- 1Professor and Associate Dean for Research, College of Health Professions, Department of Respiratory Care, Texas State University, Round Rock, TX, United States
- 2Jessie Ball Dupont Distinguished Professor of Pediatrics and Professor of Biomedical Engineering. Virginia Commonwealth University, Richmond, VA, United States
- 3College of Health Professions Department of Respiratory Care, Texas State University, San Marcos, TX, United States
The development of mesh nebulizer technology has expanded the ability to deliver medical aerosols to infants and small children via nasal cannula and prongs. Mesh nebulizers do not require compressed gas to generate aerosols and have a smaller, lighter profile facilitating placement in delivery circuits, unlike ultrasonic nebulizers. Prior to this century, aerosol delivery with the nasal interface to 1–4 kg infants or surrogate animal models was limited to low single-digit deposition. In vitro and animal studies with the enabling mesh technology increase inhaled dose by upwards of 14% when nasal continuous positive airway pressure ventilation is in use. Recently, investigations of transnasal aerosol delivery to the lung have expanded to include nasal cannula interfaces with both high and low flow oxygen administration, nasal continuous positive airway pressure therapy, and nasal noninvasive ventilation in treating respiratory distress, respiratory insufficiency, and acute respiratory failure of infants and toddlers. We will first examine the progression of testing transpulmonary delivery of medical aerosols from in vitro models to in vivo animal and human studies. Then, we will explain current and developing applications in clinical practice to view future directions and opportunities.
Manuscript’s contributions to the field
Aerosol delivery to infants and toddlers has long been problematic with low delivery efficiency and poor acceptance of mask interfaces, reducing effective administration. The authors provide a perspective on how mesh nebulizers and other technologies have transformed and enhanced the practicality of transnasal pulmonary delivery to the lung in this patient population.
Introduction
Nasal aerosol drug delivery has been around for many decades to deliver inhaled medication used for the treatment of sinusitis, allergic rhinitis, and congestion, generally incorporating particles between 10 and 100 microns that are trapped in the nose (Veldhorst-Janssen et al., 2009). Since the nose and nasal mucosa protect the lungs by capturing large inhaled particles, this filtration function may decrease the effectiveness of aerosol therapy (Phalen et al., 1989; Becquemin et al., 1991; Schwab and Zenkel, 1998). Despite these challenges, transnasal pulmonary delivery of medical aerosols is increasingly being used with patients in acute care settings, especially infants and small children who are preferential nose breathers (Ari and Fink, 2013; Ari, 2016; Ari, 2021). Through transnasal pulmonary delivery, a nebulizer is placed in line with the gas delivery system, such as high flow nasal cannula (HFNC), continuous positive airway pressure (CPAP), synchronized intermittent positive airway pressure (SiPAP), and noninvasive ventilation (NIV) to deliver aerosolized medications through nasal prong interfaces. This treatment modality also helps maintain respiratory support during aerosol treatment, improves oxygenation, avoids intubation, facilitates weaning from invasive ventilation, and improves patient tolerance and comfort during HFNC, CPAP, and NIV (Ari, 2017; Zhu et al., 2017; Biselli et al., 2018; Luo et al., 2019). According to previous research, transnasal aerosol delivery is a viable approach to administering inhaled medications such as inhaled bronchodilators, antibiotics, or surfactants to maintain respiratory support during prolonged nebulization times (Bhashyam et al., 2008; Finer et al., 2010; Ari et al., 2011; Fink, 2012; Sunbul et al., 2015; Li et al., 2019a; Corcoran et al., 2019; Sood et al., 2019; Nord et al., 2020; Ari, 2021; Ari and Moody, 2021; Bianco et al., 2021). Table 1 lists advantages, and disadvantages in transpulmonary aerosol delivery.
Successful transnasal aerosol drug delivery requires the right device, interface, nebulizer position, and optimal gas flow settings for the patient (Table 2). While both jet and mesh nebulizers have been used for transnasal aerosol delivery, jet nebulizers are less efficient than mesh nebulizers (Dugernier et al., 2017; Reminiac et al., 2017; Ari, 2019) and are operated with compressed gas that adds an additional flow of 6—10 L/min to the system, which can be problematic for infants requiring 3—10 L/min of heated humidified oxygen (Li et al., 2019a; Li and Fink, 2021).
The development of mesh nebulizer technology has expanded the ability to deliver medical aerosols to infants and small children via HFNC, nasal CPAP, and NIV. It is a product of the ability to generate aerosol without the need for compressed gas to operate like jet nebulizers and the size/weight limitations of device placement in delivery circuits, unlike ultrasonic nebulizers. Mesh nebulizers use micropump technology for aerosol production. They have several advantages such as low residual volume, controlled particle size output, consistent and improved delivery efficiency, fine particle fraction reaching into the peripheral lung, and the ability to nebulize even low medication volumes (Ari, 2014; Dugernier et al., 2017; Ari, 2021). More recent developments in mesh design and feed systems have led to lower profile devices that can operate in any orientation making placement between gas delivery circuits and infant airways more practical. Prior to this century, aerosol delivery via nasal interface to 1—4 kg infants or surrogate animal models was limited to low single-digit deposition (Fok et al., 1996; Fok et al., 1998). In vitro and animal studies with enabling mesh technology report inhaled doses upwards of 14% of nominal dose with nasal continuous positive airway pressure (Linner et al., 2015). Recently, this line of investigations has expanded to nasal cannula (both high and low flow), nasal CPAP, and NIV in treating respiratory distress, respiratory insufficiency, and acute respiratory failure in infants and toddlers.
A stated concern of transnasal pulmonary delivery is the level of drug deposited in the nasopharynx in the infant and small child. Corcoran (Corcoran et al., 2019) identified cannula flowrate as a key determinant of lung and nasal dose in an infant population, which is consistent with in vitro reports of transnasal aerosol with both infants and toddlers and merits further investigation in vivo (Li et al., 2019a).
Preterm infants and children up to 1 year of age are known to be primarily obligate nose breathers. When aerosol is administered via mask, aerosol particles emitted from the nebulizer (typically 4—5 micron) pass directly through the mask to the airway. In contrast, during transnasal pulmonary delivery, most impactive losses of larger particles occur in the gas pathway and circuit enroute to the cannula, so that aerosol exiting nasal prongs are smaller (1.6—2.4 micron) (Bass et al., 2019; Corcoran et al., 2019; Bass et al., 2022). Clark et al. (2021) identified particle size as a prime determinate of both nasal and pulmonary delivery to preterm infants and toddlers, with particles <3 micron having lower nasal deposition and greater lung deposition than larger particles. Based on in vitro modeling of upper airways ranging from 28 weeks gestational age to 9 months, an estimated 70% of 5-micron particles passing through a mask preferentially deposit in the nose compared <50% of smaller particles emitted from the cannula. So, for the infant and toddler, the case can be made that the use of high or low flow transnasal aerosol delivery with smaller inhaled particles is likely to decrease accumulation in the upper airway compared to the use of standard aerosol mask interfaces used with this population. Nevertheless, nasal deposition is proportionally higher with infants whether via nasal prongs or mask, and most administered drugs have not been approved for infants (Luo et al., 2019). The potential for local toxicity should be considered with effects beyond inefficient delivery to the lungs. Preclinical toxicology studies based on nasal delivery in two relevant species should be conducted to assure safety prior to approval of inhaled drugs being developed for infants. For other drugs, commonly administered drugs that have not been tested in relevant animal models, risks of systemic absorption and local inflammation of nasal mucosa persist.
While HFNC was primarily adopted to administer high oxygen concentrations to patients with severe respiratory distress, with flows > than 50 L/min commonly used with adults, the flows administered to infants and toddlers are much lower. In most cases, higher flows are not required to meet FiO2 in this population except in cases of severe acute respiratory distress syndrome (ARDS). For the administration of bronchodilators and prostacyclins, oxygen requirements are more readily met with lower gas flows, which are more conducive to higher transnasal pulmonary deposition. Consequently, transnasal pulmonary aerosol delivery is relevant with low, medium, and high gas flows using HFNC device setups.
In this paper, we will first examine the progression of testing transpulmonary delivery of medical aerosols from in vitro to in vivo studies. Then, we will explain current and developing applications in clinical practice to view future directions and opportunities.
In vitro lung models
Much of what we know about transpulmonary aerosol drug delivery to children today is based on the findings of in vitro studies that used a variety of models, from simple apertures mimicking nares that allow prongs to be positioned at a collecting filter to human-like anatomical models with proportionally appropriate structures in the upper and lower airways that are used for teaching. In addition to nasal cast models, which allow aerosol to be collected distal to the trachea, several upper airway casts have been described to study aerosol drug delivery to infants, pediatrics, and adults (Srichana et al., 2000; Janssens et al., 2001; Saijo et al., 2004; Minocchieri et al., 2008). While earlier in vitro models used prongs and nares and only captured inhaled doses at the nose, it is important to note that nose-only models are limited, as most infants use their mouth to release excessive pressure and flows. Training manikins with anatomically representative structures and sizes (adult, child, toddler, and infant) allow the collection of aerosol distal to the trachea. These training manikins also help to differentiate and simulate nose and mouth breathing and allow comparative aerosol delivery with variables like gas flow, prong sizes, and nasal and mouth breathing. The nasal cast models for infants and children are developed based on raw data collected from a single patient in 3D images such as CT and MRI and may only have the nose to hypopharynx path of aerosol (Deruyver et al., 2021). Also, the 3D images are converted to a 3D printing technique to build the physical nasal and airway models with various plastic or polymer materials (Salade et al., 2019; Deruyver et al., 2021). 3D printing technology has evolved significantly over the years to manufacture personalized in vitro models and improve drug delivery to patients with pulmonary diseases. Finlay and his colleagues have compared individual cast models from numerous subjects and noted a high range of variability, leading to improved models by combining measurements to develop a model that represents a “typical” idealized airway (Carrigy et al., 2015; Chen et al., 2017).
Currently, sophisticated anatomical models mimic human nasal cavities using artificial mucus layer, humidification, and flexible nose segments; however, they seldom extend through to the lower airway (Deruyver et al., 2021). Also, specific in vitro models were developed to represent specific patient populations. A 28-weeks gestational model and the premature infant nose and throat (PrINT) model of 32-weeks gestational age premature infant represent nose throat models (Minocchieri et al., 2008). Similarly, the Sophia anatomical infant nose throat (SAINT) (Janssens et al., 2001) model is an anatomically correct model of a 9-month-old infant that includes the airway from the nasal cavity down to the subglottic region. These in vitro models are used to evaluate factors affecting the administration of inhaled medications to children and determine the role of mesh nebulizers during transnasal aerosol delivery.
Many factors impact the delivery of therapeutic agents via mesh nebulizers during the transnasal delivery of aerosolized medications. Using in vitro lung models combined with representative breathing patterns shed light on transpulmonary aerosol drug delivery and helped us close the knowledge gap between the performance of the device, administration technique, and the characteristics of breathing patterns in pediatrics and infants. In these in vitro lung models, a filter is attached distally to the trachea or bronchi of the model to determine the inhaled dose during transnasal pulmonary drug delivery. Most in vitro models can be washed to quantify drugs deposited in the airway allowing a mass balance to estimate drug accumulation throughout the system. It is assumed that small aerosol particles less than 5 μm may pass to the lungs in both humans as well as these human-like anatomical models. The advantages of mesh nebulizers are to provide consistent and improved delivery efficiency, a predominantly fine-particle fraction reaching into the peripheral lung, low dead volume, and the ability to nebulize in low drug volumes. Furthermore, the size of the pores and the output rate of mesh nebulizers can be adjusted to improve aerosol delivery with different drugs. Viscous drugs such as antibiotics and some surfactants have been shown to reduce the output rate of mesh nebulizers. They may require dilution with normal saline to allow an adequate nebulization rate. Whereas clinical studies with mesh nebulizers during transnasal aerosol delivery to children are limited, in vitro studies showed higher lung deposition with mesh nebulizers than jet nebulizers in this patient population (Ari, 2019; Ari and Fink, 2021).
Previous in vitro studies on transpulmonary aerosol drug delivery used various lung models, including either nasal cavities or teaching manikins with anatomical airways to emulate the breathing patterns of preterm babies, infants, and children (Bhashyam et al., 2008; Ari et al., 2011; Sunbul et al., 2015; Alalwan et al., 2019; Li et al., 2019a; Li et al., 2019b; Li et al., 2020; Ari and Fink, 2021; Corcoran, 2021; Bass et al., 2022). It should be noted that particle size exiting the nasal prongs is consistently less than 2.6 microns, independent of prong size or gas flow. Any aerosol MMAD greater than 2 microns will rain out in the circuit or prongs, potentially obstructing the interface (Clark, 2021). Most commercial nebulizers have MMAD >4 microns. This is one reason that the placement of nebulizers before the humidifier has been advocated, as larger particles rain out in the humidifier allowing smaller particles to pass on to the patient. This technique has been observed to reduce liquid occlusion at the prongs (Sunbul et al., 2015). In vitro analysis of preterm and term infants has reported that particles >3 microns have much greater impactive losses in the nose (Clark, 2021). Therefore, aerosol passing through the nasal prongs has a greater potential for pulmonary delivery in this population than aerosol drug delivery via a face mask.
Although these lung models mimic the anatomy of infants and pediatrics, it is important to mention that patients’ nasal cavities and airways have complex structures. For instance, the human nasal cavity and upper airways vary between individuals based on differences in their age, gender, ethnicity, and disease state (Morgan et al., 1995). The upper airways are an important trap for inhaled medications (Phalen et al., 1989; Swift, 1989; Becquemin et al., 1991; Martonen, 1993; Schwab and Zenkel, 1998). Individual differences in the nasal cavity and upper airways may impact transpulmonary aerosol deposition and should be considered when characterizing aerosol drug delivery with in vitro studies. The turbinate structure in the nasal cavity changes with age and plays an important role in warming and humidifying inhaled air as well as filtration. While most in vitro studies on transpulmonary aerosol drug delivery to children administered heated humidified gas to the nasal cannula, they did not account for the humidifying function at the nose and have not simulated exhaled heat and humidity in lung models. These changes in absolute humidity can change the size of aerosol particles impacting measured delivery efficiency (Ari et al., 2016; Ari et al., 2017; Ari et al., 2018). The airway anatomy and physiology of infants are different from toddlers, older children, and adults as airway dimension, airway morphology, breathing patterns, airway resistance, and lung volume change with age (Laine-Alava and Minkkinen, 1997; Xi et al., 2012). Therefore, more studies on transnasal pulmonary delivery are warranted in infants and small children.
There are a few reports with mesh nebulizers that evaluated aerosol deposition in preterm infants (Minocchieri et al., 2008; Clark et al., 2018; Clark, 2021) For instance, Clark et al. (2018) used multiple in vitro airway models of preterm and larger infants to determine the aerosol deposition curve for both nose and lung and reported that nasal deposition in the preterm lung model was similar to a 9-month old SAINT model. Still, it was lower than deposition obtained from a 4-year-old in vitro model. A wide range of nasal and pulmonary deposition was identified and attributed to high intersubject variability (Clark et al., 2018). According to previous review articles on preterm and infant nasal models, (Xi et al., 2012; Xi et al., 2014), the curved turbinate flow passages and nasal meatus are not fully formed in preterm infants and develop with age. Low aerosol deposition obtained in the preterm lung models has been attributed to the combination of small lung volumes and rapid respiratory rates, however, changes in the ratio of inspiratory time to total breathing cycle (Ti/Ttot) and administered gas flows have a greater causal relationship. Sunbul et al. (2015) used a preterm nasal model to assess the delivery efficiency of mesh nebulizers used with HFNC, bubble CPAP, and SiPAP at two different nebulizer placements before the humidifier and near the 28 weeks gestational age model. They reported less than 2% of nebulized dose across all delivery systems and nebulizer placements tested in this study. Bianco et al. (2019) reported lung deposition up to 20% with a mesh nebulizer through eight different nasal prongs used in a preterm NT airway during CPAP and simulated preterm infant breathing. Using continuous nebulization, Moody et al. (Moody and Ari, 2020) compared the delivery efficiency of a mesh nebulizer during HFNC with a large volume jet nebulizer attached to a face mask in a pediatric lung model using two HFNC cannula designs. The findings of their study showed that continuous administration of aerosols through mesh nebulizers during HFNC led to a more than fourfold increase compared to a large volume nebulizer with a face mask (14.8% vs. 3.2%) (Moody and Ari, 2020).
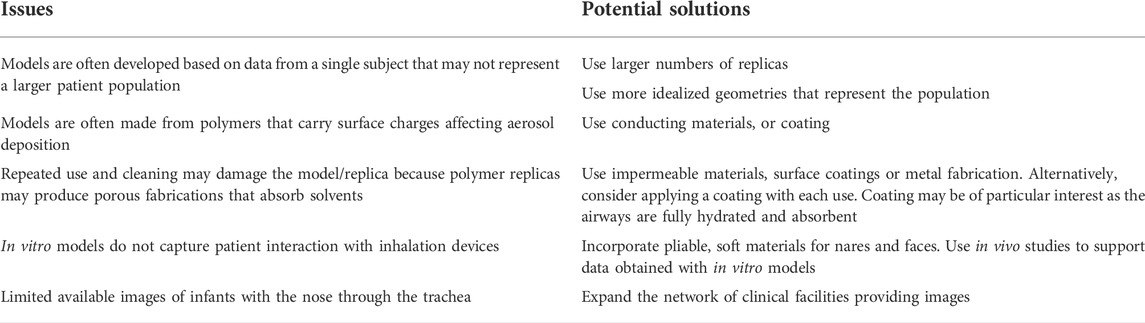
The use of nasal casts and airway replicas has several issues. Table 3 lists the issues of in vitro models and their potential solutions. For instance, they are usually developed based on data from a single subject, and it is unknown how the selected subject represents larger patient populations. Nasal casts and airway replicas are made from polymers that carry surface charge affecting aerosol deposition (Azhdarzadeh et al., 2014; Azhdarzadeh et al., 2015). Their repeated use and cleaning may damage the model/replica because polymer replicas may produce porous fabrications that absorb solvents. Also, in vitro models do not capture patient interaction with inhalation devices. Therefore, more clinical studies are warranted to validate the findings of the in vitro studies and determine patient safety and potential toxicity during transnasal pulmonary aerosol delivery.
Computational fluid dynamics models
Since nasal casts and anatomical lung models are commonly derived from a single subject, they may not be representative of an entire population. Therefore, computational fluid dynamics (CFD) models are used as an alternative approach in aerosol medicine to develop an ideal airway geometry combining key geometries of various realistic replicas and in vivo gamma scintigraphy data obtained from multiple subjects. A few studies have characterized CFD models without a nasal interface (Xi et al., 2014; Zhou et al., 2014). While CFD studies are available in the literature that characterized the progression of nasal deposition with age, (Xi et al., 2014), other studies determined nasal deposition of environmentally inhaled aerosols in infants (Storey-Bishoff et al., 2008; Javaheri et al., 2013; Tavernini et al., 2018). According to the findings of these studies, there is high inter-subject variability in nasal depositional loss of aerosols. Another CFD study by Bass et al. (2019) developed a numerically efficient CFD approach for transnasal aerosol delivery to infants and children. They reported that small aerosol particles with aerodynamic diameters of ∼1.5 μm generated with a mesh nebulizer have highly efficient lung delivery of over 90% due to low inertial depositional loss.
Animal models
There are limited animal studies on transnasal pulmonary delivery in the literature. For instance, Reminiac et al. (2017) determined the delivery efficiency of jet and mesh nebulizers attached to a face mask and HFNC, respectively, using in vitro and animal lung models. In the in vitro study, they simulated a spontaneously breathing newborn and a 9-month-old infant. At the same time, they used a spontaneously breathing macaque in the animal model to deliver aerosols with two different nebulizers (a jet nebulizer used with a face mask at 6 L/min versus a mesh nebulizer attached to HFNC at 2 and 4 L/min). Whereas the in vitro delivery efficiency of the jet nebulizer was 1.7% at 6 L/min, aerosol delivery with the mesh nebulizer was 3.3% at 2 L/min and 4.2% at 4 L/min. Transnasal aerosol delivery with the mesh nebulizer at the inlet of the humidifier administered to 1–2 kg macaques using HFNC was 0.49% and 0.85% at 2 and 4 L/min, respectively. Since it was similar to the lung deposition obtained with a jet nebulizer plus a face mask, the authors concluded that using a mesh nebulizer with HFNC is as effective as a jet nebulizer with a face mask in infants and toddlers (Reminiac et al., 2017). In a piglet model simulating administration of surfactant via nasal CPAP to preterm infants, lung deposition of 14% was reported, with nose and pharynx deposition of 19% of the nominal dose (Linner et al., 2015).
In vivo studies
While in vitro studies are not a regulatory requirement, manufacturing and pharmaceutical companies typically conduct several in vitro studies to determine emitted dose, particle size distribution, and inhaled dose of their product that are required for the regulatory applications of new aerosol devices and medications. In vitro studies are often inexpensive and speedy investigations that help us understand the reliability and quality of a product before conducting more expensive clinical studies with children. Although previous in vitro studies on transnasal aerosol drug delivery successfully assessed different devices, interfaces, delivery approaches, breathing parameters, and settings, clinical studies are always needed to confirm the findings of in vitro studies. Therefore, various methods such as gamma scintigraphy and radiotracer quantification are used to measure total, regional, and lung deposition in adults. However, scintigraphy studies are difficult to do in infants and toddlers as they are considered vulnerable populations by institutional review boards (IRB). Getting IRB approval to do such studies on children has proven challenging.
Therefore, there are a few in vivo studies on transnasal pulmonary delivery in the literature (Linner et al., 2015; Reminiac et al., 2017; Corcoran et al., 2019; Gregory et al., 2020; Nord et al., 2020). One of these studies quantified in vivo nasal deposition and lung delivery in 18 infants (3—7 kgs) using HFNC with a mesh nebulizer and found that lung deposition was 0.46% of the emitted dose at 2 L/min with 4.5% at 0.2 L/min, while nasal deposition increased from 71% to 94% with change in gas flow accounting for <40% change in nasal deposition with a >4 fold increase in lung deposition. In vivo deposition was based on proportion emitted from the nasal prongs while their in vitro analysis showed substantial drug losses in the nebulizer, HFNC delivery system, and interface (Corcoran et al., 2019).
The delivery of high-dose inhaled medications through nasal interfaces has been tested through clinical studies using nebulized liquid surfactants combined with NIV (Finer et al., 2010; Corcoran et al., 2019; Sood et al., 2019; Bianco et al., 2021). While early studies often require diluting the surfactant to allow reasonable output rates with mesh nebulizers, and require doses up to 8 fold greater than instilled to demonstrate efficacy (Bianco et al., 2021). Use of a novel mesh with smaller particles, a higher output rate without dilution has been combined with breath-synchronized nebulization (estimated inhaled dose >35% and minimal observed nasal congestion), for aerosol surfactant administration to spontaneously breathing preterm infants on nasal CPAP with doses of 200 mg/kg (MacLoughlin et al., 2017; Jardine et al., 2022).
It should be noted that the upper airway deposition at 10, 30 and 50 L/min in adults with heated humidity was reported as 34.5, 42.1, and 46.1% (<40% difference) in contrast to lung deposition of 17, 5.7, and 3.5%, (>4 fold difference) respectively (Alcoforado et al., 2019). This suggests that nasal deposition is not as highly linked to gas flow and lung delivery. Dugernier (Dugernier et al., 2017) reported similar nasal deposition/upper airway deposition 34% with mesh nebulizer via HFNC at 30 L/min. Both in vivo reports reflect upper airway deposition well below the 60—80% upper airway deposition reported with dry powder and metered dose inhalers (Rau, 2005).
Also, Morgan et al. (2015) published case studies of five infants with bronchiolitis who were administered inhaled medications through HFNC. Although no significant difference was found in pre-and post-clinical asthma scores, authors reported that infants better tolerated aerosol therapy with HFNC than a face mask. Also, an increase in heart rate was seen in infants, which may indicate the absorption of inhaled medications during treatment (Morgan et al., 2015).
Establishing in vitro and in vivo correlations
Since the anatomy of human subjects varies significantly, it is important to validate in vitro lung models. Therefore, establishing in vitro/in vivo correlations is valuable and has been done through the comparisons of in vitro lung deposition with gamma scintigraphy, pharmacokinetic and pharmacodynamic studies (Reminiac et al., 2017; Li et al., 2021). Through these studies, in vitro/in vivo correlations are determined based on the total amount of aerosol deposition in the lung, distribution of aerosol through delivery systems and interface with the use of mass balance, as well as regional distribution within various sections. Transnasal aerosol drug delivery is influenced by many factors such as particle size, velocity, nasal anatomy, and airway geometry. The nasal anatomy of human subjects has a strong impact on in vivo deposition. For instance, previous research on ten volunteers has shown 90% variability in deposition distribution not only between the upper/lower parts of the nasal cavities but also inner/outer nasal sections (Suman et al., 1999).
Current and developing applications in clinical practice
Aerosol medicine is experiencing tremendous growth with many new developments. Transnasal pulmonary drug delivery is a noninvasive method that delivers inhaled medications to the lungs and avoids the systemic effects of the medications. Therefore, it provides opportunities for a variety of therapeutic regimens for children with lung diseases. The delivery of pharmaceutical aerosols through HFNC has progressed to clinical trials.
The current mesh nebulizers available on the market generate aerosols continuously that waste aerosolized medications during patient exhalation. To overcome this challenge, the mesh nebulizer is placed before the humidifier to make the circuit and humidification chamber act as a reservoir and increase aerosol drug delivery during HFNC therapy. Recently, the intermittent nebulization that synchronizes aerosol production with a patient’s inspiratory effort has been developed and compared with continuous mesh nebulizers in both in vitro and in vivo studies (Michotte et al., 2016; Michotte et al., 2018; Li et al., 2020). While two of these studies showed higher lung deposition in adults, (Michotte et al., 2016; Michotte et al., 2018), another study did not show a significant increase in aerosol delivery with intermittent nebulization compared to continuous delivery of aerosol with mesh nebulizers unless the HFNC gas flow was set below 50% of patient inspiratory flow, at which point inhaled dose during intermittent nebulization increased up to 30% of nominal dose regardless of the nebulizer position on the HFNC circuit (Li et al., 2020).
The use of submicrometer particles combined with condensational growth techniques has been described as a strategy to increase lung dose by decreasing drug losses with HFNC systems. Previous research reported low lung deposition and high depositional losses in the delivery system and interface during transnasal pulmonary delivery (Bass et al., 2021). Recently, Bass et al. (2022) evaluated the effects of various nasal prongs on the loss of aerosolized medications in the nasal cavity of a preterm infant lung model. Their study showed that nasal prongs impact aerosol loss in the NT of simulated neonatal lung models. In the best case, the NT aerosol loss was 15–20% for the dual prongs with external or internal prongs at a 2 mm insertion depth. Innovative models for administration of dry powders through the nose have been developed and explored and show promise for the future but have yet to advance to clinical trials in infants (Spence et al., 2019; Howe et al., 2021; Howe et al., 2022). More studies on the impact of nasal cannula interfaces on children with pulmonary diseases are warranted.
Lastly, the methods and procedures for transnasal aerosol drug delivery are essential to provide effective treatments to this patient population. Currently, there is wide variation in the use of transnasal aerosol drug delivery across patient populations, which may lead to reduced benefits (Ari, 2017; Li and Fink, 2021).
Conclusion
Mesh nebulizers show great potential in transnasal aerosol drug delivery to children. Its potential and the development of this treatment modality have been explained in this paper specifically for treating infants and toddlers. Although numerous challenges still need to be overcome for successful and efficient transnasal pulmonary delivery, the increasing knowledge of this treatment approach helps us provide better patient care. While the dialogue between scientists will help improve this treatment modality and overcome its challenges, well-designed training sessions for clinicians will be essential for the correct use of mesh nebulizers during transnasal pulmonary aerosol delivery in the treatment of infants and toddlers.
Author contributions
AA conceived the idea and drafted the outline of the paper. AA, JF, and BR performed the literature search and discussed the paper’s content. All authors reviewed, revised, and approved the paper before submission.
Conflict of interest
AA served on an advisory board panel of Boehringer Ingelheim as a consultant and received a speaking fee from Aerogen Ltd and Philips Healthcare. JF is Chief Science Officer for Aerogen Pharma Corp. BR consults for Boehringer Ingelheim, Regenerx, and EpiEndo.
The reviewer MM declared a shared affiliation with the author BR to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alalwan, M. A., Fink, J. B., and Ari, A. (2019). In vitro evaluation of aerosol drug delivery with and without high flow nasal cannula in children. Pediatr. Pulmonol. 54 (12), 1968–1973. doi:10.1002/ppul.24501
Alcoforado, L., Ari, A., Barcelar, J. M., Brandao, S. C. S., Fink, J. B., and de Andrade, A. D. (2019). Impact of gas flow and humidity on trans-nasal aerosol deposition via nasal cannula in adults: a randomized cross-over study. Pharmaceutics 11 (7), E320. doi:10.3390/pharmaceutics11070320
Ari, A., and Fink, J. B. (2013). Aerosol therapy in children: Challenges and solutions. Expert Rev. Respir. Med. 7 (6), 665–672. doi:10.1586/17476348.2013.847369
Ari, A., and Fink, J. B. (2021). Delivered dose with jet and mesh nebulisers during spontaneous breathing, noninvasive ventilation and mechanical ventilation using adult lung models. ERJ Open Res. 7 (3), 00027–02021. doi:10.1183/23120541.00027-2021
Ari, A., and Moody, G. B. (2021). How to deliver aerosolized medications through high flow nasal cannula safely and effectively in the era of COVID-19 and beyond: A narrative review. Can. J. Respir. Ther. 57, 22–25. doi:10.29390/cjrt-2020-041
Ari, A., Harwood, R., Sheard, M., Dailey, P., and Fink, J. B. (2011). In vitro comparison of heliox and oxygen in aerosol delivery using pediatric high flow nasal cannula. Pediatr. Pulmonol. 46 (8), 795–801. doi:10.1002/ppul.21421
Ari, A., Harwood, R., Sheard, M., Alquaimi, M. M., Alhamad, B., and Fink, J. B. (2016). Quantifying aerosol delivery in simulated spontaneously breathing patients with tracheostomy using different humidification systems with or without exhaled humidity. Respir. Care 61 (5), 600–606. doi:10.4187/respcare.04127
Ari, A., Alwadeai, K. S., and Fink, J. B. (2017). Effects of heat and moisture exchangers and exhaled humidity on aerosol deposition in a simulated ventilator-dependent adult lung model. Respir. Care 62 (5), 538–543. doi:10.4187/respcare.05015
Ari, A., Dang, T., Al Enazi, F. H., Alqahtani, M. M., Alkhathami, A., Qoutah, R., et al. (2018). Effect of heat moisture exchanger on aerosol drug delivery and airway resistance in simulated ventilator-dependent adults using jet and mesh nebulizers. J. Aerosol. Med. Pulm. Drug Deliv. 31 (1), 42–48. doi:10.1089/jamp.2016.1347
Ari, A. (2014). Jet, ultrasonic, and mesh nebulizers: An evaluation of nebulizers for better clinical outcomes. Eurasian J. Pulmonol. 16 (1-7), 1–7. doi:10.5152/ejp.2014.00087
Ari, A. (2016). Drug delivery interfaces: A way to optimize inhalation therapy in spontaneously breathing children. World J. Clin. Pediatr. 5 (3), 281–287. doi:10.5409/wjcp.v5.i3.281
Ari, A. (2017). Aerosol drug delivery through high flow nasal cannula. Curr. Pharm. Biotechnol. 18 (11), 877–882. doi:10.2174/1389201019666171219104217
Ari, A. (2019). Effect of nebulizer type, delivery interface, and flow rate on aerosol drug delivery to spontaneously breathing pediatric and infant lung models. Pediatr. Pulmonol. 54 (11), 1735–1741. doi:10.1002/ppul.24449
Ari, A. (2021). A path to successful patient outcomes through aerosol drug delivery to children: A narrative review. Ann. Transl. Med. 9 (7), 593. doi:10.21037/atm-20-1682
Azhdarzadeh, M., Olfert, J., Vehring, R., and Finlay, W. (2014). Effect of induced charge on deposition of uniformly charged particles in a pediatric oral extrathoracic airway. Aerosol. Sci. Technol. 48 (5), 508–514. doi:10.1080/02786826.2014.896989
Azhdarzadeh, M., Olfert, J. S., Vehring, R., and Finlay, W. H. (2015). Effect of electrostatic charge on deposition of uniformly charged monodisperse particles in the nasal extrathoracic airways of an infant. J. Aerosol. Med. Pulm. Drug Deliv. 28 (1), 30–34. doi:10.1089/jamp.2013.1118
Bass, K., Boc, S., Hindle, M., Dodson, K., and Longest, W. (2019). High-efficiency nose-to-lung aerosol delivery in an infant: Development of a validated computational fluid dynamics method. J. Aerosol. Med. Pulm. Drug Deliv. 32 (3), 132–148. doi:10.1089/jamp.2018.1490
Bass, K., Farkas, D., Hassan, A., Bonasera, S., Hindle, M., and Longest, P. W. (2021). High-efficiency dry powder aerosol delivery to children: Review and application of new technologies. J. Aerosol. Sci. 153, 105692. doi:10.1016/j.jaerosci.2020.105692
Bass, K., Momin, M. A. M., Howe, C., Aladwani, G., Strickler, S., Kolanjiyil, A. V., et al. (2022). Characterizing the effects of nasal prong interfaces on aerosol deposition in a preterm infant nasal model. AAPS PharmSciTech 23 (5), 114. doi:10.1208/s12249-022-02259-z
Becquemin, M. H., Swift, D. L., Bouchikhi, A., Roy, M., and Teillac, A. (1991). Particle deposition and resistance in the noses of adults and children. Eur. Respir. J. 4 (6), 694–702.
Bhashyam, A. R., Wolf, M. T., Marcinkowski, A. L., Saville, A., Thomas, K., Carcillo, J. A., et al. (2008). Aerosol delivery through nasal cannulas: An in vitro study. J. Aerosol. Med. Pulm. Drug Deliv. 21 (2), 181–188. doi:10.1089/jamp.2007.0662
Bianco, F., Ricci, F., Catozzi, C., Murgia, X., Schlun, M., Bucholski, A., et al. (2019). From bench to bedside: in vitro and in vivo evaluation of a neonate-focused nebulized surfactant delivery strategy. Respir. Res. 20 (1), 134. doi:10.1186/s12931-019-1096-9
Bianco, F., Salomone, F., Milesi, I., Murgia, X., Bonelli, S., Pasini, E., et al. (2021). Aerosol drug delivery to spontaneously-breathing preterm neonates: Lessons learned. Respir. Res. 22 (1), 71. doi:10.1186/s12931-020-01585-9
Biselli, P., Fricke, K., Grote, L., Braun, A. T., Kirkness, J., Smith, P., et al. (2018). Reductions in dead space ventilation with nasal high flow depend on physiological dead space volume: Metabolic hood measurements during sleep in patients with COPD and controls. Eur. Respir. J. 51 (5), 1702251. doi:10.1183/13993003.02251-2017
Carrigy, N. B., Martin, A. R., and Finlay, W. H. (2015). Use of extrathoracic deposition models for patient-specific dose estimation during inhaler design. Curr. Pharm. Des. 21 (27), 3984–3992. doi:10.2174/1381612821666150820110713
Chen, J. Z., Katz, I. M., Pichelin, M., Zhu, K., Caillibotte, G., Noga, M. L., et al. (2017). Comparison of pulsed versus continuous oxygen delivery using realistic adult nasal airway replicas. Int. J. Chron. Obstruct. Pulmon. Dis. 12, 2559–2571. doi:10.2147/COPD.S141976
Clark, A., McKenna, C., and MacLoughlin, R. (2018). Aerosol delivery in term and preterm infants: The final frontier. Respir. Drug Deliv. (RDD Online) 1, 159–168.
Clark, A. R. (2021). Essentials for aerosol delivery to term and pre-term infants. Ann. Transl. Med. 9 (7), 594. doi:10.21037/atm-20-7265
Corcoran, T. E., Saville, A., Adams, P. S., Johnston, D. J., Czachowski, M. R., Domnina, Y. A., et al. (2019). Deposition studies of aerosol delivery by nasal cannula to infants. Pediatr. Pulmonol. 54 (8), 1319–1325. doi:10.1002/ppul.24326
Corcoran, T. E. (2021). Measurements of deposited aerosol dose in infants and small children. Ann. Transl. Med. 9 (7), 595. doi:10.21037/atm-20-2045
Deruyver, L., Rigaut, C., Lambert, P., Haut, B., and Goole, J. (2021). The importance of pre-formulation studies and of 3D-printed nasal casts in the success of a pharmaceutical product intended for nose-to-brain delivery. Adv. Drug Deliv. Rev. 175, 113826. doi:10.1016/j.addr.2021.113826
Dugernier, J., Hesse, M., Jumetz, T., Bialais, E., Roeseler, J., Depoortere, V., et al. (2017). Aerosol delivery with two nebulizers through high-flow nasal cannula: A randomized cross-over single-photon emission computed tomography-computed tomography study. J. Aerosol Med. Pulm. Drug Deliv. 30 (5), 349–358. doi:10.1089/jamp.2017.1366
Finer, N. N., Merritt, T. A., Bernstein, G., Job, L., Mazela, J., and Segal, R. (2010). An open label, pilot study of Aerosurf® combined with nCPAP to prevent RDS in preterm neonates. J. Aerosol. Med. Pulm. Drug Deliv. 23 (5), 303–309. doi:10.1089/jamp.2009.0758
Fink, J. B. (2012). Delivery of inhaled drugs for infants and small children: A commentary on present and future needs. Clin. Ther. 34 (11), S36–S45. doi:10.1016/j.clinthera.2012.10.004
Fok, T. F., Monkman, S., Dolovich, M., Gray, S., Coates, G., Paes, B., et al. (1996). Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 21 (5), 301–309. doi:10.1002/(SICI)1099-0496(199605)21:5<301:AID-PPUL5>3.0.CO;2-P
Fok, T. F., al-Essa, M., Dolovich, M., Rasid, F., and Kirpalani, H. (1998). Nebulisation of surfactants in an animal model of neonatal respiratory distress. Arch. Dis. Child. Fetal Neonatal. Ed. 78 (1), F3–F9. doi:10.1136/fn.78.1.f3
Gregory, T. J., Irshad, H., Chand, R., and Kuehl, P. J. (2020). Deposition of aerosolized lucinactant in nonhuman primates. J. Aerosol. Med. Pulm. Drug Deliv. 33 (1), 21–33. doi:10.1089/jamp.2018.1505
Howe, C., Momin, M. A. M., Farkas, D. R., Bonasera, S., Hindle, M., and Longest, P. W. (2021). Advancement of the infant air-jet dry powder inhaler (DPI): Evaluation of different positive-pressure air sources and flow rates. Pharm. Res. 38 (9), 1615–1632. doi:10.1007/s11095-021-03094-w
Howe, C., Momin, M. A. M., Bass, K., Aladwani, G., Bonasera, S., Hindle, M., et al. (2022). In vitro analysis of nasal interface options for high-efficiency aerosol administration to preterm infants. J. Aerosol. Med. Pulm. drug Deliv. 35 (4), 196–211. doi:10.1089/jamp.2021.0057
Janssens, H. M., de Jongste, J. C., Fokkens, W. J., Robben, S. G., Wouters, K., and Tiddens, H. A. (2001). The Sophia anatomical infant nose-throat (saint) model: A valuable tool to study aerosol deposition in infants. J. Aerosol. Med. 14 (4), 433–441. doi:10.1089/08942680152744640
Jardine, L., Lui, K., Liley, H. G., Schindler, T., Fink, J., Asselin, J., et al. (2022). Trial of aerosolised surfactant for preterm infants with respiratory distress syndrome. Arch. Dis. Child. Fetal Neonatal Ed. 107 (1), 51–55. doi:10.1136/archdischild-2021-321645
Javaheri, E., Golshani, L., and Finlay, W. H. (2013). An idealized geometry that mimics average infant nasal airway deposition. J. Aerosol. Sci. 55, 137–148. doi:10.1016/j.jaerosci.2012.07.013
Laine-Alava, M. T., and Minkkinen, U. K. (1997). Variation of nasal respiratory pattern with age during growth and development. Laryngoscope 107 (3), 386–390. doi:10.1097/00005537-199703000-00021
Li, J., and Fink, J. B. (2021). Narrative review of practical aspects of aerosol delivery via high-flow nasal cannula. Ann. Transl. Med. 9 (7), 590. doi:10.21037/atm-20-7383
Li, J., Gong, L., and Fink, J. B. (2019). The ratio of nasal cannula gas flow to patient inspiratory flow on trans-nasal pulmonary aerosol delivery for adults: An in vitro study. Pharmaceutics 11 (5), E225. doi:10.3390/pharmaceutics11050225
Li, J., Gong, L., Ari, A., and Fink, J. B. (2019). Decrease the flow setting to improve trans-nasal pulmonary aerosol delivery via "high-flow nasal cannula" to infants and toddlers. Pediatr. Pulmonol. 54 (6), 914–921. doi:10.1002/ppul.24274
Li, J., Wu, W., and Fink, J. B. (2020). In vitro comparison between inspiration synchronized and continuous vibrating mesh nebulizer during trans-nasal aerosol delivery. Intensive Care Med. Exp. 8 (1), 6. doi:10.1186/s40635-020-0293-7
Li, J., Augustynovich, A. E., Gurnani, P. K., and Fink, J. B. (2021). In-vitro and in-vivo comparisons of high versus low concentrations of inhaled epoprostenol to adult intubated patients. Respir. Res. 22 (1), 231. doi:10.1186/s12931-021-01827-4
Linner, R., Perez-de-Sa, V., and Cunha-Goncalves, D. (2015). Lung deposition of nebulized surfactant in newborn piglets. Neonatology 107 (4), 277–282. doi:10.1159/000369955
Luo, J., Duke, T., Chisti, M. J., Kepreotes, E., Kalinowski, V., and Li, J. (2019). Efficacy of high-flow nasal cannula vs standard oxygen therapy or nasal continuous positive airway pressure in children with respiratory distress: A meta-analysis. J. Pediatr. 215, 199–208. doi:10.1016/j.jpeds.2019.07.059
MacLoughlin, R., Telfer, C., Clark, A., and Fink, J. (2017). Aerosol: A novel vehicle for pharmacotherapy in neonates. Curr. Pharm. Des. 23 (38), 5928–5934. doi:10.2174/1381612823666170918122136
Martonen, T. B. (1993). Mathematical model for the selective deposition of inhaled pharmaceuticals. J. Pharm. Sci. 82 (12), 1191–1199. doi:10.1002/jps.2600821202
Michotte, J. B., Staderini, E., Le Pennec, D., Dugernier, J., Rusu, R., Roeseler, J., et al. (2016). In vitro comparison of a vibrating mesh nebulizer operating in inspiratory synchronized and continuous nebulization modes during noninvasive ventilation. J. Aerosol. Med. Pulm. Drug Deliv. 29 (4), 328–336. doi:10.1089/jamp.2015.1243
Michotte, J. B., Staderini, E., Aubriot, A. S., Jossen, E., Dugernier, J., Liistro, G., et al. (2018). Pulmonary drug delivery following continuous vibrating mesh nebulization and inspiratory synchronized vibrating mesh nebulization during noninvasive ventilation in healthy volunteers. J. Aerosol. Med. Pulm. Drug Deliv. 31 (1), 33–41. doi:10.1089/jamp.2016.1339
Minocchieri, S., Burren, J. M., Bachmann, M. A., Stern, G., Wildhaber, J., Buob, S., et al. (2008). Development of the premature infant nose throat-model (PrINT-Model): An upper airway replica of a premature neonate for the study of aerosol delivery. Pediatr. Res. 64 (2), 141–146. doi:10.1203/PDR.0b013e318175dcfa
Moody, G. B., and Ari, A. (2020). Quantifying continuous nebulization via high flow nasal cannula and large volume nebulizer in a pediatric model. Pediatr. Pulmonol. 55 (10), 2596–2602. doi:10.1002/ppul.24967
Morgan, N. J., MacGregor, F. B., Birchall, M. A., Lund, V. J., and Sittampalam, Y. (1995). Racial differences in nasal fossa dimensions determined by acoustic rhinometry. Rhinology 33 (4), 224–228.
Morgan, S. E., Mosakowski, S., Solano, P., Hall, J. B., and Tung, A. (2015). High-flow nasal cannula and aerosolized beta agonists for rescue therapy in children with bronchiolitis: A case series. Respir. Care 60 (9), e161–5. doi:10.4187/respcare.03996
Nord, A., Linner, R., Salomone, F., Bianco, F., Ricci, F., Murgia, X., et al. (2020). Lung deposition of nebulized surfactant in newborn piglets: Nasal CPAP vs Nasal IPPV. Pediatr. Pulmonol. 55 (2), 514–520. doi:10.1002/ppul.24603
Phalen, R. F., Oldham, M. J., and Mautz, W. J. (1989). Aerosol deposition in the nose as a function of body size. Health Phys. 57, 299–305. doi:10.1097/00004032-198907001-00039
Reminiac, F., Vecellio, L., Loughlin, R. M., Le Pennec, D., Cabrera, M., Vourc'h, N. H., et al. (2017). Nasal high flow nebulization in infants and toddlers: An in vitro and in vivo scintigraphic study. Pediatr. Pulmonol. 52 (3), 337–344. doi:10.1002/ppul.23509
Saijo, R., Majima, Y., Hyo, N., and Takano, H. (2004). Particle deposition of therapeutic aerosols in the nose and paranasal sinuses after transnasal sinus surgery: A cast model study. Am. J. Rhinol. 18 (1), 1–7. doi:10.1177/194589240401800101
Salade, L., Wauthoz, N., Goole, J., and Amighi, K. (2019). How to characterize a nasal product. The state of the art of in vitro and ex vivo specific methods. Int. J. Pharm. 561, 47–65. doi:10.1016/j.ijpharm.2019.02.026
Schwab, J. A., and Zenkel, M. (1998). Filtration of particulates in the human nose. Laryngoscope 108, 120–124. doi:10.1097/00005537-199801000-00023
Sood, B. G., Cortez, J., Kolli, M., Sharma, A., Delaney-Black, V., and Chen, X. (2019). Aerosolized surfactant in neonatal respiratory distress syndrome: Phase I study. Early Hum. Dev. 134, 19–25. doi:10.1016/j.earlhumdev.2019.05.005
Spence, B. M., Longest, W., Wei, X., Dhapare, S., and Hindle, M. (2019). Development of a high-flow nasal cannula and pharmaceutical aerosol combination device. J. Aerosol. Med. Pulm. Drug Deliv. 32 (4), 224–241. doi:10.1089/jamp.2018.1488
Srichana, T., Martin, G. P., and Marriott, C. (2000). A human oral-throat cast integrated with a twin-stage impinger for evaluation of dry powder inhalers. J. Pharm. Pharmacol. 52 (7), 771–778. doi:10.1211/0022357001774624
Storey-Bishoff, J., Noga, M., and Finlay, W. H. (2008). Deposition of micrometer-sized aerosol particles in infant nasal airway replicas. J. Aerosol Sci. 39, 1055–1065. doi:10.1016/j.jaerosci.2008.07.011
Suman, J. D., Laube, B. L., and Dalby, R. (1999). Comparison of nasal deposition and clearance of aerosol generated by nebulizer and an aqueous spray pump. Pharm. Res. 16 (10), 1648–1652. doi:10.1023/a:1011933410898
Sunbul, F. S., Fink, J. B., Harwood, R., Sheard, M. M., Zimmerman, R. D., and Ari, A. (2015). Comparison of HFNC, bubble CPAP and SiPAP on aerosol delivery in neonates: An in-vitro study. Pediatr. Pulmonol. 50 (11), 1099–1106. doi:10.1002/ppul.23123
Swift, D. L. (1989). Age-related scaling for aerosol and vapor deposition in the upper airways of humans. Health Phys. 57, 293–297. doi:10.1097/00004032-198907001-00038
Tavernini, S., Church, T., Lewis, D., Martin, A. R., and Finlay, W. H. (2018). Scaling an idealized infant nasal airway geometry to mimic inertial filtration of neonatal nasal airways. J. aerosol Sci. 118, 14–21. doi:10.1016/j.jaerosci.2017.12.004
Veldhorst-Janssen, N. M., Fiddelers, A. A., van der Kuy, P. H., Neef, C., and Marcus, M. A. (2009). A review of the clinical pharmacokinetics of opioids, benzodiazepines, and antimigraine drugs delivered intranasally. Clin. Ther. 31 (12), 2954–2987. doi:10.1016/j.clinthera.2009.12.015
Xi, J., Berlinski, A., Zhou, Y., Greenberg, B., and Ou, X. (2012). Breathing resistance and ultrafine particle deposition in nasal-laryngeal airways of a newborn, an infant, a child, and an adult. Ann. Biomed. Eng. 40 (12), 2579–2595. doi:10.1007/s10439-012-0603-7
Xi, J., Si, X., Zhou, Y., Kim, J., and Berlinski, A. (2014). Growth of nasal and laryngeal airways in children: implications in breathing and inhaled aerosol dynamics. Respir. Care 59 (2), 263–273. doi:10.4187/respcare.02568
Zhou, Y., Guo, M., Xi, J., Irshad, H., and Cheng, Y. S. (2014). Nasal deposition in infants and children. J. Aerosol. Med. Pulm. Drug Deliv. 27 (2), 110–116. doi:10.1089/jamp.2013.1039
Keywords: Nebulizers, aerosols, transnasal aerosol delivery, high flow nasal cannula, noninvasive ventilation, continuous positive airway pressure, infants, and children
Citation: Ari A, Rubin BK and Fink JB (2022) Mesh nebulizers enabling transnasal pulmonary delivery of medical aerosols to infants and toddlers: Roles, challenges, and opportunities. Front. Drug. Deliv. 2:995489. doi: 10.3389/fddev.2022.995489
Received: 18 July 2022; Accepted: 29 August 2022;
Published: 03 October 2022.
Edited by:
Philip Chi Lip Kwok, The University of Sydney, AustraliaReviewed by:
Mohammad Momin, Virginia Commonwealth University, United StatesHideyuki Sato, University of Shizuoka, Japan
Copyright © 2022 Ari, Rubin and Fink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arzu Ari, YXJ6dWFyaUB0eHN0YXRlLmVkdQ==
 Arzu Ari
Arzu Ari Bruce K. Rubin
Bruce K. Rubin James B. Fink
James B. Fink