- Foot and Mouth Disease Virus Laboratory, Research and Development Centre, Indian Immunologicals Limited, Hyderabad, India
Foot-and-mouth disease (FMD) is an economically important contagious disease of livestock mainly cattle, buffalo, sheep, goats, and pig. There is limited data available on pathogenesis of foot and mouth disease in goats. In the study, the sheep and goats were infected experimentally with a serotype O foot-and-mouth disease virus by different challenge routes. The sheep and goats challenged by coronary band route and coronary band and intra-dermo-lingual route exhibited FMD clinical signs at 2–5 days post challenge. Whereas intra-dermo-lingual challenged sheep and goats did not exhibit FMD clinical signs. Live virus could be isolated from blood of infected sheep and goats at 2–5 days post challenge. Viral RNA could be detected from blood of infected sheep and goats at 1–10 days post challenge. The neutralizing antibody titre was detected at 10 days post challenge and maintained up to 35 days post challenge in all infected sheep and goats. Non structural protein (NSP) antibodies were detected as early as 5–10 days post challenge and remain positive up to 35 days post challenge in the infected sheep and goats. In conclusion, the pathogenesis of sheep and goats with serotype O foot and mouth disease virus by different challenge routes could be demonstrated.
Introduction
Foot-and-Mouth Disease (FMD) is an infectious disease which causes severe economic loss to the livestock sector (1). FMD is caused by FMD virus (FMDV), a member of family Picornaviridae and genus Aphthovirus affecting all the cloven footed animals. FMDV exists as seven distinct serotypes viz., O, A, C, Asia 1, Southern African territory 1(SAT1), SAT2, and SAT3. In India, incidence of FMD is reported throughout the country with the prevalence of FMDV serotypes O, A and Asia 1 (2). Cattle and buffalo are vaccinated biannually with inactivated FMD trivalent vaccine to control FMD in India. However, sheep and goats are not included in FMD control program (3, 4). Sheep and goats play an important role in the livelihood of a large percentage of small and marginal farmers and landless laborers in India. India constitutes around 148.88 million heads of goat population and 74.26 million heads of sheep population in the world. Moreover, cattle, buffalo, sheep and goats are grazed together in India (5). FMD outbreaks in sheep and goats are reported in India (6, 7).
There is paucity of information on the role of goats in FMD epidemiology and transmission. FMD infected sheep and goats transmitted the sub-clinical infection to cattle, buffalo, sheep and goats and FMD vaccination in sheep and goats could prevent the transmission of FMD to cattle, buffalo, sheep and goats (8). Usually, sheep and goats showed mild or unapparent FMD clinical signs (9, 10). Moreover, FMD infected goats showed typical oral and foot lesions in India (11). However, there is no detailed account of the pathogenesis of the disease in these small ruminants, especially in goats. This preliminary report describes pathogenesis of sheep and goats experimentally infected with type O foot and mouth disease virus using different challenge routes.
Materials and Methods
Cell Line and Viruses
Baby Hamster kidney (BHK) and primary bovine thyroid (BTY) cells were provided by the tissue culture laboratory at Research and Development Centre, Indian Immunologicals Limited (IIL), Hyderabad. BTY cells were grown using Hely cell growth medium supplemented with 10% adult bovine serum and antibiotics cocktail (penicillin, neomycin and polymyxin). O/IND/R2/75 virus was received from the virus seed laboratory, IIL, Hyderabad.
Experimental Animals
Eight Nellore sheep and eight Osmanabadi goats of either sex (6–12 months of age) were obtained from the holding farm of IIL, Hyderabad. These animals were reared in the farm from one month of age and were screened by three rounds of testing for FMDV-non-structural protein (NSP) antibodies using PrioCHECK® FMDV NS kit (Prionics Lelystad B.V., The Netherlands). All the animals were NSP seronegative in all the three tests. Additionally, the animals were tested for the absence of virus in the oesophagopharyngeal fluids (Probang samples) thrice by virus isolation on primary bovine thyroid cells (12) followed by antigen ELISA (13) and RT-PCR (14).
Challenge Virus Preparation
Challenge virus O/IND/R2/75 was prepared and titrated by standard methods as described previously (15).
Experimental Design
One sheep and goat each were inoculated with O/IND/R2/75 cattle challenge virus by intra-dermo-lingual, coronary band and by both sites in 0.1 ml quantity in each site. The animals were monitored for 24–72 h for signs of FMD (passage 1). For a second passage, epithelial tissue collected from vesicles was triturated in 0.04 M phosphate buffer followed by centrifugation at 3000 xg. The clear supernatant was used to inoculate one sheep and goat each by intra-dermo-lingual, coronary band and by both sites in 0.1 ml quantity in each site respectively. The animals were monitored for 24–72 h for signs of FMD. Two sheep and two goats was included as unchallenged control and maintained throughout the study period. Experiments were conducted in a bio-secure animal isolation unit at IIL, Hyderabad. The studies involving animals were reviewed and approved by Institutional Animal Ethics Committee, Indian Immunologicals Limited, Hyderabad and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Department of Animal Husbandry and Dairying, Government of India.
Clinical Scoring
The sheep and goats were observed for clinical signs of disease and temperatures recorded daily. A subjective scoring system (16) was used to evaluate the progression of disease in these animals with slight modification (8).
Sample Collection and Processing
Clotted blood for serology and NSP antibody was collected at days 0, 5, 10, 15, 21, 28, and 35 post-challenge. Heparinized blood was collected daily up to 10 dpc. Heparinized blood (200 μl) was mixed with 300 μl of lysis buffer (Roche Diagnostics, Germany) for analysis by real-time RT-PCR (qRT-PCR) and stored at −70°C. Heparinized blood (1 ml) was used for virus isolation (VI) (15).
Virus Isolation
Heparinized blood samples were examined for the presence of live virus by primary bovine thyroid (BTY) cell culture inoculation (9). BTY tubes were inoculated with 250 μl sample (5 tubes per sample) and incubated in a stationary position for 30 min at 37°C. The tubes were then gently washed with 0.04 M phosphate buffer containing antibiotics and 2 ml of virus maintenance medium was added prior to incubation at 37°C on roller drums. At 24, 48, and 72 h post inoculation, cell monolayer was examined for cytopathic effect (CPE). The presence of FMDV in cultures showing CPE was confirmed using an antigen ELISA (10). BTY cell culture supernatants from samples showing no sign of CPE after 72 h were pooled and re-passaged once and the absence of FMDV was confirmed by the antigen ELISA as mentioned above (15).
Virus Neutralizing Antibody Test (VNT)
Virus neutralization tests were performed for the sera in flatbottomed tissue culture grade micro titre plates (Nunclon™, Denmark) as described previously (17). Antibody titres were expressed as the reciprocal of the final dilution of serum in the serum/virus mixture which neutralized an estimated 100 TCID50 of virus at the 50% end-point (18).
Non Structural Protein Antibody Test
Antibodies to FMDV NSP 3ABC were tested using PrioCHECK®FMDV NS kit (Prionics Lelystad B.V., The Netherlands) (19).
Quantitative Real-Time RT-PCR Assay for Detection of Viral RNA
The amount of viral RNA in blood was quantified by qRT-PCR (20). The total nucleic acid was extracted from liquid samples with MagNApure LC total nucleic acid isolation kit (Roche Diagnostics GmbH, Germany) using an automated nucleic acid robotic workstation (MagNApure LC, Roche Diagnostics GmbH, Germany). For the generation of standard curves, a FMDV RNA standard was synthesized in vitro from a plasmid containing a 79 base pair insert of the internal ribosomal entry site (IRES) of a type O FMDV (kindly provided by Dr. Donald P. King, Institute for Animal Health, UK) using a MEGAscript® T7 kit (Ambion, USA) as described previously (21) in an IQ®5 Multicolor Real-time PCR detection system (BioRad, USA). The results from all samples were analyzed using Bio-Rad iQ®5 optical system software and CT values were assigned to each reaction (18). Viral RNA was quantified using a standard curve derived from the standard RNA preparation at different concentrations (108-101) (22).
Results
Development of Clinical FMD
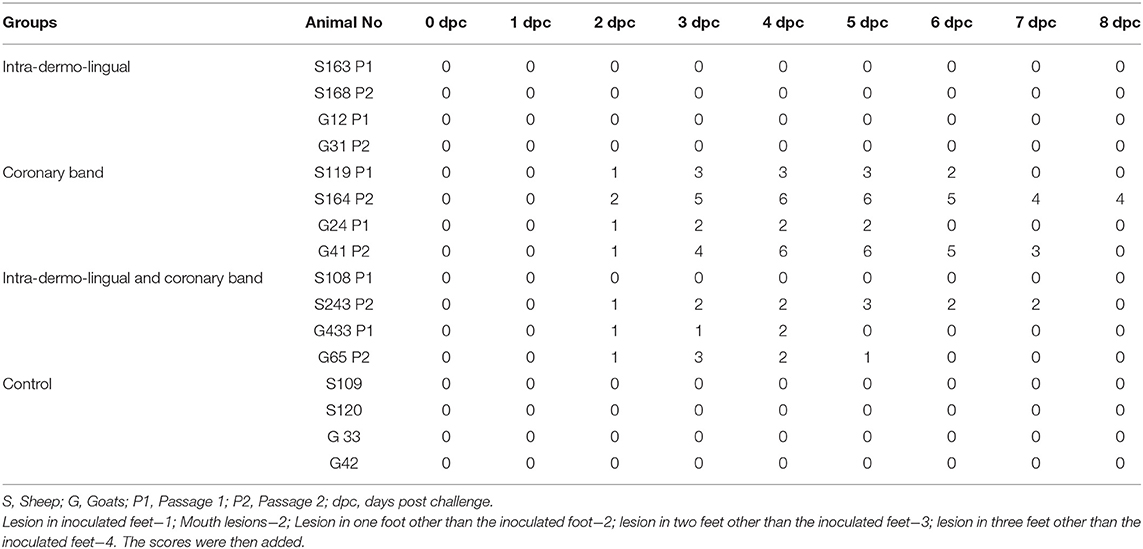
Sheep inoculated by intra-dermo-lingual route did not exhibit clinical signs of FMD in both passages. Sheep inoculated by coronary band route showed clinical signs of FMD such as inappetance, panting, pyrexia (≥40 °C) (Figure 1A), lameness and vesicles in foot and mouth at 2–5 dpc. Sheep inoculated by both intra-dermo-lingual/coronary band routes showed FMD clinical signs at 5 dpc. Goats inoculated by intra-dermo-lingual route did not exhibit clinical signs of FMD in both passages. Goats inoculated by coronary band route showed clinical signs of FMD such as inappetance, panting, pyrexia (≥40 °C) (Figure 1B), lameness and vesicles in foot and mouth at 2–5 dpc. Goats inoculated by both intra-dermo-lingual/coronary band routes also showed FMD clinical signs at 3 dpc. The unchallenged control sheep and goats did not show any FMD clinical signs (Table 1).
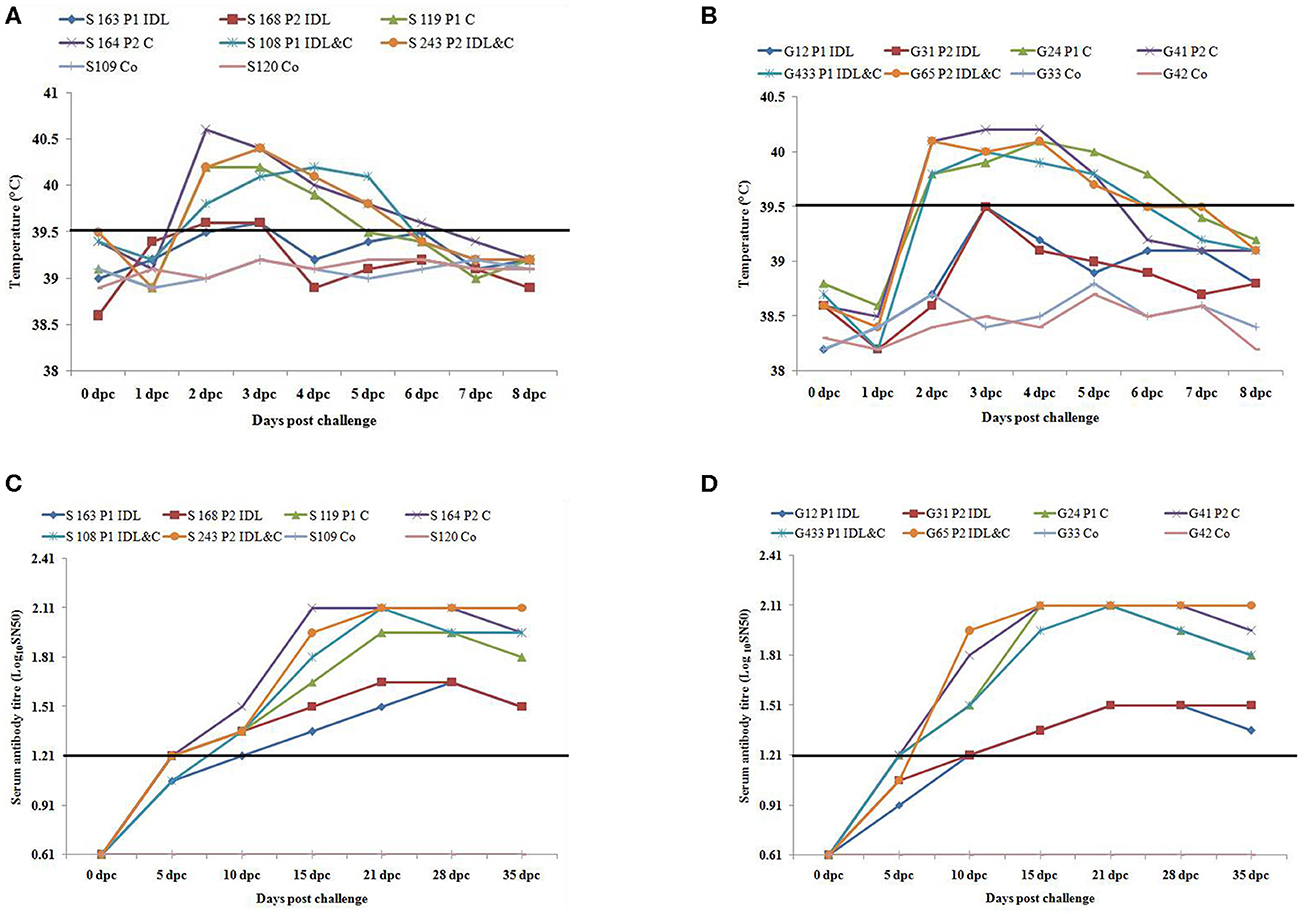
Figure 1. (A) Rectal temperature (°C) of challenged (intra-dermo-lingual (IDL), coronary band (C) and by both route) and control sheep. P1, passage 1; P2, Passage 2. Solid line indicates normal temperature of sheep. (B). Rectal temperature (°C) of challenged (intra-dermo-lingual (IDL), coronary band (C) and by both route) and control goats. P1, passage 1; P2, Passage 2. Solid line indicates normal temperature of goats. (C) Virus neutralization titres of challenged and control sheep (Expressed as the log10 reciprocal antibody dilution required for 50% neutralization of 100 tissue culture infectious units). Solid line indicates neutralizing antibody titre >1.2 log10SN50 is considered positive. (D) Virus neutralization titres of challenged and control goats (Expressed as the log10 reciprocal antibody dilution required for 50% neutralization of 100 tissue culture infectious units). Solid line indicates neutralizing antibody titre >1.2 log10SN50 is considered positive.
Detection of Virus/ Virus Nucleic Acid in Blood
In passage 1, virus could not be isolated form challenged sheep and goats (S163, S119, S108, G12, G24, and G433) irrespective of challenge routes. In passage 2, infectious virus was isolated form intra-dermal-lingual route challenged sheep (S168) and goat (G31) at 3 dpc and coronary band challenged sheep (S164) and goat (G41) at 2–5 dpc. Whereas in intra-dermal-lingual and coronary band route challenged sheep (S243) was positive for virus isolation at 4–5 dpc. In the case of intra-dermal-lingual and coronary band route challenged goat (G65) virus was isolated on 2 and 5 dpc.
Viral RNA (108.44–109.45 viral RNA copy numbers/ml of blood) was detected as early as 1 dpc from the sheep (S119P1 and S164P2) and goat (G41P2) inoculated by coronary route. Viral RNA (10 9.42-10 10.47) was detected in all the inoculated sheep between 1 and 10 dpc irrespective of challenge routes. Viral RNA (10 7.42-10 9.82) was detected in all the inoculated goats between 1 and 10 dpc. All the unchallenged control sheep and goats were negative for virus isolation and viral RNA from blood samples (Table 2).
Table 2. Virus isolation and quantification of FMD viral RNA copy numbers (Log10 RNA copy numbers/ ml of blood) from blood of challenged and control sheep and goats.
FMDV NSP Antibody Response
Four inoculated sheep (S163, S119, S108 and S243) were positive for NSP antibody on 10 dpc while other sheep NSP antibodies were observed on 15–35 dpc. Three inoculated goats (G12, G31 and G433) were positive for NSP antibody on 5 dpc while in other goats NSP antibodies were observed on 15–35 dpc. Both the unchallenged control sheep and goats were negative for NSP antibody up to 35 dpc (Table 3).
Virus Neutralizing Antibody Response
The neutralizing antibody titer was detected in all inoculated sheep and goats at 10 dpc (> 1.2 log10SN50). However, the highest neutralizing antibody titer was detected between 10 and 35 dpc (2.1 log10SN50) in sheep and goats inoculated by coronary and both by coronary and intra-dermo- lingual route. Both the unchallenged control sheep and goats had no serum neutralizing antibody titre up to 35 dpc (Figures 1C,D).
Discussion
The experiment described the preliminary results on pathogenesis and development of FMD in sheep and goats by inoculating the type O FMD virus in three different challenged routes. The development of clinical signs was observed. The viral RNA levels in blood were quantified.
The incubation period of natural FMDV infection is normally between 3 and 8 days in sheep (23), but can be as short as 24 h following experimental infection (23, 24). In the current study, lesions were evident in three sheep and four goats on the 2nd day of challenge.
Sheep and goats inoculated by coronary band route showed clinical signs of FMD such as inappetance, panting, pyrexia (≥40 °C), lameness and vesicles in foot and mouth at 2–5 dpc. This finding was in accordance with the earlier experiments in sheep (25–27) and goats (28). Hughes et al. (29) reported intra nasal inoculation of FMDV also resulted in generalized infection in sheep. Sheep and goats inoculated by both intra-dermo-lingual/coronary band routes also showed FMD clinical signs whereas sheep and goats inoculated by intra-dermo-lingual route did not produce clinical signs of FMD. It should be noted that although sheep and goats inoculated by the intra-dermo-lingual route showed no signs of generalized infection, virus could be isolated from blood samples and viral RNA was detected in blood samples up to 10 dpc. Furthermore, Lazarus, et al. (30) presented that indigenous South African goats manifested FMD clinical signs by challenging intra-dermo-lingual route with SAT1 virus pool. However, the clinical signs of FMD may be influenced by the virus strain and the breed of sheep and goats (31). In the present study, type O virus and Indian breed of sheep and goats were used. This may be the reason for intra-dermo-lingual challenged sheep and goats did not show the clinical signs of FMD.
Intra-dermo-lingual route of inoculation in cattle, dental pad/gum route of inoculation in buffalo (32) and intra dermal inoculation in the heel bulb in pigs (33) of FMDV resulted in generalized disease.
Ryan et al. (27) reported that all inoculated ewes developed viraemia at 1 dpi and viral RNA levels then peaked at 2 dpi. In the current study, viral RNA was detected as early as 1dpc, viral RNA level then peaked at 2–5 dpc from the inoculated sheep and goats. Virus was isolated from blood of inoculated sheep and goats up to 2–5 dpc as reported by Parida et al. (34).
Infection with live foot and mouth disease virus induces non structural antibody response in animals (35). Earlier studies (15, 34) revealed antibodies against 3ABC in sheep at 10 dpc. In the current experiment, all the sheep were positive for NSP antibodies on 10 dpc and continued up to the end of the experiment (35 dpc). In case of goats NSP antibodies were detected as early as 5 dpc and continued up to the end of the experiment (35 dpc). This finding was in accordance with the earlier experiment in sheep and goats (8, 36).
In the present study, neutralizing antibody titre was detected in all the inoculated sheep and goats at 10 dpc and the peak antibody titre was detected between 10 and 35 dpc. Dellers et al. (25) reported neutralizing antibodies were first detected 60 h post inoculation and initial peak titers occurred by 10th day in inoculated sheep.
In this study statistical analysis could not be carried out due to the small number of animals in each group (n = 2). In coronary band and Intra-dermo-lingual challenge group of animals received double the dose of challenge virus (0.2 ml) against the Intra-dermo-lingual challenge group (0.1 ml) and coronary band challenge group (0.1 ml), respectively. These are the limitations of this study. So, further study with increased number of animals and statistical analysis is warranted to confirm this result.
Conclusion
The sheep and goats were infected experimentally with a serotype O foot-and-mouth disease virus by different challenge routes. The sheep and goats challenged by coronary band route and coronary band and intra-dermo-lingual route exhibited FMD clinical signs at 2–5 days post challenge. Whereas intra-dermo-lingual challenged sheep and goats did not exhibit FMD clinical signs. Live virus could be isolated from blood of infected sheep and goats at 2–5 days post challenge. Viral RNA could be detected from blood of infected sheep and goats at 1–10 days post challenge. The neutralizing antibody titre was detected at 10 days post challenge and maintained up to 35 days post challenge in all infected sheep and goats. NSP antibodies were detected as early as 5–10 days post challenge and remain positive up to 35 days post challenge in the infected sheep and goats. In conclusion, the pathogenesis of sheep and goats with serotype O foot and mouth disease virus by different challenge routes could be demonstrated.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Ethics Statement
The studies involving animals were reviewed and approved by Institutional Animal Ethics Committee, Indian Immunologicals Limited, Hyderabad and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Department of Animal Husbandry and Dairying, Government of India.
Author Contributions
SV designed, coordinated, reviewed and corrected the manuscript. MM and NS performed the experiments, completed analysis and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
MM, NS, and SV were employed by the company Indian Immunologicals Limited, Hyderabad, India.
References
2. ICAR- D FMD Annual Report 2017. ICAR- Directorate on Foot-and-Mouth Disease. Nainital: Indian Veterinary Research Institute.
3. Patil PK, Bayry J, Ramakrishna C, Hugar B, Misra LD, et al. Immune response of goats against foot-and-mouth disease quadrivalent vaccine: comparison of double oil emulsion and aluminium hydroxide gel vaccine in eliciting immunity. Vaccine. (2002) 20:2781–89. doi: 10.1016/S0264-410X(02)00184-6
4. Patil PK, Bayry J, Ramakrishna C, Hugar B, Misra LD, Prabhudas K, et al. Immune responses of sheep to quadrivalent double emulsion foot-and-mouth disease vaccines: rate of development of immunity and variations among other ruminants. J Clin Microbiol. (2002) 40:4367–71. doi: 10.1128/JCM.40.11.4367-4371.2002
5. Madhanmohan M, Tresamol PV, Saseendranath MR. Immune response in goats to two commercial foot-and-mouth disease vaccines and the assessment of maternal immunity in their kids. Transbound and Emerg Dis. (2009) 56:49–53. doi: 10.1111/j.1865-1682.2008.01056.x
7. Misra N, Mishra S, Bhagwan PSK. Occurrence of an outbreak of FMD in an organized goat farm. Indian J Comp Microbiol immunol Infect Dis. (1997) 18:188.
8. Madhanmohan M, Nagendrakumar SB, Srinivasan VA. Protection against direct in-contact challenge following foot-and-mouth disease vaccination in sheep and goats: the effect on virus excretion and carrier status. Vet Res Commun. (2010) 34:285–99. doi: 10.1007/s11259-010-9353-x
9. Kitching RP, Hughes GJ. Clinical variation in foot and mouth disease. Sheep and goats. Rev Sci Tech Off Int Epiz. (2002) 21:505–12. doi: 10.20506/rst.21.3.1342
11. Shankar H, Sharma SK, Singh SV, Singh N, Gupta VK. FMD in small ruminants: some epidemiological observations. Indian Vet J. (1998) 14:21–5.
12. Snowdon WA. Growth of foot-and-mouth disease virus in monolayer cultures of calf thyroid cells. Nature. (1966) 210:1079–80. doi: 10.1038/2101079a0
13. Ferris NP, Dawson M. Routine application of enzyme-linked immunosorbent assay in comparison with complement fixation for the diagnosis of foot-and-mouth and swine vesicular diseases. Vet Microbiol. (1988) 16:201–9. doi: 10.1016/0378-1135(88)90024-7
14. Reid SM, Forsyth MA, Hutchings GH, Ferris NP. Comparison of reverse transcription polymerase chain reaction, enzyme linked immunosorbent assay and virus isolation for the routine diagnosis of foot-and-mouth disease. J Virol Methods. (1998) 70:213–17. doi: 10.1016/S0166-0934(97)00181-X
15. Madhanmohan M, Nagendrakumar SB, Lakshmi MN, Srinivasan VA. Effect of FMD vaccine antigen payload on protection, sub-clinical infection and persistence following needle challenge in sheep. Comp Immunol Microbiol Infect Dis. (2010) 33:e7–e13. doi: 10.1016/j.cimid.2009.10.001
16. Quan M, Murphy CM, Zhang Z, Alexandersen S. Determinants of early foot-and-mouth disease virus dynamics in pigs. J Comp Pathol. (2004) 131:294–307. doi: 10.1016/j.jcpa.2004.05.002
17. Golding SM, Hedger RS, Talbot P. Radial immuno-diffusion and serum neutralization techniques for the assay of antibodies to swine vesicular disease. Res Vet Sci. (1976) 20:142–7. doi: 10.1016/S0034-5288(18)33445-3
18. Karber G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Arch Exp Pathol Pharmakol. (1931) 162:480–7. doi: 10.1007/BF01863914
19. Sorensen KJ, Madsen KG, Madsen ES, Salt JS, Nqindi J, Mackay DKJ. Differentiation of infection from vaccination in foot and-mouth disease by the detection of antibodies to the non-structural proteins 3D 3AB and 3ABC in ELISA using antigens expressed in Baculovirus. Arch Virol. (1998) 143:1461–76. doi: 10.1007/s007050050390
20. Shaw AE, Reid SM, Ebert K, Hutchings GH, Ferris NP, King DP. Implementation of a one-step realtime RT-PCR protocol for diagnosis of foot-and-mouth disease. J Virol Methods. (2007) 143:81–5. doi: 10.1016/j.jviromet.2007.02.009
21. Reid SM, Ferris NP, Hutchings GH, Zhang Z, Belsham GJ, Alexandersen S. Detection of all seven serotypes of foot- and-mouth disease virus by real-time, fluorogenic reverse transcription polymerase chain reaction assay. J Virol Methods. (2002) 105:67–80. doi: 10.1016/S0166-0934(02)00081-2
22. Madhanmohan M, Nagendrakumar SB, Kumar R, Anilkumar J, Manikumar K, Yuvaraj S, et al. Clinical protection, sub-clinical infection and persistence following vaccination with extinction payloads of O1 Manisa foot-and-mouth disease monovalent vaccine and challenge in goats and comparison with sheep. Res Vet Sci. (2012) 93:1050–9. doi: 10.1016/j.rvsc.2011.10.006
24. Alexandersen S, Donaldson AI. Further studies to quantify the dose of natural aerosols of foot and mouth disease virus for pigs. Epidemiol and Infect. (2002) 128:313–23. doi: 10.1017/S0950268801006501
25. Dellers RW, Hyde JL. Response of sheep to experimental infection with foot-and-mouth disease virus. American J Vet Res. (1964) 25:469–89.
26. Burrows R. Excretion of foot-and-mouth disease virus prior to the development of lesions. Vet Record. (1968) 83:387–88.
27. Ryan E, Zhang Z, Brooks HW, Horsington J, Brownlie J. Foot-and-mouth disease virus crosses the placenta and causes death in fetal lambs. J Comp Pathol. (2007) 136:256–65. doi: 10.1016/j.jcpa.2007.03.001
28. Onozato H, Fukai K, Kitano R, Yamazoe R, Kazuki K, Yamada M, et al. Experimental infection of cattle and goats with a foot-and-mouth disease virus isolate from the 2010 epidemic in Japan. Arch Virol. (2014) 159:2901–8. doi: 10.1007/s00705-014-2135-y
29. Hughes GJ, Mioulet V, Kitching RP, Woolhouse ME, Alexandersen S, Donaldson AI. Foot-and-mouth disease virus infection of sheep: implications for diagnosis and control. Vet Record. (2002) 150:724–27. doi: 10.1136/vr.150.23.724
30. Lazarus DD, Mutowembwa PB, Sirdar MM, Rametse TM, Heath L, Opperman PA, et al. Clinical presentation of FMD virus SAT1 infections in experimentally challenged indigenous South African goats. Small Ruminant Res. (2019) 180:15–20. doi: 10.1016/j.smallrumres.2019.09.014
31. Geering WA, Foot and mouth disease in sheep. Australian Vet J. (1967) 43:485–9. doi: 10.1111/j.1751-0813.1967.tb04774.x
32. Maroudam V, Nagendrakumar SB, Madhanmohan M, Santhakumar P, Thiagarajan D, Srinivasan VA. Experimental transmission of foot-and-mouth disease among Indian buffalo (Bubalus bubalis) and from buffalo to cattle. J Comp Pathol. (2008) 139:81–5. doi: 10.1016/j.jcpa.2008.05.003
33. Parida S, Fleming L, Oh Y, Mahapatra M, Hamblin P. Reduction of foot-and-mouth disease (FMD) virus load in nasal excretions, saliva and exhaled air of vaccinated pigs following direct contact challenge. Vaccine. (2007) 25:7806–17. doi: 10.1016/j.vaccine.2007.08.058
34. Parida S, Fleming L, Oh Y, Mahapatra M, Hamblin P, Gloster J, et al. Emergency vaccination of sheep against foot-and-mouth disease: Significance and detection of subsequent sub-clinical infection. Vaccine. (2008) 26:3469–79. doi: 10.1016/j.vaccine.2008.04.026
35. Paton DJ, de Clercq K, Greine M, Dekker A, Brocchi E. Application of non-structural protein antibody tests in substantiating freedom from foot-and-mouth disease virus infection after emergency vaccination of cattle. Vaccine. (2006) 42–43:6503–12. doi: 10.1016/j.vaccine.2006.06.032
36. Madhanmohan M, Nagendrakumar SB, Santhakumar P, Thiagarajan D, Narasu ML, Srinivasan VA. Immune response in goats to different payloads of FMDV monovalent vaccine: protection against virulent challenge and development of carrier status. Indian J Microbiol. (2011) 51:88–93. doi: 10.1007/s12088-011-0101-x
Keywords: foot-and-mouth disease, serotype O, experimental infection, sheep, goats
Citation: Muthukrishnan M, Singanallur Balasubramanian N and Villuppanoor Alwar S (2020) Experimental Infection of Foot and Mouth Disease in Indian Sheep and Goats. Front. Vet. Sci. 7:356. doi: 10.3389/fvets.2020.00356
Received: 06 April 2020; Accepted: 22 May 2020;
Published: 25 June 2020.
Edited by:
Alejandra Victoria Capozzo, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Stéphan Zientara, Agence Nationale de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail (ANSES), FranceFrancois Frederick Maree, Agricultural Research Council of South Africa (ARC-SA), South Africa
Copyright © 2020 Muthukrishnan, Singanallur Balasubramanian and Villuppanoor Alwar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madhanmohan Muthukrishnan, bXV0aHVrcmlzaG5hbi5tYWRoYW5tb2hhbkBnbWFpbC5jb20=
†Present address: Nagendrakumar Singanallur Balasubramanian, FMD Risk Management Project, CSIRO Health & Bio-Security, Australian Centre for Disease Preparedness, Geelong, VIC, Australia
Srinivasan Villuppanoor Alwar, National Dairy Development Board, Hyderabad, India
Madhanmohan Muthukrishnan, Veterinary University Training and Diagnostic Centre, Tamil Nadu Veterinary and Animal Sciences University, Madurai, India
 Madhanmohan Muthukrishnan
Madhanmohan Muthukrishnan Nagendrakumar Singanallur Balasubramanian
Nagendrakumar Singanallur Balasubramanian Srinivasan Villuppanoor Alwar†
Srinivasan Villuppanoor Alwar†