- 1Department of Global Network, M & M Precision Medicine, Tokyo, Japan
- 2Drug Development Department (DITEP), Villejuif, France
- 3INSERM U981, Institut Gustave Roussy, Université Paris-Saclay, Villejuif, France
- 4Department of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- 5Division of Molecular Pharmacology, National Cancer Center, Tokyo, Japan
- 6Department of Pharmacology and Therapeutics, National Cancer Center, Tokyo, Japan
- 7Department of Omics Network, National Cancer Center, Tokyo, Japan
The classical cadherin (CDH), claudin (CLDN) and nectin families of transmembrane-type adhesion molecules are located at adherens or tight junctions in epithelial cells but diffuse to the nonjunctional cell surface in solid tumors with epithelial–mesenchymal plasticity. Human/humanized antibody-drug conjugates (ADCs) with chemical linkers and cytotoxic payloads have been developed for the treatment of malignancies. Here, the clinical development of ADCs that target CDH6, CDH17, CLDN6, CLDN18.2 and NECTIN4 is reviewed. Enfortumab vedotin is an NECTIN4-targeting antibody-drug conjugate that is approved for the treatment of urothelial cancer, whereas other ADCs or derivatives that target NECTIN4, such as bulumtatug fuvedotin, SHR-A2102 and zelenectide pevedotin, are being studied in randomized phase III clinical trials. In contrast, arcotatug tavatecan, garetatug rezetecan, sonesitatug vedotin and tecotabart vedotin are anti-CLDN18.2 ADCs in phase III clinical trials for the treatment of CLDN18.2-positive gastric or gastroesophageal junction adenocarcinomas, and raludotatug deruxtecan is an anti-CDH6 ADC in a phase II/III clinical trial for the treatment of platinum-resistant ovarian cancer. ADCs that target cell-cell adhesion molecules are a rapidly emerging class of cancer therapeutics, and bispecific ADCs and longitudinal companion diagnostics are emerging to further improve the clinical benefits of conventional ADCs.
1 Introduction
Classical cadherins (CDHs), claudins (CLDNs), junctional adhesion molecules (JAMs) and NECTINs are representative cell-cell adhesion molecules (Figure 1): classical CDHs and NECTINs are single-span transmembrane proteins that are present in adherens junctions and are tethered to cytoplasmic bundled actin filaments via the β-catenin/α-catenin complex and afadin, respectively (Gumbiner, 2005; Samanta and Almo, 2015; Takeichi, 2014); CLDNs and JAMs are tetra- and single-span transmembrane proteins, respectively, that are present in tight junctions and are tethered to cytoplasmic actin filaments via zonula occludens (ZO) proteins (Ebnet, 2017; Katoh and Katoh, 2024; Zihni et al., 2016). CDH/NECTIN and CLDN/JAM complexes network together via direct interactions of their extracellular regions and indirect interactions mediated by cytoplasmic scaffolding proteins, which dynamically regulate cell-cell adhesion, apical-basal polarity, transcription and paracellular permeability in epithelial tissues (Campbell et al., 2017; Horowitz et al., 2023; Karaman and Halder, 2018).
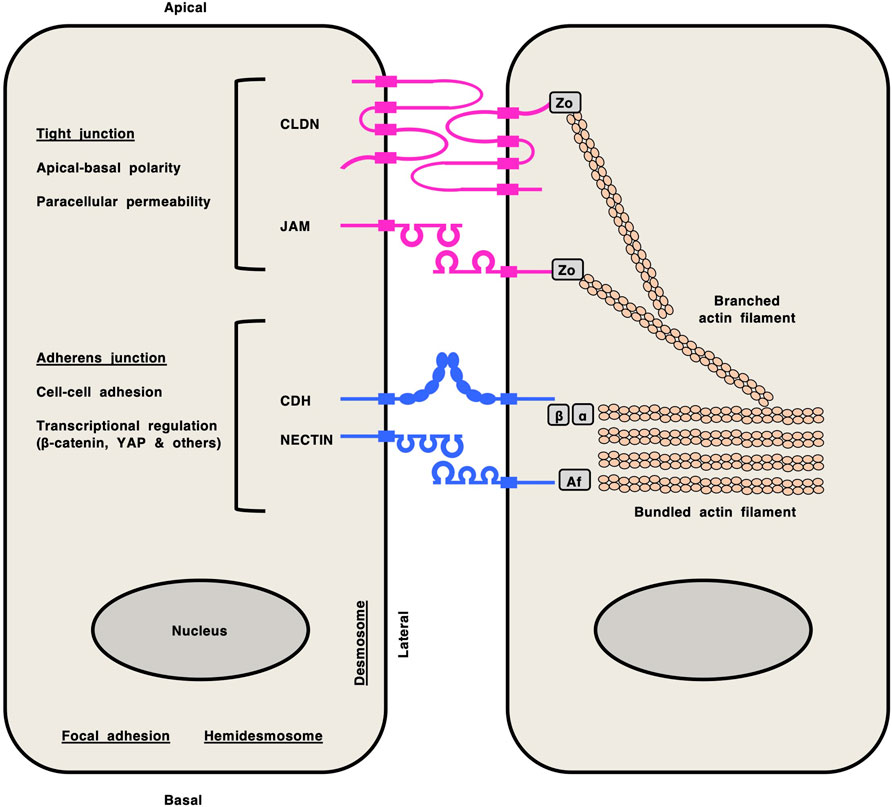
Figure 1. Cell-cell adhesion molecules in epithelial cells. Claudin (CLDN) and junctional adhesion molecule (JAM) at tight junctions are shown in pink, whereas classical cadherin (CDH) and NECTIN at adherens junctions are shown in blue. CLDNs and JAMs are tethered to cytoplasmic branched actin filaments via zonula occludens (Zo) proteins. Classical CDHs and NECTINs are tethered to cytoplasmic bundled actin filaments via the β-catenin (β)/α-catenin (α) complex and afadin (Af), respectively. Network of CLDN/JAM and CDH/NECTIN complexes via direct interactions of their extracellular regions and indirect interactions mediated by cytoplasmic scaffolding proteins regulates versatile functions in epithelial tissues, such as apical-basal polarity, paracellular permeability, cell-cell adhesion and transcription.
In contrast, antibody-drug conjugates (ADCs), each of which consists of a human/humanized monoclonal antibody (mAb), a chemical linker and a cytotoxic payload, are cutting-edge therapeutics that have been designed to increase antitumor activity and mitigate off-tumor adverse effects (Drago et al., 2021; Fu et al., 2022; Katoh and Katoh, 2020). Conventional ADCs targeting transmembrane proteins, such as human epidermal growth factor receptor 2 (HER2), MET, nectin cell adhesion molecule 4 (NECTIN4) and trophoblast cell surface antigen 2 (TROP2) (Figure 2), have been approved for the treatment of specific cancer types by the US Food and Drug Administration (FDA) and regulatory agencies in other countries (Colombo et al., 2024; Dumontet et al., 2023; FDA, 2025; Maecker et al., 2023).
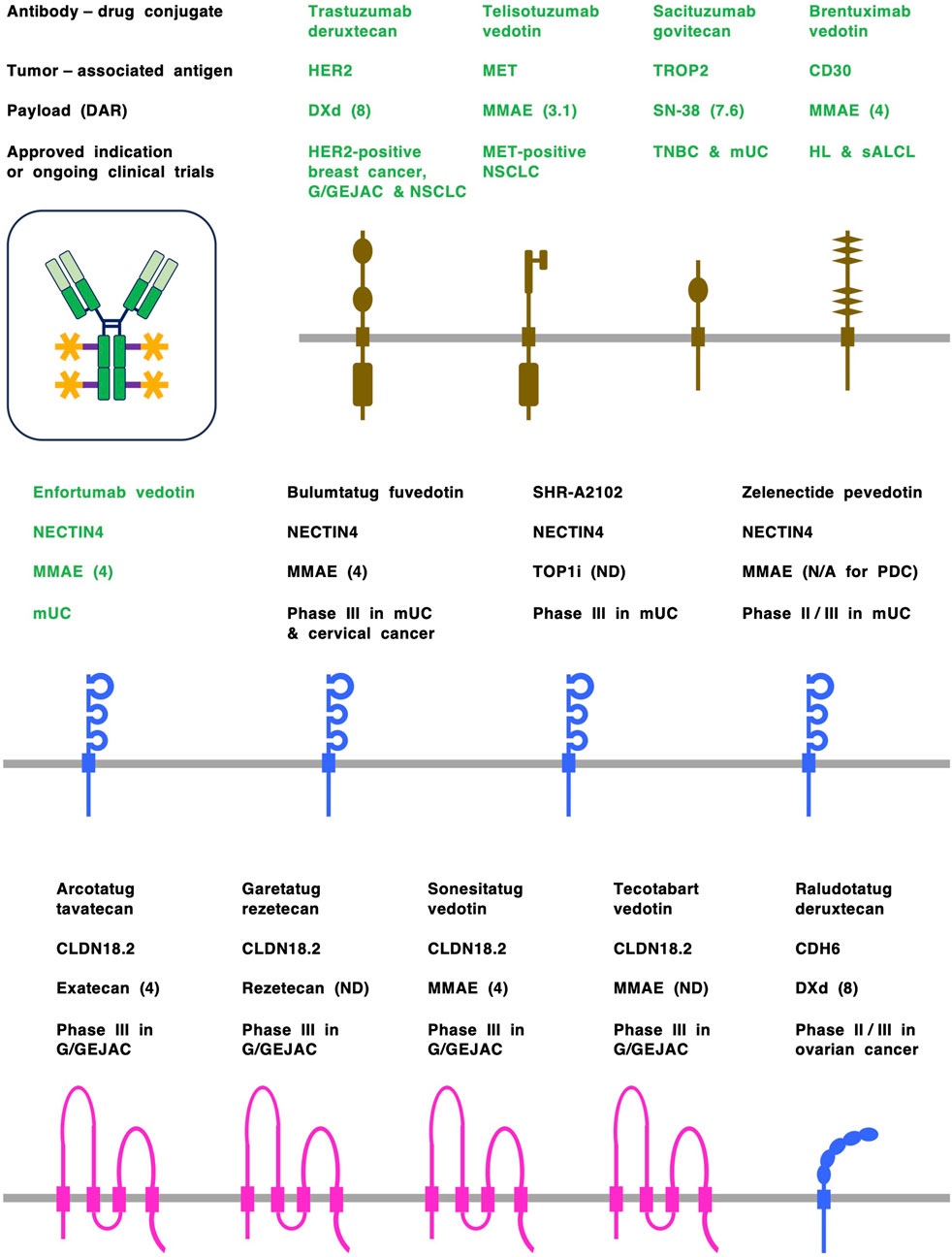
Figure 2. Antibody-drug conjugates (ADCs) and tumor-associated antigens (TAAs). ADCs are designed to preferentially deliver cytotoxic payloads to tumor cells through binding to TAAs and subsequent internalization. ADCs approved by the US Food and Drug Administration (FDA) are shown in green, whereas investigational ADCs in clinical trials are shown in black. TAAs derived from the cadherin (CDH) and NECTIN families of adrerens junction proteins are shown in blue, TAAs derived from the claudin (CLDN) family of tight junction proteins are shown in pink, and TAAs derived from receptor tyrosine kinases, etc., are shown in dark brown. Claudin 18 isoform 2 (CLDN18.2), human epidermal growth factor receptor 2 (HER2), MET, nectin cell adhesion molecule 4 (NECTIN4) and trophoblast cell surface antigen 2 (TROP2) are representative transmembrane-type TAAs on solid tumors, and CD30 is a transmembrane-type TAA on hematological malignancies. Each ADC consists of a human/humanized monoclonal antibody (mAb), a cleavable or non-cleavable chemical linker (purple) and a cytotoxic payload (orange). Monomethyl auristatin E (MMAE) is a microtubule disruptor, whereas deruxtecan (DXd), exatecan, rezetecan and SN-38 are topoisomerase I inhibitors (TOP1i). Drug-to-antibody rate (DAR) is shown in parentheses following payload. G/GEJAC, gastric or gastroesophageal junction adenocarcinoma; HL, Hodgkin’s lymphoma; mUC, metastatic urothelial cancer; N/A, not available; ND, not disclosed; NSCLC, non-small cell lung cancer; PDC, peptide-drug conjugate; sALCL, systemic anaplastic large cell lymphoma; TNBC, triple-negative breast cancer.
The epithelial–mesenchymal plasticity of solid tumors is characterized by dynamic transitions among epithelial, intermediate (quasi-epithelial and quasi-mesenchymal) and mesenchymal cell states, which confer a variety of malignant features, such as invasion and metastasis, drug resistance and immune evasion (Dongre et al., 2022; Katoh and Katoh, 2022; Yang et al., 2020). Because NECTIN4, CLDNs and classical CDHs are located at cell-cell junctions in polarized epithelial tumor cells but diffuse to nonjunctional locations in contact-naïve intermediate or mesenchymal tumor cells (Ebnet, 2017; Nakayama et al., 2024), human/humanized ADCs with cleavable linkers and cutting-edge payloads that target these adhesion molecules are expected to be active on aggressive and/or heterogenous solid tumors with a spectrum of epithelial–mesenchymal transitions in part through bystander killing effects. Here, investigational and/or FDA-approved ADCs targeting NECTIN4, CLDN18.2, CLDN6, CDH6 and CDH17 will be overviewed, and then perspectives in this field will be discussed.
2 NECTIN4-targeting ADCs and ADC derivatives
NECTIN4 is an adherens junction protein with immunoglobulin-like extracellular domains and an afadin-binding cytoplasmic tail (Reymond et al., 2001). Physiologically, it is involved in cell-cell adhesion, but this protein has also been implicated as the measles virus receptor on epithelial cells (Mühlebach et al., 2011) and as a ligand of the immune coinhibitory receptor TIGIT (T-cell immunoglobulin and ITIM domain) (Reches et al., 2020), pointing to an extended role in modulating tumor-immune interactions.
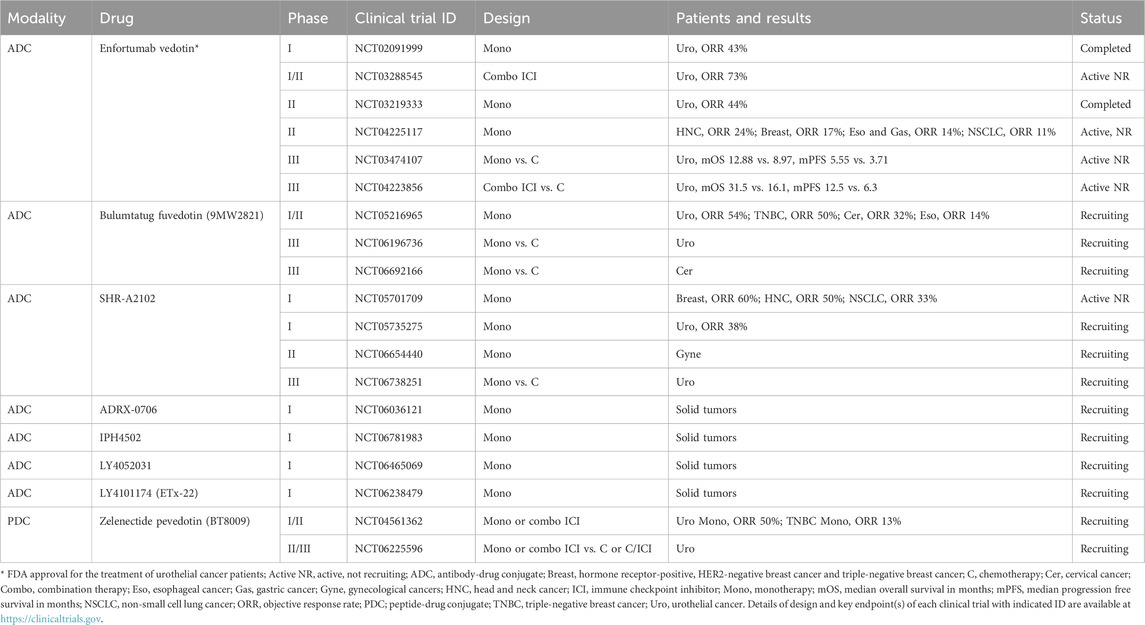
Recently, NECTIN4 was characterized as a tumor-associated antigen (TAA). Immunohistochemical surveys have demonstrated moderate-to-strong NECTIN4 expression in 60.3% of bladder cancer cases (316/524), 52.3% of breast cancer cases (342/654), 36.6% of pancreatic cancer cases (60/164), 27.0% of lung cancer cases (167/618), 24.3% of esophageal cancer cases (44/181), 18.5% of head and neck cancer (HNC) cases (25/135) and 17.8% of ovarian cancer cases (21/118) (Challita-Eid et al., 2016). Given this increased expression, anti-NECTIN4 ADCs were trialed in bladder, breast, lung and pancreatic cancers and have demonstrated responses in preclinical xenograft models (Challita-Eid et al., 2016). These preclinical results have led to clinical trials of NECTIN4-targeting ADCs or ADC derivatives as listed in Table 1.
2.1 Enfortumab vedotin
Enfortumab vedotin is the first-generation NECTIN4-targeting ADC that is composed of a fully human anti-NECTIN4 mAb, a valine-citrulline cleavable linker and a microtubule-disrupting monomethyl auristatin E (MMAE) payload with a drug-to-antibody ratio (DAR) of 4 (Challita-Eid et al., 2016).
A phase I clinical trial of enfortumab vedotin in patients with NECTIN4-expressing solid tumors who progressed on chemotherapy and/or immune checkpoint inhibitor (ICI) therapy (EV-101 study, NCT02091999) revealed manageable tolerability and clinical activities with an objective response rate (ORR) of 42.9% (48/112) and a median overall survival (mOS) of 12.3 months in metastatic urothelial cancer patients (Rosenberg et al., 2020). In this EV-101 study, NECTIN4 expression was initially required for enrollment, but was then removed owing to NECTIN4 overexpression in almost all biopsied samples derived from metastatic urothelial cancers.
A phase II clinical trial of enfortumab vedotin in locally advanced or metastatic urothelial cancer patients with previous platinum-based chemotherapy and ICI therapy (EV-201 study, NCT03219333) also showed manageable tolerability with an ORR of 44.0% (55/125) and a complete response (CR) rate of 12.0% (Rosenberg et al., 2019). FDA accelerated approval was granted for the use of enfortumab vedotin in the treatment of metastatic urothelial cancer patients on the basis of this single-arm registry trial.
In the confirmatory phase III randomized clinical trial in urothelial cancer patients with previous platinum-based and ICI therapies (EV-301 study, NCT03474107), enfortumab vedotin achieved statistically significant and clinically meaningful improvement compared with investigator-chosen chemotherapy: mOS, 12.88 months versus 8.97 months [hazard ratio (HR), 0.70; 95% confidence interval (CI), 0.56 to 0.89; P = 0.001]; and median progression-free survival (mPFS), 5.55 months versus 3.71 months [HR, 0.62; 95% CI, 0.51 to 0.75; P < 0.001] (Powles et al., 2021). FDA regular approval was then granted to enfortumab vedotin for urothelial cancer patients in advanced-line settings.
While these clinical activities of enfortumab vedotin are encouraging, the proportion of complete responses were modest, and durable remissions remain limited with many patients still progressing within 6–12 months. This would suggest that NECTIN4 targeting might improve but not transform the natural history of advanced disease.
On the basis of these landmark results of enfortumab vedotin monotherapy for urothelial cancer, phase II clinical trials for the treatment of patients with breast cancer, esophageal cancer, gastric cancer, HNC and non-small cell lung cancer (NSCLC) (EV-202 study, NCT04225117), adenoid cystic carcinoma (NCT06891560), colorectal cancer or hepatocellular carcinoma (NCT06553885), pancreatic cancer (NCT05915351) and prostate cancer (NCT04754191) are ongoing to expand the indication of enfortumab vedotin monotherapy to other types of NECTIN4-positive cancers (Table 1). In the EV-202 study, cohorts of breast cancer (Giordano et al., 2024), gastroesophageal cancer (Muro et al., 2024a), HNC (Swiecicki et al., 2025) and NSCLC (Muro et al., 2024b) revealed manageable safety profiles and ORRs of 17% (15/87), 14% (12/86), 24% (11/46) and 11% (7/66), respectively. These results of weaker efficacy (ORRs 10%–20%) in non-urothelial tumors suggest that patient selection still remains an unresolved issue as despite ubiquitous NECTIN4 expression in urothelial tumors, this biomarker-based enrichment added little predictive value. This attenuated benefit highlights the need for better predictive biomarkers beyond expression alone (e.g., differential dependency, internalization kinetics, or tumor microenvironment factors).
The most compelling development has been a new frontline standard of enfortumab vedotin plus pembrolizumab due to synergy with checkpoint inhibitors. The phase Ib/II EV-103 study (NCT03288545) showed that combining enfortumab vedotin with pembrolizumab almost doubled ORRs of 64%–73% in front-line urothelial cancer to what would be expected from either monotherapy (Hoimes et al., 2023; O'Donnell et al., 2023). Following the accelerated approval of combination therapy by the FDA, the phase III EV-302 study (NCT04223856) confirmed the major survival advantage (mOS 31.5 months vs. 16.1 months with chemotherapy) with manageable toxicity (Powles et al., 2024). Mechanistically, the synergy may reflect immunogenic cell death triggered by MMAE payload release, enhancing anti-tumor immunity.
The strength of NECTIN4 as a target is in its cell-surface abundance and accessibility, making it highly suitable for ADC delivery in selected tumors. The benefit in urothelial cancer demonstrates the therapeutic potential but the variable efficacy in non-urothelial cancers suggest need of more validated predictive biomarkers beyond expression and improvements in combinations (e.g., immune checkpoint inhibitors), next-generation payloads, or bispecific ADCs.
2.2 Other NECTIN4-targeting ADCs and derivatives
Investigational ADCs or ADC derivatives targeting NECTIN4, such as bulumtatug fuvedotin (9MW2821) (Zhang J et al., 2025; Zhou et al., 2023), ADRX-0706 (Hau et al., 2024), IPH4502 (Sen et al., 2025), LY4052031 (Gao et al., 2025), LY4101174 (ETx-22) (Rosenberg et al., 2024), SHR-A2102 (Tang et al., 2025) and zelenectide pevedotin (BT8009) (Baldini et al., 2023; Loriot et al., 2025), have also entered clinical trials (Table 1).
Bulumtatug fuvedotin is an anti-NECTIN4 ADC conjugated with MMAE (the payload) via a valine-citrulline cleavable linker that has a stabilized and homogeneous DAR of 4 (Zhou et al., 2023). A phase I/II clinical trial of bulumtatug fuvedotin for solid tumor patients (NCT05216965) revealed ORRs of 54% in urothelial cancer, 50% in triple-negative breast cancer, 32% in cervical cancer and 14% in esophageal cancer (Zhang J et al., 2025), and randomized phase III clinical trials of bulumtatug fuvedotin versus chemotherapy in urothelial cancer patients (NCT06196736) and cervical cancer patients (NCT06692166) are ongoing.
SHR-A2102 is a NECTIN4-targeting ADC with a cleavable linker and a topoisomerase I inhibitor (TOP1i) payload that led to an ORR of 38% (28/73) in urothelial cancer patients in a phase I clinical trial in solid tumor patients (NCT05735275) (Tang et al., 2025), and ORRs of 60% (31/52), 50% (6/12) and 33.1% (52/157) in breast cancer, HNC and NSCLC patients, respectively, in another phase I clinical trial (NCT05701709) (Zhong R. et al., 2025). A phase II clinical trial of SHR-A2102 in gynecological cancer patients (NCT06654440) and a randomized phase III clinical trial of SHR-A2102 versus investigator-selected chemotherapy in urothelial cancer patients (NCT06738251) are ongoing.
ADRX-0706, IPH4502, LY4052031 and LY4101174 are next-generation anti-NECTIN4 ADCs with DARs of 8 (Hau et al., 2024; Sen et al., 2025; Gao et al., 2025; Rosenberg et al., 2024) that are currently in phase I clinical trials for the treatment of solid tumors (NCT06036121, NCT06781983, NCT06465069 and NCT06238479, respectively).
Zelenectide pevedotin is an ADC derivative of the peptide-drug conjugate that consists of a NECTIN4-binding bicyclic peptide, a valine-citrulline cleavable linker and an MMAE payload with a peptide-to-drug ratio of 1 (Rigby et al., 2022). Because of its lower molecular weight of approximately 4 kDa, high systemic Cmax value and ability to perform protease-dependent cleavage in the tumor microenvironment (TME), zelenectide pevedotin was expected to improve tumor penetration, enhance clinical efficacy and mitigate adverse events. In an ongoing phase I/II clinical trial for solid tumor patients (NCT04561362), zelenectide pevedotin a revealed manageable safety profile and preliminary ORRs of 50% (4/8) in urothelial cancer patients (Baldini et al., 2023) and 13% (4/30) in triple-negative breast cancer patients (Klümper et al., 2025). A phase II/III clinical trial of zelenectide pevedotin monotherapy or combination therapy with pembrolizumab versus chemotherapy for the treatment of urothelial cancer is also ongoing (Loriot et al., 2025).
3 CLDN-targeting ADCs
The 26 human CLDN proteins encoded by 24 human genes are tight junction proteins that consist of four transmembrane domains, cytoplasmic N- and C-terminal regions, a cytoplasmic loop and two extracellular loops (Katoh and Katoh, 2024). Investigational mAbs targeting the first or second extracellular loops of CLDNs have been generated for preclinical studies, and some of them have been tested in clinical trials (Chen et al., 2023; Nakayama et al., 2024; Vonniessen et al., 2024).
The clinical benefits of the mouse/human chimeric anti-CLDN18.2 mAb zolbetuximab (IMAB362) were demonstrated in the randomized phase III clinical trials GLOW (NCT03653507) and SPOTLIGHT (NCT03504397) (Shah et al., 2023; Shitara et al., 2023), and the FDA approved zolbetuximab plus chemotherapy for the treatment of gastric or gastroesophageal junction adenocarcinoma (G/GEJAC).
The clinical activities of zolbetuximab have led to the rapid emergence of human/humanized anti-CLDN mAbs and other CLDN-targeted therapies such as ADCs, bispecific antibodies and chimeric antigen receptor T (CAR-T) cell modalities (Katoh and Katoh, 2024; Nakayama et al., 2024). CLDN18.2 and CLDN6 are representative TAAs that can be clinically targeted with ADCs (Table 2).
3.1 CLDN18.2-targeting ADCs
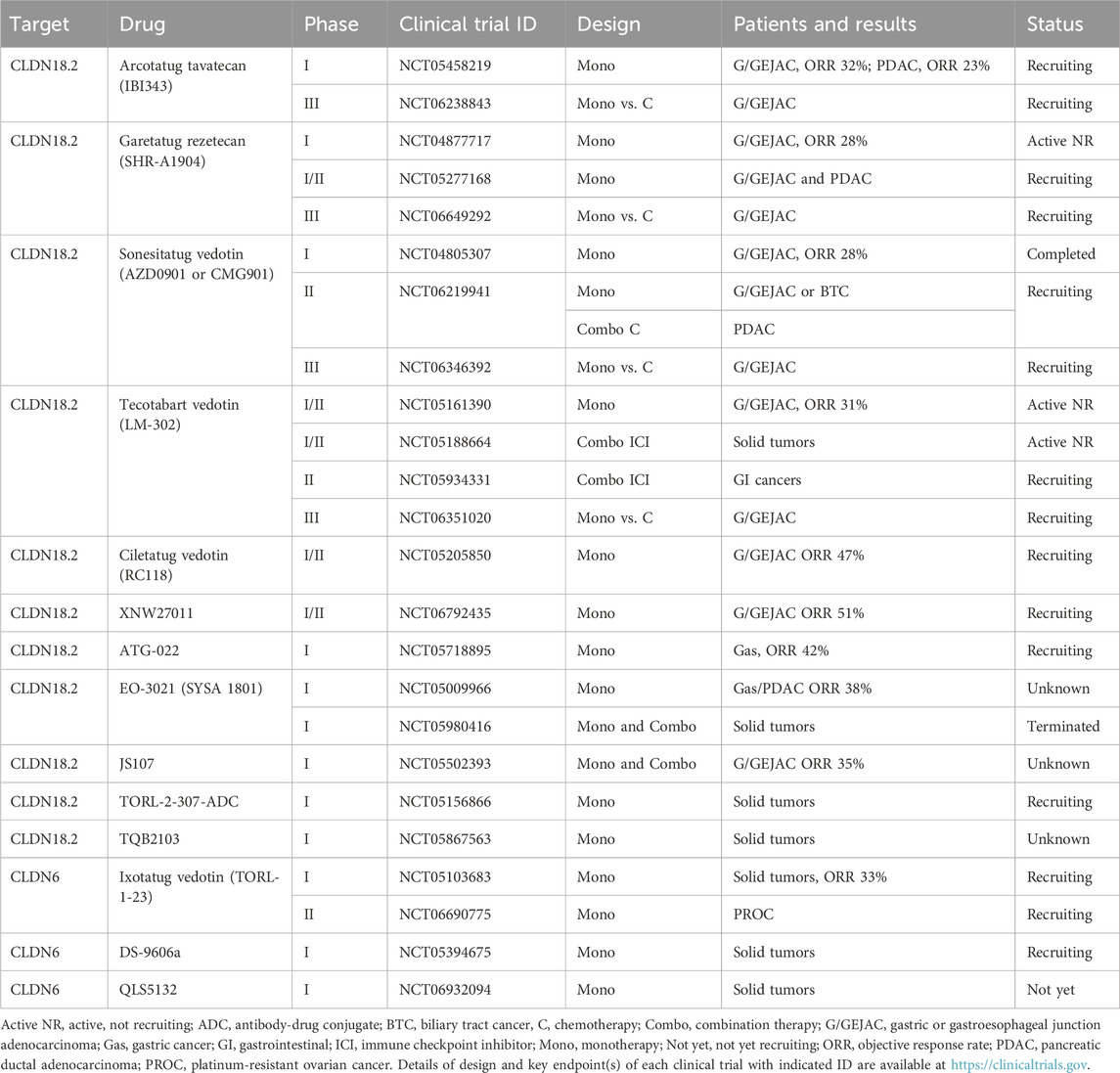
The CLDN18 gene at human chromosome 3q22.3 encodes CLDN18.1 and CLDN18.2 isoforms that are expressed in the lung and stomach, respectively, on the basis of alternative promoters (Niimi et al., 2001). CLDN18.1 and CLDN18.2 are almost identical expect for the N-terminal cytoplasmic region, the first transmembrane domain and a part of the first extracellular loop that are derived from alternative exons 1a and 1b, respectively (Katoh et al., 2024b). CLDN18.2 is orthotopically overexpressed in 27–56% of gastric adenocarcinomas (Dottermusch et al., 2019; Nakayama et al., 2024; Sahin et al., 2008) and ectopically overexpressed in 50% of esophageal adenocarcinomas (Sahin et al., 2008), 30–60% of pancreatic ductal adenocarcinomas (PDACs) (Lyu et al., 2024; Sahin et al., 2008), 10% of ovarian adenocarcinomas (Sahin et al., 2008) and 4% of NSCLCs (Micke et al., 2014).
ATG-022 (Ma et al., 2025), ciletatug vedotin (RC118) (Liu T et al., 2024), EO-3021 (CPO102 or SYSA 1801) (Wang Y. et al., 2023), JS107 (Xu R. H. et al., 2025), sonesitatug vedotin (AZD0901 or CMG901) (Xu G. et al., 2024; Ruan et al., 2025), tecotabart vedotin (LM-302, BMS-986476 or TPX-4589) (Huang W et al., 2022; Bai C et al., 2024) and TORL-2-307-ADC (O'Brien et al., 2023) are human/humanized anti-CLDN18.2 ADCs with an MMAE payload, while arcotatug tavatecan (IBI343) (Liu J. J et al., 2024; Yu X. et al., 2025b; Shen et al., 2025), garetatug rezetecan (SHR-A1904) (Xu R. H. et al., 2024), TQB2103 (Cheng X et al., 2025) and XNW27011 (Yu J. et al., 2024 and 2025) are CLDN18.2-targeting ADCs with TOP1i payloads. All of these investigational anti-CLDN18.2 ADCs are being tested in clinical trials (Table 2).
Sonesitatug vedotin consists of a humanized anti-CLDN18.2 immunoglobulin G subtype 1 (IgG1) mAb, a cleavable linker and an MMAE payload with a DAR of 4 (Xu G. et al., 2024). Sonesitatug vedotin has revealed direct killing effects on CLDN18.2-overexpressing cancer cells via MMAE-dependent cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) as well as indirect killing effects on bystander cancer cells through MMAE release from sonesitatug vedotin-internalized cells, which results in antitumor activities in preclinical models of gastric and pancreatic cancers (Xu G. et al., 2024). A phase I clinical trial of sonesitatug vedotin in patients with advanced solid tumors (KYM901 study, NCT04805307) revealed manageable safety profiles despite dose reduction and discontinuation owing to treatment-emergent adverse events in 14% and 7%, respectively, and promising clinical activities, such as a confirmed ORR of 28% (32/113) in G/GEJAC patients and a confirmed ORR of 33% (31/93) in CLDN18.2-high subgroup, which was defined as “CLDN18.2 membrane staining of 2+/3+ intensity in at least 20% of tumor cells using a diagnostic antibody EPR19202-244 instead of the standard diagnostic antibody 43-14A” (Ruan et al., 2025). A phase II clinical trial of sonesitatug vedotin monotherapy for CLDN18.2-positive G/GEJAC or biliary tract cancer patients and the combination of sonesitatug vedotin plus chemotherapy for CLDN18.2-positive PDAC patients (CLARITY-PanTumor01 study, NCT06219941) as well as a phase III randomized clinical trial of sonesitatug vedotin monotherapy versus the investigator’s choice of therapy for CLDN18.2-positive G/GEJAC patients in second- or later-line settings (CLARITY-Gastric 01 study, NCT06346392) are ongoing. The FDA’s fast-track designation was granted to sonesitatug vedotin monotherapy for the treatment of CLDN18.2-positive G/GEJAC (Rosa, 2023).
Tecotabart vedotin, which also consists of a humanized anti-CLDN18.2 IgG1 mAb, a cleavable linker and an MMAE payload, demonstrated superior antitumor efficacy to the mouse/human chimeric mAb zolbetuximab in a preclinical xenograft model of gastric cancer (Huang W et al., 2022). A phase I/II clinical trial of tecotabart vedotin for the treatment of advanced solid tumors (NCT05161390) revealed manageable safety and tolerability in a phase I part and a single-agent ORR of 31% (11/36) in CLDN18.2-positive G/GEJAC patients (Bai C et al., 2024). Phase I/II clinical trials of tecotabart vedotin plus immune checkpoint inhibitor toripalimab (NCT05188664 and NCT05934331) revealed ORR of 73% (24/33) in G/GEJAC or esophageal adenocarcinoma patients with CLDN18.2 staining of 2+/3+ intensity in at least 25% of tumor cells (Jiang et al., 2025). A phase III randomized clinical trial of tecotabart vedotin monotherapy versus the investigator’s choice of therapy for the treatment of CLDN18.2-positive G/GEJAC is ongoing (NCT06351020).
Among other CLDN18.2-targeting ADCs with an MMAE payload, phase I studies of ATG-022 (NCT05718895), EO-3021 (NCT05009966) and JS107 (NCT05502393) revealed ORRs of 42% (5/12) in gastric cancer patients (Ma et al., 2025), 38% (8/21) in gastric or pancreatic cancer patients (Wang Y. et al., 2023) and 35% (8/23) in CLDN18.2-high G/GEJAC patients (Xu R. H. et al., 2025), respectively, whereas a phase I/II clinical trial of ciletatug vedotin (NCT05205850) revealed an ORR of 47% (8/17) in CLDN18.2-positive G/GEJAC patients (Liu T et al., 2024).
In contrast, arcotatug tavatecan consists of an engineered anti-CLDN18.2 IgG1 mAb with fragment crystallizable (Fc) silencing, a cleavable linker and an exatecan payload with a DAR of 4, which was designed to reduce the risk of adverse events owing to defects in Fc-mediated ADCC (Liu J. J et al., 2024; Yu X. et al., 2025b). A phase I clinical trial of arcotatug tavatecan for the treatment of advanced solid tumors (NCT05458219) revealed manageable tolerability and clinical activity, including a single-agent ORR of 32% (32/99) in CLDN18.2-positive G/GEJAC patients (Liu J. J et al., 2024) and confirmed ORRs of 23% (10/44) versus 0% (0/12), mPFS of 5.4 versus 1.4 months and mOS of 9.1 versus 6.2 months in PDAC patients with CLDN18.2 staining in at least 60% versus less than 60% of tumor cells (Yu X. et al., 2025b). On the basis of these results, breakthrough therapy designation was granted to arcotatug tavatecan monotherapy for the treatment of CLDN18.2-positive G/GEJAC patients by the China National Medical Products Administration (NMPA), whereas fast-track designation was granted by the US FDA for the use of arcotatug tavatecan monotherapy in the treatment of PDAC (Wahner, 2024). A phase III randomized clinical trial of arcotatug tavatecan monotherapy versus the investigator’s choice of therapy (G-HOPE-001 study, NCT06238843) is recruiting G/GEJAC patients with CLDN18.2 membrane staining of 2+/3+ intensity in at least 75% of tumor cells (Shen et al., 2025).
Garetatug rezetecan, TQB2103 and XNW27011 are also anti-CLDN18.2 ADCs with TOP1i payloads. Garetatug rezetecan without disclosed DAR revealed a manageable safety profile and ORR of 28% (16/58) in CLDN18.2-positive G/GEJAC patients in a phase I clinical trial (NCT04877717) (Xu R. H. et al., 2024) and proceeded to phase I/II (NCT05277168) and phase III (NCT06649292) clinical trials. TQB2103 with a DAR of eight exhibited a favorable safety profile and ORRs of 20% (6/30) and 43% (3/7) in solid tumor patients with CLDN18.2 staining in at least 10% of tumor cells and CLDN18.2 staining of 2+/3+ intensity in at least 40% of tumor cells, respectively, in a phase I clinical trial (NCT05867563) (Cheng X et al., 2025). XNW27011, with a homogenous DAR of 8, showed a favorable safety profile in the dose escalation cohort of solid tumor patients (Yu J. et al., 2024) and ORRs of 31% (9/29), 61% (19/31) and 67% (12/18) in dose expansion cohorts of 2.4, 3.0 and 3.6 mg/kg doses, respectively, of G/GEJAC patients with CLDN18.2 staining of 2+/3+ intensity in at least 5% of tumor cells (Yu et al., 2025a) in a phase I/II clinical trial (NCT06792435). XNW27011 was licensed out to Astellas Pharma that manufactures and sells enfortumab vedotin and zolbetuximab (Plieth, 2025).
3.2 CLDN6-targeting ADCs
CLDN6 is expressed in pluripotent stem cells and fetal tissues but repressed in most adult tissues (Ben-David et al., 2013; Kong et al., 2021; Reinhard et al., 2020), whereas CLDN6 is overexpressed in 54–100% of germ cell tumors, 14–55% of ovarian cancers, 17–21% of endometrial cancers, 10–52% of gastric cancers and 6–11% of NSCLCs (Du et al., 2021; Micke et al., 2014; Qu et al., 2021; Zhang C. et al., 2021). The development of CLDN6-targeting biologics has become a trend on the basis of the potential of CLDN6 as a TAA (Katoh and Katoh, 2024).
Ixotatug vedotin (TORL-1-23) is a humanized anti-CLDN6 ADC with a conventional MMAE payload with a DAR of 4 (McDermott et al., 2023; Konecny et al., 2024), whereas AT65474 (Zhong C. et al., 2025), C6P (Yu X. et al., 2025a), DS-9606a (Patel et al., 2024), GB01-VA-PL2202 (Tsang et al., 2024), PLB-002 (Pan et al., 2025) and QLS5132 (Huang Y et al., 2025) are anti-CLDN6 ADCs with non-MMAE payloads. Among these investigational anti-CLDN6 ADCs, ixotatug vedotin, DS-9606a and QLS5132 are being tested in clinical trials (Table 2).
A phase I clinical trial of ixotatug vedotin for the treatment of advanced cancers (NCT05103683) revealed favorable safety profiles and preliminary efficacy across CLDN6-positive ovarian, endometrial and testicular cancer patients (Konecny et al., 2024). Notably, the ORR in patients treated with 0.2–3.0 mg/kg doses of ixotatug vedotin was 33% (21/64), and the ORRs in CLDN6-positive platinum-resistant ovarian cancer patients with 2.4 or 3.0 mg/kg ixotatug vedotin were 50% and 42%, respectively (Konecny et al., 2024). On the basis of promising clinical activities in the NCT05103683 clinical trial, a registry phase II clinical trial of ixotatug vedotin for the treatment of CLDN6-positive platinum-resistant epithelial ovarian cancer is ongoing (CATALINA-2 study, NCT06690775).
DS-9606a and QLS5132 are in phase I clinical trials (NCT05394675 and NCT06932094, respectively) for the treatment of advanced solid tumors (Table 2). DS-9606a revealed preliminary partial responses in four of 53 cancer patients (Patel et al., 2024), whereas QLS5132, which has a wide therapeutic window, is expected to exhibit superior efficacy and safety profiles in patients with CLDN6-positive ovarian cancers, gastric cancers, NSCLCs and other cancers (Huang Y et al., 2025).
4 CDH-targeting ADCs
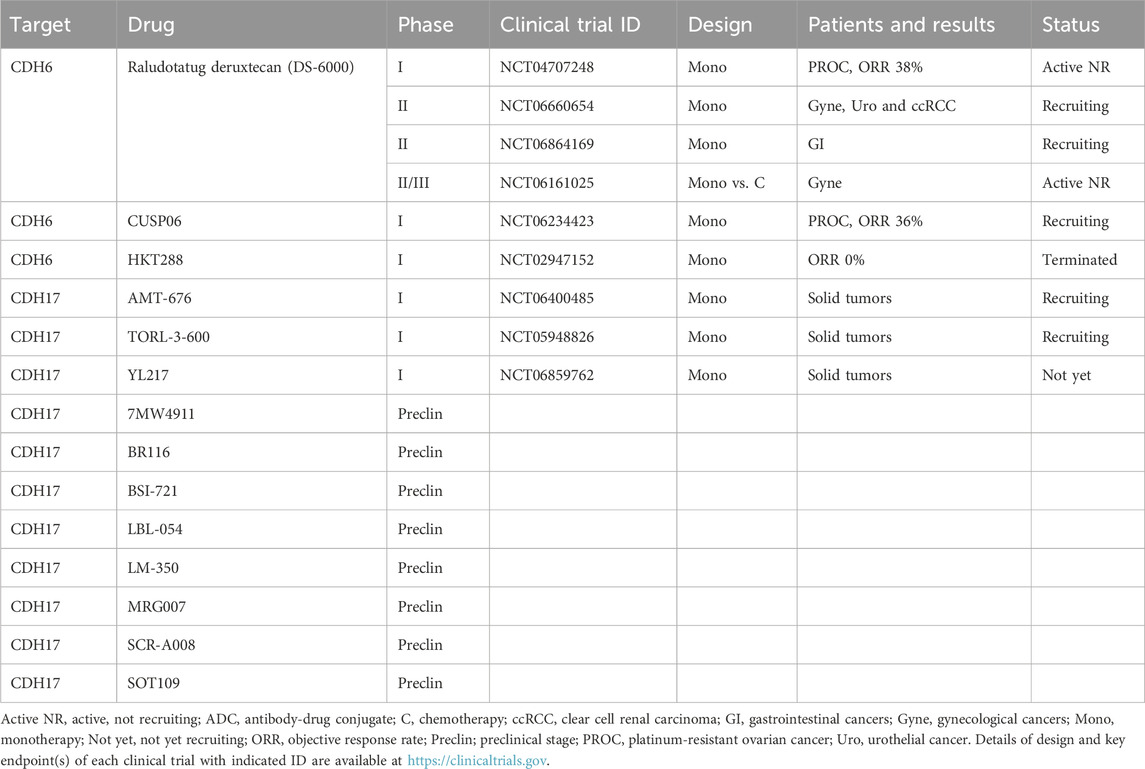
CDH1 (E-cadherin, epithelial), CDH2 (N-cadherin, neuronal), CDH3 (P-cadherin, placental), CDH4 (R-cadherin, retinal), CDH5 (VE-cadherin, vascular endothelial) and CDH6 (K-cadherin, fetal kidney) are representative classical CDHs that contain five extracellular cadherin-repeat (EC) domains, a single transmembrane domain and a cytoplasmic region interacting with armadillo-repeat proteins, such as β-catenin and p120-catenin (Gumbiner, 2005; Takeichi, 2014), whereas CDH16 (KSP-cadherin, kidney specific) and CDH17 (LI-cadherin, liver-intestine) are nonclassical CDHs with seven EC domains, a single transmembrane domain and a truncated cytoplasmic region defective in catenin-binding motifs (Kreft et al., 1997; Wendeler et al., 2004). Because CDH6 (Köbel et al., 2008; Luo S. et al., 2021; Paul et al., 1997; Sato et al., 2025) and CDH17 (Cheng G et al., 2025; Jacobsen et al., 2024; O'Brien et al., 2024) have emerged as potential TAAs, CDH6- and CDH17-targeting ADCs have entered clinical trials (Table 3).
4.1 CDH6-targeting ADCs
CDH6 is a classical CDH that interacts with αIIbβ3 or α2β1 integrins and activates α2β1 integrin signaling to promote the invasion and metastasis of ovarian cancer and renal cell carcinoma (Bartolomé et al., 2021). Because CDH6 is upregulated in endometrial cancer, gastric cancer, ovarian cancer, pancreatic cancer, papillary thyroid cancer and renal cell carcinoma, CLDN6-targeting ADCs, such as CUSP06 (AMT-707), HKT288 and raludotatug deruxtecan (R-DXd or DS-6000a), have been developed for the treatment of cancer (Bialucha et al., 2017; Patel et al., 2025; Suzuki et al., 2024).
HKT288, a first-generation anti-CDH6 ADC with a microtubule-targeting DM4 payload, has shown durable preclinical activity in 40% of patient-derived xenograft (PDX) models, especially those derived from ovarian cancer and renal cell carcinoma (Bialucha et al., 2017). HKT288 entered a phase I clinical trial in patients with epithelial ovarian cancer and renal cell carcinoma (NCT02947152), because preclinical or coclinical studies of PDX models that harbor conserved characteristics of primary tumors could predict ADC activities in clinical trials (Yagishita et al., 2023). However, HKT288 exhibited unexpected neurological toxicity, and its clinical development was discontinued (Schöffski et al., 2021).
CUSP06 and raludotatug deruxtecan are second-generation CDH6-trgeting ADCs with TOP1i payloads, exatecan and exatecan derivative deruxtecan (DXd), respectively (Patel et al., 2025; Suzuki et al., 2024). Phase I clinical trials of CUSP06 (CUSP06-1001 study, NCT06234423) and raludotatug deruxtecan (NCT04707248) revealed ORRs of 36% (5/14) (Patel et al., 2025) and 38% (13/34) (Moore et al., 2023), respectively, in platinum-resistant ovarian cancer patients. The encouraging clinical activities and manageable safety profiles observed with these clinical trials led to the fast-track designation of CUSP06 by the FDA (Sava, 2025) and the launch of a phase II/III clinical trial of raludotatug deruxtecan (NCT06161025).
4.2 CDH17-targeting ADCs
CDH17 is a nonclassical CDH that interacts with the desmosomal cadherin desmocollin-1 (DSC1) and indirectly interacts with actin filaments via the DSC1/p120-catenin complex to promote the migration and invasion of colorectal cancer (Bartolomé et al., 2024). CDH17 is upregulated in 50–100% of colorectal cancer cases, 52–73% of gastric cancer cases, 61% of mucinous ovarian cancer cases, 53% of cervical adenocarcinoma cases, and 20–31% of pancreatic cancer cases (Cheng G et al., 2025; Jacobsen et al., 2024; O'Brien et al., 2024).
Recently, CDH17-targeting ADCs, including 7MW4911 (Wang R. et al., 2025), AMT-676 (Lemech et al., 2025), BR116 (Zhao et al., 2025), BSI-721 (Hao et al., 2025), LBL-054 (Ye et al., 2025), LM-350 (Huang W et al., 2025), MRG007 (Zeng et al., 2025), SCR-A008 (Cheng G et al., 2025), SOT109 (Kalynovska et al., 2025), TORL-3-600 (O'Brien et al., 2024) and YL217 (Lian et al., 2025), have been developed: BSI-721 and TORL-3-600 are humanized ADCs with an MMAE payload, whereas 7MW4911, AMT-676, BR116, LBL-054, LM-350, MRG007, SCR-A008, SOT109 and YL217 are human/humanized ADCs with TOP1i payloads; notably, the DARs of LM-350 and SCR-A008 are 8, and the DARs of 7MW4911, AMT-676, BR116 and BSI-721 are 4. Among these investigational anti-CDH17 ADCs, AMT-676, TORL-3-600 and YL217 have entered phase I clinical trials for the treatment of advanced cancers (NCT06400485, NCT05948826 and NCT06859762, respectively).
5 Perspectives
FDA-approved ADCs have drastically changed clinical practices for the treatment of urothelial cancer (Powles et al., 2021), HER2-positive breast cancer (Hurvitz et al., 2023) and CD30-positive peripheral T-cell lymphoma (Horwitz et al., 2022) and other cancer types (Figure 2). Nevertheless, several issues remain to be overcome to further enhance the anticancer effects and reduce the off-tumor effects of ADCs. Recent progress in antibody engineering, payload diversity, chemical linkers and conjugation technologies for the tumor-specific delivery of cytotoxic drugs with DARs of 4–8 (Colombo et al., 2024; Dumontet et al., 2023; Tsuchikama et al., 2024), adverse events of FDA-approved ADCs, such as neutropenia and interstitial lung disease or pneumonitis (Ballestín et al., 2025), and resistance mechanisms (Drago et al., 2021; Khoury et al., 2023; Li S et al., 2025) have been reviewed elsewhere, whereas progress regarding bispecific ADCs and longitudinal companion diagnostics are discussed here.
5.1 Bispecific ADCs targeting adhesion molecules
Bispecific ADCs are largely classified into ADCs that bind to dual epitopes on the same TAA (biparatopic) and ADCs that bind to dual epitopes on distinct TAAs (bispecific in a narrow sense) (Tsuchikama et al., 2024). Bispecific ADCs that can specifically bind to tumor cells and be efficiently internalized and processed for payload release have been developed with promise for improved clinical benefits and mitigated adverse effects.
Receptor tyrosine kinases (RTKs) constitute the major class of TAAs for bispecific ADCs (Figure 3), and those targeting HER2 x HER2 (anbenitamab repodatecan [JSKN003], TQB2102, KM501, MEDI4276 and ZW49), EGFR x HER3 (izalontamab brengitecan [BL-B01D1]), EGFR x MET (AZD9592) and MET x MET (REGN5093-M114) have entered clinical trials (Gu et al., 2024). Among RTK-targeting bispecific ADCs, anbenitamab repodatecan (Park et al., 2024) and TQB2102 (Li J et al., 2025) have been used in randomized phase III clinical trials for HER2-positive breast cancer patients (NCT06846437 and NCT06561607, respectively), and izalontamab brengitecan (Ma et al., 2024) has been assessed in phase III clinical trials for patients with nasopharyngeal carcinoma (NCT06118333), lung cancer (NCT06382116 and NCT06500026) and other types of cancers (NCT06304974, NCT06343948, NCT06382142 and NCT06857175).
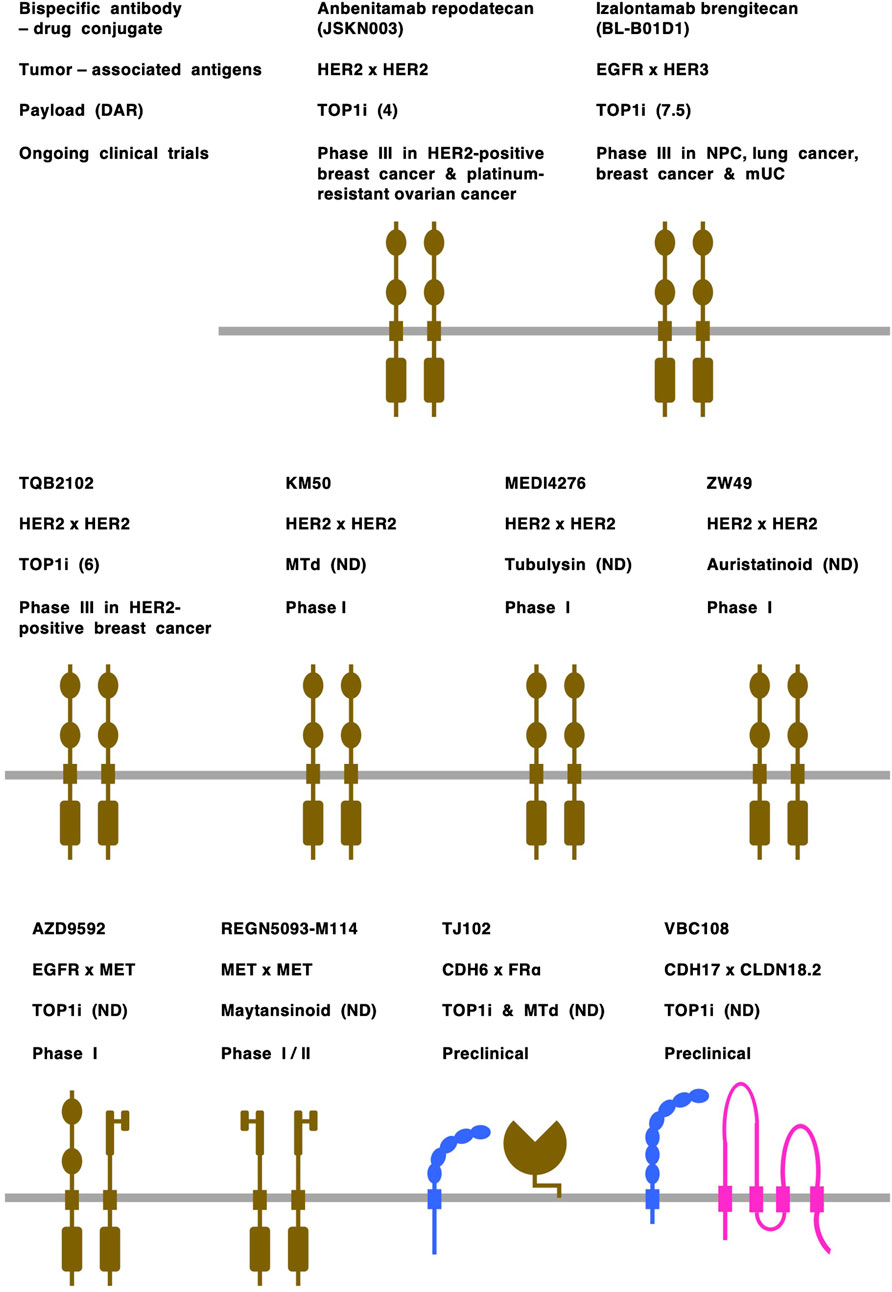
Figure 3. Bispecific antibody-drug conjugates (ADCs). Tumor-associated antigens: cadherin-6 (CHD6) and CDH17 are shown in blue; claudin 18 isoform2 (CLDN18.2) is shown in pink; and receptor tyrosine kinases, including epidermal growth factor receptor (EGFR), human EGFR2 (HER2), human EGFR3 (HER3) and MET, and folate receptor alpha (FRα) are shown in dark brown. Payloads: monomethyl auristatin E (MMAE), auristatinoid, maytansinoid and tubulysin are microtubule disruptors (MTd); other payloads are topoisomerase I inhibitors (TOP1i). Drug-to-antibody rate (DAR) is shown in parentheses following payload. mUC, metastatic urothelial cancer; ND, not disclosed; NPC, nasopharyngeal cancer.
Cell-cell adhesion molecules are emerging as a new class of TAAs for bispecific ADCs (Figure 3), and those targeting CDH3 x CDH17 (Synan et al., 2025), CDH6 x FRα (folate receptor alpha) (Zhang Y et al., 2025), CDH17 x CLDN18.2 (Wang W. V. et al., 2025) and CLDN3 x EpCAM (epithelial cell adhesion molecule) (Luo M. et al., 2025) have been shown to have anti-tumor effects on colorectal cancers, ovarian/kidney cancers, gastrointestinal cancers and solid tumors, respectively, in preclinical studies. In addition, on the basis of the overexpression of CLDN18.2 (Dottermusch et al., 2019; Nakayama et al., 2024; Sahin et al., 2008) and RTKs (Katoh et al., 2024a; Lee et al., 2012; Van Cutsem et al., 2015) in human gastric cancers, it was previously predicted that bispecific ADCs, such as CLDN18.2 x FGFR2 (fibroblast growth factor receptor 2), CLDN18.2 x HER2 and CLDN18.2 x MET, would be developed for the treatment of minor subsets of gastric cancer patients in the future (Katoh and Katoh, 2024).
Bispecific ADCs targeting adhesion molecules remain in the preclinical stage, probably owing to the lack of mechanistic understanding and clinical validation of TAA coexpression in primary tumor cells. Because TJ102, which targets both CDH6 and FRα (Zhang J et al., 2025), and VBC108, which bispecifically targets CDH17 and CLDN18.2 (Wang W. V. et al., 2025), demonstrated efficacy in mouse models and tolerability in cynomolgus monkeys, TJ102 and VBC108 are anticipated to enter clinical trials in patients with solid tumors, especially ovarian cancer and gastric cancer, respectively.
5.2 Longitudinal companion diagnostics
Immunohistochemical staining of NECTIN4 is not currently required for the use of the FDA-approved enfortumab vedotin (Klümper et al., 2023), owing to frequent NECTIN4 expression in urothelial tumors (immunohistochemical staining positive in 82.8% and strong/moderate staining in 60.3%) (Challita-Eid et al., 2016). However, because membranous immunohistochemical staining (Klümper et al., 2023), mRNA expression (Stewart et al., 2024) and gene amplification (Klümper et al., 2024) of NECTIN4 are predictive biomarkers of the enfortumab vedotin response, and because the NECTIN4 mRNA level in urothelial cancer is upregulated in luminal subtypes but downregulated in the neuroendocrine-like subtype (Chu et al., 2021), companion diagnostics of enfortumab vedotin are emerging as a hot issue for the real-world management of urothelial cancer patients.
Immunohistochemical staining of CLDN18.2 is utilized as a selection biomarker for the treatment with investigational CLDN18.2-targeting ADCs because G/GEJAC patients with 2+/3+ staining in at least 20% versus less than 20% of tumor cells revealed ORRs of 33% versus 5%, respectively, in the KYM901 study of sonesitatug vedotin (Ruan et al., 2025). CLDN18.2 staining positivities on the basis of designated diagnostic antibody, immunohistochemical staining intensity and positive cell content are inclusion criteria for phase III clinical trials of anti-CLDN18.2 ADCs, such as arcotatug tavatecan (NCT06238843), sonesitatug vedotin (NCT06346392), garetatug rezetecan (NCT06649292) and tecotabart vedotin (NCT06351020), and may be applied as companion diagnostics in the future.
In contrast, positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are medical imaging technologies that can detect primary tumors and metabolic lesions at the whole-body level (Voss, 2023; McGale et al., 2023). PET/CT hybrid imaging with radiolabeled NECTIN4-targeted bicyclic peptides, such as 68Ga-N188 and 68Ga-FZ-NR-1, was applied for urothelial cancer patients (Zhang J. et al., 2024; Sun et al., 2025), and PET/CT and SPECT/CT with radiolabeled CLDN18.2-targeted nanobodies, such as [68Ga]Ga-PMD22 and [99mTc]Tc-PHG102, respectively, were used for gastrointestinal cancer patients (Wang R. et al., 2024; Bai Z et al., 2024). In addition, CDH17-targeted hybrid imaging has been investigated in preclinical studies of PDAC and gastric cancer (Delaney et al., 2024; Mao et al., 2025). Investigational hybrid imaging features that are correlated with membranous immunohistochemical staining and can be used to detect primary, recurrent or metastatic tumor lesions are entering into clinical trials as noninvasive companion diagnostics of ADCs.
Innate and acquired resistance to ADCs are still poorly understood, but might be caused by various mechanisms, including (1) resistance to cytotoxic payloads, (2) inaccessibility of ADCs to tumor cells owing to barriers around or within the tumor microenvironment, and (3) the loss of antigens or epitopes on tumor lesions via intra- or intertumor heterogeneity, transdifferentiation and alternative splicing (Dumontet et al., 2023; Katoh et al., 2024b; Mosele et al., 2023; Nasiri et al., 2025). Immunohistochemical analyses in urothelial cancer patients receiving enfortumab vedotin treatment revealed that NECTIN4 antigen loss preferentially occurs in metastatic lesions compared with primary lesions (P < 0.001) and is associated with shortened PFS (P < 0.001) (Klümper et al., 2023). Owing to the NECTIN4 upregulation in luminal subtypes and downregulation in the neuroendocrine-like subtype as mentioned above (Chu et al., 2021), NECTIN4 antigen loss in the neuroendocrine-like subtype can induce innate resistance to enfortumab vedotin, whereas NECTIN4 antigen loss owing to transdifferentiation from the luminal to the endocrine-like subtype can lead to acquired resistance to enfortumab vedotin. In gastric cancer patients, CLDN18.2-staining intensity is decreased in peritoneal metastasis compared with primary lesion (Saito et al., 2025). Although further validation is necessary for generalization, these examples suggest that loss of TAAs, such as NECTIN4 and CLDN18.2, might occur in metastatic lesions owing to adaptation or plasticity of tumor cells in their metastatic niche. Longitudinal monitoring of TAAs before treatment, after treatment and during recurrence could lead to the optimal management of cancer via timely replacement of ADC (Figure 4).
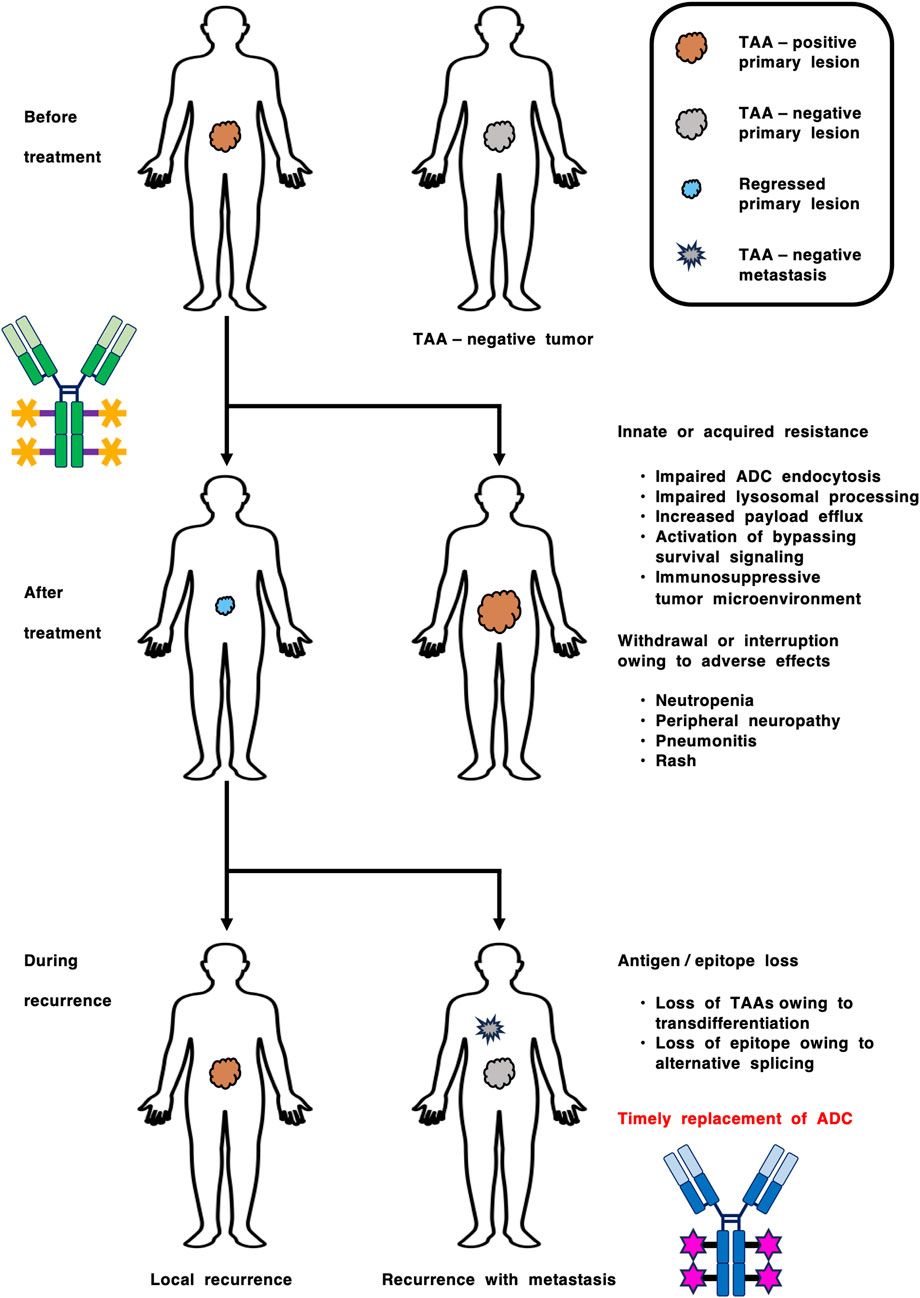
Figure 4. Longitudinal companion diagnostics for the optimization of antibody-drug conjugate (ADC) therapy. Innate and acquired resistance to ADCs are caused by various mechanisms, such as (1) inaccessibility of ADCs to tumor cells, (2) resistance to cytotoxic payloads, (3) loss of tumor-associated antigens (TAAs) owing to intra- or intertumor heterogeneity and transdifferentiation, and (4) loss of epitopes owing to alternative splicing. In contrast, adverse effects, such as neutropenia, peripheral neuropathy, pneumonitis (interstitial lung disease) and rash, lead to withdrawal or interruption of ADC therapy. Longitudinal monitoring of TAAs before treatment, after treatment and during recurrence could lead to improved clinical benefits via timely ADC replacement.
6 Conclusion
ADCs, which target cell-cell adhesion molecules, constitute a rapidly emerging class of cancer therapeutics. Enfortumab vedotin is an FDA-approved NECTIN4-targeting ADC for urothelial cancer, and other NECTIN4-targeting drugs are in phase III clinical trials. Investigational anti-CNDN18.2 ADCs for the treatment of G/GEJAC patients and anti-CLDN6 and anti-CDH6 ADCs for the treatment of ovarian cancer have also proceeded to later-stage clinical trials. NECTIN4 and CLDN18.2 are “passenger” TAAs that are expressed in primary tumors but might be dispensable in metastatic niches, whereas RTKs, such as HER2 and MET, are oncogenic TAAs that drive tumorigenesis by themselves. TAA expression in primary versus metastatic tumors and introduction of bispecific, combination or sequential strategies are hot issues in this field. Bispecific ADCs and companion diagnostics are emerging to further improve the clinical benefits of adhesion molecule-targeted ADCs.
Author contributions
MuK: Writing – original draft, Writing – review and editing. YL: Writing – review and editing. IN: Writing – review and editing. AH: Writing – review and editing. KS: Writing – review and editing. MaK: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by grants-in-aid from M. Katoh’s Fund for the Knowledge-Base and Global Network Projects (Masuko Katoh and Masaru Katoh).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, C., Xue, J., Zheng, Y., Sun, M., Ying, J., Zhou, F., et al. (2024). A phase 1/2 study of LM-302, an anti-claudin 18.2 (CLDN18.2) antibody-drug conjugate in patients with advanced gastric/gastroesophageal junction cancer. J. Clin. Oncol. 42 (16_Suppl. l), 3028. doi:10.1200/JCO.2024.42.16_suppl.3028
Bai, Z., Xie, X., Li, C., Wang, Y., Wang, Y., Li, H., et al. (2024). Claudin18.2-Targeted SPECT/CT imaging for gastric cancer: preclinical evaluation and clinical translation of the 99mTc-labeled nanobody (PHG102) radiotracer. ACS Pharmacol. Transl. Sci. 7 (8), 2465–2475. doi:10.1021/acsptsci.4c00280
Baldini, C., Goldschmidt, V., Brana, I., Doger, B., Italiano, A., Cousin, S., et al. (2023). BT8009-100: a phase I/II study of novel bicyclic peptide and MMAE conjugate BT8009 in patients (pts) with advanced malignancies associated with nectin-4 expression, including urothelial cancer (UC). J. Clin. Oncol. 41 (6_Suppl. l), 498. doi:10.1200/JCO.2023.41.6_suppl.498
Ballestín, P., López de Sá, A., Díaz-Tejeiro, C., Paniagua-Herranz, L., Sanvicente, A., López-Cade, I., et al. (2025). Understanding the toxicity profile of approved ADCs. Pharmaceutics 17 (2), 258. doi:10.3390/pharmaceutics17020258
Bartolomé, R. A., Robles, J., Martin-Regalado, Á., Pintado-Berninches, L., Burdiel, M., Jaén, M., et al. (2021). CDH6-activated αIIbβ3 crosstalks with α2β1 to trigger cellular adhesion and invasion in metastatic ovarian and renal cancers. Mol. Oncol. 15 (7), 1849–1865. doi:10.1002/1878-0261.12947
Bartolomé, R. A., Pintado-Berninches, L., Martín-Regalado, Á., Robles, J., Calvo-López, T., Ortega-Zapero, M., et al. (2024). A complex of cadherin 17 with desmocollin 1 and p120-catenin regulates colorectal cancer migration and invasion according to the cell phenotype. J. Exp. Clin. Cancer Res. 43 (1), 31. doi:10.1186/s13046-024-02956-6
Ben-David, U., Nudel, N., and Benvenisty, N. (2013). Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat. Commun. 4, 1992. doi:10.1038/ncomms2992
Bialucha, C. U., Collins, S. D., Li, X., Saxena, P., Zhang, X., Dürr, C., et al. (2017). Discovery and optimization of HKT288, a cadherin-6-targeting ADC for the treatment of ovarian and renal cancers. Cancer Discov. 7 (9), 1030–1045. doi:10.1158/2159-8290.CD-16-1414
Campbell, H. K., Maiers, J. L., and DeMali, K. A. (2017). Interplay between tight junctions and adherens junctions. Exp. Cell Res. 358 (1), 39–44. doi:10.1016/j.yexcr.2017.03.061
Challita-Eid, P. M., Satpayev, D., Yang, P., An, Z., Morrison, K., Shostak, Y., et al. (2016). Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 76 (10), 3003–3013. doi:10.1158/0008-5472.CAN-15-1313
Chen, J., Xu, Z., Hu, C., Zhang, S., Zi, M., Yuan, L., et al. (2023). Targeting CLDN18.2 in cancers of the gastrointestinal tract: new drugs and new indications. Front. Oncol. 13, 1132319. doi:10.3389/fonc.2023.1132319
Cheng, G., Zheng, H., Yu, Z., Xia, C., Guo, H., Wang, X., et al. (2025). Abstract 4328: preclinical development of SCR-A008: a CDH17-targeted ADC for gastrointestinal cancer. Cancer Res. 85 (8_Suppl. l_1), 4328. doi:10.1158/1538-7445.AM2025-4328
Cheng, X., Song, Z., Li, N., Han, L., Zhang, Y., Zheng, T., et al. (2025). First in human phase I study of TQB2103, a Claudin18.2 (CLDN18.2) targeted antibody-drug conjugate (ADC), in patients with advanced solid tumors. J. Clin. Oncol. 43 (16_Suppl. l), 3026. doi:10.1200/JCO.2025.43.16_suppl.3026
Chu, C. E., Sjöström, M., Egusa, E. A., Gibb, E. A., Badura, M. L., Zhu, J., et al. (2021). Heterogeneity in NECTIN4 expression across molecular subtypes of urothelial cancer mediates sensitivity to enfortumab vedotin. Clin. Cancer Res. 27 (18), 5123–5130. doi:10.1158/1078-0432.CCR-20-4175
Colombo, R., Tarantino, P., Rich, J. R., LoRusso, P. M., and de Vries, E. G. E. (2024). The journey of antibody-drug conjugates: lessons learned from 40 Years of development. Cancer Discov. 14 (11), 2089–2108. doi:10.1158/2159-8290.CD-24-0708
Delaney, S., Keinänen, O., Lam, D., Wolfe, A. L., Hamakubo, T., and Zeglis, B. M. (2024). Cadherin-17 as a target for the immunoPET of adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 51 (9), 2547–2557. doi:10.1007/s00259-024-06709-7
Dongre, A., Ortiz-Cuaran, S., and Korkaya, H. (2022). Editorial: the role of the EMT program in regulating the immune response in carcinoma. Front. Immunol. 13, 940164. doi:10.3389/fimmu.2022.940164
Dottermusch, M., Krüger, S., Behrens, H. M., Halske, C., and Röcken, C. (2019). Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study. Virchows Arch. 475 (5), 563–571. doi:10.1007/s00428-019-02624-7
Drago, J. Z., Modi, S., and Chandarlapaty, S. (2021). Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 18 (6), 327–344. doi:10.1038/s41571-021-00470-8
Du, H., Yang, X., Fan, J., and Du, X. (2021). Claudin 6: therapeutic prospects for tumours, and mechanisms of expression and regulation (Review). Mol. Med. Rep. 24 (3), 677. doi:10.3892/mmr.2021.12316
Dumontet, C., Reichert, J. M., Senter, P. D., Lambert, J. M., and Beck, A. (2023). Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug. Discov. 22 (8), 641–661. doi:10.1038/s41573-023-00709-2
Ebnet, K. (2017). Junctional adhesion molecules (JAMs): cell adhesion receptors with Pleiotropic functions in cell Physiology and development. Physiol. Rev. 97 (4), 1529–1554. doi:10.1152/physrev.00004.2017
FDA (2025). FDA grants accelerated approval to telisotuzumab vedotin-tllv for NSCLC with high c-Met protein overexpression. Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-telisotuzumab-vedotin-tllv-nsclc-high-c-met-protein-overexpression (Accessed June 27, 2025).
Fu, Z., Li, S., Han, S., Shi, C., and Zhang, Y. (2022). Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 7 (1), 93. doi:10.1038/s41392-022-00947-7
Gao, X., Patnaik, A., Lakhani, N. J., Call, J. A., Galsky, M. D., Garmezy, B., et al. (2025). NEXUS-01, a phase 1 study of LY4052031, an antibody-drug conjugate targeting Nectin-4 in participants with advanced or metastatic urothelial carcinoma or other solid tumors. J. Clin. Oncol. 43 (5_Suppl. l), TPS900. doi:10.1200/JCO.2025.43.5_suppl.TPS900
Giordano, A., Awan, A. A. A., Bruce, J. Y., Rugo, H. S., Diamond, J. R., Novik, Y., et al. (2024). Enfortumab vedotin (EV) in triple-negative breast cancer (TNBC) and HR+/HER2-breast cancer (BC) cohorts of EV-202. J. Clin. Oncol. 42 (16_Suppl. l), 1005. doi:10.1200/JCO.2024.42.16_suppl.1005
Gu, Y., Wang, Z., and Wang, Y. (2024). Bispecific antibody drug conjugates: making 1+1>2. Acta Pharm. Sin. B 14 (5), 1965–1986. doi:10.1016/j.apsb.2024.01.009
Gumbiner, B. M. (2005). Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6 (8), 622–634. doi:10.1038/nrm1699
Hao, X., Liu, J., Li, H., Li, J., Wang, J., Hu, H. H., et al. (2025). Development and preclinical activity of BSI-721, a novel antibody-drug conjugate (ADC) targeting cadherin 17 (CDH17) in gastrointestinal cancers. Cancer Res. 85 (8_Suppl. l_1), 4723. doi:10.1158/1538-7445.AM2025-4723
Hau, A. M., Shahmoradgoli, M., Lee, D. J., Sisson, W., Wang, A., Challita, P. P., et al. (2024). Abstract 1891: preclinical characterization of ADRX-0706: a next-generation anti-Nectin-4 antibody-drug conjugate with improved therapeutic window. Cancer Res. 84 (6_Suppl. l), 1891. doi:10.1158/1538-7445.AM2024-1891
Hoimes, C. J., Flaig, T. W., Milowsky, M. I., Friedlander, T. W., Bilen, M. A., Gupta, S., et al. (2023). Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J. Clin. Oncol. 41 (1), 22–31. doi:10.1200/JCO.22.01643
Horowitz, A., Chanez-Paredes, S. D., Haest, X., and Turner, J. R. (2023). Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 20 (7), 417–432. doi:10.1038/s41575-023-00766-3
Horwitz, S., O'Connor, O. A., Pro, B., Trümper, L., Iyer, S., Advani, R., et al. (2022). The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann. Oncol. 33 (3), 288–298. doi:10.1016/j.annonc.2021.12.002
Huang, W., Li, Y., Liu, Z., Rodon, L., Correia, S. Y., Li, Y., et al. (2022). Preclinical activity for TPX-4589 (LM-302), an antibody-drug conjugate targeting tight junction protein CLDN18.2 in solid tumors. Eur. J. Cancer 174 (Suppl. l_1), S41–S42. doi:10.1016/S0959-8049(22)00911-X
Huang, W., Li, J., Qin, X., Fei, D., and Li, R. (2025). Abstract 2868: preclinical evaluation of LM-350: an innovative anti-CDH17 antibody drug conjugate for gastrointestinal cancer therapy. Cancer Res. 85 (8_Suppl. l_1), 2868. doi:10.1158/1538-7445.AM2025-2868
Huang, Y., Sun, Z., Zhao, S., Wang, X., Zhang, L., Yang, Y., et al. (2025). Abstract 6750: QLS5132, a highly selective anti-CLDN6 ADC with broader therapeutic window. Cancer Res. 85 (8_Suppl. l_1), 6750. doi:10.1158/1538-7445.AM2025-6750
Hurvitz, S. A., Hegg, R., Chung, W. P., Im, S. A., Jacot, W., Ganju, V., et al. (2023). Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401 (10371), 105–117. doi:10.1016/S0140-6736(22)02420-5
Jacobsen, F., Pushpadevan, R., Viehweger, F., Freytag, M., Schlichter, R., Gorbokon, N., et al. (2024). Cadherin-17 (CDH17) expression in human cancer: a tissue microarray study on 18,131 tumors. Pathol. Res. Pract. 256, 155175. doi:10.1016/j.prp.2024.155175
Jiang, H., Huang, M., Wan, L., Markman, B., Pan, H., Bai, C., et al. (2025). Efficacy and safety of LM-302 (anti-claudin 18.2 ADC) in combination with anti-PD-1 therapy for advanced gastric, gastroesophageal junction cancer and esophageal adenocarcinoma: early-phase study results. J. Clin. Oncol. 43 (16_Suppl. l), 4039. doi:10.1200/JCO.2025.43.16_suppl.4039
Kalynovska, N., Hudecz, D., Hrdinka, M., Prochazkova, I., Kohelova, E., Jelinkova, L. P., et al. (2025). Abstract 5469: preclinical safety and efficacy of SOT109, an antibody-drug conjugate targeting cadherin 17 (CDH17) for the treatment of colorectal and other gastrointestinal tract tumors. Cancer Res. 85 (8_Suppl. l_1), 5469. doi:10.1158/1538-7445.AM2025-5469
Karaman, R., and Halder, G. (2018). Cell junctions in hippo signaling. Cold Spring Harb. Perspect. Biol. 10 (5), a028753. doi:10.1101/cshperspect.a028753
Katoh, M., and Katoh, M. (2020). Precision medicine for human cancers with Notch signaling dysregulation (Review). Int. J. Mol. Med. 45 (2), 279–297. doi:10.3892/ijmm.2019.4418
Katoh, M., and Katoh, M. (2022). WNT signaling and cancer stemness. Essays Biochem. 66 (4), 319–331. doi:10.1042/EBC20220016
Katoh, M., and Katoh, M. (2024). Claudin 1, 4, 6 and 18 isoform 2 as targets for the treatment of cancer (Review). Int. J. Mol. Med. 54 (5), 100. doi:10.3892/ijmm.2024.5424
Katoh, M., Loriot, Y., Brandi, G., Tavolari, S., Wainberg, Z. A., and Katoh, M. (2024a). FGFR-targeted therapeutics: clinical activity, mechanisms of resistance and new directions. Nat. Rev. Clin. Oncol. 21 (4), 312–329. doi:10.1038/s41571-024-00869-z
Katoh, M., Nakayama, I., Wainberg, Z. A., Shitara, K., and Katoh, M. (2024b). Monoclonal antibodies that target fibroblast growth factor receptor 2 isoform b and Claudin-18 isoform 2 splicing variants in gastric cancer and other solid tumours. Clin. Transl. Med. 14, e1736. doi:10.1002/ctm2.1736
Khoury, R., Saleh, K., Khalife, N., Saleh, M., Chahine, C., Ibrahim, R., et al. (2023). Mechanisms of resistance to antibody-drug conjugates. Int. J. Mol. Sci. 24, 9674. doi:10.3390/ijms24119674
Klümper, N., Ralser, D. J., Ellinger, J., Roghmann, F., Albrecht, J., Below, E., et al. (2023). Membranous NECTIN-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clin. Cancer Res. 29 (8), 1496–1505. doi:10.1158/1078-0432.CCR-22-1764
Klümper, N., Tran, N. K., Zschäbitz, S., Hahn, O., Büttner, T., Roghmann, F., et al. (2024). NECTIN4 amplification is frequent in solid tumors and predicts enfortumab vedotin response in metastatic urothelial cancer. J. Clin. Oncol. 42 (20), 2446–2455. doi:10.1200/JCO.23.01983
Klümper, N., Grünwald, V., Brägelmann, J., McKean, M., Fontana, E., Italiano, A., et al. (2025). Enhanced anti-tumor activity of zelenectide pevedotin in triple negative breast cancer (TNBC) patients (pts) with NECTIN4 gene amplification (amp). Clin. Cancer Res. 31 (12 Suppl. l), P4–21. doi:10.1158/1557-3265.SABCS24-P4-10-21
Köbel, M., Kalloger, S. E., Boyd, N., McKinney, S., Mehl, E., Palmer, C., et al. (2008). Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 5 (12), e232. doi:10.1371/journal.pmed.0050232
Konecny, G. E., Wahner Hendrickson, A. E., Winterhoff, B., Kung, A., Miller, L. L., Press, M. F., et al. (2024). Phase I, two-part, mutlicenter, first-in-human (FIH) study of TORL-1–23, a novel claudin 6 (CLDN6) targeting antibody drug conjugate (ADC) in patients with advanced solid tumors. Int. J. Gynecol. Cancer 34 (Suppl. 3), A56. doi:10.1136/ijgc-2024-IGCS.73
Kong, F. E., Li, G. M., Tang, Y. Q., Xi, S. Y., Loong, J. H. C., Li, M. M., et al. (2021). Targeting tumor lineage plasticity in hepatocellular carcinoma using an anti-CLDN6 antibody-drug conjugate. Sci. Transl. Med. 13 (579), eabb6282. doi:10.1126/scitranslmed.abb6282
Kreft, B., Berndorff, D., Böttinger, A., Finnemann, S., Wedlich, D., Hortsch, M., et al. (1997). LI-cadherin-mediated cell-cell adhesion does not require cytoplasmic interactions. J. Cell Biol. 136 (5), 1109–1121. doi:10.1083/jcb.136.5.1109
Lee, H. E., Kim, M. A., Lee, H. S., Jung, E. J., Yang, H. K., Lee, B. L., et al. (2012). MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br. J. Cancer 107 (2), 325–333. doi:10.1038/bjc.2012.237
Lemech, C. R., Joubert, W. L., Parsonson, A. O., Xu, R. H., Shinde, R., Bhave, P., et al. (2025). A phase 1, first-in-human study of AMT-676, an anti-CDH17 antibody-drug conjugate, in patients with advanced gastrointestinal tumors. J. Clin. Oncol. 43 (16_Suppl. l), TPS3161. doi:10.1200/JCO.2025.43.16_suppl.TPS3161
Li, J., Zhang, Q., Zeng, X., Zhang, W., Chen, L., Wu, J., et al. (2025). Efficacy and safety of neoadjuvant TQB2102 in women with locally advanced or early HER2-positive breast cancer: a randomized, open-label, multi-centre phase 2 trial. J. Clin. Oncol. 43 (16_Suppl. l), 591. doi:10.1200/JCO.2025.43.16_suppl.591
Li, S., Zhao, X., Fu, K., Zhu, S., Pan, C., Yang, C., et al. (2025). Resistance to antibody-drug conjugates: a review. Acta Pharm. Sin. B 15, 737–756. doi:10.1016/j.apsb.2024.12.036
Lian, W., Zong, Q., Deng, C., Zou, P., Xiao, L., Xue, T., et al. (2025). Abstract 2940: preclinical development of a next generation antibody drug conjugate (ADC) targeting CDH17 for treatment of solid tumors. Cancer Res. 85 (8_Suppl. l_1), 2940. doi:10.1158/1538-7445.AM2025-2940
Liu, J. J., Yu, Y., Zhang, J., Yang, J., Yue, J., Sun, Y., et al. (2024). 396MO Anti-claudin 18.2 (CLDN18.2) antibody-drug conjugate (ADC) IBI343 in patients (pts) with solid tumors and gastric/gastro-esophageal junction adenocarcinoma (G/GEJ AC): A phase I study. Ann. Oncol. 35 (Suppl. 1), S160. doi:10.1016/j.annonc.2024.05.311
Liu, T., Yu, Y., Ni, S., Liu, B., Li, N., Zhu, J., et al. (2024). 1456P First in human phase I/II trial of claudin 18.2 ADC RC118 in patients with advanced gastric/gastroesophageal junction cancer. Ann. Oncol. 35 (Suppl. 2), S903. doi:10.1016/j.annonc.2024.08.1522
Loriot, Y., Siefker-Radtke, A. O., Friedlander, T. W., Necchi, A., Wei, A. Z., Sridhar, S. S., et al. (2025). A phase 2/3 study of bicycle toxin conjugate zelenectide pevedotin (BT8009) targeting Nectin-4 in patients with locally advanced or metastatic urothelial cancer (la/mUC) (Duravelo-2). J. Clin. Oncol. 43 (5_Suppl. l), TPS898. doi:10.1200/JCO.2025.43.5_suppl.TPS898
Luo, S., Lin, R., Liao, X., Li, D., and Qin, Y. (2021). Identification and verification of the molecular mechanisms and prognostic values of the cadherin gene family in gastric cancer. Sci. Rep. 11 (1), 23674. doi:10.1038/s41598-021-03086-1
Luo, M., Wang, X., Yu, G., Ji, J., Li, L., and Song, F. (2025). Development of a bispecific antibody-drug conjugate targeting EpCAM and CLDN3 for the treatment of multiple solid tumors. Exp. Hematol. Oncol. 14 (1), 33. doi:10.1186/s40164-025-00624-9
Lyu, S. I., Fretter, C., Simon, A. G., Spielmann, S. M., Damanakis, A. I., Zhao, Y., et al. (2024). Extent and clinical significance of the therapy-relevant tight junction protein Claudin 18.2 in pancreatic ductal adenocarcinoma - real-world evidence. Transl. Oncol. 47, 102044. doi:10.1016/j.tranon.2024.102044
Ma, Y., Huang, Y., Zhao, Y., Zhao, S., Xue, J., Yang, Y., et al. (2024). BL-B01D1, a first-in-class EGFR-HER3 bispecific antibody-drug conjugate, in patients with locally advanced or metastatic solid tumours: a first-in-human, open-label, multicentre, phase 1 study. Lancet Oncol. 25 (7), 901–911. doi:10.1016/S1470-2045(24)00159-1
Ma, J., Cao, D., Zhao, Q., Mendis, S. R., Coward, J., Lv, J., et al. (2025). Safety and preliminary efficacy of ATG-022 in patients with advanced/metastatic gastric cancer (CLINCH). J. Clin. Oncol. 43 (4_Suppl. l), 456. doi:10.1200/JCO.2025.43.4_suppl.456
Maecker, H., Jonnalagadda, V., Bhakta, S., Jammalamadaka, V., and Junutula, J. R. (2023). Exploration of the antibody-drug conjugate clinical landscape. MAbs 15 (1), 2229101. doi:10.1080/19420862.2023.2229101
Mao, C., Li, S., Fan, R., Zhang, J., Fan, X., Shentu, Z., et al. (2025). Development and characterization of the [177Lu]Lu-labeled anti-CDH17 nanobody derivative for radioimmunotherapy in the gastric cancer xenograft model. Mol. Pharm. 22 (4), 2077–2087. doi:10.1021/acs.molpharmaceut.4c01285
McDermott, M. S. J., O'Brien, N. A., Hoffstrom, B., Gong, K., Lu, M., Zhang, J., et al. (2023). Preclinical efficacy of the antibody-drug conjugate CLDN6-23-ADC for the treatment of CLDN6-positive solid tumors. Clin. Cancer Res. 29 (11), 2131–2143. doi:10.1158/1078-0432.CCR-22-2981
McGale, J., Khurana, S., Huang, A., Roa, T., Yeh, R., Shirini, D., et al. (2023). PET/CT and SPECT/CT imaging of HER2-positive breast cancer. J. Clin. Med. 12 (15), 4882. doi:10.3390/jcm12154882
Micke, P., Mattsson, J. S., Edlund, K., Lohr, M., Jirström, K., Berglund, A., et al. (2014). Aberrantly activated claudin 6 and 18.2 as potential therapy targets in non-small-cell lung cancer. Int. J. Cancer 135 (9), 2206–2214. doi:10.1002/ijc.28857
Moore, K. N., Philipovskiy, A., Harano, K., Rini, B. I., Sudo, K., Kitano, S., et al. (2023). 745MO Raludotatug deruxtecan (R-DXd; DS-6000) monotherapy in patients with previously treated ovarian cancer (OVC): subgroup analysis of a first-in-human phase I study. Ann. Oncol. 34 (Suppl. 2), S510. doi:10.1016/j.annonc.2023.09.1924
Mosele, F., Montagnac, G., Pistilli, B., and André, F. (2023). Optimizing the potential of antibody-drug conjugates in oncology. Ann. Oncol. 34 (11), 964–967. doi:10.1016/j.annonc.2023.08.020
Mühlebach, M. D., Mateo, M., Sinn, P. L., Prüfer, S., Uhlig, K. M., Leonard, V. H., et al. (2011). Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480 (7378), 530–533. doi:10.1038/nature10639
Muro, K., Chin, K., Maron, S. B., Braiteh, F. S., Mitani, S., Hara, H., et al. (2024a). Enfortumab vedotin (EV) in previously treated gastric/esophageal cancers cohorts of EV-202. J. Clin. Oncol. 42 (16_Suppl. l), 4046. doi:10.1200/JCO.2024.42.16_suppl.4046
Muro, K., Feinstein, T., Baranda, J. C., Bonta, I., Kitazono, S., Gersten, T. A., et al. (2024b). Enfortumab vedotin (EV) in non-squamous and squamous non–small cell lung cancer (NSCLC) cohorts of EV-202. J. Clin. Oncol. 42 (16_Suppl. l), 8585. doi:10.1200/JCO.2024.42.16_suppl.8585
Nakayama, I., Qi, C., Chen, Y., Nakamura, Y., Shen, L., and Shitara, K. (2024). Claudin 18.2 as a novel therapeutic target. Nat. Rev. Clin. Oncol. 21 (5), 354–369. doi:10.1038/s41571-024-00874-2
Nasiri, F., Safarzadeh Kozani, P., Salem, F., Mahboubi Kancha, M., and Dashti Shokoohi, S. (2025). Mechanisms of antigen-dependent resistance to chimeric antigen receptor (CAR)-T cell therapies. Cancer Cell Int. 25 (1), 64. doi:10.1186/s12935-025-03697-y
Niimi, T., Nagashima, K., Ward, J. M., Minoo, P., Zimonjic, D. B., Popescu, N. C., et al. (2001). Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol. Cell Biol. 21 (21), 7380–7390. doi:10.1128/MCB.21.21.7380-7390.2001
O'Brien, N. A., McDermott, M. S. J., Zhang, J., Gong, K. W., Lu, M., Hoffstrom, B., et al. (2023). Development of a novel cldn18.2-directed monoclonal antibody and antibody-drug conjugate for treatment of cldn18.2-positive cancers. Mol. Cancer Ther. 22 (12), 1365–1375. doi:10.1158/1535-7163.MCT-23-0353
O'Brien, N. A., McDermott, M. S. J., Zhang, J., Lu, M., Gong, K. W., Hoffstrom, B., et al. (2024). TORL-3-600, a novel antibody drug conjugate directed against cadherin 17 (CDH17), has preclinical efficacy in colorectal, gastric, and pancreatic cancer. Cancer Res. 84 (6_Suppl. l), 1900. doi:10.1158/1538-7445.AM2024-1900
O'Donnell, P. H., Milowsky, M. I., Petrylak, D. P., Hoimes, C. J., Flaig, T. W., Mar, N., et al. (2023). Enfortumab vedotin with or without pembrolizumab in cisplatin-ineligible patients with previously untreated locally advanced or metastatic urothelial cancer. J. Clin. Oncol. 41 (25), 4107–4117. doi:10.1200/JCO.22.02887
Pan, H., Zhang, Q., Xiao, G., Zhang, G., Li, J., Xin, L., et al. (2025). Abstract 2945: PLB-002 is a novel Claudin 6 antibody-drug conjugate for ovarian cancer and testicular germ cell cancer. Cancer Res. 85 (8_Suppl. l_1), 2945. doi:10.1158/1538-7445.AM2025-2945
Park, J. J., Gao, B., Beecroft, C., Wilkinson, K. J., Parsonson, A., Zhang, K., et al. (2024). Safety and efficacy of JSKN003 in patients with advanced/metastatic solid tumors: a first-in-human, dose-escalation, multicenter, open-label, phase I study. J. Clin. Oncol. 42 (16_Suppl. l), 3038. doi:10.1200/JCO.2024.42.16_suppl.3038
Patel, M. R., Hamilton, E. P., Piha-Paul, S. A., Henry, J., Banerji, U., Al Hallak, M. N., et al. (2024). 610O Preliminary results from a phase I, first-in-human study of DS-9606a, a claudin 6 (CLDN6)-directed antibody-drug conjugate (ADC), in patients (pts) with tumor types known to express CLDN6. Ann. Oncol. 35 (Suppl. 2), S488–S489. doi:10.1016/j.annonc.2024.08.677
Patel, M. R., Falchook, G. S., Lee, E. K., Spira, A. I., Subbiah, V., Richardson, D. L., et al. (2025). First-in-human (FIH) phase 1 study of CUSP06, a cadherin-6 (CDH6)-directed antibody-drug conjugate (ADC), in patients with platinum-refractory/resistant ovarian cancer and other advanced solid tumors. J. Clin. Oncol. 43 (16_Suppl. l), 3042. doi:10.1200/JCO.2025.43.16_suppl.3042
Paul, R., Ewing, C. M., Robinson, J. C., Marshall, F. F., Johnson, K. R., Wheelock, M. J., et al. (1997). Cadherin-6, a cell adhesion molecule specifically expressed in the proximal renal tubule and renal cell carcinoma. Cancer Res. 57 (13), 2741–2748.
Plieth, J. (2025). ASCO 2025 – astellas's new Claudin18.2 interest. Available online at: https://www.oncologypipeline.com/apexonco/asco-2025-astellass-new-claudin182-interest (Accessed June 20, 2025).
Powles, T., Rosenberg, J. E., Sonpavde, G. P., Loriot, Y., Durán, I., Lee, J. L., et al. (2021). Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 384 (12), 1125–1135. doi:10.1056/NEJMoa2035807
Powles, T., Valderrama, B. P., Gupta, S., Bedke, J., Kikuchi, E., Hoffman-Censits, J., et al. (2024). Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N. Engl. J. Med. 390 (10), 875–888. doi:10.1056/NEJMoa2312117
Qu, H., Jin, Q., and Quan, C. (2021). CLDN6: from traditional barrier function to emerging roles in cancers. Int. J. Mol. Sci. 22 (24), 13416. doi:10.3390/ijms222413416
Reches, A., Ophir, Y., Stein, N., Kol, I., Isaacson, B., Charpak Amikam, Y., et al. (2020). Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J. Immunother. Cancer 8 (1), e000266. doi:10.1136/jitc-2019-000266
Reinhard, K., Rengstl, B., Oehm, P., Michel, K., Billmeier, A., Hayduk, N., et al. (2020). An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 367 (6476), 446–453. doi:10.1126/science.aay5967
Reymond, N., Fabre, S., Lecocq, E., Adelaïde, J., Dubreuil, P., and Lopez, M. (2001). Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J. Biol. Chem. 276 (46), 43205–43215. doi:10.1074/jbc.M103810200
Rigby, M., Bennett, G., Chen, L., Mudd, G. E., Harrison, H., Beswick, P. J., et al. (2022). BT8009; A nectin-4 targeting bicycle toxin conjugate for treatment of solid tumors. Mol. Cancer Ther. 21 (12), 1747–1756. doi:10.1158/1535-7163.MCT-21-0875
Rosa, K. (2023). CMG901 elicits responses in cldn18.2-expressing gastric/GEJ cancer. Available online at: https://www.onclive.com/view/cmg901-elicits-responses-in-cldn18-2-expressing-gastric-gej-cancer/(Accessed June 8, 2024).
Rosenberg, J. E., O'Donnell, P. H., Balar, A. V., McGregor, B. A., Heath, E. I., Yu, E. Y., et al. (2019). Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J. Clin. Oncol. 37 (29), 2592–2600. doi:10.1200/JCO.19.01140
Rosenberg, J., Sridhar, S. S., Zhang, J., Smith, D., Ruether, D., Flaig, T. W., et al. (2020). EV-101: a phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J. Clin. Oncol. 38 (10), 1041–1049. doi:10.1200/JCO.19.02044
Rosenberg, J., Sabatier, R., Viceneux, A., de La Motte Rouge, T., Champiat, S., Lebellec, L., et al. (2024). Abstract CT084: a phase 1 study of LY4101174 (ETx-22), an antibody-drug conjugate targeting nectin-4, in patients with advanced or metastatic urothelial cancer and other solid tumors (trial in progress). Cancer Res. 84 (7_Suppl. l), CT084. doi:10.1158/1538-7445.AM2024-CT084
Ruan, D. Y., Liu, F. R., Wei, X. L., Luo, S. X., Zhuang, Z. X., Wang, Z. N., et al. (2025). Claudin 18.2-targeting antibody-drug conjugate CMG901 in patients with advanced gastric or gastro-oesophageal junction cancer (KYM901): a multicentre, open-label, single-arm, phase 1 trial. Lancet Oncol. 26 (2), 227–238. doi:10.1016/S1470-2045(24)00636-3
Sahin, U., Koslowski, M., Dhaene, K., Usener, D., Brandenburg, G., Seitz, G., et al. (2008). Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin. Cancer Res. 14 (23), 7624–7634. doi:10.1158/1078-0432.CCR-08-1547
Saito, A., Ohzawa, H., Kawashima, R., Matsumoto, S., Kurashina, K., Saito, S., et al. (2025). Expression of Claudin18.2 in metastatic lesions in peritoneum of gastric cancer. J. Gastrointest. Oncol. 16 (1), 67–76. doi:10.21037/jgo-24-743
Samanta, D., and Almo, S. C. (2015). Nectin family of cell-adhesion molecules: structural and molecular aspects of function and specificity. Cell. Mol. Life Sci. 72 (4), 645–658. doi:10.1007/s00018-014-1763-4
Sato, S., Yagishita, S., Yoshida, H., Shintani, D., Ogasawara, A., Nishikawa, T., et al. (2025). Establishing a comprehensive panel of patient-derived xenograft models for high-grade endometrial carcinoma: molecular subtypes, genetic alterations, and therapeutic target profiling. Neoplasia 64, 101158. doi:10.1016/j.neo.2025.101158
Sava, J. (2025). FDA fast tracks CUSP06 in platinum-resistant ovarian cancer. Available online at: https://www.targetedonc.com/view/fda-fast-tracks-cusp06-in-platinum-resistant-ovarian-cancer (Accessed April 10, 2025).
Schöffski, P., Concin, N., Suarez, C., Subbiah, V., Ando, Y., Ruan, S., et al. (2021). A phase 1 study of a CDH6-targeting antibody-drug conjugate in patients with advanced solid tumors with evaluation of inflammatory and neurological adverse events. Oncol. Res. Treat. 44 (10), 547–556. doi:10.1159/000518549
Sen, S., Spira, A. I., Gutierrez, M., Loriot, Y., Vinceneux, A., Jensen, M., et al. (2025). A phase 1, open-label, multi-center study of the safety, tolerability, and efficacy of IPH4502 as a single agent in advanced solid tumors. J. Clin. Oncol. 43 (16_Suppl. l), TPS3159. doi:10.1200/JCO.2025.43.16_suppl.TPS3159
Shah, M. A., Shitara, K., Ajani, J. A., Bang, Y. J., Enzinger, P., Ilson, D., et al. (2023). Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat. Med. 29 (8), 2133–2141. doi:10.1038/s41591-023-02465-7
Shen, L., Shitara, K., Mei, Q., Li, C., Wei, J., Xie, X., et al. (2025). A multiregional, randomized, controlled, open-label, phase 3 study of the anti-claudin18.2 (CLDN18.2) antibody-drug conjugate (ADC) arcotatug tavatecan (IBI343) in gastric or gastroesophageal junction adenocarcinoma (G/GEJA): trial in progress. J. Clin. Oncol. 43 (16_Suppl. l), TPS4201. doi:10.1200/JCO.2025.43.16_suppl.TPS4201
Shitara, K., Lordick, F., Bang, Y. J., Enzinger, P., Ilson, D., Shah, M. A., et al. (2023). Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet 401 (10389), 1655–1668. doi:10.1016/S0140-6736(23)00620-7
Stewart, T. F., Fenton, S. E., Nazari, S., Elliott, A., Garje, R., Salmasi, A., et al. (2024). Landscape analysis and oncologic outcomes in advanced urothelial carcinoma (UC) by NECTIN4 RNA expression. J. Clin. Oncol. 42 (16_Suppl. l), 4585. doi:10.1200/JCO.2024.42.16_suppl.4585
Sun, L., Sun, Y., Zuo, K., Fan, L., Wang, X., Zhang, J., et al. (2025). Pilot study of nectin-4-targeted PET imaging agent 68Ga-FZ-NR-1 in triple-negative breast cancer from Bench to first-in-human. J. Nucl. Med. 66 (3), 473–479. doi:10.2967/jnumed.124.269024
Suzuki, H., Nagase, S., Saito, C., Takatsuka, A., Nagata, M., Honda, K., et al. (2024). Raludotatug deruxtecan, a CDH6-targeting antibody-drug conjugate with a DNA topoisomerase I inhibitor DXd, is Efficacious in human ovarian and kidney cancer models. Mol. Cancer Ther. 23 (3), 257–271. doi:10.1158/1535-7163.MCT-23-0287
Swiecicki, P. L., Yilmaz, E., Rosenberg, A. J., Fujisawa, T., Bruce, J. Y., Meng, C., et al. (2025). Phase II trial of enfortumab vedotin in patients with previously treated advanced head and neck cancer. J. Clin. Oncol. 43 (5), 578–588. doi:10.1200/JCO.24.00646
Synan, A., Wu, N. C., Velazquez, R., Gesner, T., Logel, C., Mueller, K., et al. (2025). A bispecific antibody-drug conjugate targeting pCAD and CDH17 has antitumor activity and improved tumor-specificity. MAbs 17 (1), 2441411. doi:10.1080/19420862.2024.2441411
Takeichi, M. (2014). Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 15 (6), 397–410. doi:10.1038/nrm3802
Tang, B., Sheng, X., Guo, J., Niu, H., Shen, Y., Jiang, S., et al. (2025). Nectin-4 targeted ADC, SHR-A2102, in patients with advanced or metastatic urothelial carcinoma: a phase 1 study. J. Clin. Oncol. 43 (5_Suppl. l), 657. doi:10.1200/JCO.2025.43.5_suppl.657
Tsuchikama, K., Anami, Y., Ha, S. Y. Y., and Yamazaki, C. M. (2024). Exploring the next generation of antibody-drug conjugates. Nat. Rev. Clin. Oncol. 21 (3), 203–223. doi:10.1038/s41571-023-00850-2
Van Cutsem, E., Bang, Y. J., Feng-Yi, F., Xu, J. M., Lee, K. W., Jiao, S. C., et al. (2015). HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18 (3), 476–484. doi:10.1007/s10120-014-0402-y
Vonniessen, B., Tabariès, S., and Siegel, P. M. (2024). Antibody-mediated targeting of Claudins in cancer. Front. Oncol. 14, 1320766. doi:10.3389/fonc.2024.1320766
Voss, S. D. (2023). SPECT/CT, PET/CT and PET/MRI: oncologic and infectious applications and protocol considerations. Pediatr. Radiol. 53 (7), 1443–1453. doi:10.1007/s00247-023-05597-7
Wahner, A. (2024). IBI343 Receives FDA fast track designation for advanced/metastatic PDAC. Available online at: https://www.onclive.com/view/ibi343-receives-fda-fast-track-designation-for-advanced-metastatic-pdac/(Accessed July 1, 2024).
Wang, Y., Gong, J., Lin, R., Zhao, S., Wang, J., Wang, Q., et al. (2023). First-in-human dose escalation and expansion study of SYSA1801, an antibody-drug conjugate targeting claudin 18.2 in patients with resistant/refractory solid tumors. J. Clin. Oncol. 41 (16_Suppl. l), 3016. doi:10.1200/JCO.2023.41.16_suppl.3016
Wang, R., Bai, Z., Zhong, W., Li, C., Wang, J., Xiang, J., et al. (2024). Synthesis, preclinical evaluation and pilot clinical translation of [68Ga]Ga-PMD22, a novel nanobody PET probe targeting CLDN18.2 of gastrointestinal cancer. Eur. J. Nucl. Med. Mol. Imaging 51 (12), 3731–3743. doi:10.1007/s00259-024-06808-5
Wang, R., Fang, P., Chen, X., Ji, J., Yu, D., Mei, F., et al. (2025). Overcoming multidrug resistance in gastrointestinal cancers with a CDH17-targeted ADC conjugated to a DNA topoisomerase inhibitor. Cell Rep. Med. 6, 102213. doi:10.1016/j.xcrm.2025.102213
Wang, W. V., Guan, L., Xu, M., Yin, Q., Dong, C., and Li, J. (2025). Abstract 341: VBC108: a first-in-class CDH17/CLDN18.2 targeted bispecific antibody drug conjugate (ADC) to overcome tumor heterogeneity of gastrointestinal cancers (GC, PADC, CRC, etc.). Cancer Res. 85 (8_Suppl. l_1), 341. doi:10.1158/1538-7445.AM2025-341
Wendeler, M. W., Praus, M., Jung, R., Hecking, M., Metzig, C., and Gessner, R. (2004). Ksp-cadherin is a functional cell-cell adhesion molecule related to LI-cadherin. Exp. Cell Res. 294 (2), 345–355. doi:10.1016/j.yexcr.2003.11.022
Xu, G., Liu, W., Wang, Y., Wei, X., Liu, F., He, Y., et al. (2024). CMG901, a Claudin18.2-specific antibody-drug conjugate, for the treatment of solid tumors. Cell Rep. Med. 5 (9), 101710. doi:10.1016/j.xcrm.2024.101710
Xu, R. H., Ruan, D., Luo, S., Liang, X., Niu, Z., Dang, Q., et al. (2024). 609O CLDN18.2 targeted antibody-drug conjugate (ADC), SHR-A1904, in patients (pts) with gastric/gastroesophageal junction cancer (GC/GEJC): A phase I study. Ann. Oncol. 35 (Suppl. 2), S487–S488. doi:10.1016/j.annonc.2024.08.676
Xu, R. H., Ruan, D. Y., Lin, R. B., Shan, J. Z., Nie, P., Ji, Y. H., et al. (2025). Abstract CT010: phase I dose-escalation and expansion study of JS107, a claudin 18.2 (CLDN18.2)-targeting antibody-drug conjugate (ADC), as monotherapy or in combination for patients (pts) with advanced solid tumors. Cancer Res. 85 (8_Suppl. l_2), CT010. doi:10.1158/1538-7445.AM2025-CT010
Yagishita, S., Nishikawa, T., Yoshida, H., Shintani, D., Sato, S., Miwa, M., et al. (2023). Co-clinical study of [fam-] trastuzumab deruxtecan (DS8201a) in patient-derived xenograft models of uterine Carcinosarcoma and its association with clinical efficacy. Clin. Cancer Res. 29 (12), 2239–2249. doi:10.1158/1078-0432.CCR-22-3861
Yang, J., Antin, P., Berx, G., Blanpain, C., Brabletz, T., Bronner, M., et al. (2020). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21 (6), 341–352. doi:10.1038/s41580-020-0237-9
Ye, Y., Qin, Y., Fang, X., Liu, X., Wang, W., Li, T., et al. (2025). Abstract 2934: novel hydrophilic CDH17-targeting antibody-drug conjugate exhibits anti-tumor efficacy in preclinical xenograft models. Cancer Res. 85 (8_Suppl. l_1), 2934. doi:10.1158/1538-7445.AM2025-2934
Yu, J., Sun, Y., Liu, T., Gao, S. G., Guo, Z., Liang, X., et al. (2024). 651P Phase I study of XNW27011, a novel claudin 18.2 ADC, in patients with locally advanced and/or metastatic solid tumors. Ann. Oncol. 35 (Suppl. 2), S514. doi:10.1016/j.annonc.2024.08.717
Yu, X., Wang, S., You, L., Xu, Z., Xue, Z., Mao, Y., et al. (2025a). Abstract 5460: C6P, a claudin 6 (CLDN6) directed ADC containing pyrolobenzodiazepine (PBD) for the treatment of advanced solid tumors. Cancer Res. 85 (8_Suppl. l_1), 5460. doi:10.1158/1538-7445.AM2025-5460
Yu, X., Ying, J., Li, E., Zhou, A., Sun, Y., Yue, J., et al. (2025b). Claudin18.2 (CLDN18.2) expression and efficacy in pancreatic ductal adenocarcinoma (PDAC): results from a phase I dose expansion cohort evaluating IBI343. J. Clin. Oncol. 43 (16_Suppl. l), 4017. doi:10.1200/JCO.2025.43.16_suppl.4017
Zeng, P., Wu, Q., An, Y., Feng, Y., Wang, Y., Liu, S., et al. (2025). Abstract 2877: MRG007, a cadherin 17-targeted, glycan-linked, exatecan-based antibody-drug conjugate, demonstrated potent anti-tumor activity and a good safety profile in preclinical studies. Cancer Res. 85 (8_Suppl. l_1), 2877. doi:10.1158/1538-7445.AM2025-2877
Zhang, C., Guo, C., Li, Y., Liu, K., Zhao, Q., and Ouyang, L. (2021). Identification of claudin-6 as a molecular biomarker in pan-cancer through multiple Omics Integrative analysis. Front. Cell Dev. Biol. 9, 726656. doi:10.3389/fcell.2021.726656
Zhang, J., Duan, X., Chen, X., Zhang, Z., Sun, H., Shou, J., et al. (2024). Translational PET imaging of nectin-4 expression in multiple different cancers with 68Ga-N188. J. Nucl. Med. 65 (Suppl. 1), 12S–18S. doi:10.2967/jnumed.123.266830
Zhang, J., Liu, R., Wang, S., Feng, Z., Yang, H., Gao, S., et al. (2025). Bulumtatug Fuvedotin (BFv, 9MW2821), a next-generation Nectin-4 targeting antibody-drug conjugate, in patients with advanced solid tumors: a first-in-human, open-label, multicenter, phase I/II study. Ann. Oncol. 36, 934–943. doi:10.1016/j.annonc.2025.04.009
Zhang, Y., Takeda, S., Qi, L., Huang, M., Zhang, L., Liu, W., et al. (2025). Abstract LB021: TJ102: a promising bispecific dual-payload ADC targeting CDH6 and folate receptor alpha (FRα) for the treatment of ovarian and kidney cancers. Cancer Res. 85 (8_Suppl. ment_2), LB021. doi:10.1158/1538-7445.AM2025-LB021
Zhao, X., Zhu, J., Wu, Z., Li, Y., Wu, C., Nie, L., et al. (2025). Abstract 2876: BR116: an antibody drug conjugate targeting CDH17 for gastrointestinal cancer therapy. Cancer Res. 85 (8_Suppl. l_1), 2876. doi:10.1158/1538-7445.AM2025-2876
Zhong, C., Cheng, Y. W., Wu, Y. X., Zhang, X. Y., Zhao, R. Y., Wang, Q., et al. (2025). Abstract 6741: AT65474, a highly selective anti-CLDN6 ADC with a proprietary payload. Cancer Res. 85 (8_Suppl. l_1), 6741. doi:10.1158/1538-7445.AM2025-6741
Zhong, R., Yan, M., Wang, Q., Hong, H., Zhang, Y., Hu, M., et al. (2025). Phase 1 trial of SHR-A2102, a nectin-4–directed antibody drug conjugate (ADC), in advanced solid tumors. J. Clin. Oncol. 43 (16_Suppl. l), 107. doi:10.1200/JCO.2025.43.16_suppl.107
Zhou, W., Fang, P., Yu, D., Ren, H., You, M., Yin, L., et al. (2023). Preclinical evaluation of 9MW2821, a site-specific monomethyl auristatin E-based antibody-drug conjugate for treatment of nectin-4-expressing cancers. Mol. Cancer Ther. 22 (8), 913–925. doi:10.1158/1535-7163.MCT-22-0743
Keywords: alternative splicing, fibroblast growth factor receptor, gastric cancer, gene amplification, immune checkpoint inhibitor, loss of antigen or epitope, transdifferentiation, tumor heterogeneity
Citation: Katoh M, Loriot Y, Nakayama I, Hamada A, Shitara K and Katoh M (2025) Antibody-drug conjugates targeting the cadherin, claudin and nectin families of adhesion molecules. Front. Mol. Med. 5:1661016. doi: 10.3389/fmmed.2025.1661016
Received: 07 July 2025; Accepted: 25 September 2025;
Published: 14 October 2025.
Edited by:
Yasuhito Shimada, Mie University, JapanReviewed by:
Ryan Varghese, Saint Joseph’s University, United StatesKoichi Uemura, Yokohama City University Hospital, Japan
Copyright © 2025 Katoh, Loriot, Nakayama, Hamada, Shitara and Katoh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masaru Katoh, bWthdG9oLWtrckB1bWluLmFjLmpw
 Masuko Katoh1
Masuko Katoh1 Akinobu Hamada
Akinobu Hamada Kohei Shitara
Kohei Shitara Masaru Katoh
Masaru Katoh