incidence of influenza exhibit seasonal fluctuations, with peak incidence occurring during the winter months (Doyle and Cohen, 2009). Therefore, compared to Northern hemisphere athletes, athletes residing in the Southern hemisphere will be at an increased risk for infectious URTS episodes during the preparatory phase of the Summer Games.

Table 1. Summary of five key illness prevention strategies that athletes should consider adhering to during the Summer Games.
Strategies to minimize seasonal influenza risk
• Vaccination: Advise athletes to have the influenza vaccine in autumn (April) before the influenza season as it usually takes 5–7 weeks to take effect (Schwellnus et al., 2016). Administration of the vaccine should occur during a non-competition period, or at least 2 weeks prior to competition to allow time for the development of a specific adaptive immune response and any potential side effects (Daly and Gustafson, 2011). There may be some benefit in performing moderate intensity exercise prior to vaccine administration as it has been shown to facilitate vaccine efficacy (Edwards et al., 2007, 2012) and reduce adverse reactions (Lee et al., 2018). This adjuvant strategy seems to be most successful in immunocompromised individuals (e.g., elderly) (Ranadive et al., 2014), therefore it is reasonable to suggest it may be worthwhile in elite athletes. It is considered a harmless strategy where the potential benefits could be crucial to preparation, however, further research on athletes is required to determine optimal protocols.
• Hygiene: Maintain good hygiene (refer to Table 1 for guidelines).
• Illness monitoring: Monitor illness to enable early detection and application of appropriate illness prevention strategies. Use the Jackson Common Cold Scale (Jackson et al., 1958) to monitor athlete illness. Consider monitoring household illness by adding a question alongside the Jackson Common Cold Scale (Keaney et al., 2018). Household illness monitoring is a promising strategy, although, further research in this area is required.
• Probiotic supplementation: Supplement throughout the preparatory phase (3 months) (refer to Table 1 for further guidelines).
• Zinc acetate supplementation: Supplement athletes experiencing acute URTS with zinc acetate lozenges (75 mg/day) to decrease the duration of URTS (Note: Zinc must be taken <24 h after onset of URTS and can be taken for 1–2 weeks) (Maughan et al., 2018). Excessive zinc supplementation (>150 mg/day) should be avoided as it can impair immune cell functions.
Cold Environmental Conditions
Upper respiratory tract symptoms can result from infectious (viral, bacterial, or fungal etiology) or non-infectious and inflammatory (e.g., caused by allergies, asthma and trauma to respiratory epithelial membranes) causes (Gleeson and Pyne, 2016). Southern hemisphere athletes training during winter will be exposed to cold dry air. Inhalation of cold dry air can damage airway epithelium and lead to non-infectious URTS episodes (Koskela, 2007). Athletes with asthma and allergies may be at higher risk for URTS, as winter training has been shown to increase URTS incidence among individuals with these conditions (Hyrkäs et al., 2014; Gleeson and Pyne, 2016).
Strategies to minimize cold air mediated non-infectious URTS
• Diagnose asthma and allergies: Administer the validated questionnaire Allergy Questionnaire for Athletes (AQUA) to identify athletes with asthma and allergies (Bonini et al., 2009). Confirm the diagnosis with a physician.
• Control asthma and allergies: Ensure appropriate therapeutic control of asthma and allergies and comply to World Anti-Doping Agency (WADA) regulations (Helenius and Haahtela, 2000).
• Protect airways: When practical, take extra precautions to avoid inhalation of cold dry air (below 0°C). For example, train indoors or for outdoor training use facial masks to protect airways (Walsh, 2018). It is unknown if facial mask reduce URTS incidence, however, they can attenuate cold air exercise-induced asthma which is known to elicit non-infectious URTS episodes (Beuther and Martin, 2006).
Summer Allergies and Asthma
Northern hemisphere athletes will be exposed to environmental factors and high periods of allergen load during the preparatory phase of the Summer Games, including heat and humidity, pollen, grasses, weed, mold, and dust. During exercise, high ventilation rates combined with increased exposure to environmental factors and allergens can exacerbate asthma and allergies. Prevalence of asthma and allergy is high in elite athletes, especially endurance athletes (Silva and Moreira, 2017). Exacerbations of asthma and allergies may elicit non-infectious URTS, such as runny nose, repetitive sneezing, and coughing, all of which can disrupt training and performance (Gleeson and Pyne, 2016).
Strategies to minimize summer allergy and asthma mediated non-infectious URTS
• Diagnose and control asthma and allergies (see section “Strategies to Minimize Cold Air Mediated Non-infectious URTS” for details).
• Allergen avoidance: When practical, avoid exposure to allergens (e.g., clean room and change bed lining regularly to reduce house dust mite exposure. Follow pollen forecasts and consider adapting training venues and training schedules during high pollen periods, etc.) (Silva and Moreira, 2017).
Low Ultraviolet B Exposure
Southern hemisphere athletes will be at an increased risk for Vitamin D (VD) deficiency as it is more prevalent during winter when UVB exposure and endogenous synthesis of VD are low (Backx et al., 2017). Previous research suggests that up to 50% of athletes could be considered to have an inadequate VD status, during winter training months (He et al., 2013, 2016). Some Northern hemisphere athletes may also be at risk for VD deficiency, such as indoor athletes, athletes with dark skin tone, athletes who live and train in northern latitudes (<30° or >70°) and athletes residing in countries with a poor summer season with limited sun exposure (i.e., sun exposure <20 min/day) (He et al., 2016). VD deficiency appears to be an important determinant of URTS risk in athletes (He et al., 2013, 2016). Evidence suggests an optimal serum 25(OH)D of 75 nmol/L may enhance immunity and prevent URTS (He et al., 2016).
Strategies to Maintain VD Levels
• Vitamin D recommendations for Southern hemisphere and at-risk Northern hemisphere athletes: There may be some benefit in measuring athletes serum 25(OH)D concentration, to allow more targeted VD supplementation. As outlined in recent guidelines, athletes with serum 25(OH)D concentrations <75 nmol L−1 should be supplemented with 2000–4000 IU VD3/day (Owens et al., 2018). However, the measurement of serum 25(OH)D is not always feasible (cost approximately US$255 per athletes) and may not be the most appropriate measure of an athletes VD status (Allison et al., 2018; Owens et al., 2018). Therefore, rather than measuring serum 25(OH)D, the most practical approach may be to supplement all Southern hemisphere and at-risk Northern hemisphere athletes with 1000 IU VD3/day (comply to WADA anti-doping regulations) (He et al., 2016). There is some risk for toxicity when supplementing with exogenous VD, however, previous reports suggest 1000 IU VD3/day is a safe dosage (He et al., 2016).
• General VD guidelines for Northern hemisphere athletes: Aim to acquire 15 min of non-protected (i.e., no sunscreen) sun exposure per day (He et al., 2016).
Heat Acclimation
The Summer Games are expected to be hot (>30°C) and humid (>70% relative humidity). Therefore, heat acclimation (HA) will be an integral component of the preparatory phase. Exercise immunology research suggests that heat does not pose a challenge to the immune system. Indeed, performing a one-off bout of exercise in hot conditions [28–38.7°C, 45–76% relative humidity (RH)] does not appear to exacerbate exercise induced immune perturbations, compared to temperate conditions (Mitchell et al., 2002; McFarlin and Mitchell, 2003; Niess et al., 2003; Laing S. et al., 2005; Laing S.J. et al., 2005). Similarly, HA has been shown to have negligible effects on immunity. For example, no change in white blood cell counts (Willmott et al., 2016) or inflammatory cytokines (Amorim et al., 2011; Barberio et al., 2015) has been demonstrated following HA. However, the current limitation to the HA studies discussed is that illness reports were not measured alongside immune markers. Therefore, it remains unclear how HA may impact upon athletes URTS risk. Consideration may want to be given to the acclimation status of athletes performing HA. During the preparatory phase, it is possible that acclimation status will differ between Northern and Southern hemisphere athletes; athletes residing in the Northern and Southern hemisphere will likely be seasonally acclimated and unacclimated, respectively. Future studies should assess the baseline acclimation status of athletes engaging in HA to understand whether it is associated with URTS risk.
Strategies to Maintain Athlete Health During HA
The health status of athletes should be considered when implementing HA. Athletes experiencing illness symptoms should not participate in HA as it may exacerbate illness (Casadio et al., 2017).
• Hygiene: Maintain good hygiene (e.g., remove wet clothing and have a warm shower immediately following HA sessions) (refer to Table 1 for further guidelines).
• Training load and recovery management: Heat stress adds to athletes overall training load. Carefully manage training load when training with additional heat (Walsh, 2018). Ensure adequate recovery between HA sessions, particularly if HA protocols involve prolonged exercise (≥90 min) as it can cause more severe immune perturbations than shorter duration exercise (<60 min) (Diment et al., 2015).
• Daily wellness monitoring and management: Monitor wellness to understand how individual athletes tolerate HA. A customized psychometric questionnaire (Hooper and Mackinnon, 1995) utilizing Likert scales can be used to assess indicators of wellness (e.g., sleep quality, stress, fatigue, mood, and muscle soreness) (Buchheit et al., 2013; Gallo et al., 2017). In addition to monitoring wellness during the HA period, the questionnaire should be administered during normal training weeks to establish baseline wellness data. High stress/anxiety levels and sleep deprivation have been linked to increased URTS incidence (Cohen et al., 1991, 2009). Over the HA period, apply strategies listed in Table 1 (i.e., minimize stress and anxiety and improve sleep). If athletes wellness scores are substantially reduced consider adjusting the HA protocol (e.g., reduce load) (Schwellnus et al., 2016).
• Carbohydrate (CHO) intake: Maintain day-to-day CHO availability over HA period, aim for >50% daily energy intake as CHO (Walsh, 2018).
• Hydration: Permissive dehydration is often used during HA sessions to accelerate the adaptation process (Garrett et al., 2014). Exercising in a dehydrated state does not appear to cause further exacerbation of immune perturbations, compared to euhydrated exercise (Svendsen et al., 2014; Killer et al., 2015). Therefore, permissive dehydration can be used during HA as it is unlikely to impair immunity. However, during recovery from HA, fluid replacement should be prioritized, as per current rehydration guidelines (Thomas et al., 2016).
• Probiotic supplementation: Begin supplementation at least 2-weeks before the HA block is due to commence and supplement throughout HA (refer to Table 1 for guidelines).
Long Haul Travel
During the preparatory phase, many Southern and Northern hemisphere athletes will undertake international travel to competition, heat camps and the Summer Games itself. Travel has been identified as a prominent risk factor for URTS. Increased URTS incidence and severity has been demonstrated in team sport athletes traveling to international destinations that were >5 or 11 time zones, respectively (Haywood et al., 2014; Fowler et al., 2016). Similarly, in elite endurance athletes, air travel was found to significantly increase URTS susceptibility (Svendsen et al., 2016). The current limitation to the studies discussed is that immune markers were not measured alongside illness reports. Nevertheless, in the general population simulated long haul travel has been found to induce transient immune changes that may contribute to increased URTS susceptibility (Wilder-Smith et al., 2012). Contrastingly, a recent study in master-level athletes showed that long haul travel did not impair mucosal immune responses (Stevens et al., 2018). Further research on elite athletes is required to elucidate how travel impacts upon the immune system and subsequently URTS risk.
Strategies to Maintain Athlete Health During Long Haul Travel
• Travel vaccines: Consult a physician and update athlete and support staff vaccines.
• Hand hygiene: Apply alcohol-based hand gel after touching potentially contagious objects. For example, hand gel should be used after handling airport plastic security screening trays as a recent study identified that they have the highest frequency of respiratory viruses, compared to other airport surfaces (e.g., toilets, handrails) (Ikonen et al., 2018).
• Avoid ill people: If possible, seat athletes away from ill passengers. Increased risk for infection transmission has been associated with sitting within two rows of a contagious passenger for >8 h (Mangili and Gendreau, 2005). If it is not possible to change seats, athletes should wear a disposable face mask (Walsh et al., 2011a).
• Hydration: Encourage athletes to drink plenty of water to keep well hydrated and potentially prevent mucosal membranes from drying out.
• Optimize sleep hygiene: Pre-departure, improve sleep quantity and quality (refer to Table 1 for guidelines). After long haul travel, greatest sleep disruption has been reported in the first 48 h (Stevens et al., 2018). Optimize sleep hygiene (e.g., electronic device availability, cool room temperature, caffeine, ear plugs, eye masks, etc.) to improve sleep on the first 2 days after arrival (Stevens et al., 2018).
• Recovery: Avoid flying on the same day as competition or intensive training, delay travel until at least the subsequent day (Svendsen et al., 2016).
• Probiotic supplementation: Begin supplementation at least 2-weeks before scheduled travel (refer to Table 1 for guidelines).
Competition Phase of the Summer Games
Stressors Associated With the Summer Games
During the Summer Games, both Northern and Southern hemisphere athletes will be exposed to a range of stressors. Such stressors include, intensive competition, hot and humid environmental conditions, dehydration, psychological stress, and sleep deprivation (Keaney et al., 2018; Walsh, 2018). The effect of these stressors on immunity and illness risk has been summarized in recent reviews (Keaney et al., 2018; Walsh, 2018; Williams et al., 2018). Previous studies have tended to examine each stressor in isolation when in reality athletes will be simultaneously exposed to all stressors at the Summer Games. The synergism of these stressors could potentially have a compounding effect on immunodepression, resulting in higher incidence of illness than if each stressor were applied alone. Further research is needed to understand how multiple stressors affect immunity and illness risk.
At the Summer Games, medals are often won by the smallest of margins, so even a mild illness could negatively affect results. To keep athletes healthy and minimize the potential immunodepression evoked by Summer Games stressors, athletes should consider adhering to the five key illness prevention strategies listed in Table 1. These strategies have been selected on the assumption that Summer Games athletes will adhere to fundamental principles of nutrition and sport science (e.g., macro- and micro-nutrient intake, hydration, recovery protocols, training load management, etc.). Illness prevention strategies should not replace fundamentals, but work alongside them to keep athletes healthy. In addition to these strategies, other reviews exist which provide detailed recommendations on avoiding infection and maintaining immune health in athletes (Schwellnus et al., 2016; Walsh, 2018).
Athletes at Increased Risk for Illness During the Summer Games
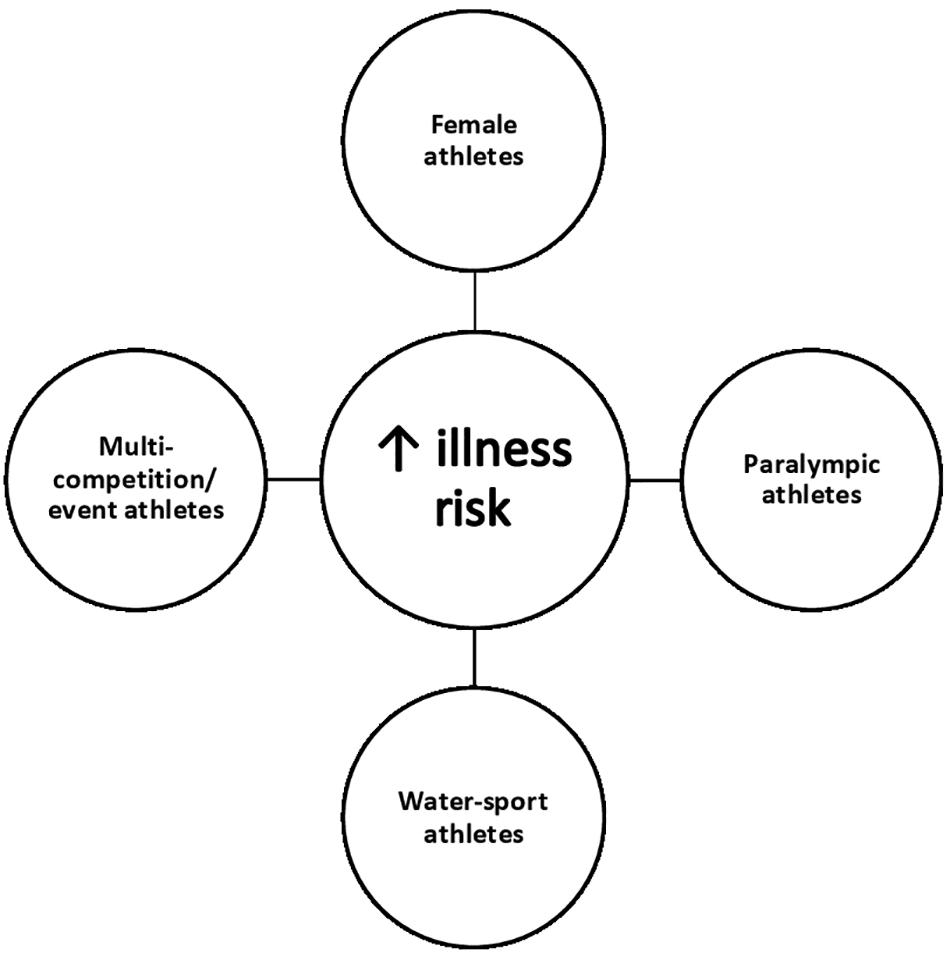
With the aim of protecting the health of athletes, the International Olympic Committee (IOC) monitored illness incidence at the London (2012) and Rio (2016) Olympic and Paralympic Games (Derman et al., 2013, 2017; Engebretsen et al., 2013; Soligard et al., 2017). At previous Summer Games 5–14% of athletes experienced at least one illness, with the highest incidence of illness affecting the respiratory tract (Derman et al., 2013, 2017; Engebretsen et al., 2013; Soligard et al., 2017). IOC reports demonstrated that the illness rates varied considerably between gender and sports (Derman et al., 2013, 2017; Engebretsen et al., 2013; Soligard et al., 2017). As summarised in Figure 1, it appears that some athletes may be more susceptible to illness during the Summer Games, namely: (1) Female athletes; (2) Paralympic athletes; (3) Water-sport athletes; and (4) Multi-competition/event athletes (i.e., athletes who compete on >1 day) (Derman et al., 2013, 2017; Engebretsen et al., 2013; Soligard et al., 2017).
Female Athletes
Data obtained at the London and Rio Summer Games demonstrated significantly higher (40–60%) illness incidence in female compared to male athletes (Engebretsen et al., 2013; Derman et al., 2017; Soligard et al., 2017). In agreement with these findings, longitudinal studies have shown that female athletes tend to be at increased risk for URTS (Gleeson et al., 2011; He et al., 2014) and experience URTS episodes for a longer duration than male athletes (He et al., 2014). Sex differences in immune variables may explain the higher illness susceptibility observed in female athletes. Differences in immune responses between males and females have largely been attributed to sex hormones and their inherent immune modulatory functions (Klein and Flanagan, 2016). Furthermore, increased URTS susceptibility in female athletes may be associated with low energy availability (LEA). Higher rates of LEA have been demonstrated in female compared to male athletes (Logue et al., 2018), and LEA has been identified as a key risk factor for illness in Olympic-level female athletes (Drew et al., 2017).
Strategies to maintain female athlete health
• Diagnose and treat LEA: In the preparatory phase, identify female athletes with LEA using the validated questionnaire Low Energy Availability in Females Questionnaire (LEAF-Q) (Melin et al., 2014). Athletes with LEA should work closely with a nutritionist to ensure daily energy intake matches training and competition demands (Logue et al., 2018).
• Supplementation: At present, a number of supplements have been proposed to alter specific aspects of the immune system and reduce athletes URTS risk (Maughan et al., 2018). However, few supplements have convincing evidence supporting their use. Currently, probiotic (refer to Table 1 for guidelines), vitamin C (0.25–1.0 g/day) (Hemilä and Chalker, 2013) or quercetin (1 g/day) (Somerville et al., 2016) are the most promising supplements in this area, although further research is needed to determine how the combined use of these supplements influence URTS risk. Athletes need for these supplements should be assessed on an individual case-by-case basis, based on several factors (e.g., URTS history, sport, nutrient status, etc.). Supplements to be used at the Summer Games should be piloted (for acceptance/compliance/safety) in an off season/preparatory phase. Ensure selected supplements are batch tested and comply to WADA regulations.
Paralympic Athletes
Paralympic athletes appear to be more susceptible to illness than able-bodied athletes. Paralympic athletes suffered almost double the amount of URTS than able bodied athletes during previous London and Rio Summer Games (Paralympics: 12–14% vs. Able-bodied: 5–7%) (Derman et al., 2013, 2017; Engebretsen et al., 2013; Soligard et al., 2017). It is difficult to ascertain why Paralympic athletes are at a heightened risk for illness, as research on Paralympic sport is limited compared to investigations of able-bodied athletes (Van Rensburg et al., 2018). Illness risk will differ between Paralympic athletes based on their disability type. Paralympic athletes with spinal cord injuries have altered autonomic control and immunity, and impaired immune function has been cited as the main reason for increased illness susceptibly in this population (Leicht et al., 2013). In addition, the use of wheelchairs by Paralympic athletes likely increases infection transmission risk, as wheelchairs pick up and carry high numbers of bacteria. Indeed, at the Rio Paralympics, the highest illness incidence rate was reported in wheelchair fencing, while wheelchair basketball was only behind Paralympic swimming in terms of illness sustained (Derman et al., 2017).
Strategies to maintain paralympic athlete health
• Hygiene: Wheelchair athletes should regularly disinfect wheelchairs, wear gloves, ensure good hand hygiene, and avoid self-inoculation by touching eyes, nose, and mouth (Walsh, 2018).
• Supplementation (see section “Strategies to Maintain Female Athlete Health” for details).
Water-Sport Athletes
Athletes involved in Water-sports may be at an increased risk for illness during the Summer Games. At previous Summer Games, the IOC identified the top 5 sports with the highest illness incidence; water-sports accounted for 2 out of 5 (sailing and synchronized swimming) and 4 out of 5 (diving, open water marathon, canoe slalom, and synchronized swimming) sports at the London (Engebretsen et al., 2013) and Rio Olympics (Soligard et al., 2017), respectively. Similarly, at the Rio Paralympics, Para-Swimming was the sport with the second highest illness incidence (Derman et al., 2017). There are two likely factors underpinning increased illness susceptibility in this population: (1) chlorine exposure for pool-athletes; and (2) water quality issues for open-water athletes. Airway disorders, including asthma and rhinitis are prevalent in pool-athletes and are often attributed to chlorine and chlorine by-products causing airway changes (Škrgat et al., 2018). As such, asthma and allergy mediated non-infectious URTS may explain why pool-sport athletes appear to be at an increased risk for illness. Alternatively, for open-water sport athletes, particularly at the Rio Summer Games, reports suggest that water quality issues (i.e., contamination with bacteria and viruses) were the primary cause of higher illness incidence (Keith, 2017).
Strategies to maintain water-sport athlete health
• Diagnose and control asthma and allergies (see section “Strategies to Minimize Cold Air Mediated Non-infectious URTS” for details).
• Supplementation (see section “Strategies to Maintain Female Athlete Health” for details).
Multi-Competition/Event Athletes
Multi-competition/event athletes may be at an increased risk for illness. Indeed, data obtained at previous Olympic games demonstrated that of the top 5 sports with the highest illness incidence the majority were multi-competition/event sports [5/5 in London: Athletics, Beach VB, Football, Sailing, and Synchronized Swimming (Engebretsen et al., 2013)] [4/5 sports in Rio: Diving, Canoe Slalom, Equestrian and Synchronized Swimming (Soligard et al., 2017)]. It is unclear why these athletes are more susceptible to illness, nonetheless it may be explained by the psychological element of having to mentally prepare for multiple events. Recent research suggests that mental state influences immunity, for example, state-anxiety and perceived psychological stress before exercise has been shown to influence immune responses to a greater extent than exercise itself (Edwards et al., 2018). In addition, a significant association between mental health (i.e., perceived stress and depression) and illness incidence has been demonstrated in athletes preparing for the Rio Olympics (Drew et al., 2017). Further studies should explore mental state to better elucidate how it affects athletes’ immunity and URTS risk.
Strategies to maintain multi-competition/event athlete health
• Manage stress and anxiety (refer to Table 1 for guidelines).
• Mindfulness practices: Mindfulness interventions such as meditation, breathing awareness, walking and yoga have the potential to alleviate psychological stress and anxiety. Recent studies have reported significant improvements to athletes’ mental state with 4–6 weeks of mindfulness training (Ajilchi et al., 2019; Chen et al., 2019). Furthermore, in wheelchair basketball players, 8 weeks of mindful mediation utilizing a smart phone app attenuated the rise in cortisol associated with a competition period (MacDonald and Minahan, 2018). However, immune responses did not appear to be influenced (MacDonald and Minahan, 2018). It is currently unclear if mindful training influences URTS risk in athletes, nevertheless in the general population a reduction in URTS incidence has been demonstrated following 8 weeks of mindful meditation (Barrett et al., 2012). Mindfulness training appears to be a promising strategy for athletes, although further investigation is warranted. Athletes planning to use mindfulness interventions at the Summer Games should pilot and optimize practices in an off season/preparatory period.
• Supplementation (see section “Strategies to Maintain Female Athlete Health” for details).
Conclusion
It is apparent that athletes will be exposed to various stressors during both the preparatory and competition phases of the Summer Games. Athletes residing in the southern hemisphere appear to be at increased risk for illness during the preparatory phase, while female, Paralympic, water-sport and multi-competition/event athletes may be more susceptible to illness during the competition phase of the Summer Games. To maintain athlete health, illness prevention strategies should be targeted to stressors and at-risk athletes. Keeping athletes healthy will contribute to optimal Olympic and Paralympic athletic performance. While the considerations and strategies outlined in this mini review are targeted for the Summer Games, many could be used for other major competitions and as such should be considered for future sporting success.
Author Contributions
All authors were involved in the conception of the manuscript. LK drafted the manuscript. FM, AK, and DD critically revised the manuscript and approved the final version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
None. The authors received no external financial support to aid the writing of this mini review.
References
Ajilchi, B., Amini, H. R., Ardakani, Z. P., Zadeh, M. M., and Kisely, S. (2019). Applying mindfulness training to enhance the mental toughness and emotional intelligence of amateur basketball players. Australas. Psychiatry 1–6. doi: 10.1177/1039856219828119
Allison, R. J., Farooq, A., Cherif, A., Hamilton, B., Close, G. L., and Wilson, M. G. (2018). Why don’t serum vitamin D concentrations associate with BMD by DXA? A case of being ‘bound’to the wrong assay? Implications for vitamin D screening. Br. J. Sports Med. 52, 522–526. doi: 10.1136/bjsports-2016-097130
Amorim, F., Yamada, P., Robergs, R., Schneider, S., and Moseley, P. (2011). Effects of whole-body heat acclimation on cell injury and cytokine responses in peripheral blood mononuclear cells. Eur. J. Appl. Physiol. 111, 1609–1618. doi: 10.1007/s00421-010-1780-4
Backx, E., Van der Avoort, C., Tieland, M., Maase, K., Kies, A., van Loon, L., et al. (2017). Seasonal variation in vitamin D status in elite athletes: a longitudinal study. Int. J. Sport Nutr. Exerc. Metab. 27, 6–10. doi: 10.1123/ijsnem.2016-0177
Barberio, M., Elmer, D., Laird, R., Lee, K., Gladden, B., and Pascoe, D. (2015). Systemic LPS and inflammatory response during consecutive days of exercise in heat. Int. J. Sports Med. 36, 262–270. doi: 10.1055/s-0034-1389904
Barrett, B., Hayney, M. S., Muller, D., Rakel, D., Ward, A., Obasi, C. N., et al. (2012). Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial. Ann. Fam. Med. 10, 337–346. doi: 10.1370/afm.1376
Bermon, S., Castell, L. M., Calder, P. C., Bishop, N. C., Blomstrand, E., Mooren, F. C., et al. (2017). Consensus statement immunonutrition and exercise. Exerc. Immunol. Rev. 23:8.
Beuther, D. A., and Martin, R. J. (2006). Efficacy of a heat exchanger mask in cold exercise-induced asthma. Chest 129, 1188–1193. doi: 10.1378/chest.129.5.1188
Bonini, M., Braido, F., Baiardini, I., Del Giacco, S., Gramiccioni, C., Manara, M., et al. (2009). AQUA: allergy questionnaire for athletes. Development and validation. Med. Sci. Sports Exerc. 41, 1034–1041. doi: 10.1249/MSS.0b013e318193c663
Buchheit, M., Simpson, B. M., Garvican-Lewis, L. A., Hammond, K., Kley, M., Schmidt, W. F., et al. (2013). Wellness, fatigue and physical performance acclimatisation to a 2-week soccer camp at 3600 m (ISA3600). Br. J. Sports Med. 47, 100–106. doi: 10.1136/bjsports-2013-092749
Burke, L. M., Hawley, J. A., Wong, S. H., and Jeukendrup, A. E. (2011). Carbohydrates for training and competition. J. Sports Sci. 29(Supp.1), S17–S27. doi: 10.1080/02640414.2011.585473
Casadio, J. R., Kilding, A. E., Cotter, J. D., and Laursen, P. B. (2017). From lab to real world: heat acclimation considerations for elite athletes. Sports Med. 47, 1467–1476. doi: 10.1007/s40279-016-0668-9
Chen, J.-H., Tsai, P.-H., Lin, Y.-C., Chen, C.-K., and Chen, C.-Y. (2019). Mindfulness training enhances flow state and mental health among baseball players in Taiwan. Psychol. Res. Behav. Manage. 12, 1–10.
Cohen, S., Doyle, W. J., Alper, C. M., Janicki-Deverts, D., and Turner, R. B. (2009). Sleep habits and susceptibility to the common cold. Arch. Intern. Med. 169, 62–67. doi: 10.1001/archinternmed.2008.505
Cohen, S., Tyrrell, D. A., and Smith, A. P. (1991). Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 325, 606–612. doi: 10.1056/NEJM199108293250903
Daly, P., and Gustafson, R. (2011). Public health recommendations for athletes attending sporting events. Clin. J. Sport Med. 21, 67–70. doi: 10.1097/JSM.0b013e31820758dc
Derman, W., Schwellnus, M., Jordaan, E., Blauwet, C. A., Emery, C., Pit-Grosheide, P., et al. (2013). Illness and injury in athletes during the competition period at the London 2012 Paralympic Games: development and implementation of a web-based surveillance system (WEB-IISS) for team medical staff. Br. J. Sports Med. 47, 420–425. doi: 10.1136/bjsports-2013-092375
Derman, W., Schwellnus, M.P., Jordaan, E., Runciman, P., Blauwet, C., Webborn, N., et al. (2017). Sport, sex and age increase risk of illness at the Rio 2016 Summer Paralympic Games: a prospective cohort study of 51 198 athlete days. Br. J. Sports Med. 52, 17–23. doi: 10.1136/bjsports-2017-097962
Diment, B. C., Fortes, M. B., Edwards, J. P., Hanstock, H. G., Ward, M. D., Dunstall, H. M., et al. (2015). Exercise intensity and duration effects on in vivo immunity. Med. Sci. Sports Exerc. 47, 1390–1398. doi: 10.1249/MSS.0000000000000562
Doyle, W. J., and Cohen, S. (2009). “Etiology of the common cold: modulating factors,” in Common Cold, eds R. Eccles and O. Weber (Berlin: Springer), 149–186. doi: 10.1007/978-3-7643-9912-2_6
Drew, M. K., Vlahovich, N., Hughes, D., Appaneal, R., Peterson, K., Burke, L., et al. (2017). A multifactorial evaluation of illness risk factors in athletes preparing for the Summer Olympic Games. J. Sci. Med. Sport. 20, 745–750. doi: 10.1016/j.jsams.2017.02.010
Edwards, J. P., Walsh, N. P., Diment, P., and Roberts, R. (2018). Anxiety and perceived psychological stress play an important role in the immune response after exercise. Exerc. Immunol. Rev. 24, 26–34. doi: 10.14195/2182-7087_ex2018_75
Edwards, K. M., Burns, V. E., Allen, L. M., McPhee, J. S., Bosch, J. A., Carroll, D., et al. (2007). Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav. Immun. 21, 209–217. doi: 10.1016/j.bbi.2006.04.158
Edwards, K. M., Pung, M. A., Tomfohr, L. M., Ziegler, M. G., Campbell, J. P., Drayson, M. T., et al. (2012). Acute exercise enhancement of pneumococcal vaccination response: a randomised controlled trial of weaker and stronger immune response. Vaccine 30, 6389–6395. doi: 10.1016/j.vaccine.2012.08.022
Engebretsen, L., Soligard, T., Steffen, K., Alonso, J. M., Aubry, M., Budgett, R., et al. (2013). Sports injuries and illnesses during the London summer olympic games 2012. Br. J. Sports Med. 47, 407–414. doi: 10.1136/bjsports-2013-092380
Fowler, P., Duffield, R., Lu, D., Hickmans, J., and Scott, T. (2016). Effects of long-haul transmeridian travel on subjective jet-lag and self-reported sleep and upper respiratory symptoms in professional rugby league players. Int. J. Sports Physiol. Perform. 11, 876–884. doi: 10.1123/ijspp.2015-0542
Fullagar, H. H., Skorski, S., Duffield, R., Hammes, D., Coutts, A. J., and Meyer, T. (2015). Sleep and athletic performance: the effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 45, 161–186. doi: 10.1007/s40279-014-0260-0
Gallo, T. F., Cormack, S. J., Gabbett, T. J., and Lorenzen, C. H. (2017). Self-reported wellness profiles of professional Australian football players during the competition phase of the season. J. Strength Cond. Res. 31, 495–502.
Garrett, A., Goosens, N., Rehrer, N., Patterson, M., Harrison, J., Sammut, I., et al. (2014). Short-term heat acclimation is effective and may be enhanced rather than impaired by dehydration. Am. J. Hum. Biol. 26, 311–320. doi: 10.1002/ajhb.22509
Gleeson, M., Bishop, N., Oliveira, M., McCauley, T., and Tauler, P. (2011). Sex differences in immune variables and respiratory infection incidence in an athletic population. Exerc. Immunol. Rev. 17, 122–135.
Gleeson, M., and Pyne, D. B. (2016). Respiratory inflammation and infections in high-performance athletes. Immunol. Cell Biol. 94, 124–131. doi: 10.1038/icb.2015.100
Haywood, B. A., Black, K. E., Baker, D., McGarvey, J., Healey, P., and Brown, R. C. (2014). Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. J. Sci. Med. Sport 17, 356–360. doi: 10.1016/j.jsams.2013.08.004
He, C.-S., Aw Yong, X. H., Walsh, N. P., and Gleeson, M. (2016). Is there an optimal vitamin D status for immunity in athletes and military personnel? Exerc. Immunol. Rev. 22, 42–64.
He, C.-S., Bishop, N., Handzlik, M. K., Muhamad, A. S., and Gleeson, M. (2014). Sex differences in upper respiratory symptoms prevalence and oral-respiratory mucosal immunity in endurance athletes. Exerc. Immunol. Rev. 20, 8–22.
He, C.-S., Handzlik, M. K., Fraser, W. D., Muhamad, A. S., Preston, H., Richardson, A., et al. (2013). Influence of vitamin D status on respiratory infection incidence and immune function during 4 months of winter training in endurance sport athletes. Exerc. Immunol. Rev. 19, 86–101.
Helenius, I., and Haahtela, T. (2000). Allergy and asthma in elite summer sport athletes. J. Allergy Clin. Immunol. 106, 444–452. doi: 10.1067/mai.2000.107749
Hemilä, H., and Chalker, E. (2013). Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 3:CD000980. doi: 10.1002/14651858.CD000980.pub4
Hooper, S. L., and Mackinnon, L. T. (1995). Monitoring overtraining in athletes. Sports Med. 20, 321–327. doi: 10.2165/00007256-199520050-00003
Hyrkäs, H., Jaakkola, M. S., Ikäheimo, T. M., Hugg, T. T., and Jaakkola, J. J. (2014). Asthma and allergic rhinitis increase respiratory symptoms in cold weather among young adults. Respir. Med. 108, 63–70. doi: 10.1016/j.rmed.2013.10.019
Ikonen, N., Savolainen-Kopra, C., Enstone, J. E., Kulmala, I., Pasanen, P., Salmela, A., et al. (2018). Deposition of respiratory virus pathogens on frequently touched surfaces at airports. BMC Infect. Dis. 18:437. doi: 10.1186/s12879-018-3150-5
Jackson, G. G., Dowling, H. F., Spiesman, I. G., and Boand, A. V. (1958). Transmission of the common cold to volunteers under controlled conditions: I. The common cold as a clinical entity. AMA Arch. Int. Med. 101, 267–278. doi: 10.1001/archinte.1958.00260140099015
Keaney, L. C., Kilding, A. E., Merien, F., and Dulson, D. K. (2018). The impact of sport related stressors on immunity and illness risk in team-sport athletes. J. Sci. Med. Sport 21, 1192–1199. doi: 10.1016/j.jsams.2018.05.014
Killer, S. C., Svendsen, I. S., and Gleeson, M. (2015). The influence of hydration status during prolonged endurance exercise on salivary antimicrobial proteins. Eur. J. Appl. Physiol. 115, 1887–1895. doi: 10.1007/s00421-015-3173-1
Klein, S. L., and Flanagan, K. L. (2016). Sex differences in immune responses. Nat. Rev. Immunol. 16:626. doi: 10.1038/nri.2016.90
Koskela, H. O. (2007). Cold air-provoked respiratory symptoms: the mechanisms and management. Int. J. Circumpolar Health 66, 91–100. doi: 10.3402/ijch.v66i2.18237
Laing, S., Blackwell, J., Gwynne, D., Walters, R., and Walsh, N. (2005). Neutrophil degranulation response to 2 hours of exercise in a 30 C environment. Aviat. Space Environ. Med. 76, 1068–1073.
Laing, S. J., Blackwell, J., Gwynne, D., Walters, R., and Walsh, N. P. (2005). Neutrophil degranulation response to 2 hours of exercise in a 30 C environment. Aviat. Space Environ. Med. 76, 1068–1073.
Lee, V., Booy, R., Skinner, S., Fong, J., and Edwards, K. (2018). The effect of exercise on local and systemic adverse reactions after vaccinations–Outcomes of two randomized controlled trials. Vaccine 36, 6995–7002. doi: 10.1016/j.vaccine.2018.09.067
Leicht, C. A., Goosey-Tolfrey, V. L., and Bishop, N. (2013). Spinal cord injury: known and possible influences on the immune response to exercise. Exerc. Immunol. Rev. 19, 144–163.
Logue, D., Madigan, S. M., Delahunt, E., Heinen, M., Mc Donnell, S.-J., and Corish, C. A. (2018). Low energy availability in athletes: a review of prevalence, dietary patterns, physiological health, and sports performance. Sports Med. 48, 73–96. doi: 10.1007/s40279-017-0790-3
MacDonald, L. A., and Minahan, C. L. (2018). Mindfulness training attenuates the increase in salivary cortisol concentration associated with competition in highly trained wheelchair-basketball players. J. Sports Sci. 36, 378–383.
Mangili, A., and Gendreau, M. A. (2005). Transmission of infectious diseases during commercial air travel. Lancet 365, 989–996. doi: 10.1016/S0140-6736(05)71089-8
Maughan, R. J., Burke, L. M., Dvorak, J., Larson-Meyer, D. E., Peeling, P., Phillips, S. M., et al. (2018). IOC consensus statement: dietary supplements and the high-performance athlete. Int. J. Sport Nutr. Exerc. Metab. 28, 104–125. doi: 10.1123/ijsnem.2018-0020
McFarlin, B. K., and Mitchell, J. B. (2003). Exercise in hot and cold environments: differential effects on leukocyte number and NK cell activity. Aviat. Space Environ. Med. 74, 1231–1236.
Melin, A., Tornberg, Å.B., Skouby, S., Faber, J., Ritz, C., Sjödin, A., et al. (2014). The LEAF questionnaire: a screening tool for the identification of female athletes at risk for the female athlete triad. Br. J. Sports Med. 48, 540–545. doi: 10.1136/bjsports-2013-093240
Mitchell, J. B., Dugas, J. P., McFarlin, B. K., and Nelson, M. J. (2002). Effect of exercise, heat stress, and hydration on immune cell number and function. Med. Sci. Sports Exerc. 34, 1941–1950. doi: 10.1249/01.MSS.0000039070.40418.90
Niess, A., Fehrenbach, E., Lehmann, R., Opavsky, L., Jesse, M., Northoff, H., et al. (2003). Impact of elevated ambient temperatures on the acute immune response to intensive endurance exercise. Eur. J. Appl. Physiol. 89, 344–351. doi: 10.1007/s00421-003-0809-3
O’Donnell, S., and Driller, M. W. (2017). Sleep-hygiene education improves sleep indices in elite female athletes. Int. J. Exerc. Sci. 10:522.
Owens, D. J., Allison, R., and Close, G. L. (2018). Vitamin D and the athlete: current perspectives and new challenges. Sports Med. 48, 3–16. doi: 10.1007/s40279-017-0841-9
Pyne, D. B., West, N. P., Cox, A. J., and Cripps, A. W. (2015). Probiotics supplementation for athletes-clinical and physiological effects. Eur. J. Sport Sci. 15, 63–72. doi: 10.1080/17461391.2014.971879
Ranadive, S. M., Cook, M., Kappus, R. M., Yan, H., Lane, A. D., Woods, J. A., et al. (2014). Effect of acute aerobic exercise on vaccine efficacy in older adults. Med. Sci. Sports Exerc. 46, 455–461. doi: 10.1249/MSS.0b013e3182a75ff2
Raysmith, B. P., and Drew, M. K. (2016). Performance success or failure is influenced by weeks lost to injury and illness in elite Australian track and field athletes: a 5-year prospective study. J. Sci. Med. Sport 19, 778–783. doi: 10.1016/j.jsams.2015.12.515
Schwellnus, M., Soligard, T., Alonso, J.-M., Bahr, R., Clarsen, B., Dijkstra, H. P., et al. (2016). How much is too much? (Part 2) international olympic committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 50, 1043–1052. doi: 10.1136/bjsports-2016-096572
Silva, D., and Moreira, A. (2017). “Asthma and allergies,” in Injuries and Health Problems in Football, eds A. Cogo, M. Bonini, and P. Onorati (Berlin: Springer), 541–561. doi: 10.1007/978-3-662-53924-8_48
Škrgat, S., Marèun, R., Kern, I., Šilar, M., Šelb, J., Fležar, M., et al. (2018). Systemic and airway oxidative stress in competitive swimmers. Respir. Med. 137, 129–133. doi: 10.1016/j.rmed.2018.03.005
Soligard, T., Steffen, K., Palmer, D., Alonso, J. M., Bahr, R., Lopes, A. D., et al. (2017). Sports injury and illness incidence in the Rio de Janeiro 2016 olympic summer games: a prospective study of 11 274 athletes from 207 countries. Br. J. Sports Med. 51, 1265–1271. doi: 10.1136/bjsports-2017-097956
Somerville, V. S., Braakhuis, A. J., and Hopkins, W. G. (2016). Effect of flavonoids on upper respiratory tract infections and immune function: a systematic review and meta-analysis. Adv. Nutr. 7, 488–497. doi: 10.3945/an.115.010538
Stevens, C. J., Thornton, H. R., Fowler, P. M., Esh, C., and Taylor, L. (2018). Long-haul northeast travel disrupts sleep and induces perceived fatigue in endurance athletes. Front. Physiol. 9:1826. doi: 10.1152/ajpregu.1999.277.4.R1152
Svendsen, I. S., Killer, S. C., and Gleeson, M. (2014). Influence of hydration status on changes in plasma cortisol, leukocytes, and antigen-stimulated cytokine production by whole blood culture following prolonged exercise. ISRN Nutr. 2014:561401. doi: 10.1155/2014/561401
Svendsen, I. S., Taylor, I. M., Tønnessen, E., Bahr, R., and Gleeson, M. (2016). Training-related and competition-related risk factors for respiratory tract and gastrointestinal infections in elite cross-country skiers. Br. J. Sports Med. 50, 509–815. doi: 10.1136/bjsports-2015-095398
Thomas, D., Erdman, K., and Burke, L. (2016). American college of sports medicine joint position statement. nutrition and athletic performance. Med. Sci. Sports Exerc. 48, 543–568.
Van Rensburg, D. C. J., Schwellnus, M., Derman, W., and Webborn, N. (2018). Illness among paralympic athletes: epidemiology, risk markers, and preventative strategies. Phys. Med. Rehabil. Clin. N. Am. 29, 185–203. doi: 10.1016/j.pmr.2018.01.003
Walsh, N. P. (2018). Recommendations to maintain immune health in athletes. Eur. J. Sport Sci. 18, 1–12. doi: 10.1080/17461391.2018.1449895
Walsh, N. P., Gleeson, M., Pyne, D. B., Nieman, D. C., Dhabhar, F. S., Shephard, R. J., et al. (2011a). Position statement part two: maintaining immune health. Exerc. Immunol. Rev. 17, 64–103.
Walsh, N. P., Gleeson, M., Shephard, R. J., Gleeson, M., Woods, J. A., Bishop, N., et al. (2011b). Position statement part one: immune function and exercise. Exerc. Immunol. Rev. 17, 6–63.
Wilder-Smith, A., Mustafa, F. B., Peng, C. M., Earnest, A., Koh, D., Lin, G., et al. (2012). Transient immune impairment after a simulated long-haul flight. Aviat. Space Environ. Med. 83, 418–423. doi: 10.3357/ASEM.3162.2012
Williams, N. C., Killer, S. C., Svendsen, I. S., and Jones, A. W. (2018). Immune nutrition and exercise: narrative review and practical recommendations. Eur. J. Sport Sci. 19, 49–61. doi: 10.1080/17461391.2018.1490458
Willmott, A. G., Hayes, M., Waldock, K. A., Relf, R. L., Watkins, E. R., James, C. A., et al. (2016). Short-term heat acclimation prior to a multi-day desert ultra-marathon improves physiological and psychological responses without compromising immune status. J. Sports Sci. 35, 2249–2256. doi: 10.1080/02640414.2016.1265142
Keywords: Olympic, Paralympic, illness, strategies, health, stressors
Citation: Keaney LC, Kilding AE, Merien F and Dulson DK (2019) Keeping Athletes Healthy at the 2020 Tokyo Summer Games: Considerations and Illness Prevention Strategies. Front. Physiol. 10:426. doi: 10.3389/fphys.2019.00426
Received: 03 November 2018; Accepted: 27 March 2019;
Published: 17 April 2019.
Edited by:
Glen Davison, University of Kent, United KingdomReviewed by:
Arwel Wyn Jones, University of Lincoln, United KingdomHelen Hanstock, Mid Sweden University, Sweden
Copyright © 2019 Keaney, Kilding, Merien and Dulson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lauren C. Keaney, TGF1cmVuLmtlYW5leUBhdXQuYWMubno=; TGF1cmVuLmtlYW5leUBocHNuei5vcmcubno=
 Lauren C. Keaney
Lauren C. Keaney Andrew E. Kilding
Andrew E. Kilding Fabrice Merien1,2
Fabrice Merien1,2 Deborah K. Dulson
Deborah K. Dulson