- Department of Biomedical Sciences, Texas A&M University College of Dentistry, Dallas, TX, United States
Background and objectives: Sleep apnea-related autonomic responses may increase cardiac arrhythmias. Ablation, cardioversion, and pharmacologic therapies for paroxysmal atrial fibrillation (AF) could benefit from adjunctive oral appliance therapy with a mouth shield (OAT+) compared to auto-adjusting positive airway pressure (APAP).
Methods: A 67-year-old male with moderate obstructive sleep apnea (OSA), AF history, three ablations, and on Carvedilol (10 mg daily) underwent home sleep recordings with APAP and with OAT+ after 4 weeks. Randomly selected premature atrial contractions (PACs; n=20) and time-linked plethysmography waves from each intervention were compared.
Results: OAT+ reduced the PAC index (-61.9%), cardiac conduction intervals (nR-R, p = 0.025; pre-PAC R-R, p = 0.003; R-PAC-R, p = 0.051; PAC R-post systolic pause-R, p < 0.001) except for a P-R interval increase (p = 0.032). PAC-associated plethysmography wave amplitudes increased with OAT+ (pre-PAC wave-1, p < 0.001; PAC wave-2, p = 0.023; post-PAC wave-3, p < 0.001).
Conclusions: OAT+ shows promise as an adjunct AF therapy in OSA patients, improving cardiac conduction and vascular function over APAP.
1 Introduction
Atrial fibrillation (AF) is highly prevalent in the U.S. and possesses a greater risk in patients with sleep disordered breathing (SDB) vs. patients without SDB (Verrier and Josephson, 2021; Goudis and Ketikoglou, 2017). AF recurrence after catheter ablation is associated with 25% increased risk in patients with obstructive sleep apnea (OSA) (Ng et al., 2011). Mounting evidence implicates repeated hypoxic episodes linked to OSA and central sleep apnea (CSA) acting as chemo-reflex triggers to enhance sympathetic nervous system (SNS) activity responses. Sympathetic over-activity may induce premature atrial contractions (PACs), excitability of cardiac pacemaker and atrial cells, tachycardia and cardiovascular stress. In an animal model, episodes of hypoxia were shown to induce pulmonary vein burst firing and reduction of negative tracheal pressure promptly restored normal sinus rhythm (Goudis and Ketikoglou, 2017).
Pharmacological and ablation therapies are effective for AF, but their efficacy is reduced by OSA. Continuous positive airway pressure (CPAP) treatment for OSA mitigates sympathetic activation, boosts vagal stimulation, and lowers the risk of AF progression and recurrence (Verrier and Josephson, 2021). However, the Sleep Apnea Cardiovascular Endpoints study found that CPAP does not prevent cardiovascular events in patients with moderate-to-severe OSA and cardiovascular disease, with CPAP users showing a non-significant increase in the hazard ratio (1.46) for new-onset AF (McEvoy et al., 2016). Additionally, a large study on adaptive servo-ventilation therapy for CSA indicated increased all-cause and cardiovascular mortality in chronic heart failure patients (Cowie et al., 2015). While CPAP is beneficial in improving blood pressure dipping (Kumagai et al., 2022), reducing the risk of cardiovascular events, enhancing cardiac function and decreasing risks of arrhythmias and pulmonary hypertension in some patients, its effectiveness is limited by poor patient adherence compared with oral appliance (OA) therapy.
OA, which advances the mandible to increase oropharyngeal space and reduce airway collapsibility, is an alternative to CPAP, a first-line treatment for mild to moderate OSA and a second-line option for severe cases or CPAP intolerance (Walsh et al., 2008). Compared to no intervention, OA improves oxygen saturation. A study in severe OSA patients without cardiac disease found OA more effective than CPAP in reducing brain natriuretic peptide levels, suggesting improved cardiac function (Hoekema et al., 2008). Notably, a patient with elevated brain natriuretic peptide levels and AF showed AF improvement with OA. The authors suggested this benefit may be due to reduced breathing effort and intrathoracic pressure, though intra-esophageal pressures, cardiac electrophysiology and hemodynamics were not analyzed.
We hypothesized that PAC temporal changes during sleep in a patient with AF and moderate OSA could reveal differences in cardiac electrophysiology between oral appliance therapy with mouth shield (OAT+) and APAP.
2 Report of case
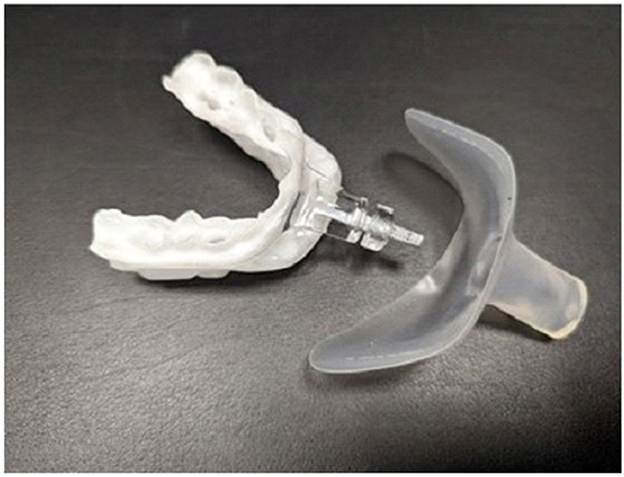
A 67-year-old Caucasian male (Body Mass Index, 27.4 kg/m2, 202 lbs) with moderate OSA (Apnea Hypopnea Index, 22 events/hour) and a history of paroxysmal AF managed with three ablations was referred for OAT+. He reported discomfort with APAP therapy using a full-face mask. His only medication is 10 mg daily of Carvedilol extended release for heart rate and blood pressure control. An oral exam revealed no contraindications including Mallampatti score =2, unobstructed nasal breathing (breathing through the nose for 1–2 min with mouth closed, awake and reclined in dental chair), >8 stable teeth per arch to support the OA, healthy gums, jaw joints and muscles. The subject was dentist-fitted with a titratable OAT+ (myTAP, AMI, Carrollton, TX; Figure 1) to reduce upper airway collapse, mouth breathing (MB) and provide comfort (Chakraborty et al., 2010).
3 Materials and methods
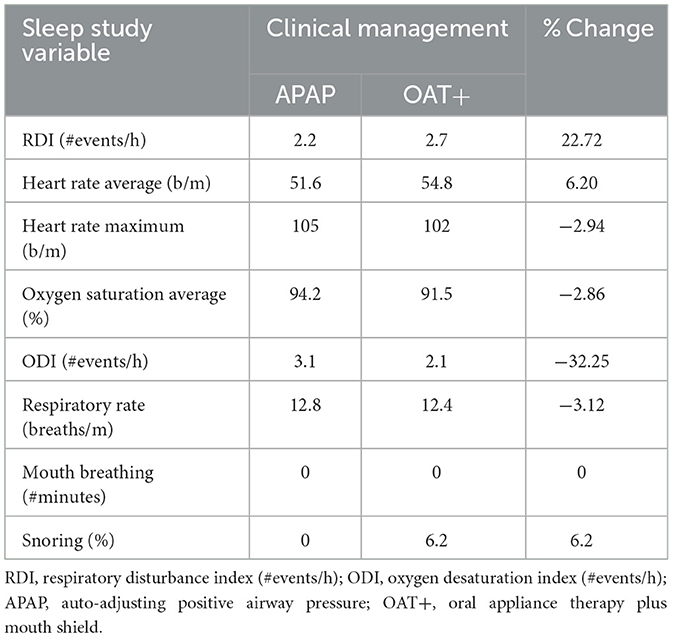
A home sleep test (HST) evaluated APAP—full-face mask efficacy 12-weeks before the introduction to OAT+ (Table 1). Following a 4-week adjustment period to OAT+, initially titrated to 60% of maximal protrusion, a second HST was performed with OAT+ at this position. Both recordings (>6 h 30 min) collected airflow, respiratory effort, snoring, electrocardiogram (ECG, 2-lead configuration), body position and pulse oximetry with a Nonin finger probe. Electroencephalogram was not recorded. Respiratory dynamics data, including RDI were obtained and analyzed using Noxturnal software and the NOX T3 recorder (NOX Medical, Reykjavík, Iceland). Apnea and hypopnea events were visually scored using revised American Academy of Sleep Medicine 2007 scoring criteria (Berry et al., 2012). RDI was defined as the sum of all apneas and hypopnea events/hour (apneas, >90% reduction in airflow from baseline; hypopneas, 30–90% airflow reduction from baseline associated with ≥3% oxygen desaturation and duration ≥10 seconds). The RDI includes the number of respiratory effort related arousal (RERA) and snore RERA events per hour of recorded time rather than sleep time, so may slightly underestimate the Apnea Hypopnea index (AHI). RERA scoring used ANS arousals based on pulse signal heart rate increases ≥5 beats/min in addition to a sequence of breaths lasting ≥10 s characterized by increasing respiratory effort or by flattening of the inspiratory portion of the nasal pressure (Mayer et al., 2020). Respiratory rate (#breaths/minute), oxygen desaturation index (ODI, #events/h with ≥3% oxygen desaturation), and SaO2 (percent), MB (#minutes; ≥3 breaths minimum duration ≥20 dB) and snore percent (snore minutes ≥20 dB/analysis duration minutes) were obtained. The recorder placement followed the manufacturer's recommended mid-thoracic montage.
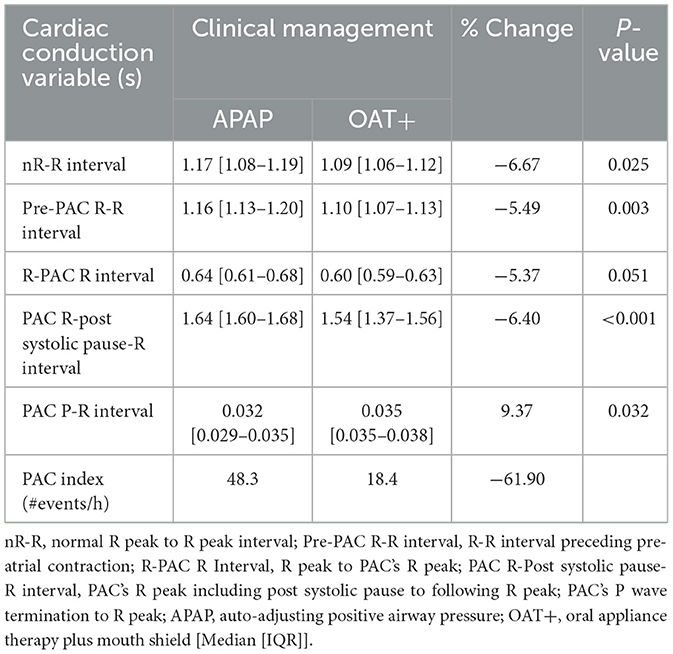
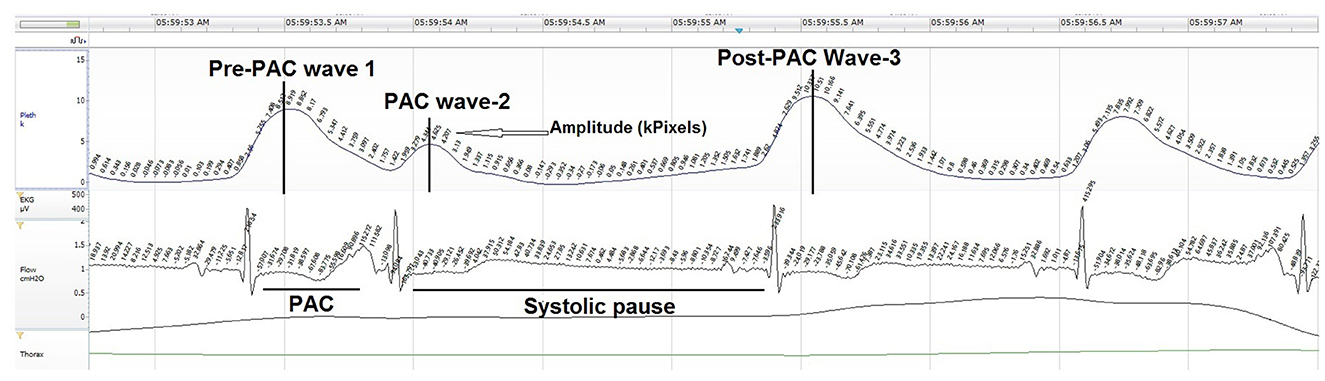
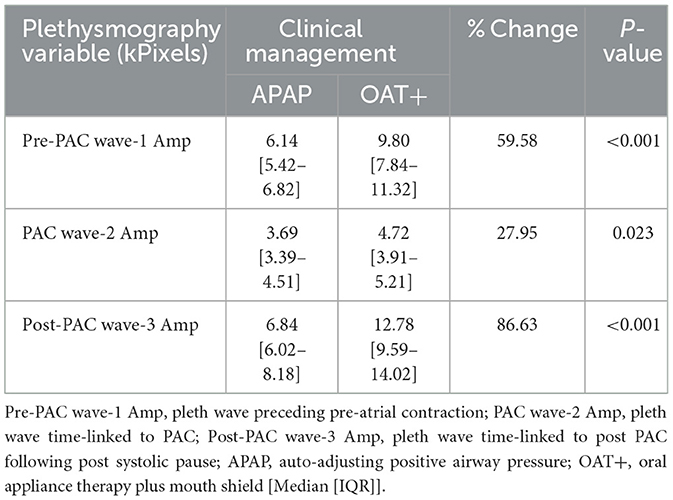
Twenty PAC events were randomly selected at similar time points from the start of each night's polygraphy ECG signal (Table 2). PACs were selected with an approximate 20–30 min interval from recording start to potentially capture NREM and REM PAC events. PACs were defined as having a coupling interval to the prior QRS complex ≤ 50% of the mean R-R interval. Manual measures of cardiac conduction and plethysmography, a non-invasive method to evaluate peripheral hemodynamic parameters by measuring changes in peak amplitude (kPixels) variation for each PAC event, with the Noxturnal software (Table 3; Figure 2).
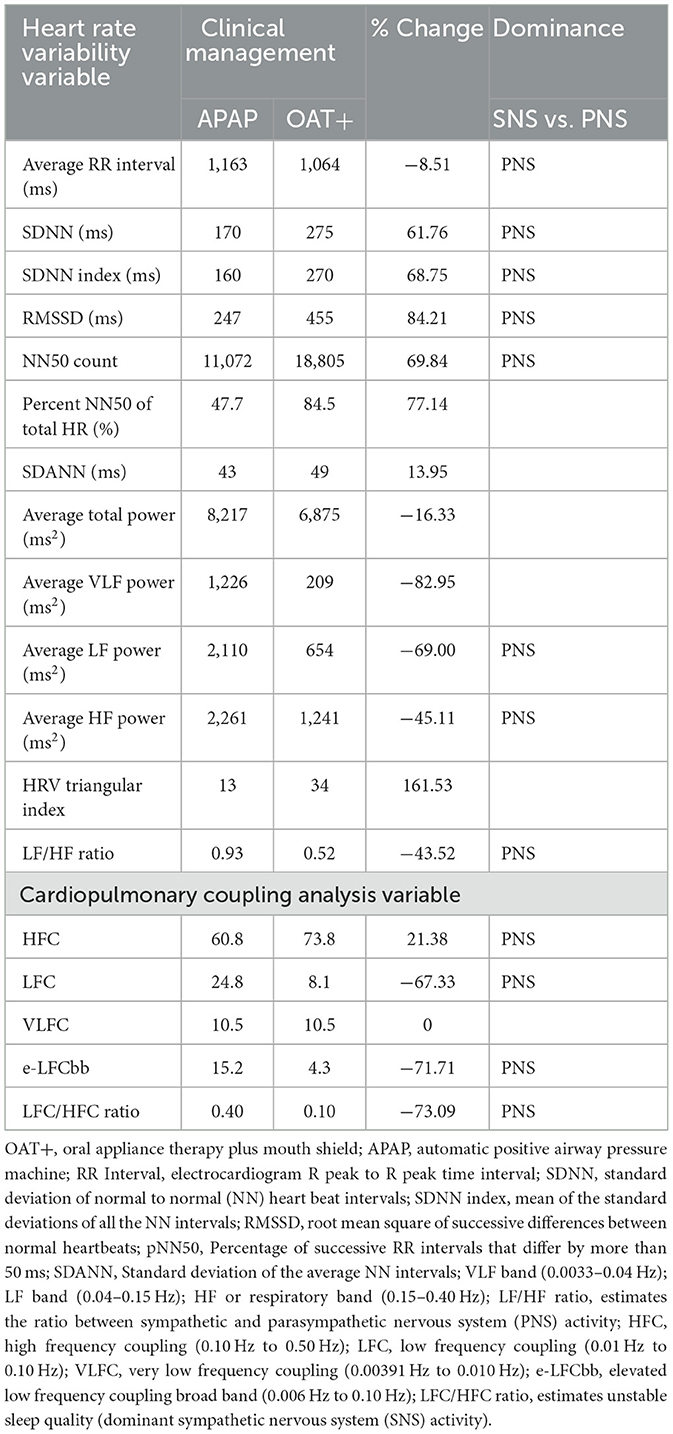
Table 3. Comparison of heart rate variability and cardiopulmonary coupling analysis responses to OAT+ vs. APAP.
RemLogic version 1.1 (Embla Systems, Inc., Thornton, CO, USA) software was used to obtain heart rate variability (HRV) and cardiopulmonary coupling (CPC) analyses results from both recordings (Table 3). CPC analysis is an automated method that uses and ECG-derived respiration (EDR) signal directly from QRS axis shifts and is designed to assess breathing dynamics that objectively measures sleep quality in patients with SDB to support our claim that OAT+ increased parasympathetic activity. CPC classification: high frequency coupling (HFC; 0.1–0.5 Hz); low frequency coupling (LFC; 0.01–0.1 Hz); Very low frequency coupling (VLFC; 0.0039–0.01 Hz) (Thomas et al., 2005).
The Mann-Whitney U Test was used for statistical comparison between interventions. The median and interquartile range [IQR] is reported.
4 Results
4.1 Home sleep test
The HST results showed increases in the RDI (22.7%), average HR (6.2%) and snoring (6.2%) with OAT+ compared with APAP. Decreases in maximum HR (−2.94), oxygen saturation (−2.86%), the oxygen desaturation index (−32.25%), and respiratory rate (−3.12%) with OAT+ compare with APAP was observed. MB was not detected with either intervention (Table 1).
4.2 Cardiac conduction
Comparison of cardiac conduction variables between OAT+ and APAP showed OAT+ significantly reduced the duration of the normal R-R interval (p = 0.025), pre-PAC R-R interval (p = 0.003), the PAC R-Post Systolic Pause-R interval (p < 0.001) and a statistical trend for reduction of the R-PAC R interval (p = 0.051), but increased the PAC P-R interval (p = 0.032) compared with APAP. OAT+ decreased the PAC index (#events/h) (−61.9%) compared with APAP (Table 2).
4.3 Heart rate variability and cardiopulmonary coupling analysis
HRV: OAT+ decreased the average R-R interval (−8.51%), average total power (−16.33%), average VLF power (−82.95%), average LF power (−69.0%), average HF power (−46.11%) and LF/HF ratio (−43.52%) compared with APAP. LF/HF ratio was lower with OAT+ (0.52) compared with APAP (0.93). OAT+ increased SDNN (61.76%), SDNN index (68.75%), RMSSD (84.21%), NN50 count (69.84%), percent NN50 of total HR (77.14%), SDANN (13.95%), and HRV Triangular index (161.53%) (Table 3).
CPC: OAT+ increased HFC (21.38%) compared with APAP. OAT+ decreased LFC (−67.33%), e-LFCbb (−71.71%) and the LFC/HFC ratio (−73.09%) compared with APAP (Table 3).
4.4 Plethysmography
OAT+ significantly increased the pre-PAC wave-1 amplitude (p < 0.001), PAC wave-2 amplitude (p = 0.023) and post-PAC wave-3 amplitude (p < 0.001) compared with APAP (Table 4).
5 Discussion
OAT+, combined with a non-selective beta-blocker, shows promise as adjunct therapy for AF patients with OSA, potentially improving cardiac conduction over full-face mask and APAP therapy. Key mechanisms include enhanced nasal airflow, increased parasympathetic activity, higher airway nitric oxide production, and reduced oxygen desaturation index, and lowering of hypoxic triggers.
A recent case report linked AF conversion to normal sinus rhythm and a stable heart rate with optimal CPAP pressure at 9 cm H2O, likely due to the reversal of intrathoracic negative pressures causing cardiac stress (Walia et al., 2016). Other factors, such as improved oxygenation and autonomic function, may have contributed, despite mild desaturations. However, conflicting reports question whether CPAP reduces intrathoracic stress as effectively as OA (Schlatzer et al., 2016).
Despite a 2.86% decrease in oxygen saturation, the 32.25% reduction in ODI and 3.12% decrease in respiratory rate, our findings suggest parasympathetic activity increased with OAT+. Our recent study found significant respiratory rate reductions in non-AF patients with mild to severe OSA using OAT+ (Schramm et al., 2024). Improved nasal airflow likely entrains delta and theta brain rhythms, synchronizing cellular networks (Fontanini and Bower, 2006), including the limbic system, which regulates breathing, heart rate, and contraction force via input from the hypothalamus and higher brain regions (Ito et al., 2014; Zelano et al., 2016; Heck et al., 2017).
Our results support the hypothesis that OAT+ reduces cardiovascular stress by mitigating snoring although 6.2% residual snoring persisted without MB. This aligns with previous findings showing OAT+ stabilizes oxygen saturation by promoting nasal breathing and reducing MB over 4–12 weeks (Schramm et al., 2024). Additionally, OA was associated with increased serum nitric oxide and improved endothelial function (Galic et al., 2016). Novel to this report, is the additional HRV and CPC results that support OAT+ increases PNS activity. Low LF/HF ratio (OAT+; 0.52) reflects PNS dominance (Salsone et al., 2018; Shaffer and Ginsberg, 2017). While increased SNS activity can cause shortening of R-R intervals, the irregular nature of AF means that shorter intervals are common due to the increased firing rate of the atria but not necessarily due to increase in SNS activity. In this case, the decrease in R-R interval duration is likely attributed to the reduction in systolic pause duration and lowered PAC index. We speculate these decreases reflect R-R interval normalization and possible reduction in intrathoracic pressure (Schlatzer et al., 2016). Furthermore, the P-R interval increase may reflect Carvedilol's mixed adrenergic blocking effects, which influence cardiac conduction based on sympathetic tone and improve cardiac efficiency by possibly reducing heart rate variability and workload (Cheng et al., 2006). The CPC HFC variable is associated with PNS dominance, stable respiration, blood pressure dipping and non-cyclic alternating patterns in the electroencephalogram (Thomas et al., 2005). The decrease in SNS dominant LFC suggest sympathetic activity down-regulation and the decreased LFC/HFC ratio, demonstrates a shift toward PNS dominance with OAT+.
The findings of this case report should be interpreted with caution because electroencephalogram (EEG) data was not collected in order to make definitive statements about the impact on NREM, REM sleep, and wake and EEG arousals. EEG sleep staging would have allowed us to use the AHI and temporally-link EEG arousals to cardiac events (i.e., increase in HR). Although we used RDI and included RERA and sRERA events, the number of respiratory events occurring during the recording vs. sleep might still be underestimated.
Although we cannot confirm increased NO levels, the rise in plethysmography amplitude likely reflects OAT+-facilitated nasal breathing in addition to Carvedilol's alpha-1 adrenergic blockade, enhancing peripheral vasodilation. These findings align with studies showing reduced arterial stiffness, measured by decreased pulse wave velocity, after 1 year of mandibular advancement device therapy (Lin et al., 2015). Further research is needed to explore OAT+ as adjunct therapy for AF patients co-morbid with OSA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The requirement of ethical approval was waived by Texas A&M University College of Dentistry IRB for the studies involving humans because it is a single-patient case report and exempt by Texas A&M University College of Dentistry IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The participant provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PS: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. ES: Formal analysis, Project administration, Supervision, Writing – review & editing. JH: Data curation, Writing – review & editing. ZG: Project administration, Validation, Writing – review & editing. JL: Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to acknowledge cardiologist, Dr. Charles German for his guidance, comments and review of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AHI, Apnea and Hypopnea Index (#events/hour); AF, Atrial fibrillation; APAP, Auto-adjusting Positive Airway Pressure; Amp, Amplitude (k-pixels); BMI, Body Mass Index (kg/m2); CPAP, Continuous positive airway pressure; CPC, Cardiopulmonary coupling; CSA, Central Sleep Apnea; e-LFCbb, Elevated low frequency coupling broad band; ECG, Electrocardiogram; HFC, High frequency coupling; HRV, Heart rate variability; HST, Home Sleep Test; LFC, Low frequency coupling; OA, Oral appliance; OAT+, Oral Appliance Therapy plus mouth shield; ODI, Oxygen Desaturation Index (#events/hour); OSA, Obstructive Sleep Apnea; PAC, Premature atrial contraction; PNS, Parasympathetic nervous system; RERA, respiratory effort related arousal; RDI, Respiratory Disturbance Index (#events/hour); SNS, Sympathetic nervous system; VLFC, Very low frequency coupling.
References
Berry, R. B., Budhiraja, R., Gottlieb, D. J., Gozal, D., Iber, C., Kapur, V. K., et al. (2012). Rules for scoring respiratory events in sleep: update of the 2007. AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J. Clin. Sleep Med. 8, 597–619. doi: 10.5664/jcsm.2172
Chakraborty, S., Shukla, D., Mishra, B., and Singh, S. (2010). Clinical updates on carvedilol: a first choice β-blocker in the treatment of cardiovascular diseases. Expert Opin. Drug Metab. Toxicol. 6, 237–250. doi: 10.1517/17425250903540220
Cheng, J., Kamiya, K., and Kodama, I. (2006). Carvedilol: molecular and cellular basis for its multifaceted therapeutic potential. Cardiovasc. Drug Rev. 19, 152–171. doi: 10.1111/j.1527-3466.2001.tb00061.x
Cowie, M. R., Woehrle, H., Wegscheider, K., Angermann, C., d'Ortho, M., Erdmann, E., et al. (2015). Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N. Engl. J. Med. 373, 1095–1105. doi: 10.1056/NEJMoa1506459
Fontanini, A., and Bower, J. (2006). Slow-waves in the olfactory system: an olfactory perspective on cortical rhythms. Trends Neurosci. 8, 429–437. doi: 10.1016/j.tins.2006.06.013
Galic, T., Bozic, J., Ivkovic, N., Gunjaca, G., Ticinovic, T. K., Dogas, Z., et al. (2016). Effects of mandibular advancement device treatment on arterial stiffness and glucose metabolism in patients with mild to moderate obstructive sleep apnea: a prospective 1 year study. Sleep Breath 20, 69–77. doi: 10.1007/s11325-015-1186-y
Goudis, C. A., and Ketikoglou, D. G. (2017). Obstructive sleep and atrial fibrillation: pathophysiology mechanisms and therapeutic implications. Int. J. Cardiol. 230, 293–300. doi: 10.1016/j.ijcard.2016.12.120
Heck, D., McAfee, S., Liu, Y., Babajani-Feremi, A., Rezaie, R., Freeman, W. J., et al. (2017). Breathing as a fundamental rhythm of brain function. Front. Neural Circuits 10:115. doi: 10.3389/fncir.2016.00115
Hoekema, A., Voors, A. A., Wijkstra, P. J., Stegenga, B., van der Hoeven, J. H., Tol, C. G., et al. (2008). Effects of oral appliances and CPAP on the left ventricle and natriuretic peptides. Int. J. Cardiol. 128, 232–239. doi: 10.1016/j.ijcard.2007.06.016
Ito, J., Roy, S., Liu, Y., Cao, Y., Fletcher, M., Lu, L., et al. (2014). Whisker barrel cortex delta oscillations and gamma power in the awake mouse are linked to respiration. Nat. Commun. 5:3572. doi: 10.1038/ncomms4572
Kumagai, H., Sawatari, H., Hoshino, T., Konishi, N., Kiyohara, Y., Kawaguchi, K., et al. (2022). Effects of continuous positive airway pressure therapy on nocturnal blood pressure fluctuation patterns in patients with obstructive sleep apnea. Int. J. Environ. Res. Public Health 19:9906. doi: 10.3390/ijerph19169906
Lin, C., Wang, H., Chiu, C., and Liaw, S. F. (2015). Effect of oral appliance on endothelial function in sleep apnea. Clin. Oral Investig. 19, 437–444. doi: 10.1007/s00784-014-1234-1
Mayer, P., Herrero Babiloni, A., Beetz, G., Marshansky, S., Kaddaha, Z., Rompré, P. H., et al. (2020). The evaluation of autonomic arousals in scoring sleep respiratory disturbances with polysomnography and portable monitor devices: a proof of concept study. Nat. Sci. Sleep 16, 443–451. doi: 10.2147/NSS.S258276
McEvoy, R. D., Antic, N. A., Heeley, E., Luo, Y., Ou, Q., Zhang, X., et al. (2016). CPAP for prevention of cardiovascular events in obstructive sleep apnea. N. Engl. J. Med. 375, 919–931. doi: 10.1056/NEJMoa1606599
Ng, C. Y., Liu, T., Shehata, M., Stevens, S., Chugh, S. S., Wang, X., et al. (2011). Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am. J. Cardiol. 108, 47–51. doi: 10.1016/j.amjcard.2011.02.343
Salsone, M., Vescio, B., Quattrone, A., Roccia, F., Sturniolo, M., Bono, F., et al. (2018). Cardiac parasympathetic index identifies subjects with adult obstructive sleep apnea: a simultaneous polysomnographic-heart rate variability study. PLoS ONE 13:e0193879. doi: 10.1371/journal.pone.0193879
Schlatzer, C., Schwar, E. I., Sievi, N. A., Clarenbach, C. F., Gaisl, T., Haegeli, L. M., et al. (2016). Intrathoracic pressure swings induced by simulated obstructive sleep apnoea promote arrhythmias in paroxysmal atrial fibrillation. EP Europace 18, 64–70. doi: 10.1093/europace/euv122
Schramm, P., Schneiderman, E., Hui, J., German, Z., Stenberg, W., and Lin, J.Y. (2024). Obstructive sleep apnea mouth breathing phenotype response to combination oral appliance therapy. Front. Sleep 3:1272726. doi: 10.3389/frsle.2024.1272726
Shaffer, F., and Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Front. Public Health 5:258. doi: 10.3389/fpubh.2017.00258
Thomas, R. J., Mietus, J. E., Peng, C. K., and Goldberger, A. L. (2005). An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep 28, 1151–1161. doi: 10.1093/sleep/28.9.1151
Verrier, R. L., and Josephson, M. E. (2021). “Cardiac arrhythmogenesis during sleep: mechanisms, diagnosis and therapy,” in Principles and Practice of Sleep Medicine, 7th Edn., eds. M. H. Kryger, T. Roth, and C. A. Goldstein (Philadelphia, PA: Elsevier), Chap. 125 Part II, Section 15, 1237–1242.e2. doi: 10.1016/B978-0-323-24288-2.00125-2
Walia, H. K., Chung, M. C., Ibrahim, S., and Mehra, R. (2016). Positive airway pressure-induced conversion of atrial fibrillation to normal sinus rhythm in severe obstructive sleep apnea. J. Clin. Sleep Med. 12, 1301–1303. doi: 10.5664/jcsm.6138
Walsh, J. H., Leigh, M. S., Paduch, A., Maddison, D., Philippe, D. L., Armstrong, J. J., et al. (2008). Evaluation of pharyngeal shape and size using anatomical optical coherence tomography in individuals with and without obstructive sleep apnea. J. Sleep Res. 17, 230–238. doi: 10.1111/j.1365-2869.2008.00647.x
Keywords: oral appliance, obstructive sleep apnea, premature atrial contraction, plethysmography, auto-adjusting positive airway pressure
Citation: Schramm P, Schneiderman E, Hui J, German Z and Lin JY (2025) Case Report: Combination oral appliance therapy acute influence on cardiac electrophysiology and hemodynamics in OSA patient with paroxysmal atrial fibrillation. Front. Sleep 4:1580381. doi: 10.3389/frsle.2025.1580381
Received: 20 February 2025; Accepted: 10 October 2025;
Published: 30 October 2025.
Edited by:
Ellie Kazemeini, Clinical Pharmacology Unit, SGS, BelgiumReviewed by:
Raichel Mary Alex, Brigham and Women's Hospital and Harvard Medical School, United StatesAynur Aliyeva, Yeditepe University, Türkiye
Copyright © 2025 Schramm, Schneiderman, Hui, German and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Preetam Schramm, c2NocmFtbUB0YW11LmVkdQ==
 Preetam Schramm
Preetam Schramm Emet Schneiderman
Emet Schneiderman Jason Hui
Jason Hui Zohre German
Zohre German