- 1Department of Hepatology, Guangdong Provincial Hospital of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2The Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Community Health Service Center of Tianhenan Street Tianhe District, Guangzhou, China
- 4Department of Hepatobiliary Surgery, Guangdong Provincial Hospital of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Background: The combined effect of hepatitis B virus infection and metabolic dysfunction-associated steatotic liver disease (MASLD) on hepatocellular carcinoma (HCC) risk remains unclear. The current study sought to elucidate the impact of MASLD on HCC progression in chronic hepatitis B (CHB) patients.
Method: This retrospective cohort study included CHB patients who had undergone liver biopsy and abdominal imaging at the Guangdong Provincial Hospital of Chinese Medicine between 2013 and 2019. We investigated the correlation between MASLD and HCC risk, and inverse probability treatment weighting (IPTW) was used to adjust for patient characteristics.
Results: A total of 1,613 patients were included, and 483 (29.9%) were diagnosed with MASLD. Over a median follow-up period of 5.02 years, 36 (2.2%) developed HCC, comprising 4.8% (23/483) of those with MASLD and 1.2% (13/1,130) of those without. Those with MASLD had a significantly higher cumulative incidence of HCC than those without (p < 0.001). The presence of MASLD was associated with a higher risk of HCC (adjusted hazard ratio [HR], 3.996; 95% confidence interval [CI], 2.007–7.959; p < 0.001). After adjustment using IPTW, the patients with MASLD retained a higher cumulative incidence of HCC (p < 0.001). Moreover, MASLD was found to be an independent risk factor for the development of HCC (adjusted HR, 10.191; 95% CI, 4.327–24.002; p < 0.001). However, among patients with MASLD, there were no significant differences in the cumulative risk of HCC between patients with and without overweight, between those with <2 and ≥2 cardiometabolic risk factors (CMRFs), between those with <3 and ≥3 CMRFs, or between those with <4 and ≥4 CMRFs (p = 0.110, p = 0.087, p = 0.066, and p = 0.490, respectively).
Conclusion: The presence of MASLD is associated with a higher risk of HCC in patients with CHB. Notably, this higher risk is present in patients with MASLD, irrespective of the presence or absence of overweight or the number of CMRFs they have.
Introduction
Hepatitis B virus (HBV) infection is a significant global public health issue. In 2019, chronic hepatitis B (CHB) affected about 296 million individuals worldwide, 820,000 of whom died from HBV-related diseases such as liver failure, cirrhosis, or hepatocellular carcinoma (HCC) (You et al., 2023). While long-term antiviral therapy with nucleos(t)ide analogues like entecavir, tenofovir disoproxil fumarate, or tenofovir alafenamide can reduce the risk of HCC occurrence in CHB patients, this treatment cannot completely eliminate the risk (Kim et al., 2022).
Non-alcoholic fatty liver disease (NAFLD) is another severe global health problem, with a worldwide prevalence of 29.8% and a prevalence in China of 29.2% (Le et al., 2019; Zhou et al., 2019). Because of the lack of a unified definition of NAFLD that incorporates key metabolic characteristics, Eslam et al. in 2020 proposed renaming NAFLD as metabolic dysfunction-associated fatty liver disease (MAFLD), defining the condition as hepatic steatosis (HS) with obesity, diabetes mellitus (DM), or other metabolic abnormalities (Eslam et al., 2020a; Eslam et al., 2020b). In 2023, a joint proposal, following the Delphi consensus, was made to redefine NAFLD as metabolic dysfunction-associated steatotic liver disease (MASLD) and include patients with HS and at least one of five cardiometabolic risk factors (CMRFs) (Rinella et al., 2023). While some studies have identified a higher risk of HCC in CHB patients who also suffered from NAFLD (Chan et al., 2017; Kanwal et al., 2018), others have found that HS correlates with a decreased risk of HCC in CHB patients (Li et al., 2021; Mak et al., 2021). These conflicting findings suggest that NAFLD has a complex relationship with CHB, especially after being redefined as MASLD. Although the presence of HS may result in the suppression of HBV viral activity and a reduction in liver damage (Tourkochristou et al., 2022), CMRFs such as DM may increase the risk of HCC (Wang et al., 2012). These findings suggest that the individual components of MASLD may have opposing effects on the clinical manifestations of CHB. In the present study, we assessed the effect of MASLD on the risk of HCC in patients with CHB and compared the differences in the effects of MASLD and NAFLD on the risk of HCC in these patients. Inverse probability treatment weighting (IPTW) was used to adjust for potential confounding factors.
Materials and methods
Population
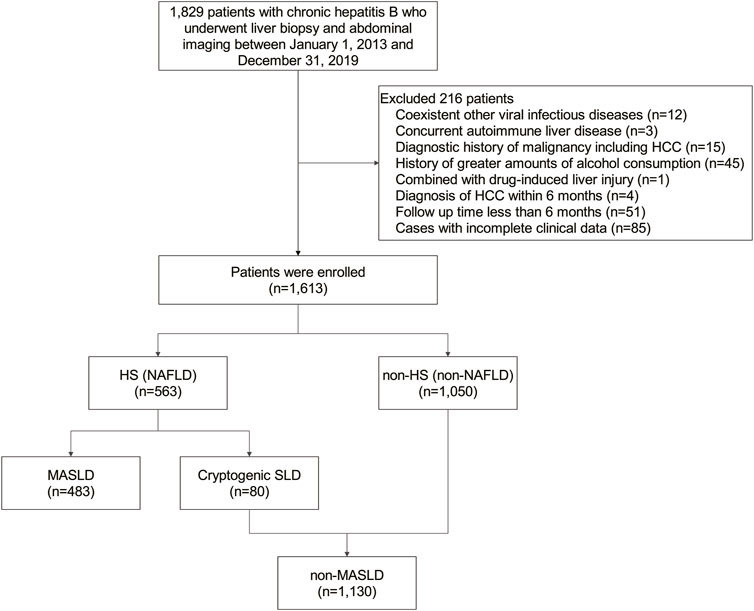
This retrospective cohort study included consecutive CHB patients who underwent liver biopsy and abdominal imaging (ultrasonography, magnetic resonance imaging [MRI], or computed tomography [CT]) at the Guangdong Provincial Hospital of Chinese Medicine from 2013 to 2019. In accordance with the multi-society Delphi consensus statement (Rinella et al., 2023), MASLD was defined as HS, diagnosed histologically or by imaging, with at least one of five CMRFs and no other discernible cause of HS. CMRFs included 1) body mass index (BMI) ≥23 kg/m2, 2) fasting glucose ≥5.6 mmol/L or type 2 DM or treatment for type 2 DM, 3) blood pressure ≥130/85 mmHg, 4) plasma triglycerides ≥1.70 mmol/L or lipid-lowering treatment, and 5) plasma high-density lipoprotein (HDL) cholesterol ≤1.0 mmol/L in males or ≤1.3 mmol/L in females, or lipid-lowering treatment. Those with no metabolic parameters and no known cause of their HS were deemed to have cryptogenic steatotic liver disease (SLD). NAFLD was defined by the presence of HS, identified through imaging or histology, with its “non-alcoholic” aspect being reflected in the subsequent inclusion and exclusion criteria (National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association, 2018).
A baseline assessment was conducted at the time of liver biopsy. We included individuals who met the following criteria: 1) serum hepatitis B surface antigen positivity for ≥6 months (Terrault et al., 2018), 2) ≥18 years regardless of sex, and 3) undergoing regular abdominal imaging procedures, such as ultrasonography, MRI, or CT. Patients were excluded if they 1) had coexistent other viral infections, including hepatitis C virus, hepatitis D virus, and human immunodeficiency virus, 2) had concurrent autoimmune liver disease, 3) consumed >210 g/week or >140 g/week of alcohol for males and females, respectively, 4) another specific etiology that could cause HS, such as drug-induced liver injury and monogenic diseases, 5) a diagnostic history of malignancy, including HCC, before the index date, 6) a follow-up time <6 months or the occurrence of HCC ≤6 months of the index date, or 7) incomplete clinical data.
The diagnostic criterion for liver biopsy is the presence of steatosis in >5% of hepatocytes (Eslam et al., 2020a). The imaging-based diagnosis of HS includes heightened echogenicity of liver parenchyma, decreased visibility of liver structures, and far-field echo attenuation on ultrasonography, a hepatic-to-splenic attenuation ratio of ≤1.0 based on CT, and a proton density fat fraction values of ≥5% on MRI (Chartampilas, 2018).
Biopsy assessment
Two specialized and experienced histopathologists assessed all liver biopsy specimens to determine the degree of steatosis (using the Brunt classification) and fibrosis (according to the Scheuer scoring system) (Scheuer, 1991; Brunt et al., 1999).
Endpoint and follow-up
This study sought to quantify the development of HCC. The date of the liver biopsy was used as the index date. The follow-up duration included time from the index date to the date of HCC diagnosis or the last recorded visit. Most patients regularly underwent HCC surveillance through abdominal imaging and semi-annual to annual monitoring of alpha-fetoprotein (AFP) levels. HCC diagnosis was determined radiologically or histologically according to practice guidelines (Zhou et al., 2023).
Ethics
This study was conducted in adherence with the ethical principles outlined in the 1975 Declaration of Helsinki and approved by the Institutional Review Board of the Guangdong Provincial Hospital of Chinese Medicine (ZE2023–194-01). Given the retrospective nature of the research, the requirement for informed consent was waived.
Statistical analysis
When comparing the baseline demographic and clinical characteristics between the two group patients, continuous variables were expressed as median (interquartile range [IQR]) and evaluated using the Mann-Whitney U test. The Chi-square test was employed to analyze categorical parameters across the two groups. Kaplan-Meier curves were used to plot the cumulative incidence rates of HCC, with intergroup differences assessed using the log-rank test. Clinicopathological factors were screened using the least absolute shrinkage and selection operator (LASSO) Cox regression (Tibshirani, 1997). These factors were then evaluated using multivariate Cox proportional hazards regression, with a bidirectional stepwise method, to identify independent risk factors for HCC.
IPTW was implemented using propensity scores to account for differences in patient characteristics across groups (Chesnaye et al., 2022). Propensity scores were determined by fitting a logistic regression model that included demographic and clinicopathological characteristics as independent variables and the occurrence of MASLD or NAFLD as the dependent variable. After IPTW, we evaluated the balance of the baseline characteristics of study patients between the groups. Weighted Kaplan-Meier curves were used to plot the cumulative incidence rates of HCC. In addition, weighted LASSO Cox regression and weighted multivariate Cox proportional hazards regression were used to identify independent risk factors for the development of HCC.
Statistical analyses were performed using R statistical software (version 4.2.3, R Foundation for Statistical Computing, Vienna, Austria). A two-sided p-value of <0.05 was used to indicate statistical significance.
Results
MASLD on HCC risk in CHB patients
Characteristics of the study population
This study included a cohort of 1,613 patients with CHB, 483 (29.9%) of whom were diagnosed with MASLD (Figure 1). Within this MASLD group, HS was confirmed in 448 patients through liver biopsy, in 13 patients via MRI, in 17 patients using CT, and in 5 patients through ultrasonography. The median (IQR) duration of follow-up was 5.02 (3.09–7.12) years. The baseline demographic and clinicopathological features of the subjects under study are outlined in Table 1. Patients in the MASLD group were predominantly older (p = 0.002) and males (p < 0.001). They exhibited significantly more CMRFs, including overweight, a history of hypertension or DM, increased fasting glucose, triglyceride, total cholesterol, and low-density lipoprotein (LDL) cholesterol levels, and decreased HDL cholesterol levels than patients in the non-MASLD group (all p < 0.001). Furthermore, patients in the MASLD group had a longer history of the use of medication, including statins (p = 0.018), metformin, and angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARBs) (both p < 0.001). MASLD patients also had significantly higher serum concentrations of albumin (ALB), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) (all p < 0.001), but a lower serum concentration of aspartate aminotransferase (AST) (p = 0.014). Interestingly, the MASLD group included fewer patients who had received antiviral treatment for ≥6 months than the non-MASLD group (p < 0.001). Notably, the MASLD group had lower baseline levels of HBV DNA and a smaller proportion of hepatitis B e antigen (HBeAg)-positive patients (p < 0.001 and p = 0.002, respectively).
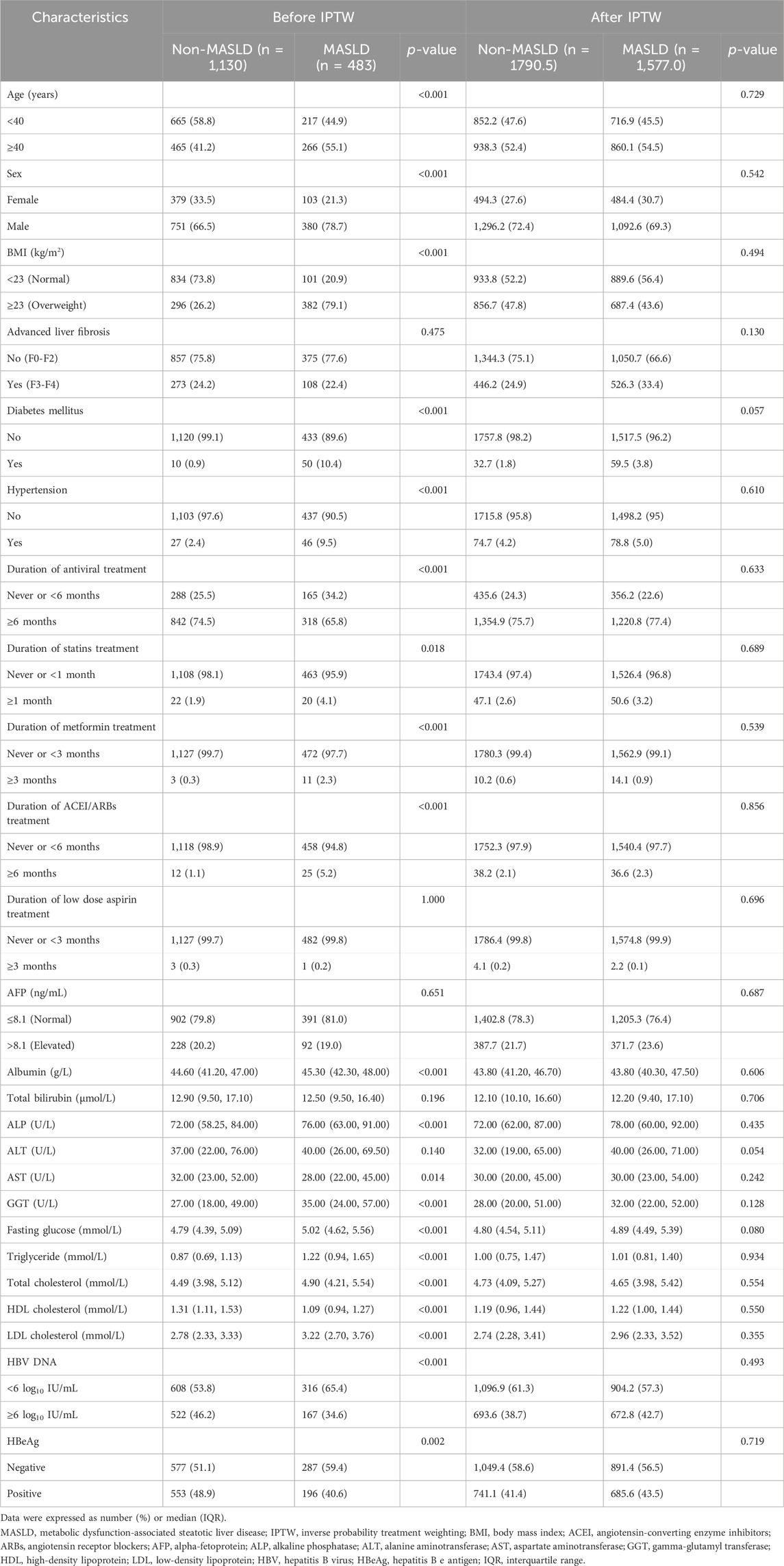
TABLE 1. Baseline clinicopathological characteristics in patients with or without MASLD before and after IPTW.
Development of HCC
During the follow-up period, HCC was diagnosed in 36 patients (2.2%). Of these, 4.8% (23/483) were in the MASLD group and 1.2% (13/1,130) were in the non-MASLD group. The 3- and 5-year cumulative incidence rates of HCC among patients in the MASLD group were 1.7% and 3.7%, respectively, markedly surpassing the 0.8% and 1.4% rates observed in the non-MASLD group (p < 0.001) (Figure 2A). MASLD patients with both advanced liver fibrosis and non-advanced liver fibrosis had a significantly higher cumulative HCC incidence than patients in the non-MASLD group (both p = 0.001) (Figures 2B, C). In addition, MASLD patients had a higher cumulative HCC incidence than non-MASLD patients regardless of whether they had received antiviral treatment for ≥6 months (p < 0.001 and p = 0.022, respectively) (Figures 2D, E). In the HS subgroup, the cumulative risk of developing HCC in patients with MASLD did not differ from that of those with cryptogenic SLD (p = 0.380) (Figure 2F). Within the MASLD subgroup, there was no significant difference in the cumulative risk of HCC between patients who had overweight (BMI ≥23 kg/m2) or not (BMI <23 kg/m2), between those with <2 and ≥2 CMRFs, between those with <3 and ≥3 CMRFs, or between those with <4 and ≥4 CMRFs (p = 0.110, p = 0.087, p = 0.066, and p = 0.490, respectively) (Figures 2G, H). In the non-MASLD subgroup, the cumulative risk of HCC in patients with cryptogenic SLD did not differ from that of those without HS (p = 0.210) (Figure 2I).
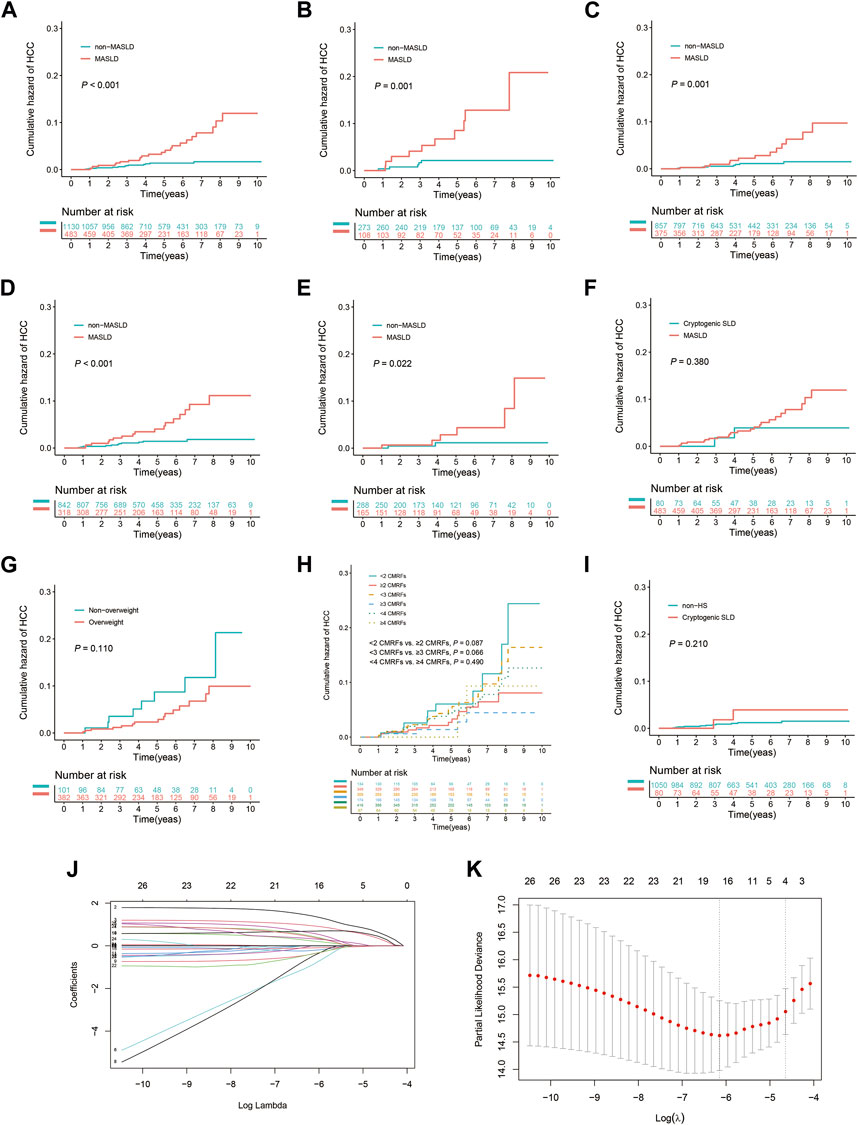
FIGURE 2. Kaplan-Meier curves and the results of LASSO Cox regression analysis prior to IPTW. (A) All patients with or without MASLD. (B) Subgroup with advanced liver fibrosis (F3–F4). (C) Subgroup without advanced liver fibrosis (F0–F2). (D) Subgroup undergoing antiviral treatment for ≥6 months. (E) Subgroup never having received antiviral treatment or undergone treatment for <6 months. (F) Patients with MASLD or cryptogenic SLD in the HS subgroup. (G) Patients with or without overweight (BMI ≥23 kg/m2) in the MASLD subgroup. (H) Patients exhibit varying levels of CMRFs in the MASLD subgroup. (I) Patients with cryptogenic SLD or without HS in the non-MASLD subgroup. (J) Selection of risk factors using LASSO Cox regression analysis. (K) The four risk factors selected using LASSO Cox regression analysis.
Four risk factors for HCC were identified using LASSO Cox regression when the lambda was set to 1 standard error: age (≥40 years), the presence of MASLD, elevated AFP, and ALB levels (Figures 2J, K). As shown in Table 2, a close association between the concomitant presence of MASLD and a high risk of HCC was identified using multivariate Cox analysis with bidirectional stepwise variable selection (adjusted hazard ratio [HR], 3.996; 95% confidence interval [CI], 2.007–7.959; p < 0.001). In addition, the following were identified as independent risk factors for HCC: age (≥40 years) (adjusted HR, 3.185; 95% CI, 1.437–7.057; p = 0.004), elevated AFP (adjusted HR, 2.488; 95% CI, 1.211–5.113; p = 0.013), and ALB levels (adjusted HR, 0.927; 95% CI, 0.861–0.997; p = 0.041).
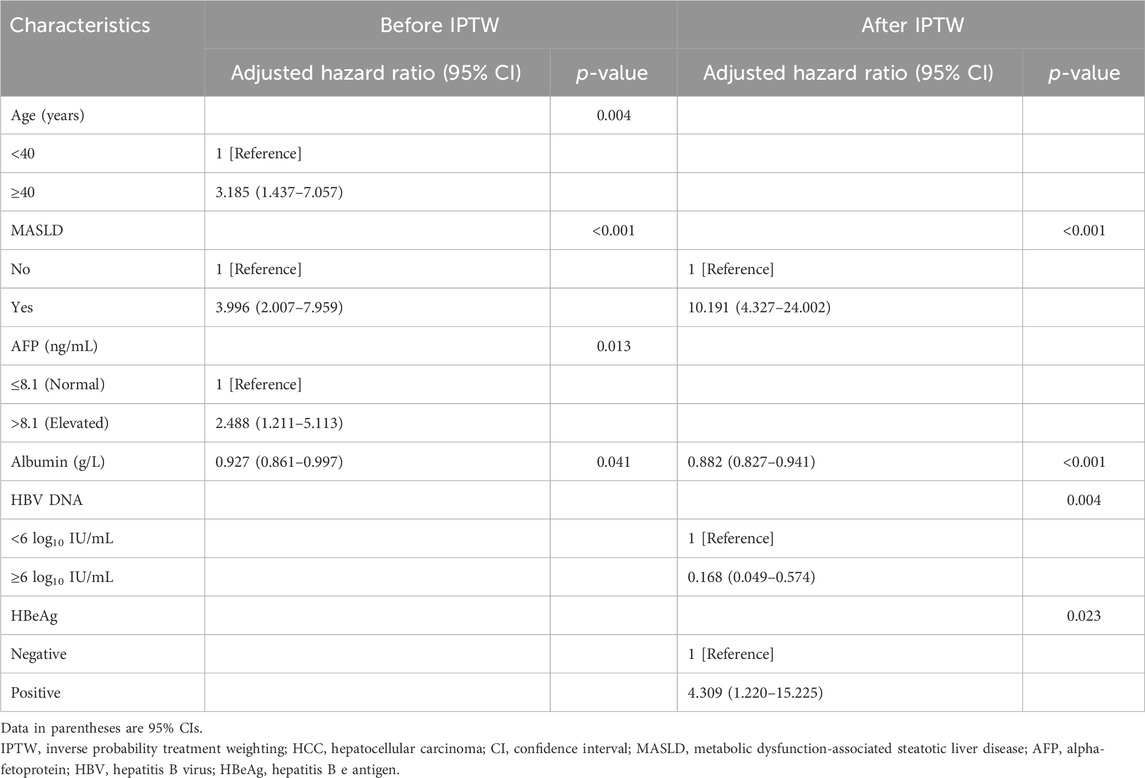
TABLE 2. Multivariate Cox analysis of the factors associated with the development of HCC before and after IPTW.
Evaluation of HCC risk after IPTW
After using IPTW to adjust for patient characteristics, baseline demographic and clinicopathological attributes, as well as medication history, including age, sex, BMI, DM, hypertension, lipid profiles, liver function indicators, HBV DNA levels, HBeAg, and the duration of antiviral, statins, metformin, and ACEI/ARBs treatment, were effectively balanced between the groups (Table 1). Consistent with the results obtained prior to IPTW matching, patients in the MASLD group exhibited a 3- and 5-year cumulative incidence rate of 4.3% and 7.5%, respectively, significantly exceeding the 0.6% and 1.0% rates in the non-MASLD group (p < 0.001) (Figure 3A). Additionally, patients in the MASLD group had a markedly elevated cumulative HCC incidence rate than those in the non-MASLD group in subgroups with advanced liver fibrosis (p = 0.001) and without advanced liver fibrosis (p = 0.001) (Figures 3B, C). Likewise, patients in the MASLD group had a significantly higher cumulative incidence of HCC than those in the non-MASLD group regardless of whether they used antiviral treatment for ≥6 months (p < 0.001 and p = 0.022, respectively) (Figures 3D, E). In the HS subgroup, the cumulative risk of HCC in patients with MASLD did not differ from that of patients with cryptogenic SLD (p = 0.380) (Figure 3F). In the MASLD subgroup, there were no significant differences in the cumulative risk of HCC between patients who had overweight and those who did not (p = 0.110), between those with <2 and ≥2 CMRFs (p = 0.087), between those with <3 and ≥3 CMRFs (p = 0.066), or between those with <4 and ≥4 CMRFs (p = 0.490) (Figures 3G, H). In the non-MASLD subgroup, the cumulative risk of HCC in patients with cryptogenic SLD did not differ from that of those without HS (p = 0.210) (Figure 3I).
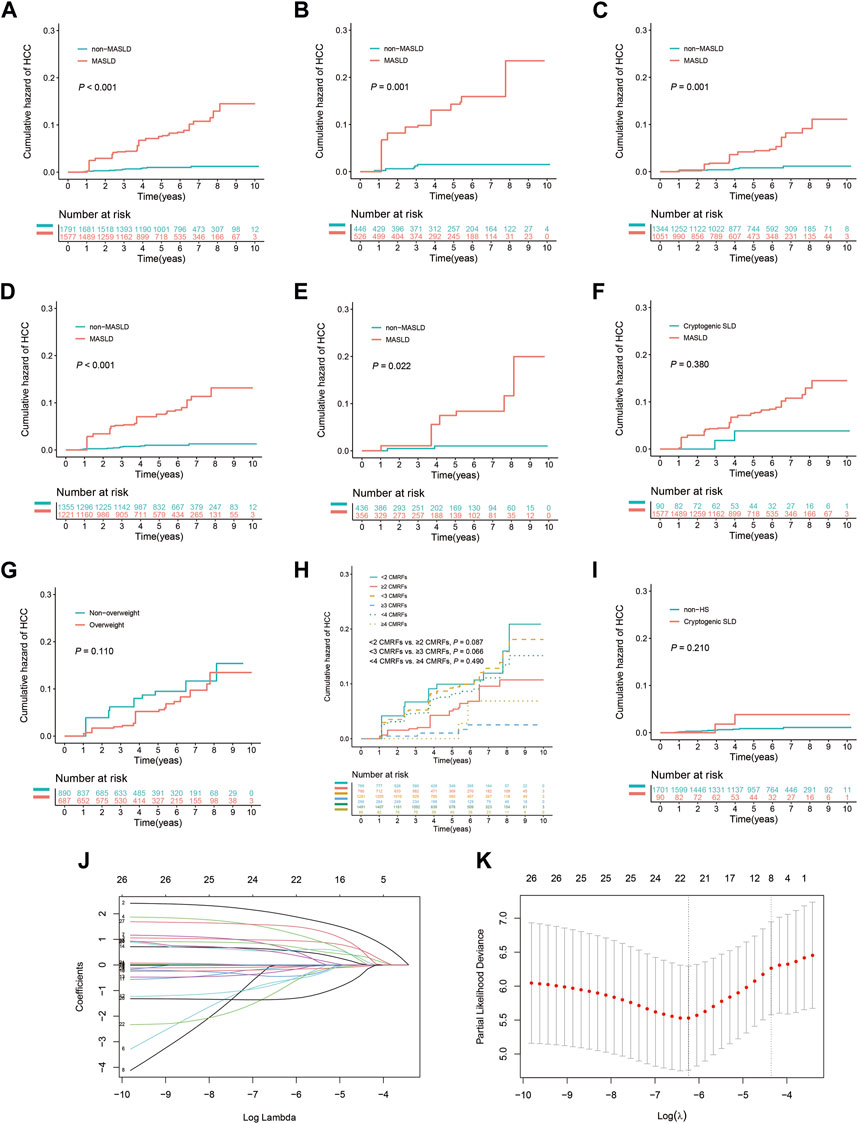
FIGURE 3. Weighted Kaplan-Meier curves and the results of weighted LASSO Cox regression analysis following IPTW. (A) All patients with or without MASLD. (B) Subgroup with advanced liver fibrosis (F3–F4). (C) Subgroup without advanced liver fibrosis (F0–F2). (D) Subgroup undergoing antiviral treatment for ≥6 months. (E) Subgroup never having received antiviral treatment or undergone treatment for <6 months. (F) Patients with MASLD or cryptogenic SLD in the HS subgroup. (G) Patients with or without overweight (BMI ≥23 kg/m2) in the MASLD subgroup. (H) Patients exhibit varying levels of CMRFs in the MASLD subgroup. (I) Patients with cryptogenic SLD or without HS in the non-MASLD subgroup. (J) Risk factors identified using weighted LASSO Cox regression analysis. (K) The eight risk factors selected using weighted LASSO Cox regression analysis.
Weighted LASSO Cox regression was employed to identify factors significantly associated with the risk of progression to HCC (Figure 3J). The identified factors were age (≥40 years), the presence of MASLD, advanced liver fibrosis, levels of ALB, ALP, and HDL cholesterol, HBV DNA concentration ≥6 log10 IU/mL, and HBeAg positivity (Figure 3K). Subsequently, these factors were further evaluated through bidirectional stepwise variable selection in a weighted multivariate Cox analysis, which identified several independent risk factors for HCC: MASLD (adjusted HR, 10.191; 95% CI, 4.327–24.002; p < 0.001), ALB levels (adjusted HR, 0.882; 95% CI, 0.827–0.941; p < 0.001), HBV DNA concentration ≥6 log10 IU/mL (adjusted HR, 0.168; 95% CI, 0.049–0.574; p = 0.004), and HBeAg positivity (adjusted HR, 4.309; 95% CI, 1.220–15.225; p = 0.023).
NAFLD on HCC risk in CHB patients
During the study, 34.9% (563/1,613) of the patients were diagnosed with NAFLD (Figure 1). The baseline demographic and clinicopathological features of these patients are shown in Supplementary Table S1. The clinical characteristics of the NAFLD and non-NAFLD groups were similar to those of the MASLD and non-MASLD groups. During the follow-up period, 4.4% (25/563) of the patients in the NAFLD group developed HCC, in contrast to 1.0% (11/1,050) in the non-NAFLD group. The 3- and 5-year cumulative incidence rates of HCC in the NAFLD group were 1.7% and 3.7%, respectively, which were significantly higher than the 0.8% and 1.2% in the non-MASLD group (p < 0.001) (Figure 4A). After IPTW adjustment, the demographic, clinicopathological features, and history of medication of the two groups were well balanced (Supplementary Table S1). The 3- and 5-year cumulative incidence rates of HCC in the NAFLD group were 2.5% and 5.2%, respectively, which significantly exceeded the 0.6% and 1.0% rates in the non-NAFLD group (p < 0.001) (Figure 4B).
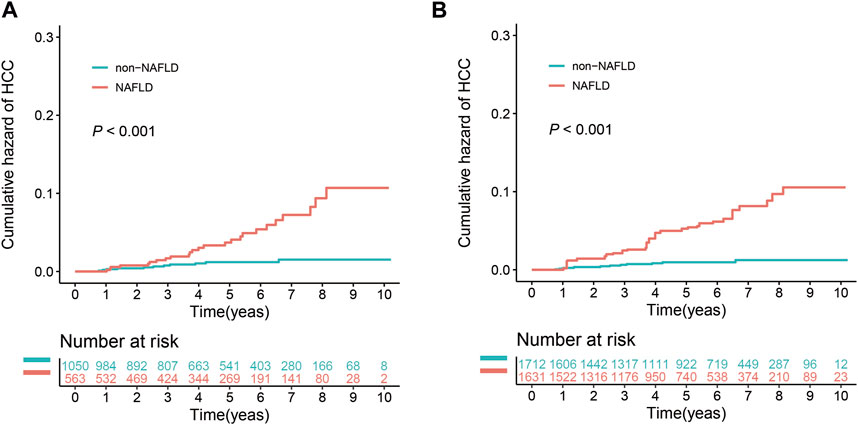
FIGURE 4. Effect of NAFLD on the cumulative risk of HCC before and after IPTW. (A) Cumulative risk of HCC before IPTW. (B) Cumulative risk of HCC after IPTW.
Discussion
This retrospective study evaluated the effect of concurrent MASLD on the incidence of HCC in Chinese patients with CHB. As far as we know, this is the first study to report the correlation between MASLD and the risk of HCC in CHB patients. This study included 1,613 CHB patients, 29.9% of whom had MASLD. This prevalence rate aligns with the MASLD prevalence in the general population as reported in a previous study (Ciardullo et al., 2023). The incidence of CMRFs, including overweight, DM, hypertension, and dyslipidemia, was higher for patients in the MASLD group than the non-MASLD group. Correspondingly, patients in the MASLD group had a longer history of using statins, metformin, and ACEI/ARBs than those in the non-MASLD group. Interestingly, patients in the MASLD group had a lower baseline level of HBV DNA and a reduced likelihood of being HBeAg-positive. Several studies have shown a decrease in viral replication among CHB patients with HS (Wang et al., 2014; Ceylan et al., 2016), yet the underlying mechanisms remain obscure, suggesting a potential interplay between HBV and CMRFs during liver disease pathogenesis (Wang and Xie, 2022). Further study is needed to explore this relationship further.
Multivariable Cox analysis identified MASLD as an independent risk factor for developing HCC. CHB patients with MASLD had a 4-fold increased risk of HCC than those without. Even after adjusting for potential confounders using IPTW score matching, MASLD persistently emerged as an independent risk factor for HCC. Similarly, van Kleef et al. reported that CHB patients with MAFLD had a 2-fold higher risk of developing HCC (van Kleef et al., 2021). Another study using the South Korean National Health Insurance System database found that after adjusting for age, gender, cirrhosis, antiviral treatment, physical activity, smoking history, and alcohol consumption, the risk of developing HCC was 1.4-fold higher in CHB patients with MAFLD (Yun et al., 2022). After applying IPTW, there was a persistent association between the risk of developing HCC in CHB patients with MASLD regardless of whether they had advanced liver fibrosis. This suggests that MASLD may contribute to HCC progression independent of liver fibrosis. These findings indicate that patients with non-advanced liver fibrosis and MASLD should undergo increased HCC surveillance. Traditionally, advanced liver fibrosis has been considered a major HCC risk factor (Fujiwara et al., 2018). However, in the current study, it was excluded from the multivariate Cox model. The independent risk factors for HCC identified were age (≥40 years), MASLD, elevated AFP, and ALB levels. Advanced liver fibrosis was excluded even after mitigating potential confounding variables using IPTW. This may be attributed to the limitations of observational studies, including potential overlooked or unknown confounders that could bias the association between advanced liver fibrosis and HCC. However, the findings also suggest that advanced liver fibrosis may not be the only or leading cause of HCC. A broader spectrum of risk factors should be considered during HCC risk assessment and management, including age, MASLD, AFP, and ALB levels.
This study also discovered that the association between MASLD and HCC risk persisted in CHB patients regardless of whether they had received antiviral treatment for more than 6 months, both before and after adjustment with IPTW. The majority of patients in this study had undergone antiviral therapy for ≥6 months, primarily with entecavir or tenofovir disoproxil fumarate. Notably, patients in the MASLD group were more likely to have never received antiviral therapy or received therapy for <6 months than to have received therapy for ≥6 months, probably because this group had lower levels of HBV replication. While antiviral drugs can inhibit viral replication, averting liver disease progression and potentially offering long-term prevention against HCC (Gordon et al., 2014), the current study found that HCC risk remained high in MASLD patients who had received long-term treatment. This is particularly true for patients with HBV DNA levels ≥6 log10 IU/mL or a positive HBeAg status, as these were identified as independent risk factors for HCC in CHB patients after adjusting for confounders with IPTW. Thus, in evaluating the risk of HCC in CHB patients who adhere to antiviral therapy, careful consideration should be given to MASLD.
Furthermore, we found that, compared to patients with CHB who had cryptogenic SLD, those with MASLD did not exhibit a significantly higher cumulative risk of developing HCC. Similarly, the cumulative risk of HCC in patients with cryptogenic SLD did not significantly differ from that of the patients without HS. This finding remained after IPTW with respect to the baseline characteristics of the patients. This suggests that the cumulative effect of multiple factors, including CMRFs and HS, mediate the effects of MASLD on the development of HCC in patients with CHB. However, the precise influence of each factor on the risk of HCC requires further investigation. Although the cumulative incidence of HCC was higher in patients with MASLD, those with overweight and those with ≥2, ≥3, or ≥4 CMRFs did not significantly influence the risk of HCC in the present sample of patients. Although overweight and CMRFs have been shown to be associated with a higher risk of HCC (Larsson and Wolk, 2007; Antwi et al., 2022), the present findings indicate that in patients with MASLD, the risk of developing HCC does not differ with varying levels of CMRFs. The findings also imply that in the presence of MASLD, the risk of HCC may also be higher in patients without overweight and in those with few CMRFs, which underscores the need for clinical vigilance.
To compare the risk of HCC in patients with CHB and either the newly named MASLD or the previously named NAFLD, we reclassified the patients according to whether they had NAFLD or not, to evaluate the effect of NAFLD on the development of HCC. The results showed that, both before and after adjustment using IPTW, the cumulative risk of HCC in the NAFLD group was significantly higher than that of the non-NAFLD group. However, after IPTW adjustment, the 3- and 5-year cumulative risks of HCC in the NAFLD group were 2.5% and 5.2%, respectively, which were lower than those in the MASLD group (4.3% and 7.5%). This suggests that the presence of MASLD, which includes a greater emphasis on metabolic parameters, is associated with a higher risk of HCC. This further underscores the importance of metabolic defects in the development of HCC.
The current study has some limitations. Its retrospective design prevented the collection of data on waist circumference, 2-h postprandial blood glucose, and glycated hemoglobin. The absence of this information could have led to an underdiagnosis of MASLD in certain patients and affected the study outcomes. Additionally, the evolving status of MASLD during the follow-up period could have changed the level of HCC risk in certain patients, a consideration not accounted for in the retrospective cohort. This limitation underscores the need for prospective studies to evaluate the correlation between MASLD and HCC risk in CHB patients while concurrently adjusting for the chronicity of MASLD. Finally, while the median follow-up duration was 5.02 years, this may not have been enough time to estimate the full impact of MASLD on HCC risk.
Conclusion
The present findings highlight the critical role of MASLD as an independent risk factor for HCC in patients with CHB. The findings were consistent when a number of subgroups were analyzed, including patients with and without advanced liver fibrosis and those undergoing antiviral therapy for <6 and ≥6 months. The higher risk of HCC in patients with CHB who have MASLD is driven by the cumulative effect of multiple factors, including CMRFs and HS. Furthermore, when compared to NAFLD, the classification MASLD places greater emphasis on metabolic factors and is associated with a higher risk of HCC. These findings suggest the importance of screening for steatosis, and especially MASLD, to reduce the risk of HCC in patients with CHB. Notably, even patients with MASLD who do not have overweight or have few CMRFs are at a high risk of HCC. Further investigations are needed to elucidate the mechanisms underlying the relationship between MASLD and HCC, which could inform the development of novel strategies for the prevention of HCC in this high-risk population.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the Guangdong Provincial Hospital of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the research is retrospective in nature, the requirement for informed consent was waived.
Author contributions
ML: Writing–original draft, Writing–review and editing, Conceptualization, Formal Analysis, Methodology. BG: Writing–original draft, Writing–review and editing. MP: Data curation, Formal Analysis, Writing–review and editing. XuC: Data curation, Writing–review and editing. HX: Data curation, Writing–review and editing. MS: Data curation, Writing–review and editing. XZ: Data curation, Writing–review and editing. FZ: Data curation, Writing–review and editing. XiC: Conceptualization, Funding acquisition, Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the XiC National Famous Traditional Chinese Medicine Expert Inheritance Studio (Teaching Letter from State Traditional Chinese Medicine Office [2022] No. 75), the Thirteenth Five-Year Plan for Major and Special Programs of the National Science and Technology of China (2018ZX10725506-003, 2018ZX10725505-004), the Clinical research projects of Guangdong Provincial Hospital of Chinese Medicine (YN10101903), the Science and Technology research project of Traditional Chinese Medicine of Guangdong Provincial Hospital of Chinese Medicine (YN2022DB04), Guangdong Traditional Chinese Medicine Administration (20231116).
Acknowledgments
We thank the Charlesworth Group (http://www.cwauthors.com.cn) for providing linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1347459/full#supplementary-material
References
Antwi S. O., Craver E. C., Nartey Y. A., Sartorius K., Patel T. (2022). Metabolic risk factors for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a prospective study. Cancers (Basel) 14 (24), Epub 2022/12/17. doi:10.3390/cancers14246234
Brunt E. M., Janney C. G., Di Bisceglie A. M., Neuschwander-Tetri B. A., Bacon B. R. (1999). Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 94 (9), 2467–2474. doi:10.1111/j.1572-0241.1999.01377.x
Ceylan B., Arslan F., Batırel A., Fincancı M., Yardımcı C., Fersan E., et al. (2016). Impact of fatty liver on hepatitis B virus replication and virologic response to tenofovir and entecavir. Turk J. Gastroenterol. 27 (1), 42–46. Epub 2015/12/17. doi:10.5152/tjg.2015.150348
Chan A. W., Wong G. L., Chan H. Y., Tong J. H., Yu Y. H., Choi P. C. (2017). Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J. Gastroenterol. Hepatol. 32 (3), 667–676. Epub 2016/08/22. doi:10.1111/jgh.13536
Chartampilas E. (2018). Imaging of nonalcoholic fatty liver disease and its clinical utility. Horm. (Athens) 17 (1), 69–81. Epub 2018/04/10. doi:10.1007/s42000-018-0012-x
Chesnaye N. C., Stel V. S., Tripepi G., Dekker F. W., Fu E. L., Zoccali C. (2022). An introduction to inverse probability of treatment weighting in observational research. Clin. Kidney J. 15 (1), 14–20. Epub 2021/08/26. doi:10.1093/ckj/sfab158
Ciardullo S., Carbone M., Invernizzi P., Perseghin G. (2023). Exploring the landscape of steatotic liver disease in the general us population. Liver Int. 43 (11), 2425–2433. Epub 2023/08/17. doi:10.1111/liv.15695
Eslam M., Newsome P. N., Sarin S. K., Anstee Q. M., Targher G., Romero-Gomez M. (2020a). A new definition for metabolic dysfunction-associated fatty liver disease: an international Expert consensus statement. J. Hepatol. 73 (1), 202–209. Epub 2020/04/08. doi:10.1016/j.jhep.2020.03.039
Eslam M., Sanyal A. J., George J. (2020b). Mafld: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158 (7), 1999–2014.e1. doi:10.1053/j.gastro.2019.11.312
Fujiwara N., Friedman S. L., Goossens N., Hoshida Y. (2018). Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 68 (3), 526–549. Epub 2017/10/06. doi:10.1016/j.jhep.2017.09.016
Gordon S. C., Lamerato L. E., Rupp L. B., Li J., Holmberg S. D., Moorman A. C. (2014). Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a us population. Clin. Gastroenterol. Hepatol. 12 (5), 885–893. Epub 2013/10/06. doi:10.1016/j.cgh.2013.09.062
Kanwal F., Kramer J. R., Mapakshi S., Natarajan Y., Chayanupatkul M., Richardson P. A. (2018). Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 155 (6), 1828–1837. e2. Epub 2018/08/23. doi:10.1053/j.gastro.2018.08.024
Kim S. K., Fujii T., Kim S. R., Nakai A., Lim Y. S., Hagiwara S. (2022). Hepatitis B virus treatment and hepatocellular carcinoma: controversies and approaches to consensus. Liver Cancer 11 (6), 497–510. Epub 2022/08/23. doi:10.1159/000525518
Larsson S. C., Wolk A. (2007). Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br. J. Cancer 97 (7), 1005–1008. Epub 2007/08/14. doi:10.1038/sj.bjc.6603932
Le M. H., Yeo Y. H., Li X., Li J., Zou B., Wu Y. (2019). 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 20 (12), 2809–2817. e28. Epub 2021/12/07. doi:10.1016/j.cgh.2021.12.002
Li J., Yang H. I., Yeh M. L., Le M. H., Le A. K., Yeo Y. H. (2021). Association between fatty liver and cirrhosis, hepatocellular carcinoma, and hepatitis B surface antigen seroclearance in chronic hepatitis B. J. Infect. Dis. 224 (2), 294–302. Epub 2020/11/29. doi:10.1093/infdis/jiaa739
Mak L. Y., Hui R. W., Fung J., Liu F., Wong D. K., Li B. (2021). Reduced hepatic steatosis is associated with higher risk of hepatocellular carcinoma in chronic hepatitis B infection. Hepatol. Int. 15 (4), 901–911. Epub 2021/06/21. doi:10.1007/s12072-021-10218-2
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee Chinese Medical Doctor Association (2018). Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update. Zhonghua Gan Zang Bing Za Zhi 26 (3), 195–203. doi:10.3760/cma.j.issn.1007-3418.2018.03.008
Rinella M. E., Lazarus J. V., Ratziu V., Francque S. M., Sanyal A. J., Kanwal F. (2023). A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 78, 1966–1986. Epub 2023/06/24. doi:10.1097/hep.0000000000000520
Scheuer P. J. (1991). Classification of chronic viral hepatitis: a need for reassessment. J. Hepatol. 13 (3), 372–374. doi:10.1016/0168-8278(91)90084-o
Terrault N. A., Lok A. S. F., McMahon B. J., Chang K. M., Hwang J. P., Jonas M. M. (2018). Update on prevention, diagnosis, and treatment of chronic hepatitis B: aasld 2018 hepatitis B guidance. Hepatology 67 (4), 1560–1599. doi:10.1002/hep.29800
Tibshirani R. (1997). The lasso method for variable selection in the Cox model. Stat. Med. 16 (4), 385–395. doi:10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3
Tourkochristou E., Assimakopoulos S. F., Thomopoulos K., Marangos M., Triantos C. (2022). Nafld and hbv interplay - related mechanisms underlying liver disease progression. Front. Immunol. 13, 965548. Epub 2022/12/05. doi:10.3389/fimmu.2022.965548
van Kleef L. A., Choi H. S. J., Brouwer W. P., Hansen B. E., Patel K., de Man R. A. (2021). Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 3 (5), 100350. Epub 2021/08/08. doi:10.1016/j.jhepr.2021.100350
Wang C., Wang X., Gong G., Ben Q., Qiu W., Chen Y. (2012). Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int. J. Cancer 130 (7), 1639–1648. Epub 2011/07/28. doi:10.1002/ijc.26165
Wang M. M., Wang G. S., Shen F., Chen G. Y., Pan Q., Fan J. G. (2014). Hepatic steatosis is highly prevalent in hepatitis B patients and negatively associated with virological factors. Dig. Dis. Sci. 59 (10), 2571–2579. Epub 2014/05/18. doi:10.1007/s10620-014-3180-9
Wang X., Xie Q. (2022). Metabolic dysfunction-associated fatty liver disease (mafld) and viral hepatitis. J. Clin. Transl. Hepatol. 10 (1), 128–133. Epub 2021/10/08. doi:10.14218/jcth.2021.00200
You H., Wang F., Li T., Xu X., Sun Y., Nan Y., et al. (2023). Guidelines for the prevention and treatment of chronic hepatitis B (version 2022). J. Clin. Transl. Hepatol. 11 (6), 1425–1442. Epub 2023/08/15. doi:10.14218/jcth.2023.00320
Yun B., Ahn S. H., Oh J., Yoon J. H., Kim B. K. (2022). Effect of metabolic dysfunction-associated fatty liver disease on liver cancer risk in a population with chronic hepatitis B virus infection: a nationwide study. Hepatol. Res. 52 (12), 975–984. Epub 2022/08/26. doi:10.1111/hepr.13830
Zhou F., Zhou J., Wang W., Zhang X. J., Ji Y. X., Zhang P. (2019). Unexpected rapid increase in the burden of nafld in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology 70 (4), 1119–1133. doi:10.1002/hep.30702
Keywords: hepatitis B, metabolic dysfunction-associated steatotic liver disease, hepatic steatosis, hepatocellular carcinoma, inverse probability treatment weighting
Citation: Lin M, Gao B, Peng M, Chen X, Xiao H, Shi M, Zhang X, Zeng F and Chi X (2024) Metabolic dysfunction-associated steatotic liver disease increases hepatocellular carcinoma risk in chronic hepatitis B patients: a retrospective cohort study. Front. Physiol. 15:1347459. doi: 10.3389/fphys.2024.1347459
Received: 01 December 2023; Accepted: 29 January 2024;
Published: 09 February 2024.
Edited by:
Giuliano Ramadori, University of Göttingen, GermanyReviewed by:
Stefano Ciardullo, University of Milano Bicocca, ItalyMei Zhang, Sichuan University, China
Copyright © 2024 Lin, Gao, Peng, Chen, Xiao, Shi, Zhang, Zeng and Chi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Chi, chixiaolingqh@163.com
 Ming Lin
Ming Lin Bowen Gao
Bowen Gao Mengnan Peng3
Mengnan Peng3 Meijie Shi
Meijie Shi Folai Zeng
Folai Zeng