- 1The Fleischer Institute for Diabetes and Metabolism, Albert Einstein College of Medicine, Bronx, NY, United States
- 2Division of Endocrinology, Department of Medicine, Albert Einstein College of Medicine, Bronx, NY, United States
- 3Department of Molecular Pharmacology, Albert Einstein College of Medicine, Bronx, NY, United States
- 4Dominick P. Purpura Department of Neuroscience, Albert Einstein College of Medicine, Bronx, NY, United States
Introduction: Prader-Willi syndrome (PWS) results from a lack of expression in several paternally inherited, imprinted contiguous genes. Among the genes inactivated in PWS, the Magel2 gene is considered a significant contributor to the etiology of the syndrome. The loss of the Magel2 gene causes abnormalities in growth and fertility and increased adiposity with altered metabolism in adulthood, which aligns with some of the pathologies observed in PWS. Given that anxiety is a prominent phenotypic behavior in PWS, we investigate the role of the Magel2 gene, particularly in hypothalamic POMC neurons innervating the medial amygdala (MeA), in the behavioral phenotypes associated with Prader-Willi Syndrome (PWS).
Methods: In this study, we used a retrograde AAV containing the Cre recombinase under the control of neuronal Pomc enhancers to genetically eliminate the Magel2 gene in MeA-innervating ARCPomc neurons.
Results: Both male and female mice lacking the Magel2 gene in MeA-innervating ARCPomc neurons display no alterations in anxiety-like behavior during the open field test, light/dark test, and elevated plus maze test in the absence of exposure to acute stress. However, male mice with a Magel2 gene deletion in these particular neurons exhibit increased stress-induced anxiety-like behavior and reduce motivation/spatial learning, while female mice do not show these behavioral changes. Our results suggest that the Magel2 gene in ARCPomc neurons, especially in males, influences the impact of stress on anxiety-like behavior and spatial learning deficits associated with a food reward.
Discussion: With the recent approval of a novel treatment for hyperphagia in PWS by the FDA that seems to target the hypothalamic melanocortin system, understanding the cellular mechanisms by which MAGEL2 in ARCPomc neurons innervating the MeA regulates emotional behaviors might help the development of new therapeutic strategies for addressing mental illness in individuals with PWS.
Introduction
Prader–Willi syndrome (PWS) is a neurogenetic disorder that results from the loss of several paternally inherited, imprinted contiguous genes in the human 15q11-q13 and mouse 7C regions (Resnick et al., 2013; Lee et al., 2000; Boccaccio et al., 1999; Manning and Holland, 2015; Butler et al., 2019). One of the defining characteristics of PWS is an insatiable appetite for food, which can cause substantial weight gain in children during the early stages of development (Cassidy et al., 2012). In addition to hyperphagia and obesity, individuals with PWS are also highly susceptible to mental health disorders (Schwartz et al., 2021). Key phenotypic PWS behaviors include temper outbursts, anxiety, obsessive-compulsive behaviors, and social cognition deficits (Schwartz et al., 2021; Kayadjanian et al., 2021). These behaviors have a significant impact on the daily functioning and quality of life for individuals with PWS and their families (Schwartz et al., 2021). Hence, it is necessary to determine which imprinted gene is responsible for these mental disorders in PWS.
Among the genes inactivated in PWS, the Magel2 gene is highly expressed in the hypothalamus (Chao et al., 2013; Kozlov et al., 2007; Maillard et al., 2016; Mercer and Wevrick, 2009). Amongst hypothalamic neurons, proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARC) play a critical role in controlling energy balance and mood disorders (Copperi et al., 2022). Intriguingly, Magel2-deficient mice have fewer ARCPomc neurons (Mercer et al., 2013) and lower levels of α-melanocyte-stimulating hormone (α-MSH) derived from POMC (Chen et al., 2020). ARCPomc axonal projections to the paraventricular and dorsomedial hypothalamus are significantly reduced by Magel2 deletion (Maillard et al., 2016). Furthermore, the basal spontaneous activity of ARCPomc neurons is lower in Magel2-null mice than in controls (Oncul et al., 2018). These prior studies support that MAGEL2 is critical for developing and maintaining ARCPomc neuronal circuits. In our prior studies (Kwon and Jo, 2020; Choi et al., 2022), we showed that optogenetic stimulation of the ARCPomc neurons - > the medial amygdala (MeA) pathway reduced acute food intake. More importantly, we also demonstrated that deleting the Magel2 gene exclusively in ARCPomc neurons that project to the MeA inhibited the onset of diet-induced obesity (DIO) in both male and female mice during a high-fat diet (HFD) feeding, which seems to be linked to an increase in physical activity (Choi et al., 2022). This finding is not consistent with the expectation that the loss of function of the PWS imprinted genes leads to the development of obesity, as observed in individuals with PWS. However, mice deficient in the paternal allele of Magel2 did not develop DIO when maintained on HFD (Bischof et al., 2007). Hence, these prior findings suggest that the Magel2 gene is unlikely to play a major role in regulating feeding behavior in PWS.
Acute emotional stressors, such as restraint and forced swim, trigger the expression of c-fos mRNA, a marker of neuronal activity, in most ARCPomc neurons (Liu et al., 2007; Lee et al., 2020) as well as in the MeA (Liu et al., 2007). Chronic stress has been shown to enhance both the intrinsic and synaptic activity of ARCPomc neurons (Fang et al., 2023). This increased activity of ARCPomc neurons induces anxiety-like behavior (Liu et al., 2007). Furthermore, the postnatal ablation of ARCPomc neurons results in an increase in anxiety-like behavior (Greenman et al., 2013). Notably, the activation of melanocortin receptor type 4 (MC4R) in the MeA elicits the anxiogenic effect (Liu et al., 2013). Given that anxiety is a prominent phenotypic behavior in PWS (Schwartz et al., 2021), it is plausible that the Magel2 gene in ARCPomc neurons, which project to the MeA, might be involved in the onset of anxiety rather than regulating feeding behavior in individuals with PWS. Hence, we sought to investigate the behavioral implications of the dysfunction of the Magel2 gene in ARCPOMC neurons that innervate the MeA and to provide new and valuable insights into the role of central MAGEL2 in the regulation of emotional behaviors.
Materials and methods
Ethics statement
All mouse care and experimental procedures were approved by the Institutional Animal Care Research Advisory Committee of the Albert Einstein College of Medicine and were performed following the guidelines described in the NIH guide for the care and use of laboratory animals. The IACUC protocol number is 00001377. Stereotaxic surgery and viral injections were performed under isoflurane anesthesia.
Animals
Rosa26-floxed-stop Cas9-eGFP (Rosa26-Cas9f, stock #026175) was purchased from the Jackson Laboratory. Mice were housed in cages at a controlled temperature (22 °C) with a constant humidity (40–60%) and a 12:12 h light–dark cycle. Mice were fed a standard chow diet with ad libitum access to water.
Viral injection
We employed neuronal Pomc enhancers 1 and 2 (nPEs) that were identified by the Rubinstein research group (de Souza et al., 2005) to induce the expression of the Cre recombinase solely in ARCPomc neurons in adult mice, a method that has proven effective in our prior studies (Jeong et al., 2018; Kwon et al., 2020). Retrograde AAV-nPEs-Cre-WPRE viruses (serotype retrograde) were made at Applied Biological Materials, Inc. (ABM). To knock down the Magel2 gene in ARCPomc neurons innervating the MeA, retrograde AAV-PGK-loxp-tdTomato-loxp-U6-mouse Magel2 sgRNA viruses were also generated at ABM. The sgRNAs target the consensus coding sequence 52264.1 region of mouse Magel2 (NM_013779.2). The sequences of Magel2 sgRNA were the following: (1) sgRNA1: cgcagctaagtacgaatctg, (2) sgRNA2: gtagggcggctatggactgc, and (3) sgRNA3: atggtccaggctccaccgct. The effectiveness of these sgRNAs was validated in our prior study (Choi et al., 2022).
A total of 84 mice were divided into four groups: 20 mice in the male control group, 20 mice in the male experimental group, 19 mice in the female control group, and 25 mice in the female experimental group; 6- and 7-week-old mice (males, ~ 20 g and females, ~18 g) were anesthetized deeply with 3% isoflurane and placed in a stereotaxic apparatus (David Kopf Instruments). A deep level of anesthesia was maintained throughout the surgical procedure. Under isoflurane anesthesia (2%), a total of 300 nL (150 + 150 nL from each) of retrograde AAV-PGK-loxp-tdTomato-loxp-U6-mouse Magel2 sgRNA viruses (titer, 1 × 1012 GC/ml) along with retrograde AAV-nPE-Cre-WPRE (titer, 1.2 × 1013 GC/ml) were bilaterally injected into the MeA of Rosa26-Cas9f mice (AP, −1.58 mm; ML, ±2 mm; DV, −5 mm). A 2.5 μl Hamilton syringe, having a 33G needle, was used to inject a volume of 50 nL viruses every 10 min. The Hamilton syringe tip was left in place for 10 min after delivering viruses in order to prevent the backflow of the viral solution up the needle track.
We conducted a systematic examination of GFP expression as an indicator of viral transfection following behavioral experiments. To achieve this, transverse brain slices were prepared as described in our prior study (Lee et al., 2015). The animals were anesthetized using isoflurane, and following decapitation, the brain was transferred into a sucrose-based solution, which was bubbled with 95% O2/5% CO2 and maintained at 3 °C. This solution comprised the following components (in mM): 248 sucrose, 2 KCl, 1 MgCl2, 1.25 KH2PO4, 26 NaHCO3, and 10 glucose. Transverse coronal brain slices (200 μm) were prepared using a vibratome (Leica VT 1000S). The brain slices were then placed on the stage of an upright, infrared-differential interference contrast microscope (Olympus BX50WI) mounted on a Gibraltar X-Y table (Burleigh), and GFP-positive cells were visualized with a 40X water immersion objective using infrared microscopy (DAGE MTI camera). Data were excluded if no GFP-positive cells were observed in the sections.
Immunostaining
Mice were anesthetized with isoflurane (3%) and transcardially perfused with 4% paraformaldehyde. Brain samples were post-fixed in 4% paraformaldehyde overnight in a cold room and then in 30% sucrose the following day. Tissues were sectioned using a cryostat at 20 μm. The sections were incubated with 0.3% Triton X-100 at room temperature (RT) for 30 min, and then blocked in PBS buffer containing 5% donkey serum, 2% bovine serum albumin and 0.15% Triton X-100 for 1 h at RT and then incubated with goat anti-GFP (NB100-1770, Novus), rabbit anti-POMC (1:1000, H-029-30, Phoenix biotech), and rabbit anti-MAGEL2 [1:2000, a gift from Dr. Tacer (Chen et al., 2020)] antibodies for overnight at a cold room, and then sections were washed three times in PBS and incubated with Alexa 488 anti-goat IgG for GFP (1:1000, 705–545-147, Jackson ImmunoResearch), Alexa 594 anti-rabbit IgG for POMC and MAGEL2 (1:1000, 711-585-152, Jackson ImmunoResearch) for 3 h at RT. After washing, the sections were stained with DAPI and mounted using VECTASHIELD medium (H-2000, Vector Lab.). Images were acquired using a Leica SP8 confocal microscope at a magnification of 20X. Using the cell count plugin in ImageJ software (version FIJI), MAGEL2-positive neurons were counted within the ARC, specifically in an area measuring 400 μm by 400 μm.
Animal behavior tests
For restraint stress, mice were restrained for 30 min in a 50 mL conical tube with the holes (0.5 cm in diameter) at the front and back for air flowing. Mice were able to move their head and limb but not their body, and unable to access food and water during the restraint. After the restraint was completed, mice were performed behavior tests immediately.
Open field exploration test
Mice were placed in the center of a chamber measured 40 cm (length) × 40 cm (width) × 40 cm (height) (Maze Engineers) and allowed to explore the chamber for 5 min freely (750 lux throughout the chamber). The center region was designated as 20 × 20 cm2. The EthoVision XT video tracking system (Noldus) was used to record the session and analyze the behavior, movement, and activity of animals. We wiped the chamber with 95% ethanol before subsequent tests to remove any scent clues (Seibenhener and Wooten, 2015). The total distance traveled and time spent in the center and outer zones of the chamber were measured.
Elevated plus maze test
Mice were placed in the central area of the maze consisting of four arms (two open and two closed arms) in the shape of a plus sign (35 cm arm in length, 5 cm arm in width, 20 cm wall in height) elevated 60 cm from the ground (Maze Engineers). Mice were placed in the center region and explored the maze for 5 min freely (750 lux throughout the chamber). The session was recorded, and the number of arm entries and the amount of time spent in the open and closed arms were analyzed with the EthoVision XT video tracking system.
Light–dark box test
The box consists of two chambers (one light, 25 cm in length × 40 cm in depth and one dark, 17.5 cm in length × 40 cm in depth, Maze Engineers). Mice were placed in the center of light chamber and allowed to explore the chambers for 5 min freely (750 lux throughout the chamber). Time spent in each compartment and crossings from one compartment to the other were measured with the video tracking system.
Conditioned place preference test
The experiment involved four phases: habituation phase, pre-conditioning test, conditioning phase, and post-conditioning test; 5 days of free access to all environments allows the mice to habituate to the apparatus having two compartments (20 cm in width × 18 cm in depth × 30 cm in height) with a corridor (20 cm in width × 7 cm in depth × 30 cm in height, Maze Engineers). The last day of habituation trial data was used as pre-conditioning test data. For conditioning phase, the compartments were divided into compartments with or without food and mice introduced into one of two compartments, with restricted access for 30 min. After 4 days of conditioning phase, preference tests were performed. Mice were introduced into the center compartment with free access to all compartments for 15 min without food. The result of the preference test was calculated using time spent in each compartment during the pre-conditioning and post-conditioning test. The CPP score was determined by subtracting the time spent in the food-paired chamber during habituation from the time spent in the same chamber on the test day.
Measurement of plasma corticosterone and norepinephrine levels
Blood samples were collected between 10:00 a.m. and 11:00 am from the retroorbital plexus using heparinized capillary tubes (VWR international, LLC). Whole blood samples were centrifuged at 2,000 × g for 10 min at 4 °C, and the plasma was separated and stored at −20 °C until use. Plasma corticosterone and norepinephrine levels were determined using two-site sandwich ELISA kits (corticosterone: Cayman, 501320; norepinephrine: MyBioSource, MBS2600834) following the manufacturer’s protocols.
Statistics
All statistical results were presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism 10.0. Two-tailed t-tests were used to calculate p-values of pair-wise comparisons. Time course comparisons between groups were analyzed using a two-way repeated-measures (RM) ANOVA with Sidak’s correction for multiple comparisons. Data were considered significantly different when the probability value is less than 0.05.
Results
Deletion of the Magel2 gene in ARCPomc neurons that innervate the MeA
We sought to determine whether knockdown of Magel2 expression in ARCPomc neurons that innervate the MeA causes an increase in anxiety-like behavior. To achieve this, we used a retrograde adeno-associated virus (AAVrg) containing the Cre recombinase under the control of neuronal Pomc enhancers (nPEs) to genetically eliminate the Magel2 gene, as we have done previously (Jeong et al., 2018; Kwon et al., 2020). We first validated our experimental approaches by injecting AAVrg-nPEs-Cre into the MeA of Rosa26-floxed-stop CRISPR-associated protein 9 (Cas9)-enhanced green fluorescent protein (eGFP) knock-in mice (Rosa26-Cas9f) (Figure 1A); 3 to 4 weeks post viral injections, double immunostaining with anti-GFP and anti-POMC antibodies revealed that a subset of ARCPomc neurons co-expressed GFP (Figure 1B). This finding indicates that these ARCPomc neurons are those that project to the MeA, further confirming our previous studies (Kwon and Jo, 2020; Choi et al., 2022). In this experimental setup, we injected AAVrg-DIO-tdTomato-U6-Magel2 sgRNA along with AAVrg-nPEs-Cre into the MeA of Rosa26-Cas9f mice to specifically knock down the expression of the Magel2 gene in ARCPomc neurons that project to the MeA (Figures 1C–E). Quantification of MAGEL2-positive cells within the ARC revealed a significant decrease in the number of MAGEL2-positive neurons (p = 0.01; Figure 1E), indicating knockdown of Magel2 in ARCPomc neurons innervating the MeA.
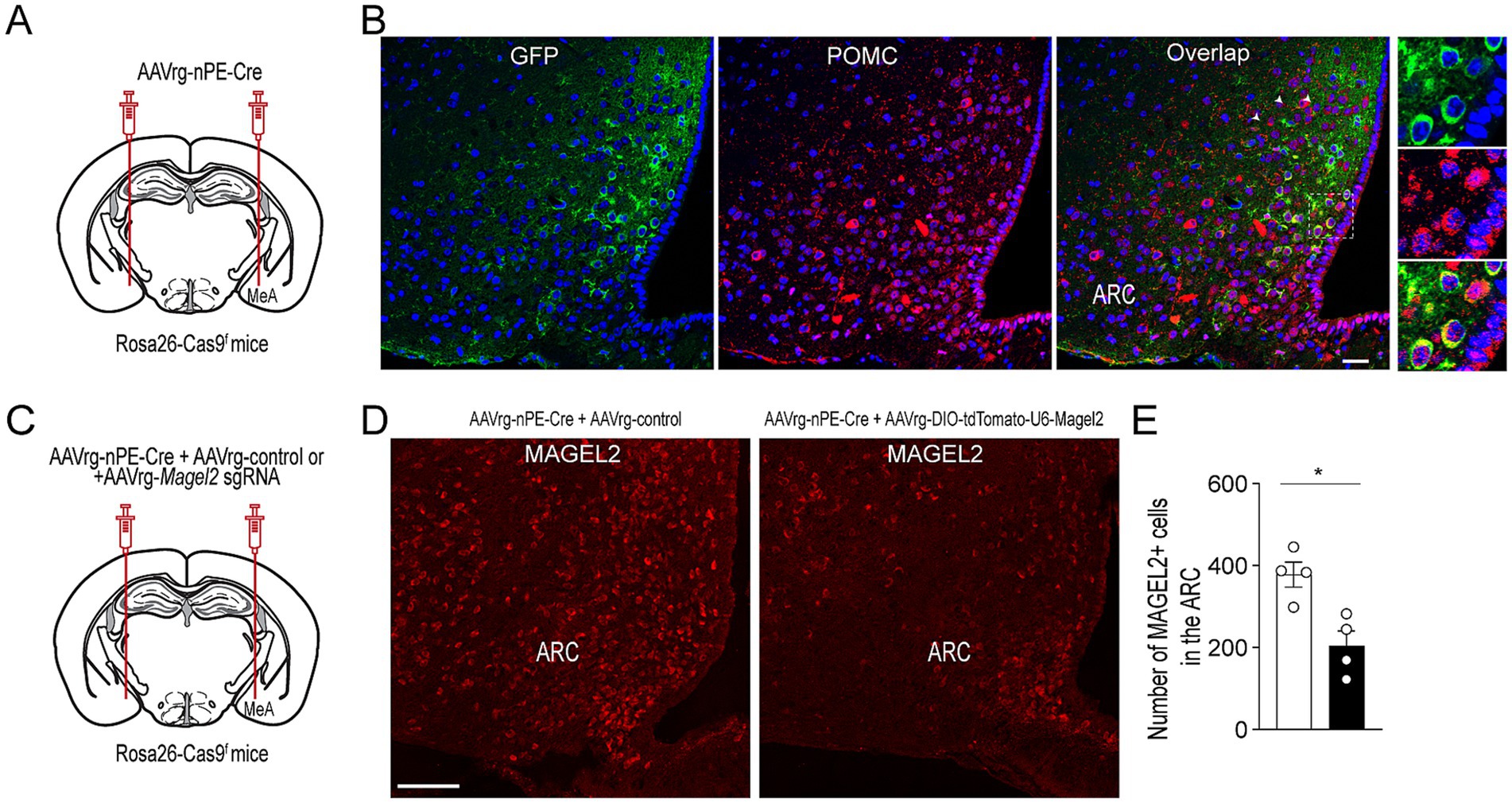
Figure 1. Selective deletion of Magel2 in MeA-projecting ARCPomc neurons. (A) Schematic illustration of the experimental condition. AAVrg-nPE-Cre viruses were injected into the MeA of Rosa26-Cas9f mice. (B) Images of confocal fluorescence microscopy showing co-expression of GFP and POMC in a subset of ARCPomc neurons within the ARC of Rosa26-Cas9f mice injected with AAVrg-nPE-Cre. Scale bar, 30 μm. (C) Schematic illustration of experimental conditions. A mixture of AAVrg-nPE-Cre viruses and either AAV-control or AAVrg-Magel2 sgRNA viruses was bilaterally injected into the MeA of Rosa26-Cas9f mice. (D,E) Images of confocal fluorescence microscopy showing the expression of MAGEL2-positive neurons in Rosa26-Cas9f mice injected with AAVrg-nPE-Cre and AAVrg-control or AAVrg-Magel2 sgRNA into the MeA (D, scale bar, 100 μm). Quantitative analysis of MAGEL2-positive neurons in the ARC (n = 4 mice per group) revealed a significant decrease in the number of MAGEL2-positive cells. Unpaired t-test, *p = 0.01. Data are presented as mean values ± SEM.
Effect of the loss of function of the Magel2 gene in MeA-innervating ARCPomc neurons on the reinforcing property of food
We conducted the conditioned place preference test to evaluate whether the reward property of food were influenced by the loss of function of the Magel2 gene. The reward effect of food was quantified by measuring the time spent in a specific chamber previously associated with food, in comparison to another environment paired with the absence of food. In the absence of the reinforcer, the time spent in these chambers provides an indication of the positive or negative affective memory that the animal associates with its food (Figure 2A). During the conditioning phase, Emmental cheese was associated with spatial information. Results revealed that male mice lacking the Magel2 gene in MeA-innervating ARCPomc neurons spent significantly less time in the compartment paired with food compared to the controls (p = 0.007; Figure 2B), while no significant difference was observed in the total distance traveled between the groups (Figure 2B). Interestingly, the experimental male mice spent more time in the neutral area than the control group on the test day (Figure 2B). This finding implies that these mice may exhibit a reduced motivation to return to the chamber associated with food rewards, which could suggest an increase in anxiety-related behaviors. In contrast to male mice, we found no significant differences between the female control and experimental groups (Figures 2C,D). In other words, the time spent in the food-paired compartment was similar across these groups.
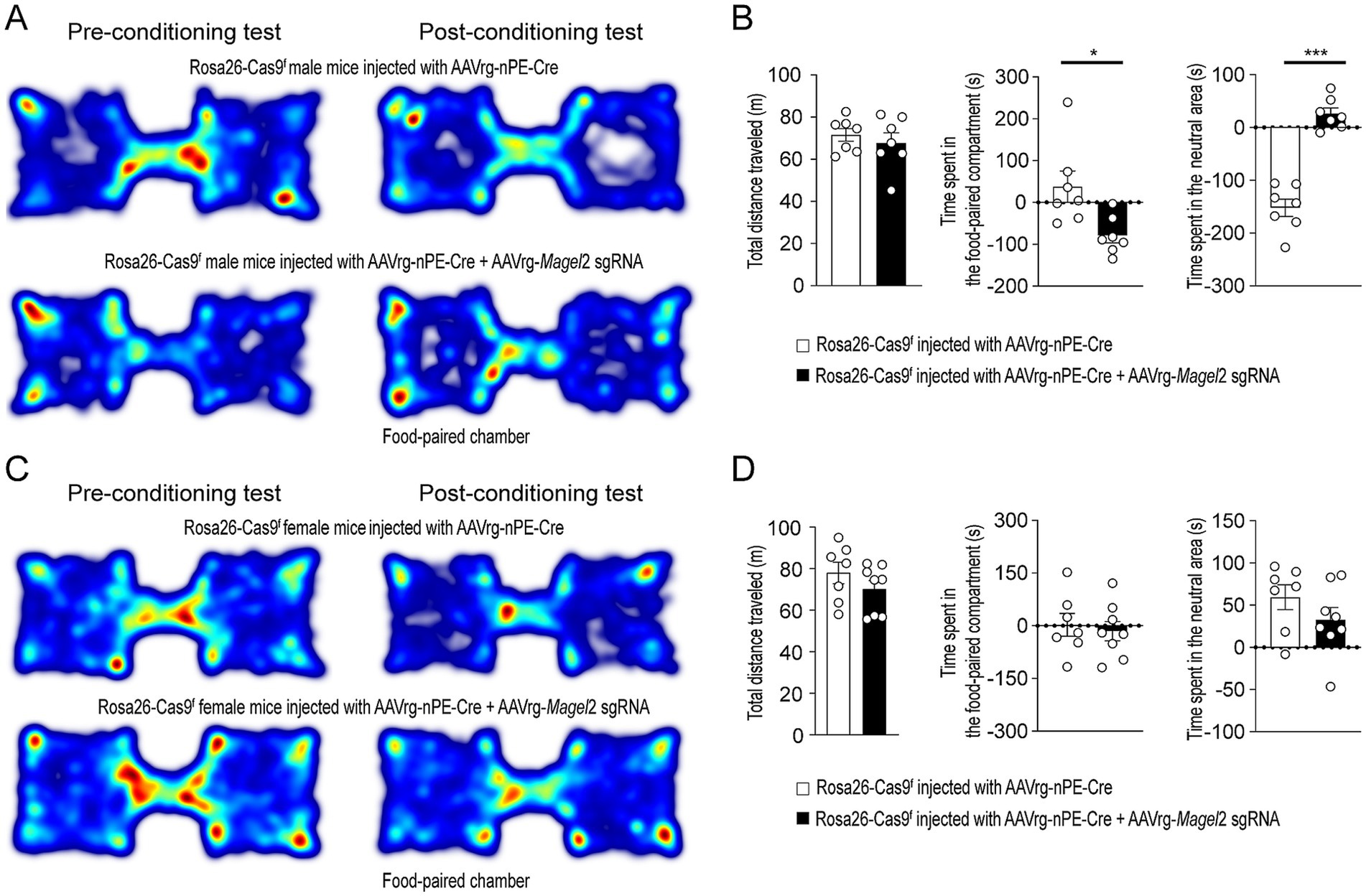
Figure 2. Knockdown of Magel2 in ARCPomc neurons causes an impairment in spatial learning associated with a food reward in male mice. (A) Representative example of the travel paths of the control (top) and the experimental (bottom) groups during the CPP test in male mice. The experimental mice showed no preference for the food-paired chamber. (B) Graphs showing the total distance (left) on the test day, the CPP score (middle), and the time spent in the neutral area (right) between the control and experimental groups (n = 7 mice vs. 7 mice, unpaired t-test, *p = 0.01; *** p < 0.0001). (C) Representative example of the travel paths of the control (top) and the experimental (bottom) groups during the CPP test in female mice. (D) Graphs showing the total distance (left), the CPP (middle), and the time spent in the neutral area (right) between the female groups (n = 7 mice vs. 8 mice). No differences in the total distance (left) on the test day and the CPP score between the control and experimental groups were observed.
Impact of acute stress on anxiety-like behavior in mice lacking Magel2 in MeA-innervating ARCPomc neurons
We then explored the impact of Magel2 gene deletion in MeA-innervating ARCPomc neurons on anxiety-like behavior as anxiety is very common in individuals with PWS (Schwartz et al., 2021; Feighan et al., 2020). We first examined if the loss of function of the Magel2 gene in these neurons influences body weight and found that there were no significant differences in body weight between the groups (Figures 3A,B). We then performed a series of experiments in male mice. Initially, we conducted an open field test, a common method for evaluating anxiety-like behavior (Seibenhener and Wooten, 2015; Sestakova et al., 2013). Behavioral elements of anxiety include decreased total locomotor activity, lower distance traveled and lower percentage of time spent in the center region, and higher percentage of time spent in the periphery zone. We observed no significant differences in the total distance traveled or the duration spent in the center zone between the control and experimental groups (Figures 3C,D), which is consistent with the prior findings with Magel2 knockout (ko) mice (Fountain et al., 2017; Mercer et al., 2009; Meziane et al., 2015). Furthermore, we conducted a light/dark (LD) box test to evaluate changes in the willingness to explore the illuminated, unprotected area, as well as an elevated plus maze (EPM) test to investigate the inherent tendency of mice to explore novel environments by offering a choice between open, unprotected maze arms and enclosed, protected arms (Bailey and Crawley, 2009). Mice deficient in the Magel2 gene within MeA-projecting ARCPomc neurons did not demonstrate any differences in these assessments compared to the control group in LD test (Figures 3E,F) and EPM (Figures 3G,H) as described in Magel2 ko mice (Mercer et al., 2009). These findings suggest no significant differences in anxiety-like behavior between the two male groups, which is not consistent with the commonly recognized anxiety associated with PWS (Feighan et al., 2020).
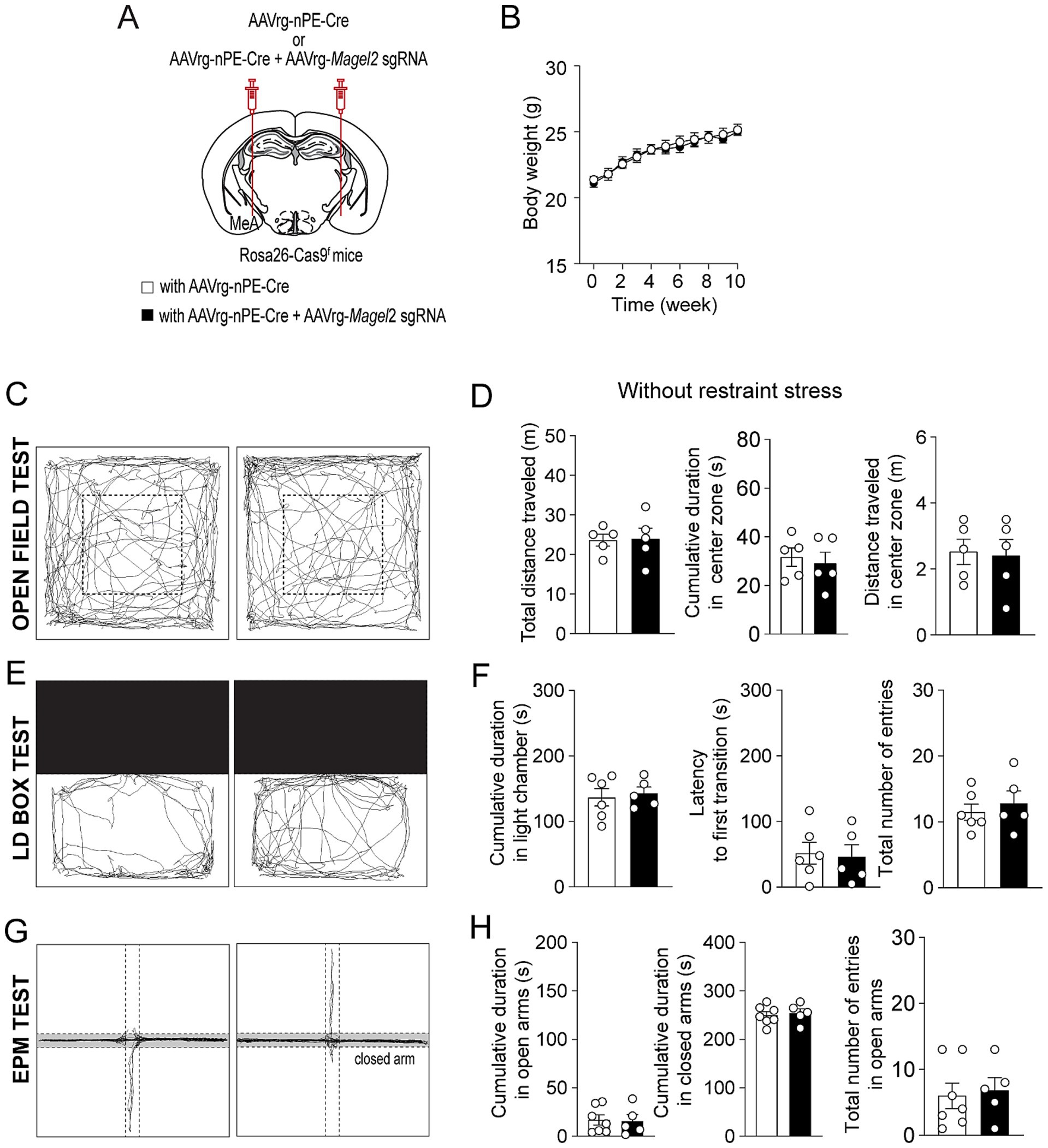
Figure 3. Loss of function of the Magel2 gene in ARCPomc neurons does not alter anxiety-like behavior in male mice. (A) A schematic illustration of the experimental condition. (B) Graph showing no significant difference in body weight between the control (n = 6 mice) and experimental (n = 7 mice) groups following viral injections. Body weight was measured weekly at 9 a.m. (C,D) Representative example of the travel paths of the control (left) and the experimental (right) groups (C). Graphs showing the total distance traveled, the time spent and the distance traveled in the inner area during the open field test (n = 5 mice vs. 5 mice). (E,F) Example of the travel paths of the control (left) and the experimental (right) groups (E). Graphs showing no significant differences in the time spent in the light chamber, the latency to first transition, and the total number of entries during the light–dark box test (n = 7 mice vs. 5 mice). (G,H) Example of the travel paths of the control (left) and the experimental (right) groups (G). Graphs showing no significant differences in the cumulative duration in the open and closed arms during the elevated plus maze test (n = 7 mice vs. 5 mice).
Individuals with PWS are highly stress-sensitive (Schwartz, n.d.). We thus examined if acute stress such as restraint affects anxiety-like behavior in male mice lacking the Magel2 gene in MeA-innervating ARCPomc neurons (Figure 4A). In the open field test, we found that, although both the control and experimental mice exhibited similar total distance traveled (Figures 4B,C), the mice lacking the Magel2 gene in MeA-innervating ARCPomc neurons spent significantly less time in the center zone compared to that of the control group following short-term restraint stress (p = 0.009; Figure 4C). Furthermore, the results of the LD box test demonstrated that the experimental mice required a significantly longer latency to make their first transition compared to the control group (p = 0.01; Figures 4D,E). However, we observed that acute stress had no impact on any parameters, including the duration spent in open arms and the frequency of entries into open arms during the EPM test (Figures 4F,G). Our findings suggest that the loss of the Magel2 gene may influence the impact of stress on anxiety-like behavior in male mice. This observed difference in the effect of stress on anxiety-like behavior could be attributed to elevated levels of stress hormones including corticosterone and norepinephrine. However, our measurements of these hormones in plasma revealed no significant differences in their levels (Figure 4H).
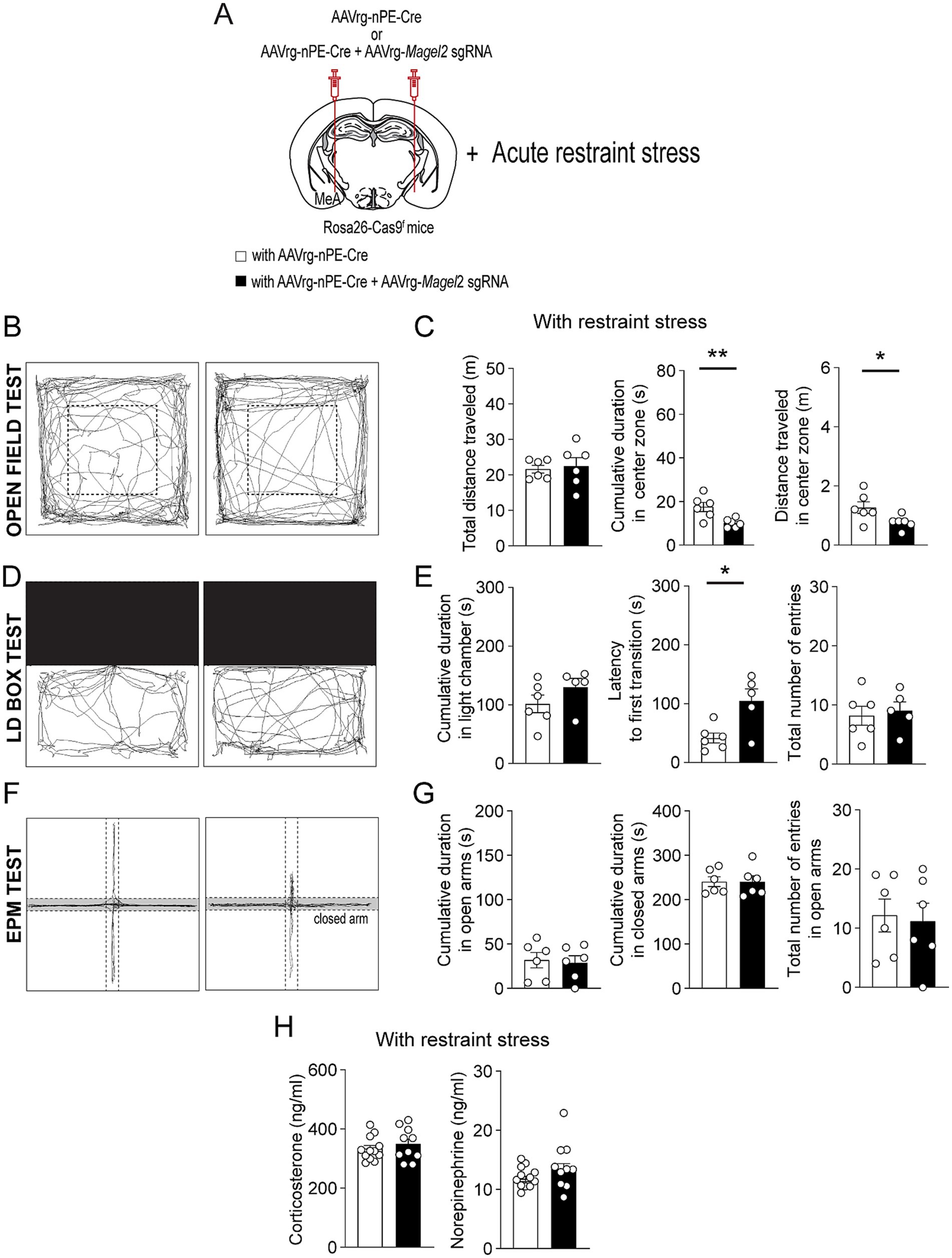
Figure 4. Loss of function of the Magel2 gene in ARCPomc neurons alters the effect of stress on anxiety-like behavior in male mice. (A) A schematic illustration of the experimental condition. Mice were restrained for 30 min (B,C) Representative example of the travel paths of the control (left) and the experimental (right) groups (B). Graphs showing the total distance traveled, the time spent, and the distance traveled in the inner area during the open field test following exposure to restraint stress for 30 min (n = 6 mice vs. 6 mice, unpaired t-test, **p = 0.009; *p = 0.04). (D,E) Representative example of the travel paths of the control (left) and the experimental (right) groups (D). Graphs showing the time spent in the light zone, the latency to first transition, and the total number of entries during the light–dark box test (n = 6 mice vs. 5 mice, unpaired t-test, *p = 0.01). There was a significant difference in the latency to first transition between the groups. (F,G) Representative example of the travel paths of the control (left) and the experimental (right) groups (F). Graphs showing the cumulative duration in the open and closed arms during the elevated plus maze test with restraint stress (n = 6 vs. 6 mice). (H) Graphs showing plasma corticosterone (left) and norepinephrine (right) levels in the control (n = 12 mice) and experimental (n = 10 mice) groups. No significant differences were observed between the groups. Data are presented as mean values ± SEM.
We also conducted same behavioral assessments on female mice. Similar to male mice, female mice lacking the Magel2 gene in ARCPomc neurons innervating the MeA exhibited no difference in body weight compared to the control mice (Figures 5A,B). Consistent with findings in Magel2 ko female mice (Meziane et al., 2015), the results from the open field test, LD box test, and EPM test in the absence of acute restraint stress exhibited no significant differences between the control and experimental mice (Figures 5C–H). Namely, both groups displayed comparable total distance traveled and time spent in the inner zone in the open field test (Figures 5C,D). No significant differences were observed in the duration spent in the light chamber or in the latency to first transition during the LD box test (Figures 5E,F). Similarly, the EPM test showed no differences in the duration spent in the open arms or the frequency of entries into the open arms (Figures 5F,G). In addition, we also found no significant difference in the open field test (Figures 6A–C), LD box test (Figures 6D,E), and EPM test (Figures 6F,G) between the two female groups following acute exposure to stress. Additionally, Measurement of plasma corticosterone and norepinephrine levels showed no significant difference between the groups (Figure 6H).
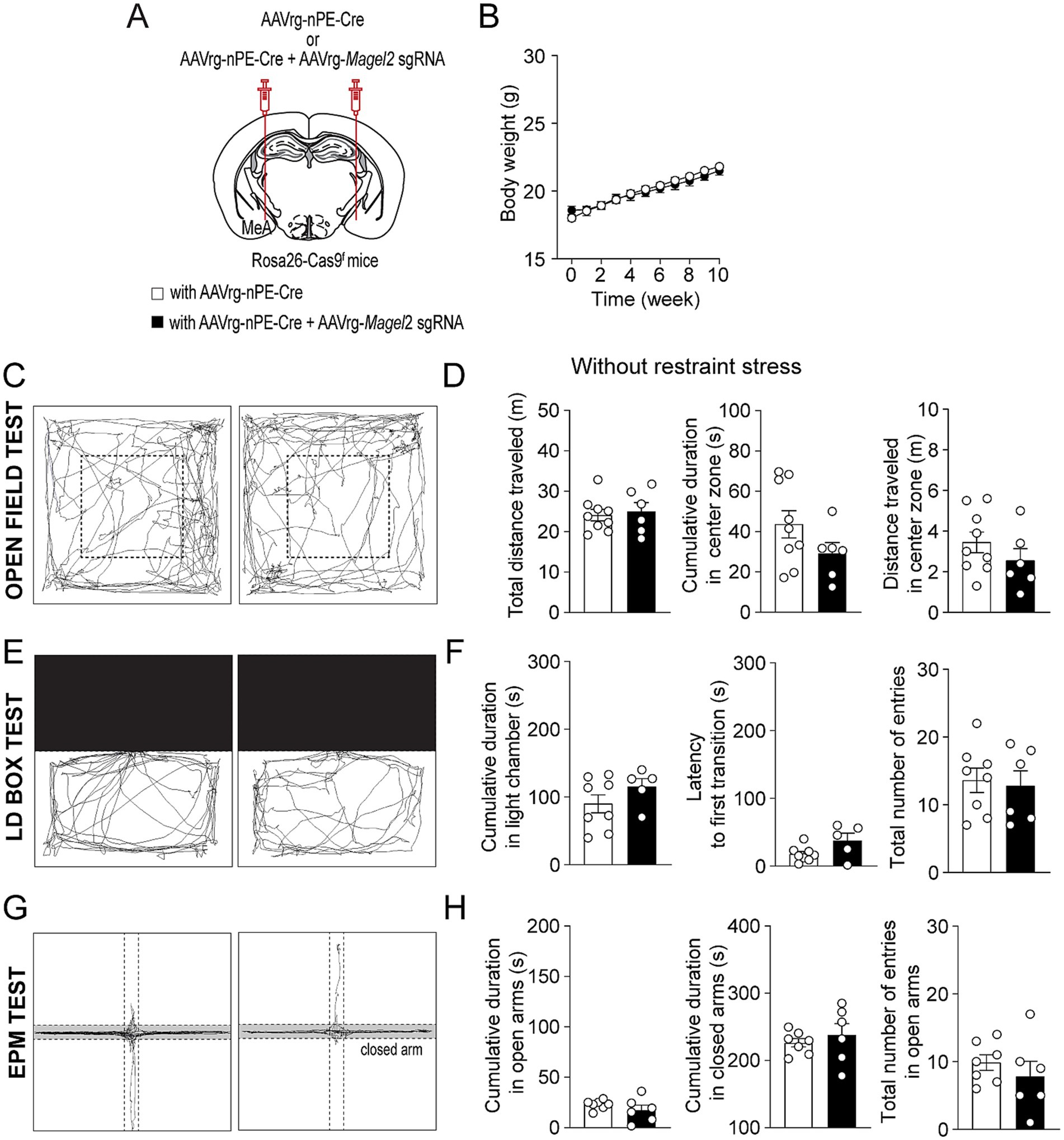
Figure 5. Deleting Magel2 in ARCPOMC neurons does not affect anxiety-like behavior in female mice. (A) A schematic illustration of the experimental condition. (B) Graph showing no difference in body weight between the control (n = 7 mice) and experimental (n = 8 mice) groups following viral injections. (C,D) Representative example of the travel paths of the control (left) and the experimental (right) groups (C). Graphs showing no significant differences in the total distance traveled, the time spent and the distance traveled in the inner area during the open field test (n = 9 mice vs. 6 mice). (E,F) Example of the travel paths of the control (left) and the experimental (right) groups (E). Graphs showing the time spent in the light chamber, the latency to transition, and the total number of entries during the light–dark box test (n = 8 mice vs. 5 mice). (G,H) Graphs showing the cumulative duration in the open and closed arms during the elevated plus maze test (n = 7 mice vs. 6 mice). No significant differences were observed between the groups.
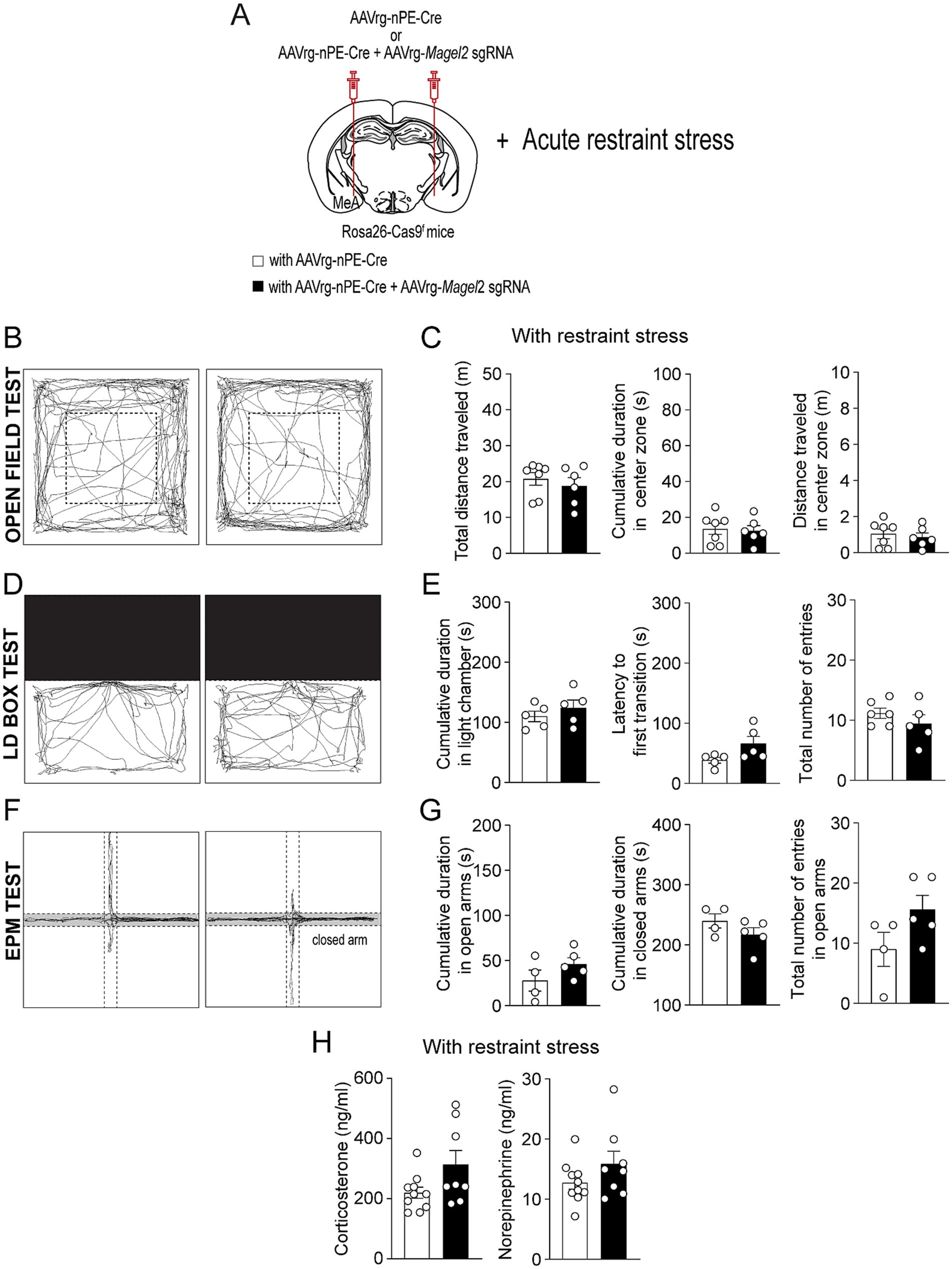
Figure 6. Deleting Magel2 in ARCPOMC neurons does not influence the impact of stress on anxiety-like behavior in female mice. (A) A schematic illustration of the experimental condition. Mice were restrained for 30 min (B,C) Representative example of the travel paths of the control (left) and the experimental (right) groups (B). Graphs showing the total distance traveled, the time spent, and the distance traveled in the inner area during the open field test following exposure to restraint stress for 30 min (n = 7 mice vs. 6 mice). (D,E) Representative example of the travel paths of the control (left) and the experimental (right) groups (D). Graphs showing the time spent in the light zone, the latency to first transition, and the total number of entries during the light–dark box test following exposure to restraint stress (n = 5 mice vs. 5 mice). (F,G) Representative example of the travel paths of the control (left) and the experimental (right) groups (F). Graphs showing the cumulative duration in the open and closed arms during the elevated plus maze test with restraint stress (n = 4 mice vs. 5 mice). (H) Graphs showing plasma corticosterone (left) and norepinephrine (right) levels in the control (n = 10 mice) and experimental (n = 8 mice) groups. Data are presented as mean values ± SEM.
Discussion
PWS is a complex neurodevelopmental disorder characterized by a range of behavioral changes, including not only hyperphagia, but also anxiety, temper outbursts, and obsessive-compulsive behavior (Schwartz et al., 2021; Feighan et al., 2020). The cellular mechanisms underlying the behavioral changes in PWS has been studied in animal models such as Magel2 ko mice (Fountain et al., 2017; Mercer et al., 2009; Meziane et al., 2015). In this study, we specifically deleted the Magel2 gene in ARCPomc neurons that innervate the MeA, given that the MeA is a critical structure involved in emotional behaviors, particularly anxiety-like behavior (Ebner et al., 2004). We found that both male and female mice lacking the Magel2 gene in MeA-innervating ARCPomc neurons did not display any significant alterations in the open field maze, LD box, and EPM assessments in the absence of restraint stress. In contrast, the male mutant mice exhibited increased anxiety-like behaviors such as a reduced time spent in the center zone and a latency to first transition, compared to their control littermates following exposure to restraint stress. Moreover, the loss of function of the Magel2 gene in these specific ARCPomc neurons resulted in decreased spatial learning that was associated with a food reward in male mice. Contrary to the male mutant mice, the female mutant mice did not show any behavioral changes with and without exposure to restraint stress. Given that individuals with PWS are highly sensitive to stress, which can lead to behavioral changes (Schwartz, n.d.), our present study may offer a novel perspective on the role of the Magel2 gene, among other imprinted genes associated with PWS, in regulating the impact of stress on anxiety-related behaviors within this population.
Hyperphagia is a primary characteristic of PWS, resulting in excessive weight gain and obesity. Intriguingly, the loss of function of the Magel2 gene did not replicate this characteristic feature in two distinct Magel2 ko mouse models, namely the LacZ knock-in allele and Magel2 deletion (Kozlov et al., 2007; Bischof et al., 2007; Mercer et al., 2009; Schaller et al., 2010). Although the male Magel2 ko mice with the LacZ knock-in allele showed a slight increase in body weight on a standard chow diet (Bischof et al., 2007), the Magel2 ko mice did not develop DIO compared to their control littermates when fed a high-fat diet (HFD). Instead the Magel2 ko mice demonstrated a reduction in body weight relative to the control mice during HFD feeding (Bischof et al., 2007). Magel2 ko mice with Magel2 deletion also showed normal weight, temperature, motor abilities, and pain sensitivity (Meziane et al., 2015). These two distinct ko mice displayed a reduction in food intake compared to the controls (Kozlov et al., 2007; Bischof et al., 2007), which seems to be partially attributed to changes in circadian output (Kozlov et al., 2007). In our prior study (Choi et al., 2022), deleting the Magel2 gene in ARCPomc neurons that project to the MeA caused a reduction in body weight in both male and female mice compared to the control littermates on HFD feeding. This was associated with increased locomotor activity particularly in the dark phase. Additionally, these mutant mice did not exhibit any differences in food intake, glucose tolerance, and insulin sensitivity (Choi et al., 2022). Hence, these prior studies suggest that the Magel2 gene, among the imprinted genes associated with PWS, might not be crucial in the development of hyperphagia and obesity.
Magel2-null mice demonstrated abnormalities in the hypothalamic endocrine axes (Tennese and Wevrick, 2011). Notably, restraint stress resulted in a significant elevation of blood corticosterone levels in both sexes compared to controls. However, while male Magel2-null mice exhibited a significant increase in corticosterone levels in response to insulin, female Magel2-null mice did not display such an increase (Tennese and Wevrick, 2011). Furthermore, Magel2 ko mice exhibited altered behavioral phenotypes, including a lack of preference for social novelty, deficits in social interaction and recognition, and diminished learning ability (Fountain et al., 2017; Mercer et al., 2009; Meziane et al., 2015). Interestingly, although anxiety is the most prevalent behavioral change in Prader–Willi syndrome (PWS) (Feighan et al., 2020), Magel2 ko mice did not exhibit anxiety-related behavior in the open field maze [32, 34] and elevated plus maze assessments in both male and female mice (Mercer et al., 2009). Contrary to these observations, it has also been demonstrated that both male and female Magel2 ko mice spent more time in the open arms of the elevated plus maze compared to the control group, indicating alterations in their exploratory behavior (Fountain et al., 2017). Interestingly, female Magel2 ko mice spent significantly less time in the closed arm compared to controls. In this study, we found that deleting the Magel2 gene in MeA-innervating ARCPomc neurons did not affect anxiety-like behavior in both male and female mice in the absence of acute stress exposure, aligning with prior findings (Mercer et al., 2009; Meziane et al., 2015). According to the foundation for Prader–Willi research (Schwartz, n.d.), individuals with PWS are highly susceptible to stress. Following short-term exposure to restraint stress, our male mutant mice exhibited reduced time spent in the central area during the open field test and demonstrated increased latency to first transition during the light–dark box test, indicating an elevation in anxiety-like behavior, whereas the female mutant mice did not display any alterations in anxiety-like behavior. In the Pavlovian fear-conditioning test, Magel2 ko mice exhibited significantly higher absolute freezing rates following the tone compared to wild-type controls, suggesting increased anxiety (Mercer et al., 2009). Hence, our current findings, along with previous ones, support the idea that the loss of function of the Magel2 gene may contribute to an enhanced impact of stress on anxiety-like behavior.
It has been shown that acute emotional stressors such as restraint and forced swim led to increased c-fos mRNA expression mainly in ARCPomc neurons rather than another important feeding-related neurons, agouti-related peptide-expressing neurons in the ARC (Liu et al., 2007). Both stressors resulted in a reduction of food intake and an increase in anxiety-like behavior, effects that were entirely inhibited by the administration of a melanocortin receptor type 4 (MC4R) antagonist. (Liu et al., 2007). Furthermore, MC4R-expressing neurons in the MeA were activated by acute stress exposure and inhibiting MC4Rs locally in the MeA abolished stress-induced anxiety-like behavior (Liu et al., 2013). We previously showed that activation of the ARCPomc → MeA pathway reduced food intake through MC4Rs (Kwon and Jo, 2020). Thus, it is possible that mice lacking the Magel2 gene in MeA-innervating ARCPomc neurons may be more susceptible to stress compared to the control groups.
Intriguingly, male mice deficient in the Magel2 gene also demonstrated a significant impairment in object recognition memory and a decrease in spatial learning abilities, whereas female mutant mice did not show these deficits (Meziane et al., 2015). In the CPP test, the animal utilizes spatial cues, such as walls with black stripes in our experimental setup, to establish a memory of the compartment associated with palatable food. Subsequently, the animal demonstrates a preference for this location based on the learned association. Our results revealed that male, but not female, mice lacking the Magel2 gene in MeA-innervating ARCPomc neurons spent considerably less time in the compartment associated with food compared to control mice, indicating a failure to exhibit CPP. Interestingly, this diminished CPP response may be partially attributed to increased time spent in the neutral compartment, suggesting reduced motivational drive in these mutant males.
The FDA has recently approved VYKAT™ XR (diazoxide choline, Soleno Therapeutics) as a treatment for hyperphagia in PWS. As MAGEL2 in ARCPomc neurons appears to be a key player in enhancing the effect of stress on anxiety-like behavior, understanding the cellular mechanisms of how MAGEL2 in ARCPOMC neurons innervating the MeA controls mental disorders will open up new therapeutic strategies for the treatment of mental illness in individuals with PWS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Institutional Animal Care Research Advisory Committee of Albert Einstein College of Medicine. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
Y-HJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SL: Data curation, Formal analysis, Investigation, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the NIH (R01 AT011653, R01 DK092246, P30 DK020541, and R03 MH137614 to Y-HJ).
Acknowledgments
We thank Drs. Patrick Potts and Klementina Fon Tacer for providing us with the MAGEL2 antibody.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bailey, K. R., and Crawley, J. N. (2009). “Anxiety-related behaviors in mice” in Methods of behavior analysis in neuroscience. Chapter 5, 2nd edition. ed. J. J. Buccafusco (Boca Raton, FL: CRC Press/Taylor & Francis).
Bischof, J. M., Stewart, C. L., and Wevrick, R. (2007). Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader-Willi syndrome. Hum. Mol. Genet. 16, 2713–2719. doi: 10.1093/hmg/ddm225
Boccaccio, I., Glatt-Deeley, H., Watrin, F., Roeckel, N., Lalande, M., and Muscatelli, F. (1999). The human MAGEL2 gene and its mouse homologue are paternally expressed and mapped to the Prader-Willi region. Hum. Mol. Genet. 8, 2497–2505. doi: 10.1093/hmg/8.13.2497
Butler, M. G., Hartin, S. N., Hossain, W. A., Manzardo, A. M., Kimonis, V., Dykens, E., et al. (2019). Molecular genetic classification in Prader-Willi syndrome: a multisite cohort study. J. Med. Genet. 56, 149–153. doi: 10.1136/jmedgenet-2018-105301
Cassidy, S. B., Schwartz, S., Miller, J. L., and Driscoll, D. J. (2012). Prader-Willi syndrome. Genet. Med. 14, 10–26. doi: 10.1038/gim.0b013e31822bead0
Chao, S. Y., Zarzabal, L. A., Walker, S. M., Herzog, C. M., Eilerman, P. A., Luce, B. K., et al. (2013). Estimating diabetes prevalence in the military health system population from 2006 to 2010. Mil. Med. 178, 986–993. doi: 10.7205/MILMED-D-13-00147
Chen, H., Victor, A. K., Klein, J., Tacer, K. F., Tai, D. J., de Esch, C., et al. (2020). Loss of MAGEL2 in Prader-Willi syndrome leads to decreased secretory granule and neuropeptide production. JCI Insight 5:e138576. doi: 10.1172/jci.insight.138576
Choi, Y., Min, H. Y., Hwang, J., and Jo, Y. H. (2022). Magel2 knockdown in hypothalamic POMC neurons innervating the medial amygdala reduces susceptibility to diet-induced obesity. Life Sci Alliance 5:e202201502. doi: 10.26508/lsa.202201502
Copperi, F., Kim, J. D., and Diano, S. (2022). Melanocortin signaling connecting systemic metabolism with mood disorders. Biol. Psychiatry 91, 879–887. doi: 10.1016/j.biopsych.2021.05.026
de Souza, F. S., Santangelo, A. M., Bumaschny, V., Avale, M. E., Smart, J. L., Low, M. J., et al. (2005). Identification of neuronal enhancers of the proopiomelanocortin gene by transgenic mouse analysis and phylogenetic footprinting. Mol. Cell. Biol. 25, 3076–3086. doi: 10.1128/MCB.25.8.3076-3086.2005
Ebner, K., Rupniak, N. M., Saria, A., and Singewald, N. (2004). Substance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc. Natl. Acad. Sci. 101, 4280–4285. doi: 10.1073/pnas.0400794101
Fang, X., Chen, Y., Wang, J., Zhang, Z., Bai, Y., Denney, K., et al. (2023). Increased intrinsic and synaptic excitability of hypothalamic POMC neurons underlies chronic stress-induced behavioral deficits. Mol. Psychiatry 28, 1365–1382. doi: 10.1038/s41380-022-01872-5
Feighan, S. M., Hughes, M., Maunder, K., Roche, E., and Gallagher, L. (2020). A profile of mental health and behaviour in Prader–Willi syndrome. J. Intellect. Disabil. Res. 64, 158–169. doi: 10.1111/jir.12707
Fountain, M. D., Tao, H., Chen, C. A., Yin, J., and Schaaf, C. P. (2017). Magel2 knockout mice manifest altered social phenotypes and a deficit in preference for social novelty. Genes Brain Behav. 16, 592–600. doi: 10.1111/gbb.12378
Greenman, Y., Kuperman, Y., Drori, Y., Asa, S. L., Navon, I., Forkosh, O., et al. (2013). Postnatal ablation of POMC neurons induces an obese phenotype characterized by decreased food intake and enhanced anxiety-like behavior. Mol. Endocrinol. 27, 1091–1102. doi: 10.1210/me.2012-1344
Jeong, J. H., Lee, D. K., Liu, S. M., Chua, S. C. Jr., Schwartz, G. J., and Jo, Y. H. (2018). Activation of temperature-sensitive TRPV1-like receptors in ARC POMC neurons reduces food intake. PLoS Biol. 16:e2004399. doi: 10.1371/journal.pbio.2004399
Kayadjanian, N., Vrana-Diaz, C., Bohonowych, J., Strong, T. V., Morin, J., Potvin, D., et al. (2021). Characteristics and relationship between hyperphagia, anxiety, behavioral challenges and caregiver burden in Prader-Willi syndrome. PLoS One 16:e0248739. doi: 10.1371/journal.pone.0248739
Kozlov, S. V., Bogenpohl, J. W., Howell, M. P., Wevrick, R., Panda, S., Hogenesch, J. B., et al. (2007). The imprinted gene Magel2 regulates normal circadian output. Nat. Genet. 39, 1266–1272. doi: 10.1038/ng2114
Kwon, E., and Jo, Y. H. (2020). Activation of the ARCPOMC→MeA projection reduces food intake. Front Neural Circuits 14:595783. doi: 10.3389/fncir.2020.595783
Kwon, E., Joung, H. Y., Liu, S. M., Chua, S. C. Jr., Schwartz, G. J., and Jo, Y. H. (2020). Optogenetic stimulation of the liver-projecting melanocortinergic pathway promotes hepatic glucose production. Nat. Commun. 11:6295. doi: 10.1038/s41467-020-20160-w
Lee, E. J., Hanchate, N. K., Kondoh, K., Tong, A. P. S., Kuang, D., Spray, A., et al. (2020). A psychological stressor conveyed by appetite-linked neurons. Sci. Adv. 6:eaay5366. doi: 10.1126/sciadv.aay5366
Lee, D. K., Jeong, J. H., Chun, S. K., Chua, S. Jr., and Jo, Y. H. (2015). Interplay between glucose and leptin signalling determines the strength of GABAergic synapses at POMC neurons. Nat. Commun. 6:6618. doi: 10.1038/ncomms7618
Lee, S., Kozlov, S., Hernandez, L., Chamberlain, S. J., Brannan, C. I., Stewart, C. L., et al. (2000). Expression and imprinting of MAGEL2 suggest a role in Prader-willi syndrome and the homologous murine imprinting phenotype. Hum. Mol. Genet. 9, 1813–1819. doi: 10.1093/hmg/9.12.1813
Liu, J., Garza, J. C., Li, W., and Lu, X. Y. (2013). Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. Int. J. Neuropsychopharmacol. 16, 105–120. doi: 10.1017/S146114571100174X
Liu, J., Garza, J. C., Truong, H. V., Henschel, J., Zhang, W., and Lu, X. Y. (2007). The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology 148, 5531–5540. doi: 10.1210/en.2007-0745
Maillard, J., Park, S., Croizier, S., Vanacker, C., Cook, J. H., Prevot, V., et al. (2016). Loss of Magel2 impairs the development of hypothalamic Anorexigenic circuits. Hum. Mol. Genet. 25, 3208–3215. doi: 10.1093/hmg/ddw169
Manning, K. E., and Holland, A. J. (2015). Puzzle pieces: neural structure and function in Prader-Willi syndrome. Diseases 3, 382–415. doi: 10.3390/diseases3040382
Mercer, R. E., Kwolek, E. M., Bischof, J. M., van Eede, M., Henkelman, R. M., and Wevrick, R. (2009). Regionally reduced brain volume, altered serotonin neurochemistry, and abnormal behavior in mice null for the circadian rhythm output gene Magel2. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 1085–1099. doi: 10.1002/ajmg.b.30934
Mercer, R. E., Michaelson, S. D., Chee, M. J., Atallah, T. A., Wevrick, R., and Colmers, W. F. (2013). Magel2 is required for leptin-mediated depolarization of POMC neurons in the hypothalamic arcuate nucleus in mice. PLoS Genet. 9:e1003207. doi: 10.1371/journal.pgen.1003207
Mercer, R. E., and Wevrick, R. (2009). Loss of magel2, a candidate gene for features of Prader-Willi syndrome, impairs reproductive function in mice. PLoS One 4:e4291. doi: 10.1371/journal.pone.0004291
Meziane, H., Schaller, F., Bauer, S., Villard, C., Matarazzo, V., Riet, F., et al. (2015). An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism. Biol. Psychiatry 78, 85–94. doi: 10.1016/j.biopsych.2014.11.010
Oncul, M., Dilsiz, P., Ates Oz, E., Ates, T., Aklan, I., Celik, E., et al. (2018). Impaired melanocortin pathway function in Prader-Willi syndrome gene-Magel2 deficient mice. Hum. Mol. Genet. 27, 3129–3136. doi: 10.1093/hmg/ddy216
Resnick, J. L., Nicholls, R. D., and Wevrick, R.G. Prader-Willi Syndrome Animal Models Working (2013). Recommendations for the investigation of animal models of Prader-Willi syndrome. Mamm. Genome 24, 165–178. doi: 10.1007/s00335-013-9454-2
Schaller, F., Watrin, F., Sturny, R., Massacrier, A., Szepetowski, P., and Muscatelli, F. (2010). A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum. Mol. Genet. 19, 4895–4905. doi: 10.1093/hmg/ddq424
Schwartz, L. PWS Mental Health Guidebook : Foundation for Prader-Willi Research. Available at: https://www.fpwr.org/pws-mental-health-guidebook#background
Schwartz, L., Caixas, A., Dimitropoulos, A., Dykens, E., Duis, J., Einfeld, S., et al. (2021). Behavioral features in Prader-Willi syndrome (PWS): consensus paper from the international PWS clinical trial consortium. J. Neurodev. Disord. 13:25. doi: 10.1186/s11689-021-09373-2
Seibenhener, M. L., and Wooten, M. C. (2015). Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. :e52434. doi: 10.3791/52434
Sestakova, N., Puzserova, A., Kluknavsky, M., and Bernatova, I. (2013). Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 6, 126–135. doi: 10.2478/intox-2013-0020
Keywords: stress, melanocortin, hypothalamus, anxiety, memory, obesity
Citation: Lee S and Jo Y-H (2025) Magel2 in hypothalamic POMC neurons influences the impact of stress on anxiety-like behavior and spatial learning associated with a food reward in male mice. Front. Neural Circuits. 19:1690406. doi: 10.3389/fncir.2025.1690406
Edited by:
Ken K. L. Yung, The Education University of Hong Kong, ChinaReviewed by:
Hui Yu, The Ohio State University, United StatesDong Kun Lee, Gyeongsang National University, Republic of Korea
Copyright © 2025 Lee and Jo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Young-Hwan Jo, eW91bmctaHdhbi5qb0BlaW5zdGVpbm1lZC5lZHU=
 Sangbhin Lee
Sangbhin Lee Young-Hwan Jo
Young-Hwan Jo