- 1Institute of Bioscience, Life Sciences Center, Vilnius University, Vilnius, Lithuania
- 2Faculty of Medicine, Translational Health Research Institute, Vilnius University, Vilnius, Lithuania
- 3Centre for Neuroscience and Neuromodulation, Institute for Medical Research, University of Belgrade, Belgrade, Serbia
Transcranial alternating current stimulation (tACS) is a non-invasive technique that modulates brain oscillatory activity in a frequency-specific manner, offering potential for improving sensory and cognitive functions. Steady-state responses (SSRs), which are periodic neural responses to rhythmic sensory stimulation, provide a robust and objective means to assess tACS effects. The present work systematically reviews the existing literature on tACS modulation of SSR. 16 studies that used either auditory (ASSR) or visual (SSVEP) SSR were included in the review. Findings indicate that tACS can enhance or suppress SSRs depending on stimulation parameters. Although ASSR studies reported mixed findings, generally, gamma tACS enhanced ASSR, whereas tACS at lower frequencies resulted in ASSR inhibition. For SSVEPs, modulation was shown to be phase- and frequency-dependent, with congruent tACS and flicker frequencies producing the most reliable effects. Despite methodological heterogeneity and inconsistent results, the reviewed evidence highlights the potential of SSRs as sensitive markers of tACS outcomes. Future studies should aim for well-planned protocols tailored to specific aims and target populations.
1 Introduction
Transcranial alternating current stimulation (tACS) is considered a promising non-invasive brain stimulation technique that rhythmically interacts with ongoing brain oscillations in a frequency-specific manner (Antal et al., 2008). By applying a weak sinusoidal current with alternating polarity through the scalp, tACS can entrain endogenous neural activity, potentially modulating sensory, cognitive, and affective processes (Antal and Paulus, 2013; Herrmann et al., 2013; Wischnewski et al., 2023). However, tACS is regarded as a relatively mild intervention, often yielding inconsistent results (Biačková et al., 2024; Chuderski and Chinta, 2024), facing reproducibility issues (Veniero et al., 2017), and being highly susceptible to individual variability (Krause and Cohen Kadosh, 2014; Zanto et al., 2021; Steinmann et al., 2022). Therefore, the technique would greatly benefit from using objective measures to assess brain activity dynamics during and after stimulation.
For this purpose, electroencephalography (EEG) or magnetoencephalography (MEG) is often utilized in tACS studies (Koninck et al., 2023). One particularly promising EEG/MEG paradigm to probe the effects of tACS is the steady-state response (SSR) - a periodic brain response elicited by rhythmic sensory stimulation (Tobimatsu et al., 1999; Brenner et al., 2009; Vialatte et al., 2010). SSRs can be evoked through various modalities, including visual (steady-state visually evoked potentials, SSVEPs), auditory (auditory steady-state responses, ASSRs), and somatosensory (steady-state somatosensory evoked potentials, SSSEPs) stimulation. These responses are highly reliable (Pang and Mueller, 2014; Roach et al., 2019; Fong et al., 2020) and frequency-specific (Tobimatsu et al., 1999; Ding et al., 2006; Roach et al., 2019), making them well-suited for evaluating the frequency-dependent effects of tACS. Studies have shown that tACS may modulate the magnitude and phase consistency of SSRs (Ruhnau et al., 2016a; Baltus et al., 2018; Ahn et al., 2021), potentially reflecting underlying mechanisms such as neural entrainment, resonance, and plasticity (Huang et al., 2021; Agboada et al., 2025).
The translation of tACS into clinical applications depends on identifying reliable biomarkers that index stimulation effects. Biomarkers serve as objective indicators of neuromodulatory efficacy, helping to validate mechanisms, monitor responses, and optimize individualized interventions (Wischnewski et al., 2023). SSRs are particularly attractive in this regard: they are highly reliable, frequency-specific, and can be easily recorded using non-invasive EEG/MEG during periodic sensory stimulation. Moreover, SSRs have been widely used in clinical research as markers of sensory and cognitive dysfunction in conditions such as schizophrenia (Thuné et al., 2016; Schielke and Krekelberg, 2022) or autism spectrum disorder (Pei et al., 2014; Seymour et al., 2020). At the same time, the mechanistic basis of both tACS and SSRs remains debated, and SSRs can theoretically have multiple biomarker roles - they could serve as index of (un)successful entrainment by tACS at individual level, but also be used as a biomarker for behavioral and clinical effects of tACS in the therapeutic context. Thus, a systematic review of how SSRs are modulated by tACS is needed to clarify their potential as biomarkers of stimulation effects.
Although the number of studies investigating SSR neuromodulation by tACS is still relatively limited, the field has grown considerably in recent years. Therefore, the present scoping review aims to synthesize the available tACS–SSR literature, critically evaluate the current empirical findings, identify methodological strengths and limitations, outline key directions and provide guidance for future research.
2 Methods
This study was conducted in line with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) (Tricco et al., 2018). Original research articles written in English that addressed tACS effects on SSR were included in the review. Conference papers were considered for inclusion only if they contained sufficient information regarding methods. Only works with a sample size of at least 10 participants were selected to ensure that included studies provide sufficient statistical reliability, since small pilot studies (n < 10) are particularly prone to inflated effect sizes and poor reproducibility (Button et al., 2013).
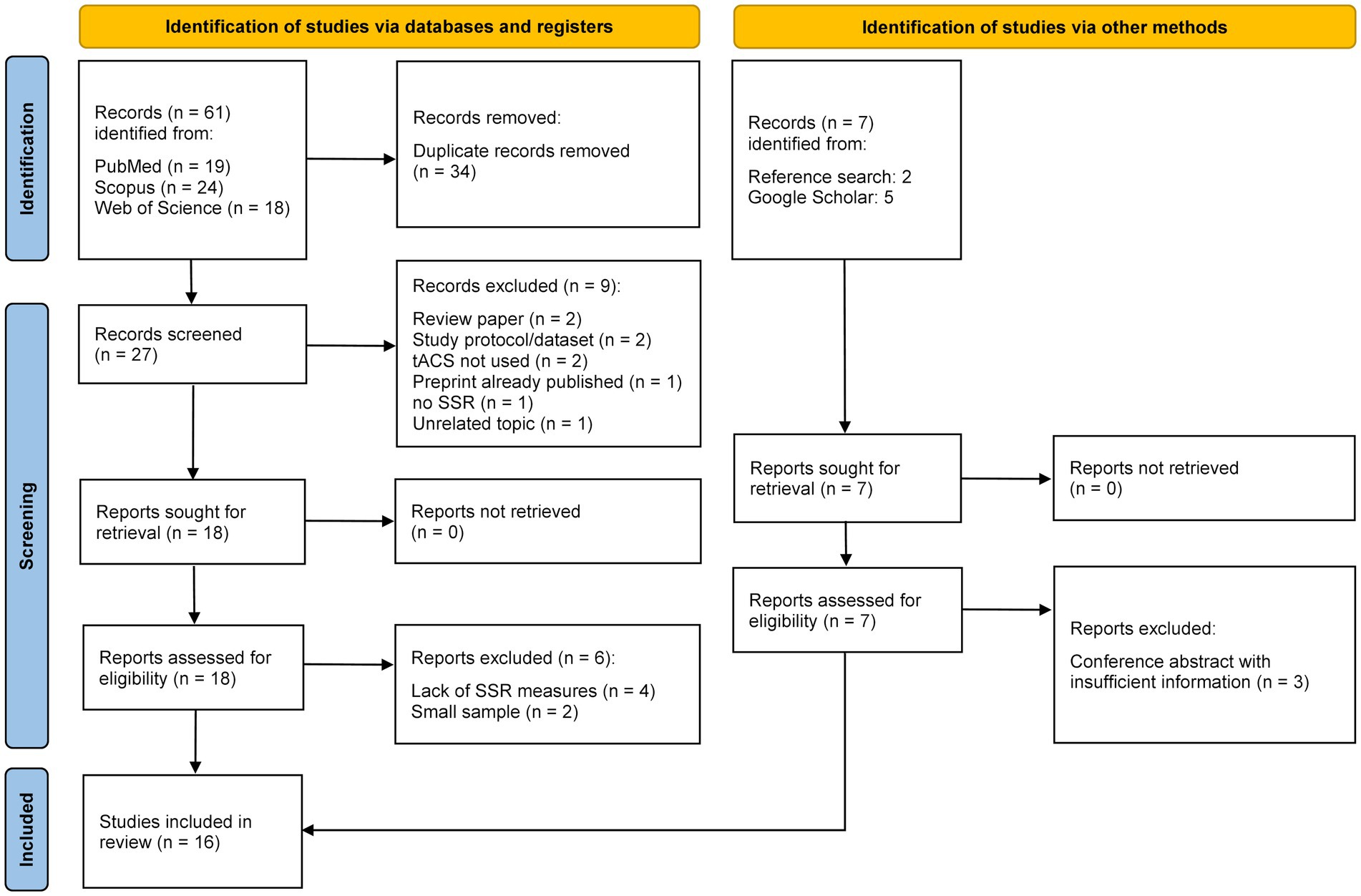
The eligibility was assessed according to PICO criteria (Table 1): (P) subjects were humans, healthy or diagnosed with neuropsychiatric disorders; (I) steady-state stimulation (visual, auditory and/or somatosensory) and tACS in any frequency were applied; (C) sham-tACS condition or SSR without tACS condition or SSR measurement pre- and post-tACS were used as control procedures; (O) changes in EEG/MEG measures (amplitude, power and/or inter-trial phase-locking) in the frequency range corresponding to steady-state stimulation frequency were assessed.
The search was carried out in February 2025. To identify relevant articles, PubMed, Scopus and Web of Science were searched for the keywords “steady state response,” “steady state evoked potential,” “sensory entrainment,” “auditory steady state response,” “ASSR,” “steady state visual evoked potential,” “steady state visually evoked potential,” “visually evoked steady state response,” “SSVEP,” “steady state somatosensory evoked potential,” “SSSEP” in combination with “tACS,” “transcranial alternating current stimulation.” To ensure that other relevant articles were not missed, bibliographies of the works identified via databases were screened, and an additional non-systematic search was carried out in Google Scholar using keywords “steady state” and “transcranial alternating current stimulation”.
After removing the duplicates, titles and abstracts of the identified articles were screened to exclude irrelevant works. Methods part and, if necessary, the whole text and supplementary materials of the remaining records were checked to be evaluated according to eligibility criteria (Table 1).
From each included study, the following information was extracted: (1) sample (type, size, age, gender composition); (2) tACS settings (montage, intensity, frequency, duration); (3) control group/condition; (4) experimental design; (5) auditory stimulation settings (frequency, intensity, duration); (6) methods to measure SSR; (7) SSR results; (8) behavioral outcomes. If the publication included multiple studies/experiments, each was analyzed separately.
3 Results
In total, the database search yielded 61 entries (Figure 1). Duplicates were removed, and titles and abstracts of 27 articles were screened. Nine papers were excluded after the initial screening: two reviews, two works in which tACS was not utilized, a study protocol, an already-published preprint (i.e., duplicate publication), a paper presenting a dataset, one work where the SSR paradigm was not used, and one study unrelated to the topic. After thoroughly screening the remaining eighteen articles, six works were excluded due to small sample sizes (n = 2) or the absence of SSR amplitude, power, or phase-locking analysis (n = 4). Seven other papers were found via bibliography search and Google Scholar, of which three were conference abstracts and were excluded due to insufficient information. A total of sixteen articles were included in the present review. Eleven of the included studies evaluated ASSR, and the remaining five assessed SSVEP. The information extracted from the studies is summarized separately for ASSR and SSVEP.
3.1 TACS-ASSR studies
3.1.1 Sample characteristics
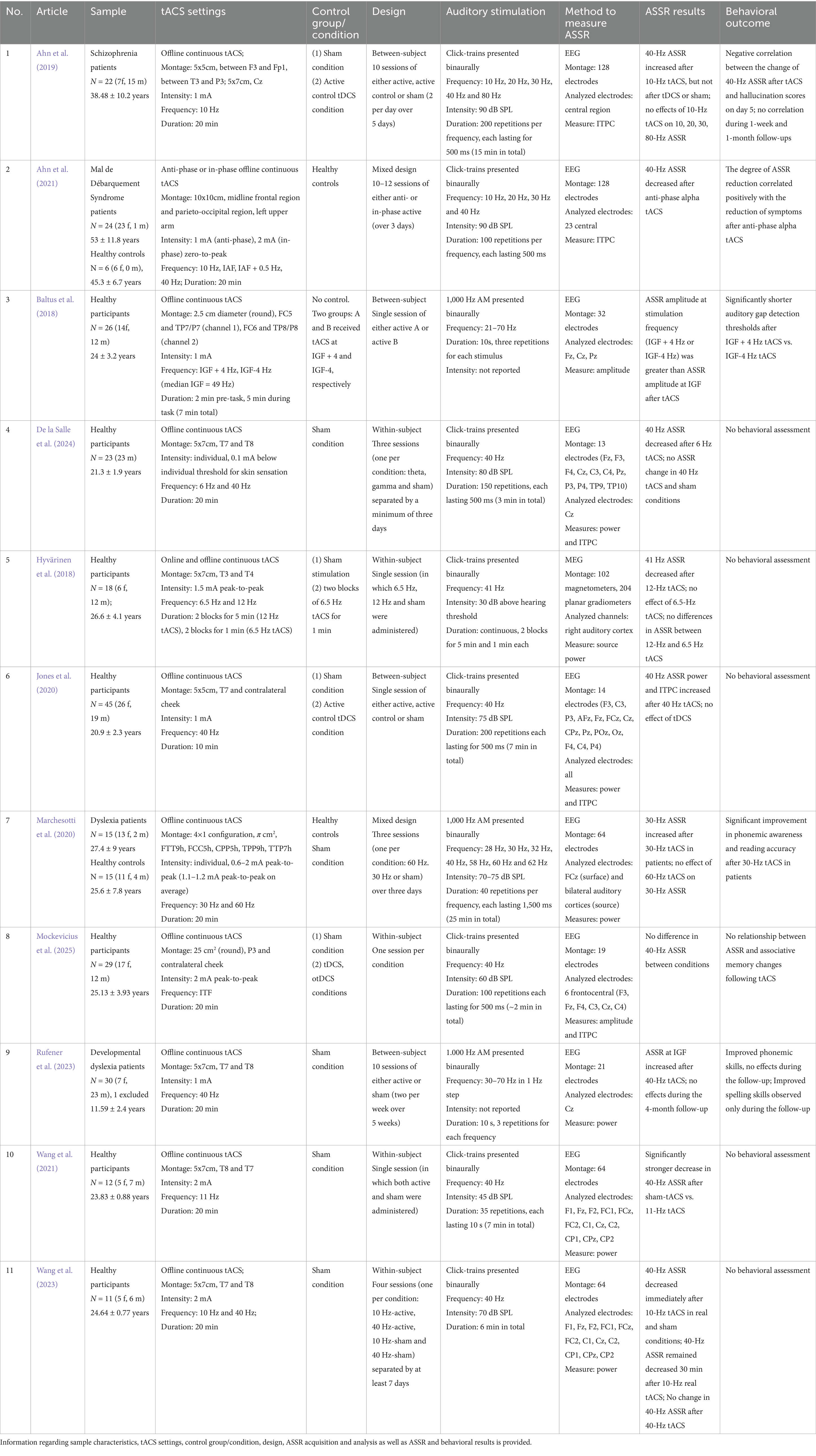
The information from the reviewed ASSR studies is presented in Table 2. Seven studies assessed tACS-induced ASSR changes only in healthy subjects (Baltus et al., 2018; Hyvärinen et al., 2018; Jones et al., 2020; Wang et al., 2021, 2023; de la Salle et al., 2024; Mockevicius et al., 2025), two studies recruited both healthy subjects and patients either with dyslexia (Marchesotti et al., 2020) or Mal de Débarquement Syndrome (MDdS) (Ahn et al., 2021), the remaining two studies involved only patients with dyslexia (Rufener et al., 2023) or schizophrenia (Ahn et al., 2019). The mean sample size in these studies was 25.1 (SD: 9.6; range: 11–45) with an average age of 26.9 (SD: 10.3; range: 11.59–51.4). Ten studies recruited adult participants, while (Rufener et al., 2023) involved minors.
3.1.2 TACS parameters
Regarding the frequency of stimulation, six studies used gamma (40, 41 Hz or at individual frequencies) tACS (Baltus et al., 2018; Jones et al., 2020; Marchesotti et al., 2020; Ahn et al., 2021; Rufener et al., 2023; Wang et al., 2023; de la Salle et al., 2024), five used alpha (10–12 Hz or at individual frequencies) tACS (Hyvärinen et al., 2018; Ahn et al., 2019, 2021; Wang et al., 2021, 2023) and three applied theta (6, 6.5 Hz or at individual frequencies) tACS (Hyvärinen et al., 2018; de la Salle et al., 2024; Mockevicius et al., 2025). The reviewed studies also applied diverse electrode montages. Eight studies targeted temporal areas, placing electrodes bilaterally (Baltus et al., 2018; Hyvärinen et al., 2018; Wang et al., 2021, 2023; Rufener et al., 2023; de la Salle et al., 2024) or over the left hemisphere (Jones et al., 2020; Marchesotti et al., 2020). Others applied tACS over the left posterior parietal cortex with the return electrode on contralateral cheek (Mockevicius et al., 2025), left frontal and temporo-parietal (Ahn et al., 2019) or frontal and parieto-occipital (Ahn et al., 2021) regions. Looking at the timing of stimulation, ten studies applied continuous tACS separately from ASSR recording (offline), while Hyvärinen et al. (2018) administered tACS and auditory stimulation simultaneously (online). On average, tACS was delivered for 15.5 min (SD: 7.3; range: 1–20) with a fixed intensity ranging from 1 to 2 mA or individually determined intensity – 0.1 mA below the individual skin sensation threshold (de la Salle et al., 2024) or between 0.6 and 2 mA peak-to-peak (Marchesotti et al., 2020).
3.1.3 ASSR parameters
The signal for ASSR assessment was acquired using EEG in 10 studies, whereas Hyvärinen et al. (2018) utilized MEG. Auditory click trains (Hyvärinen et al., 2018; Ahn et al., 2019, 2021; Jones et al., 2020; Wang et al., 2021, 2023; de la Salle et al., 2024; Mockevicius et al., 2025) or 1,000-Hz amplitude modulated tones (Baltus et al., 2018; Marchesotti et al., 2020; Rufener et al., 2023) were used, and sounds were presented binaurally. All studies delivered gamma-band auditory stimulation (30–70 Hz), however, some studies also included lower frequency (10–28 Hz) bands (Baltus et al., 2018; Ahn et al., 2019, 2021). Six studies used short-duration stimuli of 500 ms (Ahn et al., 2019, 2021; Jones et al., 2020; de la Salle et al., 2024; Mockevicius et al., 2025) or 1,500 ms (Marchesotti et al., 2020) with the number of repetitions ranging from 40 to 200 per block; others utilized sounds of 10 s for 3 repetitions (Baltus et al., 2018; Rufener et al., 2023) or 35 repetitions (Wang et al., 2021); conversely, Hyvärinen et al. (2018) used stimuli which continued throughout the whole tACS block, lasting either 1 min or 5 min.
3.1.4 ASSR and behavioral results
The reported findings of ASSR studies are categorized based on the applied frequency of tACS. Among studies that utilized gamma-band tACS, an increase in ASSR at 30 Hz (Marchesotti et al., 2020), 40 Hz (Jones et al., 2020), and at a frequency slightly below or above individual gamma frequency (IGF) (Baltus et al., 2018) was found after applying tACS at an equivalent gamma frequency. In addition, Rufener et al. (2023) reported a stronger ASSR at IGF after 40-Hz tACS. Conversely, no changes in 40-Hz ASSR were reported after 40-Hz tACS in two studies (Wang et al., 2023; de la Salle et al., 2024). Three studies showed that ASSR increase at 30 Hz (Marchesotti et al., 2020), IGF (Rufener et al., 2023) and IGF + 4 Hz (Baltus et al., 2018) was accompanied by enhanced performance in the auditory gap detection task in healthy participants (Baltus et al., 2018) or improvements in language tasks in patients with dyslexia (Marchesotti et al., 2020; Rufener et al., 2023).
When alpha-tACS was applied, suppression of 40-Hz (Ahn et al., 2021; Wang et al., 2023) and 41-Hz (Hyvärinen et al., 2018) ASSR was reported after tACS at 10 Hz (Wang et al., 2023), 12 Hz (Hyvärinen et al., 2018) or when subjects who underwent tACS at 10 Hz, individual alpha frequency (IAF) and IAF + 0.5 Hz were pooled together (Ahn et al., 2021). However, a stronger inhibitory effect of sham tACS on 40-Hz ASSR when compared to 11-Hz tACS was observed in one study (Wang et al., 2021). In addition, Wang et al. (2023) observed a comparable inhibitory effect of both sham and 10-Hz tACS on 40-Hz ASSR, however, only in real tACS condition the effect was present after 30 min following tACS. Conversely, Ahn et al. (2019) showed an opposite effect, with enhanced 40-Hz ASSR after 10-Hz tACS. Two studies that applied alpha tACS also reported the relationship between ASSR changes and behavioral measures: negative correlation was found between ASSR change and auditory hallucination score in schizophrenia (Ahn et al., 2019) and a positive relationship between ASSR reduction and symptom reduction in MdDS (Ahn et al., 2021).
Finally, different results were obtained by three works that used theta tACS. de la Salle et al. (2024) showed that 6-Hz tACS inhibited 40-Hz ASSR, whereas tACS applied at 6.5 Hz (Hyvärinen et al., 2018) and individual theta frequency (ITF) (Mockevicius et al., 2025) showed no effects on 41-Hz and 40-Hz ASSR, respectively. No association between ASSR change following tACS and performance change in associative memory was observed in Mockevicius et al. (2025), whereas other studies did not carry out behavioral assessment.
3.2 TACS-SSVEP studies
3.2.1 Sample characteristics
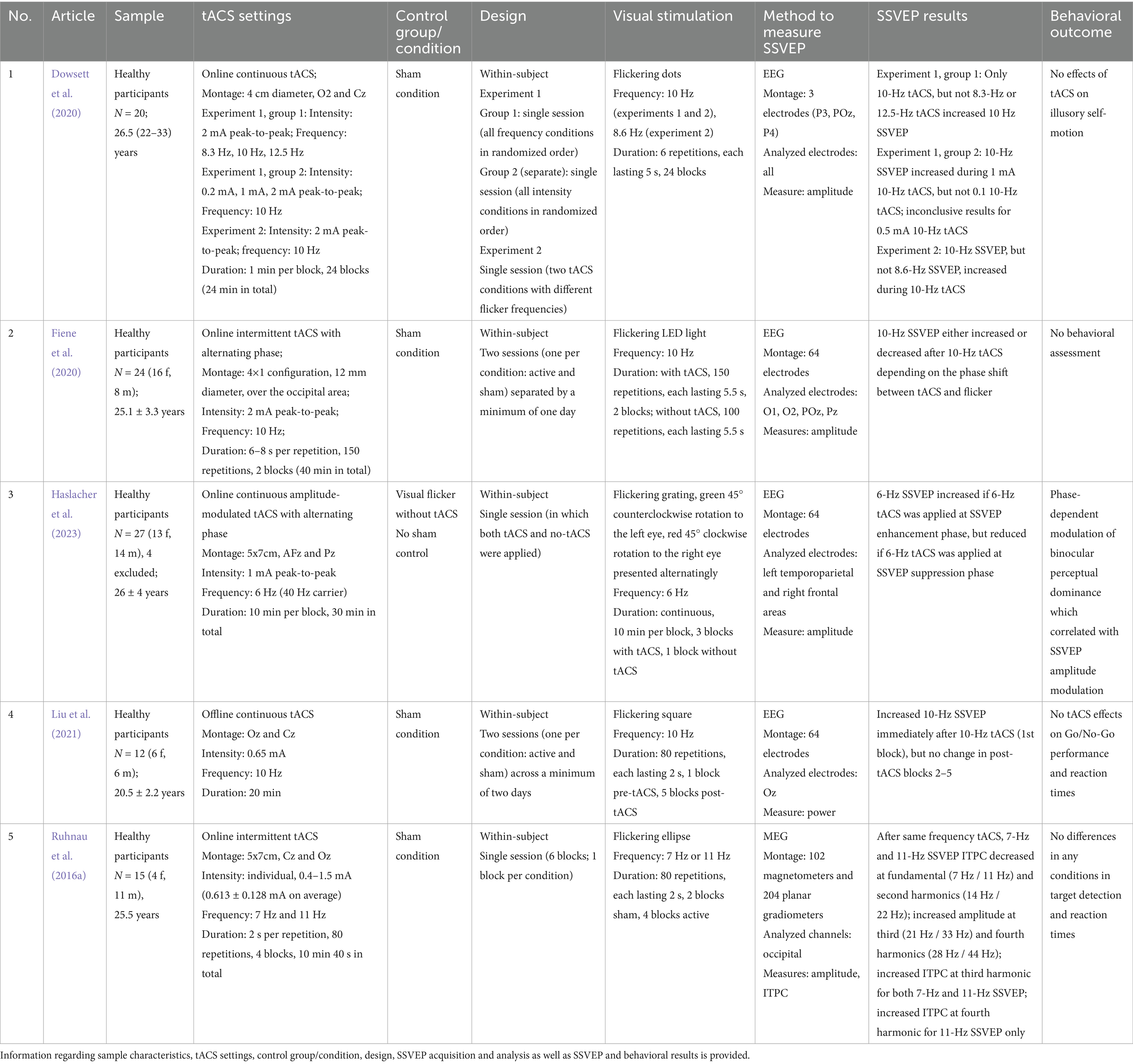
Table 3 contains the information extracted from SSVEP studies. TACS effects on SSVEP were investigated only in healthy participants. On average, 19.6 (SD: 6.2; range: 12–27) subjects participated, with the mean age of 24.7 (SD: 2.4; range: 20.5–26.5) years.
3.2.2 TACS parameters
Looking at the frequencies, four studies applied alpha-band (8.3–12.5 Hz) tACS (Ruhnau et al., 2016a; Dowsett et al., 2020; Fiene et al., 2020; Liu et al., 2021), theta (6–7 Hz) tACS was used in two works (Ruhnau et al., 2016a; Haslacher et al., 2023). Haslacher et al. (2023) used amplitude-modulated 6-Hz tACS with a 40-Hz carrier. In addition, two studies introduced an alternation of tACS phase relative to the visual flicker (Fiene et al., 2020; Haslacher et al., 2023). Two studies applied tACS online intermittently (Ruhnau et al., 2016a; Fiene et al., 2020) with tACS and visual flicker combined into simultaneous repetitions. Fiene et al. (2020) presented 150 repetitions per block, each lasting 6–8 s. Ruhnau et al. (2016a) delivered 2-s tACS pulses in 80 repetitions per block. Two studies used online continuous tACS divided into blocks lasting 1 min (Dowsett et al., 2020) or 10 min (Haslacher et al., 2023). Liu et al. (2021) used an offline continuous tACS with a duration of 20 min. Fixed intensities ranged from 0.2 to 2 mA, while individually determined intensities were set between 0.4 to 1.5 mA (Ruhnau et al., 2016a). As per electrode montages, occipital (Ruhnau et al., 2016a; Dowsett et al., 2020; Fiene et al., 2020; Liu et al., 2021) and parietal (Haslacher et al., 2023) areas were targeted. The occipital montage consisted of two electrodes in central/occipital areas (Dowsett et al., 2020; Liu et al., 2021; Ruhnau et al., 2016a) or 4×1 configuration (Fiene et al., 2020).
3.2.3 SSVEP parameters
Four studies recorded EEG and one used MEG (Ruhnau et al., 2016a). Participants watched flickering shapes or patterns on the screen (Ruhnau et al., 2016a; Dowsett et al., 2020; Liu et al., 2021; Haslacher et al., 2023) or a flickering light (Fiene et al., 2020). Dowsett et al. (2020) introduced flickering to optic flow patterns or random dot motion visual stimuli, whereas Haslacher et al. (2023) incorporated the steady-state aspect into a binocular rivalry task. Four studies used short stimuli of 2 s (Ruhnau et al., 2016a; Liu et al., 2021), 5 s (Dowsett et al., 2020) or 5.5 s (Fiene et al., 2020) presented from 6 to 150 times per block, whereas Haslacher et al. (2023) delivered continuous 10-min stimuli. Alpha (Ruhnau et al., 2016a; Dowsett et al., 2020; Fiene et al., 2020; Liu et al., 2021) and/or theta (Ruhnau et al., 2016a; Haslacher et al., 2023) stimulation frequencies were used. Three studies used flicker frequencies congruent with tACS frequency (Fiene et al., 2020; Liu et al., 2021; Haslacher et al., 2023), while two studies applied both congruent and incongruent flicker frequencies (Ruhnau et al., 2016a; Dowsett et al., 2020).
3.2.4 SSVEP and behavioral results
An increase in 10-Hz SSVEP during/after 10-Hz tACS was reported in two works (Dowsett et al., 2020; Liu et al., 2021). In addition, Dowsett et al. (2020) showed that no effects were present when tACS and SSVEP were delivered at different frequencies. Fiene et al. (2020) demonstrated that 10-Hz SSVEP can be both enhanced and inhibited by 10-Hz tACS, depending on the phase shift between tACS and the flicker. A similar phase-dependent modulation of 6-Hz SSVEP applying 6-Hz tACS was reported by Haslacher et al. (2023). Ruhnau et al. (2016a) used tACS and SSVEP at 7-Hz and 11-Hz and showed a differential modulation of SSVEP amplitude and phase-locking for different harmonics. Importantly, these effects were observed only when the same frequency was used for the flicker and tACS. Haslacher et al. (2023) showed a relationship between changes in SSVEP and binocular stimulus dominance ratio, while the other four studies did not report any significant behavioral effects.
4 Discussion
The present review shows that research combining tACS with SSR paradigms has grown substantially in recent years offering a unique opportunity for probing oscillatory brain dynamics. Since the last review on tES effects on ASSR (Griskova-Bulanova et al., 2020), the number of works investigating the modulation of ASSR using tACS has increased from 3 to 11. In addition, five tACS-SSVEP studies were featured in this review. TACS-SSR is a promising paradigm that could provide valuable insights into tACS neurophysiological and behavioral effects, yet the variability in reported effects underscores the need for a clearer mechanistic framework.
A key point concerns the neurophysiological basis of both tACS and SSRs. While entrainment of endogenous oscillations has often been proposed as the common mechanism underlying both phenomena (Thut et al., 2011), accumulating evidence suggests a more complex picture. For tACS, recent critical review of neurocognitive, physiological, and biophysical effects highlights entrainment of endogenous oscillations as a central mechanism but also presents evidence of additional effects, including shifts in neural spike timing, alterations in interregional coherence and connectivity, and even broader homeostatic or metabolic changes (Wischnewski et al., 2023). Similarly, the after-effects of tACS have been attributed to multiple forms of plasticity, such as spike-timing-dependent plasticity, spike-phase and oscillation coupling, homeostatic and state-dependent modulation (Agboada et al., 2025).
The neurophysiological basis of SSRs is likewise not unitary (Bánki et al., 2022). Several works showed a high correspondence between recorded SSRs and a synthesized waveform of linearly summated transient evoked responses to discrete stimuli (Azzena et al., 1995; Bohórquez and Özdamar, 2008; Capilla et al., 2009, 2011), suggesting that SSRs may rely on the same underlying mechanism as sensory evoked potentials and occur independently of the ongoing intrinsic oscillations. However, a larger body of evidence favors the entrainment of ongoing activity via resonant frequencies (Johnson et al., 2024) or the activation of separate rhythms by stimulation (Welle and Contreras, 2016; Duecker et al., 2021). Moreover, SSRs (1) reflect changes in neural activity in neuropsychiatric conditions (Yamasaki, 2021; Grent-‘t-Jong et al., 2023) or due to pharmacological modulation (Bale et al., 2005; Sullivan et al., 2015), (2) are associated with behavioral measures (Richard et al., 2020; Parciauskaite et al., 2021), and (3) are stronger and more synchronized at frequencies matching intrinsic individual-specific frequencies (Zaehle et al., 2010; Notbohm et al., 2016). Furthermore, prolonged rhythmic sensory stimulation has shown promise in modulating neurobiological (Martorell et al., 2019; Rodrigues-Amorim et al., 2024), neurophysiological (Lin et al., 2021; Pinardi et al., 2024) and behavioral (Sharpe et al., 2020; Cimenser et al., 2021) outcomes in both animals and humans. Together, these findings point to the potential interaction of periodic sensory stimulation with intrinsic brain oscillations.
Motivated by the notion of shared neural entrainment mechanisms hypothesized for both tACS and SSRs (Thut et al., 2011), several of the reviewed studies combined SSRs with tACS, aiming to investigate various factors related to tACS effects mechanistically. Among ASSR studies, low-frequency tACS was generally used to probe cross-frequency interactions (Hyvärinen et al., 2018; Wang et al., 2021, 2023; Mockevicius et al., 2025), while gamma tACS was utilized to study how tACS affects endogenous oscillations at a matched frequency (Jones et al., 2020; Wang et al., 2023). SSVEP paradigm was employed to evaluate the impact of tACS intensity (Dowsett et al., 2020), frequency (Ruhnau et al., 2016a; Dowsett et al., 2020; Liu et al., 2021), and phase (Fiene et al., 2020; Haslacher et al., 2023) on rhythmic brain activity. In addition, among the reviewed studies, SSRs were evaluated as biomarkers, assessing the link between SSRs and behavioral effects of tACS, including symptom severity in patients with MdDS (Ahn et al., 2021), schizophrenia (Ahn et al., 2019), and dyslexia (Marchesotti et al., 2020; Rufener et al., 2023) or sensory task performance in healthy participants (Baltus et al., 2018).
Given that tACS is oscillatory in nature, the frequency of stimulation is a fundamental parameter. The selection of tACS frequency among the reviewed studies mainly differed based on goals and the putative mechanisms of action. In ASSR studies, gamma tACS was delivered to enhance synchronized neural activity at specific gamma-range frequencies. However, the results are mixed as studies showed either a predicted increase (Baltus et al., 2018; Jones et al., 2020; Marchesotti et al., 2020; Rufener et al., 2023) or no change (Wang et al., 2023; de la Salle et al., 2024). Due to the general inhibitory role of alpha activity (Jensen and Mazaheri, 2010; Mathewson et al., 2011), potentially mediated via alpha-gamma cross-frequency interactions, alpha tACS was primarily selected to suppress gamma synchronization. While this alpha tACS effect was reported in MdDS patients (Ahn et al., 2021) as well as healthy participants (Hyvärinen et al., 2018; Wang et al., 2023), an increase in ASSR was also shown in schizophrenia patients (Ahn et al., 2019). de la Salle et al. (2024) expected increased gamma ASSR due to theta tACS based on theta-gamma phase-amplitude coupling, but the authors observed an ASSR reduction. Other studies showed no theta tACS effects on gamma ASSR (Hyvärinen et al., 2018; Mockevicius et al., 2025). Among the studies assessing SSVEP, the general aim was to enhance theta or alpha activity using a congruent tACS frequency; however, the effect was shown to depend on the phase lag between visual flicker and tACS delivered online (Fiene et al., 2020; Haslacher et al., 2023). When the frequencies of visual flicker and tACS did not match, no change in SSVEP was reported (Ruhnau et al., 2016a; Dowsett et al., 2020).
Overall, the reviewed findings suggest that tACS effects on SSRs depend on sensory and electrical stimulation frequencies. However, there is a substantial variability in the reported findings, especially among ASSR studies, which likely arose due to differences in methodology and sample characteristics. When gamma tACS was used, promising results were reported in studies with specific a priori hypotheses about disturbances of gamma oscillations in clinical groups or the relationship of gamma activity with targeted sensory processing. In two studies, the applied approaches were based on the hypothesis of reduced peak frequency of gamma oscillations in dyslexia. Marchesotti et al. (2020) showed an increase in 30-Hz ASSR after 30-Hz tACS, while Rufener et al. (2023) reported an increase in both IGF and response at IGF after 40-Hz tACS. Improvements in language task performance were also shown in both studies. In addition, Baltus et al. (2018) hypothesized that IGF can be related to auditory temporal acuity and showed that tACS applied at a frequency slightly above IGF increased ASSR at the corresponding frequency and improved auditory gap detection performance in healthy subjects. Other studies targeted the classical 40-Hz ASSR using the same frequency tACS in healthy young subjects. Of them, only Jones et al. (2020) reported an increase in ASSR, while in two studies ASSR remained unchanged (Wang et al., 2023; de la Salle et al., 2024). The latter two studies are characterized by small sample sizes (23 and 11 subjects), which could have contributed to a lack of significant effects. TACS is a relatively mild non-invasive intervention that is generally characterized by modest to moderate effect sizes (Grover et al., 2023). In addition, healthy participants are expected to be more resilient to external perturbations (Hall et al., 2020), which is likely applicable to ASSR due to its high individual stability (Van Eeckhoutte et al., 2018; Roach et al., 2019). Due to these factors, larger sample sizes may be needed to detect ASSR changes after matched frequency tACS.
Similarly, studies using low-frequency tACS and gamma ASSR also showed inconsistent outcomes. Patient sample characteristics may explain the differences in clinical studies using alpha tACS. Given that MdDS is characterized by gamma hypersynchrony, alpha entrainment by tACS may have normalized excessive gamma activity, potentially due to its inhibitory influence (Ahn et al., 2021). Conversely, since schizophrenia patients exhibit inadequate oscillatory activity in both alpha and gamma bands (Uhlhaas et al., 2008), alleviating alpha disturbances may have also contributed to improved gamma synchronization due to alpha-gamma interactions (Jensen and Mazaheri, 2010) as evidenced by increased ASSR (Ahn et al., 2019).
Theta tACS was used in three studies, showing either reduced (de la Salle et al., 2024) or unchanged (Hyvärinen et al., 2018; Mockevicius et al., 2025) ASSR. Montage choice may have contributed to mixed results. Despite the overall low focality of tACS, the induced electric field strength differs substantially across configurations (Guidetti et al., 2022), and this variability likely contributes to inconsistent outcomes. Hyvärinen et al. (2018) and De la Salle et al. (2024) applied theta tACS over bilateral temporal regions, whereas Mockevicius et al. (2025) targeted posterior parietal cortex. Since ASSR is mainly generated in the auditory cortex (Pantev et al., 1996), ASSR may have been less sensitive to tACS applied over a more distant location from its primary source. Also, authors highlight the role of individual variability as contributing to insignificant findings at the group level (Mockevicius et al., 2025). Meanwhile, the study by Hyvärinen et al. (2018), contains methodological limitations that hinder clear interpretation of the findings. Online theta and alpha tACS were applied for different durations - 1 min and 5 min, respectively. Although the authors partly accounted for this by comparing ASSR during theta and alpha tACS in 1-min time window, ASSR during alpha tACS was compared to no-tACS ASSR over the whole 5-min window, likely resulting in different signal-to-noise ratios and differences in the overall state. Both tACS conditions showed ASSR suppression descriptively, but only alpha tACS produced a statistically significant effect. However, ASSR did not differ between theta and alpha tACS conditions, suggesting no frequency-specific effects on ASSR (Hyvärinen et al., 2018).
Studies combining visual flicker with tACS were more methodologically homogeneous and reported consistent findings. All studies recruited only healthy participants and targeted posterior areas (occipital or parietal) using low frequency (alpha and/or theta) tACS, unanimously showing significant tACS effects when flicker and tACS frequencies were matched. In addition, four out of five studies used online tACS combined with visual stimulation. Three works used artifact-removal methods (Ruhnau et al., 2016a; Dowsett et al., 2020; Haslacher et al., 2023), whereas Fiene et al. (2020) analyzed uncontaminated parts of the signal immediately following online tACS. These approaches allowed for more precise and robust mechanistic investigations, such as phase-dependence of tACS-SSVEP outcomes (Fiene et al., 2020; Haslacher et al., 2023). In addition, given that tES effects are network-activity-dependent (Fertonani and Miniussi, 2017), online tACS in SSVEP studies could have contributed to more pronounced and thus more consistent effects when compared to ASSR studies that applied tACS offline. While analyzing ASSR recorded during simultaneous tACS application could be challenging due to inherently weaker gamma response as compared to low-frequency SSVEP, assessing ASSR immediately following online tACS (Fiene et al., 2020) or using non-overlapping tACS and ASSR frequencies and their harmonics (Hyvärinen et al., 2018) may be promising strategies for future online tACS-ASSR studies.
However, it is essential to mention that only one tES-SSVEP study reported significant changes in behavioral measures (Haslacher et al., 2023), likely due to the generally mechanistic nature of the studies. Firstly, only healthy participants were recruited. It has been suggested that tACS may be more beneficial for patients with psychiatric disorders as compared to healthy individuals (Lee et al., 2022). In line with this, among ASSR studies, significant behavioral effects of tACS were mainly evident in clinical populations (Ahn et al., 2019, 2021; Marchesotti et al., 2020; Rufener et al., 2023), with only Baltus et al. (2018) reporting changes in auditory perception in healthy participants. These findings fit the inverted U-shaped relationship between baseline neural activity and stimulation outcomes (Rufener et al., 2016; Ruhnau et al., 2016b; Tseng et al., 2018; Krause et al., 2022). Namely, tACS appears most effective when intrinsic oscillatory activity is suboptimal, as observed in clinical populations with reduced or dysregulated neural synchrony. In contrast, when baseline activity in healthy brains is already near optimal, tACS may have little or even disruptive effects. This principle may help reconcile why tACS robustly enhanced ASSRs in dyslexia or schizophrenia, yet yielded inconsistent effects in healthy participants. Secondly, in two studies (Fiene et al., 2020; Ruhnau et al., 2016a), the intermittent pattern of tACS might have been inadequate for behavioral modulation as it may require longer-lasting entrainment (Strüber et al., 2015). Furthermore, the differences in behavioral tasks should be taken into consideration. For example, Haslacher et al. (2023) studied binocular dominance and observed its relationship with SSVEP modulation. Conversely, Liu et al. (2021) analyzed Go/NoGo performance, showing no changes after tACS. While the former assessed lower-level perceptual processing, the Go/NoGo task in the latter study relies on multiple higher-order cognitive processes (e.g., inhibitory control, Bokura et al. (2001)), potentially making it less sensitive to tACS applied over the occipital region.
Given the complex and still-explored mechanisms underlying both tACS effects and SSRs, the heterogeneous findings are no surprise. At a neurophysiological level, tACS may interact with SSRs in multiple ways: (1) by entraining intrinsic neural oscillators at the stimulation frequency, thereby enhancing resonance phenomena, supported by cross-frequency interactions in ASSR studies and the reported relationships with behavioral measures; (2) by modulating the amplitude of repetitive evoked responses without necessarily engaging endogenous oscillators, which is in line with more consistent results when tACS frequency and phase were matched with those of SSRs; or (3) by periodically biasing membrane potential fluctuations, which can alter responsiveness to incoming rhythmic sensory input. Distinguishing these mechanisms remains challenging, yet it is critical for interpreting whether reported SSR modulations index genuine entrainment or altered evoked responses.
Taken together, the reviewed evidence suggests that tACS exerts neurophysiological effects which may be reflected in changes to SSR amplitude, power, or phase-locking, likely indexing the entrainment of intrinsic oscillations or the modulation of evoked responses. These tACS-induced changes may manifest behaviorally, e.g., as improved clinical symptoms, auditory gap detection or reading performance, which appear to be more consistent when tACS restores or enhances oscillatory activity in populations with atypical baseline activity (e.g., dyslexia, schizophrenia). These differences highlight that tACS effects are not uniform but depend on the interplay between stimulation parameters and the brain state of the participant. This mechanistic perspective can explain why SSRs may serve as both biomarkers of neural entrainment and predictors of behavioral outcomes, adding to their translational value.
The findings reviewed in the present work contribute to the existing literature by showing that tACS neurophysiological and behavioral effects depend on both extrinsic (methodology-related) and intrinsic (brain-activity-related) factors. Accordingly, future studies should focus on adapting tACS-SSR procedures to the specific aims and participant populations. When investigating tACS mechanisms of action using SSRs, online tACS would be preferred due to the possibility of applying precise experimental manipulations. Offline tACS may be suitable when assessing its potential as a biomarker of tACS-induced behavioral changes. In particular, tACS protocols aiming to improve a specific sensory or cognitive domain may include the combination of tACS with online behavioral tasks (e.g., Bjekić et al., 2022). Therefore, SSRs would be limited to offline use in these approaches. However, based on the reviewed findings, offline tACS may require a careful and theoretically grounded selection of protocol parameters as well as higher sample sizes.
In summary, this review advances the field by summarizing up to date literature on tACS-SSR interaction. This review synthesizes cross-study patterns and identifies three converging insights: (1) congruence between sensory and electrical stimulation frequencies is the most reliable determinant of tACS effects on SSRs; (2) variability in baseline oscillatory dynamics is expected to modulate the effects of tACS-SSR applications, which may result in differential outcomes across healthy and clinical populations; and (3) methodological differences, particularly online vs. offline designs, account for the variability and heterogeneity of the observed effects. Together, these insights advance a conceptual framework for using SSRs not only as mechanistic probes of entrainment but also as candidate biomarkers of tACS efficacy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AM: Methodology, Investigation, Writing – review & editing, Visualization, Formal Analysis, Writing – original draft. JB: Validation, Conceptualization, Writing – review & editing, Supervision, Writing – original draft. IG-B: Writing – review & editing, Conceptualization, Supervision, Writing – original draft, Validation, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was carried out under the EU-funded HORIZON Collaboration and Support Action TWINNIBS (101059369). JB receives institutional support from the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (451-03-66/2024-03/200015).
Acknowledgments
The authors thank Milena Spoa for her help with data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agboada, D., Zhao, Z., and Wischnewski, M. (2025). Neuroplastic effects of transcranial alternating current stimulation (tACS): from mechanisms to clinical trials. Front. Hum. Neurosci. 19:1548478. doi: 10.3389/fnhum.2025.1548478
Ahn, S., Gleghorn, D., Doudican, B., Fröhlich, F., and Cha, Y.-H. (2021). Transcranial alternating current stimulation reduces network hypersynchrony and persistent vertigo. Neuromodulation Technol. Neural Interface 24, 960–968. doi: 10.1111/ner.13389
Ahn, S., Mellin, J. M., Alagapan, S., Alexander, M. L., Gilmore, J. H., Jarskog, L. F., et al. (2019). Targeting reduced neural oscillations in patients with schizophrenia by transcranial alternating current stimulation. NeuroImage 186, 126–136. doi: 10.1016/j.neuroimage.2018.10.056
Antal, A., Boros, K., Poreisz, C., Chaieb, L., Terney, D., and Paulus, W. (2008). Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 1, 97–105. doi: 10.1016/j.brs.2007.10.001
Antal, A., and Paulus, W. (2013). Transcranial alternating current stimulation (tACS). Front. Hum. Neurosci. 7. doi: 10.3389/fnhum.2013.00317
Azzena, G. B., Conti, G., Santarelli, R., Ottaviani, F., Paludetti, G., and Maurizi, M. (1995). Generation of human auditory steadystate responses (SSRs). I: stimulus rate effects. Hear. Res. 83, 1–8. doi: 10.1016/0378-5955(94)00184-R
Bale, A. S., Adams, T. L., Bushnell, P. J., Shafer, T. J., and Boyes, W. K. (2005). Role of NMDA, nicotinic, and GABA receptors in the steady-state visual-evoked potential in rats. Pharmacol. Biochem. Behav. 82, 635–645. doi: 10.1016/j.pbb.2005.11.003
Baltus, A., Wagner, S., Wolters, C. H., and Herrmann, C. S. (2018). Optimized auditory transcranial alternating current stimulation improves individual auditory temporal resolution. Brain Stimul. 11, 118–124. doi: 10.1016/j.brs.2017.10.008
Bánki, A., Brzozowska, A., Hoehl, S., and Köster, M. (2022). Neural entrainment vs. stimulus-tracking: a conceptual challenge for rhythmic perceptual stimulation in developmental neuroscience. Front. Psychol. 13:878984. doi: 10.3389/fpsyg.2022.878984
Biačková, N., Adamová, A., and Klírová, M. (2024). Transcranial alternating current stimulation in affecting cognitive impairment in psychiatric disorders: a review. Eur. Arch. Psychiatry Clin. Neurosci. 274, 803–826. doi: 10.1007/s00406-023-01687-7
Bjekić, J., Živanović, M., Paunović, D., Vulić, K., Konstantinović, U., and Filipović, S. R. (2022). Personalized frequency modulated transcranial electrical stimulation for associative memory enhancement. Brain Sci. 12:472. doi: 10.3390/brainsci12040472
Bohórquez, J., and Özdamar, Ö. (2008). Generation of the 40-Hz auditory steady-state response (ASSR) explained using convolution. Clin. Neurophysiol. 119, 2598–2607. doi: 10.1016/j.clinph.2008.08.002
Bokura, H., Yamaguchi, S., and Kobayashi, S. (2001). Electrophysiological correlates for response inhibition in a go/NoGo task. Clin. Neurophysiol. 112, 2224–2232. doi: 10.1016/S1388-2457(01)00691-5
Brenner, C. A., Krishnan, G. P., Vohs, J. L., Ahn, W.-Y., Hetrick, W. P., Morzorati, S. L., et al. (2009). Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr. Bull. 35, 1065–1077. doi: 10.1093/schbul/sbp091
Button, K. S., Ioannidis, J. P. A., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S. J., et al. (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376. doi: 10.1038/nrn3475
Capilla, A., Pazo, P., Campo, P., and Gross, J. (2009). Steady-state and transient visual evoked potentials: are they the same? NeuroImage 47, S39–S41. doi: 10.1016/S1053-8119(09)70640-8
Capilla, A., Pazo-Alvarez, P., Darriba, A., Campo, P., and Gross, J. (2011). Steady-state visual evoked potentials can be explained by temporal superposition of transient event-related responses. PLoS One 6:e14543. doi: 10.1371/journal.pone.0014543
Chuderski, A., and Chinta, S. R. (2024). Transcranial alternating current stimulation barely enhances working memory in healthy adults: a meta-analysis. Brain Res. 1839:149022. doi: 10.1016/j.brainres.2024.149022
Cimenser, A., Hempel, E., Travers, T., Strozewski, N., Martin, K., Malchano, Z., et al. (2021). Sensory-evoked 40-Hz gamma oscillation improves sleep and daily living activities in Alzheimer’s disease patients. Front. Syst. Neurosci. 15:746859. doi: 10.3389/fnsys.2021.746859
de la Salle, S., Choueiry, J., Payumo, M., Devlin, M., Noel, C., Abozmal, A., et al. (2024). Transcranial alternating current stimulation alters auditory steady-state oscillatory rhythms and their cross-frequency couplings. Clin. EEG Neurosci. 55, 329–339. doi: 10.1177/15500594231179679
Ding, J., Sperling, G., and Srinivasan, R. (2006). Attentional modulation of SSVEP power depends on the network tagged by the flicker frequency. Cereb. Cortex 16, 1016–1029. doi: 10.1093/cercor/bhj044
Dowsett, J., Herrmann, C. S., Dieterich, M., and Taylor, P. C. J. (2020). Shift in lateralization during illusory self-motion: EEG responses to visual flicker at 10 Hz and frequency-specific modulation by tACS. Eur. J. Neurosci. 51, 1657–1675. doi: 10.1111/ejn.14543
Duecker, K., Gutteling, T. P., Herrmann, C. S., and Jensen, O. (2021). No evidence for entrainment: endogenous gamma oscillations and rhythmic flicker responses coexist in visual cortex. J. Neurosci. 41, 6684–6698. doi: 10.1523/JNEUROSCI.3134-20.2021
Fertonani, A., and Miniussi, C. (2017). Transcranial Electrical Stimulation. Neuroscientist 23, 109–123. doi: 10.1177/1073858416631966
Fiene, M., Schwab, B. C., Misselhorn, J., Herrmann, C. S., Schneider, T. R., and Engel, A. K. (2020). Phase-specific manipulation of rhythmic brain activity by transcranial alternating current stimulation. Brain Stimul. 13, 1254–1262. doi: 10.1016/j.brs.2020.06.008
Fong, D. H. C., Cohen, A., Boughton, P., Raftos, P., Herrera, J. E., Simon, N. G., et al. (2020). Steady-state visual-evoked potentials as a biomarker for concussion: a pilot study. Front. Neurosci. 14:171. doi: 10.3389/fnins.2020.00171
Grent-‘t-Jong, T., Brickwedde, M., Metzner, C., and Uhlhaas, P. J. (2023). 40-Hz auditory steady-state responses in schizophrenia: toward a mechanistic biomarker for circuit dysfunctions and early detection and diagnosis. Biol. Psychiatry 94, 550–560. doi: 10.1016/j.biopsych.2023.03.026
Griskova-Bulanova, I., Sveistyte, K., and Bjekic, J. (2020). Neuromodulation of gamma-range auditory steady-state responses: a scoping review of brain stimulation studies. Front. Syst. Neurosci. 14:41. doi: 10.3389/fnsys.2020.00041
Grover, S., Fayzullina, R., Bullard, B. M., Levina, V., and Reinhart, R. M. G. (2023). A meta-analysis suggests that tACS improves cognition in healthy, aging, and psychiatric populations. Sci. Transl. Med. 15:eabo2044. doi: 10.1126/scitranslmed.abo2044
Guidetti, M., Arlotti, M., Bocci, T., Bianchi, A. M., Parazzini, M., Ferrucci, R., et al. (2022). Electric fields induced in the brain by transcranial electric stimulation: a review of in vivo recordings. Biomedicine 10:2333. doi: 10.3390/biomedicines10102333
Hall, P. A., Erickson, K. I., Lowe, C. J., and Sakib, M. N. (2020). Quantifying cortical resilience in experimental, clinical, and epidemiological studies: a conceptually grounded method using noninvasive brain stimulation. Psychosom. Med. 82, 281–286. doi: 10.1097/PSY.0000000000000785
Haslacher, D., Narang, A., Sokoliuk, R., Cavallo, A., Reber, P., Nasr, K., et al. (2023). In vivo phase-dependent enhancement and suppression of human brain oscillations by transcranial alternating current stimulation (tACS). NeuroImage 275:120187. doi: 10.1016/j.neuroimage.2023.120187
Herrmann, C. S., Rach, S., Neuling, T., and Strüber, D. (2013). Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci. 7:279. doi: 10.3389/fnhum.2013.00279
Huang, W. A., Stitt, I. M., Negahbani, E., Passey, D. J., Ahn, S., Davey, M., et al. (2021). Transcranial alternating current stimulation entrains alpha oscillations by preferential phase synchronization of fast-spiking cortical neurons to stimulation waveform. Nat. Commun. 12:3151. doi: 10.1038/s41467-021-23021-2
Hyvärinen, P., Choi, D., Demarchi, G., Aarnisalo, A. A., and Weisz, N. (2018). tACS-mediated modulation of the auditory steady-state response as seen with MEG. Hear. Res. 364, 90–95. doi: 10.1016/j.heares.2018.03.023
Jensen, O., and Mazaheri, A. (2010). Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 4:186. doi: 10.3389/fnhum.2010.00186
Johnson, T. D., Gallagher, A. J., Coulson, S., and Rangel, L. M. (2024). Network resonance and the auditory steady state response. Sci. Rep. 14:16799. doi: 10.1038/s41598-024-66697-4
Jones, K. T., Johnson, E. L., Tauxe, Z. S., and Rojas, D. C. (2020). Modulation of auditory gamma-band responses using transcranial electrical stimulation. J. Neurophysiol. 123, 2504–2514. doi: 10.1152/jn.00003.2020
Koninck, B. P. D., Brazeau, D., Guay, S., Babiloni, A. H., and Beaumont, L. D. (2023). Transcranial alternating current stimulation to modulate alpha activity: a systematic review. Neuromodulation 26, 1549–1584. doi: 10.1016/j.neurom.2022.12.007
Krause, B., and Cohen Kadosh, R. (2014). Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front. Syst. Neurosci. 8:25. doi: 10.3389/fnsys.2014.00025
Krause, M. R., Vieira, P. G., Thivierge, J.-P., and Pack, C. C. (2022). Brain stimulation competes with ongoing oscillations for control of spike timing in the primate brain. PLoS Biol. 20:e3001650. doi: 10.1371/journal.pbio.3001650
Lee, A. R. Y. B., Yau, C. E., Mai, A. S., Tan, W. A., Ong, B. S. Y., Yam, N. E., et al. (2022). Transcranial alternating current stimulation and its effects on cognition and the treatment of psychiatric disorders: a systematic review and meta-analysis. Therapeutic Adv. Chronic Disease 13:20406223221140390. doi: 10.1177/20406223221140390
Lin, Z., Hou, G., Yao, Y., Zhou, Z., Zhu, F., Liu, L., et al. (2021). 40-Hz blue light changes hippocampal activation and functional connectivity underlying recognition memory. Front. Hum. Neurosci. 15:739333. doi: 10.3389/fnhum.2021.739333
Liu, B., Yan, X., Chen, X., Wang, Y., and Gao, X. (2021). tACS facilitates flickering driving by boosting steady-state visual evoked potentials. J. Neural Eng. 18:066042. doi: 10.1088/1741-2552/ac3ef3
Marchesotti, S., Nicolle, J., Merlet, I., Arnal, L. H., Donoghue, J. P., and Giraud, A.-L. (2020). Selective enhancement of low-gamma activity by tACS improves phonemic processing and reading accuracy in dyslexia. PLoS Biol. 18:e3000833. doi: 10.1371/journal.pbio.3000833
Martorell, A. J., Paulson, A. L., Suk, H.-J., Abdurrob, F., Drummond, G., Guan, W., et al. (2019). Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell 177, 256–271.e22. doi: 10.1016/j.cell.2019.02.014
Mathewson, K. E., Lleras, A., Beck, D. M., Fabiani, M., Ro, T., and Gratton, G. (2011). Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front. Psychol. 2:99. doi: 10.3389/fpsyg.2011.00099
Mockevicius, A., Wang, X., Bjekic, J., Zivanovic, M., Filipovic, S. R., and Griskova-Bulanova, I. (2025). Individual-specific effects of transcranial electrical stimulation on 40-Hz auditory steady-state responses. IEEE Trans. Neural Syst. Rehabil. Eng. 33, 3076–3084. doi: 10.1109/TNSRE.2025.3595925
Notbohm, A., Kurths, J., and Herrmann, C. S. (2016). Modification of brain oscillations via rhythmic light stimulation provides evidence for entrainment but not for superposition of event-related responses. Front. Hum. Neurosci. 10:10. doi: 10.3389/fnhum.2016.00010
Pang, C. Y., and Mueller, M. M. (2014). Test-retest reliability of concurrently recorded steady-state and somatosensory evoked potentials in somatosensory sustained spatial attention. Biol. Psychol. 100, 86–96. doi: 10.1016/j.biopsycho.2014.05.009
Pantev, C., Roberts, L. E., Elbert, T., Roβ, B., and Wienbruch, C. (1996). Tonotopic organization of the sources of human auditory steady-state responses. Hear. Res. 101, 62–74. doi: 10.1016/S0378-5955(96)00133-5
Parciauskaite, V., Bjekic, J., and Griskova-Bulanova, I. (2021). Gamma-range auditory steady-state responses and cognitive performance: a systematic review. Brain Sci. 11:217. doi: 10.3390/brainsci11020217
Pei, F., Baldassi, S., and Norcia, A. M. (2014). Electrophysiological measures of low-level vision reveal spatial processing deficits and hemispheric asymmetry in autism spectrum disorder. J. Vis. 14:3. doi: 10.1167/14.11.3
Pinardi, M., Schuler, A.-L., Di Pino, G., and Pellegrino, G. (2024). 40 Hz repetitive auditory stimulation promotes corticospinal plasticity. Clin. Neurophysiol. 162, 79–81. doi: 10.1016/j.clinph.2024.03.018
Richard, N., Nikolic, M., Mortensen, E. L., Osler, M., Lauritzen, M., and Benedek, K. (2020). Steady-state visual evoked potential temporal dynamics reveal correlates of cognitive decline. Clin. Neurophysiol. 131, 836–846. doi: 10.1016/j.clinph.2020.01.010
Roach, B. J., D’Souza, D. C., Ford, J. M., and Mathalon, D. H. (2019). Test-retest reliability of time-frequency measures of auditory steady-state responses in patients with schizophrenia and healthy controls. Neuroimage Clin. 23:101878. doi: 10.1016/j.nicl.2019.101878
Rodrigues-Amorim, D., Bozzelli, P. L., Kim, T., Liu, L., Gibson, O., Yang, C.-Y., et al. (2024). Multisensory gamma stimulation mitigates the effects of demyelination induced by cuprizone in male mice. Nat. Commun. 15:6744. doi: 10.1038/s41467-024-51003-7
Rufener, K. S., Oechslin, M. S., Zaehle, T., and Meyer, M. (2016). Transcranial alternating current stimulation (tACS) differentially modulates speech perception in young and older adults. Brain Stimul. 9, 560–565. doi: 10.1016/j.brs.2016.04.002
Rufener, K. S., Zaehle, T., and Krauel, K. (2023). Combined multi-session transcranial alternating current stimulation (tACS) and language skills training improves individual gamma band activity and literacy skills in developmental dyslexia. Dev. Cogn. Neurosci. 64:101317. doi: 10.1016/j.dcn.2023.101317
Ruhnau, P., Keitel, C., Lithari, C., Weisz, N., and Neuling, T. (2016a). Flicker-driven responses in visual cortex change during matched-frequency transcranial alternating current stimulation. Front. Hum. Neurosci. 10:184. doi: 10.3389/fnhum.2016.00184
Ruhnau, P., Neuling, T., Fuscá, M., Herrmann, C. S., Demarchi, G., and Weisz, N. (2016b). Eyes wide shut: transcranial alternating current stimulation drives alpha rhythm in a state dependent manner. Sci. Rep. 6:27138. doi: 10.1038/srep27138
Schielke, A., and Krekelberg, B. (2022). Steady state visual evoked potentials in schizophrenia: a review. Front. Neurosci. 16:988077. doi: 10.3389/fnins.2022.988077
Seymour, R. A., Rippon, G., Gooding-Williams, G., Sowman, P. F., and Kessler, K. (2020). Reduced auditory steady state responses in autism spectrum disorder. Mol. Autism. 11:56. doi: 10.1186/s13229-020-00357-y
Sharpe, R. L. S., Mahmud, M., Kaiser, M. S., and Chen, J. (2020). Gamma entrainment frequency affects mood, memory and cognition: an exploratory pilot study. Brain Inform 7:17. doi: 10.1186/s40708-020-00119-9
Steinmann, I., Williams, K. A., Wilke, M., and Antal, A. (2022). Detection of transcranial alternating current stimulation aftereffects is improved by considering the individual electric field strength and self-rated sleepiness. Front. Neurosci. 16:870758. doi: 10.3389/fnins.2022.870758
Strüber, D., Rach, S., Neuling, T., and Herrmann, C. S. (2015). On the possible role of stimulation duration for after-effects of transcranial alternating current stimulation. Front. Cell. Neurosci. 9:311. doi: 10.3389/fncel.2015.00311
Sullivan, E. M., Timi, P., Hong, L. E., and O’Donnell, P. (2015). Effects of NMDA and GABA-A receptor antagonism on auditory steady-state synchronization in awake behaving rats. Int. J. Neuropsychopharmacol. 18:pyu118. doi: 10.1093/ijnp/pyu118
Thuné, H., Recasens, M., and Uhlhaas, P. J. (2016). The 40-Hz auditory steady-state response in patients with schizophrenia: a meta-analysis. JAMA Psychiatr. 73, 1145–1153. doi: 10.1001/jamapsychiatry.2016.2619
Thut, G., Schyns, P. G., and Gross, J. (2011). Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front. Psychol. 2:170. doi: 10.3389/fpsyg.2011.00170
Tobimatsu, S., Zhang, Y. M., and Kato, M. (1999). Steady-state vibration somatosensory evoked potentials: physiological characteristics and tuning function. Clin. Neurophysiol. 110, 1953–1958. doi: 10.1016/S1388-2457(99)00146-7
Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 169, 467–473. doi: 10.7326/M18-0850
Tseng, P., Iu, K.-C., and Juan, C.-H. (2018). The critical role of phase difference in theta oscillation between bilateral parietal cortices for visuospatial working memory. Sci. Rep. 8:349. doi: 10.1038/s41598-017-18449-w
Uhlhaas, P. J., Haenschel, C., Nikolić, D., and Singer, W. (2008). The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 34, 927–943. doi: 10.1093/schbul/sbn062
Van Eeckhoutte, M., Luke, R., Wouters, J., and Francart, T. (2018). Stability of auditory steady state responses over time. Ear Hear. 39, 260–268. doi: 10.1097/AUD.0000000000000483
Veniero, D., Benwell, C. S. Y., Ahrens, M. M., and Thut, G. (2017). Inconsistent effects of parietal α-tACS on Pseudoneglect across two experiments: a failed internal replication. Front. Psychol. 8:952. doi: 10.3389/fpsyg.2017.00952
Vialatte, F.-B., Maurice, M., Dauwels, J., and Cichocki, A. (2010). Steady-state visually evoked potentials: focus on essential paradigms and future perspectives. Prog. Neurobiol. 90, 418–438. doi: 10.1016/j.pneurobio.2009.11.005
Wang, Y., Dong, G., Shi, L., Yang, T., Chen, R., Wang, H., et al. (2021). Depression of auditory cortex excitability by transcranial alternating current stimulation. Neurosci. Lett. 742:135559. doi: 10.1016/j.neulet.2020.135559
Wang, Y., Zhang, Y., Hou, P., Dong, G., Shi, L., Li, W., et al. (2023). Excitability changes induced in the human auditory cortex by transcranial alternating current stimulation. Neurosci. Lett. 792:136960. doi: 10.1016/j.neulet.2022.136960
Welle, C. G., and Contreras, D. (2016). Sensory-driven and spontaneous gamma oscillations engage distinct cortical circuitry. J. Neurophysiol. 115, 1821–1835. doi: 10.1152/jn.00137.2015
Wischnewski, M., Alekseichuk, I., and Opitz, A. (2023). Neurocognitive, physiological, and biophysical effects of transcranial alternating current stimulation. Trends Cogn. Sci. 27, 189–205. doi: 10.1016/j.tics.2022.11.013
Yamasaki, T. (2021). Use of VEPs as electrodiagnostic biomarkers of mild cognitive impairment. Neurol. Clin. Neurosci. 9, 3–9. doi: 10.1111/ncn3.12387
Zaehle, T., Lenz, D., Ohl, F. W., and Herrmann, C. S. (2010). Resonance phenomena in the human auditory cortex: individual resonance frequencies of the cerebral cortex determine electrophysiological responses. Exp. Brain Res. 203, 629–635. doi: 10.1007/s00221-010-2265-8
Keywords: steady state response, auditory steady state response, steady state visual evoked potential, transcranial alternating current stimulation, non-invasive brain stimulation
Citation: Mockevičius A, Bjekić J and Griškova-Bulanova I (2025) The modulation of steady-state responses by transcranial alternating current stimulation: a scoping review. Front. Syst. Neurosci. 19:1661128. doi: 10.3389/fnsys.2025.1661128
Edited by:
Michael Okun, University of Nottingham, United KingdomReviewed by:
Desmond Agboada, Universität der Bundeswehr München, GermanyMarina Fiene, University Medical Center Hamburg-Eppendorf, Germany
Copyright © 2025 Mockevičius, Bjekić and Griškova-Bulanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Inga Griškova-Bulanova, aW5nYS5ncmlza292YS1idWxhbm92YUBnZi52dS5sdA==
 Aurimas Mockevičius
Aurimas Mockevičius Jovana Bjekić
Jovana Bjekić Inga Griškova-Bulanova
Inga Griškova-Bulanova