- 1Center for Reproductive Medicine, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan, China
- 3Shandong Key Laboratory of Reproductive Medicine, Jinan, China
- 4Shandong Provincial Clinical Research Center for Reproductive Health, Jinan, China
- 5National Research Center for Assisted Reproductive Technology and Reproductive Genetics, Shandong University, Jinan, China
- 6Women and Children’s Hospital, School of Medicine, Xiamen University, Xiamen, China
Ovarian sensitivity could affect the outcome of in vitro fertilization and embryo transfer (IVF-ET). The objective of this study was to explore the relationship between the ovarian sensitivity index (OSI) and traditional ovarian response makers and observe the relationship between OSI and insulin resistance (IR). The patients enrolled in this study included 131 patients with polycystic ovary syndrome (PCOS) with IR (PCOS-IR), 52 patients with PCOS without IR (PCOS-N), 164 patients with control with IR (control-IR), 133 patients with control without IR (control-N), 295 patients with IR, 184 patients with non-IR, 183 patients with PCOS, and 297 patients with control (patients with non-PCOS). All patients received standard long protocol or the gonadotropin-releasing hormone (GnRH) antagonist protocol to induce follicular development. The two protocols downregulated the pituitary function or blocked the pituitary luteinizing hormone (LH) secretion with a GnRH antagonist. Both protocols can block premature LH surges because premature luteinization is not conducive to follicular development. All patients underwent IVF or intracytoplasmic sperm injection (ICSI). Embryo transfer was carried out according to the specific situation of each patient. The OSI was significantly reduced in patients with IR. The OSI had a significant positive relationship with anti-Müllerian hormone (AMH), antral follicle count (AFC), basal LH/follicle-stimulating hormone (FSH), dominant follicle number on trigger day, retrieved oocytes, embryo number, and high-quality embryo number. OSI had a significant negative relationship with age, body mass index (BMI), basal FSH, initial dose of Gn, and total dose of Gn. The receiver operating characteristic (ROC) curve of OSI demonstrated a better accuracy in distinguishing patients with positive pregnancy and clinical pregnancy, with an area under the curve (AUC) of 0.662 (95% CI, 0.598–0.727) and 0.636 (95% CI, 0.577–0.695), respectively. Patients could get a higher rate of dominant follicle count (p < 0.0001) through the treatment of standard long protocol when compared with GnRH antagonist protocol. The OSI has a significant correlation with traditional ovarian response markers and could be a good predictor of positive pregnancy and clinical pregnancy for patients with IR.
Introduction
Insulin resistance (IR) is defined as an impaired biological response to insulin stimulation of target tissues, mainly including the liver, muscle, and adipose tissue. IR impairs glucose metabolism and results in a compensatory increase in beta-cell insulin production and hyperinsulinemia (Freeman and Pennings, 2021). It is generally considered that Homeostatic Model Assessment of IR (HOMA-IR) ≥ 2.57 is IR. The adverse consequence of IR involves hyperglycemia, hypertension, dyslipidemia, visceral adiposity, hyperuricemia, elevated inflammatory markers, endothelial dysfunction, and a prothrombotic state (Brown et al., 2019; Deacon, 2019; Seong et al., 2019). In addition, the further development of IR may cause obesity, cardiovascular disease, non-alcoholic fatty liver disease, metabolic syndrome, and polycystic ovary syndrome (PCOS) (Freeman and Pennings, 2021).
Polycystic ovary syndrome is a common reproductive endocrine disease, which affected 8–13% of women of reproductive age (Teede et al., 2019). The main characters of PCOS include ovulatory dysfunction, hyperandrogenism, and polycystic ovary morphology on ultrasonography (Wei et al., 2017). For patients with PCOS, in vitro fertilization and embryo transfer (IVF-ET) technology is an effective method to help them get pregnant. During the IVF-ET treatment, it is important for the response of ovarian response to the controlled ovarian stimulation (COS) because the number of retrieval of oocytes was associated with the IVF-ET outcomes (Revelli et al., 2020). IR, a common complication of PCOS, is considered a key factor in the development of PCOS (Shorakae et al., 2018). A prospective observational study showed that IR not only could impair early embryo development but also reduce the implantation rate in IVF-ET (Chang et al., 2013). But there are some studies that showed that IR could impair the oocyte quality because the insulin sensitizer contributed to improved oocyte quality and increased embryonic developmental competence (Minge et al., 2008; Robker, 2008). In contrast, the ovarian reserve function of patients with PCOS is usually better (Shrikhande et al., 2020), and PCOS is also a risk factor for ovarian hyperstimulation syndrome (OHSS) (Banker and Garcia-Velasco, 2015). Therefore, it is necessary to develop an individualized COS protocol for patients with PCOS in IVF treatment.
In fact, the sensitivity of ovarian to exogenous gonadotropins [follicle-stimulating hormone (FSH), LH, hMG, or their combination] is varied from person to person (Revelli et al., 2020). Therefore, it is very important to predict the sensitivity of ovarian to exogenous gonadotropins as accurately as possible for the outcomes of COS and personalized treatment. In the current IVF treatment, the initial dose of exogenous gonadotropins is mostly based on patients, clinical parameters, such as age, body mass index (BMI), anti-Müllerian hormone (AMH), and antral follicle count (AFC). However, these clinical parameters still have certain limitations in terms of the accuracy of reflecting ovarian sensitivity (OS), and they cannot properly reflect the dynamic process of follicular growth in response to exogenous gonadotropins (Alviggi et al., 2018).
There are several prediction models used to predict the IVF-ET outcomes so far. The follicle output rate (FORT), referring to the ratio between the number of preovulatory and the pre-stimulation follicles, was positively correlated with the clinical pregnancy rate (Gallot et al., 2012). However, FORT has been shown to be negatively related to AMH, that is, patients could express a low FORT despite the presence of adequate ovarian reserve function (Genro et al., 2011). Follicle-to-oocyte index (FOI), known to be another prediction model of OS, is the ratio of the number of oocytes harvested after COS to the number of antral follicles before the start of COS [FOI = (Oocyte number/Antral follicle count) × 100]. In this study, when FOI > 50%, it is considered that the ovarian has normal sensitivity; on the contrary, FOI ≤ 50% means that the OS is not very well (Alviggi et al., 2018). The difference between FORT and FOI is that FOI could be affected by the technologies of oocyte retrieval and triggering for final oocyte maturation. So, FOI could be used alone or combined with FORT. The modified ovarian sensitivity index (OSI), MOSI [(Total number of follicles on Day 3 or Day 4 (≥6 mm)/Initial follicle-stimulating hormone dose) × 1,000], which is based on initial follicular measurements and the initial FSH dose (Camargo-Mattos et al., 2020). MOSI is mainly used to predict embryo quality and the possibility of pregnancy.
There is also a prediction model of OSI, and OSI = [(Number of retrieved oocytes/Total gonadotropin dose) × 1,000] is a maker that could reflect the potential of follicles to produce oocytes in response to exogenous gonadotropin stimulation (Revelli et al., 2020). OSI is an interesting index for patients who received IVF-ET. In addition, OSI could also predict the IVF-ET outcomes, and Huber et al. (2013) have reported that the clinical pregnancy rate is positively correlated with OSI. Compared with the OSI calculation method, the calculation methods of FOI, FORT, and MOSI all involve follicle numbers. Some studies have questioned the method of predicting OS by using follicles before oocyte retrieved and believed that antral follicle numbers could better reflect OS (Tomas et al., 1997).
In our study, we aimed to clarify the relationship between OSI and traditional ovarian response makers (i.e., age, BMI, AMH, and AFC), and at the same time, we also want to observe the relationship between OSI and IR; understand if these OSI could be a predictor index of the positive pregnancy and clinical pregnancy for patients with IR.
Materials and Methods
Patients
A total of 479 patients were enrolled from the Center for Reproductive Medicine, Shandong University in our study which included 131 patients with PCOS with IR (PCOS-IR), 52 patients with PCOS without IR (PCOS-N), 164 patients with control with IR (control-IR), 133 patients with control without IR (control-N), 295 patients with IR, 184 patients with non-IR, 183 patients with PCOS, and 297 patients with control (patients with non-PCOS). The patients with PCOS were diagnosed based on Rotterdam revised criteria after excluding patients with Cushing’s syndrome, congenital adrenal hyperplasia, and androgen-secreting tumors. The exclusion criteria of the two groups were age < 40 years old; BMI < 30 kg/m2; basal FSH level > 12 mIU/L; and systemic diseases, endometriosis, abnormal prolactin levels or thyroid function, immune diseases, recurrent abortion, and abnormal chromosomal. This study was approved by the Ethics Committee of the Reproductive Hospital Affiliated to Shandong University. Written informed consent was obtained from each patient.
Controlled Ovarian Stimulation Protocols
These patients agreed to undergo standard long protocol or the gonadotropin-releasing hormone (GnRH) antagonist (0.1 mg/ampoule) protocol to promote follicular development. In the GnRH antagonist protocol, recombinant FSH was administered on Day 3 of the menstruation cycle at a dose of 75–225 IU per day. In standard long protocol, patients were administrated GnRH agonist (GnRH-a) in the midluteal phase of the previous cycle until the day of human chorionic gonadotropin (hCG) administration. After menstruation, when the serum estradiol (E2) level was lower than 50 pg/ml, gonadotropin stimulation was started with daily use of recombinant FSH (rFSH). The dose of gonadotropins was adjusted according to the follicular growth monitored by transvaginal ultrasonography and serum E2 concentrations.
Oocyte Retrieval, in vitro Culture, Embryo Transfer, and Pregnancy Assessment
Human chorionic gonadotropin was used when at least two follicles reached 18 mm in diameter, and 36 h later, oocytes were retrieved by transvaginal ultrasound-guided follicular aspiration. All patients underwent IVF or intracytoplasmic sperm injection (ICSI) at the Center for Reproductive Medicine, Shandong University. The good quality embryos were defined as embryos developed from normally fertilized eggs with no fragmentation or not more than 1/3, no presence of multinucleation, 3–5 blastomeres at 48 h after egg retrieval, and at least 7 blastomeres by 72 h (Puissant et al., 1987).
Embryo transfer was carried out according to the specific situation of each patient. According to the quality of embryo standard: low-quality embryos have 2–7 blastomeres (fragmentation > 20%) and high-quality embryos have 8 or more blastomeres (fragmentation ≤ 20%), and we also collected the number of high-quality embryos. When plasma β-hCG > 10.0 IU/L on 14 days after embryo transfer, we considered that it was a positive pregnancy. The clinical pregnancy was diagnosed by the existence of the pregnancy sac and fetal heartbeat in the uterine cavity on 30 days after embryo transfer.
Statistical Analysis
The OSI was calculated by applying the following formula (Huber et al., 2013): (number of retrieved oocytes/total gonadotropin dose) × 1,000. The rate of the dominant follicle was calculated by the following formula: the dominant follicle count on trigger day/the follicle count on trigger day.
We used SPSS 26.0 (SPSS, Chicago, IL, United States), GraphPad Prism 8.0 (GraphPad Software, CA, United States), and R studio for the statistical analyses. Data were reported as mean ± SD. Student’s t-test was used for between-group comparisons, and Spearman correlation analysis was used for analyzing linear associations. Receiver operating characteristic (ROC) curve analysis was used for assessing the efficiency of the OSI to evaluate the positive pregnancy and clinical pregnancy. A p-value of <0.05 was regarded as statistical significance.
Results
Ovarian Sensitivity Index Was Significantly Reduced in Patients With Insulin Resistance
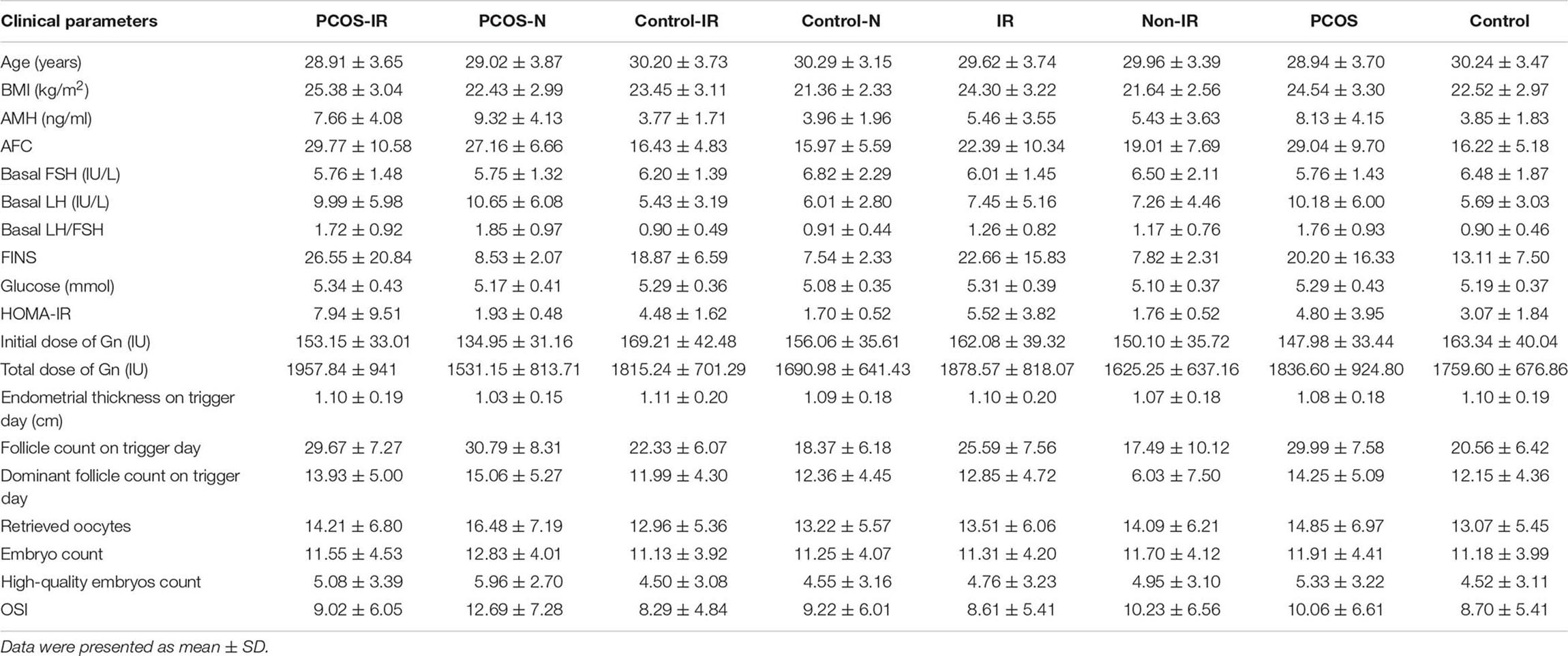
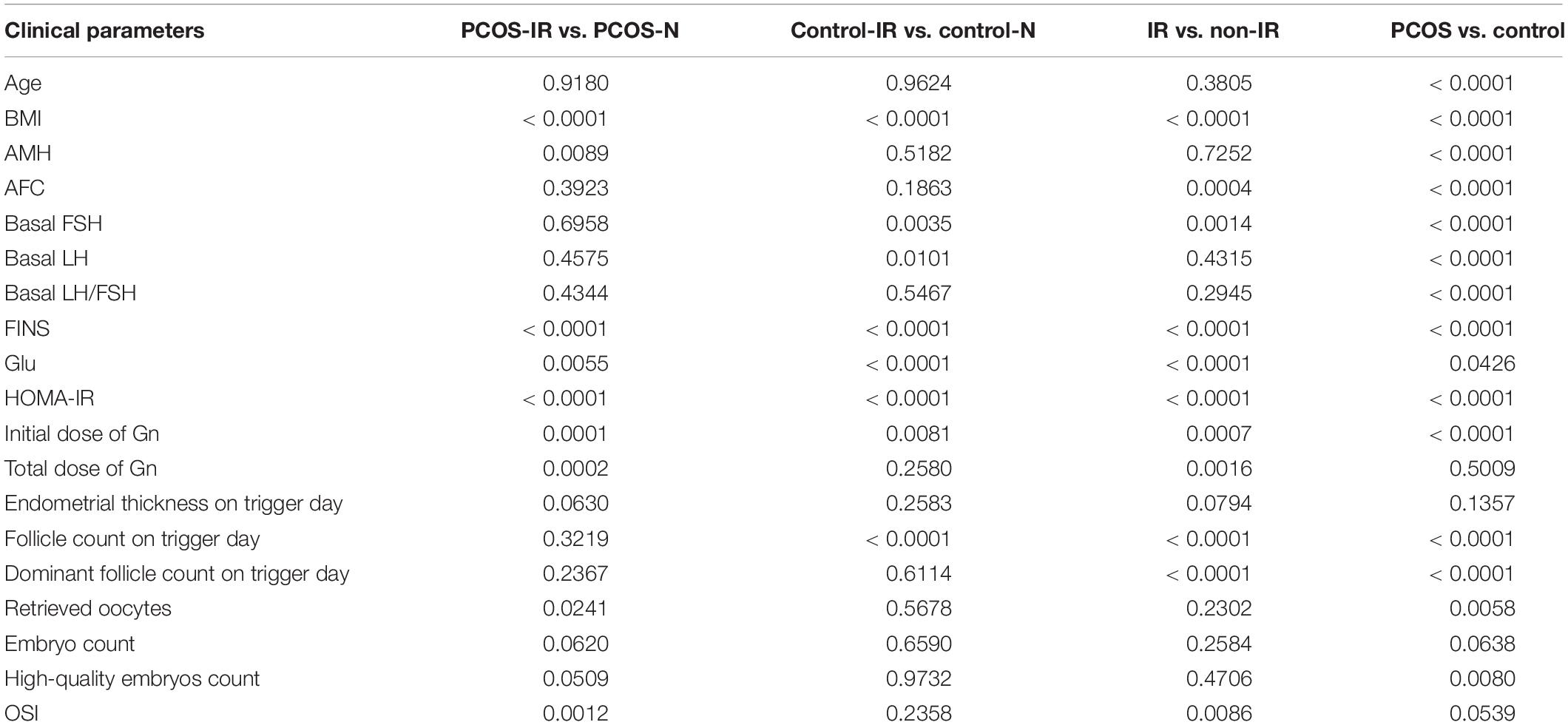
The clinical parameters of all patients are shown in Table 1. We compared the differences of OSI between patients with IR and patients with non-IR, patients with PCOS and patients with non-PCOS, patients with PCOS-IR and patients with PCOS-N, and patients with control-IR and patients with control-N (Table 2). The results showed that OSI decreased significantly in all patients with IR (p = 0.0086). Moreover, OSI also decreased significantly in patients with PCOS-IR (p = 0.0012). Although OSI was not significantly different between patients with control-IR and patients with control-N, OSI still has a downward trend in patients with control-IR (p = 0.2358). However, compared with patients with non-PCOS, OSI exhibited an increasing trend among patients with PCOS (p = 0.0539) (Figure 1).
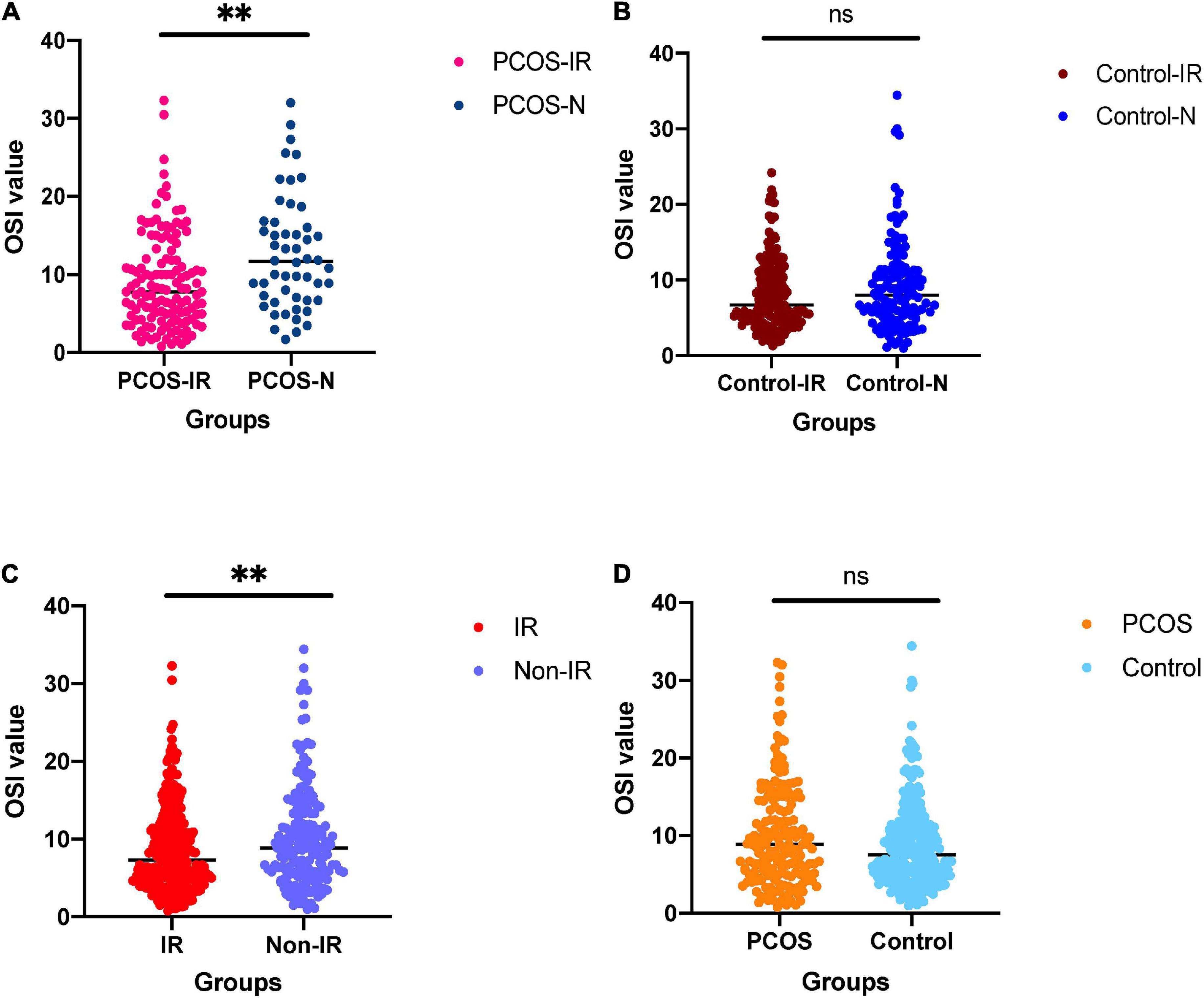
Figure 1. (A) For comparison of ovarian sensitivity index (OSI) between polycystic ovary syndrome (PCOS)-insulin resistance (IR) groups and PCOS-N group. (B) For comparison of OSI between the control-IR group and control-N group. (C) For comparison of OSI between IR group and normal group. (D) For comparison of OSI between PCOS group and control group. nsnot significant; ∗∗p < 0.01.
Analysis of the Correlation Between Ovarian Sensitivity Index With Clinical Parameters of Patients With Insulin Resistance
Pearson’s correlation analysis was performed between OSI with clinical parameters which included age, BMI, hormones level, endometrial thickness on trigger day, follicles situation on trigger day, embryos situation on trigger day, and some biomarkers. The results showed that OSI had a significant positive relationship with AMH, AFC, basal LH/FSH, follicle count on trigger day, dominant follicles number on trigger day, retrieved oocytes, embryos number, and high-quality embryos number. OSI had a significant negative relationship with age, BMI, basal FSH, initial dose of Gn, and total dose of Gn (Table 3 and Figure 2).
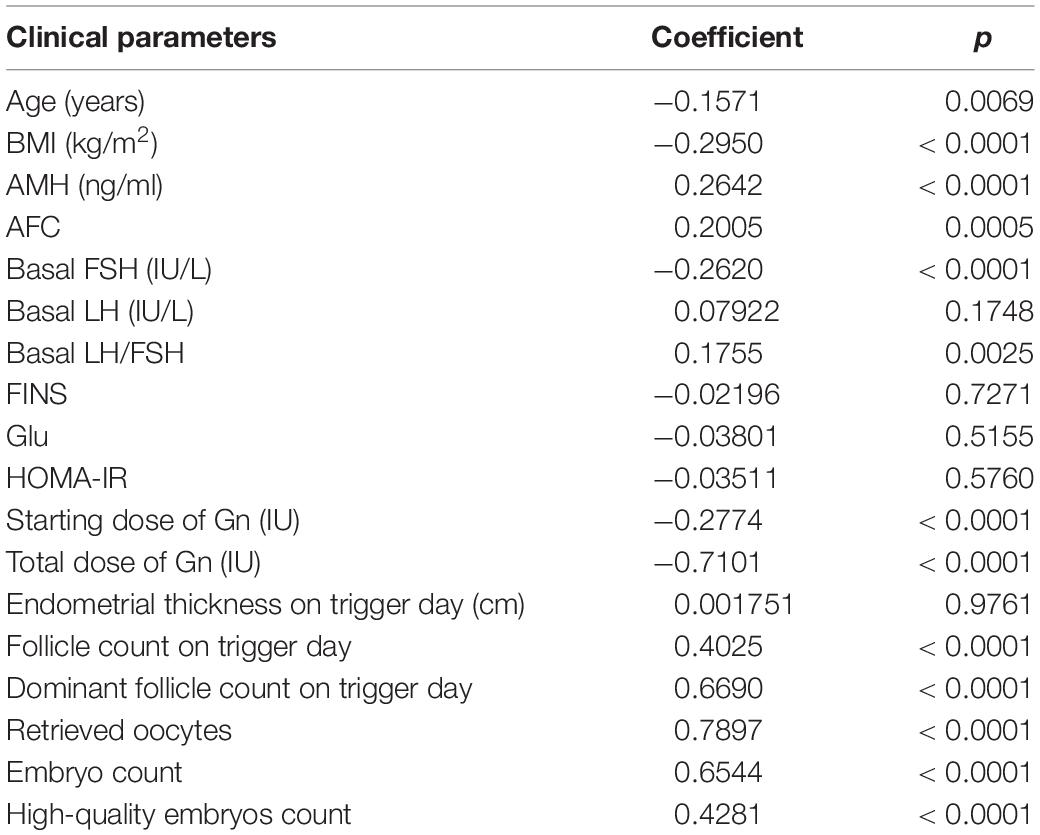
Table 3. Correlation among ovarian sensitivity index (OSI) and some clinical parameters of patients with insulin resistance (IR).

Figure 2. Correlation among OSI and clinical parameters of patients with IR. *p < 0.05; **p < 0.01; ***p < 0.001.
The Relationship of Ovarian Sensitivity Index and Positive Pregnancy and Clinical Pregnancy of Patients With Insulin Resistance
The OSI was significantly different between positive pregnancy patients and negative pregnancy patients (p < 0.0001), and it was also significantly different between clinical pregnancy patients and negative clinical pregnancy patients (p < 0.0001) (Table 4 and Figure 3). We used the ROC curve that analyzed the prediction efficiency for a positive pregnancy and clinical pregnancy of OSI. The results showed that the ROC curve of OSI demonstrated a better accuracy in distinguishing patients with positive pregnancy and clinical pregnancy, with an area under the curve (AUC) of 0.662 (95% CI, 0.598–0.727) and 0.636 (95% CI, 0.577–0.695), respectively (Table 5 and Figure 3).
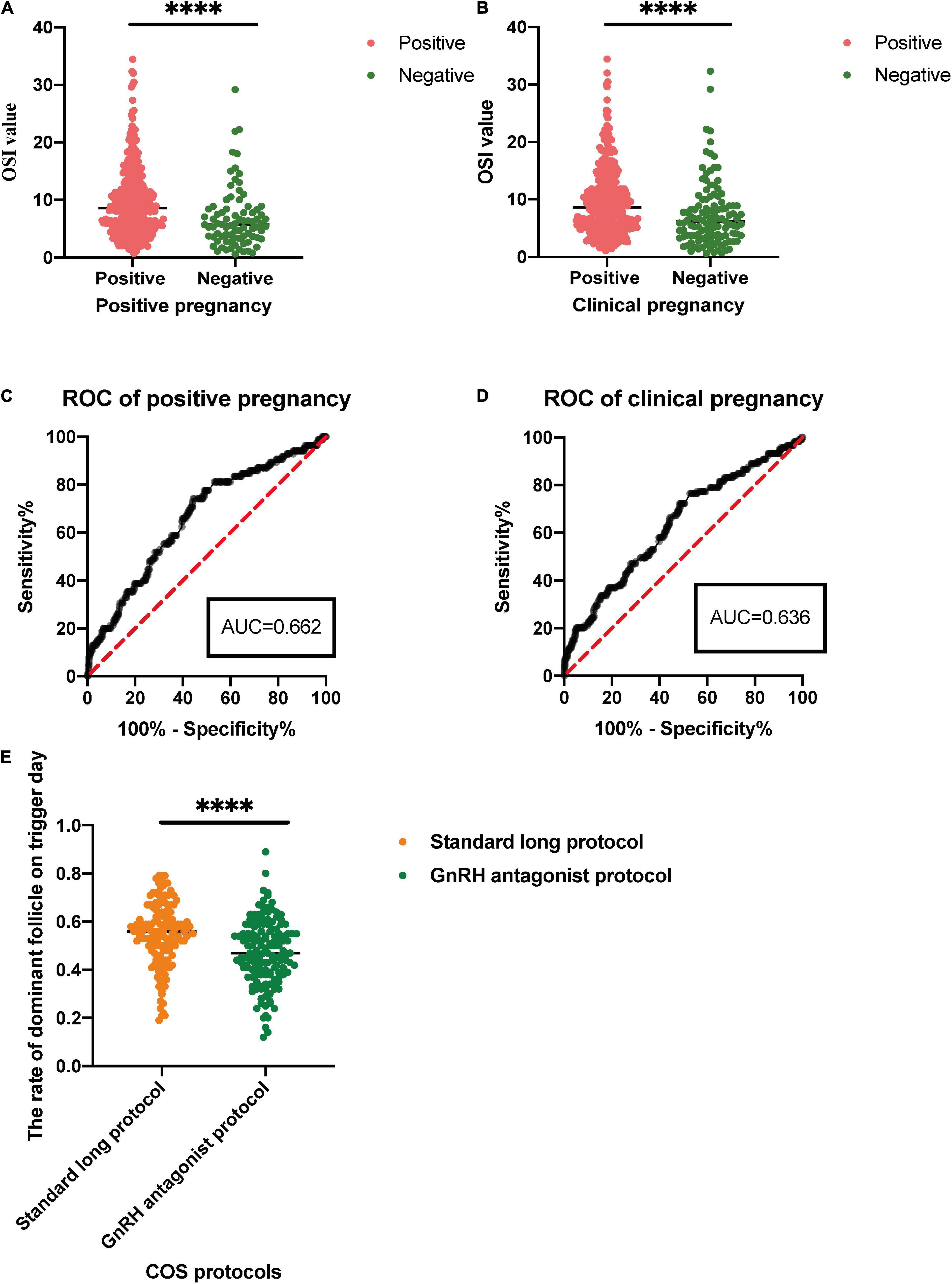
Figure 3. (A,B) For comparison of the OSI in positive pregnancy and clinical pregnancy. (C,D) For receiver operating characteristic (ROC) analysis of OSI to determine effectiveness in a positive pregnancy and clinical pregnancy. (E) For the rate of dominant follicle on trigger day in different control stimulating protocols for patients with IR. ∗∗∗∗p < 0.0001.
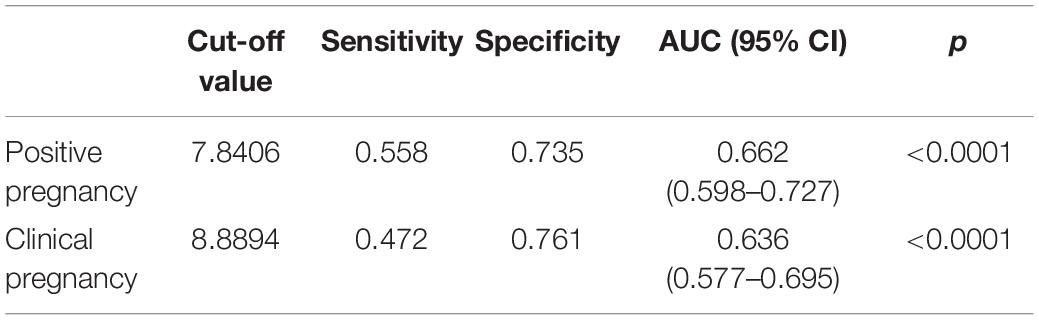
Table 5. Receiver operating characteristic analysis of OSI to determine effectiveness in a positive pregnancy and clinical pregnancy.
The Rate of Dominant Follicle on Trigger Day in Different Control Stimulating Protocols for Patients With Insulin Resistance
In this study, patients received standard long protocol and/or the GnRH antagonist protocol to induce follicular development, and we also compared the difference between these two COS protocols of patients with IR. The results showed that patients could get a higher rate of dominant follicle count (dominant follicle count on trigger day/all follicle counts on the trigger, p < 0.0001) through the treatment of standard long protocol when compared with GnRH antagonist protocol (Table 6 and Figure 3).
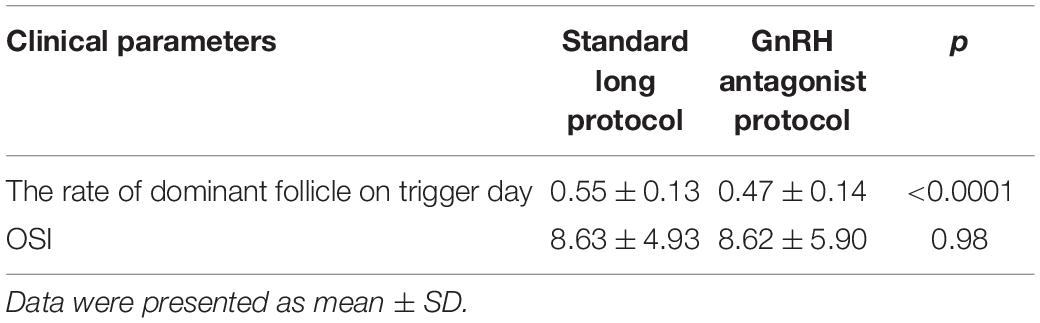
Table 6. The rate of dominant follicle on trigger day in different control stimulating protocols for patients with IR.
Discussion
In our study, compared with patients with non-IR, the OSI in the IR group and PCOS-IR group is significantly reduced. Additionally, we observed that the OSI exhibited no significant difference between patients with PCOS and patients with control, as well as between the control-IR group and control-N. Through various cross-comparisons, we believed that IR may play an important role in reducing OS. Our results were consistent with previous reports, where the OSI is negatively correlated with age and BMI, but positively correlated with AMH and AFC (Biasoni et al., 2011). In addition, the OSI is also significantly correlated with other clinical parameters. Our results showed that the OSI is negatively correlated with the basal serum FSH level, starting dose of Gn, and total dose of Gn. In addition, the OSI is positively correlated with basal serum LH/FSH, endometrial thickness on trigger day, follicle count on trigger day, dominant follicle count on trigger day, retrieved oocytes, embryo count, and high-quality embryo count. Hyperinsulinemia could impair oocyte development competence, resulting in lower rates of fertilization, embryonic development, and implantation in patients with PCOS with obesity (Qiao and Feng, 2011). Insulin binds to receptors located on granulose cells (GCs), theca cells (TCs), and oocytes to stimulate follicle recruitment, resulting in changes in the expression of multiple genes involved in meiotic/mitotic spindle dynamics and centrosome function in PCOS oocytes (Wood et al., 2007). Dumesic et al. (2008) reported that insulin may be an important mediator of oocyte developmental competence via a ligand-receptor regulating system. The compensatory response to IR is hyperinsulinemia, and increased insulin could cause an increase in androgen. Excess insulin enhances androgen production in ovarian TCs under the stimulation of LH, resulting in follicular arrest and anovulation eventually (Silvestris et al., 2018). In addition, increased androgen could result in oocytes of lower quality after maturation (Cano et al., 1997). The vicious cycle of the two factors promotes the occurrence and development of PCOS. These results suggest that we need to reduce the starting dose of Gn appropriately when we encountered such patients. However, when IR occurs in patients, the starting dose of Gn or total dose of Gn should be appropriately increased considering the possibility that the OS of patients will be reduced.
Furthermore, we observed that the OSI could demonstrate a better accuracy in distinguishing patients with positive pregnancy and clinical pregnancy. With the increase of OSI, positive pregnancy and clinical pregnancy also increase. These results could be explained by the evidence that improving blastocyst and oocyte quality can increase the rate of clinical pregnancy (Zangmo et al., 2014; Wdowiak and Filip, 2020). It is suggested that the patients should reduce the insulin level before COS, which will increase the OS and improve the positive pregnancy rate and clinical pregnancy rate. Therefore, monitoring the OSI level could contribute to the judgment of positive pregnancy and clinical pregnancy. Through evaluating the patients with IR submitted to IVF who received two COS protocols, we observed that the OSI exhibited no significant difference in these two protocols, which suggests that COS protocols would not affect OSI in IVF. This result was also in line with previous study (Revelli et al., 2020). However, the rate of the dominant follicle count was significantly different between these two COS protocols for patients with IR in our study, which provided evidence that patients with IR receiving standard long protocol are more likely to harvest more dominant follicles on trigger day under the premise of clinical medication safety. Therefore, OSI could reflect the sensitivity of the ovary to Gn and could estimate the rate of positive pregnancy and clinical pregnancy for patients under standard long protocol or GnRH antagonist protocol. Compared with other markers of ovarian response, such as FOI, FORT, and MOSI, OSI could directly reflect the Gn dosage requirement of each oocyte due to its calculation method. Moreover, the OSI level was useful to determine the COS protocol and the starting dose of Gn for patients who need to receive COS for the next cycle.
Since the number of oocytes could significantly affect the probability of obtaining a live birth with a fresh embryo transfer (Sunkara et al., 2011) and the cumulative live birth rate after transferring in utero all thawed embryos (Fatemi et al., 2013; Li et al., 2013), harvesting more oocytes also means that patients may get a higher live birth rate. In the traditional treatment of IVF-ET, age, BMI, AFC, or AMH were often used to determine the starting dose of Gn for patients to get more oocytes. However, the factors that affect the number of oocytes are not limited to these. The choices of COS protocol, the type and dose of gonadotropins, and the ovarian inherent sensitivity of each patient should take into consideration when patients receive COS treatment. The maturation process of oocytes is very complex, in theory, the larger number of oocytes we would like to harvest, the higher level of hormonal simulation should be implemented. However, the excessive hormonal simulation would bring safety problems in clinical practice, such as OHSS (Broekmans, 2019). Therefore, the number of oocytes obtained using the traditional COS protocol may not reflect the full potential of the ovary. The OSI associates the number of oocytes with the dose of Gn, which allowing us to obtain the amount of Gn required for each oocyte to develop intuitively (Biasoni et al., 2011). Along with this perspective, we considered that the OSI was very helpful for the formulation of COS protocol and the determination of the starting dose of Gn for patients.
Conclusion
Our study found that the OSI decreased significantly in patients with IR, and OSI has a significant correlation with traditional ovarian response markers and could be a good predictor of positive pregnancy and clinical pregnancy for patients with IR. In addition, the OSI had clinical guiding significance for patients to carry out the next cycle of COS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Reproductive Hospital Affiliated to Shandong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YZ and YS conceived and designed this study. ZyW collected the clinical data. YZ, YP, and ZeW contributed to statistical analysis. YZ drafted the manuscript. YZ, PL, and YS participated in the discussion and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key R&D Program of China (2021YFC2700404 and 2018YFC1003202) and Taishan scholar project special funds (No. ts201712103).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all the patients who participated in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.809419/full#supplementary-material
References
Alviggi, C., Conforti, A., Esteves, S. C., Vallone, R., Venturella, R., Staiano, S., et al. (2018). Understanding ovarian hypo-response to exogenous gonadotropin in ovarian stimulation and its new proposed Marker-The Follicle-To-Oocyte (FOI) index. Front. Endocrinol. (Lausanne) 9:589. doi: 10.3389/fendo.2018.00589
Banker, M., and Garcia-Velasco, J. A. (2015). Revisiting ovarian hyper stimulation syndrome: towards OHSS free clinic. J. Hum. Reprod. Sci. 8, 13–17. doi: 10.4103/0974-1208.153120
Biasoni, V., Patriarca, A., Dalmasso, P., Bertagna, A., Manieri, C., Benedetto, C., et al. (2011). Ovarian sensitivity index is strongly related to circulating AMH and may be used to predict ovarian response to exogenous gonadotropins in IVF. Reprod. Biol. Endocrinol. 9:112. doi: 10.1186/1477-7827-9-112
Broekmans, F. J. (2019). Individualization of FSH doses in assisted reproduction: facts and fiction. Front. Endocrinol. (Lausanne) 10:181. doi: 10.3389/fendo.2019.00181
Brown, J. C., Harhay, M. O., and Harhay, M. N. (2019). The value of anthropometric measures in nutrition and metabolism: comment on anthropometrically predicted visceral adipose tissue and blood-based biomarkers: a cross-sectional analysis. Nutr. Metab. Insights 12:1178638819831712. doi: 10.1177/1178638819831712
Camargo-Mattos, D., García, U., Camargo-Diaz, F., Ortiz, G., Madrazo, I., and Lopez-Bayghen, E. (2020). Initial ovarian sensitivity index predicts embryo quality and pregnancy potential in the first days of controlled ovarian stimulation. J. Ovarian Res. 13:94. doi: 10.1186/s13048-020-00688-7
Cano, F., García-Velasco, J. A., Millet, A., Remohí, J., Simón, C., and Pellicer, A. (1997). Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women. J. Assisted Reprod. Genet. 14, 254–261. doi: 10.1007/BF02765826
Chang, E. M., Han, J. E., Seok, H. H., Lee, D. R., Yoon, T. K., and Lee, W. S. (2013). Insulin resistance does not affect early embryo development but lowers implantation rate in in vitro maturation-in vitro fertilization-embryo transfer cycle. Clin. Endocrinol. 79, 93–99. doi: 10.1111/cen.12099
Deacon, C. F. (2019). Physiology and pharmacology of DPP-4 in glucose homeostasis and the treatment of Type 2 diabetes. Front. Endocrinol. (Lausanne) 10:80. doi: 10.3389/fendo.2019.00080
Dumesic, D. A., Padmanabhan, V., and Abbott, D. H. (2008). Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol. Surv. 63, 39–48.
Fatemi, H. M., Doody, K., Griesinger, G., Witjes, H., and Mannaerts, B. (2013). High ovarian response does not jeopardize ongoing pregnancy rates and increases cumulative pregnancy rates in a GnRH-antagonist protocol. Hum. Reprod. (Oxford, England) 28, 442–452. doi: 10.1093/humrep/des389
Freeman, A. M., and Pennings, N. (2021). “Insulin resistance,” in StatPearls [Internet]. (Treasure Island, FL: StatPearls Publishing).
Gallot, V., Berwanger da Silva, A. L., Genro, V., Grynberg, M., Frydman, N., and Fanchin, R. (2012). Antral follicle responsiveness to follicle-stimulating hormone administration assessed by the Follicular Output RaTe (FORT) may predict in vitro fertilization-embryo transfer outcome. Hum. Reprod. (Oxford, England) 27, 1066–1072. doi: 10.1093/humrep/der479
Genro, V. K., Grynberg, M., Scheffer, J. B., Roux, I., Frydman, R., and Fanchin, R. (2011). Serum anti-Müllerian hormone levels are negatively related to Follicular Output RaTe (FORT) in normo-cycling women undergoing controlled ovarian hyperstimulation. Hum. Reprod. (Oxford, England) 26, 671–677. doi: 10.1093/humrep/deq361
Huber, M., Hadziosmanovic, N., Berglund, L., and Holte, J. (2013). Using the ovarian sensitivity index to define poor, normal, and high response after controlled ovarian hyperstimulation in the long gonadotropin-releasing hormone-agonist protocol: suggestions for a new principle to solve an old problem. Fertil. Steril. 100, 1270–1276. doi: 10.1016/j.fertnstert.2013.06.049
Li, H. W., Lee, V. C., Lau, E. Y., Yeung, W. S., Ho, P. C., and Ng, E. H. (2013). Role of baseline antral follicle count and anti-Mullerian hormone in prediction of cumulative live birth in the first in vitro fertilisation cycle: a retrospective cohort analysis. PLoS One 8:e61095. doi: 10.1371/journal.pone.0061095
Minge, C. E., Bennett, B. D., Norman, R. J., and Robker, R. L. (2008). Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology 149, 2646–2656. doi: 10.1210/en.2007-1570
Puissant, F., Van Rysselberge, M., Barlow, P., Deweze, J., and Leroy, F. (1987). Embryo scoring as a prognostic tool in IVF treatment. Hum. Reprod. (Oxford, England) 2, 705–708. doi: 10.1093/oxfordjournals.humrep.a136618
Qiao, J., and Feng, H. L. (2011). Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update 17, 17–33. doi: 10.1093/humupd/dmq032
Revelli, A., Gennarelli, G., Biasoni, V., Chiadò, A., Carosso, A., Evangelista, F., et al. (2020). The Ovarian Sensitivity Index (OSI) significantly correlates with ovarian reserve biomarkers, is more predictive of clinical pregnancy than the total number of oocytes, and is consistent in consecutive IVF cycles. J. Clin. Med. 9:1914. doi: 10.3390/jcm9061914
Robker, R. L. (2008). Evidence that obesity alters the quality of oocytes and embryos. Pathophysiology 15, 115–121. doi: 10.1016/j.pathophys.2008.04.004
Seong, J., Kang, J. Y., Sun, J. S., and Kim, K. W. (2019). Hypothalamic inflammation and obesity: a mechanistic review. Arch. Pharm. Res. 42, 383–392. doi: 10.1007/s12272-019-01138-9
Shorakae, S., Ranasinha, S., Abell, S., Lambert, G., Lambert, E., de Courten, B., et al. (2018). Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin. Endocrinol. 89, 628–633. doi: 10.1111/cen.13808
Shrikhande, L., Shrikhande, B., and Shrikhande, A. (2020). AMH and its clinical implications. J. Obstet Gynaecol. Ind. 70, 337–341.
Silvestris, E., de Pergola, G., Rosania, R., and Loverro, G. (2018). Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 16:22. doi: 10.1186/s12958-018-0336-z
Sunkara, S. K., Rittenberg, V., Raine-Fenning, N., Bhattacharya, S., Zamora, J., and Coomarasamy, A. (2011). Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum. Reprod. (Oxford, England) 26, 1768–1774. doi: 10.1093/humrep/der106
Teede, H., Misso, M., Tassone, E. C., Dewailly, D., Ng, E. H., Azziz, R., et al. (2019). Anti-Müllerian hormone in PCOS: a review informing international guidelines. Trends Endocrinol. Metab. 30, 467–478. doi: 10.1016/j.tem.2019.04.006
Tomas, C., Nuojua-Huttunen, S., and Martikainen, H. (1997). Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum. Reprod. (Oxford, England) 12, 220–223. doi: 10.1093/humrep/12.2.220
Wdowiak, A., and Filip, M. (2020). The effect of myo-inositol, vitamin D3 and melatonin on the oocyte quality and pregnancy in in vitro fertilization: a randomized prospective controlled trial. Eur. Rev. Med. Pharmacol. Sci. 24, 8529–8536. doi: 10.26355/eurrev_202008_22649
Wei, D., Shi, Y., Li, J., Wang, Z., Zhang, L., Sun, Y., et al. (2017). Effect of pretreatment with oral contraceptives and progestins on IVF outcomes in women with polycystic ovary syndrome. Hum. Reprod. (Oxford, England) 32, 354–361. doi: 10.1093/humrep/dew325
Wood, J. R., Dumesic, D. A., Abbott, D. H., and Strauss, J. F. 3rd. (2007). Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J. Clin. Endocrinol. Metabol. 92, 705–713. doi: 10.1210/jc.2006-2123
Keywords: the ovarian stimulation index, insulin resistance, polycystic ovary syndrome, controlled ovarian stimulation, in vitro fertilization
Citation: Zheng Y, Pan Y, Li P, Wang Z, Wang Z and Shi Y (2022) Ovarian Sensitivity Decreased Significantly in Patients With Insulin Resistance Undergoing in vitro Fertilization and Embryo Transfer. Front. Physiol. 12:809419. doi: 10.3389/fphys.2021.809419
Received: 05 November 2021; Accepted: 13 December 2021;
Published: 11 March 2022.
Edited by:
Alfredo Ulloa-Aguirre, National Autonomous University of Mexico, MexicoReviewed by:
Fernando Larrea, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoTom Kelsey, University of St Andrews, United Kingdom
Copyright © 2022 Zheng, Pan, Li, Wang, Wang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhua Shi, shiyuhua2003@126.com
†These authors have contributed equally to this work
‡Present address: Yuhua Shi, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
 Yanjun Zheng
Yanjun Zheng Ye Pan
Ye Pan Ping Li
Ping Li Zhongyuan Wang1,4
Zhongyuan Wang1,4 Ze Wang
Ze Wang Yuhua Shi
Yuhua Shi