Abstract
Reward processing, which ensures survival, has evolved to also shape emotions, learning, and overall well-being. While traditional models of reward have focused predominantly on central neural circuits, emerging evidence underscores the role of peripheral bodily signals. This represents a new opportunity by which we may understand neurological and neuropsychiatric health. In this review, we explore the gut-brain and heart-brain interfaces in reward processing, delineating their contributions across distinct phases of reward and offering insights into their bioenergetic significance. By framing this interplay within an adaptive and clinical context, we propose new avenues for understanding and treating neuropsychiatric disorders through a mind–body medicine lens.
1 Introduction
Reward processing systems have developed to regulate internal states, promote well-being, and motivate adaptive behaviors essential for survival and reproduction (O’Connell and Hofmann, 2011). These systems, which are shaped by evolutionary pressures, organize adaptive decision-making into a series of phases that include anticipation, motivation, consumption, and a post-consummatory learning phase often referred to as satiation (Figure 1A). For example, as temperatures drop in late autumn, a mouse’s diminishing energy stores trigger hunger (anticipation) and prompt a search for food in familiar foraging sites or caches (motivation). Eating the food reward (consumption) elicits hedonic pleasure, facilitating memory consolidation (satiation) of the locations, conditions, and food availability that reinforce future behavior and enhance survival. Throughout this process, the gut communicates with the brain to coordinate internal states with behavior, first by releasing hunger signals that prompt food seeking, then by gradually shifting to satiety signals as nutrients are detected. In parallel, the heart rate decelerates in anticipation of reward, rises during energy-demanding seeking, and stabilizes following consumption (Graham and Clifton, 1966; Eubanks et al., 2002). This seamless coordination between internal systems and the brain across reward processing phases is essential for adaptive behavior and survival. However, clinical populations often exhibit dysfunctions in these stages of reward processing, manifesting as misjudgments in the value, desirability, or predictability of pleasurable outcomes (Linnet, 2014; Volkow and Morales, 2015; Serretti, 2023). And while traditional frameworks of reward processing in healthy and disordered states have predominantly focused on the central nervous mechanisms, there is much to learn about the role of bodily signals in regulating these processes.
Figure 1
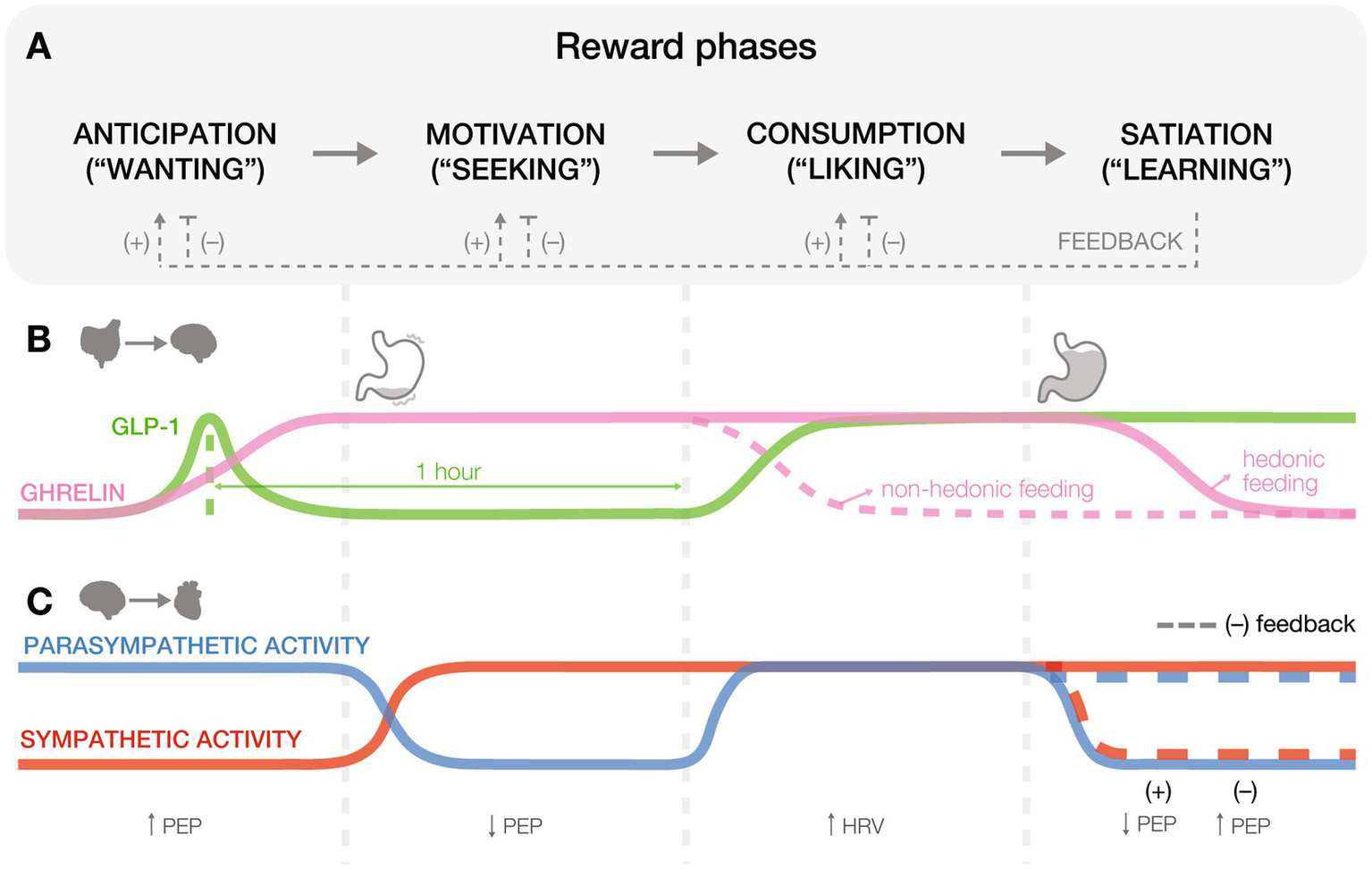
Gut and heart physiology across reward processing phases. (A) Reward processing unfolds in four phases: anticipation (“wanting”), motivation (“seeking”), consumption (“liking”), and satiation (“learning”). Positive (+) and negative (−) feedback loops emerge during the satiation phase, influencing how earlier phases are re-engaged in future cycles. Positive feedback, resulting from pleasurable stimuli, reinforces learning and increases future anticipatory, motivational, and consummatory responses toward similar rewards. In contrast, negative feedback, arising from aversive or unsatisfying stimuli, dampens the reward cycle by decreasing anticipatory, motivational, and consummatory responses to those stimuli. These feedback mechanisms function as internal updates to optimize future reward-seeking behavior based on past experiences. (B) Gut peptides influence hedonic eating and food preferences at various stages of feeding and reward. Hormones like ghrelin and glucagon-like peptide-1 (GLP-1) exhibit dynamic changes that drive food-related behaviors, balancing hunger and satiety cues. (C) Cardiac physiology responds to reward states through parasympathetic and sympathetic activity, as reflected in biomarkers like heart rate variability (HRV) and pre-ejection period (PEP).
Interoception is a mechanism evolved to support homeostasis and adaptive behavior by synergizing bodily signals (Chen et al., 2021). Given the extensive research on the roles of the gut and heart in mediating interoception, we will focus this review on these organs. The gut and heart are both essential for linking survival needs with adaptive behaviors as they each provide critical, real-time feedback to the brain about the body’s internal states (Chen et al., 2021). These organs can also directly contribute to homeostasis and reward processing, shaping an organism’s capacity to anticipate, engage with, and learn from rewarding stimuli. Governing energetic demands, visceral sensations, and autonomic control, the gut and heart work in concert with the brain to fine-tune reward processes and organize adaptive, energy-efficient decisions that optimize survival and well-being (Kimura, 2019; Liu and Bohórquez, 2022). Emerging research highlights the interplay between these peripheral systems and central reward circuits, offering new avenues to understand and treat neuropsychiatric disorders (Zheng et al., 2009; Critchley and Harrison, 2013; Seth, 2013; Karaivazoglou et al., 2024). This mini-review explores the role of gut-brain and heart-brain communication in reward processing, considering perspectives that underscore the adaptive significance of these interactions.
2 Gut-brain and heart-brain signals in reward processing
2.1 Gut-derived signals in reward processing
The gut senses diverse internal stimuli, such as nutrients, distension, and microbial metabolites, and influences reward processing through both hormonal and neural pathways. These influences fall into three domains: hormonal signaling, synaptic signaling, and microbiota (not covered here, see: González-Arancibia et al., 2019; García-Cabrerizo et al., 2021; de Wouters d’Oplinter et al., 2022). Gut epithelial enteroendocrine cells (EECs) detect these signals and communicate with the brain through multiple mechanisms (Gribble and Reimann, 2016), including slow systemic release of hormones, or rapid synaptic communication with vagal and spinal neurons through a subset of EECs known as neuropod cells (Bohórquez et al., 2015; Bellono et al., 2017; Kaelberer et al., 2018; Figures 2A–C). Together, these pathways enable the gut to modulate reward circuits across varying timescales.
Figure 2
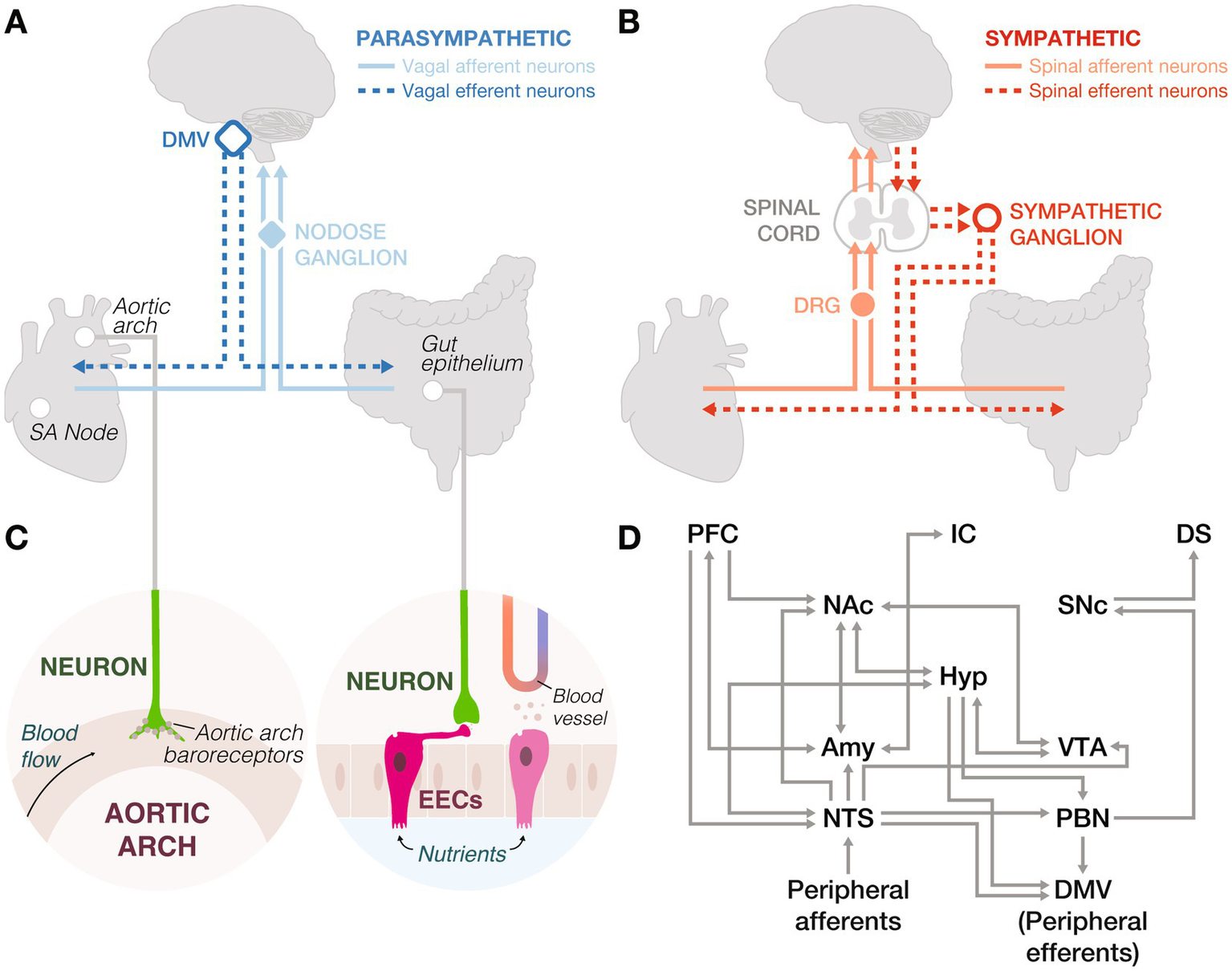
Peripheral innervation and neural circuits underlying interoception. (A) Parasympathetic nervous system: Vagal afferent neurons in the nodose ganglia transmit sensory signals from the sinoatrial (SA) node and gut epithelium to the brainstem. These signals are processed and relayed back to the body via vagal efferent neurons in the dorsal motor nucleus of the vagus (DMV). (B) Sympathetic nervous system: Spinal afferent neurons in the dorsal root ganglia (DRG) relay sensory information from the periphery to the spinal cord, which is then transmitted to the brain. Spinal efferent neurons project from the spinal cord to sympathetic ganglia, innervating peripheral organs. (C) Left: Peripheral neurons in the aortic arch express baroreceptors to detect changes in arterial pressure. Right: Enteroendocrine cells (EECs) sense nutrients, mechanical stretch, and microbial metabolites, and secrete hormones into the bloodstream. However, they can also form synapses with peripheral neurons to send fast signals to the brain. (D) Central integration of gut and heart signals involves the central autonomic network and dopaminergic systems, connecting to higher-order brain regions. This circuitry underscores the complex interplay between interoceptive inputs and reward processing. Amy: amygdala, DMV: dorsal motor nucleus of the vagus, DS: dorsal striatum, Hyp: hypothalamus, IC: insular cortex, NAc: nucleus accumbens, NTS: nucleus tractus solitarius, PBN: parabrachial nucleus, PFC: prefrontal cortex, SNc: substantia nigra pars compacta, VTA: ventral tegmental area.
Ghrelin and glucagon-like peptide-1 (GLP-1) are two key gut-derived peptides that influence reward. Ghrelin primes the stomach for food intake by stimulating gastric acid secretion and motility via vagal pathways (Masuda et al., 2000), aiding digestion and generating interoceptive signals perceived as hunger (Carlson, 1993). In rats and humans, elevated ghrelin levels track hedonic (Merkestein et al., 2012; Monteleone et al., 2012; Rigamonti et al., 2015) and caloric (Hogenkamp et al., 2013) values of an anticipated meal, enhancing food motivation (Figures 1A,B: ANTICIPATION). Interestingly, blocking GLP-1 receptors (GLP-1Rs) before feeding, when GLP-1 levels rise in anticipation, reduces food intake, suggesting an appetite-stimulating role for GLP-1 (Vahl et al., 2010). Notably, this effect was reported under highly restricted feeding schedules, raising questions about its relevance in naturalistic contexts (Williams, 2010).
During motivational phases (Figures 1A,B: MOTIVATION), ghrelin enhances reward-seeking behaviors, such as nose pokes for high-fat food pellets, independent of caloric need (Sun et al., 2004; Perello et al., 2010). In rodents, blocking ghrelin receptors abolishes these behaviors (Egecioglu et al., 2010), suggesting a role in sustaining motivational drive. Conversely, GLP-1R activation reduces the motivation to consume palatable foods (Dickson et al., 2012; Howell et al., 2019), underscoring its action as a satiety sensor (Wang et al., 2015). These mechanisms help organisms prioritize high-energy rewards when resources are abundant.
During consumption (Figures 1A,B: CONSUMPTION), ghrelin levels drop in response to caloric intake (Tschöp et al., 2000; Callahan et al., 2004), while satiety peptides such as GLP-1, cholecystokinin (CCK), and peptide YY rise to signal fullness (Murphy and Bloom, 2006). However, elevated ghrelin levels (Monteleone et al., 2012) and diminished CCK responses (Monteleone et al., 2013) during hedonic feeding can override homeostatic regulation, reinforcing consumption even in the absence of need.
Satiation, or the process of learning from hedonic pleasures of reward consumption, parallels associative and reinforcement learning strategies (Figures 1A,B: SATIATION). Although traditionally defined as reduced hunger, in reward literature, satiation more broadly refers to the integration of post-consummatory signals that inform future behavior, including food, social (Kohls et al., 2012), or musical rewards (Witek and Vuust, 2016). This expanded interpretation aligns with findings that gut-derived hormones and peripheral circuits shape learning following reward. For example, GLP-1R agonists restore impaired associative learning in humans (Hanssen et al., 2023) and reduce excitatory drive onto ventral tegmental area (VTA) dopamine neurons projecting to the nucleus accumbens (NAc; Wang et al., 2015). Similarly, optogenetic stimulation of gut-innervating vagal neurons promotes operant self-stimulation, real-time place preference, and flavor conditioning (Han et al., 2018), highlighting gut-brain contributions to reward learning.
While these mechanisms characterize systemic gut-brain communication, peripheral circuitry provides a complementary and fast synaptic pathway (Figures 2A–C). For instance, CCK-expressing EECs mediate flavor preferences through vagal afferents, while serotonin-expressing EECs drive taste aversion via spinal afferents (Bai et al., 2022; Buchanan et al., 2022), rapidly protecting organisms from ingesting harmful substances. Activation of rodent vagal neurons by nutrient-rich foods triggers mesolimbic dopamine release, reinforcing adaptive feeding (Han et al., 2018; Kim et al., 2023; McDougle et al., 2024). This gut-brain communication is also observed in zebrafish (Ye et al., 2020; Isabella et al., 2021) and flies (Kim et al., 2021; Min et al., 2021; Gao et al., 2024), highlighting conserved mechanisms to distinguish beneficial from toxic foods.
In the brain (Figure 2D), anticipation and motivation are primarily driven by the arcuate nucleus of the hypothalamus (ARC), a target of gut hormones, which receives input from the nucleus tractus solitarius (NTS) and modulates mesolimbic and mesocortical reward circuits (Rossi and Stuber, 2018). For instance, ghrelin acts on ARC neuropeptide Y neurons to release orexigenic peptides and promote feeding (Nakazato et al., 2001; Cowley et al., 2003). During consumption, hedonic value is encoded by endogenous opioids acting at “hotspots” within the NAc, orbitofrontal cortex, insular cortex, and interconnected regions of the hypothalamus, VTA, and amygdala (DiFeliceantonio et al., 2012; Morales and Berridge, 2020). GLP-1R agonists suppress intake of palatable food via receptors in the NTS and VTA, reducing synaptic strength onto NAc-projecting VTA dopamine neurons (Alhadeff and Grill, 2014; Wang et al., 2015), whereas blocking GLP-1 signaling promotes consumption of calorie-dense foods (Alhadeff et al., 2012, 2017). Satiation integrates sensory, emotional, and cognitive inputs across distributed circuits to give rise to learning via reward prediction errors (RPEs; Lokshina et al., 2021). While primarily encoded by mesolimbic dopamine, nascent evidence suggests RPEs exist across various regions. For example, amygdalar activity heightens when novel foods are paired with delayed gastrointestinal malaise, but fade in the absence of unexpected consequences, illustrating the role of postingestive feedback in learned aversions (Zimmerman et al., 2025).
Interestingly, ghrelin and GLP-1 influence non-food rewards as well. Ghrelin predicts gambling persistence in humans (Sztainert et al., 2018) and enhances alcohol’s rewarding effects in mice (Jerlhag et al., 2009). Conversely, GLP-1R activation in rodent VTA decreases the motivation to consume cocaine (Schmidt et al., 2016), alcohol (Shirazi et al., 2013), and nicotine (Tuesta et al., 2017), suggesting a conserved role for modulating non-food rewards and holding clinical therapeutic potential (Jerlhag, 2023).
Together these findings demonstrate how gut-derived signals shape central reward circuits by integrating immediate neural responses with sustained hormonal effects to balance hedonic pursuits with survival needs.
2.2 Cardiac signals in reward processing
Cardiac interoceptive signals support survival by influencing both homeostatic and hedonic choices (Figure 1C). While gut signals primarily influence natural rewards like food, cardiac fluctuations inform choices involving uncertainty, effort, or risk. These adaptive behaviors include foraging, exploration, or competition, as well as modern analogs like gambling, which engage similar neurophysiological mechanisms (Kimura, 2019; Kimura et al., 2023).
During reward anticipation (Figures 1A,C: ANTICIPATION), humans show parasympathetic-induced heart rate deceleration that orients attention towards ecologically relevant stimuli (Graham and Clifton, 1966), enhances cognitive-motor efficiency to support rapid action (Alam et al., 2023), and emerges before placing gambling bets (Sayão et al., 2021). Mediated by the central autonomic network, this deceleration primes the body for focused effort, much like an animal preparing to evade a predator or initiate a chase (Löw et al., 2008; Panitz et al., 2013), underscoring its role in reward anticipation.
As anticipation shifts to motivated behaviors (Figures 1A,C: MOTIVATION), dopaminergic activity triggers the sympathetic nervous system, shortening the pre-ejection period (PEP) in humans (Ahles et al., 2017). PEP is the interval between left-ventricular depolarization to blood ejection into the aorta and occurs following sympathetic stimulation of the heart via beta1-adrenoreceptor activation (Lanfranchi and Somers, 2010). Compared to healthy adults, individuals at risk for neuropsychiatric disorders exhibit exaggerated heart rate changes during alcohol use, gambling, or anhedonia, indicating sympathetic activation and a shortened PEP (Richter and Gendolla, 2009; Brenner and Beauchaine, 2011; Ahles et al., 2017; Silvia et al., 2020). These cardiac signals optimize energy expenditure through shared autonomic and central regulatory circuits (Critchley and Harrison, 2013), balancing between exploring new opportunities and exploiting known resources.
Cardiac signals also shape consumption and learning (Figures 1A,C: CONSUMPTION and SATIATION). For example, negative feedback, such as losing money, triggers a parasympathetic response, while positive feedback activates sympathetic pathways, reinforcing the reward experience (Dekkers et al., 2015; Kastner et al., 2017; Figures 2A,B). Causal evidence from optogenetic studies further supports this link, showing that increasing heart rate in mice induces anxiety-like behaviors in anxiety-provoking contexts (Hsueh et al., 2023). These cardiovascular signals that heighten stress and anxiety modulate decisions by shaping responses to future internal and external cues. Future studies should aim to advance from correlational findings to developing causal relationships between cardiac function and reward processing phases.
The heart-brain axis reflects evolutionary refinements aimed at maintaining cardiovascular stability and facilitating rapid adaptive responses. Parasympathetic vagal neurons (Figure 2A) and sympathetic spinal neurons (Figure 2B) mediate heart-brain communication, allowing the heart to relay sensory information about blood flow and chemical composition to the brain (Prescott and Liberles, 2022; Rajendran et al., 2024). Baroreceptors in the aortic arch, linked to vagal afferents, detect blood pressure changes (Figure 2C) and signal the brain to modulate vagal efferent neurons controlling heart rate, such as those innervating the sinoatrial node, the heart’s pacemaker (Capilupi et al., 2020). Spinal afferents trigger reflexes that elicit sympathetic activity and dopaminergic responses, supporting fight-or-flight behaviors, crucial for survival in dynamic environments (Malliani and Montano, 2002). Like the gut, peripheral afferents from the heart relay to brainstem regions, such as the NTS and parabrachial nucleus (Scott et al., 2025; Figure 2D). These cardiac signals are tightly regulated by the brain’s reward processing centers such as the VTA, amygdala, and ventral striatum (Camara et al., 2009; Lewis et al., 2021; Weinstein, 2023). This neural innervation of the heart is conserved across diverse organisms, including hermit crabs (Yazawa and Kuwasawa, 1994), flies (Dulcis and Levine, 2003), and zebrafish (Stoyek et al., 2015), underscoring its foundational role in cardiovascular regulation and adaptive behaviors.
3 Bioenergetic explanations for the body’s role in reward processing
3.1 Integration of homeostatic and hedonic mechanisms
The brain’s primary role is to maintain physiological balance by driving behaviors that restore homeostasis. This section examines how physiological signals drive reward processing and motivation, emphasizing the body’s role in adaptive behaviors for survival. Evolution has shaped the brain to prioritize rewarding stimuli that align with bodily needs. Discomfort from hunger, thirst, or social isolation motivates behaviors that restore balance. This process, known as positive alliesthesia, describes how stimuli become more rewarding when they meet homeostatic demands (Cabanac, 1971).
These adaptive mechanisms promote survival by supporting goal-directed responses to internal disruptions. Beyond food and water, social interactions maintain homeostasis, fulfilling emotional and psychological needs, contributing to “social homeostasis” across species (Matthews and Tye, 2019). In this way, these rewards similarly engage homeostatic and hedonic reward pathways to support survival and well-being (Rossi and Stuber, 2018; Matthews and Tye, 2019; Grove et al., 2022; Wee et al., 2024).
3.2 Motivational intensity theory
The classical motivational intensity theory posits that an organism’s energy expenditure is proportional to the difficulty of obtaining a reward, up until the required effort surpasses the perceived value (Richter and Gendolla, 2009; Richter et al., 2016). This framework highlights how gut-derived and cardiac signals modulate energy allocation during reward pursuit.
Gut signals influence motivational intensity by integrating physiological readiness with behavioral drive. Ghrelin enhances the perceived value of energy-rich foods and promotes effortful food-seeking (Perello et al., 2010), while GLP-1 dampens motivation for palatable (Dickson et al., 2012) and non-food rewards (Egecioglu et al., 2013a, 2013b). Beyond reward signaling, ghrelin-mediated anticipatory digestive processes represent a metabolic investment, aligning energetic costs of digestive readiness with expected intake (Masuda et al., 2000; Secor, 2009). Insufficient preparation may impair digestion or promote microbial overgrowth, underscoring ghrelin’s role as a metabolic “bet,” balancing effort with internal needs.
Cardiac responses similarly reflect the body’s energy expenditure during different phases of reward processing. Parasympathetic activity conserves energy during anticipation, preparing the body for action (Lovallo and Sollers, 2007). It acts as a real-time biomarker for prioritizing responses to environmental stimuli (Richter et al., 2016), including food (Zebunke et al., 2011), social (Zupan et al., 2016), and sexual cues (Creswell et al., 2013) across species, including pigs, dogs, and humans. During reward-seeking, sympathetic activity increases with task difficulty, but decreases when effort outweighs the reward’s value (Richter, 2010), selectively mobilizing energy.
Evolutionarily, these integrated mechanisms ensure strategic allocation of energy toward high-value rewards, optimizing resource acquisition and adaptability.
3.3 Predictive interoception coding
Predictive interoception coding provides a framework for how the brain anticipates and integrates internal bodily signals to maintain homeostasis and guide reward-related behaviors. The brain’s ability to generate internal expectations has evolved from simple reflexes to complex predictive models (Pezzulo et al., 2021). For example, false heart rate feedback can create interoceptive illusions, making participants perceive greater effort during exercise when they believe their heart rate is elevated (Iodice et al., 2019), illustrating the brain’s reliance on predicted internal states to calibrate effort. Beyond homeostasis, predictive interoception also shapes emotional and reward-based decisions (Seth, 2013). In a gambling task, participants with greater anticipatory awareness of emotional states made faster, more advantageous financial decisions (Marshall et al., 2019).
Understanding the interaction between reward circuits and the peripheral nervous system reflects how predictive mechanisms have adapted to regulate physiology, motivation, and reward seeking. This integration highlights the brain’s role in optimizing responses to internal and external challenges, enhancing survival.
4 Therapeutic implications of interoception
Neural processing of peripheral organ signals regulates stress, enhances resilience, and holds promise for neuropsychiatric treatment (Benton et al., 2021; Natterson-Horowitz et al., 2023). In humans, gut-derived signals influence emotional states and reward processing. For example, intragastric fat infusion attenuates experimentally induced sadness and dampens activity in emotion-related brain regions (Van Oudenhove et al., 2011), while striatal dopamine release during pleasurable meals predicts subjective pleasure ratings (Small et al., 2003). These psychophysical links between the gut and emotional experience underscore the promise of interoceptive therapeutics.
Originally developed for diabetes and weight management, GLP-1R agonists are now being explored for reducing cravings in addiction and mitigating symptoms of psychotic and neurocognitive disorders (Klausen et al., 2022; Leggio et al., 2023; Xie et al., 2025). Similarly, heart rate variability, a physiological marker of emotional regulation and reward sensitivity, has emerged as a therapeutic target, showing efficacy in reducing substance (Eddie et al., 2014), alcohol (Penzlin et al., 2015), and food cravings (Meule et al., 2012).
Looking ahead, interoceptive therapeutics, including trainings to enhance bodily awareness and advanced vagus nerve stimulation (VNS), hold promise for improving emotional resilience and mental health (Khalsa et al., 2017; Kim et al., 2024; Schuman-Olivier et al., 2024). Although current VNS techniques show efficacy in treating conditions like depression and anxiety, improving specificity remains a challenge. Technologies like optogenetics and targeted gene delivery aim to define cell-type-specific neuromodulation, minimizing off-target effects and enhancing efficacy (Bansal et al., 2023). Collectively, brain–body interventions hold immense potential for the treatment of various conditions, including depression, autism, and anxiety disorders (Paulus and Stein, 2010; DuBois et al., 2016; Rogers et al., 2016).
5 Conclusion
This mini-review provides an adaptive framework for understanding brain–body communication in reward processing. While focused on select interoceptive signals, other sensory inputs (e.g., gut microbiota) and organs (e.g., pancreas) also shape reward behaviors (Davis et al., 2010; Kim et al., 2023). A truly holistic view requires acknowledging multi-organ interactions, such as gut-heart-brain communication. For example, duodenal glucose infusions can lower blood pressure in healthy adults (O’Donovan et al., 2002), while gastric distension raises arterial pressure via sympathetic activation to offset digestive-related blood redistribution (Rossi et al., 1998). Such visceral signals likely modulate reward circuits by influencing physiological states. Simultaneous investigation of these systems allows for the discovery of both direct and emergent properties that govern brain–body communication, underscoring the need to integrate such complexities into a holistic view of reward processing and its role in survival and well-being.
Statements
Author contributions
MA: Data curation, Supervision Conceptualization, Visualization, Validation, Writing – original draft, Writing – review & editing. KA: Validation, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Arinel is supported by the Boehringer Ingelheim Fonds PhD Fellowship. Abdelaal is supported by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number F99NS135695 and the Howard Hughes Medical Institute Gilliam Fellowship for Advanced Studies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
Thank you to Senegal A. Mabry, whose initial conceptualization and early development of this manuscript laid the foundation for its eventual form. Thank you to the Affect and Cognition Lab and Life History Lab for their thoughtful feedback on an earlier draft. Finally, we thank the Duke Department of Neurobiology for supporting Arinel and Abdelaal.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ahles J. J. Mezulis A. H. Crowell S. E. (2017). Pre-ejection period reactivity to reward is associated with Anhedonic symptoms of depression among adolescents. Dev. Psychobiol.59, 535–542. doi: 10.1002/dev.21518
2
Alam S. Revi G. S. Kerick S. E. Yang X. Robucci R. Banerjee N. et al . (2023). Anticipatory cardiac deceleration estimates cognitive performance in virtual reality beyond tonic heart period and heart period variability. Biol. Psychol.181:108602. doi: 10.1016/j.biopsycho.2023.108602
3
Alhadeff A. L. Grill H. J. (2014). Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am. J. Phys. Regul. Integr. Comp. Phys.307, R465–R470. doi: 10.1152/ajpregu.00179.2014
4
Alhadeff A. L. Mergler B. D. Zimmer D. J. Turner C. A. Reiner D. J. Schmidt H. D. et al . (2017). Endogenous glucagon-like Peptide-1 receptor signaling in the nucleus Tractus Solitarius is required for food intake control. Neuropsychopharmacology42, 1471–1479. doi: 10.1038/npp.2016.246
5
Alhadeff A. L. Rupprecht L. E. Hayes M. R. (2012). GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus Accumbens to control for food intake. Endocrinology153, 647–658. doi: 10.1210/en.2011-1443
6
Bai L. Sivakumar N. Yu S. Mesgarzadeh S. Ding T. Ly T. et al . (2022). Enteroendocrine cell types that drive food reward and aversion. eLife11:e74964. doi: 10.7554/eLife.74964
7
Bansal A. Shikha S. Zhang Y. (2023). Towards translational optogenetics. Nat. Biomed. Eng.7, 349–369. doi: 10.1038/s41551-021-00829-3
8
Bellono N. W. Bayrer J. R. Leitch D. B. Castro J. Zhang C. O’Donnell T. A. et al . (2017). Enterochromaffin cells are gut Chemosensors that couple to sensory neural pathways. Cell170, 185–198.e16. doi: 10.1016/j.cell.2017.05.034
9
Benton M. L. Abraham A. LaBella A. L. Abbot P. Rokas A. Capra J. A. (2021). The influence of evolutionary history on human health and disease. Nat. Rev. Genet.22, 269–283. doi: 10.1038/s41576-020-00305-9
10
Bohórquez D. V. Shahid R. A. Erdmann A. Kreger A. M. Wang Y. Calakos N. et al . (2015). Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Invest.125, 782–786. doi: 10.1172/JCI78361
11
Brenner S. L. Beauchaine T. P. (2011). Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: a pilot study. Psychophysiology48, 1588–1596. doi: 10.1111/j.1469-8986.2011.01230.x
12
Buchanan K. L. Rupprecht L. E. Kaelberer M. M. Sahasrabudhe A. Klein M. E. Villalobos J. A. et al . (2022). The preference for sugar over sweetener depends on a gut sensor cell. Nat. Neurosci.25, 191–200. doi: 10.1038/s41593-021-00982-7
13
Cabanac M. (1971). Physiological role of pleasure: a stimulus can feel pleasant or unpleasant depending upon its usefulness as determined by internal signals. Science173, 1103–1107. doi: 10.1126/science.173.4002.1103
14
Callahan H. S. Cummings D. E. Pepe M. S. Breen P. A. Matthys C. C. Weigle D. S. (2004). Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict Intermeal interval in humans. J. Clin. Endocrinol. Metabol.89, 1319–1324. doi: 10.1210/jc.2003-031267
15
Camara E. Rodriguez-Fornells A. Ye Z. Münte T. F. (2009). Reward networks in the brain as captured by connectivity measures. Front. Neurosci.3, 350–362. doi: 10.3389/neuro.01.034.2009
16
Capilupi M. J. Kerath S. M. Becker L. B. (2020). Vagus nerve stimulation and the cardiovascular system. Cold Spring Harb. Perspect. Med.10:a034173. doi: 10.1101/cshperspect.a034173
17
Carlson A. J. (1993). Contributions to the physiology of the stomach.–II. The relation between the contractions of the empty stomach and the sensation of hunger. Obes. Res.1, 501–509. doi: 10.1002/j.1550-8528.1993.tb00034.x
18
Chen W. G. Schloesser D. Arensdorf A. M. Simmons J. M. Cui C. Valentino R. et al . (2021). The emerging science of Interoception: sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci.44, 3–16. doi: 10.1016/j.tins.2020.10.007
19
Cowley M. A. Smith R. G. Diano S. Tschöp M. Pronchuk N. Grove K. L. et al . (2003). The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron37, 649–661. doi: 10.1016/S0896-6273(03)00063-1
20
Creswell J. D. Pacilio L. E. Denson T. F. Satyshur M. (2013). The effect of a primary sexual reward manipulation on cortisol responses to psychosocial stress in men. Biopsychosoc. Sci. Med.75, 397–403. doi: 10.1097/PSY.0b013e31828c4524
21
Critchley H. D. Harrison N. A. (2013). Visceral influences on brain and behavior. Neuron77, 624–638. doi: 10.1016/j.neuron.2013.02.008
22
Davis J. F. Choi D. L. Benoit S. C. (2010). Insulin, leptin and reward. Trends Endocrinol. Metab.21, 68–74. doi: 10.1016/j.tem.2009.08.004
23
de Wouters d’Oplinter A. Huwart S. J. P. Cani P. D. Everard A. (2022). Gut microbes and food reward: from the gut to the brain. Front. Neurosci.16:947240. doi: 10.3389/fnins.2022.947240
24
Dekkers L. M. S. van der Molen M. J. W. Gunther Moor B. van der Veen F. M. van der Molen M. W. (2015). Cardiac and electro-cortical concomitants of social feedback processing in women. Soc. Cogn. Affect. Neurosci.10, 1506–1514. doi: 10.1093/scan/nsv039
25
Dickson S. L. Shirazi R. H. Hansson C. Bergquist F. Nissbrandt H. Skibicka K. P. (2012). The glucagon-like peptide 1 (GLP-1) analogue, Exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J. Neurosci.32, 4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012
26
DiFeliceantonio A. G. Mabrouk O. S. Kennedy R. T. Berridge K. C. (2012). Enkephalin surges in dorsal Neostriatum as a signal to eat. Curr. Biol.22, 1918–1924. doi: 10.1016/j.cub.2012.08.014
27
DuBois D. Ameis S. H. Lai M.-C. Casanova M. F. Desarkar P. (2016). Interoception in autism Spectrum disorder: a review. Int. J. Dev. Neurosci.52, 104–111. doi: 10.1016/j.ijdevneu.2016.05.001
28
Dulcis D. Levine R. B. (2003). Innervation of the heart of the adult fruit fly, Drosophila melanogaster. J. Comp. Neurol.465, 560–578. doi: 10.1002/cne.10869
29
Eddie D. Kim C. Lehrer P. Deneke E. Bates M. E. (2014). A pilot study of brief heart rate variability biofeedback to reduce craving in Young adult men receiving inpatient treatment for substance use disorders. Appl. Psychophysiol. Biofeedback39, 181–192. doi: 10.1007/s10484-014-9251-z
30
Egecioglu E. Engel J. A. Jerlhag E. (2013a). The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, Accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One8:e77284. doi: 10.1371/journal.pone.0077284
31
Egecioglu E. Engel J. A. Jerlhag E. (2013b). The glucagon-like peptide 1 analogue, Exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One8:e69010. doi: 10.1371/journal.pone.0069010
32
Egecioglu E. Jerlhag E. Salomé N. Skibicka K. P. Haage D. Bohlooly-Y M. et al . (2010). Ghrelin increases intake of rewarding food in rodents. Addict. Biol.15, 304–311. doi: 10.1111/j.1369-1600.2010.00216.x
33
Eubanks L. Wright R. A. Williams B. J. (2002). Reward influence on the heart: cardiovascular response as a function of incentive value at five levels of task demand. Motiv. Emot.26, 139–152. doi: 10.1023/A:1019863318803
34
Gao J. Zhang S. Deng P. Wu Z. Lemaitre B. Zhai Z. et al . (2024). Dietary L-Glu sensing by enteroendocrine cells adjusts food intake via modulating gut PYY/NPF secretion. Nat. Commun.15:3514. doi: 10.1038/s41467-024-47465-4
35
García-Cabrerizo R. Carbia C. O’Riordan K. Schellekens H. Cryan J. (2021). Microbiota-gut-brain axis as a regulator of reward processes. J. Neurochem.157, 1495–1524. doi: 10.1111/jnc.15284
36
González-Arancibia C. Urrutia-Piñones J. Illanes-González J. Martinez-Pinto J. Sotomayor-Zárate R. Julio-Pieper M. et al . (2019). Do your gut microbes affect your brain dopamine?Psychopharmacology236, 1611–1622. doi: 10.1007/s00213-019-05265-5
37
Graham F. K. Clifton R. K. (1966). Heart-rate change as a component of the orienting response. Psychol. Bull.65, 305–320. doi: 10.1037/h0023258
38
Gribble F. M. Reimann F. (2016). Enteroendocrine cells: Chemosensors in the intestinal epithelium. Annu. Rev. Physiol.78, 277–299. doi: 10.1146/annurev-physiol-021115-105439
39
Grove J. C. R. Gray L. A. La Santa Medina N. Sivakumar N. Ahn J. S. Corpuz T. V. et al . (2022). Dopamine subsystems that track internal states. Nature608, 374–380. doi: 10.1038/s41586-022-04954-0
40
Han W. Tellez L. A. Perkins M. H. Perez I. O. Qu T. Ferreira J. et al . (2018). A neural circuit for gut-induced reward. Cell175, 665–678.e23. doi: 10.1016/j.cell.2018.08.049
41
Hanssen R. Rigoux L. Kuzmanovic B. Iglesias S. Kretschmer A. C. Schlamann M. et al . (2023). Liraglutide restores impaired associative learning in individuals with obesity. Nat. Metab.5, 1352–1363. doi: 10.1038/s42255-023-00859-y
42
Hogenkamp P. S. Cedernaes J. Chapman C. D. Vogel H. Hjorth O. C. Zarei S. et al . (2013). Calorie anticipation alters food intake after Low-caloric but not high-caloric preloads. Obesity (Silver Spring, Md.)21, 1548–1553. doi: 10.1002/oby.20293
43
Howell E. Baumgartner H. M. Zallar L. J. Selva J. A. Engel L. Currie P. J. (2019). Glucagon-like Peptide-1 (GLP-1) and 5-Hydroxytryptamine 2c (5-HT2c) receptor agonists in the ventral tegmental area (VTA) inhibit ghrelin-stimulated appetitive reward. Int. J. Mol. Sci.20:889. doi: 10.3390/ijms20040889
44
Hsueh B. Chen R. Jo Y. Tang D. Raffiee M. Kim Y. S. et al . (2023). Cardiogenic control of affective behavioural state. Nature615, 292–299. doi: 10.1038/s41586-023-05748-8
45
Iodice P. Porciello G. Bufalari I. Barca L. Pezzulo G. (2019). An interoceptive illusion of effort induced by false heart-rate feedback. Proc. Natl. Acad. Sci. USA116, 13897–13902. doi: 10.1073/pnas.1821032116
46
Isabella A. J. Stonick J. A. Dubrulle J. Moens C. B. (2021). Intrinsic positional memory guides target-specific axon regeneration in the zebrafish vagus nerve. Development148:dev199706. doi: 10.1242/dev.199706
47
Jerlhag E. (2023). The therapeutic potential of glucagon-like peptide-1 for persons with addictions based on findings from preclinical and clinical studies. Front. Pharmacol.14:1063033. doi: 10.3389/fphar.2023.1063033
48
Jerlhag E. Egecioglu E. Landgren S. Salomé N. Heilig M. Moechars D. et al . (2009). Requirement of central ghrelin signaling for alcohol reward. Proc. Natl. Acad. Sci.106, 11318–11323. doi: 10.1073/pnas.0812809106
49
Kaelberer M. M. Buchanan K. L. Klein M. E. Barth B. B. Montoya M. M. Shen X. et al . (2018). A gut-brain neural circuit for nutrient sensory transduction. Science361:5236. doi: 10.1126/science.aat5236
50
Karaivazoglou K. Aggeletopoulou I. Triantos C. (2024). The contribution of the brain-gut Axis to the human reward system. Biomedicines12:1861. doi: 10.3390/biomedicines12081861
51
Kastner L. Kube J. Villringer A. Neumann J. (2017). Cardiac concomitants of feedback and prediction error processing in reinforcement learning. Front. Neurosci.11:598. doi: 10.3389/fnins.2017.00598
52
Khalsa S. S. Adolphs R. Cameron O. G. Critchley H. D. Davenport P. W. Feinstein J. S. et al . (2017). Interoception and mental health: a roadmap. Biol. Psychiatry3, 501–513. doi: 10.1016/j.bpsc.2017.12.004
53
Kim E. Joss D. Marin F. Anzolin A. Gawande R. Comeau A. et al . (2024). Protocol for a pilot study on the Neurocardiac mechanism of an interoceptive compassion-based heart-smile training for depression. Global Adv. Integr. Med. Health13:27536130241299389. doi: 10.1177/27536130241299389
54
Kim B. Kanai M. I. Oh Y. Kyung M. Kim E.-K. Jang I.-H. et al . (2021). Response of the microbiome–gut–brain axis in Drosophila to amino acid deficit. Nature593, 570–574. doi: 10.1038/s41586-021-03522-2
55
Kim J. S. Williams K. C. Kirkland R. A. Schade R. Freeman K. G. Cawthon C. R. et al . (2023). The gut-brain axis mediates bacterial driven modulation of reward signaling. Mol. Metab.75:101764. doi: 10.1016/j.molmet.2023.101764
56
Kimura K. (2019). Cardiac cycle modulates reward feedback processing: an ERP study. Neurosci. Lett.711:134473. doi: 10.1016/j.neulet.2019.134473
57
Kimura K. Kanayama N. Katahira K. (2023). Cardiac cycle affects risky decision-making. Biol. Psychol.176:108471. doi: 10.1016/j.biopsycho.2022.108471
58
Klausen M. K. Thomsen M. Wortwein G. Fink-Jensen A. (2022). The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br. J. Pharmacol.179, 625–641. doi: 10.1111/bph.15677
59
Kohls G. Chevallier C. Troiani V. Schultz R. T. (2012). Social ‘wanting’ dysfunction in autism: neurobiological underpinnings and treatment implications. J. Neurodev. Disord.4:10. doi: 10.1186/1866-1955-4-10
60
Lanfranchi P. A. Somers V. K. (2010). “Cardiovascular physiology: autonomic control in health and in sleep disorders” in Principles and Practice of Sleep Medicine: Fifth Edition, eds. M. H. Kryger, T. Roth and W. C. Dement (St. Louis, Missouri: Elsevier Inc.), 226–236.
61
Leggio L. Hendershot C. S. Farokhnia M. Fink-Jensen A. Klausen M. K. Schacht J. P. et al . (2023). GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med.29, 2993–2995. doi: 10.1038/s41591-023-02634-8
62
Lewis R. G. Florio E. Punzo D. Borrelli E. (2021). “The brain’s reward system in health and disease,” in Circadian clock in brain health and disease, eds. EngmannO.BrancaccioM. (Cham: Springer International Publishing), 57–69. PMID:
63
Linnet J. (2014). Neurobiological underpinnings of reward anticipation and outcome evaluation in gambling disorder. Front. Behav. Neurosci.8:100. doi: 10.3389/fnbeh.2014.00100
64
Liu W. W. Bohórquez D. V. (2022). The neural basis of sugar preference. Nat. Rev. Neurosci.23, 584–595. doi: 10.1038/s41583-022-00613-5
65
Lokshina Y. Nickelsen T. Liberzon I. (2021). Reward processing and circuit dysregulation in posttraumatic stress disorder. Front. Psych.12:559401. doi: 10.3389/fpsyt.2021.559401
66
Lovallo W. R. Sollers J. J. (2007). “Autonomic nervous system” in Encyclopedia of stress. ed. FinkG.. Second ed (New York: Academic Press), 282–289.
67
Löw A. Lang P. J. Smith J. C. Bradley M. M. (2008). Both predator and prey: emotional arousal in threat and reward. Psychol. Sci.19, 865–873. doi: 10.1111/j.1467-9280.2008.02170.x
68
Malliani A. Montano N. (2002). Emerging excitatory role of cardiovascular sympathetic afferents in pathophysiological conditions. Hypertension39, 63–68. doi: 10.1161/hy0102.099200
69
Marshall A. C. Gentsch A. Blum A.-L. Broering C. Schütz-Bosbach S. (2019). I feel what I do: relating interoceptive processes and reward-related behavior. NeuroImage191, 315–324. doi: 10.1016/j.neuroimage.2019.02.032
70
Masuda Y. Tanaka T. Inomata N. Ohnuma N. Tanaka S. Itoh Z. et al . (2000). Ghrelin stimulates gastric acid secretion and motility in rats. Biochem. Biophys. Res. Commun.276, 905–908. doi: 10.1006/bbrc.2000.3568
71
Matthews G. A. Tye K. M. (2019). Neural mechanisms of social homeostasis. Ann. N. Y. Acad. Sci.1457, 5–25. doi: 10.1111/nyas.14016
72
McDougle M. de Araujo A. Singh A. Yang M. Braga I. Paille V. et al . (2024). Separate gut-brain circuits for fat and sugar reinforcement combine to promote overeating. Cell Metab.36, 393–407.e7. doi: 10.1016/j.cmet.2023.12.014
73
Merkestein M. Brans M. A. D. Luijendijk M. C. M. de Jong J. W. Egecioglu E. Dickson S. L. et al . (2012). Ghrelin mediates anticipation to a palatable meal in rats. Obesity20, 963–971. doi: 10.1038/oby.2011.389
74
Meule A. Freund R. Skirde A. K. Vögele C. Kübler A. (2012). Heart rate variability biofeedback reduces food cravings in high food cravers. Appl. Psychophysiol. Biofeedback37, 241–251. doi: 10.1007/s10484-012-9197-y
75
Min S. Oh Y. Verma P. Whitehead S. C. Yapici N. Van Vactor D. et al . (2021). Control of feeding by piezo-mediated gut mechanosensation in Drosophila. eLife10:e63049. doi: 10.7554/eLife.63049
76
Monteleone P. Piscitelli F. Scognamiglio P. Monteleone A. M. Canestrelli B. Di Marzo V. et al . (2012). Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-Arachidonoyl-glycerol in healthy humans: a pilot study. J. Clin. Endocrinol. Metabol.97, E917–E924. doi: 10.1210/jc.2011-3018
77
Monteleone P. Scognamiglio P. Monteleone A. M. Perillo D. Canestrelli B. Maj M. (2013). Gastroenteric hormone responses to hedonic eating in healthy humans. Psychoneuroendocrinology38, 1435–1441. doi: 10.1016/j.psyneuen.2012.12.009
78
Morales I. Berridge K. C. (2020). ‘Liking’ and ‘wanting’ in eating and food reward: brain mechanisms and clinical implications. Physiol. Behav.227:113152. doi: 10.1016/j.physbeh.2020.113152
79
Murphy K. G. Bloom S. R. (2006). Gut hormones and the regulation of energy homeostasis. Nature444, 854–859. doi: 10.1038/nature05484
80
Nakazato M. Murakami N. Date Y. Kojima M. Matsuo H. Kangawa K. et al . (2001). A role for ghrelin in the central regulation of feeding. Nature409, 194–198. doi: 10.1038/35051587
81
Natterson-Horowitz B. Aktipis A. Fox M. Gluckman P. D. Low F. M. Mace R. et al . (2023). The future of evolutionary medicine: sparking innovation in biomedicine and public health. Front. Sci.1:997136. doi: 10.3389/fsci.2023.997136
82
O’Connell L. A. Hofmann H. A. (2011). The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol.519, 3599–3639. doi: 10.1002/cne.22735
83
O’Donovan D. Feinle C. Tonkin A. Horowitz M. Jones K. L. (2002). Postprandial hypotension in response to duodenal glucose delivery in healthy older subjects. J. Physiol.540, 673–679. doi: 10.1113/jphysiol.2001.013442
84
Panitz C. Wacker J. Stemmler G. Mueller E. M. (2013). Brain–heart coupling at the P300 latency is linked to anterior cingulate cortex and insula—a cardio-electroencephalographic covariance tracing study. Biol. Psychol.94, 185–191. doi: 10.1016/j.biopsycho.2013.05.017
85
Paulus M. P. Stein M. B. (2010). Interoception in anxiety and depression. Brain Struct. Funct.214, 451–463. doi: 10.1007/s00429-010-0258-9
86
Penzlin A. I. Siepmann T. Illigens B. M.-W. Weidner K. Siepmann M. (2015). Heart rate variability biofeedback in patients with alcohol dependence: a randomized controlled study. Neuropsychiatr. Dis. Treat.11, 2619–2627. doi: 10.2147/NDT.S84798
87
Perello M. Sakata I. Birnbaum S. Chuang J.-C. Osborne-Lawrence S. Rovinsky S. A. et al . (2010). Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol. Psychiatry67, 880–886. doi: 10.1016/j.biopsych.2009.10.030
88
Pezzulo G. Parr T. Friston K. (2021). The evolution of brain architectures for predictive coding and active inference. Philos. Trans. R. Soc. B Biol. Sci.377:20200531. doi: 10.1098/rstb.2020.0531
89
Prescott S. L. Liberles S. D. (2022). Internal senses of the vagus nerve. Neuron110, 579–599. doi: 10.1016/j.neuron.2021.12.020
90
Rajendran P. S. Hadaya J. Khalsa S. S. Yu C. Chang R. Shivkumar K. (2024). The vagus nerve in cardiovascular physiology and pathophysiology: from evolutionary insights to clinical medicine. Semin. Cell Dev. Biol.156, 190–200. doi: 10.1016/j.semcdb.2023.01.001
91
Richter M. (2010). Pay attention to your manipulation checks! Reward impact on cardiac reactivity is moderated by task context. Biol. Psychol.84, 279–289. doi: 10.1016/j.biopsycho.2010.02.014
92
Richter M. Gendolla G. H. E. (2009). The heart contracts to reward: monetary incentives and preejection period. Psychophysiology46, 451–457. doi: 10.1111/j.1469-8986.2009.00795.x
93
Richter M. Gendolla G. H. E. Wright R. A. (2016). “Chapter five - three decades of research on motivational intensity theory: what we have learned about effort and what we still Don’t know” in Advances in motivation science. ed. ElliotA. J. (Cambridge, MA, San Diego, CA, London, UK, and Oxford, UK: Elsevier), 149–186.
94
Rigamonti A. E. Piscitelli F. Aveta T. Agosti F. Col A. D. Bini S. et al . (2015). Anticipatory and consummatory effects of (hedonic) chocolate intake are associated with increased circulating levels of the orexigenic peptide ghrelin and endocannabinoids in obese adults. Food Nutr. Res.59:29678. doi: 10.3402/fnr.v59.29678
95
Rogers G. B. Keating D. J. Young R. L. Wong M.-L. Licinio J. Wesselingh S. (2016). From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol. Psychiatry21, 738–748. doi: 10.1038/mp.2016.50
96
Rossi P. Andriesse G. I. Oey P. L. Wieneke G. H. Roelofs J. M. M. Akkermans L. M. A. (1998). Stomach distension increases efferent muscle sympathetic nerve activity and blood pressure in healthy humans. J. Neurol. Sci.161, 148–155. doi: 10.1016/S0022-510X(98)00276-7
97
Rossi M. A. Stuber G. D. (2018). Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab.27, 42–56. doi: 10.1016/j.cmet.2017.09.021
98
Sayão A. Alves H. Furukawa E. Schultz Wenk T. Cagy M. Gutierrez-Arango S. et al . (2021). Development of a classical conditioning task for humans examining phasic heart rate responses to signaled appetitive stimuli: a pilot study. Front. Behav. Neurosci.15:639372. doi: 10.3389/fnbeh.2021.639372
99
Schmidt H. D. Mietlicki-Baase E. G. Ige K. Y. Maurer J. J. Reiner D. J. Zimmer D. J. et al . (2016). Glucagon-like Peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology41, 1917–1928. doi: 10.1038/npp.2015.362
100
Schuman-Olivier Z. Gawande R. Creedon T. B. Comeau A. Griswold T. Smith L. B. et al . (2024). Change starts with the body: interoceptive appreciation mediates the effect of mindfulness training on behavior change - an effect moderated by depression severity. Psychiatry Res.342:116230. doi: 10.1016/j.psychres.2024.116230
101
Scott K. A. Tan Y. Johnson D. N. Elsaafien K. Baumer-Harrison C. Méndez-Hernández R. et al . (2025). Mechanosensation of the heart and gut elicits hypometabolism and vigilance in mice. Nat. Metab.7, 263–275. doi: 10.1038/s42255-024-01205-6
102
Secor S. M. (2009). Specific dynamic action: a review of the postprandial metabolic response. J. Comp. Physiol. B179, 1–56. doi: 10.1007/s00360-008-0283-7
103
Serretti A. (2023). Anhedonia and Depressive Disorders. Clin Psychopharmacol Neurosci21, 401–409. doi: 10.9758/cpn.23.1086
104
Seth A. K. (2013). Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci.17, 565–573. doi: 10.1016/j.tics.2013.09.007
105
Shirazi R. H. Dickson S. L. Skibicka K. P. (2013). Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One8:e61965. doi: 10.1371/journal.pone.0061965
106
Silvia P. J. Eddington K. M. Harper K. L. Burgin C. J. Kwapil T. R. (2020). Appetitive motivation in depressive anhedonia: effects of piece-rate cash rewards on cardiac and behavioral outcomes. Motiv. Sci.6, 259–265. doi: 10.1037/mot0000151
107
Small D. M. Jones-Gotman M. Dagher A. (2003). Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage19, 1709–1715. doi: 10.1016/S1053-8119(03)00253-2
108
Stoyek M. R. Croll R. P. Smith F. M. (2015). Intrinsic and extrinsic innervation of the heart in zebrafish (anio rerio). J. Comp. Neurol.523, 1683–1700. doi: 10.1002/cne.23764
109
Sun Y. Wang P. Zheng H. Smith R. G. (2004). Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci.101, 4679–4684. doi: 10.1073/pnas.0305930101
110
Sztainert T. Hay R. Wohl M. J. A. Abizaid A. (2018). Hungry to gamble? Ghrelin as a predictor of persistent gambling in the face of loss. Biol. Psychol.139, 115–123. doi: 10.1016/j.biopsycho.2018.10.011
111
Tschöp M. Smiley D. L. Heiman M. L. (2000). Ghrelin induces adiposity in rodents. Nature407, 908–913. doi: 10.1038/35038090
112
Tuesta L. M. Chen Z. Duncan A. Fowler C. D. Ishikawa M. Lee B. R. et al . (2017). GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat. Neurosci.20, 708–716. doi: 10.1038/nn.4540
113
Vahl T. P. Drazen D. L. Seeley R. J. D’Alessio D. A. Woods S. C. (2010). Meal-anticipatory glucagon-like Peptide-1 secretion in rats. Endocrinology151, 569–575. doi: 10.1210/en.2009-1002
114
Van Oudenhove L. McKie S. Lassman D. Uddin B. Paine P. Coen S. et al . (2011). Fatty acid–induced gut-brain signaling attenuates neural and behavioral effects of sad emotion in humans. J. Clin. Invest.121, 3094–3099. doi: 10.1172/JCI46380
115
Volkow N. D. Morales M. (2015). The brain on drugs: from reward to addiction. Cell162, 712–725. doi: 10.1016/j.cell.2015.07.046
116
Wang X.-F. Liu J.-J. Xia J. Liu J. Mirabella V. Pang Z. P. (2015). Endogenous glucagon-like Peptide-1 suppresses high-fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Rep.12, 726–733. doi: 10.1016/j.celrep.2015.06.062
117
Wee R. W. S. Mishchanchuk K. AlSubaie R. Church T. W. Gold M. G. MacAskill A. F. (2024). Internal-state-dependent control of feeding behavior via hippocampal ghrelin signaling. Neuron112, 288–305.e7. doi: 10.1016/j.neuron.2023.10.016
118
Weinstein A. M. (2023). Reward, motivation and brain imaging in human healthy participants – a narrative review. Front. Behav. Neurosci.17:1123733. doi: 10.3389/fnbeh.2023.1123733
119
Williams D. L. (2010). Expecting to eat: glucagon-like Peptide-1 and the anticipation of meals. Endocrinology151, 445–447. doi: 10.1210/en.2009-1372
120
Witek M. A. G. Vuust P. (2016). Comment on Solberg and Jensenius: the temporal dynamics of embodied pleasure in music. Emp. Musicol. Rev.11, 324–329. doi: 10.18061/emr.v11i3-4.5353
121
Xie Y. Choi T. Al-Aly Z. (2025). Mapping the effectiveness and risks of GLP-1 receptor agonists. Nat. Med.31, 951–962. doi: 10.1038/s41591-024-03412-w
122
Yazawa T. Kuwasawa K. (1994). Dopaminergic acceleration and GABAergic inhibition in extrinsic neural control of the hermit crab heart. J. Comp. Physiol. A174, 65–75. doi: 10.1007/BF00192007
123
Ye L. Bae M. Cassilly C. D. Jabba S. V. Thorpe D. W. Martin A. M. et al . (2020). Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host & Microbe, 29, 179–196. doi: 10.1016/j.chom.2020.11.011
124
Zebunke M. Langbein J. Manteuffel G. Puppe B. (2011). Autonomic reactions indicating positive affect during acoustic reward learning in domestic pigs. Anim. Behav.81, 481–489. doi: 10.1016/j.anbehav.2010.11.023
125
Zheng H. Lenard N. R. Shin A. C. Berthoud H.-R. (2009). Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int. J. Obes.33, S8–S13. doi: 10.1038/ijo.2009.65
126
Zimmerman C. A. Bolkan S. S. Pan-Vazquez A. Wu B. Keppler E. F. Meares-Garcia J. B. et al . (2025). A neural mechanism for learning from delayed postingestive feedback. Nature1:10. doi: 10.1038/s41586-025-08828-z
127
Zupan M. Buskas J. Altimiras J. Keeling L. J. (2016). Assessing positive emotional states in dogs using heart rate and heart rate variability. Physiol. Behav.155, 102–111. doi: 10.1016/j.physbeh.2015.11.027
Summary
Keywords
reward, gut-brain, heart-brain, adaptive behavior, bioenergetics, interoceptive therapeutics
Citation
Arinel M and Abdelaal K (2025) The gut and heart’s role in reward processing. Front. Integr. Neurosci. 19:1479923. doi: 10.3389/fnint.2025.1479923
Received
14 August 2024
Accepted
30 May 2025
Published
02 July 2025
Volume
19 - 2025
Edited by
Steffen Schulz, Charité – Universitätsmedizin Berlin, Germany
Reviewed by
Briac Halbout, California State University, Long Beach, United States
Matthew H. Perkins, Icahn School of Medicine at Mount Sinai, United States
Magdalena Miranda, National Scientific and Technical Research Council (CONICET), Argentina
Weiyi Sun, Kyoto Prefectural University of Medicine, Japan
Updates
Copyright
© 2025 Arinel and Abdelaal.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minel Arinel, minel.arinel@duke.edu
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.