- Faculté de Médecine Timone, Institut National de la Santé et de la Recherche Médicale U751, Université de la Méditerranée, Marseille, France
Although numerous experimental data indicate that lactate is efficiently used for energy by the mature brain, the direct measurements of energy metabolism parameters during neuronal network activity in early postnatal development have not been performed. Therefore, the role of lactate in the energy metabolism of neurons at this age remains unclear. In this study, we monitored field potentials and contents of oxygen and NAD(P)H in correlation with oxidative metabolism during intense network activity in the CA1 hippocampal region of neonatal brain slices. We show that in the presence of glucose, lactate is effectively utilized as an energy substrate, causing an augmentation of oxidative metabolism. Moreover, in the absence of glucose lactate is fully capable of maintaining synaptic function. Therefore, during network activity in neonatal slices, lactate can be an efficient energy substrate capable of sustaining and enhancing aerobic energy metabolism.
Introduction
The mammalian brain is capable of utilizing various substrates and their combinations to cover energy demands. Many in vitro and in vivo studies have demonstrated that besides glucose a number of compounds, such as lactate, pyruvate, acetate, glutamate, glutamine, and others can also be oxidized by neurons (for review, Mangia et al., 2009; Zielke et al., 2009). Growing body of evidence indicate that lactate is effectively utilized as an energy substrate by activated brain neurons despite the presence of glucose (Schurr, 2006; Pellerin et al., 2007; Barros and Deitmer, 2010; Mangia et al., 2009; Zielke et al., 2009). The concept known as the astrocyte-neuron lactate shuttle hypothesis postulates that during brain activation, a major part of the increase in glucose utilization is due to the astrocytic glycolysis triggered by a massive release of glutamate from synapses, whereas neurons mostly utilize lactate released from glial cells (Pellerin and Magistretti, 1994; Pellerin et al., 2007). While this hypothesis is still disputable (Mangia et al., 2009), the overall idea of lactate utilization by activated neurons is progressively supported by the experimental results both in vitro and in vivo (Schurr, 2006; Pellerin et al., 2007; Schousboe et al., 2007; Barros and Deitmer, 2010; Magistretti, 2009; Mangia et al., 2009; Zielke et al., 2009). Importantly, it was recently directly demonstrated (Gallagher et al., 2009) that the human brain is able to aerobically utilize lactate as an energy source (see also Mangia et al., 2003; Maddock et al., 2006) and that plasma lactate is used by neurons and glia at a proportion similar to that of plasma glucose (Boumezbeur et al., 2010).
Notably, measurements in the living brain have shown that extracellular concentrations of glucose and lactate in the cortex do not correspond to those of blood plasma and the extracellular levels of lactate may be comparable to or even higher than those of glucose (Langemann et al., 2001; Abi-Saab et al., 2002; McNay and Sherwin, 2004; Cavus et al., 2005; Zilberter et al., 2010), suggesting that lactate is readily available to neurons. In vitro studies on adult animals have shown that lactate could be efficiently involved in energy metabolism during network activity in hippocampal slices (Galeffi et al., 2007) and that lactate as the only energy substrate in artificial cerebrospinal fluid (ACSF) could fully support synaptic function (Schurr et al., 1988, 1999). It has also been noted that traditionally high concentrations of glucose (10 mM or more) in ACSF are not necessary and lowering glucose to 2.5 mM, a level closer to physiological conditions, does not affect the robustness of evoked neuronal response (Schurr and Payne, 2007). However, to support aerobic metabolism, the lower glucose concentration required sufficient oxygenation of slices. If this requirement was met, lactate as an energy substrate was more efficient than glucose because under aerobic conditions glucose is less capable of increasing mitochondrial respiration than lactate (Levasseur et al., 2006; Schurr and Payne, 2007).
Lactate is commonly recognized as an important metabolic substrate during early development (Erecinska et al., 2004; Medina and Tabernero, 2005; Ward Platt and Deshpande, 2005). However, a very limited number of studies investigated the effects of lactate or other energy substrates (e.g., ketone bodies, pyruvate) on neuronal excitability. For instance, it was demonstrated that lactate and β-hydroxybutyrate (BHB) could maintain neuronal activity and the levels of high-energy phosphates in hippocampal slices of P4 and P7 rats, although these energy substrates could not support neuronal activity in older animals (Wada et al., 1997). We have shown recently that supplementing glucose in ACSF with lactate, pyruvate, or BHB considerably hyperpolarized the resting membrane potential and reversal potential of gamma-aminobutyric acid (GABA)-induced currents (Holmgren et al., 2010) in neocortical and hippocampal pyramidal cells. These supplementary energy substrates significantly affected GABAergic synaptic transmission and spontaneous network oscillations, as well (Holmgren et al., 2010). We suggested that additional energy substrates normalized energy metabolism deficiency caused by the use of traditional glucose-based ACSF.
Other studies, however, argue that glucose alone can fully cover energy needs of neonatal neurons. Recent publications (Ruusuvuori et al., 2010; Tyzio et al., 2011) claimed that the effects of energy substrates on neuronal activity were induced by the intracellular acidification, since energy substrates are weak acids. However, our study on the pH effects suggests that small acidification induced by additional energy substrates (about 0.05 pH units) should not cause strong changes in spontaneous network activity (Mukhtarov et al., 2011). The papers by Ruusuvuori et al. (2010) and Tyzio et al. (2011) also asserted that none of the energy substrates are able to augment energy metabolism when added to the glucose-based ACSF, making glucose fully sufficient for neuronal metabolic function in vitro. Therefore, the role of lactate in energy metabolism of neonatal neurons during network activity is unclear and this important issue requires further analysis and clarification.
In the present study, to explore whether lactate added to glucose actively participates in energy metabolism in neonatal neurons, and whether lactate per se can maintain synaptic function, we evoked intensive network activity in hippocampal slices of the neonatal mice. Metabolic responses were evaluated by simultaneously measuring the intrinsic NAD(P)H fluorescence and tissue oxygen consumption. We find that during neuronal activity lactate is actively utilized and that lactate considerably elevates oxidative metabolic rate and maintains synaptic efficacy in neuronal networks.
Materials and Methods
Tissue Slice Preparation
Brain slices were prepared from P4–P7 Swiss mice of both sexes. All animal protocols conformed to the French Public Health Service policy and the INSERM guidelines on the use of laboratory animals. The mouse was rapidly decapitated and the brain was removed from the skull and placed in the ice-cold ACSF oxygenated with 95% O2/5% CO2. The ACSF solution consisted of (in mmol/l): NaCl 124, KCl 2.50, NaH2PO4 1.25, NaHCO3 25, CaCl2 2.00, MgSO4 1.30, and dextrose 10, pH 7.4. Saggital slices (400 μm) were cut using a tissue slicer (Leica VT 1200s, Leica Microsystem Vertrieb GmbH, Germany). During cutting slices were submerged in an ice-cold (<6°C) cutting solution consisted of (in mmol/l): K-gluconate 140, HEPES 10, Na-gluconate 15, EGTA 0.2, NaCl 4, pH adjusted to 7.2 with KOH. Slices were transferred immediately to an oxygenated holding chamber maintained at 22°C, and allowed to recover for 2 h. Slices were then transferred to a standard round 1.5 ml recording chamber and submerged (∼2 mm) in ACSF buffer which was continuously superfused (15 ml/min) and oxygenated with 95% O2/5% CO2. The temperature in the chamber was kept at 33–34°C for all experimental conditions.
Synaptic Stimulation and Field Potential Recordings
Shaffer collateral/commissural pathway was stimulated using the DS2A isolated stimulator (Digitimer Ltd, UK) with a bipolar tungsten electrode situated in the stratum radiatum of CA1 hippocampal region. Stimulus current was adjusted using single pulses (170–240 μA, 200 μs, 0.15 Hz) to produce a local field potential (LFP) of nearly 50% of maximal amplitude. LFPs were recorded using glass microelectrodes filled with ASCF, placed in stratum pyramidale, and connected to the DAM-80 amplifier (WPI, FL, USA). An extended synaptic stimulation consisted of a 10- or 30-s stimulus train (200 μs pulses at 10 Hz) was used to generate autofluorescence reduced pyridine nucleotide response.
NAD(P)H Fluorescence Imaging
Reduced nicotinamide adenine dinucleotide phosphate (NADPH) and reduced nicotinamide adenine dinucleotide (NADH) have very similar optical properties, and therefore it is expected that NADPH also contributes to some extent to total autofluorescence signals (Klaidman et al., 1995; Shuttleworth, 2010). Changes in NAD(P)H fluorescence in hippocampal slices were monitored using a 290- to 370-nm excitation filter and a 420-nm long pass filter for the emission (Omega Optical, Brattleboro, VT, USA). The light source was the Intensiligh C-HGFI illuminator (Nikon Instruments Europe B.V., UK) equipped with a mercury arc lamp. Slices were epiilluminated and imaged through a Nikon upright microscope (FN1, Eclipse) with 4×/0.10 Nikon Plan objective. Images were acquired using a linear, cooled 12-bit CCD camera (Sensicam, PCO AG, Germany) with a 640 × 480 digital spatial resolution. Because of a low level of fluorescence emission for this fluorophore, NAD(P)H images were acquired every 600–800 ms as 8 × 8 binned images (effective spatial resolution of 80 × 60 pixels). The exposure time was adjusted to obtain fluorescence intensity between 2000 and 3000 optical intensity levels. The images were stored on a computer as 12-bit files (0–4096 dynamic range). Fluorescence intensity changes in stratum radiatum near sites of LFP and O2 recordings were measured in three to five regions of interest using ImageJ software (developed by Wayne Rasband, NIH, USA). Data were expressed as the percentage changes in fluorescence over a baseline [(ΔF/F) × 100]. Signal analysis was performed using IgorPro software (WaveMetrics, Inc, OR, USA).
Oxygen Measurements
A Clark-style oxygen microelectrode (OX-10, tip diameter 10 μm; Unisense Ltd, Denmark) was used to measure slice tissue PO2. The electrode was connected to a picoammeter (PA2000, Unisense Ltd, Denmark) and the cathode was polarized at 800 mV in normal saline at 22°C for up to 12 h before the first use. A two-point calibration (in pA) was performed following polarization by inserting the electrode in normal saline solution (at 33°C) equilibrated with either 95% O2–5% CO2 or ambient air. Calibrations were repeated after each experiment to determine the PO2 values. The oxygen electrode was positioned using motorized micromanipulator (Scientifica Ltd, UK) in the proximity to the field potential recording electrode.
Fluorescence pHi Measurements
Prior to measurements, brain slices were incubated for 15 min in 10 μM 2′,7′-bis(carboxyethyl)-5(and-6)-carboxyfluorescein, acetoxymethyl ester form (BCECF-AM; Molecular Probes, Eugene, OR, USA) dissolved in standard ACSF solution at 32–34°C. Fluorescence images were acquired using a customized digital imaging microscope. Excitation of cells at 440 and 490 nm wavelengths was achieved using a 1-nm bandwidth polychromatic light selector equipped with a 100-W xenon lamp (Polychrome II; Till Photonics, Germany). Light intensity was attenuated using neutral density filters. A dichroic mirror (495 nm; Omega Optics, USA) was used to deflect light onto the samples. Fluorescence was visualized using an upright microscope (Axioskop; Zeiss, Germany) equipped with an infinity-corrected 60× water-immersion objective (n.a. = 0.9; LumPlanFL; Olympus, USA). Fluorescent-emitted light passed to a 16-bit electron multiplying charge-coupled device digital camera system (Andor iXon EM+; Andor Technology PLC, Northern Ireland). Fluorescence signals from the BCECF loaded CA3 pyramidal cells were acquired using Andor iQ software (Andor Technology PLC, Northern Ireland). The average fluorescence intensity of each region of interest was measured (usually five to seven BCECF loaded cells for each recording). Mean background fluorescence (measured from a non-fluorescent area) was subtracted and the ratio intensities (F490/F440) were determined. The duration of excitation was 10–50 ms at 30-s sampling interval. The real pHi values were obtained from the previously published calibration curve (Holmgren et al., 2010).
Pharmacology
Drugs used were purchased from Sigma (racemic mixture of DL-3-hydroxybutyric acid sodium salt, L-lactate sodium salt, pyruvate sodium salt). Within the racemic mixture, D-BHB is the primary mediator of the physiological effects of DL-BHB, and is the only form that can function as a substrate for mitochondrial BHB dehydrogenase. Consequently, only 50% of exogenous DL-BHB is expected to be utilized (Tsai et al., 2006).
Statistical Analysis
Group measures were expressed as means ± SEM; error bars also indicate SEM. Statistical significance was assessed using the Wilcoxon’s signed paired test or Student’s paired t-test. The level of significance was set at p < 0.05.
Calculating LFP Integrals
Figure 1 clarifies the procedure of calculating LFP integrals in the train. During analysis of the LFP train (Figure 1A), the computer program separated each LFP (Figure 1B), shifted the baseline to 0 and selected the region of integration (Figure 1C, red trace). Population spikes were inverted (see blue trace) and then the integral of the whole trace was calculated.
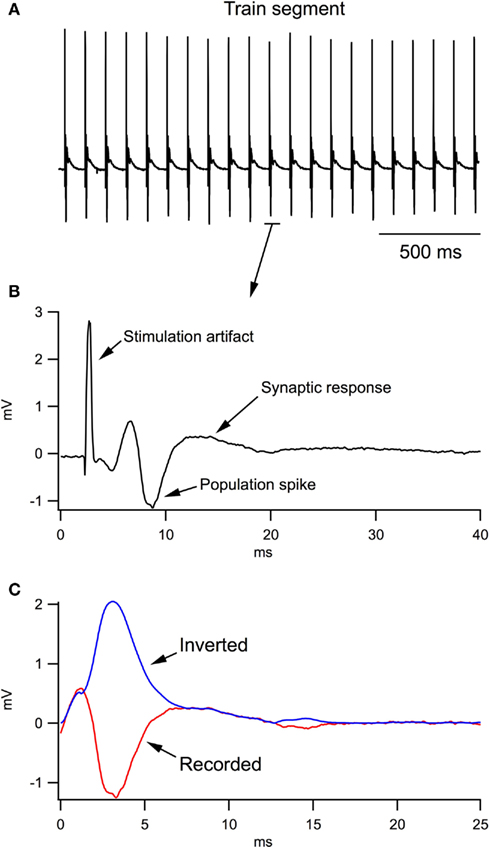
Figure 1. Analysis of local field potentials (LFPs) in response to the stimulation train. (A) A fragment of LFP train in response to a 10-Hz stimulation of Schaffer collaterals. (B) An example of one LFP in the train. (C) During computer analysis, the stimulation artifact and antidromic response were excluded from each LFP; the trace fractions related to population spikes were inverted and the resulting trace (blue trace) was integrated.
Results
Synaptic Function of Neonatal Neurons in CA1 Depends on Oxidative Metabolism
It is commonly accepted that neonatal neurons, compared to the mature cells, are much more tolerant to hypoxic conditions (Cherubini et al., 1989; Haddad and Donnelly, 1990), in part due to smaller energy needs (Kass and Lipton, 1989; Nabetani et al., 1995). In slices of mature animals, it has been shown that the efficacy of synaptic functioning (Garcia III et al., 2010) and network activity (Hajos et al., 2009) correlates strongly with the level of tissue oxygenation. Whether synaptic function in the neonatal network depends significantly on oxidative metabolism or whether its energy needs can be covered by glycolysis alone is unclear.
We used variable rates of ACSF perfusion to modify oxygen availability profile in 400 μm thick slices of P6 mice (n = 5) and measured simultaneously LFPs, induced by a single stimulation of Schaffer collaterals, and tissue oxygen levels. Firstly, we estimated the level of oxygen at different depths in the slice (Figure 2A). At a 3.25-ml/min perfusion rate, oxygen concentration strongly decreased (by about 50%) already at the slice surface. Following deeper penetration into the tissue, the oxygen level continuously declined and the slice was completely anoxic at the depth of about 200 μm. An increase of perfusion rate up to 15 ml/min allowed to maintain normoxic conditions at this depth and even deeper (Figure 2A).
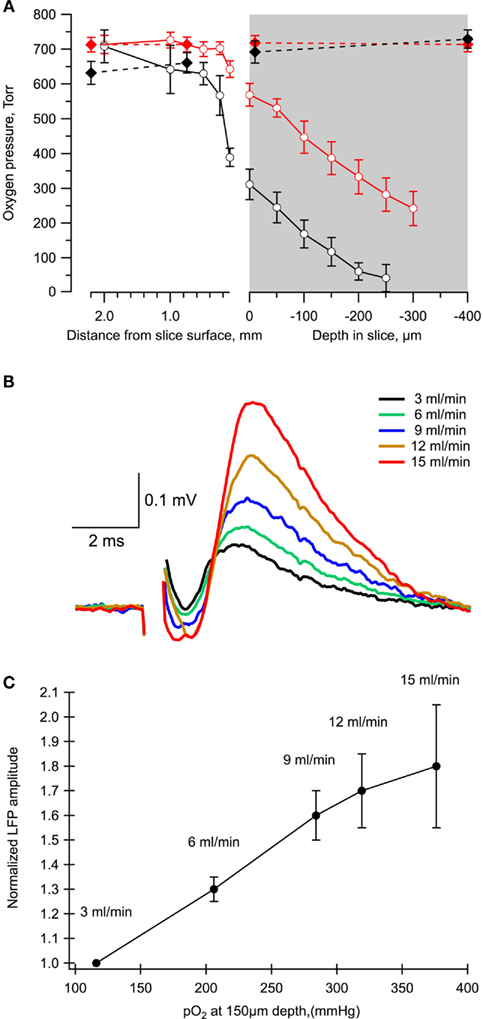
Figure 2. The level of slice oxygenation strongly affects the value of LFPs. (A) The oxygen level profile in a chamber at different rates of perfusion: 3 ml/min (black) and 15 ml/min (red). Dashed lines correspond to measurements in the absence of a slice in the chamber. Mean ± SE from five slices. (B) Examples of LFPs measured in the same slice and electrode positions at different flow rates. (C) Summary of the dependence of LFP amplitudes on the oxygen levels and perfusion rates. Mean ± SE from five slices.
Importantly, the LFP values revealed a strong dependence upon the rate of perfusion, i.e., on the oxygen levels (Figures 2B,C). Indeed, a decrease in the perfusion rate from 15 to 3.25 ml/min resulted in an about two-fold reduction of the LFP amplitude. Therefore, as in more mature neurons (Schurr and Payne, 2007; Hajos et al., 2009; Garcia III et al., 2010), the synaptic function of neonatal neurons during network activity profoundly depends on oxidative metabolism.
During Neonatal Network Activation, Lactate Modifies NAD(P)H Profile, Increases Oxygen Consumption, and Maintains Synaptic Efficacy
Here and in the experiments described below, the main experimental procedure included simultaneous measurements of field potentials, oxygen levels, and NAD(P)H fluorescence. These values were recorded both during periodic (every 7 s) single stimulations of Schaffer collaterals and following a stimulation train (10 Hz, 10-s or 30-s duration). Recordings started after stabilization of LFPs (normally about 20 min) and in most cases two trials separated by about 20 min were performed first in standard ACSF and then following a solution exchange (about a 30-min wash-in period). Importantly, in each experimental series, parameters measured in these dual trials did not differ significantly between each other, verifying that the parameters had been stabilized in control/test and the effects observed were not induced by some time-dependent variations.
If glucose alone is sufficient to cover neuronal energy requirements during network activation (Ruusuvuori et al., 2010; Tyzio et al., 2011), the addition of lactate to the extracellular solution should not be expected to modify the process of energy metabolism and synaptic functioning. We stimulated Schaffer collaterals (10 Hz, 10-s train) and measured LFPs along with corresponding changes in the oxygen and NAD(P)H levels in the CA1 region (Figure 3). Figure 3A shows original recordings of these parameters in a P5 mouse slice initially superfused with standard ACSF (10 mM glucose) and then for 30 min with a modified ACSF (5 mM glucose + 5 mM L-lactate). Addition of lactate to ACSF increased the oxygen consumption during the train stimulation and radically modified the NAD(P)H signaling: the oxidation phase was strongly increased while the overshoot practically disappeared. Note that the decrease of glucose concentration in ACSF from 10 to 5 mM alone did not induce a change in NAD(P)H signaling (n = 3, data not shown). Interestingly, although responses to a single-pulse periodic stimulation (1/7 s) and the first LFPs in response to a stimulation train were close in amplitude and had a similar slope (Figure 3B), the subsequent LFPs in the train displayed different dynamics and values, with those in the presence of lactate being significantly larger. This is confirmed by Figure 3C which shows the integrals of each LFP in the train (see Materials and Methods) and indicates that postsynaptic responses during a stimulus train are notably larger in the lactate-containing ACSF.
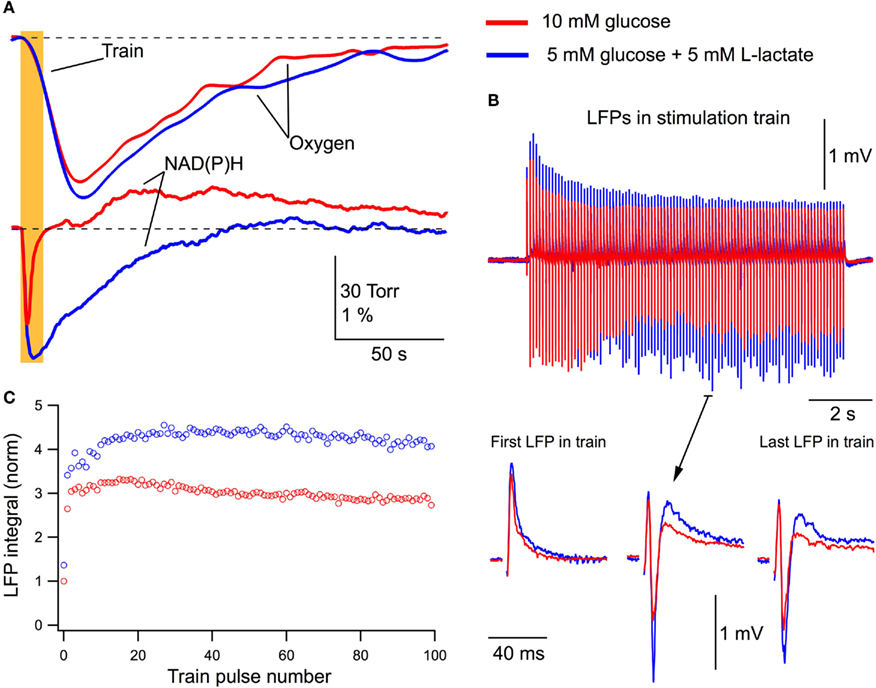
Figure 3. Lactate added to glucose increases oxygen consumption, modifies NAD(P)H signaling and amplifies synaptic function. (A) Original recordings of changes in NAD(P)H fluorescence and oxygen levels in response to a 10-Hz, 10-s stimulation of Schaffer collaterals in a slice of P5 mouse in ASCF with either 10 mM glucose (red lines) or 5 mM glucose + 5 mM lactate (blue lines). (B) LFP trains in 10 mM glucose (red) and 5 mM glucose + 5 mM lactate (blue). (C) Comparison of LFP integrals (see Figure 1) during the trains.
In five similar experiments, LFPs induced by a single stimulus in 10 mM glucose or in 5 mM glucose + 5 mM L-lactate did not differ in their integrals (p > 0.5). Meanwhile, the average LFP integrals during the stimulus train (10 Hz, 10 s) were significantly larger (p < 0.001) in the presence of lactate (Figure 4A). The average total LFP integral (summation of all LFP integrals during the train) was larger by 48 ± 14% (p < 0.02). The oxygen consumption (evaluated as an integral of a change in the oxygen level during 200 s after the train onset) increased by 31 ± 10% (p < 0.04) with the amplitude of oxygen transient increased by 17 ± 8% (p < 0.04; Figure 4B). NAD(P)H signaling in the presence of lactate revealed (Figure 4C) an about sixfold increase in the oxidation phase area and about threefold decrease in the overshoot area. From these results we conclude that lactate, even in the presence of glucose, is substantially involved in the process of oxidative metabolism, increases its efficacy, and modifies synaptic function.
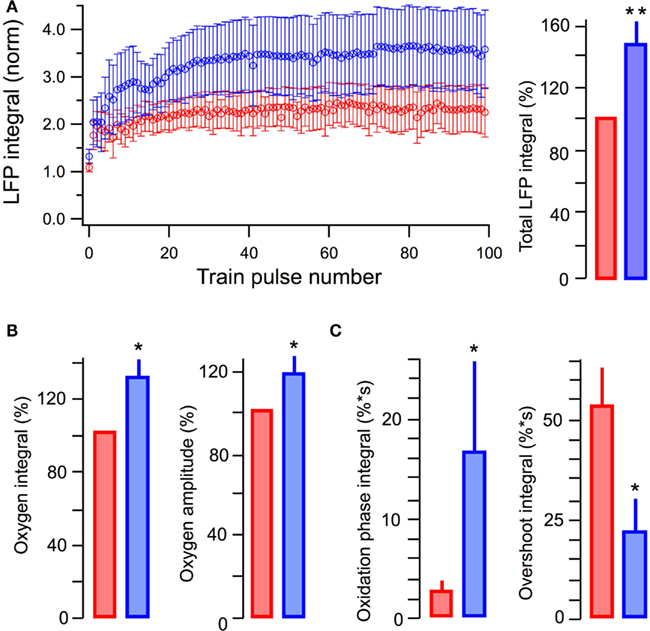
Figure 4. Summary of the effects of lactate added to glucose in ACSF. (A) Average values of LFP integrals during stimulation trains (left) and total LFP integrals (summation of LFP integrals in the train, right). (B) Average values of changes in the amplitude and integral of oxygen levels (in % to measurements in standard ACSF) induced by stimulation trains. (C) Average values in the integrals of oxidation and overshoot phases of NAD(P)H signaling. Mean ± SE from five slices.
One important question is whether in the neonatal slices, lactate can support energy metabolism and maintain synaptic function in the absence of glucose. Indeed, it has been shown in slices of mature rats that in aerobic conditions lactate is an efficient energy substrate capable for exchanging glucose (Schurr et al., 1988). Therefore, we substituted 10 mM glucose for 10 mM L-lactate in the extracellular solution and compared the effects of this solution with those of standard ACSF. In addition, for testing the efficacy of lactate comparing to glucose, we extended the duration of stimulation train to 30 s. This duration of the train was long enough to induce a progressive decay in LFP response at the end of stimulation.
Figure 5 shows an example recording from one of such experiments (P5 mouse). The integrals of LFPs for a single stimulation did not change significantly in either solution (p > 0.4; n = 10). However, the remarkable effect of lactate was revealed during the 30-s train stimulation. In the glucose-free lactate-containing solution, oxygen consumption considerably increased and the NAD(P)H profile thoroughly changed (Figure 5A), as in the case of glucose + lactate-containing solution (see Figure 3A). LFPs in the train (Figure 5B) decayed faster and population spikes disappeared earlier in the glucose-containing solution. In both solutions, LFPs stabilized on the level that was significantly larger in the lactate-containing solution. This is also evident in Figure 5C showing the LFP integrals during such a stimulus train.
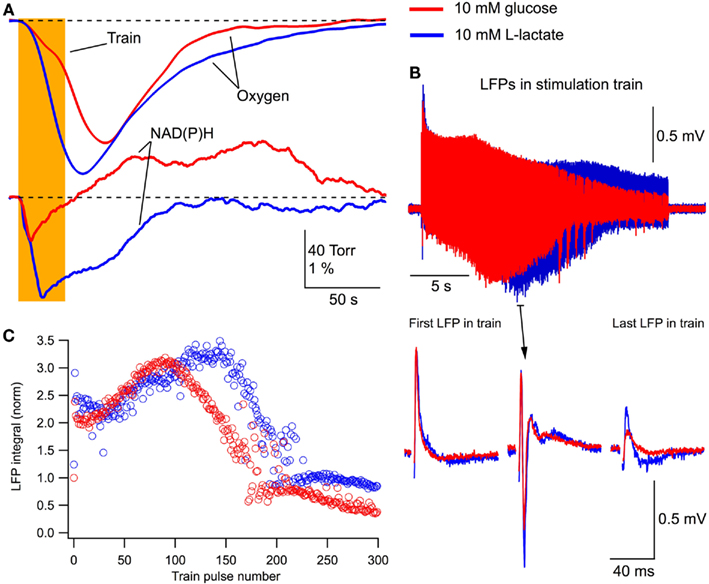
Figure 5. Lactate without glucose increases oxygen consumption, modifies NAD(P)H signaling and maintains/augments synaptic function. (A) Original recordings of changes in NAD(P)H fluorescence and oxygen levels in response to a 10-Hz, 30-s stimulation of Schaffer collaterals in a slice of P5 mouse in ASCF with either 10 mM glucose (red lines) or 10 mM lactate (blue lines). Prolonged (30 s) stimulation were used in these experiments to verify the efficacy of lactate as the energy substrate. (B) LFP trains in 10 mM glucose (red) and 10 mM lactate (blue). (C) Comparison of LFP integrals during the train.
In six similar experiments, the average LFP integrals in the stimulus train were significantly larger in the presence of lactate (p < 0.001) than those seen in glucose-ACSF (Figure 6A). The average total LFP integral in the train was larger by 39 ± 9% (p < 0.001) in the lactate-containing solution (Figure 6A, right). The oxygen consumption increased by 54 ± 10% (p < 0.001) with the amplitude of oxygen signal increased by 29 ± 5% (p < 0.001; Figure 6B). NAD(P)H signaling in the presence of lactate revealed an about fourfold larger oxidation phase area and about ninefold smaller overshoot area (Figure 6C).
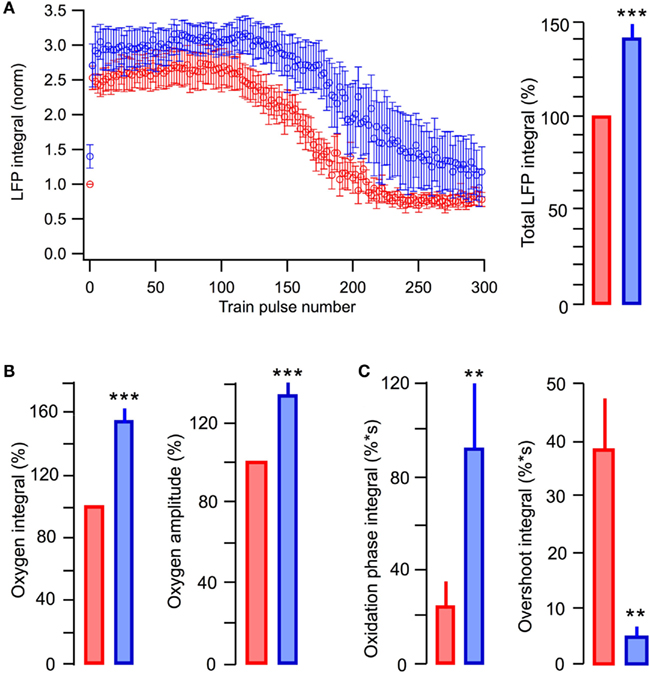
Figure 6. Summary of the effects in glucose-based and lactate-based ACSF. (A) Average values of LFP integrals during stimulation trains. (B) Average values of changes in the amplitude and integral of oxygen levels (in % to measurements in standard ACSF) induced by stimulation trains. (C) Average values in the integrals of oxidation and overshoot phases of NAD(P)H signaling. Mean ± SE from 10 slices.
Since, to our knowledge, the NAD(P)H fluorescent measurements in neonatal slices have not been performed previously, we verified that the observed changes in NAD(P)H profile were not a time-dependent artifact. For this purpose the “inverse” experiments were performed. We started the experiment with the lactate-containing solution (n = 2, data not shown) and observed a reversed modification of NAD(P)H signaling following a solution exchange to standard glucose-containing ACSF – namely, the decrease in oxidation phase and the appearance of the overshoot.
Altogether, these results indicate that in the absence of glucose, lactate is metabolized as the energy substrate and can efficiently support synaptic function.
β-Hydroxybutyrate as an Oxidative Energy Substrate
Another endogenous energy substrate, BHB, one representative of ketone bodies, is also a very important metabolic substance during the early development of postnatal brain (Erecinska et al., 2004; Nehlig, 2004; Prins, 2008). BHB, similar to lactate, can be involved in the oxidative energy metabolism (Fukao et al., 2004) and, therefore, being added to glucose, could also modify the energy metabolism parameters during neuronal network activity.
Figure 7 demonstrates experiments in which we compared the effects of standard and modified ACSF (5 mM glucose + 10 mM DL-BHB). Figure 7A shows the original recordings from a typical experiment (n = 6). In the BHB-containing solution, the oxygen consumption was enhanced considerably and the NAD(P)H overshoot practically disappeared while the NAD(P)H oxidation phase strongly increased (Figure 7Aa). However, opposite to the results seen in lactate, LFPs during the stimulus train did not change significantly (Figures 7Ab,c). In six experiments, in the presence of BHB, the oxygen consumption (Figure 7C) increased by 28 ± 12% (p < 0.03) with the amplitude of oxygen transient increased by 12 ± 7% (p < 0.04). NAD(P)H signaling in the presence of lactate revealed an about threefold larger oxidation phase area and the absence of overshoot (Figure 7D). Meanwhile, neither the LFPs in response to a single stimulus nor the average total LFP integral in the train (Figure 7B, right) changed significantly (p > 0.2 and p > 0.15, respectively). The absence of a noticeable BHB effect on LFPs in spite of the obvious enhancement of oxidative metabolism may be explained by a recently reported BHB-induced inhibition of presynaptic vesicular glutamate transport (Juge et al., 2010, see Discussion). However, profound changes in NAD(P)H profile and oxygen consumption strongly suggest that despite the presence of glucose, BHB, as well as lactate, can be effectively involved in oxidative metabolism in neonatal neurons.
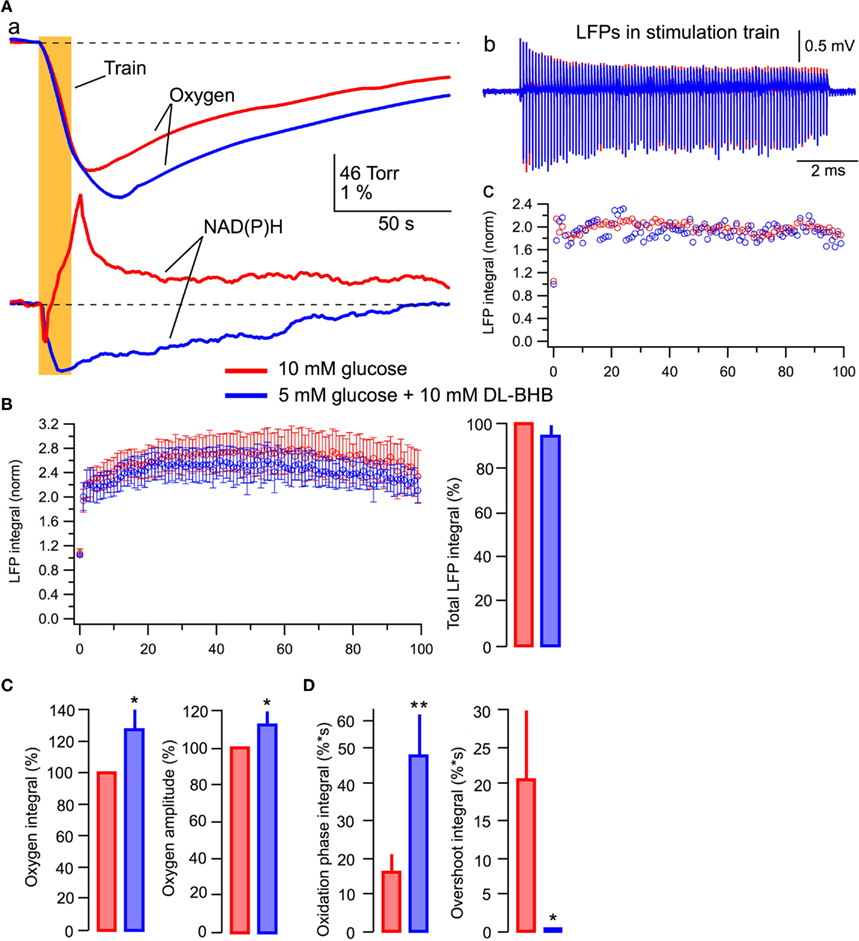
Figure 7. BHB added to glucose increases oxygen consumption and modifies NAD(P)H signaling. (Aa) Original recordings of changes in NAD(P)H fluorescence and oxygen levels in response to a 10-Hz, 10-s stimulation of Schaffer collaterals in a slice of P5 mouse; (Ab) LFP trains in 10 mM glucose (red) and 5 mM glucose + 10 mM DL-BHB (blue); (Ac) Comparison of LFP integrals during the train. (B) Average values of LFP integrals during stimulation trains. Mean ± SE from six slices. (C) Average values of changes in the amplitude and integral of oxygen levels (in % to measurements in standard ACSF) induced by stimulation trains. (D) Average values of the integrals of oxidation and overshoot phases of NAD(P)H signaling.
Pyruvate Effects Confirm that Glycolysis is Not Sufficient to Support Neuron Energy Requirements during Intense Synaptic Activity
Increase in the efficacy of synaptic function observed in the lactate-based ACSF suggests that this effect of lactate is caused by the enhancement of oxidative phosphorylation. If this is correct then pyruvate, which is believed to be the end product of neuronal aerobic glycolysis (see, however, Schurr, 2006; Schurr and Payne, 2007), should induce similar effect. Therefore, we supplemented glucose with 5 mM pyruvate in ACSF. The effects of pyruvate were similar to those of lactate (Figure 8). Pyruvate induced a strong increase in the NAD(P)H oxidation phase and almost completely eliminated the overshoot (Figures 8Aa,D). Oxygen consumption during a 30-s stimulation train was significantly increased and LFPs were considerably larger especially during the second part of the train (Figure 8Ab).
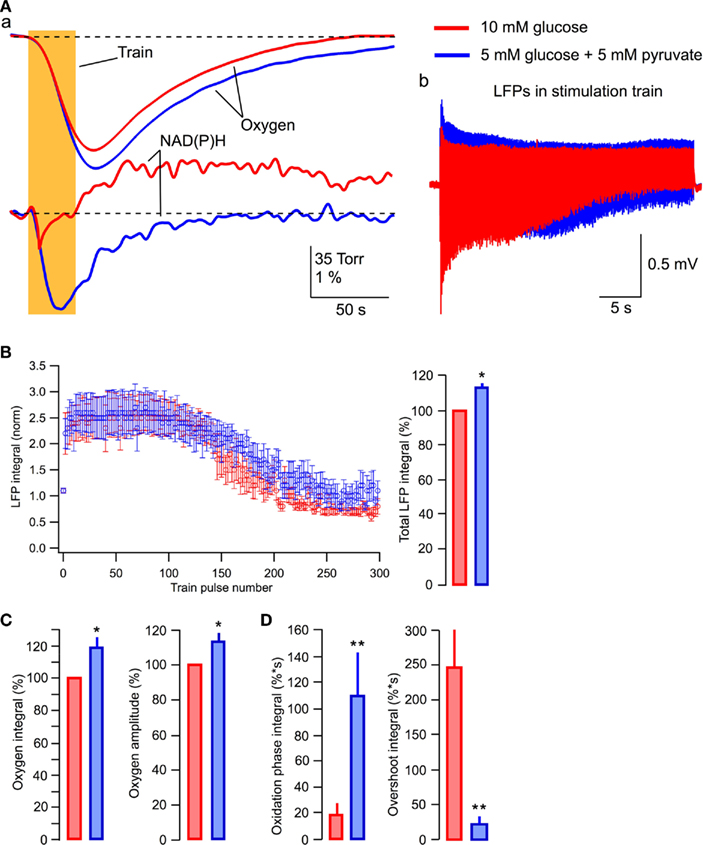
Figure 8. Pyruvate added to glucose enhances aerobic energy metabolism and synaptic integrity. (Aa) Original recordings of changes in NAD(P)H fluorescence and oxygen levels in response to a 10-Hz, 30-s stimulation of Schaffer collaterals in a slice of P6 mouse; (Ab) LFP trains in 10 mM glucose (red) and 5 mM glucose + 5 mM pyruvate (blue); (B) Average values of LFP integrals during stimulation trains. Mean ± SE from five slices. (C) Average values of changes in the amplitude and integral of oxygen levels (in % to measurements in standard ACSF) induced by stimulation trains. (D) Average values of the integrals of oxidation and overshoot phases of NAD(P)H signaling.
In five similar experiments, the average LFP integrals during the train were significantly larger in the presence of pyruvate (p < 0.001) than those seen in glucose-ACSF (Figure 8B). The average total LFP integral in the train was larger by 12 ± 3% (p < 0.04) in the pyruvate-containing solution (Figure 8B, right). The oxygen consumption increased by 19 ± 7% (p < 0.02) with the oxygen signal amplitude increased by 13 ± 5% (p < 0.02; Figure 8C). NAD(P)H signaling in the presence of pyruvate revealed approximately sixfold greater oxidation phase area and about a 12-fold smaller overshoot area (Figure 8D).
Therefore, supplementing ACSF glucose with pyruvate significantly enhances aerobic energy metabolism and synaptic integrity in neonatal neurons.
Intracellular Acidification Cannot Explain the Effects of Lactate and BHB
As we reported previously (Mukhtarov et al., 2011), the addition of lactate to the extracellular solution induced a small acidification of the intracellular pH, in average by about −0.05 pH units. Similar observations were obtained in the other recent study (Ruusuvuori et al., 2010), where authors suggested that such small changes in pH underlie the lactate’s effects on neuronal electrical activity (Ruusuvuori et al., 2010; Tyzio et al., 2011). However, our results presented in Figure 9 are difficult to reconcile with that suggestion. Figures 9A,B show the distribution of pHi in the same CA3 pyramidal cells measured initially in standard ACSF and then either after addition of lactate (Figure 9A; P4–P6, seven slices, 48 cells) or replacement of glucose with lactate (Figure 9B; six slices, 33 cells). The values of pHi differed considerably between pyramidal cells, within the range from 6.8 to 7.8. Such a wide distribution of pHi in the immature CA1 pyramidal cells has also been reported previously (Schwiening and Boron, 1994; Bevensee et al., 1996). Meanwhile, when compared with the overall range of pH values, a shift in the average pHi values resulting from the exchange of solutions is relatively small, as depicted in Figure 9A by vertical dashed lines. Clearly, in both solutions, a majority of neurons are exposed to the same range of pHi values.
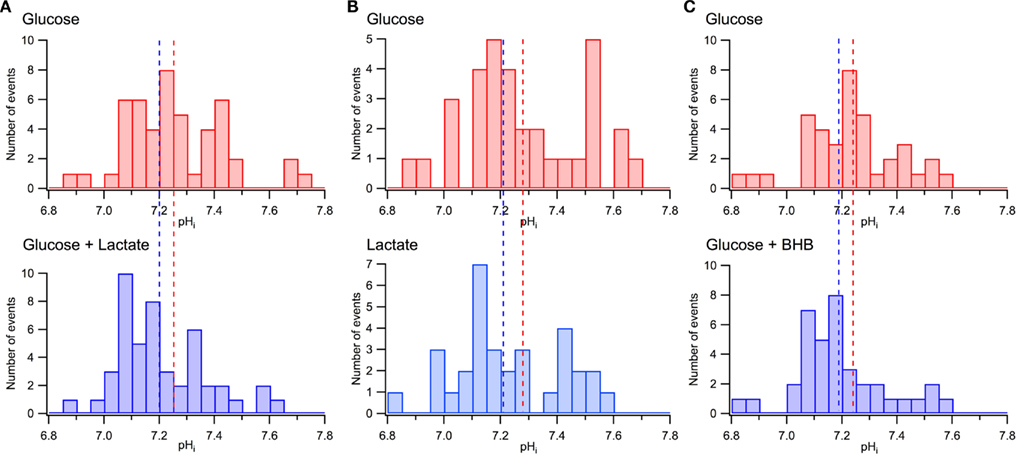
Figure 9. Minor effects of lactate and BHB on pHi distribution in CA3 pyramidal cells. (A) pHi distributions measured in the same neurons in glucose-based (10 mM) and lactate-supplemented (5 mM glucose + 5 mM lactate) ACSF. (B) pHi distributions measured in the same neurons in glucose-based (10 mM) and lactate-based (10 mM) ACSF. (C) pHi distributions measured in the same neurons in glucose-based (10 mM) and BHB-supplemented (5 mM glucose + 10 mM BHB) ASCF. In (A), (B), and (C) dashed lines indicate the average values of pHi. Note large variations of pHi in different neurons and a relatively small shift in the mean pHi values in the presence of lactate or BHB.
Similar measurements have been performed with 5 mM pyruvate supplemented to 5 mM glucose in ACSF (data not shown; eight slices, 54 cells). Compared to standard glucose-based ACSF, the pyruvate-supplemented ACSF induced a shift in the average pHi values of −0.07 units while the values of pHi differed between pyramidal cells within the range of 6.8–7.8.
Figure 9C demonstrates similar pHi distributions measured in standard ACSF and after the addition of BHB to the external solution (six slices, 38 cells). As in the case of lactate, the distributions are wide with a relatively small shift in the pHi average values following the exchange. We, therefore, conclude as previously (Mukhtarov et al., 2011) that a small shift in the average pHi alone is unlikely to contribute significantly to the energy substrates effects.
Discussion
The main finding of this study is that lactate is an efficient energy substrate in the neonatal hippocampus, especially during the intense neuronal network activity. In neonatal slices under sound aerobic conditions, whether combined with glucose or alone, lactate significantly enhances oxidative metabolism and efficiently supports synaptic function.
Lactate as the Energy Substrate
The role of lactate as an energy substrate in the mature brain is well established. Although the hypothesis of astrocyte-neuron lactate shuttle (Pellerin and Magistretti, 1994; Pellerin et al., 2007) is still debated (for review, Mangia et al., 2009), utilization of lactate by neuronal networks during their activation is strongly supported by experimental data from both in vivo and in vitro studies (Mangia et al., 2003, 2009; Maddock et al., 2006; Schurr, 2006; Pellerin et al., 2007; Barros and Deitmer, 2010; Zielke et al., 2009; Boumezbeur et al., 2010).
Multiple biochemical studies in the immature brain indicated that high levels of both lactate and ketone bodies utilization are characteristic for early postnatal development (for review, Erecinska et al., 2004). However, attempts of the direct evaluation of correlation between energy metabolism and neuronal network activity in neonatal slices have been undertaken only in a few physiological in vitro studies. Nabetani et al. (1995) has shown that population spikes in CA3 neurons of P4–P7 rats were very tolerant to oxygen deprivation but sensitive to the lack of glucose – population spikes disappeared within 15–20 min of glucose deprivation onset. Wada et al. (1997) demonstrated that population spikes in slices and concentrations of ATP and creatine phosphate in slice’s homogenate could be maintained after substitution of glucose with lactate and BHB.
Recently, Ruusuvuori et al. (2010) have concluded that lactate, being added to glucose, does not modify energy metabolism, is inefficient in oxidative phosphorylation and therefore glucose alone is able to completely satisfy neuronal energy demands. These authors confirmed the inhibitory effect of lactate on spontaneous network activity (giant depolarizing potentials, GDP; Ben-Ari et al., 2007) in neonatal slices reported previously (Holmgren et al., 2010). However, they suggested that the lactate-induced inhibition of GDPs resulted from a hypersensitivity of neuronal network activity to a small (average −0.05 pH units) lactate-induced shift in the intracellular pH. In our recent study (Mukhtarov et al., 2011), we have shown that this explanation is unlikely to be valid since GDPs remained active even after a much stronger intracellular acidification (about −0.3 pH units), with only a transient changes in GDPs frequency observed. In the present report, we substantiated our previous conclusion by demonstrating a large variation of pHi values in different pyramidal cells of the same slice region (see Figure 9): these variations exceeded by 10-fold the average shift in pHi induced by lactate or BHB.
In this study, concurrent recordings of field potentials and dynamic parameters of oxidative metabolism allowed us to analyze the correlation of neuronal electrical activity with changes in energy metabolism. Our results prove that lactate is readily utilized by neonatal neurons and that its efficiency as the aerobic energy substrate is superior to glucose. This conclusion is supported by the experiments with pyruvate supplemented to glucose in ACSF (see Figure 8). Indeed, experiments with a long-lasting periodic axon stimulation demonstrate that in both the lactate-based ACSF and pyruvate-containing ACSF, field responses significantly exceed those seen in the glucose-based ACSF and that the synaptic function is sustained for a longer time (see Figures 5, 6, and 8). This data substantiates the point that neonatal neurons are flexible in utilization of various energy substrates in order to optimize the efficacy of overall network function. It is safe to suppose that in vivo, not only the mature but also the neonatal brain utilizes the optimal combinations of substrates, depending on the activity status.
β-Hydroxybutyrate as the Energy Substrate in Neonatal Slices
Similarly to lactate, BHB is a very important metabolic compound during postnatal development (Erecinska et al., 2004; Nehlig, 2004; Prins, 2008). In our experiments, supplementing ACSF’s glucose with BHB significantly increased local oxygen consumption during network activity and strongly modified the NAD(P)H signaling profile (see Figure 7A). Therefore, in spite of the presence of glucose, BHB evidently operates as an oxidative substrate, significantly affecting energy metabolism.
It is interesting to note the apparent absence of BHB effect on the population electrical activity. In the recent paper of Juge et al. (2010), the authors found that ketone bodies can effectively compete with chloride for a binding site on the vesicular glutamate transporters in excitatory presynaptic terminals, thus inhibiting glutamate uptake to synaptic vesicles. This unexpected finding can well explain why BHB, while showing similar to lactate modifications of energy metabolism parameters, does not affect synaptic function during the train stimulation. Indeed, a relatively high concentration of the substrate (10 mM DL-BHB) could induce a partial inhibition of glutamatergic transmission that would oppose the metabolic boosting effect of BHB on transmission and apparently compensate for it. This interesting issue is outside the frame of the present study, although it well deserves further investigation.
NAD(P)H Transients during Synaptic Activity
In neonatal slices exposed to standard ACSF, the profile of NAD(P)H signaling induced by synaptic stimulation was similar to that previously reported for more mature tissue (Turner et al., 2007; Shuttleworth, 2010): the oxidation phase followed by a pronounced overshoot. The origin of overshoot phase is still a matter of debate. The overshoot has been observed in the in vivo studies on anesthetized animals (Lewis and Schuette, 1976; Dora et al., 1984) although in a smaller extent compared to slices (see, e.g., Rosenthal and Jobsis, 1971; Lothman et al., 1975; Mayevsky and Chance, 2007). In the reports on in vitro experiments, it has been suggested that the overshoot represents either rise in the cytosolic NAD(P)H associated with glycolysis (Lipton, 1973; Kasischke et al., 2004; Galeffi et al., 2011) or has a mitochondrial origin (Brennan et al., 2006, 2007). In the latter studies, however, a low solution superfusion rate was used (2 ml/min) and the oxygen consumption that could affect the results obtained (see below) was not monitored. Our data are supportive of the cytosolic/glycolitic origin of the overshoot phase of NAD(P)H signaling since the overshoot was strongly reduced following the addition of energy substrates in ACSF. Another disputable matter is the contribution of glial glycolysis to the NAD(P)H signaling (Kasischke et al., 2004; Shuttleworth, 2010). That particular issue, however, is outside the frame of this discussion.
Some Methodological Considerations on Network Activity Measurements in Slices
In acute brain slices, the neurons’ distance to the slice surface varies and their respective extracellular environments can, therefore, diverge significantly. Here, we specifically focus on the oxygen delivery to neurons since energy substrates and oxidative phosphorylation (the principal ATP provider) cannot function properly under hypoxic conditions. The importance of sufficient oxygenation and the corresponding methodological approaches have been addressed periodically in studies utilizing brain slices of adult animals. For instance, in a submerged chamber allowing precise control of oxygenation, simultaneous measurements of population responses and oxygen tension in rat’s CA1 slices (P27–P40), which were superfused from both upper and bottom sides, revealed a strong dependence of population spike amplitude on oxygen levels (Garcia III et al., 2010). Schurr et al. (1988) showed that at adequate oxygenation conditions, lactate maintained synaptic function in CA1 in the absence of glucose. The authors also lowered the concentration of glucose in ACSF down to 2.5 mM without any noticeable changes in population response (Schurr and Payne, 2007). Hajos et al. (2009) showed in slices from P14–P20 rat and mice that at the solution flow rate providing for adequate slice oxygenation (double-side perfusion), spontaneous sharp wave–ripple oscillations as well as cholinergically induced fast oscillations occurred, resembling those in the in vivo hippocampus. These oscillations disappeared at lower rates of perfusion (about 2 ml/min). Without a doubt, the oxygen level profile in a slice depends on the experimental design, e.g., the chamber construction, perfusion rate and slice fixation in the chamber, and should be verified especially in studies of multi-cellular activities to ensure the adequate conditions for energy metabolism are met.
Unfortunately, this issue has not been typically taken into account as a methodical rule and has been practically ignored in studies on neonatal tissue. In part, this could be explained by a well-established fact of higher resistance of neonatal neurons to anoxic conditions. However, the concept of survival and the term “normal functioning” should not be considered interchangeable. Indeed, due to relatively small energy demands of neonatal neurons in a quiescent state, neuronal survival may be supported for a while solely by glycolysis, which provides two molecules of ATP for each molecule of glucose, without any requirement in oxygen. This presence of anaerobic metabolism, however, does not necessarily mean that the energy-dependent processes during demanding neuronal activity would function normally without (or with reduced) aerobic metabolism that normally provides 32 molecules of ATP for each molecule of glucose (Lehninger, 2005).
Our measurements show that at the depth of 150 μm and with the flow rate of 3.25 ml/min, the oxygen level is close to zero in the neonatal submerged slices under standard experimental design conditions (1.5 ml round chamber, one-side perfusion). The oxygenation profile improved at the flow rate of 15 ml/min that we subsequently used in our experiments. Importantly, the LFPs in response to a single stimulus depend rather strongly on the real-time oxygen levels, indicating that oxidative metabolism is indeed essential for the synaptic function in neonatal neurons. Evidently, the energy substrates such as lactate and BHB, for utilization of which sufficient oxygen delivery is mandatory, can reveal their metabolic effects on slices only under adequate experimental conditions. The variability or lack of energy substrates’ effects as reported in recent papers (Kirmse et al., 2010; Ruusuvuori et al., 2010; Tyzio et al., 2011), in which low ACSF superfusion rates (1.5–3 ml/min) during multi-cellular activity measurements have been used, is likely to be attributed to the inadequate oxygen supply.
Conclusion
The brain’s energy pool is comprised of multiple energy substrates neither of which can be designated as being more or less important than the other. The rates of utilization of a specific substrate depend on the brain’s ongoing needs as well as on substrate availability, both parameters varying within rather broad limits. The brain slice preparation is an undeniably artificial system that only roughly approximates the in vivo processes. We believe, therefore, that for in vitro studies it is imperative to overcome the disregard of the fundamental brain properties. Specifically, basic properties such as parameters of energy metabolism underlying neuronal excitability must be taken into account. Our present results demonstrate that lactate and BHB considerably affect energy metabolism in neonatal slices. Therefore, glucose is not and cannot be the only player in the complicated neuronal metabolic machinery. Underestimation of the role of lactate in energy metabolism of neonatal neurons can lead to erroneous conclusions concerning the fundamental properties of the developing brain. There should be careful consideration of this fact in further in vitro experiments as well as a more diligent approach to the metabolic aspect of in vivo studies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. T. Zilberter and Dr. M. Zilberter for the valuable discussion and help in preparation of the manuscript. Yuri Zilberter was supported by the European Union Seventh Framework “MEMOLOAD” grant (HEALTH-F2-2007-201159). Yuri Zilberter is the recipient of a Contrat d’interface between INSERM and Centre Hospitalier Universitaire Necker Paris, France. For Marat Mukhtarov and Piotr Bregestovski this work was supported also by the European Union Seventh Framework Programme under grant agreement no HEALTH-F2-2008-202088 (“NeuroCypres” project).
References
Abi-Saab, W. M., Maggs, D. G., Jones, T., Jacob, R., Srihari, V., Thompson, J., Kerr, D., Leone, P., Krystal, J. H., Spencer, D. D., During, M. J., and Sherwin, R. S. (2002). Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J. Cereb. Blood Flow Metab. 22, 271–279.
Barros, L. F., and Deitmer, J. W. (2010). Glucose and lactate supply to the synapse. Brain Res. Rev. 63, 149–159.
Ben-Ari, Y., Gaiarsa, J. L., Tyzio, R., and Khazipov, R. (2007). GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 87, 1215–1284.
Bevensee, M. O., Cummins, T. R., Haddad, G. G., Boron, W. F., and Boyarsky, G. (1996). pH regulation in single CA1 neurons acutely isolated from the hippocampi of immature and mature rats. J. Physiol. 494(Pt 2), 315–328.
Boumezbeur, F., Petersen, K. F., Cline, G. W., Mason, G. F., Behar, K. L., Shulman, G. I., and Rothman, D. L. (2010). The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J. Neurosci. 30, 13983–13991.
Brennan, A. M., Connor, J. A., and Shuttleworth, C. W. (2006). NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J. Cereb. Blood Flow Metab. 26, 1389–1406.
Brennan, A. M., Connor, J. A., and Shuttleworth, C. W. (2007). Modulation of the amplitude of NAD(P)H fluorescence transients after synaptic stimulation. J. Neurosci. Res. 85, 3233–3243.
Cavus, I., Kasoff, W. S., Cassaday, M. P., Jacob, R., Gueorguieva, R., Sherwin, R. S., Krystal, J. H., Spencer, D. D., and Abi-Saab, W. M. (2005). Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann. Neurol. 57, 226–235.
Cherubini, E., Ben-Ari, Y., and Krnjevic, K. (1989). Anoxia produces smaller changes in synaptic transmission, membrane potential, and input resistance in immature rat hippocampus. J. Neurophysiol. 62, 882–895.
Dora, E., Gyulai, L., and Kovach, A. G. (1984). Determinants of brain activation-induced cortical NAD/NADH responses in vivo. Brain Res. 299, 61–72.
Erecinska, M., Cherian, S., and Silver, I. A. (2004). Energy metabolism in mammalian brain during development. Prog. Neurobiol. 73, 397–445.
Fukao, T., Lopaschuk, G. D., and Mitchell, G. A. (2004). Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fatty Acids 70, 243–251.
Galeffi, F., Foster, K. A., Sadgrove, M. P., Beaver, C. J., and Turner, D. A. (2007). Lactate uptake contributes to the NAD(P)H biphasic response and tissue oxygen response during synaptic stimulation in area CA1 of rat hippocampal slices. J. Neurochem. 103, 2449–2461.
Galeffi, F., Somjen, G. G., Foster, K. A., and Turner, D. A. (2011). Simultaneous monitoring of tissue PO(2) and NADH fluorescence during synaptic stimulation and spreading depression reveals a transient dissociation between oxygen utilization and mitochondrial redox state in rat hippocampal slices. J. Cereb. Blood Flow Metab. 31, 626–639.
Gallagher, C. N., Carpenter, K. L., Grice, P., Howe, D. J., Mason, A., Timofeev, I., Menon, D. K., Kirkpatrick, P. J., Pickard, J. D., Sutherland, G. R., and Hutchinson, P. J. (2009). The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 132, 2839–2849.
Garcia, A. J. III, Putnam, R. W., and Dean, J. B. (2010). Hyperbaric hyperoxia and normobaric reoxygenation increase excitability and activate oxygen-induced potentiation in CA1 hippocampal neurons. J. Appl. Physiol. 109, 804–819.
Haddad, G. G., and Donnelly, D. F. (1990). O2 deprivation induces a major depolarization in brain stem neurons in the adult but not in the neonatal rat. J. Physiol. 429, 411–428.
Hajos, N., Ellender, T. J., Zemankovics, R., Mann, E. O., Exley, R., Cragg, S. J., Freund, T. F., and Paulsen, O. (2009). Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur. J. Neurosci. 29, 319–327.
Holmgren, C. D., Mukhtarov, M., Malkov, A. E., Popova, I. Y., Bregestovski, P., and Zilberter, Y. (2010). Energy substrate availability as a determinant of neuronal resting potential, GABA signaling and spontaneous network activity in the neonatal cortex in vitro. J. Neurochem. 112, 900–912.
Juge, N., Gray, J. A., Omote, H., Miyaji, T., Inoue, T., Hara, C., Uneyama, H., Edwards, R. H., Nicoll, R. A., and Moriyama, Y. (2010). Metabolic control of vesicular glutamate transport and release. Neuron 68, 99–112.
Kasischke, K. A., Vishwasrao, H. D., Fisher, P. J., Zipfel, W. R., and Webb, W. W. (2004). Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305, 99–103.
Kass, I. S., and Lipton, P. (1989). Protection of hippocampal slices from young rats against anoxic transmission damage is due to better maintenance of ATP. J. Physiol. 413, 1–11.
Kirmse, K., Witte, O. W., and Holthoff, K. (2010). GABA depolarizes immature neocortical neurons in the presence of the ketone body β-hydroxybutyrate. J. Neurosci. 30, 16002–16007.
Klaidman, L. K., Leung, A. C., and Adams, J. D. Jr. (1995). High-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions. Anal. Biochem. 228, 312–317.
Langemann, H., Alessandri, B., Mendelowitsch, A., Feuerstein, T., Landolt, H., and Gratzl, O. (2001). Extracellular levels of glucose and lactate measured by quantitative microdialysis in the human brain. Neurol. Res. 23, 531–536.
Lehninger, A. L. (2005). “Principles of metabolic regulation: glucose and glycogen,” in Principles of biochemistry, 4th edn, eds D. L. Nelson and M. M. Cox (Boston, MA: W. H. Freeman), 560–600.
Levasseur, J. E., Alessandri, B., Reinert, M., Clausen, T., Zhou, Z., Altememi, N., and Bullock, M. R. (2006). Lactate, not glucose, up-regulates mitochondrial oxygen consumption both in sham and lateral fluid percussed rat brains. Neurosurgery 59, 1122–1130. [Discussion 1130–1121].
Lewis, D. V., and Schuette, W. H. (1976). NADH fluorescence, [K+]0 and oxygen consumption in cat cerebral cortex during direct cortical stimulation. Brain Res. 110, 523–535.
Lipton, P. (1973). Effects of membrane depolarization on nicotinamide nucleotide fluorescence in brain slices. Biochem. J. 136, 999–1009.
Lothman, E., Lamanna, J., Cordingley, G., Rosenthal, M., and Somjen, G. (1975). Responses of electrical potential, potassium levels, and oxidative metabolic activity of the cerebral neocortex of cats. Brain Res. 88, 15–36.
Maddock, R. J., Buonocore, M. H., Lavoie, S. P., Copeland, L. E., Kile, S. J., Richards, A. L., and Ryan, J. M. (2006). Brain lactate responses during visual stimulation in fasting and hyperglycemic subjects: a proton magnetic resonance spectroscopy study at 1.5 Tesla. Psychiatry Res. 148, 47–54.
Magistretti, P. J. (2009). Role of glutamate in neuron-glia metabolic coupling. Am. J. Clin. Nutr. 90, 875S–880S.
Mangia, S., Garreffa, G., Bianciardi, M., Giove, F., Di Salle, F., and Maraviglia, B. (2003). The aerobic brain: lactate decrease at the onset of neural activity. Neuroscience 118, 7–10.
Mangia, S., Giove, F., Tkac, I., Logothetis, N. K., Henry, P. G., Olman, C. A., Maraviglia, B., Di Salle, F., and Ugurbil, K. (2009). Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J. Cereb. Blood Flow Metab. 29, 441–463.
Mayevsky, A., and Chance, B. (2007). Oxidation-reduction states of NADH in vivo: from animals to clinical use. Mitochondrion 7, 330–339.
McNay, E. C., and Sherwin, R. S. (2004). From artificial cerebro-spinal fluid (aCSF) to artificial extracellular fluid (aECF): microdialysis perfusate composition effects on in vivo brain ECF glucose measurements. J. Neurosci. Methods 132, 35–43.
Medina, J. M., and Tabernero, A. (2005). Lactate utilization by brain cells and its role in CNS development. J. Neurosci. Res. 79, 2–10.
Mukhtarov, M., Ivanov, A., Zilberter, Y., and Bregestovski, P. (2011). Inhibition of spontaneous network activity in neonatal hippocampal slices by energy substrates is not correlated with intracellular acidification. J. Neurochem. 116, 316–321.
Nabetani, M., Okada, Y., Kawai, S., and Nakamura, H. (1995). Neural activity and the levels of high energy phosphates during deprivation of oxygen and/or glucose in hippocampal slices of immature and adult rats. Int. J. Dev. Neurosci. 13, 3–12.
Nehlig, A. (2004). Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot. Essent. Fatty Acids 70, 265–275.
Pellerin, L., Bouzier-Sore, A. K., Aubert, A., Serres, S., Merle, M., Costalat, R., and Magistretti, P. J. (2007). Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55, 1251–1262.
Pellerin, L., and Magistretti, P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U.S.A. 91, 10625–10629.
Prins, M. L. (2008). Cerebral metabolic adaptation and ketone metabolism after brain injury. J. Cereb. Blood Flow Metab. 28, 1–16.
Rosenthal, M., and Jobsis, F. F. (1971). Intracellular redox changes in functioning cerebral cortex. II. Effects of direct cortical stimulation. J. Neurophysiol. 34, 750–762.
Ruusuvuori, E., Kirilkin, I., Pandya, N., and Kaila, K. (2010). Spontaneous network events driven by depolarizing GABA action in neonatal hippocampal slices are not attributable to deficient mitochondrial energy metabolism. J. Neurosci. 30, 15638–15642.
Schousboe, A., Bak, L. K., Sickmann, H. M., Sonnewald, U., and Waagepetersen, H. S. (2007). Energy substrates to support glutamatergic and GABAergic synaptic function: role of glycogen, glucose and lactate. Neurotox. Res. 12, 263–268.
Schurr, A. (2006). Lactate: the ultimate cerebral oxidative energy substrate? J. Cereb. Blood Flow Metab. 26, 142–152.
Schurr, A., Miller, J. J., Payne, R. S., and Rigor, B. M. (1999). An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J. Neurosci. 19, 34–39.
Schurr, A., and Payne, R. S. (2007). Lactate, not pyruvate, is neuronal aerobic glycolysis end product: an in vitro electrophysiological study. Neuroscience 147, 613–619.
Schurr, A., West, C. A., and Rigor, B. M. (1988). Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 240, 1326–1328.
Schwiening, C. J., and Boron, W. F. (1994). Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na(+)-dependent Cl(−)-HCO3- exchange. J. Physiol. 475, 59–67.
Shuttleworth, C. W. (2010). Use of NAD(P)H and flavoprotein autofluorescence transients to probe neuron and astrocyte responses to synaptic activation. Neurochem. Int. 56, 379–386.
Tsai, Y. C., Chou, Y. C., Wu, A. B., Hu, C. M., Chen, C. Y., Chen, F. A., and Lee, J. A. (2006). Stereoselective effects of 3-hydroxybutyrate on glucose utilization of rat cardiomyocytes. Life Sci. 78, 1385–1391.
Turner, D. A., Foster, K. A., Galeffi, F., and Somjen, G. G. (2007). Differences in O2 availability resolve the apparent discrepancies in metabolic intrinsic optical signals in vivo and in vitro. Trends Neurosci. 30, 390–398.
Tyzio, R., Allene, C., Nardou, R., Picardo, M. A., Yamamoto, S., Sivakumaran, S., Caiati, M. D., Rheims, S., Minlebaev, M., Milh, M., Ferre, P., Khazipov, R., Romette, J. L., Lorquin, J., Cossart, R., Khalilov, I., Nehlig, A., Cherubini, E., and Ben-Ari, Y. (2011). Depolarizing actions of GABA in immature neurons depend neither on ketone bodies nor on pyruvate. J. Neurosci. 31, 34–45.
Wada, H., Okada, Y., Nabetani, M., and Nakamura, H. (1997). The effects of lactate and beta-hydroxybutyrate on the energy metabolism and neural activity of hippocampal slices from adult and immature rat. Brain Res. Dev. Brain Res. 101, 1–7.
Ward Platt, M., and Deshpande, S. (2005). Metabolic adaptation at birth. Semin. Fetal Neonatal Med. 10, 341–350.
Zielke, H. R., Zielke, C. L., and Baab, P. J. (2009). Direct measurement of oxidative metabolism in the living brain by microdialysis: a review. J. Neurochem. 109(Suppl. 1), 24–29.
Keywords: lactate, energy substrates, neonatal neurons, synaptic transmission, energy metabolism, oxygen, NAD(P)H
Citation: Ivanov A, Mukhtarov M, Bregestovski P and Zilberter Y (2011) Lactate effectively covers energy demands during neuronal network activity in neonatal hippocampal slices. Front. Neuroenerg. 3:2. doi: 10.3389/fnene.2011.00002
Received: 21 March 2011;
Accepted: 25 April 2011;
Published online: 06 May 2011.
Edited by:
Sebastian Cerdan, Instituto de Investigaciones Biomedicas Alberto Sols, SpainReviewed by:
Tibor Kristian, University of Maryland School of Medicine, USAKarl A. Kasischke, Rochester University, USA
Copyright: © 2011 Ivanov, Mukhtarov, Bregestovski and Zilberter. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Yuri Zilberter, Faculté de Médecine Timone, Institut National de la Santé et de la Recherche Médicale U751, Université de la Méditerranée, 27 Bd; Jean Moulin, 13385 Marseille Cedex 05, France, e-mail:eXVyaS56aWxiZXJ0ZXJAdW5pdm1lZC5mcg==
 Marat Mukhtarov
Marat Mukhtarov