Abstract
Introduction:
We have shown that a delayed auditory cortex neural response is associated with language ability in school-age children with autism spectrum disorder and related syndromes, with this delay exacerbated in the context of co-occurring intellectual disability (ID). As a clinical diagnosis of ID is generally not made until school age, identification of neural measures that precede a behaviorally assessed ID diagnosis would help identify young children likely to benefit from early treatment. The present study evaluated if the speed of auditory cortex neural activity (M50 latency) would predict language ability in 3-year-old children who have an existing diagnosis that is a risk factor associated with a range of later functional outcomes, including ID or developmental delay (DD), irrespective of autism spectrum disorder diagnosis.
Methods:
Thirty 3-year-old children with elevated likelihood for ID or DD (ID/DD-EL) were enrolled. Evaluable magnetoencephalography (MEG) data as well as language and cognitive ability measures were obtained from 23 participants.
Results:
A longer time to encode auditory stimuli (i.e., a delayed M50 cortical evoked response) in the left hemisphere predicted lower language ability. Left M50 latency was not associated with cognitive ability. Right hemisphere M50 latency was not associated with language or cognitive ability.
Discussion:
Present observations demonstrate that non-invasive brain imaging in conjunction with a passive auditory task (with early primary/secondary auditory cortex neural responses) can identify paths for variable language outcome in preschool children with ID/DD-EL. This lays the foundation for further investigation of these neural mechanisms as early indications for treatment as well as early signals of response to treatment.
Introduction
Auditory evoked latencies are delayed in school-age children with autism spectrum disorder (autism, hereafter) (Edgar et al., 2015a; Roberts et al., 2010), with such delays exacerbated in the context of co-occurring intellectual disability (ID). In particular, studies from our laboratory have identified in minimally verbal/non-verbal (MVNV) school-age children with autism associations between auditory cortex neural activity and both general cognitive ability and domain-specific language/communication ability (Roberts et al., 2019). A clinical diagnosis of ID is typically not made until school age, and a limitation of research in this area is identifying preschool children with an increased likelihood of a later ID diagnosis. This is of interest, as identifying early neural mechanisms that are differentially associated with variable language and cognitive outcomes in children with elevated likelihood of ID or developmental delay (ID/DD-EL) would help detect young children likely to benefit from early targeted treatment, as well as identify early signals of response to such treatment.
Multiple genetic conditions confer a risk of intellectual disability, specifically language impairment, with functional outcomes ranging from profoundly impaired to within the average range. Genetic risk factors for intellectual disability have been identified (e.g., 22q11.2 deletion syndrome and Down syndrome), with large variability in language and cognitive ability among children with known genetic diagnoses. For example, in a large group of individuals with 16p11.2 deletion, the group average Full-Scale IQ (FSIQ) was in the low average range, with only 10% meeting the criteria for an ID diagnosis (Hanson et al., 2015). In Fragile X syndrome, IQ spans the full distribution of cognitive ability and varies with the extent of FMR1 protein expression (Tassone et al., 1999). Cognitive variability among individuals with Prader–Willi syndrome is less etiologically clear and thus less predictable (Whittington and Holland, 2017).
Language impairment and an ID/DD diagnosis are also common in children with birth and prenatal complications. Similar to children with a genetic syndrome, there is substantial variability in language outcome in children born preterm (<34 weeks gestational age), with some children showing no language delay, some presenting with an early language delay but catching up to age-appropriate levels by school age, and some presenting with persistent language difficulties (Belardi et al., 2017; Chilosi et al., 2019; Dale et al., 2003; Rescorla, 2002).
The field has little insight into the neural mechanisms associated with the severity of impairment in preschool children with an elevated risk for ID/DD. Identifying valid and replicable biomarkers across heterogeneous populations (Jeste et al., 2015; Kozak and Cuthbert, 2016; Miller et al., 2016; Yee et al., 2015) that are associated with language ability before the start of kindergarten would identify children at-risk for ID/DD likely to benefit from early intervention, as well as to monitor and potentially predict treatment response. Identifying neural biomarkers across a broad range of ID/DD-EL etiologies rather than within a specific ID/DD-EL diagnostic group is needed, as traditional diagnostic categories are sometimes too limited or heterogeneously large for biological phenomena (Miller et al., 2016). Given that different supports are needed for young children with different severities of language and cognitive impairments or children who have language delay without cognitive impairment, identifying neural markers that are differentially sensitive to general cognitive ability versus language-specific ability would inform the development of targeted intervention plans.
Findings from our laboratory and others have shown atypical auditory M50 and M100 responses, recorded using magnetoencephalography (MEG), in school-age children with autism without cognitive impairment, as well as children with autism who are MVNV. These findings include delayed superior temporal gyrus (STG) auditory responses (Edgar et al., 2015a; Edgar et al., 2014; Gage et al., 2003; Naatanen and Picton, 1987; Roberts et al., 2012; Roberts et al., 2010; Roberts et al., 2019), reduced STG 40-Hz auditory steady-state total power (Wilson et al., 2007), pre-and post-stimulus pure tone STG low-and high-frequency oscillatory abnormalities (Edgar et al., 2015a), and atypical hemispheric lateralization of auditory responses (Stroganova et al., 2013).
Using procedures developed in our laboratory (Kuschner et al., 2021), M50 auditory measures have been examined in school-aged children with autism who are MVNV as well as in a broad range of children with IDD, such as Down syndrome, Bainbridge–Roper syndrome, NAA10 gene mutation, and 8p23.3 deletion (Kuschner et al., 2021; Roberts et al., 2024; Roberts et al., 2019). Roberts et al. (2019) showed that MVNV children with autism had a later M50/M100 latency compared to age-matched children with autism whose IQs were in the average range, even though in both cohorts, a later auditory evoked response latency was associated with worse language ability. Of note, MVNV children with autism often have significant cognitive impairment, with the neurophysiological mechanisms underlying language ability, independent of cognitive processes, not yet understood.
To better understand the development of auditory processes in children before they receive a diagnosis of ID/DD, the present study sought to ascertain the discriminatory potential of auditory evoked neuromagnetic response latencies with respect to language and cognitive ability in a cohort of 3-to 4-year-old children with an existing diagnosis that puts them at an increased likelihood for ID/DD. A passive auditory task was used, as such tasks provide the ability to assess auditory cortex neural function across the lifespan and a range of behavioral competence (Chen et al., 2023; Edgar et al., 2015b; Roberts et al., 2019). As the earlier auditory M50 response is more prominent than the later M100 response in younger children (Edgar et al., 2020), the present study focused on source-localized left and right hemisphere M50 auditory responses.
Materials and methods
Participants
Thirty children with ID/DD-EL were enrolled and participated in MEG and language and cognitive assessment sessions. Inclusion criteria were: (1) male or female, aged 2 years 8 months to 4 years 5 months; (2) an existing early childhood diagnosis associated with an increased likelihood of later diagnosis of ID (e.g., known genetic differences linked to IDD such as Down syndrome or 22q11.2 deletion syndrome, autism spectrum disorder, global developmental delay, neurological conditions such as cerebral palsy and prematurity) or documented developmental delay (IQ < 85); and (3) English as the primary language spoken in the home. Exclusion criteria included: (1) diagnosis of uncorrectable vision impairment (clinical blindness); (2) permanent hearing loss of greater than 25 dB; and (3) use of an implanted hearing aid. The study was approved by the Children’s Hospital of Philadelphia IRB, and all families provided written consent.
Language and cognitive assessment
For each child, language ability was assessed using the Preschool Language Scale, Fifth Edition (PLS-5) (Zimmerman et al., 2011). PLS-5 total score raw scores were obtained from the sum of receptive and expressive subscale raw scores. Cognitive ability was measured either via the full-scale IQ score from the Wechsler Preschool and Primary Scale of Intelligence Fourth Edition (WPPSI-IV) (Wechsler, 2012) or the cognitive domain standard scores from the Bayley Scales of Infant and Toddler Development Fourth Edition (Bayley-4) (Bayley and Aylward, 2019). Children who were unable to establish a baseline on the WPPSI-IV were administered the Bayley-4 Cognitive domain to ensure a developmentally appropriate assessment.
MEG data acquisition and auditory examination
Whole-head MEG data were obtained in a magnetically shielded room (Vacuumschmelze GmbH & Co. KG, Hanau, Germany) using a 275-axial gradiometer system (CTF, Coquitlam, BC) and with synthetic third-order gradiometer noise correction applied. MEG data were acquired at a sampling rate of 1,200 Hz. Before MEG acquisition, three head position indicator (HPI) coils were placed to serve as landmarks on the child’s head. The child’s head shape, anatomical landmarks (nasion, right and left preauricular points), and locations of the three HPI coils were digitized using a FastSCAN System (Polhemus, Colchester, VT, USA). The child’s head position was monitored using the three HPIs attached to the scalp with continuous head localization (essentially the magnetic detection of alternating current electrical activation of the HPI coils at non-harmonic frequencies distant from the range of brain activity). A research assistant with experience scanning young children accompanied the child and helped the parent keep the child calm and alert during the examination. A variety of strategies were used to engage the child during the scan (see details in Chen et al., 2021), including projecting an age-appropriate silent video on a screen placed above the child’s head (viewing distance 55 cm) as well as providing toys to maintain the child’s attention and focus. For each participant, otoacoustic emission (OAE) testing was performed on the same day as the MEG examination.
Auditory stimuli consisted of 500 Hz sinusoidal tones 300 ms in duration, with 10 ms onset/offset ramps. The offset-to-onset interstimulus interval varied between 600 and 2,000 ms. Stimuli were presented via a free-field speaker (Panphonics Sound Shower, Turku, Finland) at 85 dB SPL, and the distance between the free-field speaker and each participant was the same. MEG recordings were obtained while the child was awake and alert. The auditory examination lasted 8 min (~300 trials).
Magnetic source analyses
MEG data were analyzed using Brainstorm1 (Tadel et al., 2011). For each child, digitized surface points from FastSCAN representing the shape of the child’s head (>10,000 points) were used to co-register each child’s MEG data to an age-appropriate MRI template (O’Reilly et al., 2021; Richards et al., 2016) using an affine transformation that accommodated global scale differences between the child’s anatomy and the atlas.
All MEG data were band-pass filtered from 3 to 55 Hz (transition bands 1.5–3.0 Hz and 55–63.25 Hz) and with a 60 Hz power-line notch filter applied. Heartbeat and eyeblink artifacts were removed via independent component analyses (ICA). Trials with MEG activity exceeding 500 fT, due to excessive motion or magnetic noise artifact, were eliminated. Other artifacts (e.g., movement and muscle artifacts) were visually identified and removed. Artifact-free auditory evoked responses were obtained by averaging evaluable epochs from 200 ms pre-to 500 ms post-stimulus. Across all children, an average of 274 artifact-free trials were obtained. Using the auditory evoked response, whole-brain activity maps were computed using minimum norm estimation (MNE; Hämäläinen and Ilmoniemi, 1994; Hauk, 2004; Lin et al., 2006; Matsuura and Okabe, 1995), with minimum-norm imaging estimating the amplitude of brain sources constrained to the cerebral cortex and current dipoles normally oriented to the local cortical surface (Baillet et al., 2001). The auditory evoked-response activity was mapped to each child’s cortical-surface source space (~15,000 vertices; varied across infants) as a function of time. For each child, an MEG noise covariance matrix was obtained from an empty room recording obtained immediately after the child’s scan. MNE solutions were computed with normalization as part of the inverse routine, based on the noise covariance.
In each child, left-and right-hemisphere auditory source timecourses were obtained from left-and right-hemisphere auditory ROIs (Chen et al., 2023). The M50 response was first identified via the sensor magnetic field sink and the source topography (e.g., in the left hemisphere, an anterior source and posterior sink) and with left and right M50 ROIs created at the time of the peak M50 sensor activity using an amplitude threshold of 5 nAm and a cluster threshold of ≥50 neighboring cortical surface vertices. A left and right auditory source timecourse was obtained via averaging across vertices within the left and right ROI, with post-stimulus activity normalized to the baseline (i.e., providing post-stimulus Z-score measures; see Figure 1, auditory source timecourses from two representative participants). Using in-house MATLAB software, left and right peak M50 latencies, within ±30 ms surrounding the peak time point identified at the sensors, were identified in source space.
Figure 1

Histogram of PLS-5 total language standard scores and cognitive ability standard scores in ID/DD-EL children with and without an autism diagnosis. The numbers shown in the histogram indicate the number of participants for each score range.
Statistical analysis
Statistical analyses were conducted using JMP statistical software (SAS Institute Inc., 2021). The present study hypothesized that the auditory M50 latency may serve as a proxy for language and/or cognitive ability. Sex was also considered a confounder/mediator. Structural equation models (SEMs) were used to evaluate whether left and right auditory M50 response latencies predicted language and cognitive scores, respectively, while recognizing the possible influence of sex. Our models include the following paths: M50 latency predicts language/cognitive scores, sex predicts language/cognitive scores, and sex predicts M50 latency. Four SEMs were specified: Models 1 and 2 estimated the correlation between M50 latency (separate for left and right hemispheres), sex, and PLS-5 score (as an index of language ability). Models 3 and 4 estimated the correlation between M50 latency (separate for left and right hemispheres), sex, and cognitive standard score (as an index of general cognitive ability). Akaike Information Criteria (AIC) values were computed to estimate the goodness of fit of each model, with lower AIC values indicating a better-fit model.
Given that the literature has shown evidence of sex differences in language development during early childhood (see review in Rinaldi et al., 2023), we further explore whether M50 latency accounts for significant variance in language scores between female and male participants. A linear mixed model was run with the PLS score entered as a dependent variable and with M50 latency, age, sex, and the M50 latency by sex interaction term entered as fixed effect variables.
Results
Of 30 enrolled children, evaluable MEG data were available from 23 children. MEG datasets from seven children were not available/excluded due to the child being unable to tolerate the scan (N = 4), magnetic noise artifact (N = 1), or evaluable data that lacked auditory evoked response (N = 2). All children with evaluable MEG data have completed the PLS-5 assessment. Of 23 children, 13 have completed WPPSI-IV, 7 completed the Bayley-4 cognitive subscale, and 3 could not complete WPPSI-IV or Bayley-4. Tables 1, 2 shows the descriptive statistics of age, language scores, cognitive scores, and left and right auditory M50 latency between (A) female and male participants and (B) participants with and without a diagnosis of autism. Welch’s t-tests indicated that no difference was observed between male and female participants or between participants with and without autism, in any of the Table 1 measures.
Table 1
| Mean (SE) Range (Min–Max) |
Female (N = 9) | Male (N = 14) | t-test | All (N = 23) |
|---|---|---|---|---|
| Age (months) | 39.98 (1.28) 37.73–42.17 | 41.63 (4.72) 33.03–53.53 | t (16) = 1.24 p = 0.23 | 40.99 (3.80) 33.03–53.53 |
| PLS-5 Total raw score | 70.78 (8.10) 18–90 | 60.57 (7.09) 25–105 | t (18) = −0.95 p = 0.36 | 64.57 (5.34) 18–105 |
| PLS-5 Total language standard score | 95.11 (25.06) 50–118 | 82.50 (29.29) 50–141 | t (18) = −1.10 p = 0.28 | 87.43 (27.83) 50–141 |
| Cognitive ability standard score | 94.12 (8.67) 55–114 | 85.25 (7.06) 55–121 | t (15) = −0.79 p = 0.44 | 88.80 (5.42) 55–121 |
| Left M50 Latency (ms) | 138 (4.12) 122–161 | 138 (10.90) 115–189 | t (21) = 0.14 p = 0.89 | 138 (3.33) 115–189 |
| Right M50 Latency (ms) | 139 (4.91) 103–217 | 143 (4.11) 124–184 | t (10) = 0.38 p = 0.71 | 142 (4.81) 103–217 |
| Accepted number of trials | 305 (33) 152–514 | 254 (22) 89–342 | t (15) = −1.30 p = 0.21 | 274 (19) 89–514 |
Descriptive statistics between females and males.
Table 2
| Mean (SE)Range (Min-Max) | ASD (N = 7) | Non-ASD (N = 16) | t-test |
|---|---|---|---|
| PLS-5 Total language standard score | 75.86 (27.16) 50–120 | 92.50 (27.41) 50–141 | t (11.61) = 1.35 p = 0.20 |
| Cognitive ability standard score | 71.67 (25.94) 55–121 | 96.14 (20.16) 55–114 | t (7.72) = 2.06 p = 0.07 |
| Left M50 Latency (ms) | 143 (25.41) 115–189 | 135 (9.96) 123–154 | t (6.82) = −0.73 p = 0.49 |
| Right M50 Latency (ms) | 145 (8.69) 127–154 | 140 (27.29) 103–217 | t (20.07) = −0.61 p = 0.55 |
Descriptive statistics between children with and without ASD.
Figure 1 shows the distribution of language and cognitive standard scores within our cohort, separately for children with and without an autism diagnosis. Of note, the majority of children in our cohort fall within the normal range of language and cognitive ability (e.g., 75% of ID/DD-EL children without autism and 43% of ID/DD-EL children with autism have PLS-5 standard scores >85; see Figure 1).
Left and right hemisphere auditory M50 latency
The auditory M50 response component was the earliest positive response, peaking ~100–150 ms post-stimulus in young children. In children with ID/DD-EL, there was large variability in left and right hemisphere auditory M50 latency (see Tables 1, 2), consistent with broad variation in language and general cognitive abilities. Figure 2 shows auditory source timecourses from two representative children with ID/DD-EL—a child with relatively early left M50 latency and with average language performance (standard score = 92) on the PLS-5 (top panel) and a child with relatively late left M50 latency and a language score 2 SD below average (standard score = 65) on the PLS (bottom panel).
Figure 2
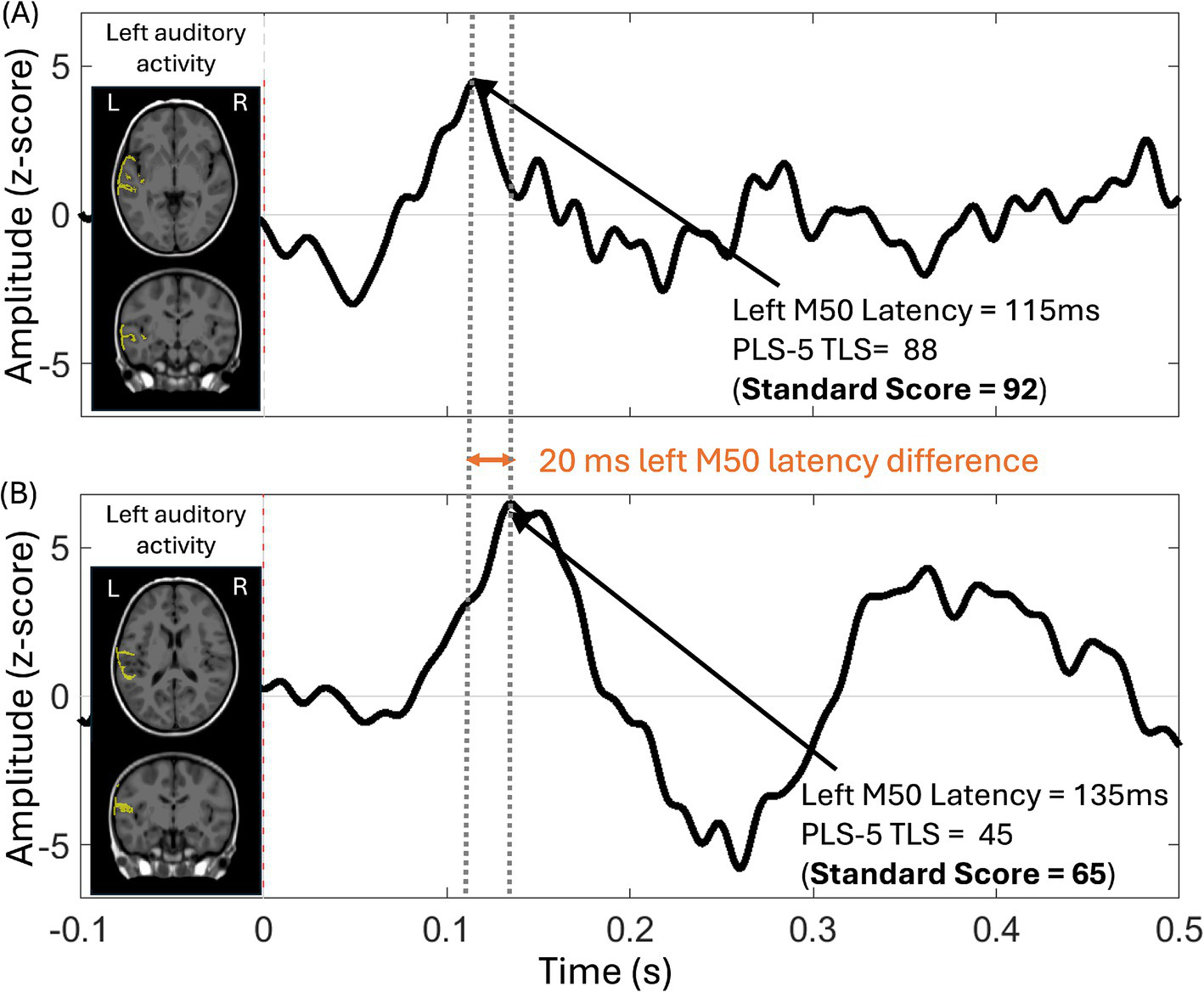
Auditory source timecourse from the left auditory cortex in two ID/DD-EL children, along with the anatomic source overlaid (yellow) on axial and coronal MRI views. The auditory source timecourse was obtained via averaging across vertices within the left auditory cortex ROI, with post-stimulus activity normalized to the baseline (auditory response amplitude expressed in Z-score on the y-axis). (A) The auditory cortex response from a child (52.8 months old) with language performance in the average range (PLS-5 Total Language Score (TLS) = 88, Standard Score = 92) is characterized by a relatively earlier M50 response. (B) The auditory cortex response from a child (42 months old) with language performance 2 SDs below average (PLS-5 TLS = 45, Standard Score = 65) is characterized by a relatively later M50 response.
Predictors of language and cognitive ability
Figure 3 shows the correlations between left and right M50 latency and PLS-5 and cognitive scores (Figures 3A,B). Only left M50 latency predicted language scores. The AICs for all four models are within a similar range as the following: 463 (left M50, sex, language), 484 (right M50, sex, language), 436 (left M50, sex, cognition), and 454 (right M50, sex, cognition). Neither left nor right M50 latency predicted cognitive scores. Sex did not mediate the relationship between M50 latency and PLS-5 or cognitive score. Figure 3C shows associations between left (top) and right (bottom) M50 latency and PLS-5 score. Of note, the standard least square test indicated language scores were significantly associated with cognitive scores (R2 = 0.86, F(1, 18) = 109.66, p < 0.0001).
Figure 3
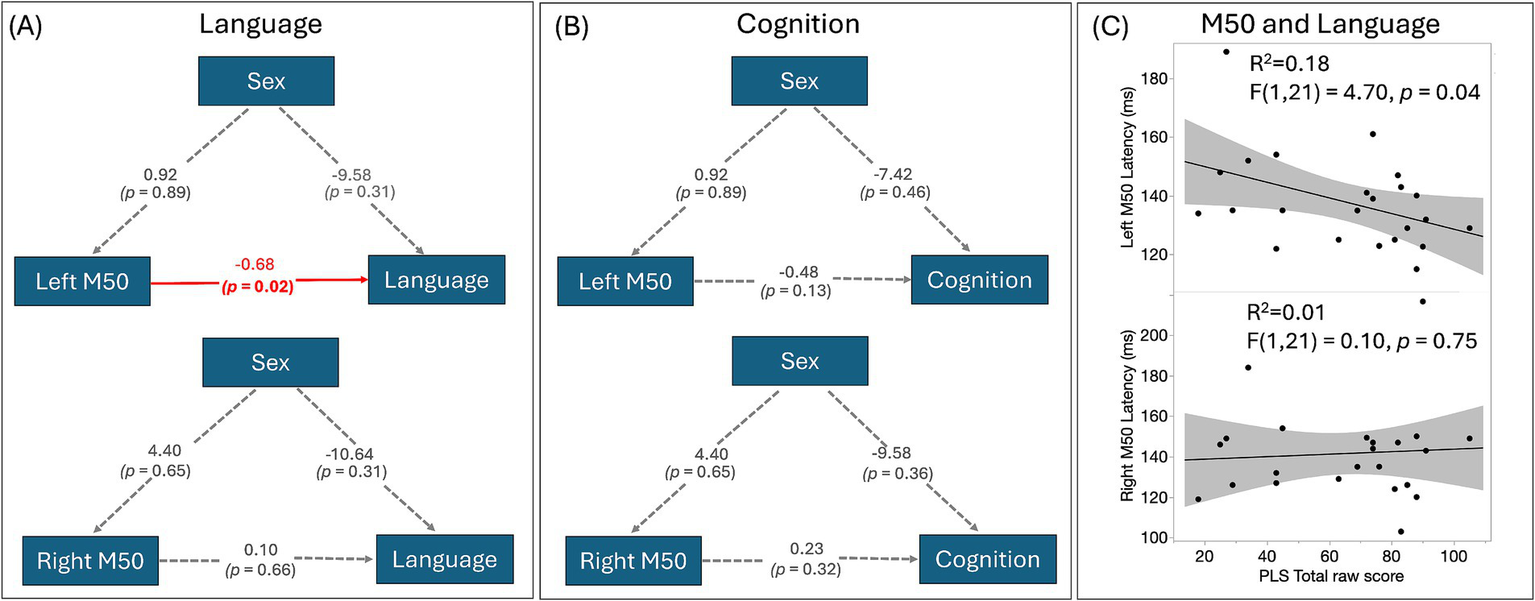
(A) SEMs of M50 latency, sex, and language. The estimates of the regressions between the factors are shown. Directed paths are shown as arrows between the factors, with a significant association highlighted in red; (B) SEMs of M50 latency, sex, and cognition show no significant associations; (C) The scatterplot between left and right M50 latency and PLS-5 score identifies a significant association between left hemisphere M50 latency and language ability, with a later M50 latency associated with a lower language ability score.
To examine differences in M50 latency and language scores associations between females and males, linear mixed model results showed an M50 latency by sex interaction (F(1,40) = 5.33, p = 0.026), with simple-effect analyses showing a relationship between M50 latency and language scores only in males.
Discussion
The present study evaluated the ability of auditory cortex neural activity to predict language and cognitive performance in 3-year-old children with ID/DD-EL. Left-hemisphere M50 latency was associated with language scores, with left M50 latency being sensitive to language but not cognitive scores. The non-significant trend between left M50 and cognitive ability can largely be accounted for by the fact that cognitive and language measures were highly correlated. The following text discusses M50 latency findings with respect to previous studies in this area.
Left hemisphere auditory M50 latency predicts language ability in children with ID/DD-EL
M50 latency indicates the time it takes auditory information to travel from the ear to the primary/secondary auditory cortex. M50 is the earliest “middle latency” cortical response identified by electrophysiological methods and is the magnetic counterpart of the mature ERP P1 component (for an extended discussion, see Chen et al., 2023; Edgar et al., 2020). Consistent with the previous literature (Kuschner et al., 2021; Roberts et al., 2024; Roberts et al., 2019), our findings show left but not right hemisphere M50 latency and language associations, with a later (more delayed) left M50 latency associated with lower language scores. Present results are consistent with studies exploring the relationship between auditory cortex neural activity and language in children 2–6 years old (Yoshimura et al., 2012; Yoshimura et al., 2014). Additionally, some studies have shown that auditory responses in infancy predict language scores later in development (Choudhury and Benasich, 2011; Musacchia et al., 2013).
A left hemisphere specialization in language processing was observed at a young age. Research has shown that rapid sound discrimination is needed for mapping meaning onto sounds, with this ability hypothesized to be associated with the maturation of the left hemisphere auditory cortex (Hickok and Poeppel, 2007). This is perhaps mirrored in the different maturation rates of left and right auditory cortices. Findings from our and other laboratories have shown that the left auditory cortex matures more slowly than the right auditory cortex between infancy and toddlerhood (Chen et al., 2023) and across the life span (Edgar et al., 2020; Paetau et al., 1995; Parviainen et al., 2019), with a slower maturation rate of the left auditory cortex likely accounting for the associations between left M50 latency and language scores, as language learning is a slow, non-linear process that starts early in life and continues throughout adolescence and adulthood. In line with findings from older children’s studies, previous findings from our typically developing infant MEG study (Chen et al., 2023) showed that a later infant auditory cortex latency (the infant “P2m” is equivalent to the “M50” in young children and adults) was associated with lower language scores in children 12–24 months old, an age when expressive language begins to emerge. This is likely because more rapid auditory processing allows infants to more efficiently process linguistically relevant acoustic information (Benasich et al., 2002). Finally, for our cohort of preschool children with ID/DD-EL, the mixed model analysis results showed stronger associations between M50 latency and language scores in male and female participants, suggesting underlying neurophysiological differences between boys and girls that likely contribute to sex differences in early stages of language development.
Present findings extend our previous findings, showing early (3–4 years of age) hemispheric specialization of the auditory cortex in language development, even in young children with an elevated likelihood of ID. Across a wide spectrum of developmental disorders, early identification and remediation of language difficulties likely lie in understanding early auditory encoding mechanisms (Musacchia et al., 2015). As the neural mechanism of early language development is better understood in at-risk young children, the field can begin to understand why some children have only delayed language, while others have delayed language and delayed cognitive ability.
Biomarkers to predict language impairment
Our laboratory has a long history of studying auditory function and language development in a wide spectrum of school-age children with autism, including verbal children without cognitive impairment, as well as minimally verbal/non-verbal children (Kuschner et al., 2021; Roberts et al., 2019). Abnormal auditory neuromagnetic M50 (and later M100) component responses reflecting primary/secondary cortex processing have been reported. Our previous findings (Roberts et al., 2019) from school-age children with autism showed a delayed M50 in both hemispheres. Present left hemisphere M50 and language associations findings indicate that in young children, abnormal auditory cortex processing is more pronounced in the left hemisphere, with this delay likely observed in both hemispheres later in development.
Present findings also indicated that a later M50 latency is sensitive to language ability, observed in both children with autism as well as in children at more general risk for cognitive impairment. Given that performance on the language and cognitive ability assessment is highly correlated, it is challenging to identify biomarkers that are specifically sensitive to language delay and independent of cognitive ability. Multi-measure batteries of tests and combinatorial measures/statistics will likely be needed.
MEG-PLAN for preschoolers with ID/DD-EL
Neuroimaging research with young children with neurodevelopmental disorders has been limited due to barriers like tolerating the loud noises of scanning and the inability to remain still during MRI scans. MEG is a non-invasive neuroimaging technique that provides whole-head measures of neural activity with millisecond temporal resolutions, which is silent, has minimal preparation time, and has head movement compensation methods that can correct for moderate head motion, making it particularly suitable for studying brain activity in young children and children with developmental differences (Chen et al., 2019). To better understand brain function across individuals with various cognitive abilities across all ages, our team has developed MEG-PLAN (Kuschner et al., 2021), a clinical and technical protocol to obtain MEG data in minimally verbal or non-verbal neurodevelopmental pediatric populations. The present study applied MEG-PLAN in this preschool-aged cohort and yielded a 77% evaluable data scan completion rate, comparable to or slightly higher than the rate of 71% in our previous school-age cohort of MVNV children with autism (Kuschner et al., 2021). Given that the present cohort was chronologically younger and many had developmental delays, the present results are encouraging.
Future directions and limitations
Present findings show an association between neural signatures of left hemisphere auditory processing and language. A future direction is to investigate whether auditory neural measures at a young age (e.g., 3 years old) contribute to the prediction of functional outcome in school-aged children (e.g., 6 years old), and thus at an age when a formal diagnosis of intellectual disability is usually established. Prospective follow-up of the current cohort will help answer the above.
Continued efforts to encourage and accommodate more inclusive scanning methodologies are warranted from scientific and sociological perspectives. The application of MEG-PLAN to younger children represents a promising avenue to achieve this inclusivity. The use of clinical and technical protocols such as MEG-PLAN across neuroimaging technologies will expand our understanding of neural processes across a broad range of phenotypic presentations and increase the generalization of results.
Although no sex difference was observed on M50 measures as well as language and cognitive scores, given exploratory findings of differential relationships between M50 and language ability in females and males, future studies with large sample sizes and age ranges are warranted to explore potential sex effects, particularly on the relationships between M50 latency and language scores in young children with ID/DD-EL.
In summary, non-invasive, quantitative, and hemisphere-specific assays of neural auditory processing offer insight into the physiological etiology of language impairment. They may also provide a targeted substrate for individualized interventions and serve as an objective brain marker for predicting later language outcomes.
Statements
Data availability statement
The MEG data for this project (de-identified) may be obtained via a Data Use Agreement with the Children’s Hospital of Philadelphia. Researchers interested in access to the data may contact Dr. Yuhan Chen at cheny4@chop.edu. Software for MEG data analysis associated with the current submission is available at https://mne.tools/stable/index.html and https://neuroimage.usc.edu/brainstorm/.
Ethics statement
The studies involving humans were approved by The Children’s Hospital of Philadelphia Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing, Project administration. LY: Project administration, Writing – review & editing. MK: Data curation, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. SW: Project administration, Writing – review & editing. VK: Writing – review & editing, Project administration. BB: Project administration, Writing – review & editing. IT: Writing – review & editing, Project administration. BW: Project administration, Writing – review & editing. DM-M: Writing – review & editing, Project administration. JE: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. EK: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. TR: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Institutes of Health U54-HD086984 to TR; P50-HD105354 to TR; R01-MH129594, R01-DC021051, and R61-MH135114 to TR, R01-HD093776, and R01-MH107506 to JE, and 3UG1HD068244-13S1 to YC (principal investigator Sara DeMauro).
Acknowledgments
The authors would like to thank the children and families who participated in this study and John Dell, Rachel Golembski, Erin Verzella, Peter Lam, Shivani Patel, and Na’Keisha Robinson.
Conflict of interest
TR discloses consulting agreements/equity positions with Prism Clinical Imaging, Proteus Neurodynamics, Fieldline Inc., Spago Nanomedicine and Westcan Proton Therapy.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1
Baillet S. Mosher J. C. Leahy R. M. (2001). Electromagnetic brain mapping. IEEE Signal Process. Mag.18, 14–30. doi: 10.1109/79.962275
2
Bayley N. Aylward G. P. (2019). Bayley scales of infant and toddler development. 4th Edn. Bloomington, MN: Pearson Education, Inc.
3
Belardi K. Watson L. R. Faldowski R. A. Hazlett H. Crais E. Baranek G. T. et al . (2017). A retrospective video analysis of canonical babbling and volubility in infants with fragile X syndrome at 9-12 months of age. J. Autism Dev. Disord.47, 1193–1206. doi: 10.1007/s10803-017-3033-4
4
Benasich A. A. Thomas J. J. Choudhury N. Leppänen P. H. (2002). The importance of rapid auditory processing abilities to early language development: evidence from converging methodologies. Dev. Psychobiol.40, 278–292. doi: 10.1002/dev.10032
5
Chen Y. Green H. L. Putt M. E. Allison O. Kuschner E. S. Kim M. et al . (2023). Maturation of auditory cortex neural responses during infancy and toddlerhood. NeuroImage275:120163. doi: 10.1016/j.neuroimage.2023.120163
6
Chen Y. Saby J. Kuschner E. Gaetz W. Edgar J. C. Roberts T. P. L. (2019). Magnetoencephalography and the infant brain. Neuroimage189, 445–458.
7
Chen Y. Slinger M. Edgar J. C. Bloy L. Kuschner E. S. Kim M. et al . (2021). Maturation of hemispheric specialization for face encoding during infancy and toddlerhood. Dev. Cogn. Neurosci.48:100918. doi: 10.1016/j.dcn.2021.100918
8
Chilosi A. M. Pfanner L. Pecini C. Salvadorini R. Casalini C. Brizzolara D. et al . (2019). Which linguistic measures distinguish transient from persistent language problems in late talkers from 2 to 4 years? A study on Italian speaking children. Res. Dev. Disabil.89, 59–68. doi: 10.1016/j.ridd.2019.03.005
9
Choudhury N. Benasich A. A. (2011). Maturation of auditory evoked potentials from 6 to 48 months: prediction to 3 and 4 year language and cognitive abilities. Clin. Neurophysiol.122, 320–338. doi: 10.1016/j.clinph.2010.05.035
10
Dale P. S. Price T. S. Bishop D. V. Plomin R. (2003). Outcomes of early language delay: I. Predicting persistent and transient language difficulties at 3 and 4 years. J. Speech Lang. Hear. Res.46, 544–560. doi: 10.1044/1092-4388(2003/044)
11
Edgar J. C. Blaskey L. Green H. L. Konka K. Shen G. Dipiero M. A. et al . (2020). Maturation of auditory cortex neural activity in children and implications for auditory clinical markers in diagnosis. Front. Psych.11:584557. doi: 10.3389/fpsyt.2020.584557
12
Edgar J. C. Khan S. Y. Blaskey L. Chow V. Y. Rey M. Gaetz W. et al . (2015a). Neuromagnetic oscillations predict evoked-response latency delays and core language deficits in autism spectrum disorders. J. Autism Dev. Disord.45, 395–405. doi: 10.1007/s10803-013-1904-x
13
Edgar J. C. Lanza M. R. Daina A. B. Monroe J. F. Khan S. Y. Blaskey L. et al . (2014). Missing and delayed auditory responses in young and older children with autism spectrum disorders. Front. Hum. Neurosci.8:417. doi: 10.3389/fnhum.2014.00417
14
Edgar J. C. Murray R. Kuschner E. S. Pratt K. Paulson D. N. Dell J. et al . (2015b). The maturation of auditory responses in infants and young children: a cross-sectional study from 6 to 59 months. Front. Neuroanat.9:131. doi: 10.3389/fnana.2015.00131
15
Gage N. M. Siegel B. Roberts T. P. (2003). Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Brain Res. Dev. Brain Res.144, 201–209. doi: 10.1016/s0165-3806(03)00172-x
16
Hämäläinen M. S. Ilmoniemi R. J. (1994). Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Comput.32, 35–42. doi: 10.1007/BF02512476
17
Hanson E. Bernier R. Porche K. Jackson F. I. Goin-Kochel R. P. Snyder L. G. et al . (2015). The cognitive and behavioral phenotype of the 16p11.2 deletion in a clinically ascertained population. Biol. Psychiatry77, 785–793. doi: 10.1016/j.biopsych.2014.04.021
18
Hauk O. (2004). Keep it simple: a case for using classical minimum norm estimation in the analysis of EEG and MEG data. NeuroImage21, 1612–1621. doi: 10.1016/j.neuroimage.2003.12.018
19
Hickok G. Poeppel D. (2007). The cortical organization of speech processing. Nat. Rev. Neurosci.8, 393–402. doi: 10.1038/nrn2113
20
Jeste S. S. Frohlich J. Loo S. K. (2015). Electrophysiological biomarkers of diagnosis and outcome in neurodevelopmental disorders. Curr. Opin. Neurol.28, 110–116. doi: 10.1097/WCO.0000000000000181
21
Kozak M. J. Cuthbert B. N. (2016). The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology53, 286–297. doi: 10.1111/psyp.12518
22
Kuschner E. S. Kim M. Bloy L. Dipiero M. Edgar J. C. Roberts T. P. L. (2021). MEG-PLAN: a clinical and technical protocol for obtaining magnetoencephalography data in minimally verbal or nonverbal children who have autism spectrum disorder. J. Neurodev. Disord.13:8. doi: 10.1186/s11689-020-09350-1
23
Lin F. H. Witzel T. Ahlfors S. P. Stufflebeam S. M. Belliveau J. W. Hämäläinen M. S. (2006). Assessing and improving the spatial accuracy in MEG source localization by depth-weighted minimum-norm estimates. NeuroImage31, 160–171. doi: 10.1016/j.neuroimage.2005.11.054
24
Matsuura K. Okabe Y. (1995). Selective minimum-norm solution of the biomagnetic inverse problem. I.E.E.E. Trans. Biomed. Eng.42, 608–615. doi: 10.1109/10.387200
25
Miller G. A. Rockstroh B. S. Hamilton H. K. Yee C. M. (2016). Psychophysiology as a core strategy in RDoC. Psychophysiology53, 410–414. doi: 10.1111/psyp.12581
26
Musacchia G. Choudhury N. A. Ortiz-Mantilla S. Realpe-Bonilla T. Roesler C. P. Benasich A. A. (2013). Oscillatory support for rapid frequency change processing in infants. Neuropsychologia51, 2812–2824. doi: 10.1016/j.neuropsychologia.2013.09.006
27
Musacchia G. Ortiz-Mantilla S. Realpe-Bonilla T. Roesler C. P. Benasich A. A. (2015). Infant auditory processing and event-related brain oscillations. J. Vis. Exp.101:e52420. doi: 10.3791/52420
28
Naatanen R. Picton T. (1987). The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology24, 375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x
29
O’Reilly C. Larson E. Richards J. E. Elsabbagh M. (2021). Structural templates for imaging EEG cortical sources in infants. Neuroimage227:117682.
30
Paetau R. Ahonen A. Salonen O. Sams M. (1995). Auditory evoked magnetic fields to tones and pseudowords in healthy children and adults. J. Clin. Neurophysiol.12, 177–185. doi: 10.1097/00004691-199503000-00008
31
Parviainen T. Helenius P. Salmelin R. (2019). Children show hemispheric differences in the basic auditory response properties. Hum. Brain Mapp.40, 2699–2710. doi: 10.1002/hbm.24553
32
Rescorla L. (2002). Language and reading outcomes to age 9 in late-talking toddlers. J. Speech Lang. Hear. Res.45, 360–371. doi: 10.1044/1092-4388(2002/028)
33
Richards J. E. Sanchez C. Phillips-Meek M. Xie W. (2016). A database of age-appropriate average MRI templates. Neuroimage124, 1254–1259.
34
Rinaldi P. Pasqualetti P. Volterra V. Caselli M. C. (2023). Gender differences in early stages of language development. Some evidence and possible explanations. J. Neurosci. Res.101, 643–653. doi: 10.1002/jnr.24914
35
Roberts T. P. Heiken K. Kahn S. Y. Qasmieh S. Blaskey L. Solot C. et al . (2012). Delayed magnetic mismatch negativity field, but not auditory M100 response, in specific language impairment [research support, N.I.H., extramural research support, non-U.S. Gov't]. Neuroreport23, 463–468. doi: 10.1097/WNR.0b013e32835202b6
36
Roberts T. P. Khan S. Y. Rey M. Monroe J. F. Cannon K. Blaskey L. et al . (2010). MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism [research support, N.I.H., extramural research support, non-U.S. Gov't]. Autism Res.3, 8–18. doi: 10.1002/aur.111
37
Roberts T. P. L. Gaetz W. C. Birnbaum C. Bloy L. Berman J. I. (2024). Towards biomarkers for autism spectrum disorder: contributions of magnetoencephalography (MEG). Adv. Neurobiol.40, 455–489. doi: 10.1007/978-3-031-69491-2_16
38
Roberts T. P. L. Matsuzaki J. Blaskey L. Bloy L. Edgar J. C. Kim M. et al . (2019). Delayed M50/M100 evoked response component latency in minimally verbal/nonverbal children who have autism spectrum disorder. Mol. Autism.10:34. doi: 10.1186/s13229-019-0283-3
39
SAS Institute Inc. (2021). JMP Pro, Version 18. Cary, NC: SAS Institute Inc.
40
Stroganova T. A. Kozunov V. V. Posikera I. N. Galuta I. A. Gratchev V. V. Orekhova E. V. (2013). Abnormal pre-attentive arousal in young children with autism spectrum disorder contributes to their atypical auditory behavior: an ERP study. PLoS One8:e69100. doi: 10.1371/journal.pone.0069100
41
Tadel F. Baillet S. Mosher J. C. Pantazis D. Leahy R. M. (2011). Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci.2011:879716. doi: 10.1155/2011/879716
42
Tassone F. Hagerman R. J. Ikle D. N. Dyer P. N. Lampe M. Willemsen R. et al . (1999). FMRP expression as a potential prognostic indicator in fragile X syndrome. Am. J. Med. Genet.84, 250–261. doi: 10.1002/(SICI)1096-8628(19990528)84:3<250::AID-AJMG17>3.0.CO;2-4
43
Wechsler D. (2012). Wechsler preschool and primary scale of intelligence. 4th Edn. San Antonio, TX: Pearson Psychological Corporation.
44
Whittington J. Holland A. (2017). Cognition in people with Prader-Willi syndrome: insights into genetic influences on cognitive and social development. Neurosci. Biobehav. Rev.72, 153–167. doi: 10.1016/j.neubiorev.2016.09.013
45
Wilson T. W. Rojas D. C. Reite M. L. Teale P. D. Rogers S. J. (2007). Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol. Psychiatry62, 192–197. doi: 10.1016/j.biopsych.2006.07.002
46
Yee C. M. Javitt D. C. Miller G. A. (2015). Replacing DSM categorical analyses with dimensional analyses in psychiatry research: the research domain criteria initiative. JAMA Psychiatry72, 1159–1160. doi: 10.1001/jamapsychiatry.2015.1900
47
Yoshimura Y. Kikuchi M. Shitamichi K. Ueno S. Remijn G. B. Haruta Y. et al . (2012). Language performance and auditory evoked fields in 2-to 5-year-old children. Eur. J. Neurosci.35, 644–650. doi: 10.1111/j.1460-9568.2012.07998.x
48
Yoshimura Y. Kikuchi M. Ueno S. Shitamichi K. Remijn G. B. Hiraishi H. et al . (2014). A longitudinal study of auditory evoked field and language development in young children. NeuroImage101, 440–447. doi: 10.1016/j.neuroimage.2014.07.034
49
Zimmerman I. L. Steiner V. G. Pond R. E. (2011). Preschool language scale, fifth edition (PLS-5). San Antonio, TX: Pearson.
Summary
Keywords
MEG, M50, IDD, language, preschool children
Citation
Chen Y, Young L, Kim M, Watson S, Kaufman V, Beal B, Tuomi I, Wang B, McDonald-McGinn DM, Edgar JC, Kuschner ES and Roberts TPL (2025) Auditory evoked neuromagnetic response latency is associated with language ability in preschoolers with an elevated likelihood of intellectual or developmental disability. Front. Integr. Neurosci. 19:1585567. doi: 10.3389/fnint.2025.1585567
Received
28 February 2025
Accepted
29 April 2025
Published
23 May 2025
Volume
19 - 2025
Edited by
Yingying Wang, University of Nebraska-Lincoln, United States
Reviewed by
Ivana Aras, SUVAG Polyclinic Zagreb, Croatia
Xiaolin Huo, Chinese Academy of Sciences (CAS), China
Lily Chamakura, Baylor College of Medicine, United States
Updates
Copyright
© 2025 Chen, Young, Kim, Watson, Kaufman, Beal, Tuomi, Wang, McDonald-McGinn, Edgar, Kuschner and Roberts.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhan Chen, cheny4@chop.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.