- 1Department of Gynecologic Oncology, First Affiliated Hospital, Bengbu Medical College, Bengbu, Anhui, China
- 2School of Medical Imaging, Bengbu Medical College, Bengbu, Anhui, China
- 3Anhui Key Laboratory of Computational Medicine and Intelligent Health, Bengbu Medical College, Bengbu, Anhui, China
Background: Previous studies have shown that heart rate variability (HRV) analysis is a sensitive indicator of chemotherapy-induced cardiotoxicity. However, most studies to date have observed long-term effects using long-term analyses. The main purpose of this study was to evaluate the acute effect of chemotherapy on the cardiac autonomic nervous system (ANS) in patients with cervical cancer (CC) by examining short-term HRV.
Methods: Fifty patients with CC admitted to the Department of Gynecology and Oncology of the First Affiliated Hospital of Bengbu Medical College were enrolled in the study. Based on their chemotherapy regimens, the patients were divided into a DC group (docetaxel + carboplatin) and a TC group (paclitaxel + carboplatin). A 5-min resting electrocardiogram (ECG) was collected before and the day after chemotherapy: the time domain (standard deviation of normal-to-normal intervals (SDNN) and root mean square of successive differences (RMSSD)) and frequency domain (low-frequency power (LF), high-frequency power (HF), and (LF/HF)) parameters were analyzed, and the differences before and after chemotherapy were compared.
Results: The results showed that SDNN, RMSSD and HF were significantly higher in the DC and TC groups after chemotherapy than before (p < 0.05, Cohen’s d > 0.5). In addition, LF was significantly higher after TC than before chemotherapy (p < 0.05, Cohen’s d > 0.3), and LF/HF was significantly lower after DC than before chemotherapy (p < 0.05, Cohen’s d > 0.5).
Conclusion: Chemotherapy combining taxane and carboplatin can increase the HRV of CC patients in the short term, and HRV may be a sensitive tool for the early detection of chemotherapy-induced cardiac ANS perturbations.
Introduction
Cervical cancer (CC) is a common gynecological cancer (Sung et al., 2021). Chemotherapy, an important means of cancer treatment, chemotherapy can effectively reduce the tumor burden of cancer patients and prolong their survival time (Monsuez et al., 2010; Regalado Porras et al., 2018; Gabani et al., 2021). Nevertheless, the toxic side effects of chemotherapy cannot be ignored. Cardiotoxicity is one of the clinically recognized side effects of chemotherapy. Chemotherapy-induced cardiotoxicity can develop over time in an acute, subacute or chronic manner, with acute or subacute cardiotoxicity appearing at any time from the start of treatment to 2 weeks after the end of treatment (Madeddu et al., 2016). A large cohort study of 2,625 cancer patients who received chemotherapy showed that the overall incidence of cardiotoxicity was 9% during a median follow-up of 5.2 years (Cardinale et al., 2015). Thus, the early detection of cardiac function in patients with chemotherapy can help to prevent and identify the occurrence of cardiotoxicity and guide and improve the direction of subsequent treatment to maximize patient cardiac safety.
Electrocardiogram (ECG) is a routine method to identify cardiotoxicity. However, the abnormalities found on ECG may be interfered with by numerous factors, and the specificity of cardiotoxicity detection is low. Imaging methods, such as echocardiography, cardiac magnetic resonance (CMR), and multigated radionuclide angiography (MUGA), are also commonly used to evaluate cardiotoxicity in clinical practice (Tan and Scherrer-Crosbie, 2012; Reuvekamp et al., 2016; Jordan and Hundley, 2019; Tak et al., 2020; Siddiqui et al., 2022). However, the accuracy of ultrasound examination is affected by the physician’s technology and image quality (Jerusalem et al., 2019), there are many contraindications in magnetic resonance examination (Christian et al., 2012), and MUGA can cause radiation damage to patients (Bikiewicz et al., 2021). As a result, imaging methods still have limitations in clinical use. Some cardiac biomarkers, such as B-type natriuretic peptide and cardiac troponin I, have also been considered for the detection of early cardiac injury during chemotherapy, but there is no consensus on the optimal time for biomarker measurement (Pudil et al., 2020; Semeraro et al., 2021). Therefore, determining the best way to assess early cardiotoxicity is still in the exploratory stage.
Currently, the mechanism of cardiac dysfunction is thought to involve abnormalities in autonomic nervous system (ANS) function (Teng et al., 2021). Heart rate variability (HRV), as an objective index to evaluate cardiac ANS regulation, is convenient and noninvasive (Patural et al., 2022; Rajendra Acharya et al., 2006). Several studies have suggested that HRV may be a sensitive indicator for evaluating chemotherapy-induced cardiotoxicity. For example, Frye et al. found that carotid artery stiffness was significantly higher and cardiovascular baroreflex sensitivity (cBRS) along with time- and frequency-domain HRV indices were significantly lower in cancer patients receiving chemotherapy compared to healthy controls; furthermore, cBRS correlated significantly with the low-frequency power of HRV (r = 0.66, p < 0.001) (Frye et al., 2018). Caru et al. compared HRV in acute lymphoblastic leukemia patients with different cumulative doses of doxorubicin. The results showed that the patients in the high-risk group had significantly altered HRV time-domain, frequency-domain and nonlinear indicators compared to the patients in the standard-risk group, suggesting that HRV is a sensitive indicator for detecting changes in cardiac ANS in patients (Caru et al., 2019).
At present, the use of HRV to detect chemotherapy-induced cardiotoxicity has been involved in a variety of cancers. However, few scholars have conducted research in this direction for CC patients. Therefore, this study used the traditional time- and frequency-domain indices of HRV to assess whether HRV can detect perturbations of the cardiac ANS in CC patients with chemotherapy and to provide new ideas for the evaluation of cardiotoxicity in CC patients receiving chemotherapy.
Methods
Subjects
The study subjects were 52 CC patients who received taxane combined with carboplatin adjuvant chemotherapy admitted to the Department of Gynecology and Oncology, the First Affiliated Hospital of Bengbu Medical College (Anhui, China), from December 2021 to October 2022.
The inclusion criteria were as follows: (1) patients with CC (pathological type of squamous and adenocarcinoma) confirmed by pathological examination; (2) patients who received chemotherapy and the chemotherapy regimen was taxane (docetaxel/paclitaxel) combined with carboplatin.
The exclusion criteria were as follows: patients with ectopic heartbeats >5% of all beats who were unsuitable for HRV analysis (2 patients were excluded).
This study was approved by the Clinical Medical Research Ethics Committee of The First Affiliated Hospital of Bengbu Medical College (Bengbu, Anhui, China) (registration number: 2021KY010). The experiments were performed in strict accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients were informed of the details of the purpose, procedures, risks and potential adverse effects of the experiment and signed an informed consent form.
Data collection
ECG data collection was performed in a quiet temperature-controlled room (23°C ± 1C). The subjects were prohibited from consuming caffeine, alcohol or other autonomic nervous system stimulants within 24 h before the test. A single-lead miniature ECG recorder (version 2.8.0, Healink-R211B, Healink Ltd., Bengbu, China) with a V6 lead was used to collect a 5 min supine resting ECG before and the day after chemotherapy. The signal bandwidth of the equipment was set to 0.67–40 Hz, and the sampling rate was 400 Hz. The patients were asked to keep quiet and breathe smoothly during the period of ECG collection.
HRV analysis
The Pan-Tompkins algorithm was used to extract the time series of the RRI. The artifacts caused by interference and ectopic heartbeat were corrected by a time-varying threshold algorithm, and then HRV time and frequency domain analysis was carried out.
The time-domain indices included the standard deviation of all normal-to-normal intervals (SDNN) and the root mean square of successive differences (RMSSD).
The frequency-domain indices included low-frequency power (LF, 0.04–0.15 Hz), high-frequency power (HF, 0.15–0.4 Hz), and the ratio of LF to HF (LF/HF).
The above analysis was performed with Kubios HRV Premium software (version 3.1.0, https://www.kubios.com, Kubios Oy, Kuopio, Finland).
Statistical analysis
The normality of all data was checked by the Shapiro-Wilk test, and the normally distributed data are expressed as the mean ± standard deviation. The nonnormally distributed data are expressed as the median (first quartile, third quartile). Independent sample t tests and chi-square tests were used to assess the differences in basic clinical information between the two groups; Mann‒Whitney U tests were used to compare the group differences in each HRV index before chemotherapy between the two groups; and paired samples t tests and Wilcoxon signed rank tests were used to examine the differences in HRV indices before and after chemotherapy between the two groups. Cohen’s d value was used to characterize the effect size of the difference in each HRV index before and after chemotherapy, with d = 0.2 considered a small effect, d = 0.5 considered a moderate effect, and d = 0.8 considered a large effect (Cohen, 1992). SPSS Statistics 26.0 (IBM Corp., Chicago, Illinois, United States of America) software was used for the above statistical analysis, and p < 0.05 (two-tailed) was considered statistically significant.
Results
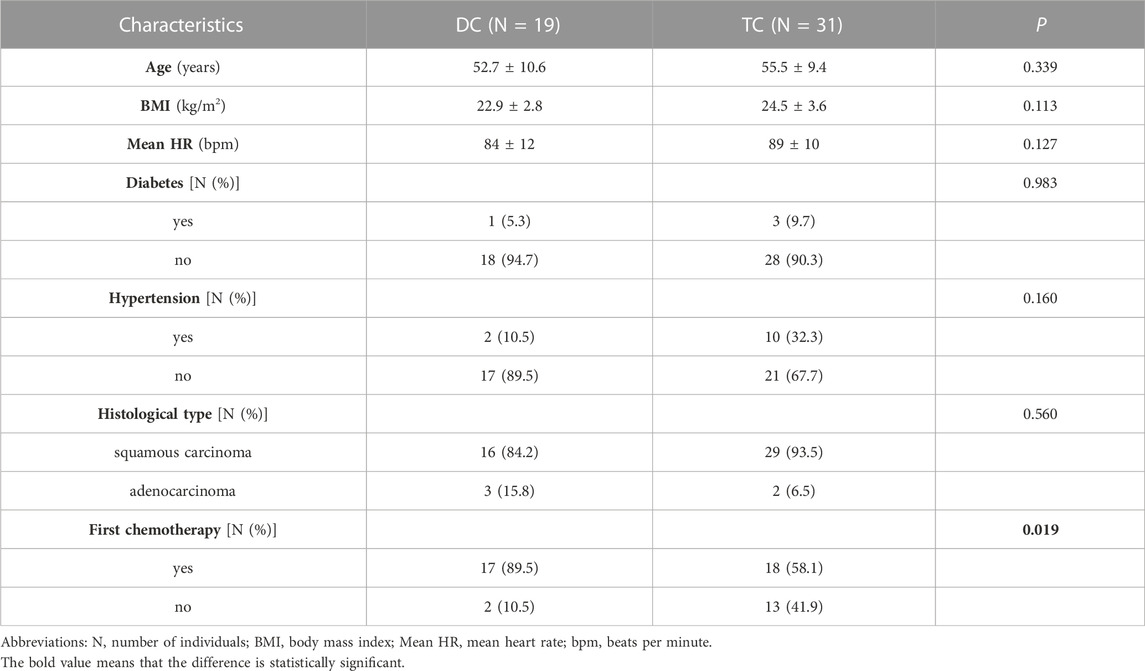
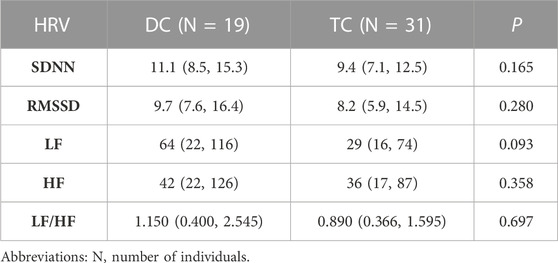
A total of 50 patients met the inclusion criteria and were divided into two groups based on chemotherapy regimen: 19 patients in the DC group (docetaxel combined with carboplatin) and 31 patients in the TC group (paclitaxel combined with carboplatin). Table 1 summarizes the basic clinical data of the two groups, and Table 2 shows the baseline HRV of the two groups. There were no significant differences in age, BMI, mean heart rate, diabetes, hypertension, histological type or baseline HRV between the two groups (p > 0.05), and there was a significant difference in whether the patients were receiving chemotherapy for the first time (p = 0.019).
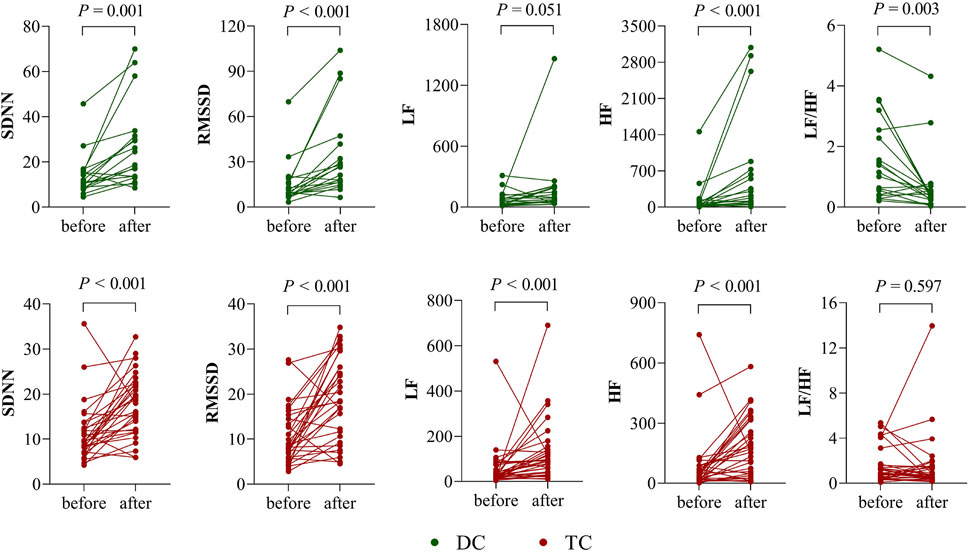
Figure 1 shows the comparison of HRV parameters before and after chemotherapy in the DC and TC groups. In the DC group, SDNN, RMSSD, and HF were significantly higher after chemotherapy than before (p < 0.05); LF/HF was significantly lower than before (p < 0.05); and LF was not significantly different before and after chemotherapy (p > 0.05). In the TC group, SDNN, RMSSD, LF, and HF were significantly higher after chemotherapy than before (p < 0.05); LF/HF was not significantly different before and after chemotherapy (p > 0.05).
The effect sizes of the differences in each HRV parameter before and after chemotherapy in the two groups are shown in Figure 2.
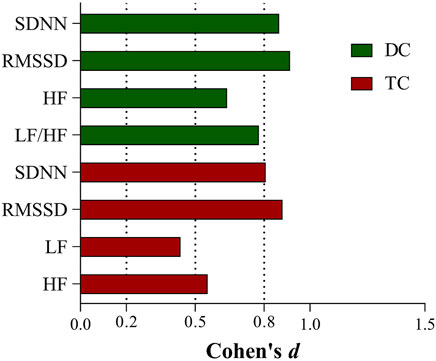
FIGURE 2. Effect sizes of HRV indicators with differences before and after chemotherapy in the DC and TC groups.
Discussion
This is a study to evaluate the effects of combined taxane and carboplatin chemotherapy on early cardiac ANS function in patients with CC. In this study, the traditional time-domain (SDNN, RMSSD) and frequency-domain (LF, HF, LF/HF) indices were used to analyze the HRV of 50 CC patients before and after chemotherapy. The results showed that significant increases in SDNN, RMSSD and HF were observed in both groups. In addition, LF increased significantly in the TC group and LF/HF decreased significantly in the DC group. The alteration of HRV predicts changes in cardiac ANS function, and the results of this study suggest that combined taxane and carboplatin chemotherapy may affect the early ANS status in CC patients.
The mechanism of the effect of taxane on cardiac function in patients has not been clarified. Because it belongs to the anti-microtubule class of drugs, it acts as an anticancer agent by promoting polymerization of tubulin, forming stable microtubules, and inhibiting cell division. However, it can damage the cytoskeleton due to its anticancer properties and impair the basic functions of cardiac endothelial cells, which in turn leads to myocardial injury (Morbidelli et al., 2016; Rosa et al., 2016). Several studies have shown that the occurrence of multiple cardiac disturbance events (including arrhythmias, bradycardia, and different degrees of atrioventricular block) can be observed during the administration of taxane (Rowinsky et al., 1991; Markman and Nazarian, 2016). The altered HRV observed in our study may be an early sign of cardiac disturbance events. There are few reports on the cardiotoxicity of carboplatin, and its major toxic side effect manifests as myelosuppression (Oun et al., 2018). However, it cannot be determined whether some of the HRV alterations observed in this study originated from the combination of carboplatin.
RMSSD is highly correlated with HF, both representing vagal activity (Picard et al., 2021). The significant increase in RMSSD and HF indicates enhanced cardiac vagal activity, whereas vagal activity stimulation shortens the atrial effective refractory period, increases spatial electrophysiological heterogeneity, and promotes early after-depolarization at the end of action potential phase 3, which may be a trigger for arrhythmias (Shen and Zipes, 2014). SDNN is generally thought to reflect the overall activity of sympathetic and vagal nerves, but the main cause of its variability in short-time recordings comes from respiratory sinus arrhythmias (RSA) (Shaffer et al., 2014; Shaffer and Ginsberg, 2017). RSA is a physiological phenomenon resulting from the regulation of the cardiac system by the vagus nerve and could be used as an indicator of vagal activity (Porges, 2007; Lubocka and Sabiniewicz, 2021). It has been found that decreased RSA is associated with reduced cardiac vagal activity in patients with paroxysmal atrial fibrillation after undergoing pulmonary vein isolation (Jungen et al., 2019). Increasing the biofeedback training of RSA can effectively enhance vagal regulation (Munafò et al., 2016). Moreover, various studies have noted that RSA is highly correlated with SDNN (Smith et al., 2013; Vieira et al., 2016). Consequently, in our study, the significant increase in SDNN after chemotherapy was most likely attributable to an increase in vagal activity rather than an alteration in overall ANS activity.
Compared to time-domain indices, the study of HRV frequency-domain indices contains a great deal of uncertainty. The physiological significance of LF has been controversial. Initially, LF was considered to reflect sympathetic activity (Pagani et al., 1997). Later, it was reported that LF represents the comodulation of the sympathetic-vagal system (Vanderlei et al., 2009). In recent years, other researchers have found that LF primarily reflects baroreflex activity (Goldstein et al., 2011; Rahman et al., 2011). This challenges the notion that LF/HF represents sympathetic-vagal balance (Billman, 2013). LF and LF/HF may not accurately reflect the state of the ANS. We observed a significant increase in LF in the TC group and a significant decrease in LF/HF in the DC group, which probably relates to the complex interaction between sympathetic and parasympathetic nerves, as well as mechanical effects caused by respiration (Billman, 2011). Additionally, in contrast to the DC group, the TC group required Cremophor EL as a solvent for the formulation of paclitaxel; evidence suggests that this solvent induces histamine release and thus causes cardiovascular stimulation (Rowinsky et al., 1991; Madeddu et al., 2016, Al-Mahayri et al., 2021), which may also be a possible reason for the elevated LF we observed in the TC group. It is worth mentioning that in our research, the effect sizes of the time-domain indicators (SDNN, RMSSD) before and after chemotherapy were greater than 0.8 in both groups. We consider the time domain is more sensitive than the frequency domain in reflecting the physiological state of the body.
In previous studies examining the effects of chemotherapeutic agents on the HRV of patients, some researchers have found no significant change in HRV in patients before and after chemotherapy. For example, Ekholm et al. included 24 BC patients previously pretreated with anthracyclines and evaluated their HRV by 24-h ambulatory ECG before and after three to four courses of docetaxel treatment. The results showed that the HRV time-domain (SDNN, NN50, RMSSD) and frequency-domain (LF, HF, VLF, LF/HF) parameters of the patients after chemotherapy were not significantly altered (Ekholm et al., 2002). In contrast to their findings, the patients in this study had significantly higher SDNN, RMSSD, and HF after chemotherapy. We suppose that, owing to the different chemotherapeutic agents from the previous studies and the heterogeneity of the study population, there will be some differences regarding the effects of HRV. Additionally, most of the present studies have used 24-h ambulatory ECGs to observe HRV in patients after several courses of treatment, and the series of changes observed may contribute to long-term cumulative drug effects. In contrast, the present study used short-term ECGs for short-term observation, and we collected 5-min ECGs before and the day after chemotherapy to analyze the HRV of patients. We considered the relatively subtle effect of chemotherapy drugs on the cardiac ANS in the short term; that is, chemotherapy drug use stimulates the patient’s cardiac vagus nerve in the short term, resulting in elevated SDNN, RMSSD and HF.
Limitations
This explorative study included a relatively small number of patients, and there was heterogeneity in the dosage of chemotherapeutic drugs. Furthermore, our study merely observed short-term changes in HRV in patients and did not indicate whether these changes are permanent. Therefore, the results of this study need to be confirmed in a prospective study with a larger sample size, homogeneous drug doses and a long-term follow-up period.
Conclusion
Our findings demonstrate that combined taxane and carboplatin chemotherapy can increase HRV in the short term for CC patients. HRV may be a sensitive tool for the early detection of cardiac ANS perturbations caused by chemotherapy with taxane combined with carboplatin. Early changes in cardiac function can be monitored clinically based on HRV alterations to prevent cardiotoxicity and myocardial injury in patients.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by This study was approved by the Clinical Medical Research Ethics Committee of The First Affiliated Hospital of Bengbu Medical College (Bengbu, Anhui, China). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization, BS and JL; methodology, YS, WG, SZ, and YW; resources, BS and JL; data curation, JL; writing—original draft preparation, WG and GL; writing—review and editing, BS and JL; supervision, JL; project administration, BS; funding acquisition, BS and SZ. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “512” Outstanding Talents Fostering Project of Bengbu Medical College (grant number BY51201312), the Natural Science Research Project of Bengbu Medical College (grant number 2020byzd013), and the Scientific Research Innovation Project of Bengbu Medical College (grant number BYKC201905).
Conflict of interest
An immediate family member of BS owns stock in HeaLink Ltd., Bengbu, China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bikiewicz, A., Banach, M., von Haehling, S., Maciejewski, M., and Bielecka-Dabrowa, A. (2021). Adjuvant breast cancer treatments cardiotoxicity and modern methods of detection and prevention of cardiac complications. Esc. Heart Fail 8 (4), 2397–2418. doi:10.1002/ehf2.13365
Billman, G. E. (2011). Heart rate variability - a historical perspective. Front. Physiol. 2, 86. doi:10.3389/fphys.2011.00086
Billman, G. E. (2013). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 4, 26. doi:10.3389/fphys.2013.00026
Cardinale, D., Colombo, A., Bacchiani, G., Tedeschi, I., Meroni, C. A., Veglia, F., et al. (2015). Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 131 (22), 1981–1988. doi:10.1161/CIRCULATIONAHA.114.013777
Caru, M., Corbin, D., Périé, D., Lemay, V., Delfrate, J., Drouin, S., et al. (2019). Doxorubicin treatments induce significant changes on the cardiac autonomic nervous system in childhood acute lymphoblastic leukemia long-term survivors. Clin. Res. Cardiol. 108 (9), 1000–1008. doi:10.1007/s00392-019-01427-9
Christian, J. B., Finkle, J. K., Ky, B., Douglas, P. S., Gutstein, D. E., Hockings, P. D., et al. (2012). Cardiac imaging approaches to evaluate drug-induced myocardial dysfunction. Am. Heart J. 164 (6), 846–855. doi:10.1016/j.ahj.2012.09.001
Ekholm, E., Rantanen, V., Syvänen, K., Jalonen, J., Antila, K., and Salminen, E. (2002). Docetaxel does not impair cardiac autonomic function in breast cancer patients previously treated with anthracyclines. Anticancer Drugs 13 (4), 425–429. doi:10.1097/00001813-200204000-00012
Frye, J. N., Sutterfield, S. L., Caldwell, J. T., Behnke, B. J., Copp, S. W., Banister, H. R., et al. (2018). Vascular and autonomic changes in adult cancer patients receiving anticancer chemotherapy. J. Appl. Physiol. (1985) 125 (1), 198–204. doi:10.1152/japplphysiol.00005.2018
Gabani, M., Castañeda, D., Nguyen, Q. M., Choi, S. K., Chen, C., Mapara, A., et al. (2021). Association of cardiotoxicity with doxorubicin and trastuzumab: A double-edged sword in chemotherapy. Cureus 13 (9), e18194. doi:10.7759/cureus.18194
Goldstein, D. S., Bentho, O., Park, M. Y., and Sharabi, Y. (2011). Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp. Physiol. 96 (12), 1255–1261. doi:10.1113/expphysiol.2010.056259
Jerusalem, G., Lancellotti, P., and Kim, S. B. (2019). HER2+ breast cancer treatment and cardiotoxicity: Monitoring and management. Breast Cancer Res. Treat. 177 (2), 237–250. doi:10.1007/s10549-019-05303-y
Jordan, J. H., and Hundley, W. G. (2019). MRI of cardiotoxicity. Cardiol. Clin. 37 (4), 429–439. doi:10.1016/j.ccl.2019.07.007
Jungen, C., Alken, F. A., Eickholt, C., Scherschel, K., Kuklik, P., Klatt, N., et al. (2019). Respiratory sinus arrhythmia is reduced after pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Arch. Med. Sci. 16 (5), 1022–1030. doi:10.5114/aoms.2019.83883
Lubocka, P., and Sabiniewicz, R. (2021). Respiratory sinus arrhythmia in children-predictable or random? Front. Cardiovasc Med. 8, 643846. doi:10.3389/fcvm.2021.643846
Madeddu, C., Deidda, M., Piras, A., Cadeddu, C., Demurtas, L., Puzzoni, M., et al. (2016). Pathophysiology of cardiotoxicity induced by nonanthracycline chemotherapy. J. Cardiovasc Med. Hagerst. 17, e12–e18. 17 Suppl 1 Special issue on Cardiotoxicity from Antiblastic Drugs and Cardioprotection. doi:10.2459/JCM.0000000000000376
Markman, T. M., and Nazarian, S. (2016). Arrhythmia and electrophysiological effects of chemotherapy: A review. Oncology 91 (2), 61–68. doi:10.1159/000446374
Monsuez, J. J., Charniot, J. C., Vignat, N., and Artigou, J. Y. (2010). Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 144 (1), 3–15. doi:10.1016/j.ijcard.2010.03.003
Morbidelli, L., Donnini, S., and Ziche, M. (2016). Targeting endothelial cell metabolism for cardio-protection from the toxicity of antitumor agents. Cardiooncology 2 (1), 3. doi:10.1186/s40959-016-0010-6
Munafò, M., Patron, E., and Palomba, D. (2016). Improving managers' psychophysical well-being: Effectiveness of respiratory sinus arrhythmia biofeedback. Appl. Psychophysiol. Biofeedback 41 (2), 129–139. doi:10.1007/s10484-015-9320-y
Oun, R., Moussa, Y. E., and Wheate, N. J. (2018). The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 47 (19), 6645–6653. doi:10.1039/c8dt00838h
Pagani, M., Montano, N., Porta, A., Malliani, A., Abboud, F. M., Birkett, C., et al. (1997). Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 95 (6), 1441–1448. doi:10.1161/01.cir.95.6.1441
Patural, H., Franco, P., Pichot, V., and Giraud, A. (2022). Heart rate variability analysis to evaluate autonomic nervous system maturation in neonates: An expert opinion. Front. Pediatr. 10, 860145. doi:10.3389/fped.2022.860145
Picard, M., Tauveron, I., Magdasy, S., Benichou, T., Bagheri, R., Ugbolue, U. C., et al. (2021). Effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: A systematic review and meta-analysis. PLoS One 16 (5), e0251863. doi:10.1371/journal.pone.0251863
Porges, S. W. (2007). The polyvagal perspective. Biol. Psychol. 74 (2), 116–143. doi:10.1016/j.biopsycho.2006.06.009
Pudil, R., Mueller, C., Čelutkienė, J., Henriksen, P. A., Lenihan, D., Dent, S., et al. (2020). Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the cardio-oncology study group of the heart failure association and the cardio-oncology council of the European society of Cardiology. Eur. J. Heart Fail 22 (11), 1966–1983. doi:10.1002/ejhf.2017
Rahman, F., Pechnik, S., Gross, D., Sewell, L., and Goldstein, D. S. (2011). Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin. Auton. Res. 21 (3), 133–141. doi:10.1007/s10286-010-0098-y
Rajendra Acharya, U., Paul Joseph, K., Kannathal, N., Lim, C. M., and Suri, J. S. (2006). Heart rate variability: A review. Med. Biol. Eng. Comput. 44 (12), 1031–1051. doi:10.1007/s11517-006-0119-0
Regalado Porras, G. O., Chávez Nogueda, J., and Poitevin Chacón, A. (2018). Chemotherapy and molecular therapy in cervical cancer. Rep. Pract. Oncol. Radiother. 23 (6), 533–539. doi:10.1016/j.rpor.2018.09.002
Reuvekamp, E. J., Bulten, B. F., Nieuwenhuis, A. A., Meekes, M. R., de Haan, A. F., Tol, J., et al. (2016). Does diastolic dysfunction precede systolic dysfunction in trastuzumab-induced cardiotoxicity? Assessment with multigated radionuclide angiography (MUGA). J. Nucl. Cardiol. 23 (4), 824–832. doi:10.1007/s12350-015-0164-x
Rosa, G. M., Gigli, L., Tagliasacchi, M. I., Di Iorio, C., Carbone, F., Nencioni, A., et al. (2016). Update on cardiotoxicity of anti-cancer treatments. Eur. J. Clin. Invest. 46 (3), 264–284. doi:10.1111/eci.12589
Rowinsky, E. K., McGuire, W. P., Guarnieri, T., Fisherman, J. S., Christian, M. C., and Donehower, R. C. (1991). Cardiac disturbances during the administration of taxol. J. Clin. Oncol. 9 (9), 1704–1712. doi:10.1200/JCO.1991.9.9.1704
Semeraro, G. C., Cipolla, C. M., and Cardinale, D. M. (2021). Role of cardiac biomarkers in cancer patients. Cancers (Basel) 13 (21), 5426. doi:10.3390/cancers13215426
Shaffer, F., and Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Front. Public Health 5, 258. doi:10.3389/fpubh.2017.00258
Shaffer, F., McCraty, R., and Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart's anatomy and heart rate variability. Front. Psychol. 5, 1040. doi:10.3389/fpsyg.2014.01040
Shen, M. J., and Zipes, D. P. (2014). Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 114 (6), 1004–1021. doi:10.1161/CIRCRESAHA.113.302549
Siddiqui, M. U., Yaacoub, Y., Hanson, H. A., Junarta, J., Pasha, A. K., and Shah, M. (2022). Echocardiographic predictors of symptomatic cardiotoxicity among patients undergoing chemotherapy: A systematic review and meta-analysis. Med. Baltim. 101 (28), e29562. doi:10.1097/MD.0000000000029562
Smith, A. L., Owen, H., and Reynolds, K. J. (2013). Heart rate variability indices for very short-term (30 beat) analysis. Part 2: Validation. J. Clin. Monit. Comput. 27 (5), 577–585. doi:10.1007/s10877-013-9473-2
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tak, T., Jaekel, C. M., Gharacholou, S. M., Dworak, M. W., and Marshall, S. A. (2020). Measurement of ejection fraction by cardiac magnetic resonance imaging and echocardiography to monitor doxorubicin-induced cardiotoxicity. Int. J. Angiol. 29 (1), 45–51. doi:10.1055/s-0039-1697921
Tan, T. C., and Scherrer-Crosbie, M. (2012). Assessing the cardiac toxicity of chemotherapeutic agents: Role of echocardiography. Curr. Cardiovasc Imaging Rep. 5 (6), 403–409. doi:10.1007/s12410-012-9163-3
Teng, A. E., Noor, B., Ajijola, O. A., and Yang, E. H. (2021). Chemotherapy and radiation-associated cardiac autonomic dysfunction. Curr. Oncol. Rep. 23 (2), 14. doi:10.1007/s11912-020-01013-7
Vanderlei, L. C., Pastre, C. M., Hoshi, R. A., Carvalho, T. D., and Godoy, M. F. (2009). Basic notions of heart rate variability and its clinical applicability. Rev. Bras. Cir. Cardiovasc 24 (2), 205–217. doi:10.1590/s0102-76382009000200018
Keywords: heart rate variability, autonomic nervous system, cervical cancer, chemotherapy, taxane
Citation: Liu J, Guan W, Sun Y, Wang Y, Li G, Zhang S and Shi B (2023) Early detection of the impact of combined taxane and carboplatin treatment on autonomic nerves in patients with cervical cancer: Measurement of heart rate variability. Front. Physiol. 14:1126057. doi: 10.3389/fphys.2023.1126057
Received: 17 December 2022; Accepted: 20 February 2023;
Published: 28 February 2023.
Edited by:
Luiz Carlos Marques Vanderlei, São Paulo State University, BrazilReviewed by:
Lihui Zheng, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaYogender Aggarwal, Birla Institute of Technology, India
Copyright © 2023 Liu, Guan, Sun, Wang, Li, Zhang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Shi, shibo@bbmc.edu.cn; Jian Liu, elitelj@126.com
†These authors have contributed equally to this work
 Jian Liu
Jian Liu Weizheng Guan
Weizheng Guan Yilin Sun
Yilin Sun Yuling Wang1
Yuling Wang1 Guangqiao Li
Guangqiao Li Bo Shi
Bo Shi