1
Department of Biochemistry, Weill Medical College of Cornell University, New York, NY, USA
2
Weill-Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD Program, New York, NY, USA
Dopaminergic (DA) neurons represent <0.01% of neurons in the human brain, but are essential for normal neurological and psychiatric function. The majority of these neurons reside in the ventral midbrain, but they exert their profound influences on brain function through projections to both the cortex and the basal ganglia. These projections secrete dopamine from small clear synaptic vesicles (SVs) in axonal varicosities. DA signaling has unique spatial and temporal characteristics as compared to the fast, focal synaptic transmission of excitatory and inhibitory neurons. However, as with fast-acting neurotransmitters, DA SVs must be locally recycled for use following exocytosis. Little is known about these DA SV recycling properties and how they might impact efficacy of DA neurotransmission. Here we used the pH-sensitive fluorescent probe synaptopHluorin to investigate SV recycling in DA neurons and compared their properties to prototypical fast neurotransmitter synapses of the hippocampus. These measurements showed that DA SVs, like hippocampal SVs, have a resting pH of ∼5.6. However, compared to hippocampal neurons, DA neurons show limited depletion of the recycling pool of vesicles as the stimulus frequency is increased from 5 to 30 Hz. Additional measurements show that exocytosis rates at this frequency are comparable between hippocampal and DA neurons. Thus, limited vesicle depletion likely arises from a stimulus frequency-dependent acceleration of DA SV endocytosis or re-acidification. Our observations imply differential regulation of endocytic–exocytic balance in DA neurons. Finally, our assay can also be used to investigate the effects of genetic and chemical modulation of the SV cycle.
The human brain contains only ∼600,000 dopaminergic (DA) neurons (Vernier et al., 2004
), which constitute <0.01% of all CNS neurons but are essential for normal neurological and psychiatric function. DA controls important mammalian functions such as voluntary movement, attention and motivation, and reward-mediated learning (Wise, 2004
). The importance of modulation of DA levels is evidenced by disease states caused by the deficiency (e.g., Parkinson’s disease) or excess (e.g., drugs of abuse) of DA signaling. DA release is typically assessed by measuring either the rate of DA neuron firing or by measuring the concentration of released DA with electrochemical techniques of amperometry and cyclic voltammetry. However, these methods generally do not directly examine the processes of vesicle fusion and retrieval or allow the study of individual presynaptic terminals. On the other hand, optical techniques using fluorescent dyes and proteins can be used to study synaptic vesicle (SV) recycling at individual presynaptic terminals (Khvotchev and Kavalali, 2008
). Such imaging techniques have been used to study vesicle recycling at several synapses such as the rodent hippocampus (Sankaranarayanan and Ryan, 2000
), olfactory bulb (Bozza et al., 2004
), and NMJ (Tabares et al., 2007
), the frog NMJ (Betz and Bewick, 1992
), the Drosophila NMJ (Poskanzer et al., 2003
), and C. elegans motor neurons (Dittman and Kaplan, 2006
). These studies have proven to be valuable in two respects. First, they have defined the time required for vesicle endocytosis (Granseth et al., 2006
; Sankaranarayanan and Ryan, 2000
), the vesicular pH (Sankaranarayanan et al., 2000
) and rate of re-acidification (Atluri and Ryan, 2006
), and vesicle pool depletion during sustained activity (Fernández-Alfonso and Ryan, 2004
). Secondly, they have provided an assay for investigating the roles of presynaptic proteins (Di Paolo et al., 2002
; Ferguson et al., 2007
; Nicholson-Tomishima and Ryan, 2004
; Poskanzer et al., 2003
), lipids (Di Paolo et al., 2004
; Kim et al., 2002
; Mani et al., 2007
), and ions (Balaji et al., 2008
; Sankaranarayanan and Ryan, 2001
) in the process of SV recycling. Finally, two groups have also demonstrated depolarization or stimulus-dependent uptake and release of FM dye in DA neurons (Jomphe et al., 2005
; Pothos et al., 1998
), implying that DA vesicle recycling is detectable with these methods.
Given the advantages of optical assays and their potential for complementing electrochemical measurements of DA release, we aimed to establish an optical assay to study kinetics of the different steps in vesicle recycling. Here we report the first such measurements at DA presynaptic terminals. We modified a primary culture method for DA neurons and used a combination of synaptopHluorin (spH, Sankaranarayanan and Ryan, 2000
) and FM 4-64 dye uptake and re-release assays (Ryan and Smith, 1995
) to examine SV recycling. We found that spH is resident both on the axonal surface and within recycling vesicles, and that expression of the exogenous protein does not alter native rates of vesicle exocytosis. We also found that DA SVs are acidic, with an internal pH of ∼5.6. DA neurons demonstrated an easily detectable increase in fluorescence in response to electrical stimulation and a decline in fluorescence after the stimulus train, implying that the assay was capable of detecting DA vesicle fusion and retrieval. We then compared DA neuron properties to those of hippocampal neurons and found that increasing the stimulus frequency six-fold from 5 to 30 Hz causes limited depletion of the recycling vesicle pool in DA neurons. We also found no difference in the rates of vesicle exocytosis, suggesting that faster endocytosis or re-acidification may be the reason for limited DA vesicle depletion at 30 Hz. Our findings demonstrate the feasibility of optical measurements of vesicle recycling in live DA neurons and identify a novel feature of DA endocytic–exocytic coupling. These results can be used as a basis for investigation of the effects of pharmacological or genetic perturbations at DA synapses.
Cell Culture, Transfection, and Experimental Conditions
Weill Medical College’s Institutional Animal Care and Use Committee (IACUC) approved all animal experiments. Dissociated primary DA neuron cultures were prepared as previously described (Rayport et al., 1992
; St-Gélais et al., 2004
), with the modifications stated below. DA neurons were dissected from the ventral midbrain (substantia nigra and ventral tegmental area) of post-natal day one Sprague-Dawley rats and placed in ice-cold phosphate buffered saline (1× PBS). Brain pieces were incubated with papain (Worthington Biochemical Corp., NJ, USA) for 10 min at 35°C under humidified oxygenation. Cells were then dissociated, washed twice, and plated in the following medium (v/v): 60% Neurobasal-A, 30% Basal Media Eagle, 10% fetal bovine serum (Atlanta Biologicals, GA, USA). The growth medium was supplemented with 1× B-27, 2 mM GlutaMAX-1, and 10 ng/mL glial cell line-derived neurotrophic factor (Millipore, MA, USA). Neurons were either plated onto rat astrocyte monolayers or poly-ornithine coated coverslips at a density of 200,000 cells/cm2. At 1.5 days in vitro (DIV), neurons were transfected with the spH plasmid (a gift from Dr. James Rothman, Yale University) using calcium phosphate-mediated gene transfer. Experiments were typically conducted at 14–40 DIV under previously described buffer and stimulus conditions (Sankaranarayanan and Ryan, 2000
), with a perfusion rate of ∼1.5 mL/min. Bafilomycin A1 (Calbiochem/EMD Biosciences, CA, USA) was used at a concentration of 0.3–1 μM. All reagents were from Invitrogen Corp., CA, USA, unless otherwise specified.
Immunocytochemistry
Neurons expressing spH that were used for experimentation were always verified to be dopaminergic by post-experiment immunostaining for DA neuron-specific marker tyrosine hydroxylase (TH). Cultures were fixed for 15 min with PBS containing 4% paraformaldehyde (EMS, PA, USA) and 4% sucrose (w/v). They were permeabilized in PBS containing 0.2% Triton (v/v) for 10 min. After a 30-min incubation in blocking solution (10% BSA in PBS, w/v), cells were incubated for 1 h with a monoclonal anti-TH antibody (Calbiochem, CA, USA) at 1:1000 dilution in PBS containing 1% BSA (w/v). After a 5-min wash in the same medium, cells were incubated for 1 h with 1:500 dilutions of Alexa Fluor® 546 goat anti-mouse antibody, and an anti-GFP, Alexa Fluor® 488 conjugate (Invitrogen, CA, USA). Cells were washed two times for 5 min prior to imaging. All steps were completed at room temperature.
Confocal Microscopy and Analysis
Laser-scanning fluorescence images were acquired using a custom-built laser-scanning microscope, through a 40 × 1.3 numerical aperture Zeiss Fluar objective. Specimens were illuminated with ∼45 μW of the 488 nm line of an argon ion laser that was rapidly shuttered during all non-data-acquiring periods using acousto-optic or electro-optic modulation. The sequential scanning time for a single frame was ∼1 s, and the time courses of spH and FM responses were measured from time-lapse images taken every 3 s. Images were acquired for at least 15 s prior to the start of the stimulus (time 0 s in all experiments, unless otherwise specified). Quantitative measurements of fluorescence intensity at individual presynaptic terminals were obtained by averaging square regions of interest (ROI) of side length 0.44 μm (four pixels). Individual ROIs were selected by hand, and the optical center of mass used to center the measurement box was computed over a slightly larger area (typically 16 × 16 pixels). Each experiment represents the average behavior of 10–80 presynaptic terminals.
Wide-Field Microscopy and Analysis
A subset of spH experiments was conducted with a highly sensitive, back-illuminated EM-CCD camera (iXon+ Model # DU-897E-BV, Andor Corp., CT, USA). An epifluorescence microscope (Zeiss Axiovert 200) was modified to use laser illumination. The 488-nm argon ion laser beam was sufficiently expanded to get near uniform illumination, and was shuttered using acousto-optic modulation in all non-data-acquiring periods. Fluorescence excitation and collection were done through a 40× 1.3 NA Fluar Zeiss objective using a 515–560 nm emission filter and a 510-nm dichroic filter. Images were acquired every 500 ms, with integration times of 50–80 ms. Images were acquired for at least 15 s prior to the start of the stimulus (time 0 s in all experiments, unless otherwise specified). Circular ROIs with a diameter of 2.5 μm were used for measurement of fluorescence intensities at presynaptic varicosities.
Fluorescence Response Analysis
In the FM experiment (Figure 1
D), responses were normalized to the maximum change in fluorescence (ΔF) observed with stimulus-driven re-release of FM dye.
The spH responses in Figures 3 and 4
were normalized to the size of the recycling pool of vesicles, which was measured as the ΔF elicited by a large stimulus (1500–4500 AP) in the presence of bafilomycin A1, a v-type ATPase inhibitor that blocks vesicle re-acidification (Sankaranarayanan and Ryan, 2001
). The spH response saturated under these conditions and subsequent stimulation did not elicit a further increase in fluorescence. This saturation indicates that all recycling vesicles have undergone at least one round of fusion; thus, the ΔF in this experiment offers a measure of the size of the recycling pool.
If we encountered spontaneous vesicle alkalinization during measurement of exocytosis rates, a simple linear correction was applied to account for it, as described previously (Sankaranarayanan and Ryan, 2001
). If the linear correction was inadequate or excessive, ostensibly because it was not a good approximation for alkalinization rates that varied over time, we excluded such boutons from analysis.
To establish dissociated primary cultures with a high percentage of DA neurons for our studies, we first optimized dissection, culture, and transfection techniques. We dissected the ventral midbrain region (substantia nigra and ventral tegmental area) of p0-1 rats. We used a method gleaned from previously described dissection and culture techniques (Rayport et al., 1992
; St-Gélais et al., 2004
), with two key modifications. First, we dissected a very narrow (<1 mm) region in the ventral midbrain in order to minimize contamination with non-DA neurons from adjoining regions. With this parsimonious dissection in a large number of animals, we were able to obtain 60–80% DA neurons in our cultures. We also plated very high density cultures (200,000 cells/cm2), since a large fraction of neurons did not survive transfection or the ∼2-week incubation period required for synapse maturation. With these steps, we have both sufficient numbers of surviving cultures and a high percentage of DA neurons, ensuring experimental efficiency. We then optimized a strategy for transient transfection of spH into DA neurons, ultimately using calcium-phosphate gene transfer at 1.5 DIV (see Section ‘Materials and Methods’). The transfected probe spH is a fusion protein consisting of a pH-sensitive mutant of GFP (pKa ∼7.1, Sankaranarayanan et al., 2000
) fused to the lumenal end of integral vesicle membrane protein VAMP-2 (Miesenböck et al. 1998
). spH fluorescence is quenched when exposed to the internal acidic vesicle pH but undergoes an increase upon exocytosis and exposure to the external buffer at pH 7.4 (Sankaranarayanan et al., 2000
). The fluorescence is quenched again upon endocytosis and re-acidification (Sankaranarayanan and Ryan, 2000
).
SynaptopHluorin is Expressed at Presynaptic Terminals of Dopaminergic Neurons without Perturbation of Native Vesicle Exocytosis
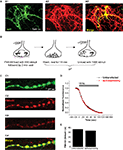
We found that spH was expressed in the soma, dendrites, and axons of DA neurons (Figure 1
A). Within the axon, spH co-localized with areas where FM dye could be loaded and released in an activity-dependent manner (Figures 1
B, 1
C1–C4); this implies that spH was present at presynaptic terminals containing recycling vesicles. Since the spH assay involves transient expression of an exogenous protein, we wanted to ensure that it did not perturb native vesicle recycling properties in these neurons. We therefore compared stimulus-driven FM dye unloading between transfected and untransfected DA neurons, and found close overlap between the FM release traces (Figure 1
D). The time constants obtained by first-order exponential fit to the traces were also similar (Figure 1
E). Our results imply that spH expression does not alter the rate of vesicle exocytosis, similar to previously reported results in hippocampal neurons (Sankaranarayanan and Ryan, 2000
).
Figure 1. SynaptopHluorin is expressed at presynaptic terminals of dopaminergic neurons without perturbation of native vesicle exocytosis. (A) SynaptopHluorin (spH), a fusion protein consisting of VAMP-2 tagged with pHluorin (GFP modified for greater pH sensitivity, Miesenböck et al., 1998
), was transfected into primary cultures of DA neurons dissected from the ventral midbrain of neonatal rats. spH is expressed in both the soma and neurites. Shown here is spH (A1) in a DA neuron identified by immunostaining for DA-specific marker tyrosine hydroxylase (A2). Merge (A3). Scale bar, 5 μm. (B) Protocol for FM dye loading and unloading. Neurons were stimulated at 10 Hz for 60 s in the presence of FM4-64 dye. The dye remained in the bath for 2 min after the end of the stimulus to allow complete endocytosis, and was then rapidly removed by a 4-min wash with 1 mM Advasep-7, a dye chelator. After another 10-min wash, neurons were stimulated for 100 s at 10 Hz to release loaded dye, and the kinetics of dye release were measured. (C) In DA neurons, spH (C1) is present at presynaptic terminals that contain recycling synaptic vesicles. This was verified by the stimulus-dependent uptake (C2) and release (C3) of tracer dye FM4-64 as described in (B). spH and FM4-64 co-localization (C4). Scale bar, 2 μm. (D) spH expression does not alter the rate of vesicle exocytosis. The average kinetics of FM4-64 release are similar in untransfected (black) and spH-expressing DA neurons (red). (E) The average time constants obtained by fitting each FM decay to a first-order exponential were similar. Data averaged from seven DA and seven hippocampal neurons, each with 10–50 presynaptic terminals. Error bars are SEM.
The Internal Vesicle pH and Synaptic Distribution of spH are Similar Between DA and Hippocampal Synapses
We then examined the relative abundance of spH on the axonal surface and within the lumen of SVs. Our motivation was to determine the surface fraction of spH under normal circumstances, since this parameter can be altered by changing rates of exocytosis or endocytosis (Dittman and Kaplan, 2006
). We also wanted to determine the vesicular pH, since DA-modulating drugs such as amphetamine are thought to act in part by altering this parameter (Sulzer et al., 2005
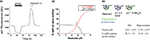
). Analysis of fluorescence changes resulting from transient application of pH 5.5 buffer (quenches surface spH fluorescence) and of pH 7.4 NH4Cl (de-quenches vesicular spH fluorescence) can be used to calculate both surface fraction and vesicular pH (Figure 2
A3; Mitchell and Ryan, 2004
; Sankaranarayanan et al., 2000
). Such experiments (Figure 2
) revealed that ∼30% of spH at DA presynaptic terminals was present on the presynaptic surface. The remaining ∼70% of spH was present within vesicles with an average pH of 5.62. The acidic vesicle pH also implied that a ∼25-fold change in fluorescence would result from deprotonation of spH during exocytosis (based on Henderson–Hasselbalch calculations, Sankaranarayanan et al., 2000
), making it feasible to detect fusion and retrieval events using the spH assay. Finally, similar experiments in hippocampal neurons revealed no significant differences in spH surface fraction and vesicular pH between the two cell types (Figure 2
A3).
Figure 2. The internal vesicle pH and synaptic distribution of spH are similar between DA and hippocampal synapses. (A1) Representative example of the change in spH fluorescence at presynaptic terminals upon exposure to buffer containing 25 mM MES at pH 5.5 and 50 mM NH4Cl at pH 7.4. Exposure to pH 5.5 quenches spH fluorescence on the axon surface, and application of NH4Cl raises the intra-vesicular pH to 7.4, thereby dequenching vesicular spH fluorescence, as shown graphically in (A3). Traces represent the average from 20 boutons in a single neuron. (A2) Measurement of surface fraction and vesicular pH. The fluorescence changes in the pH 5.5 quenching (red) and NH4Cl dequenching (black) experiments from (A1) were used to calculate spH surface fraction, using equations described previously (Mitchell and Ryan, 2004
). The two independent measurements (acid quench and NH4Cl dequench) in the same set of boutons converged at the value of 32% surface fraction and vesicular pH of 5.75 (arrow). (A3) Similar experiments were also conducted in hippocampal neurons and revealed that the internal vesicle pH and synaptic distribution of spH are similar between DA and hippocampal synapses. n = 8 DA neurons and n = 6 hippocampal neurons. Data compared using Student’s t-test. Error bars are SEM.
Increasing Stimulus Frequency from 5 to 30 Hz Causes Limited Depletion of DA Synaptic Vesicles
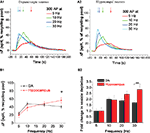
As expected, we found that stimulus-driven changes in spH fluorescence were readily detectable in DA neurons. We examined the response to sustained stimulation (300 stimuli) over a six-fold range of frequencies (Figure 3
A1). A similar experiment in hippocampal neurons revealed that vesicle depletion increased as stimulus frequency was increased from 5 to 30 Hz (Figure 3
A2), in agreement with our previous measurements (Fernández-Alfonso and Ryan, 2004
). The stimulus-driven increase in spH fluorescence corresponds to an increase in the number of spH molecules in an alkaline environment, which arises from a greater number of spH molecules on both the synaptic surface as well as in recently endocytosed but not yet re-acidified vesicles. We refer to this as ‘depletion’ as it corresponds to the loss of vesicles in a usable state. In order to calibrate the increase in spH fluorescence with respect to the spH fluorescence that would correspond to the total recycling pool becoming alkaline, we made use of the vesicular proton pump blocker bafilomycin. Action potential stimulation in the presence of bafilomycin traps every vesicle that undergoes exocytosis in the alkaline state (Sankaranarayanan and Ryan, 2001
). Thus spH signals obtained during stimulation in the absence of bafilomycin can be normalized to the maximal value of spH fluorescence achieved during continuous stimulation in the presence of bafilomycin. Comparison of DA (Figure 3
A1) and hippocampal neurons (Figure 3
A2) showed that DA neurons had less depletion during 30 Hz stimulation. In hippocampal neurons increasing the stimulus frequency from 5 to 30 Hz resulted in a ∼2.8-fold increase in vesicle pool depletion (Figure 3
B2). In contrast, DA neurons showed a smaller 1.8-fold increase in vesicle pool depletion under the same conditions (Figure 3
B2). At the stimulus frequency of 30 Hz, ∼41% of the recycling vesicle pool was depleted in DA neurons, as compared to ∼57% in hippocampal neurons (Figure 3
B1). Two potential mechanisms could explain this finding of limited DA depletion, namely slower exocytosis of vesicles, or faster endocytosis or re-acidification after release. Regardless of the specific mechanism, these results suggest that DA neurons differ from hippocampal neurons in their endocytic–exocytic coupling at 30 Hz.
Figure 3. Increasing stimulus frequency from 5 to 30 Hz causes limited depletion of DA synaptic vesicles. (A1, A2) Average increase in presynaptic spH fluorescence due to 300 stimuli at 5- (red), 10- (green), 20- (blue), or 30 Hz (cyan) in dopaminergic (A1) and hippocampal (A2) neurons. Arrows indicate the end of the stimulus, which began at time 0 s in all trials. Responses are normalized to the size of the total recycling pool of vesicles, which is measured by the maximum, saturating ΔF in the presence of bafilomycin (See Section ‘Materials and Methods’). (B1) With a 30 Hz 300 AP stimulus, DA neurons (black squares) have limited depletion of the recycling vesicle pool as compared to hippocampal neurons (red circles). *p < 0.05. (B2) With a six-fold increase in stimulus frequency from 5 to 30 Hz, DA neurons (black bars) show an ∼1.7-fold increase in vesicle depletion as compared to ∼2.8-fold in hippocampal neurons (red bars). Data from B1 were normalized to spH vesicle pool depletion at 5 Hz. **p < 0.005. Data are the average from 10, 11, 16, and 14 DA neuron experiments and 10, 8, 13, and 8 hippocampal neuron experiments at 5, 10, 20, and 30 Hz, respectively. Error bars are SEM.
The Rate of Exocytosis with a 30 Hz Stimulus is Similar in DA and Hippocampal Neurons
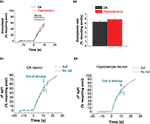
In order to determine whether lower vesicle pool depletion at 30 Hz was due to slower exocytosis in DA neurons, we proceeded to directly examine the rates of exocytosis. We determined the rate of exocytosis by measuring the spH response in the presence of bafilomycin A1 (Baf), a v-type ATPase inhibitor that blocks vesicle re-acidification (Sankaranarayanan and Ryan, 2001
). In the presence of bafilomycin, activity-dependent increase in fluorescence corresponds to vesicle exocytosis, and the rate of this increase is the rate of exocytosis. We found that during the 10 s stimulus, the rate of exocytosis at 30 Hz was similar between hippocampal and DA neurons (Figures 4
A1,A2). Our observations suggest that slower DA exocytosis does not account for the limited DA vesicle pool depletion observed at 30 Hz. Thus, we infer that lower DA vesicle depletion is due to faster endocytosis or re-acidification. Of note, while there was no difference in the exocytic rate during the first 10 s between DA and hippocampal neurons, we observed a slight deviation between the traces at later time points (data not shown). On average, DA neurons reached ∼90% of the maximal value at 50 s, while hippocampal neurons reached 100% by this time. This difference could result from a slight difference in either the re-use kinetics or in the late phase of exocytosis. Finally, we compared the average responses in the absence or presence of bafilomycin in DA (Figure 4
B1) and hippocampal neurons (Figure 4
B2). The difference between the traces represents vesicles that have been both internalized and acidified, i.e., it represents a fraction of the vesicles that have been internalized. Not surprisingly, this pool is not significantly larger in DA neurons; this is because for brief stimuli, a large fraction of internalized vesicles can be expected to be in the alkaline state and therefore not accounted for by measuring the difference between ‘baf’ and ‘no baf’ responses. Future experiments that utilize higher sensitivity methods can help determine the rate of DA vesicle re-acidification and using that parameter, the rate of DA endocytosis. In summary, the difference in pool depletion at 30 Hz (Figure 3
) and the similar exocytic rates (Figures 4
A1,A2) suggest that DA endocytic–exocytic coupling differs from that in hippocampal neurons at 30 Hz, likely due to faster DA endocytosis or re-acidification.
Figure 4. The rate of exocytosis with a 30 Hz stimulus is similar in DA and hippocampal neurons. (A1) Average exocytosis at 30 Hz in DA (black) and hippocampal (red) neurons. The increase in fluorescence with 30 Hz activity was measured in the presence of bafilomycin. There is close overlap between the exocytosis traces in DA and hippocampal neurons during activity at 30 Hz. Data averaged from six DA and eight hippocampal neurons. Each experiment consists of 10–50 presynaptic terminals from one neuron. Error bars are SEM. (A2) The rate of exocytosis at 30 Hz [slope from linear fitting of traces from (A1)] is similar between DA (black bar) and hippocampal (red bar) neurons. (B1) The average spH response in DA neurons to a 30 Hz stimulus in the absence (green) or presence (blue) of bafilomycin. Green arrows mark the end of the stimulus in the absence of bafilomycin. In the presence of bafilomycin, the stimulus was applied for 120–150 s. The difference between the ‘baf’ and ‘no baf’ traces represents vesicles that have been both internalized and acidified. Data averaged from five DA neurons. Error bars are SEM. (B2) The average spH response in hippocampal neurons to a 30 Hz stimulus in the absence (green) or presence (blue) of Bafilomycin. Data averaged from eight hippocampal neurons. Error bars are SEM.
Our results are relevant to the study of DA synaptic physiology for two reasons: first, due to establishment of an optical assay for easy detection of vesicular release of DA, and second, due to identification of the novel phenomenon of limited vesicle pool depletion at 30 Hz that likely results from faster DA endocytosis or re-acidification.
To establish this assay, we first optimized cell preparation and transfection techniques to generate cultures with sufficient numbers of spH-expressing DA neurons. The second component of establishing the assay was to verify that the transfected probe spH was targeted to SVs, did not alter native vesicle recycling, and could provide a robust signal during vesicle exocytosis. Our first observation was that spH was expressed in both the soma and neurites of DA neurons (Figure 1
A). Since VAMP-2 is a SV membrane protein (Baumert et al., 1989
) and part of the SNARE complex that mediates fusion between two membranes (Sollner et al., 1993
), it could be expressed at all cellular sites of vesicle fusion-somatic and axonal. DA neurons have been shown to release DA from the soma and dendrites (Geffen et al., 1976
), with vesicular fusion thought to be partially responsible for somatodendritic DA release (Chen and Rice, 2001
; Li et al., 2005
; Nirenberg et al., 1996
; Rice et al., 1997
). Thus, it was not surprising to observe spH expression in the soma of DA neurons. Indeed, this somatic expression of spH could be used in future experiments to clarify the extent and mechanism of somatodendritic vesicular release. Our present study however, focused on axonal release. We found spH at axon terminals, which were identified based on diameter (<5 μm), en-passant morphology (example in Figure 1
C1; the ‘beads on string’ pattern of presynaptic terminals along an axon), and stimulus-dependent loading and unloading of FM dye (Figure 1
C). Since we did not observe changes in the rate of vesicle exocytosis (Figures 1
D,E) due to spH expression, we infer that native vesicle release is not altered.
We then measured the vesicular pH in DA neurons with two goals in mind. First, the magnitude of fluorescence changes from vesicle fusion depends on the internal vesicle pH relative to the pKa of spH (Sankaranarayanan et al., 2000
). We therefore wanted to assess whether the vesicular pH was sufficiently acidic to generate detectable change in fluorescence with as few as 20 stimuli, as in hippocampal neurons (Sankaranarayanan and Ryan, 2000
). There is also a biological interest in measuring DA vesicular pH, both for comparison with other monoaminergic cell types and for assessing the effects of pharmacological agents such as amphetamine, which are thought to acutely enhance DA release in part by collapsing the pH gradient of vesicles (Sulzer et al., 2005
). Our measurements reveal a pH of 5.62, which agrees well with previous measurements of vesicular pH in catecholamine-containing vesicles in both chromaffin secretory vesicle preparations (Pollard et al., 1979
; Sulzer and Rayport, 1990
) and in intact chromaffin cells (Pothos et al., 2002
). Having the ability to measure the pH in small SVs of intact DA neurons, it will now be of great interest to determine how clinically relevant drugs such as amphetamine and methamphetamine can modify vesicular pH with both acute (Mosharov et al., 2003
) and chronic (Markov et al., 2008
) exposure. The same experiments also revealed that ∼30% of spH was resident on the DA axonal surface, whereas ∼24% of spH is resident on the hippocampal axonal surface (Figure 2
). Under our experimental conditions, DA surface fraction was higher than that in hippocampal neurons but not significantly so. Nevertheless, it is possible that the surface spH level in DA neurons is enhanced due to their tonic firing at 4–5 Hz (Grace and Bunney, 1984
; Grace and Onn, 1989
), which results in exocytosis and greater surface deposition of spH. Such differences may be revealed with blocking or augmentation of activity with agents such as tetrodotoxin and calcium, respectively. The phenomenon of surface spH expression is also known to occur in C. elegans motor neurons (Dittman and Kaplan, 2006
). Alterations in the synaptic surface levels of spH were found in mutant worms with defects in vesicle exocytosis and endocytosis (Dittman and Kaplan, 2006
), implying that this parameter could also be examined in mutants with altered DA release properties. In summary, DA vesicle pH and surface spH expression are not significantly different from hippocampal neurons, implying that it should be possible to detect signals resulting from release of a small number of DA vesicles.
Our next set of experiments sought to characterize the DA SV cycle and identify its novel features by comparing DA responses to those in hippocampal neurons. In DA neurons, we found a smaller rise in fluorescence with a 30 Hz stimulus, implying that fewer vesicles are either waiting to be internalized or have been internalized but not yet re-acidified after their release (Figure 3
B). Two potential mechanisms could explain this finding, namely slower exocytosis of vesicles, or faster endocytosis or re-acidification after release. We found no difference in the rates of exocytosis in DA and hippocampal neurons (Figures 4
A1,A2), leading us to infer that either faster endocytosis or re-acidification occurs in DA neurons during activity at 30 Hz. Future experiments that directly measure the rate of vesicle re-acidification can clarify whether faster endocytosis or re-acidification occurs in DA neurons.
Our results are interesting given the context of DA neuron firing and synaptic properties. DA neurons are known to fire tonically at 4–5 Hz (Grace and Bunney, 1984
; Grace and Onn, 1989
). They also demonstrate phasic activity known as ‘bursting’, which is defined as a firing frequency of 12.5 Hz or greater (Grace and Bunney, 1983
). Given this broad range of activity regimes, it is unlikely that there exists a specific adaptation in the vesicle cycle for stimuli at ∼30 Hz which causes limited vesicle depletion at this frequency. Instead, it may be that these adaptations emerge at ∼30 Hz and persist with increasing stimulus frequency in order to rapidly replenish the releasable pool of DA vesicles and thereby maintain fidelity of neurotransmission during burst firing. DA modulation of circuit function is achieved in part through secretion of DA from small clear SVs in axonal varicosities. These DA terminals differ in two important ways from their fast-acting neurotransmitter counterparts. First, since they do not always secrete focally onto defined postsynaptic clusters of receptors (Descarries et al., 2008
), one might expect the presynaptic organization of the exocytosis machinery to differ from typical GABAergic or glutamatergic nerve terminals. Second, as DA typically acts on a much slower timescale than GABA or glutamate (Greengard, 2001
), one might also expect that the presynaptic machinery has adapted in kind. We hope to use this initial characterization of DA vesicle recycling to help open the door to detailed molecular and physiological comparisons of fast versus slow acting neurotransmitter-based nerve terminals. Finally these approaches should also be well suited for studying mutations in genes such as α-synuclein that are known to both affect DA release and be implicated in neurological and psychiatric diseases (Maroteaux et al., 1988
; Singleton et al., 2003
; Spillantini et al., 1997
).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Ricky Kwan for outstanding technical contributions and members of the Ryan laboratory and Dr. David Sulzer for valuable discussions and critical reading of this manuscript. This project was supported by NIH-GM07739 and a Ruth L. Kirschstein Pre-doctoral fellowship (Meera Mani), NIH-NS036942 (Meera Mani, Timothy A. Ryan), and NIH-DA010154 (Meera Mani, Timothy A. Ryan).
Ferguson, S. M., Brasnjo, G., Hayashi, M., Wolfel, M., Collesi, C., Giovedi, S., Raimondi, A., Gong, L. W., Ariel, P., Paradise, S., O’toole, E., Flavell, R., Cremona, O., Miesenbock, G., Ryan, T. A., and De Camilli, P. (2007). A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science 316, 570–574.
Singleton, A. B., Farrer, M., Johnson, J., Singleton, A., Hague, S., Kachergus, J., Hulihan, M., Peuralinna, T., Dutra, A., Nussbaum, R., Lincoln, S., Crawley, A., Hanson, M., Maraganore, D., Adler, C., Cookson, M. R., Muenter, M., Baptista, M., Miller, D., Blancato, J., Hardy, J., and Gwinn-Hardy, K. (2003). Alpha-synuclein locus triplication causes Parkinson’s disease. Science 302, 841.