- 1Division of Academic Neurosurgery, Department of Clinical Neurosciences, Addenbrooke’s Hospital, University of Cambridge, Cambridge, United Kingdom
- 2Neurosciences Critical Care Unit, Addenbrooke’s Hospital, University of Cambridge, Cambridge, United Kingdom
- 3Department of Paediatric Intensive Care, Addenbrooke’s Hospital, University of Cambridge, Cambridge, United Kingdom
Introduction: A strong association exists between hyperglycemia and outcome in pediatric traumatic brain injury (TBI). Herein, we describe observations of serum markers of glucose metabolism in a cohort of pediatric TBI patients and how these variables are related to parameters of intracranial pathophysiology.
Methods: A retrospective analysis was performed on pediatric severe TBI patients admitted to Addenbrookes Hospital Paediatric Intensive Care Unit (PICU) between January 2001 and December 2013. Demographic, outcome, systemic physiological, and cerebral autoregulatory data were extracted for patients who had received continuous invasive monitoring (ICM+, Cambridge Enterprise, Cambridge, UK). Data were analyzed using a mixed linear model.
Results: Forty-four patients with an average age of 12.2 years were admitted to the PICU with a TBI requiring invasive neurosurgical monitoring. Thirty-two patients (73%) survived, with favorable outcomes in 62%. The mean (SD) intracranial pressure (ICP) was 17.6 + 9.0 mmHg, MAP was 89.7 + 9.0 mmHg, and pressure-reactivity index (PRx) was −0.01 + 0.23 a.u. The mean (SD) serum lactate was 2.2 (3.3) mmol/L. and the mean (SD) serum glucose was 6.1 (1.6) mmol/L. Early hyperglycemia was strongly associated with both PRx (Pearson correlation 0.351, p < 0.001) and ICP (Pearson correlation 0.240, p = 0.002) death (p = 0.021) and impaired cerebral autoregulation (p = 0.02). There was a strong association between ICP and serum lactate (p = 0.001).
Conclusion: Increases in systemic glucose are associated with impaired cerebrovasular autoregulation after severe pediatric TBI. Moreover, deranged blood glucose is a marker of poor prognosis. Further studies are required to delineate putative mechanisms of hyperglycemia induced cerebral harm.
Introduction
In the context of trauma, primary brain injury occurs due to cellular and extracellular matrix disruption from direct mechanical forces at the time of the traumatic incident. Primary brain injury then initiates a complex cascade of secondary molecular and vascular mechanisms culminating in inflammation, edema, impaired cerebral autoregulation, blood–brain barrier disruption, intracranial hypertension, reduced cerebral perfusion, and ultimately neuronal cell death. Secondary brain injury persists for weeks and may contribute to a further loss of potentially viable cerebral tissue, ultimately worsening neurological outcome (1). While primary brain injury is unpredictable and irreversible, the sequelae of secondary brain injury may be modified by prevention or minimization of recognized exacerbating systemic insults, such as hypotension, hypoxia, and hyperglycemia (2).
Hyperglycemia occurs frequently in the pediatric traumatic brain injury (TBI) population and the occurrence of elevated blood glucose values has been linked to increased mortality and worse neurological outcomes (3–6). While it is not entirely clear whether this association is due to a direct deleterious effect of hyperglycemia or simply a marker of illness severity, there is an increasing body evidence from the adult TBI population that ascribe a putative pathological role to raised blood glucose (2).
In a retrospective observational study of adult TBI our group has recently demonstrated an association between elevated blood glucose and impaired cerebral pressure-reactivity index (PRx) (7). Cerebral pressure reactivity is a fundamental component of cerebral autoregulation, whereby cerebrovascular resistance is altered in response to changes in cerebral perfusion pressure (CPP) (8). The PRx is a surrogate measure of cerebral pressure reactivity and calculated as the correlation between arterial blood pressure (MAP) and intracranial pressure (ICP) (9). A negative correlation implies active pressure reactivity while a positive correlation implies a “pressure passive,” impaired pressure reactivity. Previous studies have shown that a positive PRx is associated with disturbance of cerebral autoregulation (10, 11). Importantly, PRx has also been demonstrated to independently predict outcome after TBI in children (12, 13).
While epidemiological studies have demonstrated that the incidence of hospitalization and fatal brain injury is disproportionately high in children (14), somewhat surprisingly, the interplay between glycemia, markers of secondary neurological insult, and outcome remains poorly described in the pediatric population. The primary objective of this study was to determine associations between systemic glucose, cerebral pressure reactivity (PRx), and outcome in a cohort of pediatric TBI patients. Secondary objectives were to determine associations between systemic lactate, PRx and ICP.
Materials and Methods
Patients
This is a retrospective observational study of all pediatric patients admitted to an Intensive Care Unit in Addenbrooke’s Hospital, Cambridge with severe TBI from January 2001 to December 2015 inclusive. Consecutive patients with a clinical need for ICP monitoring were included for analysis. The insertion of an intracranial monitoring device is part of routine clinical practice and as such did not require ethical approval. The analysis of data within this study for the purposes of service evaluation was approved by the Cambridge University Hospital NHS Trust, Audit and Service Evaluation Department (Ref: 2143) and did not require ethical approval or patient consent.
Inclusion criteria were as follows: (1) TBI-related pathology, confirmed on CT or MRI, (2) severe injury (GCS < 8) failing to demonstrate significant early clinical improvement (i.e., poor neurology on sedation hold), and (3) requirement for invasive monitoring of ICP and mean arterial pressure (MAP). Patients were excluded if there was suspicion of non-accidental injury. Multi-modality monitoring was commenced at the earliest possible opportunity following arrival to the ICU and was terminated when sedation was lifted and the child either began to waken or died.
Pre-hospital data were recorded from the ambulance service records. Hypoxia was defined as saturations under 96% on arrival of the crew. Hypotension was defined as age-specific hypotension on arrival of the crew.
Patients were managed according to current TBI guidelines (15). Interventions were aimed at keeping ICP < 20 mmHg using a tiered treatment protocol of positioning, sedation, muscle paralysis, moderate hyperventilation, ventriculostomy, osmotic agents, and induced hypothermia. CPP was maintained >50–60 mmHg using intravenous fluids, vasopressors, and inotropes. Glucose management was achieved with a continuous intravenous insulin infusion targeting a blood glucose between 6 and 10 mmol/L. Clinical outcome was determined using the Glasgow Outcome Scale at 6 months (1—death, 2—persistent vegetative state, 3—severe disability, 4—moderate disability, 5—good recovery) (16). A favorable outcome was defined as a GOS ≥ 4.
Data Acquisition and Analyses
Intracranial pressure was monitored with an intraparenchymal microsensor inserted into the right frontal cortex (Codman ICP MicroSensor, Codman and Shurtleff, Raynham, MA, USA) and arterial blood pressure (MAP) was monitored in the radial or femoral artery with a zero level at the right atrium (Baxter Healthcare CA, USA; Sidcup, UK). End-tidal carbon dioxide (CO2) data were collected from the ventilator.
Data were sampled at 100 Hz with proprietary data acquisition software (ICM+, Cambridge Enterprise, Cambridge, UK) and stored for subsequent analysis. Data were collected on each day of invasive monitoring until the fifth day post ictus. Cerebrovascular PRx was calculated as a moving Pearson correlation coefficient between 30 consecutive, 10-s averaged values of MAP and corresponding ICP signals (with 80% overlap of data). Averages over 10 s were used to suppress the influence of the pulse and respiratory frequency wave components.
Arterial blood samples were taken at 8 a.m. and 8 p.m. daily for the measurement of arterial glucose and lactate. All blood samples were analyzed by the Core Biochemical Assay Laboratory at Addenbrookes Hospital, Cambridge. Meso Scale Diagnostics (MD, USA) assays were standardized against an approved reference preparation (IFCC calibration). Six hours of time-averaged cerebrovascular data (CPP, ICP, PRx, and end-tidal CO2) were assessed for each measurement of arterial glucose and lactate.
Statistical Analyses
Raw data were screened and cleared of artifacts and then examined for normality prior to analysis. The cohort was dichotomized into survivors and non-survivors. Ordinal data are presented as medians (IQR) and continuous data as means (SD). Differences in physiological values between survivors and non-survivors were interrogated with the Mann–Whitney U-test. The significance level was set to 0.05, and all tests were two-tailed and unadjusted for multiple comparisons. Bivariate correlation analyses (Pearson coefficient) were calculated between glucose, CPP, ICP, and PRx. Correlations are zero-order and unadjusted for multiple comparisons.
A repeated measures mixed effect model was generated to evaluate associations between systemic glucose and lactate, and intracerebral biochemical and pressure parameters with plasma glucose and lactate as covariates, time as a fixed effect and patient ID as a random effect. The model was adjusted for the following; age, injury characteristics (hypotensive and hypoxic episodes), ICP, PRx, and outcome. All data analyses were performed on SPSS version 21.0 software (SPSS Inc., Chicago, IL, USA). All statistical tests were performed with α ≤ 0.05 (two-tailed).
Results
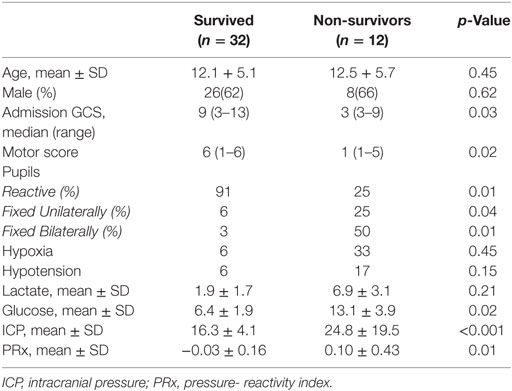
A total of 44 patients with an average age of 12.2 years were admitted to the Paediatric Intensive Care Unit with a TBI requiring invasive neuro-monitoring (Table 1). Thirty-two patients (73%) were alive at 6 months, and 27 (62%) were deemed to have a favorable outcome. Thirty patients (68%) sustained an isolated head injury with the others having poly trauma. The incidence of poly trauma had no significant impact on outcome. All ICP wires were placed after sedation hold demonstrated poor neurology. This was usually within 6 h of injury. No patients were excluded on the basis of the timing of ICP insertion. Prior to the injury two children had mild learning disabilities, and one had attention deficit hyperactivity disorder. All patients were maintained at normothermia, none of them were cooled to hypothermia. Five patients had external ventricular drain inserted. Two patients had a decompressive craniectomy. 82% of patients had vassopressor/inotrope support. 64% of patients had insulin infusions in an attempt to control glucose levels. Mean physiologic monitoring values during the first 5 days since ictus are shown in Table 2. The mean (SD) ICP was 17.6 (9.0) mmHg, MAP was 89.7 (9.0) mmHg and PRx was −0.01 (0.22) a.u. The mean (SD) serum lactate was 2.2 (3.3) mmol/L. The mean (SD) serum glucose was 6.1 (1.6) mmol/L. The observed evolution of serum glucose and lactate over the first 5 days post-TBI has been visualized in Figures 1A,B.
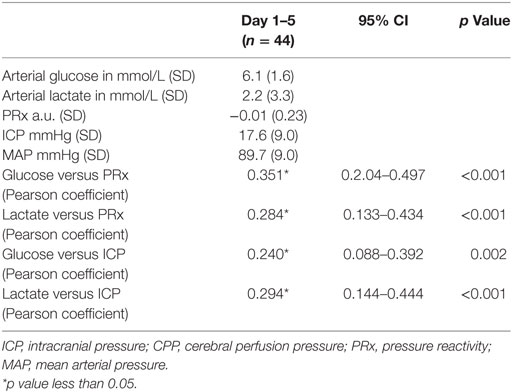
Table 2. Mean values (SD) and correlations of physiologic variables during intracranial monitoring during the first 5 days since ictus.
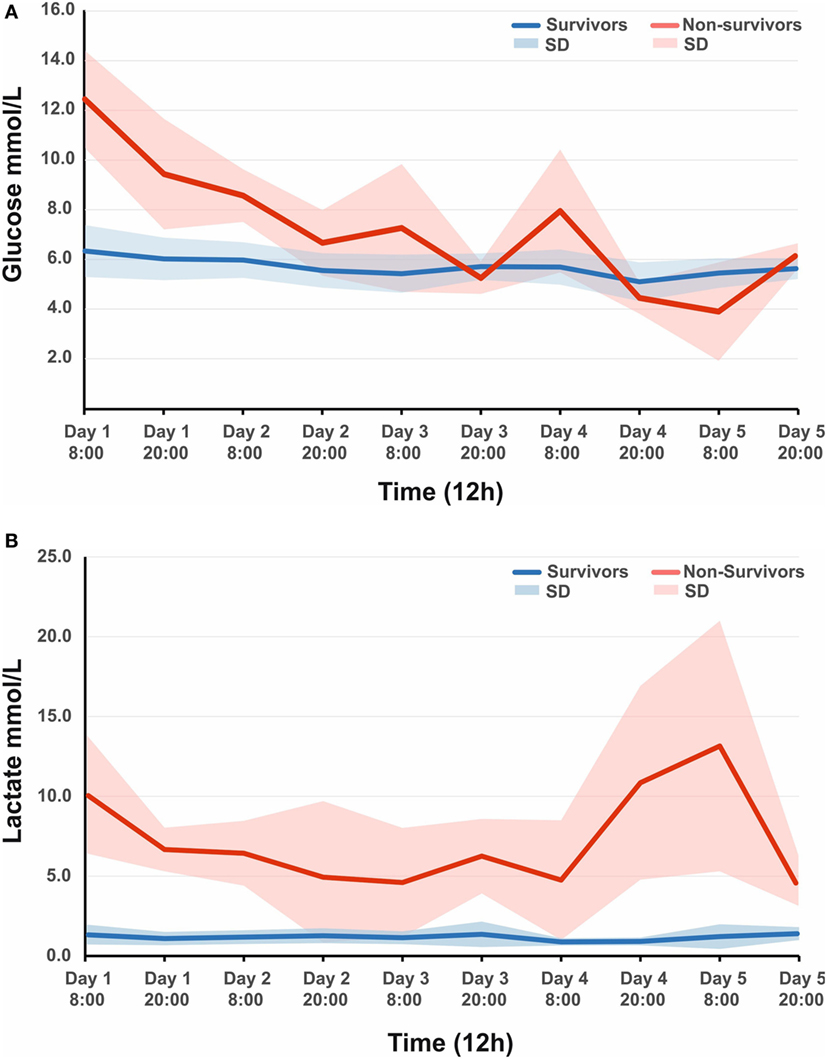
Figure 1. (A) Observed mean arterial glucose (SD) in pediatric traumatic brain injury (TBI) patients during the first 5 days since ictus, stratified by fatal outcome. (B) Observed mean arterial lactate (SD) in pediatric TBI patients during the first 5 days since ictus, stratified by fatal outcome.
Comparison of Demographic and Physiological Parameters between Survivors and Non-Survivors
Demographic and physiological data for survivors and non-survivors are presented in Table 1. The admission GCS in those who survived was 9 (3–13) at presentation compared to 3 (3–9) in non-survivors (p = 0.03). More significantly, the motor score was 6 (1–6) in survivors and 1 (1–5) in non-survivors (p = 0.02). The majority of survivors had bilateral reactive pupils (91%) versus 25% in non-survivors (p = 0.01). Only 6% of survivors had a unilaterally fixed pupil compared to 25% of non-survivors (p = 0.04), whereas 3% of survivors had bilaterally fixed pupils versus 50% of non-survivors (p = 0.01). There was no significant difference between the incidence of pre-hospital hypoxia or hypotension between survivors and non-survivors (7 versus 33%; p = −0.45 and 7 versus 17%; p = 0.15, respectively).
Correlations between Systemic Glucose, Cerebral Autoregulation, and Outcome
Mean arterial glucose concentration for each patient during the first 5 days from ictus was significantly correlated with mean PRx (Pearson correlation 0.351, p < 0.001; Table 2; Figure 2) and mean ICP (Pearson correlation 0.240, p = 0.002; Table 2). To account for the repeated measures for each patient in each outcome group, a linear mixed effects model analysis was used. The linear mixed effects model fitting identified a significant effect of PRx (p = 0.016) and non-survivors (p = 0.021) on arterial glucose. The interaction of these two factors (PRx versus Outcome) was non-significant (p = 0.124). Further modeling showed no additional contribution to model fit from age (p = 0.886), hypoxia and hypotension on admission (p = 0.408 and p = 0.488), or ICP (p = 0.593). Only daily mean arterial glucose concentration during the first 2 days post-TBI correlated significantly with mean PRx in the mixed effects model (p < 0.001), i.e., increases in systemic glucose correlated with a worse state of cerebral pressure reactivity.
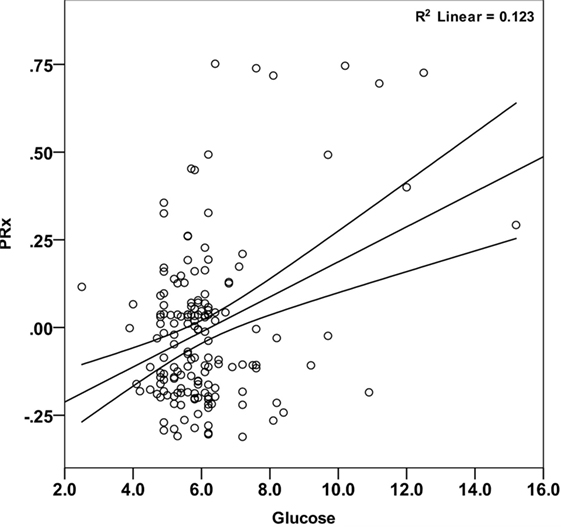
Figure 2. Correlation between mean arterial glucose and PRx in pediatric traumatic brain injury patients during the first 5 days since ictus. There was a significant positive relationship between blood glucose and PRx (Pearson correlation = 0.351; p < 0.001). Each data point represents the mean of the available arterial glucose concentration and PRx measurements during the first 5 days since ictus.
Correlations between Systemic Lactate, Cerebral Autoregulation, and Outcome
Mean arterial glucose concentration for each patient during the first 5 days from ictus was significantly correlated with mean PRx (Pearson correlation 0.284, p < 0.001; Table 2) and mean ICP (Pearson correlation 0.294, p < 0.001; Table 2). The linear mixed effects model fitting did not demonstrate correlations between serum lactate and PRx (p = 0.108) and hypoxia on admission (p = 0.87). However, a significant effect of ICP (p = 0.001) and hypotension on admission (p = 0.013) on arterial lactate was observed. The temporal evolution of lactate did not differ between fatal and non-fatal patients after adjustment in the mixed model (p = 0.318).
Discussion
The key finding of this observational study is a significant positive relationship between arterial glucose concentration and cerebral pressure reactivity in pediatric TBI patients. Secondary observations are (i) a strong association between increasing blood glucose and mortality and (ii) a positive relationship between serum lactate and ICP.
Cerebral autoregulation is the physiologic mechanism that protects the brain against detrimental variations in cerebral blood flow (17), it is frequently impaired following severe TBI and is associated with worse functional outcomes following pediatric TBI (18, 19). Independently, poor glycemic control has been demonstrated to be linked to worse outcomes in pediatric TBI (20). Interestingly, the CHiP study (Control of hyperglycemia in pediatric intensive care) demonstrated no difference in outcome when comparing tight glycemic control versus conventional methods (21). Nevertheless, this study included multiple pathologies including trauma. By using the cerebral perfusion index as a surrogate measure of cerebral autoregulation, this is the first study to report an association between glycemia and impaired cerebral autoregulation in a pediatric TBI population. These findings are consistent with the association between glycemia and cerebral pressure reactivity in an adult TBI (7) and strengthen the external validity of these results as children are less likely to have any pre-existing systemic vascular pathology that could result in impaired cerebral autoregulation (22).
Traumatic brain injury initiates a dramatic systemic stress response with the release of cortisol, catecholamines, and glucagon leading to excessive hepatic gluconeogenesis and peripheral insulin resistance. The hyperglycemia attributed to these metabolic derangements is further exacerbated by therapeutic interventions, such as the administration of exogenous catecholamines and enteral or parenteral nutrition. Resultantly, hyperglycemia occurs frequently in patients with TBI, even in pediatric patients who were glucose tolerant prior to the traumatic insult (3–6). Consistent with these previous studies, we report an association between elevated blood glucose concentration and mortality. Despite these findings, there remains a lack of evidence guiding the management of hyperglycemia in the pediatric TBI population which is reflected in a marked disparity in insulin regimens and glucose targets worldwide (23). Furthermore, it remains unclear whether hyperglycemia is truly deleterious or is simply a marker of illness severity; however, it is generally accepted the extremes of hyperglycemia (>10 mmol/L) should be avoided (24).
Manifold putative mechanisms whereby glucose causes neurological harm have been described including induction of oxidative stress pathways (25, 26), generation of pro-inflammatory transcription factors (27), and disruption of blood–brain barrier integrity (28) predisposing to oedma and cell death (29, 30). It has also been suggested that hyperglycemia may impair vascular function; exogenous glucose reduces regional cerebral blood flow (31, 32) which may be linked to impaired endothelial function and smooth muscle vasoconstriction (33, 34). While we acknowledge that association does not prove causation, our findings support the concept that hyperglycemia post-TBI may contribute to impaired cerebral autoregulation.
Finally, we report an association between ICP and serum lactate. While this may just be a marker of severity of illness, the concept that lactate, or rather acidosis, may alter ICP merits consideration. The presence of high levels of lactate within the cerebrovascular circulation can result in localized vasodilation, extravasation, and worsening edema with a potential increase in ICP (35, 36). Furthermore, the ubiquitous disruption of blood–brain barrier integrity following TBI allows the inappropriate passage of lactate into the brain parenchyma where it can exert an osmotic load and contribute to brain edema (37). Future mechanistic studies employing tracer labeled lactate and cerebral microdialysis are required to help clarify this relationship further.
Limitations
The current study has several important limitations. Primarily, it is possible that the observed relationships could be due to the effect of a confounding physiologic variable. Additionally, serum glucose levels were collected only twice daily. Since severe TBI in pediatric patients is relatively rare, strong multi-center collaborative groups are required to evaluate mechanisms underlying the relationships observed with the ultimate aim of guiding clinical practice to improve patient outcomes.
Conclusion
Consistent with data from the adult TBI population, we have demonstrated that elevations in blood glucose may impair cerebrovascular reactivity in pediatric TBI, supporting the requirement for adequate glucose control in the first few days post insult. Further studies are warranted to delineate mechanisms linking glycemia and impaired cerebral autoregulation.
Ethics Statement
The analysis of data within this study for the purposes of service evaluation was approved by the Cambridge University Hospital NHS Trust, Audit and Service Evaluation Department (Ref: 2143) and did not require ethical approval or patient consent.
Author Contributions
AY designed the study, performed the analysis, and wrote the paper, HA, JD, and MGuilfoyle performed the analysis, HF provided patient care and directed the methods, MGarnett provided patient care and directed the methods, MC provided supervision of the analysis, PS provided supervision of the analysis, MP provided expertise on glucose control, SA designed the project and provided supervision of the analysis, and PH designed the study and oversaw the project.
Conflict of Interest Statement
ICM+ software is licensed by Cambridge Enterprise Ltd.; PS and MC have financial interest in a part of the licensing fee. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FC declared a shared affiliation, though no other collaboration, with the authors to the handling editor, who ensured that the process nevertheless met the standards of a fair and objective review.
Funding
We gratefully acknowledge financial support as follows. Research support: the Medical Research Council (MRC, Grant Nos. G0600986 ID79068 and G1002277 ID98489) and the National Institute for Health Research Biomedical Research Centre (NIHR BRC) Cambridge (Neuroscience Theme; Brain Injury and Repair Theme). Authors’ support: PH—NIHR Research Professorship, Academy of Medical Sciences/Health Foundation Senior Surgical Scientist Fellowship and NIHR Cambridge BRC. AY is supported by an NIHR Academic Clinical Fellowship. JD is supported by a Woolf Fisher Scholarship.
References
1. Algattas H, Huang JH. Traumatic brain injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int J Mol Sci (2014) 15(1):309–41. doi:10.3390/ijms15010309
2. Jauch-Chara K, Oltmanns KM. Glycemic control after brain injury: boon and bane on the brain. Neuroscience (2014) 283:202–9. doi:10.1016/j.neuroscience.2014.04.059
3. Smith RL, Lin JC, Adelson PD, Kochanek PM, Fink EL, Wisniewski SR, et al. Relationship between hyperglycemia and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med (2012) 13(1):85–91. doi:10.1097/PCC.0b013e3182192c30
4. Elkon B, Cambrin JR, Hirshberg E, Bratton SL. Hyperglycemia: an independent risk factor for poor outcome in children with traumatic brain injury*. Pediatr Crit Care Med (2014) 15(7):623–31. doi:10.1097/PCC.0000000000000170
5. Chong SL, Harjanto S, Testoni D, Ng ZM, Low CY, Lee KP, et al. Early hyperglycemia in pediatric traumatic brain injury predicts for mortality, prolonged duration of mechanical ventilation, and intensive care stay. Int J Endocrinol (2015) 2015:719476. doi:10.1155/2015/719476
6. Chiaretti A, Piastra M, Pulitanò S, Pietrini D, De Rosa G, Barbaro R, et al. Prognostic factors and outcome of children with severe head injury: an 8-year experience. Childs Nerv Syst (2002) 18(3–4):129–36. doi:10.1007/s00381-002-0558-3
7. Donnelly J, Czosnyka M, Sudhan N, Varsos GV, Nasr N, Jalloh I, et al. Increased blood glucose is related to disturbed cerebrovascular pressure reactivity after traumatic brain injury. Neurocrit Care (2015) 22(1):20–5. doi:10.1007/s12028-014-0042-4
8. Czosnyka M, Smielewski P, Piechnik S, Steiner LA, Pickard JD. Cerebral autoregulation following head injury. J Neurosurg (2001) 95(5):756–63. doi:10.3171/jns.2001.95.5.0756
9. Czosnyka M, Smielewski P, Kirkpatrick P, Piechnik S, Laing R, Pickard JD. Continuous monitoring of cerebrovascular pressure-reactivity in head injury. Acta Neurochir Suppl (1998) 71:74–7.
10. Budohoski KP, Czosnyka M, de Riva N, Smielewski P, Pickard JD, Menon DK, et al. The relationship between cerebral blood flow autoregulation and cerebrovascular pressure reactivity after traumatic brain injury. Neurosurgery (2012) 71(3):652–60; discussion 660–651. doi:10.1227/NEU.0b013e318260feb1
11. Lang EW, Lagopoulos J, Griffith J, Yip K, Yam A, Mudaliar Y, et al. Cerebral vasomotor reactivity testing in head injury: the link between pressure and flow. J Neurol Neurosurg Psychiatry (2003) 74(8):1053–9. doi:10.1136/jnnp.74.8.1053
12. Nagel C, Diedler J, Gerbig I, Heimberg E, Schuhmann MU, Hockel K. State of cerebrovascular autoregulation correlates with outcome in severe infant/pediatric traumatic brain injury. Acta Neurochir Suppl (2016) 122:239–44. doi:10.1007/978-3-319-22533-3_48
13. Young AM, Donnelly J, Czosnyka M, Jalloh I, Liu X, Aries MJ, et al. Continuous multimodality monitoring in children after traumatic brain injury-preliminary experience. PLoS One (2016) 11(3):e0148817. doi:10.1371/journal.pone.0148817
14. Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control (2010).
15. Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents – second edition. Pediatr Crit Care Med (2012) 13(Suppl 1):S1–82. doi:10.1097/PCC.0b013e31823f435c
16. Brooks DN, Hosie J, Bond MR, Jennett B, Aughton M. Cognitive sequelae of severe head injury in relation to the Glasgow outcome scale. J Neurol Neurosurg Psychiatry (1986) 49(5):549–53. doi:10.1136/jnnp.49.5.549
17. Miller JD, Stanek A, Langfitt TW. Concepts of cerebral perfusion pressure and vascular compression during intracranial hypertension. Prog Brain Res (1972) 35:411–32. doi:10.1016/S0079-6123(08)60102-8
18. Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol (2008) 38(4):225–34. doi:10.1016/j.pediatrneurol.2007.09.012
19. Brady KM, Shaffner DH, Lee JK, Easley RB, Smielewski P, Czosnyka M, et al. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics (2009) 124(6):e1205–12. doi:10.1542/peds.2009-0550
20. Seyed Saadat SM, Bidabadi E, Seyed Saadat SN, Mashouf M, Salamat F, Yousefzadeh S. Association of persistent hyperglycemia with outcome of severe traumatic brain injury in pediatric population. Childs Nerv Syst (2012) 28(10):1773–7. doi:10.1007/s00381-012-1753-5
21. Macrae D, Pappachan J, Grieve R, Parslow R, Nadel S, Schindler M, et al. Control of hyperglycaemia in paediatric intensive care (CHiP): study protocol. BMC Pediatr (2010) 10:5. doi:10.1186/1471-2431-10-5
22. Maas AI, Murray GD, Roozenbeek B, Lingsma HF, Butcher I, McHugh GS, et al. Advancing care for traumatic brain injury: findings from the IMPACT studies and perspectives on future research. Lancet Neurol (2013) 12(12):1200–10. doi:10.1016/S1474-4422(13)70234-5
23. Bell MJ, Adelson PD, Hutchison JS, Kochanek PM, Tasker RC, Vavilala MS, et al. Differences in medical therapy goals for children with severe traumatic brain injury-an international study. Pediatr Crit Care Med (2013) 14(8):811–8. doi:10.1097/PCC.0b013e3182975e2f
24. NICE-SUGAR Study Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group and the Canadian Critical Care Trials Group, Finfer S, Chittock D, Li Y, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients with traumatic brain injury: long-term follow-up of a subgroup of patients from the NICE-SUGAR study. Intensive Care Med (2015) 41(6):1037–47. doi:10.1007/s00134-015-3757-6
25. Godoy DA, Di Napoli M, Rabinstein AA. Treating hyperglycemia in neurocritical patients: benefits and perils. Neurocrit Care (2010) 13(3):425–38. doi:10.1007/s12028-010-9404-8
26. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab (2000) 85(8):2970–3. doi:10.1210/jcem.85.8.6854
27. Dhindsa S, Tripathy D, Mohanty P, Ghanim H, Syed T, Aljada A, et al. Differential effects of glucose and alcohol on reactive oxygen species generation and intranuclear nuclear factor-kappaB in mononuclear cells. Metabolism (2004) 53(3):330–4. doi:10.1016/j.metabol.2003.10.013
28. Ennis SR, Keep RF. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab (2007) 27(9):1573–82. doi:10.1038/sj.jcbfm.9600454
29. Diaz-Parejo P, Ståhl N, Xu W, Reinstrup P, Ungerstedt U, Nordström CH. Cerebral energy metabolism during transient hyperglycemia in patients with severe brain trauma. Intensive Care Med (2003) 29(4):544–50. doi:10.1007/s00134-003-1669-3
31. Duckrow RB, Beard DC, Brennan RW. Regional cerebral blood flow decreases during hyperglycemia. Ann Neurol (1985) 17(3):267–72. doi:10.1002/ana.410170308
32. Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA (2013) 309(1):63–70. doi:10.1001/jama.2012.116975
33. Ward ME, Yan L, Angle MR. Modulation of rat pial arteriolar responses to flow by glucose. Anesthesiology (2002) 97(2):471–7. doi:10.1097/00000542-200208000-00026
34. Lott ME, Hogeman C, Herr M, Gabbay R, Sinoway LI. Effects of an oral glucose tolerance test on the myogenic response in healthy individuals. Am J Physiol Heart Circ Physiol (2007) 292(1):H304–10. doi:10.1152/ajpheart.00940.2005
35. Hutchinson PJ, Gupta AK, Fryer TF, Al-Rawi PG, Chatfield DA, Coles JP, et al. Correlation between cerebral blood flow, substrate delivery, and metabolism in head injury: a combined microdialysis and triple oxygen positron emission tomography study. J Cereb Blood Flow Metab (2002) 22(6):735–45. doi:10.1097/00004647-200206000-00012
36. Dalkara T, Alarcon-Martinez L. Cerebral microvascular pericytes and neurogliovascular signaling in health and disease. Brain Res (2015) 1623:3–17. doi:10.1016/j.brainres.2015.03.047
Keywords: brain, injury, acute, glucose, lactate
Citation: Young AMH, Adams H, Donnelly J, Guilfoyle MR, Fernandes H, Garnett MR, Czosnyka M, Smielewski P, Plummer M, Agrawal S and Hutchinson PJ (2017) Glycemia Is Related to Impaired Cerebrovascular Autoregulation after Severe Pediatric Traumatic Brain Injury: A Retrospective Observational Study. Front. Pediatr. 5:205. doi: 10.3389/fped.2017.00205
Received: 09 March 2017; Accepted: 06 September 2017;
Published: 25 September 2017
Edited by:
Saskia N. De Wildt, Radboud University Nijmegen, NetherlandsReviewed by:
Frederick Robert Carrick, Bedfordshire Centre for Mental Health Research in Association with University of Cambridge, United KingdomEnno Wildschut, Sophia Children’s Hospital, Netherlands
Copyright: © 2017 Young, Adams, Donnelly, Guilfoyle, Fernandes, Garnett, Czosnyka, Smielewski, Plummer, Agrawal and Hutchinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam M. H. Young, YXkyNzZAY2FtLmFjLnVr
 Adam M. H. Young
Adam M. H. Young Hadie Adams
Hadie Adams Joseph Donnelly
Joseph Donnelly Mathew R. Guilfoyle1
Mathew R. Guilfoyle1 Marek Czosnyka
Marek Czosnyka