A commentary on
Coding of stereoscopic depth information in visual areas V3 and V3A
by Anzai, A., Chowdhury, S. A., and DeAngelis, G. (2011). J. Neurosci. 31, 10270–10282
In frontal eye animals, one main cue to depth is absolute disparity, the difference between the two retinal coordinates of a given object. The cortical mechanisms underlying absolute disparity processing are rather well understood in primates. Disparity-tuned neurons can be found as early as primary visual area V1, as well as in many extra-striate areas. However, disparity judgments are imprecise when based only on absolute disparity, and are greatly enhanced when a second disparity is available as a reference in the visual field. The neural mechanisms mediating these “relative disparity” computations remain poorly understood despite a number of important studies that have addressed this topic in the past 10 years. In particular, the cortical origin of the disparity interactions is a matter of debate. Population measurements (fMRI and high-density EEG) in humans have indicated that area V3A in the “where” pathway along the dorsal cortex could be a candidate (Backus et al., 2001; Tsao et al., 2003; Cottereau et al., 2011, in press) while primate single cell recordings (see e.g., Umeda et al., 2007) have suggested a progressive encoding of relative disparity in the “what” pathway along the ventral cortex (V1–V2v–V3v–V4–IT). Until recently, no single cell measurements of relative disparity processing in macaque area V3A had been made, so a comparison with the human results was therefore impossible.
Fortunately, with their study published in July, Anzai et al. (2011) provided the missing piece of the puzzle. Using a center-surround paradigm, they characterized the disparity processing of neurons in monkey areas V3 and V3A. Following the analysis techniques employed in previous single-unit papers, at each recording site they first measured the disparity-tuning curve (i.e., the firing rate as a function of the center disparity) for a surround localized in the fixation plane (zero disparity). They then measured the tuning curves with the surround at two other disparities (−0.5° and 0.5°). Finally, they tested if a change in the surround position shifted the tuning curve by the same amount while keeping its shape unchanged. Rather surprisingly, the “phase shifts” of tuning curves were not sufficient to support an invariant processing of disparity in V3A. These results are different from those obtained in macaque ventral area V4 (Umeda et al., 2007). Nonetheless, another form of relative disparity was observed: the surround position significantly modulated the curve amplitudes (Anzai et al., 2011; Figure 5). Interestingly, this effect was also found at the population level in one recent imaging study (Cottereau et al., in press), which specifically tested how different surround positions changed the responses to a modulation of the central disk between 0 and 16 arc min. Responses in V3A kept their global shape unchanged but their amplitudes were modified by the surround disparity. The correspondence between the studies suggests that the “gain” modulations observed in single units may be consistent across extended cortical areas. Otherwise they would cancel out at the population level. The Anzai multi-unit results are in agreement with this hypothesis as they show that within V3A, neighboring cells share the same disparity properties (Anzai et al., 2011; Figure 9). Consistent gain effects across several multi-unit recordings sites have been reported in another context (Durand et al., 2010).
What could be the role of disparity interaction in area V3A? In his conclusion, Anzai suggested that they may not be specific to 3D processing, but rather reflect general surface segmentation properties. However, Tsao et al. (2003) did not find activation to orientation-defined checkerboard in V3A. When they compared the responses elicited by one versus two disparity-defined transparent planes containing the same number of dots, Backus et al. (2001) obtained bigger activations in V3A for two planes than one. They had specifically designed their stimuli without any contiguous borders, so that the effects they observed were not linked to local discontinuities in the display. Thus, the V3A responses cannot be exclusively devoted to surface segmentation. Interestingly, in our recent center-surround study (Cottereau et al., in press), my group and I showed that the gain modulation in V3A is proportional to the disparity difference between center and surround, being larger for big differences, and suppressed when the difference is zero (iso-disparity suppression). These neural computations could therefore provide an effective framework to support visually guided actions such as reaching or grasping (Melmoth and Grant, 2006). The gain modulation in V3A would produce a signal proportional to the disparity difference between hand and object whose minimization could permit an on-going monitoring of the desired action. Interestingly V3A projects directly or indirectly to the anterior intra-parietal area (AIP), which is selective for grasp in monkeys.
In conclusion, disparity interactions may take different forms in the two classical cortical pathways of primate brain (see Figure 1). In the ventral areas, they would be encoded as relative disparity, based on the phase shift of the neurons’ disparity-tuning curves. This could serve as an invariant representation of the objects in a 3D visual scene (“what is the object?”). In dorsal areas, interactions would be associated with a gain modification of the absolute disparity-tuning. This process could guide actions visually by providing an on-going estimate of the disparity difference separating hand and object (“where is the object?”). However, these two different mechanisms do not imply that disparity processing in one pathway is not interacting with processing in the other pathway. In monkeys, a recent study (Verhoef et al., 2011) used functional connectivity to demonstrate that areas IT and AIP are communicating during 3D-shape perception. The same sort of cortical connections could be used by the primate brain when performing relative disparity processing. The exact nature of these processes should be addressed in future studies on stereopsis.
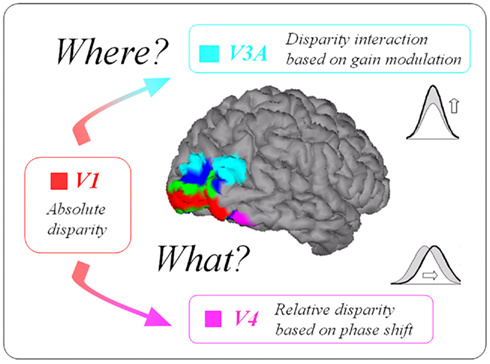
Figure 1. Different processes for disparity interaction in the primate brain. While interactions would be based on gain modulation in the dorsal pathway, phase shift of the disparity-tuning curves would be observed in the ventral pathway.
Acknowledgments
This work was supported by National Eye Institute grant R01 EY018875 and a Walt and Lilly Disney Amblyopia Research Award from Research to Prevent Blindness.
References
Anzai, A., Chowdhury, S. A., and DeAngelis, G. (2011). Coding of stereoscopic depth information in visual areas V3 and V3A. J. Neurosci. 31, 10270–10282.
Backus, B. T., Fleet, D. J., Parker, A. J., and Heeger, D. J. (2001). Human cortical activity correlates with stereoscopic depth perception. J. Neurophysiol. 86, 2054–2058.
Cottereau, B. R., McKee, S. P., Ales, J. M., and Norcia, A. M. (2011). Disparity-tuned population responses from visual cortex. J. Neurosci. 31, 954–965.
Cottereau, B. R., McKee, S. P., Ales, J. M., and Norcia, A. M. (in press). Disparity-specific spatial interactions: evidence from EEG source imaging. J. Neurosci.
Durand, J. B., Trotter, Y., and Celebrini, S. (2010). Privileged processing of the straight-ahead direction in primate area V1. Neuron 66, 126–137.
Melmoth, D. R., and Grant, S. (2006). Advantages of binocular vision for the control of reaching and grasping. Exp. Brain Res. 171, 371–388.
Tsao, D. Y., Vanduffel, W., Sasaki, Y., Fize, D., Knutsen, T. A., Mandeville, J. B., Wald, L. L., Dale, A. M., Rosen, B. R., Van Essen, D. C., Livingstone, M. S., Orban, G. A., and Tootell, R. B. (2003). Stereopsis activates V3A and caudal intraparietal areas in macaques and humans. Neuron 39, 555–568.
Umeda, K., Tanabe, S., and Fujita, I. (2007). Representation of stereoscopic depth based on relative disparity in macaque area V4. J. Neurophysiol. 98, 241–252.
Citation: Cottereau BR (2011) Disparity context processing in the primate brain: and if the question was both “what” and “when”…. Front. Hum. Neurosci. 5:152. doi: 10.3389/fnhum.2011.00152
Received: 27 September 2011;
Accepted: 10 November 2011;
Published online: 01 December 2011.
Copyright: © 2011 Cottereau. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: b.cottereau@stanford.edu
