- 1Division of Cardiology, Departments of Internal Medicine and Genetic Diagnosis Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Key Laboratory of Genetics and Molecular Mechanism of Cardiological Disorders, Wuhan, China
Background: Long QT syndrome (LQTS) is an inherited malignant arrhythmia syndrome that poses a risk of sudden death. Variants in the Potassium Voltage-Gated Channel Subfamily H Member 2 (KCNH2) gene are known to cause Long QT syndrome through an autosomal dominant inheritance pattern. However, as of now, there have been no reports of any KCNH2 variant leading to Long QT syndrome exhibiting incomplete penetrance that is influenced by gender.
Methods: Whole-exome sequencing (WES) was conducted on the proband to identify pathogenic variants. Subsequently, Sanger sequencing was employed to validate the identified likely pathogenic variants in all family members.
Results: We analyzed a pedigree spanning three-generations afflicted by Long QT syndrome. WES revealed a novel KCNH2 missense variant (p.Val630Gly, c.1889 T>G) as the causative factor for the family’s phenotype. Within this family, all three male carriers of the KCNH2 variant carriers exhibited the Long QT syndrome phenotype: one experienced sudden death during sleep, another received an implantable cardioverter defibrillator (ICD), and a younger man displayed a prolonged QTc interval without any instances of syncope or malignant arrhythmia to date. Interestingly, the middle-aged female carrier showed no Long QT Syndrome phenotype. However, her offspring, diagnosed with Turner syndrome (45, X) and also a carrier of this variant, experienced frequent syncope starting at 12 years old and was diagnosed with Long QT syndrome, leading to an ICD implantation when she was 15 years old. These observations suggest that the manifestation of Long QT syndrome associated with this KCNH2 variant exhibits incomplete penetrance influenced by gender within this family, indicating potential protective mechanisms against the syndrome in females affected by this variant.
Conclusion: Our investigation has led to the identification of a novel pathogenic KCNH2 variant responsible for Long QT syndrome within a familial context characterized by gender-selective, incomplete penetrance. This discovery highlights a unique pathogenic inheritance pattern for the KCNH2 gene associated with Long QT syndrome, and could potentially shed light on the distinct penetrance behaviors and patterns of the KCNH2 gene. This discovery broadens our exploration of the KCNH2 gene in cardiac arrhythmias, highlighting the intricate genetic dynamics behind Long QT syndrome.
Introduction
Long QT syndrome (LQTS) is an inherited arrhythmia syndrome, which characterized by a prolonged corrected QT interval on the Electrocardiogram (ECG) without structural heart disease and drug influence (Priori et al., 2013; Schwartz and Ackerman, 2013). LQTS is associated with malignant arrhythmias that can lead to syncope and sudden death (Schwartz, 2021). Most patients with LQTS have pathogenic variants in genes encoding ion channels or their regulating proteins (Wilde et al., 2022). The three most common subtypes of LQTS are distinguished based on characteristic features and genotype-phenotype relationships, which have been widely described (Tester et al., 2005; Kapplinger et al., 2009).
LQT2, accounting for approximately 30% of LQTS cases, exhibits high penetrance (Wilde et al., 2022). Loss-of-function variants in potassium voltage-gated channel subfamily H member 2 (KCNH2) gene are responsible for LQTS2 (Wilde et al., 2022). To date, a significant number of pathogenic or likely pathogenic KCNH2 variants have been reported, with the majority showing no gender-specific differences in morbidity. However, select studies have reported that females may possess a heightened risk of developing morbidity and life-threatening arrhythmias associated with LQTS2 (Mazzanti et al., 2018; Ke et al., 2023).
Turner syndrome is a rare genetic disorder characterized by features such as female hypergonadotropic hypogonadism, dysplasia, infertility, endocrine and a spectrum of endocrine and metabolic syndromes (Gravholt et al., 2019). This condition arises from the complete or partial absence of one X-chromosome, leading to the pathogenesis of Turner syndrome (Barr and Bertram, 1949). Consequently, individuals with Turner syndrome may exhibit features typically associated with “maleness” (Steiner and Saenger, 2022). Additionally, there is an increased risk of cardiovascular diseases in these patients, including hypertension, coronary heart disease, heart failure, and aortic disease (Mortensen et al., 2018).
In this study, we identified a novel pathogenic KCNH2 variant through whole exome sequencing in a three-generation pedigree. This variant causes LQTS with a novel-gender selective, incomplete penetrance inheritance pattern.
Methods
Participant recruitment
We identified a three-generation pedigree with multiple individuals suffering from LQTS. This family was recruited at Tongji Hospital in 2021, with the Institutional Review Board of Tongji Hospital granting approval for the study. Additionally, we recruited 500 healthy, unrelated Han Chinese individuals’ as controls from those undergoing routine health examinations. Informed consent, including consent for portraits, was obtained from all participants. ECGs were collected from all family members. Echocardiography and 24-h Holter monitoring were specifically performed for the proband. Furthermore, program-controlled telemetry for the implantable cardioverter defibrillator (ICD) of the proband was conducted regularly. The QT intervals were measured using the Tangent method, and the Bazett correction formula was applied to calculate the rate-corrected QT (QTc) (Vink et al., 2018). The diagnosis of LQTS was determined based on the Schwartz scoring scale (Priori et al., 2015; Westphal et al., 2020).
Whole exome sequencing
Genomic DNA was extracted from the leukocytes of peripheral blood samples using the QIAamp DNA Mini Kit (Qiagen, Germany). Following extraction, the DNA underwent fragmentation through sonification. The fragmented DNA’s tail ends were subsequently repaired, and adapters were ligated using the Agilent SureSelect Human All Exon V6 Kit (Agilent, Santa Clara, CA). These prepared libraries were then amplified and sequenced on the Illumina HiSeq platform (Illumina, California, America).
Bioinformatics analysis
The sequence reads were mapped to the GRCh37 human reference genome using the Burrows–Wheeler Aligner (BWA) (Li and Durbin, 2010). Coverage analysis was performed using BWA, while the Genome Analysis Toolkit (GATK) (McKenna et al., 2010) was used for marking duplicates, sorting BAM files, recalibrating base quality scores, and variant calling. Variants were then annotated using ANNOVAR and reported following the Human Genome Variation Society nomenclature guidelines (http://www.hgvs.org/mutnomen). Their evaluation was based on the American College of Medical Genetics (ACMG) standards (Richards et al., 2015). Given that LQTS is a rare inherited condition, variants displaying minor allele frequencies greater than 1% were excluded, as they were deemed unlikely to be deleterious. Population allele frequency data for variants were obtained from databases such as the 1000 Genomes Project (http://browser.1000genomes.org/), the Exome Aggregation Consortium (ExAC) (http://exac.broadinstitute.org/), the Exome Sequencing Project (ESP) (https://evs.gs.washington.edu/EVS/), and the Genome Aggregation Database (gnomAD) (https://gnomad.broadinstitute.org/). Variants also underwent filtering against our exome database of 500 healthy Han Chinese controls to further refine the analysis. The pathogenicity of all variants was assessed through databases like the Human Gene Mutation Database (HGMD) (http://www.hgmd.cf.ac.uk/ac/index.php) and ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar). Additionally, all missense variants were evaluated based on their effect on the function of the coding protein using SIFT, PolyPhen2 HDIV, PolyPhen2 HVAR, LRT, MutationTaster, MutationAssessor, FATHMM, PROVEAN, MetaSVM, MetaLR, VEST, M-CAP, CADD, GERP++, DANN, fathmm-MKL, Eigen, GenoCanyon, fitCons, PhyloP and SiPhy scores. (Chen et al., 2020). Protein structure of mutation was predicted using Pymol. Briefly, the pdb file (A0PJW5) of KCNH2 was downloaded from Alphafold Protein Structure Database (https://alphafold.ebi.ac.uk/) and mutagenesis as well as plot was generated with Pymol.
Sanger sequencing validation
Sanger sequencing was conducted using Applied Biosystems 3500xl capillary sequencer (Applied Biosystems, Foster City, USA) to validate all variants identified as likely pathogenic and associated with the phenotypes observed in this pedigree. Furthermore, each variant confirmed as likely pathogenic was subjected to Sanger sequencing in a group of 500 healthy controls for further validation.
Statistical analysis
Statistical analysis was performed using the SPSS software, version 26.0. Group differences were assessed with the Pearson chi-square test, and a p-value <0.05 was considered statistically significant.
Results
Case presentation
In our study, we identified a three-generation Chinese family presenting multiple cases of LQTS exhibiting gender-selective incomplete penetrance (Figure 1). The proband (III-3), a 15-year-old female with short stature and skeletal dysplasia, has a medical history of Turner syndrome and diabetes (Figures 2A,B). She has been on regular estrogen and metformin therapy. The proband reported symptoms of palpitations and amaurosis over 3 years, accompanied by four episodes of syncope—two occurring during the day with cold sweats and two at night, accompanied by urinary incontinence. Upon admission, a 12-lead electrocardiogram (ECG) revealed a prolonged QTc interval of 485 m for the proband. Further, a 24-h Holter monitor recorded a maximum QTc interval of 518 m, with alterations in the T wave observed across multiple leads (Figure 2C). Echocardiography examination showed no abnormalities, with a left ventricular dimension of 40 mm and an ejection fraction of 61%. Therefore, the diagnosis of this patient was considered as LQTS.
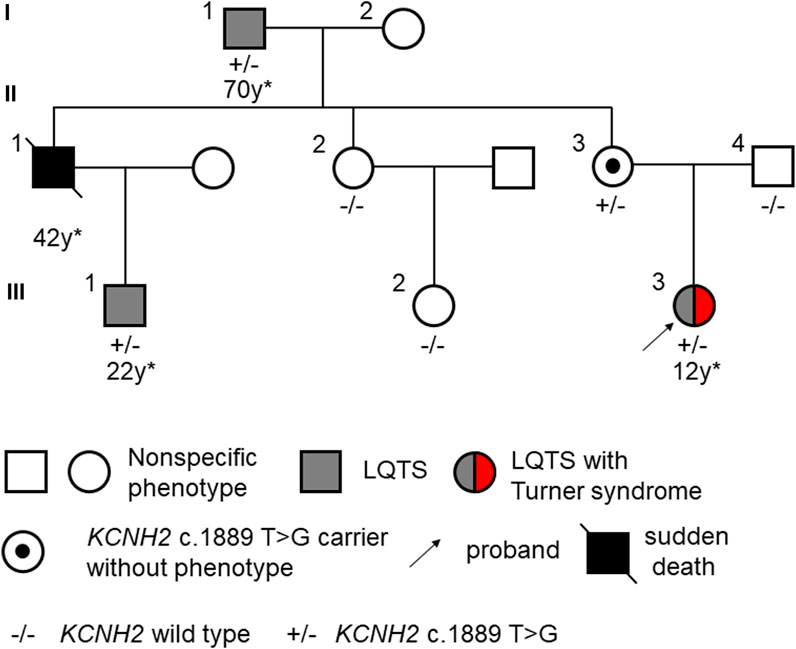
Figure 1. Pedigree of LQTS displaying gender-selective incomplete penetrance. The asterix (*) indicates the age at the first onset of a malignant event or diagnosis of LQTS.

Figure 2. Phenotypes of the proband affected by Turner syndrome. (A) Short stature compared to a reference female model, whose height is 160 cm on the right side of the panel; (B) Skeletal dysplasia caused by Turner syndrome; (C) An ECG demonstrating a prolonged QTc interval with T-wave alterations in multiple leads.
The proband’s grandfather (I-1) began to experience syncope at the age of 70 and received an implantable cardioverter defibrillator (ICD) 2 years later. The proband’s uncle (II-1) suffered multiple syncope episodes starting at the age of 42, but did not undergo any specific treatment. Tragically, he passed away in his sleep that same year. Furthermore, this man’s son (III-1), who had never shown symptoms of palpitation, amaurosis, or syncope, was discovered to have a prolonged QTc interval of 498 m with notched T waves at the age of 22 (Supplementary Figure S1). Meanwhile, the proband’s grandmother (I-2), aunt (II-2), mother (II-3), father (II-4) and cousin sister (III-2) showed no specific symptoms, displaying normal QTc intervals in their 12-lead ECGs. No history of specific cardiology diseases was found in the paternal lineage of the proband. The clinical characteristics of the family members are listed in Table 1.
Genetic screening
Whole exome sequencing was conducted on the proband, revealing a chromosomal pattern of 45, X, which is consistent with the diagnosis of Turner syndrome. Moreover, a novel missense variant in the KCNH2 gene (p.Val630Gly, c.1889 T>G) was identified and deemed responsible for the LQTS phenotype observed in this family (Figure 3). This variant has not previously been reported in the 1000 Genomes Project, ExAC, gnomAD, ESP, HGMD, or ClinVar databases, nor was it found in the 500 healthy controls. The missense constraint metrics of KCNH2 from gnomAD database (missense Z score = 2.48) is quite high, indicating that KCNH2 is intolerance to missense variants. Additionally, this variant is predicted to be deleterious by in silico algorithm (Supplementary Table S1). Protein structure predictions suggest that this variant might alter the structure of KCNH2 protein (Figure 4). Sanger sequencing confirmed the presence of this variant in all relevant family members. The proband’s grandfather (I-1), uncle (II-1), mother (II-3), and cousin brother (III-1) were identified as carriers of the KCNH2 variant. In contrast, the genotype of the proband’s grandmother (I-2), aunt (II-2), father (II-4) and cousin sister (III-2) were validated as wild type (Table 1; Figure 3). Additionally, whole exome sequencing performed on the proband’s grandfather (I-1), mother (II-3), and cousin brother (III-1) revealed no other pathogenic variants in KCHN2 or any other genes known to be associated with LQTS. The KCNH2 gene is recognized as a known causative gene of LQTS. The identified variant co-segregated with the LQTS phenotype in the proband with Turner syndrome and all male family members, except for the proband’s mother. Interestingly, no LQTS phenotypes were observed in the only ‘confirmed’ female carrier of this variant. These findings suggest the existence of protective mechanisms that shields females from LQTS associated with this variant, indicating a gender-selective incomplete penetrance in this pedigree.
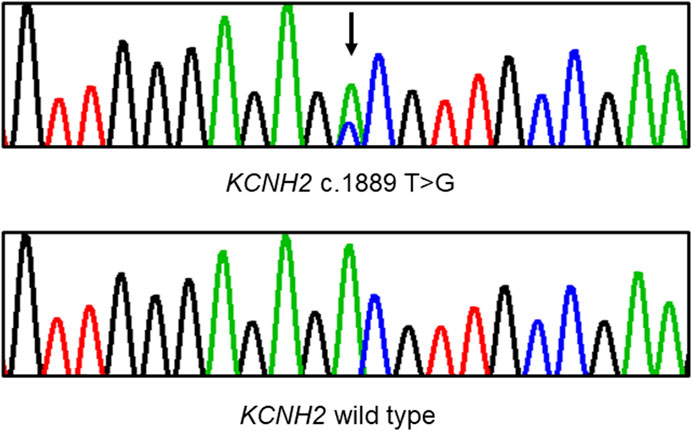
Figure 3. Sanger sequencing demonstrates a heterozygous missense variant in the KCNH2 gene within the pedigree. A black arrow indicates the position of the mutation.
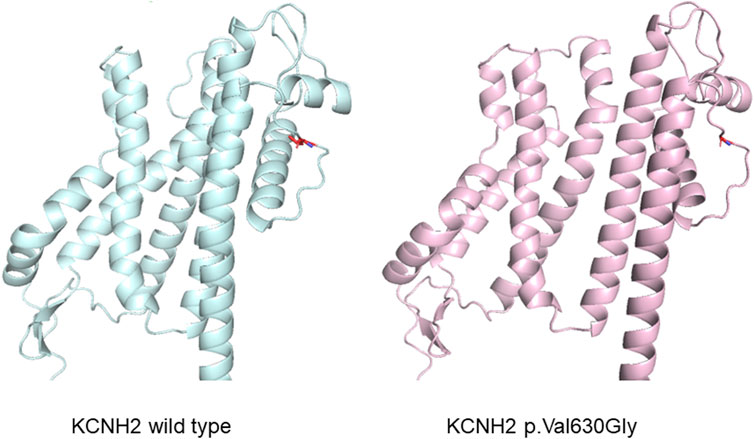
Figure 4. Protein structure prediction illustrating an alteration in the structure of the KCNH2 protein caused by the variant.
Treatment and follow-up
The ICD was implanted in the proband at the age of 15. Additionally, 10 mg propranolol has been taken for three times daily. In the seventh month after ICD implantation, 52 occurrences of ventricular flutter and 40 episodes of ventricular fibrillation were documented, resulting in five defibrillation events. She was admitted to hospital and diagnosed as electrolyte disturbance (hypokalemia) caused by diarrhea. She was treated with potassium chloride solution, suitable fluid supplement. The ventricular arrythmia attenuated and she recovered promptly. She kept on regular taking propranolol to control the ventricular arrythmia. After that, several occurrences of ventricular flutter, but no defibrillation events again in the first-year follow-up.
Discussion
This study discovered a novel missense variant in KCNH2 gene (p.Val630Gly, c.1889T>G) through whole exome sequencing in a three-generation pedigree affected by LQTS. Among the family members carrying this variant, all male carriers and one proband with Turner syndrome exhibited LQTS phenotypes, while the female carrier displayed no abnormalities. Therefore, a novel gender-selective, incomplete penetrance inheritance mode was observed in this pedigree.
The KCNH2 gene located on chromosome seven which encodes the α-subunit of the voltage-gated K+ channel Kv11.1, which comprises six α-helical transmembrane segments (S1-S6). The segments from S1 to S4 constitute the voltage sensor domain, and S5 to S6 form the pore domain (Sanguinetti et al., 1995; Trudeau et al., 1995; Wang and MacKinnon, 2017). This gene is responsible for the rapid delayed rectifier current IKr (Wilde et al., 2022). Loss-of-function variants in KCNH2 can decrease the amplitude of IKr, leading to a prolonged ventricular action potential duration (Ono et al., 2020). Previous studies have shown that more than 90% of variants in KCNH2 disrupt the intracellular transport or trafficking of the Kv11.1 channel to the cell surface membrane (Ono et al., 2020). In our study, referencing the SMART database (http://smart.embl-heidelberg.de/), the identified KCNH2 variant (p.Val630Gly, c.1889 T>G) is associated with a transmembrane region in S5-S6, potentially affecting the spatial structure and functionality of the pore domain.
Previous studies have highlighted gender differences in the manifestation of LQTS. Notably, a comprehensive follow-up study including 1,710 LQTS patients indicated that females are at a higher risk of experiencing life-threatening arrhythmic events (Mazzanti et al., 2018). Additionally, a case report outlined a pedigree with a putative KCNH2 mutation that exclusively affected females (Ke et al., 2023). Our research similarly identified a family with LQTS carrying a KCNH2 variant, which exhibited gender-selective incomplete penetrance. Contrary to expectations, female carriers of the KCNH2 variant did not exhibit LQTS phenotypes; instead, male carriers and those with Turner syndrome (45, X) demonstrated typical LQTS phenotypes. Previous studies suggested that this gender-selective pattern might be influenced by androgen levels, considering the differential impact of sex hormones on potassium currents (Grouthier et al., 2021; Ke et al., 2023). However, our findings challenge this theory, as the Turner syndrome patient, despite undergoing regular estrogen therapy for years, began experiencing arrhythmias at a younger age compared to all male patients in the study. This observation leads us to speculate that the presence of complete female sex chromosomes (XX) could offer protective mechanisms against the development of LQTS in carriers of this variant. Supporting this, published data indicate that male LQTS2 patients with mutations in the pore region are at a higher risk of cardiac event than those with non-pore mutations (Platonov et al., 2018). This observation may partially explain the gender-selective incomplete penetrance inheritance pattern observed in this family’s pedigree.
A previous study demonstrated that most LQTS2 patients start showing symptoms around puberty (Wilde et al., 2022). In this family we studied, male patients began experiencing arrhythmias at an older age. However, we observed a trend where the onset of malignant arrhythmias or sudden cardiac death occurred progressively earlier with each generation. Despite this, the proband (III-3), who has Turner syndrome, started suffering from malignant arrhythmias at a significantly younger age than other family members, displaying symptoms more frequently. Prior studies have shown that Turner syndrome patients are at a higher risk of cardiovascular diseases, including hypertension, congenital heart disease, coronary heart disease, and aortic disease (De Groote et al., 2017; Klaskova et al., 2017; Mortensen et al., 2018). This suggests that Turner syndrome may cause latent cardiac structural changes, potentially hastening the onset of LQTS in the proband or affected individuals.
Limitation
This study identified a LQTS pedigree exhibiting a gender-selective incomplete penetrance inheritance pattern. However, a limitation of our study is the absence of a second female carrier of the variant within this pedigree. Future research should include functional studies on this variant to deepen our understanding.
Conclusion
This study identified a novel missense variant in the KCNH2 gene, linked to LQTS in a Chinese Han family/pedigree, characterized by gender-selective incomplete penetrance. This suggests the existence of protective mechanisms that may shield female carriers from the onset of LQTS. The discovery of this variant introduces a novel pathogenic inheritance mode for KCNH2 associated LQTS, offering valuable insights into the gene’s penetrance mechanisms. This contribution enriches our understanding of the genetic factors influencing LQTS and highlights the importance of considering gender in genetic studies of inherited arrhythmias.
Data availability statement
The data presented in the study are deposited in the following depository: https://pan.baidu.com/s/1-SEpn19b49lPxVfkZr5uyw acess code: 5dpt
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Tongji Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
PC: Funding acquisition, Investigation, Resources, Data curation, Formal Analysis, Methodology, Project administration, Software, Writing–original draft. ZZ: Data curation, Investigation, Methodology, Software, Writing–review and editing. HW: Investigation, Writing–review and editing, Project administration. LW: Investigation, Writing–review and editing, Conceptualization, Funding acquisition, Resources, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study received financial support from the National Natural Science Foundation of China (NSFC) under project number Nos. 82170283, 82100510, 81600236). The Staff Starting Foundation in Tongji Hospital (2020HGRY013).
Acknowledgments
The authors wish to express their sincere gratitude to all the participants involved in this study for their valuable contribution.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1409459/full#supplementary-material
References
Barr, M. L., and Bertram, E. G. (1949). A morphological distinction between neurones of the male and female, and the behaviour of the nucleolar satellite during accelerated nucleoprotein synthesis. Nature 163, 676. doi:10.1038/163676a0
Chen, P., Li, Z., Nie, J., Wang, H., Yu, B., Wen, Z., et al. (2020). MYH7B variants cause hypertrophic cardiomyopathy by activating the CaMK-signaling pathway. Sci. China Life Sci. 63, 1–16. doi:10.1007/s11427-019-1627-y
De Groote, K., Devos, D., Van Herck, K., De Wolf, D., Van der Straaten, S., Rietzschel, E., et al. (2017). Increased aortic stiffness in prepubertal girls with Turner syndrome. J. Cardiol. 69, 201–207. doi:10.1016/j.jjcc.2016.03.006
Gravholt, C. H., Viuff, M. H., Brun, S., Stochholm, K., and Andersen, N. H. (2019). Turner syndrome: mechanisms and management. Nat. Rev. Endocrinol. 15, 601–614. doi:10.1038/s41574-019-0224-4
Grouthier, V., Moey, M. Y. Y., Gandjbakhch, E., Waintraub, X., Funck-Brentano, C., Bachelot, A., et al. (2021). Sexual dimorphisms, anti-hormonal therapy and cardiac arrhythmias. Int. J. Mol. Sci. 22, 1464. doi:10.3390/ijms22031464
Kapplinger, J. D., Tester, D. J., Salisbury, B. A., Carr, J. L., Harris-Kerr, C., Pollevick, G. D., et al. (2009). Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 6, 1297–1303. doi:10.1016/j.hrthm.2009.05.021
Ke, Z., Li, C., Bai, G., Tan, L., Wang, J., Zhou, M., et al. (2023). KCNH2 mutation c.3099_3112del causes congenital long QT syndrome type 2 with gender differences. Clin. (Sao Paulo) 78, 100285. doi:10.1016/j.clinsp.2023.100285
Klaskova, E., Zapletalova, J., Kapralova, S., Snajderova, M., Lebl, J., Tudos, Z., et al. (2017). Increased prevalence of bicuspid aortic valve in Turner syndrome links with karyotype: the crucial importance of detailed cardiovascular screening. J. Pediatr. Endocrinol. Metab. 30, 319–325. doi:10.1515/jpem-2016-0301
Li, H., and Durbin, R. (2010). Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595. doi:10.1093/bioinformatics/btp698
Mazzanti, A., Maragna, R., Vacanti, G., Monteforte, N., Bloise, R., Marino, M., et al. (2018). Interplay between genetic substrate, QTc duration, and arrhythmia risk in patients with long QT syndrome. J. Am. Coll. Cardiol. 71, 1663–1671. doi:10.1016/j.jacc.2018.01.078
McKenna, A., Hanna, M., Banks, E., Sivachenko, A., Cibulskis, K., Kernytsky, A., et al. (2010). The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. doi:10.1101/gr.107524.110
Mortensen, K. H., Young, L., De Backer, J., Silberbach, M., Collins, R. T., Duijnhouwer, A. L., et al. (2018). Cardiovascular imaging in Turner syndrome: state-of-the-art practice across the lifespan. Heart 104, 1823–1831. doi:10.1136/heartjnl-2017-312658
Ono, M., Burgess, D. E., Schroder, E. A., Elayi, C. S., Anderson, C. L., January, C. T., et al. (2020). Long QT syndrome type 2: emerging strategies for correcting class 2 KCNH2 (hERG) mutations and identifying new patients. Biomolecules 10, 1144. doi:10.3390/biom10081144
Platonov, P. G., McNitt, S., Polonsky, B., Rosero, S. Z., Kutyifa, V., Huang, A., et al. (2018). Risk stratification of type 2 long-QT syndrome mutation carriers with normal QTc interval: the value of sex, T-wave morphology, and mutation type. Circ. Arrhythm. Electrophysiol. 11, e005918. doi:10.1161/CIRCEP.117.005918
Priori, S. G., Blomstrom-Lundqvist, C., Mazzanti, A., Blom, N., Borggrefe, M., Camm, J., et al. (2015). ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). Endorsed by: association for European paediatric and congenital cardiology (AEPC). Eur. Heart J. 36, 2793–2867. doi:10.1093/eurheartj/ehv316
Priori, S. G., Wilde, A. A., Horie, M., Cho, Y., Behr, E. R., Berul, C., et al. (2013). HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 10, 1932–1963. doi:10.1016/j.hrthm.2013.05.014
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and genomics and the association for molecular pathology. Genet. Med. 17, 405–424. doi:10.1038/gim.2015.30
Sanguinetti, M. C., Jiang, C., Curran, M. E., and Keating, M. T. (1995). A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81, 299–307. doi:10.1016/0092-8674(95)90340-2
Schwartz, P. J. (2021). 1970-2020: 50 years of research on the long QT syndrome-from almost zero knowledge to precision medicine. Eur. Heart J. 42, 1063–1072. doi:10.1093/eurheartj/ehaa769
Schwartz, P. J., and Ackerman, M. J. (2013). The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur. Heart J. 34, 3109–3116. doi:10.1093/eurheartj/eht089
Steiner, M., and Saenger, P. (2022). Turner syndrome: an update. Adv. Pediatr. 69, 177–202. doi:10.1016/j.yapd.2022.03.004
Tester, D. J., Will, M. L., Haglund, C. M., and Ackerman, M. J. (2005). Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2, 507–517. doi:10.1016/j.hrthm.2005.01.020
Trudeau, M. C., Warmke, J. W., Ganetzky, B., and Robertson, G. A. (1995). HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269, 92–95. doi:10.1126/science.7604285
Vink, A. S., Neumann, B., Lieve, K. V. V., Sinner, M. F., Hofman, N., El Kadi, S., et al. (2018). Determination and interpretation of the QT interval. Circulation 138, 2345–2358. doi:10.1161/CIRCULATIONAHA.118.033943
Wang, W., and MacKinnon, R. (2017). Cryo-EM structure of the open human ether-a-go-go-related K(+) channel hERG. Cell 169, 422–430. doi:10.1016/j.cell.2017.03.048
Westphal, D. S., Burkard, T., Moscu-Gregor, A., Gebauer, R., Hessling, G., and Wolf, C. M. (2020). Reclassification of genetic variants in children with long QT syndrome. Mol. Genet. Genomic Med. 8, e1300. doi:10.1002/mgg3.1300
Keywords: kcnh2, Long QT syndrome, gender selective, turner syndrome, incomplete penetrance
Citation: Chen P, Zampawala Z, Wang H and Wang L (2024) Exploring the impact of a KCNH2 missense variant on Long QT syndrome: insights into a novel gender-selective, incomplete penetrance inheritance mode. Front. Genet. 15:1409459. doi: 10.3389/fgene.2024.1409459
Received: 30 March 2024; Accepted: 08 May 2024;
Published: 30 May 2024.
Edited by:
Xinxiu Xu, Cincinnati Children’s Hospital Medical Center, United StatesReviewed by:
Wenjuan Zhu, Stanford University, United StatesYin Dechun, Harbin Medical University, China
Copyright © 2024 Chen, Zampawala, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luyun Wang, wangluyun@tjh.tjmu.edu.cn
 Peng Chen
Peng Chen Zainul Zampawala
Zainul Zampawala Hong Wang
Hong Wang Luyun Wang
Luyun Wang