- 1Department of Emergency Medicine, Chung-Kang Branch, Cheng Ching Hospital, Taichung, Taiwan
- 2Department of Physical Education, Fu Jen Catholic University, New Taipei, Taiwan
- 3Research and Development Center for Physical Education, Health, and Information Technology, Fu Jen Catholic University, New Taipei, Taiwan
- 4Department of Obstetrics and Gynecology, Chung-Kang Branch, Cheng Ching Hospital, Taichung, Taiwan
- 5Department of Public Health and Institute of Public Health, Chung Shan Medical University, Taichung, Taiwan
- 6Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung, Taiwan
Background: Over the past few decades, gout and diseases like metabolic syndrome (MetS) have become more prevalent. Attempts have been made in Taiwan to identify the genes responsible for gout. A few gene loci, among them SLC2A9, have been identified using Taiwan Biobank (TWB) data. We, therefore, examined whether MetS could also account for the association between polymorphism SLC2A9 rs3733591 and gout.
Methods: The final analysis consisted of 73,558 subjects, of whom 2,709 had gout. To estimate the likelihood of gout occurrence based on rs3733591 and MetS, we used logistic regression models.
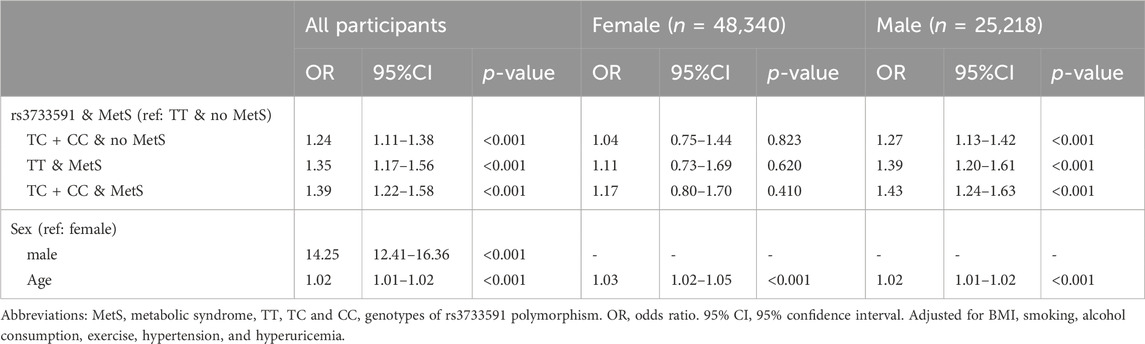
Results: Rs3733591-TC + CC compared to TT genotype was associated with gout (OR, 1.15; 95% CI, 1.06–1.25). Also associated with gout was MetS (OR, 1.21; 95% CI, 1.10–1.33). A significant interaction was seen between rs3733591 and MetS (p-value = 0.039). Using rs3733591-TT/no MetS as the reference group, the ORs (95% CI) for gout was 1.24 (1.11–1.38) for TC + CC/no MetS, 1.35 (1.17–1.56) for TT/MetS, and 1.39 (1.22–1.58) for TC + CC/MetS. However, subgroup analysis defined by sex showed no significant associations in women.
Conclusion: In summary, metabolic syndrome and SLC2A9 rs3733591 genotypes were interactively associated with gout in Taiwanese men, but not women.
Introduction
Gout is the most common form of inflammatory arthritis with the worst affected groups being elderly people, men, and racial and ethnic minorities (Singh and Gaffo, 2020). Recent years have seen an increase in the prevalence of gout and other diseases such as MetS and obesity (Pascart and Lioté, 2019). Taiwan is one of the countries where men are more likely to suffer from gout (Singh and Gaffo, 2020). About 1%–6.8% of people across the globe (Dehlin et al., 2020) and 3.8% in Taiwan (5.2% in men and 2.3% in women) are affected by gout (Kuo et al., 2013; Cox et al., 2021). It is mostly caused by hyperuricemia (elevated blood urate concentrations). Urate-lowering medications have been frequently used to treat the disease, though further efforts are needed to improve adherence to these medications (Pascart and Lioté, 2019; Dehlin et al., 2020). Previous epidemiological studies have also linked gout with MetS among Taiwanese (Wei et al., 2015) and US adults (Yoo et al., 2011). Furthermore, studies in other populations (RHO et al., 2004; Choi et al., 2007; Yoo et al., 2011; Jung et al., 2018) indicated that patients with gout had a greater prevalence of MetS than the general population.
As noted above, gout is linked to serum urate, whose heritability is estimated at 40%–70% as previously described (Wilk et al., 2000; Yang et al., 2005; Nath et al., 2007; Narang et al., 2019). According to our knowledge, several genes have been studied in relation to hyperurecemia and gout. Among them is the SLC2A9 gene, which is one of the key urate transporters in the kidney and gut that regulate serum urate. It is found on chromosome 4p16 and encodes the glucose transporter 9 (GLUT9), which is crucial for urate reuptake (Stiburkova et al., 2012; Pavelcova et al., 2020). Compared to men, variation in SLC2A9 is believed to have the greatest effect on serum urate in women (i.e., approximately 6% as opposed to 2% in men) (Riches, 2012). Topless et al. observed that the SLC2A9 variant affected serum urate more in premenopausal women than in postmenopausal women (Topless et al., 2015). So far, the SLC2A9 gene has shown the strongest association with uric acid levels among Asians but not much has been done to investigate it in Taiwan. Moreover, replication studies have shown conflicting results on SLC2A9 polymorphisms in gout and serum urate (Lukkunaprasit et al., 2020).
SLC2A9 rs3733591 is among polymorphisms found to be common in people with hyperurecemia and gout (Tu et al., 2010; Zhang et al., 2016; WT et al., 2018; Pavelcova et al., 2020) even though contrary results have also been published. For instance, an earlier study on the Minnan population did not find an association between the variant and gout risk (Zheng et al., 2016). In another study, Hollis-Moffatt et al. were not able to replicate associations of rs3733591 with gout in Caucasians and Western/Eastern Polynesians (Hollis-Moffatt et al., 2011). In the authors’ view, the variant has a weaker effect on gout in these populations than it does in Asian populations. According to Hung et al., the variant did not appear to increase gout susceptibility in Vietnamese populations (Hung et al., 2022).
There is evidence suggesting a link between gout and metabolic syndrome (González-Senac et al., 2014; Eun et al., 2022; Eun et al., 2023). Both conditions share common risk factors, such as obesity, insulin resistance, hypertension, and dyslipidemia. Exploring candidate genes and their variants linked to serum urate levels aids in regulating the metabolic processes involved in both urate production and removal (Köttgen et al., 2013): This knowledge could potentially inform strategies for treating and preventing gout.
The genetic basis of gout in the Taiwanese population has been a subject of investigation, with efforts focused on identifying relevant genetic loci. However, research thus far, particularly utilizing data TWB, has only yielded a limited number of identified loci, notably including SLC2A9. Despite these efforts, large-scale replication studies confirming genome-wide significant associations in this population remain scarce (Ko, 2022). A case-control study (Tu et al., 2018), albeit with limited sample size, aimed to determine the positive predictive value for gout in Taiwan. This study recruited individuals from both Han Chinese and indigenous populations across various communities through hospital settings. Notably, the study found that rs3733591 was not associated with gout in the indigenous population, in contrast to its association in the Han population. Surprisingly, despite its potential relevance, research focusing on this SNP in the context of gout in Taiwan has been minimal. Given the gap in understanding the role of rs3733591 in gout within the Taiwanese population, we sought to address this by leveraging the extensive data available from the TWB. Thus, we examined the association between polymorphism rs3733591 and gout, while also assessing whether this association could be attributed to MetS.
Materials and methods
Study design and participants
This study utilized data from the TWB resource, where demographic, lifestyle, biochemistry, and genotype data were collected through biobank questionnaires. At the beginning of the study, data from 76,229 participants were available. Exclusion criteria included subjects with incomplete information (n = 2,671). Ultimately, 73,558 subjects were analyzed, 2,709 of whom had gout. Informed written consent was obtained from all TWB participants at the time of recruitment. The institutional Review Board of Cheng-Ching General Hospital approved this study (HP210007). All methods were performed in accordance with the relevant guidelines and regulations.
Genotyping and quality control
Whole-genome genotyping was performed on the Biobank participants using the Axiom Genome-Wide Array Plate chip system (Affymetrix Inc., Santa Clara, CA, United States). This study examined SLC2A9 rs3733591 of the urate transporter gene. During quality control, we ensured that the variant rs3733591 had a call rate of >95 percent, a p-value of >0.00001 for the Hardy-Weinberg equilibrium test, and a minor allele frequency of >0.01.
Disease definition and covariates
The outcome of our interest was self-reported gout, with exposures including rs3733591 and MetS, which was defined according to the revised National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) criteria as proposed by the Health Promotion Administration in Taiwan (Health Promotion Administration, 2007; Chen et al., 2020). MetS was defined as having at least three of the following conditions: (1) hypertension (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg); (2) abdominal obesity (waist circumference: men ≥90cm, women ≥80 cm); (3) low high-density lipoprotein cholesterol (men <40 mg/dL, women <50 mg/dL); (4) hyperglycemia (fasting blood glucose ≥100 mg/dL) or (5) triglyceride ≥150 mg/dL. Hyperlipidemia was defined based on a self-report questionnaire or triglyceride and total cholesterol levels of over 150 mg/dL and 200 mg/dL, respectively. The adjusted variables included sex, body mass index (BMI, determined as weight divided by height squared (kg/m2), smoking, drinking, exercise, hypertension, and hyperuricemia. Those who have smoked continuously for at least 6 months are classified as current smokers and those who have quit smoking for at least 6 months as former smokers. Those who consumed more than 150 mL/week for 6 months were considered current drinkers, and those who stopped drinking for 6 months were considered former drinkers. Exercise was defined as engaging in physical activity for 30 min or more at least three times a week. Historically, hyperuricemia was defined as uric acid levels over 6 mg/dL in females and 7 mg/dL in males.
Statistical analysis
Both continuous and categorical variables were tested using Student's t-test and Chi-square test. In order to estimate the likelihood of gout based on rs3733591 and MetS, we used logistic regression. There was also a test to determine whether rs3733591 interacts with MetS. The statistical software used in this study is SAS 9.4 (SAS Institute, Cary, NC, United States) and PLINK 1.90 beta.
Results
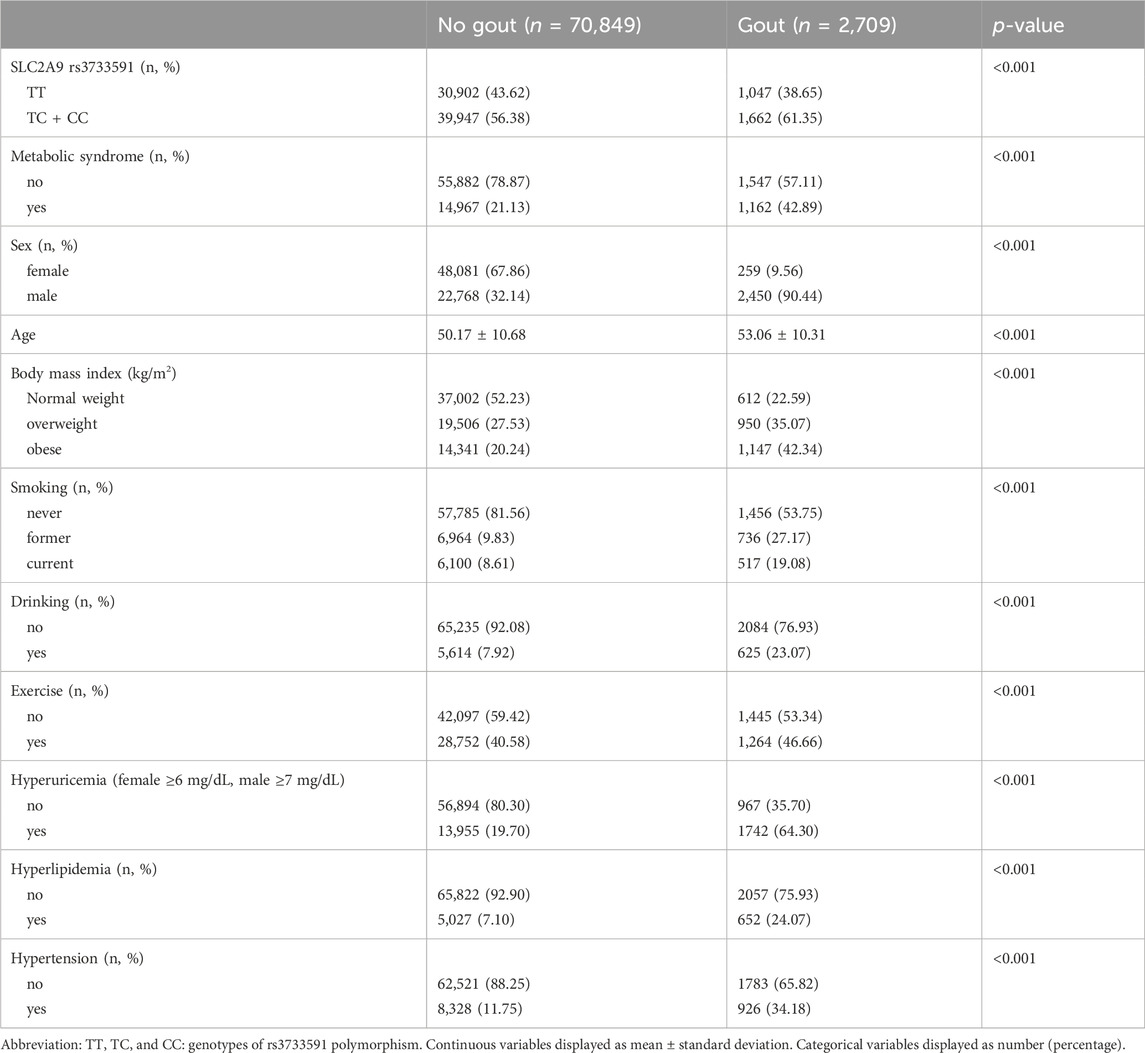
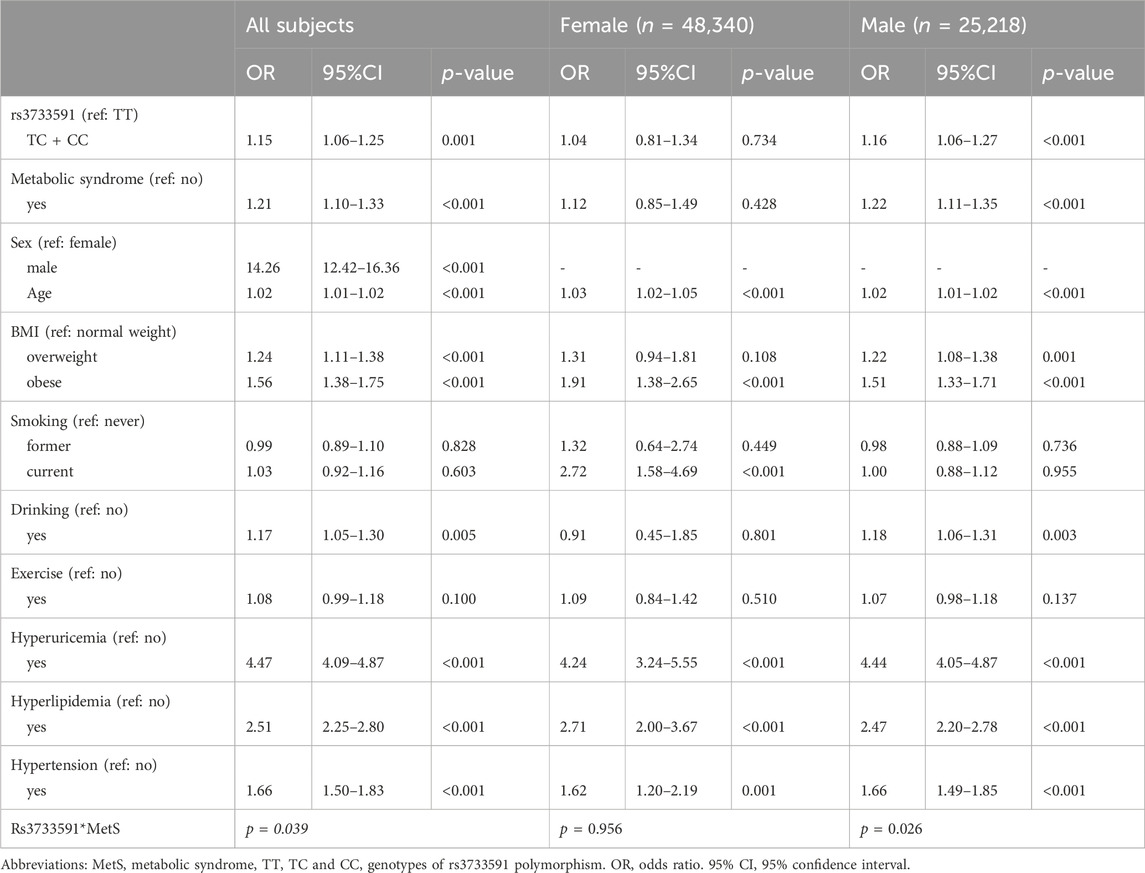
Table 1 outlines the characteristics of subjects from TWB. Among the subjects, 70,849 were healthy and 2,709 had gout. Among subjects with gout, 259 (9.56%) were females and 2,450 (90.44%) were males. As shown in Table 2, factors significantly associated with gout were rs3733591-TC + CC compared to TT genotype (OR, 1.15; 95% CI, 1.06–1.25), MetS (OR, 1.21; 95% CI, 1.10–1.33), male compared to female sex (OR, 14.26 95% CI, 12.42–16.36), hyperuricemia (OR, 4.47.95% CI, 4.09–4.87), hyperlipidemia (OR, 2.51, 95% CI, 2.25–2.80), and hypertension (OR, 1.66, 95% CI, 1.50–1.83), respectively. The sex-related risk of gout among study subjects is also shown. Compared to the TT genotype, the OR (95% CI) for gout in TC + CC males was 1.16 (1.06–1.27) and 1.04 (0.81–1.34) in TC + CC females. Hyperuricemia, increasing age, hyperlipidemia, hypertension, and obesity were all associated with gout in both men and women. There was an interaction between variant rs3733591 and MetS (p-value = 0.039). Due to interaction, we further stratified our analyses using rs3733591-TT/no MetS as the reference group, as shown in Table 3. In the general population, the ORs (95% CI) were 1.24 (1.11–1.38) for TC + CC/no MetS, 1.35 (1.17–1.56) for TT/MetS, and 1.39 (1.22–1.58) for TC + CC/MetS. In a sex-based stratification, female subjects showed no significant associations, unlike their male counterparts (Table 3).
Discussion
Gout, a prevalent ailment among native Taiwanese, has been linked to numerous loci associated with urate concentrations. In our investigation, we sought to ascertain the impact of MetS and the presence of SLC2A9, a gene strongly linked to urate concentrations, on gout development in Taiwan. Our findings revealed that in the general model, the presence of SLC2A9 rs3733591-TC + CC genotypes, compared to rs3733591-TT, along with MetS, exhibited independent associations with gout. Notably, gender disparities emerged regarding MetS and the rs3733591-TC + CC genotype, with significant odds ratios observed solely among men.
The C-allele of SNP rs3733591 has been identified as the risk allele for gout among Han Chinese and Solomon Islanders (Tu et al., 2010), underscoring its significance as a genetic determinant of uric acid concentrations and gout in Taiwan. According to Tu et al., this variant is an important genetic checkpoint for uric acid concentrations and gout in Taiwan. As seen earlier, rs3733591 is believed to have a weaker effect in Caucasians and Polynesians than it does in Asians (Hollis-Moffatt et al., 2011). Despite these, our logistic regression model revealed a significant interaction between the variant and MetS. However, in stratified analyses, this interaction demonstrated a more pronounced association with gout in men than in women, warranting further elucidation of underlying mechanisms.
The genetic variant, rs3733591 resides within the SLC2A9 gene. A comprehensive examination spanning the entire SLC2A9 locus would have been imperative to ascertain whether rs3733591 ranks among the primary SNPs associated with gout and serum urate levels at this locus. The complexity of the SLC2A9 locus, potentially harboring multiple distinct signals of association attributable to various causal variants and compounded by population structure, underscores the need for thorough investigation.
While acknowledging the intricacies of the SLC2A9 locus and the possibility of multiple independent signals of association, our study sought to validate and expand upon previous findings. However, to evaluate the significance of rs3733591 as a principal variant within the SLC2A9 gene linked to gout and serum urate levels, we employed AI (artificial intelligence) models (data not presented) that encompassed seven SLC2A9 loci (rs3733591, rs16890979, rs1014290, rs6449213, rs6855911, rs12498742, rs3775948) previously associated with these conditions. Our analysis demonstrated that rs3733591 ranked among the top three SNPs in terms of relative importance within the Gradient Boosting champion model. This helps alleviate concerns regarding potential artefacts arising from complex linkage disequilibrium (LD) patterns at this locus.
Both our general and sex-stratified models indicated that hyperuricemia was associated with a four-fold increased risk of gout. This aligns with previous findings highlighting the requisite presence of hyperuricemia and distinct inflammatory mechanisms for gout development (Chen et al., 2018). Acute inflammation in gout patients ensues from the accumulation of monosodium urate (MSU) crystals, which then interact with macrophages to trigger activation of the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome and release of Interleukin-1 β (IL-1β), which is linked to gout flare (Chen et al., 2018).
Furthermore, our analysis revealed no gender disparities in certain gout risk factors such as obesity, hypertension, and advancing age. Notably, alcohol consumption demonstrated a significant association with gout solely among men, despite previous indications (Wang et al., 2013) suggesting a dose-dependent effect of alcohol consumption on gout development.
In the present investigation, the absence of data regarding medication utilization represents a notable limitation. Furthermore, the unavailability of dietary information, including purine intake, within our chosen data source complicates the assessment of potential dietary influences on our findings. Additionally, the duration of illness was not ascertainable from the questionnaires administered within the Biobank database. Moreover, our identification of gout relied solely on self-reported data, which introduces the possibility of misclassification and may not fully capture the prevalence of gout within the broader population. Lastly, the inherent limitations of a cross-sectional study design impede the establishment of causal relationships among the variables under examination.
Conclusion
In conclusion, we found that MetS and rs3733591 genotypes exhibited a stronger interactive association with gout in Taiwanese men but not in women. Understanding the discovery, potential causality, and linkage disequilibrium (LD) patterns of rs3733591 and other variants within the SLC2A9 gene is crucial for elucidating their roles in gout susceptibility and metabolic syndrome, as well as their interactions with environmental factors. Further research, including functional studies and replication in diverse populations, is needed to validate and characterize these genetic associations comprehensively.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available from Taiwan Biobank but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding authors upon reasonable request and with permission of Taiwan Biobank. Requests to access these datasets should be directed to liawyp@csmu.edu.tw.
Ethics statement
The study protocol followed the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Board of Cheng-Ching General Hospital (HP210007).
Author contributions
C-NL: Conceptualization, Methodology, Writing–original draft. C-CH: Conceptualization, Data curation, Validation, Writing–review and editing. P-CH: Conceptualization, Methodology, Validation, Writing–review and editing. C-HH: Conceptualization, Formal Analysis, Validation, Writing–review and editing. ONN: Conceptualization, Formal Analysis, Methodology, Supervision, Validation, Writing–review and editing. Y-PL: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by Cheng-Ching General Hospital and the National Science and Technology Council (MOST 111-2121-M-040-002, MOST 111-2811-M-040-001, NSTC 112-2121-M-040-002, NSTC 112-2811-M-040-001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Chen, C.-J., Tseng, C.-C., Yen, J.-H., Chang, J.-G., Chou, W.-C., Chu, H.-W., et al. (2018). ABCG2 contributes to the development of gout and hyperuricemia in a genome-wide association study. Sci. Rep. 8, 3137. doi:10.1038/s41598-018-21425-7
Chen, Y.-F., Lin, Y.-A., Yeh, W.-C., Tsao, Y.-C., Li, W.-C., Fang, W.-C., et al. (2020). The association between metabolic syndrome and elevated Alanine Aminotransferase levels in an indigenous population in Northern Taiwan: a Community-based and cross-sectional study. Evidence-Based Complementary Altern. Med. 2020, 6612447. doi:10.1155/2020/6612447
Choi, H. K., Ford, E. S., Li, C., and Curhan, G. (2007). Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition examination Survey. Arthritis Care & Res. Official J. Am. Coll. Rheumatology 57, 109–115. doi:10.1002/art.22466
Cox, P., Gupta, S., Zhao, S. S., and Hughes, D. M. (2021). The incidence and prevalence of cardiovascular diseases in gout: a systematic review and meta-analysis. Rheumatol. Int. 41, 1209–1219. doi:10.1007/s00296-021-04876-6
Dehlin, M., Jacobsson, L., and Roddy, E. (2020). Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 16, 380–390. doi:10.1038/s41584-020-0441-1
Eun, Y., Han, K., Lee, S. W., Kim, K., Kang, S., Lee, S., et al. (2022). Increased risk of incident gout in young men with metabolic syndrome: a nationwide population-based cohort study of 3.5 million men. Front. Med. 9, 1010391. doi:10.3389/fmed.2022.1010391
Eun, Y., Han, K., Lee, S. W., Kim, K., Kang, S., Lee, S., et al. (2023). Altered risk of incident gout according to Changes in metabolic syndrome Status: a nationwide, population-based cohort study of 1.29 million young men. Arthritis & Rheumatology 75, 806–815. doi:10.1002/art.42381
González-Senac, N. M., Bailén, R., Torres, R. J., De Miguel, E., and Puig, J. G. (2014). Metabolic syndrome in primary gout. Nucleosides, Nucleotides Nucleic Acids 33, 185–191. doi:10.1080/15257770.2013.853785
Hollis-Moffatt, J. E., Gow, P. J., Harrison, A. A., Highton, J., Jones, P. B., Stamp, L. K., et al. (2011). The SLC2A9 nonsynonymous Arg265His variant and gout: evidence for a population-specific effect on severity. Arthritis Res. Ther. 13, R85–R88. doi:10.1186/ar3356
Hung, P. Q., Canh, N. X., and Duong, N. T. (2022). Study on association between SLC2A9 rs3733591 and Gout susceptibility in 481 Vietnamese individuals. Vietnam J. Sci. Technol. Eng. 64, 39–42. doi:10.31276/vjste.64(1).39-42
Jung, J. H., Song, G. G., Ji, J. D., Lee, Y. H., Kim, J.-H., Seo, Y. H., et al. (2018). Metabolic syndrome: prevalence and risk factors in Korean gout patients. Korean J. Intern. Med. 33, 815–822. doi:10.3904/kjim.2016.062
Ko, Y.-L. (2022). Genetics of hyperuricemia and gout: Insights from recent genome-wide association studies and Mendelian randomization studies.
Köttgen, A., Albrecht, E., Teumer, A., Vitart, V., Krumsiek, J., Hundertmark, C., et al. (2013). Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 45, 145–154. doi:10.1038/ng.2500
Kuo, C.-F., Yu, K.-H., See, L.-C., Chou, I.-J., Ko, Y.-S., Chang, H.-C., et al. (2013). Risk of myocardial infarction among patients with gout: a nationwide population-based study. Rheumatology 52, 111–117. doi:10.1093/rheumatology/kes169
Lukkunaprasit, T., Rattanasiri, S., Turongkaravee, S., Suvannang, N., Ingsathit, A., Attia, J., et al. (2020). The association between genetic polymorphisms in ABCG2 and SLC2A9 and urate: an updated systematic review and meta-analysis. BMC Med. Genet. 21, 210. doi:10.1186/s12881-020-01147-2
Narang, R. K., Topless, R., Cadzow, M., Gamble, G., Stamp, L. K., Merriman, T. R., et al. (2019). Interactions between serum urate-associated genetic variants and sex on gout risk: analysis of the UK Biobank. Arthritis Res. Ther. 21, 13. doi:10.1186/s13075-018-1787-5
Nath, S. D., Voruganti, V. S., Arar, N. H., Thameem, F., Lopez-Alvarenga, J. C., Bauer, R., et al. (2007). Genome scan for determinants of serum uric acid variability. J. Am. Soc. Nephrol. 18, 3156–3163. doi:10.1681/ASN.2007040426
Pascart, T., and Lioté, F. (2019). Gout: state of the art after a decade of developments. Rheumatology 58, 27–44. doi:10.1093/rheumatology/key002
Pavelcova, K., Bohata, J., Pavlikova, M., Bubenikova, E., Pavelka, K., and Stiburkova, B. (2020). Evaluation of the influence of genetic variants of SLC2A9 (GLUT9) and SLC22A12 (URAT1) on the development of hyperuricemia and gout. J. Clin. Med. 9, 2510. doi:10.3390/jcm9082510
Rho, Y.-H., Choi, S.-J., Lee, Y.-H., Ji, J.-D., Choi, K.-M., Baik, S.-H., et al. (2004). Prevalence of the metabolic syndrome in patients with gout. J. Korean Rheumatism Assoc., 349–357. doi:10.3346/jkms.2005.20.6.1029
Riches, P. L. (2012). “Chapter 7 - genetics of gout,” in Gout & other crystal Arthropathies. Editor R. Terkeltaub (Philadelphia: W.B. Saunders), 85–93.
Singh, J. A., and Gaffo, A. (2020). Gout epidemiology and comorbidities. Seminars Arthritis Rheumatism 50, S11–S16. doi:10.1016/j.semarthrit.2020.04.008
Stiburkova, B., Taylor, J., Marinaki, A. M., and Sebesta, I. (2012). Acute kidney injury in two children caused by renal hypouricaemia type 2. Pediatr. Nephrol. 27, 1411–1415. doi:10.1007/s00467-012-2174-0
Topless, R. K., Flynn, T. J., Cadzow, M., Stamp, L. K., Dalbeth, N., Black, M. A., et al. (2015). Association of SLC2A9 genotype with phenotypic variability of serum urate in pre-menopausal women. Front. Genet. 6, 313. doi:10.3389/fgene.2015.00313
Tu, H.-P., Chen, C.-J., Tovosia, S., Ko, A.M.-S., Lee, C.-H., Ou, T.-T., et al. (2010). Associations of a non-synonymous variant in SLC2A9 with gouty arthritis and uric acid levels in Han Chinese subjects and Solomon Islanders. Ann. rheumatic Dis. 69, 887–890. doi:10.1136/ard.2009.113357
Tu, H.-P., Min-Shan Ko, A., Lee, S.-S., Lee, C.-P., Kuo, T.-M., Huang, C.-M., et al. (2018). Variants of ALPK1 with ABCG2, SLC2A9, and SLC22A12 increased the positive predictive value for gout. J. Hum. Genet. 63, 63–70. doi:10.1038/s10038-017-0368-9
Wang, M., Jiang, X., Wu, W., and Zhang, D. (2013). A meta-analysis of alcohol consumption and the risk of gout. Clin. Rheumatol. 32, 1641–1648. doi:10.1007/s10067-013-2319-y
Wei, C.-Y., Sun, C.-C., Wei, J.C.-C., Tai, H.-C., Sun, C.-A., Chung, C.-F., et al. (2015). Association between hyperuricemia and metabolic syndrome: an epidemiological study of a labor force population in Taiwan. BioMed Res. Int. 2015, 369179. doi:10.1155/2015/369179
Wilk, J. B., Djousse, L., Borecki, I., Atwood, L. D., Hunt, S. C., Rich, S. S., et al. (2000). Segregation analysis of serum uric acid in the NHLBI Family Heart Study. Hum. Genet. 106, 355–359. doi:10.1007/s004390000243
Wt, W. R., Mahfudzah, A., Nazihah, M., Tan, H., Wg, W. S., Amanda Jane, P. G., et al. (2018). Association of solute carrier family 2, member 9 (SLC2A9) genetic variant rs3733591 with gout in a Malay sample set. Med. J. Malays. 73, 307–310.
Yang, Q., Guo, C.-Y., Cupples, L. A., Levy, D., Wilson, P. W., and Fox, C. S. (2005). Genome-wide search for genes affecting serum uric acid levels: the Framingham Heart Study. Metabolism 54, 1435–1441. doi:10.1016/j.metabol.2005.05.007
Yoo, H.-G., Lee, S.-I., Chae, H.-J., Park, S. J., Lee, Y. C., and Yoo, W.-H. (2011). Prevalence of insulin resistance and metabolic syndrome in patients with gouty arthritis. Rheumatol. Int. 31, 485–491. doi:10.1007/s00296-009-1304-x
Zhang, X., Yang, X., Wang, M., Li, X., Xia, Q., Xu, S., et al. (2016). Association between SLC2A9 (GLUT9) gene polymorphisms and gout susceptibility: an updated meta-analysis. Rheumatol. Int. 36, 1157–1165. doi:10.1007/s00296-016-3503-6
Zheng, C., Yang, H., Wang, Q., Rao, H., and Diao, Y. (2016). Association analysis of five SNP variants with gout in the Minnan population in China. Turk J. Med. Sci. 46, 361–367. doi:10.3906/sag-1409-58
Keywords: metabolic syndrome, gout, polymorphism, genetics, osteoarthritis
Citation: Lin C-N, Ho C-C, Hsieh P-C, Hsiao C-H, Nfor ON and Liaw Y-P (2024) Polymorphism rs3733591 of the SLC2A9 gene and metabolic syndrome affect gout risk in Taiwan Biobank subjects. Front. Genet. 15:1374405. doi: 10.3389/fgene.2024.1374405
Received: 22 January 2024; Accepted: 03 April 2024;
Published: 16 April 2024.
Edited by:
Yoon Shin Cho, Hallym University, Republic of KoreaReviewed by:
Megan Patricia Leask, University of Otago, New ZealandHong Li, Southern Medical University, China
Copyright © 2024 Lin, Ho, Hsieh, Hsiao, Nfor and Liaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yung-Po Liaw, liawyp@csmu.edu.tw; Oswald Ndi Nfor, nforoswald2@yahoo.com
 Chun-Nan Lin1
Chun-Nan Lin1 Oswald Ndi Nfor
Oswald Ndi Nfor Yung-Po Liaw
Yung-Po Liaw