- 1Faculty of Science and Engineering, Waseda University, Shinjuku, Tokyo, Japan
- 2Research Institute of Electrical Communication, Tohoku University, Sendai, Japan
Neuronal networks in animal brains are considered to realize specific filter functions through the precise configuration of synaptic weights, which are autonomously regulated without external supervision. In this study, we employ a single Hodgkin–Huxley-type neuron with autapses as a minimum model to computationally investigate how spike-timing-dependent plasticity (STDP) adjusts synaptic weights through recurrent feedback. The results show that the weights undergo oscillatory potentiation or depression with respect to autaptic delay and high-frequency stimulation. Our findings suggest that the STDP-mediated modulation of autaptic weights, governed by autaptic delay and input frequency, may serve as a mechanism for promoting network-level synchronization in neural systems if the network contains neurons with autapses.
1 Introduction
A neuron is believed to act as a filter that integrates input signals and attempts to transmit the signals to the postsynaptic neurons depending on the timing and magnitude of the input. While signals are structured as spike patterns based on the synaptic connectivity, the synaptic weights between neurons change gradually in response to the spike patterns (Hebb, 1949; Bi and Poo, 1998). When postsynapse neurons receive neurotransmitters, intracellular calcium-dependent protein kinases and Rho GTPases are activated, followed by the reorganization of the actin cytoskeleton (Lee et al., 2024, 2009; Murakoshi et al., 2011). This causes a transient increase in spine volume, and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors inserted into the expanding spine enhance synaptic weight continuously (Lee et al., 2024; Matsuzaki et al., 2001, 2004; Murakoshi and Yasuda, 2011). On the other hand, if the synaptic weight decreases, spike signals are no longer effectively transferred to the postsynaptic neurons; hence, the connection becomes comparatively insignificant. The overall circuit connectivity thus changes dynamically. Such autonomous adjustment undergoes in individual synapses, resulting in a local circuit with a sophisticated connectivity that works as a specific filter.
An autapse is a synaptic structure in which the axon of a neuron forms a connection onto its own dendrites and has been identified in multiple brain regions (Gilson et al., 2009; Loos and Glaser, 1972; Karabelas and Purrura, 1980; Bekkers and Stevens, 1991). For example, in the mammalian cortex, although autapses are approximately one-third as abundant as synapses, they are observed in 80% of the neurons (Lübke et al., 1996) and considered to contribute to temporal tuning, gain control, and network synchronization (Guo et al., 2016; Ma et al., 2015; Protachevicz et al., 2020; Jiang et al., 2012). Thus far, computational simulation has predicted that, if the network contains neurons with autapses, autapses with specific propagation delays can enhance global-network-wide synchronization in the absence of synaptic plasticity (Sillito et al., 1994; Kerr et al., 2013). However, autaptic signals may reenter a presynaptic neuron within 5 milliseconds (Patolsky et al., 2006; Uchino et al., 2022), suggesting that the autaptic weight may be autonomously modulated depending on the neuron's spiking activity. Understanding how neurons regulate autaptic weights offers insights into the coordination of recurrent connections and the autonomous emergence of synaptic configurations that enable specific filter functions.
Here, we report our computational simulations to investigate how a single neuron with autapses adjusts the autaptic weights through spike-timing-dependent plasticity (STDP) (Bi and Poo, 1998; Markram et al., 1997; Kopysova and Debanne, 1998; Song et al., 2000; Letzkus et al., 2006; Gilson et al., 2009; Feldman, 2012). This neuron model was configured with multiple autapses bearing different transmission delays (Lubenov and Siapas, 2008) and was driven by input spike trains generated from a Poisson process at defined frequencies, mimicking presynaptic activity. All autaptic weights were updated according to the STDP rule. We show that the potentiation and depression of autapic weights depend on the interplay between input frequency and transmission delay, offering a delay- and frequency-dependent selection mechanism. We further discuss how such selection may contribute to the synchronization of neuronal firing.
2 Computational model
A Hodgkin–Huxley-type model of a cortical neuron was used to simulate the neuronal dynamics (Hodgkin and Huxley, 1952; Pospischil et al., 2008; Hattori et al., 2020). The time evolution of the membrane potential V(t) was calculated as
where Cm is the specific capacitance of the cell membrane, V the membrane potential, INa the sodium current, IKd the potassium current, IM the slow non-inactivating potassium current responsible for spike-frequency adaptation, Ileak the leakage current, Isyn the synaptic current, and Iapp the current applied additionally to the cell. In the simulation, Cm was assumed to be 1.0 μF/cm2 (Pospischil et al., 2008). Numerical integrations were performed using the Euler method with a time step of less than 0.04 ms.
The current terms are given by
where ḡNa, ḡKd, ḡM, and gleak indicate the maximum sodium conductance, the maximum potassium conductance, the maximum slow non-inactivating potassium conductance, and the leakage conductance, respectively. In the simulation, ḡNa, ḡKd, ḡM, and gleak were set to 56 mS/cm2, 6 mS/cm2, 75 μS/cm2, and 20.5 μS/cm2, respectively (Pospischil et al., 2008). Moreover, the reversal potential for sodium current ENa, potassium channels EK, and leakage channels Eleak were set to 50, −90, and −70.3 mV, respectively (Pospischil et al., 2008). The functionals m, h, and n are given by
where the voltage-dependent functions αx(V) and βx(V) obey the following equations (Pospischil et al., 2008; Hattori et al., 2020):
The functional p is given by the following equations (Pospischil et al., 2008):
The synaptic current Isyn is the current introduced by AMPA and N-methyl-D-aspartate (NMDA) receptors and is given by
where wi and Vsyn indicate the synaptic weight and the synaptic reversal potential, respectively (Hattori et al., 2020; Borges et al., 2017). We assumed Vsyn = 0 mV (Yamamoto et al., 2016). The suffix i denotes the i-th synaptic connection. In the case of an autapse, the synaptic current Isyn, i was fed back to the neuron after a delay of τdelay, corresponding to the time required for an action potential to propagate through the axon and transmitted through the autapse. The AMPA conductance gAMPA, i and the NMDA conductance gNMDA, i are given by
where N indicates the number of synaptic connections, and tAP indicates the time the neuron fires. We assumed τrec, AMPA = 200ms, τinact, AMPA = 5ms, USE, AMPA = 0.7, τrec, NMDA = 200ms, τinact, NMDA = 55ms, USE, NMDA = 0.03, Vsyn = 0mV, and [Mg2+] = 1.0mM (Hattori et al., 2020).
The synaptic (autaptic) weight wi is updated according to the following equations representing STDP (Borges et al., 2017):
where Δt represents the time difference between the firing of the postsynaptic neuron and the arrival time of a synaptic current from the presynaptic neuron, namely, Δt = tAP−tEPSC. Hence, Δt takes both positive and negative values. In the case of an autapse, the pre- and postsynaptic neurons are identical. We assumed that A1 = 1.0, A2 = 0.5, τ1 = 1.8ms, and τ2 = 6.0ms (Borges et al., 2017). We also assumed that wi is bound to a maximum of one and a minimum of zero. The learning rate η was set to 10−3. All autaptic weights were initialized to 0.5 before stimulation and limited to the range of 0–1.
The applied current Iapp models synaptic inputs from other neurons (Dayan and Abbott, 2005; Miller, 2018) and is defined as
where tinput denotes the onset time of an input event, drawn from an exponential distribution, and Papp is the transmission intensity of the input current and is set to 10−2. The time constants were set to τrise = 0.2ms and τfall = 5.3ms (Yamamoto et al., 2016). The synaptic inputs were generated as a Poisson process with a specific mean frequency. The input spike frequency was changed from 200 spikes/s to 5000 spikes/s. For each condition, simulations were repeated 50 times using independently generated inputs, and the results were averaged. The fixed parameters used in the present simulation are summarized in Supplementary Table 1.
3 Results and discussion
We first investigated how the synaptic weights of STDP-regulated autapses are modulated when a neuron receives external spike inputs. The simulation model is illustrated in Figure 1A. A single neuron with 60 autapses, each with a unique autaptic delay (1–60 ms), was simulated. A spike train Iapp, modeling input spikes from other neurons, was applied for 5 s, and the time evolution of synaptic weights of autapses was analyzed.
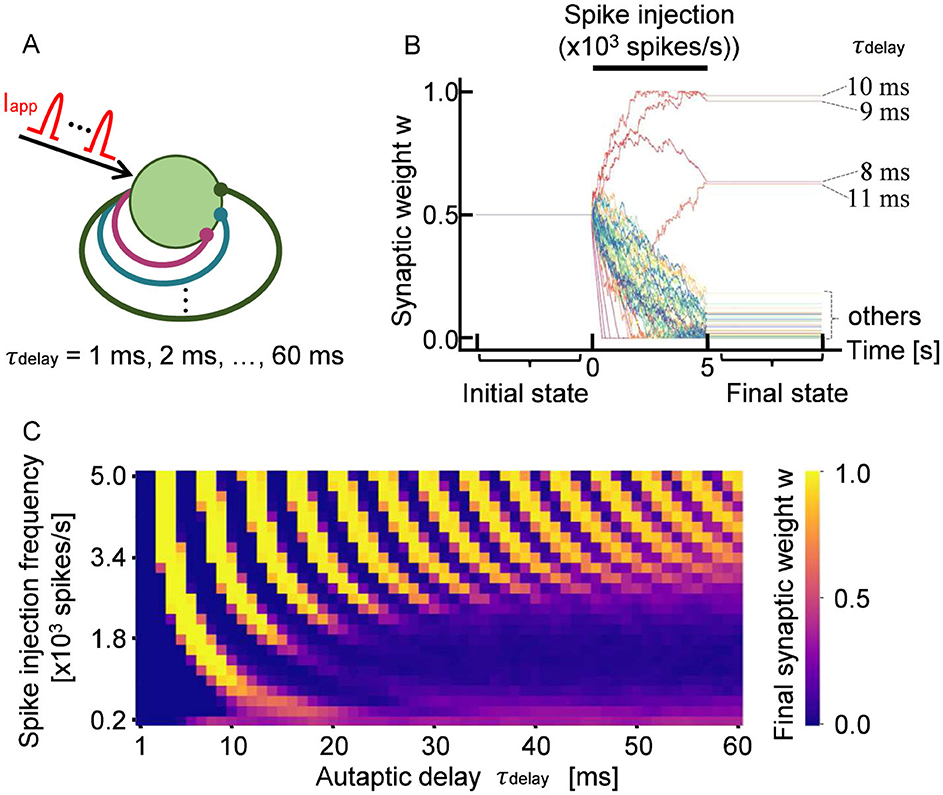
Figure 1. (A) Schematic of a neuron with 60 autapses, each with a distinct propagation delay, τdelay. The neuron receives stochastic input spikes Iapp with a mean frequency of 103 spikes per second. (B) Representative time evolution of synaptic weights w during 5 s of stimulation. Each trace represents an individual weight that was updated in response to neuronal firing. The autaptic delay τdelay is annotated for autapses that were potentiated. (C) Final synaptic weights of autapses. The observed stripe pattern indicates that STDP potentiates or depresses autapses depending on their propagation delay.
Figure 1B shows a representative example of how the synaptic weights of STDP-regulated autapses evolve in response to external inputs. The synaptic weight set to be 0.5 at the starting point was updated during the spike injection. Minor fluctuations observed in individual synaptic weights originated from the variability in spike timing caused by stochasticity in Iapp. Notably, it was found that autapses with specific delays tended to be selectively potentiated when the input frequency was fixed. This property remained consistent across multiple simulations using different random seeds for generating Iapp. As shown in Figure 1B, autapses with delays of approximately 10 ms (8 ms < τdelay < 12 ms) were robustly potentiated by input stimulation with a frequency of 103 spikes per second. In contrast, autapses with shorter (τdelay < 8 ms) or longer (τdelay>12 ms) delays tended to be depressed under the same stimulation condition.
A similar trend was also observed when input stimulation was delivered at other frequencies. Specifically, the evolution of the weights could be classified into three categories: (i) those that gradually potentiated and reached saturation, (ii) those that gradually depressed and stabilized at low values, and (iii) those that fluctuated within an intermediate range (τdelay = 8 ms and τdelay = 11 ms). Figure 1C shows the final synaptic weights for each autaptic delay and input frequency, with each of the three categories represented with different colors. The simulation was performed fifty times with different spike trains, and the average eventual synaptic weight was taken. As shown in Figure 1C, the eventual synaptic weight shows a periodicity with alternating patterns of potentiation and depression depending on the autaptic delay. Thus, STDP modulates the weights of autapses in a delay-dependent manner, enabling the specific selection of autaptic connections.
In STDP, spike timing is the key to connection strength modulation. Therefore, the interspike interval (ISI) is an essential indicator when discussing changes in neuron connection strength.Figure 2A shows the relationship between the ISI and the spike injection frequency. The ISI is long at a low frequency and asymptotically approaches the minimum value as the spike injection frequency increases. The minimum value is attributed to the refractory period of the neuron. In the following analyses, we use the ISI rather than the spike injection frequency because the ISI can be compared directly with the autaptic delay on the temporal axis, as shown in Figure 2B.
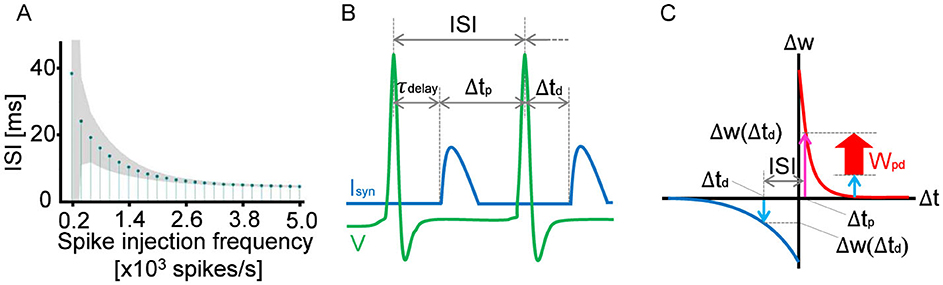
Figure 2. (A) Relationship between the average ISI of a neuron with 60 autapses and the average spike injection frequency. The shaded area of the lollipop chart indicates the standard deviation. (B) Schematic showing how to determine Δtp and Δtd for the given ISI and autaptic delay. Δtp is defined as the duration from the arrival time of the former feedback synaptic current Isyn to the subject spike (the second spike in the figure), whereas Δtd is defined as the duration from the spike to the arrival time of the latter synaptic current. In the case of τdelay ≤ ISI, Δtd is equivalent to −τdelay and Δtp is equivalent to ISI−τdelay, as shown in the schematic. In the case of τdelay > ISI, Δtd and Δtp can be determined accordingly. The potentiation and depression of the synaptic weight can be estimated using the STDP curve, as shown in (C). At every spike timing, the synaptic weight is updated in accordance with the balance between Δw (Δtp) and Δw (Δtd). If Δw(Δtp) > Δw(Δtd), the synaptic weight is potentiated. Otherwise, the synaptic weight is depressed.
Every spike injection updates synaptic weights, and it is the STDP curve that determines whether the synaptic weight is potentiated or depressed. In the case of autapses, input signals are assumed to be feedback signals via the autaptic connection. As shown in Figure 2B, the feedback synaptic current Isyn arrives with the autaptic delay τdelay after the first spike, depolarizing the membrane potential. Therefore, Δtp is defined as the duration from the arrival time of Isyn to the second spike. Δtd is also defined as the duration from the second spike to the subsequent arrival time of Isyn, which is equivalent to τdelay in the case of ISI>τdelay. As shown in Figure 2C, the potentiation component Δw(Δtp) was evaluated using the STDP curve. Similarly, the depression component Δw(Δtd) was estimated as a negative value. Since these potentiation and depression components are competing in STDP, the synaptic weight must be updated depending on the balance between Δw(Δtp) and Δw(Δtd); namely, Wpd = Δw(Δtp)+Δw(Δtd). As a result, if τdelay is close to the ISI but does not exceed the ISI, the synaptic weight of the autapse is potentiated. This is because the neuron fires immediately after Isyn arrives. In contrast, if τdelay exceeds the ISI but is close to the ISI, the synaptic weight of the autapse is depressed because the neuron fires immediately before Isyn arrives.
As shown in Figure 2C, Wpd is constant at a constant ISI. This condition is fulfilled at a high spike injection frequency because, as shown in Figure 2A, the fluctuation in ISI decreases with increasing spike injection frequency. Therefore, as shown in Figure 1C, the oscillation in the autapse selection becomes clear in the high-frequency region. In contrastingly, depression is dominant at a low frequency because of the asymmetric characteristics in the potentiation and depression of the STDP curve.
The STDP autapses undergo selections according to the autaptic delay, particularly at a high spike injection frequency, as shown in Figure 1C. Although the selection was made only on the local autapses, it was reported that a single autapse may induce network synchronization. Yilmaz et al. (2016) simulated a neuronal network containing a single autapse and reported that network synchronization occurs depending on the autaptic delay and conductance. Moreover, Wang et al. (2015) reported that the autaptic delay inducing network synchronization resulted in discrete values. Similar results were obtained by the simulation of a more complicated network. Note that the autaptic delay and conductance were controlled artificially in the simulation reported previously, where neuronal plasticity was not modeled. In this work, neuronal plasticity was realized in the presented simulation, where the selection was made spontaneously in a spike-timing-dependent manner. The above results suggest that the selection of autapses regulates the network activity such as network synchronization through neuronal plasticity.
It was reported in Graupner and Brunel (2012) that, in physiological calcium concentrations, not the STDP function like the one shown in Figure 2C but the depression-potentiation-depression (DPD) function is realistic. Thus, the final synaptic weights of autapses were calculated by using the DPD function proposed in Graupner and Brunel (2012). Supplementary Figure 1 shows that the autaptic weights undergo oscillatory potentiation or depression with respect to autaptic delay and high-frequency stimulation. This is because the balance between potentiation and depression changes periodically with increasing autaptic delay. The small discrepancy between Figure 1C and Supplementary Figure 1 is due to the difference of the STDP curve used in the present simulations.
On the other hand, it was reported that the STDP function like the one shown in Figure 2C is usually unapplicable in physiological calcium conditions (Inglebert et al., 2020) but is applicable only when the postsynaptic neuron fires at a high frequency (>10 Hz). As shown in Figure 2A, the average ISI of the neuron with sixty autapses is shorter than 40 ms, which corresponds to the average firing frequency higher than 25 Hz. Hence, it is conjectured that the STDP function like the one shown in Figure 2C is applicable to the neuron with sixty autapses.
The simulation reported previously (Borges et al., 2017) employed η = 10−3 as the coefficient to update synaptic weights in the simulation. In this work, the same coefficient was used accordingly. However, it is suggested that a smaller coefficient (η≃10−6) is realistic because the changes in synaptic weight that real neurons undergo in response to a single input are more gradual (Bi and Poo, 1998). The decrease in η by three orders of magnitude may retard the change in autaptic weight markedly. Nevertheless, if we assume a longer spike injection duration (e.g., 2 h), the autaptic weights are conjectured to converge into the same values, suggesting that the present simulation can be considered an accelerated test.
As shown in Figure 1C, clear periodic differences in connection strength were observed by assuming autapses with a wide range of propagation delays. However, in the case of cultured neurons and neurons in vivo, autapses with a propagation delay time of 60 ms are not realistic because the time scale is very long. Given that the signal conduction velocity along dendrites is approximately 0.16 m/s and that the average dendritic length of neurons with autapses is about 330 μm, the corresponding autaptic delay is estimated to be 5 ms or less (Patolsky et al., 2006; Uchino et al., 2022). In Figure 3A, we show how the final synaptic weights of STDP-regulated autapses with delays ranging from 0.5 to 5 ms as a function of input stimulation frequency. Clearly, synaptic weights were either potentiated or depressed depending on the autaptic delay. Autapses with delays shorter than 2 ms were consistently depressed because of the refractory period of a neuron (Yeomans, 1978), suggesting that short-delay autapses are insignificant. Even among autapses with delays exceeding 2 ms, synaptic depression was dominant when the input signal frequency was below 2,000 spikes per second. Although this simulation did not include autapses with delays longer than 5 ms, the results within this range closely align with those presented in Figure 1C. This indicates that the effects of individual autapses on the potentiation/depression of synaptic weights are independent. Figure 3B further reveals that the autapses with delays slightly shorter than the average ISI are preferentially potentiated. These findings suggest that the autapses with delays between 2 and 5 ms are most effective in promoting network synchronization if it is induced by autaptic feedback.
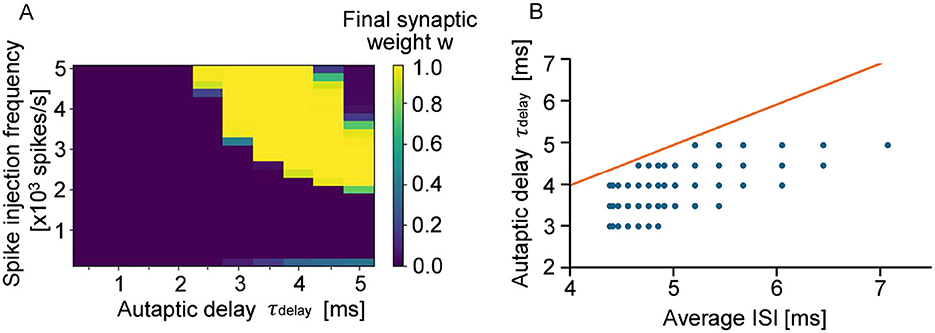
Figure 3. (A) Eventual synaptic weight of autapses with autaptic delays of 0.5–5 ms. The bright pixels show potentiated autapses, whereas the dark pixels show depressed autapses. (B) Relationship between ISI and autaptic delay. The red line shows that the ISI and the autaptic delay are equivalent. The blue dots show the autapses whose synaptic weights are potentiated and almost saturated in the stimmulation.
As reported in Boudkkazi et al. (2007) and Boudkkazi et al. (2011), the synaptic delay increases with decreasing presynaptic release probability by 1–2 ms. The synaptic delay also increases with increasing duration and amplitude of the presynaptic action potential by 1–2 ms. If these conditions are applied for the presented simulations, the autaptic delay corresponding to the horizontal axis of Figures 1C, 3A and Supplementary Figure 1 may shift by 1–2 ms accordingly.
4 Conclusions
In this study, we analyzed how STDP modulates the synaptic weights of a single neuron with autapses by computational simulation. We found that synaptic weights of autapses exhibit oscillatory patterns of potentiation and depression depending on the autaptic delay. The oscillation was also found to vary with the frequency of input stimulation. In particular, focusing on the physiologically realistic range of autaptic delays (τdelay < 5 ms), our results indicate that autapses with a specific delay are selectively potentiated under high-frequency inputs, suggesting their role in regulating network dynamics such as network synchronization. Taken together, STDP modulates the synaptic weights of autapses in a delay-dependent manner, enabling the specific selection of autaptic connections depending on the input spike frequency.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RO: Writing – review & editing, Software, Investigation, Writing – original draft, Data curation, Visualization, Validation, Formal analysis, Methodology. MI: Software, Writing – original draft, Investigation, Data curation, Formal analysis, Validation, Conceptualization, Visualization, Methodology. KH: Investigation, Conceptualization, Writing – review & editing, Software, Visualization, Formal analysis. HY: Funding acquisition, Resources, Writing – review & editing, Supervision, Project administration, Validation. TT: Project administration, Validation, Methodology, Conceptualization, Supervision, Investigation, Writing – original draft, Resources, Funding acquisition, Writing – review & editing, Visualization, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Cooperative Research Project Program of Research Institute of Electrical Communication (RIEC), Tohoku University and JSPS KAKENHI Grant Numbers JP20K19925, JP21H05164, and JP24H02333 and partly by Advanced Research Infrastructure for Materials and Nanotechnology in Japan (ARIM) and Design & Engineering by Joint Inverse Innovation for Materials Architecture (DEJI2MA) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncir.2025.1646317/full#supplementary-material
References
Bekkers, J. M., and Stevens, C. F. (1991). Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc. Nat. Acad. Sci. 88, 7834–7838. doi: 10.1073/pnas.88.17.7834
Bi, G. Q., and Poo, M. M. (1998). Synaptic modifications in cultured hippocampalneurons: Dependence on spike timing, synaptic strength, and postsynapticcell type. J. Neurosci. 18, 10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998
Borges, R. R., Borges, F. S., Lameu, E. L., Batista, A. M., Iarosz, K. C., Caldas, I. L., et al. (2017). Spike timing-dependentplasticity induces non-trivial topology in the brain. Neural Netw. 88, 58–64. doi: 10.1016/j.neunet.2017.01.010
Boudkkazi, S., Carlier, E., Ankri, N., Caillard, O., Giraud, P., Fronzaroli-Molinieres, L., et al. (2007). Release-dependent variations in synaptic latency: a putative code for short- and long-term synaptic dynamics. Neuron 56, 1048–1060. doi: 10.1016/j.neuron.2007.10.037
Boudkkazi, S., Fronzaroli-Molinieres, L., and Debanne, D. (2011). Presynaptic action potential waveform determines cortical synaptic latency. J. Physiol. 589, 1117–1131. doi: 10.1113/jphysiol.2010.199653
Dayan, P., and Abbott, L. F. (2005). Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems (Cambridge, MA: MIT Press).
Feldman, D. E. (2012). The spike-timing dependence of plasticity. Neuron. 75, 556–571. doi: 10.1016/j.neuron.2012.08.001
Gilson, M., Burkitt, A. N., Grayden, D. B., Thomas, D. A., and vanHemmen, J. L. (2009). Emergence of network structure due to spike-timing-dependentplasticity in recurrent neuronal netowork IV: structuring synaptic pathways among recurrent connections. Biol. Cybern. 101, 427–444. doi: 10.1007/s00422-009-0346-1
Graupner, M., and Brunel, N. (2012). Calcium-based plasticity model explains sensitivity of synaptic changes to spike pattern, rate, and dendritic location. Proc. Nat. Acad. Sci. 109, 3991–3996. doi: 10.1073/pnas.1109359109
Guo, D., Chen, M., Perc, M., Wu, S., Xia, C., Zhang, Y., et al. (2016). Firing regulation of fast-spiking interneurons by autaptic inhibition. Europhys. Lett. 114:30001. doi: 10.1209/0295-5075/114/30001
Hattori, K., Hayakawa, T., Nakanishi, A., Ishida, M., Yamamoto, H., Hirano-Iwata, A., et al. (2020). Contribution of AMPA and NMDA receptors in the spontaneous firing patterns of single neurons in autaptic culture. BioSystems 198:104278. doi: 10.1016/j.biosystems.2020.104278
Hebb, D. O. (1949). The Organization of Behavior: A Neuropsychological Theory (Hoboken, NJ: John Wiley &Sons).
Hodgkin, A. L., and Huxley, A. F. (1952). A quantitative description of membranecurrent and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544. doi: 10.1113/jphysiol.1952.sp004764
Inglebert, Y., Aljadeff, J., Brunel, N., and Debanne, D. (2020). Synaptic plasticity rules with physiological calcium levels. Proc. Nat. Acad. Sci. 117, 33639–33648. doi: 10.1073/pnas.2013663117
Jiang, M., Zhu, J., Liu, Y., Yang, M., Tian, C., Jiang, S., et al. (2012). Enhancement of asynchronous release from fast-spiking interneuron in human and rat epileptic neocortex. PLoS Biol. 10:1001320. doi: 10.1371/journal.pbio.1001324
Karabelas, A. B., and Purrura, D. P. (1980). Evidence for autapses in the substantia nigra. Brain Res. 2, 467–473. doi: 10.1016/0006-8993(80)90935-X
Kerr, R. R., Burkitt, A. N., Thomas, D. A., Gilson, M., and Grayden, D. B. (2013). Delay selection by spike-timing-dependent plasticity in recurrent networks of spiking neurons receiving oscillatory inputs. PLoS Comput. Biol. 9, 1–19. doi: 10.1371/journal.pcbi.1002897
Kopysova, I. L., and Debanne, D. (1998). Critical role of axonal A-type K+ channels and axonal geometry in the gating of action potential propagation along CA3 pyramidal cell axons: A simulation study. J Neurosci. 18, 7436–7451. doi: 10.1523/JNEUROSCI.18-18-07436.1998
Lee, C. T., Bell, M., Bonilla-Quintana, M., and Rangamani, P. (2024). Biophysical modeling of synaptic plasticity. Annu. Rev. Biophys. 53, 397–426. doi: 10.1146/annurev-biophys-072123-124954
Lee, S. J. R., Escobedo-Lozoya, Y., Szatmari, E. M., and Yasuda, R. (2009). Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458, 299–304. doi: 10.1038/nature07842
Letzkus, J. J., Kampa, B. M., and Stuart, G. J. (2006). Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J. Neurosci. 26, 10420–10429. doi: 10.1523/JNEUROSCI.2650-06.2006
Loos, H. V. D., and Glaser, E. M. (1972). Autapses in neocortex cerebri: synapses between a pyramidal cell's axon and its own dendrites. Brain Res. 48, 355–360. doi: 10.1016/0006-8993(72)90189-8
Lubenov, E. V., and Siapas, A. G. (2008). Decoupling through synchrony in neuronal circuits with propagation delays. Neuron. 58, 118–131. doi: 10.1016/j.neuron.2008.01.036
Lübke, J., Markram, H., Frotscher, M., and Sakmann, B. (1996). Frequency and dendritic distribution of autapses established by layer 5 pyramidal neurons in the developing rat neocortex: comparison with synaptic innervation of adjacent neurons of the same class. J. Neurosci. 178, 3209–3218. doi: 10.1523/JNEUROSCI.16-10-03209.1996
Ma, J., Song, X., Tang, J., and Wang, C. (2015). Wave emitting and propagation induced by autapse in a forward feedback neuronal network. Neurocomputing 167, 378–389 doi: 10.1016/j.neucom.2015.04.056
Markram, H., Lübke, J., Frotscher, M., and Sakmann, B. (1997). Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215. doi: 10.1126/science.275.5297.213
Matsuzaki, M., Ellis-Davies, G. C., Nemoto, T., Miyashita, Y., Iino, M., Kasai, H., et al. (2001). Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 4, 1086–1092. doi: 10.1038/nn736
Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. R., and Kasai, H. (2004). Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766. doi: 10.1038/nature02617
Murakoshi, H., Wang, H., and Yasuda, R. (2011). Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 472, 100–104. doi: 10.1038/nature09823
Murakoshi, H., and Yasuda, R. (2011). Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci. 35, 135–143. doi: 10.1016/j.tins.2011.12.002
Patolsky, F., Timko, B. P., Yu, G., Fang, Y., Greytak, A. B., Zheng, G., et al. (2006). Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science 313, 1100–1104. doi: 10.1126/science.1128640
Pospischil, M., Toledo-Rodriguez, M., Monier, C., Piwkowska, Z., Bal, T., Frégnac, Y., et al. (2008). Minimal Hodgkin-Huxleytype models for different classes of cortical and thalamic neurons. Biol. Cybern. 99, 427–441. doi: 10.1007/s00422-008-0263-8
Protachevicz, P. R., Iarosz, K. C., Caldas, I. L., Antonopoulos, C. G., Batista, A. M., and Kurths, J. (2020). Influence of autapses on synchronization in neural networks with chemical synapses. Front. Syst. Neurosci. 14:604563. doi: 10.3389/fnsys.2020.604563
Sillito, A. M., Jones, H. E., Gerstein, G. L., and West, D. C. (1994). Feature-linked synchronization of thalamic relay cell firing induced by feedback from the visual cortex. Nature 369, 479–482. doi: 10.1038/369479a0
Song, S., Miller, K. D., and Abbott, L. F. (2000). Competitive Hebbian learning through spike-timing dependent synaptic plasticity. Nat. Neurosci. 3, 919–926. doi: 10.1038/78829
Uchino, K., Tanaka, Y., Kawaguchi, S., Kubota, K., Watanabe, T., Katsurabayashi, S., et al. (2022). Establishment of autaptic culture with human-induced pluripotent stem cell-derived astrocytes. iScience. 25:104762. doi: 10.1016/j.isci.2022.104762
Wang, Q., Gong, Y., and Wu, Y. (2015). Autaptic self-feedback-induced synchronization transitions in Newman-Watts neuronal network with time delays. Eur. Physi. J. B. 88, 103–108. doi: 10.1140/epjb/e2015-60164-8
Yamamoto, H., Kubota, S., Chida, Y., Morita, M., Moriya, S., Akima, H., et al. (2016). Niwano M. Size-dependent regulation of synchronized activity in living neuronal networks. Phys. Rev. E. 94, 012407. doi: 10.1103/PhysRevE.94.012407
Yeomans, J. S. (1978). The absolute refractory periods of self-stimulation neurons. Physiol. Behav. 22, 911–919. doi: 10.1016/0031-9384(79)90336-6
Keywords: autapse, STDP, recurrent connection, selection rule, autaptic delay, network-level synchronization
Citation: Onda R, Ishida M, Hattori K, Yamamoto H and Tanii T (2025) Spike-timing-dependent plasticity offers delay-gated oscillatory potentiation for autaptic weights. Front. Neural Circuits 19:1646317. doi: 10.3389/fncir.2025.1646317
Received: 13 June 2025; Accepted: 28 July 2025;
Published: 25 August 2025.
Edited by:
Nicolangelo Iannella, University of Oslo, NorwayReviewed by:
Dominique Debanne, Unité de Neurobiologie des canaux Ioniques et de la Synapse (UNIS), FranceVeli Baysal, Bartin University, Türkiye
Copyright © 2025 Onda, Ishida, Hattori, Yamamoto and Tanii. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takashi Tanii, dGFuaWlAd2FzZWRhLmpw
 Risa Onda1
Risa Onda1 Kouhei Hattori
Kouhei Hattori Hideaki Yamamoto
Hideaki Yamamoto Takashi Tanii
Takashi Tanii