- Department of Translational Research, Academic Radiology, University of Pisa, Pisa, Italy
The aim of this systematic review was to evaluate the state of the art of radiomics in testicular imaging by assessing the quality of radiomic workflow using the Radiomics Quality Score (RQS) and the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2). A systematic literature search was performed to find potentially relevant articles on the applications of radiomics in testicular imaging, and 6 final articles were extracted. The mean RQS was 11,33 ± 3,88 resulting in a percentage of 31,48% ± 10,78%. Regarding QUADAS-2 criteria, no relevant biases were found in the included papers in the patient selection, index test, reference standard criteria and flow-and-timing domain. In conclusion, despite the publication of promising studies, radiomic research on testicular imaging is in its very beginning and still hindered by methodological limitations, and the potential applications of radiomics for this field are still largely unexplored.
1. Introduction
Radiomics is defined as the process of obtaining high-dimensional data from medical images as quantitative features (1). In recent years, a growing interest in this field led to a rising number of applications in many different medical imaging fields (2). Radiomics has gained importance especially in the field of precision medicine, which aims to tailor treatments based on specific characteristics, including genetical and phenotypical ones (3). The huge number of quantitative features obtained through Radiomics may be selected and used for classification, prediction and prognosis of different neoplasms, providing additional information about tumor phenotype and gene expression pattern (4, 5). However, application of radiomics is still mainly limited to research setting for many reasons, mostly due to the lack of reproducibility and repeatability of the results, often associated with heterogeneities in the several steps of radiomics workflow (6). Thus, effective evaluation criteria and standardization of radiomic workflows are needed, ranging from the data collection to the model building (7). An attempt to standardize research in radiomics was made by Lambin et al., who developed the Radiomics Quality Score (RQS) for quality assessment of radiomics studies (8). Undoubtedly, oncologic imaging is the main field for radiomics application, including testicles (9–13).
Testicular cancer is a relative rare disease (representing only the 1% of neoplasm and the 5% of urological tumors in males) and it is predominant in young/middle-aged males (14, 15). Many imaging techniques can be used for the evaluation of testicles in different clinical scenarios, before and after therapy (surgery, chemotherapy or radiotherapy), and include ultrasound (US), magnetic resonance imaging (MRI), computed tomography (CT) and fluorodeoxyglucose-positron emission tomography (FDG-PET) (16). The characterization of histologic type is of extreme importance as different tumors (e.g., seminoma germ cell tumor and non-seminoma germ cell tumor) present different prognosis and treatment. Moreover, orchidectomy is still the standard of care for testicular cancer, although it may have a negative impact on reproduction (17). In this setting, alternative non-invasive methods of diagnosis had been proposed to avoid unnecessary surgery, including MRI for the identification and differentiation of benign scrotal lesions, but it still may be inconclusive (18). Additionally, imaging plays an important role in the diagnostic framework of male infertilities, as a relationship between US testicular volume and testicular steroidogenic function has already been demonstrated (19, 20). However, the lack of standardized method to calculate US testicular volume and validated reference ranges has prompted the search for other reliable quantitative US parameters (21). The aim of this systematic review is to evaluate the state of the art of radiomics in testicular imaging by assessing the quality of radiomic workflow.
2. Materials and methods
2.1. Literature search
To identify records of interest, two reviewers (S.C.F. and M.F.) independently performed a systematic literature search for potentially relevant articles about Radiomics applications in testicular imaging.
The examined medical literature archives were PubMed, Scopus, and Web of Science, using the following search terms: testicular AND radiomics. Filters were applied to include only original research published in English. No restrictions in country of publication, study design, and outcomes were applied. The last search was run in September 2022. Duplicates have been removed and all the selected articles were initially screened reviewing the titles and abstracts. After the screening, the authors read the full text of the studies and any disagreement was overcome by discussion to reach a mutual agreement. From each study, the following data were extracted: publication year, number of patients, study design, study aim, journal topic, and professional role of the first author.
2.2. Study evaluation
The methodological quality of the included studies was carried out by two readers (M.F. and L.C.) using the Radiomics Quality Score (RQS), as proposed by Lambin et al., and the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2), as proposed by Whiting et al. (8, 22). Conflicts between the two reviewers were resolved in consensus together with a third reviewer (S.C.F).
Radiomics Quality Score (RQS) is a tool made up of 16 items, categorized by Park et al. into 6 domains, with different possible scores in relation to its importance (23). According to the RQS, the summed total score ranges from −8 to 36. To calculate percentages, a score of 0% was assign to studies with summed score from −8 up to 0, while a score of 100% was defined for summed score of 36. QUADAS-2 criteria were used to assess presence of relevant biases, including the following domains: patient selection, index test, reference standard and flow and timing. For each domain, the risk of biases and concerns regarding the applicability of the review question were scored as low, high or unclear (in case of insufficient data). You may insert up to 5 heading levels into your manuscript as can be seen in “Styles” tab of this template. These formatting styles are meant as a guide, as long as the heading levels are clear, Frontiers style will be applied during typesetting.
3. Results
After the exclusion of duplicates (19) and unrelated papers (2), 6 articles were finally included in the review. The study selection flow-chart is resumed in Figure 1.
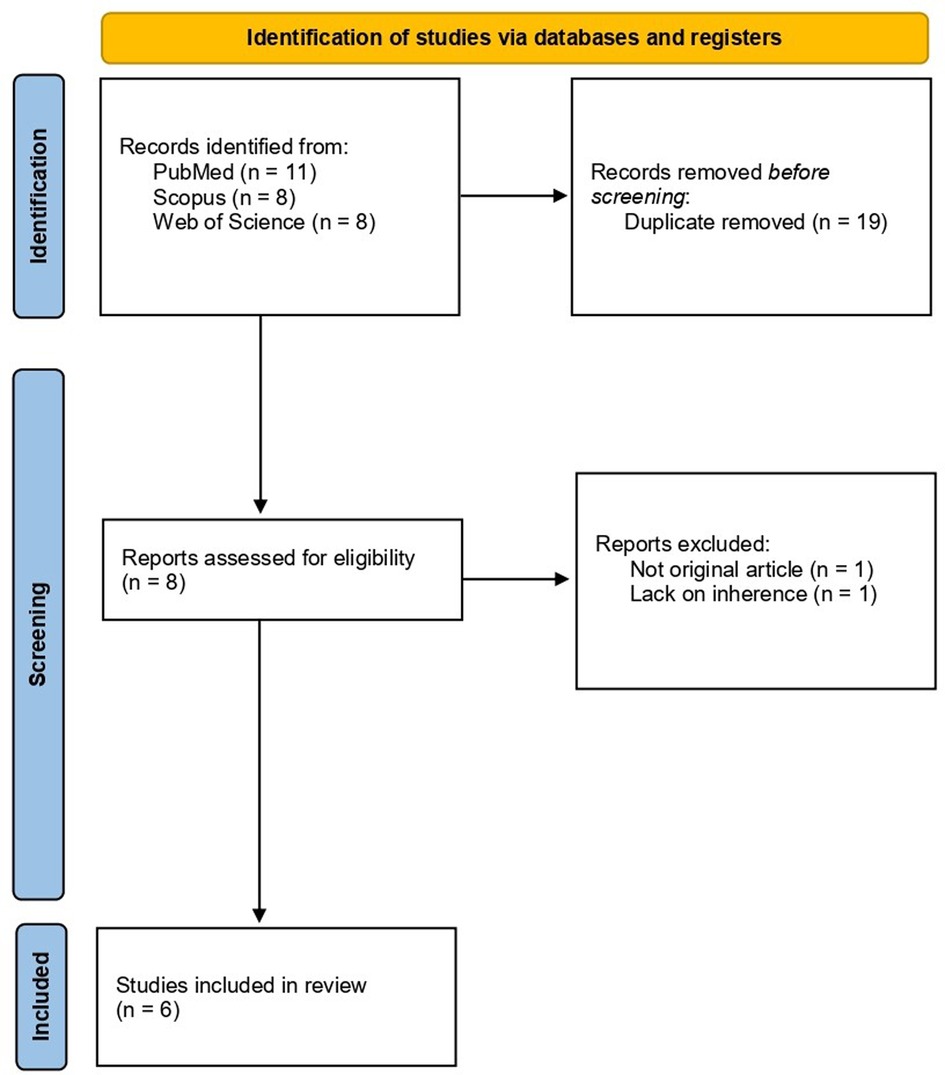
Figure 1. Study selection process flow-diagram according to PRISMA statement 2020 (24).
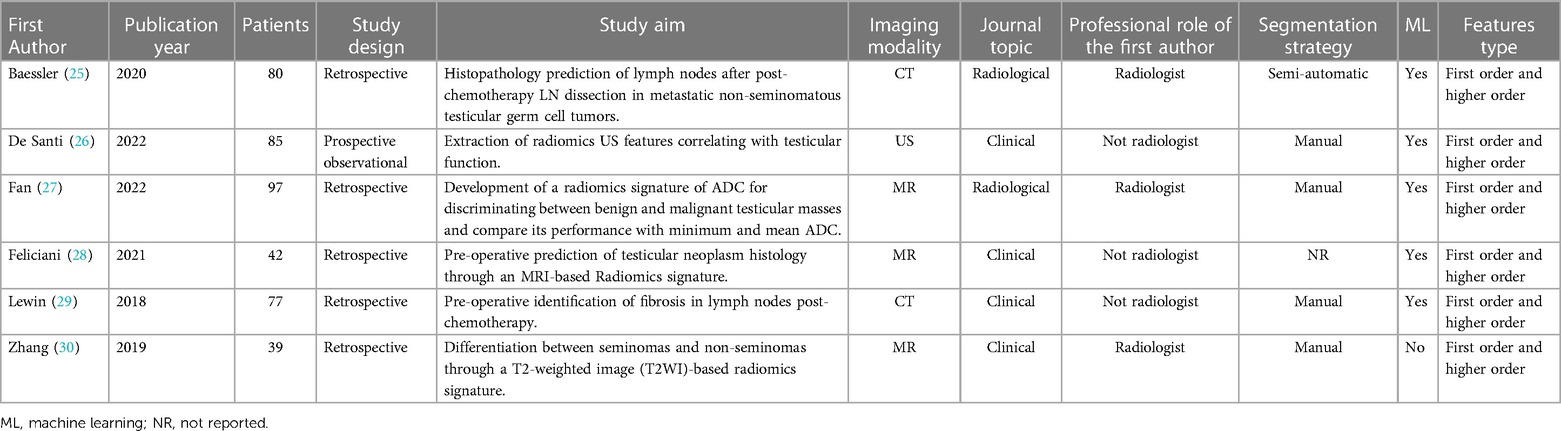
Out of 6 articles, none were published before 2018, four (66%; 4/6) were published in clinical journals and only two (33%; 2/6) in radiological journals. The mean patient number was 70 ± 23,86 (range 39–97). Many of the studies had a retrospective study design (83%; 5/6), while only one was a prospective observational study (17%; 1/6). In half of the papers included in the review, the first author was a radiologist. Most of the articles addressed the oncologic topic (83%; 5/6), focusing on differential diagnosis between benign and malignant testicular masses or prediction of lymph nodes histopathology after chemotherapy. Only one article investigated potential role of radiomics as gonadal function biomarker. Characteristics of included articles are resumed in Table 1.
Overall, the included articles achieved a mean RQS total of 11,33 ± 3,88 (range 6–18) and a percentage of 31,48% ± 10,78% (range 16,67%–50%). Imaging protocols, features reduction, discrimination statistics and comparison to gold standard are well-documented in all the included articles. Validation without retraining was performed in all the studies, even though only on dataset from the same institute. Half of the studies reported multiple segmentation, to analyze feature robustness to segmentation variabilities, while only two provided more holistic models combining radiomics with clinical variables. None of the studies performed phantom studies or imaging at multiple time-points. Similarly, no study reported cut-off analyses, calibration statistics, decision curve or cost-effectiveness analysis. Finally, none of the included articles made code and data publicly available to facilitate reproducibility of the study. The detailed RQS score for all included articles for each RQS item is shown in Table 2.
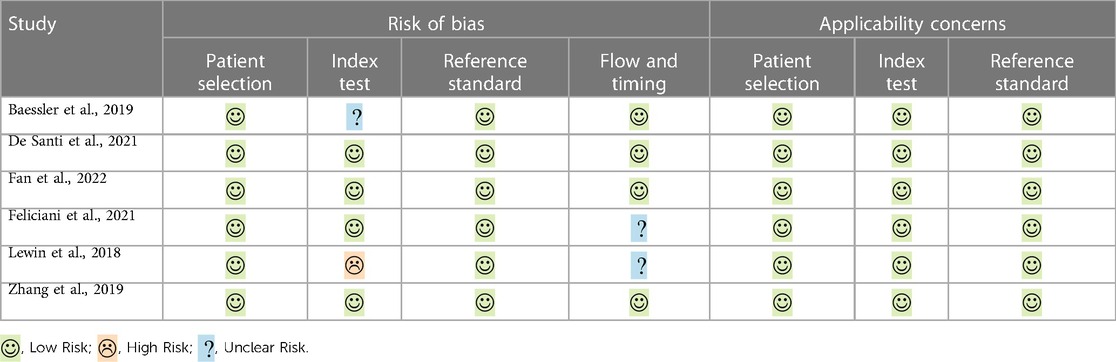
The risk of bias and applicability concerns according to the QUADAS-2 are summarized in Table 3 and Figure 2. All studies were rated as low risk regarding patients' selection and reference standard interpretation. Regarding test interpretation, 4 studies (66%; 4/6) were rated as low, and two studies as unclear (unclear data). Finally, regarding flow and timing of the study, 2 studies (33%; 2/6) were considered unclear.
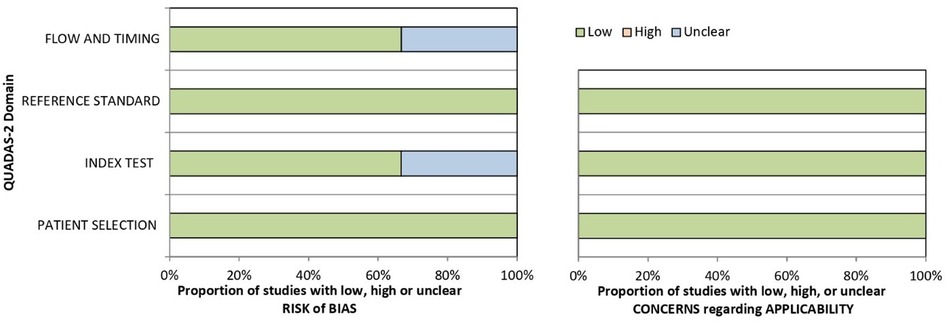
Figure 2. Risk of bias and concerns regarding applicability histograms according to QUADAS-2 for included papers.
4. Discussion
Radiomics is a new engineering approach based on automated high-throughput extraction of quantitative features from medical images (2). Radiomics-based models may empower radiology to overcome the limit of radiologists' visual interpretation.
Despite promising studies, the potential applications of radiomics for testicular imaging are still largely unexplored. Indeed, testicular tumors are relatively rare, as they represent only the 1% of neoplasm and the 5% of urological tumors in males (14, 15). Coherently, the number of patients enrolled in the included studies was very low (range 39–97). However, it is conceivable that the interest in radiomics application will increase in the following years considering the estimated rise by 24% of testicular cancer incidence in the years from 2005 to 2025 (31). In the preoperative setting, it will become more and more important to precisely identify patients with testicular benignities, accounting for approximately the 20% of testicular masses, to avoid inappropriate radical inguinal orchiectomy (32, 33). Aimed at accurately discriminating between benign and malignant masses, Fan et al. developed an ADC-based radiomics signature and compared its classification performance with that of minimum and mean ADC (27). No statistically significant difference was found for this parameter between benign and malignant testicular masses (34), probably because the mean ADC does not take into account the whole lesion heterogeneity. Conversely, ADC-based radiomics signature provided an optimal performance in validation cohort (AUC 0.868). However, to solely discriminate between benign and malignant masses may not be enough in patients unwilling to undergo orchiectomy. In this cohort of patients, the therapeutic strategy usually relies on histological subtype.
The most common testicular cancers are testicular germ tumors (TGCTs), accounting for approximately the 90%–95%, and are split into two categories: seminomas (SGTs) and non-seminomas (NSGTs) (35). The main difference between SGTs and NSGTs lies in the different sensitivity to radio- and chemo-therapy. Invasive procedures, such as biopsy, are not currently recommended in order to avoid tumor spread (33). Thus, imaging may have a role to differentiate the two histological subtypes and decide the optimal therapeutic strategy (36). To exploit all the potential value of MRI in this clinical setting, Zhang et al. applied radiomics to T2-weighted (T2W) sequence to differentiate SGTs and NSGTs. A radiomics signature with five different features achieved an AUC of 0.979 (30). Feliciani et al. extended the work of Zhang by investigating the diagnostic performance of MRI and radiomics model in differentiating between TGCTs and testicular non germ cells tumors (TNGCTs). T2W-based radiomics model achieved an overall accuracy of 89% in differentiating these two categories. Also, an optimal performance in discriminating between SGCTs and NSGCTs was confirmed (28).
Another relevant difference between SGCTs and NGCTs is that nearly half of NSGCTs already show metastases at the time of first diagnosis (37). Currently, the standard of care for these patients is chemotherapy followed by post-chemotherapy retroperitoneal lymph node dissection (pcRPLND) of residual nodal masses with measurements > 1 cm and oncologic markers plateau or normalization (38). However, after pcRPLND, viable cancer is detected in only 15% and teratoma in 40% of patients, while the remaining show fibrotic or necrotic tissues (39). Radiomics may provide imaging biomarkers indicating which patient would actually benefit from pcRPLND in order to reduce overtreatment of young patients.
Baessler et al. trained a machine learning classifier to differentiate “benign” (fibrotic/necrotic) from “malignant” (viable cancer/teratoma) lymph nodes on contrast-enhanced CT in patients with NSGCT post-chemotherapy. The classifier achieved an accuracy of 0.81 and outperformed the commonly used “size” criterion (0.68) (25). The model performance in discriminating fibrotic or necrotic lymhp nodes from neoplastic ones may be further improved through the combination of radiomics features with already established clinical biomarkers. Indeed, Lewin et al. optimized a radiomics-based classifier performance by adding clinical variables to the model, such as pre-chemotherapy biomarkers, and achieved the best algorithm performance (AUC 0.88) (29).
Beyond oncology, radiomics have also demonstrated the potential role in providing valuable and reliable imaging biomarkers of gonadal function. Specifically, radiomics may empower US as an in vivo imaging measurement of testicular function. The unavailability of standard methods and reference ranges for US testicular volume measurement prompts to look for other parameters, such as testicular echostructure (40–42) However, despite promising results, the clinical applicability of testis echostructure was limited by the operator dependency of its measurement and by the lack of a widely accepted quantitative measure (43).
De Santi et al. designed a prospective observational study to correlate objective US features with both spermato- and steroido-genesis. First, the authors demonstrated that US texture features significantly predict visually defined inhomogeneity, providing for the first time a reliable mathematical quantification of a subjective US evaluation. Second, thirteen US texture features significantly predict sperm concentration, total sperm number, progressive motility, total motility and sperm morphology, while no significant correlation was found with total testosterone serum levels. Finally, at classification analysis, US textural features were validated as parameters able to classify patients' accordingly to semen parameters alterations (26). However, despite the growing interest in radiomics, the methodology's complexity and the uncertain quality of these studies are significantly slowing down the implementation of these techniques in the clinical practice (44, 45).
The methodological quality of the 6 included studies was assessed using RQS and QUADAS-2. The mean RQS was 11,33 ± 3,88 (range 6–18) resulting in a percentage of 31,48% ± 10,78% (range 16,67%–50%). This percentage is slightly higher compared to the average result (18.87%) reported by Spadarella et al. in a systematic review of RQS applications. However, it would be premature to draw firm conclusions from this difference, as it could be largely explained by the limitations of the RQS tool itself and particularly by its lack of reproducibility (46–48). Regarding the items of RQS, it's worth pointing out many key points particularly given the fact that radiomics application in testicular imaging is in its very beginning. First, none of the studies have performed an external validation without retraining. The lack of external validations significantly questions the credibility of the model performance if transposed to real clinical practice (8). As the number of studies regarding this topic increases, it can be expected that more and more institutes will be interested in collaborating leading to increased number of external validations. Unfortunately, none of the studies have provide public code or data to facilitate the reproducibility of the studies and, most importantly, starting to promote further research in this clinical setting. Finally, one study out of six presented a prospective observational design. Prospective studies provide the highest level of evidence regarding the real clinical value of radiomics also providing essential information about its cost-effectiveness ratio.
Regarding QUADAS-2 criteria, no relevant biases were found in the included papers in the patient selection, reference standard criteria and flow-and-timing domains.
In conclusion, radiomics application in testicular imaging is a promising area of research, despite being in its early stages, and should be pursued to improve the accuracy of testicular tumor diagnosis, staging, and treatment planning. Compared to other oncologic diseases, invasive procedures such as testicular biopsy are not recommended, and currently represent a limitation in the pre-operative characterization of testicular cancer and therapy planning. Radiomics has already been shown able to fill this gap when applied to cross-sectional imaging, but it could be even more useful if employed in ultrasound, which represents a first-line diagnostic imaging technique. However, clinical implementation is hindered by methodological limitations, including the lack of external validation and the prevalence of retrospective studies. Large-scale multicenter studies and prospective designs are needed to overcome these limitations and push this field forward.
Author contributions
SCF, GAr, FV: designed and wrote the first draft of the manuscript. SCF, MF, LC and LT: worked on methodology and formal analysis. SCF, GAr, MF, IA, GAg, revised and edited the manuscript. EN, DC and GAr: supervised and administered the study. All authors contributed to manuscript revision, read, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This report was conducted within a study funded by the ProCancer-I Project under the European Union's Horizon 2020 research and innovation programme (Grant agreement No 952159).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. (Mar. 2012) 48(4):441–6. doi: 10.1016/j.ejca.2011.11.036
2. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. (2016) 278(2):563–77. doi: 10.1148/radiol.2015151169
3. Jameson JL, Longo DL. Precision medicine—personalized, problematic, and promising. N Engl J Med. (2015) 327(23):2229–34. doi: 10.1056/NEJMsb1503104
4. Keek SA, Leijenaar RT, Jochems A, Woodruff H. Theranostics and precision medicine special feature: review article a review on radiomics and the future of theranostics for patient selection in precision medicine. Br J Radiol. (2018) 91(1091):20189004. doi: 10.1259/bjr.20189004
5. Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. (2014) 5:4006. doi: 10.1038/ncomms5006
6. Xue C, Zhou Y, Lo GG, Wong OL, Yu SK, Cheung KY, et al. Reliability of radiomics features due to image reconstruction using a standardized T2-weighted pulse sequence for MR-guided radiotherapy: an anthropomorphic phantom study. Magn Reson Med. (2021) 85(6):3434–46. doi: 10.1002/mrm.28650
7. Miles K. Radiomics for personalised medicine: the long road ahead. Br J Cancer. (2020) 122(7):929–30. doi: 10.1038/s41416-019-0699-8
8. Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. (2017) 14(12):749–62. doi: 10.1038/nrclinonc.2017.141
9. Katabathina VS, Vargas-Zapata D, Monge RA, Nazarullah A, Ganeshan D, Tammisetti V, et al. Testicular germ cell tumors: classification, pathologic features, imaging findings, and management. Radiographics. (2021) 41(6):1698–716. doi: 10.1148/RG.2021210024
10. Aringhieri G, Fanni SC, Febi M, Colligiani L, Cioni D, Neri E. The role of radiomics in salivary gland imaging: a systematic review and radiomics quality assessment. Diagnostics (Basel). (2022) 12(12):3002. doi: 10.3390/diagnostics12123002
11. Eslami P, Parmar C, Foldyna B, Scholtz JE, Ivanov A, Zeleznik R, et al. Radiomics of coronary artery calcium in the framingham heart study. Radiol Cardiothorac Imaging. (2020) 2(1):e190119. doi: 10.1148/ryct.2020190119
12. Romei C, Fanni SC, Volpi F, Milazzo A, D'Amore CA, Colligiani L, et al. New updates of the imaging role in diagnosis, staging, and response treatment of malignant pleural mesothelioma. Cancers (Basel). (2021) 13(17):4377. doi: 10.3390/cancers13174377
13. Ponsiglione A, Stanzione A, Spadarella G, Baran A, Cappellini LA, Lipman KG, et al. Ovarian imaging radiomics quality score assessment: an EuSoMII radiomics auditing group initiative. Eur Radiol. (2023) 33(3):2239–47. doi: 10.1007/s00330-022-09180-w
14. Cheng L, Albers P, Berney DM, Feldman DR, Daugaard G, Gilligan T, et al. Testicular cancer. Nat Rev Dis Primers. (2018) 4(1):29. doi: 10.1038/s41572-018-0029-0
15. Batool A, Karimi N, Wu XN, Chen SR, Liu YX. Testicular germ cell tumor: a comprehensive review. Cell Mol Life Sci. (2019) 76(9):1713–27. doi: 10.1007/s00018-019-03022-7
16. Berney DM, Cree I, Rao V, Moch H, Srigley JR, Tsuzuki T, et al. An introduction to the WHO 5th edition 2022 classification of testicular tumours. Histopathology. (2022) 81(4):459–66. doi: 10.1111/his.14675
17. Petersen PM, Skakkebaek NE, Vistisen K, Rørth M, Giwercman A. Semen quality and reproductive hormones before orchiectomy in men with testicular cancer. J Clin Oncol. (1999) 17(3):941–7. doi: 10.1200/JCO.1999.17.3.941
18. Tsili AC, Bertolotto M, Rocher L, Turgut AT, Dogra V, Seçil M, et al. Sonographically indeterminate scrotal masses: how MRI helps in characterization. Diagn Interv Radiol. (2018) 24(4):225–36. doi: 10.5152/dir.2018.17400
19. Spaggiari G, Granata ARM, Santi D. Testicular ultrasound inhomogeneity is an informative parameter for fertility evaluation. Asian J Androl. (2020) 22(3):302–8. doi: 10.4103/aja.aja_67_19
20. Condorelli R, Calogero AE, la Vignera S. Relationship between testicular volume and conventional or nonconventional sperm parameters. Int J Endocrinol. (2013) 2013:145792. doi: 10.1155/2013/145792
21. Fedder J. Prevalence of small testicular hyperechogenic foci in subgroups of 382 non-vasectomized, azoospermic men: a retrospective cohort study. Andrology. (2017) 5(2):248–55. doi: 10.1111/andr.12291
22. Whiting PF, Rutjes AW, Westwood , M E, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
23. Park JE, Kim HS, Kim D, Park SY, Kim JY, Cho SJ, et al. A systematic review reporting quality of radiomics research in neuro-oncology: toward clinical utility and quality improvement using high-dimensional imaging features. BMC Cancer. (2020) 20(1):29. doi: 10.1186/s12885-019-6504-5
24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J. (2009) 339:b2700. doi: 10.1136/bmj.b2700
25. Baessler B, Nestler T, Pinto Dos Santos D, Paffenholz P, Zeuch V, Pfister D, et al. Radiomics allows for detection of benign and malignant histopathology in patients with metastatic testicular germ cell tumors prior to post-chemotherapy retroperitoneal lymph node dissection. Eur Radiol. (2020) 30(4):2334–45. doi: 10.1007/s00330-019-06495-z
26. De Santi B, Spaggiari G, Granata AR, Romeo M, Molinari F, Simoni M, et al. From subjective to objective: a pilot study on testicular radiomics analysis as a measure of gonadal function. Andrology. (2022) 10(3):505–17. doi: 10.1111/andr.13131
27. Fan C, Sun K, Min X, Cai W, Lv W, Ma X, et al. Discriminating malignant from benign testicular masses using machine-learning based radiomics signature of appearance diffusion coefficient maps: comparing with conventional mean and minimum ADC values. Eur J Radiol. (2022) 148:110158. doi: 10.1016/j.ejrad.2022.110158
28. Feliciani G, Mellini L, Carnevale A, Sarnelli A, Menghi E, Piccinini F, et al. The potential role of MR based radiomic biomarkers in the characterization of focal testicular lesions. Sci Rep. (2021) 11(1):3456. doi: 10.1038/s41598-021-83023-4
29. Lewin J, Dufort P, Halankar J, O'Malley M, Jewett MAS, Hamilton RJ, et al. Applying radiomics to predict pathology of postchemotherapy retroperitoneal nodal masses in germ cell tumors. JCO Clin Cancer Inform. (2018) 2:1–12. doi: 10.1200/CCI.18.00004
30. Zhang P, Feng Z, Cai W, You H, Fan C, Lv W, et al. T2-weighted image-based radiomics signature for discriminating between seminomas and nonseminoma. Front Oncol. (2019) 9:1330. doi: 10.3389/fonc.2019.01330
31. Le Cornet C, Lortet-Tieulent J, Forman D, Béranger R, Flechon A, Fervers B, et al. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur J Cancer. (2014) 50(4):831–9. doi: 10.1016/j.ejca.2013.11.035
32. Heidenreich A, Paffenholz P, Nestler T, Pfister D. European Association of urology guidelines on testis cancer: important take home messages. Eur Urol Focus. (2019) 5(5):742–4. doi: 10.1016/j.euf.2019.08.002
33. Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, et al. Guidelines on testicular cancer: 2015 update. Eur Urol. (2015) 68(6):1054–68. doi: 10.1016/j.eururo.2015.07.044
34. Xu Q, Zhu Q, Liu H, Chang L, Duan S, Dou W, et al. Differentiating benign from malignant renal tumors using T2- and diffusion-weighted images: a comparison of deep learning and radiomics models versus assessment from radiologists. J Magn Reson Imaging. (2022) 55(4):1251–9. doi: 10.1002/jmri.27900
35. Tsili AC, Sylakos A, Ntorkou A, Stavrou S, Astrakas LG, Sofikitis N, et al. Apparent diffusion coefficient values and dynamic contrast enhancement patterns in differentiating seminomas from nonseminomatous testicular neoplasms. Eur J Radiol. (2015) 84(7):1219–26. doi: 10.1016/j.ejrad.2015.04.004
36. Tsili AC, Sofikitis N, Stiliara E, Argyropoulou MI. MRI Of testicular malignancies. Abdom Radiol. (2019) 44(3):1070–82. doi: 10.1007/s00261-018-1816-5
37. Klepp O, Flodgren P, Maartman-Moe H, Lindholm CE, Unsgaard B, Teigum H, et al. Early clinical stages (CSl, CSlMk+ and CS2A) of non-seminomatous testis cancer value ofpre-and post-orchiectomy serum tumor marker information in prediction of retroperitoneal lymph node metastases. Ann Oncol. (1990) 1(4):281–8. doi: 10.1093/oxfordjournals.annonc.a057749
38. Kollmannsberger C, Daneshmand S, So A, Chi KN, Murray N, Moore C, et al. Management of disseminated nonseminomatous germ cell tumors with risk-based chemotherapy followed by response-guided postchemotherapy surgery. J Clin Oncol. (2010) 28(4):537–42. doi: 10.1200/JCO.2009.23.0755
39. Steyerberg EW, Keizer HJ, Fosså SD, Sleijfer DT, Toner GC, Schraffordt Koops H, et al. Prediction of residual retroperitoneal mass histology after chemotherapy for metastatic nonseminomatous germ cell tumor: multivariate analysis of individual patient data from six study groups. J Clin Oncol. (1995) 13(5):1177–87. doi: 10.1200/JCO.1995.13.5.1177
40. Lotti F, Frizza F, Balercia G, Barbonetti A, Behre HM, Calogero AE, et al. The European academy of andrology (EAA) ultrasound study on healthy, fertile men: clinical, seminal and biochemical characteristics. Andrology. (2020) 8(5):1005–20. doi: 10.1111/andr.12808
41. Cocuzza MS, Tiseo BC, Srougi V, Wood GJA, Cardoso JPGF, Esteves SC, et al. Diagnostic accuracy of physical examination compared with color Doppler ultrasound in the determination of varicocele diagnosis and grading: impact of urologists’ experience. Andrology. (2020) 8(5):1160–6. doi: 10.1111/andr.12797
42. D'Andrea S, Martorella A, Castellini C, Cordeschi G, Totaro M, Parisi A, et al. Clinical and seminal parameters associated with testicular microlithiasis and its severity in males from infertile couples. Hum Reprod. (2021) 36(4):891–8. doi: 10.1093/humrep/deaa354
43. Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. (2015) 21(1):56–83. doi: 10.1093/humupd/dmu042
44. Pinto dos Santos D, Dietzel M, Baessler B. A decade of radiomics research: are images really data or just patterns in the noise? Eur Radiol. (2021) 31:1–4. doi: 10.1007/s00330-020-07108-w
45. Koçak B, Cuocolo R, dos Santos DP, Stanzione A, Ugga L. Must-have qualities of clinical research on artificial intelligence and machine learning. Balkan Med J. (2023) 40:3–12. doi: 10.4274/balkanmedj.galenos.2022.2022-11-51
46. Spadarella G, Stanzione A, Akinci D'Antonoli T, Andreychenko A, Fanni SC, et al. Systematic review of the radiomics quality score applications: an EuSoMII radiomics auditing group initiative. Eur Radiol. (2023) 33(3):1884–94. doi: 10.1007/s00330-022-09187-3
47. Stanzione A, Gambardella M, Cuocolo R, Ponsiglione A, Romeo V, Imbriaco M. Prostate MRI radiomics: a systematic review and radiomic quality score assessment. Eur J Radiol. (2020) 129:109095. doi: 10.1016/j.ejrad.2020.109095
Keywords: testicular imaging, radiomics, radiomics quality score, germ cell tumors, seminoma, nonseminoma, gonadal function
Citation: Fanni SC, Febi M, Colligiani L, Volpi F, Ambrosini I, Tumminello L, Aghakhanyan G, Aringhieri G, Cioni D and Neri E (2023) A first look into radiomics application in testicular imaging: A systematic review. Front. Radiol. 3:1141499. doi: 10.3389/fradi.2023.1141499
Received: 10 January 2023; Accepted: 27 March 2023;
Published: 17 April 2023.
Edited by:
Alessandro Stefano, Institute of Bioimaging and Molecular Physiology, National Research Council (CNR), ItalyReviewed by:
Sirio Cocozza, University of Naples Federico II, ItalyMarco Rengo, Sapienza University of Rome, Italy
© 2023 Fanni, Febi, Colligiani, Volpi, Ambrosini, Tumminello, Aghakhanyan, Aringhieri, Cioni and Neri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Febi bS5mZWJpQHN0dWRlbnRpLnVuaXBpLml0
Specialty Section: This article was submitted to Artificial Intelligence in Radiology, a section of the journal Frontiers in Radiology
 Salvatore C. Fanni
Salvatore C. Fanni Maria Febi
Maria Febi Leonardo Colligiani
Leonardo Colligiani Gayane Aghakhanyan
Gayane Aghakhanyan Giacomo Aringhieri
Giacomo Aringhieri Dania Cioni
Dania Cioni