- Anatomy, Department of Medicine, University of Fribourg, Fribourg, Switzerland
The hexa-EF-hand Ca2+-binding protein calretinin (CR) is predominantly expressed in specific neurons of the central and peripheral nervous system. However, CR expression is also observed in non-neuronal cells, e.g., during embryonic development and in mesothelioma cells. Of the 6 EF-hand domains, 5 are functional; the first 4 domains form 2 pairs showing high cooperativity within a pair that results in non-linear modulation of intracellular Ca2+ signals by CR. EF-hand domain 5 has a low affinity and represents the identified interaction site with CR-binding partners present in mouse cerebellar granule cells. CR binding to other targets including the pore-forming α1 subunit of the Ca2+ channel CaV2.1, as well as to huntingtin indicates additional Ca2+ sensor functions besides the well-known Ca2+-buffering functions. The absence of CR in cerebellar granule cells of CR−/− mice results in increased excitability and altered firing of Purkinje cells and promotes cerebellar 160-Hz oscillations impairing motor coordination. The putative role of CR in neuroprotection is still highly discussed. Altogether, CR emerges as a multi-functional protein also associated with development, i.e., cell proliferation, differentiation, and cell death.
Basic Facts About Calretinin (CR)
Calretinin (CR; human gene symbol: CALB2), calbindin D-28k (CB; CALB1) and secretagogin (SCGN; SCGN) represent the 3 members of the hexa-EF-hand protein family, also named the calbindin sub-family [see recent reviews on CR (Camp et al., 2009), CB (Schmidt, 2012) and SCGN (Alpar et al., 2012)]. They all contain 6 structural motifs named EF-hand Ca2+-binding domains. Each domain consists of an alpha-helix of approximately 10 amino acids, a Ca2+-chelating loop of 12 amino acids and a second alpha-helix that is oriented perpendicular to the first one (for more details on the EF-hand structure, see (Schwaller, 2010). CR (Mr 31 kDa) initially discovered in the retina, thus the name: calcium + retina = CR consists of 271 amino acids in many species and is highly conserved; the number of amino acids varies from 269 (e.g., Gallus gallus; Chicken) to 273 (e.g., Monodelphis domestica; Gray short-tailed opossum). CR is also expressed in zebrafish (Danio rerio) and an invertebrate ortholog named calbindin 53E (Cbp53E; previously calbindin-32) exists in Drosophila melanogaster that shares the highest sequence identity with CR (Reifegerste et al., 1993). In CR, the first 5 EF-hand domains are capable of binding Ca2+ ions, while the sixth one is inactive (Stevens and Rogers, 1997; Schwaller et al., 1997). Moreover the Ca2+-binding affinity for site 5 is very low (KD: 36 μM) (Faas et al., 2007) indicating that this site is expected to be rarely in the Ca2+-bound form in the cytoplasmic compartment except in microdomains close to Ca2+ channels. The other 4 functional Ca2+-binding sites form 2 similar pairs likely consisting of domains 1 and 2, as well as 3 and 4 showing strong cooperativity within a pair (Faas et al., 2007). The apparent KD (KD, app) for the 4 sites is 1.4–1.5 μM with high cooperativity (nH of 1.9; for more details on CR's properties, see Table 1). This property results in non-linear Ca2+ regulation in cells due to the presence of CR. In a situation when the intracellular Ca2+ concentration [Ca2+]i is at resting (basal) levels of 50–100 nM, then upon a brief and limited increase in [Ca2+]i, CR behaves like a typical slow-onset buffer (EGTA). However, if the same increase occurs at elevated [Ca2+]i, in the order of 1 μM, when the first site of a pair is in the Ca2+-bound form, cooperativity sets in and CR functions almost like the fast buffer BAPTA (for more details on this behavior, e.g., on the spatiotemporal patterns of IP3-evoked Ca2+ signals, see Dargan et al. (2004) or on CR's role modeled for a train of intracellular Ca2+ signals, see Figure 3 in (Schwaller, 2009). Thus, the Ca2+-binding kinetics of CR strongly depends on [Ca2+]i levels at the time when another increase in [Ca2+]i occurs. Besides these novel properties of Ca2+ binding in a protein, first described for CR, several studies in the 90's reported CR to undergo considerable Ca2+-dependent conformational changes, which indicated that CR might also have “Ca2+ sensor” functions like the prototypical sensor calmodulin (CaM). Results in support of CR acting as a Ca2+ sensor are presented in Section III.
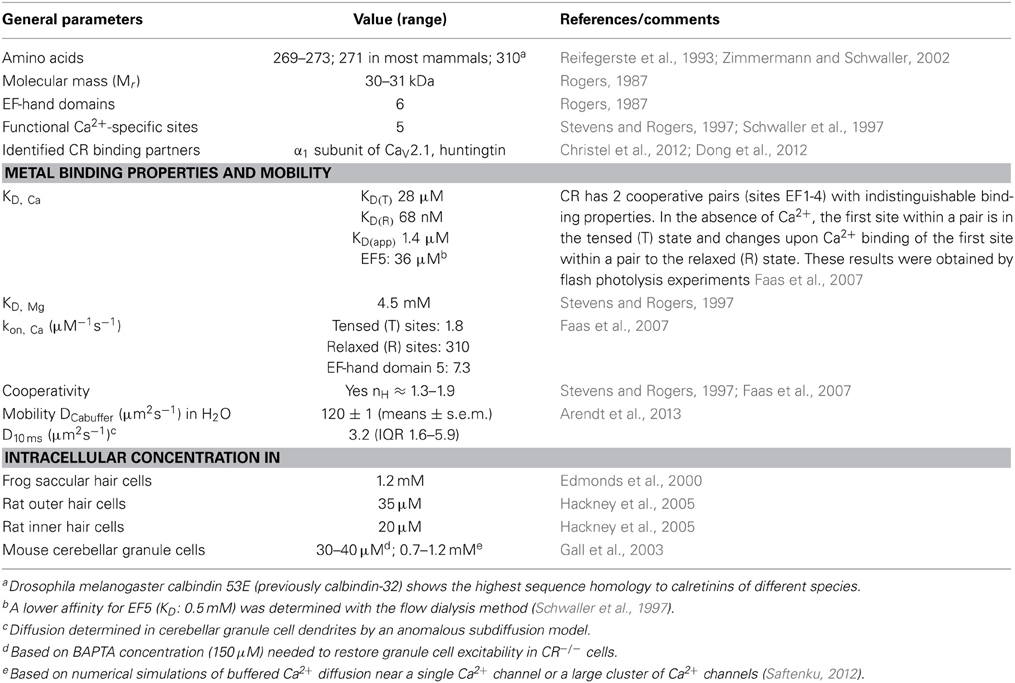
Table 1. Properties of calretinin (modified from Schwaller, 2009, 2010, 2012).
Up to date, no structural data of full-length CR have been reported. However, the NMR structure of the N-terminal 100 amino acids of rat CR (Palczewska et al., 2001) embracing EF-hand domains 1 and 2 are very similar to the NMR solution structure of the corresponding domains in rat CB (Kojetin et al., 2006). Together with the similar results from limited proteolysis experiments obtained with CR and CB, this suggests that the overall structure of hexa-EF-hand proteins might be rather similar.
Regulation of Calretinin Expression
Still relatively little is known on the mechanisms of regulation of CR expression in various tissues; altered CR expression levels have been reported as the consequence of experimental manipulations or are associated with certain diseases in humans and/or animal models of these diseases [for more details, see Schwaller, 2009, 2010, 2012]. Based on the substantial sequence homology in the promoter region including the TATA and CAAT boxes of the human CALB2 and mouse Calb2 gene (Strauss et al., 1997), it is reasonable to assume that CR expression is regulated in a similar manner in the two species, although species differences in CR expression have been reported before. Neuron-specific “CR-like” expression of a luciferase reporter gene in cortical cultures is achieved in the presence of the mouse Calb2 promoter region from −115/+54. The 5′ region of this promoter fragment (−115/−71) selectively binds a nuclear protein present in cerebellar granule cells and contains an “AP2-like” element (−90/−80 bp; Figure 1). This element is essential for the neuron-specific reporter expression (Billing-Marczak et al., 2002). The same “AP2-like” element doesn't affect transcriptional activity in human colon carcinoma and mesothelioma cells indicating that CR expression in neurons and non-neuronal cell types is differently regulated (Billing-Marczak et al., 2004). In human colon cancer cells, CR expression is downregulated by butyrate (Marilley et al., 2001), a substance derived from intestinal fermentation of dietary fibers by bacteria. Butyrate, a known modulator of histone acetylation, inhibits the cell cycle and leads to enterocyte differentiation. Of the several putative butyrate-responsive elements (BREs) present in the human CALB2 promoter, two elements embracing the TATA box act as butyrate-sensitive repressor elements in colon cancer cells, but not in cells of mesothelial origin (Figure 1; Haner et al., 2010). This supports the notion of cell type-specific CALB2 regulation. The rat Calb2 promoter region contains 3 binding motifs for the transcription factor LEF1/TCF that binds to β-catenin via its N-terminal region (Figure 1); β-catenin, not directly binding to DNA, contains a strong transactivation domain and is highly expressed in thalamic neurons. Down-regulation of β-catenin by its negative regulator Axin2 significantly reduces CR expression in cultured rat thalamic neurons indicating that β-catenin is a positive regulator of the Calb2 gene (Wisniewska et al., 2012). In addition, several transcripts exist from the human CALB2 gene (Schwaller et al., 1995), which are present in several colon cancer cell lines (Gander et al., 1996) and in tumor tissue from primary colon tumors (Schwaller et al., 1998). One splice variant with deletion of exons 8 and 9 results in a truncated protein named CR-22k of 192 amino acids, which is expressed in certain tumors (Schwaller et al., 1998); the other transcript (deletion of exon 8) is currently known to exist only at the level of mRNA (Schwaller et al., 1995) and might have a function as an RNA molecule, possibly as a target for micro (mi)RNA or acting as a long non-coding (lnc) RNA.
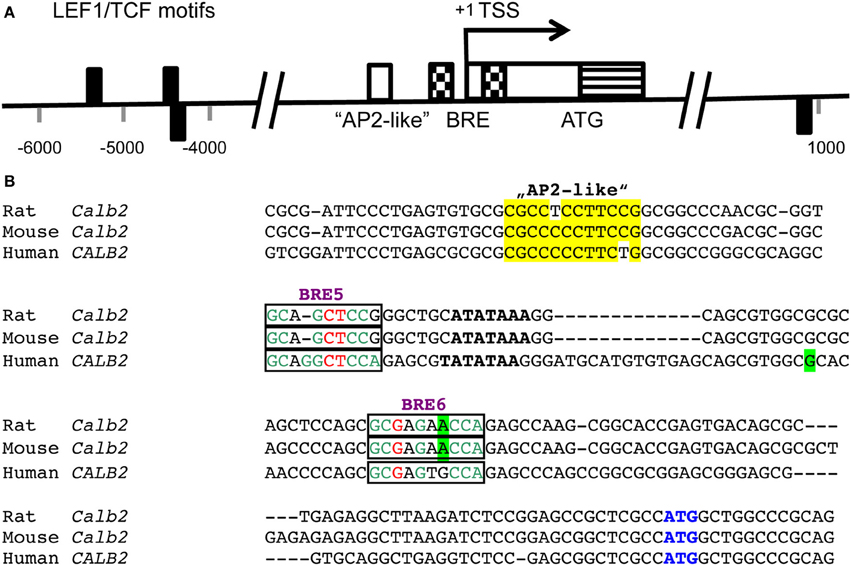
Figure 1. (A) Positions of LEF1/TCF motifs (black rectangles) are present within conserved (human/rat) non-coding regions flanking the transcription start site (TSS; pos. +1) of the CALB2/Calb2 genes and are depicted either above or below the axis depending on the strand (modified from Wisniewska et al., 2012). The consensus sequence recognized by the DNA-binding domain of LEF1/TCFs is WWCAAAG (W is either A or T). Nucleotide numbering is shown with respect to the TSS. In the region −90/−80 of the mouse Calb2 gene, an “AP2-like” element (white box) is responsible for neuron-specific expression of the transcript (Billing-Marczak et al., 2002). A bipartite butyrate-responsive element (BRE; checkered boxes) surrounds the TATA box and the TSS (Haner et al., 2010). The non-coding part of exon 1 is shown as a white box and the coding region including the ATG start codon as a striped box. (B) Sequence comparison of the rat, mouse and human Calb2 gene around the TSS. The “AP2-like” region is boxed in yellow. The TATA box is marked in bold and the BREs 5 and 6 acting as butyrate-responsive repressors in colon cancer cells are boxed. The most highly conserved nucleotides in the BREs (consensus sequence: GCGGGCTCCA) are shown in green, the less conserved ones in red and the nucleotides not conforming to the consensus sequence are shown in black. The TSS (+1) is boxed in green. The start codon ATG is marked in blue.
The Identification of Calretinin-Interacting Targets Supports CR's Role as a Ca2+ Sensor
The finding that purified CR undergoes significant Ca2+-dependent conformational changes in vitro (Kuznicki et al., 1995; Schwaller et al., 1997), together with the observation that CR immunoreactivity in chick brainstem auditory neurons changes from diffuse cytosolic staining to intense localized staining beneath the plasma membrane, which occurs together with the onset of spontaneous activity (Hack et al., 2000), suggested already in the late 90's that CR might have additional Ca2+-sensor functions (Billing-Marczak and Kuznicki, 1995). Furthermore, CR was shown to be present in membrane fractions of rat cerebellum (Winsky and Kuznicki, 1995) and to bind to cytoskeletal elements in WiDr colon cancer cells (Marilley and Schwaller, 2000). In support of the presence of CR targets, CR translocates from the cytosol to the nucleus in a vitamin D3-dependant and/or butyrate-dependent way in colon cancer cells in vitro, also indicating that CR might have nuclear interaction partners (Schwaller and Herrmann, 1997). Recently, in two studies CR targets were identified (Christel et al., 2012; Dong et al., 2012) and moreover, the interacting domain of CR that leads to a decreased Ca2+-dependent mobility of CR in cerebellar granule cells was identified (Arendt et al., 2013). A yeast-two-hybrid screen with CR as bait, identified a consensus, strongly basic peptide sequence H(R/K)HRRR(E/D) as a putative CR-binding (CRB) motif (Christel et al., 2012). This motif is present in multiple copies (CRB1-5) in the cytoplasmic linker region between domains II and III of the channel-forming alpha 1 subunit of the high-voltage activated Ca2+ channel CaV2.1 (P/Q type). This channel is regulated in a dual fashion by Ca2+ ions, showing both, Ca2+-dependent inactivation (CDI) and Ca2+-dependent facilitation (CDF); both mechanisms influence synaptic plasticity in the nervous system. In cells expressing CaV2.1 in vitro, co-expression of CR inhibits CDI and enhances CDF via a direct interaction with the α12.1 subunit. The channel subunit α12.1 co-immunoprecipitates with CR antibodies using either extracts from HEK293T cells transfected with CR and CaV2.1 or mouse cerebellar extracts. In mouse cerebellum, both CR and CaV2.1 are strongly expressed in granule cells and the absence of CR in CR−/− mice causes impairment in motor control (Schiffmann et al., 1999). This impairment is essentially caused by CR's absence in granule cells, since the motor phenotype can be rescued by selective re-expression of CR in granule cells (Bearzatto et al., 2006). Thus, the direct modulation of CaV2.1 by CR affects intracellular Ca2+ signaling and probably also neuronal excitability via a mechanism that is different from CR's previously proven Ca2+ buffering function as discussed in Section IV. The interaction between CR and interacting partners likely including CaV2.1 was studied in granule cells by fluorescence recovery after photobleaching (FRAP). The diffusion of fluorescence-labeled CR molecules is much slower than the one of freely diffusible molecules (fluorescein dextrans) of comparable size (Arendt et al., 2013). Moreover, during a burst of action potentials (APs) that leads to an increase in dendritic [Ca2+]i, CR's mobility is further decreased, indicative of Ca2+-dependent interactions. Addition of a peptide consisting of EF-hand 5 of CR to granule cells, considerably increases CR's mobility implicating that the CR interactions occur mainly via the region of EF-hand 5, the Ca2+-binding site with very low affinity. Estimations on the density (concentration) of CaV2.1 channels and CR in granule cells indicate that channel numbers are too low to account for the strong effect on CR's mobility implicating additional, yet unidentified CR-binding partners in these neurons. A binding partner interacting with CR was found to be huntingtin (Htt), identified by tandem affinity purification (Dong et al., 2012). Binding to CR is even stronger with a mutant form of Htt characterized by a polyglutamine (polyQ) region that is typical for Huntington's disease (HD). In neuronal cultures, CR colocalizes with Htt and a CR/Htt complex can be isolated by co-immunoprecipitation. In CR-overexpressing HEK293 cells, levels of phosphorylated AKT (p-AKT) are increased. At the functional level, CR overexpression reduces mHtt-related H2O2 cytotoxicity in various HD in vitro models. This might be directly linked to CR's capacity to decrease [Ca2+]i in these cells and/or to indirectly increase levels of p-AKT considered as a pro-survival signal. On the other hand, CR down-regulation by shRNA enhances mHtt-mediated neuronal cell death. Based on their findings, the authors conclude that “CR may be a potential therapeutic target for treatment of HD.” A link between CR and p-AKT was reported before; increased expression levels of CR strongly correlate with increased resistance to asbestos cytotoxicity in immortalized Met-5A mesothelial cells (Henzi et al., 2009). This protective effect is abrogated in the presence of phosphatidylinositol 3-kinase (PI3K) inhibitors, in support of the above findings that increased PI3K/AKT signaling (increased p-AKT) caused by CR up-regulation favors cell survival. Thus, in the case of CR-expressing mesothelial and mesothelioma cells, CR or more precisely its down-regulation, might be viewed as a potential new target/strategy for malignant mesothelioma therapy (Blum and Schwaller, 2013).
The Effect of Calretinin on Intracellular Ca2+ Signaling
The particular Ca2+-binding properties of CR together with its mobility differently affect intracellular Ca2+ signals, however only to a measurable extent, if present at a sufficiently high concentration, typically in the range of tens of μM in neurons. Generally, lower CR concentrations (≈1 μM) don't affect Ca2+ signals and e.g., don't protect PC12 cells against Ca2+ overload induced by ionophore treatment or trophic factor deprivation (Kuznicki et al., 1996). Effects of CR on Ca2+ signals are often deduced from comparing signals in neurons from WT and CR−/− mice (Schmidt et al., 2013) or when overexpressing or down-regulating CR in cell culture models (Billing-Marczak et al., 1999; D'Orlando et al., 2001; Pecze et al., 2013). CR's particular properties, i.e., its low Ca2+ occupancy at resting [Ca2+]i together with the high co-operativity resulting in non-linear binding properties in a setting in which neurotransmitter release depends supralinearly on Ca2+ (e.g., in parallel-fiber (PF) terminals onto Purkinje cells) result in considerable nanodomain Ca2+-buffering by CR. As a consequence, a minor increase in the amplitude of AP-evoked Ca2+ signals in CR−/− PF boutons results in a considerably higher release probability (Schmidt et al., 2013). CR-deficient cerebellar granule cells are characterized by faster APs and, when electrically stimulated to generate repetitive spike discharges, show enhanced frequency increase with injected currents, i.e., increased excitability (Gall et al., 2003). The excitability can be reverted to the situation seen in WT cells, by loading the cells with the fast buffer BAPTA (150 μM) strongly indicating that the “fast” Ca2+ buffering function of CR is most likely responsible for limiting granule cell excitability. From these experiments it was also deduced that the CR concentration in these neurons is in the order of 40 μM, based on CR's 4 high-affinity Ca2+-binding sites; this estimation is in line with modeling studies on CR function (Roussel et al., 2006). However, other models taking into account CR's cooperativity of Ca2+ binding resulting in a delayed equilibration with Ca2+ predict the concentration of CR to be even higher, in the order of 0.7–1.2 mM (Saftenku, 2012), a value estimated to be present in frog saccular hair cells (Edmonds et al., 2000). However, in this model the modulation of the main voltage-activated Ca2+ channel in granule cells, CaV2.1, by CR (Christel et al., 2012) was not taken into account. Thus, the precise concentration of CR in granule cells has to be determined yet, possibly by a in situ calibration method as previously used for the determination of the concentration of CB (≈40 μM) in hippocampal granule cells (Muller et al., 2005) or of PV in DG basket cells (11.9 ± 1.6 μ M) or in cerebellar basket cells (563 ± 66 μ M) (Eggermann and Jonas, 2012). The particular biophysical properties of CR also acting as a slow-onset Ca2+ buffer are best appreciated from studies in Xenopus oocytes (Dargan et al., 2004). Photo-release of inositol 1,4,5 triphosphate (IP3) evokes Ca2+ signals that are differently modulated by endogenous or synthetic Ca2+ buffers (Dargan and Parker, 2003). In the presence of slow buffers such as PV or EGTA, global Ca2+ signals are fragmented into local Ca2+ puffs, resulting from Ca2+ release from discrete clusters of IP3 receptors, while low concentrations of fast buffers (CB, BAPTA) decrease the amplitude of Ca2+ signals and favor “globalization” of spatially uniform Ca2+ signals, in particular, at high [IP3]. Interestingly, puffs are observed in the presence of CR at low stimulation intensities, i.e., at low [IP3], an effect never occurring in the presence of CB or BAPTA. Thus, under conditions of small elevations in [Ca2+]i from resting Ca2+ levels, CR has properties of a slow Ca2+ buffer such as PV or EGTA.
Calretinin Expression is Linked to Neuronal Development
The detailed analyses of temporal and spatial expression of CR in the brain is the major focus of this Frontiers series, has been summarized in several papers and reviews Arai et al. (1991); Jacobowitz and Winsky (1991); Hof et al. (1999); Barinka and Druga (2010) and is thus not covered in this mini-review. Recent findings on CR expression (often transient) and neurogenesis are briefly summarized. Olfactory receptor neurons are generated throughout lifetime and are characterized by a short period of CR expression just before these neurons are fully mature (Wei et al., 2013), yet the functional significance is currently unknown. Also mouse adult hippocampal neurogenesis, more precisely, the early postmitotic stage of dentate gyrus (DG) granule cell development is characterized by transient CR expression (Brandt et al., 2003). This stage coincides with onset of differentiation and absence of CR in the immature early postmitotic granule cells of CR−/− mice (systematic name: Calb2tm1Map) “causes an early loss in proliferative capacity of the subgranular zone that is maintained into adult age, when it has a further impact on the migration/survival of newborn granule cells” (Todkar et al., 2012). Interestingly, when in WT mice newborn cells are functionally integrated in the DG granule cell network, CR expression stops and is changed to CB, the typical marker for adult DG granule cells. The functional consequences of this swap from CR to CB expression for granule cell physiology are currently unknown, as well as the mechanisms that lead to such a change. Nonetheless, it indicates Ca2+-binding protein-specific functions that cannot be shared and/or substituted even by apparently very similar proteins such as CB and CR.
What is the Physiological Role of Calretinin?
As was previously reported for CB (Schmidt, 2012), CR certainly has more than one function, depending on various parameters including cell type (neurons vs. non-excitable cells including tumor cells), stages of development (adult vs. developmental stages) and probably also different neuronal subtypes. For some proposed roles of CR, e.g., a role in neuroprotection, the proportion of reports, mostly obtained in correlative studies, in favor or against such a role is almost 50:50 [for more details, see (Schwaller, 2009, 2010)], clearly necessitating more studies directly addressing this putative function of CR. Here, just the most important results obtained in CR−/− mice are summarized. The decreased LTP in the DG is thought to be the result of an increased excitatory drive from CR-depleted mossy cells onto hilar interneurons (Schurmans et al., 1997). Most findings on the function of CR are derived from studies in the cerebellum, where CR is expressed in cerebellar granule cells. Their increased excitability in the absence of CR (Gall et al., 2003) is linked to the altered firing properties (Cheron et al., 2000) and likely Ca2+ homeostasis of Purkinje cells and the emergence of cerebellar 160-Hz oscillations (Cheron et al., 2004) that result in impairment in motor coordination (Schiffmann et al., 1999), for more details, see Schwaller (2009). Thus, CR expression in granule cells appears necessary for correct computation that is crucial for the coding and storage of information in the cerebellum. Of note, there is currently no data available on CR's function in cortical interneurons, e.g., derived from CR−/− mice. I am also not aware that anybody has attempted to manipulate CR expression levels of cortical interneurons, e.g., by shRNA and to investigate the functional consequences of CR down-regulation.
In CR-expressing mesothelial cells, CR down-regulation causes a G1 block and in mesothelioma-derived cells leads to apoptosis via strong activation of the intrinsic caspase 9-dependent pathway (Blum and Schwaller, 2013). A rather similar effect is also seen in CR-expressing colon cancer cells (Gander et al., 1996) indicating a role for CR in cell cycle regulation, proliferation, possibly differentiation and cell death. These findings are in line with transient CR expression during development, whether in the nervous system or in other tissues including mesenchymal tissue (Gangji et al., 1994). In summary, we have just started to unravel the likely many functions of CR in different settings and there are still plenty of interesting aspects on CR's function(s) to be discovered.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I would like to thank Walter-Vincent Blum, Thomas Henzi, and Laszlo Pecze, University of Fribourg, Switzerland and the reviewers for their helpful comments that resulted in the final manuscript.
References
Alpar, A., Attems, J., Mulder, J., Hokfelt, T., and Harkany, T. (2012). The renaissance of Ca2+-binding proteins in the nervous system: secretagogin takes center stage. Cell. Signal. 24, 378–387. doi: 10.1016/j.cellsig.2011.09.028
Arai, R., Winsky, L., Arai, M., and Jacobowitz, D. M. (1991). Immunohistochemical localization of calretinin in the rat hindbrain. J. Comp. Neurol. 310, 21–44. doi: 10.1002/cne.903100105
Arendt, O., Schwaller, B., Brown, E. B., Eilers, J., and Schmidt, H. (2013). Restricted diffusion of calretinin in cerebellar granule cell dendrites implies Ca2+-dependent interactions via its EF-hand 5 domain. J. Physiol. 591, 3887–3899. doi: 10.1113/jphysiol.2013.256628
Barinka, F., and Druga, R. (2010). Calretinin expression in the mammalian neocortex: a review. Physiol. Res. 59, 665–677.
Bearzatto, B., Servais, L., Roussel, C., Gall, D., Baba-Aissa, F., Schurmans, S., et al. (2006). Targeted calretinin expression in granule cells of calretinin-null mice restores normal cerebellar functions. FASEB J. 20, 380–382. doi: 10.1096/fj.05-3785fje
Billing-Marczak, K., Buzanska, L., Winsky, L., Nowotny, M., Rudka, T., Isaacs, K., et al. (2002). AP2-like cis element is required for calretinin gene promoter activity in cells of neuronal phenotype differentiated from multipotent human cell line DEV. Biochim. Biophys. Acta 1577, 412–420. doi: 10.1016/S0167-4781(02)00443-8
Billing-Marczak, K., and Kuznicki, J. (1995). Calretinin -sensor or buffer–function still unclear. Pol. J. Pharmacol. 1173–1178. doi: 10.1267/ahc.06008
Billing-Marczak, K., Przybyszewska, M., and Kuznicki, J. (1999). Measurements of [Ca2+] using fura-2 in glioma C6 cells expressing calretinin with GFP as a marker of transfection: no Ca2+-buffering provided by calretinin. Biochim. Biophys. Acta 1449, 169–177. doi: 10.1016/S0167-4889(99)00010-5
Billing-Marczak, K., Zieminska, E., Lesniak, W., Lazarewicz, J. W., and Kuznicki, J. (2004). Calretinin gene promoter activity is differently regulated in neurons and cancer cells. Role of AP2-like cis element and zinc ions. Biochim. Biophys. Acta 1678, 14–21. doi: 10.1016/j.bbaexp.2004.01.004
Blum, W., and Schwaller, B. (2013). Calretinin is essential for mesothelioma cell growth/survival in vitro: a potential new target for malignant mesothelioma therapy? Int. J. Cancer J. Int. du cancer 133, 2077–2088. doi: 10.1002/ijc.28218
Brandt, M. D., Jessberger, S., Steiner, B., Kronenberg, G., Reuter, K., Bick-Sander, A., et al. (2003). Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol. Cell. Neurosci. 24, 603–613. doi: 10.1016/S1044-7431(03)00207-0
Camp, A., Wijesinghe, J., and Calretinin, R. (2009). Modulator of neuronal excitability. Int. J. Biochem. Cell Biol. 41, 2118–2121. doi: 10.1016/j.biocel.2009.05.007
Cheron, G., Gall, D., Servais, L., Dan, B., Maex, R., and Schiffmann, S. N. (2004). Inactivation of calcium-binding protein genes induces 160 Hz oscillations in the cerebellar cortex of alert mice. J. Neurosci. 24, 434–441. doi: 10.1523/JNEUROSCI.3197-03.2004
Cheron, G., Schurmans, S., Lohof, A., d'Alcantara, P., Meyer, M., Draye, J. P., et al. (2000). Electrophysiological behavior of Purkinje cells and motor coordination in calretinin knock-out mice. Prog. Brain Res. 124, 299–308. doi: 10.1016/S0079-6123(00)24024-7
Christel, C. J., Schär, R., Wang, S., Henzi, T., Kreiner, L., Grabs, D., et al. (2012). Calretinin regulates Ca2+-dependent inactivation and facilitation of CaV2.1 Ca2+ channels through a direct interaction with the α12.1 subunit. J. Biol. Chem. 287, 39766–39775. doi: 10.1074/jbc.M112.406363
Dargan, S. L., and Parker, I. (2003). Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J. Physiol. 553, 775–788. doi: 10.1113/jphysiol.2003.054247
Dargan, S. L., Schwaller, B., and Parker, I. (2004). Spatiotemporal patterning of IP3-mediated Ca2+ signals in Xenopus oocytes by Ca2+-binding proteins. J. Physiol. 556, 447–461. doi: 10.1113/jphysiol.2003.059204
Dong, G., Gross, K., Qiao, F., Ferguson, J., Callegari, E. A., Rezvani, K., et al. (2012). Calretinin interacts with huntingtin and reduces mutant huntingtin-caused cytotoxicity. J. Neurochem. 123, 437–446. doi: 10.1111/j.1471-4159.2012.07919.x
D'Orlando, C., Fellay, B., Schwaller, B., Salicio V., Bloc, A., Gotzos, V., et al. (2001). Calretinin and calbindin D-28k delay the onset of cell death after excitotoxic stimulation in transfected P19 cells. Brain Res. 909, 145–158. doi: 10.1016/S0006-8993(01)02671-3
Edmonds, B., Reyes, R., Schwaller, B., and Roberts, W. M. (2000). Calretinin modifies presynaptic calcium signaling in frog saccular hair cells. Nat. Neurosci. 3, 786–790. doi: 10.1038/77687
Eggermann, E., and Jonas, P. (2012). How the ‘slow’ Ca2+ buffer parvalbumin affects transmitter release in nanodomain-coupling regimes. Nat. Neurosci. 15, 20–22. doi: 10.1038/nn.3002
Faas, G. C., Schwaller, B., Vergara, J. L., and Mody, I. (2007). Resolving the fast kinetics of cooperative binding: Ca2+ buffering by calretinin. PLoS Biol. 5:e311 doi: 10.1371/journal.pbio.0050311
Gall, D., Roussel, C., Susa, I., D'Angelo, E., Rossi, P., Bearzatto, B., et al. (2003). Altered neuronal excitability in cerebellar granule cells of mice lacking calretinin. J. Neurosci. 23, 9320–9327.
Gander, J. C., Gotzos, V., Fellay, B., and Schwaller, B. (1996). Inhibition of the proliferative cycle and apoptotic events in WiDr cells after down-regulation of the calcium-binding protein calretinin using antisense oligodeoxynucleotides. Exp. Cell Res. 225, 399–410. doi: 10.1006/excr.1996.0191
Gander, J.-C., Bustos-Castillo, M., Stüber, D., Hunziker, W., Celio, M. R., and Schwaller, B. (1996). The calcium-binding protein calretinin-22k, an alternative splicing product of the calretinin gene is expressed in several colon adenocarcinoma cell lines. Cell Calcium. 20, 63–72. doi: 10.1016/S0143-4160(96)90051-2
Gangji, V., Bastianelli, E., Rooze, M., and Pochet, R. (1994). Transient Calretinin Expression during Intervertebral Disc Formation of the Chick Embryo. Dev. Growth Differ. 36, 621–628. doi: 10.1111/j.1440-169X.1994.00621.x
Hack, N. J., Wride, M. C., Charters, K. M., Kater, S. B., and Parks, T. N. (2000). Developmental changes in the subcellular localization of calretinin. The Journal of neurosci. 20, RC67.
Hackney, C. M., Mahendrasingam, S., Penn, A., and Fettiplace, R. (2005). The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J. Neurosci. 25, 7867–75. doi: 10.1523/JNEUROSCI.1196-05.2005
Haner, K., Henzi, T., Pfefferli, M., Kunzli, E., Salicio V., and Schwaller B. (2010). A bipartite butyrate-responsive element in the human calretinin (CALB2) promoter acts as a repressor in colon carcinoma cells but not in mesothelioma cells. J Cell Biochem. 109, 519–531. doi: 10.1002/jcb.22429
Henzi, T., Blum, W. V., Pfefferli, M., Kawecki, T. J., Salicio, V., and Schwaller, B. (2009). SV40-induced expression of calretinin protects mesothelial cells from asbestos cytotoxicity and may be a key factor contributing to mesothelioma pathogenesis. Am. J. Pathol. 174, 2324–2336. doi: 10.2353/ajpath.2009.080352
Hof, P. R., Glezer, I. I., Conde, F., Flagg, R. A., Rubin, M. B., Nimchinsky, E. A., et al. (1999). Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J. Chem. Neuroanat. 16, 77–116. doi: 10.1016/S0891-0618(98)00065-9
Jacobowitz, D. M., and Winsky, L. (1991). Immunocytochemical localization of calretinin in the forebrain of the rat. J. Comp. Neurol. 304, 198–218. doi: 10.1002/cne.903040205
Kojetin, D. J., Venters, R. A., Kordys, D. R., Thompson, R. J., Kumar, R., and Cavanagh, J. (2006). Structure, binding interface and hydrophobic transitions of Ca2+-loaded calbindin-D(28K). Nat. Struct. Mol. Biol. 13, 641–647. doi: 10.1038/nsmb1112
Kuznicki, J., Isaacs, K. R., and Jacobowitz, D. M. (1996). The expression of calretinin in transfected PC12 cells provides no protection against Ca2+-overload or trophic factor deprivation. Biochim. Biophys. Acta 1313, 194–200. doi: 10.1016/0167-4889(96)00089-4
Kuznicki, J., Wang, T.-C. L., Martin, B. M., Winsky, L., and Jacobowitz, D. M. (1995). Localization of Ca2+-dependent conformational changes of calretinin by limited tryptic proteolysis. Biochem. J. 308, 607–619.
Marilley, D., and Schwaller, B. (2000). Association between the calcium-binding protein calretinin and cytoskeletal components in the human colon adenocarcinoma cell line WiDr. Exp Cell Res. 259, 12–22. doi: 10.1006/excr.2000.4942
Marilley, D., Vonlanthen, S., Gioria, A., and Schwaller, B. (2001). Calretinin and calretinin-22k increase resistance towards sodium butyrate-induced differentiation in CaCo-2 colon adenocarcinoma cells. Exp Cell Res. 268, 93–103. doi: 10.1006/excr.2001.5261
Muller, A., Kukley, M., Stausberg, P., Beck, H., Muller, W., and Dietrich, D. (2005). Endogenous Ca2+ buffer concentration and Ca2+ microdomains in hippocampal neurons. J. Neurosci. 25, 558–565. doi: 10.1523/JNEUROSCI.3799-04.2005
Palczewska, M., Groves, P., Ambrus, A., Kaleta, A., Kover, K. E., Batta, G., et al. (2001). Structural and biochemical characterization of neuronal calretinin domain I-II (residues 1-100). Comparison to homologous calbindin D28k domain I-II (residues 1-93). Eur. J. Biochem. 268, 6229–6237. doi: 10.1046/j.0014-2956.2001.02575.x
Pecze, L., Blum, W., and Schwaller, B. (2013). Mechanism of capsaicin receptor TRPV1-mediated toxicity in pain-sensing neurons focusing on the effects of Na+/Ca2+ fluxes and the Ca2+-binding protein calretinin. Biochim. Biophys. Acta 1833, 1680–1691. doi: 10.1016/j.bbamcr.2012.08.018
Reifegerste, R., Grimm, S., Albert, S., Lipski, N., Heimbeck, G., Hofbauer, A., et al. (1993). An invertebrate calcium-binding protein of the calbindin subfamily: protein structure, genomic organization, and expression pattern of the calbindin-32 Gene of Drosophila. J. Neurosci. 13, 2186–2198.
Rogers, J. H. (1987). Calretinin: a gene for a novel calcium-binding protein expressed principally in neurons. J. Cell Biol. 105, 1343–1353. doi: 10.1083/jcb.105.3.1343
Roussel, C., Erneux, T., Schiffmann, S. N., and Gall, D. (2006). Modulation of neuronal excitability by intracellular calcium buffering: from spiking to bursting. Cell Calcium 39, 455–466. doi: 10.1016/j.ceca.2006.01.004
Saftenku, E. E. (2012). Effects of calretinin on Ca2+ signals in cerebellar granule cells: implications of cooperative Ca2+ binding. Cerebellum 11, 102–120. doi: 10.1007/s12311-011-0263-4
Schiffmann, S. N., Cheron, G., Lohof, A., d'Alcantara, P., Meyer, M., Parmentier, M., et al. (1999). Impaired motor coordination and Purkinje cell excitability in mice lacking calretinin. Proc. Natl. Acad. Sci. U.S.A. 96, 5257–5262. doi: 10.1073/pnas.96.9.5257
Schmidt, H. (2012). Three functional facets of calbindin D-28k. Front. Mol. Neurosci. 5:25. doi: 10.3389/fnmol.2012.00025
Schmidt, H., Brachtendorf, S., Arendt, O., Hallermann, S., Ishiyama, S., Bornschein, G., et al. (2013). Nanodomain coupling at an excitatory cortical synapse. Curr. Biol. 23, 244–249. doi: 10.1016/j.cub.2012.12.007
Schurmans, S., Schiffmann, S. N., Gurden, H., Lemaire, M., Lipp, H.-P., Schwam, V., et al. (1997). Impaired LTP induction in the dentate gyrus of calretinin-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 94, 10415–10420. doi: 10.1073/pnas.94.19.10415
Schwaller, B. (2009). The continuing disappearance of “pure” Ca2+ buffers. Cell. Mol. Life Sci. 66, 275–300. doi: 10.1007/s00018-008-8564-6
Schwaller, B. (2010). Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2:a004051. doi: 10.1101/cshperspect.a004051
Schwaller, B. (2012). The Regulation of a Cell's Ca2+ Signaling Toolkit: The Ca2+ Homeostasome. Adv. Exp. Med. Biol. 740, 1–25. doi: 10.1007/978-94-007-2888-2_1
Schwaller, B., and Herrmann, B. (1997). Regulated redistribution of calretinins in WiDr cells. Cell Death Differ. 4, 325–333. doi: 10.1038/sj.cdd.4400240
Schwaller, B., Celio, M. R., and Hunziker, W. (1995). Alternative splicing of calretinin mRNA leads to different forms of calretinin. Eur. J. Biochem. 230, 424–430. doi: 10.1111/j.1432-1033.1995.0424h.x
Schwaller, B., Durussel, I., Jermann, D., Herrmann, B., and Cox, J. A. (1997). Comparison of the Ca2+-binding properties of human recombinant calretinin-22k and calretinin. J. Biol. Chem. 272, 29663–29671. doi: 10.1074/jbc.272.47.29663
Schwaller, B., Meyer-Monard, S., Gander, J.-C., Pugin, P., Celio, M. R., and Ludwig, C. (1998). The calcium-binding protein calretinin-22k is detectable in the serum and specific cells of cancer patients. Anticancer Res. 18, 3361–3367.
Stevens, J., and Rogers, J. H. (1997). Chick calretinin: purification, composition, and metal binding activity of native and recombinant forms. Protein Expr. Purif. 9, 171–181. doi: 10.1006/prep.1996.0677
Strauss, K. I., Kuznicki, J., Winsky, L., Kawagoe, J. I., Hammer, M., and Jacobowitz, D. M. (1997). The mouse calretinin gene promoter region: structural and functional components. Brain Res. Mol. Brain Res. 49, 175–187. doi: 10.1016/S0169-328X(97)00143-5
Todkar, K., Scotti, A. L., and Schwaller, B. (2012). Absence of the calcium-binding protein calretinin, not of calbindin D-28k, causes a permanent impairment of murine adult hippocampal neurogenesis. Front. Mol. Neurosci. 5:56. doi: 10.3389/fnmol.2012.00056
Wei, H., Lang, M. F., and Jiang, X. (2013). Calretinin is expressed in the intermediate cells during olfactory receptor neuron development. Neurosci. lett. 542, 42–46. doi: 10.1016/j.neulet.2013.03.022
Winsky, L., and Kuznicki, J. (1995). Distribution of calretinin, calbindin D28k and parvalbumin in subcellular fractions of rat cerebellum: effects of calcium. J. Neurochem. 65, 381–388. doi: 10.1046/j.1471-4159.1995.65010381.x
Wisniewska, M. B., Nagalski, A., Dabrowski, M., Misztal, K., and Kuznicki, J. (2012). Novel beta-catenin target genes identified in thalamic neurons encode modulators of neuronal excitability. BMC Genomics 13:635. doi: 10.1186/1471-2164-13-635
Keywords: calretinin, calcium signaling, calcium sensor, calcium buffer, neuron excitability
Citation: Schwaller B (2014) Calretinin: from a “simple” Ca2+ buffer to a multifunctional protein implicated in many biological processes. Front. Neuroanat. 8:3. doi: 10.3389/fnana.2014.00003
Received: 23 December 2013; Paper pending published: 09 January 2014;
Accepted: 19 January 2014; Published online: 05 February 2014.
Edited by:
Filip Barinka, University of Regensburg, GermanyReviewed by:
Hartmut Schmidt, University of Leipzig, GermanyPing Liu, University of Connecticut Health Center, USA
Copyright © 2014 Schwaller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beat Schwaller, Anatomy, Department of Medicine, University of Fribourg, Route Albert-Gockel 1, CH-1700 Fribourg, Switzerland e-mail:YmVhdC5zY2h3YWxsZXJAdW5pZnIuY2g=
 Beat Schwaller
Beat Schwaller