- 1Department of Oncology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
- 2Department of Respiratory and Critical Care Medicine, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
- 3Department of Pathology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
The prevalence of Anaplastic Lymphoma Kinase gene (ALK) fusion is about 5% among patients with lung adenocarcinoma, underscoring the importance of pinpointing distinct fusion variants for optimizing treatment approaches. This is the first reported case of a 74-year-old female with stage IV lung adenocarcinoma, featuring a novel Kinesin Family Member 13A (KIF13A)-ALK fusion, identified via next-generation sequencing (NGS) and confirmed with fluorescence in situ hybridization (FISH). Initially undergoing chemotherapy and then crizotinib, she achieved a partial response (PR) before progressing with multiple bone metastases. However, subsequent treatment with alectinib as a third-line option yielded positive results. A stable disease state persisted for an impressive 31 months of progression-free survival (PFS), accompanied by minimal toxicity symptoms. Up until now, a remarkable near 4-year span of overall survival (OS) has been consistently observed and monitored. This report of a KIF13A-ALK fusion case benefit significantly from alectinib with extensive follow-up. The case diversifies the array of ALK fusion partners and holds clinical relevance in refining therapeutic choices for KIF13A-ALK fusion-associated lung cancer.
Introduction
The therapeutic landscape of lung adenocarcinoma has undergone a profound shift, catalyzed by the discovery of diverse gene fusions that drive tumorigenesis. Among these, Anaplastic Lymphoma Kinase gene (ALK) rearrangements have emerged as pivotal targets for precision medicine due to their central role in oncogenesis. These well-described driver mutations exist in approximately 5% of non-small-cell lung cancers (NSCLCs) (Devarakonda et al., 2015). Patients with ALK fusions can experience substantial benefits from ALK tyrosine-kinase inhibitors (TKIs) like alectinib. Notably, it is imperative to recognize that distinct ALK fusion partners generate heterogeneous responses to ALK TKIs (Chuang et al., 2021; Sun et al., 2021). As a result, identifying specific fusion variants assumes paramount importance in refining treatment strategies. Kinesin Family Member 13A (KIF13A), a constituent of the kinesin superfamily of motor proteins, has garnered attention for its active participation in intracellular transport and cargo trafficking within cells (Zhang et al., 2018). Within the scope of this study, we present a noteworthy discovery: the identification of an unreported fusion involving KIF13A and ALK in a patient with lung adenocarcinoma. This KIF13A-ALK fusion displays exceptional sensitivity to alectinib treatment, leading to prolonged survival and an enduring favorable clinical response. In addition to advancing our understanding of the phenomenon, this report underscores the promising therapeutic implications for patients carrying this fusion.
Case report
A 60-year-old non-smoking Chinese female was admitted to our hospital in October 2019 with a one-month history of cough, sputum production, and dyspnea. Computed tomography (CT) scan revealed pulmonary lesions in the left main bronchus and lower right lobe invading mediastinum, concomitant with widespread metastases involving multiple mediastinal lymph nodes, the liver, and multiple bones. Diagnostic bronchoscopy revealed an uneven and congestive polypoid mass with luminal obstruction of the right principal bronchus, electrocautery based on bronchoscopy was applied for central airway obstruction (Figures 1A, B). Chest CT and pathological examination of a bronchoscopic biopsy showed poorly differentiated invasive adenocarcinoma. (Figures 2A, B). Immunohistochemical (IHC) analysis revealed positive staining for Napsin A and thyroid transcription factor 1 (TTF1) which have been widely used as sensitive markers of pulmonary adenocarcinoma (2) (Figures 2C, D). The patient’s condition was classified as Stage IV (cT4N3M1c) adenocarcinoma.
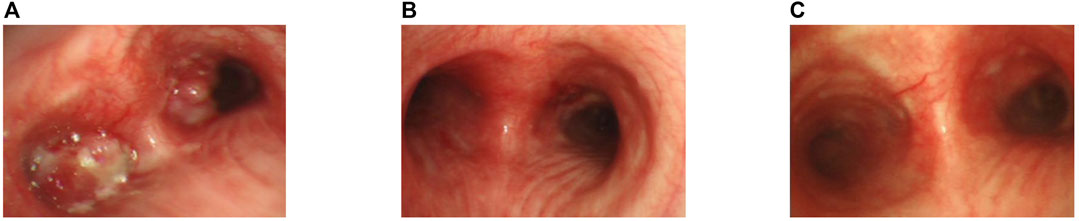
FIGURE 1. Bronchoscopic view of the patient. (A) Bronchoscopic view at the carinal level, illustrating obstructive masses within the bilateral bronchi and the left main bronchus.(B) After the apply of bronchoscopy electrocautery for central airway obstruction.(C) Two months following the commencement of alectinib treatment.
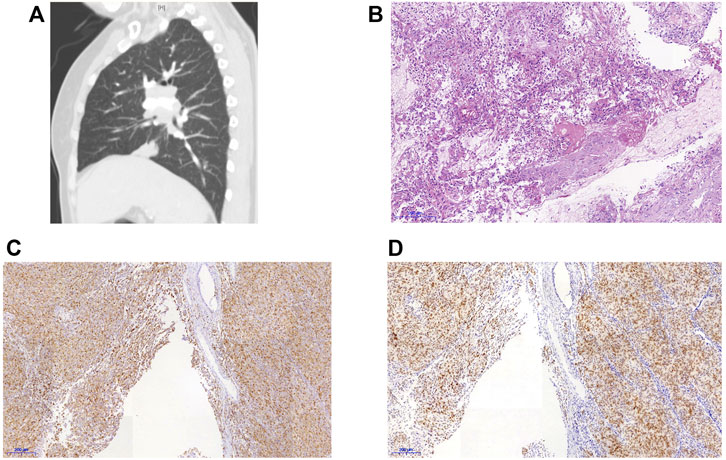
FIGURE 2. CT scan and lung biopsies of the reported patient. (A) CT findings of the patient (B)HE staining of the biopsy specimen, 40×. (C) Immunohistochemical staining for Napsin A. (D) Immunohistochemical staining for thyroid transcription factor-1 (TTF-1).
The tumor tissue acquired during biopsy was sent for genomic testing by next-generation sequencing (NGS) based on a pan-cancer 1021-gene panel containing whole exons and selected introns of 288 genes and selected regions of 733 genes, a novel KIF13A-ALK fusion was identified (abundance: 11.4%). This fusion encompassed exons 1-18 of KIF13A on chromosome 6 and exons 20-29 of ALK on chromosome 3 (Figures 3A, B). Notably, no concurrent driver alterations, including those in EGFR, ROS1, BRAF, KRAS, MET, or RET genes, were detected. Furthermore, immunocytochemistry (IHC) and fluorescence in situ hybridization (FISH) were also performed to verify the above mutation (Figures 3C, D).
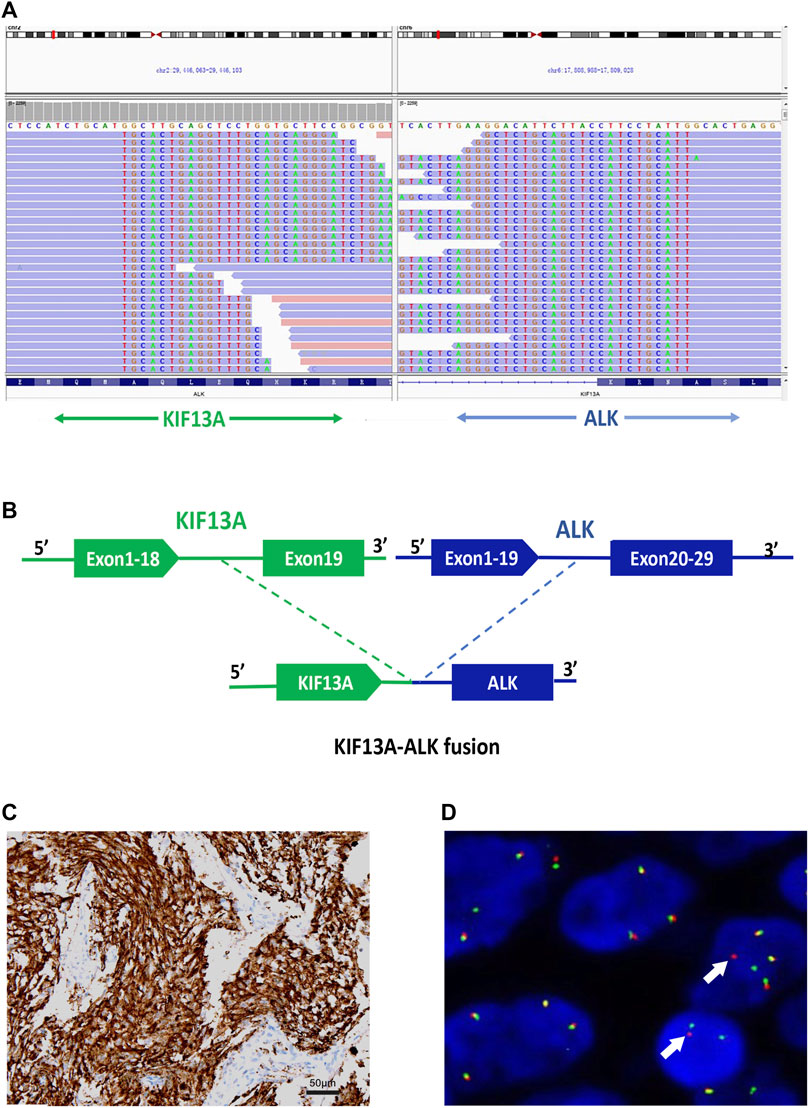
FIGURE 3. Identification of the KIF13A-ALK fusion (A) KIF13A-ALK fusion was validated manually using the Integrated Genomics Viewer; (B) A novel intergenic region between exons 1-18 of KIF13A on chromosome 6 and exons 20-29 of ALK on chromosome 2 fusion variant was identified. (C) Validation of ALK expression via IHC staining (40×), (D) Fluorescence in situ hybridization (FISH) analysis utilizing the ALK split apart probe, demonstrating distinct split signals (indicated by arrows) suggestive of a rearrangement of the ALK gene.
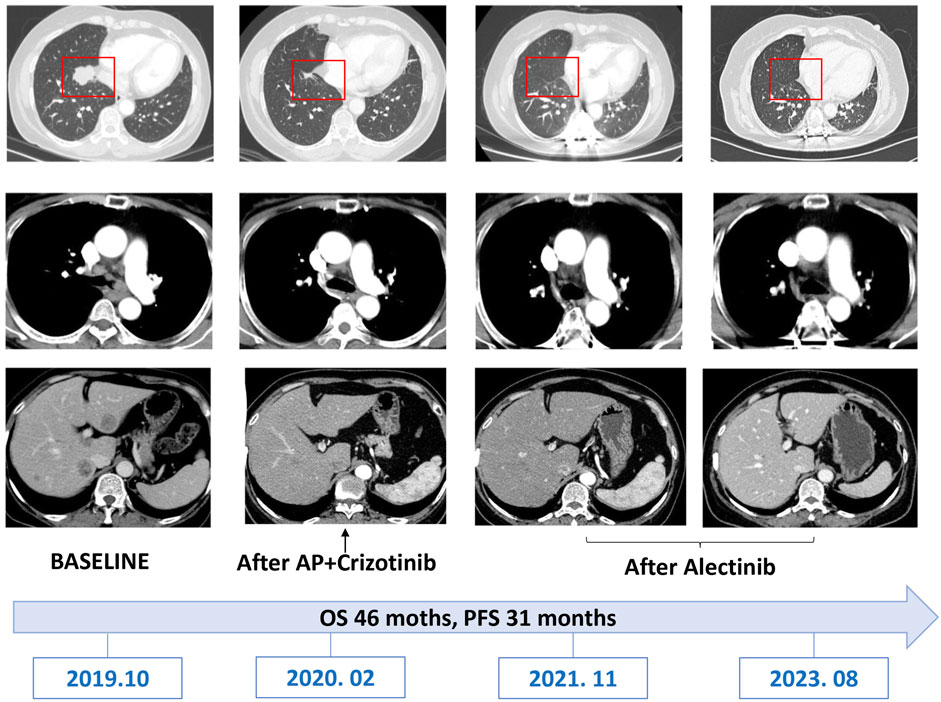
Given the novelty of this ALK fusion, its specific response to ALK tyrosine kinase inhibitors (TKIs) remained uncertain, further accentuated by the patient’s lack of insurance coverage for ALK TKIs. After considering the patient’s preferences, a therapeutic approach comprising six cycles of first-line chemotherapy with pemetrexed (500 mg/m2 every 3 weeks) and cisplatin (75 mg/m2 every 3 weeks) was initiated in October 2019. Notably, complete resolution of symptoms was achieved. Subsequent evaluations via CT scan and bronchoscopy in December evidenced a partial response (PR) across all lesions, accompanied by nausea and a reduction in white blood cell count (2.2 × 109/L) classified as grade 2 according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Regrettably, following the completion of six cycles of chemotherapy, the patient experienced disease progression (PD) attributed to the emergence of additional bone metastases. In response, the patient was transitioned to crizotinib at a dose of 250 mg twice daily. However, after 1 month of crizotinib treatment, imaging studies revealed further progression of bone metastases. Consequently, crizotinib was discontinued, and the patient was subsequently administered alectinib therapy (600 mg twice daily) in conjunction with radiation therapy for thoracic vertebral metastasis.
Two months following the commencement of alectinib treatment, the patient had a PR in all lesions including mediastinal lymph nodes and liver metastases. These promising clinical responses were consistently observed in subsequent follow-up assessments (Figures 1C, 4). Notably, metabolic activity in bone metastases subsided, and the absence of new lesions was confirmed. As of the current stage of manuscript preparation (August 2023), the patient continues to undergo regular follow-up visits, maintaining a state of stable disease during alectinib treatment. Impressively, the patient has achieved a progression-free survival (PFS) exceeding 31 months. Importantly, no significant drug-related adverse events have been observed to date.
Discussion
To our best understanding, this is the first report documenting a case of stage IV lung adenocarcinoma characterized by a previously unreported KIF13A-ALK fusion, identified through NGS, FISH and IHC staining. Furthermore, this study provides the initial clinical substantiation of a patient harboring the KIF13A-ALK fusion who benefits from alectinib.
Kinesin family member 13A (KIF13A) constitutes a member of the kinesin-3 subfamily of microtubule-based motor proteins, engaging in the vesicular transport from the trans-Golgi network to the plasma membrane. Widely expressed in the central nervous system, KIF13A plays an indispensable role in the normal development of intervertebral discs. The fusion protein, KIF13A-ALK, retains coiled-coil domains (CCDs) within its N-terminal region, a feature detected in our case (Nakagawa et al., 2000). It is noteworthy that, as observed in other fusion configurations, this KIF13A CCD is projected to be integral for the activation of the ALK oncogene via homodimerization and kinase activation. Notably, oncogenic fusion between KIF13A (exon 18) and the RET gene has been reported in a distinct case report (Zhang et al., 2018); however, the fusion event with ALK remains undocumented. It is prudent to regard KIF13A-ALK as an oncogenic genetic alteration, meriting inclusion in the catalog of ALK fusion variants.
ALK fusion events have unequivocally demonstrated their significance as critical targetable oncogenic drivers. The most prevalent ALK fusion partner is EML4-ALK (88.9%) (Solomon et al., 2014). The expanding adoption of NGS-based testing has augmented the identification of less common ALK fusions, resulting in the documentation of over 90 diverse ALK fusion protein partners (Ou et al., 2020). However, there persists an ongoing debate surrounding the imperative question of whether infrequent ALK fusion variants warrant targeted therapies, given the disparate responses exhibited by diverse ALK fusion variants to ALK tyrosine kinase inhibitors (TKIs) (Chuang et al., 2021; Sun et al., 2021).
Historically, crizotinib held precedence as the favored first-line treatment for ALK-positive NSCLC patients, as it demonstrated superior treatment responses, extended overall survival, and prolonged progression-free survival (Ou et al., 2020). In light of uncertainties regarding the response profile of new variants to ALK TKIs, compounded by economic considerations, we opted for chemotherapy as the initial therapeutic strategy. Although the patient displayed a clinical response subsequent to chemotherapy, disease progression manifested after the completion of six cycles. Regrettably, the patient’s condition deteriorated within a month of commencing crizotinib treatment, aligning with previous reports that highlighted the rapid emergence of resistance and consequent tumor relapse as limitations of crizotinib (Chuang and Neal, 2015). Following crizotinib resistance, the patient was transitioned to alectinib therapy. The second-generation inhibitor alectinib exhibited superiority over crizotinib, characterized by lower mean inhibitory concentrations (CI50) against native ALK kinase and broader coverage against ALK resistance mutations (Samacá-Samacá et al., 2023). Besides, clinicians have previously reported that ALK-positive NSCLS patients achieved long-lasting responses from the use of alectinib (Camidge et al., 2019). Our case demonstrated the potential sensitivity of the new KIF13A-ALK fusion variant to alectinib, as opposed to crizotinib. Moreover, the patient’s sustained long-lasting response (progression-free survival exceeding 31 months) suggests a rationale for considering alectinib as the optimal therapeutic selection for KIF13A-ALK fusion-positive cases.
In conclusion, our report stands as the pioneer documentation of the novel KIF13A-ALK fusion variant, thereby enriching the spectrum of recognized ALK fusions. This contribution also bears valuable implications for therapeutic decision-making in this rare subtype. Subsequent work should further elucidate the biological characteristics and optimal treatment interventions for lung cancers bearing the KIF13A-ALK fusion gene.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by the Beijing Tsinghua Changgung Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft. CC: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Writing–review and editing. JY: Investigation, Software, Writing–original draft. JZ: Investigation, Methodology, Writing–original draft. MZ: Conceptualization, Data curation, Investigation, Methodology, Writing–original draft. HL: Methodology, Writing–original draft. XM: Conceptualization, Supervision, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. None.
Acknowledgments
We thank the patients; the nurses and clinical staff who are providing care for the patients in Beijing Tsinghua Changgung Hospital. We would like to also thank the patient for providing written, informed consent for the inclusion of the case details presented in this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALK, Anaplastic lymphoma kinase; CCDs, coiled-coil domains; CT, Computed tomography; FFPE, formalin-fixed paraffin-embedded; FISH, fluorescence in situ hybridization; IHC, Immunohistochemical; KIF13A, Kinesin Family Member 13A; NGS, next-generation sequencing; NSCLCs, non-small-cell lung cancers; OS, overall survival; PFS, progression-free survival; PD, disease progression; PR, partial response; TKIs, tyrosine-kinase inhibitors; TTF1, thyroid transcription factor 1.
References
Camidge, D. R., Dziadziuszko, R., Peters, S., Mok, T., Noe, J., Nowicka, M., et al. (2019). Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J. Thorac. Oncol. 14 (7), 1233–1243. doi:10.1016/j.jtho.2019.03.007
Chuang, C. H., Chen, H. L., Chang, H. M., Tsai, Y. C., Wu, K. L., Chen, I. H., et al. (2021). Systematic review and network meta-analysis of anaplastic lymphoma kinase (ALK) inhibitors for treatment-naïve ALK-positive lung cancer. Cancers 13 (8), 1966. doi:10.3390/cancers13081966
Chuang, J. C., and Neal, J. W. (2015). Crizotinib as first line therapy for advanced ALK-positive non-small cell lung cancers. Transl. Lung Cancer Res. 4 (5), 639–641. doi:10.3978/j.issn.2218-6751.2015.03.06
Devarakonda, S., Morgensztern, D., and Govindan, R. (2015). Genomic alterations in lung adenocarcinoma. Lancet. Oncol. 16 (7), e342–e351. doi:10.1016/s1470-2045(15)00077-7
Nakagawa, T., Setou, M., Seog, D., Ogasawara, K., Dohmae, N., Takio, K., et al. (2000). A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell 103 (4), 569–581. doi:10.1016/s0092-8674(00)00161-6
Ou, S. I., Zhu, V. W., and Nagasaka, M. (2020). Catalog of 5' fusion partners in ALK-positive NSCLC circa 2020. JTO Clin. Res. Rep. 1 (1), 100015. doi:10.1016/j.jtocrr.2020.100015
Samacá-Samacá, D., Prieto-Pinto, L., Peréz, A. Y., Valderrama, C., and Hernández, F. (2023). Alectinib for treating patients with metastatic ALK-positive NSCLC: systematic review and network metanalysis. Lung cancer Manag. 12 (2), Lmt59. doi:10.2217/lmt-2022-0018
Solomon, B. J., Mok, T., Kim, D. W., Wu, Y. L., Nakagawa, K., Mekhail, T., et al. (2014). First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 371 (23), 2167–2177. doi:10.1056/NEJMoa1408440
Sun, K., Nie, L., Nong, L., and Cheng, Y. (2021). Primary resistance to alectinib in a patient with STRN-ALK-positive non-small cell lung cancer: a case report. Thorac. cancer 12 (12), 1927–1930. doi:10.1111/1759-7714.13983
Keywords: anaplastic lymphoma kinase (ALK fusion), case report, kinesin family member 13A (KIF13A), lung adenocarcinoma, alectinib
Citation: Mo Z, Cai C, Yao J, Zhao J, Zhang M, Liu H and Mu X (2023) First case report of a novel KIF13A-ALK fusion in a lung adenocarcinoma patient and response to alectinib with a 4-year follow-up. Front. Genet. 14:1289346. doi: 10.3389/fgene.2023.1289346
Received: 05 September 2023; Accepted: 01 December 2023;
Published: 13 December 2023.
Edited by:
Liang Cheng, Harbin Medical University, ChinaReviewed by:
Shuhua Wei, Peking University Third Hospital, ChinaAnne Mc Leer, Centre Hospitalier Universitaire de Grenoble, France
Xiao-Jun Wang, Gansu Provincial Hospital, China
Copyright © 2023 Mo, Cai, Yao, Zhao, Zhang, Liu and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cunliang Cai, Y2NsdG9tQDE2My5jb20=
 Zheng Mo
Zheng Mo Cunliang Cai
Cunliang Cai Jingjing Yao3
Jingjing Yao3