- 1Clinical Medical College of Acupuncture-Moxibustion and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2School of Physical Education and Health, Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Department of Acupuncture-Moxibustion and Tuina, Shaanxi University of Chinese Medicine, Xianyang, China
Background: Recent epidemiological studies and animal experiments have highlighted the significant role of oxidative stress in the development of osteoporosis (OP). The provision of antioxidants is widely considered a fundamental strategy to combat free radical-induced stress, inhibit oxidative damage, and potentially reverse the adverse effects of oxidative stress on bone health. However, there is no consensus in the scientific literature regarding the practical effectiveness of antioxidants in OP prevention and treatment. Some studies have not shown a clear connection between antioxidant supplementation and decreased OP risk. Therefore, it is essential to clarify the potential causal relationship between antioxidants and the development of OP.
Methods: The study utilized the inverse variance weighted (IVW) approach as the primary analytical method in the Mendelian Randomization (MR) framework to investigate the causal effects of five exogenous and six endogenous antioxidants on the risk of OP. To thoroughly assess potential pleiotropic effects and heterogeneity among the data analyzed, the MR-Egger intercept test was employed, and Cochran’s Q statistic was calculated.
Results: In the evaluation of exogenous antioxidants, single-directional two-sample MR analyses did not reveal any statistically significant relationship between these agents and the risk of OP. Regarding endogenous antioxidants, bidirectional two-sample MR analyses were conducted, which generally indicated that most genetically regulated endogenous antioxidants had no significant association with the onset risk of OP. A significant causal relationship was found between OP and serum albumin levels (β: −0.0552, 95%CI: −0.0879 to −0.0225, p
Conclusion: The research uncovers OP as a possible determinant contributing to a decrement in serum albumin levels, and further suggests a potentially intimate relationship between the downward trajectory of serum albumin concentrations and the advancement of the OP disease process.
1 Introduction
In 1993, the World Health Organization (WHO) classified osteoporosis (OP) as a systemic skeletal disease. OP is characterized by low bone density, deterioration of bone tissue microstructure, increased bone fragility, and susceptibility to fracture (Genant et al., 1999). As a severe, chronic, progressive, and asymptomatic metabolic bone disease, OP is a major public health concern worldwide (LeBoff et al., 2022; Moore et al., 2023). The statistical evidence indicates that over 200 million people worldwide suffer from OP. Women over the age of 50 are particularly affected by this condition, with almost one-third being affected. Additionally, about one-fifth of men experience fractures related to osteoporosis during their lifetime (Sozen et al., 2017; Gregson et al., 2022).
In recent years, OP has become a pressing emerging health issue that requires prompt attention. It imposes considerable economic and caregiving burdens on societal healthcare resources and individual households (Mottaghi and Nasri, 2021). The pathophysiology of OP involves a dysregulation in bone metabolism, characterized by an excessive rate of bone resorption over bone formation. This, in turn, results in reduced bone mass and declining bone mineral density (BMD) (Liang et al., 2022). Historically, estrogen deficiency has been identified as a key trigger for OP. However, recent epidemiological and experimental animal studies have highlighted the significant role of oxidative stress in the OP disease process. This underscores the close relationship between bone density and the oxidative state within the organism (Manolagas, 2010; Zhang et al., 2023).
Normally, intracellular reactive oxygen species (ROS) are effectively scavenged by the body’s antioxidant systems to maintain cellular redox homeostasis (de Almeida et al., 2022). ROS act as dual-effect molecules, serving as crucial secondary messengers involved in regulating essential life processes such as apoptosis, differentiation, and proliferation at appropriate concentrations. This has significant implications for the dynamic balance between osteoblasts and osteoclasts (Zhu et al., 2022; Endale et al., 2023). However, studies have shown that aging, inflammatory responses and estrogen deficiency can increase the production of ROS. When the rate of ROS generation exceeds the body’s intrinsic antioxidant clearance capacity, it results in a state of oxidative stress (Mohamad et al., 2020; Zhu et al., 2022; Iantomasi et al., 2023) Excessive accumulation of ROS can suppress osteoblast differentiation and proliferation, while promoting osteoclast differentiation. This can cause structural disruption of bone tissue and subsequent loss of bone mass (Kimball et al., 2021). Additionally, it is noteworthy that the decline in estrogen levels often coincides with elevated levels of pro-inflammatory factors. This further exacerbates ROS production and consequently elevates the risk of OP (Zhou et al., 2021).
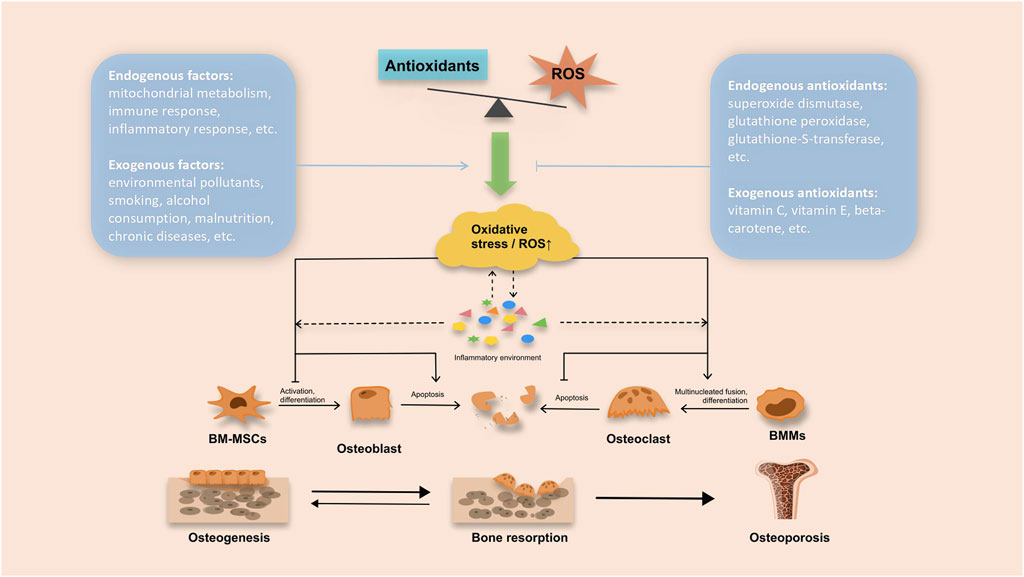
Research has shown that supplementing with antioxidants is a key approach to reducing the impact of free radicals, preventing oxidative damage, and countering the negative effects of oxidative stress on bone health (Damani et al., 2022; Davan et al., 2023). Figure 1 illustrates the oxidative stress and antioxidant mechanisms involved in the development of OP. Dietary exogenous antioxidants mainly comprise carotenoids, retinol, vitamin C, and vitamin E. On the other hand, endogenous constituents primarily consist of albumin, bilirubin, and key antioxidant enzymes such as Catalase (CAT), Superoxide Dismutase (SOD), Glutathione Peroxidase (GPX), and Glutathione S-transferase (GST) (Behera and Malik, 1989; Jomová et al., 2023). Research has shown that osteoporotic rats have notably depleted serum glutathione levels (Yalin et al., 2012; Ameen et al., 2020). Furthermore, increasing the GSH/GSSG ratio can help mitigate ROS-induced oxidative injury to osteoblasts by activating the PI3K/Akt-Nrf2 signaling cascade (Casati et al., 2019). However, when examining the relationship between antioxidant biomarkers and OP, conflicting results have been reported. One systematic appraisal found lower SOD activity in postmenopausal women with OP (Zhou et al., 2016), while a case-control investigation showed significantly higher plasma SOD activity in osteoporotic cases compared to controls (Malekian et al., 2023). The effects of dietary-exogenous antioxidants and their metabolites on OP also remain inconsistent. Numerous studies have demonstrated that exogenous antioxidants, including carotenoids, vitamin C, and vitamin E, can reduce the risk of OP (Chavan et al., 2007; Yang et al., 2008; Xu et al., 2016). In studies investigating exogenous antioxidant metabolites, Maggio observed plasma antioxidant levels in patients with osteoporosis, noting consistently lower mean plasma levels of vitamin C, vitamin E, and vitamin A in osteoporosis patients compared to the control group (Maggio et al., 2003). Additionally, Zhang et al. observed a U-shaped correlation between plasma retinol concentration and BMD, suggesting that an appropriate plasma retinol concentration may contribute to improving bone mineral status (Zhang et al., 2022). However, some studies have yielded conflicting conclusions. For instance, Barker et al.’s study failed to find a significant correlation between serum β-carotene levels and the risk of hip fractures in elderly women, indicating a lack of association between β-carotene and reduced OP or fracture risk (Barker et al., 2005). Furthermore, a substudy of the Women’s Health Initiative (WHI) examined the associations between serum levels of α-tocopherol and γ-tocopherol, dietary and total vitamin E intake, and BMD in postmenopausal women. It found no significant correlation between dietary or total vitamin E intake, serum tocopherol concentrations, and BMD (Wolf et al., 2005). Consequently, the scientific community has not yet reached a consensus on the precise role of antioxidants in the prevention and treatment of osteoporosis.
Observational studies often face complexities in discerning a direct causal relationship between antioxidants and OP due to confounding factors, potential reverse causality, limitations imposed by sample size, and measurement errors (Sekula et al., 2016). To overcome these issues, Mendelian Randomization (MR) research emerges as an ideal methodology due to its unique theoretical framework and practical advantages. This technique uses naturally occurring genetic Instrumental Variables (IVs) to indirectly infer causality between genetically determined biological exposures and health outcomes. In this study, we will use external or exogenous antioxidants and related metabolites as the exposure variable and OP as the outcome variable. Using a unidirectional two-sample MR design, we investigated whether there is a causal link between exogenous antioxidants and OP incidence. Additionally, employing a bidirectional two-sample MR approach, we assessed the relationship between endogenous antioxidants and the risk of OP. These analyses aim to provide insights into the causal relationships between antioxidants and OP, contributing to the understanding of their roles in the pathogenesis and potential prevention strategies for OP.
2 Materials and methods
2.1 Research design
Figures 2–4 provide a schematic overview of the MR design used in this study. A unidirectional two-sample MR approach was employed to evaluate the potential causal association between absolute circulating levels of exogenous antioxidants and their corresponding metabolites and the risk of OP. Additionally, a bidirectional two-sample MR strategy was utilized to investigate the causal relationship between endogenous antioxidants and OP incidence. The analysis of the MR was based on three fundamental assumptions. Firstly, the genetic variants used should be associated with the exposure. Secondly, the outcome should only be influenced by the exposure. Finally, the genetic variants should be independent of any measured or unmeasured confounders. The data used in this study were obtained from publicly available sources and were accompanied by ethical approvals and informed consent in each of the original studies from which they were derived (Richmond and Davey Smith, 2021).
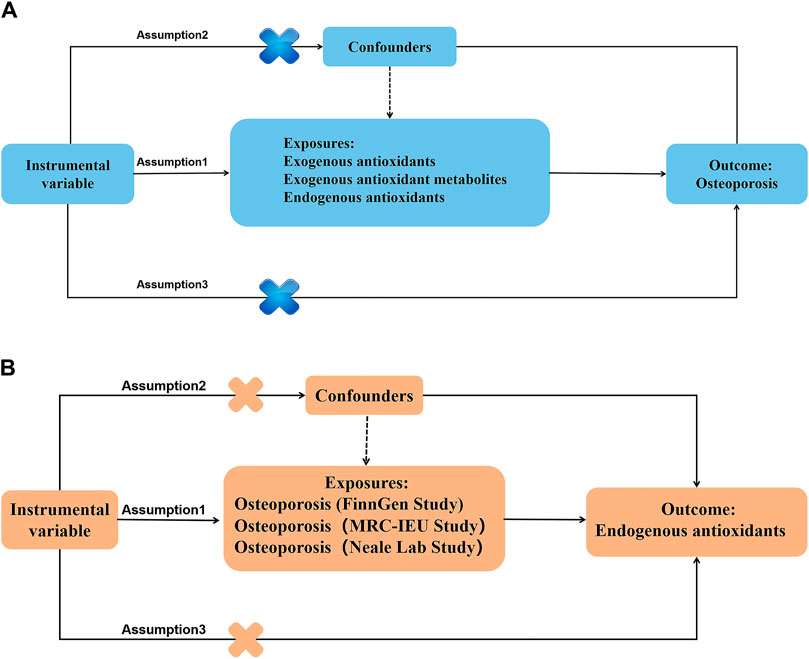
Figure 2. Main design of the study. (A) The MR hypothesis diagram of antioxidants on osteoporosis. (B) The MR hypothesis diagram of osteoporosis on endogenous antioxidants.
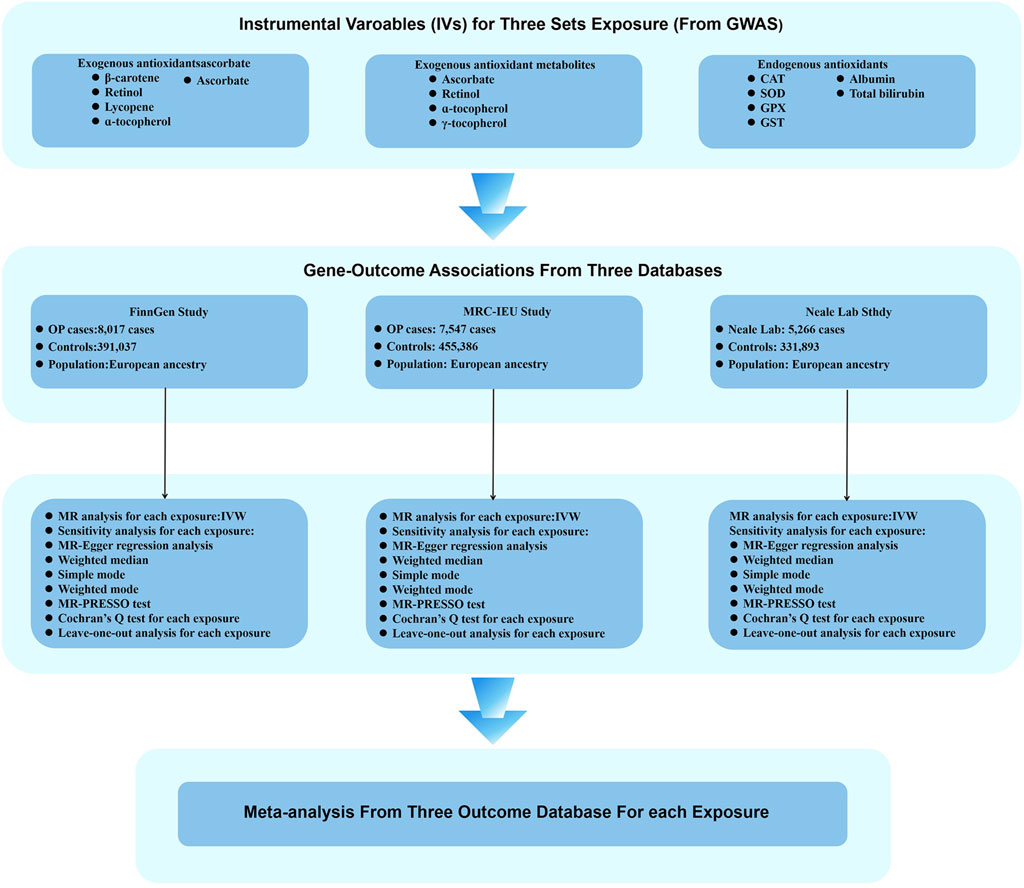
Figure 3. Flow chart illustrating the MR study design investigating the relationship between antioxidants and OP. During the exposure variable identification phase, SNPs for exogenous antioxidants, exogenous antioxidant metabolites, and endogenous antioxidants were used as IVs. In the analytical phase of assessing associations between genes and outcomes, three databases were employed as outcome variables: the FinnGen study, the MRC-IEU study, and the Neale Laboratory study. Ultimately, a meta-analysis of the MR analysis results led to the inference of a causal relationship between antioxidants and OP.
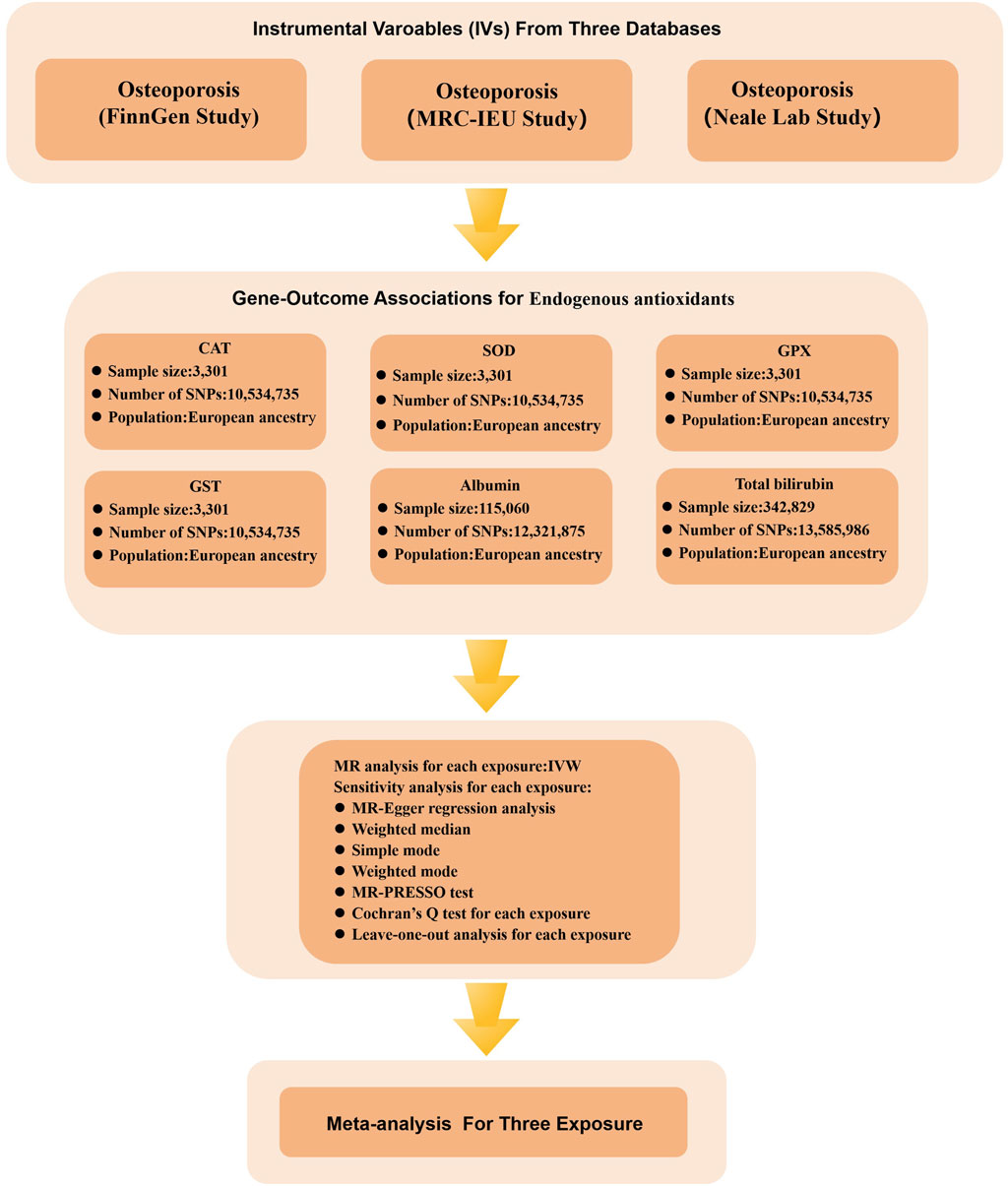
Figure 4. Flow chart illustrating the MR study design investigating the relationship between OP and endogenous antioxidants. During the exposure variable identification phase, data from three distinct sources of OP were utilized: the FinnGen Study, the MRC-IEU Study, and the Neale Lab Study. In the gene-outcome association analysis phase, six different endogenous antioxidants were employed as outcome variables. Ultimately, a meta-analysis of the MR analysis results led to the inference of a causal relationship between OP and endogenous antioxidants.
2.2 Selection of genetic instruments
This study focuses on five central dietary antioxidants and six endogenous antioxidants. The concentrations of exogenous antioxidants in blood and their related metabolites were evaluated, and their concentrations in plasma or serum were quantified using relative units. The study employed various exogenous and endogenous antioxidants, including ascorbate, β-carotene, lycopene, α-tocopherol, γ-tocopherol, retinol, CAT, SOD, GPX, GST, albumin, and total Bilirubin. The Genome-wide Association Studies (GWAS) data for OP were obtained from the GWAS database (http://gwas-api.mrcieu.ac.uk/) and the FinnGen consortium (https://www.finngen.fi/en).
To obtain IVs that satisfy the three critical assumptions necessary for robust and reliable MR analysis, we implemented a series of stringent quality control measures:
• Firstly, we identified genome-wide significant SNPs independently associated with each exposure, ensuring a direct genetic influence on the studied trait without residual confounding.
• Secondly, we eliminated linkage disequilibrium among SNPs to avoid inflated statistical associations due to non-independent genetic variants.
• Thirdly, we utilized the “get_f” functionality provided by the Get_MR software toolkit (https://github.com/HaobinZhou/Get_MR). This tool assists in computing both the squared correlation coefficient (R2) and the F-statistic, enabling us to discard weak instrumental variables. Specifically, we retained SNPs with F-statistics greater than 10 to strengthen the reliability of our genetic instruments.
• Fourthly, to ensure accurate results and mitigate issues related to ambiguous allele phasing, we also removed palindromic variants that exhibited intermediate allele frequencies.
These measures enhance the validity of our MR approach by ensuring that the selected SNPs robustly represent genetic proxies for antioxidant exposure, thereby minimizing the potential for biased or spurious results.
2.3 Data sources and SNPs selection for exogenous antioxidant biomarkers
This study extracted ten distinct SNPs related to the metabolism or biological action of ascorbate (vitamin C) from a contemporary GWAS (Zheng et al., 2020). To explore the genetic basis of β-carotene levels, we referred to a GWAS involving 2,344 participants enrolled in the Nurses’ Health Study, which yielded three genetic variants significantly associated (p
2.4 Data sources and SNPs selection for exogenous antioxidant metabolite biomarkers
Exogenous antioxidant metabolites are products formed through the metabolic processing of exogenous antioxidants within the body. Exogenous antioxidants, such as vitamins C and E, carotenoids, and lycopene, which are ingested through the diet, are transformed into various metabolites through digestion and metabolic actions. These metabolites exert antioxidant effects within the body, helping to neutralize free radicals, reduce oxidative stress, and thereby protect cells from damage (Moussa et al., 2019). In a meticulous review of peer-reviewed GWAS data, we identified a set of genetic variants displaying genomewide significance (p
2.5 Data sources and SNPs selection for endogenous antioxidant biomarkers
Six genetic predictors of endogenous antioxidants were extracted from recent state-of-the-art global genomic studies. The INTERVAL project performed a comprehensive quantitative assessment of 3,622 plasma proteins in 3,301 healthy participants, revealing 27 genetic variants associated with CAT, 23 with SOD, 22 with GPX, and 14 with GST expression (p
2.6 Data sources and SNPs selection for osteoporosis
The OP dataset was compiled by the MRC-IEU using data from the UK Biobank (Sudlow et al., 2015). It comprises 462,933 European individuals, of which 7,547 are cases and 455,386 are controls and covers 9,851,867 SNPs (ukb-b-12141). Additionally, OP-associated SNPs were obtained from the integrated dataset of the Neale Lab Consortium, which included 337,159 Europeans, of which 5,266 were cases and 331,893 were controls and covered 10,894,596 SNPs (ukb-a-87). In addition, the OP dataset of the FinnGen Consortium consists of 399,054 Europeans, including 8,017 cases and 391,037 controls. The dataset contains 19,173,961 SNPs(The dataset is publicly available at the Google Cloud Storage location gs://finngen-public-data-r10/summary_stats/finngen_R10_M13_OSTEOPOROSIS.gz).
2.7 Statistical analysis
Statistical analyses were conducted using R version 4.3.1 and software packages “TwoSampleMR,” “MRPRESSO,” and “meta.” The Inverse Variance Weighted (IVW) approach was used as the primary analytical method for MR to investigate the causal relationship between genetic variants associated with exposures and the outcome (Boehm and Zhou, 2022). To guarantee the robustness and dependability of the MR findings, we validated the consistency of these associations using supplementary methods, including MR-Egger, Weighted Median, Simple Mode, and Weighted Mode techniques (Boehm and Zhou, 2022). We evaluated heterogeneity using Cochran’s Q statistic, with a p-value
The study presented the change in natural log-transformed concentrations of β-carotene and retinol (using μg/dL and μmol/L as units respectively), α-tocopherol (measured in mg/L), lycopene (expressed in μg/dL), and ascorbate (represented in μmol/L) about the risk of OP. The absolute levels of exogenous antioxidants or their corresponding metabolite concentrations when increased tenfold were also considered. In the bidirectional analysis that examined the relationship between endogenous antioxidants and OP, we quantified the effect of a one-standard-deviation (SD) increment in the genetically predicted changes of endogenous antioxidants on the risk of OP as OR. We also assessed the potential influence of OP on the concentrations of six specific endogenous antioxidants, which were conveyed by the β coefficients.
In this study, we conducted separate MR analyses using exogenous and endogenous antioxidants as exposure factors and OP data from the MRC-IEU, Neale Lab, and FinnGen datasets as outcome variables, and subsequently meta-analysed the results of the individual studies to obtain a composite estimate of the risk of OP associated with each type of antioxidant exposure. Inversely, we also performed the corresponding MR analyses and meta-analyses using OP as an exposure factor from these three dataset sources with endogenous antioxidants as the outcome variable to derive an estimate of the possible pooled effect of OP status on endogenous antioxidant levels. During the process, we quantified the extent of heterogeneity among the estimates across different studies by computing the I2 statistic and using Cochran’s Q test to assess its significance. Depending on the results of heterogeneity testing, we used either a fixed-effect model for meta-analysis in the absence of significant heterogeneity or switched to a random-effects model when substantial heterogeneity was observed.
To mitigate the occurrence of spurious statistically significant results during multiple testing, commonly known as Type I errors, we adopted the Bonferroni correction method for multiple comparisons (VanderWeele and Mathur, 2018). Following this methodological principle, we set the significance threshold at 0.0011 (0.05/45) to determine the presence of an association between the examined variables. Results with a p-value below this threshold are considered robust and compelling evidence supporting the presence of an association. Conversely, if the p-value falls within the range of 0.0011–0.05, it is considered preliminary indicative evidence suggesting a potential causal relationship.
To ensure that our MR study had sufficient statistical power to detect the anticipated causal effect, we utilized the online MR Power Calculator developed by Simon Briscoe. When the outcome variable is continuous, Eq. 1 is used for the calculation:
Equation 1 describes the statistical power calculation used in our study, where Φ is the cumulative distribution function of the standard normal distribution, N is the sample size, R2 is the proportion of variance explained by the genetic instruments, β is the estimated effect size, σ2 is the variance of the error term, and z1−α/2 is the critical value from the normal distribution corresponding to a significance level α.
3 Results
3.1 Screening of genetic instruments
Table 1 provides a summary of the genetic instrumental attributes for exogenous and endogenous antioxidants about OP risk. All selected genetic IVs in this investigation exhibited F-statistics surpassing the threshold of 10, indicating their robustness as surrogates for antioxidant exposures. These strong instruments effectively minimized bias in the IVs estimation, thereby enabling more credible causal inferences.
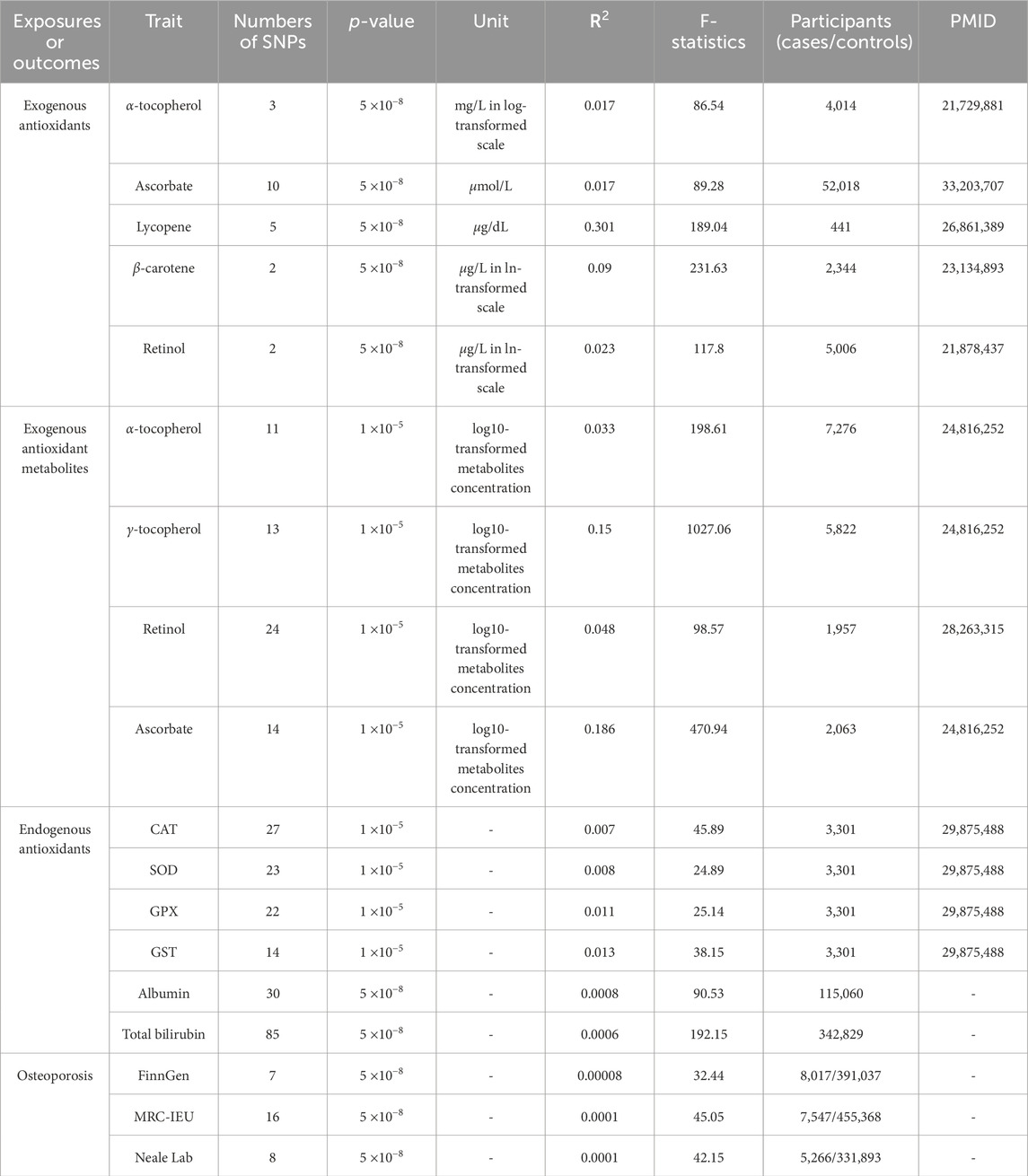
Table 1. Comprehensive information on studies and associated datasets utilized in the present investigation.
3.2 Causal impacts of exogenous antioxidants on osteoporosis risk
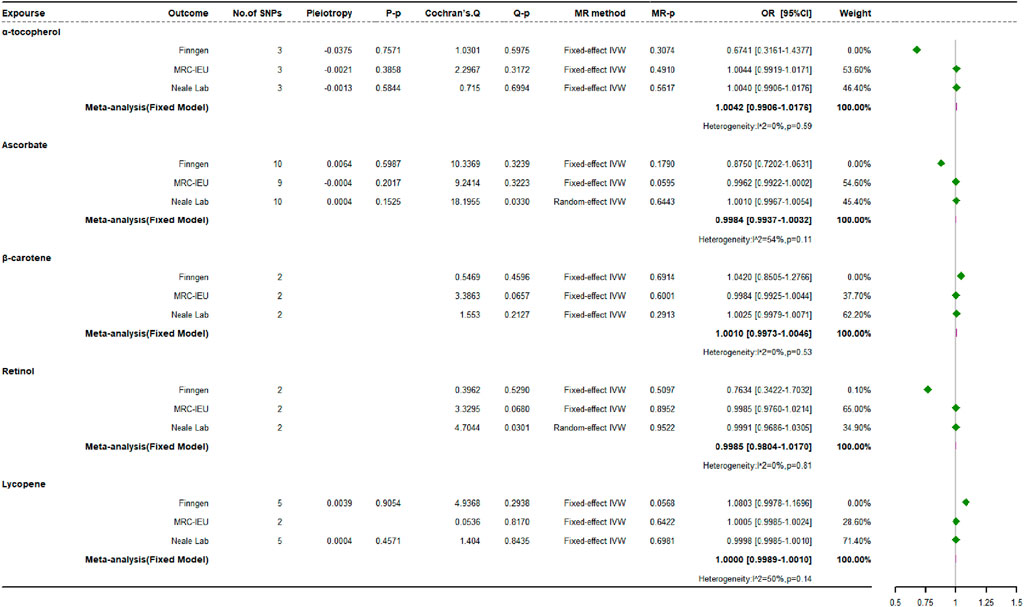
The primary IVW analysis did not reveal any significant association between genetically derived absolute levels of exogenous antioxidants and the risk of OP in any of the three databases examined (Figure 5). For the MR analysis with only two SNPs, the results of the likelihood ratio method were generally consistent with the results of the main analysis. After natural log transformation, the OR per unit increment was 1.0010 (95% CI: 0.9973 to 1.0046) for β-carotene, 0.9985 (95% CI: 0.9804 to 1.0170) for retinol, 1.0000 (95% CI: 0.9989 to 1.0010) for lycopene per 1 μg/dL, and 0.9984 (95% CI: 0.9937 to 1.0032) for ascorbate per 1 μmol/L. For each 1 mg/dL increase in α-tocopherol, the OR was 1.0042 (95% CI: 0.9906 to 1.0176).
Multiple MR methods were used to assess the impact of three antioxidants with at least three SNPs, including ascorbate, α-tocopherol, and lycopene. The methods used included MR-Egger regression, weighted median estimation, simple mode, and weighted mode analyses. The results obtained from these methods were concordant. Although no significant heterogeneity was observed across studies using Cochran’s Q test for the association between exogenous antioxidants and OP, mild heterogeneity was detected during MR analyses when ascorbate and retinol data from the Neale Lab source were utilized. However, the instrumental SNPs showed no signs of directional pleiotropy, as evidenced by MR-Egger regression analyses. The MR-PRESSO tests confirmed that there is no horizontal pleiotropy affecting the relationship between exogenous antioxidant levels and OP risk. The conclusions were further strengthened by sensitivity analyses using a Leave-one-out strategy, which showed that the observed causal relationships were not dependent on any individual SNP. Therefore, these findings are robust to the influence of specific genetic alterations.
3.3 Causal impacts of exogenous antioxidant metabolites on osteoporosis risk
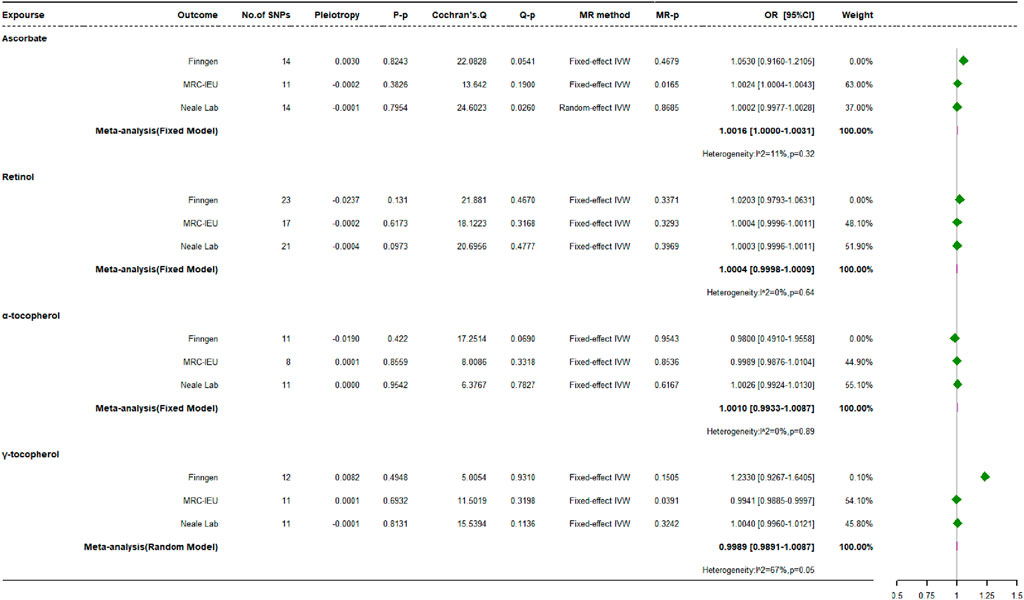
Analogous conclusions emerged from our analysis of exogenous antioxidant metabolites (Figure 6), reinforcing the findings for their parent compounds. In-depth scrutiny across three distinct databases uniformly revealed that the majority of genetically regulated exogenous antioxidant metabolites displayed no substantial correlation with the occurrence of OP. A notable exception was discovered within the MRC-IEU dataset, suggesting that genetically elevated levels of γ-tocopherol might confer protection against OP (OR: 0.9941, 95% CI: 0.9885 to 0.9970, p = 0.0391, power = 3.8%).
Various MR techniques, such as MR-Egger, weighted median, simple mode, and weighted mode approaches, were used to confirm the associations between exogenous antioxidants and OP risk. Although Cochran’s Q statistic indicated heterogeneity in the relationship between ascorbate and OP when analyzing Neale Lab data, the remaining collective analyses showed consistency. No evidence of directional pleiotropy among the IVs was detected via MR-Egger regression, nor were anomalous SNPs indicative of horizontal pleiotropy uncovered by MR-PRESSO. Additionally, the leave-one-out sensitivity analysis, which excluded one SNP at a time, confirmed that the majority of the observed causal relations were not critically dependent on any singular genetic variant.
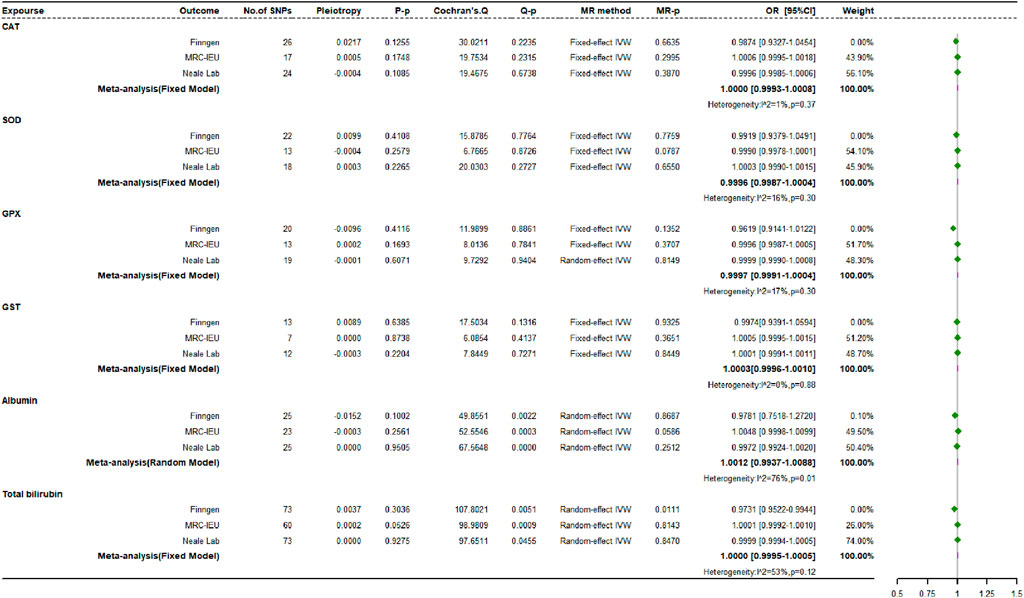
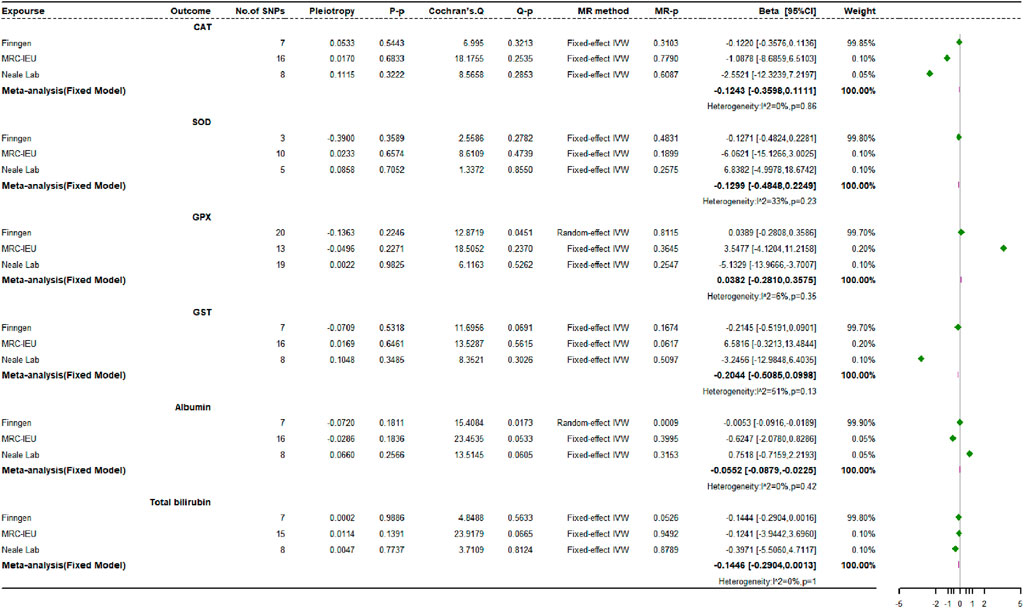
3.4 Causal impacts of endogenous antioxidants on osteoporosis risk
Figure 7 shows the MR estimates for the causal relationships between six different endogenous antioxidants and OP. Analyses across three independent databases suggest that most genetically driven endogenous antioxidants do not have significant associations with OP risk. In the FinnGen study, an exception was observed where albumin showed a positive correlation with OP incidence (OR: 1.2587, 95% CI: 1.0009 to 1.5829, p = 0.0491, power = 8.3%), while total bilirubin demonstrated a potentially protective effect, inversely correlated with OP risk (OR: 0.9753, 95% CI: 0.9522 to 0.9990, p = 0.0411, power = 2.8%).
Using various MR techniques, including MR-Egger, weighted median, simple mode, and weighted mode strategies, we replicated the association between endogenous antioxidants and OP. Our results were consistent with the IVW approach. In the MR analyses of all three datasets, albumin, and serum total bilirubin exhibited heterogeneity, while GPX showed heterogeneity when analyzing OP data from the Neale Laboratory repository. The MR-Egger regression analyses did not reveal any evidence of directional multidirectionality between IVs. Using the FinnGen dataset, MR-PRESSO identified outlier SNPs for albumin (rs28929474) and total bilirubin (rs76895963) associated with OP susceptibility. After removing these outliers, updated MR analysis showed a change in the causal relationship between albumin and OP. This indicates that the previously observed positive correlation disappeared after removing potential bias (OR: 0.9781, 95% CI: 0.7518 to 1.272, p = 0.8687, power = 2.8%). Meanwhile, the OR estimate for total bilirubin remained statistically significant with no significant change (OR: 0.9731, 95% CI: 0.9522 to 0.9944, p = 0.0111, power = 2.9%). Furthermore, leave-one-out sensitivity analysis confirmed that most of the causal associations were not solely determined by individual genetic variations.
3.5 Causal impact of osteoporosis on endogenous antioxidants
In the bidirectional MR analysis, we observed a statistically significant causal effect of OP on albumin (β: −0.0552, 95% CI: −0.0879 to −0.0225, p = 0.0009, power = 100%), which maintained significance even after adjusting for the Bonferroni correction threshold (Figure 8). However, we did not identify any causal connections between OP and the other endogenous antioxidants under scrutiny.
Various MR methods, such as MR-Egger regression, the weighted median technique, simple mode, and weighted mode evaluations, were used to investigate the potential causal link between OP and endogenous antioxidants. The results were consistent with those obtained from the IVW method. Statistical heterogeneity, as evaluated by Cochran’s Q test, was significant only in the relationship between OP cases sourced from the FinnGen dataset and the biomarkers GPX and albumin. Other genetic and phenotypic correlation analyses did not provide enough evidence to infer heterogeneity. It is important to note that MR-PRESSO analysis did not detect any outlier SNPs that could plausibly cause horizontal pleiotropy. It should be emphasized that the leave-one-out sensitivity assessment robustly confirmed that most observed causal relationships were not exclusively contingent upon any specific genetic polymorphism.
4 Discussion
In the present research, we utilized a dual-sample MR strategy to conduct a comprehensive examination of the causal relationship between both extrinsic and intrinsic antioxidants and OP. Despite the results indicating that neither the consumption of exogenous antioxidants, which include ascorbate, tocopherol, carotenoids, retinol, and lycopene, nor augmented activities within the intrinsic antioxidant defense system, composed of enzymes such as CAT, SOD, GPX, GST, serum albumin, and total bilirubin, showed a statistically discernible causal link with OP risk, our study disclosed a noteworthy observation suggesting that OP itself might act as a causative agent contributing to reduced total albumin concentrations. Additionally, exploratory analyses of data from the FinnGen and MRC-IEU repositories suggested a potential beneficial role of genetically high γ-tocopherol levels and increased serum total bilirubin in mitigating OP risk. Nonetheless, when extending our analysis through a meta-analysis incorporating data from three separate databases, we did not obtain uniform supportive evidence across these larger datasets.
In the course of biological activities, especially within cellular energy metabolism and the biosynthesis of adenosine triphosphate (ATP), the production of ROS as a byproduct of oxygen reduction is unavoidable. An overabundance of ROS can provoke oxidative stress, a condition that is broadly accepted as a key contributor to the advancement of a broad spectrum of age-related and degenerative diseases, OP being one among them (León-Reyes et al., 2023). A multitude of studies highlights the core position occupied by oxidative stress in the senescence process and the pathophysiology of OP. Antioxidants serve a vital protective function by countering ROS, preserving cellular redox equilibrium, and thereby effectively shielding cells from oxidative injury caused by ROS, thereby offering the potential for suppressing the development of OP (Damani et al., 2022; Davan et al., 2023).
Albumin is the most abundant protein in plasma and serves as an important endogenous antioxidant reservoir in response to chronic oxidative stress (Atukeren et al., 2010). Its antioxidant properties are mainly derived from the exposed thiol moiety at the Cys34 site. During oxidative stress, Cys34 can form disulfide bridges with free cysteine or glutathione, which alters the tertiary structure of human serum albumin (HSA) and effectively eliminates ROS such as hydrogen peroxide, peroxynitrite, superoxide radicals, and hypochlorous acid (Kawakami et al., 2006; Belinskaia et al., 2021). Previous observational research has presented varying perspectives on the relationship between albumin and OP risk. For instance, in a large study of 21,121 individuals with a mean age of 61 years, Afshinnia et al. found that higher serum albumin levels were negatively correlated with OP prevalence, indicating a decline in OP incidence with rising albumin (Afshinnia et al., 2016). In contrast, Rico et al. discovered in a case-control study that serum albumin levels were significantly lower in elderly women with OP compared to healthy counterparts (Rico et al., 2009). However, some studies have questioned the independent correlation between serum albumin and BMD. For instance, a study conducted in Rancho Bernardo reported a slight positive correlation between serum albumin and BMD, which disappeared after adjusting for age (Lunde et al., 1998). On the other hand, a different study conducted on healthy postmenopausal women did not find any significant correlation between serum albumin and BMD (D’Erasmo et al., 1999). The preliminary analysis of the FinnGen database in the current study suggests a potential positive association between albumin and the onset of OP. However, after removing outliers using MR-PRESSO, the causal relationship between albumin and OP no longer had statistical significance. The reverse MR analysis revealed a new causal pathway, indicating that OP may reduce serum albumin levels. It is hypothesized that this reduction in albumin levels may be linked to oxidative stress, poor nutritional status, and chronic inflammation during the course of OP (Vaughan et al., 2012; Cauley et al., 2016; Tugba et al., 2019; Xu et al., 2022). Extensive research has highlighted the significant role of oxidative stress in the pathogenesis of osteoporosis (Manolagas, 2010; Zhang et al., 2023). Oxidative stress has been identified as a significant contributor to hypoalbuminemia (Chojkier, 2005), with the potential to induce molecular modifications in human serum albumin, including carbonylation and the formation of advanced oxidation protein products and advanced glycation end-products (Michelis et al., 2010). Studies have demonstrated that the cobalt binding capacity of albumin is diminished in individuals diagnosed with osteoporosis, reflecting oxidative damage and a decline in albumin functionality (Tugba et al., 2019). Albumin, an essential protein in the bloodstream, is significantly correlated with muscle mass and strength in elderly individuals with osteoporosis, suggesting a potential nutritional link (Xu et al., 2022). Poor nutritional status is prevalent among the elderly, particularly those diagnosed with osteoporosis, which may contribute to hypoalbuminemia (Xu et al., 2022). Additionally, the synthesis of albumin may be inhibited due to the body’s response to inflammation (Vaughan et al., 2012; Cauley et al., 2016). Overall, our study provides new insights for clinical practice by establishing a causal link between serum albumin and OP. Monitoring changes in serum albumin levels could serve as a biomarker for evaluating disease progression and treatment responsiveness in OP (Fujii et al., 2018). In the future clinical handling of OP, measuring serum albumin levels could provide valuable information for early disease risk detection, tracking disease progression, and devising personalized therapeutic regimens, in addition to standard bone density measurements. However, this proposition requires further substantiation through extensive prospective studies and clinical trials to verify its practicality and effectiveness in OP management.
Bilirubin was previously considered a cytotoxic byproduct. Despite this, bilirubin has been shown to hinder osteoblast function, including growth, differentiation, and mineralization (Turkeli et al., 2008). Recent research, however, has shown that it has antioxidant potential, sometimes even surpassing certain vitamin E analogs in combating lipid peroxidation (Wu et al., 1994; Žiberna et al., 2021). A cross-sectional study of 918 postmenopausal women found a positive correlation between elevated total bilirubin levels and improved BMD as well as reduced risk of osteoporosis (Bian et al., 2013). The impact of α-tocopherol and γ-tocopherol, which are key forms of vitamin E found in the human body and diet and possess antioxidant and anti-inflammatory qualities, on skeletal metabolism remains unclear (Macdonald et al., 2004; Hamidi et al., 2012). MacDonald et al.’s research suggests that excess vitamin E intake may negatively correlate with BMD (Macdonald et al., 2004). However, another study suggests that γ-tocopherol may promote bone formation in postmenopausal women by modulating bone turnover processes (Hamidi et al., 2012). Our study, which utilized MR analyses on FinnGen and MRC-IEU datasets, has preliminarily discovered a potentially significant association between genetically higher levels of γ-tocopherol and serum total bilirubin levels, and a reduced risk of OP. This suggests a positive role for these compounds in bone health, potentially aiding in the prevention of OP due to their potent antioxidant and anti-inflammatory characteristics. Although our FinnGen and MRC-IEU analyses revealed an association, attempts to generalize this finding across multiple independent databases through a meta-analysis did not yield strong and consistent statistical support. This inconclusiveness could result from several factors, including population-specific genetic heterogeneity, sample size constraints, potential confounders, and complex interplays between environmental factors and study outcomes.
In recent MR investigations, researchers have not been able to establish a significant causal link between circulating bilirubin concentrations and either extraskeletal bone mineral density (eBMD) or the incidence of fractures. This suggests that bilirubin may not be a direct, pivotal factor affecting OP susceptibility (Zhao et al., 2021). Li’s study suggests that antioxidants β-carotene and γ-tocopherol may potentially mitigate OP risk. However, the robustness of these claims requires more rigorous examination. Li’s study used a meta-analytic approach to enhance the persuasiveness of the conclusions. However, the credibility and broad applicability of these outcomes were limited by reliance on just two discrete datasets. Of particular relevance, the extensive osteoporosis-centric research endeavor, the GEFOS database, did not reveal any statistically discernible disparities in OP risk between β-carotene and γ-tocopherol levels (Li et al., 2023).
In this research, we leveraged the extensive capabilities of large-scale MR techniques, utilizing aggregated data from GWAS involving 1,178,298 cases and controls, to pioneer a systematic inquiry into the potential causal links between exogenous antioxidants—those obtained through dietary supplements or pharmaceutical ingestion—and endogenous antioxidants with the risk of OP. A prime virtue of our methodology is the employment of genetic proxies instead of conventional interventions, thereby mitigating potential risks associated with direct antioxidant administration and mitigating issues of reverse causality between exposures and outcomes, thus enhancing the precision of causal reasoning. Additionally, the GWAS subjects in this investigation were predominantly of European descent, and we applied meticulous genomic control procedures uniformly across all studies. This greatly diminishes biases resulting from population stratification and genomic inflation effects, thereby strengthening the internal validity of our findings. In pursuit of further validation and consistency, we conducted a meta-analysis of data from three major databases. Our statistical heterogeneity assessments demonstrated that the merged findings across these three databases displayed a relatively low degree of heterogeneity, which partly attests to the robustness of our study’s conclusions.
In this study, we employ MR to explore the causal relationship between antioxidants and the risk of OP. However, it is crucial to acknowledge several limitations. Firstly, one significant limitation not addressed directly in our analysis is the intake of exogenous antioxidants, which was not measured through dietary consumption frequencies or 24-h dietary recalls. This omission might have influenced the observed associations, as dietary antioxidant intake could modulate the effects of genetically predicted antioxidant levels. Future studies should incorporate detailed dietary assessments to better control for this potential confounder. Secondly, the selection of single nucleotide polymorphisms (SNPs) in our study involved different criteria, including various p-value thresholds (e.g., p
5 Conclusion
This MR study used a large dataset and rigorous genetic analysis to provide substantial evidence for exploring the causal relationship between antioxidants and OP risk in Europeans. Although the study could not definitively confirm a statistically significant causal link between exogenous and endogenous antioxidants and OP risk, it reveals that osteoporosis may contribute to reduced serum albumin levels. This suggests that a decrease in serum albumin levels may be strongly associated with the progression of OP. Furthermore, this study indicates that monitoring dynamic fluctuations in serum albumin levels can serve as an effective biomarker for evaluating treatment efficacy and tracking the progression of OP in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YL: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Software, Visualization, Writing–original draft. HQ: Data curation, Methodology, Resources, Validation, Visualization, Writing–original draft. XH: Data curation, Formal Analysis, Software, Writing–original draft. GL: Supervision, Writing–review and editing, Investigation, Validation. HP: Supervision, Writing–review and editing, Conceptualization, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Guangdong Province (Grant No. 2024A515012151), the Guangdong Provincial Key Laboratory of Fangzheng (Grant No. 2022B212010012-3) and the Characteristic Innovation Project of Guangdong Ordinary Colleges and Universities (Grant No. 2021KTSCX237).
Acknowledgments
The authors acknowledge the participants and investigators of the UK Biobank study and FinnGen study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1411148/full#supplementary-material
References
Afshinnia F., Wong K. K., Sundaram B., Ackermann R. J., Pennathur S. (2016). Hypoalbuminemia and osteoporosis: reappraisal of a controversy. J. Clin. Endocrinol. Metabolism 101, 167–175. doi:10.1210/jc.2015-3212
Ameen O., Yassien R. I., Naguib Y. M. (2020). Activation of foxo1/sirt1/rankl/opg pathway may underlie the therapeutic effects of resveratrol on aging-dependent male osteoporosis. BMC Musculoskelet. Disord. 21, 375. doi:10.1186/s12891-020-03389-w
Atukeren P., Aydin S., Uslu E., Gumustas M., Cakatay U. (2010). Redox homeostasis of albumin in relation to alpha-lipoic acid and dihydrolipoic acid. Oxidative Med. Cell. Longev. 3, 206–213. doi:10.4161/oxim.3.3.11786
Barker M. E., McCloskey E., Saha S., Gossiel F., Charlesworth D., Powers H. J., et al. (2005). Serum retinoids and -carotene as predictors of hip and other fractures in elderly women. J. Bone Mineral Res. 20, 913–920. doi:10.1359/jbmr.050112
Behera D., Malik S. K. (1989). Combination chemotherapy in inoperable lung cancer–a trial of 2 regimens. Indian J. Chest Dis. Allied Sci. 31, 21–24.
Belinskaia D. A., Voronina P. A., Shmurak V. I., Jenkins R. O., Goncharov N. V. (2021). Serum albumin in health and disease: esterase, antioxidant, transporting and signaling properties. Int. J. Mol. Sci. 22, 10318. doi:10.3390/ijms221910318
Bian L.-Q., Li R.-Z., Zhang Z.-Y., Jin Y.-J., Kang H.-W., Fang Z.-Z., et al. (2013). Effects of total bilirubin on the prevalence of osteoporosis in postmenopausal women without potential liver disease. J. Bone Mineral Metabolism 31, 637–643. doi:10.1007/s00774-013-0452-y
Boehm F. J., Zhou X. (2022). Statistical methods for mendelian randomization in genome-wide association studies: a review. Comput. Struct. Biotechnol. J. 20, 2338–2351. doi:10.1016/j.csbj.2022.05.015
Bowden J., Del Greco M. F., Minelli C., Zhao Q., Lawlor D. A., Sheehan N. A., et al. (2018). Improving the accuracy of two-sample summary-data mendelian randomization: moving beyond the nome assumption. Int. J. Epidemiol. 48, 728–742. doi:10.1093/ije/dyy258
Casati L., Pagani F., Limonta P., Vanetti C., Stancari G., Sibilia V. (2019). Beneficial effects of -tocotrienol against oxidative stress in osteoblastic cells: studies on the mechanisms of action. Eur. J. Nutr. 59, 1975–1987. doi:10.1007/s00394-019-02047-9
Cauley J. A., Barbour K. E., Harrison S. L., Cloonan Y. K., Danielson M. E., Ensrud K. E., et al. (2016). Inflammatory markers and the risk of hip and vertebral fractures in men: the osteoporotic fractures in men (mros). J. Bone Mineral Res. 31, 2129–2138. doi:10.1002/jbmr.2905
Chavan S. N., More U., Mulgund S., Saxena V., Sontakke A. N. (2007). Effect of supplementation of vitamin c and e on oxidative stress in osteoporosis. Indian J. Clin. Biochem. 22, 101–105. doi:10.1007/bf02913324
Chojkier M. (2005). Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J. Clin. Gastroenterology 39, S143–S146. doi:10.1097/01.mcg.0000155514.17715.39
D’Adamo C. R., D’Urso A., Ryan K. A., Yerges-Armstrong L. M., Semba R. D., Steinle N. I., et al. (2016). A common variant in the setd7 gene predicts serum lycopene concentrations. Nutrients 8, 82. doi:10.3390/nu8020082
Damani J. J., De Souza M. J., VanEvery H. L., Strock N. C. A., Rogers C. J. (2022). The role of prunes in modulating inflammatory pathways to improve bone health in postmenopausal women. Adv. Nutr. 13, 1476–1492. doi:10.1093/advances/nmab162
Davan I., Fakurazi S., Alias E., Ibrahim N. I., Hwei N. M., Hassan H. (2023). Astaxanthin as a potent antioxidant for promoting bone health: an up-to-date review. Antioxidants Basel, Switz. 12, 1480. doi:10.3390/antiox12071480
de Almeida A. J. P. O., de Oliveira J. C. P. L., da Silva Pontes L. V., de Souza Júnior J. F., Gonçalves T. A. F., Dantas S. H., et al. (2022). Ros: basic concepts, sources, cellular signaling, and its implications in aging pathways. Oxidative Med. Cell. Longev. 2022, 1225578–1225623. doi:10.1155/2022/1225578
D’Erasmo E., Pisani D., Ragno A., Raejntroph N., Letizia C., Acca M. (1999). Relationship between serum albumin and bone mineral density in postmenopausal women and in patients with hypoalbuminemia. Hormone Metabolic Res. = Hormon- Und Stoffwechselforschung = Hormones Metabolisme 31, 385–388. doi:10.1055/s-2007-978760
Endale H. T., Tesfaye W., Mengstie T. A. (2023). Ros induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 11, 1226044. doi:10.3389/fcell.2023.1226044
Fujii R., Ueyama J., Aoi A., Ichino N., Osakabe K., Sugimoto K., et al. (2018). Oxidized human serum albumin as a possible correlation factor for atherosclerosis in a rural Japanese population: the results of the yakumo study. Environ. Health Prev. Med. 23, 1. doi:10.1186/s12199-017-0690-z
Genant H. K., Cooper C., Poor G., Reid I., Ehrlich G., Kanis J., et al. (1999). Interim report and recommendations of the world health organization task-force for osteoporosis. Osteoporosis 10, 259–264. doi:10.1007/s001980050224
Gregson C. L., Armstrong D. J., Bowden J., Cooper C., Edwards J., Gittoes N. J. L., et al. (2022). UK clinical guideline for the prevention and treatment of osteoporosis. Archives Osteoporos. 17, 58. doi:10.1007/s11657-022-01061-5
Hamidi M. S., Corey P. N., Cheung A. M. (2012). Effects of vitamin e on bone turnover markers among us postmenopausal women. J. Bone Mineral Res. 27, 1368–1380. doi:10.1002/jbmr.1566
Hendrickson S. J., Hazra A., Chen C., Eliassen A. H., Kraft P., Rosner B. A., et al. (2012). β-Carotene 15,15'-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of European descent. Am. J. Clin. Nutr. 96, 1379–1389. doi:10.3945/ajcn.112.034934
Iantomasi T., Romagnoli C., Palmini G., Donati S., Falsetti I., Miglietta F., et al. (2023). Oxidative stress and inflammation in osteoporosis: molecular mechanisms involved and the relationship with micrornas. Int. J. Mol. Sci. 24, 3772. doi:10.3390/ijms24043772
Jomová K., Raptova R., Alomar S. Y., Alwasel S., Nepovimova E., Kuca K., et al. (2023). Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Archives Toxicol. 97, 2499–2574. doi:10.1007/s00204-023-03562-9
Kawakami A., Kubota K., Yamada N., Tagami U., Takehana K., Sonaka I., et al. (2006). Identification and characterization of oxidized human serum albumin. A slight structural change impairs its ligand-binding and antioxidant functions. FEBS J. 273, 3346–3357. doi:10.1111/j.1742-4658.2006.05341.x
Kimball J. S., Johnson J. P., Carlson D. A. (2021). Oxidative stress and osteoporosis. J. Bone Jt. Surg. Am. Volume 103, 1451–1461. doi:10.2106/JBJS.20.00989
LeBoff M. S., Greenspan S. L., Insogna K. L., Lewiecki E. M., Saag K. G., Singer A. J., et al. (2022). The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 33, 2049–2102. doi:10.1007/s00198-021-05900-y
León-Reyes G., Argoty-Pantoja A. D., Becerra-Cervera A., López-Montoya P., Rivera-Paredez B., Velázquez-Cruz R. (2023). Oxidative-stress-related genes in osteoporosis: a systematic review. Antioxidants Basel, Switz. 12, 915. doi:10.3390/antiox12040915
Li H., Chen L., Yuan C., Yang H., Ma Z., Zuo J. (2023). Diet-derived antioxidants and osteoporosis: a mendelian randomization study. PloS One 18, e0293145. doi:10.1371/journal.pone.0293145
Liang B., Burley G., Lin S., Shi Y.-C. (2022). Osteoporosis pathogenesis and treatment: existing and emerging avenues. Cell. Mol. Biol. Lett. 27, 72. doi:10.1186/s11658-022-00371-3
Long T., Hicks M., Yu H.-C., Biggs W. H., Kirkness E. F., Menni C., et al. (2017). Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat. Genet. 49, 568–578. doi:10.1038/ng.3809
Lunde A. V., Barrett-Connor E., Morton D. J. (1998). Serum albumin and bone mineral density in healthy older men and women: the rancho bernardo study. Osteoporos. Int. 8, 547–551. doi:10.1007/s001980050097
Macdonald H. M., New S. A., Golden M. H., Campbell M. K., Reid D. M. (2004). Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am. J. Clin. Nutr. 79, 155–165. doi:10.1093/ajcn/79.1.155
Maggio D., Barabani M., Pierandrei M., Polidori M. C., Catani M., Mecocci P., et al. (2003). Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J. Clin. Endocrinol. Metabolism 88, 1523–1527. doi:10.1210/jc.2002-021496
Major J. M., Yu K., Wheeler W., Zhang H., Cornelis M. C., Wright M. E., et al. (2011). Genome-wide association study identifies common variants associated with circulating vitamin e levels. Hum. Mol. Genet. 20, 3876–3883. doi:10.1093/hmg/ddr296
Malekian S., Mirghafourvand M., Najafipour F., Ostadrahimi A., Ghassab-Abdollahi N., Farshbaf-Khalili A. (2023). The associations between bone mineral density and oxidative stress biomarkers in postmenopausal women. Korean J. Fam. Med. 44, 95–101. doi:10.4082/kjfm.22.0022
Manolagas S. C. (2010). From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 31, 266–300. doi:10.1210/er.2009-0024
Michelis R., Kristal B., Snitkovsky T., Sela S. (2010). Oxidative modifications impair albumin quantification. Biochem. Biophysical Res. Commun. 401, 137–142. doi:10.1016/j.bbrc.2010.09.027
Mohamad N.-V., Ima-Nirwana S., Chin K.-Y. (2020). Are oxidative stress and inflammation mediators of bone loss due to estrogen deficiency? a review of current evidence. Endocr. Metabolic Immune Disord. - Drug Targets 20, 1478–1487. doi:10.2174/1871530320666200604160614
Mondul A. M., Yu K., Wheeler W., Zhang H., Weinstein S. J., Major J. M., et al. (2011). Genome-wide association study of circulating retinol levels. Hum. Mol. Genet. 20, 4724–4731. doi:10.1093/hmg/ddr387
Moore A. E., Dulnoan D., Voong K., Ayis S., Mangelis A., Gorska R., et al. (2023). The additive effect of vitamin k supplementation and bisphosphonate on fracture risk in post-menopausal osteoporosis: a randomised placebo controlled trial. Archives Osteoporos. 18, 83. doi:10.1007/s11657-023-01288-w
Mottaghi P., Nasri P. (2021). Antioxidant and bone; protect your future: a brief review. Iran. J. Public Health 50, 1783–1788. doi:10.18502/ijph.v50i9.7049
Moussa Z., Judeh Z., Ahmed S. A. (2019). “Nonenzymatic exogenous and endogenous antioxidants,” in Free radical medicine and biology. doi:10.5772/intechopen.87778
Richmond R. C., Davey Smith G. (2021). Mendelian randomization: concepts and scope. Cold Spring Harb. Perspect. Med. 12, a040501. doi:10.1101/cshperspect.a040501
Rico H., Revilla M., Villa L. F., Hernandez E. R., Fernandez J. P. (2009). Crush fracture syndrome in senile osteoporosis: a nutritional consequence? J. Bone Mineral Res. 7, 317–319. doi:10.1002/jbmr.5650070311
Sekula P., Del Greco M., Pattaro C., Köttgen A. (2016). Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. 27, 3253–3265. doi:10.1681/ASN.2016010098
Shin S.-Y., Fauman E. B., Petersen A.-K., Krumsiek J., Santos R., Huang J., et al. (2014). An atlas of genetic influences on human blood metabolites. Nat. Genet. 46, 543–550. doi:10.1038/ng.2982
Sozen T., Ozisik L., Calik Basaran N. (2017). An overview and management of osteoporosis. Eur. J. Rheumatology 4, 46–56. doi:10.5152/eurjrheum.2016.048
Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., et al. (2015). UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Med. 12, e1001779. doi:10.1371/journal.pmed.1001779
Sun B. B., Maranville J. C., Peters J. E., Stacey D., Staley J. R., Blackshaw J., et al. (2018). Genomic atlas of the human plasma proteome. Nature 558, 73–79. doi:10.1038/s41586-018-0175-2
Tugba A., Alisik M., Koyuncu P., Nacir B., Ayhan F. (2019). Fri0476 ischemia modified albumin in osteoporosis. Ann. Rheumatic Dis. 78, 934. doi:10.1136/annrheumdis-2019-eular.8042
Turkeli M., Dursun H., Albayrak F., Okçu N., Uyanik M. H., Uyanik A., et al. (2008). Effects of cirrhosis on bone mineral density and bone metabolism. Eurasian J. Med. 40, 18–24.
VanderWeele T. J., Mathur M. B. (2018). Some desirable properties of the bonferroni correction: is the bonferroni correction really so bad? Am. J. Epidemiol. 188, 617–618. doi:10.1093/aje/kwy250
Vaughan V. C., Martin P., Lewandowski P. A. (2012). Cancer cachexia: impact, mechanisms and emerging treatments. J. Cachexia, Sarcopenia Muscle 4, 95–109. doi:10.1007/s13539-012-0087-1
Verbanck M., Chen C.-Y., Neale B., Do R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698. doi:10.1038/s41588-018-0099-7
Wolf R. L., Cauley J. A., Pettinger M., Jackson R. D., LaCroix A. Z., LeBoff M. S., et al. (2005). Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the women’s health initiative. Am. J. Clin. Nutr. 82, 581–588. doi:10.1093/ajcn.82.3.581
Wu T.-W., Fung K.-P., Yang C.-C. (1994). Unconjugated bilirubin inhibits the oxidation of human low density lipoprotein better than trolox. Life Sci. 54, PL477–PL481. doi:10.1016/0024-3205(94)90140-6
Xu B., Guo Z.-L., Jiang B., Zhang K., Zhu W., Lian X., et al. (2022). Factors affecting sarcopenia in older patients with chronic diseases. Ann. Palliat. Med. 11, 972–983. doi:10.21037/apm-22-201
Xu J., Song C., Song X., Zhang X., Li X. (2016). Carotenoids and risk of fracture: a meta-analysis of observational studies. Oncotarget 8, 2391–2399. doi:10.18632/oncotarget.13678
Yalin S., Sagir O., Comelekoglu U., Berköz M., Eroglu P. (2012). Strontium ranelate treatment improves oxidative damage in osteoporotic rat model. Pharmacol. Rep. 64, 396–402. doi:10.1016/s1734-1140(12)70780-6
Yang Z., Zhang Z., Penniston K. L., Binkley N., Tanumihardjo S. A. (2008). Serum carotenoid concentrations in postmenopausal women from the United States with and without osteoporosis. Int. J. Vitam. Nutr. Res. 78, 105–111. doi:10.1024/0300-9831.78.3.105
Zhang C., Li H., Li J., Hu J., Yang K., Tao L. (2023). Oxidative stress: a common pathological state in a high-risk population for osteoporosis. Biomed. Pharmacother. 163, 114834. doi:10.1016/j.biopha.2023.114834
Zhang X., Huang J., Zhou Y., Hong Z., Lin X., Chen S., et al. (2022). Vitamin a nutritional status is a key determinant of bone mass in children. Nutrients 14, 4694. doi:10.3390/nu14214694
Zhao J., Zhang M., Quan Z., Deng L., Li Y., He B. (2021). Systematic influence of circulating bilirubin levels on osteoporosis. Front. Endocrinol. 12, 719920. doi:10.3389/fendo.2021.719920
Zheng J.-S., Luan J., Sofianopoulou E., Imamura F., Stewart I. D., Day F. R., et al. (2020). Plasma vitamin c and type 2 diabetes: genome-wide association study and mendelian randomization analysis in european populations. Diabetes Care 44, 98–106. doi:10.2337/dc20-1328
Zhou Q., Zhu L., Zhang D., Li N., Li Q., Dai P., et al. (2016). Oxidative stress-related biomarkers in postmenopausal osteoporosis: a systematic review and meta-analyses. Dis. Markers 2016, 7067984–7068012. doi:10.1155/2016/7067984
Zhou X., Yuan W., Xiong X., Zhang Z., Liu J., Zheng Y., et al. (2021). Ho-1 in bone biology: potential therapeutic strategies for osteoporosis. Front. Cell Dev. Biol. 9, 791585. doi:10.3389/fcell.2021.791585
Zhu C., Shen S., Zhang S., Huang M., Zhang L., Chen X. (2022). Autophagy in bone remodeling: a regulator of oxidative stress. Front. Endocrinol. 13, 898634. doi:10.3389/fendo.2022.898634
Keywords: oxidative stress, endogenous antioxidant, exogenous antioxidant, osteoporosis, albumin, Mendelian Randomization analysis
Citation: Li Y, Qi H, Huang X, Lu G and Pan H (2024) Exogenous and endogenous antioxidants in osteoporosis risk: causal associations unveiled by Mendelian Randomization analysis. Front. Physiol. 15:1411148. doi: 10.3389/fphys.2024.1411148
Received: 02 April 2024; Accepted: 16 May 2024;
Published: 31 May 2024.
Edited by:
Gael Y. Rochefort, Université de Tours, FranceReviewed by:
Lin Bo, Second Affiliated Hospital of Soochow University, ChinaDongsheng Di, Huazhong University of Science and Technology, China
Rafael Velazquez-Cruz, National Institute of Genomic Medicine (INMEGEN), Mexico
Copyright © 2024 Li, Qi, Huang, Lu and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huashan Pan, phs681011@126.com; Gang Lu, lg20202110148@126.com
 Yuancheng Li
Yuancheng Li Huaqian Qi
Huaqian Qi Xin Huang2
Xin Huang2 Gang Lu
Gang Lu Huashan Pan
Huashan Pan