- 1Department of Biology, Fairfield University, Fairfield, CT, United States
- 2Department of Nutritional Sciences, University of Connecticut, Storrs, CT, United States
- 3Department of Exercise and Nutrition Sciences, The State University of New York Plattsburgh, Plattsburgh, NY, United States
Lipid metabolism contributes to the regulation of leukocyte activity and immune responses, and may serve as a therapeutic target in the pathophysiology and clinical management of autoimmune disorders. In addition to lipid-lowering properties, statins have been shown to exert anti-inflammatory and immunomodulatory effects within the context of autoimmunity. Importantly, autoimmune incidence and lipid markers differ between men and women, suggesting that the relationship between lipid metabolism and immune function may vary by sex. Therefore, we investigated whether a predictive, sex-specific relationship exists between serum lipids, statin use, and antinuclear antibodies (ANA)—a routine clinical marker of autoimmunity and immune dysfunction—in U.S. men and women (>20 years old; n = 1,526) from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Within this population, a greater proportion of women were positive for ANA (ANA+) and had higher ANA titers, as compared to men. While we did not observe statistical differences in average total cholesterol, LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C), or triglyceride levels in ANA positive (ANA+) vs. ANA negative (ANA–) men or women, we observed that a greater proportion of ANA+ women had high total cholesterol levels (>240 mg/dL) when compared to ANA+ men (13.0 vs. 9.0%), and that a greater percentage of ANA+ women had low HDL-C as compared to ANA+ men (29.2 vs. 19.6%). However, in logistic regression models, total cholesterol, LDL-C, and HDL-C levels were not able to predict ANA status, whereas elevated serum triglycerides (150 to < 200 mg/dL) were significantly less likely to be ANA+ vs. ANA– (OR 0.33; 95% CI 0.11–0.92) in men only. Interestingly, women who reported taking statins have significantly lower odds of being ANA+ (OR 0.25; 95% CI 0.09–0.76), whereas no significant association between statin use and ANA status was observed in men. Together, our findings provide novel insight into the relationship between lipid metabolism and autoimmunity by elucidating the limited, albeit sex-specific utility of routine clinical serum lipid levels to predict ANA status at the population level, while further identifying a sex-specific and protective role for statins in predicting ANA status in women.
Introduction
Leukocyte selection, activation, and expansion is tightly regulated to ensure appropriate pathogen defense, immune surveillance of tumor cells, and resolution of inflammation to promote tissue healing and homeostasis (1–3). Compromised coordination of the mechanisms underlying immune responses can lead to impaired immunity, chronic inflammatory disorders, and autoimmune disease (4, 5). In autoimmune conditions, auto-reactive lymphocytes evade elimination during selection by lymphoid tissues and/or fail to be effectively regulated systemically, leading to the production of autoantibodies and inappropriate immune activation in response to self-antigens (6). Approximately 8% of the U.S. population −78% of whom are women—is affected by autoimmune diseases such as systemic lupus erythematous (SLE), multiple sclerosis, rheumatoid arthritis (RA), and type 1 diabetes mellitus, with the prevalence of many autoimmune conditions reported to be rapidly increasing (7–11). Autoimmune disorders are often complex to diagnose and treat, have a high economic burden, and can lead to chronic disability and death (8, 12). Apart from health complications directly associated with autoimmune conditions, SLE and RA patients—particularly premenopausal women—exhibit accelerated atherosclerosis and increased risk of cardiovascular morbidity and death (13, 14). Individuals with autoimmune disorders are similarly at increased risk for certain secondary autoimmune disorders, cancer, and infectious disease—including risk of COVID-19 (15–18). Thus, it is essential to develop effective prognostic tools and therapeutic strategies to minimize risk of developing advanced autoimmune complications (19).
Currently, antinuclear antibodies (ANA)—the most common type of autoantibodies—serve as routine clinical biomarkers in the diagnosis of autoimmune disorders (20). While a positive ANA status does not constitute a diagnosis of autoimmunity in the absence of additional measures, studies have demonstrated that the presence of ANA is evident in individuals who later develop autoimmune disease for years prior to the emergence of other clinical symptoms (21, 22). Accordingly, ANA positivity is associated with risk factors of autoimmunity, including smoking, age, infection, ethnicity, and sex (23–25). ANA positivity has additionally been shown to be more prevalent in lipid-related disorders and patients with severe coronary atherosclerosis as compared to healthy controls, and serves as a significant predictor of cardiovascular events and death (26, 27). ANA have additionally been identified in COVID-19 patients, with some reports indicating that patients who are positive for ANA have worse COVID-19 prognoses (28, 29). These findings have important implications for the use of ANA as a routine, preventative screening tool, and warrant the investigation of additional physiological parameters that influence ANA status to better improve the specificity and predictability of developing autoimmune disorders and associated comorbidities based on early ANA measures (30).
While the etiology of autoimmune disorders remains largely unknown, a growing body of evidence suggests that lipid metabolism plays an important role in regulating leukocyte activity and global immune responses, with implications for autoimmunity etiology, pathophysiology, and ANA status (5, 31). Animal studies have demonstrated that cholesterol-enriched atherogenic diets may induce and/or exasperate autoimmune-like conditions, whereas HDL-mediated efflux suppresses B cell expansion and autoantibody production (5, 32). Atherogenic lipid profiles have been associated with future RA development (33), while multiple studies have observed dyslipidemias and dysfunctional HDL in SLE, RA, and psoriasis patients (34–38). Further, lipid-lowering statins have anti-inflammatory effects in SLE and RA patients (39, 40), and may protect against RA development (41). These findings suggest that lipid metabolism is a promising target to mitigate autoimmune development and complications; however, the predictive relationship between clinical lipid and autoimmune biomarkers in the general population remains to be elucidated.
Therefore, we investigated the association between serum lipids, statin use, and ANA status in men and women from the National Health and Nutrition Examination Surveys (NHANES) 1999–2004, which is representative of the general U.S. population. Given the established differences in lipid metabolism (42, 43), ANA status (23, 24), and autoimmune incidence between men and women (11), we further evaluated whether the relationship between clinical lipid markers and ANA status was sex-specific, as this may help to better inform prognostic and therapeutic practices.
Materials and Methods
Study Design and Population
NHANES is an annual, cross-sectional survey of the non-institutionalized, civilian U.S. population conducted by the Center for Disease Control National Center for Health Sciences. This survey program collects data through interviews and physical examinations from ~5,000 individuals across 15 different U.S. states each year, and is considered to be nationally representative of the general U.S. population. Participant data from NHANES 1999–2000, 2001–2002, 2003–2004 were used for secondary data analysis in this study, which included adult men and women ≥20 years old. ANA data was available for a random subsample of participants within these survey cycles. The final sample size for our analyses consisted of 1,526 individuals, determined by the availability of data for all variables of interest. NHANES protocols for each survey cycle were approved by the National Center for Health Statistics Research Ethics Review Board. All participants provided informed consent. NHANES information, protocols, and datasets are available online at: https://www.cdc.gov/nchs/nhanes/.
Collection of Biological Samples, Anthropometric Measurements, and Survey Data
Health assessments and collection of fasting blood samples were performed in NHANES mobile examination center laboratories. Blood samples were analyzed for fasting serum lipid levels and ANA positivity and titers. For serum lipids, blood samples collected in mobile examination center laboratories were processed and stored at −20 °C until shipment to the Johns Hopkins University Lipoprotein Analytical Laboratory for analysis. Serum cotinine—a biomarker of smoking status (44)—was additionally measured by isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry (ID HPLC-APCI MS/MS). Body weight and height were measured to determine body mass index (BMI), whereas waist circumference was measured using a non-flexible tape. Participants provided self-reported data on education level, race/ethnicity, and statin usage to trained NHANES interviewers.
Fasting Serum Lipids
Serum was isolated from fasting blood samples to determine clinical lipid profiles, including total cholesterol, LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C), and triglycerides. Enzymatic assays were used to directly measure serum total cholesterol and triglycerides, whereas serum HDL-C was measured via direct immunoassays or enzymatic assays following serum depletion of apolipoprotein B-containing lipoproteins using heparin-Mn2+ precipitation. LDL-C was estimated using the Friedewald equation: LDL-C (mg/dL) = total cholesterol (mg/dL) − HDL-C (mg/dL) − triglycerides (mg/dL)/5 (45). All serum lipid measures are expressed as mg/dL. To assess the distribution of serum lipid levels between ANA positive and ANA negative participants, the following clinically-relevant lipid categories were used: total cholesterol (Optimal: <200 mg/dL; Borderline High: 200 to <240 mg/dL; High: >240 mg/dL), LDL-C (Optimal: <100 mg/dL; Near/Above Optimal: 100 to <130 mg/dL; Borderline High: 130 to <160 mg/dL, High: 160 to <190 mg/dL, Very High: >190 mg/dL), HDL-C (High/Optimal: Men: ≥40 mg/dL, Women: ≥50 mg/dL; Low: Men: <40 mg/dL, Women: <50 mg/dL), and triglycerides (Optimal: <150 mg/dL; Borderline High: 150 to <200 mg/dL; High: ≥200 mg/dL) (43, 46).
Antinuclear Antibodies
A subsample of stored serum samples collected in NHANES 1999–2004 surveys were assessed for immunoglobulin G (IgG) autoantibodies against human nuclear antigens. Autoantibodies were detected using a HEp-2 cell immunofluorescence assay from INOVA Diagnostics (San Diego, CA, USA). Staining intensity of autoantibodies was ranked from 0 to 4+, with samples undergoing staining analysis and interpretation by a minimum of two independent experienced evaluators (24). Samples were compared against autoimmune and healthy control reference serum samples. To ensure assay precision, analysis was automatically repeated for a minimum of every 50th sample. Repeated analysis was additionally performed for titering of all samples with staining intensity of 3+ and 4+, tittering of randomly selected samples, and samples where there were potential technical issues or discrepancy in interpretation between evaluators. Samples with staining intensities of 3+ and 4+ further underwent immunoprecipitation analysis for the presence of autoantibodies, in addition to autoantibody titer assessment by serial dilution to concentrations of 1:80, 1:160, 1:320, 1:640, and 1:1,280. Individuals were considered to be ANA positive (ANA+) with total ANA staining intensity of >3+ at each titer concentration (≥1:80), whereas individuals with total ANA staining intensity of <2+ were considered to be ANA negative (ANA–), in line with previous studies and reports from ANA reference laboratories (7, 24, 25).
Statistical Analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). In order to account for the complex probability sample of NHANES, SAS SURVEY procedures and appropriate sample weights were used for all analyses. To determine sex effects in the relationship between ANA positivity and serum lipids, all analyses were performed separately for men and women. Descriptive statistics and distribution of serum lipids levels across groups were reported as counts and weighted percentages for categorical variables and means and standard errors for continuous variables. Differences in mean values for continuous variables were determined by one-way ANOVA and Tukey HSD post-hoc comparisons, whereas differences in counts for categorical variables were determined by Chi-square or Fisher's Exact tests. The association between blood lipid levels and ANA positivity was assessed using multiple logistic regression, with analyses adjusted for age, race/ethnicity, education, serum cotinine, BMI, waist circumference, statin use, and survey cycle. For logistic regression models of the association between statin use and ANA positivity, the results were reported for both minimally adjusted and fully adjusted analyses, with minimally adjusted analyses controlling for age and fully adjusted analyses controlling for age, race/ethnicity, education, serum cotinine, BMI, waist circumference, and survey cycle. For all analyses, a P < 0.05 was considered statistically significant.
Results
Descriptive Characteristics of Men and Women by ANA Status
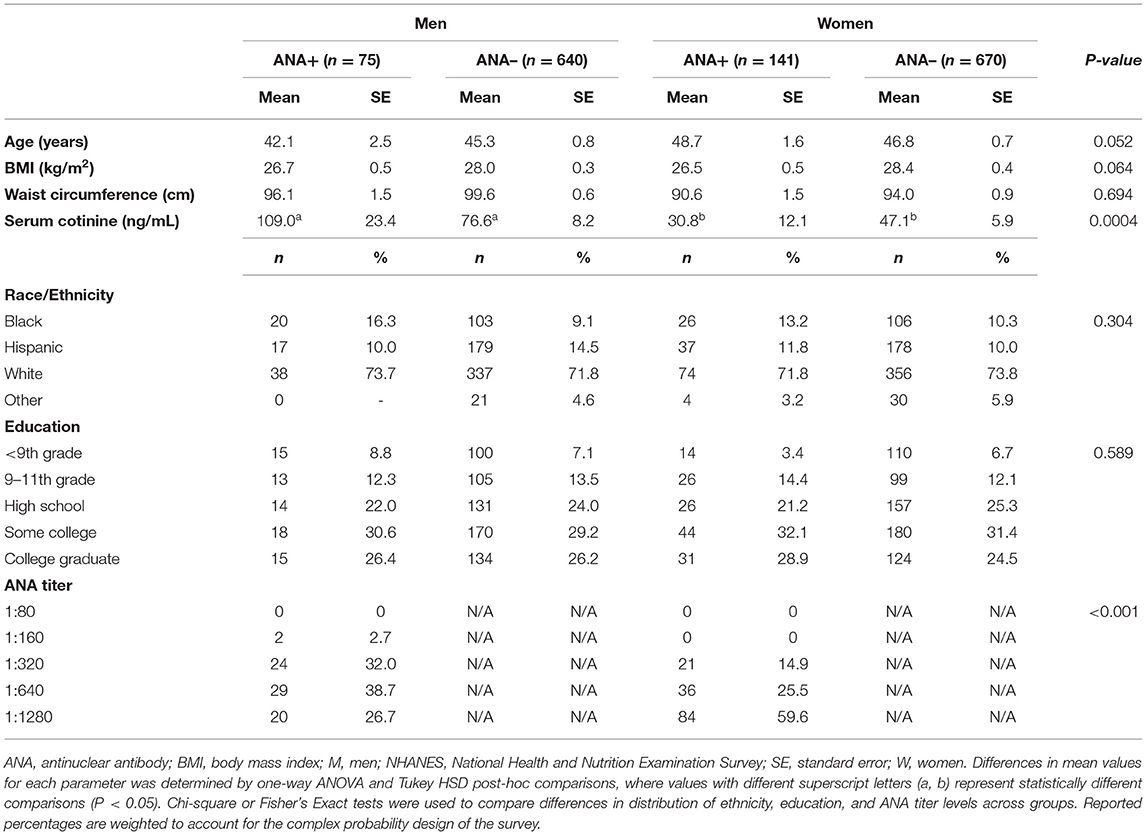
Population characteristics according to sex and ANA status are presented in Table 1. The NHANES study population (n = 1,526) consisted of a greater number of women (n = 811, 53.1%) than men (n = 715, 46.9%). We additionally observed a slightly greater proportion of women who were ANA+ (17.4%) as compared to men (11.7%), in line with previous studies (24, 47). Accordingly, for those men and women who were ANA+, women tended to have higher antibody titers within higher sensitivity ranges for autoimmune disease diagnosis (48), as evidenced by a greater number of women having positive ANA titers of 1:320 or greater. We further evaluated parameters that are associated with increased incidence of autoimmunity and elevated ANA, including age, anthropometrics, smoking, and race/ethnicity (24, 49–51). Waist circumference did not significantly differ between men and women or by ANA status, whereas statistical trends were observed for age (P = 0.052) and BMI (P = 0.064), with ANA+ men trending toward a younger age, and ANA+ men and women trending toward a lower BMI. Regardless of ANA status, men had significantly greater mean serum cotinine levels as compared to ANA+ and ANA– women, suggesting a more prevalent smoking status among men, on average. Further, we did not observe overall sex-specific differences in the distribution of education level or race/ethnicity or across ANA+ and ANA– groups. While the overall study population, regardless of sex and ANA status, consisted of a larger proportion of White individuals (53.1%), followed by Hispanic (27.1%), Black (16.8%), and Other race/ethnicities (3.6%), it is important to note that Black men and women represented a greater proportion of ANA+ individuals as compared to ANA– groups (Black Men: 26.7% ANA+ vs. 16.1% ANA–; Black Women: 18.4% ANA+ vs. 15.8% ANA–), and that a greater percentage of Hispanic men were ANA– (14.5%) as compared to women (10.0%).
Serum Lipid Profiles of Men and Women by ANA Status
We further evaluated the distribution of serum lipid levels across clinical ranges by sex and ANA status to determine whether trends exist between markers of autoimmunity and dyslipidemias. In comparing average serum lipid levels, there were few differences between ANA+ vs. ANA– men and women (Table 2). Total serum cholesterol, LDL-C, and triglyceride levels were similar between men and women, regardless of ANA status. As expected, HDL-C levels were higher in women as compared to men in both ANA+ and ANA– groups; however, differences in HDL-C between ANA+ vs. ANA– men (P = 0.074) and ANA+ vs. ANA– women (P = 0.383) were not significant. When comparing the distribution of clinical lipid ranges by sex and ANA status, we observed significant differences in total cholesterol and HDL-C, in addition to a statistical trend in LDL-C distributions (P = 0.068) and no group differences in clinical triglyceride range distributions. The majority of men and women—regardless of ANA status—had optimal total cholesterol levels (<200 mg/dL), and a greater proportion of ANA+ women had high total cholesterol levels (>240 mg/dL) when compared to ANA+ men (13.0 vs. 9.0%). Further, a greater percentage of women had low HDL-C as compared to men—regardless of ANA group. ANA+ women had low HDL-C as compared to ANA+ men (29.2 vs. 19.6%), as did ANA– women vs. ANA– (35.8 vs. 27.6%). We additionally observed that a smaller proportion of ANA+ men and women were taking statins compared to ANA– groups, with a greater proportion of ANA+ men reporting statin use compared to ANA+ women.
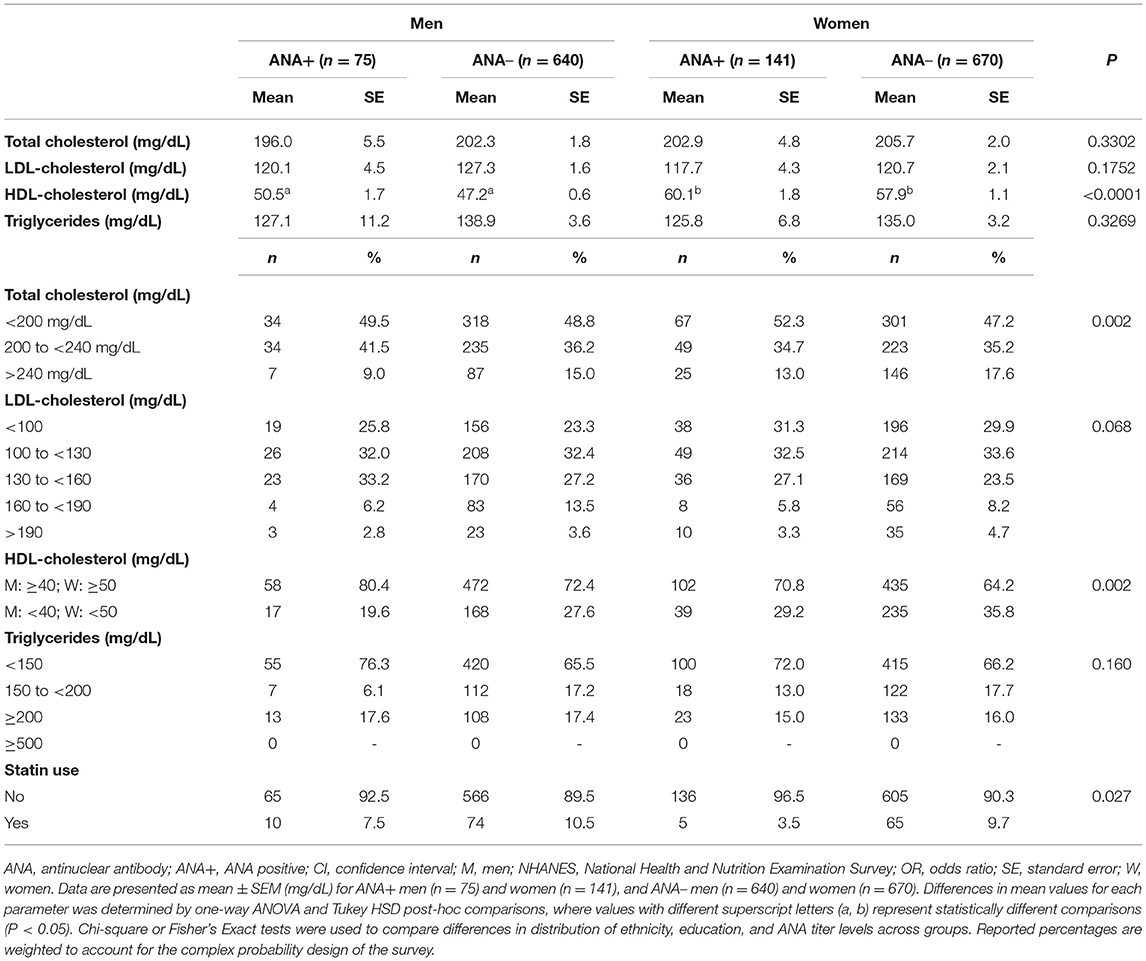
Table 2. Distribution of serum lipids in ANA+ and ANA– men and women in NHANES 19992004 (n = 1,526).
Serum Lipid Profiles Predict ANA Status to a Limited Extent in Men
We next set out to determine whether serum lipid markers could predict the odds of testing positive for ANA using logistic regression, and whether the predictive potential of lipid markers varied between men and women. After adjusting for age, race/ethnicity, education, serum cotinine, BMI, waist circumference, statin use, and survey cycle, the odds ratios (OR) for ANA positivity were not impacted by total cholesterol, LDL-C, or HDL-C levels in either men or women (Table 3). Conversely, we observed a 67% lower odds of ANA positivity in men with elevated triglycerides (150 to <200 mg/dL), whereas this relationship was not observed in men with optimal (<150 mg/dL) or high (≥200 mg/dL) triglycerides, nor was there an association between triglycerides and ANA positivity in women.
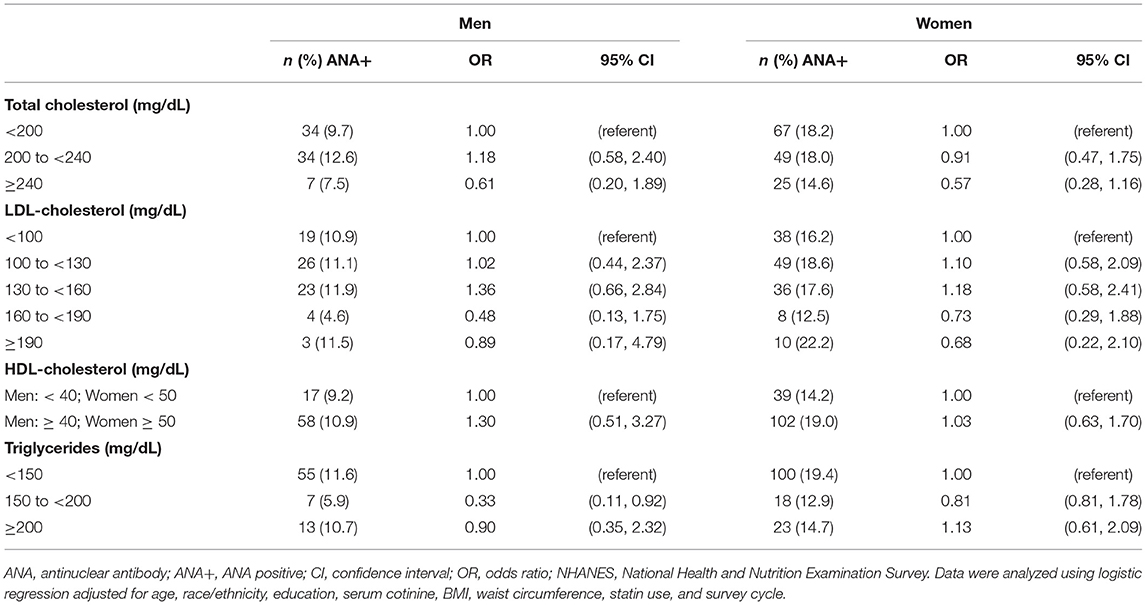
Table 3. Association between blood lipids and ANA positivity among adults ≥20 years of age in NHANES 1999–2004 (n = 1,526).
Statin Use Predicts ANA Status in Women, but Not Men
Given the anti-inflammatory and protective effects of statins in autoimmunity (39–41), we evaluated whether statin use impacts the odds of testing ANA+ in a general population. Interestingly, we found that women who reported taking statins had significantly lower odds of being ANA+ as compared to women who did not take statins in both age-adjusted (71% reduced risk) and fully-adjusted (75% reduced risk) models (Table 4). This observation was sex-dependent, as no significant associations between statin use and ANA prevalence was identified in men.
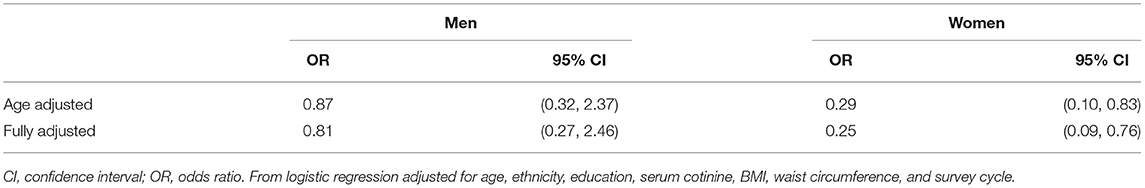
Table 4. Odds of ANA positivity among adult (≥20 years of age) statin users vs. non-users in NHANES 1999–2004 (n = 1,526).
Discussion
Lipid metabolism plays a significant role in regulating leukocyte activation and immune responses, which may have important implications in the pathophysiology and treatment of autoimmune diseases (5, 32, 40). Given the well-established sex differences in lipid metabolism, ANA status, and autoimmune disease risk (24, 52), as well as the increased risk of cardiovascular events and death in ANA positive individuals (26, 27), it is essential to evaluate the relationship between serum lipids and autoimmune markers in men vs. women, as this may inform risk assessment and therapeutic interventions. Further, it is important to determine whether routine clinical biomarkers have predictive utility in evaluating the lipid-autoimmune relationship, which has yet to be investigated at the population level. In this study, we observed sex-specific differences in the distribution of total cholesterol and HDL-cholesterol across clinical ranges between ANA+ and ANA– individuals, but that serum lipids have limited, albeit sex-specific, capacity to predict ANA status. Importantly, statin usage significantly reduced the odds of being ANA+ in women only. Together, these findings provide valuable insight into the relationship between lipid metabolism and autoimmunity at the population level by highlighting the limitations and sex-specific conditions of utilizing routine clinical biomarkers to predict ANA status. Moreover, we identified a clear sex-specific and protective role for statins in predicting ANA status, which warrants further investigation to evaluate the potential clinical and therapeutic implications.
Differences in ANA status and autoimmune prevalence between men and women are well-documented (23, 53). Approximately 78% of the 23.5 million individuals in the United States with autoimmune disorders are women, while ANA have been reported to be more prevalent in women in the general population as compared to men (11, 24). Our findings are consistent with these previous studies, in that we observed a greater proportion of women who were ANA+ (17.4%) as compared to men (11.7%). We additionally found that ANA+ women had higher ANA titers as compared to men, each having ANA titers of 1:320 or greater. While ANA can be detected in healthy populations as a result of exposure to pathogens or self-antigens during cellular apoptosis and turnover (54)—including SARS-CoV2 infection (28), ANA titers of 1:160 or greater may have a higher specificity and sensitivity in the diagnosis of autoimmune disorders (48). Differences in autoimmune disease risk between men and women may be attributable to sex hormone-mediated effects on immune cells, as well as differences in the regulation of acute and chronic immune responses (11). Estrogen receptor α (ERα) has been implicated in the suppression of T regulatory (Treg) cells, which protect against autoimmune dysfunction and pro-inflammatory T helper 1 (TH1), TH2, and TH17-mediated responses (55, 56). Females additionally display greater antibody production and TH2 cell-biased responses to infection and vaccination, whereas TH1 cell-biased responses are observed in males (57). Accordingly, autoimmune disorders that disproportionately affect women are associated with high autoantibody levels, as well as antibody-mediated and chronic pathologies (11). Thus, it is essential to consider sex as a significant confounding factor when investigating immune function and pathologies (58).
In evaluating the relationship between serum lipids and ANA status in men and women, we controlled for additional factors that are associated with ANA status and autoimmune risk, including age, BMI, smoking status, and race/ethnicity (24, 49, 59). In evaluating a subset of 4,754 individuals (≥12 years old) in NHANES 1999–2004, Satoh et al. (24) found that the ANA prevalence increased with age, and that African Americans were more likely to be ANA+ compared to White individuals. Increased risk of autoimmunity with aging is thought to be attributable to thymic involution and immunosenescence of T and B cells (60, 61), whereas the underlying cause of autoimmune disparities across racial/ethnic groups may be attributable to genetic and environmental factors (62). Interestingly, there was trend toward ANA+ men having a lower mean age, which corresponds with recent reports that immune aging is accelerated to a greater extent in men (63, 64). Further, while overall sex-specific differences in the distribution of race/ethnicity or education level across ANA+ and ANA– groups did not reach statistical significance, it is important to note that Black men and women represented a greater proportion of ANA+ individuals as compared to ANA– groups (Men: 26.7% ANA+ vs. 16.1% ANA–; Women: 18.4% ANA+ vs. 15.8% ANA–), and that a greater percentage of Hispanic men were ANA– (14.5%) as compared to women (10.0%), suggesting that sex may differentially impact autoimmune risk across racial/ethnic groups. We further observed differences in smoking status between ANA and sex groups, where men had significantly greater mean serum cotinine levels as compared to women, regardless of ANA status. While a study by Young et al. (65) did not find a relationship between cigarette smoking and ANA prevalence, smoking has been associated with a greater risk of developing SLE and RA (50, 66, 67). Obesity has additionally been linked to immune dysfunction and autoimmune disease (4, 68, 69), yet we did not observe significant statistical differences in mean BMI between ANA+ and ANA– groups for men or women.
Similar to ANA status and autoimmune disease risk, sex differences have been observed in various aspects of lipid metabolism (11, 23, 42, 70). Men are typically reported to have higher total cholesterol, LDL-C, and triglycerides as compared to premenopausal women, whereas lipid levels tend to equalize in men and postmenopausal women, which correspond to sex-specific differences in cardiovascular disease risk (70–72). In addition to having higher HDL-C as compared to men, women exhibit distinct HDL particle profiles and patterns of HDL-medicated cholesterol efflux (42, 43). Although the etiology underlying sex-specific variations in lipid markers remains to be elucidated, differences in sex hormones, body composition, and metabolism of fatty acids and complex lipids are thought to be involved (71, 73–75). Consistent with these studies, we observed significantly higher HDL-C levels in women as compared to men that was independent of ANA status, while we did not observe differences in total cholesterol, LDL-C, or triglyceride levels between men and women.
Evidence from animal studies and clinical trials demonstrates that lipid metabolism plays a direct and significant role in autoimmune disease risk, development, and treatment, suggesting that sex-specific lipid profiles may contribute to the sexual dimorphism of autoimmunity and subsequent risk of cardiovascular disease (5, 14, 27, 32, 52). Cell and animal-based studies demonstrate that cellular cholesterol loading is associated with serum hypercholesterolemia and a lower threshold to pro-inflammatory T cell activation, whereas lipid efflux via HDL-mediated efflux suppresses T and B cell expansion, inflammation, and autoantibody production (5, 32, 76, 77). Accordingly, we have recently demonstrated that serum lipids can differentially predict leukocyte subset counts in NHANES 1999–2004 in a sex-dependent manner (52), whereas various other population-based studies utilizing different cohorts and datasets have similarly identified predictive relationships between serum lipids and apolipoproteins vs. leukocytes counts (78–80). Further, atherogenic dyslipidemias and dysfunctional HDL are observed in SLE, RA, and psoriasis patients (34–38). In this current study, we observed that a greater proportion of ANA+ women had high total cholesterol levels (>240 mg/dL) when compared to ANA+ men (13.0 vs. 9.0%). We additionally observed that a greater percentage of ANA+ women had low HDL-C as compared to ANA+ men (29.2 vs. 19.6%), although differences in HDL-C between ANA+ vs. ANA– status in men and women did not reach significance. Despite variability in clinical lipid ranges between ANA+ and ANA– men and women, total cholesterol, LDL-C, and HDL-C levels did not predict ANA status in adjusted models. While HDL is known to mediate autoimmune dysfunction and inflammation (5, 32), immunomodulatory properties of HDL are most often attributable to functional components of the HDL particles (e.g., lipidome, proteome, and cholesterol-accepting capacity) that are independent of HDL-C levels (5, 81–83), which could explain why stronger associations between HDL-C and ANA status were not observed. Thus, further research is warranted to evaluate the extent to which degrees of hypercholesterolemia and HDL function may influence leukocyte cholesterol metabolism, activity, and autoantibody production in human populations, and whether additional routine clinical markers could be utilized to develop stronger predictive models.
Despite a lack of predictive associations between serum cholesterol markers and ANA status, we found that elevated serum triglycerides levels (150 to <200 mg/dL) were associated with a reduced odds of being ANA positive—an association that was only found in men, and not women. Hypertriglyceridemia is a result of increased lipogenesis in the liver and reduced systemic lipolysis, leading to greater secretion and circulation of triglyceride-rich very low-density lipoproteins (VLDL), respectively (84, 85). Intriguingly, our findings are in contrast with previous studies which have shown that elevated serum triglycerides can promote deposition of lipids in metabolic and lymphoid tissues, inducing direct activation of leukocytes by fatty acids, in addition to indirect immune activation in response to tissue dysfunction and inflammation that may exasperate autoimmune dysfunction (4, 86–88). We additionally did not observe a dose-dependent relationship between ANA and triglycerides, as no association was observed between ANA status and serum triglyceride levels ≥200 mg/dL. Given that our study was based on secondary analysis of NHANES data, we are not able to identify mechanisms to explain these effects, or rule out residual confounding factors. Our analysis was additionally limited by a relatively small sample size (n = 7 for men with triglyceride levels within 150 to <200 mg/dL). Thus, further investigation is warranted to confirm and explore these observations. Additionally, while regulation of systemic lipolysis varies between men and women (89), the mechanisms underlying sex-specific associations between ANA and serum triglycerides are unclear, and warrant further study.
In line with the associations between dyslipidemias and autoimmune disease risk (34–38), lipid-lowering statins have been shown to exert protective and anti-inflammatory effects within the context of autoimmunity (5, 39–41, 90, 91). Statins have been shown to improve clinical outcomes in SLE and RA patients, and have been reported to reduce the risk of developing RA (39–41). Interestingly, we observed a sex-specific effect in evaluating the relationship between statin use and ANA status, in that women who reported taking statins had significantly lower odds of being ANA+, whereas no significant association between statin use and ANA status was observed in men. While the mechanism underlying this observation is unclear, it has been reported that statins may have variable efficacy in men vs. women in regards to cardiovascular disease risk (92, 93). Women have also reported greater side effects in taking statins as compared to men, including muscle symptoms indicative of statin-induced myopathy (94, 95). These findings warrant further investigation as to whether statins have greater immunomodulatory properties in women, which would have significant implications for clinical care and autoimmune disease treatment.
In conclusion, the findings from this study provide insight into the relationship between lipid metabolism and autoimmunity by elucidating the limited, albeit sex-specific utility of routine clinical serum lipid levels to predict ANA status at the population level. Most importantly, our study identifies a sex-specific and protective role for statins in predicting ANA status in women, who are most likely to be diagnosed with autoimmune conditions and experience statin intolerance (11, 94). Given the strength of preclinical evidence that points to a significant role for lipoproteins in modulating leukocyte activity and autoimmune outcomes, further clinical and population-based studies to evaluate the utility of serum lipids or other routine lipid biomarkers in predicting autoimmune outcomes, as well as elucidate potential therapeutic opportunities and limitations of statins in treating rheumatic diseases, is warranted.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Ethics Statement
The studies involving human participants were reviewed and approved by the National Center for Health Statistics Research Ethics Review Board. The participants provided their written informed consent.
Author Contributions
CA: conceptualization, data analysis, and drafted the manuscript. TV: statistical methodology and analysis, review, and contribution of writing to the manuscript. CA and TV: data interpretation. Both authors approve of the final manuscript.
Funding
Open access publication fees were supported by University of Connecticut start-up funds to CA.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ANA, antinuclear antibodies; BMI, body mass index; ERα, Estrogen receptor α; HDL-C, HDL-cholesterol; ID HPLC-APCI MS/MS, isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry; IgG, immunoglobulin G; LDL-C, LDL-cholesterol; NHANES, National Health and Nutrition Examination Survey; RA, rheumatoid arthritis; SLE, systemic lupus erythematous; TH1, T helper 1 cells; TH2, T helper 1 cells; TH17, T helper 1 cells; Treg, T regulatory cells.
References
1. Schett G, Neurath MF. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun. (2018) 9:3261. doi: 10.1038/s41467-018-05800-6
2. Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. (2015) 21:687–92. doi: 10.1158/1078-0432.CCR-14-1860
3. Heinzel S, Marchingo JM, Horton MB, Hodgkin PD. The regulation of lymphocyte activation and proliferation. Curr Opin Immunol. (2018) 51:32–8. doi: 10.1016/j.coi.2018.01.002
4. Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. (2016) 7:66–75. doi: 10.3945/an.115.010207
5. Andersen CJ. Impact of dietary cholesterol on the pathophysiology of infectious and autoimmune disease. Nutrients. (2018) 10:764. doi: 10.3390/nu10060764
6. Rosenblum MD, Remedios KA, Abbas AK. Mechanisms of human autoimmunity. J Clin Invest. (2015) 125:2228–33. doi: 10.1172/JCI78088
7. Dinse GE, Jusko TA, Whitt IZ, Co CA, Parks CG, Satoh M, et al. Associations between selected xenobiotics and antinuclear antibodies in the national health and nutrition examination survey, 1999-2004. Environ Health Perspect. (2016) 124:426–36. doi: 10.1289/ehp.1409345
8. Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am J Manag Care. (2014) 20:S128–35.
9. Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. (2017) 66:241–55. doi: 10.2337/db16-0806
10. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. (2013) 19:S15–20.
11. Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. (2008) 173:600–9. doi: 10.2353/ajpath.2008.071008
12. Ocampo-Piraquive V, Nieto-Aristizabal I, Canas CA, Tobon GJ. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. (2018) 14:1043–53. doi: 10.1080/1744666X.2018.1538789
13. Durante A, Bronzato S. The increased cardiovascular risk in patients affected by autoimmune diseases: review of the various manifestations. J Clin Med Res. (2015) 7:379–84. doi: 10.14740/jocmr2122w
14. Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the framingham study. Am J Epidemiol. (1997) 145:408–15. doi: 10.1093/oxfordjournals.aje.a009122
15. Somers EC, Thomas SL, Smeeth L, Hall AJ. Are individuals with an autoimmune disease at higher risk of a second autoimmune disorder? Am J Epidemiol. (2009) 169:749–55. doi: 10.1093/aje/kwn408
16. Li CM, Chen Z. Autoimmunity as an etiological factor of cancer: the transformative potential of chronic type 2 inflammation. Front Cell Dev Biol. (2021) 9:664305. doi: 10.3389/fcell.2021.664305
17. Maddur MS, Vani J, Lacroix-Desmazes S, Kaveri S, Bayry J. Autoimmunity as a predisposition for infectious diseases. PLoS Pathog. (2010) 6:e1001077. doi: 10.1371/journal.ppat.1001077
18. Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of Covid-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. (2020) annrheumdis-2020-219394. doi: 10.1136/annrheumdis-2020-219394
19. Maecker HT, Lindstrom TM, Robinson WH, Utz PJ, Hale M, Boyd SD, et al. New tools for classification and monitoring of autoimmune diseases. Nat Rev Rheumatol. (2012) 8:317–28. doi: 10.1038/nrrheum.2012.66
20. Sur LM, Floca E, Sur DG, Colceriu MC, Samasca G, Sur G. Antinuclear antibodies: marker of diagnosis and evolution in autoimmune diseases. Lab Med. (2018) 49:e62–e73. doi: 10.1093/labmed/lmy024
21. Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. (2003) 349:1526–33. doi: 10.1056/NEJMoa021933
22. Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC, Harley JB, et al. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. (2007) 56:2344–51. doi: 10.1002/art.22665
23. Parks CG, Miller FW, Satoh M, Chan EK, Andrushchenko Z, Birnbaum LS, et al. Reproductive and hormonal risk factors for Antinuclear Antibodies (Ana) in a representative sample of U.S. Women. Cancer Epidemiol Biomarkers Prev. (2014) 23:2492–502. doi: 10.1158/1055-9965.EPI-14-0429
24. Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. (2012) 64:2319–27. doi: 10.1002/art.34380
25. Dinse GE, Parks CG, Meier HCS, Co CA, Chan EKL, Jusko TA, et al. Prescription medication use and antinuclear antibodies in the United States, 1999-2004. J Autoimmun. (2018) 92:93–103. doi: 10.1016/j.jaut.2018.05.006
26. Liang KP, Kremers HM, Crowson CS, Snyder MR, Therneau TM, Roger VL, et al. Autoantibodies and the risk of cardiovascular events. J Rheumatol. (2009) 36:2462–9. doi: 10.3899/jrheum.090188
27. Grainger DJ, Bethell HW. High titres of serum antinuclear antibodies, mostly directed against nucleolar antigens, are associated with the presence of coronary atherosclerosis. Ann Rheum Dis. (2002) 61:110–4. doi: 10.1136/ard.61.2.110
28. Peker BO, Sener AG, Kaptan Aydogmus F. Antinuclear Antibodies (Anas) detected by Indirect Immunofluorescence (Iif) method in Acute Covid-19 infection; future roadmap for laboratory diagnosis. J Immunol Methods. (2021) 499:113174. doi: 10.1016/j.jim.2021.113174
29. Muratori P, Lenzi M, Muratori L, Granito A. Antinuclear antibodies in Covid 19. Clin Transl Sci. (2021) 14:1627–8. doi: 10.1111/cts.13026
30. Soto ME, Hernandez-Becerril N, Perez-Chiney AC, Hernandez-Rizo A, Telich-Tarriba JE, Juarez-Orozco LE, et al. Predictive value of antinuclear antibodies in autoimmune diseases classified by clinical criteria: analytical study in a specialized health institute, one year follow-up. Results Immunol. (2015) 5:13–22. doi: 10.1016/j.rinim.2013.10.003
31. Bagchi S, Genardi S, Wang CR. Linking Cd1-Restricted T Cells with autoimmunity and dyslipidemia: lipid levels matter. Front Immunol. (2018) 9:1616. doi: 10.3389/fimmu.2018.01616
32. Ito A, Hong C, Oka K, Salazar JV, Diehl C, Witztum JL, et al. Cholesterol accumulation in Cd11c(+) immune cells is a causal and targetable factor in autoimmune disease. Immunity. (2016) 45:1311–26. doi: 10.1016/j.immuni.2016.11.008
33. van Halm VP, Nielen MM, Nurmohamed MT, van Schaardenburg D, Reesink HW, Voskuyl AE, et al. Lipids and inflammation: serial measurements of the lipid profile of blood donors who later developed rheumatoid arthritis. Ann Rheum Dis. (2007) 66:184–8. doi: 10.1136/ard.2006.051672
34. Szabo MZ, Szodoray P, Kiss E. Dyslipidemia in systemic lupus erythematosus. Immunol Res. (2017) 65:543–50. doi: 10.1007/s12026-016-8892-9
35. McMahon M, Grossman J, Skaggs B, Fitzgerald J, Sahakian L, Ragavendra N, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. (2009) 60:2428–37. doi: 10.1002/art.24677
36. Kim JY, Lee EY, Park JK, Song YW, Kim JR, Cho KH. Patients with rheumatoid arthritis show altered lipoprotein profiles with dysfunctional high-density lipoproteins that can exacerbate inflammatory and atherogenic process. PLoS ONE. (2016) 11:e0164564. doi: 10.1371/journal.pone.0164564
37. Holzer M, Wolf P, Curcic S, Birner-Gruenberger R, Weger W, Inzinger M, et al. Psoriasis alters Hdl composition and cholesterol efflux capacity. J Lipid Res. (2012) 53:1618–24. doi: 10.1194/jlr.M027367
38. McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. (2006) 54:2541–9. doi: 10.1002/art.21976
39. Artola RT, Mihos CG, Santana O. Effects of statin therapy in patients with systemic lupus erythematosus. South Med J. (2016) 109:705–11. doi: 10.14423/SMJ.0000000000000561
40. Li GM, Zhao J, Li B, Zhang XF, Ma JX, Ma XL, et al. The anti-inflammatory effects of statins on patients with rheumatoid arthritis: a systemic review and meta-analysis of 15 randomized controlled trials. Autoimmun Rev. (2018) 17:215–25. doi: 10.1016/j.autrev.2017.10.013
41. Jick SS, Choi H, Li L, McInnes IB, Sattar N. Hyperlipidaemia, statin use and the risk of developing rheumatoid arthritis. Ann Rheum Dis. (2009) 68:546–51. doi: 10.1136/ard.2008.091967
42. Catalano G, Duchene E, Julia Z, Le Goff W, Bruckert E, Chapman MJ, et al. Cellular Sr-Bi and Abca1-mediated cholesterol efflux are gender-specific in healthy subjects. J Lipid Res. (2008) 49:635–43. doi: 10.1194/jlr.M700510-JLR200
43. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
44. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. (2016) 1236. doi: 10.3390/ijerph13121236
45. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. (1972) 18:499–502. doi: 10.1093/clinchem/18.6.499
46. Expert Expert Panel on Detection E Treatment Treatment of High Blood Cholesterol in A. Executive Summary of the Third Report of the National Cholesterol Education Program (Ncep) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
47. Li QZ, Karp DR, Quan J, Branch VK, Zhou J, Lian Y, et al. Risk factors for Ana positivity in healthy persons. Arthritis Res Ther. (2011) 13:R38. doi: 10.1186/ar3271
48. Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in “Healthy” individuals. Arthritis Rheum. (1997) 40:1601–11. doi: 10.1002/art.1780400909
49. Xavier RM, Yamauchi Y, Nakamura M, Tanigawa Y, Ishikura H, Tsunematsu T, et al. Antinuclear antibodies in healthy aging people: a prospective study. Mech Ageing Dev. (1995) 78:145–54. doi: 10.1016/0047-6374(94)01532-Q
50. Majka DS, Holers VM. Cigarette smoking and the risk of systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis. (2006) 65:561–3. doi: 10.1136/ard.2005.046052
51. Kamen DL. Environmental influences on systemic lupus erythematosus expression. Rheum Dis Clin North Am. (2014) 40:401–12, vii. doi: 10.1016/j.rdc.2014.05.003
52. Andersen CJ, Vance TM. Gender dictates the relationship between serum lipids and leukocyte counts in the National Health and Nutrition Examination Survey 1999(-)2004. J Clin Med. (2019) 8:365. doi: 10.3390/jcm8030365
53. Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. (2014) 35:347–69. doi: 10.1016/j.yfrne.2014.04.004
54. Suurmond J, Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest. (2015) 125:2194–202. doi: 10.1172/JCI78084
55. Mohammad I, Starskaia I, Nagy T, Guo J, Yatkin E, Vaananen K, et al. Estrogen receptor alpha contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci Signal. (2018) 11:eaap9415. doi: 10.1126/scisignal.aap9415
56. Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. (2018) 19:665–73. doi: 10.1038/s41590-018-0120-4
57. Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. (2015) 109:9–15. doi: 10.1093/trstmh/tru167
58. Klein SL. Immune cells have sex and so should journal articles. Endocrinology. (2012) 153:2544–50. doi: 10.1210/en.2011-2120
59. Harel-Meir M, Sherer Y, Shoenfeld Y. Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol. (2007) 3:707–15. doi: 10.1038/ncprheum0655
60. Ma S, Wang C, Mao X, Hao Y. B cell dysfunction associated with aging and autoimmune diseases. Front Immunol. (2019) 10:318. doi: 10.3389/fimmu.2019.00318
61. Coder B, Su DM. Thymic involution beyond T-cell insufficiency. Oncotarget. (2015) 6:21777–8. doi: 10.18632/oncotarget.4970
62. Molokhia M, McKeigue P. Risk for rheumatic disease in relation to ethnicity and admixture. Arthritis Res. (2000) 2:115–25. doi: 10.1186/ar76
63. Shapiro JR, Li H, Morgan R, Chen Y, Kuo H, Ning X, et al. Sex-specific effects of aging on humoral immune responses to repeated influenza vaccination in older adults. NPJ Vaccines. (2021) 6:147. doi: 10.1038/s41541-021-00412-6
64. Marquez EJ, Chung CH, Marches R, Rossi RJ, Nehar-Belaid D, Eroglu A, et al. Sexual-dimorphism in human immune system aging. Nat Commun. (2020) 11:751. doi: 10.1038/s41467-020-14396-9
65. Young KA, Terrell DR, Guthridge JM, Kamen DL, Gilkeson GS, Karp DR, et al. Smoking is not associated with autoantibody production in systemic lupus erythematosus patients, unaffected first-degree relatives, nor healthy controls. Lupus. (2014) 23:360–9. doi: 10.1177/0961203314520838
66. Barbhaiya M, Tedeschi SK, Lu B, Malspeis S, Kreps D, Sparks JA, et al. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the nurses' health study cohorts. Ann Rheum Dis. (2018) 77:196–202. doi: 10.1136/annrheumdis-2017-211675
67. Freemer MM, King TE Jr, Criswell LA. Association of smoking with dsDNA autoantibody production in systemic lupus erythematosus. Ann Rheum Dis. (2006) 65:581–4. doi: 10.1136/ard.2005.039438
68. Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. (2014) 13:981–1000. doi: 10.1016/j.autrev.2014.07.001
69. Andersen CJ. High BMI: a new determinant of impaired rubella immunity during pregnancy? Obesity. (2018) 26:1390. doi: 10.1002/oby.22286
70. Swiger KJ, Martin SS, Blaha MJ, Toth PP, Nasir K, Michos ED, et al. Narrowing sex differences in lipoprotein cholesterol subclasses following mid-life: the Very Large Database of Lipids (Vldl-10b). J Am Heart Assoc. (2014) 3:e000851. doi: 10.1161/JAHA.114.000851
71. Seidell JC, Cigolini M, Charzewska J, Ellsinger BM, Bjorntorp P, Hautvast JG, et al. Fat distribution and gender differences in serum lipids in men and women from four European Communities. Atherosclerosis. (1991) 87:203–10. doi: 10.1016/0021-9150(91)90022-U
72. Goh VH, Tong TY, Mok HP, Said B. Differential impact of aging and gender on lipid and lipoprotein profiles in a cohort of healthy Chinese Singaporeans. Asian J Androl. (2007) 9:787–94. doi: 10.1111/j.1745-7262.2007.00294.x
73. Palmisano BT, Zhu L, Eckel RH, Stafford JM. Sex differences in lipid and lipoprotein metabolism. Mol Metab. (2018) 15:45–55. doi: 10.1016/j.molmet.2018.05.008
74. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90
75. Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it's not just about sex hormones. J Clin Endocrinol Metab. (2011) 96:885–93. doi: 10.1210/jc.2010-2061
76. Armstrong AJ, Gebre AK, Parks JS, Hedrick CC. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J Immunol. (2010) 184:173–83. doi: 10.4049/jimmunol.0902372
77. Bietz A, Zhu H, Xue M, Xu C. Cholesterol metabolism in T cells. Front Immunol. (2017) 8:1664. doi: 10.3389/fimmu.2017.01664
78. Lai YC, Woollard KJ, McClelland RL, Allison MA, Rye KA, Ong KL, et al. The association of plasma lipids with white blood cell counts: results from the multi-ethnic study of atherosclerosis. J Clin Lipidol. (2019) 13:812–20. doi: 10.1016/j.jacl.2019.07.003
79. Tucker B, Sawant S, McDonald H, Rye KA, Patel S, Ong KL, et al. The association of serum lipid and lipoprotein levels with total and differential leukocyte counts: results of a cross-sectional and longitudinal analysis of the UK Biobank. Atherosclerosis. (2021) 319:1–9. doi: 10.1016/j.atherosclerosis.2020.12.016
80. Liu Y, Kong X, Wang W, Fan F, Zhang Y, Zhao M, et al. Association of peripheral differential leukocyte counts with dyslipidemia risk in Chinese patients with hypertension: insight from the China stroke primary prevention trial. J Lipid Res. (2017) 58:256–66. doi: 10.1194/jlr.P067686
81. Vaisar T, Tang C, Babenko I, Hutchins P, Wimberger J, Suffredini AF, et al. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J Lipid Res. (2015) 56:1519–30. doi: 10.1194/jlr.M059089
82. Andersen CJ, Fernandez ML. Dietary approaches to improving atheroprotective HDL functions. Food Funct. (2013) 4:1304–13. doi: 10.1039/c3fo60207a
83. Andersen CJ, Blesso CN, Lee J, Barona J, Shah D, Thomas MJ, et al. Egg consumption modulates HDL lipid composition and increases the cholesterol-accepting capacity of serum in metabolic syndrome. Lipids. (2013) 48:557–67. doi: 10.1007/s11745-013-3780-8
84. Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. (2012) 32:2104–12. doi: 10.1161/ATVBAHA.111.241463
85. Willecke F, Scerbo D, Nagareddy P, Obunike JC, Barrett TJ, Abdillahi ML, et al. Lipolysis, and not hepatic lipogenesis, is the primary modulator of triglyceride levels in streptozotocin-induced diabetic mice. Arterioscler Thromb Vasc Biol. (2015) 35:102–10. doi: 10.1161/ATVBAHA.114.304615
86. van de Woestijne AP, Monajemi H, Kalkhoven E, Visseren FL. Adipose tissue dysfunction and hypertriglyceridemia: mechanisms and management. Obes Rev. (2011) 12:829–40. doi: 10.1111/j.1467-789X.2011.00900.x
87. Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. (2008) 79:101–8. doi: 10.1016/j.plefa.2008.09.016
88. Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, et al. Obesity accelerates thymic aging. Blood. (2009) 114:3803–12. doi: 10.1182/blood-2009-03-213595
89. Santosa S, Jensen MD. The sexual dimorphism of lipid kinetics in humans. Front Endocrinol. (2015) 6:103. doi: 10.3389/fendo.2015.00103
90. Aprahamian T, Bonegio R, Rizzo J, Perlman H, Lefer DJ, Rifkin IR, et al. Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol. (2006) 177:3028–34. doi: 10.4049/jimmunol.177.5.3028
91. Jury EC, Isenberg DA, Mauri C, Ehrenstein MR. Atorvastatin restores Lck expression and lipid raft-associated signaling in T cells from patients with systemic lupus erythematosus. J Immunol. (2006) 177:7416–22. doi: 10.4049/jimmunol.177.10.7416
92. Karp I, Chen SF, Pilote L. Sex differences in the effectiveness of statins after myocardial infarction. CMAJ. (2007) 176:333–8. doi: 10.1503/cmaj.060627
93. Plakogiannis R, Arif SA. Women versus men: is there equal benefit and safety from statins? Curr Atheroscler Rep. (2016) 18:6. doi: 10.1007/s11883-016-0562-9
94. Karalis DG, Wild RA, Maki KC, Gaskins R, Jacobson TA, Sponseller CA, et al. Gender differences in side effects and attitudes regarding statin use in the understanding statin use in America and gaps in patient education (Usage) study. J Clin Lipidol. (2016) 10:833–41. doi: 10.1016/j.jacl.2016.02.016
Keywords: serum lipids, cholesterol, statins, autoimmunity, NHANES, antinuclear antibodies (ANA), sex
Citation: Andersen CJ and Vance TM (2022) Sex-Specific Associations Between Serum Lipids, Antinuclear Antibodies, and Statin Use in National Health and Nutrition Examination Surveys 1999–2004. Front. Med. 9:887741. doi: 10.3389/fmed.2022.887741
Received: 07 March 2022; Accepted: 26 April 2022;
Published: 26 May 2022.
Edited by:
Chris Wincup, King's College Hospital NHS Foundation Trust, United KingdomReviewed by:
Rachita Nanda, All India Institute of Medical Sciences Raipur, IndiaAnna Kathleen Coussens, The University of Melbourne, Australia
Copyright © 2022 Andersen and Vance. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine J. Andersen, Y2F0aGVyaW5lLmFuZGVyc2VuQHVjb25uLmVkdQ==
†Present Address: Catherine J. Andersen, Department of Nutritional Sciences, University of Connecticut, Storrs, CT, United States
 Catherine J. Andersen
Catherine J. Andersen Terrence M. Vance
Terrence M. Vance