- 1Centre de Recherche Cerveau et Cognition, Université de Toulouse-UPS, Toulouse, France
- 2Centre National de la Recherche Scientifique, Toulouse, France
- 3Department of Psychology, University of California, Riverside, CA, USA
- 4Department of Ophthalmology, Hopital CHU Purpan, Toulouse, France
Age related macular degeneration (AMD) is a visual disease that affects elderly population. It entails a progressive loss of central vision whose consequences are dramatic for the patient’s quality of life. Current rehabilitation programs are restricted to technical aids based on visual devices. They only temporarily improve specific visual functions such as reading skills. Considering the rapid increase of the aging population worldwide, it is crucial to intensify clinical research on AMD in order to develop simple and efficient methods that improve the patient’s visual performances in many different contexts. One very promising approach to face this challenge is based on perceptual learning (PL). Through intensive practice, PL can induce neural plasticity in sensory cortices and result in long-lasting enhancements for various perceptual tasks in both normal and visually impaired populations. A growing number of studies showed how appropriate PL protocols improve visual functions in visual disorders, namely amblyopia, presbyopia or myopia. In order to successfully apply these approaches to more severe conditions such as AMD, numerous challenges have to be overcome. Indeed, the overall elderly age of patients and the reduced cortical surface that is devoted to peripheral vision potentially limit neural plasticity in this population. In addition, ocular fixation becomes much less stable because patients have to rely on peripheral fixation spots outside the scotoma whose size keeps on evolving. The aim of this review article is to discuss the recent literature on this topic and to offer a unified approach for developing new rehabilitation programs of AMD using PL. We argue that with an appropriate experimental and training protocol that is adapted to each patient needs, PL can offer fascinating opportunities for the development of simple, non-expensive rehabilitation approaches a large spectrum of visual functions in AMD patients.
Introduction
Age-related macular degeneration (AMD) is the leading cause of visual impairments in elderly population in western countries and affects several million of people worldwide. Starting progressively over 50 years, the end-stage AMD results in loss of central vision, usually in both eyes, with retinal scotomas extending beyond 20° of diameter (Cheung and Legge, 2005). It therefore has severe consequences for the patient autonomy and quality of life. Currently, no standard rehabilitation procedure exists (Amoaku et al., 2012). When treatments are available, they are only rarely able to reverse vision loss and are usually associated with adverse side effects (Falavarjani and Nguyen, 2013). Most of the rehabilitation programs focus on improving reading skills. These approaches generally involve vision technicians who teach the patients how to use visual aids like magnifiers, scroll text or prisms. If these devices partially help the patients to overcome their handicap, they do not improve their perceptual abilities. Moreover, training requires constant supervision, which in practice is very difficult to achieve with most of the patients.
In the recent past, new rehabilitation approaches with training based on eccentric viewing, oculomotor control and perceptual learning (PL) appeared. These approaches were quite successful at improving peripheral reading in patients (for a review see Pijnacker et al., 2011). In particular, PL seems to be very promising. This technique produces effective sensory improvement in performing perceptual tasks by training (Sagi, 2011). It can be used in clinical practice and does not require supervision of experts, nor expensive equipment. Moreover, PL mechanisms are based on long-term modifications in sensory cortex guided by neural plasticity. The aim of this review article is to describe how PL can be used to improve vision in AMD patients. In the following, we first present recent results obtained with PL in normal population or with patients suffering from central vision deficits (see the “Perceptual Learning as a Tool to Improve Visual Functions” Section), then we describe and discuss the results of very recent studies that applied PL techniques in AMD patients (see the “Perceptual Learning in AMD Patients” Section).
Perceptual Learning as a Tool to Improve Visual Functions
Perceptual Learning in Normal Population
The efficacy of PL has been shown in all sensory modalities, with the majority of studies focusing on visual learning. Training has been shown to improve visual perception for a variety of low-level tasks including grating detection (De Valois, 1977; Fiorentini and Berardi, 1980, 1981; Mayer, 1983), motion direction discrimination (Ball and Sekuler, 1982, 1987; Ball et al., 1983), visual search (Sireteanu and Rettenbach, 1995; Ahissar and Hochstein, 1996; Ellison and Walsh, 1998) and texture discrimination (Karni and Sagi, 1991, 1993). While the underlying neural mechanisms remain controversial (see “Perceptual Learning and Cortical Plasticity in AMD” Section), vision scientists usually agree that PL of basic visual stimulus properties induces modifications of cortical processing in early visual system, e.g., in the striate (Karni and Sagi, 1991, 1993; Saarinen and Levi, 1995; Pourtois et al., 2008) and/or extrastriate (Ahissar and Hochstein, 1996) visual cortex. In this context, the question of the specificity of PL has long been considered as an important drawback for its application in visual rehabilitation. To result in a general improvement of visual perception, the neural changes induced by PL should reach beyond early visual areas, producing modification in higher-level, non-retinotopic cortical regions (Ahissar and Hochstein, 1997; Zhang et al., 2010). Interestingly, recent studies demonstrated that specific experimental designs actually permit to transfer the improvement obtained in a low level training task (i.e., contrast detection) to higher-level visual functions (i.e., Visual Acuity (VA), Contrast Sensitivity (CS)) in both normal and visually impaired population (Levi et al., 1997; Polat et al., 2004; Tan and Fong, 2008). Other studies have also demonstrated that, under precise conditions, learning could transfer to different retinal locations. Specifically, Xiao et al. (2008) showed that with a double training paradigm, in which one retinal location is trained with a task (contrast discrimination) and another retinal position is trained with a different task (orientation discrimination), performances on the first task also increased in the second position that was not trained on this task. This result supports the involvement of higher-level visual areas that enable the transfer of learning across spatial positions. Finally, recent studies suggest that specificity of learning and amount of transfer are directly related to the difficulty of the training (Hung and Seitz, 2014). Transfer of learning seems absent for difficult trials and restored with easy trials, consistent with the reverse hierarchy theory (Ahissar and Hochstein, 1997, 2000) stating that difficult tasks induce a shrinking of the attentional window and an increase of learning specificity. Altogether, the aforementioned studies suggest that, with the appropriate experimental protocol, PL can induce general and long-lasting improvements in a number of visual abilities. For these reasons, PL has been rapidly proposed as a rehabilitation procedure in patients suffering from visual impairments.
Perceptual Learning in Population with Central Vision Deficits
In the last 20 years, PL has been applied to the re-education of patients with central vision deficits (Levi and Polat, 1996; Levi et al., 1997). A multitude of techniques have attempted to improve vision in a variety of visual conditions (see Campana and Maniglia, 2015, for a recent research topic on practical applications of PL). A number of these studies used a low-level task based on a collinear facilitation paradigm. In this task, participants have to detect a low-contrast Gabor patch between two Gabor patches aligned collinearly (Polat and Sagi, 1993, 1994a). The flanking elements facilitate detection performances from early sensory mechanisms that are likely to involve horizontal connections in primary visual cortex (Gilbert and Wiesel, 1985, 1992; Ts’o et al., 1986; Grinvald et al., 1994; Polat and Norcia, 1996). Through PL, collinear facilitation can be improved and enhances in turn higher visual abilities that rely on inputs from earlier stages (Polat and Sagi, 1994b). This protocol has proven its efficacy in treating amblyopia, a developmental visual anomaly that reduces vision in one eye. For example, Polat et al. (2004) showed that this training improved CS and VA in the amblyopic eye, with results persisting after up to 1 year. Hussain et al. (2012) trained amblyopic patients on a flanked letter identification task and reported a reduction of critical spacing between letters in the amblyopic eye. The collinear facilitation paradigm has also been used to treat presbyopia (Polat, 2009), a common age-related visual disease that affects most of the population over 50 years and myopia (Tan and Fong, 2008). In both cases, results showed improvement of CS and VA. In conclusion, PL paradigms, based on simple training over a reduced period of time (1–2 months) can improve visual performances in a variety of visual deficits. However, all these PL paradigms target pathologies characterized by an intact retina, with the final goal of improving central vision after the emergence of optic (presbyopia and myopia) or cortical (amblyopia) abnormalities. In cases of more severe visual disease, such as a retinal damage in AMD, foveal restoration is unlikely. Therefore, PL must accomplish a different goal, that is, training a new retinal location to become the functional substitute of the fovea called Preferred Retinal Locus (PRL) and located in the peripheral retina, usually close to the scotoma. Consequently, an effective rehabilitation program for AMD must take into account the structural differences between foveal and peripheral vision.
Perceptual Learning in Peripheral Vision
Peripheral vision is constrained by its anatomy. The cortical surface devoted to the processing of the peripheral field is indeed very small when compared to that dedicated to central vision. As a result, CS, orientation discrimination, letter acuity (Johnson et al., 1978), word identification speed (Latham and Whitaker, 1996), among others, are reduced in the periphery (Strasburger et al., 2011). Collinear facilitation, the aforementioned training configuration used for vision restoration (see the previous section), emerges at larger target-to-flanker distances in peripheral vision (Lev and Polat, 2011; Maniglia et al., 2011), in agreement with cortical magnification. Its spatial frequency tuning (Maniglia et al., 2015a) and spatial range (Maniglia et al., 2015b) are also different from those observed in foveal vision. Similarly, in the periphery, we experience visual crowding, i.e., the difficulty to identify peripheral targets when surrounded by flanking elements (Bouma, 1970). While almost absent in foveal vision, crowding represents a strong limitation in peripheral reading. Numerous PL studies aimed at improving peripheral visual abilities during crowding (Chung, 2007; Maniglia et al., 2011; Hussain et al., 2012; Chung and Truong, 2013), texture discrimination (Karni and Sagi, 1991), letter recognition (Chung et al., 2004, 2005) and reading speed (Chung et al., 2004; Yu et al., 2010a). Because VA and reading are the most impaired functions in peripheral vision of elderly people, these studies could provide a stepping-stone to develop rehabilitative protocols for AMD patients. Chung (2007) used a peripheral (10° of eccentricity) crowded letter identification task and reported an improvement in the task of 88% and a reduction of crowding extent of 38%, but no transfer to reading speed. Hussain et al. (2012) trained normal participants and amblyopic patients on crowded letter identification, with both groups showing similar improvements in the crowding reduction but no transfer to VA. Regarding reading speed, a number of studies showed that through various training protocols, such as letter identification (Chung et al., 2004; Lee et al., 2010; Yu et al., 2010a,b) word/non-word tasks (Yu et al., 2010a) and Rapid Serial Visual Presentation (RSVP; Chung et al., 2004), PL increased peripheral reading speed in healthy participants. For example, Chung et al. (2004) used a trigram letter-recognition task to increase the peripheral visual span (the number of characters that can be recognized during a fixation) that consequently improves reading speed. After four daily sessions, participants improved their peripheral reading speed by 41%. Yu et al. (2010a) compared three types of training for peripheral reading speed: RSVP, trigram letter-recognition and lexical-decision training method. RSVP is a paradigm in which words are showed one per time in rapid sequence in the same location, avoiding the implication of eye movements (Rubin and Turano, 1994). Participants in the first group improved their reading speed by 72%, the second group improved it by 54%, while participants trained with the lexical-decision improved it by 39%, which is significantly lower than the RSVP group. Bernard et al. (2012) investigated whether training on a trigram letter-recognition task can be optimized by using triplets of letters that are frequently used in the participants’ native language. However, results were not different from those obtained in the study by Chung et al. (2004). Maniglia et al. (2011) tested a collinear facilitation paradigm at intermediate eccentricities (4°), and showed that practice on this configuration also improved untrained visual functions such as peripheral CS and reduced crowding, in agreement with recent hypotheses supporting the idea that collinear facilitation and crowding might share similar neural substrates (Lev and Polat, 2011). This may have important implications for the rehabilitation of low-vision patients who must use peripheral vision to perform tasks (such as e.g., reading) that are usually processed by the fovea in normal sighted participants.
Perceptual Learning in AMD
Overview
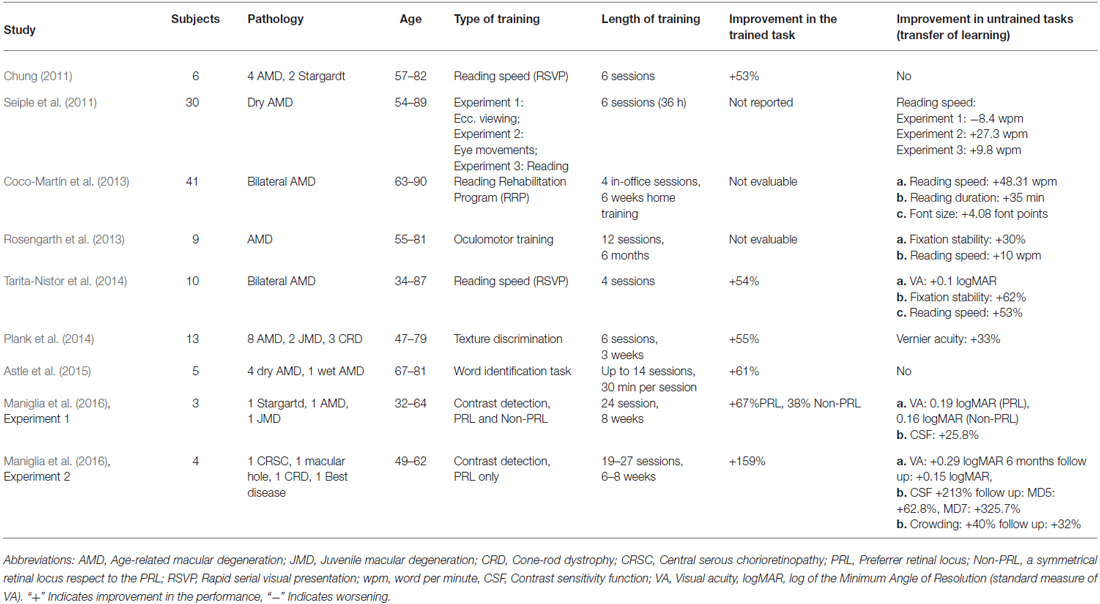
So far, only a few studies investigated the application of PL in AMD patients. Previous rehabilitative approaches in AMD: (i) focused on exercises aimed at improving muscle control, eye movements and fixation (Nilsson et al., 2003), cognitive tasks (Watson et al., 1992) or used reading rehabilitation programs (RRP; Coco-Martín et al., 2013); (ii) stemmed from the optometry field rather than visual science; (iii) stressed the role of low vision therapists and the use of magnification devices; and (iv) lacked a theoretical framework of PL on neural plasticity. Chung (2011) can be considered as the first study in which a typical PL protocol (namely RSVP) was used to improve visual functions in AMD patients (see Table 1 for more details about this study and others that used PL on AMD population). Results showed an improvement in reading speed, with nonetheless an important inter-individual variability. However, no transfer of learning was observed for critical letter size, VA and fixation stability. This study had several limitations such as the absence of a control group, the identification of the PRL based on a task that differed from the one used during training (fixation vs. reading), the discrepancy between the monocular PRL profiling and the binocular training and the limited ecological value of the training task (RSVP prevents eye movements). It nonetheless demonstrated for the first time the possibility of improving residual vision in AMD through PL. Seiple et al. (2011) compared three rehabilitation protocols in AMD patients: (i) visual awareness and eccentric view training; (ii) eye movement training; and (iii) RSVP. In their sample of patients, only the eye movement training produced improvements in reading speed. This is consistent with the hypothesis that oculomotor control in patients without central vision plays a key role during reading. However, in contrast with Chung (2011), Seiple et al. (2011) did not find improvement in reading speed with RSVP. Methodological differences between the two studies can account for this discrepancy. Chung (2011) tested the patients binocularly while Seiple et al. (2011) did it monocularly, and their measures of reading speed differed (continuous text for Seiple et al., 2011; RSVP for Chung, 2011). Tarita-Nistor et al. (2014) used a paradigm similar to Chung (2011) but based on smaller words, near the reading acuity limit. The rationale was that PL is more effective when stimuli are presented around participants’ threshold, thereby inducing greater focus on the task (Tsodyks and Gilbert, 2004; Seitz and Watanabe, 2005; Sagi, 2011). Moreover, near threshold training generalizes to untrained visual functions (Polat, 2009), unlike above threshold training (Chung et al., 2012). Tarita-Nistor et al. (2014) reported improvement in reading speed (54%, similar to Chung, 2011), as well as binocular VA (from 0.54 to 0.44 LogMAR on average) and monocular VA in the better eye (but not in the worse). As reported earlier, RSVP eliminates the need for eye movements but it does represent an unusual situation outside experimental settings. Consequently, Tarita-Nistor et al. (2014) tested reading acuity and maximum reading speed with continuous text and found an improvement in both indexes (although no changes in critical print size). Finally, fixation stability improved after training in both eyes (62% in the better eye and 58% in the worse). These results highlight the importance of a PL training tailored on each patient’s thresholds, in order to maximize the effect of training and promote transfer to other visual abilities. However, the main limitations of this study are the absence of a control group (although authors used a test-retest sample of five patients, finding no changes in the measured visual abilities) and the lack of monitoring of the PRL position during training. Astle et al. (2015) trained AMD patients in a word identification task and reported a significant improvement in reading speed and baseline performances. Improvements were correlated with age with younger participants performing better than the elderly. Overall, learning curves for controls and patients were similar. The latter were however able to complete less blocks because of fatigue. In Astle et al. (2015), AMD patients were not trained in their PRL but rather at a fixed eccentricity (10°) and “asked to fixate the center of the fixation cross so that the end of the limbs appeared to extend equal distances into the peripheral visual field, even though the center of the fixation cross itself was not visible to any of the participants (i.e., it fell within the scotoma)”, a demanding task that probably explains the reduced number of trials that patients were able to complete during each session. Rosengarth et al. (2013) trained AMD patients using an oculomotor task, reporting improvements in reading speed (10 words/min) and fixation stability (30%). These improvements were however stronger between pre- and mid-test measurements than between pre- and post-training. Similarly, Plank et al. (2014) trained AMD patients with a classic Texture-Discrimination Task (TDT) in their PRL. At the end of the training, patients improved in the trained task of about 55%, with transfer of learning to Vernier Acuity. One of the reasons why little transfer is observed after TDT training might be due to the high specificity of learning for this task (Karni and Sagi, 1991). Similarly, Chung (2011) did not observe transfer of learning after training reading speed on a RSVP display, another task that might not represent an ideal transfer probe. Recently, Maniglia et al. (2016) trained AMD patients with a collinear facilitation configuration similar to the one used to treat amblyopia and myopia, a protocol that permits a better generalization of learning. Results showed that patients improved their performances not only in the trained task but also in transfer tasks such as CSF, VA and crowding reduction. Importantly, follow up tests demonstrated that this transfer of learning was retained 6 months after the end of the training.
Perceptual Learning and Cortical Plasticity in AMD
PL aims at improving perceptual functions by promoting neural plasticity. However, it cannot always be assumed that PL-related improvements observed in normal participants can be found in AMD patients as well because of the age at which AMD usually emerges (>65 years old). Older participants might indeed show smaller training-related improvements because of reduced neural plasticity (Sunness et al., 2004; Smirnakis et al., 2005). In general, detrimental effects of age on visual abilities have been reported for several perceptual attributes. However, numerous studies showed PL-induced improvements in healthy elderly population, such as in texture discrimination (Andersen et al., 2010), motion discrimination (Ball and Sekuler, 1987; Bower and Andersen, 2012), orientation discrimination (DeLoss et al., 2014), Vernier acuity (Fahle and Daum, 1997), CS (DeLoss et al., 2015) and VA (Polat, 2009) among others. For example, Andersen et al. (2010) showed that texture discrimination significantly improved for elderly trained with near threshold stimuli, with a 3-month follow up showing retention of the improvement. Yu et al. (2010b) tested elderly population (55–76 years old) with the same task as Chung et al. (2004) and showed that perceptual improvements were smaller than those observed in younger participants. The difference in performance does not seem related to the training duration (Richards et al., 2006) but rather to the day-to-day lapse in learning effect absent in younger participants. Moreover, unlike in younger participants, learning in elderly participants was more specific and led to an improved reading speed only at the trained location and for the print size used during the training. This result is consistent with the idea that aging brain presents reduced neural plasticity. Astle et al. (2014) investigated the effect of age on PL: using a peripheral word identification task, the authors showed that older normal sighted participants improved more than younger participants and that the amount of learning was correlated with age and initial performance. The improvement in reading speed was significantly greater in the older group that reached the performance level of younger participants at the end of the training. This result is encouraging in the perspective of PL trainings for AMD since the average age of this clinical population leans on the older elderly side. These different studies show that even though PL in elderly population is far from being well understood, this method might offer interesting perspectives regarding rehabilitation strategies for patients suffering from AMD.
Nonetheless, a further concern is that we cannot assume that residual vision in AMD patients is the same as in normal population for a series of reasons: (1) the retinal lateral connections might be affected by the macular degeneration; and (2) the spontaneous cortical reorganization taking place after the lesion might produce differences in perceptual and training effects in AMD patients with respect to healthy participants. From the electrophysiological and neuroimaging perspectives, spontaneous cortical reorganization in AMD is a controversial topic: earlier electrophysiology studies agreed upon consistent evidence for cortical reorganization after retinal lesion (Darian-Smith and Gilbert, 1994; Gilbert, 1998). Using animal models such as cat and monkey, these topographic reorganizations following retinal lesions were hypothesized to arise from long-range horizontal connections formed by cortical pyramidal cells that undergo rapid and exuberant sprouting and pruning in response to removal of sensory input that can account for the topographic reorganization following retinal lesions (for review see Gilbert and Li, 2012). However, these views on spontaneous cortical reorganization were not conclusively supported by a study combining fMRI and electrophysiology in macaque (Smirnakis et al., 2005). In addition, recent neuroimaging data in human led to controversial results regarding whether and to what extent spontaneous neural plasticity exists in the lesion projection zone (LPZ), the region of the cortex formerly activated by foveal inputs. Using a large sample of patients under passive viewing, no evidence for cortical reorganization and remapping of V1 was found after retinal lesion (Baseler et al., 2011). Other studies used an active task (e.g., where patients had to detect some visual properties in the stimuli) and found significant BOLD activations in the LPZ but it is still unclear whether these activations reflected a real remapping of V1 (Baker et al., 2005; Dilks et al., 2009, 2014) or rather the unmasking of pre-existing cortico-cortical feedback inputs that lost their feed-forward input balancing from the LPZ (for a review Masuda et al., 2008; Wandell and Smirnakis, 2009). Also, smaller silent zones have been reported at the posterior pole of the occipital cortex during active compared to passive conditions possibly involving task-dependent signals from higher cortical areas Liu et al. (2010). These controversial views are also reflected in psychophysical studies. For example, Chung (2013) measured the psychophysical critical space of crowding in AMD patients, in particular the radial-tangential anisotropy, a trademark characteristic of crowding for which its spatial extension is about twice as large in the radial vs. the tangential meridian. Results showed that this anisotropy is absent in their PRL, with the shape of crowding resembling that of normal participants’ fovea rather than of periphery. Chung (2013) hypothesized that this finding might reflect a spontaneous reorganization based on the re-referencing of the oculomotor system towards the PRL rather than the fovea. Nandy and Tjan (2012) suggested that the anisotropic shape of peripheral crowding depends on saccade-confounded image statistics, since normal saccades are radial with respect to the fovea. If the PRL becomes the new reference for eye movements, the absence of radial saccades towards the fovea should reduce crowding. Consistently with this hypothesis, several studies showed a re-referencing of eye movements towards the PRL in AMD patients (White and Bedell, 1990; Whittaker et al., 1991). Finally, Chung (2013) suggested that the cortical site for spontaneous reorganization might be localized within visual areas usually associated with crowding, i.e., V1 (Nandy and Tjan, 2012), V2 (Freeman and Simoncelli, 2011), V3 (Tyler and Likova, 2007; Bi et al., 2009) or V4 (Motter, 2006). On the other hand, an article by Haun and Peli (2015) reported similar performances for AMD patients and controls in a peripheral ladder contour detection task. Since this task is probably based on the visual integration mechanisms supporting crowding (Field et al., 1993; Pelli et al., 2004; May and Hess, 2007), this result disagrees with a spontaneous cortical reorganization around the PRL.
Recently, two articles (Rosengarth et al., 2013; Plank et al., 2014) investigated PL in AMD patients and its neural correlates with fMRI. In the first study, Rosengarth et al. (2013) used an oculomotor training and reported no significant changes in BOLD signal between pre and post test in early visual (V1, V2 and V3) or higher level associative areas (LOC, fusiform gyrus, ITG). They nonetheless found positive correlation between fixation stability and BOLD signal. Similarly, Plank et al. (2014) used a TDT and showed a positive correlation between BOLD signal in early visual cortex and fixation stability at baseline as well as a positive correlation between the amount of learning and fixation stability at baseline. As reported earlier, TDT (and probably oculomotor training) might not be an ideal task to induce transfer of learning and plasticity effects, which could partially explain the lack of functional changes observed in the aforementioned studies.
Challenges in the Use of Perceptual Learning in AMD Patients
PL in AMD patients suffers from practical and theoretical limitations such as the elderly age of patients, the lack of independence in transportation to reach clinical/lab facilities or the limited control of experimental conditions during potential at-home sessions. All these limitations concur in making clinical research with this population a challenge. It explains why one of the main goals in visual rehabilitation is to reduce the overall amount of training sessions and to develop effective at-home training paradigms. Previous studies on PL preferred to schedule training sessions over consecutive days, with the rationale that learning needs a sustained investment. However, a recent study by Chung and Truong (2013) investigating the effect of training frequency on a crowding task in normal and amblyopic participants, showed that improvement did not depend on the training frequency but rather on the number of sessions. In particular, PL literature shows that spaced practice, a training procedure that includes a break between trials (Donovan and Radosevich, 1999) produces greater improvements and longer duration, most likely because of the sleep consolidation process between sessions (Karni and Sagi, 1993). Overall, these results work in favor of applying PL in cases in which patients cannot reach the labs more than once per week.
Regarding the theoretical challenges, the application of vision models tested in healthy participants to clinical population is not always straightforward. Protocols that successfully led to performance improvements in normal participants might not be suitable for AMDs, because of overall poorer performance of AMD patients’ peripheral vision or as a consequence of partial spontaneous cortical reorganization (Chung, 2013). Another point of concern seems to be the retinal location at which the patients should be trained. The process leading to the emergence of the PRL is poorly understood and it is sill unclear why it takes longer to develop a PRL in patients than in controls with simulated central scotoma (Kwon et al., 2013). While several authors proposed the possibility of choosing a more favorable or sensitive spot (Nilsson, 1990; Nilsson et al., 2003) as a new PRL (a TRL, a trained retinal locus), a systematic monitoring is necessary, since there is no certainty that patients will retain the TRL after the end of the training. Similarly, a regular supervision of the scotoma size is a crucial step in order to control that the new PRL does not fall into the expanding scotoma. Moreover, patients might use different PRL to process different tasks and/or stimuli of different sizes. Finally, similarly to what is observed in normal participants (Hung and Seitz, 2014), the difficulty of the task seems to play a role in defining the amount of improvement that can be reached (Tarita-Nistor et al., 2014) which in addition to the duration of the sessions/blocks should therefore be adjusted for each patient’s need and possibility. Another issue with AMD research is the small number of patients tested and the common absence of a proper control group. As reported above, practical difficulties do not help recruiting a significant number of patients and samples are often rather inhomogeneous (Goodrich et al., 2004). It results in different outcomes from studies with similar protocols. Furthermore, the use of simulated central scotoma with an artificial disk in normal sighted participants as a control condition might not be the solution due to the functional difference with a physical retinal scotoma, of which the patient might not be totally aware. Actually, retinal lesions might not always induce dense scotomas, leaving insulas of residual vision that might not be well simulated by artificial scotomas (undefined borders). Moreover, the overall elderly age of AMD patients increases the risk that they also develop other health pathologies, either physical or mental. Even more, AMD patients seem in general more prone to have comorbidity, some of which can be life-threatening (Zlateva et al., 2007). This makes it challenging to induce improvement when targeting MD alone or to isolate the training contribution to MD reduction from the deleterious effects of other concomitant pathologies.
Finally, all these training protocols have the need for follow-up tests and regular post training monitoring, facing the difficulty of distinguishing the natural decay of visual abilities, due to age, disease and comorbidity, from the lack of effectiveness of the training.
Perspectives
PL has recently become a focus of interest for its clinical applications and it stands as a promising tool to improve visual abilities in clinical population. However, as reported in the previous section, AMD patients present special needs and challenges that require the training to be as comfortable and effective as possible. New approaches to PL showed how specificity and lack of transfer can be overcome by new theoretical paradigms, such as double training (Xiao et al., 2008) or manipulation of exogenous attention (Szpiro and Carrasco, 2015). Both these two approaches induce transfer of learning from one retinal location to another and offer fascinating perspectives for more advanced forms of visual training that might improve visual abilities in different retinal spots as it would be the case in patients with multiple PRLs. Another potentially effective approach would be to focus on tasks that are known for inducing transfer of learning in peripheral vision as well (Polat et al., 2004; Polat, 2009; Maniglia et al., 2011, 2016). Finally, a more integrated PL paradigm that takes into account not only basic features or visual abilities (VA, crowding reduction, contrast sensitivity function (CSF)) but also their interactions with higher level functions during an active visual task (e.g., attention, cognitive control) to guide the re-referencing of the oculomotor system towards the PRL would probably be more beneficial on the long run.
Finally, brain stimulation, alone or coupled with PL, has been recently used to improve perceptual abilities in normal and visually impaired populations (Terney et al., 2008; Thompson et al., 2008; Olma et al., 2013; Camilleri et al., 2014b). In particular, transcranial magnetic stimulation (TMS), a technique based on the induction of a transient magnetic field that depolarizes the membrane of the stimulated neurons, has been shown to enhance CS in both normal sighted and amblyopic patients when applied over early visual areas (Thompson et al., 2008). Similarly, transcranial direct current stimulation (tDCS), a technique that consists in delivering weak (1.0–2.0 mA) electrical currents in targeted brain regions to induce modulation of cortical excitability, seems to improve motion perception (Olma et al., 2013) and line bisection (Sunwoo et al., 2013) in stroke patients and to reduce surround suppression (Spiegel et al., 2012) in normal participants. Neural modulation via brain stimulation therefore appears as a promising technique to improve perceptual function in visually impaired population, especially when considering that the neural basis of the observed improvement seems to rely on a long-lasting form of plasticity similar to long term potentiation (Stagg and Nitsche, 2011). Even more promising are results coming from recent studies in which brain stimulation is combined with a training protocol. Coupling PL with a stimulation protocol can increase visual abilities and reduce the number of training sessions necessary to observe a significant improvement, both in normal and visually impaired population (Camilleri et al., 2014a; Campana et al., 2014). As reported earlier, reducing training sessions would make PL-based programs more feasible for AMD patients with limited transportation options. Most of the trainings reported in the present review involved a large number of sessions, spanning over weeks or months, thereby causing discomfort and inducing some participants to abandon the protocol because of logistical reasons. Moreover, long trainings might be ineffective in cases of rapidly expanding degenerations. In particular, a form of non invasive transcranial electric stimulation (tES), namely transcranial Random Noise Stimulation (tRNS) may optimize PL effect by modulating synchronization of neural activity and inducing excitation (Moss et al., 2004) that in turn are associated with neuroplasticity (Grenier et al., 2001; Ponomarenko et al., 2008; Terney et al., 2008). The advantage of tRNS over tDCS is that it does not induce homeostasis in the targeted neurons because of its random frequency of stimulation (Terney et al., 2008). Few studies so far investigated the effect of tRNS on visual PL (Fertonani et al., 2011; Pirulli et al., 2013; Camilleri et al., 2014a; Campana et al., 2014). Specifically, Fertonani et al. (2011) showed how tRNS resulted in the highest amount of improvement in orientation discrimination with respect to other tES protocols. Campana et al. (2014) recently reported faster learning and greater transfer to VA and CS when PL was coupled with tRNS, both in normal sighted and visually impaired (myopia, amblyopia) population. Specifically, 2 weeks (8 sessions) of PL-tRNS combinations seem to induce the same amount of improvement in VA and CS as a 2 months (24 sessions) classic PL training (Camilleri et al., 2014a; Campana et al., 2014).
Conclusion
With the increasingly aging population, AMD is destined to become an even more common problem in western countries. Different approaches to the problem seem to focus on partial solutions or to demand a serious amount of investment both in terms of time and effort from the patient. PL seems to be a promising technique because it is easy to use (it does not require low vision therapists), affordable (it does not require expensive equipment) and comfortable (participants are trained with demanding but not uncomfortable session). Moreover PL does not just produce a temporary increase of performance, but it also promotes neural plasticity and cortical reorganization. Even more, combined approaches coupling PL with brain stimulation (electric or magnetic) promise to reduce the time needed for a significant improvement, delineating scenarios in which few weeks of training can produce long lasting changes in visual functions. They should contribute to develop efficient and appropriate rehabilitation programs to increase visual abilities and therefore quality of life of AMD patients. Figure 1 summarizes rehabilitation approaches for potential benefits.
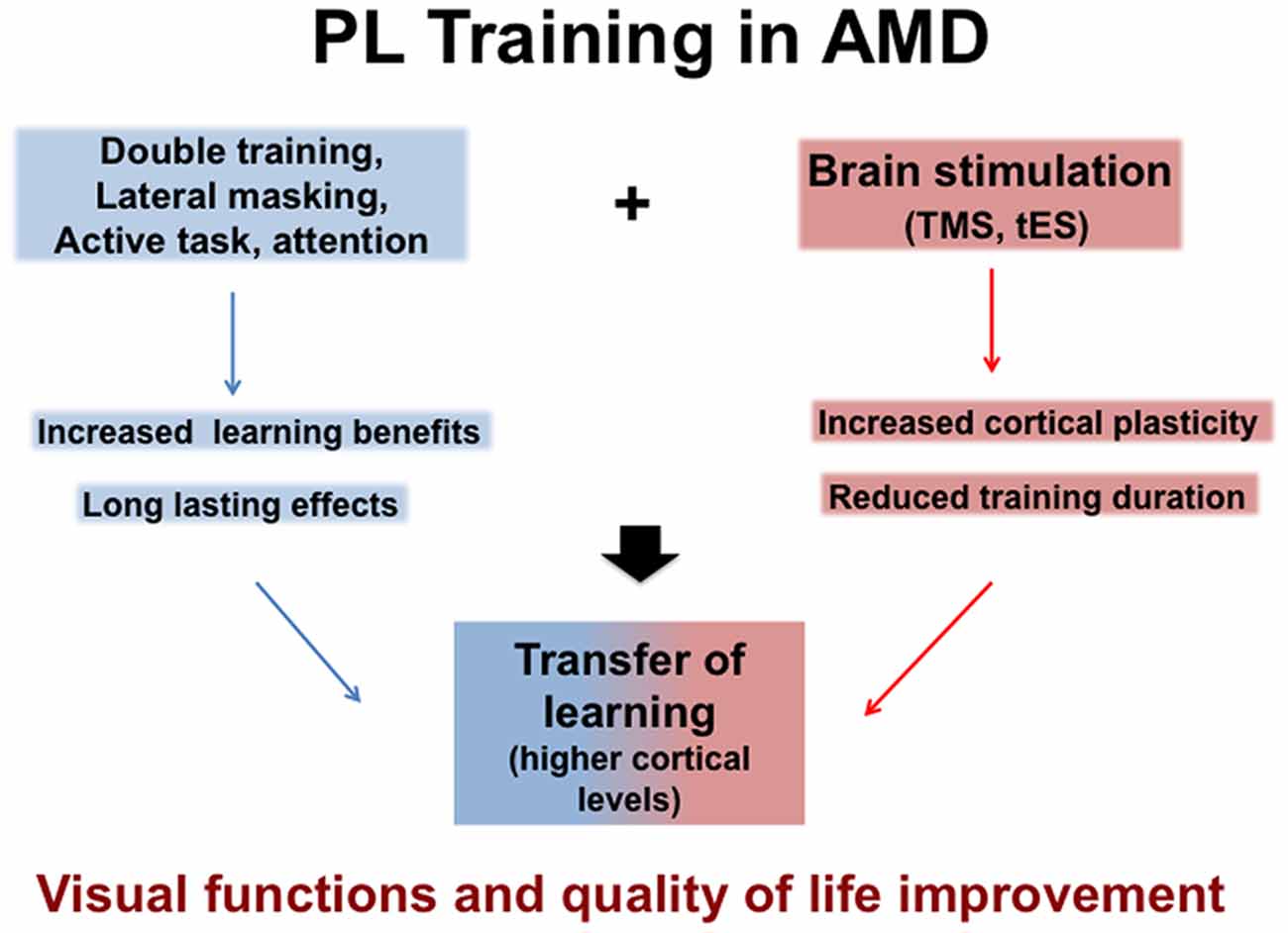
Figure 1. Potential benefits of Perceptual Learning (PL) in Age Macular Degeneration (AMD) either alone or associated with brain stimulation, Transcranial Magnetic Stimulation (TMS) or transcranial Electric Stimulation (tES) that both facilitate Transfer learning (see text).
Author Contributions
All authors partipated in the writing of this review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
MM was supported by the “Fondation de France (Fouassier; 2013 00039351 and 2014 00048124)” and “Fondation de l’Avenir (AP-RMA-2015-003)”. Authors would like to thank Russell Cohen Hoffing for english proofreading.
References
Ahissar, M., and Hochstein, S. (1996). Learning pop-out detection: Specificities to stimulus characteristics. Vision Res. 36, 3487–3500.
Ahissar, M., and Hochstein, S. (1997). Task difficulty and the specificity of perceptual learning. Nature 387, 401–406.
Ahissar, M., and Hochstein, S. (2000). The spread of attention and learning in feature search: Effects of target distribution and task difficulty. Vision Res. 40, 1349–1364.
Amoaku, W., Blakeney, S., Freeman, M., Gale, R., Johnston, R., Kelly, S. P., et al. (2012). Action on AMD. Optimising patient management: Act now to ensure current and continual delivery of best possible patient care. Eye (Lond) 26, S2–S21.
Andersen, G. J., Ni, R., Bower, J. D., and Watanabe, T. (2010). Perceptual learning, aging and improved visual performance in early stages of visual processing. J. Vis. 10:4.
Astle, A. T., Blighe, A. J., Webb, B. S., and McGraw, P. V. (2014). The effect of aging on crowded letter recognition in the peripheral visual field. Invest. Ophthalmol. Vis. Sci. 55, 5039–5045.
Astle, A. T., Blighe, A. J., Webb, B. S., and McGraw, P. V. (2015). The effect of normal aging and age-related macular degeneration on perceptual learning. J. Vis. 15:16.
Baker, C. I., Peli, E., Knouf, N., and Kanwisher, N. G. (2005). Reorganization of visual processing in macular degeneration. J. Neurosci. 25, 614–618.
Ball, K., and Sekuler, R. (1982). A specific and enduring improvement in visual motion discrimination. Science 218, 697–698.
Ball, K., and Sekuler, R. (1987). Direction-specific improvement in motion discrimination. Vision Res. 27, 953–965.
Ball, K., Sekuler, R., and Machamer, J. (1983). Detection and identification of moving targets. Vision Res. 23, 229–238.
Baseler, H. A., Gouws, A., Haak, K. V., Racey, C., Crossland, M. D., Tufail, A., et al. (2011). Large-scale remapping of visual cortex is absent in adult humans with macular degeneration. Nat. Neurosci. 14, 649–655.
Bernard, J. B., Arunkumar, A., and Chung, S. T. L. (2012). Can reading-specific training stimuli improve the effect of perceptual learning on peripheral reading speed? Vision Res. 66, 17–25.
Bi, T., Cai, P., Zhou, T., and Fang, F. (2009). The effect of crowding on orientation-selective adaptation in human early visual cortex. J. Vis. 9, 13.1–13.10.
Bower, J. D., and Andersen, G. J. (2012). Aging, perceptual learning and changes in efficiency of motion processing. Vision Res. 61, 144–156.
Camilleri, R., Pavan, A., Ghin, F., Battaglini, L., and Campana, G. (2014a). Improvement of uncorrected visual acuity and contrast sensitivity with perceptual learning and transcranial random noise stimulation in individuals with mild myopia. Front. Psychol. 5:1234.
Camilleri, R., Pavan, A., Ghin, F., and Campana, G. (2014b). Improving myopia via perceptual learning: is training with lateral masking the only (or the most) efficacious technique? Atten. Percept. Psychophys. 76, 2485–2494.
Campana, G., Camilleri, R., Pavan, A., Veronese, A., and Lo Giudice, G. (2014). Improving visual functions in adult amblyopia with combined perceptual training and transcranial random noise stimulation (tRNS): a pilot study. Front. Psychol. 5:1402.
Campana, G., and Maniglia, M. (2015). Editorial: improving visual deficits with perceptual learning. Front. Psychol. 6:491.
Chung, S. T. L. (2007). Learning to identify crowded letters: does it improve reading speed? Vision Res. 47, 3150–3159.
Chung, S. T. L. (2011). Improving reading speed for people with central vision loss through perceptual learning. Invest. Ophthalmol. Vis. Sci. 52, 1164–1170.
Chung, S. T. L. (2013). Cortical reorganization after long-term adaptation to retinal lesions in humans. J. Neurosci. 33, 18080–18086.
Cheung, S.-H., and Legge, G. E. (2005). Functional and cortical adaptations to central vision loss. Vis. Neurosci. 22, 187–201.
Chung, S. T. L., Legge, G. E., and Cheung, S. H. (2004). Letter-recognition and reading speed in peripheral vision benefit from perceptual learning. Vision Res. 44, 695–709.
Chung, S. T. L., Levi, D. M., and Tjan, B. S. (2005). Learning letter identification in peripheral vision. Vision Res. 45, 1399–1412.
Chung, S. T. L., Li, R. W., and Levi, D. M. (2012). Learning to identify near-acuity letters, either with or without flankers, results in improved letter size and spacing limits in adults with amblyopia. PLoS One 7:e35829.
Chung, S. T. L., and Truong, S. R. (2013). Learning to identify crowded letters: does the learning depend on the frequency of training? Vision Res. 77, 41–50.
Coco-Martín, M. B., Cuadrado-Asensio, R., López-Miguel, A., Mayo-Iscar, A., Maldonado, M. J., and Pastor, J. C. (2013). Design and evaluation of a customized reading rehabilitation program for patients with age-related macular degeneration. Ophthalmology 120, 151–159.
Darian-Smith, C., and Gilbert, C. D. (1994). Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature 368, 737–740.
De Valois, K. K. (1977). Spatial frequency adaptation can enhance contrast sensitivity. Vision Res. 17, 1057–1065.
DeLoss, D. J., Watanabe, T., and Andersen, G. J. (2014). Optimization of perceptual learning: effects of task difficulty and external noise in older adults. Vision Res. 99, 37–45.
DeLoss, D. J., Watanabe, T., and Andersen, G. J. (2015). Improving vision among older adults: Behavioral training to improve sight. Psychol. Sci. 26, 456–466.
Dilks, D. D., Baker, C. I., Peli, E., and Kanwisher, N. (2009). Reorganization of visual processing in macular degeneration is not specific to the “preferred retinal locus”. J. Neurosci. 29, 2768–2773.
Dilks, D. D., Julian, J. B., Peli, E., and Kanwisher, N. (2014). Reorganization of visual processing in age-related macular degeneration depends on foveal loss. Optom. Vis. Sci. 91, e199–e206.
Donovan, J. J., and Radosevich, D. J. (1999). A meta-analytic review of the distribution of practice effect: now you see it, now you don’t. J. Appl. Psychol. 84, 795–805.
Ellison, A., and Walsh, V. (1998). Perceptual learning in visual search: some evidence of specificities. Vision Res. 38, 333–345.
Fahle, M., and Daum, I. (1997). Visual learning and memory as functions of age. Neuropsychologia 35, 1583–1589.
Falavarjani, K. G., and Nguyen, Q. D. (2013). Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 27, 787–794.
Fertonani, A., Pirulli, C., and Miniussi, C. (2011). Random noise stimulation improves neuroplasticity in perceptual learning. J. Neurosci. 31, 15416–15423.
Field, D. J., Hayes, A., and Hess, R. F. (1993). Contour integration by the human visual system: evidence for a local “association field”. Vision Res. 33, 173–193.
Fiorentini, A., and Berardi, N. (1980). Perceptual learning specific for orientation and spatial frequency. Nature 287, 43–44.
Fiorentini, A., and Berardi, N. (1981). Learning in grating waveform discrimination: specificity for orientation and spatial frequency. Vision Res. 21, 1149–1158.
Freeman, J., and Simoncelli, E. P. (2011). Metamers of the ventral stream. Nat. Neurosci. 14, 1195–1201.
Gilbert, C. D., and Wiesel, T. N. (1985). Intrinsic connectivity and receptive field properties in visual cortex. Vision Res. 25, 365–374.
Gilbert, C. D., and Wiesel, T. N. (1992). Receptive field dynamics in adult primary visual cortex. Nature 356, 150–152.
Goodrich, G., Kirby, J., Oros, T., Wagstaff, P., McDevitt, B., Hazan, J., et al. (2004). Goldilocks and the three training models: A comparison of three models of low vision reading training on reading efficiency. Vis. Impair. Res. 6, 135–152.
Grenier, F., Timofeev, I., and Steriade, M. (2001). Focal synchronization of ripples (80–200 Hz) in neocortex and their neuronal correlates. J. Neurophysiol. 86, 1884–1898.
Grinvald, A., Lieke, E. E., Frostig, R. D., and Hildesheim, R. (1994). Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J. Neurosci. 14, 2545–2568.
Haun, A. M., and Peli, E. (2015). Similar sensitivity to ladder contours in macular degeneration patients and controls. PLoS One 10:e0128119.
Hung, S.-C., and Seitz, A. R. (2014). Prolonged training at threshold promotes robust retinotopic specificity in perceptual learning. J. Neurosci. 34, 8423–8431.
Hussain, Z., Webb, B. S., Astle, A. T., and McGraw, P. V. (2012). Perceptual learning reduces crowding in amblyopia and in the normal periphery. J. Neurosci. 32, 474–480.
Johnson, C. A., Keltner, J. L., and Balestrery, F. (1978). Effects of target size and eccentricity on visual detection and resolution. Vision Res. 18, 1217–1222.
Karni, A., and Sagi, D. (1991). Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc. Natl. Acad. Sci. U S A 88, 4966–4970.
Kwon, M., Nandy, A. S., and Tjan, B. S. (2013). Rapid and persistent adaptability of human oculomotor control in response to simulated central vision loss. Curr. Biol. 23, 1663–1669.
Latham, K., and Whitaker, D. (1996). A comparison of word recognition and reading performance in foveal and peripheral vision. Vision Res. 36, 2665–2674.
Lee, H.-W., Kwon, M., Legge, G. E., and Gefroh, J. J. (2010). Training improves reading speed in peripheral vision: is it due to attention? J. Vis. 10:18.
Lev, M., and Polat, U. (2011). Collinear facilitation and suppression at the periphery. Vision Res. 51, 2488–2498.
Levi, D. M., and Polat, U. (1996). Neural plasticity in adults with amblyopia. Proc. Natl. Acad. Sci. U S A 93, 6830–6834.
Levi, D. M., Polat, U., and Hu, Y. S. (1997). Improvement in Vernier acuity in adults with amblyopia: practice makes better. Invest. Ophthalmol. Vis. Sci. 38, 1493–1510.
Liu, T., Cheung, S.-H., Schuchard, R. A., Glielmi, C. B., Hu, X., He, S., et al. (2010). Incomplete cortical reorganization in macular degeneration. Invest. Ophthalmol. Vis. Sci. 51, 6826–6834.
Maniglia, M., Pavan, A., Aedo-Jury, F., and Trotter, Y. (2015a). The spatial range of peripheral collinear facilitation. Sci. Rep. 5:15530.
Maniglia, M., Pavan, A., and Trotter, Y. (2015b). The effect of spatial frequency on peripheral collinear facilitation. Vision Res. 107, 146–154.
Maniglia, M., Pavan, A., Cuturi, L. F., Campana, G., Sato, G., and Casco, C. (2011). Reducing crowding by weakening inhibitory lateral interactions in the periphery with perceptual learning. PLoS One 6:e25568.
Maniglia, M., Pavan, A., Sato, G., Contemori, G., Montemurro, S., Battaglini, L., et al. (2016). Perceptual learning leads to long lasting visual improvement in patients with central vision loss. Restor. Neurol. Neurosci. 34, 697–720.
Masuda, Y., Dumoulin, S. O., Nakadomari, S., and Wandell, B. A. (2008). V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cereb. Cortex 18, 2483–2493.
May, K. A., and Hess, R. F. (2007). Ladder contours are undetectable in the periphery: a crowding effect? J. Vis. 7, 9.1–9.15.
Moss, F., Ward, L. M., and Sannita, W. G. (2004). Stochastic resonance and sensory information processing: a tutorial and review of application. Clin. Neurophysiol. 115, 267–281.
Motter, B. C. (2006). Modulation of transient and sustained response components of V4 neurons by temporal crowding in flashed stimulus sequences. J. Neurosci. 26, 9683–9694.
Nandy, A. S., and Tjan, B. S. (2012). Saccade-confounded image statistics explain visual crowding. Nat. Neurosci. 15, 463–469.
Nilsson, U. L. (1990). Visual rehabilitation with and without educational training in the use of optical aids and residual vision. A prospective study of patients with advanced age-related macular degeneration. Clin. Vis. Sci. 6, 3–10.
Nilsson, U. L., Frennesson, C., and Nilsson, S. E. G. (2003). Patients with AMD and a large absolute central scotoma can be trained successfully to use eccentric viewing, as demonstrated in a scanning laser ophthalmoscope. Vision Res. 43, 1777–1787.
Olma, M. C., Dargie, R. A., Behrens, J. R., Kraft, A., Irlbacher, K., Fahle, M., et al. (2013). Long-term effects of serial anodal tDCS on motion perception in subjects with occipital stroke measured in the unaffected visual hemifield. Front. Hum. Neurosci. 7:314.
Pelli, D. G., Palomares, M., and Majaj, N. J. (2004). Crowding is unlike ordinary masking: distinguishing feature integration from detection. J. Vis. 4, 1136–1169.
Pijnacker, J., Verstraten, P., van Damme, W., Vandermeulen, J., and Steenbergen, B. (2011). Rehabilitation of reading in older individuals with macular degeneration: a review of effective training programs. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 18, 708–732.
Pirulli, C., Fertonani, A., and Miniussi, C. (2013). The role of timing in the induction of neuromodulation in perceptual learning by transcranial electric stimulation. Brain Stimul. 6, 683–689.
Plank, T., Rosengarth, K., Schmalhofer, C., Goldhacker, M., Brandl-Rühle, S., and Greenlee, M. W. (2014). Perceptual learning in patients with macular degeneration. Front. Psychol. 5:1189.
Polat, U. (2009). Making perceptual learning practical to improve visual functions. Vision Res. 49, 2566–2573.
Polat, U., Ma-Naim, T., Belkin, M., and Sagi, D. (2004). Improving vision in adult amblyopia by perceptual learning. Proc. Natl. Acad. Sci. U S A 101, 6692–6697.
Polat, U., and Norcia, A. M. (1996). Neurophysiological evidence for contrast dependent long-range facilitation and suppression in the human visual cortex. Vision Res. 36, 2099–2109.
Polat, U., and Sagi, D. (1993). Lateral interactions between spatial channels: suppression and facilitation revealed by lateral masking experiments. Vision Res. 33, 993–999.
Polat, U., and Sagi, D. (1994a). The architecture interactions of perceptual spatial interactions. Vision Res. 34, 73–78.
Polat, U., and Sagi, D. (1994b). Spatial interactions in human vision: From near to far experience-dependent cascades fo connections. Proc. Natl. Acad. Sci. U S A 91, 1206–1209.
Ponomarenko, A. A., Li, J. S., Korotkova, T. M., Huston, J. P., and Haas, H. L. (2008). Frequency of network synchronization in the hippocampus marks learning. Eur. J. Neurosci. 27, 3035–3042.
Pourtois, G., Rauss, K. S., Vuilleumier, P., and Schwartz, S. (2008). Effects of perceptual learning on primary visual cortex activity in humans. Vision Res. 48, 55–62.
Richards, E., Bennett, P. J., and Sekuler, A. B. (2006). Age related differences in learning with the useful field of view. Vision Res. 46, 4217–4231.
Rosengarth, K., Keck, I., Brandl-Rühle, S., Frolo, J., Hufendiek, K., Greenlee, M. W., et al. (2013). Functional and structural brain modifications induced by oculomotor training in patients with age-related macular degeneration. Front. Psychol. 4:428.
Rubin, G. S., and Turano, K. (1994). Low vision reading with sequential word presentation. Vision Res. 34, 1723–1733.
Saarinen, J., and Levi, D. M. (1995). Perceptual learning in vernier acuity: what is learned? Vision Res. 35, 519–527.
Seiple, W., Grant, P., and Szlyk, J. P. (2011). Reading rehabilitation of individuals with AMD: relative effectiveness of training approaches. Invest. Ophthalmol. Vis. Sci. 52, 2938–2944.
Seitz, A., and Watanabe, T. (2005). A unified model for perceptual learning. Trends Cogn. Sci. 9, 329–334.
Sireteanu, R., and Rettenbach, R. (1995). Perceptual learning in visual search: fast, enduring, but non-specific. Vision Res. 35, 2037–2043.
Smirnakis, S. M., Brewer, A. A., Schmid, M. C., Tolias, A. S., Schüz, A., Augath, M., et al. (2005). Lack of long-term cortical reorganization after macaque retinal lesions. Nature 435, 300–307.
Spiegel, D. P., Hansen, B. C., Byblow, W. D., and Thompson, B. (2012). Anodal transcranial direct current stimulation reduces psychophysically measured surround suppression in the human visual cortex. PLoS One 7:e36220.
Stagg, C. J., and Nitsche, M. A. (2011). Physiological basis of transcranial direct current stimulation. Neuroscientist 17, 37–53.
Strasburger, H., Rentschler, I., and Jüttner, M. (2011). Peripheral vision and pattern recognition: a review. J. Vis. 11:13.
Sunness, J. S., Liu, T., and Yantis, S. (2004). Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology 111, 1595–1598.
Sunwoo, H., Kim, Y. H., Chang, W. H., Noh, S., Kim, E. J., and Ko, M. H. (2013). Effects of dual transcranial direct current stimulation on post-stroke unilateral visuospatial neglect. Neurosci. Lett. 554, 94–98.
Szpiro, S. F. A., and Carrasco, M. (2015). Exogenous attention enables perceptual learning. Psychol. Sci. 26, 1854–1862.
Tan, D. T. H., and Fong, A. (2008). Efficacy of neural vision therapy to enhance contrast sensitivity function and visual acuity in low myopia. J. Cataract Refract. Surg. 34, 570–577.
Tarita-Nistor, L., Brent, M. H., Steinbach, M. J., Markowitz, S. N., and González, E. G. (2014). Reading training with threshold stimuli in people with central vision loss: a feasibility study. Optom. Vis. Sci. 91, 86–96.
Terney, D., Chaieb, L., Moliadze, V., Antal, A., and Paulus, W. (2008). Increasing human brain excitability by transcranial high-frequency random noise stimulation. J. Neurosci. 28, 14147–14155.
Thompson, B., Mansouri, B., Koski, L., and Hess, R. F. (2008). Brain plasticity in the adult: modulation of function in amblyopia with rTMS. Curr. Biol. 18, 1067–1071.
Ts’o, D. Y., Gilbert, C. D., and Wiesel, T. N. (1986). Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J. Neurosci. 6, 1160–1170.
Wandell, B. A., and Smirnakis, S. M. (2009). Plasticity and stability of visual field maps in adult primary visual cortex. Nat. Rev. Neurosci. 10, 873–884.
Watson, G. R., Wright, V., and De l’Aune, W. (1992). The efficacy of comprehension training and reading practice for print readers with macular loss. J. Vis. Impairm. Blindn. 86, 37–43.
White, J. M., and Bedell, H. E. (1990). The oculomotor reference in humans with bilateral macular disease. Invest. Ophthalmol. Vis. Sci. 31, 1149–1161.
Whittaker, S. G., Cummings, R. W., and Swieson, L. R. (1991). Saccade control without a fovea. Vision Res. 31, 2209–2218.
Xiao, L. Q., Zhang, J. Y., Wang, R., Klein, S. A., Levi, D. M., and Yu, C. (2008). Complete transfer of perceptual learning across retinal locations enabled by double training. Curr. Biol. 18, 1922–1926.
Yu, D., Cheung, S. H., Legge, G. E., and Chung, S. T. L. (2010a). Reading speed in the peripheral visual field of older adults: does it benefit from perceptual learning? Vision Res. 50, 860–869.
Yu, D., Legge, G. E., Park, H., Gage, E., and Chung, S. T. L. (2010b). Development of a training protocol to improve reading performance in peripheral vision. Vision Res. 50, 36–45.
Zhang, J. Y., Zhang, G. L., Xiao, L.-Q., Klein, S. A., Levi, D. M., and Yu, C. (2010). Rule-based learning explains visual perceptual learning and its specificity and transfer. J. Neurosci. 30, 12323–12328.
Keywords: AMD, perceptual learning, peripheral vision, cortical plasticity, brain stimulation
Citation: Maniglia M, Cottereau BR, Soler V and Trotter Y (2016) Rehabilitation Approaches in Macular Degeneration Patients. Front. Syst. Neurosci. 10:107. doi: 10.3389/fnsys.2016.00107
Received: 16 September 2016; Accepted: 12 December 2016;
Published: 27 December 2016.
Edited by:
Emmanuel Bui Quoc, Assistance Publique - Hopitaux De Paris, FranceReviewed by:
Mark W. Greenlee, University of Regensburg, GermanyWioletta Joanna Waleszczyk, Nencki Institute of Experimental Biology, Poland
Copyright © 2016 Maniglia, Cottereau, Soler and Trotter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcello Maniglia, bWFyY2VsbG8ubWFuaWdsaWFAZ21haWwuY29t
Yves Trotter, eXZlcy50cm90dGVyQGNucnMuZnI=
 Marcello Maniglia
Marcello Maniglia Benoit R. Cottereau
Benoit R. Cottereau Vincent Soler
Vincent Soler Yves Trotter
Yves Trotter