- 1The First Clinical Medical College of Shaanxi University of Traditional Chinese Medicine, Xianyang, China
- 2Department of Clinical Medicine, Hengyang Medical School, University of South China, Hengyang, China
- 3Shaanxi Province Rehabilitation Hospital, Xi’an, China
- 4The Affiliated Hospital of Shaanxi University of Traditional Chinese Medicine, Xianyang, China
Objective: Evidence from observational studies suggests that Sjögren’s syndrome (SS) may contribute to an elevated risk of Parkinson’s disease (PD) and dementia. However, few studies have been undertaken to summarize and assess the consistency of the data quantitatively. Therefore, we evaluated the risk of dementia and PD in SS patients through a systematic review and meta-analysis approach.
Methods: Two reviewers independently conducted a systematic search of PubMed, Embase, and Web of Science databases (updated to February 14, 2022) to identify published literature on the association between SS and dementia or PD. The risk estimates of dementia or PD in patients with SS were pooled using fixed or random-effects models.
Results: Of the 631 studies initially searched, 10 were eventually included. Pooled results suggested that the risk of developing dementia significantly increased in patients with SS (HR = 1.24, 95% CI: 1.15–1.33, P < 0.001), and such risk in females with SS was similar to that in males. The risk of PD was 1.36 times higher in SS (HR = 1.36, 95% CI: 1.23–1.50, P < 0.001). The association between SS and PD risk appeared to occur primarily in female patients (female: HR = 1.28, 95% CI: 1.21–1.35; P < 0.001 vs. male: HR = 1.00, 95% CI: 0.87–1.16, P = 0.962, respectively). No significant effect of age was observed on the risk of developing PD and dementia in SS patients.
Conclusion: Our study supports that people with SS are at higher risk of PD and dementia than the general population. Further studies are needed to elucidate the underlying mechanisms and to assess whether interventions for SS have the potential to affect dementia and PD development.
Objective
Sjögren’s syndrome (SS) is an autoimmune disorder that plagues 35 million people worldwide. Its main clinical manifestations include dry eyes and dry mouth as well as lymphocytic infiltration of glandular tissues (Sjögren, 1933). SS is characterized by reduced salivary and lacrimal gland function, lymphocytic infiltration, elevated pro-inflammatory cytokines, and circulating autoantibodies. Seventy percent of patients experienced significant fatigue, which greatly disrupted their daily lives (Odani and Chiarini, 2019; Mæland et al., 2021). Among 30–50% of SS patients, extra-glandular symptoms involving the skin, joints, lungs, neurological system, and kidneys, as well as malignant lymphoma were observed (Fox, 2005; Malladi et al., 2012; Ramos-Casals et al., 2015). Recent studies have revealed that the prevalence of neurological manifestations of primary SS (pSS) ranges from 8 to 49% (Margaretten, 2017), such as cognitive dysfunction and dementia (Delalande et al., 2004; Blanc et al., 2013).
Since the beginning of the twenty first century, population aging has become a prominent issue, challenging all countries in the world (Beard et al., 2016). China now ranks first in the world regarding the number of older adults and the rate of aging (Wu et al., 2021). Dementia and PD have emerged as the most common neurodegenerative diseases threatening the health of the age (Jellinger, 2003), with high incidence, serious hazards, and irreversible neuronal damage to the brain (Hindle, 2010). The treatment of these diseases also places a heavy burden on families and society (Alzheimer’s Association, 2017; Kwon et al., 2017). This predicament is compounded by the dim prospect for the research and development of medication targeting such diseases.
An association between neuropsychiatric symptoms and autoimmune diseases has been increasingly noted. Several observational studies have explored the association between SS and dementia/PD. Blanc et al. (2013) reported that of 25 patients with SS, 15 suffered from cognitive impairment and 5 developed dementia, revealing the risk of dementia in patients with SS. Several large sample longitudinal cohort studies with long-term follow-up (9–15 years) also confirmed that baseline SS is significantly associated with elevated risks of PD and dementia (Wu et al., 2017; Liliang et al., 2018; Chen et al., 2019; Hsu et al., 2020). Although there is evidence of an association between SS and PD and dementia, no meta-analysis has been conducted to quantitatively summarize and examine data consistency for higher-quality evidence.
Therefore, the present study aimed to quantify the association between SS and dementia/PD. To achieve this end, data were collected to (1) investigate the comprehensive results of the association between SS and dementia, and that between SS and PD; (2) validate the main results by analyzing the results in different subgroups and determining the sources of heterogeneity; (3) test the robustness of the results through sensitivity analysis and assess the potential for publication bias.
Methods
Search strategy
The study was reported according to the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-p) statement (Moher et al., 2015). We systematically searched PubMed, Embase, and Web of Science for relevant literature on SS and dementia/PD from inception to February 14, 2022. The following keywords were used: “SS AND PD”, “SS AND Alzheimer’s disease OR dementia.” No restrictions were imposed on the publication type or language of the journal. The detailed search strategy is provided in Supplementary Table 1.
Inclusion and exclusion criteria
Studies were eligible for inclusion if they were longitudinal case-control studies or cohort studies, entailed a clear definition of SS, dementia, and PD, or applied standard clinical diagnostic criteria to identify relevant cases. Included studies also should meet the following criteria: assessing the association between SS and dementia as well as SS and PD, or reporting effect estimates and corresponding 95% confidence intervals (CIs). The studies should also include relevant data to calculate hazard ratios (HRs) for the associations between SS and dementia/PD. We excluded reviews, conference abstracts, commentaries, reprinted literature, and studies with duplicated or incomplete data. To avoid omitting any studies, we manually searched the literature cited in the reference lists of included studies.
Data extraction
The final screening results were compared by two authors (WZZ and SZ) based on the same inclusion and exclusion criteria, and any disagreements were resolved by third-party authors. An MS Excel form was created to record essential data from the included studies, including first author, year of publication, study type, country, sample size, the mean age of samples, exposure definition, factors adjusted for the outcome, eligible subgroups, and follow-up periods. All authors approved the final version of the template. Two independent reviewers (WZZ and SZ) extracted data using the pre-determined form, and any discrepancies were addressed through discussion. The corresponding authors of the included literature were also contacted for further information when necessary.
Quality assessment
Two independent reviewers (WZZ and SZ) scored the quality of the included case-control and cohort studies, following the Newcastle-Ottawa Quality Assessment Scale. Studies were rated as high-quality if scored between 7 and 9, medium-quality (5∼6), and low-quality (≤4) (Stang, 2010).
Statistical methods
The HRs were pooled to examine the relationship between SS and PD/dementia risk (Higgins et al., 2003). Heterogeneity was considered acceptable when I2 < 50% and p > 0.05, and a fixed-effects model was used for analysis. A random-effects model was applied when I2 ≥ 50% or p ≤ 0.05. HR, an effective indicator for meta-analysis, was used to calculate pooled effect values, and forest plots were drawn. Further subgroup analyses were conducted based on the available data from the included studies. Sensitivity analysis was performed to evaluate the stability of the results. Publication bias was assessed using Begg’s and Egger’s tests and graphing funnel plots. All statistical analyses were done using Stata 16.0 software.
Results
Study selection
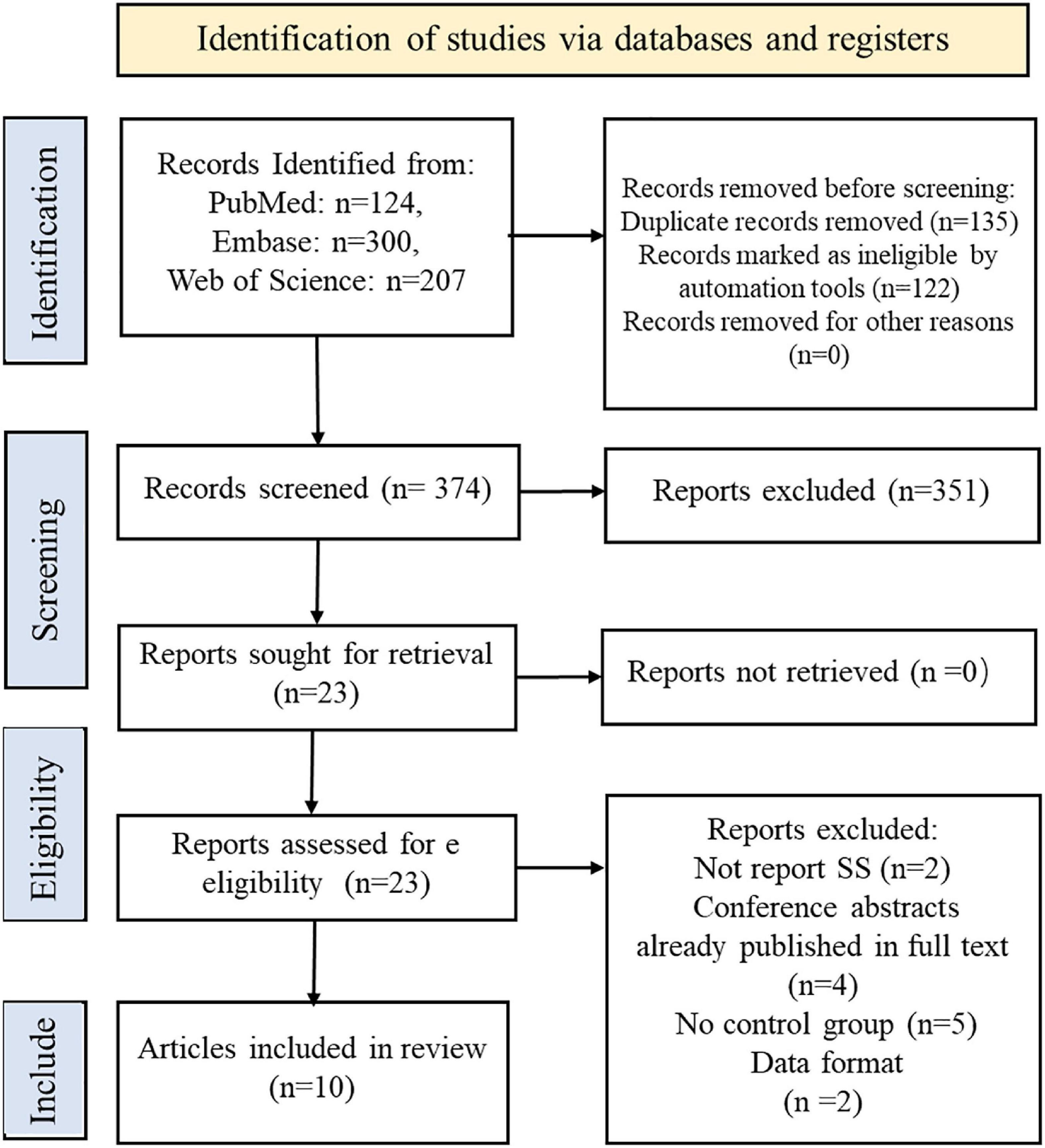
The screening process is summarized in the flow chart (Figure 1). We identified 631 records from PubMed, Embase, Web of Science databases according to a preformulated search strategy. After reviewing titles and abstracts, we excluded 135 studies with duplication and 122 irrelevant studies. The remaining 351 excluded studies were reviews, systematic reviews, animal experiments, and conference abstracts. After further reviewing the full text, 13 studies were excluded due to a lack of control groups or incomplete data. Ultimately, 10 studies were included in this meta-analysis (Wu et al., 2017; Chang et al., 2018; Chen et al., 2018, 2019; Liliang et al., 2018; Lin et al., 2018; Hou et al., 2019; Ju et al., 2019; Hsu et al., 2020; Park et al., 2021).
Study characteristics
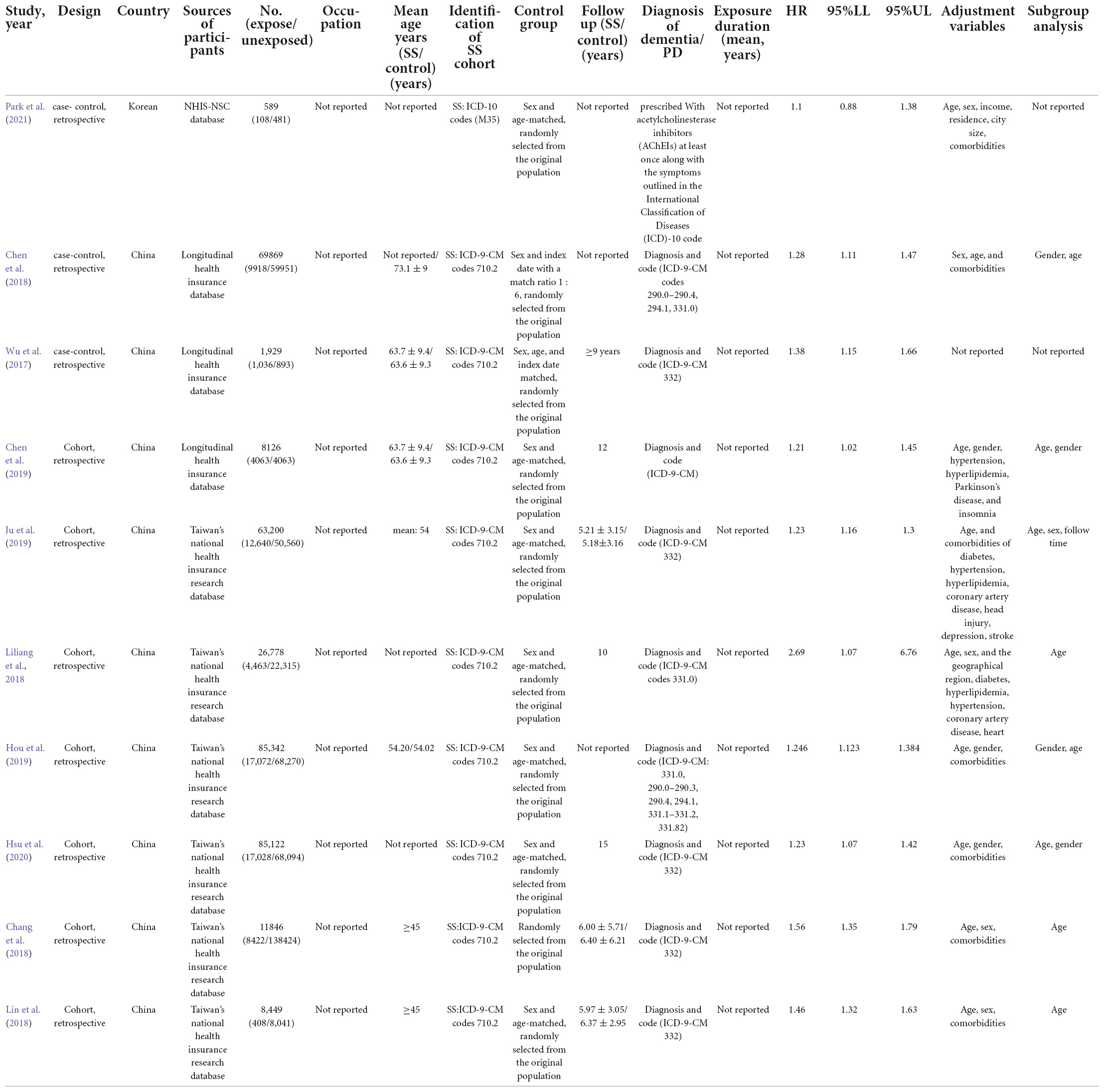
All included studies were published between 2017 and 2021. Of the 10 included studies, three were case-control studies (Wu et al., 2017; Chen et al., 2018; Park et al., 2021) and seven were cohort studies (Chang et al., 2018; Liliang et al., 2018; Lin et al., 2018; Chen et al., 2019; Hou et al., 2019; Ju et al., 2019; Hsu et al., 2020). One was conducted in South Korea (Park et al., 2021), and nine reported the scenarios in China (Wu et al., 2017; Chang et al., 2018; Chen et al., 2018, 2019; Liliang et al., 2018; Lin et al., 2018; Hou et al., 2019; Ju et al., 2019; Hsu et al., 2020). Participant sample sizes ranged from 1,929 to 85,342, with an overall mean age from 45 to 63.7 years. Participants diagnosed with Alzheimer’s disease, dementia, or PD at baseline were not included in any of the cohort studies or randomized controlled trials. Six of the 10 studies (Wu et al., 2017; Liliang et al., 2018; Chen et al., 2019; Hou et al., 2019; Ju et al., 2019; Hsu et al., 2020) were explicitly designed to assess the association between SS and dementia or PD. In comparison, in the remaining four studies (Chang et al., 2018; Chen et al., 2018; Lin et al., 2018; Park et al., 2021), SS was only part of their analysis to assess the relationship between autoimmune disease and dementia/PD. The definitions of dementia and PD varied between studies. Nine Taiwan, China-based studies used the ICD-9 standard, developed by neurologists based on the (UK) Parkinson’s Disease (PD) Society Brain Bank Clinical Diagnostic Criteria (Higgins et al., 2003; Stang, 2010; Moher et al., 2015; Wu et al., 2017; Chang et al., 2018; Chen et al., 2018, 2019; Liliang et al., 2018; Lin et al., 2018; Hou et al., 2019; Ju et al., 2019; Hsu et al., 2020), and one Korean study followed the ICD-10 classification standard (Park et al., 2021). All eligible studies were assessing dementia, PD, or SS as an outcome event. Seven studies specified the duration of follow-up, ranging from 5.18 to 15 years (Higgins et al., 2003; Stang, 2010; Moher et al., 2015; Wu et al., 2017; Chang et al., 2018; Liliang et al., 2018; Lin et al., 2018; Hou et al., 2019; Ju et al., 2019; Hsu et al., 2020). One study mentioned the follow-up but did not specify the duration (Chen et al., 2019), and the follow-up duration was missed in two studies (Chen et al., 2018; Park et al., 2021). The main characteristics of these studies are summarized in Table 1.
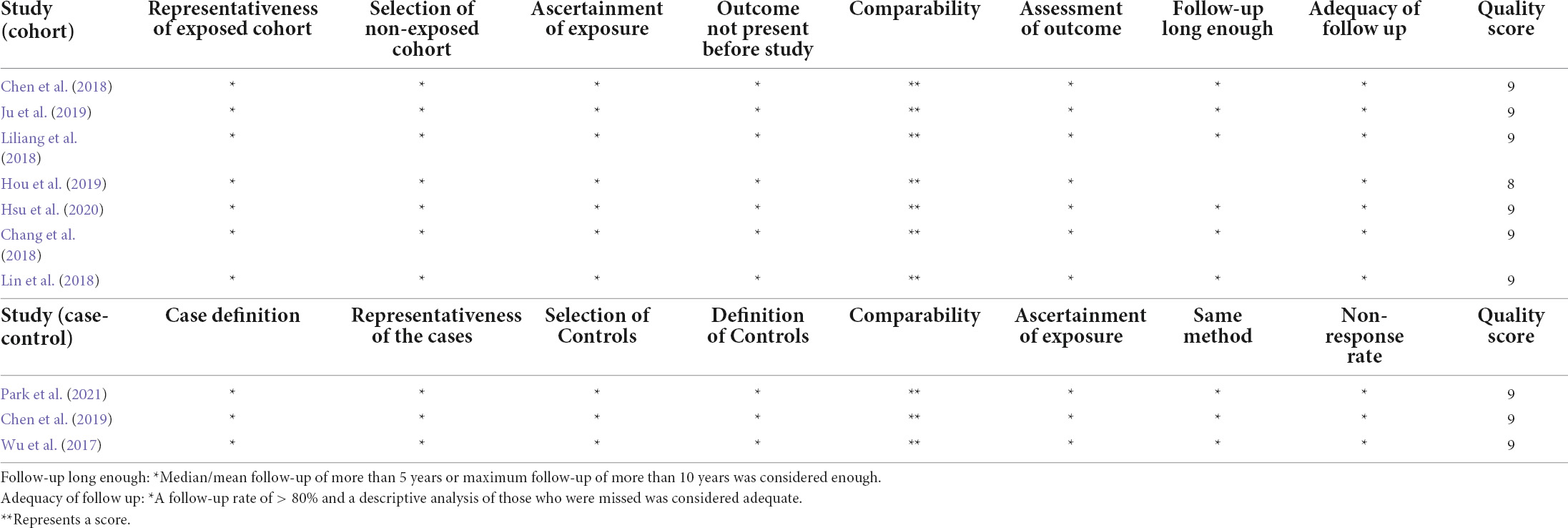
The Newcastle-Ottawa Quality Assessment Scale scores for the included studies are shown in Table 2. All 10 included studies were considered as high-quality; all of them scored 9, except for the study by Hou et al. (2019), which was rated as 8 because the specific follow-up time was not reported.
Association between Sjögren’s syndrome and dementia
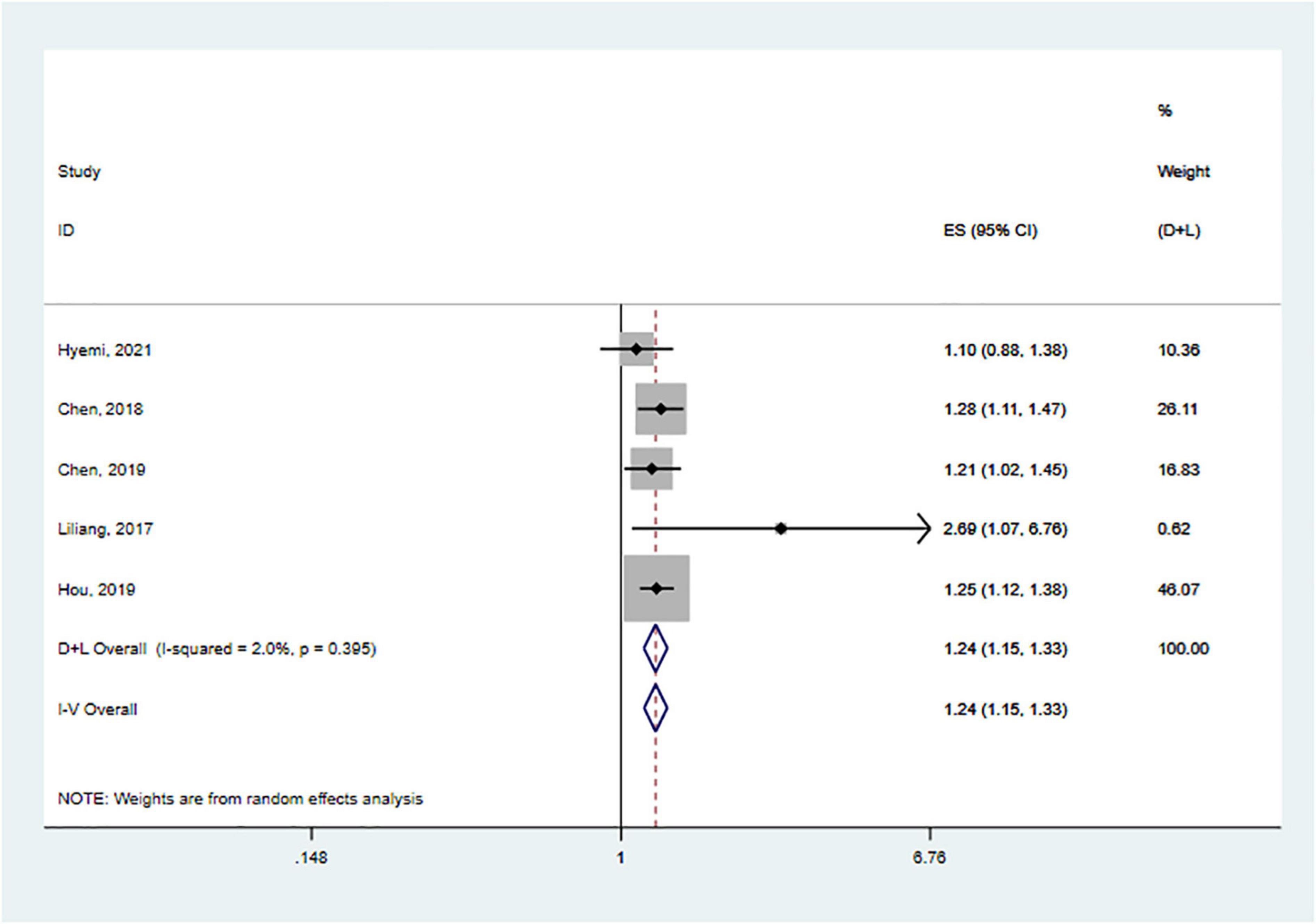
We performed meta-analyses and calculated pooled effect estimates for five studies that included 190 704 subjects (Chen et al., 2018, 2019; Liliang et al., 2018; Hou et al., 2019; Park et al., 2021). Heterogeneity between included studies was subtle [I2 = 2.0%, p (heterogeneity) = 0.395]. Therefore, a fixed-effects model was used to pool the effect size of each study to determine the association between SS and the risk of dementia (Figure 2). The overall pooled results showed that SS was associated with an increased risk of dementia (HR = 1.24, 95% CI: 1.15–1.33, p < 0.001).
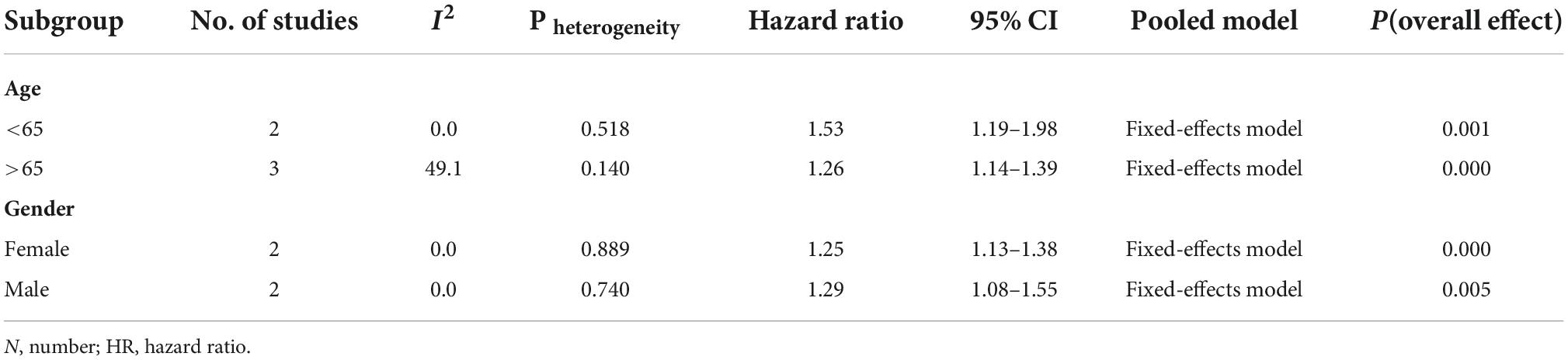
Our subgroup analyses were conducted by age (<65 and >65) and sex (male and female). Corresponding subgroup analyses of follow-up time and comorbidities on risk were not performed due to the lack of data on the effect (Table 3).
In terms of stratified analysis for age, people with SS had a 26% higher relative risk of dementia than those without SS among those over 65 (HR = 1.26; 95% CI: 1.14–1.39; p = 0.004), and this risk was similar in patients with SS under 65 years of age (HR = 1.53, 95% CI: 1.19–1.98; p < 0.001). Two studies were included in the subgroup analysis by sex (Chen et al., 2018; Hou et al., 2019). Females with SS had a similar risk of developing PD as males. (Females: HR = 1.25; 95% CI: 1.13–1.38; p < 0.001 vs. Male: HR = 1.29; 95% CI: 1.08–1.55; p = 0.005, respectively).
Evaluation for publication bias and sensitivity analysis
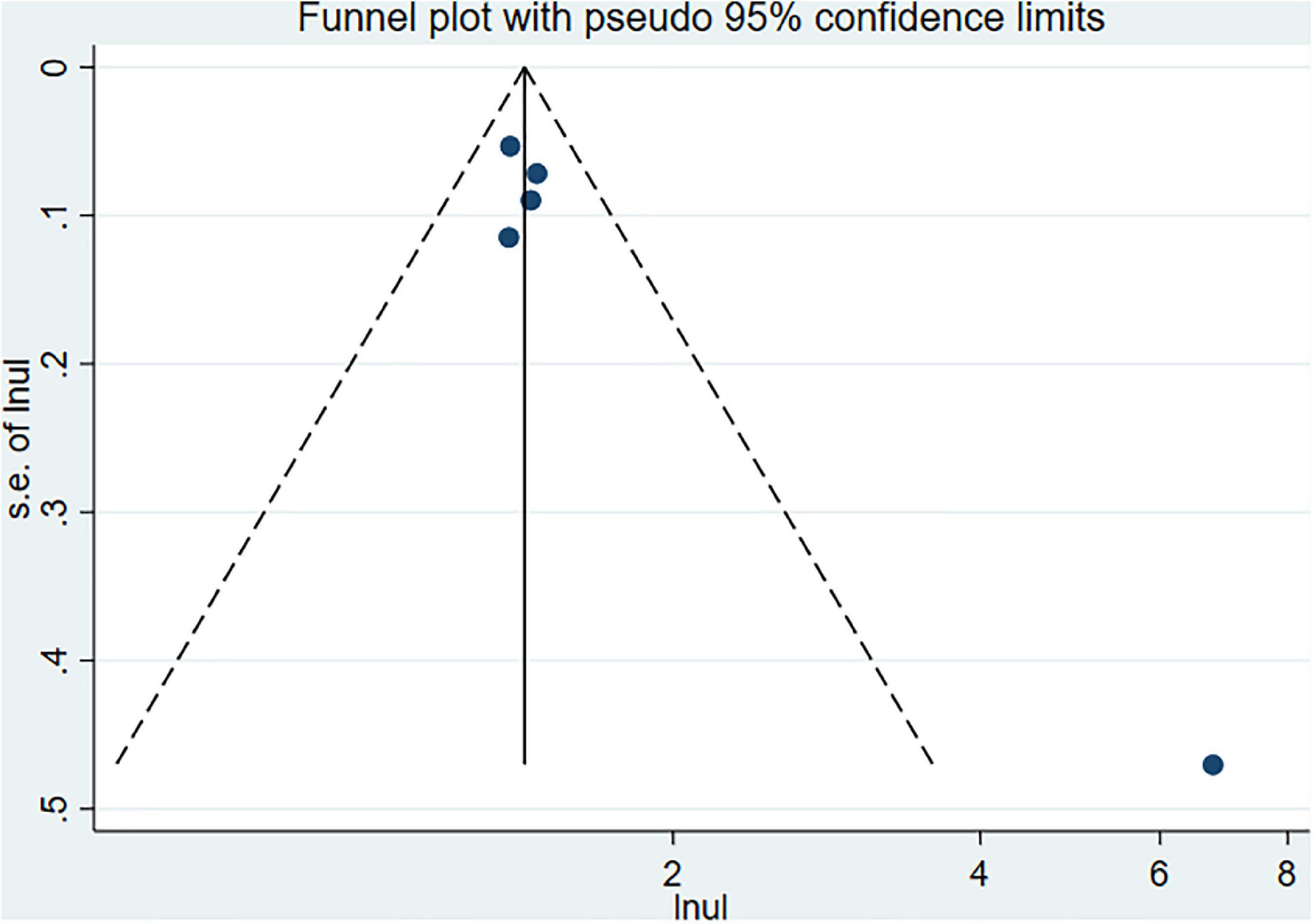
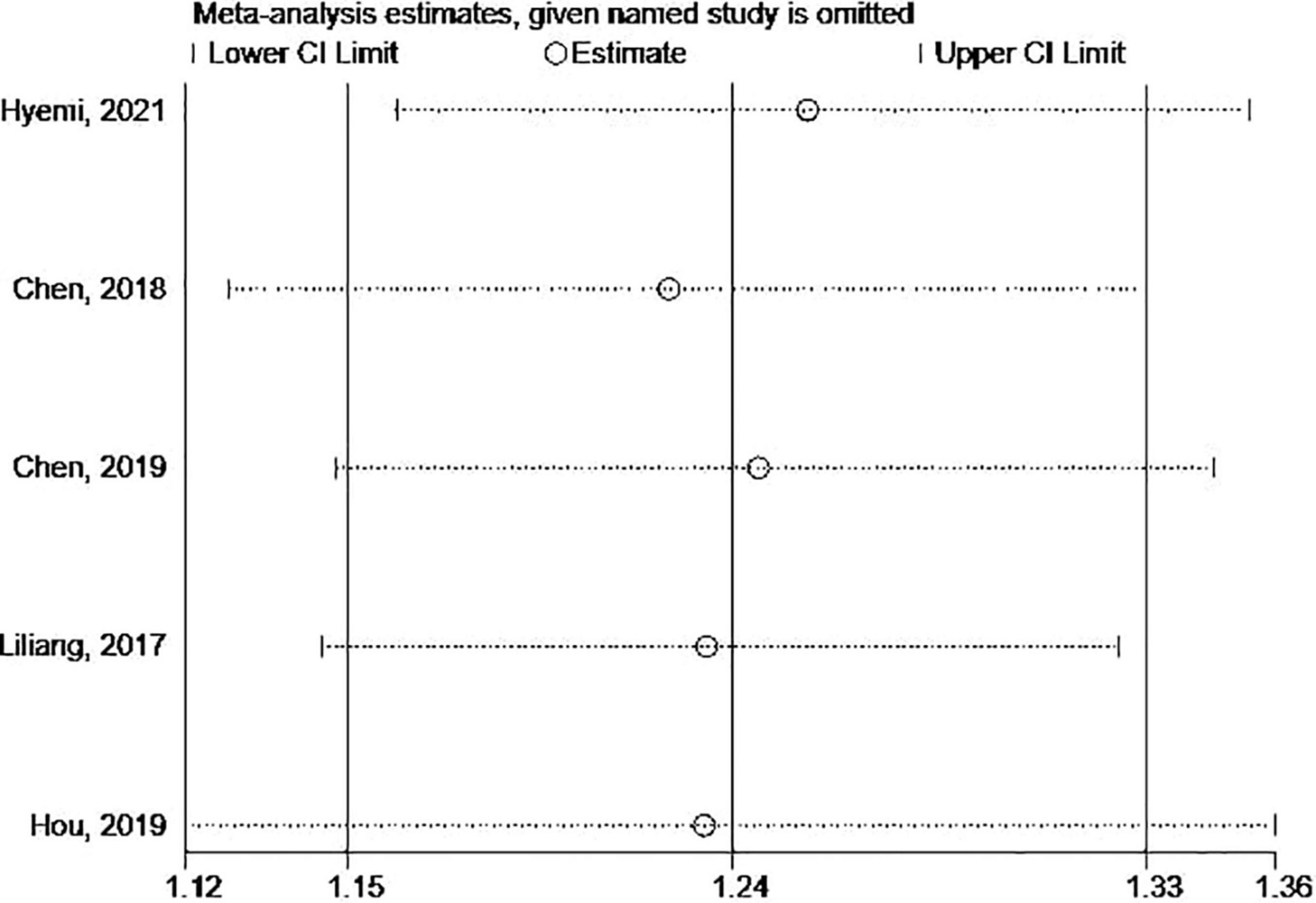
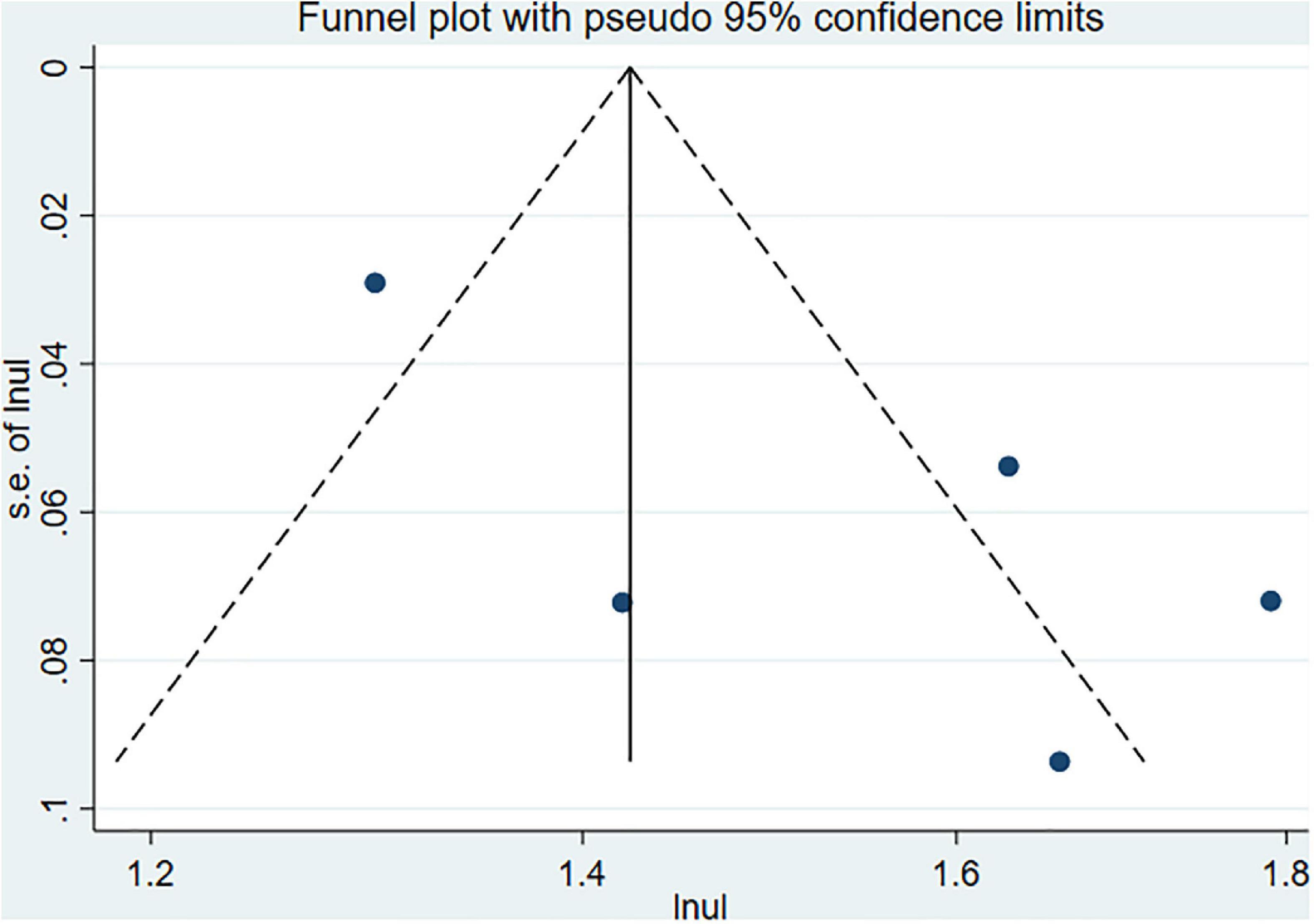
The Begg’s and Egger’s tests and funnel plot showed no publication bias in the current studies (Begg = 1.000, Egger = 0.446) (Figure 3). Furthermore, sensitivity analysis of the pooled results showed that individual studies did not substantially affect the association between SS and dementia (Figure 4).
Association between Sjögren’s syndrome and Parkinson’s disease
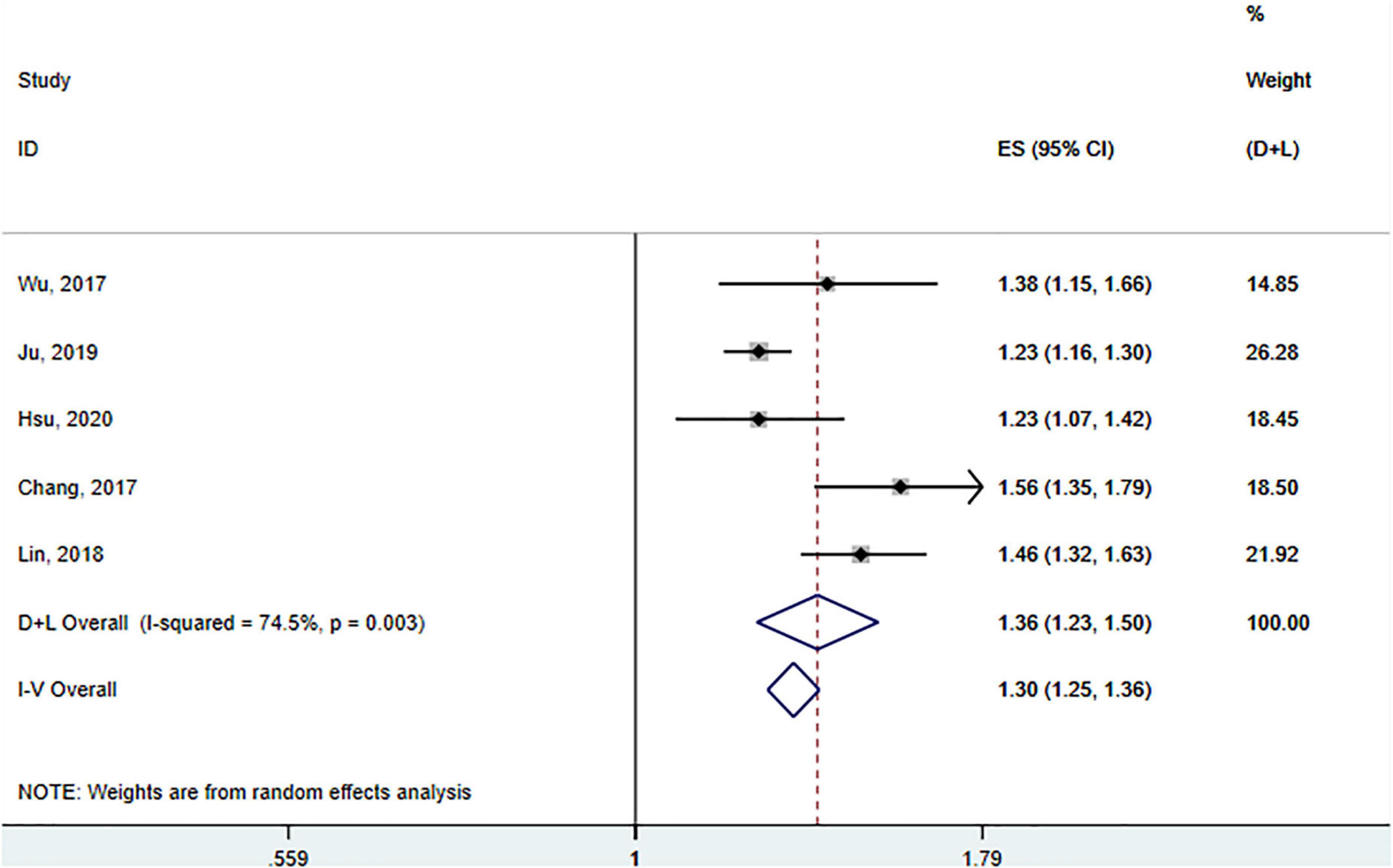
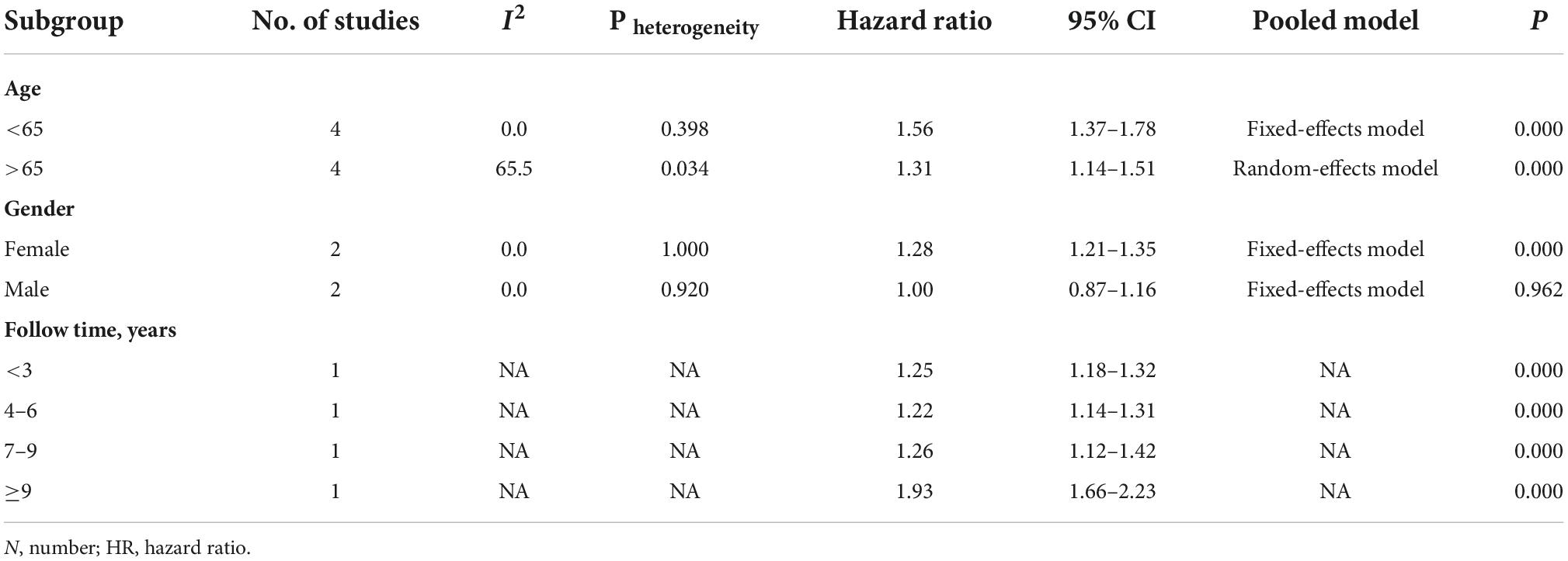
A total of five studies involving 163 522 participants (Wu et al., 2017; Chang et al., 2018; Lin et al., 2018; Ju et al., 2019; Hsu et al., 2020) assessed the association between SS and the risk of PD. Tests for heterogeneity showed large heterogeneity between studies (I2 = 74.5%, p (heterogeneity) = 0.003), so a random-effects model was adopted. Pooled results showed that SS was significantly associated with a subsequent increased risk of PD (HR = 1.36; 95% CI: 1.23–1.50, p < 0.001) (Figure 5).
We conducted a meta-analysis of subgroups according to age, sex, and duration of follow-up (Table 4). In the subgroup analysis based on age, the risk of developing PD was 1.56 and 1.31 times higher in SS patients under and over 65 than in non-SS patients (Chang et al., 2018; Lin et al., 2018; Ju et al., 2019; Hsu et al., 2020), respectively (<65: HR = 1.56; 95% CI: 1.37–1.78 vs. > 65: HR = 1.31; 95% CI: 1.14–1.51, respectively), indicating that age had no significant effect on the increased risk.
In the subgroup analysis by sex, two cohort studies (Ju et al., 2019; Hsu et al., 2020) examined the risk of subsequent PD in male and female SS patients. The pooled HR for PD was 1.28 (95% CI: 1.21–1.35; p = 0.000) in female SS patients and 1.00 (95% CI: 0.87–1.16, p = 0.962) in male SS patients. These results revealed that the correlation between SS and PD risk seemed to be applicable primarily to female patients.
Only Park et al. (2021) assessed the association between the duration of follow-up on SS and subsequent PD risk. The result showed that the association between SS and PD risk was higher for long-term follow-up (≥9 years) than for follow-up periods of less than 9 years.
Evaluation for publication bias and sensitivity analysis
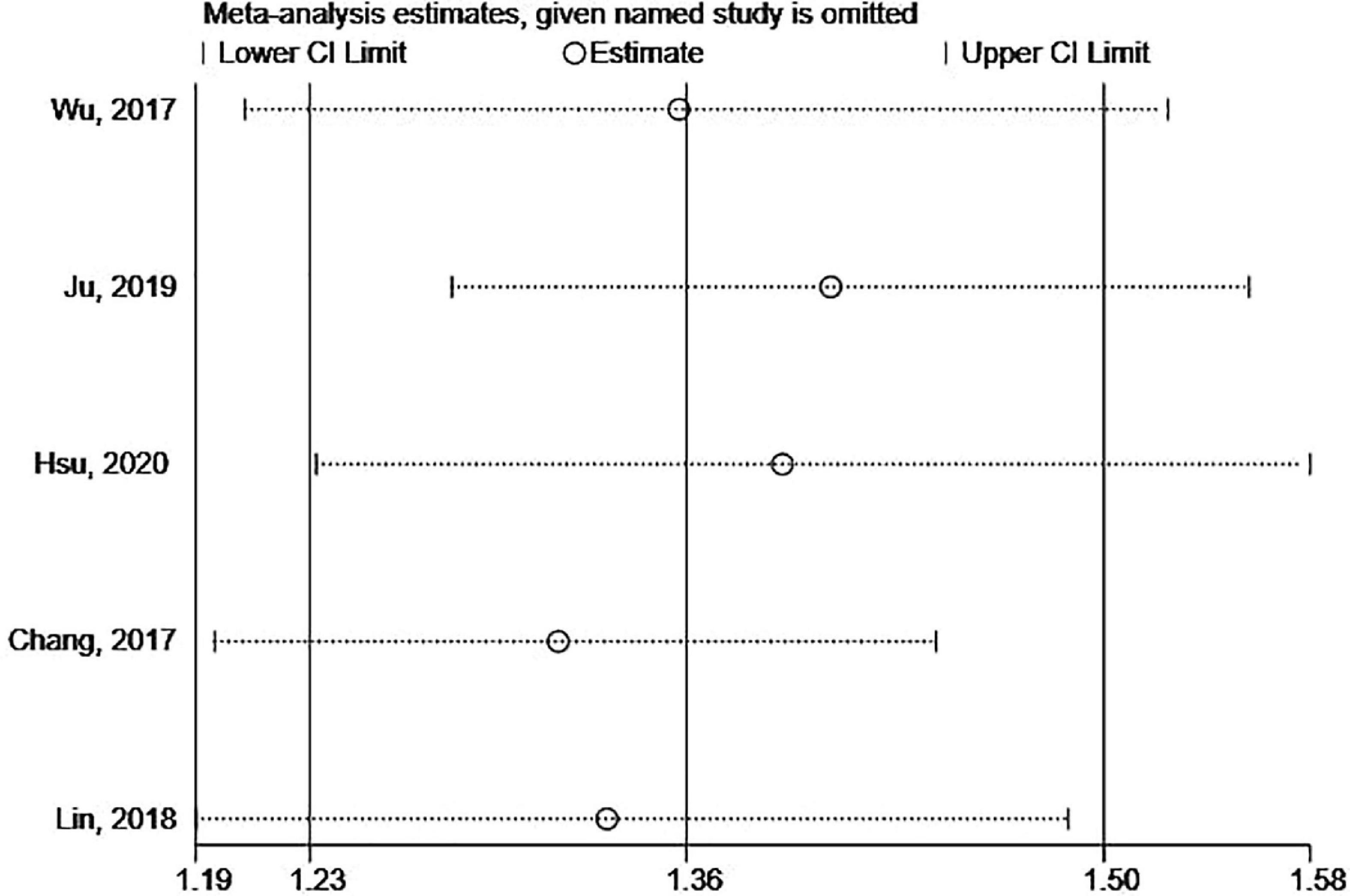
Begg’s and Egger’s tests as well as funnel plots were used to assess potential publication bias. No potential publication bias was present (Begg = 0.806 and Egger = 0.242) (Figure 6). The robustness of the results was assessed by deleting each study in turn. The exclusion of any individual study did not affect the conclusions (Figure 7).
Discussion
Main findings
To our knowledge, this meta-analysis is the first to comprehensively investigate and quantify the association between SS and the risk of dementia/PD. We found a 1.24-fold greater risk of dementia in people with SS compared to the general population; age and gender appear to have no significant effect on this risk. The risk of PD in SS patients was 1.36 times higher in SS individuals. This elevated risk, interestingly, appeared to be prevalent solely in female individuals. Age had no significant effect on the risk of developing PD in SS patients. Based on the fact that over 10,000 patients with both SS and dementia or both SS and PD were involved, the study provided convincing information for the association between SS and PD.
Influence of Sjögren’s syndrome on dementia
In recent years, the prevalence of dementia has been rising at an alarming rate in our aging population (Raz et al., 2016). Dementia mainly includes Alzheimer’s disease, Lewy body dementia, frontotemporal dementia, vascular dementia, and mixed dementia (Marson et al., 2021). It is estimated that 46.8 million people worldwide were living with dementia in 2015. This number is expected to reach 131.5 million by 2050 (Timoszuk et al., 2018). Amyloid-beta (Aβ) peptides and neuroinflammation are the most notable indicators of the disease (Leng and Edison, 2021). Although the molecular mechanisms of tissue damage in these dementias are yet to be fully understood, neuroinflammation and other specific changes in these neurodegenerative diseases, have become the focus of new research (Hensley, 2010; Calsolaro and Edison, 2016). The risk of PD as a comorbidity in patients with SS, one of the most common inflammatory rheumatic diseases with a prevalence between 1:100 and 1:1,000 (Witte, 2019), has received increasing attention (Liliang et al., 2018; Chen et al., 2019; Hou et al., 2019). Since the higher risk of dementia in SS patients may produce significant public health consequences, it is necessary to explore the possibility of SS as an early manifestation of dementia and obtain the potential benefits of early treatment with immunomodulatory or immunosuppressive drugs at an early stage.
The influence of neuroinflammation on dementia remains a controversial topic. The interaction between SS and dementia is becoming more obvious, yet the factors that contribute to progression are unclear. Vasculitis, autoantibodies, immune complex deposition, and cellular inflammation are potential pathways through which SS may elevate the risk of dementia, resulting in nerve damage, cognitive impairment, and initial dementia (Tobón et al., 2012). Previous studies have suggested that inflammation may be a significant event in the pathophysiology of dementia (Newcombe et al., 2018; Webers et al., 2020). Recent studies have found that SS can produce local and systemic inflammation, which in turn leads to a large elevation in IL-1β and TNF-α in vivo (Lisi et al., 2011, 2012) and directly or indirectly causes neuronal damage, followed by AD (Ye et al., 2013). Furthermore, Choi et al. (2018) observed that damage to hippocampal neurogenesis in the early stages of AD may increase the vulnerability of hippocampal neurons, resulting in more severe cognitive impairment and neuronal loss in the later stages of AD. Animal experiments by Vom Berg demonstrated that the natural antibody ustekinumab inhibited the pro-inflammatory cytokines IL-12 and IL-23, which are associated with the accumulation of amyloid, and improved cognitive performance in mice (Sue and Griffin, 2013). Although there is no direct evidence on the occurrence of neuroinflammation in the human AD brain, the findings above suggest that neuroinflammation and altered neurogenesis are linked in AD models. However, further studies are needed to clarify the exact causal relationship between these two phenomena.
Noteworthily, Hou et al. (2019) observed that SS patients with a combination of any other comorbidities (diabetes, hypertension, cardiovascular disease, stroke, and severe mental illnesses) had a higher risk of dementia than non-SS patients without comorbidities. This is consistent with the findings of Chen et al. (2019) who found that SS patients with co-occurring hypertension, PD, and insomnia were more likely to develop dementia. Some studies have confirmed that SS patients are more likely to develop hyperlipidemia, hypertension, and other disorders (Ramos-Casals et al., 2007; Pérez-De-Lis et al., 2010). Many dementias, on the other hand, are associated with diabetes, metabolic syndrome, and cardiovascular disease, suggesting that underlying disease factors may skew the association between SS and increased risk of dementia. This may explain why patients with SS who have comorbidities are more likely to develop dementia. Furthermore, there is no association between substantial cognitive impairment (including dementia) and the use of cardiovascular or hypertensive medications in people with these comorbidities (Hou et al., 2019).
Influence of Sjögren’s syndrome on Parkinson’s disease
PD is the second most common neurodegenerative disease after Alzheimer’s disease, and it is expected to place an increasing medical and economic burden on society as the population ages (de Lau and Breteler, 2006). Typical PD symptoms are resting tremor, rigidity, bradykinesia, postural instability (Aarsland et al., 2017), and pathology characterized by degeneration and death of dopaminergic neurons in the substantia nigra (Obeso et al., 2008). Visser et al. (1993) first reported a case of hemiparkinsonism (partial Parkinson’s syndrome) in a patient who presented with both signs and symptoms of the primary SS in 1993. Mochizuki et al. (1997) discovered a man diagnosed with Parkinson’s syndrome who also had SS. His PD was believed to be caused by SS. More recently, Kchaou et al. (2015) concluded that neuroinflammatory processes appear to exist between two completely different diseases, PD and pSS, based on observations of pathophysiological findings in PD patients. A growing number of researchers recognized the risk of PD as a comorbidity in SS patients, and several immune system-mediated mechanisms have been proposed to account for the possible pathogenic mechanism of autoantibodies inducing dopaminergic cell death (Kunas et al., 1995; Monahan et al., 2015). SS and other autoimmune diseases form a spectrum of systemic symptoms ranging from organ-specific to multi-organ involvement. Patients suffer from a significant symptom-related burden, followed by a drop in health-related quality of life and productivity (Meijer et al., 2009). Therefore, health education and risk factor modification are essential for this patient group.
It is crucial to determine the causal relationship between SS and PD. In several of the included studies, all patients with PD were diagnosed after an episode of SS (Wu et al., 2017; Chang et al., 2018; Lin et al., 2018; Ju et al., 2019; Hsu et al., 2020). In the study by Ju et al. (2019), there was a 93% greater risk of PD with SS when the follow-up duration was longer than 9 years compared to subjects without SS. As a result, the available evidence suggests that SS is a risk factor for PD rather than a shared risk factor or a factor with an inverse correlation.
SS is known to be mediated by the interaction of genetic, epigenetic, and environmental factors causing immune dysregulation, which leads to an aggressive autoimmune disease affecting the central nervous system (CNS) and peripheral nervous system (Morgen et al., 2004), often exhibiting a higher female bias (Chatzis et al., 2021). Our study also found that the correlation between SS and PD risk appears to occur primarily in female patients, as opposed to male SS patients, which may be explained by that higher urate levels in men diminish the risk of PD, whereas this is not the case in women (Ascherio et al., 2009; O’Reilly et al., 2010). Autopsy analyses and experimental animal studies of human PD patients have shown that increased pro-inflammatory factors in the brain lead to neuronal degeneration and the development of PD (Wang et al., 2015). Furthermore, both anticardiolipin and anti-β2-glycoprotein-I levels have been reported to be higher in SS patients who develop PD (Mochizuki et al., 1997; Hassin-Baer et al., 2007). Inflammation may thus be a critical factor in the development of PD in patients with SS, yet neuroinflammation has been a controversial topic in the pathogenesis of PD (Figueiredo-Pereira et al., 2015; Renaud et al., 2015). A recent review explored the evidence for autoimmune involvement in PD and proposed targeted inflammatory therapy as a novel neuroprotective approach (Moehle and West, 2015).
Ju et al. (2019) explored the effect of immunosuppression on the risk of PD in patients with autoimmune diseases, including SS, and found that the risk of PD was significantly higher in participants with SS who received hydroxychloroquine, compared to those without SS (HR = 1.46, 95% CI: 1.34–1.59), but the risk of PD in SS participants receiving non-hydroxychloroquine immunosuppressive therapy was relatively low (HR = 0.86; 95% CI: 0.73–1.01). It is, therefore, justifiable to assume that non-hydroxychloroquine immunosuppression plays a crucial role in lowering the risk of PD. The neuroprotective potential of hydroxychloroquine has been debated, and its efficacy in treating systemic lupus erythematosus (Ruiz-Irastorza et al., 2010) and rheumatoid arthritis (Suarez-Almazor et al., 2000) has been widely recognized. Still, its efficacy in the treatment of SS remains unclear. In a study by Fox et al. (1996) on hydroxychloroquine in the treatment of SS, regular use of hydroxychloroquine was found to be effective in treating SS, providing relief from fatigue and arthralgia. However, several studies have indicated that taking hydroxychloroquine for SS had no therapeutic effect for people with pSS when compared to placebo treatment (Kruize et al., 1996). Immunotherapy-related neurological disorders have been progressively documented in recent years (Villoslada et al., 2008; Prior, 2015; General-López, 2018). Future research should concentrate on the association between autoimmune disease and PD, as well as the relation between immunosuppression and PD.
Limitations
There are still some limitations to our meta-analysis. First, we could not distinguish whether SS was primary or secondary to another autoimmune disease in our meta-analysis. Failure to fully elucidate the clinical severity of SS and the subtypes of dementia and PD may lead to inaccurate estimates of their genuine association. Secondly, most of the eligible studies were conducted in Taiwan region (China), which may lead to geographical bias and relatively high overlap of included samples. Therefore, it is unclear whether this association holds for other races and regions in the world. In addition, none of the 10 included studies assessed the effect of monitoring bias on outcomes. Since subjects with SS typically experienced more medical visits than those without SS, they were more likely to be diagnosed with dementia or PD. In this way, the risk of dementia or PD for subjects with SS may be skewed higher in the overall analysis results. Finally, all original studies were designed retrospectively and their data collection did not consider the need for specific scientific research, thus limiting the completeness and homogeneity of the data.
Conclusion
The results of this study reveal that patients with SS are at significantly elevated risk of developing PD and dementia and that regular neurological screening of SS patients may be warranted. More prospective studies from different regions are necessary to elucidate the underlying mechanisms and to further characterize the impact of SS on development of PD and dementia.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
Z-ZW, M-SL, and ZS developed the protocol, participated in the literature search, extracted data, and drafted the manuscript. X-LZ and M-LZ was responsible for the analysis and interpretation of the data. KX contributed to statistical expertise. FZ supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (81873387), the National key talents Project of traditional Chinese Medicine (2019), This study was also supported by the foundation of the training Project of Young and Middle-aged Science and Technology leaders in Xianyang (Major Scientific and Technological Innovation Project) (2019k01-52), and the training Program of Shaanxi University of Traditional Chinese Medicine (2017SZKY-018). The funding agents play no role in study design, data collection, and data analyses.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnint.2022.1027044/full#supplementary-material
References
Aarsland, D., Creese, B., Politis, M., Chaudhuri, K. R., Ffytche, D. H., Weintraub, D., et al. (2017). Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 13, 217–231. doi: 10.1038/nrneurol
Alzheimer’s Association (2017). 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 10:e47–e92.
Ascherio, A., LeWitt, P. A., Xu, K., Eberly, S., Watts, A., Matson, W. R., et al. (2009). Parkinson study group DATA TOP investigators. urate as a predictor of the rate of clinical decline in Parkinson disease. Arch. Neurol. 66, 1460–1468.
Beard, J. R., Officer, A., de Carvalho, I. A., Sadana, R., Pot, A. M., Michel, J. P., et al. (2016). The world report on aging and health: A policy framework for healthy aging. Lancet 387, 2145–2154. doi: 10.1016/S0140-6736(15)00516-4
Blanc, F., Longato, N., Jung, B., Kleitz, C., Di Bitonto, L., Cretin, B., et al. (2013). Cognitive dysfunction and dementia in primary sjögren’s syndrome. ISRN Neurol. 2013:501327. doi: 10.1155/2013/501327
Calsolaro, V., and Edison, P. (2016). Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 12, 719–732. doi: 10.1016/j.jalz.2016.02.010
Chang, C. C., Lin, T. M., Chang, Y. S., Chen, W. S., Sheu, J. J., Chen, Y. H., et al. (2018). Autoimmune rheumatic diseases and the risk of Parkinson’s disease: A nationwide population-based cohort study in Taiwan. Ann. Med. 50, 83–90. doi: 10.1080/07853890.2017.1412088
Chatzis, L. G., Goules, A. V., and Tzioufas, A. G. (2021). Searching for the “X factor” in Sjögren’s syndrome female predilection. Clin. Exp. Rheumatol. 39, 206–214.
Chen, K. T., Chen, Y. C., Fan, Y. H., Lin, W. X., Lin, W. C., Wang, Y. H., et al. (2018). Rheumatic diseases are associated with a higher risk of dementia: A nation-wide, population-based, case-control study. Int. J. Rheum. Dis. 21, 373–380. doi: 10.1111/1756-185X.13246
Chen, H. H., Perng, W. T., Chiou, J. Y., Wang, Y. H., Huang, J. Y., and Wei, J. C. (2019). Risk of dementia among patients with Sjogren’s syndrome: A nationwide population-based cohort study in Taiwan. Semin. Arthritis. Rheum. 48, 895–899. doi: 10.1016/j.semarthrit
Choi, S. H., Bylykbashi, E., Chatila, Z. K., Lee, S. W., Pulli, B., Clemenson, G. D., et al. (2018). Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361:eaan8821. doi: 10.1126/science.aan8821
de Lau, L. M., and Breteler, M. M. (2006). Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535. doi: 10.1016/S1474-4422(06)70471-9
Delalande, S., de Seze, J., Fauchais, A. L., Hachulla, E., Stojkovic, T., Ferriby, D., et al. (2004). Neurologic manifestations in primary Sjogren syndrome: A study of 82 patients. Medicine 83, 280–291.
Figueiredo-Pereira, M. E., Rockwell, P., Schmidt-Glenewinkel, T., and Serrano, P. (2015). Neuroinflammation and J2 prostaglandins: Linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Front. Mol. Neurosci. 7:104. doi: 10.3389/fnmol.2014.00104
Fox, R. I., Dixon, R., Guarrasi, V., and Krubel, S. (1996). Treatment of primary Sjögren’s syndrome with hydroxychloroquine: A retrospective, open-label study. Lupus 5:S31–S36.
General-López, R. C. (2018). Immunotherapy for neurological diseases, present, and future. Farm. Hosp. 42, 251–260. doi: 10.7399/fh.11031
Hassin-Baer, S., Levy, Y., Langevitz, P., Nakar, S., and Ehrenfeld, M. (2007). Anti-beta2-glycoprotein I in Sjogren’s syndrome is associated with parkinsonism. Clin. Rheumatol. 26, 743–747. doi: 10.1007/s10067-006-0398-8
Hensley, K. (2010). Neuroinflammation in Alzheimer’s disease: Mechanisms, pathologic consequences, and potential for therapeutic manipulation. J. Alzheimers Dis. 21, 1–14. doi: 10.3233/JAD-2010-1414
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ. 327, 557–560. doi: 10.1136/bmj.327.7414.557
Hindle, J. V. (2010). Ageing, neurodegeneration, and Parkinson’s disease. Age Ageing 39, 156–161. doi: 10.1093/ageing/afp223
Hou, T. Y., Hsu, H. C., Lin, T. M., Chang, Y. S., Chen, W. S., Kuo, P. I., et al. (2019). Higher risk of dementia in primary Sjogren’s syndrome. Ann. Clin. Transl. Neurol. 6, 633–641. doi: 10.1002/acn3.737
Hsu, H. C., Hou, T. Y., Lin, T. M., Chang, Y. S., Chen, W. S., Kuo, P. I., et al. (2020). Higher risk of Parkinson’s disease in patients with primary Sjögren’s syndrome. Clin. Rheumatol. 39, 2999–3007. doi: 10.1007/s10067-020-05053-z
Jellinger, K. A. (2003). Alpha-synuclein pathology in Parkinson’s and Alzheimer’s disease brain: Incidence and topographic distribution–a pilot study. Acta. Neuropathol. 106, 191–201. doi: 10.1007/s00401-003-0725-y
Ju, U. H., Liu, F. C., Lin, C. S., Huang, W. Y., Lin, T. Y., Shen, C. H., et al. (2019). Risk of Parkinson disease in Sjögren syndrome administered ineffective immunosuppressant therapies: A nationwide population-based study. Medicine 98:e14984. doi: 10.1097/MD.0000000000014984
Kchaou, M., Ben Ali, N., Hmida, I., Fray, S., Jamoussi, H., Jalleli, M., et al. (2015). Parkinsonism, and sjögren’s syndrome: A fortuitous association or a shared immunopathogenesis? Case Rep. Med. 2015:432910. doi: 10.1155/2015/432910
Kruize, A. A., Hené, R. J., Kallenberg, C. G., van Bijsterveld, O. P., van der Heide, A., Kater, L., et al. (1996). Hydroxychloroquine treatment for primary Sjögren’s syndrome: A two year double blind crossover trial. Ann. Rheum. Dis. 52, 360–364. doi: 10.1136/ard.52.5.360
Kunas, R. C., McRae, A., Kesselring, J., and Villiger, P. M. (1995). Antidopaminergic antibodies in a patient with a complex autoimmune disorder and rapidly progressing Parkinson’s disease. J. Allergy. Clin. Immunol. 6, 688–690. doi: 10.1016/s0091-6749(95)70268-7
Kwon, M. J., Kim, J. H., Kim, T., and Lee, S. B. (2017). Pharmacological intervention of early neuropathy in neurodegenerative diseases. Pharmacol. Res. 119, 169–177. doi: 10.1016/j.phrs.2017.02.003
Leng, F., and Edison, P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 17, 157–172. doi: 10.1038/s41582-020-00435-y
Liliang, P. C., Liang, C. L., Lu, K., Yang, S. N., Hsieh, M. T., Tai, Y. C., et al. (2018). The population-based study suggests an increased risk of Alzheimer’s disease in Sjögren’s syndrome. Clin. Rheumatol. 37, 935–941. doi: 10.1007/s10067-017-3940-y
Lin, T. M., Chen, W. S., Sheu, J. J., Chen, Y. H., Chen, J. H., and Chang, C. C. (2018). Autoimmune rheumatic diseases increase dementia risk in middle-aged patients: A nationwide cohort study. PLoS One 13:e0186475. doi: 10.1371/journal.pone.0186475
Lisi, S., Sisto, M., Lofrumento, D. D., and D’Amore, M. (2011). Sjögren’s syndrome autoantibodies provoke changes in gene expression profiles of inflammatory cytokines triggering a pathway involving TACE/NF-κB. Lab. Invest. 92, 615–624. doi: 10.1038/labinvest
Lisi, S., Sisto, M., Lofrumento, D. D., and D’Amore, M. (2012). Sjögren’s syndrome autoantibodies provoke changes in gene expression profiles of inflammatory cytokines triggering a pathway involving TACE/NF-κB. Lab. Invest. 92, 615–624.
Malladi, A. S., Sack, K. E., Shiboski, S. C., Shiboski, C. H., Baer, A. N., Banushree, R., et al. (2012). primary sjögren’s syndrome as a systemic disease: A study of participants enrolled in an international Sjögren’s syndrome registry. Arth. Care Res. 64, 911–918. doi: 10.1002/acr.21610
Margaretten, M. (2017). neurologic manifestations of primary sjögren syndrome. Rheum. Dis. Clin. North Am. 43, 519–529. doi: 10.1016/j.rdc.2017.06.002
Marson, F., Lasaponara, S., and Cavallo, M. (2021). A scoping review of neuromodulation techniques in neurodegenerative diseases: A useful tool for clinical practice? Medicine 57:215. doi: 10.3390/medicina57030215
Meijer, J. M., Meiners, P. M., Huddleston Slater, J. J., Spijkervet, F. K., Kallenberg, C. G., Vissink, A., et al. (2009). Health-related quality of life, employment and disability in patients with Sjogren’s syndrome. Rheumatology 48, 1077–1082.
Mochizuki, H., Okano, M., Masaki, T., Nagata, N., and Kamakura, K. (1997). [A case of Sjögren’s syndrome with a high titer of anticardiolipin antibody that developed as parkinsonism]. Rinsho Shinkeigaku 37, 57–59.
Moehle, M. S., and West, A. B. (2015). M1 and M2 immune activation in Parkinson’s Disease: Foe and ally? Neuroscience 302, 59–73. doi: 10.1016/j.neuroscience.2014.11.018
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4:1. doi: 10.1186/2046-4053-4-1
Monahan, A. J., Warren, M., and Carvey, P. M. (2015). Neuroinflammation and peripheral immune infiltration in Parkinson’s disease: An autoimmune hypothesis. Cell Transplant. 17, 363–372.
Morgen, K., McFarland, H. F., and Pillemer, S. R. (2004). Central nervous system disease in primary Sjogren’s syndrome: The role of magnetic resonance imaging. Semin. Arth. Rheum. 34, 623–630.
Mæland, E., Miyamoto, S. T., Hammenfors, D., Valim, V., and Jonsson, M. V. (2021). Understanding fatigue in sjögren’s syndrome: Outcome measures, biomarkers and possible interventions. Front. Immunol. 12:703079. doi: 10.3389/fimmu.2021.703079
Newcombe, E. A., Camats-Perna, J., Silva, M. L., Valmas, N., Huat, T. J., and Medeiros, R. (2018). Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflamm. 15:276.
Obeso, J. A., Rodríguez-Oroz, M. C., Benitez-Temino, B., Blesa, F. J., Guridi, J., Marin, C., et al. (2008). Functional organization of the basal ganglia: Therapeutic implications for Parkin son’s disease. Mov. Disord 23:S548–S559.
Odani, T., and Chiarini, J. A. (2019). Targeting primary Sjögren’s syndrome. Mod. Rheum. 29, 70–86. doi: 10.1080/14397595.2018.1546268
O’Reilly, E. J., Gao, X., Weisskopf, M. G., Chen, H., Schwarzschild, M. A., Spiegelman, D., et al. (2010). Plasma urate and Parkinson’s disease in women. Am. J. Epidemiol. 172, 666–670.
Park, H., Yim, D. H., Ochirpurev, B., Eom, S. Y., Choi, I. A., Ju, G., et al. (2021). Association between dementia and systemic rheumatic disease: A nationwide population-based study. PLoS One 16:e0248395. doi: 10.1371/journal.pone.0248395
Pérez-De-Lis, M., Akasbi, M., Sisó, A., Diez-Cascon, P., Brito-Zerón, P., Diaz-Lagares, C., et al. (2010). Cardiovascular risk factors in primary Sjögren’s syn-drome: A case-control study in 624 patients. Lupus 19, 941–948. doi: 10.1177/0961203310367504
Prior, P. L. (2015). Immunotherapy applied to neuropsychiatric disorders: A new perspective of treatment. J. Mol. Neurosci. 57, 139–141. doi: 10.1007/s12031-015-0587-5
Ramos-Casals, M., Brito-Zerón, P., Seror, R., Bootsma, H., Bowman, S. J., Dörner, T., et al. (2015). Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS task force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology 54, 2230–2238. doi: 10.1093/rheumatology/kev200
Ramos-Casals, M., Brito-Zerón, P., Sisó, A., Vargas, A., Ros, E., Bove, A., et al. (2007). High prevalence of serum metabolic alterations in primary Sjögren’s syndrome: Influence on clinical and immunological expression. J. Rheumatol. 34, 754–761.
Raz, L., Knoefel, J., and Bhaskar, K. (2016). The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 36, 172–186. doi: 10.1038/jcbfm
Renaud, J., Nabavi, S. F., Daglia, M., Nabavi, S. M., and Martinoli, M. G. (2015). Epigallocatechin-3-gallate, a promising molecule for Parkinson’s disease? Rejuvenation Res. 18, 257–269. doi: 10.1089/rej.2014.1639
Ruiz-Irastorza, G., Ramos-Casals, M., Brito-Zeron, P., and Khamashta, M. A. (2010). Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: A systematic review. Ann. Rheum. Dis. 69, :20–28. doi: 10.1136/ard.2008.101766
Sjögren, H. (1933). Zur kenntnis der keratoconjunctivitis sicca. Acta. Ophthalmol. 3, 1–39. doi: 10.1111/j.1755-3768.1935.tb04186.x
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Suarez-Almazor, M. E., Belseck, E., Shea, B., Homik, J., Wells, G., and Tugwell, P. (2000). Antimalarials for rheumatoid arthritis. Cochrane Database Syst. Rev. 2000:Cdc000959.
Sue, W., and Griffin, T. (2013). Neuroinflammation cytokine signaling and Alzheimer’s disease. N. Engl. J. Med. 368, 770–771. doi: 10.1056/NEJMcibr121454
Timoszuk, M., Bielawska, K., and Skrzydlewska, E. (2018). Evening primrose (oenothera biennis) biological activity dependent on chemical composition. Antioxidants 7:108. doi: 10.3390/antiox7080108
Tobón, G. J., Pers, J. O., Devauchelle-Pensec, V., and Youinou, P. (2012). Neurological disorders in primary sjögren’s syndrome. Autoimmune Dis. 2012:645967. doi: 10.1155/2012/645967
Villoslada, P., Moreno, B., Melero, I., Pablos, J. L., Martino, G., Uccelli, A., et al. (2008). Immunotherapy for neurological diseases. Clin. Immunol. 128, 294–305. doi: 10.1016/j.clim.2008.04.003
Visser, L. H., Koudstaal, P. J., and van de Merwe, J. P. (1993). Hemiparkinsonism in a patient with primary Sjögren’s syndrome. A case report and a review of the literature. Clin. Neurol. Neurosurg. 95, 141–145. doi: 10.1016/0303-8467(93)90009-6
Wang, Q., Liu, Y., and Zhou, J. (2015). Neuroinflammation in Parkinson’s disease and its potential as a therapeutic target. Transl. Neurodegener. 4:19. doi: 10.1186/s40035-015-0042-0
Webers, A., Heneka, M. T., and Gleeson, P. A. (2020). The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 98, 28–41. doi: 10.1111/imcb.12301
Witte, T. (2019). Sjögren-Syndrom [Sjögren’s syndrome]. Z. Rheumatol. 78, 511–517. doi: 10.1007/s00393-019-0625-8
Wu, L., Huang, Z., and Pan, Z. (2021). The spatiality and driving forces of population aging in China. PLoS One 16:e0243559. doi: 10.1371/journal.pone.0243559
Wu, M. C., Xu, X., Chen, S. M., Tyan, Y. S., Chiou, J. Y., Wang, Y. H., et al. (2017). Impact of Sjogren’s syndrome on Parkinson’s disease: A nationwide case-control study. PLoS One 12:e0175836. doi: 10.1371/journal.pone.0175836
Keywords: Sjögren’s syndrome, Parkinson’s disease, dementia, systematic review, meta-analysis, risk factor
Citation: Wang Z-Z, Liu M-S, Sun Z, Zhang X-L, Zhang M-L, Xiong K and Zhou F (2022) Risk of dementia or Parkinson’s disease in the presence of Sjögren’s syndrome: A systematic review and meta-analysis. Front. Integr. Neurosci. 16:1027044. doi: 10.3389/fnint.2022.1027044
Received: 24 August 2022; Accepted: 19 October 2022;
Published: 07 November 2022.
Edited by:
Steffen Schulz, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Pingyi Xu, First Affiliated Hospital of Guangzhou Medical University, ChinaIvan G. Milanov, Multiprofile Hospital for Active Treatment in Neurology and Psychiatry St. Naum, Bulgaria
Guigang Li, Huazhong University of Science and Technology, China
Copyright © 2022 Wang, Liu, Sun, Zhang, Zhang, Xiong and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Zhou, MzYyODExNzE1QHFxLmNvbQ==
†These authors have contributed equally to this work
 Zhen-Zhi Wang
Zhen-Zhi Wang Meng-Si Liu2†
Meng-Si Liu2†