- 1Sleep and Brain Plasticity Centre, Department of Neuroimaging, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), King's College London, London, United Kingdom
- 2Sleep Disorder Centre, Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy
- 3Sleep Disorders Centre, Department of Medicine and Surgery, Parma University Hospital and Mario Giovanni Terzano Interdepartmental Centre for Sleep Medicine, University of Parma, Parma, Italy
- 4Sleep Disorders Centre, Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom
- 5BRAIN Centre, Institute of Psychiatry Psychology and Neuroscience, King's College London, London, United Kingdom
- 6Department for Sleep Disorders, Psychiatric Clinic Vrapce, Zagreb, Croatia
- 7Department of Clinical Research Center for Neurodegenerative Diseases and the Aging Brain, University of Bari ‘Aldo Moro' Pia Fondazione “Card. G. Panico” Tricase (LE), Tricase, Italy
- 8School of Basic and Medical Biosciences, Faculty of Life Science and Medicine, King's College London, London, United Kingdom
- 9IRCCS Neuromed Istituto Neurologico Mediterraneo Sleep Medicine Center Pozzilli IS and International Medical University UNICAMILLUS, Rome, Italy
- 10Department of Neurology, Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom
- 11Healthy Brain Ageing Program, The Brain and Mind Centre, University of Sydney, Sydney, NSW, Australia
- 12Minnesota Regional Sleep Disorders Center, and Departments of Psychiatry, Hennepin County Medical Centre and University of Minnesota Medical School, Minneapolis, MN, United States
Background: REM sleep behavior disorder (RBD) is characterized by loss of normal muscle atonia during REM sleep, often associated with dream enactment behaviors, and is typically a prodromal neurodegenerative condition in middle-aged and older adults. However, emerging case reports and case series suggest that not all RBD presentations follow this trajectory, particularly in younger individuals.
Case presentation: A case of 7-year history of vivid, immersive dreaming perceived as continuous with waking life, accompanied by persistent dream-reality confusion, is described. The patient frequently engaged in reality-testing behaviors and reported significant cognitive fatigue. Video-polysomnography confirmed REM sleep without atonia and a concordant dream re-enactment episode. Neuropsychiatric evaluation ruled out dissociative or psychotic disorders, and no evidence of neurodegenerative disease was observed.
Conclusion: This presented case illustrates a potentially distinct, non-neurodegenerative REM parasomnia phenotype that underscores the need to expand current parasomnia classifications to better capture the diverse cognitive and metacognitive dimensions of REM sleep disorders. Moreover, potential mechanisms underlying the main features of this case, including immersive dreaming and persistent dream-reality confusion, are discussed in relation to hypothesized dysfunction in melanin-concentrating hormone (MCH) signaling.
1 Introduction
REM sleep is a discrete neurophysiological state defined by dyssynchronous cortical activation, not that dissimilar to wakefulness, and with muscular atonia (McCarley, 2007). A substantial body of work supports REM sleep's role in procedural memory consolidation, emotional regulation, higher cognition and generative mentation, commonly experienced as dreaming (Stickgold and Walker, 2013). In patients with REM sleep behavior disorder (RBD), patients can be observed re-enacting their dreams due to loss of muscular atonia (due to presumed underlying pathological process in the brainstem region), which otherwise defines this state (Schenck and Mahowald, 2002). Patients with RBD are known to be at significantly increased risk of developing alpha-synucleinopathies, including Parkinson's disease and Dementia with Lewy bodies (Iranzo et al., 2006). Moreover, idiopathic RBD (iRBD) is increasingly viewed as a prodromal, or an early stage, of such disorders, particularly in middle-aged and older adults (Postuma et al., 2009).
However, not all patients with RBD-like symptoms follow this trajectory. It has been noted that a smaller subgroup, often younger, and without neurodegenerative signs, can present with REM sleep without atonia (RSWA), dream enactment, and vivid dreaming, and yet they can remain clinically and cognitively stable over years (Stores, 2008). A more recent case series involving children, adolescents, and young adults found no evidence of neurodegeneration, but did identify a diverse range of comorbidities, including neurodevelopmental disorders (Shukla et al., 2019). Historical pediatric reports remind us that REM-sleep motor phenomena can present outside prodromal neurodegeneration. In particular, early case series described REM-sleep motor disorder in children, underscoring that RBD-spectrum features may occur in younger patients without progressive neurological disease (Sheldon and Jacobsen, 1998). Within this context, our case adds a cognitive-metacognitive phenotype (epic dreaming with dream–reality confusion) to the non-neurodegenerative end of the spectrum. The nosological position of these individuals remains uncertain. Their presentation invites important questions about the breadth of the RBD spectrum and the specificity of its association with synucleinopathy across the lifespan.
Historically, aside from RBD, other dream-related disorders have similarly captured the interest of sleep researchers. For instance, one pathology that is yet to be officially included into any official disorder classification includes reports of abnormal persistence of dream content into waking life (Rassin et al., 2001). In such cases patients have been known to describe dreams of such clarity and realism that they become phenomenologically indistinguishable from lived experience (Mazzoni and Loftus, 1996). This phenomenon, termed dream-reality confusion, has been reported as a feature of several parasomnias (Tuisku, 2020; Gnoni et al., 2020). It has been also reported in narcolepsy-spectrum syndromes, and, more controversially, in dissociative and psychotic conditions (Wamsley et al., 2014). In its most disabling form, dream-reality confusion compels individuals to engage in elaborate reality-testing rituals, thus, undermining their confidence in memory and agency, with profound effects on the quality of their life (Rassin et al., 2001).
The concept of “epic dreaming” similarly remains underexplored. It refers to immersive, continuous dream narratives that, while emotionally neutral and mundane, are subjectively experienced as mentally and physically exhausting (Lin, 2023; Yeh and Schenck, 2000; Schenck and Mahowald, 1995). Unlike nightmares or lucid dreams, epic dreams have been described by affected individuals as notable for their content's ordinariness and their sustained narrative structure (Lin, 2023). Patients often report awakening unrefreshed, with a sense that the night has passed in unrelenting mental activity. Taken together, where these cognitive-affective symptoms intersect with RSWA and dream re-enactments, they may be taken to suggest a broader and more heterogeneous REM parasomnia phenotype than currently represented in the International Classification of Sleep Disorders (ICSD-3; Högl et al., 2018; Sateia, 2014). At present, parasomnia classification is anchored predominantly in behavioral and motor phenomena, with less consideration given to the cognitive and metacognitive correlates of REM disruption (Mahowald and Schenck, 2005).
The present study reports a case with persistent dream reality re-enactment, immersive epic dreaming, and polysomnographically confirmed RSWA with dream re-enactment. The case is of particular interest due to the temporal consistency of the symptoms, the absence of psychiatric comorbidity, and the phenomenological richness of the patient's account. Importantly, RSWA and dream enactment were captured in a polysomnographic study conducted prior to pharmacological intervention. The patient's dreams were reported as being commonly devoid of emotional salience but retained with such fidelity that they repeatedly disrupted post-sleep functioning (see Appendix for some examples). Reality-monitoring deficits were particularly prominent and intrusive.
In exploring this presentation, we draw attention to emerging, albeit theoretical, work on REM-associated memory suppression. Animal studies have proposed that melanin-concentrating hormone (MCH) neurons, which are active during REM sleep, may play a role in attenuating the encoding of REM sleep mentation, possibly by modulating hippocampal plasticity (Izawa et al., 2019). If such mechanisms are indeed operative in humans, dysfunction within this system may permit inappropriate consolidation of dream content, contributing to intrusive post-REM recall, and dream re-enactment (Torterolo et al., 2011). These considerations, whilst speculative, however, advance frameworks that may be valuable in guiding future empirical investigation.
2 Case presentation
A right-handed woman in her late 30s presented with a 7-year history of frequent, immersive dreaming experiences indistinguishable from waking reality. The dreams, which occurred four to six times per week, were typically mundane, non-stereotyped, and centered on routine professional or domestic tasks (see Table 1; Supplement for Supplementary Table S1 and Dream Vignette). Notably, they were experienced as continuous with wakefulness. For example, she reported significant post-awakening confusion, often unable to determine whether specific events or conversations had occurred during sleep or wakefulness. Functional impact was marked, the patient routinely consulted digital records (e.g., text messages, emails) to verify whether actions or communications had actually taken place.
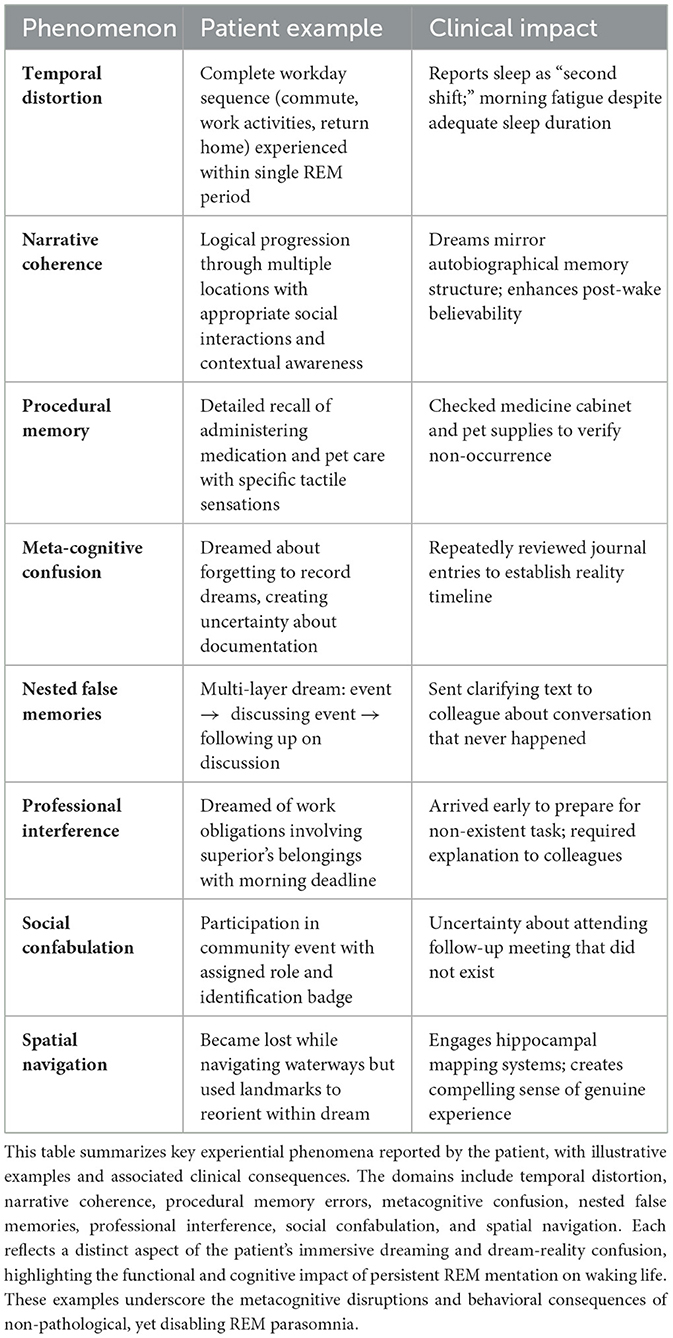
Table 1. Phenomenological dimensions of immersive dreaming and dream-reality confusion in the present case.
The patient denied any history of trauma, neurologic or psychiatric disorders, or substance use. There was no reported history or clinical suspicion of a prior sleep disorder. Her developmental history included mildly delayed ambulation and persistent childhood tactile hypersensitivity, with no formal diagnosis. Medical history was notable for polycystic ovary syndrome and gastrointestinal dysmotility, treated with prucalopride. She was not taking any psychotropic medications at the time of referral. Cognitive screening with the Addenbrooke's Cognitive Examination (ACE) yielded a perfect score of 100/100, indicating intact global cognitive functioning.
An overnight video-polysomnography (vPSG) was performed prior to pharmacological intervention. Total sleep time was 409 min with preserved sleep architecture. Sleep efficiency was high at 96.3%, with 14.5 min of wake after sleep onset (WASO), The Epworth Sleepiness Scale score of 12/24, arousal index of 14.7 events/hour, apnea–hypopnea index (AHI) of 1.3 events/hour, and periodic limb movement index (PLMI) of 5.1 events/hour were recorded. Stage N1 sleep comprising 12.5% of total sleep time (TST), N2 48.4%, and N3 20.0% were reported. REM sleep accounted for 22.3% of total sleep time. REM latency was 98 min, and sleep latency was 4.5 min. A 15-day actigraphy assessment preceding the vPSG showed a stable and regular sleep–wake pattern, with an average time in bed of 9 h and 11 min, and average sleep duration of 7 h and 44 min, with infrequent and brief napping. A multiple sleep latency test (MSLT) demonstrated sleep onset in all four nap opportunities, with a mean sleep latency of 9.7 min and no sleep-onset REM periods (SOREMPs). Notably, tonic and phasic electromyographic (EMG) activity during REM epochs was observed, consistent with RSWA. During one of the dream-enactment episodes that was recorded during REM, the patient appeared to self-soothe by stroking her head (Supplementary Video). Upon awakening, she reported dreaming about being upset and then, of being comforted, demonstrating concordance between dream content and observed motor behavior.
Trials of pharmacological treatment included prolonged-release melatonin (2 mg nocte) and, subsequently, vortioxetine (15 mg once daily). Both led to significant reduction in dream-reality confusion, improved overall cognitive functioning, and reduced post-awakening confusion. However, symptoms of immersive and vivid dreaming remained. Structured neuropsychological assessment revealed no evidence of dissociation, psychosis, or cognitive impairment. Similarly, neurological evaluation did not show anything of note. Screening for psychotic symptoms was negative, with a score of 0 on the psychosis module of the Mini International Neuropsychiatric Interview (MINI), and no evidence of delusional ideation or hallucinations on clinical interview.
3 Discussion
Presented is a distinct REM parasomnia case characterized by young-adult onset RSWA with RBD, dream enactment, persistent dream-reality confusion, and immersive dream narrative (see Supplementary for further examples). The absence of trauma, affective illness, or neurodegenerative disease distinguishes this presentation from PTSD or other trauma-associated parasomnia and classical idiopathic RBD (iRBD; Husain et al., 2001). At the time of initial evaluation, conducted 7 years after symptom onset, there was no evidence of parkinsonism or cognitive impairment. Consistent with this, a case series of 12 young patients with iRBD, including five children and seven adolescents or young adults (ages 18–25), also found no clinical signs suggestive of neurodegenerative disease at the time of evaluation, despite the presence of complex and heterogeneous clinical profiles (Shukla et al., 2019). Together, these observations raise the possibility that early-onset RSWA/iRBD may comprise a clinically and biologically distinct entity, phenotypically similar to classical RBD, yet etiologically independent of the prodromal neurodegenerative pathway that characterizes late-onset forms.
Clinically, dream-reality confusion in this context carries significant functional consequences. While phenomenologically similar to narcolepsy and dissociative states, our patient did not exhibit hypnagogic hallucinations or cataplexy. Patient's immersive, emotionally neutral, and predominantly mundane, dreams also differ from the affect-laden mentation observed in nightmare disorder or trauma-related REM disturbances (Levin and Nielsen, 2007). Thus, rather than indicating emotional overload, these dreams may suggest an atypical persistence of internally generated REM mentation into waking cognition (Wamsley and Stickgold, 2010). Arguably, the clinical features observed in this case may be interpreted as consistent with a variant of epic dreaming, potentially overlapping with the atypical end of the RBD spectrum.
One possible mechanistic explanation could involve disruption in the post-REM processing of dream content. Under normal conditions, REM-associated dreams, despite their vividness, are commonly easily forgotten (Nir and Tononi, 2010). Convergent animal work indicates that a subset of melanin-concentrating hormone (MCH) neurons in lateral hypothalamus is selectively active during REM sleep and supports forgetting of hippocampus-dependent memories (Izawa et al., 2019; Jego et al., 2013; Hassani et al., 2009). Using fiber photometry in freely behaving mice, Izawa et al. (2019) showed REM-linked activation of MCH neurons; chemogenetic and optogenetic, state-specific inhibition during REM impaired forgetting without altering gross sleep architecture (Izawa et al., 2019). Conversely, activating MCH neurons promoted forgetting (Izawa et al., 2019). Translating cautiously, a relative failure of REM-active MCH signaling in humans could allow unusually persistent encoding of REM mentation, aligning with our patient's intrusive recall and dream–reality confusion (Izawa et al., 2019; Luppi et al., 2024).
So far any direct evidence for this mechanistic scaffold in humans is currently lacking (see Figure 1). In the present case, the suggestion of impaired dream forgetting remains a hypothesis that would require dedicated functional imaging or neurochemical studies to support. Nonetheless, this theoretical framework may offer a useful lens through which to interpret similar cases.
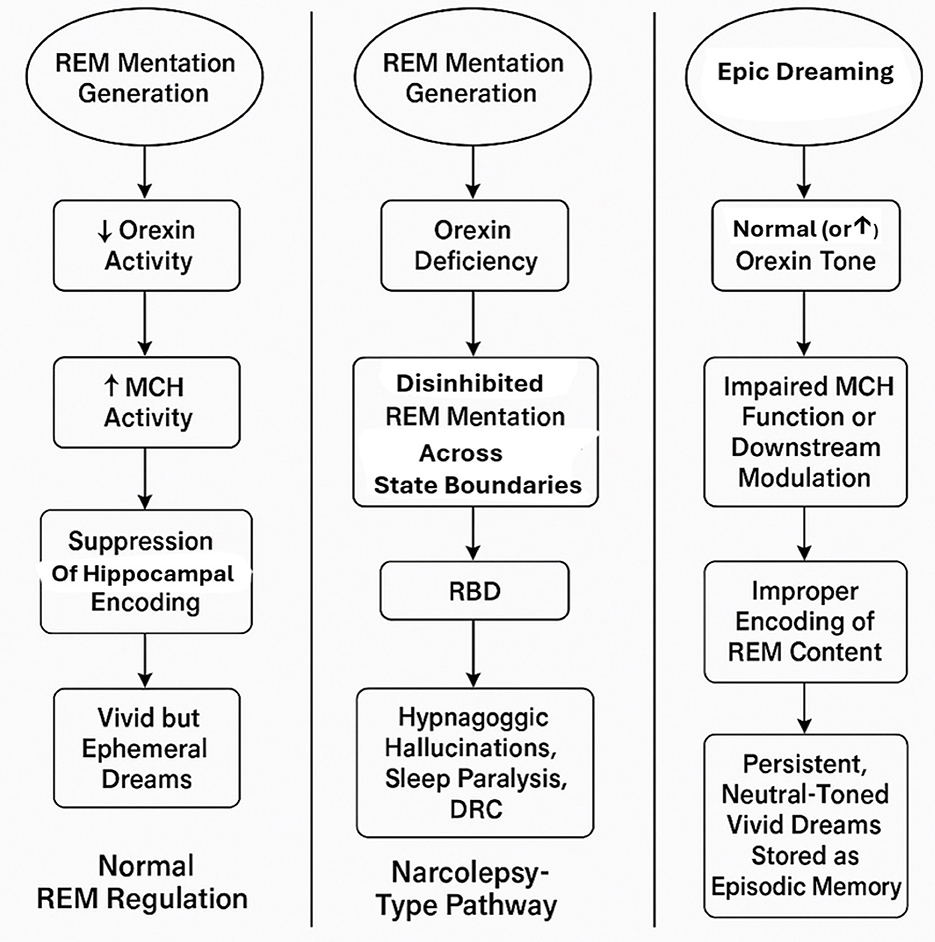
Figure 1. Integrated model of REM mentation regulation and pathways to dream-reality confusion (DRC). This schematic illustrates three hypothesized pathways linking REM mentation, orexin and melanin-concentrating hormone (MCH) signaling, hippocampal encoding, and the development of dream-reality confusion (DRC). (Left panel) Normal REM regulation: reduced orexin activity during REM sleep enables MCH-mediated suppression of hippocampal encoding, resulting in vivid but typically ephemeral dreams. (Middle panel) Narcolepsy-type pathway: orexin deficiency permits disinhibited REM mentation across wake-sleep boundaries, contributing to REM sleep behavior disorder (RBD), hypnagogic hallucinations, sleep paralysis, and DRC. (Right panel) Epic dreaming phenotype (present case): despite preserved orexin tone, impaired MCH function or downstream modulation is hypothesized to allow abnormal encoding of REM content into episodic memory, leading to persistent, neutral-toned immersive dreams and pronounced dream-reality confusion. Moreover, theoretically, increased orexin tone, whether state-dependent (e.g., stress, sleep deprivation) or trait-related, could similarly lead to impaired MCH function.
Past studies have highlighted the putative role of thalamocortical oscillations and sleep spindle dynamics in memory consolidation and dream recall (Steriade and Timofeev, 2003). Although spindle activity was not assessed in our patient, reduced spindle density has been linked to heightened dream recall and nightmare frequency in individuals with fragmented sleep, plausibly reducing ‘gating' of REM mentation into waking memory (Picard-Deland et al., 2018). We note this as a target for future longitudinal studies, alongside mechanistic hypotheses involving MCH–hippocampal pathways (Luppi et al., 2024). If present, such disruptions may facilitate the persistence of dream content into waking consciousness. Given our patient's childhood sensory vulnerabilities and evidence of fragmented sleep, a contribution of altered spindle regulation remains a plausible, although unconfirmed, element.
Partial improvement with vortioxetine in this case is potentially suggestive of serotonergic modulation of REM-related phenomena. Vortioxetine's pharmacological profile includes serotonergic reuptake inhibition and receptor modulation, which may influence prefrontal and limbic circuits involved in emotional contextualization and memory gating (Sanchez et al., 2015). However, while it is unclear whether vortioxetine exerts direct effects on REM circuitry, its partial effect may also reflect enhanced executive control or cognitive flexibility, rather than a direct modulation of RSWA or dream processing mechanisms (McIntyre et al., 2014). Classically, serotonergic reuptake blockade increases synaptic 5-HT and suppresses REM sleep (longer REM latency, reduced REM percentage) across patients and healthy volunteers (Wichniak et al., 2017). In healthy men, vortioxetine (20–40 mg) delayed REM onset and reduced REM time in a dose-dependent manner, with a profile distinct from paroxetine, likely reflecting its multimodal actions (e.g., 5-HT_3 antagonism; Wilson et al., 2015). Recent adolescent vPSG data similarly report increased REM latency and decreased REM percentage after vortioxetine treatment (Mlyncekova et al., 2023). Beyond macro-architecture, serotonergic input can facilitate spinal motoneuronal excitability via 5-HT-dependent mechanisms, which could, in principle, interact with REM atonia circuits (Perrier and Cotel, 2015). Importantly, several studies associate serotonergic antidepressants with increased RSWA in some patients; thus any anti-confusional benefit from vortioxetine should be interpreted as primarily cognitive/affective unless serial PSG demonstrates normalization of REM motor atonia (McCarter et al., 2015; Lee et al., 2016) In our case, vortioxetine improved dream–reality confusion whereas immersive dreaming persisted, aligning with a cognitive rather than purely motor mechanism. We did not quantify sleep spindles or repeat PSG following vortioxetine initiation; future longitudinal assessments will examine spindle metrics and RSWA indices in parallel with symptom change. Importantly, we here advance that even if distinct mechanisms underlying this specific phenotype remain unclear, they align with emerging views that RBD-like phenomena may arise not solely from neurodegeneration but also from more subtle neurochemical imbalances (Jiménez-Jiménez et al., 2021).
More broadly, this case emphasizes that RBD as a REM parasomnia can present with disabling cognitive and affective features even in the absence of violent or injurious behavior, and possibly, without any association to narcolepsy, nor to any other neurological disorders, including a neurodegenerative process such as alpha-synucleinopathy. However, long-term follow-up would be needed to fully address this issue, as neurodegenerative disorders often evolve slowly over decades, with the cardinal sleep manifestations, e.g., RBD and its spectrum disorders, emerging early in the clinical course. Current diagnostic systems, such as the ICSD-3-TR (AAoS Medicine, 2023), emphasize motor manifestations while neglecting metacognitive disturbances like dream-reality confusion (Avidan and Kaplish, 2010). Expanding diagnostic criteria to reflect the cognitive and affective dimensions of REM sleep dysregulation may allow for earlier recognition and more nuanced treatment strategies. Encouraging the systematic reporting and phenotypic characterization of similar cases will be essential to inform and support future revisions of diagnostic frameworks.
From a therapeutic standpoint, effective interventions for such presentations remain elusive. Neither melatonin nor serotonergic antidepressants yielded full resolution in our patient. Cognitive-behavioral strategies targeting reality monitoring may offer promise but require empirical validation. Future therapies might aim to modulate REM memory encoding directly, pending advances in our understanding of these processes.
In conclusion, we describe an idiopathic and non-neurodegenerative case of RBD, with features of epic dreaming, and with marked cognitive and affective symptoms not currently captured by existing nosologies. While supported by objective evidence of RSWA, the hallmark features lie in the persistence, content, and behavioral impact of REM sleep mentation. This case underscores the need for refined clinical frameworks and targeted research into the cognitive consequences of REM dysregulation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because the patient provided written informed consent for the publication of clinical details and supplementary materials, including video recordings. This report was prepared in accordance with the ethical guidelines and case reporting standards of King's College London and Guy's and St Thomas' NHS Foundation Trust, both of which require explicit, documented patient consent for the publication of identifiable health information. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NB: Data curation, Formal analysis, Writing – original draft. MM: Data curation, Formal analysis, Writing – original draft. CM: Writing – review & editing. SH: Data curation, Formal analysis, Methodology, Visualization, Writing – review & editing. KI: Visualization, Writing – review & editing. JB: Data curation, Formal analysis, Methodology, Writing – review & editing. AS: Writing – review & editing. VG: Writing – review & editing. PD: Conceptualization, Supervision, Validation, Writing – review & editing. AR: Conceptualization, Supervision, Validation, Writing – review & editing. AN: Writing – review & editing. SN: Conceptualization, Supervision, Validation, Writing – review & editing. CS: Conceptualization, Supervision, Validation, Writing – review & editing. DO: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. MP: Conceptualization, Resources, Supervision, Validation, Writing – review & editing. IR: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded in whole, or in part, by the Wellcome Trust (103952/Z/14/Z). For the purpose of open access, the author IR has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission. This article represents independent research in part funded by the NIHR Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London.
Acknowledgments
A special gratitude goes to all our patients at the Sleep Disorders Centre's, Guy's and St Thomas' Hospital, London, and to all current and past Sleep and Brain Plasticity Centre (King's College London) team members and colleagues.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frsle.2025.1659300/full#supplementary-material
Supplementary Table S1 | Phenomenological Matrix.
Supplementary Video S1 | Dream enactment behaviour in a patient with dream-reality confusion. This segment of video-polysomnography (vPSG) illustrates a brief episode, where patient performs repetitive movements with the right arm (touching her hair and forehead), then lifts her left arm and finally moves her legs (first she adducts her legs and then extends them, and finally rotates the pelvis and the lower limbs to the right). The episode lasted approximately 8 seconds. At EEG level the ongoing activity is completely masked by motion artifacts. The ECG trace documented a mild acceleration of heart rate during the hypermotor manifestation. The montage includes the following channels, displayed from top to bottom: electrooculography (EOG; E1–M1 and E2–M2), electroencephalography (EEG; F3–M2, F4–M1, C3–M2, C4–M1, O1–M2, O2–M1), electrocardiography (ECG-LA–ECG-RA), submental electromyography (EMG; CHIN1–CHINz, CHIN2–CHINz, CHIN1–CHIN2), bilateral anterior tibialis EMG (RLEG+-RLEG, LLEG+-LLEG), airflow (XFlow), snore and flow digital recordings (Snore_DR and Flow_DR), thoracic and abdominal effort plethysmography (Chest and Abdomen), pulse oximetry (SpO2, %), and pulse rate (PR, bpm). Notably, the submental and limb EMG channels reveal elevated and sustained muscle activity during REM sleep, consistent with REM sleep without atonia.
Abbreviations
BPM, beats per minute; EEG, electroencephalography; EOG, electrooculography; EMG, electromyography; REM, rapid eye movement; vPSG, video-polysomnography; %, percentage.
References
AAoS Medicine. (2023). International Classification of Sleep Disorders—Third Edition (ICSD-3). AASM, Darien (IL).
Avidan, A. Y., and Kaplish, N. (2010). The parasomnias: epidemiology, clinical features, and diagnostic approach. Clin. Chest Med. 31, 353–370. doi: 10.1016/j.ccm.2010.02.015
Gnoni, V., Higgins, S., Nesbitt, A. D., Wasserman, D., Duncan, I., Birdseye, A., et al. (2020). Cotard parasomnia: le delire de negation that occur during the sleep-wake dissociation? J. Clin. Sleep Med. 16, 971–976. doi: 10.5664/jcsm.8430
Hassani, O. K., Lee, M. G., and Jones, B. E. (2009). Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep–wake cycle. Proc. Natl. Acad. Sci. U.S.A. 106, 2418–2422. doi: 10.1073/pnas.0811400106
Högl, B., Stefani, A., and Videnovic, A. (2018). Idiopathic REM sleep behaviour disorder and neurodegeneration—an update. Nat. Rev. Neurol. 14, 40–55. doi: 10.1038/nrneurol.2017.157
Husain, A. M., Miller, P. P., and Carwile, S. T. (2001). REM sleep behavior disorder: potential relationship to post-traumatic stress disorder. J. Clin. Neurophysiol. 18, 148–157. doi: 10.1097/00004691-200103000-00005
Iranzo, J. L., Molinuevo, J., Santamaría, M., Serradell, M. J., Martí, F., Valldeoriola, et al. (2006). Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 5, 572–577. doi: 10.1016/S1474-4422(06)70476-8
Izawa, S., Chowdhury, S., Miyazaki, T., Mukai, Y., Ono, D., Inoue, R., et al. (2019). REM sleep–active MCH neurons are involved in forgetting hippocampus-dependent memories. Science 365, 1308–1313. doi: 10.1126/science.aax9238
Jego, S., Glasgow, S. D., Herrera, C. G., Ekstrand, M., Reed, S. J., Boyce, R., et al. (2013). Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat. Neurosci. 16, 1637–1643. doi: 10.1038/nn.3522
Jiménez-Jiménez, F. J., Alonso-Navarro, H., Garcia-Martin, E., and Agúndez, J. A. (2021). Neurochemical features of rem sleep behaviour disorder. Journal of personalized medicine 11, 880. doi: 10.3390/jpm11090880
Lee, K., Baron, K., Soca, R., and Attarian, H. (2016). The prevalence and characteristics of REM sleep without atonia (RSWA) in patients taking antidepressants. J. Clin. Sleep Med. 12, 351–355. doi: 10.5664/jcsm.5582
Levin, R., and Nielsen, T. A. (2007). Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol. Bull. 133:482. doi: 10.1037/0033-2909.133.3.482
Lin, C. (2023). An investigation on the nature of epic dreaming—is it nightmare or vivid dream. advances in education. Humanit. Soc. Sci. Res. 4, 332–332. doi: 10.56028/aehssr.4.1.332.2023
Luppi, P. H., Chancel, A., Malcey, J., Cabrera, S., Fort, P., Maciel, R. M., et al. (2024). Which structure generates paradoxical (REM) sleep: the brainstem, the hypothalamus, the amygdala or the cortex? Sleep Med. Rev. 74:101907. doi: 10.1016/j.smrv.2024.101907
Mahowald, M. W., and Schenck, C. H. (2005). Insights from studying human sleep disorders. Nature 437, 1279–1285. doi: 10.1038/nature04287
Mazzoni, G. A., and Loftus, E. F. (1996). When dreams become reality. Conscious. Cogn. 5, 442–462. doi: 10.1006/ccog.1996.0027
McCarley, R. W. (2007). Neurobiology of REM and NREM sleep. Sleep Med. 8, 302–330. doi: 10.1016/j.sleep.2007.03.005
McCarter, S. J., St Louis, E. K., Sandness, D. J., Arndt, K., Erickson, M., Tabatabai, G., et al. (2015). Antidepressants increase REM sleep muscle tone in patients with and without REM sleep behavior disorder. Sleep 38, 907–917. doi: 10.5665/sleep.4738
McIntyre, R. S., Lophaven, S., and Olsen, C. K. (2014). A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int. J. Neuropsychopharmacol. 17, 1557–1567. doi: 10.1017/S1461145714000546
Mlyncekova, Z., Hutka, P., Visnovcova, Z., Ferencova, N., Kovacova, V., Macejova, A., et al. (2023). Effects of vortioxetine on sleep architecture of adolescents with major depressive disorder. Clocks Sleep 5, 627–638. doi: 10.3390/clockssleep5040042
Nir, Y., and Tononi, G. (2010). Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn. Sci. 14, 88–100. doi: 10.1016/j.tics.2009.12.001
Perrier, J. F., and Cotel, F. (2015). Serotonergic modulation of spinal motor control. Curr. Opin. Neurobiol. 33, 1–7. doi: 10.1016/j.conb.2014.12.008
Picard-Deland, C., Carr, M., Paquette, T., and Nielsen, T. (2018). Sleep spindles are altered in early-but not late-onset nightmare recallers. Sleep Med. 52, 34–42. doi: 10.1016/j.sleep.2018.07.015
Postuma, R., Gagnon, J., Vendette, M., Fantini, M., Massicotte-Marquez, J., Montplaisir, J., et al. (2009). Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 72, 1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e
Rassin, E., Merckelbach, H., and Spaan, V. (2001). When dreams become a royal road to confusion: realistic dreams, dissociation, and fantasy proneness. J. Nerv. Ment. Dis. 189, 478–481. doi: 10.1097/00005053-200107000-00010
Sanchez, C., Asin, K. E., and Artigas, F. (2015). Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol. Ther. 145, 43–57. doi: 10.1016/j.pharmthera.2014.07.001
Sateia, M. J. (2014). International classification of sleep disorders-third edition: highlights and modifications. Chest 146, 1387–1394. doi: 10.1378/chest.14-0970
Schenck, C. H., and Mahowald, M. W. (1995). A disorder of epic dreaming with daytime fatigue, usually without polysomnographic abnormalities, that predominantly affects women. 1995:137.
Schenck, C. H., and Mahowald, M. W. (2002). REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 25, 120–138. doi: 10.1093/sleep/25.2.120
Sheldon, S. H., and Jacobsen, J. (1998). REM-sleep motor disorder in children. J. Child Neurol. 13, 257–260. doi: 10.1177/088307389801300603
Shukla, G., Gupta, A., Chakravarty, K., Joseph, A. A., Ravindranath, A., Mehta, M., et al. (2019). Rapid eye movement (REM) sleep behavior disorder and REM sleep with atonia in the young. Can. J. Neurol. Sci. 47, 100–108. doi: 10.1017/cjn.2019.302
Steriade, M., and Timofeev, I. (2003). Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron 37, 563–576. doi: 10.1016/S0896-6273(03)00065-5
Stickgold, R., and Walker, M. P. (2013). Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci. 16, 139–145. doi: 10.1038/nn.3303
Stores, G. (2008). Rapid eye movement sleep behaviour disorder in children and adolescents. Dev. Med. Child Neurol. 50, 728–732. doi: 10.1111/j.1469-8749.2008.03071.x
Torterolo, P., Lagos, P., and Monti, J. M. (2011). Melanin-concentrating hormone: a new sleep factor? Front. Neurol. 2:14. doi: 10.3389/fneur.2011.00014
Tuisku, K. S. (2020). Nils; partinen, markku; paunio, tiina. Dreaming and parasomnias – a case with severe parasomnia overlap disorder and its treatment. Psychiatr. Fenn. 51, 92–107.
Wamsley, E., Donjacour, C. E., Scammell, T. E., Lammers, G. J., and Stickgold, R. (2014). Delusional confusion of dreaming and reality in narcolepsy. Sleep 37, 419–422. doi: 10.5665/sleep.3428
Wamsley, E. J., and Stickgold, R. (2010). Dreaming and offline memory processing. Curr. Biol. 20, R1010–R1013. doi: 10.1016/j.cub.2010.10.045
Wichniak, A., Wierzbicka, A., Walecka, M., and Jernajczyk, W. (2017). Effects of antidepressants on sleep. Curr. Psychiatry Rep. 19:63. doi: 10.1007/s11920-017-0816-4
Wilson, S., Højer, A. M., Buchberg, J., Areberg, J., and Nutt, D. J. (2015). Differentiated effects of the multimodal antidepressant vortioxetine on sleep architecture: Part 1, a pharmacokinetic/pharmacodynamic comparison with paroxetine in healthy men. J. Psychopharmacol. 29, 1085–1091. doi: 10.1177/0269881115599387
Keywords: REM sleep behavior disorder, dream-reality confusion, immersive dreaming, parasomnia, REM sleep without atonia, melanin-concentrating hormone (MCH), memory consolidation, sleep cognition
Citation: Biabani N, Mulas M, Mutti C, Higgins S, Ilic K, Benson J, Santic A, Gnoni V, Drakatos P, Romigi A, Nesbitt AD, Naismith SL, Schenck CH, O'Regan D, Puligheddu M and Rosenzweig I (2025) A non-neurodegenerative REM parasomnia with immersive dreaming and dream-reality confusion: a case report. Front. Sleep 4:1659300. doi: 10.3389/frsle.2025.1659300
Received: 03 July 2025; Accepted: 25 August 2025;
Published: 11 September 2025.
Edited by:
Stuart F. Quan, Harvard Medical School, United StatesReviewed by:
Pablo Torterolo, Universidad de la República, UruguayArturo Garay, Norberto Quirno Medical Education and Clinical Research Center (CEMIC), Argentina
Copyright © 2025 Biabani, Mulas, Mutti, Higgins, Ilic, Benson, Santic, Gnoni, Drakatos, Romigi, Nesbitt, Naismith, Schenck, O'Regan, Puligheddu and Rosenzweig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivana Rosenzweig, aXZhbmEuMS5yb3Nlbnp3ZWlnQGtjbC5hYy51aw==
†Joint Authors
 Nazanin Biabani
Nazanin Biabani Martina Mulas1,2†
Martina Mulas1,2† Carlotta Mutti
Carlotta Mutti Katarina Ilic
Katarina Ilic Valentina Gnoni
Valentina Gnoni Panagis Drakatos
Panagis Drakatos Andrea Romigi
Andrea Romigi Sharon L. Naismith
Sharon L. Naismith Carlos H. Schenck
Carlos H. Schenck David O'Regan
David O'Regan Ivana Rosenzweig
Ivana Rosenzweig