- 1Department of Biological Sciences, Korea Advanced Institute of Science and Technology, Daejeon, South Korea
- 2KAIST Institute for the BioCentury, Korea Advanced Institute of Science and Technology, Daejeon, South Korea
Specific behavioral patterns are expressed by complex combinations of muscle coordination. Tremors are simple behavioral patterns and are the focus of studies investigating motor coordination mechanisms in the brain. T-type Ca2+ channels mediate intrinsic neuronal oscillations and rhythmic burst spiking, and facilitate the generation of tremor rhythms in motor circuits. Despite substantial evidence that T-type Ca2+ channels mediate pathological tremors, their roles in physiological motor coordination and behavior remain unknown. Here, we review recent progress in understanding the roles that T-type Ca2+ channels play under pathological conditions, and discuss the potential relevance of these channels in mediating physiological motor coordination.
Introduction
Unraveling the mechanisms that underlie specific motor patterns is a challenging issue in neuroscience. Motor coordination is a complex process that determines the timing and sequence of both activation and relaxation of a huge number of muscles (reviewed by Mauk et al., 2000; De Zeeuw et al., 2011). More than one gigahertz of information processing is required for the optimal execution of even the simplest motor behavior, such as holding a cup.
Tremors are one of the simplest forms of motor coordination and are characterized by involuntary rhythmic movements of either the whole body or of body parts (reviewed by Findley, 1995; Grimaldi and Manto, 2008). Low amplitude tremor, also known as physiological tremor, exist in humans and animals during normal states and may function to help behavioral control (Elble et al., 1984). High amplitude tremors that interfere with voluntary movements are observed in pathological conditions (reviewed by Deuschl et al., 2001). These pathological tremors are classified into different types, and are associated with specific neural mechanisms and circuits (Table 1) (reviewed by Wilms et al., 1999; Deuschl et al., 2001).
T-type Ca2+ channels (CaV3.1, 3.2, and 3.3) modulate both physiological and pathological rhythms in the brain (Crunelli et al., 1989; reviewed by Huguenard, 1996). These channels mediate the generation of low-threshold spikes (LTS) in response to hyperpolarizing membrane potentials elicited by inhibitory inputs. LTS regulate neural oscillations, resonance, and synchrony (Llinas and Yarom, 1986; Crunelli et al., 1989; Kim et al., 2001, 2011; Mangoni et al., 2006) (Figure 1, left). Pharmacological and genetic studies show that T-type Ca2+ channels are also involved in the generation of pathological tremors (Sinton et al., 1989; Handforth et al., 2010). However, the precise roles of T-type Ca2+ channels in physiological tremors and in normal motor coordination remain unknown. Here, we propose potential roles for T-type Ca2+ channels in physiological motor functions based on their pathological roles.
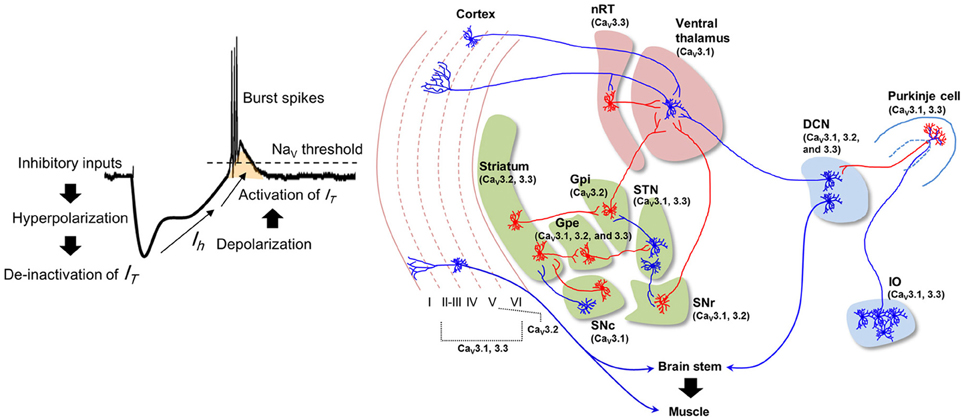
Figure 1. T-type Ca2+ channels and pathological tremor pathways. Potentiation of T-type Ca2+ conductance and the generation of low-threshold spikes (LTS) (left). Hyperpolarization and subsequent depolarization by HCN channel-mediated currents (Ih) open T-type Ca2+ channels. IT: T-type calcium current; NaV: voltage-gated sodium channel. Neural pathways involved in pathological tremors (right). Blue indicates excitatory neurons, and red indicates inhibitory neurons. The blue regions represent the olivocerebellar pathway, the green regions represent basal ganglia circuits, and the red regions indicate thalamocortical pathways. The T-type Ca2+ channel isoforms (CaV3.1, 3.2, and 3.3) expressed in these regions are indicated (Lein et al., 2006). IO: Inferior olive; DCN: deep cerebellar nuclei; SNc: substantia nigra compacta; Gpe: globus pallidus externa; Gpi: globus pallidus interna; STN: subthalamic nucleus; SNr: substantia nigra reticulata; nRT: nucleus reticularis of the thalamus.
Roles of T-type Ca2+ Channels in Generating Pathological Tremors
T-type Ca2+ Channels in Essential Tremor
Essential tremor is the most common form of movement disorder (Kurtzke, 1982; reviewed by Louis, 2005) and is characterized by postural and kinetic tremors at 4–12 Hz (Bain et al., 2000; Brennan et al., 2002). Harmaline is a plant-derived metabolite (Lutomski et al., 1974) that induces ET-like tremors and tremor-related neural oscillations in both humans and animals (Battista et al., 1969; de Montigny and Lamarre, 1973; Llinas and Volkind, 1973; Ahmed and Taylor, 2012). Since harmaline binds to various ion channels, including glutamate receptors, GABA receptors, and voltage-gated Ca2+ channels (Du et al., 1997; Glennon et al., 2000; Splettstoesser et al., 2005), specific molecular mechanism that underlies harmaline tremor have been unclear.
Inferior olive (IO) neurons are also implicated in the generation of harmaline tremor. IO lesions reduce harmaline tremors in rats (Simantov et al., 1976). Harmaline also alters the intrinsic properties of IO neurons: it increases LTS and amplifies sub-threshold oscillations (STOs). Both of these properties are dependent upon the conductance of T-type Ca2+ channels (Llinas and Yarom, 1986; Crunelli et al., 1989; Park et al., 2010). A non-selective T-type Ca2+ channel inhibitor, 1-octanol, reduces harmaline-induced tremors in rats (Sinton et al., 1989), supporting the role of T-type Ca2+ channels in harmaline-induced tremor.
There are three distinct isoforms of T-type Ca2+ channels, CaV3.1, 3.2, and 3.3 (Cribbs et al., 1998; Perez-Reyes et al., 1998; Lee et al., 1999). CaV3.1 is the major isoform expressed in the IO, Purkinje cells, and deep cerebellar nuclei (DCN) (Figure 1, right) (Talley et al., 1999; Lein et al., 2006). CaV3.1−/− mice treated with harmaline (dose: 9 mg/kg) do not display behavioral tremors, 4–10 Hz tremor-related oscillation in the olivocerebellar pathway, or STOs in IO neurons (Park et al., 2010). Patch clamp recording revealed that harmaline inhibits the activation of CaV3.1 channels while also promoting their de-inactivation. These effects result in a net potentiation of CaV3.1 channels under physiological conditions (Park et al., 2010).
Other ionic mechanisms and their interactions with CaV3.1 channel could also contribute to the generation of harmaline tremor. A Ca2+ activated K+ channel isoform (KCa1.1) is known to form a complex with CaV3 channels and be activated in response to CaV3—mediated calcium influx (Rehak et al., 2013). Interaction between CaV3 and Ca2+ activated K+ channels could possibly be involved in the generation of STO and its exaggeration during harmaline tremor. Hyperpolarization-activated cation channel is another candidate. Activation of this channel could contribute to slow rebound potential in STOs. This channel is also involved in rhythmic thalamic oscillation and the blockade of the channel ameliorates oscillation induced by hyperpolarizing current injection into IO neurons (Bal and McCormick, 1997). Investigation of harmaline tremor and tremor rhythm in Ca2+ activated K+ channel or hyperpolarization-activated cation channel knockout mice is required to test these possibilities.
GABA-A1 receptor knockout mice (a1−/−) are a genetic model of essential tremor. These mutant mice display ~25 Hz ET-like tremors (Kralic et al., 2005). A subset of non-selective T-type Ca2+ channel antagonists (ethosuximide, zonisamide, ECN, KYS05064, and NNC 55-0396) ameliorate both a1−/− mouse tremors and harmaline tremor (Handforth et al., 2010). While CaV3.1 knockout mice display reduced harmaline tremor (Park et al., 2010), double knockout mice (CaV3.1−/− and a1−/−) exhibit exacerbated tremor behavior (Chang et al., 2011). Thus, these two distinct animal models of essential tremor (a1−/− and harmaline-induced tremors) likely result from different mechanisms, such as the involvement of distinct T-type Ca2+ channel isoforms (e.g., Harmaline tremor by CaV3.1 and a1−/− tremor by CaV3.2 or 3.3). The heterogeneity of essential tremor is well-described by clinical studies (Kovach et al., 2001; Louis et al., 2007). Future studies are necessary to determine how the other T-type isoforms contribute to essential tremor.
T-type Ca2+ Channels in Parkinson Tremors
Resting tremor is one of the most detrimental symptoms experienced by Parkinson's disease (PD) patients. PD is caused by a dopamine deficiency in the brain. Rhythmic stimulation of the motor cortex via subcortical pathway is thought to underlie resting tremor of PD patients (Plenz and Kital, 1999; Magnin et al., 2000; Chan et al., 2011). There are several hypotheses about the origin of the tremor.
The basal ganglia circuit hypothesis states that rhythmic burst firings of neurons in subthalamic nuclei (STN) underlie the resting tremor in PD patients (Magnin et al., 2000; Chan et al., 2011). Suppressing STN activity using deep brain stimulation ameliorates resting tremor in PD patient (Kumar et al., 1998; Sturman et al., 2004; Amtage et al., 2008). STN burst activity is associated with the activation of T-type Ca2+ channels (Beurrier et al., 1999; Tai et al., 2011). Moreover, pharmacological inhibition of T-type Ca2+ channels in the STN rescues locomotor deficits in rat PD models, while effect on the resting tremor was not accessed (Tai et al., 2011). Isoforms of T-type Ca2+ channels that express in STN (CaV3.1 and 3.3) (Figure 1) could possibly play a role in the generation of resting tremor in PD patients.
The ventrolateral (VL) thalamus is another candidate for PD resting tremor. Dopamine deficiency in PD would result in the activation of globus pallidus interna (Gpi) and substantia nigra reticulata (SNr) neurons that provides GABAergic input to the VL thalamus (Vitek, 2002). Subsequent hyperpolarization of the neurons may induce rhythmic LTS in VL thalamocortical (TC) neurons and thus generates the resting tremor. Consistently, tremor-related rhythmic LTS is observed in VL thalamic neurons (Zirh et al., 1998; Magnin et al., 2000; Pifl et al., 2012). These results suggest that CaV3.1 expressing in VL thalamus (Figure 1) could be involved in the tremor generation. However, some other studies in PD patients also reports that LTS in VL neurons do not coincide with ongoing resting tremor (Zirh et al., 1998; Magnin et al., 2000). Thus, the role of thalamic burst firing in the resting tremor is still controversial.
A third hypothesis is the “dimmer-switch model” which states that core tremor activities are expressed by the cerebello-thalamo-cortical circuit (Figure 1, right) (Helmich et al., 2012). Hyperactivity in the cerebellum of PD patients is reported (Ghaemi et al., 2002; Timmermann et al., 2002), which is concomitant with the activation of sensory and motor cortices responsible for hand exhibiting resting tremor (Timmermann et al., 2004; Pollok et al., 2009). Deep brain stimulation of the STN or Gpi, or the administration of Levodopa normalizes cerebellar activity and improves tremor in PD patients (Payoux et al., 2008; Wu et al., 2009). This may be due to the ventral intermediate thalamus (which receives excitatory inputs from the cerebellum) acting as a critical relay station in the cerebello-thalamo-cortical circuit (Lenz et al., 1995; Tarsy et al., 2008). Consistently, PD resting tremor is suppressed by stimulation of the ventral intermediate thalamus, with decreased blood flow in cerebellar cortex (Deiber et al., 1993).
In spite of these evidences that T-type Ca2+ channels are involved in neuronal burst activity and oscillations in PD tremor circuits, there is no direct evidence that links T-type Ca2+ channels to resting tremor. One of the main obstacles on defining the role of T-type Ca2+ channels in PD tremor is a lack of robust rodent models that display resting tremor (Potashkin et al., 2010). Developing a robust resting tremor model and modulating T-type Ca2+ channels in the model might unravel the mechanism of PD resting tremor.
T-type Ca2+ Channels in Palatal Tremor
Palatal tremor, also called palatomyoclonus, is characterized by rhythmic movement of soft palate and sometimes of other muscles (Deuschl et al., 2001). Hypertrophy of IO neurons has been proposed as a pathologic substrate of the tremor (Deuschl et al., 2000; Pearce, 2008). This condition develops after lesions in the brainstem or cerebellum, manifesting as tremor in body parts contralateral to the region of damage in both human and animals (De Zeeuw et al., 1998; Deuschl et al., 2000). Studies of hemicerebellectomized animals reveal that hypertrophic IO neurons show failure in after-depolarization of action potential and have decreased numbers of GABAergic boutons in their dendrites (Ruigrok et al., 1990; De Zeeuw et al., 1998).
Because GABAergic input to IO neuron modulates electrotonic coupling of IO neurons (Sotelo et al., 1986; Leznik et al., 2002), it can be inferred that synchrony between IO neurons would be enhanced in the hypertrophic IO (Deuschl et al., 2000). This might entrain larger range of IO neurons with synchronized STOs, resulting rhythmic activations required for palatal tremor (Deuschl et al., 2000).
CaV3.1 could also be involved in palatal tremor generation, in consideration of its role in STOs (Llinas and Yarom, 1986; Choi et al., 2010; Park et al., 2010). However, the contribution of CaV3.1 would be different from the case of harmaline tremor. Potentiation of the CaV3.1 channel is required for harmaline tremor (Park et al., 2010). Hyperpolarization of IO neurons contributes CaV3.1 potentiation which amplifies STOs and subsequently generates tremor rhythm. (Park et al., 2010). In palatal tremor, however, hypertrophic IO neurons seem to be depolarized, meaning that CaV3.1 might not be potentiated (Crunelli et al., 1989). Instead, increased firing rate with enhanced synchrony in IO neurons could, when combined with basal STOs generate a rhythmic activity for palatal tremor. Investigation of palatal tremor in CaV3.1−/− mice and changes in the CaV3.1 activity may shed light on the differential mechanisms of IO-dependent tremor generation by T-type Ca2+ channel.
The Role of T-type Ca2+ Channels in Generating Physiological Motor Functions
While their association with pathological tremors (Table 1) is well-understood, whether T-type Ca2+ channels contribute to physiological motor functions is unclear. Both CaV3.1−/− (Park et al., 2010) and CaV3.2−/− mice (Choi et al., 2006) do not have significant motor defects. In addition, overexpression of the CaV3.1 gene in mouse brain does not result in motor dysfunction (Ernst et al., 2009). These results might be due to compensatory expressions between CaV3 channels, or the possibility that motor defects in CaV3 mutants are not be able to be examined by conventional motor tests. Below, we summarize the potential roles of T-type Ca2+ channels in physiological motor behavior and describe how to study these potential roles.
Generation of Physiological Tremors by T-type Ca2+ Channels
Physiological tremors are induced by extrinsic factors such as gravity force (Marsden et al., 1969; Young and Hagbarth, 1980), or by central mechanisms such as a tremor rhythm pacemaker in the brain (Hagbarth et al., 1983; Vallbo and Wessberg, 1993; Llinas and Pare, 1994). 8–12 Hz component of physiological tremor is associated with central mechanisms because this component is unaffected by extrinsic factors (Elble and Randall, 1976; Vallbo and Wessberg, 1993).
The intrinsic rhythmicity of IO neurons may be one of these central mechanisms (de Montigny and Lamarre, 1973; Llinas and Pare, 1994; Findley, 1995), since the frequencies of STOs are around 10 Hz (Llinas and Yarom, 1986; Chorev et al., 2007; Khosrovani et al., 2007). These frequencies are similar to those of physiological tremors of humans and animals (Elble and Randall, 1976; Elble et al., 1984; Vallbo and Wessberg, 1993). Moreover, vibrissal movement generated at around 10 Hz (Fukuda et al., 1989) is abolished by electrolytic lesions of the IO in rats (Semba and Komisaruk, 1984), supporting that STOs in IO neurons could be the origin of the physiological tremor.
While the frequencies of STOs are ~10 Hz, the average firing rate of IO neuron is about 1 Hz, suggesting that individual IO neurons are insufficient to generate signal for 10 Hz physiological tremor (Keating and Thach, 1997; Lang et al., 1999; Chorev et al., 2007). However, presence of gap junction in IO and property of their connectivity with descending motor pathway support that STOs in IO neurons could be responsible for physiological tremor. Gap junctions synchronize IO neurons with a 10 Hz STO rhythm (Long et al., 2002; Van Der Giessen et al., 2008), ensuring that some IO neurons fire with the 10 Hz cycle at the population level (Chorev et al., 2007; Park et al., 2010). Since multiple IO neurons innervate a DCN neuron via Purkinje cells (Van der Want et al., 2004), an individual DCN neuron may receive 10Hz rhythmic input, as well as pharmacologically induced synchronization of IO rhythmic activity evoke rhythmic modulation of DCN firing with same frequency (Lamarre et al., 1971; Park et al., 2010). The 10 Hz oscillation in DCN may recruit brainstem nuclei (e.g., the red nucleus and the lateral reticular formation) and motor neurons, resulting in 10 Hz physiological tremor (Figure 2, left).
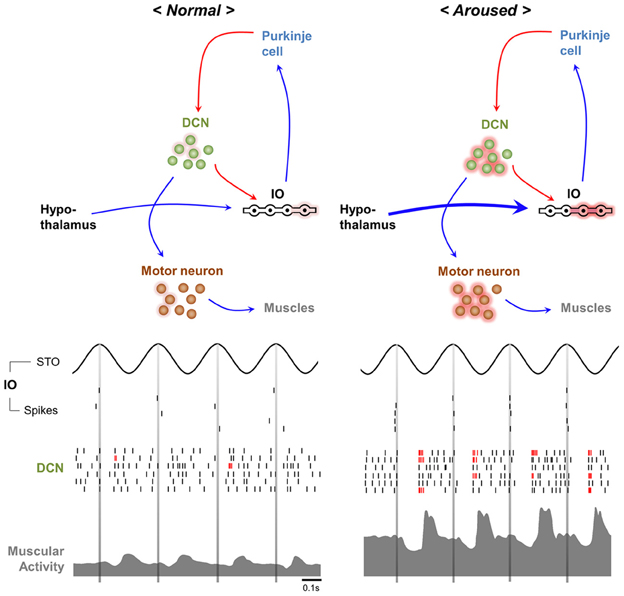
Figure 2. Hypothetical mechanisms underlying normal and arousal-enhanced physiological tremors. Physiological tremor generation (left). Adjacent IO neurons share ~10 Hz STOs via gap junctions, which results in ~10 Hz rhythms in the population of cells. Action potentials propagate from IO neurons to Purkinje cells and then to the DCN, thereby causing inhibitory responses and then rebound excitation in DCN neurons. The rhythmicity of the IO neuron population is converted into the rhythmicity of individual DCN neurons, which results in 10 Hz rhythmic muscle contractions. Increased excitatory inputs from hypothalamic neurons depolarize the membrane potentials of IO neurons upon arousal (right), which increases the ~10 Hz rhythmic LTS (red bars) in DCN neurons and muscle contractions.
One simple way to link STOs and physiological tremor would be to examine physiological tremors in CaV3.1−/− mice that lack STOs of IO neurons (Park et al., 2010). Unfortunately, physiological tremors are not well documented in mice. One study reported that 20–35 Hz forelimb vibration may reflect physiological tremor in mice (Kralic et al., 2005). However, the 20–35 Hz vibration could be an artifact of resonant frequencies in the recording system of the study. Application of more sensitive techniques, such as electromagnetic or optoelectronic detection methods (Grimaldi and Manto, 2008), might be necessary for future studies on the mechanism of physiological tremors in mice.
Arousal-Induced Enhancement of Physiological Tremors
Physiological tremors in both humans and animals are amplified in response to various alerting stimuli such as anger, novelty, or stress (Günther et al., 1983; Duan et al., 1996; Klein, 2002; Siniscalchi et al., 2013). The enhanced physiological tremor is probably important for providing optimal muscular coordination during arousal. Because the hypothalamus controls arousal (Lin et al., 1988; Adamantidis et al., 2007; Tsunematsu et al., 2011) and its projections to IO neurons are revealed by anterograde tracing with AAV virus (Lein et al., 2006), we here propose that arousal-induced physiological tremors could be dependent upon the excitation of IO neurons (Figure 2, right) by hypothalamic input.
Increased excitatory input to the IO may enhance synchrony between IO neurons, since infusion of a glutamate receptor antagonist into the IO decreases complex spike synchrony in the mediolateral direction (Lang, 2002). Otherwise, the increased firing rate of IO neurons might also raises the probability of synchronous firing among IO neurons. Hypersynchronous IO firing would be translated into rhythmic LTS in individual DCN neurons (Hoebeek et al., 2010; De Zeeuw et al., 2011) that might cause high amplitude physiological tremor during arousal (Witter et al., 2013) (Figure 2, right).
Among the three isoforms of T-type Ca2+ channels expressed in DCN neurons (Figure 1), CaV3.1 is thought to serve a role in the tremor, as CaV3.1 is responsible for generating LTS with multiple sodium spikes in DCN neurons (Molineux et al., 2006). Analysis of muscular activities of CaV3.1−/− mice in response to novel contexts is required to address this possibility.
Modulation of Movement Initiation Timing
In humans movement initiation is connected to the timing of physiological tremor in some respects (Travis, 1929; Goodman and Kelso, 1983). For example, the 10 Hz periodicity of motor initiation timing is observed in various parts of human body (Harter and White, 1968). While this restriction may reduce the temporal precision of movement initiation (Figure 3, upper) (Lakie and Combes, 2000; Lakie, 2010), physiological tremor also helps overcome muscular friction prior to action initiation and permits more powerful and faster movements (Adamovich et al., 1994; de Rugy and Sternad, 2003). Modulation of Cav3.1 in IO neurons hypothesized to be involved in physiological tremor and is expected to affect the kinetics of movement inhibition.
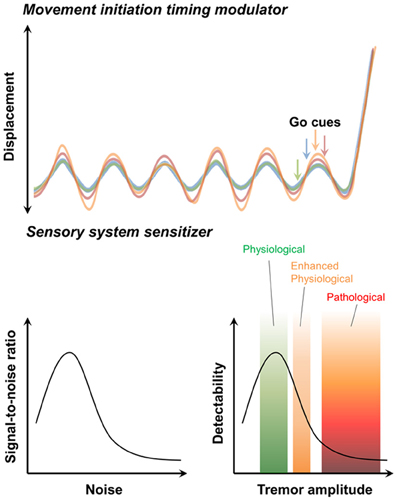
Figure 3. Possible contribution of physiological tremors to motor function. The movement initiation timing modulator hypothesis (upper). Initiation of voluntary movement in response to “Go” cues depends on the phase of the ongoing physiological tremor. The sensory system sensitivity hypothesis (lower). The stochastic resonance signature (left). “Noise” caused by physiological tremors increases the ability to detect signals. By contrast, pathological tremors decrease the ability of the sensory system to detect signals (right).
The basal ganglia-thalamocortical circuit is important for movement initiation in both rodents and primates (Yin and Knowlton, 2006; Bédard and Sanes, 2011). Studies show that the GABAergic outputs of medium spiny neurons expressing dopamine receptor 1 or 2 in the dorsal striatum play inhibitory or facilitatory roles, respectively, in movement initiation (Kravitz and Kreitzer, 2012). However, in vivo imaging study shows that the both types of medium spiny neurons are activated during movement initiation in mice (Cui et al., 2013). Therefore, the role of medium spiny neurons in movement initiation is still controversial (Surmeier, 2013).
During resting states, there is increased inhibition of VL neurons by the basal ganglia, which in turn raises the threshold for the onset of movement initiation signals in the thalamocortical pathway. VL neurons also receive excitatory signals from DCN through monosynaptic connections between them (Shinoda et al., 1985, 1993; Lein et al., 2006). Consistent with their supposed role in generating 10 Hz DCN rhythms (Figure 2, left), T-type Ca2+ channels may also play a role in movement initiation. 10 Hz rhythmic signals in DCN neurons may reduce the action potential threshold in VL neurons. It will be necessary to selectively inhibit DCN-VL circuits in future studies.
Physiological Tremors Provide Preparation for Emergent Motor Responses
Emergent motor responses are critical for the survival of animals in nature. The unexpectancy hypothesis of IO function (Devor, 2002) states that IO neurons reliably respond to unexpected motor disturbances. One example is the increased IO neuronal excitability when cat misses a step on a ladder by unexpected rung down (Andersson and Armstrong, 1987). Consistent with this idea, IO neuronal excitability decreases after rodents learn the timing of air puffs (Kim et al., 1998). As with hypothalamic control of the IO, unexpected external stimuli could activate hypothalamic arousal pathways and amplify 10 Hz physiological tremors through the action of CaV3.1 in the DCN (Figure 2, right). This enhanced tremor may help overcome inertial resistances and synchronize muscles when emergent motor responses are required (Greene, 1972).
Meanwhile, T-type Ca2+ channel also could contribute to the generation of emergent movement through a DCN-dependent mechanism. LTS in DCN neurons is proposed to provide synchronous and strong output to descending motor pathways (De Zeeuw et al., 2011). Recently, a study with optogenetic modulation of Purkinje cells reveals that induced LTS in DCN neurons can evoke emergent movement (Witter et al., 2013). Cessation of induced Purkinje cell ensemble activity induces rebound activity in DCN and timed movement whose amplitude is dependent on the degree of Purkinje cell activation. As CaV3.1 is responsible for generating LTS with multiple sodium spikes in DCN neurons (Molineux et al., 2006), analysis of emergent motor responses generated by CaV3.1−/− mice would help to access this idea.
Sensory Sensitization Hypothesis
Studies of human sensory perception suggest that physiological tremors can facilitate sensory functions. For example, eyeball tremors sensitize visual function (Hennig et al., 2002). Intentional suppression of physiological limb tremors reduces visual cue-tracking abilities (Daneault et al., 2011). Moreover, artificial vibrations of foot muscles, which could mimic sensory feedback by physiological tremors, increase somatosensory sensitivity (Liu et al., 2002).
Common sense suggests that proprioceptive feedback signals resulted from physiological tremors act as “noise” that might interfere with sensation. However, the stochastic resonance theory (McNamara et al., 1988; Wiesenfeld and Moss, 1995) states that moderate levels of “noise” actually facilitate signal detection in nervous system (Figure 3, lower) (Douglass et al., 1993; Levin and Miller, 1996). Therefore, by providing moderate noise, physiological tremors could enhance sensory detection (Figure 3, lower) and subsequently raise motor performances. The role of CaV3.1 in the IO in generating physiological tremor would be critical in this process.
T-type Ca2+ channels in the thalamus might also be associated with sensory sensitization. VL thalamocortical relay neurons may receive tremor signals generated by the cerebellum through DCN-VL connections. Rhythmic activation of the VL neurons activates GABAergic nRT neurons through reciprocal connections between TC and nRT neurons (Huguenard and Prince, 1994). Activation of nRT neurons can in turn induces rhythmic inhibition and thus LTS in VL thalamic neurons, while non-specifically inhibit TC neurons with other modalities. This may increase sensory coding in TC neurons: intensifying VL thalamic input with LTS while filtering out weak intensity sensory stimuli from other thalamic nuclei (Pinault and Deschênes, 2001). The role of LTS in sensory processing is controversial (Beurrier et al., 1999; Perez-Reyes, 2003), and studies evaluating sensory processing in the knockout mice of CaV3.1 knockout mice would verify this hypothesis.
Muscular Activity Changes According to Emotion
Emotions play an essential role in modulating motor functions. Anger or panic increases physiological tremor (Duan et al., 1996; Klein, 2002; Siniscalchi et al., 2013) and can severely impair motor coordination in human (Parker et al., 1993; Allgulander et al., 2003). In the patient with dystonia which is characterized by sustained synchronous muscle contractions and twisting body parts (Herz, 1944), dystonic symptoms become exaggerated in response to fear or stress (Burgyone et al., 2004; Jabusch and Altenmuller, 2004; Calderon et al., 2011)
The hypothalamus is activated by either fear or stress (Porter, 1952; Yokoo et al., 1990; Tsunematsu et al., 2011) and connections between the hypothalamus and the IO (Lein et al., 2006) lead some to speculate that the activation of IO neurons may mediate increased dystonic responses as a result of fear or stress. Subsequent activation of LTS in DCN neurons by synchronous inputs from IO neurons may increase muscular synchrony and symptoms of dystonia (Figure 2, right). Because CaV3.1 channel majorly generate LTS in DCN neurons, knockout of CaV3.1, or the application of T-type Ca2+ channel blockers in the DCN may ameliorate emotion-dependent motor symptoms.
Conclusion
T-type Ca2+ channels are expressed in neurons that comprise motor circuits, but the roles of these channels in physiological motor functions remain unknown. Because T-type Ca2+ channels are involved in generating pathological tremors, we propose that these channels may also play important roles in various physiological motor functions by enhancing physiological tremors or muscle tone. Optogenetic techniques (Boyden et al., 2005; Deisseroth, 2010) may be useful for identifying the neural circuits and cell types that underlie each of these physiological motor functions. Once the neural circuits are defined, then isoform-specific knockdowns of T-type Ca2+ channels (Park et al., 2010) can be applied to identified circuits. Future studies into physiological tremors and T-type Ca2+ channels using advanced technologies will improve our understanding of the neural mechanisms underlying higher motor coordination.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Research Foundation (NRF) and the KAIST Future Systems Healthcare Project from the Ministry of Science, ICT and Future Planning (No. 20120008795, 2012K001117)
References
Adamantidis, A. R., Zhang, F., Aravanis, A. M., Deisseroth, K., and de Lecea, L. (2007). Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424. doi: 10.1038/nature06310
Adamovich, S., Levin, M., and Feldman, A. (1994). Merging different motor patterns: coordination between rhythmical and discrete single-joint movements. Exp. Brain Res. 99, 325–337. doi: 10.1007/BF00239599
Ahmed, A., and Taylor, N. (2012). The analysis of drug-induced tremor in mice. Br. J. Pharmacol. Chemother. 14, 350–354. doi: 10.1111/j.1476-5381.1959.tb00255.x
Allgulander, C., Bandelow, B., Hollander, E., Montgomery, S., Nutt, D., Okasha, A., et al. (2003). WCA recommendations for the long-term treatment of generalized anxiety disorder. CNS Spectr. 8, 53.
Amtage, F., Henschel, K., Schelter, B., Vesper, J., Timmer, J., Lucking, C. H., et al. (2008). Tremor-correlated neuronal activity in the subthalamic nucleus of Parkinsonian patients. Neurosci. Lett. 442, 195–199. doi: 10.1016/j.neulet.2008.06.087
Andersson, G., and Armstrong, D. M. (1987). Complex spikes in Purkinje cells in the lateral vermis (b zone) of the cat cerebellum during locomotion. J. Physiol. 385, 107–134.
Axelrad, J. E., Louis, E. D., Honig, L. S., Flores, I., Ross, G. W., Pahwa, R., et al. (2008). Reduced Purkinje cell number in essential tremor: a postmortem study. Arch. Neurol. 65, 101. doi: 10.1001/archneurol.2007.8
Bain, P., Brin, M., Deuschl, G., Elble, R., Jankovic, J., Findley, L., et al. (2000). Criteria for the diagnosis of essential tremor. Neurology 54, S7.
Bal, T., and McCormick, D. A. (1997). Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current I h. J. Neurophysiol. 77, 3145–3156.
Battista, A., Goldstein, M., Nakatani, S., and Anagnoste, B. (1969). Drug induced changes of abnormal movements in monkeys with central nervous system lesions. Stereotact. Funct. Neurosurg. 31, 135–144. doi: 10.1159/000103474
Bédard, P., and Sanes, J. N. (2011). Basal ganglia-dependent processes in recalling learned visual-motor adaptations. Exp. Brain Res. 209, 385–393. doi: 10.1007/s00221-011-2561-y
Bergman, H., Wichmann, T., and Delong, M. R. (1990). Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249, 1436–1438. doi: 10.1126/science.2402638
Beurrier, C., Congar, P., Bioulac, B., and Hammond, C. (1999). Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J. Neurosci. 19, 599–609.
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., and Deisseroth, K. (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268. doi: 10.1038/nn1525
Brennan, K. C., Jurewicz, E. C., Ford, B., Pullman, S. L., and Louis, E. D. (2002). Is essential tremor predominantly a kinetic or a postural tremor? A clinical and electrophysiological study. Mov. Disord. 17, 313–316. doi: 10.1002/mds.10003
Britton, T., Thompson, P., Day, B., Rothwell, J., Findley, L., and Marsden, C. (2004). Modulation of postural wrist tremors by magnetic stimulation of the motor cortex in patients with Parkinson's disease or essential tremor and in normal subjects mimicking tremor. Ann. Neurol. 33, 473–479. doi: 10.1002/ana.410330510
Brooks, D., Playford, E., Ibanez, V., Sawle, G., Thompson, P., Findley, L., et al. (1992). Isolated tremor and disruption of the nigrostriatal dopaminergic system an 18F−dopa PET study. Neurology 42, 1554–1554. doi: 10.1212/WNL.42.8.1554
Burgyone, K., Aduri, K., Ananth, J., and Parameswaran, S. (2004). The use of antiparkinsonian agents in the management of drug-induced extrapyramidal symptoms. Curr. Pharm. Des. 10, 2239–2248. doi: 10.2174/1381612043384123
Calderon, D. P., Fremont, R., Kraenzlin, F., and Khodakhah, K. (2011). The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat. Neurosci. 14, 357–365. doi: 10.1038/nn.2753
Chan, C. S., Glajch, K. E., Gertler, T. S., Guzman, J. N., Mercer, J. N., Lewis, A. S., et al. (2011). HCN channelopathy in external globus pallidus neurons in models of Parkinson's disease. Nat. Neurosci. 14, 85–92. doi: 10.1038/nn.2692
Chang, K. Y., Park, Y. G., Park, H. Y., Homanics, G. E., Kim, J., and Kim, D. (2011). Lack of CaV3.1 channels causes severe motor coordination defects and an age-dependent cerebellar atrophy in a genetic model of essential tremor. Biochem. Biophys. Res. Commun. 410, 19–23. doi: 10.1016/j.bbrc.2011.05.082
Choi, S., Na, H., Kim, J., Lee, J., Lee, S., Kim, D., et al. (2006). Attenuated pain responses in mice lacking CaV3. 2 T−type channels. Genes Brain Behav. 6, 425–431. doi: 10.1111/j.1601-183X.2006.00268.x
Choi, S., Yu, E., Kim, D., Urbano, F. J., Makarenko, V., Shin, H. S., et al. (2010). Subthreshold membrane potential oscillations in inferior olive neurons are dynamically regulated by P/Q-and T-type calcium channels: a study in mutant mice. J. Physiol. 588, 3031–3043. doi: 10.1113/jphysiol.2009.184705
Chorev, E., Yarom, Y., and Lampl, I. (2007). Rhythmic episodes of subthreshold membrane potential oscillations in the rat inferior olive nuclei in vivo. J. Neurosci. 27, 5043–5052. doi: 10.1523/JNEUROSCI.5187-06.2007
Cribbs, L. L., Lee, J. H., Yang, J., Satin, J., Zhang, Y., Daud, A., et al. (1998). Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ. Res. 83, 103–109. doi: 10.1161/01.RES.83.1.103
Crunelli, V., Lightowler, S., and Pollard, C. E. (1989). A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J. Physiol. 413, 543–561.
Cui, G., Jun, S. B., Jin, X., Pham, M. D., Vogel, S. S., Lovinger, D. M., et al. (2013). Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–241. doi: 10.1038/nature11846
Daneault, J. F., Carignan, B., and Duval, C. (2011). Finger tremor can be voluntarily reduced during a tracking task. Brain Res. 1370, 164–174. doi: 10.1016/j.brainres.2010.11.047
de Montigny, C., and Lamarre, Y. (1973). Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res. 53, 81–95. doi: 10.1016/0006-8993(73)90768-3
de Rugy, A., and Sternad, D. (2003). Interaction between discrete and rhythmic movements: reaction time and phase of discrete movement initiation during oscillatory movements. Brain Res. 994, 160–174. doi: 10.1016/j.brainres.2003.09.031
Deiber, M. P., Pollak, P., Passingham, R., Landais, P., Gervason, C., Cinotti, L., et al. (1993). Thalamic stimulation and suppression of parkinsonian tremor. Evidence of a cerebellar deactivation using positron emission tomography. Brain 116, 267–279. doi: 10.1093/brain/116.1.267
Deuschl, G., Raethjen, J., Baron, R., Lindemann, M., Wilms, H., and Krack, P. (2000). The pathophysiology of parkinsonian tremor: a review. J. Neurol. 247, 33–48. doi: 10.1007/PL00007781
Deuschl, G., Raethjen, J., Lindemann, M., and Krack, P. (2001). The pathophysiology of tremor. Muscle Nerve 24, 716–735. doi: 10.1002/mus.1063
Devor, A. (2002). The great gate: control of sensory information flow to the cerebellum. Cerebellum 1, 27–34. doi: 10.1080/147342202753203069
De Zeeuw, C. I., Hoebeek, F. E., Bosman, L. W., Schonewille, M., Witter, L., and Koekkoek, S. K. (2011). Spatiotemporal firing patterns in the cerebellum. Nat. Rev. Neurosci. 12, 327–344. doi: 10.1038/nrn3011
De Zeeuw, C. I., Hoogenraad, C. C., Koekkoek, S., Ruigrok, T. J., Galjart, N., and Simpson, J. I. (1998). Microcircuitry and function of the inferior olive. Trends Neurosci. 21, 391–400. doi: 10.1016/S0166-2236(98)01310-1
Douglass, J. K., Wilkens, L., Pantazelou, E., and Moss, F. (1993). Noise enhancement of information transfer in crayfish mechanoreceptors by stochastic resonance. Nature 365, 337–340. doi: 10.1038/365337a0
Du, W., Aloyo, V. J., and Harvey, J. A. (1997). Harmaline competitively inhibits [3H]MK-801 binding to the NMDA receptor in rabbit brain. Brain Res. 770, 26–29. doi: 10.1016/S0006-8993(97)00606-9
Duan, Y. F., Winters, R., McCabe, P. M., Green, E. J., Huang, Y., and Schneiderman, N. (1996). Behavioral characteristics of defense and vigilance reactions elicited by electrical stimulation of the hypothalamus in rabbits. Behav. Brain Res. 81, 33–41. doi: 10.1016/S0166-4328(96)00042-3
Elble, R. J., and Randall, J. E. (1976). Motor-unit activity responsible for 8-to 12-Hz component of human physiological finger tremor. J. Neurophysiol. 39, 370–383.
Elble, R. J., Schieber, M. H., and Thach, W. T. Jr. (1984). Activity of muscle spindles, motor cortex and cerebellar nuclei during action tremor. Brain Res. 323, 330–334. doi: 10.1016/0006-8993(84)90308-1
Ernst, W. L., Zhang, Y., Yoo, J. W., Ernst, S. J., and Noebels, J. L. (2009). Genetic enhancement of thalamocortical network activity by elevating α1G-mediated low-voltage-activated calcium current induces pure absence epilepsy. J. Neurosci. 29, 1615–1625. doi: 10.1523/JNEUROSCI.2081-08.2009
Findley, L. J. (1996). Classification of tremors. J. Clin. Neurophysiol. 13, 122–132. doi: 10.1097/00004691-199603000-00003
Fukuda, M., Yamamoto, T., and Llinas, R. (1989). Simultaneous recordings from Purkinje cells in rat cerebellum, and their relation to movement. Neurosci. Res. Suppl. 9, 99.
Ghaemi, M., Raethjen, J., Hilker, R., Rudolf, J., Sobesky, J., Deuschl, G., et al. (2002). Monosymptomatic resting tremor and Parkinson's disease: a multitracer positron emission tomographic study. Mov. Disord. 17, 782–788. doi: 10.1002/mds.10125
Glennon, R. A., Dukat, M., Grella, B., Hong, S., Costantino, L., Teitler, M., et al. (2000). Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A), dopamine (D(2) and benzodiazepine receptors. Drug Alcohol Depend. 60, 121–132. doi: 10.1016/S0376-8716(99)00148-9
Goodman, D., and Kelso, J. A. S. (1983). Exploring the functional significance of physiological tremor: a biospectroscopic approach. Exp. Brain Res. 49, 419–431. doi: 10.1007/BF00238783
Grimaldi, G., and Manto, M. (2008). Tremor: from pathogenesis to treatment. Synth. Lect. Biomed. Eng. 3, 1–212. doi: 10.2200/S00129ED1V01Y200807BME020
Guiot, G. (1958). Le traitement des syndromes parkinsoniens par la destruction du pallidum interne. Neurochirurgia 1, 94–98.
Günther, H., Brunner, R., and Klussmann, F. (1983). Spectral analysis of tremorine and cold tremor electromyograms in animal species of different size. Pflügers Arch. 399, 180–185. doi: 10.1007/BF00656712
Hagbarth, K. E., Jessop, J., Eklund, G., and Wallin, E. (1983). The Piper rhythm–a phenomenon related to muscle resonance characteristics? Acta Physiol. Scand. 117, 263–271. doi: 10.1111/j.1748-1716.1983.tb07205.x
Hallett, M., and Dubinsky, R. M. (1993). Glucose metabolism in the brain of patients with essential tremor. J. Neurol. Sci. 114, 45–48. doi: 10.1016/0022-510X(93)90047-3
Halliday, D., Conway, B., Farmer, S., Shahani, U., Russell, A., and Rosenberg, J. (2000). Coherence between low-frequency activation of the motor cortex and tremor in patients with essential tremor. Lancet 355, 1149–1153. doi: 10.1016/S0140-6736(00)02064-X
Handforth, A., Homanics, G. E., Covey, D. F., Krishnan, K., Lee, J. Y., Sakimura, K., et al. (2010). T-type calcium channel antagonists suppress tremor in two mouse models of essential tremor. Neuropharmacology 59, 380–387. doi: 10.1016/j.neuropharm.2010.05.012
Harter, M. R., and White, C. (1968). Periodicity within reaction time distributions and electromyograms. Q. J. Exp. Psychol. 20, 157–166. doi: 10.1080/14640746808400144
Hassler, R., Riechert, T., Mundinger, F., Umbach, W., and Ganglberger, J. (1960). Physiological observations in stereotaxic operations in extrapyramidal motor disturbances. Brain 83, 337–350. doi: 10.1093/brain/83.2.337
Hellwig, B., and Häußler, S. Schelter, B., Lauk, M., Guschlbauer, B., Timmer, J. et al. (2001). Tremor-correlated cortical activity in essential tremor. Lancet 357, 519–523. doi: 10.1016/S0140-6736(00)04044-7
Helmich, R. C., Hallett, M., Deuschl, G., Toni, I., and Bloem, B. R. (2012). Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain 135, 3206–3226. doi: 10.1093/brain/aws023
Hennig, M. H., Kerscher, N. J., Funke, K., and Wörgötter, F. (2002). Stochastic resonance in visual cortical neurons: does the eye-tremor actually improve visual acuity? Neurocomputing 44, 115–120. doi: 10.1016/S0925-2312(02)00371-5
Herz, E. (1944). Dystoniai. Histological review; analysis of dystonic symptoms and physiologic mechanisms involved. Arch. Neurol. Psychiatry 51, 305–318. doi: 10.1001/archneurpsyc.1944.02290280003001
Herzog, J., Hamel, W., Wenzelburger, R., Pötter, M., Pinsker, M. O., Bartussek, J., et al. (2007). Kinematic analysis of thalamic versus subthalamic neurostimulation in postural and intention tremor. Brain 130, 1608–1625. doi: 10.1093/brain/awm077
Hirsch, E. C., Mouatt, A., Faucheux, B., Bonnet, A. M., Javoy-Agid, F., Graybiel, A. M., et al. (1992). Dopamine, tremor, and Parkinson's disease. Lancet 340, 125. doi: 10.1016/0140-6736(92)90457-E
Hoebeek, F. E., Witter, L., Ruigrok, T. J. H., and De Zeeuw, C. I. (2010). Differential olivo-cerebellar cortical control of rebound activity in the cerebellar nuclei. Proc. Natl. Acad. Sci. U.S.A. 107, 8410–8415. doi: 10.1073/pnas.0907118107
Huguenard, J. (1996). Low-threshold calcium currents in central nervous system neurons. Annu. Rev. Physiol. 58, 329–348. doi: 10.1146/annurev.ph.58.030196.001553
Huguenard, J., and Prince, D. (1994). Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. J. Neurosci. 14, 5485–5502.
Jabusch, H. C., and Altenmuller, E. (2004). Anxiety as an aggravating factor during onset of focal dystonia in musicians. Med. Probl. Perform. Art. 19, 75–81.
Jasper, H. H., Shacter, D. G., and Montplaisir, J. (1970). The effect of local cooling upon spontaneous and evoked electrical activity of cerebral cortex. Can. J. Physiol. Pharmacol. 48, 640–652. doi: 10.1139/y70-094
Jellinger, K. (1999). Post mortem studies in Parkinson's disease–is it possible to detect brain areas for specific symptoms? J. Neural Transm. Suppl. 56, 1. doi: 10.1007/978-3-7091-6360-3_1
Keating, J., and Thach, W. (1997). No clock signal in the discharge of neurons in the deep cerebellar nuclei. J. Neurophysiol. 77, 2232–2234.
Khosrovani, S., Van Der Giessen, R., De Zeeuw, C., and De Jeu, M. (2007). In vivo mouse inferior olive neurons exhibit heterogeneous subthreshold oscillations and spiking patterns. Proc. Natl. Acad. Sci. U.S.A. 104, 15911–15916. doi: 10.1073/pnas.0702727104
Kim, D., Song, I., Keum, S., Lee, T., Jeong, M. J., Kim, S. S., et al. (2001). Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking α1G T-type Ca2+ channels. Neuron 31, 35–45. doi: 10.1016/S0896-6273(01)00343-9
Kim, J., Woo, J., Park, Y. G., Chae, S., Jo, S., Choi, J. W., et al. (2011). Thalamic T-type Ca2+ channels mediate frontal lobe dysfunctions caused by a hypoxia-like damage in the prefrontal cortex. J. Neurosci. 31, 4063–4073. doi: 10.1523/JNEUROSCI.4493-10.2011
Kim, J. J., Krupa, D. J., and Thompson, R. F. (1998). Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science 279, 570–573. doi: 10.1126/science.279.5350.570
Kim, J. S., Park, J. W., Kim, W. J., Kim, H. T., Kim, Y. I., and Lee, K. S. (2006). Disappearance of essential tremor after frontal cortical infarct. Mov. Disord. 21, 1284–1285. doi: 10.1002/mds.20894
Klien, H. (1907). Zur pathologie der kontinuierlichen rhythmischen Krämpfe der Schlingmuskulatur (2 Fälle von Erweichungsherden im Kleinhirn). Neurologisches Centralblatt 26, 245–254.
Kovach, M. J., Ruiz, J., Kimonis, K., Mueed, S., Sinha, S., Higgins, C., et al. (2001). Genetic heterogeneity in autosomal dominant essential tremor. Genet. Med. 3, 197–199. doi: 10.1097/00125817-200105000-00009
Krack, P., Hamel, W., Mehdorn, H. M., and Deuschl, G. (1999). Surgical treatment of Parkinson's disease. Curr. Opin. Neurol. 12, 417–425. doi: 10.1097/00019052-199908000-00008
Kralic, J. E., Criswell, H. E., Osterman, J. L., O'Buckley, T. K., Wilkie, M. E., Matthews, D. B., et al. (2005). Genetic essential tremor in gamma-aminobutyric acidA receptor alpha1 subunit knockout mice. J. Clin. Invest. 115, 774–779. doi: 10.1172/JCI23625
Kravitz, A. V., and Kreitzer, A. C. (2012). Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology 27, 167–177. doi: 10.1152/physiol.00004.2012
Kumar, R., Lozano, A. M., Kim, Y. J., Hutchison, W. D., Sime, E., Halket, E., et al. (1998). Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson's disease. Neurology 51, 850–855. doi: 10.1212/WNL.51.3.850
Kurtzke, J. F. (1982). The current neurologic burden of illness and injury in the United States. Neurology 32, 1207–1207. doi: 10.1212/WNL.32.11.1207
Lakie, M. (2010). The influence of muscle tremor on shooting performance. Exp. Physiol. 95, 441–450. doi: 10.1113/expphysiol.2009.047555
Lakie, M., and Combes, N. (2000). There is no simple temporal relationship between the initiation of rapid reactive hand movements and the phase of an enhanced physiological tremor in man. J. Physiol. 523, 515–522. doi: 10.1111/j.1469-7793.2000.t01-2-00515.x
Lamarre, Y., de Montigny, C., Dumont, M., and Weiss, M. (1971). Harmaline-induced rhythmic activity of cerebellar and lower brain stem neurons. Brain Res. 32, 246. doi: 10.1016/0006-8993(71)90174-0
Lang, E. J. (2002). GABAergic and glutamatergic modulation of spontaneous and motor-cortex-evoked complex spike activity. J. Neurophysiol. 87, 1993–2008. doi: 10.1152/jn.00477.2001
Lang, E. J., Sugihara, I., Welsh, J. P., and Llinás, R. (1999). Patterns of spontaneous Purkinje cell complex spike activity in the awake rat. J. Neurosci. 19, 2728–2739.
Lapresle, J. (1979). Rhythmic palatal myoclonus and the dentato-olivary pathway. J. Neurol. 220, 223–230. doi: 10.1007/BF00314146
Lee, J. H., Daud, A. N., Cribbs, L. L., Lacerda, A. E., Pereverzev, A., Klöckner, U., et al. (1999). Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J. Neurosci. 19, 1912–1921.
Lenz, F., Normand, S., Kwan, H., Andrews, D., Rowland, L., Jones, M., et al. (1995). Statistical prediction of the optimal site for thalamotomy in parkinsonian tremor. Mov. Disord. 10, 318–328. doi: 10.1002/mds.870100315
Lein, E. S., Hawrylycz, M. J., Ao, N., Ayres, M., Bensinger, A., Bernard, A., et al. (2006). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. doi: 10.1038/nature05453
Levin, J. E., and Miller, J. P. (1996). Broadband neural encoding in the cricket cereal sensory system enhanced by stochastic resonance. Nature 380, 165–168. doi: 10.1038/380165a0
Leznik, E., Makarenko, V., and Llinás, R. (2002). Electrotonically mediated oscillatory patterns in neuronal ensembles: an in vitro voltage-dependent dye-imaging study in the inferior olive. J. Neurosci. 22, 2804–2815.
Lin, J. S., Sakai, K., and Jouvet, M. (1988). Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology 27, 111–122. doi: 10.1016/0028-3908(88)90159-1
Liu, W., Lipsitz, L. A., Montero-Odasso, M., Bean, J., Kerrigan, D. C., and Collins, J. J. (2002). Noise-enhanced vibrotactile sensitivity in older adults, patients with stroke, and patients with diabetic neuropathy. Arch. Phys. Med. Rehabil. 83, 171–176. doi: 10.1053/apmr.2002.28025
Llinas, R., and Pare, D. (1994). Role of intrinsic neuronal oscillations and network ensembles in the genesis of normal and pathological tremors. Neurol. Dis. Ther. 30, 7–36.
Llinas, R., and Volkind, R. (1973). The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp. Brain Res. 18, 69–87. doi: 10.1007/BF00236557
Llinas, R., and Yarom, Y. (1986). Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J. Physiol. 376, 163–182.
Long, M. A., Deans, M. R., Paul, D. L., and Connors, B. W. (2002). Rhythmicity without synchrony in the electrically uncoupled inferior olive. J. Neurosci. 22, 10898–10905.
Louis, E., Vonsattel, J., Honig, L., Ross, G., Lyons, K., and Pahwa, R. (2006). Neuropathologic findings in essential tremor. Neurology 66, 1756–1759. doi: 10.1212/01.wnl.0000218162.80315.b9
Louis, E. D. (2005). Essential tremor. Lancet Neurol. 4, 100–110. doi: 10.1016/S1474-4422(05)00991-9
Louis, E. D., Faust, P. L., Vonsattel, J. P. G., Honig, L. S., Rajput, A., Robinson, C. A., et al. (2007). Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 130, 3297–3307. doi: 10.1093/brain/awm266
Lutomski, J., Malek, B., and Stachowiak, Z. (1974). Pharmacochemical investigation of the raw materials from passiflora genus. 1. New method of chromatographic separation and fluorometric-planimetric determination of alkaloids and flavonoids in harman raw materials (author's transl)]. Planta Med. 26, 311. doi: 10.1055/s-0028-1099393
Magnin, M., Morel, A., and Jeanmonod, D. (2000). Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience 96, 549–564. doi: 10.1016/S0306-4522(99)00583-7
Mangoni, M. E., Traboulsie, A., Leoni, A. L., Couette, B., Marger, L., Le Quang, K., et al. (2006). Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3. 1/α1G T-type calcium channels. Circ. Res. 98, 1422–1430. doi: 10.1161/01.RES.0000225862.14314.49
Marsden, C., Meadows, J., Lange, G., and Watson, R. (1969). The role of the ballistocardiac impulse in the genesis of physiological tremor. Brain 92, 647–662. doi: 10.1093/brain/92.3.647
Mauk, M., Medina, J., Nores, W., and Ohyama, T. (2000). Cerebellar function: coordination, learning or timing? Curr. Biol. 10, R522–R525. doi: 10.1016/S0960-9822(00)00584-4
McNamara, B., Wiesenfeld, K., and Roy, R. (1988). Observation of stochastic resonance in a ring laser. Phys. Rev. Lett. 60, 2626–2629. doi: 10.1103/PhysRevLett.60.2626
Miyagishima, T., Takahashi, A., Kikuchi, S., Watanabe, K., Hirato, M., Saito, N., et al. (2007). Effect of ventralis intermedius thalamotomy on the area in the sensorimotor cortex activated by passive hand movements: fMR imaging study. Stereotact. Funct. Neurosurg. 85, 225–234. doi: 10.1159/000103261
Molineux, M. L., McRory, J. E., McKay, B. E., Hamid, J., Mehaffey, W. H., Rehak, R., et al. (2006). Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proc. Natl. Acad. Sci. U.S.A. 103, 5555–5560. doi: 10.1073/pnas.0601261103
Park, Y. G., Park, H. Y., Lee, C. J., Choi, S., Jo, S., Choi, H., et al. (2010). CaV3. 1 is a tremor rhythm pacemaker in the inferior olive. Proc. Natl. Acad. Sci. U.S.A. 107, 10731–10736. doi: 10.1073/pnas.1002995107
Parker, G., Hadzi-Pavlovic, D., Brodaty, H., Boyce, P., Mitchell, P., Wilhelm, K., et al. (1993). Psychomotor disturbance in depression: defining the constructs. J. Affect. Disord. 27, 255–265. doi: 10.1016/0165-0327(93)90049-P
Paulus, W., and Jellinger, K. (1991). The neuropathologic basis of different clinical subgroups of Parkinson's disease. J. Neuropathol. Exp. Neurol. 50, 743. doi: 10.1097/00005072-199111000-00006
Payoux, P., Remy, P., Miloudi, M., Houeto, J.-L., Stadler, C., Bejjani, B.-P., et al. (2008). Contrasting changes in cortical activation induced by acute high-frequency stimulation within the globus pallidus in Parkinson's disease. J. Cereb. Blood Flow Metab. 29, 235–243. doi: 10.1038/jcbfm.2008.107
Pearce, J. (2008). Palatal myoclonus (syn. palatal tremor). Eur. Neurol. 60, 312–315. doi: 10.1159/000159929
Perez-Reyes, E. (2003). Molecular physiology of low-voltage-activated T-type calcium channels. Physiol. Rev. 83, 117–161. doi: 10.1152/physrev.00018.2002
Perez-Reyes, E., Cribbs, L. L., Daud, A., Lacerda, A. E., Barclay, J., Williamson, M. P., et al. (1998). Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature 391, 896–900. doi: 10.1038/36110
Pifl, C., Kish, S. J., and Hornykiewicz, O. (2012). Thalamic noradrenaline in Parkinson's disease: deficits suggest role in motor and non-motor symptoms. Mov. Disord. 27, 1618–1624. doi: 10.1002/mds.25109
Pinault, D., and Deschenes, M. (2001). Anatomical evidence for a mechanism of lateral inhibition in the rat thalamus. Eur. J. Neurosci. 10, 3462–3469. doi: 10.1046/j.1460-9568.1998.00362.x
Pinto, A. D., Lang, A. E., and Chen, R. (2003). The cerebellothalamocortical pathway in essential tremor. Neurology 60, 1985–1987. doi: 10.1212/01.WNL.0000065890.75790.29
Plenz, D., and Kital, S. T. (1999). A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 400, 677–682. doi: 10.1038/23281
Pollok, B., Makhloufi, H., Butz, M., Gross, J., Timmermann, L., Wojtecki, L., et al. (2009). Levodopa affects functional brain networks in Parkinsonian resting tremor. Mov. Disord. 24, 91–98. doi: 10.1002/mds.22318
Porter, R. (1952). Alterations in electrical activity of the hypothalamus induced by stress stimuli. Am. J. Physiol. Legacy Content 169, 629–637.
Potashkin, J. A., Blume, S. R., and Runkle, N. K. (2010). Limitations of animal models of Parkinson's disease. Parkinsons Dis. 2011, 658083. doi: 10.4061/2011/658083
Rehak, R., Bartoletti, T. M., Engbers, J. D., Berecki, G., Turner, R. W., and Zamponi, G. W. (2013). Low voltage activation of KCa1. 1 current by Cav3-KCa1. 1 complexes. PLoS ONE 8:e61844. doi: 10.1371/journal.pone.0061844
Ruigrok, T., de Zeeuw, C., and Voogd, J. (1990). Hypertrophy of inferior olivary neurons: a degenerative, regenerative or plasticity phenomenon. Eur. J. Morphol. 28, 224.
Semba, K., and Komisaruk, B. (1984). Neural substrates of two different rhythmical vibrissal movements in the rat. Neuroscience 12, 761–774. doi: 10.1016/0306-4522(84)90168-4
Shinoda, Y., Futami, T., and Kakei, S. (1993). Input-output organization of the ventrolateral nucleus of the thalamus. Stereotact. Funct. Neurosurg. 60, 17–31. doi: 10.1159/000100587
Shinoda, Y., Futami, T., and Kano, M. (1985). Synaptic organization of the cerebello-thalamo-cerebral pathway in the cat. II. Input-output organization of single thalamocortical neurons in the ventrolateral thalamus. Neurosci. Res. 2, 157–180. doi: 10.1016/0168-0102(85)90010-0
Simantov, R., Snyder, S. H., and Oster-Granite, M. L. (1976). Harmaline-induced tremor in the rat: abolition by 3-acetylpyridine destruction of cerebellar climbing fibers. Brain Res. 114, 144. doi: 10.1016/0006-8993(76)91016-7
Siniscalchi, M., McFarlane, J., Kauter, K., Quaranta, A., and Rogers, L. (2013). Cortisol levels in hair reflect behavioural reactivity of dogs to acoustic stimuli. Res. Vet. Sci. 94, 49–54. doi: 10.1016/j.rvsc.2012.02.017
Sinton, C. M., Krosser, B. I., Walton, K. D., and Llinás, R. R. (1989). The effectiveness of different isomers of octanol as blockers of harmaline-induced tremor. Pflügers Arch. Eur. J. Physiol. 414, 31–36. doi: 10.1007/BF00585623
Sotelo, C., Gotow, T., and Wassef, M. (1986). Localization of glutamic−acid−decarboxylase−immunoreactive axon terminals in the inferior olive of the rat, with special emphasis on anatomical relations between GABAergic synapses and dendrodendritic gap junctions. J. Comp. Neurol. 252, 32–50. doi: 10.1002/cne.902520103
Splettstoesser, F., Bonnet, U., Wiemann, M., Bingmann, D., and Busselberg, D. (2005). Modulation of voltage-gated channel currents by harmaline and harmane. Br. J. Pharmacol. 144, 52–58. doi: 10.1038/sj.bjp.0706024
Sturman, M. M., Vaillancourt, D. E., Metman, L. V., Bakay, R. A. E., and Corcos, D. M. (2004). Effects of subthalamic nucleus stimulation and medication on resting and postural tremor in Parkinson's disease. Brain 127, 2131–2143. doi: 10.1093/brain/awh237
Surmeier, D. J. (2013). Neuroscience: to go or not to go. Nature 494, 178–179. doi: 10.1038/nature11856
Tai, C. H., Yang, Y. C., Pan, M. K., Huang, C. S., and Kuo, C. C. (2011). Modulation of subthalamic T-type Ca2+ channels remedies locomotor deficits in a rat model of Parkinson disease. J. Clin. Invest. 121, 3289. doi: 10.1172/JCI46482
Talley, E. M., Cribbs, L. L., Lee, J. H., Daud, A., Perez-Reyes, E., and Bayliss, D. A. (1999). Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J. Neurosci. 19, 1895–1911.
Tarsy, D., Papavassiliou, E., Lyons, K. E., and Pahwa, R. (2008). “Thalamic Deep Brain Stimulation for Parkinson's Disease Tremor,” in Deep brain stimulation in neurological and psychiatric disorders. (Springer), 229–241.
Thach, W. T., Goodkin, H., and Keating, J. (1992). The cerebellum and the adaptive coordination of movement. Annu. Rev. Neurosci. 15, 403–442. doi: 10.1146/annurev.ne.15.030192.002155
Timmermann, L., Gross, J., Butz, M., Kircheis, G., Haussinger, D., and Schnitzler, A. (2004). Pathological oscillatory coupling within the human motor system in different tremor syndromes as revealed by magnetoencephalography. Neurol. Clin. Neurophysiol. 26.
Timmermann, L., Gross, J., Dirks, M., Volkmann, J., Freund, H. J., and Schnitzler, A. (2002). The cerebral oscillatory network of parkinsonian resting tremor. Brain 126, 199–212. doi: 10.1093/brain/awg022
Travis, L. E. (1929). The relation of voluntary movements to tremors. J. Exp. Psychol. 12, 515. doi: 10.1037/h0073785
Tsunematsu, T., Kilduff, T. S., Boyden, E. S., Takahashi, S., Tominaga, M., and Yamanaka, A. (2011). Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J. Neurosci. 31, 10529–10539. doi: 10.1523/JNEUROSCI.0784-11.2011
Vallbo, A., and Wessberg, J. (1993). Organization of motor output in slow finger movements in man. J. Physiol. 469, 673–691.
Van Der Giessen, R. S., Koekkoek, S. K., van Dorp, S., De Gruijl, J. R., Cupido, A., Khosrovani, S., et al. (2008). Role of olivary electrical coupling in cerebellar motor learning. Neuron 58, 599–612. doi: 10.1016/j.neuron.2008.03.016
Van der Want, J., Wiklund, L., Guegan, M., Ruigrok, T., and Voogd, J. (2004). Anterograde tracing of the rat olivocerebellar system with phaseolus vulgaris leucoagglutinin (PHA−L). Demonstration of climbing fiber collateral innervation of the cerebellar nuclei. J. Comp. Neurol. 288, 1–18. doi: 10.1002/cne.902880102
Vitek, J. L. (2002). Mechanisms of deep brain stimulation: excitation or inhibition. Mov. Disord. 17, S69–S72. doi: 10.1002/mds.10144
Vitek, J. L., Wichmann, T., and Delong, M. R. (1994). Current concepts of basal ganglia neurophysiology relative to tremorgenesis. Neurol. Dis. Ther. 30, 37.
Wiesenfeld, K., and Moss, F. (1995). Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs. Nature 373, 33–36. doi: 10.1038/373033a0
Wills, A., Jenkins, I., Thompson, P., Findley, L., and Brooks, D. (1994). Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomoraphic study. Ann. Neurol. 36, 636–642. doi: 10.1002/ana.410360413
Witter, L., Canto, C. B., Hoogland, T. M., de Gruijl, J. R., and De Zeeuw, C. I. (2013). Strength and timing of motor responses mediated by rebound firing in the cerebellar nuclei after Purkinje cell activation. Front. Neural Circuits 7:133. doi: 10.3389/fncir.2013.00133
Wu, T., Wang, L., Chen, Y., Zhao, C., Li, K., and Chan, P. (2009). Changes of functional connectivity of the motor network in the resting state in Parkinson's disease. Neurosci. Lett. 460, 6–10. doi: 10.1016/j.neulet.2009.05.046
Yin, H. H., and Knowlton, B. J. (2006). The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 7, 464–476. doi: 10.1038/nrn1919
Yokoo, H., Tanaka, M., Yoshida, M., Tsuda, A., Tanaka, T., and Mizoguchi, K. (1990). Direct evidence of conditioned fear-elicited enhancement of noradrenaline release in the rat hypothalamus assessed by intracranial microdialysis. Brain Res. 536, 305–308. doi: 10.1016/0006-8993(90)90039-E
Young, R. R., and Hagbarth, K. E. (1980). Physiological tremor enhanced by manoeuvres affecting the segmental stretch reflex. J. Neurol. Neurosurg. Psychiatry 43, 248–256. doi: 10.1136/jnnp.43.3.248
Keywords: T-type Ca2+ channels, tremors, motor coordination, inferior olive, thalamocortical neurons
Citation: Park Y-G, Kim J and Kim D (2013) The potential roles of T-type Ca2+ channels in motor coordination. Front. Neural Circuits 7:172. doi: 10.3389/fncir.2013.00172
Received: 19 February 2013; Accepted: 06 October 2013;
Published online: 28 October 2013.
Edited by:
G. J. Augustine, KIST, South KoreaReviewed by:
Michael Nitabach, Yale University School of Medicine, USAChris I. De Zeeuw, Erasmus Medical Center, Netherlands
Copyright © 2013 Park, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daesoo Kim, Department of Biological Sciences, KAIST Institute for the BioCentury, Korea Advanced Institute of Science and Technology, 291 Daehak-Ro, Daejeon 305-701, South Korea e-mail:ZGFlc29vQGthaXN0LmFjLmty
 Young-Gyun Park
Young-Gyun Park Jeongjin Kim
Jeongjin Kim Daesoo Kim
Daesoo Kim