- 1Manchester Skull Base Unit, Salford Royal Hospital, Manchester, United Kingdom
- 2Department of Otolaryngology, University of Pittsburgh, Pittsburgh, PA, United States
Immune-mediated inner ear disease (IMIED) is emerging in our understanding as a cause of sensorineural hearing loss (SNHL). It poses a considerable diagnostic challenge due to the lack of specific tests and diagnostic biomarkers as well as clinical features that overlap with other audiovestibular disorders. Cases may involve isolated inner ear dysfunction or occur in the context of systemic autoimmune diseases. IMIED pathogenesis involves the interplay of autoantibodies, cytotoxic T cells, and innate immune mechanisms. Corticosteroid responsiveness is a defining feature of IMIED, but refractory cases may require alternative immunosuppressive agents. Although emerging immunosuppressive regimens demonstrate potential efficacy, larger trials are warranted to establish diagnostic and therapeutic guidelines. While symptomatic treatments including hearing aids and cochlear implants are beneficial in patients with IMIED associated hearing loss, future strategies focus on preventing irreversible inner ear damage and preserving hearing by developing innovative immunomodulatory strategies.
1 Introduction
Traditionally, the pathological mechanisms underpinning many audiovestibular conditions have remained largely unknown and diagnosis has been made purely on symptomatic features. However, serological biomarkers have linked some of these symptoms to systemic or localized autoimmune conditions. Research has also shed light on the role the inner ear immune system plays in maintaining homeostasis and response to injury and pathogens (Keithley, 2022). Interestingly, while the normal immune response in the inner ear can sometimes cause damage, there are cases where individuals experience either an exaggerated immune response, that can cause more damage compared to their normal peers, or have an abnormal immune response and suffer damage in situations where their normal peers would not. The latter category includes patients that have immune systems that fail to recognize self-antigens resulting in autoimmunity. The field is now being redefined as a spectrum of immune mediated inner ear disease (IMIED) that encompasses a normal response, to an abnormal response to an autoimmune response. In this review we discuss the implications for diagnosis and management of the abnormal and autoimmune response in the inner ear.
2 Definition
Autoimmune inner ear disease (AIED) is classically defined based on a presentation of progressive or fluctuating bilateral hearing loss with or without vestibular dysfunction secondary to an uncontrolled immune system response, typified by the loss of immunological tolerance to self-antigens (Ciorba et al., 2018). This classical definition is useful in alerting the treating clinician as to when an autoimmune cause should be suspected but relies on identifying the uncontrolled immune response in terms of positive serum biomarkers. Given the lack of validated biomarkers for AIED coupled with the difficulties of inner ear tissue sampling, the diagnosis is restricted to a limited population. It ignores the practicality that many patients can potentially benefit from treatment with immunomodulatory agents. With new knowledge of the immune function in the inner ear it is now time to redefine and expand the definition of AIED to encompass IMIED.
IMIED is sensorineural hearing loss or peripheral vestibular dysfunction, where there is an aberrant response by a component of the patient's own immune system resulting in inner ear dysfunction. It is important to note that this definition does not include the many inner ear conditions that have an immune basis for their pathogenesis but rather those patients that have abnormalities in their immune system that cause them to have disease when other “normal” patients do not. Diseases like vestibular schwannoma, chronic suppurative otitis media and noise induced hearing loss have immune responses that contribute to inner ear dysfunction; however, this is likely the normal response to insult (Sagers et al., 2019; Sai et al., 2022; Xia et al., 2022).
IMIED encompasses conditions that we are now recognizing as occurring in the ear, where overactive immune cells are causing injury through classical autoimmune mechanisms but additionally also cause disease through mechanisms outside the typical loss of self-tolerance and the failure to distinguish between self and non-self-antigens. Accordingly, we now know that immune disease can present in the ear as non-progressive and non-fluctuating symptoms which is outside the traditional clinical definition of AIED. Patients presenting only with inner ear disease are typically classified as primary AIED, whereas those where inner ear disease that occurs in the context of systemic autoimmune disease are classified as secondary AIED (Aftab et al., 2010). This again is an oversimplification that suggests either only the ear or the whole body is affected by the autoimmune condition, when in reality, primary AIED patients likely have manifestations that may have gone undetected or have led to generalized conditions that have not been recognized as autoimmune. Examples include skin rashes, arthritis, diabetes, and vascular disease. For the purposes of this review, many of the discussion points will change between AEID and IMIED reflecting the blurred lines between these old and new definitions. The novel IMIED definition presented here represents a more pragmatic definition in that it suggests whether an inner ear condition should be treated with immunomodulators or not.
3 Historical perspective
The field of immunology has its origins in the late nineteenth century with the discovery by Metchnikff of phagocytic cells, that later became known to have a central role in the innate immune system. Subsequently, Behrig and Ehrlich's discovery of antibodies established the basis for adaptive immunity. The concept of autoimmunity emerged in 1938 following Dameshak and Schwartz's work on hemolytic anemia (Ahsan, 2023).
In 1958, Lehnhardt was the first to propose that anti-cochlear antibodies could explain bilateral hearing loss in patients with delayed hearing loss in the contralateral ear (Lehnhardt, 1958). Later, in 1979, McCabe documented a series of 18 patients with progressive bilateral hearing loss. After ruling out infectious and neoplastic causes, hearing improvements were demonstrated with an immunomodulatory treatment using corticosteroids and cyclophosphamide (McCabe, 1979).
The inner ear was once thought to be an “immune-privileged” site largely due to the tight junctions of the stria vascularis that form the blood-labyrinth barrier (BLB) (Harris, 1984, 1983). It was suggested that if this barrier was compromised and inner ear antigens were released, the immune system would treat them as foreign (Harris et al., 1985). However, later research revealed that the inner ear can actively process antigens, release cytokines, and recruit immune cells from the systemic circulation (Harris et al., 1997).
Harris and Sharp further demonstrated antibodies against inner ear antigens in the serum of patients with progressive sensorineural hearing loss suggesting that these antibodies may be mechanistically linked to hearing loss (Harris and Sharp, 1990). This was followed by confirmation that the presence of a circulating antibody to a 68 KDa component of a bovine inner ear extract correlates with progression of hearing loss and response to corticosteroid in patients with idiopathic, progressive, bilateral sensorineural hearing loss. The 68 kDa protein was subsequently identified as heat shock protein 70 (HSP-70), which is widely expressed in human tissues, including the inner ear, further supporting a pathogenic role for these antibodies in hearing loss (Moscicki et al., 1994). These seminal studies established AIED, and the broader concept of IMIED, as a potentially treatable cause of sensorineural hearing loss that could be defined by both functional and potentially biochemical biomarkers.
4 Epidemiology
Estimating the true prevalence of IMIED is challenging due to the absence of definitive diagnostic tests or imaging and its clinical presentation that overlaps with other audiovestibular disorders. Currently, the prevalence of AIED, based on its classical definition, is estimated at 15 cases per 100,000 people, translating to ~45,000 cases annually in the United States. AIED is reported to be more common in women between in their third to sixth decade (Ciorba et al., 2018). This estimate is based on the incidence of AIED being significantly lower than the rate of sudden SNHL, that occurs at a rate of 5–20 cases per 100,000 per year (George and Pradhan, 2009). The progression from initial symptoms to treatment-resistant disease, requiring rehabilitative strategies such as hearing aids, typically takes about 3 years. With a U.S. population of 300 million, the point prevalence of AIED is estimated at around 45,000 individuals (Vambutas and Pathak, 2016). However, the actual incidence and prevalence of IMIED, if redefined as proposed here, are likely much higher. This discrepancy highlights the need for improved diagnostics and broader recognition of IMIED within the Otolaryngology and Primary Care communities.
In the United States, it is estimated that 15–20 million people have immune-mediated diseases, and individuals with IMIED are likely a significant, underrecognized subset of this group (Roberts and Erdei, 2020). The actual prevalence may be even higher, as many cases of progressive hearing loss or vestibular dysfunction currently labeled as idiopathic or hereditary may have an immune-related origin. IMIED can therefore be categorized into two groups: secondary cases, where a systemic immune disease or specific trigger is documented, and primary cases, where no systemic immune condition, symptoms, or clear trigger is identified. Most patients fall into the primary category, which aligns with the classical definition of AIED. This underscores the substantial work needed to better understand and address these conditions (Harris and Weisman, 2007).
5 The inner ear immune system, autoimmunity and immune mediated injury
Autoimmunity refers to the immune system's coordinated response against the body's own healthy cells and tissues. While self-reactive antibodies and T cells are naturally present in all healthy individuals, autoimmune disease occurs when this self-recognition becomes pathological and leads to immune attacks against the body's own tissues. This happens due to a breakdown in immunological tolerance, where self-antigens are no longer ignored by the immune system. The causes of autoimmunity are complex and involve a combination of genetic, environmental, and hormonal factors. Autoimmune diseases vary widely in severity, ranging from asymptomatic biochemical abnormalities to severe organ failure, and can be categorized as either organ-specific or systemic (Pisetsky, 2023).
At its core, autoimmunity arises from an overactive immune system, triggered by the loss of self-tolerance, leading to cellular damage in ways that would not occur without the underlying trigger. Advances in whole genome sequencing and genome-wide association studies (GWAS) are shedding light on the genetic factors contributing to these diseases (Hocking and Buckner, 2022). For instance, a gain-of-function mutation in TLR7 has been linked to increased activation of TLR7 and B cells, resulting in childhood-onset systemic lupus erythematosus (SLE). However, many common autoimmune diseases are polygenic, involving multiple genetic factors (Brown et al., 2022; Grimbacher et al., 2016).
The inner ear is isolated from the systemic circulation by the BLB, which is characterized by non-fenestrated capillaries with tight junctions. This anatomical barrier ensures the separation of cochlear and vestibular tissues and fluids from the rest of the circulatory system (Nyberg et al., 2019). However, the inner ear interacts with the systemic immune system via the circulation and lymph to respond to homeostatic perturbations, including noise trauma and infection (Keithley, 2022).
The immune system of the inner ear comprises both innate and adaptive components. Following noise-induced trauma or middle ear infections, studies have shown that elements of these systems, including T cells, B cells, natural killer (NK) cells, macrophages, and neutrophils, are actively recruited to the cochlea (Rai et al., 2020). A key aspect of the inner ear's innate immunity is the presence of resident macrophages, which originate from the yolk sac and fetal liver. These macrophages maintain their population through self-renewal and the infiltration of circulating monocytes (Hough et al., 2022; Kishimoto et al., 2019; Miwa and Okano, 2022; Hu et al., 2018; Francis and Cunningham, 2017). These inner ear macrophages include the “cochlear macrophages” in the spiral ligament and the “perivascular macrophage-like melanocytes” in the stria vascularis (Zhang et al., 2012). The stria vascularis therefore acts as important interface between inner ear structures and the immune system and is also an important site of cytokine signaling within the inner ear (Samaha et al., 2021).
Cochlear macrophages have diverse roles including phagocytosis, antigen presentation and cytokine secretion (Liu and Xu, 2024). Similar to macrophages in other organs, cochlear macrophages can be broadly classified into M1 and M2 subtypes. M1-polarized macrophages are pro-inflammatory, secreting interleukin-12 (IL-12) and tumor necrosis factor-alpha (TNF-α) to promote inflammation and recruit additional immune cells. In contrast, M2-polarized macrophages secrete interleukin-4 (IL-4) and interleukin-10 (IL-10) and are primarily involved in wound healing and tissue repair (Miwa and Okano, 2022). The M1/M2 dichotomy is however likely to be an over simplification, and it has been demonstrated that cochlear macrophages are predominantly multi-marker M1/M2 mixed lineage macrophages (Bedeir et al., 2022). It is known that inhibition of pro-inflammatory M1 macrophages or induction of M2 macrophages results in attenuated experimentally induced inflammatory bowel disease (Zhu et al., 2016). It is thus speculated that similar class switching may be an important mechanism of IMIED (Miwa and Okano, 2022). There is evidence that cochlear trauma activates resident cochlear macrophages, switching them to a pro-inflammatory M1 state and in turn stimulating the recruitment other immune cells into the cochlea (Frye et al., 2017). A major component of the inner ear immune response, for example to noise trauma, is the secretion of tumor necrosis factor (TNF-α), interleukin 1β (IL-1β) and IL-6 thought to be by cochlear macrophages (Landegger et al., 2019). There is therefore increasing evidence that macrophages are important effectors of cochlear damage via their pro-inflammatory and fibrogenic functions (Navegantes et al., 2017).
Evidence suggests that resident cochlear macrophages also have a neuroprotective role, migrating to the synaptic region of inner hair cells in response to noise-induced synaptopathy. These macrophages are both essential and sufficient for restoring synaptic function following noise exposure (Manickam et al., 2023). It is therefore likely cochlear macrophages play multiple context dependent roles within the cochlea and can ultimately be both protective and detrimental to cochlear function.
Neutrophils play a critical role in the immune response to chronic suppurative otitis media (CSOM) (Khomtchouk et al., 2021). Two-photon intravital microscopy has shown that, following intracochlear lipopolysaccharide inoculation in mice, neutrophils are recruited to the cochlea via ICAM-1 expression in the spiral ligament (Bae et al., 2021). However, despite the well-established link between CSOM and sensorineural hearing loss (SNHL), it is macrophages rather than neutrophils that mediate inner ear damage. In a validated Pseudomonas aeruginosa CSOM mouse model, macrophage infiltration of the cochlea was found to correlate with outer hair cell loss. This suggests that monocyte-to-macrophage differentiation within the cochlea is a key driver of immune-mediated damage (Xia et al., 2022).
Lymphocytes have also been detected in surgically obtained human cochleae, and evidence of T-lymphocyte/macrophage interactions supports the hypothesis that antigen-presenting activity occurs within the inner ear (Liu and Rask-Andersen, 2019). In mouse models, lymphocyte populations increase 4–7 days after noise exposure, neutrophils peak at 1 day, and myeloid cells reach their maximum levels after 14 days (Rai et al., 2020). It has also been proposed that interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) upregulate ICAM-1 expression on the spiral modiolar vein, facilitating the recruitment of systemic immune cells into the inner ear (Suzuki and Harris, 1995).
The endolymphatic sac (ES) plays a dual role in maintaining inner ear fluid homeostasis and contributing to innate immunity through the expression of genes such as TLR4, TLR7, beta-defensin, and lactoferrin. Research indicates that the ES serves as the primary antigen-processing site of the inner ear and acts as its primary immunological defense organ (Kämpfe Nordström et al., 2019). The ES also houses various immune cells, including macrophages, monocytes, and lymphocytes, specifically T cells and B cells containing IgG, IgM, and IgA (Keithley, 2022). However to date, the precise spatiotemporal coordination of the inner ear's immune response remains incompletely understood.
5.1 Pathophysiology of IMIED
The pathophysiology of IMIED remains poorly understood due to the lack of histological samples from living patients. Much of the current knowledge is derived from animal models, including genetically modified mice, rats, and guinea pigs (Goodall and Siddiq, 2015). IMIED can generally be classified as either cell-mediated, involving mature T cells, macrophages, and neutrophils, or humoral, driven by antigen-specific antibodies produced by B cells.
Cell-mediated IMIED has been modeled in rats by inducing labyrinthitis through the transfer of autoreactive T cells, which led to inflammation in naïve recipients (Gloddek et al., 1997). Additional studies using radiolabeled lymphocytes demonstrated their migration to the inner ear in response to antigenic stimuli mediated by ICAM-1 expression (Gloddek et al., 1991). Humoral mechanisms have been explored by sensitizing guinea pigs with Keyhole Limpet Hemocyanin (KLH), resulting in anti-KLH antibodies that correlated with hair cell and supporting cell damage in the inner ear (Harris, 1983). It is therefore evident that both cell mediated and humoral mechanisms are likely important in the pathophysiology of IMIED. However, although these models are useful as mechanistic descriptions, they were unable to be used to design therapeutic interventions, as a similar efficacy of immunomodulation, by protecting against hearing loss with etanercept treatment, was not achieved in humans with AIED (Wang et al., 2018; Cohen et al., 2005).
Like other autoimmune conditions, AIED occurs in part as a result of a loss of self-tolerance with the development of proinflammatory T cell and autoantibody production. Tolerance is defined as the prevention of an immune response against a defined antigen, for example self-antigens. Immune tolerance is maintained by a number of mechanisms including the elimination or suppression of autoreactive cells, regulatory immune cells and the maintenance of immune privileged sites such as the eye and brain (Sykes, 2007). Failures in these mechanisms can result in autoimmunity. Although thousands of cochlear proteins have been identified, including 4,435 in a quantitative proteomic study of the mouse cochlea (Miao et al., 2021), the specific inner ear antigens implicated in AIED remain largely unknown.
Some studies have suggested that IgG antibodies target inner ear proteins like cochlin, β-tectorin, and HSP-70 in patients with idiopathic sensorineural hearing loss (Tebo et al., 2006). This points to a potential Type II hypersensitivity reaction involving complement activation, antibody-dependent cellular cytotoxicity, or anti-receptor activity (Warrington et al., 2011). However, the pathogenic role of these autoantibodies, particularly HSP-70, remains unclear (Yeom et al., 2003).
Additional postulated mechanisms of inner ear cellular damage in IMIED include the direct activation of the complement system, action of cytotoxic T cells (type IV response) whereby T cells can cause cell lysis via the release of cytotoxins and additionally release proinflammatory cytokines TNF-α and IFN-γ leading to the recruitment and activation of macrophages and immune complex deposition (type III immune response) which cause a vasculitis of inner ear vessels resulting in cochlear and vestibular damage especially if the labyrinthine artery, the major blood supply of the inner ear, is affected. Ultimately, these processes lead to audiovestibular dysfunction via several mechanisms, including hair cell and supporting cell apoptosis, increased vascular permeability, loss of the blood labyrinthine barrier with potential subsequent exposure of the inner ear to environmental toxins, and the disruption of electrochemical gradients by damage to the stria vascularis (Goodall and Siddiq, 2015). Histological findings from mouse models and human temporal bones with AIED have revealed cochlear vasculitis, otospongiosis, endolymphatic hydrops, and degeneration of the Organ of Corti and spiral ganglion neurons (Schwartz et al., 1992; Kamakura et al., 2017).
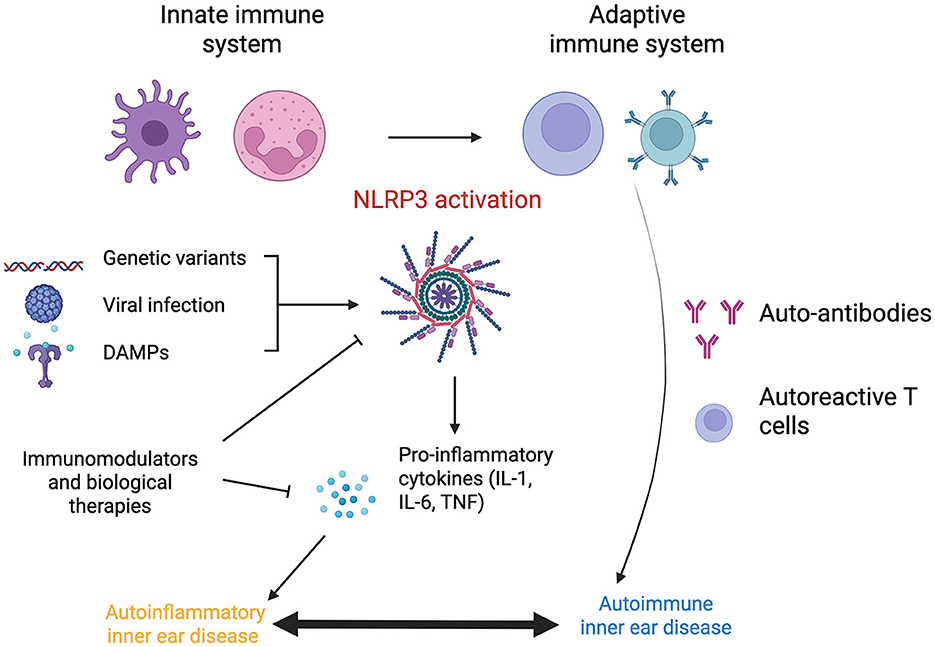
The triggers for autoimmunity in IMIED remain speculative but may involve molecular mimicry from viral or bacterial infections and exposure to pathogen-associated molecular patterns (PAMPs), including endotoxin or lipopolysaccharide, activate both innate and adaptive immune responses (Cusick et al., 2012; Gauthier et al., 2022) (Figure 1).
Genetic predispositions also play a role in IMIED. Variants in immune-related genes have been linked to autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus, suggesting similar mechanisms may underlie IMIED (Lenz et al., 2015). Specific HLA haplotypes, including HLA-B27, B35, and A1-B8-DR3, have been associated with AIED (Psillas et al., 2021, 2022). Environmental factors, such as female sex hormones, smoking, microbiome dysbiosis, and vitamin deficiencies, may also modulate susceptibility to IMIED (Nusbaum et al., 2020; Pisetsky, 2023). However, these triggers remain challenging to define given the diversity of potential insults the inner ear encounters over a lifetime (Touil et al., 2023).
5.2 Types of immune mediated inner ear diseases
AIED, characterized by a loss of self-tolerance, can present as an isolated condition in ~70% of cases, where the inner ear is exclusively affected, or as part of a systemic autoimmune disorder (Table 1). While some pathophysiological mechanisms of AIED have been elucidated, the underlying etiology of primary inner ear immune conditions remains largely unknown, apart from their association with the described HLA haplotypes. It is plausible that other, yet unidentified, genetic or epigenetic factors contribute to the development of AIED. The application of next-generation sequencing technologies offers hope for identifying these candidates in the future (Wajda et al., 2021).
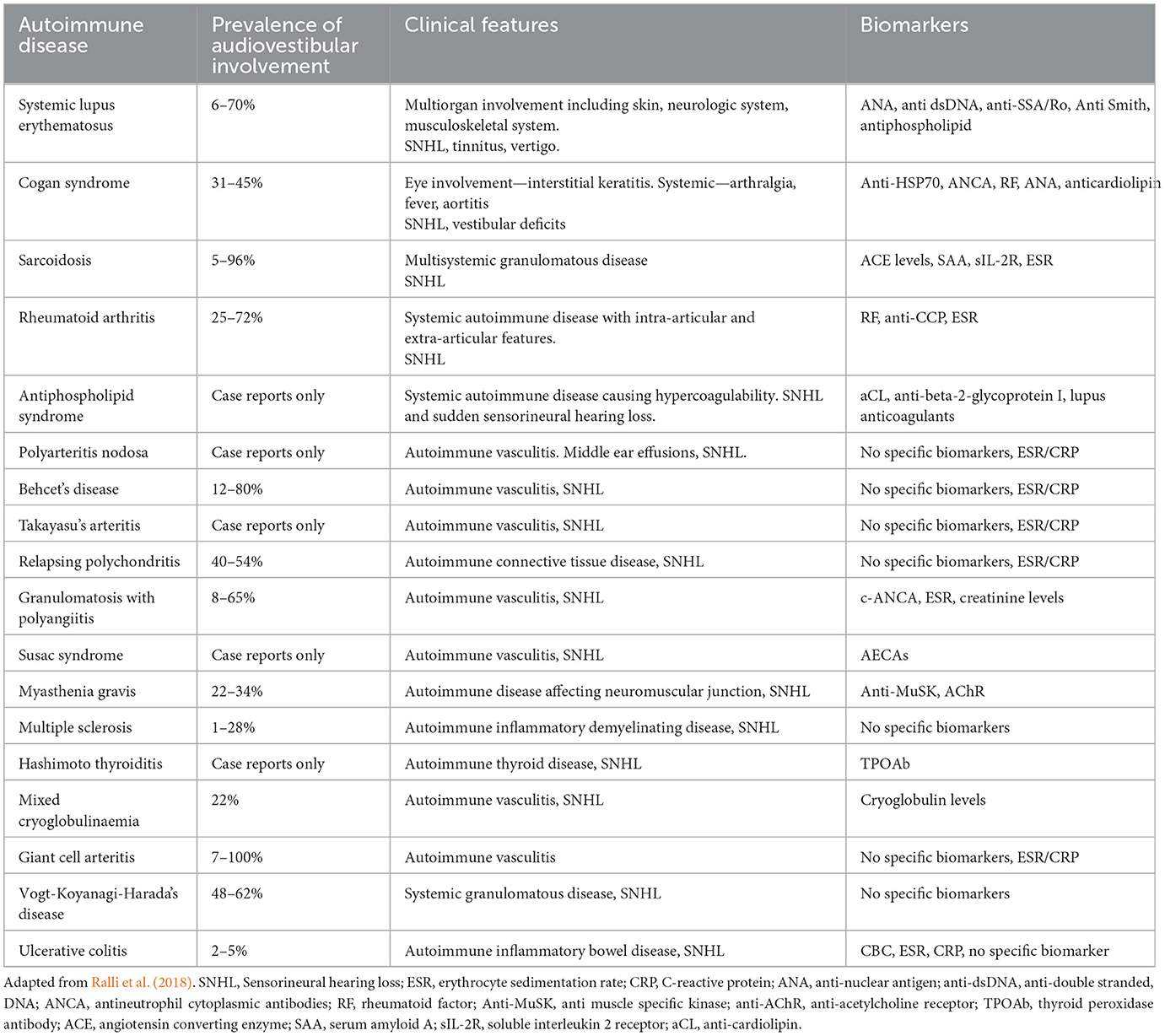
Table 1. Summary of systemic autoimmune diseases that have been reported to be associated with audiovestibular dysfunction with the prevalence of audiovestibular symptoms and relevant biomarkers.
Emerging research indicates that IMIED, where self-tolerance is preserved but the immune system exhibits hyperactivity or a gain-of-function response, may also be implicated in inner ear pathology. The inner ear immune system reacts to disruptions in cochlear homeostasis, such as those caused by noise or infection, by initiating an immune response (Liu and Xu, 2024). It is likely that IMIED arises from environmental triggers, which may remain undefined, interacting with a genetically susceptible background. Conversely, in individuals with a non-permissive genetic background, the same environmental triggers might elicit a controlled immune response that does not harm the host.
5.2.1 Autoinflammatory hearing loss
An overactive or injurious immune-mediated inflammatory response has been identified as a potential common pathway for hearing loss associated with various conditions, including noise exposure, aging, tumors, chronic suppurative otitis media, and auto-inflammatory diseases (Sagers et al., 2019; Xia et al., 2022). Autoinflammatory hearing loss, characterized by dysregulation of the innate immune system, is driven by monocyte activation and does not require specific antibody or T cell responses (Nakanishi et al., 2020).
A central player in autoinflammatory diseases is the NLRP3 inflammasome, a key effector in cellular inflammation. The NLRP3 inflammasome consists of the cytosolic pattern recognition receptor NLR family pyrin domain containing 3, caspase-1, and an apoptosis-associated speck-like protein containing a CARD (ASC). It is increasingly recognized as a potential mechanism for hearing loss in various disease states (Gregory et al., 2023). Activation of the NLRP3 inflammasome in cochlear macrophages, triggered by a wide range of danger signals, has been shown to be a key driver in hearing loss associated with CSOM (Schiel et al., 2024).
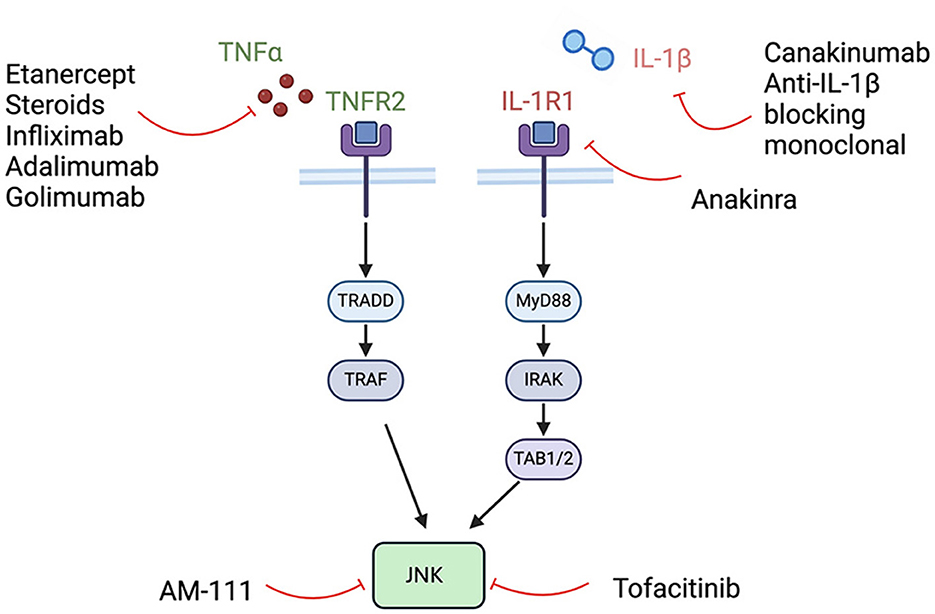
NLRP3 activation leads to the oligomerization of ASC and the activation of caspase-1. This process results in the maturation of the pro-inflammatory cytokines pro-IL-1β and pro-IL-18, converting them into their active forms. Caspase-1 also facilitates the formation of pores in the plasma membrane, enabling the secretion of IL-1β and IL-18, which can induce pyroptosis, a form of inflammatory cell death. The TNF and IL-1 pathways converge on c-Jun N-terminal kinase (JNK), a downstream effector that controls cellular functions, including survival and apoptosis (Dhanasekaran and Reddy, 2017) (Figures 2, 3).
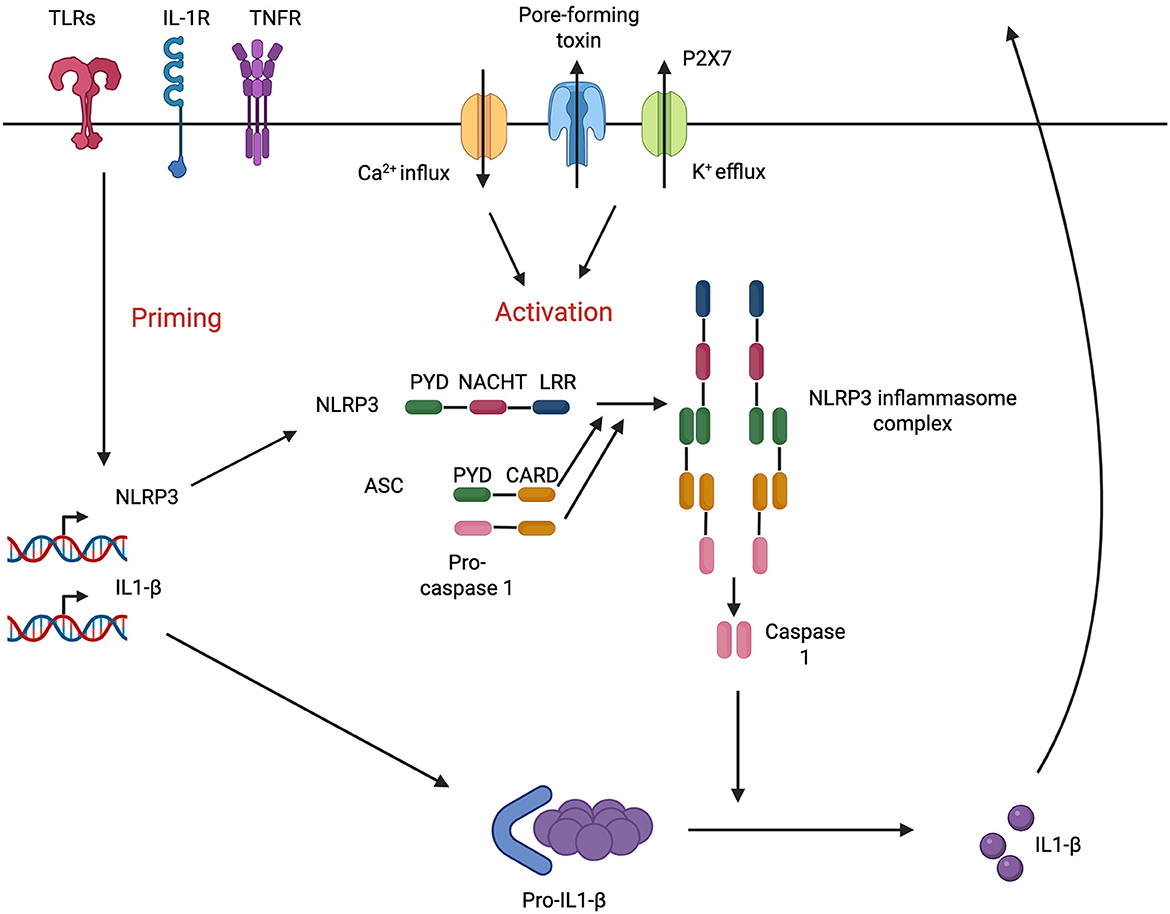
Figure 2. NLRP3 pathway in cochlear macrophages. Adapted from Nakanishi et al. (2017).
Mutations activating the NLRP3 inflammasome are known to cause a spectrum of autoinflammatory diseases called cryopyrin-associated periodic syndromes (CAPS), which include familial cold autoinflammatory syndrome, Muckle-Wells syndrome, and neonatal-onset multisystem inflammatory disease. These conditions are characterized by IL-1β mediated inflammation affecting the skin, joints, eyes, and central nervous system, often with hearing loss (Nakanishi et al., 2020). A mutation in NLRP3 has also been implicated in non-syndromic hearing loss, specifically DFNA34, with some evidence showing that hearing loss can be reversed or reduced with anakinra treatment (Nakanishi et al., 2017). In a recent case at our institution, a patient with bilateral fluctuating hearing loss and no formal systemic immune diagnosis was found to carry a variant in NOD2, a gene associated with Crohn's disease and Blau syndrome and a known inhibitor of the NLRP3 inflammasome (Mao et al., 2022). Subsequent treatment with anakinra resulted in stabilization of the patient's hearing.
Given the growing evidence of the NLRP3 pathway's role in inflammation and hearing loss, there is increasing clinical interest in developing NLRP3 inhibitors. These inhibitors are likely to play an increasingly important role in managing IMIED in the future (El-Sharkawy et al., 2020; Vande Walle and Lamkanfi, 2024).
6 Clinical presentation and diagnosis of IMIED
AIED has classically been described as presenting with bilateral sensorineural hearing loss and has been separated from sudden sensorineural hearing loss as developing more slowly, with an onset over 3 to 90 days (Bovo et al., 2009). However, this distinction is outdated, as both autoimmune conditions and IMIED can manifest as sudden hearing loss, while non-immune conditions may evolve over longer periods. IMIED should therefore be considered in patients with sudden, rapidly progressive, or fluctuating sensorineural hearing loss, particularly when there is a response to steroids or signs of immune involvement in other organ systems.
AIED often begins unilaterally and progresses to bilateral hearing loss in 80% of cases. The progression tends to be non-linear, with fluctuating hearing levels, though some patients may experience non-progressive hearing loss. Vestibular symptoms such as vertigo and disequilibrium are common, affecting ~50% of patients. These may be accompanied by horizontal-torsional nystagmus, reflecting asymmetric vestibular input characteristic of peripheral vestibulopathy. Early in the disease, vestibular signs may be intermittent, but over time, progressive involvement can lead to bilateral vestibular loss and the absence of nystagmus due to symmetric dysfunction (Mijovic et al., 2013). Additional otological symptoms include aural fullness and tinnitus. In the early stages of IMIED, when hearing fluctuations coincide with aural pressure and tinnitus, a differential diagnosis of Meniere's disease should be considered. It is possible that a subset of Meniere's patients have an underlying immune etiology. Studies have shown that some Meniere's patients have elevated levels of autoantibodies or circulating immune complexes against inner ear antigens, and epidemiological data suggest a higher prevalence of concurrent autoimmune conditions in this population (Lopez-Escamez et al., 2023; Kim et al., 2014).
Ultimately, it is crucial for clinicians to recognize the clinical features of IMIED, which should prompt further investigation of the immune system and the initiation of immunomodulatory therapies. We recommend considering any newly diagnosed sudden sensorineural hearing loss (SNHL) or vestibular dysfunction that improves with immunomodulation as a possible case of IMIED, with their future response to steroid treatment either confirming or excluding the diagnosis. While a personal or family history of autoimmune disease strengthens the diagnosis, it is not an absolute requirement for diagnosis.
6.1 Diagnosis and work up of IMIED
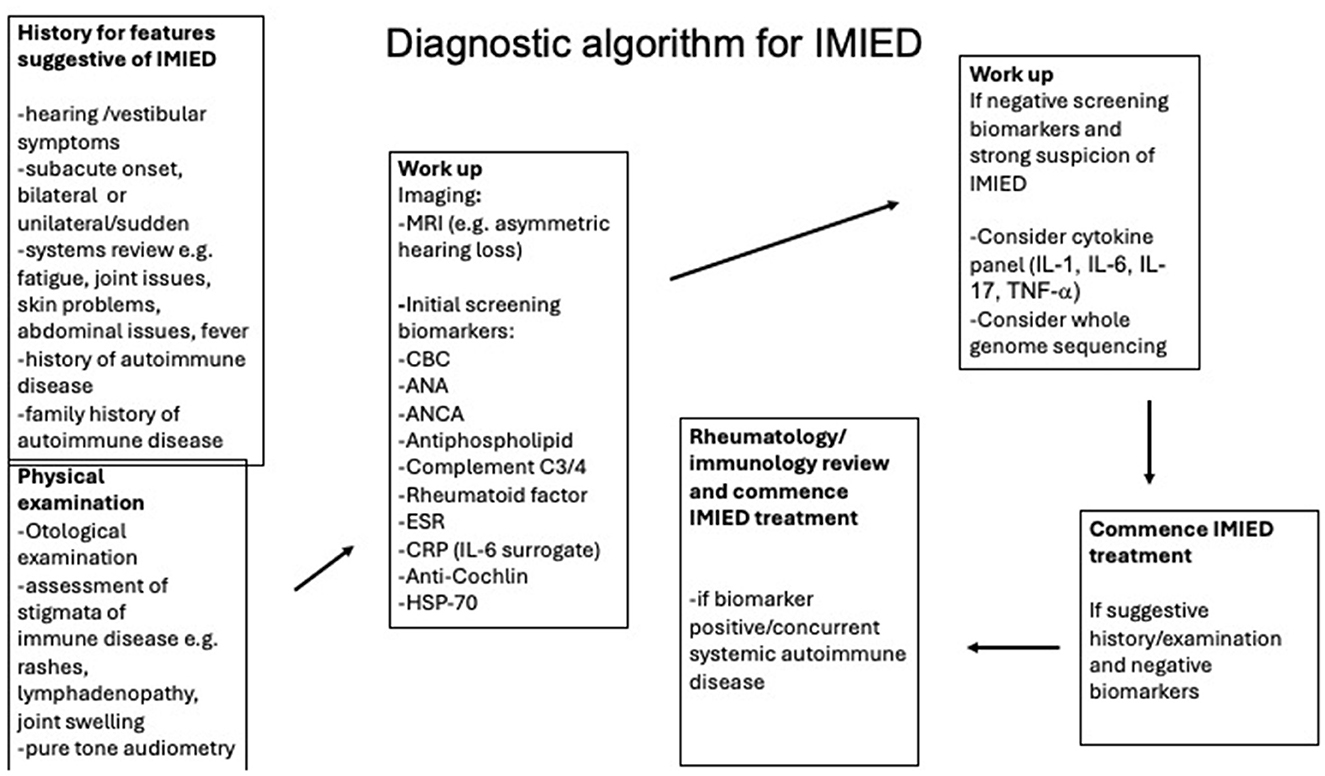
A proposed diagnostic algorithm for immune-mediated inner ear disease (IMIED) is shown in Figure 4, although it is important to note that no definitive biomarker, audiological test, or imaging modality currently exists for diagnosing IMIED. As a result, the diagnosis is based on the clinical history and the patient's response to immunosuppression. The classic definition of AIED involves idiopathic, progressive, bilateral sensorineural hearing loss of 30 dB or more at one or more frequencies (ranging from 250 to 8,000 Hz), with deterioration in at least one ear within the last 3 months. This deterioration is typically defined by a threshold shift of ≥15 dB at one frequency, 10 dB at two or more consecutive frequencies, or a significant change in speech discrimination scores (Moscicki et al., 1994). Additionally, a demonstrable improvement in hearing after systemic steroids is often considered a key diagnostic criterion (Matsuoka and Harris, 2013). However, if applied strictly, this legacy definition may lead to underdiagnosis and the failure to treat many patients who would benefit from immunomodulatory therapy.
Ultimately, IMIED is believed to have an underlying immune cause (which may not always be identifiable) and typically involves hearing loss or vestibular dysfunction that can be reversed or improved with immunomodulation in the short term. The pattern of hearing loss can vary significantly, with different tonal frequencies affected, and can involve high, mid, or low tones, or even lead to more significant speech discrimination issues. Tympanometry results are usually normal unless middle ear disease is present, which may be associated with systemic immune conditions.
In cases where central pathology is suspected, gadolinium-enhanced MRI may be used for exclusion. Vestibular testing can help identify underlying dysfunction, revealing potential abnormalities in caloric testing, video head impulse test (vHIT), and cervical vestibular evoked myogenic potentials (cVEMPs) (Mendis et al., 2022).
Our recommended standard workup for suspected IMIED includes serological testing, including:
• Full blood count with differential
• Antinuclear antibody (ANA)
• Antineutrophil cytoplasmic antibodies (ANCA)
• Antiphospholipids
• Rheumatoid factor (RF)
• Complement C3/C4
• Anti-cochlin, anti-HSP70 antibodies
• Erythrocyte sedimentation rate
However, the role of these biomarkers remains controversial due to their low diagnostic value for both inner ear and immune-related conditions. For example, the HSP70 antibody was initially thought to correlate with steroid responsiveness, but later studies revealed it was present in a wide range of disease states, including in healthy individuals (Tebo et al., 2006). Similarly, the creation of anti-HSP70 antibodies in mouse models did not lead to hearing loss (Trune et al., 1998), though anti-HSP70 antibodies might still be useful for diagnosing Cogan's syndrome (Bonaguri et al., 2014).
A cytokine panel may also be ordered to check for elevated IL-1, IL-6, IL-17, and TNF-α levels in the peripheral blood, although these cytokine profiles do not currently correlate with changes in the inner ear (Quan et al., 2021). The cytokine profile might, however, guide therapeutic decisions. For example, a patient in our clinic with fluctuating hearing loss was found to have low serum IL-2R levels, which prompted a trial of anti-TNFα therapy. Low IL-2R levels are considered a favorable biomarker for anti-TNFα response, as they indicate reduced T-cell activation and lower systemic immune activity (Kuuliala et al., 2006).
Other potential biomarkers under investigation include Cochlin (a protein associated with hereditary deafness DFNA9), anti-cochlin antibodies, myelin p0, β-tectorin, and COCH5B2 proteins. However, these markers have limited sensitivity and specificity, and none are used in routine clinical practice (Boulassel et al., 2001; Matsuoka and Harris, 2013). Similarly, tests like lymphocyte migration inhibition assays and lymphocyte transformation tests have undetermined diagnostic accuracy (Mijovic et al., 2013).
Given the greater understanding of genetic causes of autoimmunity and IMIED we now recommend the use of whole genome sequencing in selected patients, particularly in those with other features of autoimmune disease that is currently undiagnosed.
Due to the limitations of current diagnostic criteria and the lack of definitive biomarkers, we propose a new classification system that better reflects the diversity of clinical presentations in IMIED. This framework would help clinicians identify more patients as “possible IMIED” and then trial immunomodulatory treatments to further confirm the diagnosis. Although the presence of diagnostic biomarkers is not necessary for treatment, clinical criteria should guide the initiation of immunomodulatory therapies (Box 1).
Box 1. Proposed diagnostic criteria for Immune Mediated Inner Ear Disease (IMIED).
Certain IMIED – SNHL or peripheral vestibular dysfunction that reverses with immunomodulatory treatment and relapses with withdrawal of immunomodulatory treatment and reverses again with recommencement of immunomodulatory treatment.
Probable IMIED - SNHL or peripheral vestibular dysfunction that reverses with immunomodulatory treatment and relapses with withdrawal of immunomodulatory treatment.
Possible IMIED - SNHL or peripheral vestibular dysfunction that reverses with immunomodulatory treatment or fluctuating SNHL or peripheral vestibular dysfunction or SNHL or peripheral vestibular dysfunction in the presence of a detected immune marker.
7 Treatment approaches
The management of IMIED requires a multidisciplinary approach, bringing together specialists from various fields, including otologists, audiologists, immunologists, and potentially rheumatologists and dermatologists. This collaboration is crucial as 15–30% of IMIED patients may present with concurrent systemic immune features or develop these features over time (Mijovic et al., 2013). The involvement of rheumatology and immunology is particularly important to ensure appropriate clinical management, prescribing, and ongoing workup for immunomodulatory therapies.
From the neurotologist's perspective, the primary focus of management revolves around addressing hearing and vestibular impairments. However, many patients with IMIED also experience tinnitus, which can significantly impact their quality of life. Tinnitus management in IMIED often occurs alongside efforts to improve hearing function and includes traditional tinnitus management approaches. For concomitant vestibulopathy, treatment follows a similar multidisciplinary approach, aligning with how other vestibulopathies are managed in neurotology (Mendis et al., 2022). The collaborative care model ensures that patients receive comprehensive and coordinated treatment, optimizing both their hearing and balance while addressing the underlying immune dysregulation that causes the disease.
7.1 Pharmacological interventions
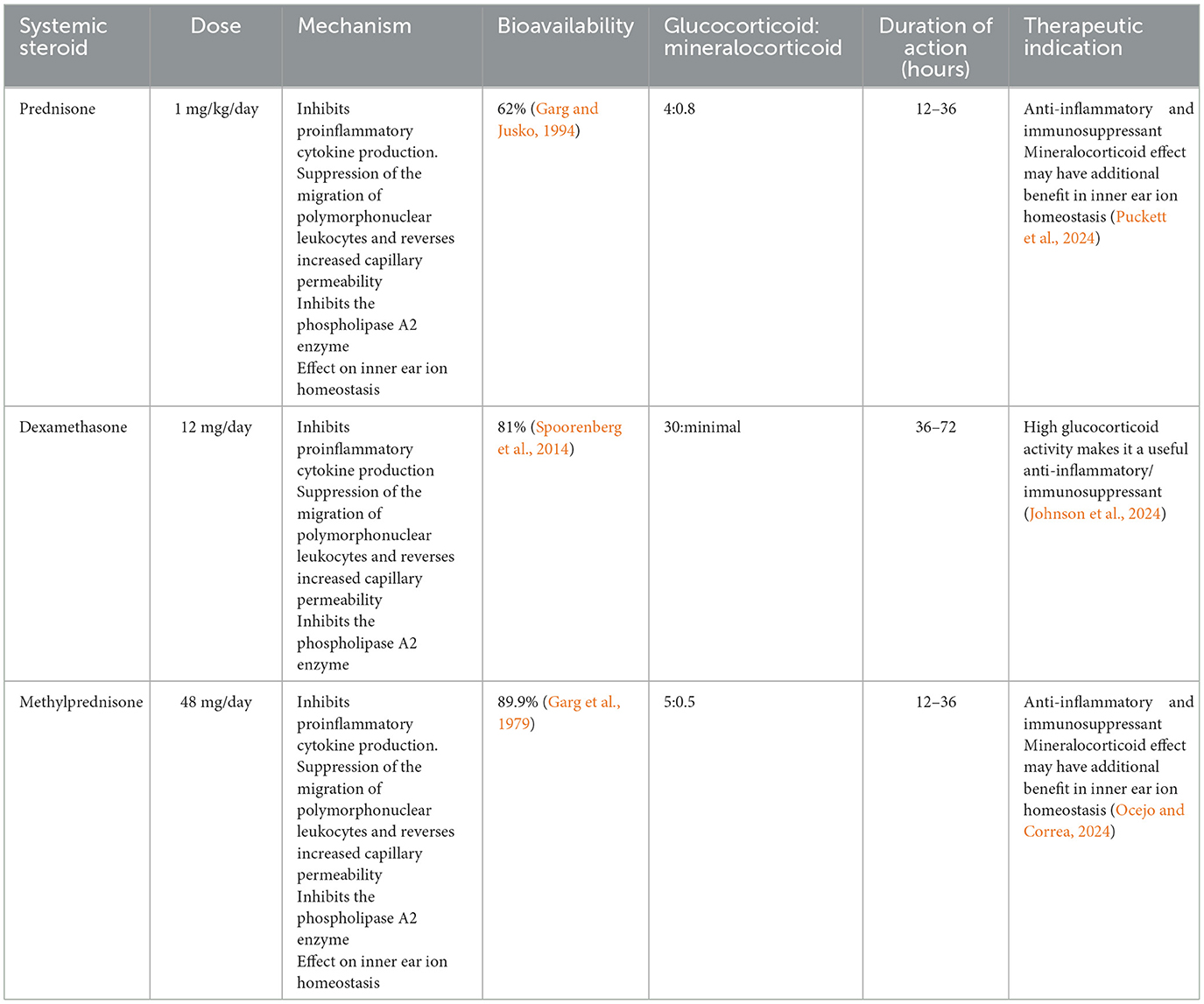
Corticosteroids are the first-line treatment for IMIED, primarily due to their effectiveness, affordability, and wide availability. They have an initial response rate of 50–70% in IMIED cases (Sakano and Harris, 2018). The action of corticosteroids is believed to be mediated via glucocorticoid receptors in the inner ear, which leads to downregulation of cytokines, inflammation, and antibody production (Breslin et al., 2020). Steroids with mineralocorticoid effects (e.g., prednisone and methylprednisolone) may also help by improving ion homeostasis in the inner ear, offering additional benefits in steroid-responsive hearing loss (Trune et al., 2006) (Tables 2, 3).
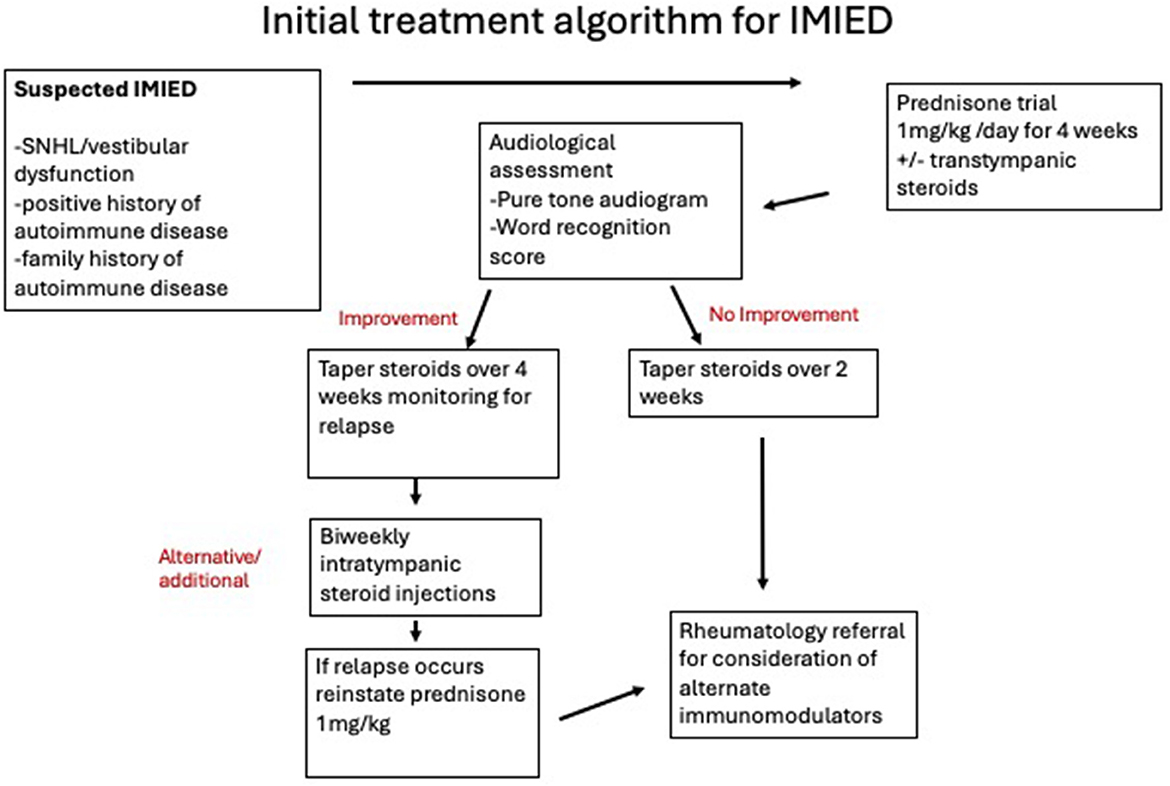
7.1.1 Steroid protocol
A typical initial steroid regimen involves a trial of 1 mg/kg (up to 60 mg) of prednisone for 4 weeks. If no response is observed, the steroid is tapered over 2 weeks. If a response is noted, the dose is tapered over 4 weeks, carefully monitoring for relapse. In the event of relapse, the dose is reinstated to 60 mg and the patient is reviewed by an immunologist for possible switch to another immunomodulatory agent (Alexander et al., 2009; Rauch, 1997). In some cases, patients may remain on low-dose maintenance steroids, potentially combined with other therapies or pulsed high-dose steroids for short-term relapses.
Despite their initial effectiveness, long-term steroid use raises concerns due to side effects such as mood changes, mania, depression, weight gain, insomnia, and a rare risk of hip necrosis (Liu et al., 2017). In a study of 116 patients on a 1-month course of prednisone, 6% had to discontinue due to adverse effects, with hyperglycemia being the most common. Chronic side effects include osteoporosis, diabetes, cardiovascular issues, gastrointestinal disturbances, immunosuppression, and psychiatric disturbances (Liu et al., 2017).
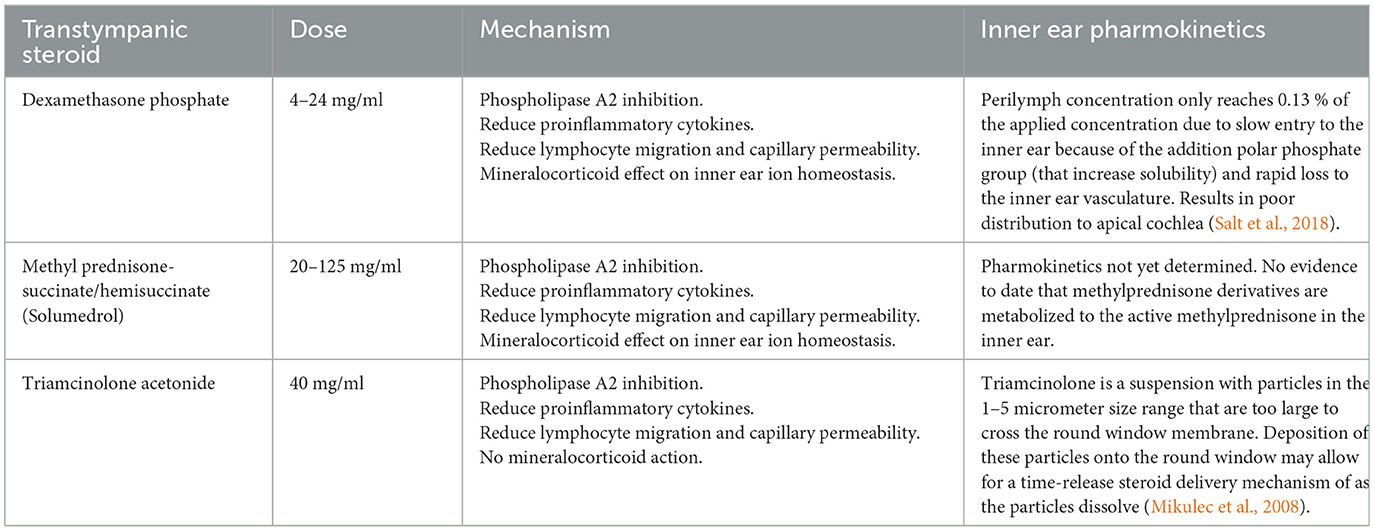
7.1.2 Transtympanic steroids
With an understanding of pharmacokinetics to the inner ear, we routinely co-prescribe transtympanic steroids with oral steroids. Overall, steroids delivered through the middle ear and via the round window lead to larger steroid concentration in the perilymph and cochlear tissue when compared with systemic administration where the blood labyrinthine barrier needs to be overcome (Wang et al., 2018; Yang et al., 2008). Transtympanic steroids enter through the round window and, as expected, demonstrate a significant base to apex gradient with a higher concentration at the base in perilymph and inner ear tissue (Creber et al., 2018). The basal regions of the cochlear are exposed to five hundred fold the steroid concentration compared to the apex, with the overall concentration being around two fold higher in the perilymph with transtympanic delivery (Salt and Hirose, 2018; Bird et al., 2007, 2011). In comparison oral steroids, demonstrate an apex to base gradient with higher concentrations at the apex (Creber et al., 2018). Interestingly, despite the large differences and gradients between transtympanic and systemic delivery, there is similar levels of glucocorticoid receptor activation across the regions of the inner ear (Creber et al., 2018). In short, the two modes of delivery should not be seen as alternatives, rather they should be seen as complementary. If the aim to maximize steroid concentration in the inner ear tissue for a more “complete cochlear coverage,” both routes of delivery should be used, until further research is performed. What is not known is whether the increased concentration of steroids in the inner ear is clinically significant over one route alone. Matsuoka et al. reported that 50% (15/30 AIED patients) demonstrated hearing improvement with intratympanic steroids, although it is uncertain from the report if these patients received other treatments (Matsuoka and Harris, 2013). García-Berrocal et al. (2006) demonstrated 6/11 patients with AIED treated with intratympanic methylprednisone showed improved hearing thresholds. Additionally, there have been small case series reporting that intratympanic steroids can be effective in the management of AIED induced by immune checkpoint inhibitors and adoptive cell immunotherapy for cancer (Zibelman et al., 2016; Johnson et al., 2009).
Commercially available steroid solutions of methylprednisolone, dexamethasome and triamcinolone are formulated with ~8.8 mg/ml benzyl alcohol which has been shown to increase round window penetration (Mikulec et al., 2008). There is controversy over which steroid is the best use for transtympanic delivery. Unfortunately, the majority of clinical studies in this area do not take advantage of the highest available doses. When considering steroid injection via the transtympanic route, factors such as available dosage, anti-inflammatory potency, and round window membrane permeability should be taken into consideration. Anecdotally, methylprednisolone may be uncomfortable to patients in the middle ear and has traditionally been diluted with sodium bicarbonate prior to injection (Dallan et al., 2006; Berjis et al., 2016). Dexamethasone is the most studied but likely the poorest of the options for round window penetration (Salt and Plontke, 2018). If selecting for the highest dose commercially available to deliver the highest anti-inflammatory effect with the best likely round window penetration then the choice should be triamcinolone at 24 mg/ml, followed by methylprednisolone at 80 mg/ml. Higher concentrations may also be available outside of the United States and change the decision making to favor a different choice so clinicians should be aware of the doses available and anti-inflammatory potency of the steroids available to them. Unfortunately, most of the comparison studies in this area are non-randomized or non-blinded and have failed to consistently show clinically significant differences (Emami et al., 2023). A proposed initial treatment algorithm is shown in Figure 5.
7.1.3 Cytotoxic chemotherapy and biologic agents
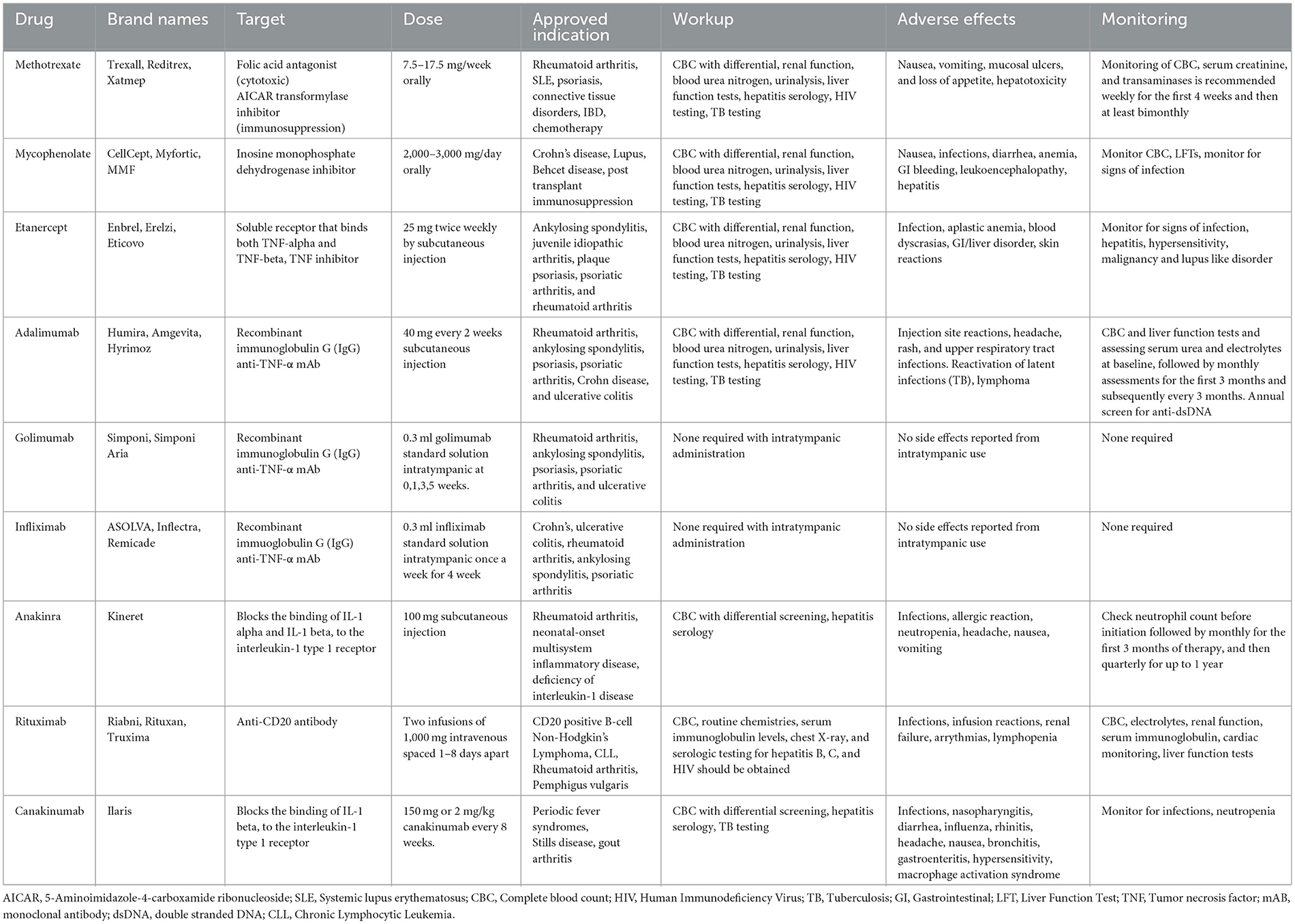
Beyond oral and intra-tympanic steroid treatments, cytotoxic chemotherapeutic agents (e.g., methotrexate, mycophenolate, and azathioprine) and biologics have been trialed in IMIED (Table 4, Figure 3).
Methotrexate, an antifolate antimetabolite and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) inhibitor, is a disease modifying treatment that is used in a number of autoimmune conditions. It works through several mechanisms, including TNF, IL-1 inhibition, IL-10 upregulation and modulation of NFkB and immune cell adenosine signaling (Friedman and Cronstein, 2019). A randomized control trial of 67 patients with AIED examined whether methotrexate could be used to maintain hearing improvement achieved with prednisone. However, methotrexate was not more effective than placebo in this regard (Harris et al., 2003). A combination of methotrexate and azathioprine demonstrated low and mid tone improvement in hearing in a patient with AIED (Huang et al., 2023). Mycophenolate is an immunosuppressant primarily via its inhibition of de novo purine synthesis in lymphocytes. Clinically it is used to prevent organ transplant rejection and autoimmune conditions including Crohn's disease, lupus, and small vessel vasculitides. Although not studied in larger trials, there have been reports of the successful use of mycophenolate as a steroid sparing agent in Cogan's syndrome (Hautefort et al., 2009).
Cytotoxic chemotherapeutic agents however carry significant systemic risks including increased risk of lymphoma, myelosuppression and teratogenicity (Katherine et al., 2023). There is therefore increasing interest in biologic agents including TNF-α inhibitors (etanercept, infliximab, adalimumab, and golimumab), CD20 inhibitors (rituximab) and IL-1 inhibitors (anakinra and canakinumab) (Figure 3). These classes of immunotherapies are likely to have the greatest impact in the near future for the treatment of IMIED.
TNF alpha is a cytokine that plays a critical role in cellular inflammation and the regulation of immune cells. Additionally, it acts an endogenous pyrogen that can induce fever, apoptosis and inhibit tumor growth. Aberrant TNF signaling has been implicated in several disease states including autoimmune conditions such as rheumatoid arthritis, ankylosing spondylitis and inflammatory bowel disease (Jang et al., 2021). TNF is not detected in the normal cochlea but can be upregulated by cellular stress, including aging and acoustic trauma and hence TNF alpha has been shown to play a role in SNHL, possibly by altering cochlear blood flow (Arpornchayanon et al., 2013). Intracochlear perfusion of KLH causes an influx of immune cells, alteration in cochlear blood flow and an increase in TNF alpha which was reversed on administration of etanercept, a TNF alpha antagonist (Satoh et al., 2002). Etanercept is a human fusion protein composed of the Fc portion of IgG1 and extracellular ligand-binding domain of the TNF p75 receptor. It is currently indicated for the treatment of autoimmune diseases including rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis (Jang et al., 2021). It is a common first biologic agent choice for AIED, and our preferred first option, given it is widely used, inexpensive and can be administered by injection 1-2 times per week without the requirement to monitor drug levels. Before starting therapy, Hepatitis B and Quantiferon testing are performed. There is an increased risk of infection, allergic reactions and induced psoriasis and patients should be cautioned that resistance may develop requiring treatment discontinuation. In the future it is hoped there will be biomarkers that may be used to predict response, as shown by the use of HLA Cw06 testing for the response to ustekinumab (Chiu et al., 2014). A retrospective case series of 12 AIED patients refractory to corticosteroids treated with etanercept twice weekly for 6 months demonstrated improvement or stabilization of hearing in 92% of patients (Rahman et al., 2001). However, a further cohort study contradicted these results, demonstrating that etanercept was no better than placebo for treating AIED hearing loss (Cohen et al., 2005). Cogan syndrome has been primarily treated with corticosteroids although more recently infliximab, an anti-TNF therapy, has demonstrated hearing improvement in 89% of treated patients (Padoan et al., 2019). Other anti-TNF alpha therapies that have been used with limited success in small AIED case series include Adalimumab and Golimumab (Balouch et al., 2022).
IL-1 is a family of 11 cytokines, including IL-1α and IL-1β, that plays a central role in the regulation of inflammatory and immune responses. Patients with IMIED who fail to respond to corticosteroids have been shown to have higher plasma levels of IL-1β than steroid-responsive patients. The mechanistic link between IL-1 and SNHL is uncertain, but IL-1β triggered immune cell infiltration has been suggested (Hashimoto et al., 2005). Additionally, IL-1β increases expression of metalloproteinase-9 in the cochlea, which may cause compromise of the blood labyrinth barrier and stria vascularis (Wu et al., 2017). IL-1β and IL-18 are downstream products of the NLRP3 inflammasome, discussed above, and so IL-1β inhibition has a promising role for macrophage mediated inner ear hearing loss (Xia et al., 2022). Anakinira, an IL-1 receptor antagonist, is currently approved for use in rheumatoid arthritis and neonatal multisystem inflammatory disease. It requires daily injection and may cause thrombocytopenia requiring a full blood count check 4 weeks after starting therapy. It is recommended to order a Hepatitis B panel before starting treatment. Although there is an increased risk of infections with Anakinra use, the half-life of the drug is short, so on treatment discontinuation there is a quick reconstitution of the immune system. In a phase I/II efficacy trial of 10 patients with corticosteroid resistant IMIED, 7 patients treated with anakinra demonstrated improvements in pure tone average and word recognition score with decreased levels of IL-1β correlating with hearing levels (Vambutas et al., 2014). Anakinra has also been used successfully to treat hearing loss in patients with Muckle-Wells syndrome. In a prospective cohort study with 10 patients treated with Anakinra, 5/20 ears showed improvement in hearing thresholds (Kuemmerle-Deschner et al., 2015). This study also demonstrated improvement in 6/26 ears in patients treated with Canakiumab, another IL-1 receptor antagonist.
Although promising, there are multiple aspects of biologic therapies for IMIED that warrant further exploration. These include the study of novel classes of use of biologics, including JNK inhibitors such as tofacitinib and AM-111, in addition to optimizing dosage and administration routes for current therapies (Hu et al., 2021; Balouch et al., 2022).
7.2 Hearing aids and cochlear implants
Hearing aids are the primary treatment for patients with unsalvageable hearing loss due to Immune-Mediated Inner Ear Disease (IMIED). They should be offered to adults whose hearing loss significantly impacts their ability to communicate, hear environmental sounds, or enjoy music. Studies have shown that AIED often leads to cochlear inflammation, which can progress to fibrosis and ossification (Hoistad et al., 1998). One study found that 53.3% of AIED patients had cochlear fibrosis or ossification (Kamakura et al., 2017). This structural damage can severely limit hearing recovery, making hearing aids an essential tool in managing these patients.
Given the risk of cochlear fibrosis, a T2-weighted MRI should be included in the work-up for cochlear implantation to assess the extent of damage and predict potential outcomes. Intracochlear fibrosis can reduce the effectiveness of cochlear implants (Hoistad et al., 1998). In addition, patients with AIED often have an increased risk of immunocompromise, which warrants careful consideration of the risk of meningitis and the need for pneumococcal vaccines prior to surgery.
During surgery, patients on chronic steroid therapy may experience secondary adrenal insufficiency, which could require a preinduction steroid dose to mitigate the risk of adrenal crisis (Goh et al., 2022). Cochlear implant outcomes for AIED patients are generally comparable to those of the general population; however, some patients have experienced progressive deterioration in hearing outcomes. This deterioration appears to correlate with fluctuations in cochlear impedances and the ongoing activity of the systemic disease (Goh et al., 2022). These findings suggest that synaptopathy or dysfunction in the spiral ganglion may play a role in the progressive hearing loss observed in these patients.
8 Conclusions
IMIED is an important, yet often underdiagnosed cause of medically treatable sensorineural hearing loss. Its diagnosis is challenging due to the absence of specific diagnostic tests, and it relies heavily on clinical presentation and disease course, which can overlap with other audiovestibular disorders. While most patients experience disease isolated to the inner ear, up to a third may present with hearing loss as part of a systemic autoimmune disease. A key feature of IMIED is the characteristic response to corticosteroids, which is a determining factor in our new classification. However, some patients may become refractory to steroids, requiring alternative immunosuppressive treatments.
Current evidence suggests that autoantibodies, cytotoxic T cells, or innate immune system mechanisms, or a combination of these, mediate IMIED. With a deeper understanding of the disease's pathological mechanisms, there is hope that the development of novel biomarkers, particularly serum markers that correlate with treatment response, will emerge and become clinically useful.
Although immunosuppressive treatments typically yield positive results for IMIED patients, large, multicenter randomized trials are needed to better evaluate the role of corticosteroids and emerging immunomodulatory drugs. It is critical to establish evidence-based diagnostic and therapeutic guidelines for the management of IMIED. While hearing aids and cochlear implants provide symptomatic relief for patients, the ultimate goal is that novel immunomodulatory treatments will prevent irreversible inner ear damage and preserve hearing in these patients.
Author contributions
PK: Writing – review & editing, Writing – original draft. PS: Funding acquisition, Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Carr Foundation.
Conflict of interest
PK and PS are topic editors for the Frontiers research topic “Innovating to Shape the Future of Audiology and Otology.” This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aftab, S., Semaan, M. T., Murray, G. S., and Megerian, C. A. (2010). Cochlear implantation outcomes in patients with autoimmune and immune-mediated inner ear disease. Otol Neurotol. 31, 1337–42. doi: 10.1097/MAO.0b013e3181f0c699
Ahsan, H. (2023). Origins and history of autoimmunity—A brief review. Rheumatol. Autoimmun. 3, 9–14. doi: 10.1002/rai2.12049
Alexander, T. H., Weisman, M. H., Derebery, J. M., Espeland, M. A., Gantz, B. J., Gulya, A. J., et al. (2009). Safety of high-dose corticosteroids for the treatment of autoimmune inner ear disease. Otol. Neurotol. 30, 443–448. doi: 10.1097/MAO.0b013e3181a52773
Arpornchayanon, W., Canis, M., Ihler, F., Settevendemie, C., and Strieth, S. (2013). TNF-α Inhibition using etanercept prevents noise-induced hearing loss by improvement of cochlear blood flow in vivo. Int. J. Audiol. 52, 545–52. doi: 10.3109/14992027.2013.790564
Bae, S. H., Yoo, J. E., Choe, Y. H., Kwak, S. H., Choi, J. Y., Jung, J., et al. (2021). Neutrophils infiltrate into the spiral ligament but not the stria vascularis in the cochlea during lipopolysaccharide-induced inflammation. Theranostics 11, 2522–2533. doi: 10.7150/thno.49121
Balouch, B., Meehan, R, Suresh, A, Zaheer, H. A., Jabir, A. R., Qatanani, A. M., et al. (2022). Use of Biologics for Treatment of Autoimmune Inner Ear Disease. Am. J. Otolaryngol. 43:103576. doi: 10.1016/j.amjoto.2022.103576
Bedeir, M. M., Ninoyu, Y., Nakamura, T., Tsujikawa, T., and Hirano, S. (2022). Multiplex immunohistochemistry reveals cochlear macrophage heterogeneity and local auditory nerve inflammation in cisplatin-induced hearing loss. Front. Neurol. 13:1015014. doi: 10.3389/fneur.2022.1015014
Berjis, N., Soheilipour, S., Musavi, A., and Hashemi, S. M. (2016). Intratympanic dexamethasone injection vs. methylprednisolone for the treatment of refractory sudden sensorineural hearing loss. Adv. Biomed. Res. 5:111. doi: 10.4103/2277-9175.184277
Bird, P. A., Begg, E. J., Zhang, M., Keast, A. T., Murray, D. P., and Balkany, T. J. (2007). Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol. Neurotol. 28, 1124–30. doi: 10.1097/MAO.0b013e31815aee21
Bird, P. A., Murray, D. P., Zhang, M., and Begg, E. J. (2011). Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to cochlear perilymph. Otol. Neurotol . 32, 933–36. doi: 10.1097/MAO.0b013e3182255933
Bonaguri, C., Orsoni, J., Russo, A., Rubino, P., Bacciu, S., Lippi, G., et al. (2014). Cogan's syndrome: anti-Hsp70 antibodies are a serological marker in the typical form. Isr. Med. Assoc. J. 16, 285–288.
Boulassel, M. R., Tomasi, J. P., Deggouj, N., and Gersdorff, M. (2001). COCH5B2 is a target antigen of anti-inner ear antibodies in autoimmune inner ear diseases. Otol. Neurotol. 22, 614–618. doi: 10.1097/00129492-200109000-00009
Bovo, R., Ciorba, A., and Martini, A. (2009). The diagnosis of autoimmune inner ear disease: evidence and critical pitfalls. Eur. Arch. Otorhinolaryngol. 266, 37–40. doi: 10.1007/s00405-008-0801-y
Breslin, N. K., Varadarajan, V. V., Sobel, E. S., and Haberman, R. S. (2020). Autoimmune inner ear disease: a systematic review of management. Laryngosc. Investig. Otolaryngol. 5, 1217–1226. doi: 10.1002/lio2.508
Brown, G. J., Canete, P. F., Wang, H., Medhavy, A., Bones, J., Roco, J. A., et al. (2022). TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356. doi: 10.1038/s41586-022-04642-z
Chiu, H. -Y., Wang, T. S., Chan, C. –C., Cheng, Y. -P., Lin, S. -J., and Tsai, T.-F. (2014). Human leucocyte antigen-Cw6 as a predictor for clinical response to ustekinumab, an interleukin-12/23 blocker, in Chinese patients with psoriasis: a retrospective analysis. Br. J. Dermatol. 171, 1181–88. doi: 10.1111/bjd.13056
Ciorba, A., Corazzi, V., Bianchini, C., Aimoni, C., Pelucchi, S., Skarżyński, H. P., et al. (2018). Autoimmune Inner Ear Disease (AIED): a diagnostic challenge. Int. J. Immunopathol. Pharmacol. 32:2058738418808680. doi: 10.1177/2058738418808680
Cohen, S., Shoup, A., Weisman, M. H., and Harris, J. (2005). Etanercept treatment for autoimmune inner ear disease: results of a pilot placebo-controlled study. Otol. Neurotol . 26, 903–7. doi: 10.1097/01.mao.0000185082.28598.87
Creber, N. J., Eastwood, H. T., Hampson, A. J., Tan, J., and O'Leary, S. J. (2018). A comparison of cochlear distribution and glucocorticoid receptor activation in local and systemic dexamethasone drug delivery regimes. Hear. Res. 368, 75–85. doi: 10.1016/j.heares.2018.03.018
Cusick, M. F., Libbey, J. E., and Fujinami, R. S. (2012). Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 42, 102–111. doi: 10.1007/s12016-011-8294-7
Dallan, I., Bruschini, L., Nacci, A., Fattorp, B., Traino, A. C., Rognini, F., et al. (2006). Transtympanic steroids in refractory sudden hearing loss. personal experience. Acta. Otorhinolaryngol. Ital. 26, 14–19.
Dhanasekaran, D. N., and Reddy, E. P. (2017). JNK-signaling: a multiplexing hub in programmed cell death. Genes Cancer 8, 682–94. doi: 10.18632/genesandcancer.155
El-Sharkawy, L. Y., Brough, D., and Freeman, S. (2020). Inhibiting the NLRP3 inflammasome. Molecules 25:5533. doi: 10.3390/molecules25235533
Emami , H., Tajdini, A., Amirzargar, B., Habibi, S., Varpaei, H. A., Gholami , R., et al. (2023). Intratympanic triamcinolone or dexamethasone in sudden sensory neural hearing loss: a randomized clinical trial. Indian J. Otolaryngol. Head Neck Surg.75, 3545–52. doi: 10.1007/s12070-023-04032-5
Francis, S. P., and Cunningham, L. L. (2017). Non-autonomous cellular responses to ototoxic drug-induced stress and death. Front. Cell Neurosci. 11:252. doi: 10.3389/fncel.2017.00252
Friedman, B., and Cronstein, B. (2019). Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine 86, 301–7. doi: 10.1016/j.jbspin.2018.07.004
Frye, M. D., Yang, W., Zhang, C., Xiong, B., and Hu, B. H. (2017). Dynamic activation of basilar membrane macrophages in response to chronic sensory cell degeneration in aging mouse cochleae. Hear. Res. 344, 125–34. doi: 10.1016/j.heares.2016.11.003
García-Berrocal, J. R., Ibáñez, A., Rodríguez, A., González-García, J. A., Verdaguer, J. M., Trinidad, A., et al. (2006). Alternatives to systemic steroid therapy for refractory immune-mediated inner ear disease: a physiopathologic approach. Eur. Arch. Otorhinolaryngol. 263, 977–82. doi: 10.1007/s00405-006-0096-9
Garg, D. C., Wagner, J. G., Sakmar, E., Weidler, D. J., and Albert, K. S. (1979). Rectal and oral absorption of methylprednisolone acetate. Clin. Pharmacol. Ther. 26, 232–39. doi: 10.1002/cpt1979262232
Garg, V., and Jusko, W. J. (1994). Bioavailability and reversible metabolism of prednisone and prednisolone in man. Biopharm. Drug Disposit. 15, 163–72. doi: 10.1002/bdd.2510150208
Gauthier, A. E., Rotjan, R. D., and Kagan, J. C. (2022). Lipopolysaccharide detection by the innate immune system may be an uncommon defence strategy used in nature. Open Biol. 12:220146. doi: 10.1098/rsob.220146
George, D. L., and Pradhan, S. (2009). Idiopathic sensorineural hearing disorders in adults—a pragmatic approach. Nat. Rev. Rheumatol. 5, 505–12. doi: 10.1038/nrrheum.2009.150
Gloddek, B., Gloddek, J., and Arnold, W. (1997). Induction of an inner-ear-specific autoreactive T-cell line for the diagnostic evaluation of an autoimmune disease of the inner ear. Ann. N. Y. Acad. Sci. 830, 266–276. doi: 10.1111/j.1749-6632.1997.tb51897.x
Gloddek, B., Ryan, A. F., and Harris, J. P. (1991). Homing of lymphocytes to the inner ear. Acta Otolaryngol. 111, 1051–1059. doi: 10.3109/00016489109100755
Goh, X., Muzaffar, J., and Bance, M. (2022). Cochlear implantation in systemic autoimmune disease. Curr. Opin. Otolaryngol. Head Neck Surg. 30:291. doi: 10.1097/MOO.0000000000000839
Goodall, A. F., and Siddiq, M. A. (2015). Current understanding of the pathogenesis of autoimmune inner ear disease: a review. Clin Otolaryngol. 40, 412–429. doi: 10.1111/coa.12432
Gregory, G. E., Munro, K. J., Couper, K. N., Pathmanaban, O. N., and Brough, D. (2023). The NLRP3 inflammasome as a target for sensorineural hearing loss. Clin. Immunol. 249:109287. doi: 10.1016/j.clim.2023.109287
Grimbacher, B., Warnatz, K., Yong, P. F. K., Korganow, A. S., and Peter, H. H. (2016). The crossroads of autoimmunity and immunodeficiency: lessons from polygenic traits and monogenic defects. J. Allergy Clin. Immunol. 137, 3–17. doi: 10.1016/j.jaci.2015.11.004
Harris, J. P. (1983). Immunology of the inner ear: response of the inner ear to antigen challenge. Otolaryngol. Head Neck Surg. 91, 18–32. doi: 10.1177/019459988309100105
Harris, J. P. (1984). Immunology of the inner ear: evidence of local antibody production. Ann. Otol. Rhinol. Laryngol. 93, 157–62. doi: 10.1177/000348948409300211
Harris, J. P., Heydt, J., Keithley, E. M., and Chen, M. C. (1997). Immunopathology of the inner ear: an update. Ann. N.Y. Acad. Sci. 830, 166–78. doi: 10.1111/j.1749-6632.1997.tb51888.x
Harris, J. P., Low, N. C., and House, W. F. (1985). Contralateral hearing loss following inner ear injury: sympathetic cochleolabyrinthitis? Am. J. Otol. 6, 371–77.
Harris, J. P., and Sharp, P. A. (1990). Inner ear autoantibodies in patients with rapidly progressive sensorineural hearing loss. Laryngoscope 100, 516–24. doi: 10.1288/00005537-199005000-00015
Harris, J. P., and Weisman, M. H. (2007). Head and Neck Manifestations of Systemic Disease. Routledge, 53–63.
Harris, J. P., Weisman, M. H., Derebery, J. M., Espeland, M. A., Gantz, B. J., Julianna Gulya, A., et al. (2003). Treatment of corticosteroid-responsive autoimmune inner ear disease with methotrexate: a randomized controlled trial. JAMA 290, 1875–83. doi: 10.1001/jama.290.14.1875
Hashimoto, S., Billings, P., Harris, J. P., Firestein, G. S., and Keithley, E. M. (2005). Innate immunity contributes to cochlear adaptive immune responses. Audiol. Neurootol. 10, 35–43. doi: 10.1159/000082306
Hautefort, C., Loundon, N., Montchilova, M., Marlin, S., Garabedian, E. N., and Ulinski, T. (2009). Mycophenolate mofetil as a treatment of steroid dependent cogan's syndrome in childhood. Int. J. Pediatr. Otorhinolaryngol. 73, 1477–79. doi: 10.1016/j.ijporl.2009.06.025
Hocking, A. M., and Buckner, J. H. (2022). Genetic basis of defects in immune tolerance underlying the development of autoimmunity. Front. Immunol. 13:972121. doi: 10.3389/fimmu.2022.972121
Hoistad, D. L., Schachern, P. A., and Paparella, M. M. (1998). Autoimmune sensorineural hearing loss: a human temporal bone study. Am. J. Otolaryngol. 19, 33–39. doi: 10.1016/S0196-0709(98)90063-1
Hough, K., Verschuur, C. A., Cunningham, C., and Newman, T. A. (2022). Macrophages in the Cochlea; an immunological link between risk factors and progressive hearing loss. Glia 70, 219–38. doi: 10.1002/glia.24095
Hu, B. H., Zhang, C., and Frye, M. D. (2018). Immune cells and non-immune cells with immune function in mammalian cochleae. Hear. Res. 362, 14–24. doi: 10.1016/j.heares.2017.12.009
Hu, X., Li, J., Fu, M., Zhao, X., and Wang, W. (2021). The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct. Target Ther. 6, 1–33. doi: 10.1038/s41392-021-00791-1
Huang, K., Lin, H., and Lin, C„ Wu, P. (2023). Relapsing autoimmune inner ear disease with significant response to methotrexate and azathioprine combination therapy: a case report and mini literature review. Medicine 102:e33889. doi: 10.1097/MD.0000000000033889
Jang, D., Lee, A., Shin, H., Song, H., Park, J. H., Kang, T., et al. (2021). The role of Tumor Necrosis Factor Alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 22:2719. doi: 10.3390/ijms22052719
Johnson, D. B., Lopez, M. J., and Kelley, B. (2024). Dexamethasone. In StatPearls. Treasure Island (FL): StatPearls Publishing.
Johnson, L. A., Morgan, R. A., Dudley, M. E., Cassard, L., Yang, J. C., Marybeth S. Hughes, M. S., et al. (2009). Gene therapy with human and mouse T-Cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–46. doi: 10.1182/blood-2009-03-211714
Kamakura, T., Lee, D. J., Herrmann, B. S., and Nadol, J. B. (2017). Histopathology of the human inner ear in the cogan syndrome with cochlear implantation. Audiol. Neurootol. 22, 116–123. doi: 10.1159/000477534
Kämpfe Nordström, C., Danckwardt-Lillieström, N., Laurell, G., Liu, W., and Rask-Andersen, H. (2019). The human endolymphatic sac and inner ear immunity: macrophage interaction and molecular expression. Front. Immunol. 9:3181. doi: 10.3389/fimmu.2018.03181
Katherine, D., James, M., James, H., Rohan, A., Philip, A., Saiju, J., et al. (2023). Mycophenolate is better tolerated than azathioprine in myasthenia gravis. J. Neurolo. Neurosurg. Psychiatry 94, A19–A19. doi: 10.1136/JNNP-2023-ABN.56
Khomtchouk, K. M., Joseph, L. I., Khomtchouk, B. B., Kouhi, A., Massa, S., Xia, A., et al. (2021). Treatment with a neutrophil elastase inhibitor ofloxacin reduces P. aeruginosa burden in a mouse model of chronic suppurative otitis media. NPJ Biofilms Microbiomes 7:31. doi: 10.1038/s41522-021-00200-z
Kim, S. H., Kim, J. Y., Lee, H. J., Gi, M., Kim, B. G., and Choi, J. Y. (2014). Autoimmunity as a candidate for the etiopathogenesis of Meniere's disease: detection of autoimmune reactions and diagnostic biomarker candidate. PLoS ONE 9:e111039. doi: 10.1371/journal.pone.0111039
Kishimoto, I., Okano, T., Nishimura, K., Motohashi, T., and Omori, K. (2019). Early development of resident macrophages in the mouse cochlea depends on yolk sac hematopoiesis. Frontiers in Neurology 10:1115. doi: 10.3389/fneur.2019.01115
Kuemmerle-Deschner, J. B., Koitschev, A., Tyrrell, P. N., Plontke, S. K., Deschner, N., Hansmann, S., et al. (2015). Early detection of sensorineural hearing loss in muckle-wells-syndrome. Pediatr. Rheumatol. Online J. 13:43. doi: 10.1186/s12969-015-0041-9
Kuuliala, A., Nissinen, R., Kautiainen, H., Repo, H., and Leirisalo-Repo, M. (2006). Low circulating soluble interleukin 2 receptor level predicts rapid response in patients with refractory rheumatoid arthritis treated with infliximab. Ann. Rheum. Dis. 65, 26–29. doi: 10.1136/ard.2004.034728
Landegger, L. D., Vasilijic, S., Fujita, T., Soares, V. Y., Seist, R., Xu, L., et al. (2019). Cytokine levels in inner ear fluid of young and aged mice as molecular biomarkers of noise-induced hearing loss. Front. Neurol. 10:977. doi: 10.3389/fneur.2019.00977
Lehnhardt, E. (1958). [Sudden hearing disorders occurring simultaneously or successively on both sides]. Z. Laryngol. Rhinol. Otol. 37, 1–16.
Lenz, T. L., Deutsch, A. J., Han, B., Hu, X., Okada, Y., Eyre, S., et al. (2015). Widespread non-additive and interaction effects within HLA loci modulate the risk of autoimmune diseases. Nat. Genet. 47, 1085–1090. doi: 10.1038/ng.3379
Liu, L. H., Zhang, Q. Y., Sun, W., Li, Z. R., and Gao, F. Q. (2017). Corticosteroid-induced Osteonecrosis of the Femoral Head: Detection, Diagnosis, and Treatment in Earlier Stages. Chin Med J (Engl). 130, 2601–2607. doi: 10.4103/0366-6999.217094
Liu, W., and Rask-Andersen, H. (2019). Super-resolution immunohistochemistry study on CD4 and CD8 cells and the relation to macrophages in human cochlea. J. Otol. 14, 1–5. doi: 10.1016/j.joto.2018.11.010
Liu, Y., and Xu, K. (2024). Macrophage-related immune responses in inner ear: a potential therapeutic target for sensorineural hearing loss. Front. Neurosci. 17:4. doi: 10.3389/fnins.2023.1339134
Lopez-Escamez, J. A., Vela, J., and Frejo, L. (2023). Immune-related disorders associated with Meniere's disease: a systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 169, 1122-1131. doi: 10.1002/ohn.386
Manickam, V., Gawande, D. Y., Stothert, A. R., Clayman, A. C., Batalkina, L., Warchol, M. E., et al. (2023). Macrophages promote repair of inner hair cell ribbon synapses following noise-induced cochlear synaptopathy. J. Neurosci. 43, 2075–89. doi: 10.1523/JNEUROSCI.1273-22.2023
Mao, L., Dhar, A., Meng, G., Fuss, I., Montgomery-Recht, K., Yang, Z., et al. (2022). Blau syndrome NOD2 mutations result in loss of NOD2 cross-regulatory function. Front. Immunol. 13:988862. doi: 10.3389/fimmu.2022.988862
Matsuoka, A. J., and Harris, J. P. (2013). Autoimmune inner ear disease: a retrospective review of forty-seven patients. Audiol. Neuro-Otol. 18, 228–39. doi: 10.1159/000351289
McCabe, B. F. (1979). Autoimmune sensorineural hearing loss. Ann. Otol. Rhinol. Laryngol. 88, 585–589. doi: 10.1177/000348947908800501
Mendis, S., Longley, N., Morley, S., Korres, G., and Kaski, D. (2022). Autoimmune vestibulopathy—A case series. Brain Sci. 12:306. doi: 10.3390/brainsci12030306
Miao, L., Zhang, J., Yin, L., and Pu, Y. (2021). TMT-based quantitative proteomics reveals cochlear protein profile alterations in mice with noise-induced hearing loss. Int. J. Environ. Res. Public Health 19:382. doi: 10.3390/ijerph19010382
Mijovic, T., Zeitouni, A., and Colmegna, I. (2013). Autoimmune sensorineural hearing loss: the otology-rheumatology interface. Rheumatology. 52, 780–789. doi: 10.1093/rheumatology/ket009
Mikulec, A. A., Hartsock, J. J., and Salt, A. N. (2008). Permeability of the round window membrane is influenced by the composition of applied drug solutions and by common surgical procedures. Otol. Neurotol. 29, 1020–26. doi: 10.1097/MAO.0b013e31818658ea
Miwa, T., and Okano, T. (2022). Role of inner ear macrophages and autoimmune/autoinflammatory mechanisms in the pathophysiology of inner ear disease. Front. Neurol. 13:861992. doi: 10.3389/fneur.2022.861992
Moscicki, R. A., San Martin, J. E., Quintero, C. H., Rauch, S. D., Nadol, J. B., and Bloch, K. J. (1994). Serum antibody to inner ear proteins in patients with progressive hearing loss. Correlation with disease activity and response to corticosteroid treatment. JAMA 272, 611–16. doi: 10.1001/jama.1994.03520080053043
Nakanishi, H., Kawashima, Y., Kurima, K., Chae, J. J., Ross, A. M., Pinto-Patarroyo, G., et al. (2017). NLRP3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss DFNA34 responsive to anakinra therapy. Proc. Natl. Acad. Sci. U.S.A. 114, E7766–E7775. doi: 10.1073/pnas.1702946114
Nakanishi, H., Prakash, P., Ito, T., Kim, H. J., Brewer, C. C., Harrow, D., et al. (2020). Genetic hearing loss associated with autoinflammation. Front. Neurol. 11:141. doi: 10.3389/fneur.2020.00141
Navegantes, K. C., de Souza Gomes, R., Tártari Pereira, P. A., Czaikoski, P. G., Azevedo, C. H. M., and Monteiro, M. C. (2017). Immune modulation of some autoimmune diseases: the critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Translat. Med. 15:36. doi: 10.1186/s12967-017-1141-8
Nusbaum, J. S., Mirza, I., Shum, J., Freilich, R. W., Cohen, R. E., Pillinger, M. H., et al. (2020). Sex differences in systemic lupus erythematosus: epidemiology, clinical considerations, and disease pathogenesis. Mayo Clin. Proc. 95, 384–394. doi: 10.1016/j.mayocp.2019.09.012
Nyberg, S., N. Abbott, J., Shi, X., Steyger, P. S., and Dabdoub, A. (2019). Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci. Translat. Med. 11:eaao0935. doi: 10.1126/scitranslmed.aao0935
Ocejo, A, and Correa, R. (2024). Methylprednisolone. In StatPearls. Treasure Island (FL): StatPearls Publishing.
Padoan, R., Cazzador, D., Pendolino, A. L., Felicetti, M., Pascalis, S. D., Zanoletti, E., et al. (2019). Cogan's syndrome: new therapeutic approaches in the biological era. Expert Opin. Biol. Ther. 19, 781–88. doi: 10.1080/14712598.2019.1611779
Pisetsky, D. S. (2023). Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 19, 509–524. doi: 10.1038/s41581-023-00720-1
Psillas, G., Binos, P., Dimas, G. G., Daniilidis, M., and Constantinidis, J. (2021). Human Leukocyte Antigen (HLA) influence on prognosis of autoimmune hearing loss. Audiol. Res. 11, 31–37. doi: 10.3390/audiolres11010004
Psillas, G., Dimas, G. G., Savopoulos, C., and Constantinidis, J. (2022). Autoimmune hearing loss: a diagnostic challenge. J. Clin. Med. 11:4601. doi: 10.3390/jcm11154601
Puckett, Y., Gabbar, A., and Bokhari, A. A. (2024). Prednisone. In StatPearls. Treasure Island (FL): StatPearls Publishing.
Quan, W., An, J., Li, G., Qian, G., Jin, M., Feng, C., et al. (2021). Th cytokine profile in childhood-onset systemic lupus erythematosus. BMC Pediatr. 21:187. doi: 10.1186/s12887-021-02659-3
Rahman, M. U., Poe, D. S., and Choi, H. K. (2001). Etanercept therapy for immune-mediated cochleovestibular disorders: preliminary results in a pilot study. Otol. Neurotol. 22:619. doi: 10.1097/00129492-200109000-00010
Rai, V., Wood, M. B., Feng, H., Schabla, N. M., Tu, S., and Zuo, J. (2020). The immune response after noise damage in the cochlea is characterized by a heterogeneous mix of adaptive and innate immune cells. Sci. Rep. 10:15167. doi: 10.1038/s41598-020-72181-6
Ralli, M., D'Aguanno, V., Di Stadio, V., De Virgilio, V., Croce, A., Longo, L., et al. (2018). Audiovestibular symptoms in systemic autoimmune diseases. J. Immunol. Res. 2018:5798103. doi: 10.1155/2018/5798103
Rauch, S. D. (1997). Clinical management of immune-mediated inner-ear disease. Ann. N. Y. Acad. Sci. 830, 203–210. doi: 10.1111/j.1749-6632.1997.tb51891.x
Roberts, M. H., and Erdei, E. (2020). Comparative United States autoimmune disease rates for 2010-2016 by sex, geographic region, and race. Autoimmun. Rev. 19:102423. doi: 10.1016/j.autrev.2019.102423
Sagers, J. E., Sahin, M. I., Moon, I., Ahmed, S. G., Stemmer-Rachamimov, A., Brenner, G. J., et al. (2019). NLRP3 inflammasome activation in human vestibular schwannoma: implications for tumor-induced hearing loss. Hear. Res. 381:107770. doi: 10.1016/j.heares.2019.07.007
Sai, N., Yang, Y.-Y, Ma, L., Liu, D., Jiang, Q.-Q., Guo, W.-W, et al. (2022). Involvement of NLRP3-inflammasome pathway in noise-induced hearing loss. Neur. Regener. Res. 17, 2750–54. doi: 10.4103/1673-5374.339499
Sakano, H., and Harris, J. P. (2018). Emerging options in immune-mediated hearing loss. Laryngosc. Investig. Otolaryngol. 4, 102–108. doi: 10.1002/lio2.205
Salt, A. N., Hartsock, J. J., Piu, F., and Hou, J. (2018). Dexamethasone and dexamethasone-phosphate entry into perilymph compared for middle ear applications in guinea pigs. Audiol. Neurootol. 23, 245–57. doi: 10.1159/000493846
Salt, A. N., and Hirose, K. (2018). Communication pathways to and from the inner ear and their contributions to drug delivery. Hear. Res. 362, 25–37. doi: 10.1016/j.heares.2017.12.010
Salt, A. N., and Plontke, S. K. (2018). Pharmacokinetic principles in the inner ear: influence of drug properties on intratympanic applications. Hear. Res. 368:28. doi: 10.1016/j.heares.2018.03.002
Samaha, N. L., Almasri, M. M., Johns, J. D., and Hoa, M. (2021). Hearing restoration and the stria vascularis: evidence for the role of the immune system in hearing restoration. Curr. Opin. Otolaryngol. Head Neck Surg. 29, 373–84. doi: 10.1097/MOO.0000000000000738
Satoh, H., Firestein, G. S., Billings, P. B., Harris, J. P., and Keithley, E. M. (2002). Tumor necrosis factor-alpha, an initiator, and etanercept, an inhibitor of cochlear inflammation. Laryngoscope 112, 1627–34. doi: 10.1097/00005537-200209000-00019
Schiel, V., Bhattacharya, R., Gupta, A., Eftekharian, K., Xia, A., and Santa Maria, P. L. (2024). Targeting the NLRP3 inflammasome in cochlear macrophages protects against hearing loss in chronic suppurative otitis media. J. Neuroinflamm. 21:223. doi: 10.1186/s12974-024-03212-6
Schwartz, I., McMenomey, S. O., Russell, N. J., Morton, J. I., and Trune, D. R. (1992). Stria vascularis ultrastructural pathology in the C3H/lpr autoimmune strain mouse: a potential mechanism for immune-related hearing loss. Otolaryngol. Head Neck Surg. 106, 288–295. doi: 10.1177/019459989210600317
Spoorenberg, S. M. C., Deneer, V. H., Grutters, J. C., Pulles, A. E., Voorn, G. P., Rijkers, G. T., et al. (2014). Pharmacokinetics of oral vs. intravenous dexamethasone in patients hospitalized with community-acquired pneumonia. Br. J. Clin. Pharmacol. 78, 78–83. doi: 10.1111/bcp.12295
Suzuki, M., and Harris, J. P. (1995). Expression of intercellular adhesion molecule-1 during inner ear inflammation. Ann. Otol. Rhinol. Laryngol. 104:69–75. doi: 10.1177/000348949510400111
Sykes, M. (2007). Immune tolerance: mechanisms and application in clinical transplantation. J. Intern. Med. 262, 288–310. doi: 10.1111/j.1365-2796.2007.01855.x
Tebo, A. E., Szankasi, P., Hillman, T. A., Litwin, C. M., and Hill, H. R. (2006). Antibody reactivity to heat shock protein 70 and inner ear-specific proteins in patients with idiopathic sensorineural hearing loss. Clin. Exp. Immunol. 146, 427–432. doi: 10.1111/j.1365-2249.2006.03227.x
Touil, H., Mounts, K., and De Jager, P. L. (2023). Differential impact of environmental factors on systemic and localized autoimmunity. Front. Immunol. 14:1147447. doi: 10.3389/fimmu.2023.1147447
Trune, D. R., Kempton, J. B., and Gross, N. D. (2006). Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hear. Res. 212, 22–32. doi: 10.1016/j.heares.2005.10.006
Trune, D. R., Kempton, J. B., Mitchell, C. R., and Hefeneider, S. H. (1998). Failure of elevated heat shock protein 70 antibodies to alter cochlear function in mice. Hear. Res. 116, 65–70. doi: 10.1016/s0378-5955(97)00198-6
Vambutas, A, Lesser, M., Mullooly, V., Pathak, S., Zahtz, G., Rosen, L., et al. (2014). Early efficacy trial of anakinra in corticosteroid-resistant autoimmune inner ear disease. J. Clin. Invest. 124, 4115–22. doi: 10.1172/JCI76503
Vambutas, A., and Pathak, S. (2016). AAO: autoimmune and autoinflammatory (disease) in otology: what is new in immune-mediated hearing loss. Laryngo. Invest. Otolaryngol. 1, 110–15. doi: 10.1002/lio2.28
Vande Walle, L., and Lamkanfi, M. (2024). Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets. Nat. Rev. Drug. Discov. 23, 43–66. doi: 10.1038/s41573-023-00822-2
Wajda, A., Sivitskaya, L., and Paradowska-Gorycka, A. (2021). Application of NGS technology in understanding the pathology of autoimmune diseases. J. Clin. Med. 10:3334. doi: 10.3390/jcm10153334
Wang, Y., Han, L., Diao, T., Jing, Y., Wang, L., Zheng, H., et al. (2018). A comparison of systemic and local dexamethasone administration: from perilymph/cochlea concentration to cochlear distribution. Hear. Res. 370, 1–10. doi: 10.1016/j.heares.2018.09.002
Warrington, R., Watson, W., Kim, H. L., and Antonetti, F. R. (2011). An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 7:S1. doi: 10.1186/1710-1492-7-S1-S1
Wu, J., Han, W., Chen, X., Guo, W., Liu, K., Wang, R., et al. (2017). Matrix Metalloproteinase-2 and−9 Contribute to Functional Integrity and Noise-induced Damage to the Blood-Labyrinth-Barrier. Molecular Medicine Reports 16, 1731–38. doi: 10.3892/mmr.2017.6784
Xia, A., Thai, A., Cao, Z., Chen, X., Chen, J., Bacacao, J., et al. (2022). Chronic suppurative otitis media causes macrophage-associated sensorineural hearing loss. J. Neuroinflamm. 19:224. doi: 10.1186/s12974-022-02585-w
Yang, J., Wu, H., Zhang, P., Hou, D.-M., Chen, J., and Zhang, S.-G. (2008). The pharmacokinetic profiles of dexamethasone and methylprednisolone concentration in perilymph and plasma following systemic and local administration. Acta Oto-Laryngologica 128, 496–504. doi: 10.1080/00016480701558906
Yeom, K., Gray, J., Nair, T. S., Arts, H. A., Telian, S. A., Disher, M. J-, et al. (2003). Antibodies to HSP-70 in normal donors and autoimmune hearing loss patients. Laryngoscope 113, 1770–1776. doi: 10.1097/00005537-200310000-00020
Zhang, W, Dai, M., Fridberger, A., Hassan, A., Degagne, J., Neng, L., et al. (2012). Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc. Natl. Acad. Sci. U.S.A. 109, 10388–93. doi: 10.1073/pnas.1205210109
Zhu, Y., Li, X., Chen, J., Chen, T., Shi, Z., Lei, M., et al. (2016). The pentacyclic triterpene lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. Int. Immunopharmacol. 30, 74–84. doi: 10.1016/j.intimp.2015.11.031
Keywords: hearing loss, immunity, macrophages, autoimmunity, inner ear
Citation: Kullar P and Santa Maria P (2025) Advances in immune mediated inner ear disease. Front. Audiol. Otol. 3:1624303. doi: 10.3389/fauot.2025.1624303
Received: 07 May 2025; Accepted: 18 July 2025;
Published: 18 August 2025.
Edited by:
Sofia Waissbluth, Pontificia Universidad Católica de Chile, ChileReviewed by:
Adam Kaufman, University of Maryland, United StatesKengo Yamamoto, Kitasato University Graduate School of Medical Sciences, Japan
Copyright © 2025 Kullar and Santa Maria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Santa Maria, c2FudGFtYXJpYXBAdXBtYy5lZHU=
 Peter Kullar
Peter Kullar Peter Santa Maria
Peter Santa Maria