- 1Department of Biomedical Sciences, School of Medicine, Debre Markos University, Debre Markos, Ethiopia
- 2Department of Human Physiology, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 3Department of Biomedical Sciences, School of Medicine, Injbara University, Injbara, Ethiopia
Background: Obstructive sleep apnea results from intermittent airway collapse during sleep. Despite its health risks, the prevalence and associated factors of OSA among hypertensive patients in Ethiopia remain unexplored.
Objective: This study assessed the prevalence and associated factors of high-risk OSA among hypertensive patients in referral hospitals within the Amhara Region, Northwest Ethiopia, in 2022.
Methods: A cross-sectional study was conducted in selected referral hospitals from 21 April to 14 June 2022. A systematic random sampling technique was employed. Data were collected through structured, pretested, interviewer-administered questionnaires, file reviews, and physical examinations. Data were entered in Epi-Data 4.6 and analyzed using Stata 14. Logistic regression was performed, and variables with p < 0.05 were regarded as significantly associated with high-risk OSA.
Results: Of the 412 participants (97% response rate), the mean age was 58.95 ± 12.6 years, with 55.1% being female. The prevalence of high-risk OSA was determined to be 43.93% (95% CI: 39.2–48.8). Significant factors included age > 65 years (AOR = 8.00, 95% CI: 4.48–14.14), diabetes mellitus (AOR = 4.7, 95% CI: 2.48–8.94), male sex (AOR = 4.2, 95% CI: 2.38–7.45), and large neck circumference (AOR = 3.13, 95% CI: 1.27–7.74).
Conclusion: High-risk OSA is prevalent among hypertensive patients, particularly in older males and those with diabetes or a large neck circumference. Routine OSA screening should be integrated into hypertension care. Future studies should utilize gold-standard tools and explore cause-and-effect relationships.
Introduction
Obstructive sleep apnea (OSA) is a sleep disorder characterized by repeated upper airway obstruction during sleep due to intermittent airway collapse (Bacci et al., 1992). This condition arises from factors that reduce the pharyngeal space, leading to disrupted airflow (Dempsey et al., 2010). Common symptoms include snoring, daytime sleepiness, restles sleep, morning fatigue, and headaches (Phillips and O'Driscoll, 2013). The gold standard for diagnosis is polysomnography; however, in its absence, standardized assessment tools such as the Berlin and STOP-BANG Questionnaires are effective for risk assessment (Tintinger et al., 2011; Chung et al., 2008, 2016). OSA severity is classified by the apnea-hypopnea index (AHI) as mild (5–15 events/h), moderate (16–30 events/h), or severe (>30 events/h) (Prejbisz et al., 2014).
OSA is a significant global health concern, affecting approximately one billion individuals (Prejbisz et al., 2014). It impacts 4–9% of middle-aged men and 1–2% of middle-aged women, with prevalence reaching 20–30% in obese individuals (Phillips and O'Driscoll, 2013). Among adults aged 30–69 years, an estimated 936 million have mild to severe OSA, while 425 million have moderate to severe OSA (Hedner et al., 2006). Epidemiological studies reveal varying prevalence rates: 73% in Sweden (Hedner et al., 2006), 58.1% in Brazil (Bacci et al., 1992), 24% in India (Kareem et al., 2020), 70.5% in China (Cai et al., 2017), 32.38% in Thailand (Jinchai et al., 2020), 42.1% in Tanzania (Pallangyo et al., 2021), and 52% in hypertensive individuals in Nigeria (Akintunde et al., 2012).
Key risk factors for OSA include obesity, chronic illnesses, smoking, and alcohol consumption (Tintinger et al., 2011; Lin et al., 2012; Kasai et al., 2012; Aurora and Punjabi, 2013; Abuyassin et al., 2015). Severe OSA (AHI >30) significantly elevates the risk of cardiovascular events such as myocardial infarction, stroke, hypertension, and arrhythmias (Korostovtseva et al., 2011; Kario, 2009; Shah et al., 2010). Chronic hypoxia in OSA leads to renal damage, increasing the risk of chronic kidney disease (Fu et al., 2016). Additionally, OSA contributes to metabolic dysregulation, oxidative stress, heightened sympathetic activity, lipolysis, inflammation, and insulin resistance, exacerbating type 2 diabetes and obesity (Gaines et al., 2018). It is also linked to secondary and resistant hypertension (Kario, 2009). Overall, OSA is associated with reduced quality of life, substantial morbidity, and increased cardiovascular mortality risk, with affected individuals being 4–5 times more likely to experience fatal cardiovascular events (Tintinger et al., 2011; Young et al., 2008). Additionally, mortality rates are higher in the severe OSA group (41%) compared to the mild and moderate OSA group (29%) (Won et al., 2013). Continuous positive airway pressure (CPAP) remains the primary treatment, significantly reducing OSA-related complications (Litvin et al., 2013).
Although OSA is known to reduce the quality of life and increase morbidity and mortality, research on OSA in Africa is limited, and there are no documented studies in Ethiopia examining its prevalence and associated factors among hypertensive patients. This study seeks to fill this gap by providing valuable baseline data for future research and offering a foundation for policymakers to address this critical health challenge.
Methods and materials
Study setting and period
The study was conducted from April 21 to June 14, 2022, at Felege Hiwot, Debre Markos, and Debre Tabor referral hospitals in the Amhara Region, Ethiopia. These hospitals serve over 25 million people, including about 2,500 hypertensive patients receiving follow-up care (unpublished data).
Study design and period
An institutional-based cross-sectional study was conducted among hypertensive patients in the Amhara region in selected referral hospitals in Ethiopia. Of 423 invited patients, 412 completed the questionnaire, but 11 were excluded due to incomplete responses. The source population included all hypertensive patients in follow-up care (every 3 months) at the selected hospitals, while the study population comprised those attending Felege Hiwot, Debre Markos, and Debre Tabor referral hospitals during data collection.
Inclusion and exclusion criteria
Hypertensive patients (BP >140/90 mmHg), aged ≥18 years, who gave voluntary consent were included. However, critically ill patients or those with COPD or psychiatric disorders were excluded.
Sample size determination
The sample size was calculated using a single population proportion formula.
Assumption
n = sample size, P = proportion of the risk of OSA = 50% since the study on the risk of OSA among visually hypertensive patients was not conducted in the study area, d = Margin of sampling error tolerated-5% (0.05), α = Critical value at 95% confidence interval of certainty (1.96).
Sampling procedure
A combination of simple and systematic random sampling was used. Three hospitals (Felege Hiwot, Debre Markos, and Debretabor Referral Hospitals) were randomly selected by the lottery method. A sampling frame of hypertensive patients was created from chronic disease clinic records. Systematic random sampling was applied, selecting every 2nd patient (K = 6, derived from 2,500 hypertensive patients divided by a sample size of 423). The starting point (patient 2) was chosen by lottery, and participants were proportionally allocated across hospitals for representativeness.
Study variables
Dependent variable
High risk of obstructive sleep apnea (yes/no).
Independent variables
Socio-demographic factors
Age, sex, residence, religion, education, occupation.
Behavioral factors
Smoking, Alcohol consumption.
Clinical factors
Heart failure, Kidney failure, Type 2 Diabetes Mellitus.
Anthropometric measurements
Body Mass Index (BMI), Neck circumference, Waist circumference, Physical inactivity, Family history of snoring.
Data collection procedure and data collection tools
Data were collected using the STOP-BANG questionnaire, an eight-item tool assessing OSA risk based on snoring, daytime sleepiness, observed apnea, hypertension, BMI, age (≥50), neck circumference, and male gender (Chiu et al., 2017; Abumuamar et al., 2018). It is administered by an interviewer, takes 2–5 min, and has over 80% sensitivity but <50% specificity, effectively identifying high-risk individuals (Abumuamar et al., 2018; Luo et al., 2014). A STOP-BANG score of 0 to 2 indicates a low risk of obstructive sleep apnea, a score of 3 to 4 suggests an intermediate risk, and a score of 5 to 8 reflects a high risk of the condition (Yang and Chung, 2013 #186). Additionally, data were gathered through medical chart reviews and physical examinations, which included measurements of weight, height, neck circumference, and waist circumference. Weight was assessed using a digital scale (±0.5 kg) while participants wore light clothing and no shoes, and height was measured with a stadiometer, ensuring participants stood erect, barefoot, with their heels against a wall or board (Douketis et al., 2005).
BMI was calculated as weight divided by height squared (kg/m2). Neck circumference was measured with non-elastic tape at the cricothyroid level, and waist circumference at the midpoint between the lower costal margin and the iliac crest was recorded to the nearest 0.5 cm (Douketis et al., 2005). Clinical factors were obtained from the patient file.
Data processing and analysis
After data collection, the dataset was cleaned, edited, and entered into Epi Data 4.6, then exported to Stata 14.0 for analysis. Descriptive statistics summarized the study population. Bi-variable and multivariable logistic regressions examined associations, with variables (p < 0.25) from the bivariable analysis included in the multivariable model. Statistical significance was set at p < 0.05. Multicollinearity among selected independent variables was checked, and the variance inflation 1.58 factor was found to be acceptable (<2), and the Hosmer-Lemeshow test (χ2 = 17.95, p = 0.16) confirmed a good model fit. A post-hoc power analysis was conducted to assess the study's statistical power to identify significant associations between high-risk OSA and its determinants among hypertensive patients, and with a final sample size of 412, an observed odds ratio of 1.5, and a significance level of 0.05, the calculated power was 0.847. The sensitivity analysis was conducted using Cook's distance and leverage, which would be below the typical threshold of 1.
Data quality assurance
A 1-day training session was held for data collectors and supervisors by the principal investigator, covering data collection methods, patient interaction, and maintaining privacy and confidentiality. Two trained diploma nurses collected data from the target population, with daily reviews by the supervisor and principal investigator to ensure consistency and completeness. A pretest was conducted at Tibebe Geon Specialized Hospital with 30 participants (5% of the total sample size) before the main data collection. During the interview, all participants showed a clear understanding of the questionnaire.
Results
Socio-demographic characteristics of study participants
A total of 412 hypertensive patients participated, with a response rate of 97%. The mean age was 58.95 ± 12.6 years. Of the participants, 227 (55.1%) were female, and 260 (63.1%) lived in urban areas. Most identified as Orthodox Christians and were married. Additionally, 144 (37.95%) were illiterate, and 163 (39.56%) were farmers (Table 1).
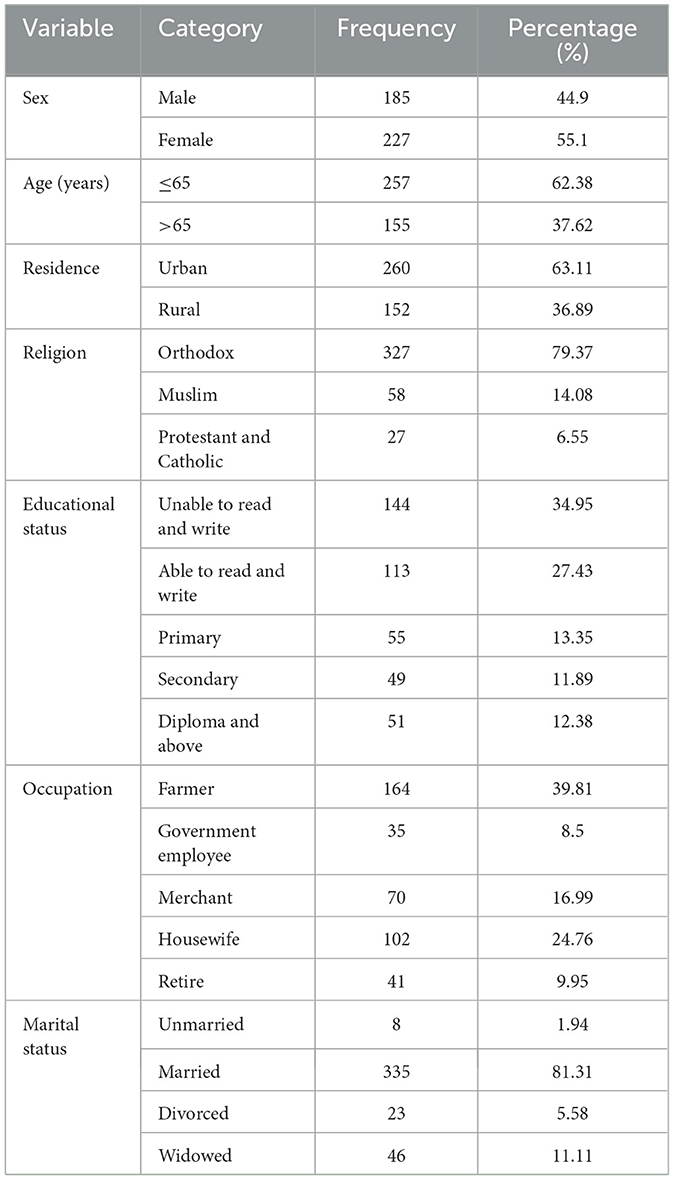
Table 1. Socio-demographic characteristics of hypertensive patients in selected referral hospitals, Amhara region, Ethiopia, 2022(n = 412).
Clinical and behavioral characteristics of study participants
Among hypertensive patients, 102 (24.76%) were also diabetic. The majority, 408 (99%), were non-smokers, and 130 (28.1%) had a history of alcohol consumption. Most participants, 310 (75.24%), were physically inactive. Only 54 (13.11%) had a family history of snoring. The average weight was 67.1 ± 11.7 kg, height 1.61 ± 0.07 m, neck circumference 35.6 ± 3.9 cm, and waist circumference 91.6 ± 10.9 cm. The average BMI was 26 ± 4.25, while mean systolic and diastolic blood pressures were 132 ± 26.1 mmHg and 80.37 ± 9.56 mmHg, respectively (Table 2).
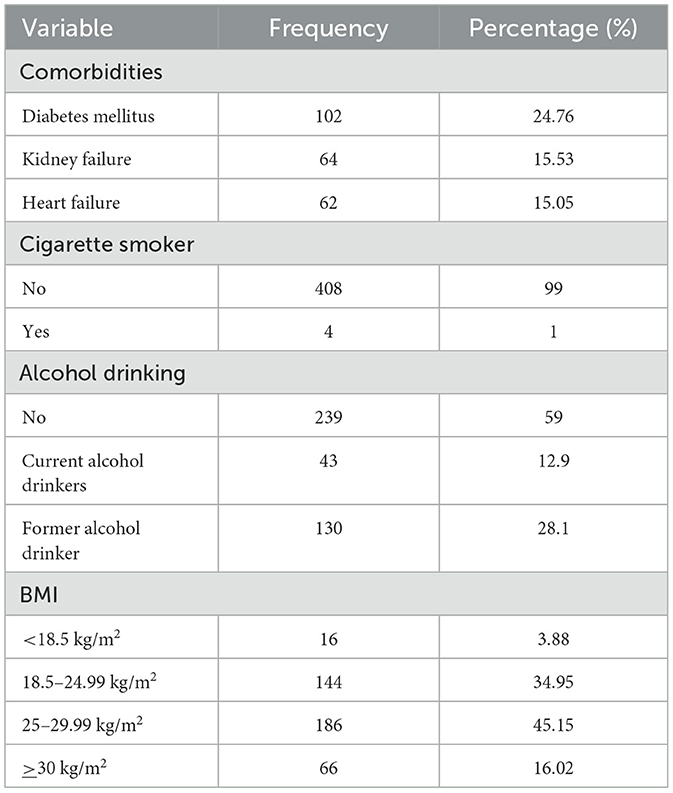
Table 2. Clinical and behavioral characteristics of hypertensive patients in selected referral hospitals, Amhara region, Ethiopia, 2022 (n = 412).
Findings of post-hoc power analysis
The post-hoc power analysis demonstrated that the power to detect significant associations between key determinants and high-risk OSA was 0.85, surpassing the typical 0.80 threshold for adequate power and confirming that the sample size provided enough power for prevalence estimation, ensuring the statistical robustness of the observed high-risk OSA prevalence rates.
Prevalence of risk of obstructive sleep apnea
Among hypertensive participants, 43.93% (95% CI: 39.52–48.8) were at high risk of OSA, while 56.07% (95% CI: 51.2–60.8) were at low risk.
Subgroup analysis results: high vs. low risk of OSA
The risk of obstructive sleep apnea (OSA) was significantly influenced by various demographic and clinical factors: males had a higher risk than females (61.1 vs. 29.95%), with low risk more prevalent in females (70.05%) than males (38.9%); individuals over 65 years exhibited a higher risk (74.84%) compared to those 65 years or younger (25.3%), who had a greater proportion in the low-risk category (74.7 vs. 25.16%); urban residents had a slightly higher high-risk rate (47.3%) and slightly lower low-risk rate (52.7%) than rural residents (38.16 and 61.84%, respectively). Diabetic patients were more likely to be at high risk (74.5%) than non-diabetics (33.9%), with non-diabetics more commonly in the low-risk group (66.1 vs. 25.5%); respondents with renal failure (64%) or heart failure (58%) had a higher risk than those without these conditions (40.23 and 42.4%, respectively), while the latter had greater low-risk proportions (59.77 and 58.6%, respectively); alcohol drinkers showed a higher risk (47.95%) than non-drinkers (39.38%), who had a greater proportion of low-risk individuals (60.62 vs. 52.05%); a family history of snoring was associated with a much higher risk of OSA (85.98%) than no family history (16.13%); individuals with a BMI ≥30 kg/m2 had a high risk (65.15%), whereas those with BMI 18.5–24.9 kg/m2 (76.39%) and <18.5 kg/m2 (68.75%) had low-risk; those with neck circumference >40 cm had a high risk (77.8%) compared to those with ≤ 40 cm (61.18%) who were had low-risk; participants with waist circumference >102 cm had a higher risk (69.74%) than those with ≤ 102 cm, who more frequently had a low risk (61.9 vs. 30.26%); and individuals with systolic blood pressure (SBP) >140 mmHg had a higher OSA risk (55%), while those with SBP <140 mmHg had low risk (58.74%; Table 3).
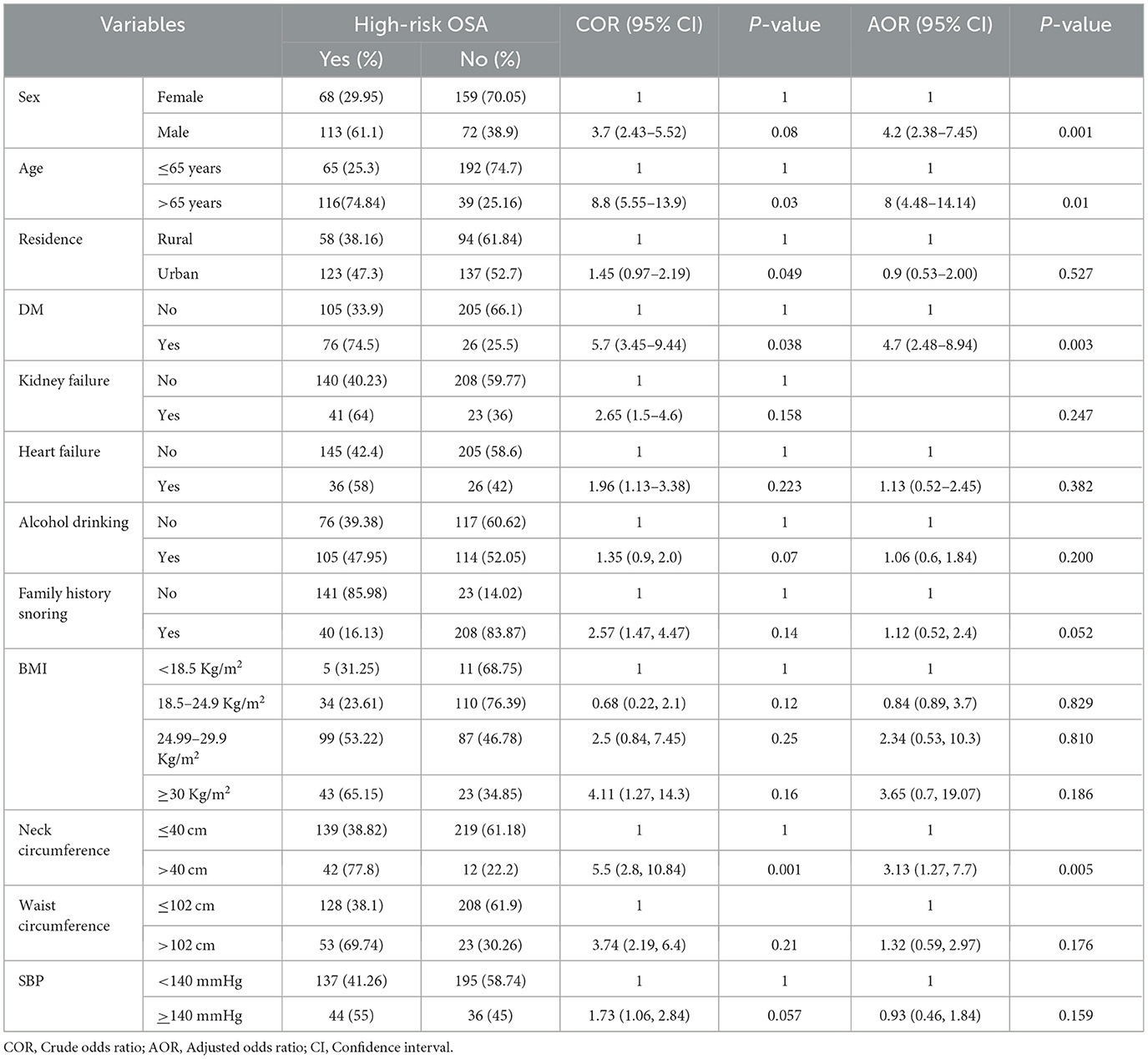
Table 3. Bivariable and multivariable binary logistic regression analysis of factors associated with high risk of OSA among hypertensive patients in selected referral hospitals, Amhara region, Ethiopia, 2022(n = 412).
Factors associated with the risk of OSA
In the bivariable binary logistic regression analysis, factors such as age, sex, residence, diabetes mellitus, heart failure, kidney failure, systolic blood pressure, family history of snoring, alcohol consumption, BMI, neck circumference, and waist circumference were associated with an increased risk of obstructive sleep apnea (OSA). However, in the multivariable analysis, significant associations with a high risk of OSA were being over 65 years old, male, having diabetes mellitus, and having a large neck circumference.
Those over 65 had an 8fold higher risk of OSA compared to those under 65 (AOR = 8.00, 95% CI: 4.48–14.14). Individuals with diabetes were 4.7 times more likely to be at high risk for OSA (AOR = 4.7, 95% CI: 2.48–8.94). Men had a 4.2-fold higher likelihood of OSA risk than women (AOR = 4.2, 95% CI: 2.38–7.45). Additionally, those with a neck circumference over 40 cm had 3.13 times higher odds of OSA risk (AOR = 3.13, 95% CI: 1.27–7.74; Table 3).
In our data, the sensitivity analysis revealed that the maximum values of Cook's distance and leverage were found to be extremely less than one (0.03–0.2).
Discussion
This study assessed the risk of obstructive sleep apnea (OSA) and associated factors among hypertensive patients in referral hospitals within the Amhara Regional State.
The prevalence of high-risk OSA in this study was 43.93% (95% CI: 39.2–48.8), which was higher than the study done in Thailand at 32.38% (Jinchai et al., 2020) and India at 23.8% (Kareem et al., 2020). This discrepancy may be due to the larger sample size in this study compared to the Thailand and India study, which focused on younger hypertensive patients under 35 years with 118 participants. The higher illiteracy rate and limited education and healthcare access among participants likely contributed to an overestimation of high-risk obstructive sleep apnea prevalence. This study, with a larger sample and primarily older participants, may account for the difference.
Conversely, the prevalence observed in this study was lower than in studies conducted in Nigeria (52%) (Akintunde et al., 2012), Brazil (58.1%) (Bacci et al., 1992), Sweden (73%) (Hedner et al., 2006), and China (70.5% ) (Cai et al., 2017). These differences may stem from variations in assessment tools. This study used the STOP-BANG questionnaire, a practical screening tool, while studies in Sweden and China used polysomnography, the gold standard for OSA diagnosis, and those in Nigeria and Brazil used the Berlin questionnaire. The study populations may also have contributed to these variations; for example, the Chinese study had a larger sample with mostly male participants, while this study had a smaller sample with more female participants.
This finding aligns closely with a study conducted in Tanzania, which reported a prevalence of 42.1% (Pallangyo et al., 2021).
Age was found to be a significant factor, with older individuals having eight times higher odds of being at high risk, as supported by studies in Nigeria (Akintunde et al., 2012) and Brazil (Bacci et al., 1992). This association may be due to anatomical changes in the upper airway, increased pharyngeal resistance, airway oscillations, and sleep instability with aging (Levy et al., 1996).
The study found that males were 4.2 times more likely to have a high risk of OSA than females, aligning with studies from Sweden (Bacci et al., 1992) and China (Cai et al., 2017). This difference may be due to sex hormones like progesterone, which helps maintain airway patency in females by enhancing airway dilator muscle activity during sleep (Sigurð*ardóttir et al., 2022). Additionally, males tend to have more fat around the neck, increasing airway load and promoting pharyngeal collapse during sleep (Whittle et al., 1999).
Comorbid diabetes mellitus (DM) was linked to a 4.7-fold higher risk of high-risk OSA, as supported by studies in Nigeria (Akintunde et al., 2012) and Tanzania (Pallangyo et al., 2021). This association may be due to DM-induced peripheral autonomic neuropathy, which impairs upper airway muscle function, mechanoreceptor activation, and ventilatory control, leading to reduced airway diameter and increased airway obstruction risk (Vinik et al., 2003).
Finally, a large neck circumference was strongly linked to high-risk OSA, as supported by studies in Nigeria (Akintunde et al., 2012), Tanzania (Pallangyo et al., 2021), and India (Kareem et al., 2020). This association is likely due to subcutaneous fat in the neck, which increases airway pressure and promotes pharyngeal collapse during sleep.
This study relied on participants' recall for many questions, which may introduce recall bias. As a cross-sectional study, it cannot establish cause-and-effect relationships. Moreover, the absence of a confirmatory polysomnography test may have led to an inaccurate estimation of OSA risk.
Conclusion and recommendations
Nearly half of the hypertensive patients in this study were at high risk for obstructive sleep apnea (OSA), with significant associations observed for male gender, diabetes mellitus, age over 65, and neck circumference above 40 cm. These findings highlight the need for routine OSA screening in referral hospitals, particularly for hypertensive patients presenting with symptoms like daytime sleepiness, fatigue, or snoring. Future research should employ gold-standard screening methods and stronger study designs to clarify causal links while also considering factors such as medication use, physical activity, diet, sleep duration, and daytime sleepiness. We further recommend using validated tools, random sampling when feasible, and including control groups from non-conflict areas to enhance data quality and generalizability.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by ethical clearance was obtained from the institutional review board (IRB) at the University of Gondar, College of Medicine and Health Science (ref. no SOM/1492/2022). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MK: Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. BA: Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. DC: Conceptualization, Software, Validation, Writing – original draft, Writing – review & editing. KA: Formal analysis, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. BA: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to acknowledge the study participants, data collectors, and supervisors for their willingness, valuable support, and assistance during this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AOR, Adjusted Odds Ratio; BMI, Body Mass Index; COR, Crude Odds Ratio; DBP, Diastolic Blood Pressure; DM, Diabetes mellitus; Kg, Kilogram; Kg/m2, Kilogram per meter square; OSA, Obstructive sleep apnea; SBP, Systolic Blood Pressure; WHO, World Health Organization.
References
Abumuamar, A. M., Dorian, P., Newman, D., and Shapiro, C. M. (2018). The STOP-BANG questionnaire shows an insufficient specificity for detecting obstructive sleep apnea in patients with atrial fibrillation. J. Sleep Res. 27:e12702. doi: 10.1111/jsr.12702
Abuyassin, B., Sharma, K., Ayas, N. T., and Laher, I. (2015). Obstructive sleep apnea and kidney disease: a potential bidirectional relationship? J. Clin. Sleep Med. 11, 915–924. doi: 10.5664/jcsm.4946
Akintunde, A. A., Okunola, O. O., Oluyombo, R., Oladosu, Y. O., and Opadijo, O. G. OSnoring obstructive sleep apnoea syndrome among hypertensive Nigerians: prevalence clinical correlates. Pan Afr. Med. J. (2012). 11, 1071–1078.
Aurora, R. N., and Punjabi, N. M. (2013). Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir. Med. 1, 329–338. doi: 10.1016/S2213-2600(13)70039-0
Bacci, M. R., Emboz, J. N. M., Alves, B., Veiga, G. L. D., Murad, N., Meneghini, A., et al. (1992). Obstructive sleep apnea syndrome and sleep quality in hypertensive patients. Rev. Assoc. Med. Bras. 63, 1055–1060. doi: 10.1590/1806-9282.63.12.1055
Cai, A., Zhou, Y., Zhang, J., Zhong, Q., Wang, R., Wang, L., et al. (2017). Epidemiological characteristics and gender-specific differences of obstructive sleep apnea in a Chinese hypertensive population: a cross-sectional study. BMC Cardiovasc. Disord. 17, 1–7. doi: 10.1186/s12872-016-0447-4
Chiu, H. Y., Chen, P. Y., Chuang, L. P., Chen, N. H., Tu, Y. K., Hsieh, Y. J., et al. (2017). Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med. Rev. 36, 57–70. doi: 10.1016/j.smrv.2016.10.004
Chung, F., Abdullah, H. R., and Liao, P. (2016). STOP-bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 149, 631–638. doi: 10.1378/chest.15-0903
Chung, F., Yegneswaran, B., Liao, P., Chung, S. A., Vairavanathan, S., Islam, S., et al. (2008). STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 108, 812–821. doi: 10.1097/ALN.0b013e31816d83e4
Dempsey, J. A., Veasey, S. C., Morgan, B. J., and O'Donnell, C. P. (2010). Pathophysiology of sleep apnea. Physiol. Rev. 90, 47–112. doi: 10.1152/physrev.00043.2008
Douketis, J. D., Paradis, G., Keller, H., and Martineau, C. (2005). Canadian guidelines for body weight classification in adults: application in clinical practice to screen for overweight and obesity and to assess disease risk. CMAJ 172, 995–998. doi: 10.1503/cmaj.045170
Fu, Q., Colgan, S. P., and Shelley, C. S. (2016). Hypoxia: the force that drives chronic kidney disease. Clin. Med. Res. 14, 15–39. doi: 10.3121/cmr.2015.1282
Gaines, J., Vgontzas, A. N., Fernandez-Mendoza, J., and Bixler, E. O. (2018). Obstructive sleep apnea and the metabolic syndrome: the road to clinically meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med. Rev. 42, 211–219. doi: 10.1016/j.smrv.2018.08.009
Hedner, J., Bengtsson-Boström, K., Peker, Y., Grote, L., Råstam, L., Lindblad, U., et al. (2006). Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur. Respir. J. 27, 564–570. doi: 10.1183/09031936.06.00042105
Jinchai, J., Khamsai, S., Chattakul, P., Limpawattana, P., Chindaprasirt, J., Chotmongkol, V., et al. (2020). How common is obstructive sleep apnea in young hypertensive patients? Intern. Emerg. Med. 15, 1005–1010. doi: 10.1007/s11739-019-02273-3
Kareem, O., Tanvir, M., and Bader, G. N. (2020). Prevalence of high-risk obstructive sleep apnoea by Berlin questionnaire in patients with hypertension: a study from a tertiary care hospital. Sleep Sci. Pract. 4, 1–9. doi: 10.1186/s41606-020-00052-0
Kario, K. (2009). Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertens. Res. 32, 428–432. doi: 10.1038/hr.2009.56
Kasai, T., Floras, J. S., and Bradley, T. D. (2012). Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 126, 1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813
Korostovtseva, L. S., Sviryaev, Y. V., Zvartau, N. E., Konradi, A. O., and Kalinkin, A. L. (2011). Prognosis and cardiovascular morbidity and mortality in a prospective study of hypertensive patients with obstructive sleep apnea syndrome in St Petersburg, Russia. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 17, Cr146–53. doi: 10.12659/MSM.881448
Levy, P., Pepin, J., Malauzat, D., Emeriau, J., and Leger, J. (1996). Is sleep apnea syndrome in the elderly a specific entity? Sleep 19, S29–38. doi: 10.1093/sleep/19.suppl_3.S29
Lin, Y. N., Li, Q. Y, and Zhang, X. J. (2012). Interaction between smoking and obstructive sleep apnea: not just participants. Chin. Med. J. 125, 3150–3156.
Litvin, A. Y., Sukmarova, Z. N., Elfimova, E. M., Aksenova, A. V., Galitsin, P. V., Rogoza, A. N., et al. (2013). Effects of CPAP on “vascular” risk factors in patients with obstructive sleep apnea and arterial hypertension. Vasc. Health Risk Manag. 9, 229–235. doi: 10.2147/VHRM.S40231
Luo, J., Huang, R., Zhong, X., Xiao, Y., and Zhou, J. (2014). STOP-bang questionnaire is superior to Epworth sleepiness scales, Berlin questionnaire, and STOP questionnaire in screening obstructive sleep apnea-hypopnea syndrome patients. Chin. Med. J. 127, 3065–3070. doi: 10.3760/cma.j.issn.0366-6999.20133003
Pallangyo, P., Mgopa, L. R., Mkojera, Z., Komba, M., Millinga, J., Misidai, N., et al. (2021). Obstructive sleep apnea and associated factors among hypertensive patients attending a tertiary cardiac centre in Tanzania: a comparative cross-sectional study. Sleep Sci. Pract. 5, 1–11. doi: 10.1186/s41606-021-00069-z
Phillips, C. L., and O'Driscoll, D. M. (2013). Hypertension, and obstructive sleep apnea. Nat. Sci. Sleep 5, 43–52. doi: 10.2147/NSS.S34841
Prejbisz, A., Florczak, E., Pregowska-Chwała, B., Klisiewicz, A., Kuśmierczyk-Droszcz, B., Zieliński, T., et al. (2014). Relationship between obstructive sleep apnea and markers of cardiovascular alterations in never-treated hypertensive patients. Hypertens. Res. 37, 573–579. doi: 10.1038/hr.2014.43
Shah, N. A., Yaggi, H. K., Concato, J., and Mohsenin, V. (2010). Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath 14, 131–136. doi: 10.1007/s11325-009-0298-7
Sigurð*ardóttir, E. S., Gislason, T., Benediktsdottir, B., Hustad, S., Dadvand, P., Demoly, P., et al. (2022). Female sex hormones and symptoms of obstructive sleep apnea in European women of a population-based cohort. PLoS ONE 17:e0269569. doi: 10.1371/journal.pone.0269569
Tintinger, G. R., Pretorius, L., and Labadarios, D. (2011). Obstructive sleep apnoea and obesity. South Afr. J. Clin. Nutr. 24, 174–177. doi: 10.1080/16070658.2011.11734384
Vinik, A. I., Maser, R. E., Mitchell, B. D., and Freeman, R. (2003). Diabetic autonomic neuropathy. Diabetes Care 26, 1553–1579. doi: 10.2337/diacare.26.5.1553
Whittle, A. T., Marshall, I., Mortimore, I. L., Wraith, P. K., Sellar, R. J., Douglas, N. J., et al. (1999). Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax 54, 323–328. doi: 10.1136/thx.54.4.323
Won, C. H., Chun, H. J., Chandra, S. M., Sarinas, P. S., Chitkara, R. K., Heidenreich, P. A., et al. (2013). Severe obstructive sleep apnea increases mortality in patients with ischemic heart disease and myocardial injury. Sleep Breath 17, 85–91. doi: 10.1007/s11325-012-0653-y
Yang, Y., and Chung, F. (2013). A screening tool of obstructive sleep apnea: STOP-bang questionnaire. Sleep Med. Clin. 8, 65–72.
Keywords: hypertension, obstructive sleep apnea, associated factors, Ethiopia, breathing
Citation: Kassaw M, Ayal BM, Chilot D, Abebaw K and Ashenef B (2025) Prevalence and determinants of high-risk obstructive sleep apnea among hypertensive patients in referral hospitals of the Amhara Region, Northwest Ethiopia, 2022. Front. Sleep 4:1554653. doi: 10.3389/frsle.2025.1554653
Received: 02 January 2025; Accepted: 24 April 2025;
Published: 16 May 2025.
Edited by:
Dalva Poyares, Federal University of São Paulo, BrazilReviewed by:
Bilgay Izci Balserak, University of Illinois Chicago, United StatesRaichel Mary Alex, Brigham and Women's Hospital and Harvard Medical School, United States
Copyright © 2025 Kassaw, Ayal, Chilot, Abebaw and Ashenef. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baye Ashenef, YmF5ZWFzaGVuYWZpNzdAZ21haWwuY29t
 Meseret Kassaw1
Meseret Kassaw1 Bezawit Mulat Ayal
Bezawit Mulat Ayal Kassa Abebaw
Kassa Abebaw Baye Ashenef
Baye Ashenef