- 1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Clinical Research Institute, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Rehabilitation, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 4China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Background and Purpose: Non-alcoholic fatty liver disease (NAFLD) and cognitive impairment are common aging-related disorders. This study aims to explore the changes of cognitive function in middle-aged and elderly population with NAFLD from a Jidong impairment cohort.
Methods: A total of 1,651 middle-aged and elderly participants (>40 years) without cognitive impairment were recruited into the current study in 2015 and were followed up until to 2019. Abdominal ultrasonography was used for diagnosis of NAFLD. Global cognitive function was assessed with the Mini-Mental State Examination (MMSE). Cognitive impairment was defined as a score <18 for illiterates, a score <21 for primary school graduates, and a score <25 for junior school graduates or above. Multivariable regression analysis was performed to evaluate the association between NAFLD and the four-year cognitive changes.
Results: Out of 1,651 participants, 795 (48.2%) of them had NAFLD in 2015. Cognitive impairment occurred in 241 (14.6%) participants in 2019. Patients with NAFLD had higher 4-year incidence of cognitive impairment than non-NAFLD patients did (17.7 vs. 11.7%, p < 0.001). Multivariable linear regression analysis showed significant association of baseline NAFLD with lower MMSE score in 2019 (β = −0.36, p < 0.05). Multivariable logistic analysis found that the adjusted odds ratio (OR) with 95% confidence interval (CI) of baseline NAFLD was 1.45 (1.00–2.11) for cognitive impairment in 2019 (p = 0.05). We also identified effects of baseline NAFLD on subsequent cognitive impairment as modified by age (interaction p < 0.01) and carotid stenosis (interaction p = 0.05) but not by gender.
Conclusions: NAFLD is associated with cognitive decline, especially in middle-aged and with carotid stenosis population.
Introduction
Cognitive impairment is a leading cause of disability worldwide and imposes a heavy public and economic burden (1). The prevalence of cognitive impairment ranges from 9.7 to 23.3% among subjects aged 65 years or older in China (2). It is necessary to identify and to control its risk factors. Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease with a prevalence of 20–42% in China (3, 4). Previous studies demonstrated that NAFLD is not only confined to the liver but also harmful to the extra-hepatic multisystem (5, 6). Patients with NAFLD had reduced total cerebral brain volume, higher white mater hyperintensities, and more lacunar infarction than those without NAFLD (7–10).
A population with NAFLD were more likely to have impaired cognitive performance as compared with a healthy population (11–14). However, most of the previous studies were cross-sectional, which cannot assess the long-term effect of NAFLD on cognitive function (15, 16). In addition, it was also unexplored whether the effect of NAFLD on subsequent cognitive impairment is modified by age, gender and carotid stenosis. Therefore, we hypothesized that NAFLD had an independent detrimental effect on subsequent cognitive function. In current study, our aim was to evaluate the association between NAFLD and the longitudinal cognitive changes from 2015 to 2019 in middle-aged and elderly population from a Jidong cognitive impairment cohort.
Methods
Study Participants
Participants were derived from a Jidong cognitive impairment cohort study in 2015 and were followed for 4 years until 2019. A detailed information about this cohort were found in previous publication (17). Briefly, this cohort was established in April 2012 and the follow-up will continue until December 2024. This community-based study aims to investigate the incidence of cognitive impairment and its prognostic factors. Participants who met the following criteria were enrolled into the study: age 40 years or older, no history of dementia, and signed informed consent as provided. In accordance with the declaration of Helsinki, the study was approved by the Ethics Committee of Kailuan General Hospital of Tangshan City and the Medical Ethics Committee, Staff Hospital, Jidong Oilfield Branch, China National Petroleum Corporation.
Data Collection
Baseline information including demographic characteristics, medical history, and biochemical variables were collected by a series of face-to-face standardized questionnaires, clinical examinations, and laboratory tests. Medical history included metabolic syndrome, hypertension, diabetes, hyperlipidemia, cardiovascular adverse events, and carotid stenosis. Hypertension was defined as self-reported history, any current use of antihypertensive drug, or a diagnosis of hypertension in a healthcare examination. Diabetes was defined as fasting glucose level ≥7.0 mmol/L, any current use of glucose-lowering drugs, or a self-reported history. Hyperlipidemia was defined as serum levels of triglyceride ≥1.7 mmol/L, total cholesterol ≥5.72 mmol/L, high-density lipoprotein ≤ 0.9 mmol/L, current use of lipid-lowering therapy, or a self-reported history. Cardiovascular adverse event included stroke, coronary heart disease, and heart failure. Metabolic syndrome was assessed using standardized definition (18). Carotid stenosis was defined with ultrasonography, that the internal carotid artery peak systolic velocity was >125 cm/s (19). Height, weight, and waist circumference of the participants were measured while in a relaxed standing. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
NAFLD Diagnosis
The diagnosis of NAFLD in this study was based on the guideline proposed by the Asia-Pacific Working Party (20). According to criteria, NAFLD was diagnosed on the presence of at least 2 of the following characteristics in the absence of other liver diseases or excessive alcohol: diffusely increased echogenicity of liver relative to kidney or spleen, hepatic vascular blurring and deep attenuation signs. Excessive alcohol consumption in this study was defined as >20 g/day for men and 10 g/day for women. Liver ultrasound examination was performed by using a B-mode Doppler sonography machine with a 3.5 MHz probe (ACUSON X300, Siemens, Germany). Imaging interpretation was accomplished by two expert radiologists who were unaware of the patients' health data.
Outcome Measure
Follow-up was done by a face-to-face interview. All participants experienced the Chinese version of Mini-Mental State Examination (MMSE), so as to assess global cognitive status and to evaluate cognitive impairment incidence. MMSE, one of the most common and popular global cognitive examination, is used to evaluate five cognitive domains: orientation, registration, attention and calculation, recall, and language (21). The MMSE scores ranged from 0 to 30 points, and the higher scores indicated better cognitive functioning. Cognitive impairment was defined as the education-based cutoffs of MMSE score: <18 for illiterates, <21 for primary school graduates, and <25 for junior school graduates or above (21).
Statistical Analysis
Continuous variables were presented as means ± standard deviations and categorical variables as frequency (percentage). The baseline characteristics of NAFLD group and healthy group were compared using independent sample t-tests, while the Mann-Whitney U tests were used for continuous variables, and the Chi-squared tests for categorical variables. The associations of NAFLD, with MMSE score and cognitive impairment, were assessed via multivariable linear and logistic regression analysis, respectively. In the first model, we just adjusted the sex, age, and educational levels. In the second model, we further adjusted the following covariates which have been demonstrated to be associated with cognitive function: BMI, hypertension, diabetes mellitus, hyperlipidemia, and carotid artery disease. The interaction of NAFLD with age, sex and carotid stenosis were also analyzed using a multivariate logistic model. p < 0.05 was considered to be statistically significant (2-sided). All analyses were performed with SAS version 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
Study Participants and Characteristics
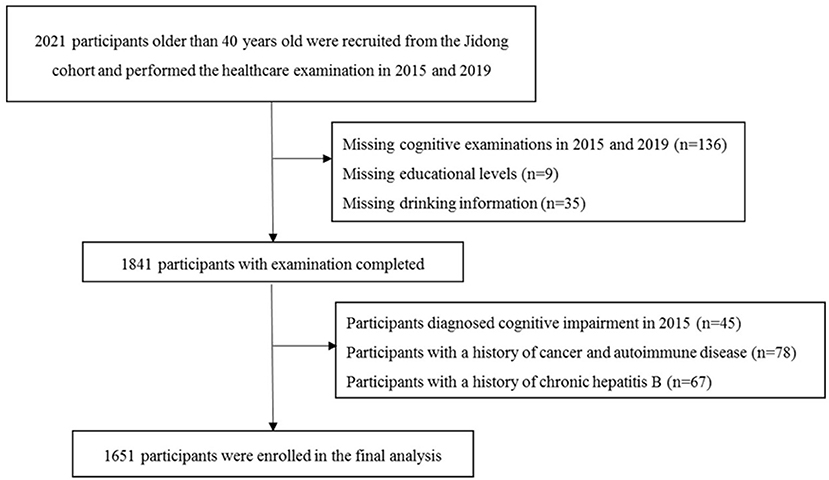
In total, there were 2,021 participants recruited into the Jidong study and had their healthcare examination in 2015 and 2019. We excluded 180 participants (8.9%) due to missing data, such as educational level, drinking habits, and cognitive performance; 145 (7.2%) due to a history of tumor, autoimmune disease, and chronic hepatitis B; and 45 (2.2%) due to a history of cognitive impairment. Finally, 1,651 participants were included into the current study (Figure 1). The baseline demographic characteristics and clinical features of the participants included and excluded in the final analysis were not significantly different, except the participants who were included were younger and had a higher educational level (Supplementary Table 1).
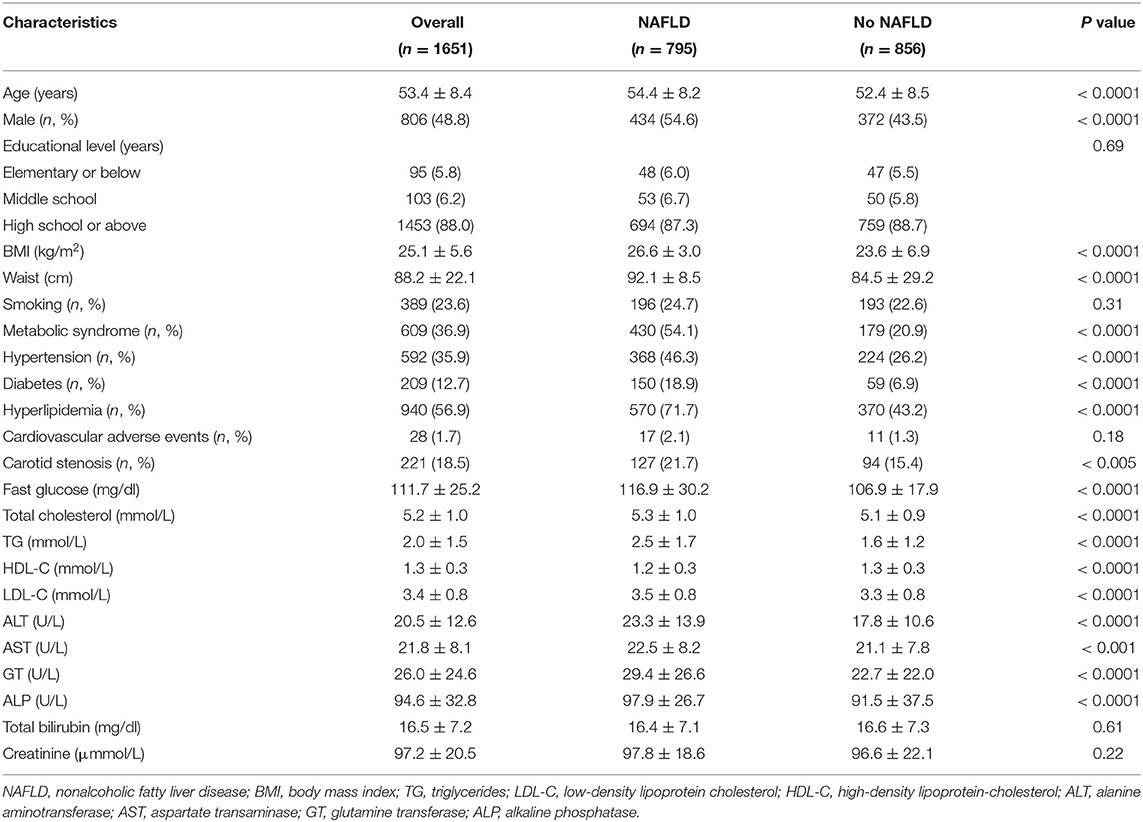
Non-alcoholic fatty liver disease (NAFLD) occurred in 795 participants (48.2%) in 2015 alone. Baseline characteristics are summarized in Table 1. Participants with NAFLD were older, more likely to be male, have higher BMI, higher levels of liver enzymes, blood glucose, serum lipid, higher proportion of history of diabetes mellitus, hypertension, hyperlipidemia, and carotid artery disease, as compared with those without NAFLD. Educational level and history of cardiovascular-adverse events were not significantly different between participants with or without NAFLD.
Cognitive Function Measures in 2015 and in 2019 for Patients With NAFLD
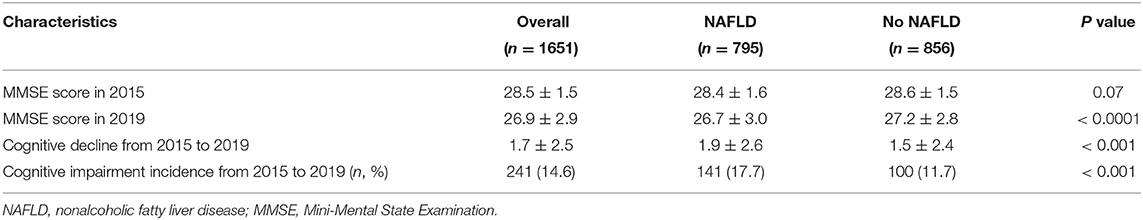
Table 2 shows the cognitive performance in 2015 and in 2019 of the participants with or without NAFLD. No significant difference was found between groups on cognitive performance in 2015 (28.4 ± 1.6 vs. 28.6 ± 1.5, p = 0.07). Participants with NAFLD had higher proportion of cognitive impairment in 2019 (26.7 ± 3.0 vs. 27.2 ± 2.8, p < 0.0001) and faster cognitive decline from 2015 to 2019 (1.9 ± 2.6 vs. 1.5 ± 2.4, p < 0.001) than those participants without NAFLD. Cognitive impairment occurred in 241 (14.6%) participants in 2019. Patients with NAFLD had higher 4-year incidence of cognitive impairment than non-NAFLD patients did (17.7 vs. 11.7%, p < 0.001) (Table 2).
Association Between NAFLD and Cognitive Function
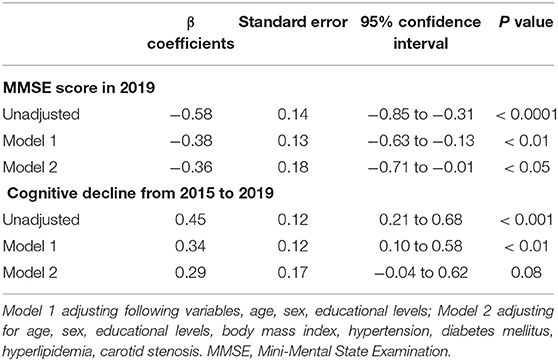
Unadjusted analysis showed baseline NAFLD was significantly associated with lower MMSE score in 2019 (β = 0.58, p < 0.0001) and cognitive function declined from 2015 to 2019 (β = 0.45, p < 0.001). Multivariable analysis has still demonstrated significant association of baseline NAFLD with lower MMSE score in 2019 (β = −0.36, p < 0.05). We also found the trend of NAFLD with cognitive function decline from 2015 to 2019 (β = 0.29, p = 0.08) (Table 3).
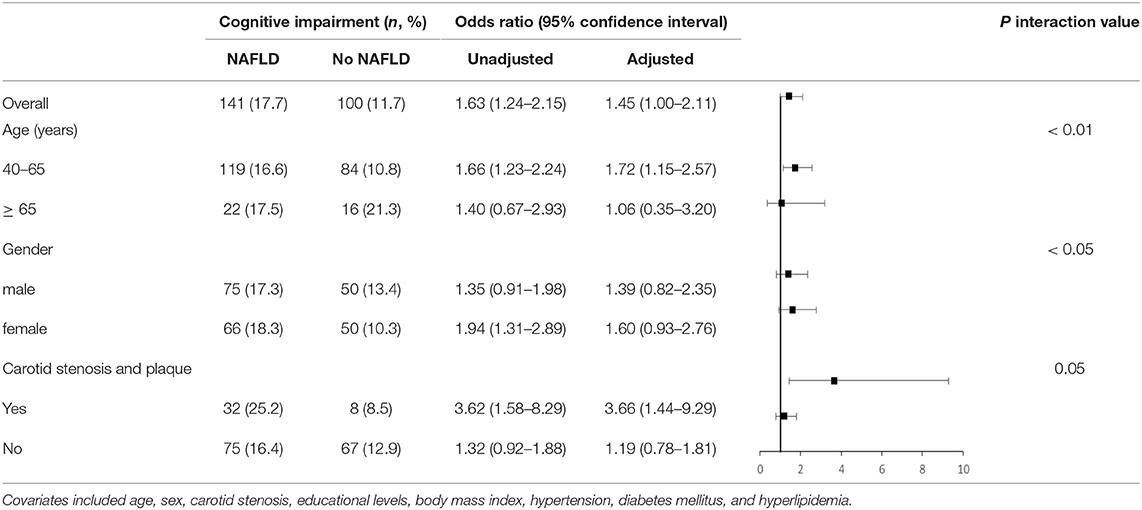
The unadjusted odds ratios (ORs) with 95% confidence interval (CI) of NAFLD were 1.47 (1.10–1.96) for cognitive impairment in 2019 (p < 0.001). The adjusted ORs with 95% CI were 1.45 (1.00–2.11) for cognitive impairment (p = 0.05) (Table 4).
Subgroup Analysis According to Age, Gender, and Carotid Stenosis
In 2019, we assessed association of baseline NAFLD with cognitive in participants stratified by age, sex, and carotid stenosis (Table 4). The adjusted ORs with 95% CI were 1.72 (1.06–2.21) in middle-aged participants (40–65 years) and 1.06 (0.35–3.20) in elderly participants (≥ 65 years) (interaction p < 0.01). The adjusted ORs with 95% CI were 3.66 (1.44–9.29) in participants with carotid stenosis and 1.19 (0.78–1.81) in those without carotid stenosis (interaction p = 0.05). In addition, we did not identify statistically significant interaction between sex and NAFLD for cognitive impairment.
Discussion
We found that a 4-year incidence of cognitive impairment was about 15.0% in our whole cohort study, but was ~18.0% in participants with NAFLD. Patients with NAFLD had 1.5-time increased risk of a subsequent 4-year cognitive impairment as compared to those without NAFLD. We also identified effects of baseline NAFLD on subsequent cognitive impairment as significantly modified by age and carotid stenosis but not by gender. To our knowledge, this is the first longitudinal study that investigated the relationship between NAFLD and cognitive function in a large community-dwelling of middle-aged and elderly population.
In the present study, NAFLD population had a higher 4-year incidence of cognitive impairment than the general population did. Also, the multivariable analysis of our study illustrated that NAFLD was independently associated with subsequent cognitive function, which was consistent with previous studies (11–14). Sang and colleagues firstly identified that NAFLD was significantly associated with worse cognitive performance in a large population-based study which contained 4,254 subjects (12). In a 3-year follow-up study, Elliott and colleagues demonstrated that patients with NAFLD had faster cognitive decline compared to the general population (11). However, other studies have failed to demonstrate such a relationship (15, 16). It remains open to question whether the relationship is an epiphenomenon. As liver is a regulator of systemic metabolic homeostasis and plays a critical role in the metabolism of glucose and lipid, greater risk for metabolic diseases (such as hypertension, diabetes, and hyperlipidemia) was found among patients with NAFLD in our study, which are related to both NAFLD and cognition (14, 22).
In the general population, few studies demonstrated that NAFLD is a risk factor for cognitive impairment (13, 23). In the multivariable analysis of our study, we found patients with NAFLD had a 1.5 folded-up risk of the subsequent 4-year cognitive impairment as compared to those without NAFLD. The mechanisms underlying the association of NAFLD with cognition are hard to elucidate. Insulin resistance was speculated as one of the possible mechanisms (24, 25). Pre-clinical experiment in the murine NAFLD model showed that insulin resistance has led the impaired activity of neurotransmitters enzymes and has limited the energy production accompanied by oxidative and endoplasmic reticulum stress, thereby, triggering a cascade of neurodegenerative changes (26–29). In addition, previous study found that patients with NAFLD had lower scavenging efficiency of central Amyloid-beta (Aβ), which is implicated with synaptic loss and neuronal degeneration (30). The low-density lipoprotein receptor-related protein-1 (LRP1) is one of the transporters involved in the clearance of Aβ out of the brain. Reduced LRP1 expression in patients with NAFLD leads to decreased peripheral Aβ protein clearance, which ultimately exacerbates amyloid load (25, 30). Moreover, liver can also regulate neuroendocrine system by synthesizing and secreting hepatic factors (31). For example, Irisin can accelerate neuroregeneration by promoting the production of neurotrophic factors (32), while the fetuin-A is a neuroprotective and anti-inflammatory molecule (33). The expression of both hepatokines decreases when fibrosis and fatty liver degeneration occurs (31).
Few studies have explored the effects of NAFLD on cognitive impairment as modified by age, gender, and carotid stenosis. In the present study, baseline NAFLD was associated with risk of a subsequent 4-year cognitive impairment in middle-aged but not in elderly population. A possible explanation is that the elderly was with more metabolic comorbidities than the middle-aged group, consequently, blunted the effect of NAFLD on cognition. We also identified the stronger effect of NAFLD on cognitive impairment in participants with carotid stenosis. Liver steatosis is characterized by a pro-inflammatory state, which promotes atherosclerosis and endothelial dysfunction, and, finally, induces micro- and macrovascular structural alterations, thereby, increasing the risk for vascular dementia (24, 34, 35). Previous studies confirmed that women had a higher incidence of dementia compared with men (36, 37). However, we did not find the interaction of gender and of NAFLD for cognitive impairment, which may be caused by the small sample size. It will be interesting in the future to observe the effects of gender, as well as other factors, on the association between NAFLD and cognitive impairment in a study with larger sample size.
Our study has several limitations. First, as age and educational level are associated with cognition (38), participants included in our analysis were generally younger and had a higher educational level compared with participants excluded, which may lead to an underestimation of cognitive impairment incidence. Second, we did not perform liver biopsy in this dwelling population. However, a meta-analysis found that ultrasound diagnosis of NAFLD had 84.8% sensitivity and 93.6% specificity compared with histology, suggesting that ultrasound is a reliable method for NAFLD diagnosis (39). In addition, because this was a post hoc analysis of an existing database, we were limited by the available data and were not able to explore further the effect of the severity of liver function and of liver fibrosis on cognitive decline. Finally, MMSE, which was used to assess cognitive status in this study, is a simple screening test and has a ceiling effect to identify cognitive decline (40). To detect cognitive dysfunctions more sensitively and to evaluate the characteristic of impaired cognitive domains in NAFLD population, other systematic cognitive tests, such as the Montreal Cognitive Assessment scale (MoCA), should be performed in the future research.
Non-alcoholic fatty liver disease (NAFLD) was associated with subsequent cognitive function, especially in middle-aged and with carotid stenosis population. Our findings suggested that NAFLD, which can be improved through lifestyle changes, can be a target for prevention of cognitive impairment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Kailuan General Hospital of Tangshan City and the Medical Ethics Committee, Staff Hospital, Jidong Oilfield Branch, China National Petroleum Corporation. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YZ had full access to all of the data in the study, takes responsibility for the integrity of the data, and the accuracy of the data analysis. QL contributed to the study concept and drafted the paper. CL and YZ revised the manuscript for important intellectual content. FH and XD performed statistical analysis. All authors contributed to the article and approved the submitted version.
Funding
Funding for this study was provided by the National Key R&D Program of China (2018YFC2002300, 2018YFC2002302, and 2020YFC2004102) and the National Natural Science Foundation of China (81972144, 31872785, and 81972148).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate all the members who participated in the Jidong cognitive impairment cohort study (CICS).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.738835/full#supplementary-material
References
1. Pan Y, Li H, Wardlaw J, Wang YJ. A new dawn of preventing dementia by preventing cerebrovascular diseases. BMJ. (2020) 371:m3692. doi: 10.1136/bmj.m3692
2. Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. (2020) 19:81–92. doi: 10.1016/S1474-4422(19)30290-X
3. Li Z, Xue J, Chen P, Chen L, Yan S, Liu L. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta-analysis of published studies. J Gastroenterol Hepatol. (2014) 29:42–51. doi: 10.1111/jgh.12428
4. Fung J, Lee C, Chan M, Seto W, Lai C, Yuen M, et al. High prevalence of non-alcoholic fatty liver disease in the Chinese—results from the Hong Kong liver health census. Liver Int. (2015) 35:542–9. doi: 10.1111/liv.12619
5. Colognesi M, Gabbia D, De Martin S. Depression and Cognitive Impairment-Extrahepatic Manifestations of NAFLD and NASH. Biomedicines. (2020) 8:7. doi: 10.3390/biomedicines8070229
6. Maher J, Schattenberg JJG. Nonalcoholic fatty liver disease in 2020. Gastroenterology. (2020) 158:1849–50. doi: 10.1053/j.gastro.2020.04.013
7. VanWagner L, Terry J, Chow L, Alman A, Kang H, Ingram K, et al. Nonalcoholic fatty liver disease and measures of early brain health in middle-aged adults: The CARDIA study. Obesity Silver Spring. (2017) 25:642–51. doi: 10.1002/oby.21767
8. Kwak M, Kim K, Seo H, Chung G, Yim J, Kim D. Non-obese fatty liver disease is associated with lacunar infarct. Liver Int. (2018) 38:1292–9. doi: 10.1111/liv.13663
9. Weinstein G, Zelber-Sagi S, Preis SR, Beiser AS, DeCarli C, Speliotes EK, et al. Association of nonalcoholic fatty liver disease with lower brain volume in healthy middle-aged adults in the framingham study. JAMA Neurol. (2018) 75:97–104. doi: 10.1001/jamaneurol.2017.3229
10. Jang H, Kang D, Chang Y, Kim Y, Lee JS, Kim KW, et al. Non-alcoholic fatty liver disease and cerebral small vessel disease in Korean cognitively normal individuals. Sci Rep. (2019) 9:1814. doi: 10.1038/s41598-018-38357-x
11. Elliott C, Frith J, Day CP, Jones DE, Newton JL. Functional impairment in alcoholic liver disease and non-alcoholic fatty liver disease is significant and persists over 3 years of follow-up. Dig Dis Sci. (2013) 58:2383–91. doi: 10.1007/s10620-013-2657-2
12. Seo SW, Gottesman RF, Clark JM, Hernaez R, Chang Y, Kim C, et al. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology. (2016) 86:1136–42. doi: 10.1212/WNL.0000000000002498
13. Filipović B, Marković O, Ðurić V, Filipović B. Cognitive changes and brain volume reduction in patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol. (2018) 18:9638797. doi: 10.1155/2018/9638797
14. Weinstein AA, de Avila L, Paik J, Golabi P, Escheik C, Gerber L, et al. Cognitive performance in individuals with non-alcoholic fatty liver disease and/or type 2 diabetes mellitus. Psychosomatics. (2018) 59:567–74. doi: 10.1016/j.psym.2018.06.001
15. Weinstein G, Davis-Plourde K, Himali JJ, Zelber-Sagi S, Beiser AS, Seshadri S. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: the Framingham Study. Liver Int. (2019) 39:1713–21. doi: 10.1111/liv.14161
16. Labenz C, Kostev K, Kaps L, Galle PR, Schattenberg JM. Incident dementia in elderly patients with nonalcoholic fatty liver disease in Germany. Dig Dis Sci. (2020) 4:1. doi: 10.1007/s10620-020-06644-1
17. Song D, Wang X, Wang S, Ge S, Ding G, Chen X, et al. Jidong cognitive impairment cohort study: objectives, design, and baseline screening. Neural Regen Res. (2020) 15:1111–9. doi: 10.4103/1673-5374.266070
18. Gu D, Reynolds K, Wu X, Jing C, Jiang HJTL. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. (2005) 365:1398–405. doi: 10.1016/S0140-6736(05)66375-1
19. Touboul P, Hennerici M, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. (2007) 23:75–80. doi: 10.1159/000097034
20. Farrell G, Chitturi S, Lau G, Sollano J. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. (2007) 22:775–7. doi: 10.1111/j.1440-1746.2007.05002.x
21. Katzman R, Zhang M, Ouang-Ya Q, Wang Z, Liu W, Yu E, et al. A Chinese version of the mini-mental state examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. (1988) 41:971–8. doi: 10.1016/0895-4356(88)90034-0
22. Tuttolomondo A, Petta S, Casuccio A, Maida C, Corte VD, Daidone M, et al. Reactive hyperemia index (RHI) and cognitive performance indexes are associated with histologic markers of liver disease in subjects with non-alcoholic fatty liver disease (NAFLD): a case control study. Cardiovasc Diabetol. (2018) 17:28. doi: 10.1186/s12933-018-0670-7
23. Gerber Y, VanWagner L, Yaffe K, Terry J, Rana J, Reis J, et al. Non-alcoholic fatty liver disease and cognitive function in middle-aged adults: the CARDIA study. BMC Gastroenterol. (2021) 21:96. doi: 10.1186/s12876-021-01681-0
24. Lombardi R, Fargion S, Fracanzani AL. Brain involvement in non-alcoholic fatty liver disease (NAFLD): a systematic review. Dig Liver Dis. (2019) 51:1214–22. doi: 10.1016/j.dld.2019.05.015
25. Bassendine M, Taylor-Robinson S, Fertleman M, Khan M, Neely D. Is Alzheimer's Disease a liver disease of the brain? J Alzheimers Dis. (2020) 75:1–14. doi: 10.3233/JAD-190848
26. de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. (2009) 10:1049–60. doi: 10.3233/JAD-2009-0984
27. Ghareeb DA, Hafez HS, Hussien HM, Kabapy NF. Non-alcoholic fatty liver induces insulin resistance and metabolic disorders with development of brain damage and dysfunction. Metab Brain Dis. (2011) 26:253–67. doi: 10.1007/s11011-011-9261-y
28. De Felice F, Lourenco M, Ferreira ST. How does brain insulin resistance develop in Alzheimer's disease? Alzheimers Dement. (2014) 10:S26–32. doi: 10.1016/j.jalz.2013.12.004
29. Kim D, Krenz A, Toussaint L, Maurer K, Robinson S, Yan A, et al. Non-alcoholic fatty liver disease induces signs of Alzheimer's disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J Neuroinflammation. (2016) 13:1. doi: 10.1186/s12974-015-0467-5
30. He Z, Guo J, McBride J, Narasimhan S, Kim H, Changolkar L, et al. Amyloid-β plaques enhance Alzheimer's brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Med. (2018) 24:29–38. doi: 10.1038/nm.4443
31. Chung HS, Choi KM. Organokines in disease. Adv Clin Chem. (2020) 94:261–321. doi: 10.1016/bs.acc.2019.07.012
32. Zsuga J, Tajti G, Papp C, Juhasz B, Gesztelyi R. FNDC5/irisin, a molecular target for boosting reward-related learning and motivation. Med Hypotheses. (2016) 90:23–28. doi: 10.1016/j.mehy.2016.02.020
33. Mukhopadhyay S, Mondal S, Kumar M, Dutta D. Proinflammatory and antiinflammatory attributes of fetuin-a: a novel hepatokine modulating cardiovascular and glycemic outcomes in metabolic syndrome. Endocr Pract. (2014) 20:1345–51. doi: 10.4158/EP14421.RA
34. Long M, Wang N, Larson M, Mitchell G, Palmisano J, Vasan R, et al. Nonalcoholic fatty liver disease and vascular function: cross-sectional analysis in the Framingham heart study. Arterioscler Thromb Vasc Biol. (2015) 35:1284–91. doi: 10.1161/ATVBAHA.114.305200
35. Harada P, Bensenõr I, Drager L, Goulart A, Mill J, Lotufo PJA. Non-alcoholic fatty liver disease presence and severity are associated with aortic stiffness beyond abdominal obesity: The ELSA-Brasil. Atherosclerosis. (2019) 284:59–65. doi: 10.1016/j.atherosclerosis.2019.02.005
36. Yuan J, Zhang Z, Wen H, Hong X, Hong Z, Qu Q, et al. Incidence of dementia and subtypes: A cohort study in four regions in China. Alzheimers Dement. (2016) 12:262–71. doi: 10.1016/j.jalz.2015.02.011
37. Ferretti M, Martinkova J, Biskup E, Benke T, Gialdini G, Nedelska Z, et al. Sex and gender differences in Alzheimer's disease: current challenges and implications for clinical practice: position paper of the dementia and cognitive disorders panel of the european academy of neurology. Eur J Neurol. (2020) 27:928–43. doi: 10.1111/ene.14174
38. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
39. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati F, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. (2011) 54:1082–90. doi: 10.1002/hep.24452
Keywords: longitudinal study, non-alcoholic fatty liver disease, cognitive impairment, middle-aged and older populations, risk factors
Citation: Liu Q, Liu C, Hu F, Deng X and Zhang Y (2022) Non-alcoholic Fatty Liver Disease and Longitudinal Cognitive Changes in Middle-Aged and Elderly Adults. Front. Med. 8:738835. doi: 10.3389/fmed.2021.738835
Received: 05 October 2021; Accepted: 26 November 2021;
Published: 17 January 2022.
Edited by:
Vered Hermush, Technion Israel Institute of Technology, IsraelReviewed by:
Revital Feige Gross Nevo, Beit Rivka Geriatric Rehabilitation Center, IsraelNoa Stern, Laniado Hospital, Israel
Dvorah Sara Shapiro, Shaare Zedek Medical Center, Israel
Copyright © 2022 Liu, Liu, Hu, Deng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumei Zhang, zhangyumei95@aliyun.com
 Qi Liu
Qi Liu Chang Liu1
Chang Liu1 Xuan Deng
Xuan Deng Yumei Zhang
Yumei Zhang