- 1Center for Sleep and Vigilance Disorders, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 2Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium
- 3EctoSense, Leuven, Belgium
- 4Respiratory Service and Sleep Unit, Araba University Hospital, Bioaraba Scientific Institute, Ciberes, Vitoria-Gasteiz, Spain
- 5ResMed Science Center, Basel, Switzerland
- 6ResMed Science Center, Saint-Priest, France
- 7Bioaraba, Neurosciences-Sleep Disorders, Osakidetza Basque Health Service, Araba University Hospital, Sleep Unit, Vitoria-Gasteiz, Spain
- 8Interdisciplinary Center of Sleep Medicine, Charité-Universitätsmedizin Berlin, Berlin, Germany
Obstructive sleep apnea (OSA) is a prevalent condition that negatively impacts cardiovascular, metabolic and mental health. A high proportion of individuals with OSA remain undiagnosed and incur significant healthcare costs. The gold standard OSA diagnostic is in-lab polysomnography, but this is costly and time-consuming. Home sleep apnea tests (HSATs), including cardiorespiratory polygraphy and peripheral artery tonometry technology, provide an alternative. Advances in HSAT technology include non-invasive, easy-to-use medical devices that could allow unobtrusive, accessible, multi-night, cost-effective diagnosis and management of sleep-disordered breathing. One type of these devices is based on determination of peripheral arterial tone, and use photoplethysmography signals from the finger (oxygen saturation, pulse wave amplitude and pulse rate). The devices contain algorithms that use these data to generate the traditional metrics required by the American Academy of Sleep Medicine. They can be used to record sleep parameters over multiple nights at home, and can also provide information on total sleep time (TST) and sleep stages (including time spent in rapid eye movement sleep). The combination of objective measures (apnea-hypopnea index, oxygen desaturation index, respiratory disturbance index, TST) and subjective measures (symptoms and other patient-reported outcome measures) could facilitate the development of a personalized therapeutic plan for OSA patients. It is anticipated that the streamlined digital pathway facilitated by new peripheral artery tone-based technology could contribute to reducing the underdiagnosis of OSA, accelerating access to appropriate treatment, and the optimization of OSA therapy.
1. Introduction
Obstructive sleep apnea (OSA) is the most common form of sleep-disordered breathing (SDB). It is characterized by partial or complete upper airway obstructions that are associated with intermittent hypoxia and transient arousals. The global prevalence of OSA in middle-aged adults has been estimated to be nearly one billion, with approximately half of these having moderate-to-severe disease with an indication for treatment (Benjafield et al., 2019).
OSA results in increased sympathetic activity, oxidative stress, inflammation, endothelial and metabolic dysfunction, and is associated with a variety of cardiovascular, cerebrovascular and metabolic diseases, and increased all-cause mortality (Nieto et al., 2000; Peppard et al., 2000; Kendzerska et al., 2014; Kent et al., 2015; Linz et al., 2015; Reutrakul and Mokhlesi, 2017; Xie et al., 2017; Mehra et al., 2022; Salari et al., 2022). Untreated OSA also contributes to occupational and traffic accidents (Bioulac et al., 2017; Hirsch Allen et al., 2020) and absence from work (Lallukka et al., 2014), and has a negative impact on cognitive function (Gnoni et al., 2023) and quality of life (Kerner and Roose, 2016; Vinnikov et al., 2017; Alomri et al., 2021; Legault et al., 2021).
A high proportion of individuals with OSA remain undiagnosed (Young et al., 1997; Kapur et al., 2002). This is relevant from a health system perspective because a person with OSA has been estimated to have double the annual healthcare costs than someone without OSA (Kapur et al., 1999). Furthermore, the diagnosis and treatment of OSA are associated with positive economic benefit (Wickwire, 2021; Mattila et al., 2022; Sterling et al., 2023).
2. OSA diagnosis
The current gold standard for diagnosing OSA is in-laboratory polysomnography (PSG). PSG is a costly and time-consuming process that requires highly trained personnel for set-up and scoring, and therefore has limited availability. PSG is essential in specific patient groups (e.g., those with comorbidities), but the majority of individuals do not require PSG for diagnosis of OSA. PSG is subject to the first-night effect and although it can be performed over multiple nights and at home, this is resource intensive and not feasible in the majority of cases, which limits its ability to detect night-to-night variability in SDB parameters (Newell et al., 2012). Therefore, there is a need for OSA diagnostic tests that are more widely available, cost effective and can be used for timely multi-night sleep testing, allowing healthcare professionals to take care of all individuals referred for evaluation or management of OSA. As a result, home sleep apnea testing (HSAT) has become a routine approach for individuals with suspected OSA. HSAT does not require supervision, is less expensive than PSG and allows replication of sleep patterns under “usual” conditions. Many PSG-validated HSAT devices are available (e.g., level 3 cardiorespiratory polygraphy) that provide adequate apnea-hypopnea index (AHI) estimation according to the American Academy of Sleep Medicine (AASM) criteria for sleep apnea diagnosis (Kapur et al., 2017; Rosen et al., 2018). However, use of total recording time rather than total sleep time (TST) to calculate respiratory indices may lead to important underestimation of event rates (Escourrou et al., 2015; Massie et al., 2022a). According to the AASM practical guidelines, both polygraphy and peripheral artery tonometry-based HSATs can be used for the diagnosis of sleep apnea (American Academy of Sleep Medicine, 2023). There are two Conformité Europénne (CE) mark and US Food and Drug Administration-approved, commercially available peripheral artery tonometry-based HSAT devices (NightOwl® and WatchPAT®).
2.1. Photoplethysmography and peripheral arterial tonometry for detection of respiratory events
Reflectance-based photoplethysmography (PPG) detects pulsatile changes in blood volume in peripheral tissues and has been defined as an important technology in sleep monitoring devices (Ryals et al., 2023). Peripheral artery tonometry refers to the determination of peripheral arterial vascular tone (the net balance between vasoconstriction and vasodilation) using PPG data. Peripheral artery tonometry measures pulsatile volume changes in the digital vascular bed that are densely innervated (Schnall et al., 1999; Zou et al., 2004). In the context of OSA, there is increased sympathetic nervous system activity near the end of a respiratory event (obstructive apnea). The associated release of norepinephrine increases tone in the peripheral arteries, resulting in vasoconstriction and a reduction in the volume of blood displaced between systole and diastole. By measuring this relative change in blood volume, sudden changes in peripheral arterial tone that occur in response to respiratory events can be detected (O'Donnell et al., 2002). These pulse wave amplitude drops have been shown to be an important biomarker of cardiometabolic risk and outcomes (Hirotsu et al., 2020; Strassberger et al., 2021; Solelhac et al., 2023).
Peripheral artery tonometry-based devices combine information on changes in arterial volume with oxygen saturation (SpO2; both from the PPG signal) with data on peripheral arterial tone and heart rate (Yalamanchali et al., 2013; Massie et al., 2018; Van Pee et al., 2022; Lyne et al., 2023). During recording, a respiratory event is typically detected by analyzing the co-occurrence of one or more of the following events: oxygen desaturation; vasoconstriction (decreased peripheral artery tonometry signal); and increased pulse rate. Based on these data, peripheral artery tonometry devices contain proprietary algorithms that generate the traditional metrics required by the AASM Manual for the Scoring of Sleep and Associated Events (e.g., AHI, respiratory disturbance index) (American Academy of Sleep Medicine, 2023). The two currently available devices, NightOwl® and WatchPAT®, have different proprietary algorithms and technical specifications. Both have been validated against PSG (Zou et al., 2006; Massie et al., 2018; Van Pee et al., 2022), and generally show good agreement with PSG for parameters such as the AHI and OSA severity (O'Brien et al., 2012; Yalamanchali et al., 2013; Camilon et al., 2014; Choi et al., 2018; Ioachimescu et al., 2020; Van Pee et al., 2022). Furthermore, information on sleep (e.g., TST, time spent in rapid eye movement [REM] sleep, wake time) can also be estimated using peripheral artery tonometry-based devices (Hedner et al., 2011; Massie et al., 2018; Zhang et al., 2020).
2.2. Detection of central sleep apnea and REM sleep using peripheral artery tonometry-based devices
Although OSA is the predominant sleep apnea subtype, central sleep apnea (CSA) is another important form of SDB (Dempsey, 2019). The mechanisms underlying these two types of sleep apnea are different, because CSA is characterized by a lack of respiratory drive, while OSA result from a partial or complete obstruction of the upper airways. In PSG, cessation of respiratory drive or effort can be inferred from the abdominal and thoracic respiratory effort belts. This information is not currently available from peripheral artery tonometry-based devices, but could be detected using fingertip PPG data. The fingertip PPG signal inherently contains respiratory information because blood flow to body extremities is influenced by alterations in thoracic pressure throughout the respiratory cycle (Ryals et al., 2023). Therefore, the PPG signal amplitude oscillates in synchrony with the respiratory cycle. This amplitude modulation can be isolated to retain a signal representing respiratory effort. The respiratory effort signal can then be used to classify respiratory events as being of an obstructive or central nature. Use of PPG has recently been shown to provide useful data for the detection of CSA in individuals with suspected sleep apnea (Sommermeyer et al., 2012; Massie et al., 2023).
Approximately 10%−36% of individuals with sleep apnea have REM-predominant OSA (Alzoubaidi and Mokhlesi, 2016), whereby SDB events are more pronounced during REM sleep (Varga and Mokhlesi, 2019). These individuals are at high risk for common OSA comorbidities, including atherosclerosis, hypertension, metabolic syndrome and diabetes (Mokhlesi et al., 2014; Acosta-Castro et al., 2018; Ljunggren et al., 2022). In order to properly define this phenotype, it is essential to be able to classify REM sleep with sufficient accuracy. Vasoconstrictions and oxygen desaturations detected in peripheral artery tonometry and SpO2 signal traces, respectively, show a different temporal pattern between REM and non-REM sleep (Lavie et al., 2000; Dvir et al., 2002; Herscovici et al., 2007; Choi et al., 2016). Furthermore, pulse rate low frequency power has been shown to increase in REM sleep (Chouchou and Desseilles, 2014). This means that PPG-based techniques can be used to detect REM sleep (Lavie et al., 2000; Zhang et al., 2020), although peripheral artery tonometry-based HSAT has lower sensitivity for REM detection than PSG (Massie et al., 2022b).
Overall, the ability of peripheral artery tonometry-based HSAT devices to detect REM sleep and their potential to differentiate between central and obstructive respiratory events increase the utility and application of these devices across a range of SDB types. They may also have clinical usefulness in individuals with comorbidities such as atrial fibrillation (Tauman et al., 2020; Jensen et al., 2023) and chronic obstructive pulmonary disease (Hansson et al., 2023).
2.3. Multi-night sleep testing
A key advantage of a peripheral artery tonometry-based approach is that it provides a convenient and low-cost option for multi-night testing. This is important because evaluating SDB over multiple nights provides a greater amount of data on respiratory parameters. This may help to achieve a correct diagnosis, and could allow evaluation of the evolution of sleep-related breathing disorders over time during the application of appropriate therapy. Peripheral artery tonometry devices are small, and therefore allow more natural (e.g., less supine) sleep due to the lack of cables compared with PSG. Furthermore, there is a large body of evidence showing that there is substantial night-to-night variation in sleep-related respiratory events, meaning that a single night of monitoring may be insufficient to allow reliable determination of sleep apnea severity at the individual level, resulting in misclassification in a substantial proportion of people (Punjabi et al., 2020; Roeder et al., 2020; Lechat et al., 2022). Furthermore, emerging evidence suggests that large night-to-night variability in sleep apnea severity (based on the AHI) is a predictor of uncontrolled hypertension (Lechat et al., 2023), and that sleep data from a single night of recording performed worse than multi-night testing with respect to cardiovascular risk prediction (Lechat et al., 2023). For data from multiple nights of sleep testing (at least three nights in total, including one night on the weekend), some experts believe that it is probably best to use the highest AHI value recorded to provide guidance regarding treatment initiation, rather than the average AHI. However, studies are needed to validate this approach. In summary, the multi-night monitoring capability of peripheral artery tonometry devices allows patient sleep trajectories over time to be determined in an accessible and acceptable manner, providing a clearer understanding of sleep habits and allowing better shared decision-making and more personalized therapy (Hrubos-Strøm et al., 2023; Lisik et al., 2023).
3. OSA diagnostic and management workflow using peripheral artery tonometry-based devices
HSAT workflow is simple and can be implemented remotely. However, a wider consideration is how new, multi-night, low-touch tools such as peripheral artery tonometry devices can be incorporated into the SDB patient pathway in a way that maximizes benefits for the patient (optimizing diagnosis and therapy), for healthcare professionals (time saving, reduced sleep lab workload, patient-centered management), and for the healthcare system (cost savings, resource optimization). As well as diagnosis, use of simple, compact HSAT devices could contribute to improving the efficiency of ongoing management of OSA therapies, including oral appliances and positive airway pressure therapies. An integrated and personalized diagnostic and therapeutic digital pathway can be facilitated by the use of objective diagnostic measures (AHI, oxygen desaturation index, sleep time, hypoxic burden), subjective measures such as symptoms and patient-reported outcome measures, and the monitoring of therapy efficacy (Figure 1).
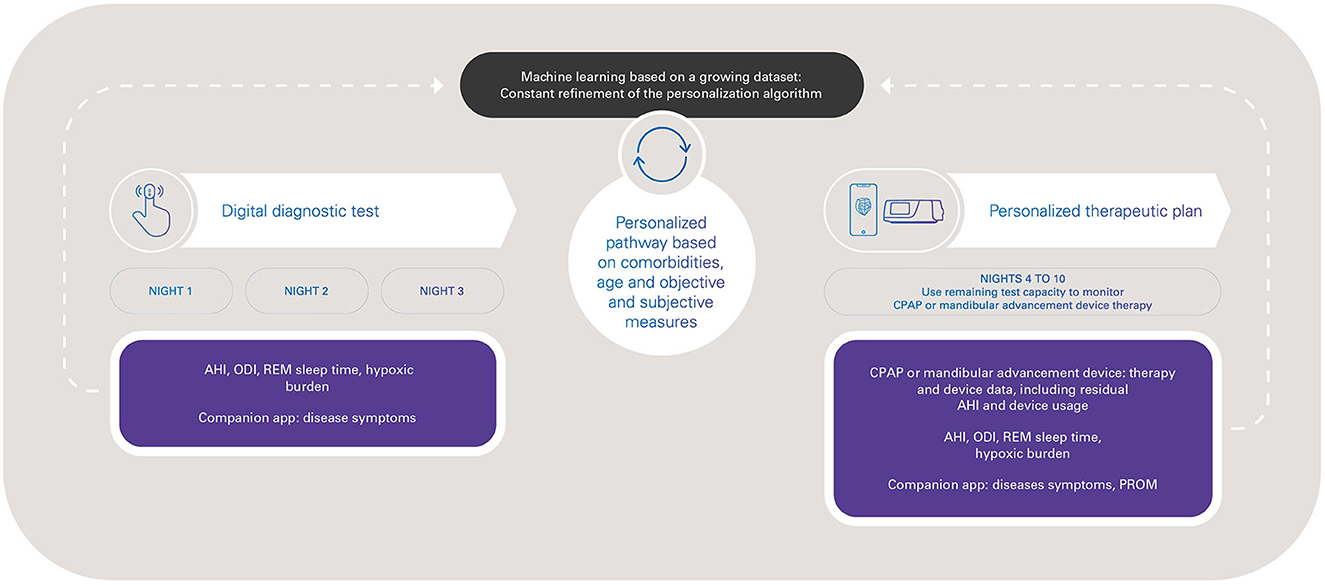
Figure 1. Toward a digital pathway for obstructive sleep apnea with peripheral arterial tone-based monitoring, from diagnosis to therapy management. AHI, apnea-hypopnea index; CPAP, continuous positive airway pressure; ODI, oxygen desaturation index; PROM, patient-reported outcome measures; REM, rapid eye movement.
For healthcare professionals, HSAT with a device that uses peripheral artery tonometry (such as NightOwl® and WatchPAT®) is considered to be less time consuming, allowing more efficient patient management using a digital pathway without any loss of diagnostic accuracy. Having a solution that can be implemented remotely also allows more patients to be reached, especially those who do not have easy access to a sleep laboratory or sleep physician. In addition, the COVID-19 pandemic highlighted the value of being able to continue healthcare evaluations and treatment monitoring without face-to-face interaction between healthcare professionals and patients (Bouloukaki et al., 2023).
Accurate, multi-night sleep testing information is a key component that can help to drive much-needed personalized approaches to the diagnosis and treatment of sleep apnea (Arnardottir et al., 2022). While OSA may superficially be considered as a single disease, there are a variety of diverse clinical manifestations (or phenotypes) (Zinchuk et al., 2017; Duong-Quy et al., 2022). The presence of different OSA phenotypes means that a personalized, approach to the diagnosis and treatment of OSA is required to optimize clinical outcomes for individual patients (McNicholas and Korkalainen, 2023). The ability to detect different sleep apnea phenotypes such as REM-predominant OSA and CSA makes peripheral artery tonometry-based devices valuable tools for facilitating this type of personalized treatment.
Another important consideration is the patient experience, which is becoming increasingly recognized as a key measure of health system performance (Jamieson Gilmore et al., 2023). There are a number of features that would likely result in a good experience for individuals being investigated for SDB using peripheral artery tonometry-based devices. These include the ability to perform sleep testing over multiple nights in the home environment, simple device set-up, quick and reliable event analysis. This approach is also ideally suited to facilitate a P4 medicine approach to OSA—Predict; Prevent; Personalize; Participate (Lim et al., 2017). Early and effective diagnosis of OSA in otherwise healthy individuals would allow the implementation of lifestyle interventions and early treatment that could contribute to prevention of common OSA comorbidities (i.e., primary prevention) (Yim-Yeh et al., 2010), facilitate personalization of therapy options, and allow the individual to participate in the diagnosis and monitoring of their condition. Furthermore, simplicity and flexibility are important, especially for the disabled, the elderly and for people who are less familiar with new technologies.
4. Discussion
It has long been recognized that there is a lack of healthcare resources to meet the clinical demands of individuals with sleep apnea or suspected sleep apnea (Flemons et al., 2004; Pack, 2004). Nevertheless, effective and timely diagnosis of OSA plays an important role in preventing or limiting the negative health impacts of this condition. Peripheral artery tonometry-based wearable sleep testing devices that can be self-administered by the patient and scored automatically using validated artificial intelligence and machine learning-based algorithms have the potential to fill an important gap in healthcare service provision, improve access to diagnostic sleep studies and provide a cost-effective solution for sleep apnea diagnosis and monitoring.
Compared with conventional PSG, the benefits of peripheral artery tonometry-based wearable sleep testing devices include ease of performing evaluations over multiple nights. In addition, there will be savings in clinical staff time by avoiding complicated inventory, on-site desktop software updates, and cleaning and sterilization/disinfection procedures. However, these HSAT devices do not record a direct measurement of flow so it is not possible to distinguish between apneas and hypopneas (although both are counted), and there is no EEG-based sleep-staging (although information on sleep stages can be obtained by other means). Furthermore, there are some settings where use of peripheral artery tonometry may not be the most appropriate option. For example, device performance could be adversely impacted by alternations in the sympathetic response or impaired perfusion at the peripheral tissue, such as during treatment with adrenergic system modulators (e.g., alpha-adrenergic antagonists) (Zou et al., 2010) and in individuals with clinically relevant peripheral vascular disease. Thus, although alternative approaches to sleep apnea assessment might be more appropriate in these groups, use of peripheral artery tonometry-based devices to address the unmet need for better approaches to OSA diagnosis for the majority of individuals would allow in-demand sleep laboratory services to be prioritized for more complex individuals (Fietze et al., 2022).
4.1. Looking to the (not too distant) future
Sleep medicine is a rapidly developing field, but the prevalence of OSA is growing and the number of sleep specialists is inadequate to meet the increasing need. This highlights the need for initiatives such as new tools and telehealth to provide safe, effective clinical care to an expanding group of patients (O'Donnell et al., 2020). The move toward greater utilization of telemedicine solutions was accelerated during the COVID-19 pandemic due to lockdowns and social distancing requirements (Monaghesh and Hajizadeh, 2020). It makes sense to capitalize on this momentum to improve the diagnosis and management of SDB, and simple, wearable devices based on measurement of peripheral arterial tone, such as NightOwl® and WatchPAT®, can make an important contribution to this. For instance, peripheral arterial tone-based HSATs can provide primary care professionals with easy tools to diagnose OSA. These cloud-based multiple-night HSAT technologies can be beneficial for communities without major medical center for SDB management thus promoting equitable SDB identification, diagnosis, and treatment (Gueye-Ndiaye et al., 2023). Moreover, the technologies provide the possibility of OSA screening in large populations and enable new approaches for a simplified and automated OSA diagnostic procedure and treatment follow-up. HSATs, wearable technologies and advances in telemedicine may also help to strengthen inter-departmental collaboration, thus improving the overall care of patients with OSA (Mahoney, 2020; McNicholas and Pevernagie, 2022).
Overall, the possibility of integrating diagnostic, device therapy and patient clinical data is attractive, and facilitates a more holistic approach to patient management. New-generation wearable devices that record a variety of signals to provide information on sleep stage/quality, arousals, sleep position, and a variety of SDB metrics (such as hypoxic burden) (Trzepizur et al., 2022) will provide a more complete picture to inform clinical decision making throughout the patient journey. Better understanding of patient phenotypes will allow specific characteristics to be linked to treatment outcomes (Mazzotti et al., 2019). The variety of accurate data obtained from new connected devices could be used to inform both diagnostics and clinical decision making based on sleep-related breathing parameters, age, symptoms and risks (Hajipour et al., 2023), and in accordance with current clinical recommendations and guidelines (Patil et al., 2019; Randerath et al., 2021; Grote et al., 2023). The new capabilities provided by new technologies and innovations bring new capabilities, such as use of the same device to diagnose sleep apnea and then monitor therapy compliance and efficacy. For example, one currently available peripheral artery tonometry-based device (NightOwl®) has 10 nights of battery capacity. The ability to record over 10 consecutive nights could allow three nights for diagnostic studies (AHI, oxygen desaturation index, hypoxic burden and patient-reported outcome measures) followed by sevn nights to implement and monitor a personalized treatment plan, including assessment of changes in hypoxic burden and patient-reported outcomes (Figure 1). This could contribute to reducing the underdiagnosis of OSA, accelerating access to appropriate treatment, and optimization of OSA therapy.
Author contributions
DZ: Conceptualization, Writing—original draft, Writing—review and editing. SV: Conceptualization, Writing—original draft, Writing—review and editing. CE: Writing—original draft, Writing—review and editing. DE-T: Writing—original draft, Writing—review and editing, Conceptualization. FL: Conceptualization, Writing—original draft, Writing—review and editing. MA: Conceptualization, Writing—original draft, Writing—review and editing. IF: Writing—original draft, Writing—review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
FL and DE-T are employees of ResMed. SV is an employee of EctoSense, a ResMed subsidiary.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acosta-Castro, P., Hirotsu, C., Marti-Soler, H., Marques-Vidal, P., Tobback, N., Andries, D., et al. (2018). REM-associated sleep apnoea: prevalence and clinical significance in the HypnoLaus cohzort. Eur. Respir. J. 52, 1702484. doi: 10.1183/13993003.02484-2017
Alomri, R. M., Kennedy, G. A., Wali, S. O., Alhejaili, F., and Robinson, S. R. (2021). Association between nocturnal activity of the sympathetic nervous system and cognitive dysfunction in obstructive sleep apnoea. Sci. Rep. 11, 11990. doi: 10.1038/s41598-021-91329-6
Alzoubaidi, M., and Mokhlesi, B. (2016). Obstructive sleep apnea during rapid eye movement sleep: clinical relevance and therapeutic implications. Curr. Opin. Pulm. Med. 22, 545–554. doi: 10.1097/MCP.0000000000000319
American Academy of Sleep Medicine (2023). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 3. Darien, IL, American Academy of Sleep Medicine.
Arnardottir, E. S., Islind, A. S., Óskarsdóttir, M., Ólafsdóttir, K. A., August, E., Jónasdóttir, L., et al. (2022). The Sleep Revolution project: the concept and objectives. J. Sleep Res. 31, e13630. doi: 10.1111/jsr.13630
Benjafield, A. V., Ayas, N. T., Eastwood, P. R., Heinzer, R., Ip, M. S. M., Morrell, M. J., et al. (2019). Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir. Med. 7, 687–698. doi: 10.1016/S2213-2600(19)30198-5
Bioulac, S., Micoulaud-Franchi, J. A., Arnaud, M., Sagaspe, P., Moore, N., Salvo, F., et al. (2017). Risk of motor vehicle accidents related to sleepiness at the wheel: a systematic review and meta-analysis. Sleep 40, zsx134. doi: 10.1093/sleep/zsx134
Bouloukaki, I., Pataka, A., Mauroudi, E., Moniaki, V., Fanaridis, M., Schiza, S. E., et al. (2023). Impact of the COVID-19 pandemic on positive airway pressure adherence and patients' perspectives in Greece: the role of telemedicine. J. Clin. Sleep Med. doi: 10.5664/jcsm.10664. [Epub ahead of print].
Camilon, P. R., Nguyen, S. A., Camilon, M. P., and Gillespie, M. B. (2014). WatchPAT versus polysomnography: a meta-analysis. Otolaryngol.–Head Neck Surg. 151(1_suppl), P265. doi: 10.1177/0194599814541629a405
Choi, E., Park, D. H., Yu, J. H., Ryu, S. H., and Ha, J. H. (2016). The severity of sleep disordered breathing induces different decrease in the oxygen saturation during rapid eye movement and non-rapid eye movement sleep. Psychiatry Investig. 13, 652–658. doi: 10.4306/pi.2016.13.6.652
Choi, J. H., Lee, B., Lee, J. Y., and Kim, H. J. (2018). Validating the watch-PAT for diagnosing obstructive sleep apnea in adolescents. J. Clin. Sleep Med. 14, 1741–1747. doi: 10.5664/jcsm.7386
Chouchou, F., and Desseilles, M. (2014). Heart rate variability: a tool to explore the sleeping brain? Front. Neurosci. 8, 402. doi: 10.3389/fnins.2014.00402
Dempsey, J. A. (2019). Central sleep apnea: misunderstood and mistreated! F1000Res. 8, F1000. doi: 10.12688/f1000research.18358.1
Duong-Quy, S., Nguyen-Huu, H., Hoang-Chau-Bao, D., Tran-Duc, S., Nguyen-Thi-Hong, L., Nguyen-Duy, T., et al. (2022). Personalized medicine and obstructive sleep apnea. J. Pers. Med. 12, 2034. doi: 10.3390/jpm12122034
Dvir, I., Adler, Y., Freimark, D., and Lavie, P. (2002). Evidence for fractal correlation properties in variations of peripheral arterial tone during REM sleep. Am. J. Physiol. Heart Circ. Physiol. 283, H434–439. doi: 10.1152/ajpheart.00336.2001
Escourrou, P., Grote, L., Penzel, T., McNicholas, W. T., Verbraecken, J., Tkacova, R., et al. (2015). The diagnostic method has a strong influence on classification of obstructive sleep apnea. J. Sleep Res. 24, 730–738. doi: 10.1111/jsr.12318
Fietze, I., Laharnar, N., Bargiotas, P., Basoglu, O. K., Dogas, Z., Drummond, M., et al. (2022). Management of obstructive sleep apnea in Europe - a 10-year follow-up. Sleep Med. 97, 64–72. doi: 10.1016/j.sleep.2022.06.001
Flemons, W. W., Douglas, N. J., Kuna, S. T., Rodenstein, D. O., and Wheatley, J. (2004). Access to diagnosis and treatment of patients with suspected sleep apnea. Am. J. Respir. Crit. Care Med. 169, 668–672. doi: 10.1164/rccm.200308-1124PP
Gnoni, V., Mesquita, M., O'Regan, D., Delogu, A., Chakalov, I., Antal, A., et al. (2023). Distinct cognitive changes in male patients with obstructive sleep apnoea without co-morbidities. Front. Sleep 2, 1097946. doi: 10.3389/frsle.2023.1097946
Grote, L., Anderberg, C-. P., Friberg, D., Grundström, G., Hinz, K., Isaksson, G., et al. (2023). National knowledge-driven management of obstructive sleep apnea—The Swedish approach. Diagnostics 13, 1179. doi: 10.3390/diagnostics13061179
Gueye-Ndiaye, S., Williamson, A. A., and Redline, S. (2023). disparities in sleep-disordered breathing: upstream risk factors, mechanisms, and implications. Clin. Chest Med. 44, 585–603. doi: 10.1016/j.ccm.2023.03.012
Hajipour, M., Baumann, B., Azarbarzin, A., Allen, A. J. H., Liu, Y., Fels, S., et al. (2023). Association of alternative polysomnographic features with patient outcomes in obstructive sleep apnea: a systematic review. J. Clin. Sleep Med. 19, 225–242. doi: 10.5664/jcsm.10298
Hansson, D., Andersson, A., Vanfleteren, L., Andelid, K., Zou, D., Hedner, J., et al. (2023). Clinical impact of routine sleep assessment by peripheral arterial tonometry in patients with COPD. ERJ Open Res. 9, 00458–2022. doi: 10.1183/23120541.00458-2022
Hedner, J., White, D. P., Malhotra, A., Herscovici, S., Pittman, S. D., Zou, D., et al. (2011). Sleep staging based on autonomic signals: a multi-center validation study. J. Clin. Sleep Med. 7, 301–306. doi: 10.5664/JCSM.1078
Herscovici, S., Pe'er, A., Papyan, S., and Lavie, P. (2007). Detecting REM sleep from the finger: an automatic REM sleep algorithm based on peripheral arterial tone (PAT) and actigraphy. Physiol. Meas. 28, 129–140. doi: 10.1088/0967-3334/28/2/002
Hirotsu, C., Betta, M., Bernardi, G., Marques-Vidal, P., Vollenweider, P., Waeber, G., et al. (2020). Pulse wave amplitude drops during sleep: clinical significance and characteristics in a general population sample. Sleep 43, zsz322. doi: 10.1093/sleep/zsz322
Hirsch Allen, A. J., Peres, B., and Ayas, N. T. (2020). Obstructive sleep apnea severity and the risk of occupational injury: a prospective observational cohort. Lung 198, 283–287. doi: 10.1007/s00408-020-00325-6
Hrubos-Strøm, H., Bergqvist, J., and Zou, D. (2023). Longitudinal management and a decision-aid tool in treatment-resistant sleep apnea. Curr. Sleep Med. Rep. 9, 133–139. doi: 10.1007/s40675-023-00257-6
Ioachimescu, O. C., Allam, J. S., Samarghandi, A., Anand, N., Fields, B. G., Dholakia, S. A., et al. (2020). Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J. Clin. Sleep Med. 16, 1663–1674. doi: 10.5664/jcsm.8620
Jamieson Gilmore, K., Corazza, I., Coletta, L., and Allin, S. (2023). The uses of patient reported experience measures in health systems: a systematic narrative review. Health Policy 128, 1–10. doi: 10.1016/j.healthpol.2022.07.008
Jensen, M. H., Dalgaard, F., Rude Laub, R., Gottlieb, V., Nielsen, O. W., Hansen, J., et al. (2023). Prevalence of sleep apnea in unselected patients with atrial fibrillation by a home-monitoring device: the DAN-APNO study. Int. J. Cardiol. Heart Vasc. 47, 101219. doi: 10.1016/j.ijcha.2023.101219
Kapur, V., Blough, D. K., Sandblom, R. E., Hert, R., de Maine, J. B., Sullivan, S. D., et al. (1999). The medical cost of undiagnosed sleep apnea. Sleep 22, 749–755. doi: 10.1093/sleep/22.6.749
Kapur, V., Strohl, K. P., Redline, S., Iber, C., O'Connor, G., Nieto, J., et al. (2002). Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 6, 49–54. doi: 10.1055/s-2002-32318
Kapur, V. K., Auckley, D. H., Chowdhuri, S., Kuhlmann, D. C., Mehra, R., Ramar, K., et al. (2017). Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 13, 479–504. doi: 10.5664/jcsm.6506
Kendzerska, T., Gershon, A. S., Hawker, G., Leung, R. S., and Tomlinson, G. (2014). Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med. 11, e1001599. doi: 10.1371/journal.pmed.1001599
Kent, B. D., McNicholas, W. T., and Ryan, S. (2015). Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. J. Thorac. Dis. 7, 1343–1357. doi: 10.3978/j.issn.2072-1439.2015.08.11
Kerner, N. A., and Roose, S. P. (2016). Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am. J. Geriatr. Psychiatry 24, 496–508. doi: 10.1016/j.jagp.2016.01.134
Lallukka, T., Kaikkonen, R., Härkänen, T., Kronholm, E., Partonen, T., Rahkonen, O., et al. (2014). Sleep and sickness absence: a nationally representative register-based follow-up study. Sleep 37, 1413–1425. doi: 10.5665/sleep.3986
Lavie, P., Schnall, R. P., Sheffy, J., and Shlitner, A. (2000). Peripheral vasoconstriction during REM sleep detected by a new plethysmographic method. Nat. Med. 6, 606. doi: 10.1038/76135
Lechat, B., Loffler, K. A., Reynolds, A. C., Naik, G., Vakulin, A., Jennings, G., et al. (2023). High night-to-night variability in sleep apnea severity is associated with uncontrolled hypertension. NPJ Digit Med 6, 57. doi: 10.1038/s41746-023-00801-2
Lechat, B., Naik, G., Reynolds, A., Aishah, A., Scott, H., Loffler, K. A., et al. (2022). Multinight prevalence, variability, and diagnostic misclassification of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 205, 563–569. doi: 10.1164/rccm.202107-1761OC
Legault, J., Thompson, C., Martineau-Dussault, M., André, C., Baril, A. A., Martinez Villar, G., et al. (2021). Obstructive sleep apnea and cognitive decline: a review of potential vulnerability and protective factors. Brain Sci. 11, 706. doi: 10.3390/brainsci11060706
Lim, D. C., Sutherland, K., Cistulli, P. A., and Pack, A. I. (2017). P4 medicine approach to obstructive sleep apnoea. Respirology 22, 849–860. doi: 10.1111/resp.13063
Linz, D., Woehrle, H., Bitter, T., Fox, H., Cowie, M. R., Böhm, M., et al. (2015). The importance of sleep-disordered breathing in cardiovascular disease. Clin. Res. Cardiol. 104, 705–718. doi: 10.1007/s00392-015-0859-7
Lisik, D., Pires, G. N., and Zou, D. (2023). Perspective: Systematic review and meta-analysis in obstructive sleep apnea – what is lacking? Sleep Med. 111, 54–61. doi: 10.1016/j.sleep.2023.09.006
Ljunggren, M., Naessén, T., Theorell-Haglöw, J., Franklin, K. A., and Lindberg, E. (2022). Rapid eye movement sleep apnea and carotid intima thickness in men and women: a SHE-MUSTACHE cohort study. J. Sleep Res. 31, e13599. doi: 10.1111/jsr.13599
Lyne, C. J., Hamilton, G. S., Turton, A. R. E., Stupar, D., and Mansfield, D. R. (2023). Validation of a single-use and reusable home sleep apnea test based on peripheral arterial tonometry compared to laboratory polysomnography for the diagnosis of obstructive sleep apnea. J. Clin. Sleep Med. 19, 1429–1435. doi: 10.5664/jcsm.10568
Mahoney, M. F. (2020). Telehealth, telemedicine, and related technologic platforms: current practice and response to the COVID-19 pandemic. J. Wound Ostomy Continence Nurs. 47, 439–444. doi: 10.1097/WON.0000000000000694
Massie, F., Mendes de Almeida, D., Dreesen, P., Thijs, I., Vranken, J., and Klerkx, S. (2018). An evaluation of the NightOwl home sleep apnea testing system. J. Clin. Sleep Med. 14, 1791–1796. doi: 10.5664/jcsm.7398
Massie, F., Van Pee, B., and Bergmann, J. (2022a). Correlations between home sleep apnea tests and polysomnography outcomes do not fully reflect the diagnostic accuracy of these tests. J. Clin. Sleep Med. 18, 871–876. doi: 10.5664/jcsm.9744
Massie, F., Van Pee, B., Vits, S., Verbraecken, J., and Bergmann, J. (2022b). Phenotyping REM OSA by means of peripheral arterial tone-based home sleep apnea testing and polysomnography: a critical assessment of the sensitivity and specificity of both methods. J. Sleep Res. 31, e13481. doi: 10.1111/jsr.13481
Massie, F., Vits, S., Khachatryan, A., Van Pee, B., Verbraecken, J., Bergmann, J., et al. (2023). Central sleep apnea detection by means of finger photoplethysmography. IEEE J. Transl. Eng Health Med. 11, 126–136. doi: 10.1109/JTEHM.2023.3236393
Mattila, T., Hasala, H., Kreivi, H. R., Avellan-Hietanen, H., Bachour, A., Herse, F., et al. (2022). Changes in the societal burden caused by sleep apnoea in Finland from 1996 to 2018: a national registry study. Lancet Reg. Health Eur. 16, 100338. doi: 10.1016/j.lanepe.2022.100338
Mazzotti, D. R., Keenan, B. T., Lim, D. C., Gottlieb, D. J., Kim, J., Pack, A. I., et al. (2019). Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am. J. Respir. Crit. Care Med. 200, 493–506. doi: 10.1164/rccm.201808-1509OC
McNicholas, W. T., and Korkalainen, H. (2023). Translation of obstructive sleep apnea pathophysiology and phenotypes to personalized treatment: a narrative review. Front. Neurol. 14, 1239016. doi: 10.3389/fneur.2023.1239016
McNicholas, W. T., and Pevernagie, D. (2022). Obstructive sleep apnea: transition from pathophysiology to an integrative disease model. J. Sleep Res. 31, e13616. doi: 10.1111/jsr.13616
Mehra, R., Chung, M. K., Olshansky, B., Dobrev, D., Jackson, C. L., Kundel, V., et al. (2022). Sleep-disordered breathing and cardiac arrhythmias in adults: mechanistic insights and clinical implications: a scientific statement from the American Heart Association. Circulation 146, e119–e136. doi: 10.1161/CIR.0000000000001082
Mokhlesi, B., Finn, L. A., Hagen, E. W., Young, T., Hla, K. M., Van Cauter, E., et al. (2014). Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am. J. Respir. Crit. Care Med. 190, 1158–1167. doi: 10.1164/rccm.201406-1136OC
Monaghesh, E., and Hajizadeh, A. (2020). The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health 20, 1193. doi: 10.1186/s12889-020-09301-4
Newell, J., Mairesse, O., Verbanck, P., and Neu, D. (2012). Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples. Psychiatry Res. 200, 795–801. doi: 10.1016/j.psychres.2012.07.045
Nieto, F. J., Young, T. B., Lind, B. K., Shahar, E., Samet, J. M., Redline, S., et al. (2000). Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283, 1829–1836. doi: 10.1001/jama.283.14.1829
O'Brien, L. M., Bullough, A. S., Shelgikar, A. V., Chames, M. C., Armitage, R., Chervin, R. D., et al. (2012). Validation of Watch-PAT-200 against polysomnography during pregnancy. J. Clin. Sleep Med. 8, 287–294. doi: 10.5664/jcsm.1916
O'Donnell, C., Ryan, S., and McNicholas, W. T. (2020). The impact of telehealth on the organization of the health system and integrated care. Sleep Med. Clin. 15, 431–440. doi: 10.1016/j.jsmc.2020.06.003
O'Donnell, C. P., Allan, L., Atkinson, P., and Schwartz, A. R. (2002). The effect of upper airway obstruction and arousal on peripheral arterial tonometry in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 166, 965–971. doi: 10.1164/rccm.2110072
Pack, A. I. (2004). Sleep-disordered breathing. Access is the issue. Am. J. Respir. Crit. Care Med. 169, 666–667. doi: 10.1164/rccm.2401008
Patil, S. P., Ayappa, I. A., Caples, S. M., Kimoff, R. J., Patel, S. R., Harrod, C. G., et al. (2019). Treatment of adult obstructive sleep apnea with positive airway pressure: an american academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 15, 335–343. doi: 10.5664/jcsm.7640
Peppard, P. E., Young, T., Palta, M., and Skatrud, J. (2000). Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 342, 1378–1384. doi: 10.1056/NEJM200005113421901
Punjabi, N. M., Patil, S., Crainiceanu, C., and Aurora, R. N. (2020). Variability and misclassification of sleep apnea severity based on multi-night testing. Chest 158, 365–373. doi: 10.1016/j.chest.2020.01.039
Randerath, W. J., Herkenrath, S., Treml, M., Grote, L., Hedner, J., Bonsignore, M. R., et al. (2021). Evaluation of a multicomponent grading system for obstructive sleep apnoea: the Baveno classification. ERJ Open Res. 7, 00928–02020. doi: 10.1183/23120541.00928-2020
Reutrakul, S., and Mokhlesi, B. (2017). Obstructive sleep apnea and diabetes: a state-of-the-art review. Chest 152, 1070–1086. doi: 10.1016/j.chest.2017.05.009
Roeder, M., Bradicich, M., Schwarz, E. I., Thiel, S., Gaisl, T., Held, U., et al. (2020). Night-to-night variability of respiratory events in obstructive sleep apnoea: a systematic review and meta-analysis. Thorax 75, 1095–1102. doi: 10.1136/thoraxjnl-2020-214544
Rosen, I. M., Kirsch, D. B., Carden, K. A., Malhotra, R. K., Ramar, K., Aurora, R. N., et al. (2018). Clinical use of a home sleep apnea test: an updated american academy of sleep medicine position statement. J. Clin. Sleep Med. 14, 2075–2077. doi: 10.5664/jcsm.7540
Ryals, S., Chiang, A., Schutte-Rodin, S., Chandrakantan, A., Verma, N., Holfinger, S., et al. (2023). Photoplethysmography-new applications for an old technology: a sleep technology review. J. Clin. Sleep Med. 19, 189–195. doi: 10.5664/jcsm.10300
Salari, N., Khazaie, H., Abolfathi, M., Ghasemi, H., Shabani, S., Rasoulpoor, S., et al. (2022). The effect of obstructive sleep apnea on the increased risk of cardiovascular disease: a systematic review and meta-analysis. Neurol. Sci. 43, 219–231. doi: 10.1007/s10072-021-05765-3
Schnall, R. P., Shlitner, A., Sheffy, J., Kedar, R., and Lavie, P. (1999). Periodic, profound peripheral vasoconstriction–a new marker of obstructive sleep apnea. Sleep 22, 939–946.
Solelhac, G., Sanchez-de-la-Torre, M., Blanchard, M., Berger, M., Hirotsu, C., Imler, T., et al. (2023). Pulse wave amplitude drops index: a biomarker of cardiovascular risk in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 207, 1620–1632. doi: 10.1164/rccm.202206-1223OC
Sommermeyer, D., Zou, D., Grote, L., and Hedner, J. (2012). Detection of sleep disordered breathing and its central/obstructive character using nasal cannula and finger pulse oximeter. J. Clin. Sleep Med. 8, 527–533. doi: 10.5664/jcsm.2148
Sterling, K. L., Cistulli, P. A., Linde-Zwirble, W., Malik, A., Benjafield, A. V., Malhotra, A., et al. (2023). Association between positive airway pressure therapy adherence and health care resource utilization in patients with obstructive sleep apnea and type 2 diabetes in the United States. J. Clin. Sleep Med. 19, 563–571. doi: 10.5664/jcsm.10388
Strassberger, C., Zou, D., Penzel, T., Fietze, I., Hedner, J., Ficker, J. H., et al. (2021). Beyond the AHI-pulse wave analysis during sleep for recognition of cardiovascular risk in sleep apnea patients. J. Sleep Res. 30, e13364. doi: 10.1111/jsr.13364
Tauman, R., Berall, M., Berry, R., Etzioni, T., Shrater, N., Hwang, D., et al. (2020). Watch-PAT is useful in the diagnosis of sleep apnea in patients with atrial fibrillation. Nat. Sci. Sleep 12, 1115–1121. doi: 10.2147/NSS.S278752
Trzepizur, W., Blanchard, M., Ganem, T., Balusson, F., Feuilloy, M., Girault, J. M., et al. (2022). Sleep apnea-specific hypoxic burden, symptom subtypes, and risk of cardiovascular events and all-cause mortality. Am. J. Respir. Crit. Care Med. 205, 108–117. doi: 10.1164/rccm.202105-1274OC
Van Pee, B., Massie, F., Vits, S., Dreesen, P., Klerkx, S., Bijwadia, J., et al. (2022). A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep 45, zsac028. doi: 10.1093/sleep/zsac028
Varga, A. W., and Mokhlesi, B. (2019). REM obstructive sleep apnea: risk for adverse health outcomes and novel treatments. Sleep Breath. 23, 413–423. doi: 10.1007/s11325-018-1727-2
Vinnikov, D., Blanc, P. D., Alilin, A., Zutler, M., and Holty, J. C. (2017). Fatigue and sleepiness determine respiratory quality of life among veterans evaluated for sleep apnea. Health Qual. Life Outcomes 15, 48. doi: 10.1186/s12955-017-0624-x
Wickwire, E. M. (2021). Value-based sleep and breathing: health economic aspects of obstructive sleep apnea. Fac. Rev. 10, 40. doi: 10.12703/r/10-40
Xie, C., Zhu, R., Tian, Y., and Wang, K. (2017). Association of obstructive sleep apnoea with the risk of vascular outcomes and all-cause mortality: a meta-analysis. BMJ Open 7, e013983. doi: 10.1136/bmjopen-2016-013983
Yalamanchali, S., Farajian, V., Hamilton, C., Pott, T. R., Samuelson, C. G., Friedman, M., et al. (2013). Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol. Head Neck Surg. 139, 1343–1350. doi: 10.1001/jamaoto.2013.5338
Yim-Yeh, S., Rahangdale, S., Nguyen, A. T., Jordan, A. S., Novack, V., Veves, A., et al. (2010). Obstructive sleep apnea and aging effects on macrovascular and microcirculatory function. Sleep 33, 1177–1183. doi: 10.1093/sleep/33.9.1177
Young, T., Evans, L., Finn, L., and Palta, M. (1997). Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 20, 705–706. doi: 10.1093/sleep/20.9.705
Zhang, Z., Sowho, M., Otvos, T., Sperandio, L. S., East, J., Sgambati, F., et al. (2020). A comparison of automated and manual sleep staging and respiratory event recognition in a portable sleep diagnostic device with in-lab sleep study. J. Clin. Sleep Med. 16, 563–573. doi: 10.5664/jcsm.8278
Zinchuk, A. V., Gentry, M. J., Concato, J., and Yaggi, H. K. (2017). Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med. Rev. 35, 113–123. doi: 10.1016/j.smrv.2016.10.002
Zou, D., Grote, L., Eder, D. N., Peker, Y., and Hedner, J. (2004). Obstructive apneic events induce alpha-receptor mediated digital vasoconstriction. Sleep 27, 485–489. doi: 10.1093/sleep/27.3.485
Zou, D., Grote, L., Eder, D. N., Radlinski, J., and Hedner, J. (2010). A double-blind, crossover study of Doxazosin and Enalapril on peripheral vascular tone and nocturnal blood pressure in sleep apnea patients. Sleep Med. 11, 325–328. doi: 10.1016/j.sleep.2009.10.004
Keywords: diagnosis, peripheral artery tonometry, precision medicine, sleep disordered breathing, telehealth, HSAT, night-to-night variability
Citation: Zou D, Vits S, Egea C, Ehrsam-Tosi D, Lavergne F, Azpiazu M and Fietze I (2023) A new approach to streamline obstructive sleep apnea therapy access using peripheral arterial tone-based home sleep test devices. Front. Sleep 2:1256078. doi: 10.3389/frsle.2023.1256078
Received: 10 July 2023; Accepted: 21 September 2023;
Published: 06 November 2023.
Edited by:
Dalva Poyares, Federal University of São Paulo, BrazilReviewed by:
Brandon Nokes, University of California, San Diego, United StatesStuart F. Quan, Harvard Medical School, United States
Copyright © 2023 Zou, Vits, Egea, Ehrsam-Tosi, Lavergne, Azpiazu and Fietze. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florent Lavergne, ZmxvcmVudC5sYXZlcmduZUByZXNtZWQuZnI=
 Ding Zou
Ding Zou Steven Vits2,3
Steven Vits2,3 Carlos Egea
Carlos Egea Florent Lavergne
Florent Lavergne Mikel Azpiazu
Mikel Azpiazu Ingo Fietze
Ingo Fietze