- 1Department of Dermatology, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Tianjin, China
- 2Dermatology Hospital, Southern Medical University, Guangzhou, China
- 3Dermatology Service, Veterans Affairs Medical Center San Francisco, Department of Dermatology, University of California San Francisco, San Francisco, CA, United States
Because of the crucial role of epidermal permeability barrier in regulation of cutaneous and extracutaneous functions, great efforts have been made to identify and develop the regimens that can improve epidermal permeability barrier function. Studies have demonstrated that oral administration of natural ingredients can improve epidermal permeability barrier in various skin conditions, including inflammatory dermatoses and UV-irradiation. Moreover, topical applications of some natural ingredients can also accelerate the repair of epidermal permeability barrier after acute barrier disruption and lower transepidermal water loss in the intact skin. Natural ingredient-induced improvements in epidermal permeability barrier function can be attributable to upregulation of keratinocyte differentiation, lipid production, antioxidant, hyaluronic acid production, expression of aquaporin 3 and sodium-hydrogen exchanger 1. In this review, we summarize the benefits of topical natural ingredients in epidermal permeability barrier in normal skin with or without acute barrier disruption and the underlying mechanisms.
1 Introduction
Over the last decades, the regulatory role of epidermal permeability barrier in cutaneous and extracutaneous function has been well appreciated. Disruption of epidermal permeability barrier increases epidermal lipid and DNA syntheses (Feingold, 1991; Proksch et al., 1993). Similarly, barrier disruption increases release and synthesis of proinflammatory cytokines in the epidermis in addition to the increases in the density of Langerhans cells and mast cells in the dermis (Wood et al., 1992; Proksch et al., 1996; Proksch and Brasch, 1997; Wood et al., 1997; Lin et al., 2013). Moreover, compromised epidermal permeability barrier increases cutaneous inflammatory response to stimuli (Nishijima et al., 1997). Furthermore, disruption of epidermal permeability barrier function increases circulating levels of proinflammatory cytokines (Hu et al., 2017). Thus, prolonged, sustained cutaneous inflammation can induce chronic, systemic inflammation, which has been linked to the development of a variety of disorders, including type 2 diabetes, obesity, cardiovascular diseases and Alzheimer’s disease (Man and Elias, 2019). Conversely, improvement in epidermal permeability barrier function lowers expression levels of proinflammatory cytokines in both the skin and the circulation (Wood et al., 1994; Wood et al., 1996; Hu et al., 2017). In addition, defective epidermal permeability barrier function is associated with colonization of cutaneous Staphylococcus aureus (Na et al., 2012; Jinnestål et al., 2014). This line of evidence demonstrates the crucial role of competent epidermal permeability barrier in the maintenance of normal health condition.
Because of the regulatory role of epidermal permeability barrier in human health, approaches that can improve epidermal permeability barrier have been widely employed in the prevention and the treatment of some health conditions, such as eczematous dermatitis and psoriasis (Man et al., 2018; Man et al., 2019a; Man et al., 2019b; Park et al., 2021; Ní Chaoimh et al., 2023). Although oral administration of substances is suitable for improvement of epidermal permeability barrier in a larger surface area of the body, higher dose of active ingredients is usually required to achieve the benefits in epidermal function as compared to topical treatments. In addition, oral intake of substances can increase the risk of adverse events for the digestive system, kidney, and the liver. In contrast, topical applications of substances usually do not cause extracutaneous adverse events. Moreover, some body sites such as the hands and the face are often subject to external insults, resulting in disruption of epidermal permeability barrier. It is more appropriate to use topical products to improve epidermal permeability barrier function in these vulnerable body sites. Among a variety of ingredients that can improve epidermal permeability barrier, natural ingredients exhibit several advantages, such as lower cost, higher safety and availability, compared to synthetic chemicals. In this review, we brief the topical natural ingredients that benefit epidermal permeability barrier in normal skin with or without acute barrier disruption and the underlying mechanisms (Table 1).
2 Natural ingredients that accelerate epidermal permeability barrier recovery after acute barrier disruption
The stratum corneum is the major site of epidermal permeability barrier, which is largely determined by the quality and quantity of a mixture of lipids, including ceramides, cholesterol and fatty acids (Man et al., 1996; Feingold and Elias, 2014). Of course, structural proteins are also the determinant for epidermal permeability barrier function (Haftek et al., 2021). Agents that stimulate keratinocyte differentiation and lipid production can improve epidermal permeability barrier homeostasis (Man et al., 2006; Schmuth et al., 2008). Correspondently, single topical application of an optimal lipid mixture (cholesterol, fatty acids and ceramide at a molar ratio of 3:1:1, any of these lipids can be 3) accelerates permeability barrier recovery by over 30% compared to the vehicle-treated controls in a murine model with acute barrier disruption with topical acetone treatment (Man et al., 1996). Similarly, single topical application of an optimal lipid mixture (cholesterol, ceramides, palmitate and linoleate = 3:1:1:1) accelerates barrier recovery 6 h after barrier disruption with tape-stripping in both aged mice and aged humans (Zettersten et al., 1997). Notably, free fatty acid-dominant lipid mixture delays permeability barrier recovery in aged mice although it accelerates recovery in young mice (Man et al., 1996; Zettersten et al., 1997). Such delayed barrier recovery is possibly due to the excessive content of medium and long chain fatty acids (C14-C18) in the epidermis because aged epidermis already has higher content of these fatty acids in comparison to young epidermis (Ghadially et al., 1995). It is known that excessive fatty acid delays permeability barrier recovery (Man et al., 1996). Moreover, topical application of an optimal lipid mixture (the molar ratio of cholesterol, ceramide, palmitate and linoleate = 4.3:2.3:1:1.08) improves barrier recovery in young mice following acute barrier disruption with either acetone or petrolatum ether or tape-stripping and some detergents, such as 15% N-laurosarcosine free acid and 10% dodecylbenzenesulphuric acid, but not with 10% sodium dodecyl sulphate nor 50% ammonium lauryl sulphosuccinate (Yang et al., 1995). The differential effects of optimal lipid mixture on skin treated with different detergents can be attributable to the extent of skin damage induced by these detergents. Either 10% sodium dodecyl sulphate or 50% ammonium lauryl sulphosuccinate requires 15 min to effectively disrupt the permeability barrier. In contrast, 10% dodecylbenzenesulphuric acid and 15% laurosarcosine free acid only require 5 and 6 min, respectively, to disrupt the permeability barrier (Yang et al., 1995). The longer the skin exposes to detergent, the more severe damage can be induced. Hence, optimal lipid mixture may not benefit barrier repair in sodium dodecyl sulphate- or ammonium lauryl sulphosuccinate-treated skin. Nonetheless, this line evidence indicates that the impact of optimal lipid mixture on epidermal permeability barrier homeostasis varies with age and the type of insults.
In our daily lives, our hands often contact a variety of detergents, which can disrupt epidermal permeability barrier and induce dermatitis. Following topical application of 17% sodium lauryl sulfate for 7 h, twice-daily applications of product containing either ceramide 1 or ceramide 3 for 2 days induce 12% reduction in transepidermal water loss rates (TEWL), an indicator of epidermal permeability barrier, while product containing both ceramide 1 and 3 lowers TEWL by 32% compared to the vehicle-treated site in humans (Huang and Chang, 2008). Taken together, this evidence demonstrates the benefit of stratum corneum lipids in epidermal permeability barrier homeostasis.
In addition to the stratum corneum lipids, topical plant oils also improve epidermal permeability barrier homeostasis after acute barrier disruption. For example, single topical application of sunflower oil accelerates barrier recovery by 31% and 16%, respectively, 1 and 5 h after barrier disruption with tape-stripping in mice whereas mustard oil, olive oil and soybean oil significantly delay barrier recovery (Darmstadt et al., 2002). The benefit of sunflower oil on permeability barrier homeostasis has also been demonstrated in humans with barrier disruption. Following challenges with 14% sodium lauryl sulfate, topical sunflower oil significantly lowers TEWL in human skin (Lodén and Andersson, 1996). However, neither borage oil nor shea butter lowers TEWL in human skin following the treatment with 14% sodium lauryl sulfate. Collectively, some plant oils accelerate barrier repair following acute barrier disruption.
Several studies have shown the benefits of herbal extract in epidermal permeability barrier homeostasis. In hairless mice, topical applications of 0.1% apigenin, a bioflavonoid found in chamomile tea and a variety of other plants, for 9 days markedly accelerate barrier recovery 4 h after barrier disruption with tape-stripping (Hou et al., 2013). Likewise, topical applications of 2% hesperidin, a dietary flavone glycoside mainly derived from citrus species, for 6 days accelerate barrier recovery by 37% and 23%, respectively, 2 and 4 h after barrier disruption with tape-stripping in young mice (Hou et al., 2012). Similar effects were also observed in aged mice (Man et al., 2015). Moreover, topical applications of extract of an herbal mixture (Radix Paeonlae rubra, Cat Nut, Phelloden Dron, Rhizoma Alismatis, Angelica sinensis and Glabrous Greenbrier) twice daily for 6 days significantly accelerate barrier recovery 2 and 4 h after barrier disruption with tape-stripping (Man et al., 2011). In addition, topical application of herbal extract improves barrier recovery when applied to barrier disrupted skin with tape-stripping. Following barrier disruption with tape-stripping, topical application of water extract of Agrimonia pilosa leaves does-dependently accelerates barrier recovery up to 30 h after application in mice (Nam et al., 2017). Topical cannabis sativa L. extract lowers TEWL by 20%–30% in mouse skin treated with 1% sodium lauryl sulfate (Zagórska-Dziok et al., 2021). Thus, a pile of evidence shows that topical extract of natural ingredients accelerates epidermal permeability barrier repair following acute barrier disruption in both the murine and human skin.
3 Natural ingredients that attenuate the disruption of epidermal permeability barrier by external stimuli
In addition to acceleration of permeability barrier recovery, attenuation of barrier abnormalities induced by insults is another approach to mitigate the negative impacts of external insults on epidermal permeability barrier. Some natural ingredients can diminish the abnormalities in epidermal permeability barrier induced by stimuli. For example, either topical or systemic administrations of glucocorticoids can delay epidermal permeability barrier recovery (Denda et al., 2000; Kao et al., 2003), whereas topical co-applications of 0.05% clobetasol and 2% hesperidin twice daily for 9 days override clobetasol-induced delay in barrier recovery 4 h after barrier disruption with tape-stripping, in addition to correction of clobetasol-induced elevation in skin surface pH (Man et al., 2014). Repeated exposure of the skin to detergents contributes to the development of eczema (Hamnerius et al., 2018; Jindal and Pandhi, 2020; Teo et al., 2023), likely resulting from the disruption of epidermal permeability barrier (Príborský et al., 1992; Okuda et al., 2002; Wolf and Parish, 2012). Pretreatment of the skin with some oils, such as palm fruit oil, soybean oil and rapeseed oil, largely prevents sodium lauryl sulfate-induced elevation in TEWL, evidenced by 22%–62% reductions in TEWL vs. untreated controls (Schliemann-Willers et al., 2002). Dishwashing liquid can irritate the skin and increase TEWL (Astner et al., 2006). Washing dishes with dishwashing liquid containing 0.1%–0.7% of Chamomilla recutita extract lowers TEWL by up to 40% in comparison to that without Chamomilla recutita extract (Wasilewski et al., 2016). Hence, topical applications of some natural ingredients can mitigate epidermal permeability barrier abnormalities induced by external stimuli.
4 Natural ingredients that enhance epidermal permeability barrier in intact skin
Compromised epidermal permeability barrier function increases cutaneous inflammatory response to stimuli and bacterial colonization (Nishijima et al., 1997; Scharschmidt et al., 2009; Na et al., 2012; Jinnestål et al., 2014). Therefore, enhancement of epidermal permeability barrier function can decrease cutaneous inflammation and infections when subjected to external insults. Several natural ingredients can enhance baseline epidermal permeability barrier function in the intact skin. In murine model, topical applications of an aqueous extract of Melissa officinalis leaves at a concentration of 5 mg/mL every other day induce a significant reduction in TEWL on day 12 (p < 0.05), with a further reduction on day 28 (p < 0.0001) (Sipos et al., 2021). Likewise, topical applications of pinus halepensis bark extract twice daily for 3 days markedly lower TEWL (p < 0.05) (Zoumpliou et al., 2014). Similarly, topical applications of 0.2% or 0.5% of N-palmitoyl serinol (NPS), a commensal bacterial metabolite, twice-daily for 1 week lower basal TEWL and accelerate barrier recovery by 20% (Wen et al., 2021). Moreover, the benefit of natural ingredients in epidermal permeability barrier function has also been demonstrated in humans. For example, topical applications of centella asiatica extract twice daily for 4 weeks decrease TEWL on the palm, with a comparable efficacy to ceramide (Anggraeni et al., 2021). However, other study did not show significant changes in TEWL on either the palm or the dorsal hand following topical applications of centella asiatica extract twice daily for 4 weeks (Damayanti Umborowati et al., 2021). In addition, topical extract of ashwagandha root twice daily for 60 days lowers TEWL by 40% (Narra et al., 2023). Topical treatments with product containing extract of hippophae rhmanoides berries also lower TEWL by 28% by 2 weeks and 50% reduction by 8 weeks in comparison to the placebo-treated skin (Khan et al., 2011). Impressively, a single topical application of coffee bean extract induces over 25% reduction in TEWL compared to untreated skin (Zofia et al., 2020). Regarding the influence of aloe vera on epidermal permeability barrier, the results are inconclusive although it is widely used in skin care products. One study showed that topical applications of cream containing 10% of aloe leaf extract decreased TEWL by 56% 27 h after application and by 65% 15 days after applications in humans (Laneri et al., 2020). Similarly, TEWL on the dorsal hand, but not on the palm, was decreased by topical applications of cream containing aloe extract twice daily for 4 weeks (Damayanti Umborowati et al., 2021). However, other studies did not show the benefit of topical aloe extract in epidermal permeability barrier function (Akhtar et al., 2011; Dal'Belo et al., 2006). In contrast, topical aloe extract increases epidermal permeability in vitro (Fox et al., 2015; Sharma et al., 2015). Thus, additional studies are needed to validate the benefit of topical aloe extract in epidermal permeability barrier. Apparently, some natural ingredients have long-lasting effects on epidermal permeability barrier function. For example, topical application of a product containing a mixture of coconut oil, Macadamia nut oil and Mangifera indica seed oil dramatically decreased TEWL within 1 hour after application (11.04 g/m2/hr vs. 9.6 g/m2/hr). A further decrease in TEWL was observed even 1 week after stopping application of the product (9.76 g/m2/hr vs. 7.88 g/m2/hr) (Samadi et al., 2021). Taken together, this line of evidence illustrates that topical applications of some natural ingredients enhance epidermal permeability barrier function in the intact skin.
5 Mechanisms by which natural ingredients improve epidermal permeability barrier
As aforementioned, epidermal permeability barrier is primarily determined by stratum corneum lipids and differentiation marker-related proteins. Natural ingredients improve epidermal permeability barrier mainly via direct or indirect regulation of epidermal lipid metabolism and keratinocyte differentiation.
5.1 Upregulation of epidermal lipid production
The critical role of three key stratum corneum lipids, including cholesterol ceramides, and fatty acids, in epidermal permeability barrier function has been well demonstrated in both humans and murine models (Feingold, 1991; Mao-Qiang et al., 1993; Mao-Qiang et al., 1995a; Yang et al., 1995; Zettersten et al., 1997; Huang and Chang, 2008). Topical application of stratum corneum lipid accelerates the formation of lamellar bilayers, a critical structure for epidermal permeability barrier, in the intercellular space of the stratum corneum (Mao-Qiang et al., 1995b; Yang et al., 1995; Zettersten et al., 1997), while some natural ingredients can upregulate expression levels of mRNA for enzymes required for epidermal lipid synthesis. The synthesis of three key barrier-related lipids requires their respective enzymes, 3-hydroxy-3-methyl glutaryl-CoA (HMGCoA), serine–palmitoyl transferase 1(SPT1), and fatty acid synthase (FAS), which all are rate-limiting enzymes in the early step of respective lipid synthesis pathway. Topical treatments of mouse skin with apigenin increase expression levels of mRNA for HMGCoA, SPT1, and FAS, accompanied by acceleration of lamellar body formation and secretion, a critical event to deliver lipids to the stratum corneum (Hou et al., 2013).
Chronologically-aged skin displays delayed permeability barrier recovery after barrier disruption (Wang et al., 2020), in part, due to reduction in epidermal lipid synthesis (Ghadially et al., 1995). Correspondingly, mRNA levels for all three key lipid synthetic enzymes are lower in the epidermis of the aged skin compared to that of the young skin (Man et al., 2015). Topical treatments with hesperidin significantly increase expression levels of mRNA for HMGCoA, SPT1 and FAS in the aged mouse epidermis (Man et al., 2015), suggesting that improvement in epidermal permeability barrier function by hesperidin is attributable, at least in part, to the upregulation of epidermal lipid production. Moreover, upregulation of epidermal lipid synthesis can also account for the improvement in epidermal permeability barrier by the extract of an herbal mixture, which increases expression levels of mRNA for SPT1 and fatty acid 2-hydroxylase by over 2-fold in the mouse epidermis, following twice-daily applications for 6 days (Man et al., 2011).
The signaling pathways involved in the increased lipid production by natural ingredients are not clear. But evidence suggests that some natural ingredient-induced improvement in epidermal permeability barrier is attributable to the upregulation of expression of peroxisome proliferator-activated receptors (PPAR). Previous studies showed that activation of PPARs improves epidermal permeability barrier via stimulation of epidermal lipid production (Schmuth et al., 2004; Man et al., 2006; Nazari et al., 2011). Hesperidin can increase expression levels of PPARγ in vitro and in vivo (Nazari et al., 2011; Ghorbani et al., 2012; Elshazly et al., 2018; Meng et al., 2018). In addition, either topical ursolic acid or oleanolic acid, which both accelerate permeability barrier in mice (Lim et al., 2007), increases PPARα expression in keratinocyte cultures (Lee et al., 2006; Lim et al., 2007). Thus, natural ingredient-induced increase in PPAR expression can contribute to the improved epidermal permeability barrier function.
The formation of lamellar bilayers requires lamellar bodies to deliver the lipids from the stratum granulosum to the stratum corneum, while maturation of lamellar bodies and their cargo content requires ATP-binding cassette A12 (ABCA12), a transmembrane glycosylceramide transporter (Mao-Qiang et al., 1995a; Lefévre et al., 2003; Smyth et al., 2008; Hotz et al., 2023). Humans with ABCA12 mutation exhibit defective epidermal permeability barrier (Lefévre et al., 2003; Hotz et al., 2023). Topical treatments with natural ingredients, such as hesperidin and extract of an herbal mixture, induce up to 8-fold increases in expression levels of ABCA12 mRNA (Man et al., 2011; Hou et al., 2012; Man et al., 2015). Additionally, maturation of lamellar bilayers in the stratum corneum is critical to form competent permeability barrier. Both secretory phospholipase and beta-glucocerebrosidase are required for maturation of lamellar bilayers (Holleran et al., 1994; Man et al., 1996; Hanley et al., 1997; Chan et al., 2011). Treatment of the skin with hesperidin increases the activity of epidermal β-glucocerebrosidase and accelerates the maturation of lamellar bilayers in mice (Man et al., 2014; Man et al., 2015). Taken together, increases in epidermal lipid production, lamellar body formation and maturation of lamellar bilayers account for the improved epidermal permeability barrier function by topical natural ingredients.
5.2 Stimulation of keratinocyte differentiation
During the terminal differentiation, the plasma membrane of keratinocytes is replaced by the cornified envelope, consisting of ∼80% loricrin, 8% small proline-rich proteins and 6% filaggrin, crosslinked by transglutaminase (Candi et al., 2005). The cornified envelope covalently binds to a monolayer of lipids, mainly ω-acylated-hydroxy-ceramides, forming the corneocyte-bound lipid envelope, which is an important structure for epidermal permeability barrier function (Elias et al., 2014). Therefore, regulation of differentiation marker-related proteins can affect epidermal permeability barrier function. Apigenin can upregulate expression levels of filaggrin in the mouse epidermis and expression levels of both filaggrin protein and mRNA in keratinocyte cultures (Hou et al., 2013). Similarly, hesperidin increases expression levels of filaggrin and loricrin proteins in both the aged and young mouse skin (Hou et al., 2012; Man et al., 2015). In parallel, mRNA levels for filaggrin, loricrin and involucrin are also increased in keratinocytes cultured with hesperidin (Man et al., 2015). Likewise, extract of royal jelly (a product of honeybees) at a concentration of 40 μm significantly increases expression levels of filaggrin mRNA and protein in vitro (Gu et al., 2017). Moreover, water extract of aloe vera increases filaggrin and involucrin expression in keratinocyte cultures (Razia et al., 2021). Additionally, agrimonia pilosa leaf extract increases transglutaminase activity by almost 100% over the controls in keratinocyte cultures (Nam et al., 2017). Hence, stimulation of keratinocyte differentiation is another mechanism accounting for the improvement in epidermal permeability barrier function by topical natural ingredients.
5.3 Anti-oxidative stress
Oxidative stress has been linked to compromised epidermal permeability barrier in some cutaneous conditions such as dermatitis and UV irradiation (Rojo de la Vega et al., 2017; Bertino et al., 2020; Yang et al., 2022). Accordingly, administrations of antioxidants improve epidermal permeability barrier function (Kuriyama et al., 2002; Masaki, 2010; Campos et al., 2014). Some natural ingredients exhibit potent antioxidant capacity. Previous study showed that extract of cannabis sativa L. scavenged 40% of the 1,1-diphenyl-2-picrylhydrazyl radical and increased superoxide dismutase activity by ≈ 70%, in addition to a reduction in intracellular reactive oxygen species in keratinocyte cultures (Zagórska-Dziok et al., 2021). Similarly, coffee bean extract increases superoxide dismutase activity by 50% in vitro (Zofia et al., 2020). Aqueous extract of agrimonia Pilosa also exhibits radical-scavenging property with IC50 value of as low as 5.6 μg/mL (Zhu et al., 2009). Reduction in intracellular reactive oxygen species was also observed in keratinocyte cultures following the treatment with extract of kombucha berry leaves (Ziemlewska et al., 2022). Thus, antioxidant of the natural ingredients is an additional mechanism accounting for the improvement in epidermal permeability barrier function.
5.4 Others
Several other mechanisms can also contribute to the natural ingredient-induced improvement in epidermal permeability barrier function. First, the epidermis expresses antimicrobial peptides, including cathelicidin-related peptide, which is packaged within and secreted by lamellar bodies (Oren et al., 2003; Braff et al., 2005). Cathelicidin-related peptide is required for and regulated by epidermal permeability barrier function (Aberg et al., 2008). Topical applications of either apigenin or hesperidin or extract of an herbal mixture markedly increased expression levels of cathelicidin-related peptide in the mouse epidermis (Man et al., 2011; Hou et al., 2012; Hou et al., 2013). Second, maturation of lamellar bilayers, a critical structure for epidermal permeability barrier, requires enzymatic processing of the lipids in the stratum corneum (Holleran et al., 1994; Mao-Qiang et al., 1995b; Chan et al., 2011). The optimal pH for those lipid processing enzymes is ≈ 5 (Takagi et al., 1999). Both sodium/hydrogen exchanger 1 (NHE1) and acidic secretory phospholipase A2 are the key regulator of the stratum corneum pH. Either inhibition of sPLA2-I or NHE1 deficiency delays epidermal permeability barrier recovery following acute barrier disruption (Mao-Qiang et al., 1993; Mao-Qiang et al., 1995a; Mao-Qiang et al., 1996; Fluhr et al., 2001; Behne et al., 2002; Fluhr et al., 2004). Topical hesperidin increases expression levels of epidermal NHE1 and sPLA2 (sPLA2g2f) mRNA by over 60% in mice (Man et al., 2015). While rosmarinic acid activates NHE1, Melissa officinalis leaf extract upregulates expression levels of NHE1 protein and mRNA in keratinocyte cultures (Jung et al., 2022). Third, hyaluronic acid can stimulate keratinocyte differentiation and lipid production, resulting in improvement in epidermal permeability barrier homeostasis (Bourguignon et al., 2006; Bourguignon et al., 2013). Extract of hippophae rhamnoides at a concentration of 10 μg/mL significantly increases expression levels of hyaluronan synthase mRNA and protein in keratinocyte cultures (Yao et al., 2021). Aloe vera extract also increases hyaluronic acid production and hyaluronan synthase expression in keratinocyte cultures (Razia et al., 2021). Moreover, aquaporin-3 (AQP3) is a water-, glycerol-, and hydrogen peroxide-transporter expressed in the epidermis. AQP3 deficiency delays permeability barrier recovery (Hara et al., 2002), while overexpression of AQP3 accelerates permeability barrier recovery (Qin et al., 2011). Extract of either hippophae rhamnoides or aloe vera or royal jelly can significantly increase aquaporin 3 expression in keratinocyte cultures (Gu et al., 2017; Razia et al., 2021; Yao et al., 2021). Additionally, transient receptor potential ion channel 3 (TRPV3) also regulates epidermal permeability barrier. Mice with TRPV3 deficiency display defective epidermal permeability barrier (Cheng et al., 2010). Treatment of keratinocytes with extract of agrimonia pilosa leaves induces over 4-fold increases in TRPV3 activity (Nam et al., 2017). Taken together, this bulk of evidence demonstrates that natural ingredients improve epidermal permeability barrier function via multiple mechanisms.
6 Potential clinical applications of natural ingredients in the management of skin conditions with compromised permeability barrier function
Because of the crucial role of epidermal permeability dysfunction in some dermatoses (Elias and Schmuth, 2009; Ye et al., 2014; Hatano and Elias, 2023), strategy that improve epidermal permeability barrier has been deployed in the treatment and prevention of skin disorders with defective permeability barrier. Previous studies showed that topical applications of emollient containing petrolatum, glycerol and sunflower seed oil, prevent the relapse of psoriasis (Man et al., 2019a). Similarly, topical applications of product containing Spa water and urea prevent the relapse of psoriasis following the treatment with glucocorticoids (Seité et al., 2009). Atopic dermatitis is another common skin disorder with defective epidermal permeability barrier. Topical applications of sunflower seed oil, which is known to improve epidermal permeability barrier (Darmstadt et al., 2002), reduce the risk for the development of atopic dermatitis in infants with high risk of atopic dermatitis (Simpson et al., 2014). Moreover, topical applications of a mixture of cholesterol, fatty acids and ceramides at a molar ratio of 1:1:3 for 28 days improve Severity Scoring for Atopic Dermatitis, pruritus and sleep habit scores in patients with atopic dermatitis, with comparable efficacy to fluticasone (Sugarman and Parish, 2009). Furthermore, topical applications of either beeswax or honey markedly lower TEWL in humans (Pavlačková et al., 2020). In parallel, topical treatments of either atopic dermatitis or psoriasis with honey mixture (honey, beeswax and olive oil at a ratio of 1:1:1) improve disease severity and reduce the dose of glucocorticoids (Al-Waili, 2003). Likewise, topical applications of a mixture of honey, beeswax and olive oil at a ratio of 4:1:2 improve diaper dermatitis (El Sakka et al., 2013). Taken together, the bulk of evidence indicates topical natural ingredients can improve epidermal permeability barrier function and alleviate skin disorders with defective epidermal permeability barrier. The benefits of topical natural ingredients on epidermal permeability barrier and possible clinical application are illustrated in Figure 1.
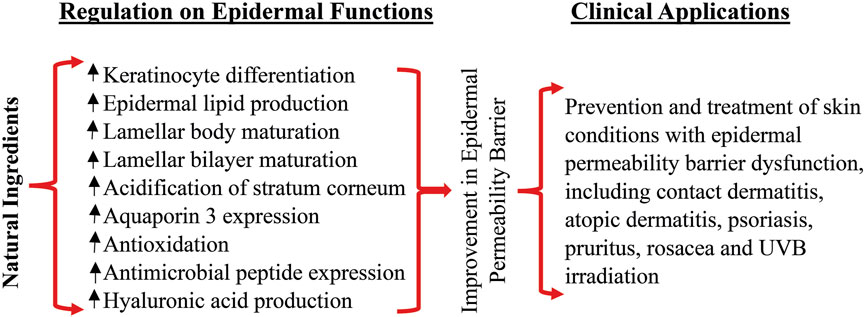
FIGURE 1. Benefits of topical natural ingredients on epidermal permeability barrier and clinical applications.
In summary, a variety of natural ingredients used alone or in combination can improve epidermal permeability barrier function in normal intact skin or following acute barrier disruption. The underlying mechanisms by which natural ingredients improve epidermal permeability barrier function include stimulation of keratinocyte differentiation, lipid production, antioxidant, activation of TRPV3, and upregulation of AQP3 and hyaluronic acid production. Topical applications of natural ingredients can accelerate the repair of epidermal permeability barrier after acute damage and enhance the permeability barrier to prevent the penetration of harmful substances into the skin, which is particularly important for some body sites such as the hands that are vulnerable to damage and exposure to harmful substances. However, available evidence cannot draw a conclusion which ingredient is superior to the others in term of efficacy because of lacking the data of side-by-side comparison.
Author contributions
DL: Formal Analysis, Funding acquisition, Methodology, Writing–original draft, Data curation, Investigation, Validation. DL: Formal Analysis, Investigation, Methodology, Writing–original draft. JZ: Writing–review and editing. LZ: Writing–review and editing, Formal Analysis, Funding acquisition, Supervision. M-QM: Formal Analysis, Writing–review and editing, Conceptualization, Methodology, Writing–original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Health Bureau of Tianjin City (TJW2022QN088).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aberg K. M., Man M. Q., Gallo R. L., Ganz T., Crumrine D., Brown B. E., et al. (2008). Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J. Invest. Dermatol 128, 917–925. doi:10.1038/sj.jid.5701099
Akhtar N., Khan B. A., Khan M. S., Mahmood T., Khan H. M. S., Iqbal M., et al. (2011). Formulation development and moiturising effects of a topical cream of aloe vera extract. World academy of science, engineering and technology. Int. J. Pharm. Pharm. Sci. 5, 128–136. doi:10.5281/zenodo.1073543
Akhtar N., Zaman A., Ali A., Mahmood T., Khan H. M. S., Khan B. A., et al. (2012). Effects of emblica officinalis extract cream on human skin trans-epidermal water loss measured with non invasive probe. J. Pharm. Altern. Med. 1, 32–37.
Al-Waili N. S. (2003). Topical application of natural honey, beeswax and olive oil mixture for atopic dermatitis or psoriasis: partially controlled, single-blinded study. Complement. Ther. Med. 11, 226–234. doi:10.1016/s0965-2299(03)00120-1
Anggraeni S., Umborowati M. A., Damayanti D., Endaryanto A., Prakoeswa C. R. S. (2021). Role of Centella asiatica and ceramide in skin barrier improvement: a double blind clinical trial of Indonesian batik workers. J. Basic Clin. Physiol. Pharmacol. 32, 589–593. doi:10.1515/jbcpp-2020-0510
Astner S., Burnett N., Rius-Díaz F., Doukas A. G., González S., Gonzalez E. (2006). Irritant contact dermatitis induced by a common household irritant: a noninvasive evaluation of ethnic variability in skin response. J. Am. Acad. Dermatol 54, 458–465. doi:10.1016/j.jaad.2005.11.1099
Atrux-Tallau N., Romagny C., Padois K., Denis A., Haftek M., Falson F., et al. (2010). Effects of glycerol on human skin damaged by acute sodium lauryl sulphate treatment. Arch. Dermatol Res. 302, 435–441. doi:10.1007/s00403-009-1021-z
Behne M. J., Meyer J. W., Hanson K. M., Barry N. P., Murata S., Crumrine D., et al. (2002). NHE1 regulates the stratum corneum permeability barrier homeostasis. Microenvironment acidification assessed with fluorescence lifetime imaging. J. Biol. Chem. 277, 47399–47406. doi:10.1074/jbc.M204759200
Bertino L., Guarneri F., Cannavò S. P., Casciaro M., Pioggia G., Gangemi S. (2020). Oxidative stress and atopic dermatitis. Antioxidants 9, 196. doi:10.3390/antiox9030196
Bourguignon L. Y., Ramez M., Gilad E., Singleton P. A., Man M. Q., Crumrine D. A., et al. (2006). Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J. Invest. Dermatol 126, 1356–1365. doi:10.1038/sj.jid.5700260
Bourguignon L. Y., Wong G., Xia W., Man M. Q., Holleran W. M., Elias P. M. (2013). Selective matrix (hyaluronan) interaction with CD44 and RhoGTPase signaling promotes keratinocyte functions and overcomes age-related epidermal dysfunction. J. Dermatol Sci. 72, 32–44. doi:10.1016/j.jdermsci.2013.05.003
Braff M. H., Di Nardo A., Gallo R. L. (2005). Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J. Invest. Dermatol 124, 394–400. doi:10.1111/j.0022-202X.2004.23443.x
Campos P. M., Gianeti M. D., Mercurio D. G., Gaspar L. R. (2014). Synergistic effects of green tea and ginkgo biloba extracts on the improvement of skin barrier function and elasticity. J. Drugs Dermatol 13, 1092–1097.
Candi E., Schmidt R., Melino G. (2005). The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340. doi:10.1038/nrm1619
Chan A., Holleran W. M., Ferguson T., Crumrine D., Goker-Alpan O., Schiffmann R., et al. (2011). Skin ultrastructural findings in type 2 Gaucher disease: diagnostic implications. Mol. Genet. Metab. 104, 631–636. doi:10.1016/j.ymgme.2011.09.008
Cheng X., Jin J., Hu L., Shen D., Dong X. P., Samie M. A., et al. (2010). TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141, 331–343. doi:10.1016/j.cell.2010.03.013
Dal'Belo S. E., Gaspar L. R., Maia Campos P. M. (2006). Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin. Res. Technol. 12, 241–246. doi:10.1111/j.0909-752X.2006.00155.x
Damayanti Umborowati M. A., Anggraeni S., Prakoeswa C. R. (2021). The role of aloe vera and Centella asiatica to the improvement of skin barrier function in Indonesian batik workers. Indian J. Med. Forensic Med. Toxicol. 15, 2805–2811. doi:10.37506/ijfmt.v15i3.15731
Darmstadt G. L., Mao-Qiang M., Chi E., Saha S. K., Ziboh V. A., Black R. E., et al. (2002). Impact of topical oils on the skin barrier: possible implications for neonatal health in developing countries. Acta Paediatr. 91, 546–554. doi:10.1080/080352502753711678
Denda M., Tsuchiya T., Elias P. M., Feingold K. R. (2000). Stress alters cutaneous permeability barrier homeostasis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R367–R372. doi:10.1152/ajpregu.2000.278.2.R367
Elias P. M., Gruber R., Crumrine D., Menon G., Williams M. L., Wakefield J. S., et al. (2014). Formation and functions of the corneocyte lipid envelope (CLE). Biochim. Biophys. Acta 1841, 314–318. doi:10.1016/j.bbalip.2013.09.011
Elias P. M., Schmuth M. (2009). Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 9, 265–272. doi:10.1007/s11882-009-0037-y
El Sakka A., Abdulrhman M., Shehata I. H. (2013). Comparison between topical application of honey, bees wax and olive oil propolis extract and nystatin for treatment of diaper dermatitis in infants. Int. J. Paediatr. Child. Health 1, 39–42.
Elshazly S. M., Abd El Motteleb D. M., Ibrahim IAAE (2018). Hesperidin protects against stress induced gastric ulcer through regulation of peroxisome proliferator activator receptor gamma in diabetic rats. Chem. Biol. Interact. 291, 153–161. doi:10.1016/j.cbi.2018.06.027
Feingold K. R. (1991). The regulation of epidermal lipid synthesis by permeability barrier requirements. Crit. Rev. Ther. Drug Carr. Syst. 8, 193–210.
Feingold K. R., Elias P. M. (2014). Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Biophys. Acta 1841, 280–294. doi:10.1016/j.bbalip.2013.11.007
Fluhr J. W., Behne M. J., Brown B. E., Moskowitz D. G., Selden C., Mao-Qiang M., et al. (2004). Stratum corneum acidification in neonatal skin: secretory phospholipase A2 and the sodium/hydrogen antiporter-1 acidify neonatal rat stratum corneum. J. Invest. Dermatol 122, 320–329. doi:10.1046/j.0022-202X.2003.00204.x
Fluhr J. W., Gloor M., Lehmann L., Lazzerini S., Distante F., Berardesca E. (1999). Glycerol accelerates recovery of barrier function in vivo. Acta Derm. Venereol. 79, 418–421. doi:10.1080/000155599750009825
Fluhr J. W., Kao J., Jain M., Ahn S. K., Feingold K. R., Elias P. M. (2001). Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J. Invest. Dermatol 117, 44–51. doi:10.1046/j.0022-202x.2001.01399.x
Fox L. T., Gerber M., du Preez J. L., du Plessis J., Hamman J. H. (2015). Skin permeation enhancement effects of the gel and whole-leaf materials of Aloe vera, Aloe marlothii and Aloe ferox. J. Pharm. Pharmacol. 67, 96–106. doi:10.1111/jphp.12311
Ghadially R., Brown B. E., Sequeira-Martin S. M., Feingold K. R., Elias P. M. (1995). The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J. Clin. Invest. 95, 2281–2290. doi:10.1172/JCI117919
Ghorbani A., Nazari M., Jeddi-Tehrani M., Zand H. (2012). The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: involvement of PPARγ-dependent mechanism. Eur. J. Nutr. 51, 39–46. doi:10.1007/s00394-011-0187-2
Gu L., Zeng H., Maeda K. (2017). 10-Hydroxy-2-Decenoic acid in royal jelly extract induced both filaggrin and amino acid in a cultured human three-dimensional epidermis model. Cosmetics 4, 48. doi:10.3390/cosmetics4040048
Haftek M., Roy D. C., Liao I. C. (2021). ARTICLE: evolution of skin barrier science for healthy and compromised skin. J. Drugs Dermatol 20, s3–s9. doi:10.36849/JDD.2021.589a
Hamnerius N., Svedman C., Bergendorff O., Björk J., Bruze M., Pontén A. (2018). Wet work exposure and hand eczema among healthcare workers: a cross-sectional study. Br. J. Dermatol 178, 452–461. doi:10.1111/bjd.15813
Hanley K., Jiang Y., Crumrine D., Bass N. M., Appel R., Elias P. M., et al. (1997). Activators of the nuclear hormone receptors PPARalpha and FXR accelerate the development of the fetal epidermal permeability barrier. J. Clin. Invest. 100, 705–712. doi:10.1172/JCI119583
Hara M., Ma T., Verkman A. S. (2002). Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 277, 46616–46621. doi:10.1074/jbc.M209003200
Hatano Y., Elias P. M. (2023). Outside-to-inside,” “inside-to-outside,” and “intrinsic” endogenous pathogenic mechanisms in atopic dermatitis: keratinocytes as the key functional cells involved in both permeability barrier dysfunction and immunological alterations. Front. Immunol. 14, 1239251. doi:10.3389/fimmu.2023.1239251
Holleran W. M., Ginns E. I., Menon G. K., Grundmann J. U., Fartasch M., McKinney C. E., et al. (1994). Consequences of beta-glucocerebrosidase deficiency in epidermis. Ultrastructure and permeability barrier alterations in Gaucher disease. J. Clin. Invest. 93, 1756–1764. doi:10.1172/JCI117160
Hotz A., Kopp J., Bourrat E., Oji V., Süßmuth K., Komlosi K., et al. (2023). Mutational spectrum of the ABCA12 gene and genotype-phenotype correlation in a cohort of 64 patients with autosomal recessive congenital ichthyosis. Genes (Basel). 14, 717. doi:10.3390/genes14030717
Hou M., Man M., Man W., Zhu W., Hupe M., Park K., et al. (2012). Topical hesperidin improves epidermal permeability barrier function and epidermal differentiation in normal murine skin. Exp. Dermatol 21, 337–340. doi:10.1111/j.1600-0625.2012.01455.x
Hou M., Sun R., Hupe M., Kim P. L., Park K., Crumrine D., et al. (2013). Topical apigenin improves epidermal permeability barrier homoeostasis in normal murine skin by divergent mechanisms. Exp. Dermatol 22, 210–215. doi:10.1111/exd.12102
Hu L., Mauro T. M., Dang E., Man G., Zhang J., Lee D., et al. (2017). Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J. Invest. Dermatol 137, 1277–1285. doi:10.1016/j.jid.2017.01.007
Huang H. C., Chang T. M. (2008). Ceramide 1 and ceramide 3 act synergistically on skin hydration and the transepidermal water loss of sodium lauryl sulfate-irritated skin. Int. J. Dermatol 47, 812–819. doi:10.1111/j.1365-4632.2008.03687.x
Jindal R., Pandhi D. (2020). Hand hygiene practices and risk and prevention of hand eczema during the COVID-19 pandemic. Indian Dermatol Online J. 11, 540–543. doi:10.4103/idoj.IDOJ_448_20
Jinnestål C. L., Belfrage E., Bäck O., Schmidtchen A., Sonesson A. (2014). Skin barrier impairment correlates with cutaneous Staphylococcus aureus colonization and sensitization to skin-associated microbial antigens in adult patients with atopic dermatitis. Int. J. Dermatol 53, 27–33. doi:10.1111/ijd.12198
Jung S. W., Park G. H., Kim E., Yoo K. M., Kim H. W., Lee J. S., et al. (2022). Rosmarinic acid, as an NHE1 activator, decreases skin surface pH and improves the skin barrier function. Int. J. Mol. Sci. 23, 3910. doi:10.3390/ijms23073910
Kao J. S., Fluhr J. W., Man M. Q., Fowler A. J., Hachem J. P., Crumrine D., et al. (2003). Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J. Invest. Dermatol 120, 456–464. doi:10.1046/j.1523-1747.2003.12053.x
Khan B. A., Akhtar N., Mahmood T., Khan H. M. S., Zaman S. U., Rasul A., et al. (2011). In-vivo study of stratum corneum water content and transepideramal water loss using a newly formulated topical cream of hippophae rhamnoides fruit extract. Afr. J. Pharm. Pharmacol. 5, 1092–1095.
Khan H., Akhtar N., Ali A. (2014). Effects of cream containing Ficus carica L. Fruit extract on skin parameters: in vivo evaluation. Indian J. Pharm. Sci. 76, 560–564.
Kuriyama K., Shimizu T., Horiguchi T., Watabe M., Abe Y. (2002). Vitamin E ointment at high dose levels suppresses contact dermatitis in rats by stabilizing keratinocytes. Inflamm. Res. 51, 483–489. doi:10.1007/pl00012416
Laneri S., Di Lorenzo R. M., Bernardi A., Sacchi A., Dini I. (2020). Aloe barbadensis: a plant of nutricosmetic interest. Nat. Product. Commun. 15, 1934578X2093274–6. doi:10.1177/1934578x20932744
Lee H. K., Nam G. W., Kim S. H., Lee S. H. (2006). Phytocomponents of triterpenoids, oleanolic acid and ursolic acid, regulated differently the processing of epidermal keratinocytes via PPAR-alpha pathway. Exp. Dermatol 15, 66–73. doi:10.1111/j.0906-6705.2005.00386.x
Lefévre C., Audebert S., Jobard F., Bouadjar B., Lakhdar H., Boughdene-Stambouli O., et al. (2003). Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum. Mol. Genet. 12, 2369–2378. doi:10.1093/hmg/ddg235
Lim S. W., Hong S. P., Jeong S. W., Kim B., Bak H., Ryoo H. C., et al. (2007). Simultaneous effect of ursolic acid and oleanolic acid on epidermal permeability barrier function and epidermal keratinocyte differentiation via peroxisome proliferator-activated receptor-alpha. J. Dermatol 34, 625–634. doi:10.1111/j.1346-8138.2007.00344.x
Lin T. K., Man M. Q., Santiago J. L., Park K., Roelandt T., Oda Y., et al. (2013). Topical antihistamines display potent anti-inflammatory activity linked in part to enhanced permeability barrier function. J. Invest. Dermatol 133, 469–478. doi:10.1038/jid.2012.335
Lodén M., Andersson A. C. (1996). Effect of topically applied lipids on surfactant-irritated skin. Br. J. Dermatol 134, 215–220. doi:10.1111/j.1365-2133.1996.tb07604.x
Maia Campos P. M., Mercurio D., O Melo M., Closs-Gonthier B. (2017). Cichorium intybus root extract: a “vitamin D-like” active ingredient to improve skin barrier function. J. Dermatol. Treat. 28, 78–81. doi:10.1080/09546634.2016.1178695
Man G., Hu L. Z., Elias P. M., Man M. Q. (2018). Therapeutic benefits of natural ingredients for atopic dermatitis. Chin. J. Integr. Med. 24, 308–314. doi:10.1007/s11655-017-2769-1
Man G., Mauro T. M., Kim P. L., Hupe M., Zhai Y., Sun R., et al. (2014). Topical hesperidin prevents glucocorticoid-induced abnormalities in epidermal barrier function in murine skin. Exp. Dermatol 23, 645–651. doi:10.1111/exd.12480
Man G., Mauro T. M., Zhai Y., Kim P. L., Cheung C., Hupe M., et al. (2015). Topical hesperidin enhances epidermal function in an aged murine model. J. Invest. Dermatol 135, 1184–1187. doi:10.1038/jid.2014.486
Man M., Hupe M., Mackenzie D., Kim H., Oda Y., Crumrine D., et al. (2011). A topical Chinese herbal mixture improves epidermal permeability barrier function in normal murine skin. Exp. Dermatol 20, 285–288. doi:10.1111/j.1600-0625.2010.01205.x
Man M. Q., Choi E. H., Schmuth M., Crumrine D., Uchida Y., Elias P. M., et al. (2006). Basis for improved permeability barrier homeostasis induced by PPAR and LXR activators: liposensors stimulate lipid synthesis, lamellar body secretion, and post-secretory lipid processing. J. Invest. Dermatol 126, 386–392. doi:10.1038/sj.jid.5700046
Man M. Q., Elias P. M. (2019). Could inflammaging and its sequelae Be prevented or mitigated? Clin. Interv. Aging 14, 2301–2304. doi:10.2147/CIA.S235595
Man M. Q., Hu L. Z., Elias P. M. (2019a). Herbal medicines prevent the development of atopic dermatitis by multiple mechanisms. Chin. J. Integr. Med. 25, 151–160. doi:10.1007/s11655-015-2438-1
Man M. Q., Ye L., Hu L., Jeong S., Elias P. M., Lv C. (2019b). Improvements in epidermal function prevent relapse of psoriasis: a self-controlled study. Clin. Exp. Dermatol 44, 654–657. doi:10.1111/ced.13888
Man M. Q. M., Feingold K. R., Thornfeldt C. R., Elias P. M. (1996). Optimization of physiological lipid mixtures for barrier repair. J. Invest. Dermatol 106, 1096–1101. doi:10.1111/1523-1747.ep12340135
Mao-Qiang M., Brown B. E., Wu-Pong S., Feingold K. R., Elias P. M. (1995a). Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch. Dermatol 131, 809–816. doi:10.1001/archderm.131.7.809
Mao-Qiang M., Elias P. M., Feingold K. R. (1993). Fatty acids are required for epidermal permeability barrier function. J. Clin. Invest. 92, 791–798. doi:10.1172/JCI116652
Mao-Qiang M., Feingold K. R., Jain M., Elias P. M. (1995b). Extracellular processing of phospholipids is required for permeability barrier homeostasis. J. Lipid Res. 36, 1925–1935. doi:10.1016/s0022-2275(20)41111-3
Mao-Qiang M., Jain M., Feingold K. R., Elias P. M. (1996). Secretory phospholipase A2 activity is required for permeability barrier homeostasis. J. Invest. Dermatol 106, 57–63. doi:10.1111/1523-1747.ep12327246
Masaki H. (2010). Role of antioxidants in the skin: anti-aging effects. J. Dermatol Sci. 58, 85–90. doi:10.1016/j.jdermsci.2010.03.003
Meng C., Guo Z., Li D., Li H., He J., Wen D., et al. (2018). Preventive effect of hesperidin modulates inflammatory responses and antioxidant status following acute myocardial infarction through the expression of PPAR-γ and Bcl-2 in model mice. Mol. Med. Rep. 17, 1261–1268. doi:10.3892/mmr.2017.7981
Milani M., Sparavigna A. (2017). The 24-hour skin hydration and barrier function effects of a hyaluronic 1%, glycerin 5%, and Centella asiatica stem cells extract moisturizing fluid: an intra-subject, randomized, assessor-blinded study. Clin. Cosmet. Investig. Dermatol 10, 311–315. doi:10.2147/CCID.S144180
Na S. Y., Roh J. Y., Kim J. M., Tamang M. D., Lee J. R. (2012). Analysis of colonization and genotyping of the exotoxins of Staphylococcus aureus in patients with atopic dermatitis. Ann. Dermatol 24, 413–419. doi:10.5021/ad.2012.24.4.413
Nam Y. R., Kim H. J., Kim Y. M., Chin Y. W., Bae H. S., Kim W. K., et al. (2017). Agrimonia pilosa leaf extract accelerates skin barrier restoration by activation of transient receptor potential vanilloid 3. J. Dermatol Sci. 86, 255–258. doi:10.1016/j.jdermsci.2017.03.003
Narra K., Naik S. K., Ghatge A. S. (2023). A study of efficacy and safety of ashwagandha (withania somnifera) lotion on facial skin in photoaged healthy adults. Cureus 15, e36168. doi:10.7759/cureus.36168
Nawrot J., Budzianowski J., Nowak G., Micek I., Budzianowska A., Gornowicz-Porowska J. (2021). Biologically active compounds in stizolophus balsamita inflorescences: isolation, phytochemical characterization and effects on the skin biophysical parameters. Int. J. Mol. Sci. 22, 4428. doi:10.3390/ijms22094428
Nazari M., Ghorbani A., Hekmat-Doost A., Jeddi-Tehrani M., Zand H. (2011). Inactivation of nuclear factor-κB by citrus flavanone hesperidin contributes to apoptosis and chemo-sensitizing effect in Ramos cells. Eur. J. Pharmacol. 650, 526–533. doi:10.1016/j.ejphar.2010.10.053
Ní Chaoimh C., Lad D., Nico C., Puppels G. J., Wong XFCC, Common J. E., et al. (2023). Early initiation of short-term emollient use for the prevention of atopic dermatitis in high-risk infants-The STOP-AD randomised controlled trial. Allergy 78, 984–994. doi:10.1111/all.15491
Nishijima T., Tokura Y., Imokawa G., Seo N., Furukawa F., Takigawa M. (1997). Altered permeability and disordered cutaneous immunoregulatory function in mice with acute barrier disruption. J. Invest. Dermatol 109, 175–182. doi:10.1111/1523-1747.ep12319282
Okuda M., Yoshiike T., Ogawa H. (2002). Detergent-induced epidermal barrier dysfunction and its prevention. J. Dermatol Sci. 30, 173–179. doi:10.1016/s0923-1811(02)00106-8
Oren A., Ganz T., Liu L., Meerloo T. (2003). In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp. Mol. Pathol. 74, 180–182. doi:10.1016/s0014-4800(02)00023-0
Park G., Moon B. C., Choi G., Lim H. S. (2021). Cera flava alleviates atopic dermatitis by activating skin barrier function via immune regulation. Int. J. Mol. Sci. 22, 7531. doi:10.3390/ijms22147531
Pavlačková J., Egner P., Slavík R., Mokrejš P., Gál R. (2020). Hydration and barrier potential of cosmetic matrices with bee products. Molecules 25, 2510. doi:10.3390/molecules25112510
Príborský J., Takayama K., Príborská Z., Mühlbachová E., Nagai T. (1992). The influence of detergents on skin barrier properties. Pharmacol. Toxicol. 70, 344–346. doi:10.1111/j.1600-0773.1992.tb00484.x
Proksch E., Brasch J. (1997). Influence of epidermal permeability barrier disruption and Langerhans' cell density on allergic contact dermatitis. Acta Derm. Venereol. 77, 102–104. doi:10.2340/0001555577102104
Proksch E., Brasch J., Sterry W. (1996). Integrity of the permeability barrier regulates epidermal Langerhans cell density. Br. J. Dermatol 134, 630–638. doi:10.1046/j.1365-2133.1996.66828.x
Proksch E., Holleran W. M., Menon G. K., Elias P. M., Feingold K. R. (1993). Barrier function regulates epidermal lipid and DNA synthesis. Br. J. Dermatol 128, 473–482. doi:10.1111/j.1365-2133.1993.tb00222.x
Qin H., Zheng X., Zhong X., Shetty A. K., Elias P. M., Bollag W. B. (2011). Aquaporin-3 in keratinocytes and skin: its role and interaction with phospholipase D2. Arch. Biochem. Biophys. 508, 138–143. doi:10.1016/j.abb.2011.01.014
Razia S., Park H., Shin E., Shim K. S., Cho E., Kim S. Y. (2021). Effects of aloe vera flower extract and its active constituent isoorientin on skin moisturization via regulating involucrin expression: in vitro and molecular docking studies. Molecules 26, 2626. doi:10.3390/molecules26092626
Rojo de la Vega M., Krajisnik A., Zhang D. D., Wondrak G. T. (2017). Targeting NRF2 for improved skin barrier function and photoprotection: focus on the achiote-derived apocarotenoid bixin. Nutrients 9, 1371. doi:10.3390/nu9121371
Samadi A., Nasrollahi S. A., Rostami M. N., Rezagholi Z., Abolghasemi F., Firooz A. (2021). Long-term effects of two 24-hour moisturizing products on skin barrier structure and function: a biometric and molecular study. Health Sci. Rep. 4, e308. doi:10.1002/hsr2.308
Savić V.L., Nikolić V. D., Arsić I. A., Stanojević L. P., Najman S. J., Stojanović S., et al. (2015). Comparative study of the biological activity of allantoin and aqueous extract of the comfrey root. Phytother. Res. 29, 1117–1122. doi:10.1002/ptr.5356
Scharschmidt T. C., Man M. Q., Hatano Y., Crumrine D., Gunathilake R., Sundberg J. P., et al. (2009). Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J. Allergy Clin. Immunol. 124, 496–506. doi:10.1016/j.jaci.2009.06.046
Schliemann-Willers S., Wigger-Alberti W., Kleesz P., Grieshaber R., Elsner P. (2002). Natural vegetable fats in the prevention of irritant contact dermatitis. Contact Dermat. 46, 6–12. doi:10.1034/j.1600-0536.2002.460102.x
Schmuth M., Haqq C. M., Cairns W. J., Holder J. C., Dorsam S., Chang S., et al. (2004). Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J. Invest. Dermatol 122, 971–983. doi:10.1111/j.0022-202X.2004.22412.x
Schmuth M., Jiang Y. J., Dubrac S., Elias P. M., Feingold K. R. (2008). Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J. Lipid Res. 49, 499–509. doi:10.1194/jlr.R800001-JLR200
Seité S., Khemis A., Rougier A., Ortonne J. P. (2009). Emollient for maintenance therapy after topical corticotherapy in mild psoriasis. Exp. Dermatol 18, 1076–1078. doi:10.1111/j.1600-0625.2009.00903.x
Sharma K., Mittal A., Chauhan N. (2015). Aloe vera as penetration enhancer. Int. J. Drug Dev. Res. 7, 31–43.
Shu X., Wang L., Qu R., Li L., Wang X. (2023). Jade material in vitro and in vivo: a study on the anti-inflammatory and repair efficacy of jade material on the skin. Int. J. Cosmet. Sci. 45, 177–186. doi:10.1111/ics.12829
Simpson E. L., Chalmers J. R., Hanifin J. M., Thomas K. S., Cork M. J., McLean W. H., et al. (2014). Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 134, 818–823. doi:10.1016/j.jaci.2014.08.005
Sipos S., Moacă E. A., Pavel I. Z., Avram Ş., Crețu O. M., Coricovac D., et al. (2021). Melissa officinalis L. Aqueous extract exerts antioxidant and antiangiogenic effects and improves physiological skin parameters. Molecules 26, 2369. doi:10.3390/molecules26082369
Smyth I., Hacking D. F., Hilton A. A., Mukhamedova N., Meikle P. J., Ellis S., et al. (2008). A mouse model of harlequin ichthyosis delineates a key role for Abca12 in lipid homeostasis. PLoS Genet. 4, e1000192. doi:10.1371/journal.pgen.1000192
Souza C., de Freitas L. A. P., Maia Campos PMBG (2017). Topical formulation containing beeswax-based nanoparticles improved in vivo skin barrier function. AAPS PharmSciTech 18, 2505–2516. doi:10.1208/s12249-017-0737-x
Sugarman J. L., Parish L. C. (2009). Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J. Drugs Dermatol 8, 1106–1111.
Takagi Y., Kriehuber E., Imokawa G., Elias P. M., Holleran W. M. (1999). β-Glucocerebrosidase activity in mammalian stratum corneum. J. Lipid Res. 40, 861–869. doi:10.1016/s0022-2275(20)32121-0
Teo H. G., Lim T. H., Bujang M. A., Kiing J. W., Muniandy P. (2023). Prevalence of occupational hand eczema among healthcare workers and its associated risk factors in a tertiary hospital in sarawak during covid-19 pandemic. Indian J. Dermatol 68, 121. doi:10.4103/ijd.ijd_803_22
Wang Z., Man M. Q., Li T., Elias P. M., Mauro T. M. (2020). Aging-associated alterations in epidermal function and their clinical significance. Aging (Albany NY) 12, 5551–5565. doi:10.18632/aging.102946
Wasilewski T., Seweryn A., Krajewski M. (2016). Improvement in the safety of use of hand dishwashing liquids through the addition of hydrophobic plant extracts. J. Surfactants Deterg. 19, 1315–1326. doi:10.1007/s11743-016-1868-x
Wen S., Ye L., Liu D., Yang B., Man M. Q. (2021). Topical N-palmitoyl serinol, a commensal bacterial metabolite, prevents the development of epidermal permeability barrier dysfunction in a murine model of atopic dermatitis-like skin. Can. J. Vet. Res. 85, 201–204.
Wolf R., Parish L. C. (2012). Effect of soaps and detergents on epidermal barrier function. Clin. Dermatol 30, 297–300. doi:10.1016/j.clindermatol.2011.08.021
Wood L. C., Elias P. M., Calhoun C., Tsai J. C., Grunfeld C., Feingold K. R. (1996). Barrier disruption stimulates interleukin-1 alpha expression and release from a pre-formed pool in murine epidermis. J. Invest. Dermatol 106, 397–403. doi:10.1111/1523-1747.ep12343392
Wood L. C., Elias P. M., Sequeira-Martin S. M., Grunfeld C., Feingold K. R. (1994). Occlusion lowers cytokine mRNA levels in essential fatty acid-deficient and normal mouse epidermis, but not after acute barrier disruption. J. Invest. Dermatol 103, 834–838. doi:10.1111/1523-1747.ep12413597
Wood L. C., Jackson S. M., Elias P. M., Grunfeld C., Feingold K. R. (1992). Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J. Clin. Invest. 90, 482–487. doi:10.1172/JCI115884
Wood L. C., Stalder A. K., Liou A., Campbell I. L., Grunfeld C., Elias P. M., et al. (1997). Barrier disruption increases gene expression of cytokines and the 55 kD TNF receptor in murine skin. Exp. Dermatol 6, 98–104. doi:10.1111/j.1600-0625.1997.tb00154.x
Yang J., Zeng J., Lu J. (2022). Mechanisms of ultraviolet-induced melasma formation: a review. J. Dermatol 49, 1201–1210. doi:10.1111/1346-8138.16542
Yang L., Mao-Qiang M., Taljebini M., Elias P. M., Feingold K. R. (1995). Topical stratum corneum lipids accelerate barrier repair after tape stripping, solvent treatment and some but not all types of detergent treatment. Br. J. Dermatol 133, 679–685. doi:10.1111/j.1365-2133.1995.tb02738.x
Yao Q., Jia T., Qiao W., Gu H., Kaku K. (2021). Unsaturated fatty acid-enriched extract from Hippophae rhamnoides seed reduces skin dryness through up-regulating aquaporins 3 and hyaluronan synthetases 2 expressions. J. Cosmet. Dermatol 20, 321–329. doi:10.1111/jocd.13482
Ye L., Lv C., Man G., Song S., Elias P. M., Man M. Q. (2014). Abnormal epidermal barrier recovery in uninvolved skin supports the notion of an epidermal pathogenesis of psoriasis. J. Invest. Dermatol 134, 2843–2846. doi:10.1038/jid.2014.205
Zagórska-Dziok M., Bujak T., Ziemlewska A., Nizioł-Łukaszewska Z. (2021). Positive effect of cannabis sativa L. Herb extracts on skin cells and assessment of cannabinoid-based hydrogels properties. Molecules 26, 802. doi:10.3390/molecules26040802
Zettersten E. M., Ghadially R., Feingold K. R., Crumrine D., Elias P. M. (1997). Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. J. Am. Acad. Dermatol 37, 403–408. doi:10.1016/s0190-9622(97)70140-3
Zhu L., Tan J., Wang B., He R., Liu Y., Zheng C. (2009). Antioxidant activities of aqueous extract from Agrimonia pilosa Ledeb and its fractions. Chem. Biodivers. 6, 1716–1726. doi:10.1002/cbdv.200800248
Ziemlewska A., Nizioł-Łukaszewska Z., Zagórska-Dziok M., Bujak T., Wójciak M., Sowa I. (2022). Evaluation of cosmetic and dermatological properties of kombucha-fermented berry leaf extracts considered to Be by-products. Molecules 27, 2345. doi:10.3390/molecules27072345
Zofia N. Ł., Aleksandra Z., Tomasz B., Martyna Z. D., Magdalena Z., Zofia H. B., et al. (2020). Effect of fermentation time on antioxidant and anti-ageing properties of green coffee kombucha ferments. Molecules 25, 5394. doi:10.3390/molecules25225394
Keywords: topical, natural ingredients, transepidermal water loss, epidermis, barrier, permeability
Citation: Lei D, Liu D, Zhang J, Zhang L and Man M-Q (2024) Benefits of topical natural ingredients in epidermal permeability barrier. Front. Physiol. 14:1275506. doi: 10.3389/fphys.2023.1275506
Received: 10 August 2023; Accepted: 07 December 2023;
Published: 04 January 2024.
Edited by:
Sandrine Dubrac, Innsbruck Medical University, AustriaReviewed by:
Corinne Leprince, INSERM UMR1291 Institut Toulousain des Maladies Infectieuses et Inflammatoires, FranceElia Ranzato, Università del Piemonte Orientale, Italy
Copyright © 2024 Lei, Liu, Zhang, Zhang and Man. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Litao Zhang, emhhbmdsaXRhb0BtZWRtYWlsLmNvbS5jbg==; Mao-Qiang Man, bXFtYW5AaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Dongyun Lei1†
Dongyun Lei1† Litao Zhang
Litao Zhang Mao-Qiang Man
Mao-Qiang Man