Corrigendum: Analysis of risk factors for changes of left ventricular function indexes in Chinese patients with gout by echocardiography
- 1Department of Rheumatology and Immunology, Clinical Medical College, The First Affiliated Hospital of Chengdu Medical College, Chengdu, China
- 2Department of Ultrasound, Clinical Medical College, The First Affiliated Hospital of Chengdu Medical College, Chengdu, China
Background: Echocardiographic data investigating the association between left ventricular (LV) function and gout is still limited.
Purpose: To analyze the association of echocardiographic parameters based on two-dimentional speckle tracking analysis with clinically related indicators in patients with gout, and to provide a clinical basis for the early diagnosis and treatment of cardiovascular disease in patients with gout.
Methods: This study collected gout patients who visited the outpatient and inpatient departments of the first affiliated hospital of chengdu medical college from November 2019 to December 2020. Spearman correlation test was performed to analyze the correlation coefficients between the laboratorial indicators with echocardiographic parameters. And the logistic regression analysis was performed to evaluate the independent effects.
Results: The results of multivariate logistic regression showed that fasting plasma glucose (FPG) was a risk factor for the decrease in absolute value of global longitudinal strain [GLS (OR = 2.34; 95% CI, 1.01–5.39; p = 0.04)], Urea was a risk factor for absolute reduction in GCS (OR = 1.40; 95% CI, 1.07–1.85; p = 0.02), age (OR = 1.09, 95% CI, 1.04–1.16; p = 0.001), and hypertension (OR = 8.35; 95% CI, 1.83–38.02; p = 0.006) were risk factors for increased E/Em. High urea levels were significantly related with high risks of LVH (OR = 1.59, 95% CI, 1.04–2.43; p = 0.03) and enlargement of LAVI (OR = 1.68, 95% CI, 1.01–2.80; p = 0.04).
Conclusion: Our study found that elevated urea and FPG were risk factors for subclinical LV myocardial dysfunction in patients with gout, which might provide a theoretical basis for the early diagnosis and treatment of heart disease in clinical practice.
1 Introduction
Gout is the most common form of inflammatory arthritis characterized by deposition of monosodium urate monohydrate (MSU) in synovial fluid and other tissues, with a prevalence of 1.1% in mainland China, and 6.8% of the population worldwide (Neogi, 2011; Liu et al., 2015; Dehlin et al., 2020). Previous studies has reported that cardiovascular events are positively associated with gout. The relationship between SUA and cardiovascular events is also complex and requires further study. To better understand the relationship between SUA and cardiovascular events and to develop effective prevention and treatment strategies, we must take into account the widespread (and often underestimated) conditions such as incorrect lifestyle, obesity, and insulin resistance that can harm SUA levels while increasing cardiometabolic risk (Maloberti et al., 2023). A recent observational evidence demonstrated that patients with gout were found to have a higher burden of co-morbidities and a higher rate of all-cause hospitalization compared to patients without gout (Carnicelli et al., 2020). Another study showed that the frequency of gout attacks was associated with an odds ratio (OR) of 1.18 for myocardial infarction (Chen et al., 2007). In patients with gout, hyperuricemia mainly upregulates xanthine oxidase activity leading to increased oxidative stress and monosodium urate crystal-mediated inflammation that affects the pathophysiological changes of cardiovascular disease (Eisen et al., 2013; Pan et al., 2014a). But study have shown that hyperuricemia is not the only risk factor for left ventricular (LV) dysfunction in gout patients (Lin et al., 2015).
In recent years, echocardiographic techniques has been rapidly developed as a novel auxiliary technique for assessing the structure and function of the heart. One of which is speckle tracking, which is principally based on the analysis of speckles during the cardiac cycle (Mondillo et al., 2011). This technique can be used to study global myocardial deformation via global longitudinal strain (GLS) and global circumferential strain (GCS) assessment (Reant et al., 2010; Zhang et al., 2018). This method trackes the scattered spots evenly distributed in the myocardium in the two-dimensional gray-scale image and the movement of its geometric position frame by frame, and applys spatial and temporal image processing algorithms to obtain tissue motion information, thereby obtaining multiple motion parameters in longitudinal, radial and circumferential directions. Moreover, it overcomes the angle dependence and can more accurately reflect the myocardial movement and evaluate the myocardial strain capacity (Langeland et al., 2005). Therefore, it can serve as a powerful quantitative tool to assess global and regional LV function. In addition, measurement of this index is now possible on-line during the ultrasound examination, with no requirement for post-processing.
Although study has not found significant change on LV structure and systolic function in gout patients, there were evidences suggested that the severity of gout can lead to LV diastolic dysfunction and subclinical systolic dysfunction (Pan et al., 2014b; Naghavi et al., 2016). In addition, echocardiographic data investigating the association between LV function and gout is still limited. Speckle-tracking echocardiography–derived measurements have been validated against sonomicrometry and tagged MRI, showing high feasibility and reproducibility (Amundsen et al., 2006). The purpose of this study was to analyze the association of echocardiographic parameters based on two-dimentional speckle tracking analysis with clinically related indicators in patients with gout, and to provide a clinical basis for the early diagnosis and treatment of cardiovascular disease in patients with gout.
2 Methods
2.1 Subjects and study design
This study collected gout patients who visited the outpatient and inpatient departments of the first affiliated hospital of chengdu medical college from November 2019 to December 2020. The inclusion criteria: 1) diagnosis of gout based on the definition of the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR); 2) have not received any related therapies (within 3 months) to reduce uric acid, blood pressure, and glucose; 3) agree to sign the informed consent. The exclusion criteria were: 1) patients with secondary gout caused by malignant tumor, radiotherapy, chemotherapy, or blood system diseases; 2) with previous valvular heart disease, ischemic cardiomyopathy, dilated or hypertrophic cardiomyopathy, coronary artery disease, chronic lung disease, or severe anemia; 3) with arrhythmia, pacemaker implantation, or previous history of cardiac surgery; 4) with autoimmune diseases such as rheumatoid arthritis and ankylosing spondyloarthritis (excluding immune-related indicators such as rheumatoid factor and anti-citrulline peptide autoantibodies). Fifty-one healthy adults were recruited from the same hospital’s health management center as a control group, screened through history, physical examination, and laboratory tests to exclude any individuals with conditions such as hypertension and diabetes, and echocardiography was performed on them. This study was approved by the Institutional Review Board of our hospital and written informed consent was obtained from all subjects participating in the study.
2.2 Echocardiography
All subjects received two-dimensional echocardiographic examinations at rest in the left lateral decubitus position using a EPIQ 7C color Doppler ultrasound diagnostic apparatus (1–5 MHz, Philips, Washington, United States) and S5-1 broadband phased array transducer. According to the current guidelines of the American Society of Echocardiography (Mitchell et al., 2019), early diastolic peak of mitral valve orifice (E) were measured at the mitral valve tip by pulse-wave tissue Doppler imaging (TDI) in the apical four-chamber view (Figure 1A). The peak early diastolic mitral annular velocity (Em) were obtained at the mitral valve tip by pulse-wave TDI at the septal site of the mitral annulus (Figure 1B). The E/Em ratio was used as an index of LV filling pressure (Nagueh et al., 2016; Oh et al., 2020). The left ventricular end-diastolic diameter (LVDd), end-diastolic septal thickness (IVST), and end-diastolic left ventricular posterior wall thickness (PWT) were measured for 3 continuously cardiac cycles, and calculated the average value of 3 measures. The left ventricular mass (LVM) was calculated according to the formula recommended by the American Society of Echocardiography (ASE) (Lang, 2016): LVM(g) = 0.8 × 1.04×[(IVST + PWT + LVDd)3-LVDd3] + 0.6. After standardized the LVM by body surface area (BSA), we calculated the left ventricular mass index (LVMI) as LVMI(g/m2) = LVMI/BSA. Left ventricular hypertrophy (LVH) was diagnosed as LVMI>95 g/m2 in females and LVMI>115 g/m2 in males (Lang, 2016). The left atrial volume index (LAVI) was calcualted and left ventricular ejection fraction (LVEF), which represented the LV systolic function, was calculated. Two-dimensional dynamic images of three apical views (four-, two- and three-chamber) were recorded, and all echocardiographic measures were obtained through consecutive three heart cycles. Speckle-tracking analysis was performed using Qlab software. Two experienced ultrasound physicians independently measured the GLS and GCS, and the intra-group correlation coefficients were > 0.90 for both GLS and GCS which indicated for a good consistency. GLS and GCS values were calculated respectively as the means of the global longitudinal strains and global circumferential strain of each apical view (Figures 1C, D). LAVI enlargement was defined as LAVI > 34 mL/m2. The absolute decrease in GLS was defined as GLS > −20%, and the absolute decrease in GCS was defined as GCS > −30%. The increase in E/Em was defined as E/Em > 14 (Lang et al., 2015).
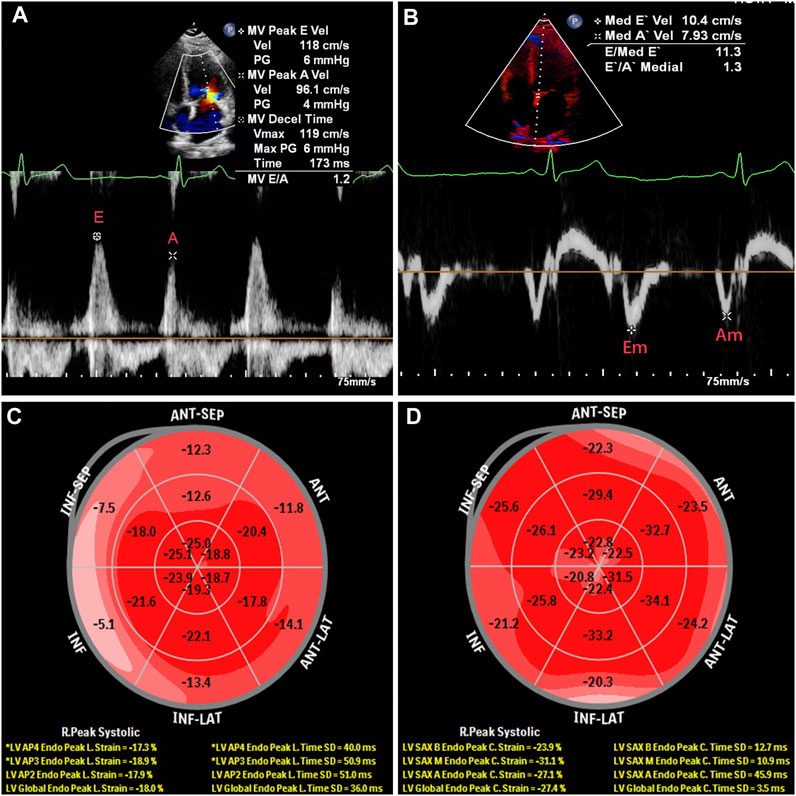
Figure 1. Echocardiography of a 34-year-old male patient diagnosed with gout without secondary hypertension and diabetes. (A), early diastolic peak of mitral valve orifice; (B), the peak early diastolic mitral annular velocity; (C), global longitudinal strain (GLS); (D), global circumferential strain (GCS).
2.3 Laboratory
3 mL of fasting blood was collected from the patient (no food and drink for at least 8 h overnight) in the morning, and examined with an automatic hematology analyzer (Japan, SYSMEX, xn-9000) on the day of echocardiographic examination. The erythrocyte sedimentation rate was analyzed (Italy, VITAL diagnostics, MONITOR-100). Laboratorial indicators related to renal function, lipid metabolism, and blood glucose were measured by an automatic biochemical analyzer (Japan, Hitachi Limited, 7600). The data of red blood cell (RBC), hemoglobin (HGB), platelets (PLT), neutrophils (NE), lymphocytes (LY), Urea, cystatin C (Cys-C), β2-microglobulin (β2-MG), total protein (TP), albumin (ALB), albumin/globulin (A/G), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A-I (ApoA-I), apolipoprotein B (ApoB), lipoprotein (a) (Lp(a)), fasting plasma glucose (FPG), glycated hemoglobin (HBA1C), average blood glucose (AG), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) were recorded for analysis.
2.4 Statistical analysis
All the data were analyzed by SPSS Software v22.0 (IBM Corporation, Armonk, NY, United States). Normally distributed data was presented in mean ± standard deviation. We perform a normality test on the selected variable by performing a probe analysis and checking the normality option. According to the Kolmogorov-Smirnov and Shapiro-Wilk tests, GLS, GCS, E/Em, LVEF, LVMI and LAVI all agree with the hypothesis of normal distribution. The intra-class correlation coefficient (ICC) was calculated to evaluate the consistency of the GLS and GCS measurements between the two ultrasound physicians (ICC > 0.75 indicates a good consistency). Spearman correlation test was utilized to examine the correlation coefficients between laboratorial indicators and echocardiographic parameters. Furthermore, logistic regression analysis was applied to assess the independent effects of the laboratorial indicators on changes in GLS, GCS, LVMI, LAVI and E/Em, respectively, while controlling for other covariates. The variable selection method for building the regression models was a stepwise approach, which was performed after the initial correlation analysis. A p-value < 0.05 was considered statistical significant.
3 Results
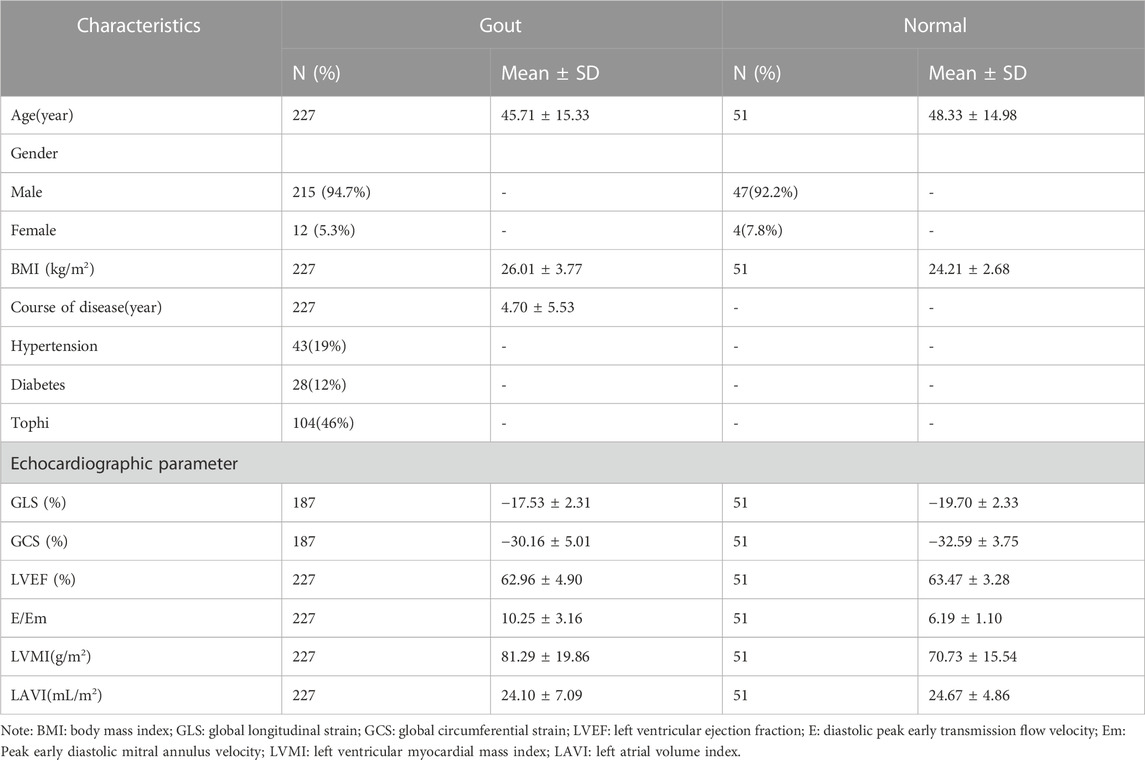
A total of 227 cases including 215 males (94.7%) and 12 females (5.3%) were included in the study, of which, 40 patients cannot obtain GLS and GCS due to poor image resolution. The average age of the included patients were 45.71 ± 15.33 years old. The baseline characteristics of included patients were summarized in Table 1. Among these patients, 164 had decreased GLS, 96 had decreased GCS, 20 had increased E/Em, 16 had LVH, and 16 had increased LAVI. Table 2 shows the statistical results between the complete sample and the reduced sample using GLS and GCS. The results show that there are no significant differences in Age, BMI and Course of disease between the two groups (p > 0.05).
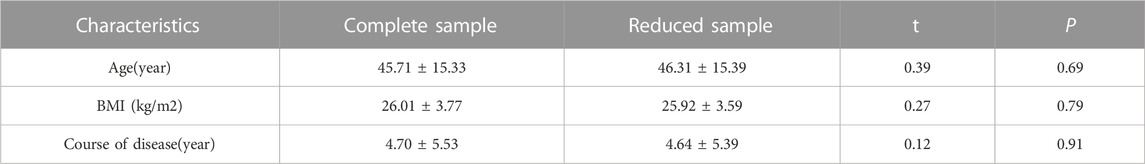
Table 2. Comparison of basic characteristics of the complete sample and the reduced sample using GLS and GCS.
Compared with the normal group, the absolute values of GLS and GCS in the gout group were lower, E/Em and LVMI were larger, while LVEF and LAVI were not different between the two groups (The p-values of GLS, E/Em and LVMI were all < 0.001, and the p-values of GCS were 0.001. The p-values of LVEF and LAVI were all > 0.05).
Table 3 presents the results of a univariate analysis of variables with statistical differences and variables of clinical significance, where GLS, GCS, E/Em, LVH and LAVI were used as dependent variables, while laboratory indicators served as independent variables. This analysis aimed to examine the individual associations between each laboratory indicator and the dependent variables. Table 4, on the other hand, summarizes the results of further multivariate logistic regression analysis. In this analysis, we included the laboratory indicators that were found to be significantly associated with the dependent variables in the univariate analysis. The purpose of multivariate regression was to evaluate the combined effects of these laboratory indicators on GLS, GCS, and E/Em, while adjusting for potential confounding factors. By performing multivariate logistic regression analysis, we were able to assess the independent contributions of the laboratory indicators, while controlling for other variables, and obtain more comprehensive and accurate conclusions regarding their relationships with GLS, GCS, and E/Em, LVH and LAVI. This approach allowed us to account for potential confounders and better understand the association between the laboratory indicators and the dependent variables of interest. The results showed that FPG was a risk factor for the decrease in absolute value of GLS (OR = 2.34; 95% CI, 1.01–5.39; p = 0.04); urea was a risk factor of absolute reduction in GCS (OR = 1.40; 95% CI, 1.07–1.85; p = 0.02); age (OR = 1.09, 95% CI, 1.04–1.16; p = 0.001), and hypertension (OR = 8.35; 95% CI, 1.83–38.02; p = 0.006) were risk factors for increased E/Em. High urea levels were significantly related with high risks of LVH (OR = 1.59, 95% CI, 1.04–2.43; p = 0.03) and enlargement of LAVI (OR = 1.68, 95% CI, 1.01–2.80; p = 0.04).
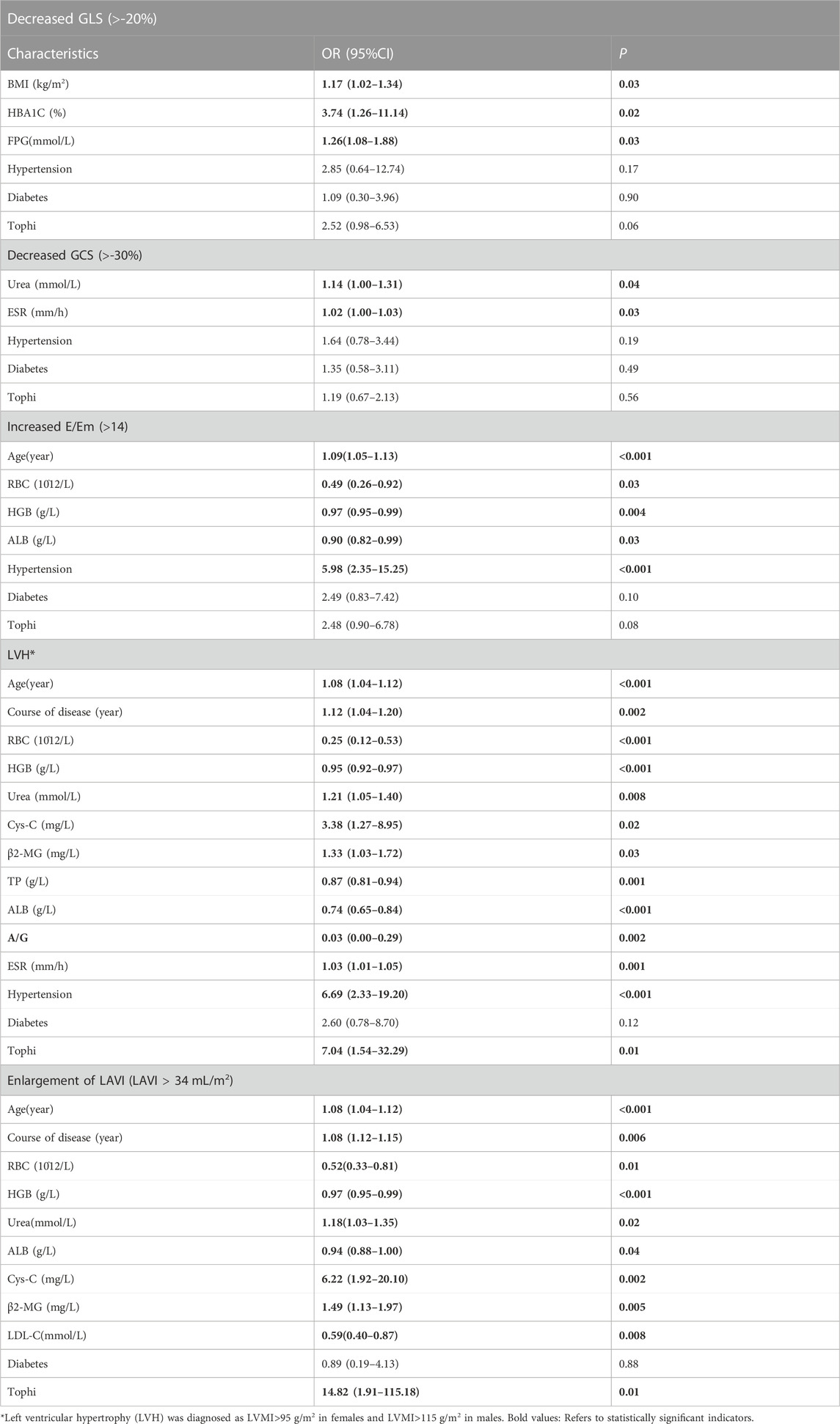
Table 3. Univariate binary logistic regression analysis of GLS, GCS, E/Em, LVH and LAVI in gout patients.

Table 4. Multivariate binary logistic regression analysis results of GLS, GCS, E/Em, LVH and LAVI in gout patients.
4 Discussion
Our findings showed that decreased GLS and GCS, LVH, and enlargement of LAVI were associated with urea levels. Previous evidence suggested that left atrial remodeling was an important value in predicting the risk and mortality of cardiovascular events, and left atrial enlargement played an important role in the development of heart failure (Welles et al., 2012; Inoue et al., 2021). Also, LAVI is a robust indicator of diastolic dysfunction independent of left ventricular loading conditions (Douglas, 2003; Singh et al., 2017). The left atrium maintains the normal function of the heart by regulating the filling process of the left ventricle and affecting the rhythm of myocardial contraction and diastole. In addition, the left atrium also has reservoir, conduit, and reinforcement functions, and is able to adaptively change its structural and mechanical properties to maintain its normal function (Pathan et al., 2017). Enlargement of the left atrium is considered to be one of the important markers of the development of heart disease, especially closely related to the development of heart failure (Ye et al., 2020). A number of studies have shown that high urea levels were associated with adverse cardiovascular outcomes, and elevated urea could predict renal hypoperfusion, a renal hemodynamic state that may be associated with reduced cardiac output (Jujo et al., 2017; Carnicelli et al., 2020). Taken all these together, our study suggested that elevated urea might be a good predictor of changes in myocardial subclinical systolic function in gout patients. In addition, urea is also considered as a risk factor for diastolic dysfunction and left ventricular hypertrophy in gout patients. This might related to the activation of a complex neurohormonal mechanism that stimulates the release of vasopressin and activates the renal sympathetic nervous system (Fang et al., 2017). Yanwei Du et al. have also demonstrated that high urea could lead to hypertrophy cardiomyocytes in mouse (Du et al., 2014). They found that the deletion of urea channel protein would not only lead to the accumulation of urea in mouse cardiomyocytes, and also lead to the increase of myocardial reactive oxygen species production and increased oxidative stress in mouse. In addition, the increase of oxidative stress from reactive oxygen species was suggested to associate with myocardial fibrosis, myocardial hypertrophy, LV remodeling, impaired myocardial contractility, and decreased cardiac function (Herrmann et al., 2012).
Our study also showed that GLS was positively correlated with FPG, and LVEF was negatively correlated with FPG. After the logistic regression analysis, we found that increased FPG was a risk factor for absolute decrease of GLS. Diabetes could result in the structural abnormalities of the heart (Zhao et al., 2020; Turkes et al., 2021). And it is an important risk factor for heart failure, suggesting that glycemic control may influence the development of heart failure (Gilbert and Krum, 2015). Elevated FPG has been shown to be prediabetic, and also a risk factor for heart failure in non-diabetic patients (Dauriz et al., 2017). Poor glycemic control in diabetic patients may cause cardiomyocyte hypertrophy, collagen deposition and cross-linking, and further lead to increased LV mass and enhanced myocardial oxidative stress, all of which may lead to impaired myocardial function (Harada et al., 2017). This is also consistent with our findings that elevated FPG might be a significant predictor of subclinical systolic dysfunction in gout patients.
It has been found that gout can cause changes in LV diastolic function, and patients with gout can have diastolic dysfunction before systolic dysfunction (Pan et al., 2014b). E/Em is a sensitive indicator of LV diastolic function, which increases as LV diastolic function decreases (Dudziak et al., 2016). Our findings demonstrated that age and hypertension were risk factors for increased E/Em, suggesting that patients with gout might have an increased risk of LV diastolic dysfunction with age and secondary hypertension. Hypertension is considered to be an important factor in the development of LV hypertrophy (Aronow, 2017). Previously, the cardiac structural change of LV hypertrophy was thought to be related with hypertension, but recent studies showed that metabolic syndrome was also associated with the development of LV remodeling (Homan et al., 2019). Several hypotheses including angiotensin II stimulation, cardiomyocyte hypertrophy, high insulin levels, and the role for the sympathetic nervous system have been proposed for the explanation of LV remodeling. Whereas it is difficult to determine which mechanisms was conclusive as these mechanisms were often jointly found in patients with gout. Hyperuricemia and elevated uric acid levels were potential risk factors for hypertension, which was a major cause of cardiovascular disease (Stewart et al., 2019). Therefore, it is necessary to monitor the chanegs of blood pressure in gout patients to reduce the risk of LV remodeling. In addition, gout was associated with an increased risk of heart failure in the elderly patients, hence early changes in cardiac function should be especially noted in elderly patients when clinical interventions were performed in patients with gout (Colantonio et al., 2020).
This study has several limitations that need to be acknowledged. Firstly, it is a single-center study, which may introduce regional bias and limit the generalizability of the research findings. Conducting multi-center or cross-regional studies would be necessary to validate the stability and applicability of the results. Secondly, the sample size is relatively small, which may affect the statistical power and reliability of the study. Further expansion of the sample size is needed to enhance the credibility and generalizability of the research. Thirdly, the majority of patients in this study are males, with a relatively small number of females included. Considering the physiological differences between genders, gender factors may interfere with the associations between cardiac ultrasound parameters and laboratory indicators. Future studies should strive for a better balance in gender distribution to explore the influence of gender on the research results. Additionally, there may be issues with data quality in this study. Although efforts have been made to ensure the accuracy of data collection and processing, measurement errors or incomplete data records may still exist. This could potentially impact the reliability and interpretation of the results. Furthermore, there are unmeasured confounding factors in our study. Apart from the potential factors we have considered, there may be other unanalyzed factors that could influence the results. This may lead to the inability to completely eliminate the possibility of confounding. Lastly, we acknowledge the potential statistical issues when conducting multiple tests. Since we have performed multiple correlation analyses, there is an increased chance of discovering chance results. Therefore, future research needs to employ more rigorous statistical methods to validate our findings.
In conclusion, our study found that elevated urea and FPG were risk factor for subclinical LV myocardial dysfunction in patients with gout, which might provide a theoretical basis for the early diagnosis and treatment of heart disease in clinical practice. While caution must be taken in generalising these findings to female patients due to potential gender differences in this study, they provide a valuable theoretical basis for the early diagnosis and treatment of heart disease in patients with gout in clinical practice (Redon et al., 2019). Besides, older patients with gout and hypertension should be close monitored to prevent the occurrence of cardiovascular events.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the Affiliated Hospital of Chengdu Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WD: Conceptualization, Funding acquisition, Writing–original draft, Writing–review and editing. DL: Data curation, Writing–original draft, Writing–review and editing. JH: Data curation, Writing–original draft, Writing–review and editing. HL: Data curation, Writing–original draft, Writing–review and editing. XX: Data curation, Writing–original draft, Writing–review and editing. JL: Funding acquisition, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Basic Application Research Project of Sichuan Science and Technology Department (No. 2022NSFSC0692). Scientific research project of Sichuan Medical Association (Grant number S18011); and Project of Chengdu Medical College (Grant number 18Z168).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amundsen B. H., Helle-Valle T., Edvardsen T., Torp H., Crosby J., Lyseggen E., et al. (2006). Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J. Am. Coll. Cardiol. 47, 789–793. doi:10.1016/j.jacc.2005.10.040
Aronow W. S. (2017). Hypertension and left ventricular hypertrophy. Ann. Transl. Med. 5, 310. doi:10.21037/atm.2017.06.14
Carnicelli A. P., Clare R., Chiswell K., Lytle B., Bjursell M., Perl S., et al. (2020). Comparison of characteristics and outcomes of patients with heart failure with preserved ejection fraction with versus without hyperuricemia or gout. Am. J. Cardiol. 127, 64–72. doi:10.1016/j.amjcard.2020.04.026
Chen S. Y., Chen C. L., Shen M. L. (2007). Severity of gouty arthritis is associated with Q-wave myocardial infarction: a large-scale, cross-sectional study. Clin. Rheumatol. 26, 308–313. doi:10.1007/s10067-006-0292-4
Colantonio L. D., Saag K. G., Singh J. A., Chen L., Reynolds R. J., Gaffo A., et al. (2020). Gout is associated with an increased risk for incident heart failure among older adults: the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study. Arthritis Res. Ther. 22, 86. doi:10.1186/s13075-020-02175-2
Dauriz M., Targher G., Temporelli P. L., Lucci D., Gonzini L., Nicolosi G. L., et al. (2017). GISSI-HF investigators. Prognostic impact of diabetes and prediabetes on survival outcomes in patients with chronic heart failure: a post-hoc analysis of the GISSI-HF (gruppo italiano per lo studio della Sopravvivenza nella insufficienza cardiaca-heart failure) trial. J. Am. Heart Assoc. 6, e005156. doi:10.1161/JAHA.116.005156
Dehlin M., Jacobsson L., Roddy E. (2020). Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 16, 380–390. doi:10.1038/s41584-020-0441-1
Douglas P. S. (2003). The left atrium: a biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J. Am. Coll. Cardiol. 42, 1206–1207. doi:10.1016/s0735-1097(03)00956-2
Du Y., Meng Y., Zhu J., Kang L., Jia X., Guo L., et al. (2014). Quantitative proteomic study of myocardial mitochondria in urea transporter B knockout mice. Proteomics 14, 2072–2083. doi:10.1002/pmic.201400123
Dudziak M., Rawicz-Zegrzda D., Jankowska M., Dębska-Ślizień A., Wołyniec Z., Rutkowski B. (2016). Tissue Doppler imaging and intima-media thickness as noninvasive methods of cardiovascular risk stratification in patients after kidney transplantation. Transpl. Proc. 48, 1535–1538. doi:10.1016/j.transproceed.2015.12.132
Eisen A., Benderly M., Goldbourt U., Haim M. (2013). Is serum uric acid level an independent predictor of heart failure among patients with coronary artery disease? Clin. Cardiol. 36, 110–116. doi:10.1002/clc.22083
Fang X., Pan C., Chen Y., Sun M., Zhang Z., Jiang L., et al. (2017). Assessment of subclinical left ventricular changes in essential hypertensive patients with hyperuricemia: a three-dimensional speckle-tracking echocardiography study. Clin. Exp. Hypertens. 39, 93–99. doi:10.1080/10641963.2016.1210626
Gilbert R. E., Krum H. (2015). Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet 385, 2107–2117. doi:10.1016/S0140-6736(14)61402-1
Harada M., Izawa A., Hidaka H., Nakanishi K., Terasawa F., Motoki H., et al. (2017). Study Project on Prevention of Metabolic Syndrome among Children, Adolescents, and Young Adults in Shinshu. Importance of cystatin C and uric acid levels in the association of cardiometabolic risk factors in Japanese junior high school students. J. Cardiol. 69, 222–227. doi:10.1016/j.jjcc.2016.03.013
Herrmann R., Sandek A., von Haehling S., Doehner W., Schmidt H. B., Anker S. D., et al. (2012). Risk stratification in patients with chronic heart failure based on metabolic-immunological, functional and haemodynamic parameters. Int. J. Cardiol. 156, 62–68. doi:10.1016/j.ijcard.2010.10.028
Homan E. A., Reyes M. V., Hickey K. T., Morrow J. P. (2019). Clinical overview of obesity and diabetes mellitus as risk factors for atrial fibrillation and sudden cardiac death. Front. Physiol. 9, 1847. doi:10.3389/fphys.2018.01847
Inoue K., Khan F. H., Remme E. W., Ohte N., García-Izquierdo E., Chetrit M., et al. (2021). Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc Imaging 23, 61–70. doi:10.1093/ehjci/jeaa415
Jujo K., Minami Y., Haruki S., Matsue Y., Shimazaki K., Kadowaki H., et al. (2017). Persistent high blood urea nitrogen level is associated with increased risk of cardiovascular events in patients with acute heart failure. Esc. Heart Fail 4, 545–553. doi:10.1002/ehf2.12188
Lang (2016). Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of, cardiovascular imaging. Eur. Heart J. Cardiovasc Imaging 17, 412. doi:10.1093/ehjci/jew041
Lang R. M., Badano L. P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., et al. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc Imaging 16, 233–270. doi:10.1093/ehjci/jev014
Langeland S., D'hooge J., Wouters P. F., Leather H. A., Claus P., Bijnens B., et al. (2005). Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation 112, 2157–2162. doi:10.1161/CIRCULATIONAHA.105.554006
Lin J. C., Lin C. L., Chen M. C., Chang P. J., Chang S. T., Chung C. M., et al. (2015). Gout, not hyperuricemia alone, impairs left ventricular diastolic function. Arthritis Res. Ther. 17, 323. doi:10.1186/s13075-015-0842-8
Liu R., Han C., Wu D., Xia X., Gu J., Guan H., et al. (2015). Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed. Res. Int. 2015, 762820. doi:10.1155/2015/762820
Maloberti A., Mengozzi A., Russo E., Cicero A. F. G., Angeli F., Agabiti Rosei E., et al. (2023). The results of the urrah (uric acid right for heart health) project: a focus on hyperuricemia in relation to cardiovascular and kidney disease and its role in metabolic dysregulation. High. Blood Press Cardiovasc Prev. 30, 411–425. doi:10.1007/s40292-023-00602-4
Mitchell C., Rahko P. S., Blauwet L. A., Canaday B., Finstuen J. A., Foster M. C., et al. (2019). Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the american society of echocardiography. J. Am. Soc. Echocardiogr. 32, 1–64. doi:10.1016/j.echo.2018.06.004
Mondillo S., Galderisi M., Mele D., Cameli M., Lomoriello V. S., Zacà V., et al. (2011). Echocardiography Study Group of the Italian Society of Cardiology (Rome, Italy). Speckle-tracking echocardiography: a new technique for assessing myocardial function. J. Ultrasound Med. 30, 71–83. doi:10.7863/jum.2011.30.1.71
Naghavi M., Yen A. A., Lin A. W., Tanaka H., Kleis S. (2016). New indices of endothelial function measured by digital thermal monitoring of vascular reactivity: data from 6084 patients registry. Int. J. Vasc. Med. 2016, 1348028. doi:10.1155/2016/1348028
Nagueh S. F., Smiseth O. A., Appleton C. P., Byrd B. F., Dokainish H., Edvardsen T., et al. (2016). Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 29, 277–314. doi:10.1016/j.echo.2016.01.011
Oh J. K., Miranda W. R., Bird J. G., Kane G. C., Nagueh S. F. (2020). The 2016 diastolic function guideline: is it already time to revisit or revise them? JACC Cardiovasc Imaging 13, 327–335. doi:10.1016/j.jcmg.2019.12.004
Pan K. L., Lin J. C., Lin C. L., Chen M. C., Chang P. J., Hsiao J. F., et al. (2014a). The effects of gout on left atrial volume remodelling: a prospective echocardiographic study. Rheumatol. Oxf. 53, 867–874. doi:10.1093/rheumatology/ket444
Pan K. L., Lin J. C., Lin C. L., Chen M. C., Chang S. T., Chung C. M., et al. (2014b). Impact of gout on left atrial function: a prospective speckle-tracking echocardiographic study. PLoS One 9, e108357. doi:10.1371/journal.pone.0108357
Pathan F., D'Elia N., Nolan M. T., Marwick T. H., Negishi K. (2017). Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J. Am. Soc. Echocardiogr. 30, 59–70. doi:10.1016/j.echo.2016.09.007
Reant P., Dijos M., Donal E., Mignot A., Ritter P., Bordachar P., et al. (2010). Systolic time intervals as simple echocardiographic parameters of left ventricular systolic performance: correlation with ejection fraction and longitudinal two-dimensional strain. Eur. J. Echocardiogr. 11, 834–844. doi:10.1093/ejechocard/jeq084
Redon P., Maloberti A., Facchetti R., Redon J., Lurbe E., Bombelli M., et al. (2019). Gender-related differences in serum uric acid in treated hypertensive patients from central and east european countries: findings from the blood pressure control rate and cardiovascular risk profile study. J. Hypertens. 37, 380–388. doi:10.1097/HJH.0000000000001908
Singh A., Addetia K., Maffessanti F., Mor-Avi V., Lang R. M. (2017). LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging 10, 735–743. doi:10.1016/j.jcmg.2016.08.014
Stewart D. J., Langlois V., Noone D. (2019). Hyperuricemia and hypertension: links and risks. Integr. Blood Press Control 24 (12), 43–62. doi:10.2147/IBPC.S184685
Turkes G. F., Uysal S., Demir T., Demiral Y., Pamuk B. O., Yılmaz H., et al. (2021). Associations between bioavailable vitamin D and remnant cholesterol in patients with type 2 diabetes mellitus. Cureus 13, e13248. doi:10.7759/cureus.13248
Welles C. C., Ku I. A., Kwan D. M., Whooley M. A., Schiller N. B., Turakhia M. P. (2012). Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J. Am. Coll. Cardiol. 59, 673–680. doi:10.1016/j.jacc.2011.11.012
Ye Z., Miranda W. R., Yeung D. F., Kane G. C., Oh J. K. (2020). Left atrial strain in evaluation of heart failure with preserved ejection fraction. J. Am. Soc. Echocardiogr. 33, 1490–1499. doi:10.1016/j.echo.2020.07.020
Zhang X., Lu Q., Zhang Z., Chen Y., Wang Y., Wang Y., et al. (2018). Value of three-dimensional speckle tracking echocardiography to assess left ventricular function in hyperuricemia patients. Clin. Rheumatol. 37, 2539–2545. doi:10.1007/s10067-018-4132-0
Keywords: left ventricular function, gout, echocardiography, uric acid, risk factors
Citation: Dang W, Luo D, Hu J, Luo H, Xu X and Liu J (2023) Analysis of risk factors for changes of left ventricular function indexes in Chinese patients with gout by echocardiography. Front. Physiol. 14:1280178. doi: 10.3389/fphys.2023.1280178
Received: 19 August 2023; Accepted: 15 November 2023;
Published: 23 November 2023.
Edited by:
Tarik Kivrak, Firat University, TürkiyeReviewed by:
Caroline Sabin, University College London, United KingdomAlessandro Maloberti, University of Milano Bicocca, Italy
Copyright © 2023 Dang, Luo, Hu, Luo, Xu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Liu, liujiansh@126.com
†These authors have contributed equally to this work
 Wantai Dang
Wantai Dang Danling Luo2†
Danling Luo2†