- 1Program in Biochemistry, Vassar College, Poughkeepsie, NY, United States
- 2Department of Biology, Program in Neuroscience and Behavior, Vassar College, Poughkeepsie, NY, United States
Traumatic brain injury (TBI) induces a wide range of neurodegenerative symptoms, yet effective treatment strategies remain limited. Emerging evidence suggests that post-TBI recovery recapitulates aspects of early brain development, highlighting the potential for developmental molecular mechanisms to inform therapeutic interventions. The transcription factor Otx2 is critical for early brain and sensory organ development, as well as the maintenance of retinal and neural function in adulthood. Notably, the transfer of Otx2 homeoprotein into parvalbumin-expressing (PV+) GABAergic interneurons is essential for opening and closing critical periods of plasticity across vertebrates. Here, we investigate the acute regulation of Otx2 mRNA following TBI in adult zebra finches (ZF) to evaluate its potential as a target for future study and therapeutic manipulation in neural repair. Adult ZFs sustained unilateral hemispheric brain injuries, and qPCR was used to quantify Otx2 mRNA expression at 24 hours and 1 week post-injury in both males and females. Our findings reveal a significant downregulation of Otx2 mRNA expression following injury, highlighting Otx2 as a potential target for further investigation and manipulation. These results provide insight into the molecular response to brain injury and suggest a potential link between developmental pathways and post-injury plasticity.
1 Introduction
Traumatic brain injury (TBI) is a significant public health burden worldwide (Dewan et al., 2019; Flanagan, 2015) and remains one of the leading causes of injury-related death and disability (Maas et al., 2008). TBIs can result in substantial long-term health consequences, including increased risks of developing Alzheimer’s Disease, Parkinson’s Disease, chronic traumatic encephalopathy, and nearly doubling the risk of suicide (Bielanin et al., 2023). Despite this prevalence and burden, effective treatment options remain limited (Watson et al., 2016). While there have been few advances in clinical management, extensive research has focused on identifying therapeutic targets with potential for clinical application (Tabet et al., 2022; Bruhns et al., 2022; Kim et al., 2022; Song et al., 2023).
For decades, researchers have noted parallels between the stages of normal neuronal development and recovery from brain injury (Cramer and Chopp, 2000). Specifically, motor function recovery across vertebrates mirrors the developmental processes of those functions (Cramer and Chopp, 2000; Berenbaum and Hines, 1992; Teitelbaum et al., 1969; Wolgin et al., 1980). Post-injury plasticity involves molecular and cellular events akin to those occurring during normal brain development (Nudo, 2003; Nudo, 2013). Neurons undergo similar stages of growth and reorganization during recovery, suggesting that targeting developmental pathways could enhance therapeutic outcomes. We propose that effective treatments for brain injury should activate these normal developmental processes to promote recovery. Studying neuroplasticity during early recovery phases may yield valuable insights into the brain’s restorative mechanisms (Song et al., 2023; Cramer and Chopp, 2000).
Songbirds provide a valuable model for studying the connections between development and recovery from injury. The songbird brain contains a specialized system of interconnected nuclei responsible for singing and song learning, known as the song system. Estrogens play a critical role in the development and sexual differentiation of song control nuclei in songbirds by influencing neuronal growth and connectivity, particularly during early life stages (Gurney and Konishi, 1980; Schlinger and Arnold, 1992; Cornil et al., 2006). Juvenile songbirds learn to sing during a sensitive period involving two sequential phases—sensory and sensorimotor learning—both of which depend on auditory experience (Mooney, 2009). This naturally occurring framework provides a model for investigating whether developmental organizational patterns re-emerge after injury. Notably, sites of neuronal recovery extend beyond the confines of the song system, suggesting the involvement of broader, more robust regulatory mechanisms. Previous work has identified recovery-related activity in regions outside the song system, revealing striking similarities in the underlying mechanisms (Wynne et al., 2008; Duncan and Saldanha, 2011). Additionally, recent research has identified genes undergoing epigenetic regulation during song learning, offering promising targets for understanding recovery processes in the adult brain (Diddens et al., 2021). For this report, we will focus on the role of orthodenticle homeobox 2 Otx2, which has been previously identified during song nuclei development (Diddens et al., 2021) and among vertebrate development in general as it relates to coordination of A-P and D-V patterning by regulating the expression of key morphogenetic signals (Puelles et al., 2003; Puelles et al., 2004; Vernay et al., 2005; Bernard and Prochiantz, 2016; Sugiyama et al., 2009). Beyond its developmental role, Otx2 regulates perineuronal net (PNN) activity, which modulates plasticity and closes sensitive periods for vocal learning in songbirds (Cornez et al., 2017). Our research has found that following brain injury, PNNs exhibit a brief period of increased plasticity that may facilitate recovery from injury (Talwalkar et al., 2024), however how changes in Otx2 expression might trigger PNN formation and enhance neuronal plasticity remain unknown.
To begin investigating Otx2’s role following TBI, we examined its expression at two time points post-injury. The two timepoints selected have previously been identified as critical windows for steroid-mediated neuroprotection in the zebra finch brain, during which local estrogen synthesis particularly by reactive glia plays a key role in promoting neuronal survival and limiting apoptosis following injury (Duncan and Saldanha, 2011; Wynne et al., 2008; Pedersen et al., 2017). We observed a decrease in Otx2 mRNA expression, suggesting a potential role in post-TBI recovery. Given the well-documented neuroprotective effects of estrogen in the zebra finch brain (Saldanha, 2020; Holloway and Clayton, 2001), we also tested whether estrogen availability influences injury-induced Otx2 mRNA expression. These findings highlight Otx2 as a promising candidate for future studies exploring its potential as a therapeutic target following damage to the brain.
2 Methods
2.1 Animals
Adult (> 90 days post hatching) Australian zebra finches were originally obtained from a breeder (Magnolia Bird Farms; Anaheim, CA) and group housed in the animal facility at Vassar College. The Vassar College Institutional Animal Care and Use Committee approved all animal procedures. All subjects were pseudo-randomly assigned to groups at the time of testing. Birds were provided with food and water ad libitum and maintained under a 14:10 light: dark cycle.
2.2 Penetrating brain injury
Prior to injury, 4% Lidocaine hydrochloride (Lidocaine Plus, Barboursville, WV) was applied to the top of the subject’s head. All subjects were then anesthetized with 5% isoflurane and maintained at 2–3.5% (Henry Schein Animal Health, Dublin, OH). Subjects were then positioned in a stereotaxic apparatus (Stoelting, Wood Dale, IL) with the head angled downward at 45°. When the cranium was exposed, a bore needle was used to create a bilateral craniotomy. The injury was targeted to the entopallial nucleus (2 mm anterior to the pineal, 3 mm lateral to the midline, 3 mm ventral to the dural surface) because this area lacks detectable steroid hormone/aromatase expression in the absence of injury in zebra finches (Wynne et al., 2008; Saldanha et al., 2000; Shen et al., 1995; Walters et al., 2011). A 22 s Hamilton syringe (Hamilton Company, Reno, NV) was positioned at the surface of the brain and lowered 3-mm to target the area. Subjects (n = 5–6 per treatment group, with equal males and females) were pseudo-randomly assigned to receive either Fadrozole or vehicle. The needle remained in position for 60 s. The scalp incision was sealed with Collodion Flexible (EM Science, Gibbstown, NJ) or Vetbond Tissue Adhesive (3 M, Saint Paul, MN) and birds were allowed to recover under a heat lamp until they were returned to the aviary. For sham animals, the cranium was exposed, but no craniotomy or injection was performed.
2.3 Fadrozole administration
Birds were administered either fadrozole (Sigma-Aldrich, St. Louis, MO, USA) or vehicle (saline) via intracranial injection at the time of surgery. Each bird received 5 μL of either fadrozole (10 mg/mL; 50 μg per injection) suspended in vehicle or 5 μL of vehicle alone. Injections were delivered at a rate of 2.5 μL/s (Wynne et al., 2008; Cook et al., 2019), and the needle was left in place for 90 s following infusion. This protocol has been previously shown to significantly reduce estrogen levels in brain tissue surrounding the injection site (Walters et al., 2011; Charlier et al., 2011; Pedersen et al., 2016).
2.4 cDNA synthesis and qPCR
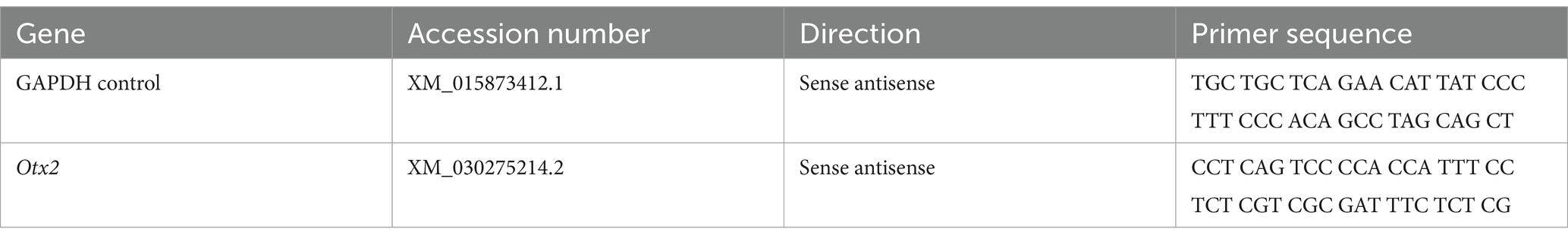
Following an overdose of isoflurane, zebra finches were rapidly decapitated, and brain tissue was immediately extracted. One telencephalic lobe was dissected and homogenized in 1 mL of TRIzol reagent for RNA extraction. Individual birds and treatments were treated as biological replicates for all statistical analyses. Total RNA was isolated according to the manufacturer’s protocol. RNA quality and purity were assessed using a NanoDrop One spectrophotometer (NanoDrop, Wilmington, DE), and only samples with 260/280 absorbance ratios greater than 1.85 were included in downstream analyses. While 260/280 ratios are commonly used to evaluate RNA purity, we acknowledge that RNA Integrity Numbers (RIN) or gel electrophoresis were not performed, and this represents a limitation of the current study. For each qPCR experiment, 1 μg of total RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). qPCR reactions were performed using 1 μL of resulting cDNA and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, CA) in a total volume of 10 μL. Reactions were carried out in 96-well optical plates, with each sample run in duplicate on a QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific Inc.). Amplicons targeted exonic regions of the genes listed in Table 1.
2.5 Statistical analysis
The delta threshold cycle number (ΔCt) method was used for quantification of the qPCR data using the detection threshold (Ct) for each target gene less the Ct for the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). GAPDH was chosen as a reference because of its stability as a reference gene in songbird brain and has been previously validated (Zinzow-Kramer et al., 2014; Rensel and Schlinger, 2020). Comparative Ct measurements give a relative expression difference between samples, where a lower number means greater mRNA expression. All statistical analyses were performed on ΔCt values, and are presented as means ± SEM. Data were analyzed using a two-way ANOVA with treatment and sex as between-subject variables in GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Post hoc comparisons following significant main effects or interactions were conducted using Sidak’s multiple comparisons test to control for Type I error. A significance level of α = 0.05 was used for all analyses.
3 Results
3.1 Experiment 1: Otx2 expression following injury at 24 h and 1 week post injury
Otx2 expression was measured at two timepoints (24 h (24 h) and 1 week(1 W)) following injury. Two-way ANOVA revealed a significant effect of injury on Otx2 expression at 1 week (Figure 1C), but not for 24 h (Figure 1A); F1W(1, 15) = 6.847, p = 0.019; F24h(1, 15) = 0.192, p = 0.192, but not of sex F1W(1, 15) = 3.751, p = 0.072; F24h(1, 15) = 2.315, p = 0.149, and no overall interaction between sex and injury: F1W(1, 15) = 2.429, p = 0.140; F24h(1, 15) = 0.118, p = 0.735. These data suggest that Otx2 mRNA expression is decreased following injury and may allow for increased neuronal plasticity following injury.
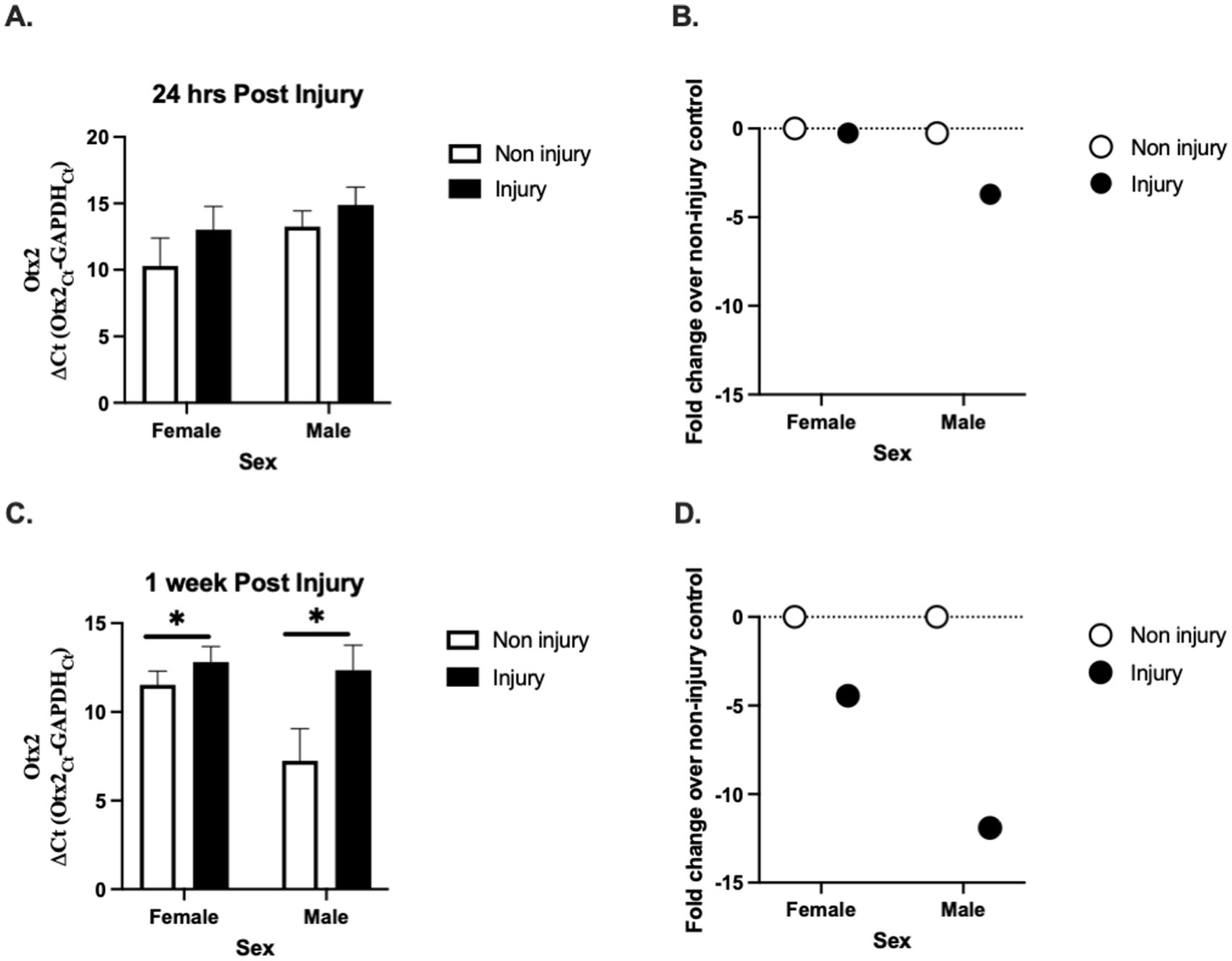
Figure 1. Effect of injury on Otx2 mRNA expression in the adult zebra finch (ZF) brain. Otx2 mRNA expression in adult male and female ZFs at 24 h (A) and 1 week (C) relative to GAPDH (ΔCt values ± SEM) following injury. Mean ΔCt values are presented where a lower number means greater mRNA expression. 1 week following injury Otx2 mRNA expression was significantly lower compared to sham controls. There was no effect of sex at either timepoint. For a better clarity, fold change (B,D) values ± SEM over uninjured controls are also presented. The dashed line represents uninjured controls (controls were set to 1 for fold change calculation). n = 5 per sex and per treatment * Denotes a significant effect of injury at p < 0.05. These data suggest that Otx2 mRNA expression is decreased following injury and may allow for increased neuronal plasticity following injury (B,D).
3.2 Experiment 2: homeodomain expression following injury and circulating estrogen depletion
Following injury, Otx2 mRNA expression (Figure 2A) was not altered by circulating estrogen levels; F(1, 20) = 0.07, p = 0.794 in males or females F(1, 20) = 0.729, p = 0.403 with no interaction between the two F(1, 20) = 0.04, p = 0.843. Fold change calculations show no difference in expression (Figure 2B).
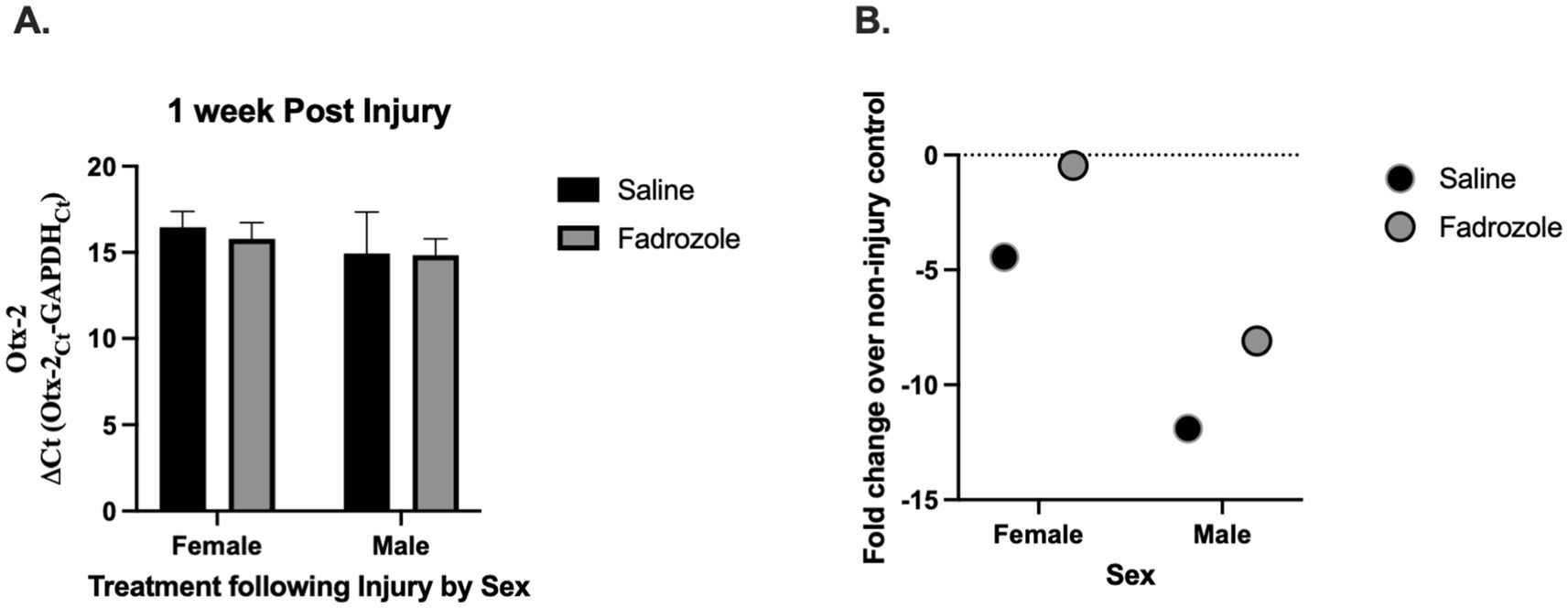
Figure 2. Effect of estrogen depletion on Otx2 mRNA expression following injury. Otx2 (A) mRNA expression relative to GAPDH (ΔCt values ± SEM) in adult male and female zebra finches at 1 week post injury. Fadrozole (aromatase inhibitor) treatment had no significant effect at 1 week. Mean ΔCt values are presented where a lower number means greater mRNA expression and fold change values. (B) Over injured controls are presented for clarity. The dashed line represents injured controls (controls were set to 1 for fold change calculation). n = 5–6 per sex and per treatment.
4 Discussion
Our study examined the role of Otx2 mRNA in the zebra finch brain following traumatic brain injury (TBI). We observed a significant decrease in Otx2 mRNA expression post-injury (Figure 1A), suggesting an involvement in neuronal response to TBI and highlighting it as a potential target for further investigation. Despite the known neuroprotective effects of estrogens in the zebra finch brain, reduced estrogen levels had no effect on Otx2 mRNA expression following injury. This suggests that Otx2 mRNA regulation following TBI occurs independently of estrogen signaling.
4.1 Otx2 and critical period plasticity
Otx2 is a transcription factor essential for neuronal regulation and has been identified as a key regulator in closing critical periods of plasticity in both developing and adult brains (Sugiyama et al., 2009; Reichelt et al., 2019; Beurdeley et al., 2012). These critical periods represent windows of heightened neural plasticity during which environmental stimuli strongly shape circuit formation. Reopening such periods after injury may be important for enabling neural repair and reorganization (Hensch, 2005; Takesian and Hensch, 2013), a phenomenon also observed in mammalian models, suggesting an evolutionarily conserved mechanism (Fawcett et al., 2019; Pizzorusso et al., 2006).
While our interpretation is informed by Otx2’s established role in critical period closure (Sugiyama et al., 2009; Beurdeley et al., 2012; Spatazza et al., 2013), the transcriptional pathways involved may diverge following injury in the adult zebra finch brain (Spatazza et al., 2013; Di Nardo et al., 2018). Furthermore, we only examined Otx2 mRNA expression at two timepoints. These timepoints represented pivotal times associated with estrogen mediated neuroprotection, but later timepoints may be necessary to fully understand the role of Otx2 in neuronal recovery. Future studies incorporating functional and behavioral analyses are necessary to clarify the role of Otx2 in injury-induced neuroplasticity and critical periods. It is important to note that mRNA expression does not always correlate with protein abundance due to post-transcriptional regulation, mRNA stability, and translational control; thus, without protein-level validation, the observed downregulation of Otx2 mRNA remains an incomplete indicator of functional change. Notably, Otx2 mRNA expression was unaffected by estrogen depletion, suggesting that its regulation may occur independently of estrogen signaling in this context. Estrogens are known to influence the growth and connectivity of song nuclei during the critical period, increasing HVC volume and promoting synaptogenesis (Fusani and Gahr, 2006; Herrmann and Arnold, 1991; Charlier et al., 2010; Brenowitz, 2015). After injury, local estrogen production is upregulated through aromatase-expressing glial cells, a response that supports neuronal survival and repair (Saldanha, 2020; Lee et al., 2007; Duncan, 2020). Further research incorporating functional, behavioral, and hormonal measures will be necessary to determine whether Otx2 downregulation supports or impairs recovery after TBI.
5 Conclusion
Our findings highlight Otx2 as potential target for further study into the mechanisms and therapeutic strategies surrounding neuronal injury. Future research should focus on elucidating the molecular mechanisms underlying this process and exploring therapeutic strategies that harness critical period plasticity to promote recovery from neuronal injuries (Hensch, 2005; Bavelier et al., 2010). By drawing on developmental parallels, we may be able to design interventions that optimize recovery outcomes and improve the quality of life for individuals with brain injuries.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AT: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. KD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Vassar College’s Lucy Maynard Salmon Research Fund and the Patricia Shoer Goldman-Rakic 59 Professorship fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bavelier, D., Levi, D. M., Li, R. W., Dan, Y., and Hensch, T. K. (2010). Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J. Neurosci. 30, 14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010
Berenbaum, S. A., and Hines, M. (1992). Early androgens are related to childhood sex-typed toy preferences. Psychol. Sci. Public Interest 3, 203–206. doi: 10.1111/j.1467-9280.1992.tb00028.x
Bernard, C., and Prochiantz, A. (2016). Otx2-PNN interaction to regulate cortical plasticity. Neural Plast. 2016:7931693. doi: 10.1155/2016/7931693
Beurdeley, M., Spatazza, J., Lee, H. H. C., Sugiyama, S., Bernard, C., Di Nardo, A. A., et al. (2012). Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J. Neurosci. 32, 9429–9437. doi: 10.1523/JNEUROSCI.0394-12.2012
Bielanin, J. P., Metwally, S. A. H., and Paruchuri, S. S. (2023). Sun D. An overview of mild traumatic brain injuries and emerging therapeutic targets. Neurochem. Int. 172:105655. doi: 10.1016/j.neuint.2023.105655
Brenowitz, E. A. (2015). Transsynaptic trophic effects of steroid hormones in an avian model of adult brain plasticity. Front. Neuroendocrinol. 37, 119–128. doi: 10.1016/j.yfrne.2014.09.003
Bruhns, R. P., Sulaiman, M. I., Gaub, M., Bae, E. H., Davidson Knapp, R. B., Larson, A. R., et al. (2022). Angiotensin-(1-7) improves cognitive function and reduces inflammation in mice following mild traumatic brain injury. Front. Behav. Neurosci. [Internet] 16:903980. doi: 10.3389/fnbeh.2022.903980
Charlier, T. D., Newman, A. E., Heimovics, S. A., Po, K. W., Saldanha, C. J., and Soma, K. K. (2011). Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J. Neuroendocr. 23, 742–753. doi: 10.1111/j.1365-2826.2011.02170.x
Charlier, T. D., Po, K. W. L., Newman, A. E. M., Shah, A. H., Saldanha, C. J., and Soma, K. K. (2010). 17β-estradiol levels in male zebra finch brain: combining Palkovits punch and an ultrasensitive radioimmunoassay. Gen. Comp. Endocrinol. 167, 18–26. doi: 10.1016/j.ygcen.2010.02.002
Cook, S., Hung, V., and Duncan, K. A. (2019). Crosstalk between estrogen withdrawal and NFκB signaling following penetrating brain injury. Neuroimmunomodulation 25, 193–200. doi: 10.1159/000493506
Cornez, G., Madison, F. N., Van der Linden, A., Cornil, C., Yoder, K. M., Ball, G. F., et al. (2017). Perineuronal nets and vocal plasticity in songbirds: a proposed mechanism to explain the difference between closed-ended and open-ended learning. Dev. Neurobiol. 77, 975–994. doi: 10.1002/dneu.22485
Cornil, C. A., Ball, G. F., and Balthazart, J. (2006). Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 1126, 2–26. doi: 10.1016/j.brainres.2006.07.098
Cramer, S. C., and Chopp, M. (2000). Recovery recapitulates ontogeny. Trends Neurosci. 23, 265–271. doi: 10.1016/S0166-2236(00)01562-9
Dewan, M. C., Rattani, A., Gupta, S., Baticulon, R. E., Hung, Y. C., Punchak, M., et al. (2019). Estimating the global incidence of traumatic brain injury. J. Neurosurg. 130, 1080–1097. doi: 10.3171/2017.10.JNS17352
Di Nardo, A. A., Fuchs, J., Joshi, R. L., Moya, K. L., and Prochiantz, A. (2018). The physiology of homeoprotein transduction. Physiol. Rev. 98, 1943–1982. doi: 10.1152/physrev.00018.2017
Diddens, J., Coussement, L., Frankl-Vilches, C., Majumdar, G., Steyaert, S., Ter Haar, S. M., et al. (2021). DNA methylation regulates transcription factor-specific neurodevelopmental but not sexually dimorphic gene expression dynamics in zebra finch telencephalon. Front. Cell Dev. Biol. 9:9. doi: 10.3389/fcell.2021.583555
Duncan, K. A. (2020). Estrogen formation and inactivation following TBI: what we know and where we could go. Front. Endocrinol. 11:11. doi: 10.3389/fendo.2020.00345
Duncan, K. A. A., and Saldanha, C. J. J. (2011). Neuroinflammation induces glial aromatase expression in the uninjured songbird brain. J. Neuroinflammation 8:81. doi: 10.1186/1742-2094-8-81
Fawcett, J. W., Oohashi, T., and Pizzorusso, T. (2019). The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat. Rev. Neurosci. 20, 451–465. doi: 10.1038/s41583-019-0196-3
Flanagan, S. R. (2015). Invited commentary on centers for disease control and prevention report to congress: traumatic brain injury in the United States: epidemiology and rehabilitation. Arch. Phys. Med. Rehabil. 96, 1753–1755. doi: 10.1016/j.apmr.2015.07.001
Fusani, L., and Gahr, M. (2006). Hormonal influence on song structure and organization: the role of estrogen. Neuroscience 138, 939–946. doi: 10.1016/j.neuroscience.2005.08.041
Gurney, M. E., and Konishi, M. (1980). Hormone induced sexual differentiation of brain and behavior in zebra finches. Science 208, 1380–1382.
Hensch, T. K. (2005). Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888. doi: 10.1038/nrn1787
Herrmann, K., and Arnold, A. P. (1991). Lesions of HVc block the developmental masculinizing effects of estradiol in the female zebra finch song system. J. Neurobiol. 22, 29–39. doi: 10.1002/neu.480220104
Holloway, C. C., and Clayton, D. F. (2001). Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat. Neurosci. 4, 170–175. doi: 10.1038/84001
Kim, J. T., Kim, T. Y., Youn, D. H., Han, S. W., Park, C. H., Lee, Y., et al. (2022). Human embryonic stem cell-derived cerebral organoids for treatment of mild traumatic brain injury in a mouse model. Biochem. Biophys. Res. Commun. 635, 169–178. doi: 10.1016/j.bbrc.2022.10.045
Lee, D. W., Fernando, G., Peterson, R. S., Allen, T. A., and Schlinger, B. A. (2007). Estrogen mediation of injury-induced cell birth in neuroproliferative regions of the adult zebra finch brain. Dev. Neurobiol. 67, 1107–1117. doi: 10.1002/dneu.20399
Maas, A. I., Stocchetti, N., and Bullock, R. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741. doi: 10.1016/S1474-4422(08)70164-9
Mooney, R. (2009). Neurobiology of song learning, current opinion in neurobiology. NIH Public Access 19, 654–660. doi: 10.1016/j.conb.2009.10.004
Nudo, R. J. (2003). Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys. Med. Rehabil. Clin. N. Am. 14, S57–S76. doi: 10.1016/S1047-9651(02)00054-2
Nudo, R. J. (2013). Recovery after brain injury: mechanisms and principles. Front. Hum. Neurosci. 7:887. doi: 10.3389/fnhum.2013.00887
Pedersen, A. L., Brownrout, J. L., and Saldanha, C. J. (2017). Central administration of indomethacin mitigates the injury-induced upregulation of aromatase expression and estradiol content in the zebra finch brain. Endocrinology 158, 2585–2592. doi: 10.1210/en.2017-00346
Pedersen, A. L., Nelson, L. H., and Saldanha, C. J. (2016). Centrally synthesized estradiol is a potent anti-inflammatory in the injured zebra finch brain. Endocrinology 157, 2041–2051. doi: 10.1210/en.2015-1991
Pizzorusso, T., Medini, P., Landi, S., Baldini, S., Berardi, N., and Maffei, L. (2006). Structural and functional recovery from early monocular deprivation in adult rats. Proc. Natl. Acad. Sci. USA 103, 8517–8522. doi: 10.1073/pnas.0602657103
Puelles, E., Acampora, D., Lacroix, E., Signore, M., Annino, A., Tuorto, F., et al. (2003). Otx dose-dependent integrated control of antero-posterior and dorso-ventral patterning of midbrain. Nat. Neurosci. 6, 453–460. doi: 10.1038/nn1037
Puelles, E., Annino, A., Tuorto, F., Usiello, A., Acampora, D., Czerny, T., et al. (2004). Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development 131, 2037–2048. doi: 10.1242/dev.01107
Reichelt, A. C., Hare, D. J., Bussey, T. J., and Saksida, L. M. (2019). Perineuronal Nets: Plasticity, Protection, and Therapeutic Potential. Trends Neurosci. 42, 458–470. doi: 10.1016/j.tins.2019.04.003
Rensel, M. A., and Schlinger, B. A. (2020). The stressed brain: regional and stress-related corticosterone and stress-regulated gene expression in the adult zebra finch (Taeniopygia guttata). J. Neuroendocrinol. 32:e12852. doi: 10.1111/jne.12852
Saldanha, C. J. (2020). Estrogen as a neuroprotectant in both sexes: stories from the bird brain. Front. Neurol. 11:497. doi: 10.3389/fneur.2020.00497
Saldanha, C. J., Tuerk, M. J., Kim, Y. H., Fernandes, A. O., Arnold, A. P., and Schlinger, B. A. (2000). Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J. Comp. Neurol. 423, 619–630. doi: 10.1002/1096-9861(20000807)423:4<619::AID-CNE7>3.0.CO;2-U
Schlinger, B. A., and Arnold, A. P. (1992). Circulating estrogens in a male songbird originate in the brain. Proc. Natl. Acad. Sci. USA 89, 7650–7653. doi: 10.1073/pnas.89.16.7650
Shen, P., Schlinger, B. A., Campagnoni, A. T., and Arnold, A. P. (1995). An atlas of aromatase mRNA expression in the zebra finch brain. J. Comp. Neurol. Psychol. 360, 172–184. doi: 10.1002/cne.903600113
Song, Y., Sun, Z. F., Sun, W. Z., Luo, M. L., Du, Y. J., Jing, J., et al. (2023). Neuroplasticity following stroke from a functional laterality perspective: a fNIRS study. Brain Topogr. 36, 283–293. doi: 10.1007/s10548-023-00946-z
Spatazza, J., Lee, H. H. C., di, A., Tibaldi, L., Joliot, A., Hensch, T. K., et al. (2013). Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep. 3, 1815–1823. doi: 10.1016/j.celrep.2013.05.014
Sugiyama, S., Prochiantz, A., and Hensch, T. K. (2009). From brain formation to plasticity: insights on Otx2 homeoprotein. Dev. Growth Differ. 51, 369–377. doi: 10.1111/j.1440-169X.2009.01093.x
Tabet, M., El-Kurdi, M., Haidar, M. A., Nasrallah, L., Reslan, M. A., Shear, D., et al. (2022). Mitoquinone supplementation alleviates oxidative stress and pathologic outcomes following repetitive mild traumatic brain injury at a chronic time point. Exp. Neurol. 351:113987. doi: 10.1016/j.expneurol.2022.113987
Takesian, A. E., and Hensch, T. K. (2013). Balancing plasticity/stability across brain development. Prog. Brain Res. 207, 3–34. doi: 10.1016/B978-0-444-63327-9.00001-1
Talwalkar, A., Haden, G., and Duncan, K. A. (2024). Chondroitin sulfate proteoglycans mRNA expression and degradation in the zebra finch following traumatic brain injury. J. Chem. Neuroanat. 138:102418. doi: 10.1016/j.jchemneu.2024.102418
Teitelbaum, P., Cheng, M. F., and Rozin, P. (1969). Development of feeding parallels its recovery after hypothalamic damage. J. Comp. Physiol. Psychol. 67, 430–441. doi: 10.1037/h0027288
Vernay, B., Koch, M., Vaccarino, F., Briscoe, J., Simeone, A., Kageyama, R., et al. (2005). Otx2 regulates subtype specification and neurogenesis in the midbrain. J. Neurosci. 25, 4856–4867. doi: 10.1523/JNEUROSCI.5158-04.2005
Walters, B. J., Alexiades, N. G., and Saldanha, C. J. (2011). Intracerebral estrogen provision increases cytogenesis and neurogenesis in the injured zebra finch brain. Dev. Neurobiol. 71, 170–181. doi: 10.1002/dneu.20839
Watson, H. R., Ghani, M., and Correll, T. (2016). Treatment options for individuals with PTSD and concurrent TBI: a literature review and case presentation. Curr. Psychiatry Rep. 18:63. doi: 10.1007/s11920-016-0699-9
Wolgin, D. L., Hein, A., and Teitelbaum, P. (1980). Recovery of forelimb placing after lateral hypothalamic lesions in the cat: parallels and contrasts with development. J. Comp. Physiol. Psychol. 94, 795–807. doi: 10.1037/h0077836
Wynne, R. D., Maas, S., and Saldanha, C. J. (2008). Molecular characterization of the injury-induced aromatase transcript in the adult zebra finch brain. J. Neurochem. 105, 1613–1624. doi: 10.1111/j.1471-4159.2008.05256.x
Wynne, R. D., Walters, B. J., Bailey, D. J., and Saldanha, C. J. (2008). Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia 56, 97–105. doi: 10.1002/glia.20594
Keywords: avian, neuroprotection, injury, homeoprotein, Otx2 gene
Citation: Talwalkar A and Duncan KA (2025) Otx2 mRNA expression is downregulated following traumatic brain injury in zebra finches. Front. Neural Circuits. 19:1591983. doi: 10.3389/fncir.2025.1591983
Edited by:
Jiandi Wan, University of California, Davis, United StatesReviewed by:
Xubin Hou, Niigata University, JapanDonald Joseph, University of Pennsylvania, United States
Copyright © 2025 Talwalkar and Duncan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelli A. Duncan, a2VkdW5jYW5AdmFzc2FyLmVkdQ==
 Adam Talwalkar
Adam Talwalkar Kelli A. Duncan
Kelli A. Duncan