- 1Institute of Chemistry, University of Sargodha, Sargodha, Pakistan
- 2College of Pharmacy, University of Sargodha, Sargodha, Pakistan
- 3Centre of Excellence in Molecular Biology, University of the Punjab, Lahore, Pakistan
- 4School of Health and Life Sciences, Teesside University, Middlesbrough, United Kingdom
- 5Department of Mechanical Engineering, Khalifa University, Abu Dhabi, United Arab Emirates
The screening of hair follicles, dermal papilla cells, and keratinocytes through in vitro, in vivo, and histology has previously been reported to combat alopecia. Ficus benghalensis has been used conventionally to cure skin and hair disorders, although its effect on 5α-reductase II is still unknown. Currently, we aim to analyze the phytotherapeutic impact of F. benghalensis leaf extracts (FBLEs) for promoting hair growth in rabbits along with in vitro inhibition of the steroid isozyme 5α-reductase II. The inhibition of 5α-reductase II by FBLEs was assessed by RP-HPLC, using the NADPH cofactor as the reaction initiator and Minoxin (5%) as a positive control. In silico studies were performed using AutoDock Vina to visualize the interaction between 5α-reductase II and the reported phytoconstituents present in FBLEs. Hair growth in female albino rabbits was investigated by applying an oral dose of the FBLE formulation and control drug to the skin once a day. The skin tissues were examined by histology to see hair follicles. Further, FAAS, FTIR, and antioxidants were performed to check the trace elements and secondary metabolites in the FBLEs. The results of RP-HPLC and the binding energies showed that FBLEs reduced the catalytic activity of 5α-reductase II and improved cell proliferation in rabbits. The statistical analysis (p < 0.05 or 0.01) and percentage inhibition (>70%) suggested that hydroalcoholic FBLE has more potential in increasing hair growth by elongating hair follicle’s anagen phase. FAAS, FTIR, and antioxidant experiments revealed sufficient concentrations of Zn, Cu, K, and Fe, together with the presence of polyphenols and scavenging activity in FBLE. Overall, we found that FBLEs are potent in stimulating hair follicle maturation by reducing the 5α-reductase II action, so they may serve as a principal choice in de novo drug designing to treat hair loss.
Highlights
1) Study of the potential of Ficus benghalensis leaves for hair regrowth via in vitro, in silico, and in vivo studies.
2) The crude extract constituents inhibit steroid enzyme 5α-reductase II activity by binding with its active site.
3) Hair analeptics were prepared instead of direct application of crude extracts to avoid any fungal infection.
4) Photomicrographs of skin biopsies show a hair growth cycle, e.g., anagen and telogen.
1 Introduction
The term androgenic alopecia (AGA) refers to the patterned loss of scalp hair in men and women due to heredity and hormonal factors (Abbas et al., 2021). Hair is an indispensable structure of the body that guards the scalp, adorns human personality, and performs multiple functions such as insulation, attraction, and tangibility (Koch et al., 2020). The hair follicle (HF) cycle passes through telogen (resting phase), catagen (regression phase), and anagen (growth phase), where pigmentation and hair shaft synthesis take place (Tamura et al., 2018). Factors causing AGA are androgen hormonal imbalance, stress, genetic disorders, malnutrition, chemotherapy, 5α-reductase II (SRD5AII) overactivity, thyroid malfunctioning, drug addiction, ageing, and malignancy (Richards et al., 2008).
Treatment of AGA has been an open debate in clinical dermatology for many years. The risk alleles associated with AGA are located at chromosome 20p11.22 (Richards et al., 2008). “Trichoscopy” has been introduced as the first method to diagnose AGA in women (Rakowska et al., 2008). Many therapies are now available to combat baldness, like hair transplant through bioengineering (Asakawa et al., 2012), HF regeneration by rearranging stem cells (Toyoshima et al., 2012), platelet-rich plasma (PRP) (Cervantes et al., 2018), and synthetic drugs minoxidil and finasteride (Roy et al., 2008; Gregoriou et al., 2010). However, the worst dermatological effects of drugs have been observed, such as scaling, itching, and dermatitis (Choi et al., 2018). A recent study claims that ceria nanozyme-integrated microneedles can reshape the perifollicular microenvironment by reducing oxidative stress and regenerate hair at the balding site (Yuan et al., 2021). A correlation was observed between plasma micronutrients, vitamin deficiency, and hair density in non-genetic patients with AGA by direct colorimetric tests and flame atomic absorption spectrophotometry (FAAS) (Kondrakhina et al., 2020).
Human 5α-reductase (SDR5A) has three types of functional enzymes coded by the SRD5A gene. Among these, steroid isozyme SDR5AII (optimum pH; 5.0–5.5) plays a key role in the production of AGA by catalyzing the conversion of testosterone into more active dihydrotestosterone (DHT). The enzyme is sufficiently found in the prostate gland, epididymis, genital skin, and seminal vesicles, while the brain and liver contain fewer amounts. Moreover, DHT overexpression also causes acne, prostate cancer, and benign prostate hyperplasia (Srivilai et al., 2019). Histological studies in DHT-treated mice show delayed hair regrowth and shortened anagen (Fu et al., 2021). Computational and mutagenesis studies explained the binding interaction of SRD5AII′ to finasteride, demonstrating how the drug inhibits this integral membrane enzyme (Xiao et al., 2020).
To cure alopecia, herbs can provide nutrition, enhance scalp blood circulation, and cease DHT and SRD5A response (Kaur et al., 2020). The use of Trigonella foenum-graecum and Eclipta alba promoted hair growth in rats more efficiently than the drug (Roy et al., 2008; Imtiaz et al., 2017). Microwave-assisted solvent extraction and GC-MS reveal organic compounds in F. benghalensis that show an anti-inflammatory and vasodilator response (Jayasree Radhakrishnan and Venkatachalam, 2020). Under chronic stress, a high corticosterone level elongates the telogen phase due to the suppressive expression of Gas6 (Choi et al., 2021). Interestingly, the neuromodulator effect of the methanol bark extract of F. benghalensis boosted memory and learning behavior and reduced stress in rats (Malik et al., 2020).
Here, we aimed to elucidate the effect of FBLEs in stimulating cell proliferation to promote hair growth (HG) in rabbits and inhibit SRD5AII activity without leaving undesired secondary effects.
2 Materials and Methods
2.1 Extract Preparation
Fresh leaves of the F. benghalensis (5 kg) were collected from Chakwal, Pakistan. The plant was authenticated by a taxonomist from the Department of Botany, University of Sargodha (UOS), Pakistan. The leaves were washed and dried in the air, followed by the production of coarse powder using a mechanical grinder. Powdered leaves (20 g) were soaked in 200 ml of either solvent—petroleum ether (PE), ethyl acetate (EtOAc), and 70% v/v aqueous ethanol (aq. EtOH) labeled as FBLE1-3. The suspensions were placed on an orbital shaker for 5 days. Later, the mixtures were filtered, subjected to evaporation, concentrated using porous aluminum foil, and finally kept at 4°C until further use (Handa et al., 2008).
2.2 In Vitro 5α-Reductase II Study for Hair Growth
The inhibition of the catalytic activity of SRD5AII was studied by initially mincing 4 g of the prostate gland of a male goat and crushing it under liquid nitrogen. The tissue was further homogenized using a handled homogenizer in 20 ml of sodium phosphate buffer (SPB) (pH 6.5, 50 mM) containing 1 mM EDTA (200 µl), 100 mM sucrose (64 ml), 1 mM sodium azide (200 µl), and a protease inhibitor tablet (Roche Pharma, Mannheim, Germany). Homogenate was centrifuged at 15,000 rpm for 20 min at 4°C, separating the supernatant for further use as a crude enzyme source. The soluble protein was tested by Bradford assay (Rehman et al., 2009).
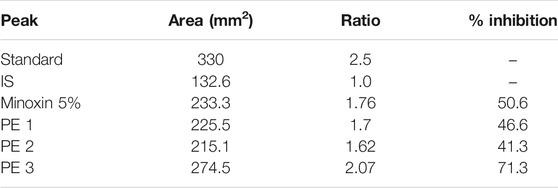
For the reverse-phase high-performance liquid chromatography (RP-HPLC; Shimadzu, Japan), Testovirone ampoule (testosterone enanthate, 250 mg/ml; Bayer Pharma, Leverkusen, Germany) was taken as standard. Six reaction mixtures (RMs) were prepared as positive control (PC), reaction control (standard), complete reaction (internal standard, IS), and others with FBLEs (1–3) (Table 1). The RMs were incubated at 37°C for 30 min, and the reaction was terminated by adding 2 ml of EtOAc followed by the addition of 150 µl prednisolone 250 μg/ml ethanol as an IS. The RMs were mixed for 1 min and centrifuged at 5,000 rpm (10 min, 4°C). Subsequently, the water phase was frozen at −80°C, and the organic layer was decanted and evaporated. The concentrated residue (1.5 ml) was later diluted in 3 ml methanol. Finally, RMs were syringe filtered (0.2 µm) before HPLC run to avoid contamination (Sher et al., 2015; Kumar et al., 2011).
The RP-HPLC was performed using Chromatographic Column C18-ODS type (250 mm × 4.6 mm), maintaining system conditions at 40°C and flow rate 10 μl/min for 30 min, while absorbance was recorded at 254 nm. Isocratic elution was performed, making a mobile phase with methanol and water (80:20) and filtering it to remove any contamination. The percentage inhibition of SRD5AII was observed from the peak height and the peak area ratio (r) applying the following formula (Kumar et al., 2011):
2.3 Molecular Docking
To determine the ligand–receptor binding interactions, we docked SD5ARII with minoxidil and reported bioactive constituents: (-)-Gallocatechin, Rhein, and Mucusoside of FBLEs using AutoDock Vina and AutoDock Tools 1.5.6. The X-ray crystal structure of the target mammalian’s SD5ARII with a resolution of 2.8 Å was retrieved from Protein Data Bank (PDB ID: 7BW1). After docking, the binding energies (BEs) were noted, and the lowest values were chosen to view the interactions in PyMol (Bhaskara Rao et al., 2014; Hassan et al., 2020; Bilal et al., 2021; Rehman et al., 2021).
2.4 In Vivo Study for HG
The female albino rabbits (6–11 months old; 1–2 kg) were purchased from the University of Veterinary and Animal Sciences, Lahore, Pakistan. Prior to proceeding further, rabbits were authorized by the ethical committee of the College of Pharmacy, UOS, providing approval no. 70B18 IAEC/UOS. Animals were acclimatized for 3 days providing standard food and water in an animal housing facility at the College of Pharmacy, UOS. Rabbits were divided into eight groups, shaved at the dorsal side area (5 × 4 cm), using a razor, and left for 24 h to observe any edema or erythema. HG formulations (500 µl) were sprayed once a day on the nude area, and eventually HG was observed. On the 28th day, rabbits were sacrificed, and skin biopsies were performed while skin tissues were kept in 10% formalin for histological study (Roy et al., 2008; Imtiaz et al., 2017). Ten hair strands were randomly plucked every week, and an average length of each hair was noted in mm using a digital Vernier caliper (Adhirajan et al., 2003). Histology was performed using subcutaneous hematoxylin and eosin staining (Lin et al., 2015) to analyze HFs, observed at ×10 magnification using a digital microscope (Bresser, Rhede, Germany).
2.4.1 Hair Growth Formulations
The HG formulations were prepared by mixing dilute FBLEs (10 ml), EtOH (7.5 ml), and 32 ml of distilled water, followed by ultrasonication. Later, it was syringe filtered (0.24 µm) to avoid any microbial infection on the animal skin and noted pH. Minoxin® 5% (Brookes Pharma, Karachi, Pakistan) was used as PC, whereas PE, EtOAc, and 70% aq. EtOH were used as negative controls (NCs) (Imtiaz et al., 2017).
2.5 Qualitative Phytochemical Analysis
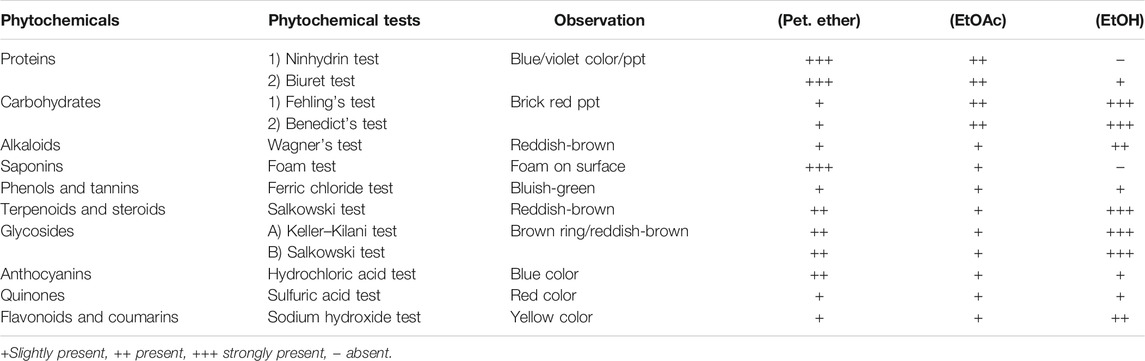
Phytochemical analysis was performed to analyze various phytoconstituents in crude extracts like phenolic compounds and tannins, alkaloids, saponins and glycosides, terpenoids and steroids, anthocyanins, flavonoids, coumarins, and quinones (Harborne, 1998). The results of qualitative analysis were noted with color change, foamy appearance, or precipitates formation.
2.6 Characterization of FBLEs
The FBLEs were analyzed for calcium, iron, zinc, and potassium using FAAS. The powdered leaves (50 mg) were digested using 15 ml of HCl and HNO3 (3:1) to extract metal ions. The acid mixture was poured into the beaker having powdered leaves and placed on a hot plate until the sample got mixed thoroughly. The acidic mixture was allowed to cool, then filtered, and finally diluted up to 100.0 ml using distilled water. Stock solutions of 3, 6, 9, 12, and 15 ppm of metal salts were prepared for mineral detection, and concentrations were noted in mg/g (Ahmad et al., 2014). Chemical characterization of FBLEs was done through Fourier transform infrared (FTIR) spectroscopy. The greasy extracts were dried using KBr pellets and analyzed in the mid-range, 4,000–600 cm-1 (Gopukumar et al., 2016).
2.6 Antioxidant Activity
Total polyphenol content (TPC) was estimated by modifying a method (Abbas et al., 2021), taking gallic acid as a standard, and FBLEs (50 mg) diluted in 5 ml methanol were used to determine TPC. The absorbance was noted at 765 nm in triplicate. Total flavonoid content (TFC) was estimated by aluminum chloride colorimetric assay, taking catechin as a standard, using FBLE dilutions prepared for TPC. Each reading was measured in triplicate at 510 nm.
2.7 DPPH Assay
Radical scavenging activity (RSA) was assessed using DPPH (1,1-diphenyl-2-picrylhydrazyl) assay, taking 100 µl FBLE dilutions, added to 3 ml of DPPH (0.1 mM), followed by incubation at room temperature in the dark for 30 min. The absorbance was recorded in triplicate at 517 nm while taking 3 ml of DPPH as control (Abbas et al., 2021). Data were recorded as % RSA, and IC50 (minimum inhibitory concentration) was calculated using MS Excel 2016. The percentage of RSA was calculated using the following formula;
2.8 Statistical Analysis
The statistical analysis was scrutinized by one-way ANOVA via Tukey test using SigmaPlot ver. 14.0. The Pearson correlation coefficient (p) was applied to correlate HG results among all test groups at significance level p < 0.05 or highly significant p < 0.01 using SPSS version 21.
3 Results
3.1 Percentage Yield
The percentage yield of FBLEs (PE, EtOAc, and 70% aq. EtOH) was calculated as 3.9%, 4.8%, and 20.6%, respectively.
3.2 In Vitro Enzyme Study
The quantified protein from the male goat prostate gland was 7.99 mg/ml of BSA. In the RP-HPLC charts, a prominent peak (b) at retention time (RT) around 6 min was entitled as the testosterone’s peak (Figure 5D). Results are justified by the peak height intensity (mAU), which determines how much testosterone has been left behind in the RM. The results of (r) and peak height revealed more unconverted testosterone in hydroalcoholic FBLE, favoring sufficient percentage inhibition for SRD5AII, than Minoxin 5% (Table 2). No nicotinamide adenine dinucleotide hydrogen phosphate (NADPH) was added in the “reaction control” to compare the peak height of the PE 1-3 and PC. However, the minimum peak height a) observed in the complete reaction with IS indicated more testosterone conversion to DHT. A delayed peak c) refers to SRD5AII due to the hydrophobic nature of both steroid enzyme and stationary phase.
3.3 Molecular Docking
Docking studies showed a strong binding interaction of Rhein (BE; −7.4 kcal/mol) with the enzyme’s active sites, revealing a prompt inhibitory potential against SD5ARII. In comparison, the binding energies (Bes) of phytoconstituents of crude FBLEs showed significant interactions with the receptor protein than Minoxidil. Figure 1 and Table 3 show protein–ligand interactions and values of their BEs in Kcal/mol and type of binding interaction.
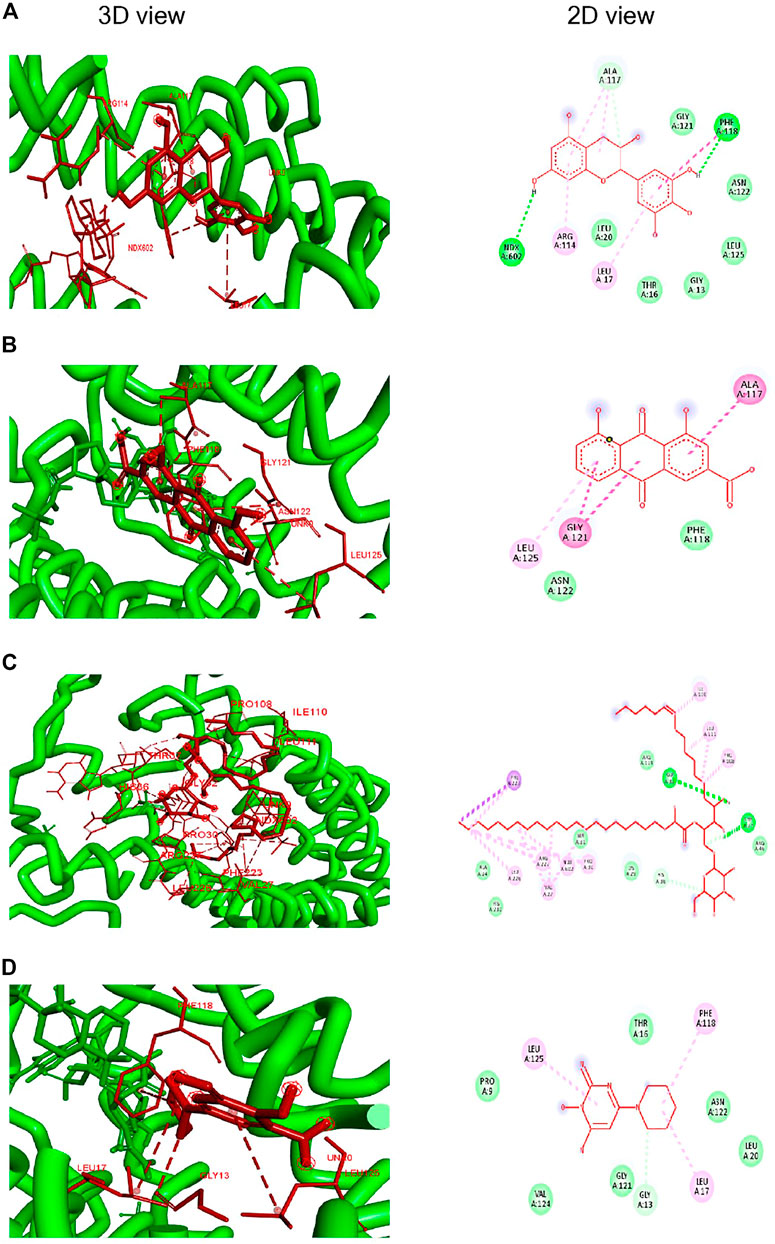
FIGURE 1. Molecular docking of SD5ARII with the ligands. (A)–(–Gallocatechin); (B) Rhein; (C) Mucusasoide, and (D) Minoxidil.
3.4 In Vivo Analysis
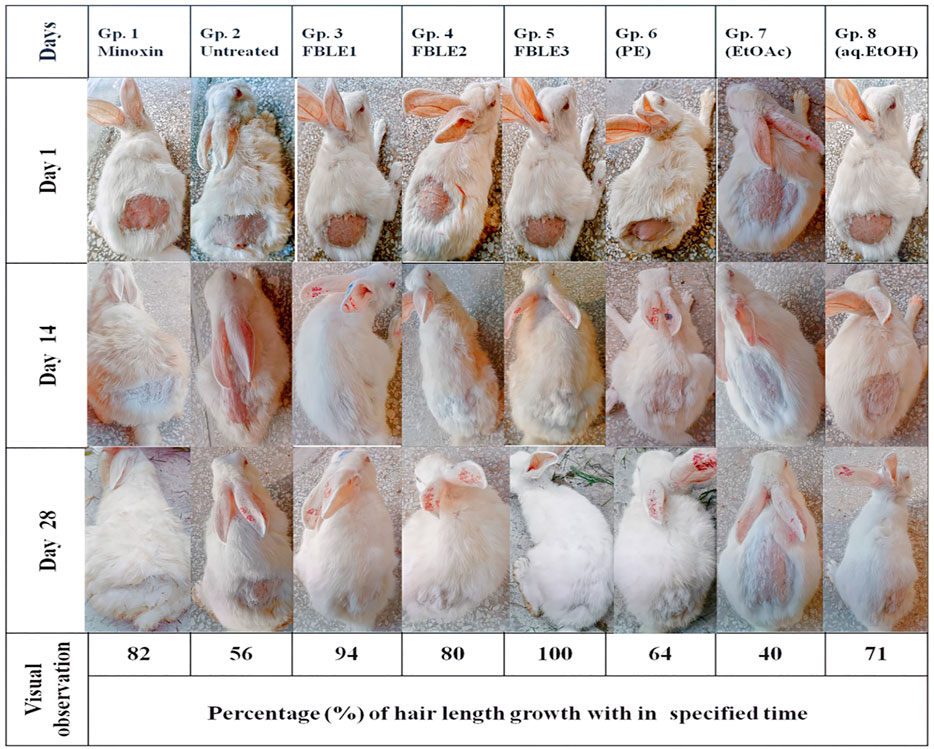
No skin irritation was observed in the rabbits, and rapid hair regrowth was noticed in the first week that varied subsequently Figure 2. The hair texture appeared to be rough and weak in PC and NCs but smooth in others. The average hair length confirms that weekly HG was approximately 1–2 mm Figure 3. The Pearson correlation (p < 0.05) revealed an exceptional HG rate in group 5. From the photomicrographs of the longitudinal section, HF phases and DPCs were observed in treated tissues (Figure 4). The pH outcome was 7.6, 4.3, and 6.4 for FBLE1-3, respectively.
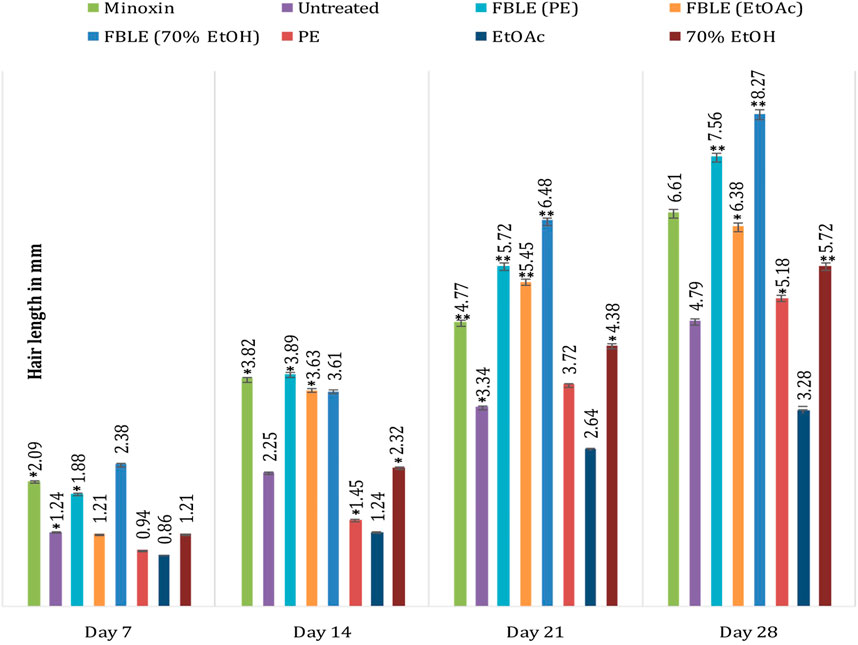
FIGURE 3. Improvement in hair growth within due time shows the weekly increase in hair length. Mean ± SD * Significant at p < 0.05; **p < 0.01.
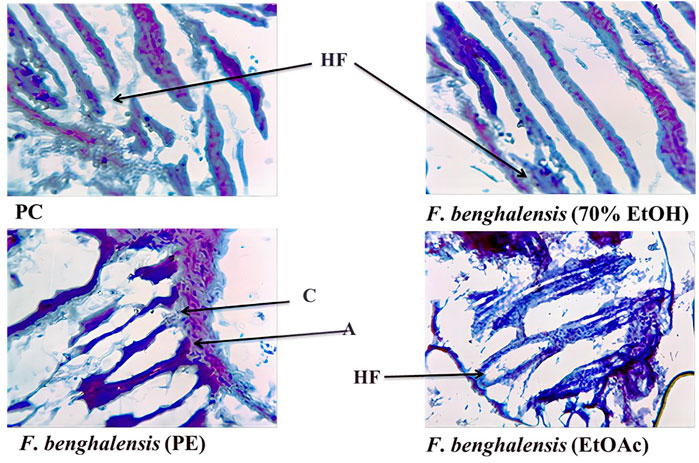
FIGURE 4. Photomicrographs of the longitudinal section of skin biopsies under digital microscope × 10 resolution; PC (Minoxin 5%) and crude FBLE extracts depict HF= Hair Follicle, anagen (A), and C= catagen (B).
3.5 Qualitative Phytochemical Analysis
The screening of the preliminary phytochemicals has shown the presence of carbohydrates, proteins, phenolic compounds, flavonoids, alkaloids, saponins, glycosides, steroids, and tannins. The results of all phytochemicals are represented in Table 4.
3.6 FAAS and FTIR Analysis
FAAS showed the mineral concentrations in mg/g (copper = 13; zinc = 9; iron = 23.4; potassium = 21.8) found in powdered leaves. In FTIR fingerprints, peaks are attributed to stretching and bending vibrations, characterizing functional groups in essential metabolites (Figures 5A,C).
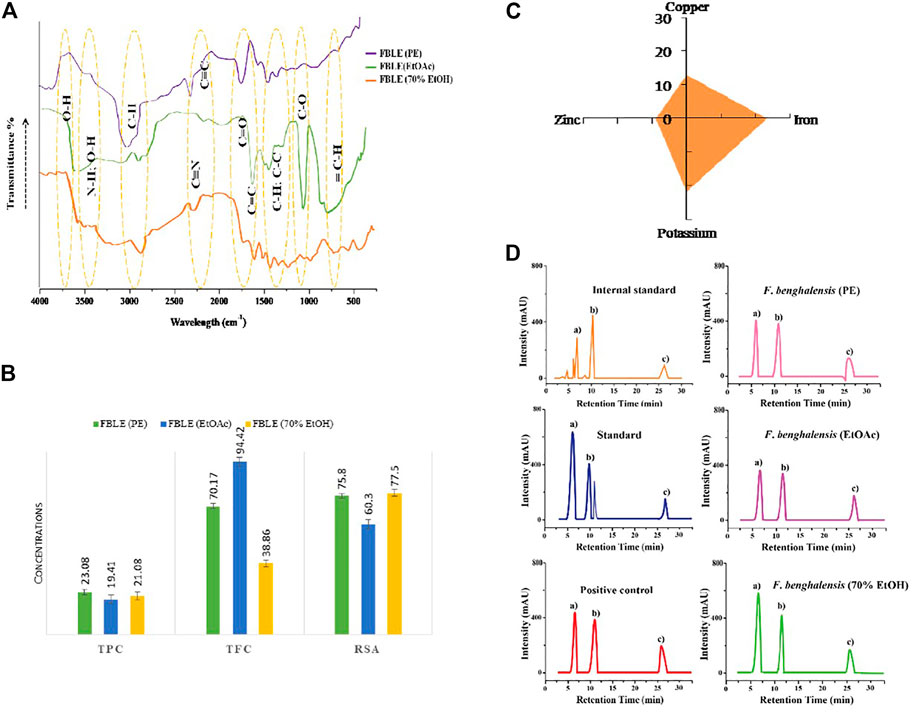
FIGURE 5. Characterization of FBLEs and RP-HPLC analysis. In (A), FTIR fingerprints show the functional groups; (B) presence of essential minerals in dried leaves; (C) depicts antioxidants present in FBLEs. Values are mean ± SD. (D) demonstrates how much FBLEs are active in limiting SRD5AII activity. The peak (B) in RP-HPLC graphs indicates the availability of testosterone in RMs; the same peak is represented by (A) in the graph labeled as “IS”.
3.7 Antioxidant Activity
FBLEs showed concentrations for TPC, TFC, and RSA, presented in Figure 5B.
3.8 DPPH Scavenging Activity
Different concentrations of FBLEs against DPPH were tested to find the inhibitory potential of antioxidants present in the crude extracts. It has been observed that %RSA increased as the concentrations of FBLEs increased. Data were recorded as mean ± SD. Table 5 shows the maximum %RSA and IC50 values for FBLEs.
4 Discussion
Androgenic alopecia (AGA), a leading disorder in both genders, plays a role in building psychological trauma (Koch et al., 2020), demanding a detailed study to cope with the disease. In the current study, in vitro and in vivo effects of FBLEs were tested for promoting HG in rabbits. Previously, females suffering from AGA were tested for aromatase mRNA levels by RT-PCR from plucked hair strands from the top of the scalp. Most women had low levels of aromatase mRNA and high levels for SRD5AI, II, and III (Sánchez et al., 2018). The in vitro evaluation of valproic acid in cultured human DPC decreased the level of β-catenin and increased anagen induction in mice (Jo et al., 2013).
The prostate gland holds sufficient amount of isozyme SRD5AII, and its homogenate explains the phenomena of testosterone’s conversion into DHT in RM (Steers, 2001). To study the inhibitory activity of SRD5AII, 17 hydroalcoholic Thai plant extracts were examined using HPLC analysis (Kumar et al., 2011). The in vitro study describes limiting the conversion of testosterone to DHT by lowering the catalytic activity of SRD5AII. In RP-HPLC, the peak intensity indicates more testosterone level in the RMs and thus more inhibition of SRD5AII by Ocimum basilicum (Kumar et al., 2011); in comparison, FBLEs were found to inhibit SRD5AII (Figure 5D; Table 2) significantly. We assumed that the peak at (RT = 11.8 min) is castor oil present in the standard and testosterone. In silico molecular docking of phytochemicals of FBLEs was performed to observe the inhibition of acetylcholinesterase against Alzheimer’s (Hassan et al., 2020).
The proliferation of dermal papillae cells (DPCs) and keratinocytes contributes a pivotal role in extending HF anagen while protecting the skin tissues (Madaan et al., 2017; Bejaoui et al., 2021). DPCs, the specialized markers, are cultured as a therapeutic tool during hair constitution assays to expand HFs and sustain hair inductivity (Yang and Cotsarelis, 2010). The in vivo studies claim that HG promoted the potential of shikimic acid and cilostazol on C57BL/6 mouse and ex vivo DPC proliferation of human HF by upregulating vasodilation and HG factors through kinase assays (Choi et al., 2018; Choi et al., 2019). Eclipta alba has imparted more HG in rats than minoxidil (Roy et al., 2008). The FBLEs promoted cell proliferation and prolonged anagen, indicated by an increase in the HG rate (Figures 3,4). Researchers have reported that a minor acidic pH is essential for healthy HG (Dias et al., 2014); pH results in the range between 4 and 8 for FBLE1-3 favor the study.
A lack of minerals could risk for producing alopecia in women (Almohanna et al., 2019); AGA male patients had insufficient zinc and copper in hair, serum, and urine (Ozturk et al., 2014). Concentrations in powdered leaves (0.01–0.6 mg/100 g) were found to be sufficient (as daily recommended dose) (Figure 5C) as compared to other species of Ficus (Wangkheirakpam and Laitonjam, 2012). The DPPH assay was chosen as a reliable method to find the potential of antioxidants to convert DPPH solution into non-radical DPPH. The hydro-alcoholic bark extract of F. benghalensis showed TPC (23.2 ± 0.6), TFC (100.24 ± 4.21), and RSA (45.73 ± 1.17) µg/ml (Wangkheirakpam and Laitonjam, 2012), respectively. A significant %RSA (85.20 ± 0.96%) was shown by the fruit extract of F. auriculata (Shahinuzzaman et al., 2021). Our findings revealed competitive outcomes for antioxidants in FBLEs mentioned in Table 5 and Figure 5B. Leaves of F. benghalensis yield β-amyrin along with psoralen, β-sitosterol, bergapten, friedelin, glycyl-d-asparagine lupeol, quercetin-3-galactoside, rutin, and taraxosterol all come under the classes of phytoconstituents (Murti et al., 2010). Further, a qualitative assessment of secondary metabolites and reported compounds of FBLEs we used for in silico analysis favors the confirmation of the presence of essential components in the FBLEs.
Altogether, FBLEs prepared using maceration upregulated HG in rabbits without leaving skin rash and reducing the biocatalytic potential of SRD5AII, thus depriving the conversion of testosterone to more potent DHT. The study has shown the possible binding interaction of phytochemicals with SRD5AII, inhibiting the enzyme’s activity. Histological findings depicted clear anagen in the particularly hydroalcoholic FBLE-treated group, validating visually enhanced HG in the animal model. Hence, the conclusion favors that phytoconstituents of F. benghalensis could serve as future drug candidates.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the author, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by The Pharmacy Research Ethics Committee (PREC), under approval no. 70B18 IAEC/UOS. Approval has been obtained from the local institutional review boards. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
SN and MFUR conceived, planned, and supervised the experimental work. JI collect and compile the data and performed analysis. FB interpret the spectra of all techniques. MM and SH helped in writing draft of manuscript and performed basic in silico studies. MA and HB have performed the computational studies as recommended by the reviewers, have validated the data analysis and manuscript review.
Funding
The authors acknowledge Khalifa University of Science and Technology (KUST) for the KU-KAIST Joint Research Center (Project code: 8474000220-KKRJC-2019-Health1) research funding. We also acknowledge Sandooq Al Watan LLC and Aldar Properties for the research funding (SWARD Program - AWARD Project code: 8434000391 - EX2020-044).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are thankful for the supportive role of Biogen and Maraday Pharma, Rawat, Pakistan.
References
Abbas, A., Naqvi, S. a. R., Rasool, M. H., Noureen, A., Mubarik, M. S., and Tareen, R. B. (2021). Phytochemical Analysis, Antioxidant and Antimicrobial Screening of Seriphidium Oliverianum Plant Extracts. Dose-response 19, 15593258211004739. a publication of International Hormesis Society. doi:10.1177/15593258211004739
Adhirajan, N., Ravi Kumar, T., Shanmugasundaram, N., and Babu, M. (2003). In Vivo and In Vitro Evaluation of Hair Growth Potential of Hibiscus Rosa-Sinensis Linn. J. Ethnopharmacol 88, 235–239. doi:10.1016/s0378-8741(03)00231-9
Ahmad, A., Husain, A., Mujeeb, M., Siddiqui, N. A., Damanhouri, Z. A., and Bhandari, A. (2014). Physicochemical and Phytochemical Standardization with HPTLC Fingerprinting of Nigella Sativa L. Seeds. Pak J. Pharm. Sci. 27, 1175–1182.
Almohanna, H. M., Ahmed, A. A., Tsatalis, J. P., and Tosti, A. (2019). The Role of Vitamins and Minerals in Hair Loss: a Review. Dermatol. Ther. (Heidelb) 9, 51–70. doi:10.1007/s13555-018-0278-6
Asakawa, K., Toyoshima, K. E., Ishibashi, N., Tobe, H., Iwadate, A., Kanayama, T., et al. (2012). Hair Organ Regeneration via the Bioengineered Hair Follicular Unit Transplantation. Sci. Rep. 2, 424–427. doi:10.1038/srep00424
Bejaoui, M., Taarji, N., Saito, M., Nakajima, M., and Isoda, H. (2021). Argan (Argania Spinosa) Press Cake Extract Enhances Cell Proliferation and Prevents Oxidative Stress and Inflammation of Human Dermal Papilla Cells. J. Dermatol. Sci. 103, 33–40. doi:10.1016/j.jdermsci.2021.06.003
Bhaskara Rao, K. V., Ojha, V., PreetiKumar, G., Kumar, G., and Karthik, L. (2014). Phytochemical Composition and Antioxidant Activity ofFicus benghalensis(Moraceae) Leaf Extract. J. Biologically Active Prod. Nat. 4, 236–248. doi:10.1080/22311866.2014.936902
Bilal, S., Mudassir Hassan, M., Fayyaz ur Rehman, M., Nasir, M., Jamil Sami, A., and Hayat, A. (2021). An Insect Acetylcholinesterase Biosensor Utilizing WO3/g-C3n4 Nanocomposite Modified Pencil Graphite Electrode for Phosmet Detection in Stored Grains. Food Chem. 346, 128894. doi:10.1016/j.foodchem.2020.128894
Cervantes, J., Perper, M., Wong, L. L., Eber, A. E., Villasante Fricke, A. C., Wikramanayake, T. C., et al. (2018). Effectiveness of Platelet-Rich Plasma for Androgenetic Alopecia: a Review of the Literature. Skin Appendage Disord. 4, 1–11. doi:10.1159/000477671
Choi, H. I., Kim, D. Y., Choi, S. J., Shin, C. Y., Hwang, S. T., Kim, K. H., et al. (2018). The Effect of Cilostazol, a Phosphodiesterase 3 (PDE3) Inhibitor, on Human Hair Growth with the Dual Promoting Mechanisms. J. Dermatol. Sci. 91, 60–68. doi:10.1016/j.jdermsci.2018.04.005
Choi, M., Choi, S. J., Jang, S., Choi, H. I., Kang, B. M., Hwang, S. T., et al. (2019). Shikimic Acid, a Mannose Bioisostere, Promotes Hair Growth with the Induction of Anagen Hair Cycle. Sci. Rep. 9, 17008–8. doi:10.1038/s41598-019-53612-5
Choi, S., Zhang, B., Ma, S., Gonzalez-Celeiro, M., Stein, D., Jin, X., et al. (2021). Corticosterone Inhibits GAS6 to Govern Hair Follicle Stem-Cell Quiescence. Nature 592, 428–432. doi:10.1038/s41586-021-03417-2
Fu, D., Huang, J., Li, K., Chen, Y., He, Y., Sun, Y., et al. (2021). Dihydrotestosterone-induced Hair Regrowth Inhibition by Activating Androgen Receptor in C57BL6 Mice Simulates Androgenetic Alopecia. Biomed. Pharmacother. 137, 111247. doi:10.1016/j.biopha.2021.111247
Gavazzoni Dias, M. F., De Almeida, A. M., Cecato, P. M., Adriano, A. R., and Pichler, J. (2014). The Shampoo pH Can Affect the Hair: Myth or Reality. Int. J. Trichology 6, 95–99. doi:10.4103/0974-7753.139078
Gopukumar, S., Alexander, P., Jainamboo, M., and Praseetha, P. (2016). Phytochemical Screening and FT-IR Analysis of Ficus Benghalensis Fruits. Int. J. Pharmacognosy Phytochem. Res. 8, 1529–1534.
Gregoriou, S., Papafragkaki, D., Kontochristopoulos, G., Rallis, E., Kalogeromitros, D., and Rigopoulos, D. (20102010). Cytokines and Other Mediators in Alopecia Areata. Mediators Inflamm. 2010, 928030. doi:10.1155/2010/928030
Handa, S., Khanuja, S., Longo, G., and Rakesh, D. (2008). Extraction Technologies for Medicinal and Aromatic Plants: Earth. Environ. Mar. Sci. Tech. 12, 1–10.
Harborne, A. (1998). Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. springer science & business media.
Hassan, H. A., Allam, A. E., Abu-Baih, D. H., Mohamed, M. F. A., Abdelmohsen, U. R., Shimizu, K., et al. (2020). Isolation and Characterization of Novel Acetylcholinesterase Inhibitors from Ficus Benghalensis L. Leaves. RSC Adv. 10, 36920–36929. doi:10.1039/d0ra06565j
Imtiaz, F., Islam, M., Saeed, H., Saleem, B., Asghar, M., and Saleem, Z. (2017). Impact of Trigonella Foenum-Graecum Leaves Extract on Mice Hair Growth. Pakistan J. Zoolog. 49. doi:10.17582/journal.pjz/2017.49.4.1405.1412
Jayasree Radhakrishnan, A., and Venkatachalam, S. (2020). A Holistic Approach for Microwave Assisted Solvent Extraction of Phenolic Compounds from Ficus Benghalensis Fruits and its Phytochemical Profiling. J. Food Process Eng. 43, e13536. doi:10.1111/jfpe.13536
Jo, S. J., Choi, S. J., Yoon, S. Y., Lee, J. Y., Park, W. S., Park, P. J., et al. (2013). Valproic Acid Promotes Human Hair Growth in In Vitro Culture Model. J. Dermatol. Sci. 72, 16–24. doi:10.1016/j.jdermsci.2013.05.007
Kaur, A., Singh, T. G., Dhiman, S., Arora, S., and Babbar, R. (2020). Novel Herbs Use Din Cosmetics for Skin an D Hair Care: A Review. Plant Arch. 20, 3784–3793.
Koch, S. L., Tridico, S. R., Bernard, B. A., Shriver, M. D., and Jablonski, N. G. (2020). The Biology of Human Hair: A Multidisciplinary Review. Am. J. Hum. Biol. 32, e23316. doi:10.1002/ajhb.23316
Kondrakhina, I. N., Verbenko, D. A., Zatevalov, A. M., Gatiatulina, E. R., Nikonorov, A. A., Deryabin, D. G., et al. (2020). A Cross-Sectional Study of Plasma Trace Elements and Vitamins Content in Androgenetic Alopecia in Men. Berlin: Biological Trace Element Research.
Kumar, T., Chaiyasut, C., and Suttajit, M. (2011). Screening of Steroid 5-reductase Inhibitory Activity and Total Phenolic Content of Thai Plants. J. Med. Plants Res. 5, 1265–1271.
Lin, C. M., Yuan, Y. P., Chen, X. C., Li, H. H., Cai, B. Z., Liu, Y., et al. (2015). Expression of Wnt/β-Catenin Signaling, Stem-Cell Markers and Proliferating Cell Markers in Rat Whisker Hair Follicles. J. Mol. Histol. 46, 233–240. doi:10.1007/s10735-015-9616-5
Madaan, A., Joshi, V., Kishore, A., Verma, R., Singh, A. T., Jaggi, M., et al. (2017). In Vitro hair Growth Promoting Effects of Naringenin and Hesperetin on Human Dermal Papilla Cells and Keratinocytes. Am. J. Dermatol. Venereol. 6, 51–57.
Malik, H., Javaid, S., Fawad Rasool, M., Samad, N., Rizwan Ahamad, S., Alqahtani, F., et al. (2020). Amelioration of Scopolamine-Induced Amnesic, Anxiolytic and Antidepressant Effects of Ficus Benghalensis in Behavioral Experimental Models. Medicina (Kaunas) 56, 144. doi:10.3390/medicina56030144
Murti, K., Kumar, U., Lambole, V., and Gajera, V. (2010). Ethnopharmacological Properties of Ficus Benghalensis Linn: A Review. Pharmacologyonline 2, 717–726.
Ozturk, P., Kurutas, E., Ataseven, A., Dokur, N., Gumusalan, Y., Gorur, A., et al. (2014). BMI and Levels of Zinc, Copper in Hair, Serum and Urine of Turkish Male Patients with Androgenetic Alopecia. J. Trace Elem. Med. Biol. 28, 266–270. doi:10.1016/j.jtemb.2014.03.003
Rakowska, A., Slowinska, M., Kowalska-Oledzka, E., Olszewska, M., and Rudnicka, L. (2008). Trichoscopy Criteria for Diagnosing Female Androgenic Alopecia. Nat. Prec, 1. doi:10.1038/npre.2008.1913.1
Rehman, F. U., Aslam, M., Tariq, M. I., Shaheen, A., Sami, A. J., Naveed, N. H., et al. (2009). Isolation of Cellulolytic Activities from Tribolium castaneum (Red Flour Beetle). Afr. J. Biotechnol. 8.
Rehman, M. F. u., Akhter, S., Batool, A. I., Selamoglu, Z., Sevindik, M., Eman, R., et al. (2021). Effectiveness of Natural Antioxidants against SARS-CoV-2? Insights from the In-Silico World. Antibiotics 10, 1011. doi:10.3390/antibiotics10081011
Richards, J. B., Yuan, X., Geller, F., Waterworth, D., Bataille, V., Glass, D., et al. (2008). Male-pattern Baldness Susceptibility Locus at 20p11. Nat. Genet. 40, 1282–1284. doi:10.1038/ng.255
Roy, R. K., Thakur, M., and Dixit, V. K. (2008). Hair Growth Promoting Activity of Eclipta alba in Male Albino Rats. Arch. Dermatol. Res. 300, 357–364. doi:10.1007/s00403-008-0860-3
Sánchez, P., Serrano-Falcón, C., Torres, J. M., Serrano, S., and Ortega, E. (2018). 5α-Reductase Isozymes and Aromatase mRNA Levels in Plucked Hair from Young Women with Female Pattern Hair Loss. Arch. Dermatol. Res. 310, 77–83. doi:10.1007/s00403-017-1798-0
Shahinuzzaman, M., Akhtar, P., Amin, N., Ahmed, Y., Anuar, F. H., Misran, H., et al. (2021). New Insights of Phenolic Compounds from Optimized Fruit Extract of Ficus Auriculata. Sci. Rep. 11, 12503. doi:10.1038/s41598-021-91913-w
Sher, M., Hameed, A., Noreen, S., Fayyaz-Ur-Rehman, M., Hussain, M. A., and Bukhari, S. N. (2015). Extraction, Purification and Characterization of the Crystallin Protein of Cataractous Eye Lens Nucleus. Analyst 140, 6392–6397. doi:10.1039/c5an01212k
Srivilai, J., Minale, G., Scholfield, C. N., and Ingkaninan, K. (2019). Discovery of Natural Steroid 5 Alpha-Reductase Inhibitors. Assay Drug Dev. Technol. 17, 44–57. doi:10.1089/adt.2018.870
Tamura, Y., Takata, K., Eguchi, A., and Kataoka, Y. (2018). In Vivo monitoring of Hair Cycle Stages via Bioluminescence Imaging of Hair Follicle NG2 Cells. Sci. Rep. 8, 393–412. doi:10.1038/s41598-017-18763-3
Toyoshima, K. E., Asakawa, K., Ishibashi, N., Toki, H., Ogawa, M., Hasegawa, T., et al. (2012). Fully Functional Hair Follicle Regeneration through the Rearrangement of Stem Cells and Their Niches. Nat. Commun. 3, 784–812. doi:10.1038/ncomms1784
Wangkheirakpam, S. D., and Laitonjam, W. S. (2012). Comparative Study of Leaves of Ficus Pomifera Wall., Ficus Hispida Linn. And Ficus Religiosa Linn. Biochemical Contents, Minerals Trace Elements 3, 184–188.
Xiao, Q., Wang, L., Supekar, S., Shen, T., Liu, H., Ye, F., et al. (2020). Structure of Human Steroid 5α-Reductase 2 with Anti-androgen Drug Finasteride. Res. Sq 11, 5430. doi:10.21203/rs.3.rs-40159/v1
Yang, C. C., and Cotsarelis, G. (2010). Review of Hair Follicle Dermal Cells. J. Dermatol. Sci. 57, 2–11. doi:10.1016/j.jdermsci.2009.11.005
Keywords: androgenic alopecia, Ficus benghalensis, 5α-reductase, dihydrotestosterone, Minoxin, RP-HPLC
Citation: Iltaf J, Noreen S, Rehman MFu, Ghumman SA, Batool F, Mehdi M, Hasan S, Ijaz B, Akram MS and Butt H (2021) Ficus benghalensis as Potential Inhibitor of 5α-Reductase for Hair Growth Promotion: In Vitro, In Silico, and In Vivo Evaluation. Front. Pharmacol. 12:774583. doi: 10.3389/fphar.2021.774583
Received: 12 September 2021; Accepted: 01 November 2021;
Published: 07 December 2021.
Edited by:
Claudio Ferrante, University of Studies G. d'Annunzio Chieti and Pescara, ItalyReviewed by:
Jen-Tsung Chen, National University of Kaohsiung, TaiwanSengul Uysal, Erciyes University, Turkey
Copyright © 2021 Iltaf, Noreen, Rehman, Ghumman, Batool, Mehdi, Hasan, Ijaz, Akram and Butt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sobia Noreen, c29iaWEubm9yZWVuQHVvcy5lZHUucGs=
 Jawaria Iltaf
Jawaria Iltaf Sobia Noreen
Sobia Noreen Muhammad Fayyaz ur Rehman
Muhammad Fayyaz ur Rehman Shazia Akram Ghumman
Shazia Akram Ghumman Fozia Batool1
Fozia Batool1 Muhammad Mehdi
Muhammad Mehdi Bushra Ijaz
Bushra Ijaz