- 1Department of Immunology, School of Medicine, University of Washington, Seattle, WA, United States
- 2Center for Innate Immunity and Immune Disease, University of Washington, Seattle, WA, United States
- 3Washington National Primate Research Center, Seattle, WA, United States
- 4Department of Microbiology, School of Medicine, University of Washington, Seattle, WA, United States
- 5Department of Cell Biology, University of Miami Miller School of Medicine, Miami, FL, United States
- 6Department of Comparative Medicine, School of Medicine, University of Washington, Seattle, WA, United States
- 7Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, United States
Zika virus (ZIKV) is a mosquito-borne flavivirus that causes an acute febrile illness. ZIKV can be transmitted between sexual partners and from mother to fetus. Infection is strongly associated with neurologic complications in adults, including Guillain-Barré syndrome and myelitis, and congenital ZIKV infection can result in fetal injury and congenital Zika syndrome (CZS). Development of an effective vaccine is imperative to protect against ZIKV vertical transmission and CZS. Recombinant Vesicular Stomatitis virus (rVSV) is a highly effective and safe vector for the delivery of foreign immunogens for vaccine purposes. Here, we evaluate an rVSV vaccine expressing the full length pre-membrane (prM) and ZIKV envelope (E) proteins (rVSVΔM-ZprME), shown to be immunogenic in murine models of ZIKV infection, for its capacity to induce immune responses in nonhuman primates. Moreover, we assess the efficacy of the rVSVΔM-ZprME vaccine in the protection of pigtail macaques against ZIKV infection. Administration of the rVSVΔM-ZprME vaccine was safe, but it did not induce robust anti-ZIKV T-cell responses, IgM or IgG antibodies, or neutralizing antibodies in most animals. Post ZIKV challenge, animals that received the rVSVΔM control vaccine lacking ZIKV antigen had higher levels of plasma viremia compared to animals that received the rVSVΔM-ZprME vaccine. Anti-ZIKV neutralizing Ab titers were detected in a single animal that received the rVSVΔM-ZprME vaccine that was associated with reduced plasma viremia. The overall suboptimal ZIKV-specific cellular and humoral responses post-immunization indicates the rVSVΔM-ZprME vaccine did not elicit an immune response in this pilot study. However, recall antibody response to the rVSVΔM-ZprME vaccine indicates it may be immunogenic and further developments to the vaccine construct could enhance its potential as a vaccine candidate in a nonhuman primate pre-clinical model.
Introduction
Zika virus (ZIKV) is a pathogenic arbovirus comprised of three structural proteins, pre-membrane (prM), capsid (C) and envelope (E), and seven non-structural proteins (1). The ZIKV E protein is the primary glycoprotein present on the virion surface that mediates cellular receptor binding and viral entry into susceptible cells (2). Cellular immunity and antibody responses are robustly generated against ZIKV E, thus making E a major target of vaccine development and therapeutic interventions (3–5). Aedes mosquitoes are the primary vector for ZIKV transmission and despite low-level transmission rates since the 2015-2016 epidemic, the risk of recurrent outbreaks due to waning population immunity and expansion of the insect vector range, underscore the threat that new outbreaks of ZIKV infection will occur. As recent as 2019, the Rajasthan State of India reported cases of ZIKV infection (6). In adults, ZIKV infection is associated with neurologic complications, including Guillain-Barré syndrome and myelitis, while vertical transmission in pregnancy can lead to congenital Zika syndrome (CZS) (7–10). Thus, an effective vaccine to protect against ZIKV infection and disease is imperative. However, currently there is no U.S. Food and Drug Administration (FDA)-approved ZIKV vaccine, making the development of vaccines to combat ZIKV disease an urgent objective.
Several ZIKV vaccines are undergoing clinical evaluation that include a candidate DNA vaccine now in a multi-site Phase 2/2b clinical trial in healthy adults and adolescents (NCT03110770) in the United States, Central and South America (11). The DNA-based vaccine encoding ZIKV prM and E proteins of the ZIKV H/PF/2013 strain induces neutralizing antibody responses that are protective against ZIKV infection (12). Other ZIKV vaccine candidates being evaluated include i) a purified inactivated ZIKV vaccine (VLA1601) in a Phase I clinical trial in flavivirus-naïve adults (NCT03425149), which uses the IXIARO Japanese encephalitis vaccine platform (13), ii) a live attenuated chimeric Zika vaccine (rZIKV/D4Δ30-713) in a Phase 3 clinical trial in Brazil (NCT03611946), which expresses ZIKV E in a dengue virus type 4 backbone (14), and iii) a mRNA 1325 vaccine (NCT03014089) encoding the prME glycoprotein of the ZIKV H/PF/2013 strain (15). While nucleic acid ZIKV vaccine candidates are at the forefront of clinical evaluation, several others utilizing different immunogen delivery platforms are in development. One such platform is a viral vector, recombinant vesicular stomatitis virus (rVSV), that provides a unique approach to induction of cellular and humoral immune responses through the intracellular synthesis of specific viral antigens at high levels. VSV is a single stranded, negative-sense RNA virus belonging to the Rhabdoviridae family that expresses five protein products, with the VSV G glycoprotein mediating broad cellular tropism that includes targeting malignant cells. The genetic flexibility to engineer VSV, as demonstrated with oncolytic virotherapy, combined with low pre-existing immunity in humans makes rVSV an attractive platform for vaccine applications (16–18). Indeed, an rVSVΔG-ZEBOV vaccine (ERVEBO®, Merck), approved by the U.S. FDA, has been shown to be safe, highly immunogenic, and effective against Ebola virus infection and disease following a clinical trial during the 2014-2016 outbreak in Guinea (19–22). In addition, the rVSV vaccine platform has been evaluated in models of influenza A virus and SARS-CoV-2 infection (6, 23–26).
An rVSV lacking the viral matrix (M) protein (rVSVΔM) is an ideal vector for vaccine applications, as it exhibits attenuated viral replication due to its inability to block host mRNA export or translation, while still maintaining a highly immunogenic infection cleared by the host (27, 28). In mice, an rVSVΔM-based ZIKV vaccine candidate expressing the full length ZIKV prM and E proteins (VSVΔM-ZprME) was shown to produce high levels of neutralizing antibody (Ab) to the ZIKV envelope region and generate ZIKV-specific cytotoxic T lymphocyte (CTL) activity (29). Furthermore, Betancourt et al., showed maternal Abs from VSVΔM-ZprME vaccinated female mice protected neonatal offspring from lethal ZIKV challenge after birth. Here, we evaluated rVSVΔM-ZprME as a candidate ZIKV vaccine in a preclinical nonhuman primate model. This pilot study assessed vaccine safety, immunogenicity and prophylactic efficacy of a candidate rVSVΔM-ZprME vaccine in an established adult pigtail macaque model of acute ZIKV infection (30). This study addresses the need for an effective ZIKV vaccine that protects against disease from ZIKV infection in adults, with the goal of producing relevant data to advance pre-clinical evaluation of rVSVΔM-ZprME in macaque pregnancy models of ZIKV vertical transmission and fetal injury.
Methods
Nonhuman primate study design
The rVSV constructs were generated and characterized as previously described (29). The viral vector is a recombinant Vesicular Stomatitis virus (rVSV) lacking the viral M protein and containing the Zika virus (Puerto Rico strain 2015) prME transgene inserted 5’ to the viral G gene (Figure 1A). Six female pigtail macaques (7- 12 yrs) that were seronegative for ZIKV, West Nile virus (WNV), dengue virus (DENV), chikungunya virus (CHKV), and yellow fever virus (YFV) flaviviruses were enrolled in the study (Tables S1, S2). All animals received two intramuscular injections of 1×107 PFU of either rVSVΔM-ZprME (n=4) or rVSVΔM (n=2) in the quadricep muscle of the leg at weeks 0 and 4, and a higher boost immunization of 2×107 PFU given at week 8 (Figure 1B). The intramuscular inoculation route and prime-boost strategy were chosen based on the immunization regimen commonly employed in nonhuman primate models and for direct translation for clinical trials. At 10 weeks post-prime immunization, animals underwent heterologous virus challenge via subcutaneous administration of 1×105 plaque forming units (PFU) of ZIKV FSS13025 (GenBank no. MH368551) in five separate 100 µl injections administered into the bilateral forearm at different sites.
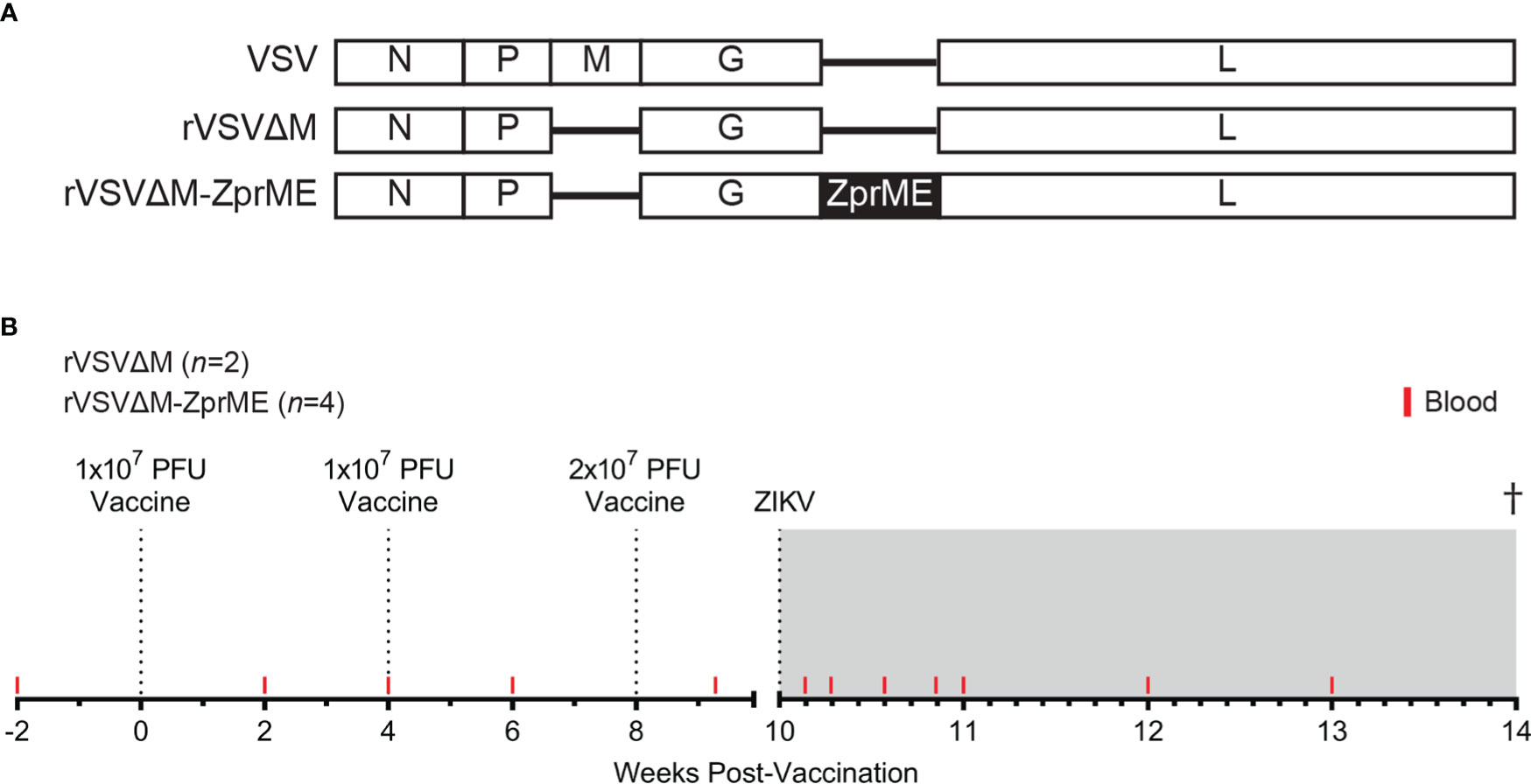
Figure 1 Vaccine vector schematic and NHP vaccine study design. (A) Schematic showing the VSV (-) strand RNA genome and recombinant VSV vaccine constructs. The VSV M gene is deleted from the rVSV vector (rVSVΔM). The rVSVΔM-ZprME construct includes a ZIKV pre-membrane envelope (prME) transgene (B) NHP vaccine study design and blood sample collection time-points. Six female pigtail macaques were enrolled in the study and received intramuscular injections of either rVSVΔM-ZprME (n=4) or rVSVΔM (n=2) in a prime-boost strategy at Weeks 0, 4 and 8 and are indicated by the dotted lines. The second boost immunization given at week 8 was administered at twice the original dose. All animals were subcutaneously challenged at 10 weeks with total dose of 5×105 PFU ZIKV FSS13025. The study ended 27-30 days post-challenge. The ZIKV phase is the shaded grey region.
Care and use of pigtail macaques
All animals used in this study were housed at the Washington National Primate Research Center (WaNPRC), as accredited by the American Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The University of Washington’s Institutional Animal Care and Use Committee (IACUC) approved all experiments (IACUC Protocol Number 4158-10) and were in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and Animal Welfare. For timepoints involving experimental procedures, animals were sedated with 10 mg/kg ketamine and dexmeditomidine sedative intramuscular (Ketaset® Henry Schein).
Clinical observations, blood chemistries and postmortem exam
Animal care and welfare was monitored daily by veterinary staff and animal technicians throughout the course of the study. Animals were weighed and rectal temperatures collected following sedation at each sampling timepoint. On days of immunization, whole blood was collected prior to vaccine administration. Serum, plasma, and peripheral blood mononuclear cells (PBMCs) were isolated from whole blood as previously described (30). Serum chemistry analysis was performed on pre-immunization sera collected 2 weeks before prime, sera collected at 4, 8 and 10 weeks post-prime immunization, pre-challenge, 2 days post-challenge, and sera collected at weekly intervals through the end of study (7-30 dpi). SST samples were spun at 1100 x g for 10 min at room temperature and isolated sera submitted to Research Testing Services at the University of Washington Department of Laboratory Medicine. Samples were run on a chemistry panel with 17 analytes that included electrolytes and a comprehensive metabolic panel (CMP) to monitor liver and kidney function, such as alkaline phosphatase (ALP), alanine transaminase (ALT), and aspartate aminotransferase (AST) (Table S3). At the end of the study the animals were humanely euthanized and a complete postmortem exam was performed. Representative samples from all tissues and organs were preserved in 10% neutral buffered formalin, processed routinely, embedded in paraffin, sectioned at 3-5um, Hematoxylin and eosin stained, and evaluated by light microscopy.
Serology for WNV, CHIKV, DENV and ZIKV in nonhuman primates
All animals were pre-screened and negative for i) CHV-1 (B virus), ii) SIV, iii) SRV, and iv) STLV prior to assignment to the study. We used a viral-specific diagnostic assay to screen animals for ZIKV, DENV, CHIKV, WNV and YFV IgG to assist with animal study assignment (Table S2). Ultraviolet-inactivated serum collected from naïve animals was run according to the manufacturer’s (Xpress Biosystems, Frederick MD) instructions as previously described (31). Diluted serum samples were added to the wells of the ELISA plate coated with viral antigens and incubated at 37°C for 45 min. Wells were washed 5x and peroxidase conjugate was added to each well and incubated for 45 min at 37°C. Following incubation, the plates were washed 5 times followed by the addition of 2,2’-azino-bis (3-ethylbenzothiazoline-6- sulphonic acid)-peroxidase substrate to each well. The plates were incubated at room temperature for 30 min and the absorbance of the colorimetric reaction in each well was read within 15 min on a plate reader at 405 nm.
ZIKV plasma viral loads
Plasma was isolated from whole blood collected at -5, 1, 2, 4, 6, 7, 14, and 21 days post-challenge, and at necropsy (days 27-30). Viral RNA load was assessed in plasma using a ZIKV-specific RT-qPCR assay, as previously described (30). RNA was isolated using the QIAamp Viral RNA Mini Kit (Qiagen). Plasma RNAs were concentrated using the RNA Clean & Concentrator Kit (Zymo Research) and eluted in 12 µl nuclease-free water. The iScript Select cDNA Synthesis Kit (Bio-Rad) was used for gene-specific cDNA synthesis with an input of 10 µl RNA sample. Viral RNA was quantified using the TaqMan Universal PCR Master Mix (Applied Biosystems) and a 7300 Real-Time PCR System (Applied Biosystems) using a ZIKV prM-specific primer/probe set.
PRNT assay
NHP sera collected at baseline (-2 weeks), and weeks 2, 4, 6, 8 and 10 post-prime immunization were tested in PRNT assay for neutralizing antibody production. The highest serum dilution reducing plaque numbers by 50% (PRNT50) were determined with a limit of detection (LOD) of 1:50. The assay was repeated three times. ZIKV strain used was ZIKV FSS13025 (Cambodia, 2010).
Enzyme-linked immunosorbent assay
NHP sera and/or plasma was assessed for anti-VSV Glycoprotein (G) and anti-ZIKV envelope (E) IgG titers by an Enzyme-Linked Immunosorbent Assay (ELISA) and for anti-ZIKV E IgM titers using a ZIKA Detect™ 2.0 IgM Capture ELISA Kit (InBios, Lot# BM6156) by the WaNPRC Pathogen Detection Services Laboratory (PDSL) (32). For anti-VSV G and anti-ZIKV E IgG, samples were diluted 1:100 and/or 1:200 in a blocking buffer (5% w/v nonfat dried milk (Bio-Rad Laboratories) and 0.5% v/v Tween-20) and added in triplicate to a high-binding 96-well plate (Costar) pre-coated with either recombinant VSV G (Alpha Diagnostics VSIG15-R) or ZIKV E (Fitzgerald Industries International, 30-1932). Plasma IgG specific for VSV G or ZIKV E protein were detected by a Horseradish peroxidase-linked antibody (ThermoFisher) via a color change reaction upon addition of the SureBlue Reserve substrate (KPL). Reaction was stopped after 30 minutes with 1N HCl (VWR) and absorbance at 450nm was measured on a EMax plate reader (Molecular Devices). A standard curve (MyBioSource) was to calculate anti-VSV G IgG levels via a linear interpolation performed in Microsoft Excel. For anti-ZIKV IgM, UV-inactivated NHP sera and/or plasma was diluted 1:100 and 50 µl added to the three assay wells, ZIKV antigen (Zika Ag), Cross-reactive Control Antigen (CCA) and Normal Cell Antigen (NCA), per sample.
IFN-γ Enzyme-linked immunosorbent spot (ELISPOT) assay
Antigen-specific T-cells secreting IFN-γ in the PBMC were detected using a Human IFN-γ Single-Color ELISPOT (ImmunoSpot, Shaker Heights, Cleveland, OH), per the manufacturer’s protocol. Briefly, cryopreserved PBMC cells were thawed, and 1 x 105 cells were stimulated for 24 hours in duplicate with 8 Zika Envelope peptide pools (15-mers with 12 amino acid overlap) (NR-50553, BEI Resources, Manassas, VA) at a concentration of 1 μg/mL per peptide. DMSO was used as a negative control and Phorbol 12-myristate 13-acetate (PMA) and Ionomycin (Sigma-Aldrich, St. Louis, MO) were used as positive controls. Spots were counted on an Immunospot Analyzer with CTL Immunospot Profession Software (Cellular Technology Ltd., Shaker Heights, Cleveland, OH). Spot forming cells (SFC) were computed following DMSO subtraction and were considered positive if the number of SFC was > 20 SFC per 1 x 106 cells.
Immunophenotyping
Whole blood was assessed for viability with a live/dead stain (Life Technologies) and stained with a panel of antibodies in brilliant stain buffer (BD Biosciences) to identify immune cells, as described previously (30): Beckman Coulter: NKG2A (Z199); Biolegend: CD16 (3G8), CD20 (2H7), CD4 (OKT4), HLA-DR (L243), CD14 (M5E2), CD28 (CD28.2), CD40 (5C3), CD69 (FN50); BD Biosciences: CD11b (ICRF44), CD3 (Sp34-2), CD11c (S-HCL-3), CD45 (D058-1283); and eBioscience: CD123 (6H6), CD8 (RPA-T8), CD95 (DX2). Cells were then re-suspended in 1% paraformaldehyde and samples were acquired on a LSRII (BD Biosciences) using FACS Diva software (version 8). Samples were analyzed using FlowJo software version 9.9.4 (FlowJo, LLC). All events were first gated on FSC singlets, CD45+ leukocytes, live, and then mononuclear cells according to FSC-A and SSC-A profiles. CD3+ T-cells were gated in CD4+ and CD8+ populations and into naïve (CD95-CD28+), central memory (CM) (CD95+CD28+) and effector memory (EM) (CD95+CD28-). Cellular activation of T-cells were measured using CD69 that met a minimum threshold of ≥100 cells/gate.
Results
A recombinant VSV-based ZIKV vaccine previously shown to be protective in mice (29) was evaluated in a pre-clinical nonhuman primate (NHP) model to assess vaccine immunogenicity and protection against ZIKV infection. The attenuated rVSVΔM-ZprME vaccine construct lacks the VSV membrane (M) gene and carries a transgene encoding ZIKV prM and E proteins from a 2015 ZIKV Puerto Rico strain (Figure 1A). A total of six adult female pigtail macaques received a prime-boost vaccine regimen of rVSVΔM-ZprME (n=4) or the control rVSVΔM viral vector (n=2) without the ZIKV transgene spaced 4 weeks apart (Figure 1B). Increased levels of ZIKV-specific binding antibody responses were not detected after the first two immunizations (Figures 3A, B); therefore, a third immunization at twice the original dose was administered 8 weeks post-prime immunization.
rVSVΔM-ZprME vaccine safety profile in nonhuman primates
Vaccine safety assessment was performed to evaluate the overall health of the animals following vaccination and through the entirety of the study. All animals remained clinically normal with unremarkable bloodwork throughout the course of the study. There were no abnormal changes in serum blood chemistries measured that included a metabolic panel to evaluate liver and kidney function (Figures 2A–C; Table S2). A single animal (R10117) had elevated blood AST levels at 2 days post-ZIKV infection corresponding to peak viremia (Figure S2). Vaccinated rVSVΔM-ZprME and rVSVΔM control animals had stable weight throughout the study and did not show signs of fever following immunization, with rectal temperatures ranging from 98.2°F to 102.6°F (average 100.3°F vaccine phase) (Figures 2D, E). Body temperature became slightly elevated, but within a normal range, post-ZIKV challenge (average 101.2°F challenge phase) for both rVSVΔM-ZprME and rVSVΔM control animals, with temperature fluctuations among individual animals. A complete gross exam and histologic evaluation of representative samples from all tissues and organ systems was performed at necropsy. In all animals, typical pre-existing lesions that are present in most captive macaques and consistent with inflammatory bowel disease (IBD) were identified; the diagnosis was mild to moderate lymphoplasmacytic, histiocytic and eosinophilic gastro-entero-colitis with enteric villar blunting and fusion (data not shown). Additionally, all animals had commensal infections with gastric spiral bacteria and large intestinal spirochetosis. Finally, Z08064 had pre-existing, moderate and subclinical, hepatic secondary amyloidosis; secondary to chronic inflammation from IBD with mis-metabolism of acute-phase reactive proteins and subsequent hepatic sinusoidal deposition. Secondary amyloidosis is commonly seen in captive pigtail macaques, the disease is slowly progressive, and in this animal, it was relatively early in the disease course. Thus overall, the vaccine was well tolerated in the animals and displayed a normal safety profile and there was no gross or histologic evidence of changes due to the viral challenge.
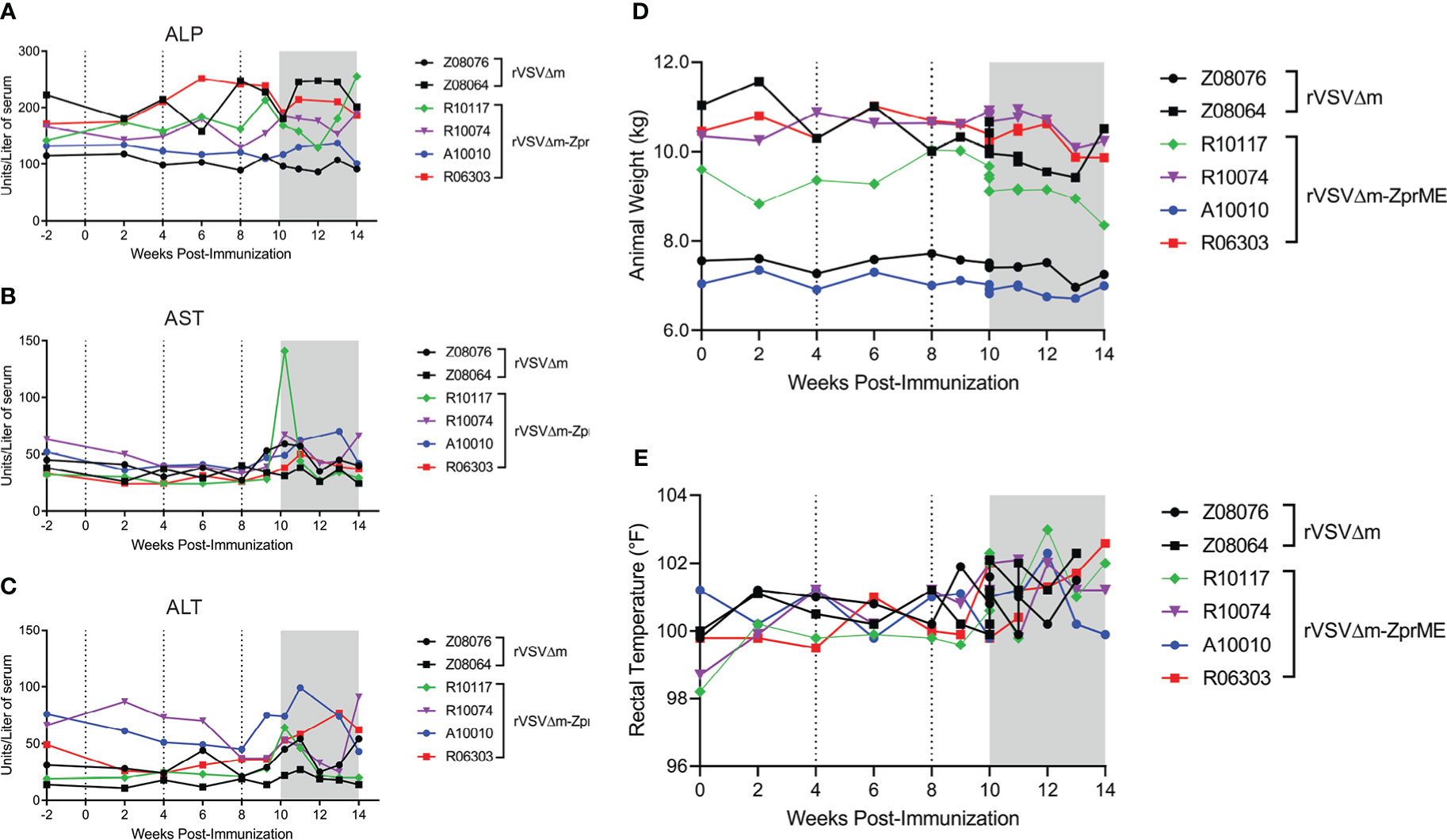
Figure 2 Clinical parameters of study animals and serum chemistry. Serum chemistry measurements for (A) alkaline phosphatase (ALP) (B) aspartate transaminase (AST) and (C) alanine transaminase (ALT) liver enzymes. Points on the plot represent individual animals for rVSVΔM (black) and rVSVΔM-ZprME (colored) vaccine regimens. (D) Animal weight (kg) and (E) body temperatures in Fahrenheit (°F) measured by rectal thermometer over the study period. (A–D) Dotted lines indicate immunizations and the ZIKV phase is the shaded grey region.
Evaluation of rVSVΔM-ZprME vaccine immunogenicity
As antibodies are important for protection from ZIKV infection (33), we first evaluated humoral immune responses elicited by the rVSVΔM-ZprME vaccine. Pre- and post-immunization sera/plasma were analyzed for IgM and IgG binding antibodies against the ZIKV envelope (Env) protein by ELISA. Three immunizations with rVSVΔM-ZprME were insufficient at inducing IgM or IgG binding antibodies to ZIKV Env (Figures 3A, B). Plaque reduction neutralization test (PRNT) was next used to assess the production of ZIKV-specific neutralizing antibodies (NAb) in sera following vaccination. As expected, sera from animals that received the control rVSVΔM vector did not exhibit ZIKV neutralization. One of the four animals vaccinated with the rVSVΔM-ZprME vector (R10117) had detectable NAb (200) following the second boost immunization (Figure 3C). We next evaluated ZIKV Env-specific cellular responses in peripheral blood mononuclear cells (PBMC) collected prior to vaccination and after the 3rd immunization, as measured by IFN-γ activity by ELISPOT. No ZIKV-specific T-cells were induced in response to rVSVΔM-ZprME vaccination following the second boost immunization (Table S4). Collectively, these results demonstrate that rVSVΔM-ZprME did not robustly induce anti-ZIKV humoral or cellular immunity in all animals.
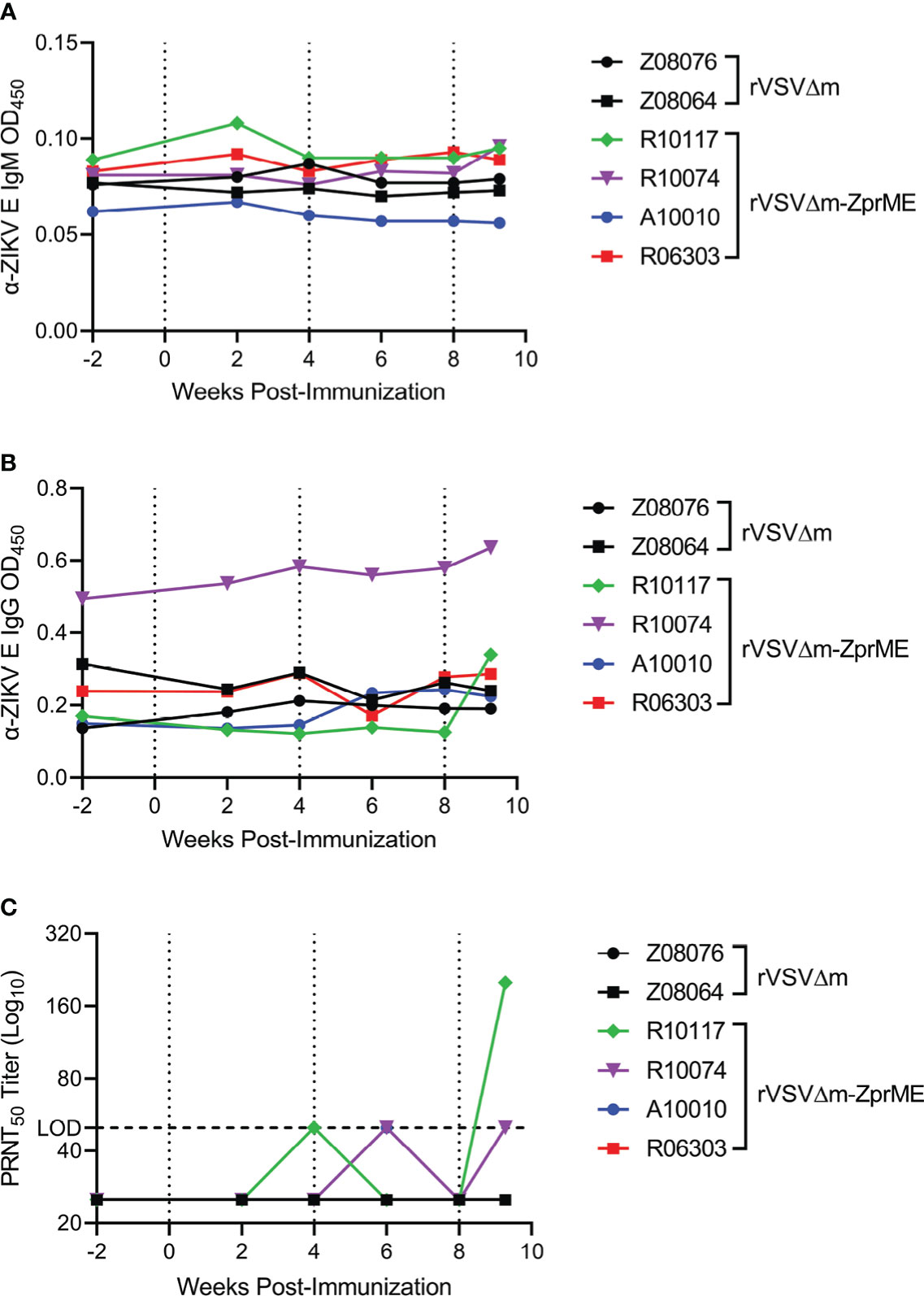
Figure 3 ZIKV-specific Ig titers and ZIKV neutralization. Anti (α)-ZIKV Env-specific (A) IgM and (B) IgG responses in all animals were measured by ELISA (OD450 nm). Samples were diluted 1:100 to run for each assay OD, Optical Density (C) Sera were diluted 1:50 and evaluated for virus neutralization by PRNT50 assay against ZIKV FSS13025 (Cambodia, 2010). The PRNT50 50 lower limit neutralization value is marked by the dashed line. Neutralization tests were carried out in three independent assays. (A–C) Pre-immunization sera, serum samples collected biweekly after immunization, and pre-challenge samples were assayed. The data points on the plots represent individual animals for rVSVΔM (black) and rVSVΔM-ZprME (colored) vaccine regimens. Immunizations are indicated by the dotted lines.
To ensure the vaccine was successfully delivered to the macaques and able to induce an immune response we further evaluated anti-vector IgG responses against fusion glycoprotein (G protein) of VSV and found all control rVSVΔM and rVSVΔM-ZprME vaccinated animals developed an anti-VSV-G-specific IgG response to the vaccine vector after a single immunization (Figure S1). In the absence of ZIKV-specific cellular responses, we next evaluated non-specific responses to determine if the vaccine similarly stimulated T cells in whole blood. Activation of CD8+ T cells was detected in 3/6 animals (1 rVSVΔM and 2 rVSVΔM-ZprME vaccinated animals) 4 weeks after prime immunization and to a greater extent 4 weeks after the boost immunization (Figure S2) and suggests non-specific activation of memory CD8+ T-cells. These results show that overall both rVSVΔM and rVSVΔM-ZprME constructs induced cellular and humoral responses to the VSV vaccine construct; however, the rVSVΔM-ZprME vaccine only induced weak humoral immunity against ZIKV.
Assessment of plasma viremia and ZIKV IgG and IgM responses of vaccinated animals following ZIKV challenge
To assess the efficacy of the vaccine, all animals were challenged subcutaneously with heterologous ZIKV FSS13025 14 days after the second boost immunization (Week 8) and monitored over a 28-day period. This virus strain causes less robust infection in macaques compared to America lineage isolates (34) and was selected as we have previously shown that infection in a pigtail macaque model of pregnancy results in severe fetal brain lesions (31). The relative efficacy of the vaccine was evaluated by measuring the ZIKV load in plasma. In animals receiving the rVSVΔM control vector, ZIKV RNA was detected in the plasma in both animals at day 2 and in 1 of the animals at day 4 post-challenge (Figure 4A), a result that is consistent with our previous findings (31). In contrast, only 1/4 of the animals vaccinated with the rVSVΔM-ZprME vector had detectable levels of plasma viremia (R10074) at days 1 and 2 post-challenge and at levels that were lower compared to the rVSVΔM control animals (Figure 4A). Analysis of anti-ZIKV IgM responses post-challenge revealed that all vaccinated and control animals developed responses to the ZIKV infection starting 7-14 days after challenge (Figure 4B), indicative of a primary and/or secondary immune response to infection. Analysis of anti-ZIKV IgG antibodies revealed that 3/4 rVSVΔM-ZprME vaccinated and 0/2 rVSVΔM control animals mounted a response 14-21 days post challenge (Figure 4C) and is suggestive of a recall antibody response to the rVSVΔM-ZprME vaccine. Anti-ZIKV Env-specific cellular IFN-γ activity was not detected in any of the rVSVΔM control or rVSVΔM-ZprME vaccine animals 14 days post-challenge (Table S4), nor was there robust non-specific cellular activation post-challenge (Figure S2). These results demonstrate that the low levels of immunogenicity elicited in some animals by rVSVΔM-ZprME was capable of reducing ZIKV viremia following heterologous virus challenge.
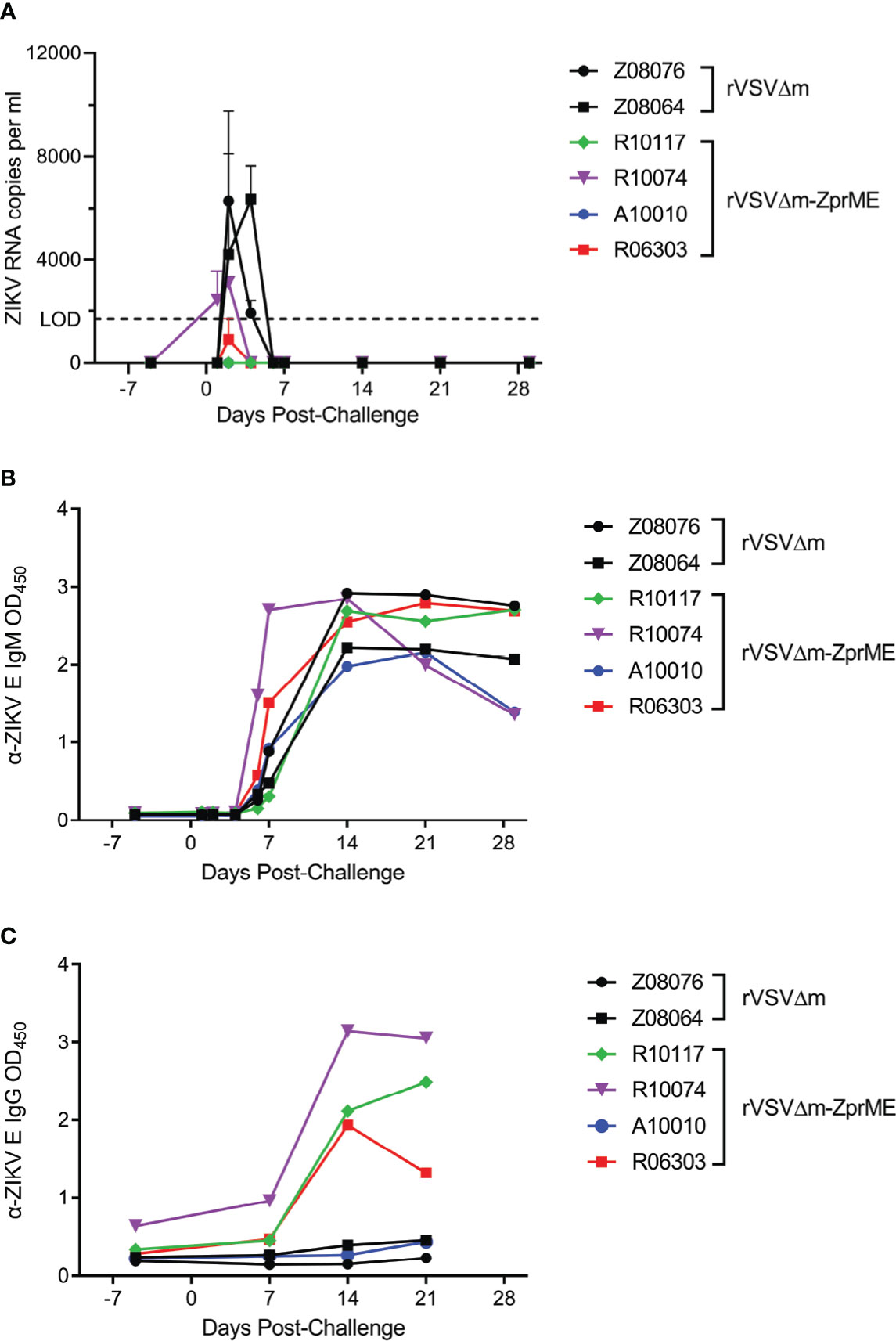
Figure 4 rVSVΔM-ZprME efficacy assessment. (A) ZIKV RNA levels in plasma were measured using a ZIKV prME-specific qRT-PCR assay. The limit of detection (LOD) of the assay (1700) is marked by the dashed line. Anti (α)-ZIKV Env-specific (B) IgM and (C) IgG responses in all animals were measured by ELISA (OD450 nm). Pre-challenge and post-ZIKV challenge samples were diluted 1:100 and run for each assay. OD, Optical Density. (A–C) The data points on the plots represent individual animals for rVSVΔM (black) and rVSVΔM-ZprME (colored) vaccine regimens.
Discussion
An effective Zika virus (ZIKV) vaccine that elicits both robust CD8+ T cell activity and a strong ZIKV-specific neutralizing antibody response is needed to protect against ZIKV infection and disease. Such a public health measure would impact the overall ZIKV infection rate of new epidemics and counter Guillain-Barré syndrome and congenital Zika syndrome (CZS) linked with ZIKV infection. The emergence of SARS-CoV-2 in 2020 shifted vaccine efforts toward the development of a COVID-19 vaccine and stymied ZIKV vaccine clinical trial endeavors. Nevertheless, efforts to develop a ZIKV vaccine are imperative to address the underlying threat of future ZIKV epidemics and several candidates have progressed to clinical trials. This pilot rVSV ZIKV vaccine study expands ZIKV vaccine research by applying the VSV vector vaccine platform. VSV is an attractive viral vector for vaccine development as it can infect a variety of cell types, has routinely displayed a well-tolerated safety profile, and can withstand breaks in supply chain operations (35–38).
Here, we evaluated rVSVΔM-ZprME as a candidate ZIKV vaccine for its safety, immunogenicity, and efficacy in nonhuman primates. We found that pigtail macaques immunized with a prime-boost vaccine regimen did not develop overt malaise or adverse effects to the vaccine or to the experimental infections. This was evidenced by the absence of fever and observable healthy behavior of the animals overall and lack of significant postmortem findings. The animals also exhibited normal weight and serum chemistries throughout the study. Thus, our findings confirm rVSVΔM-ZprME provides a well-tolerated safety profile in pigtail macaques. All animals developed a robust VSV-specific IgG response to the vaccine vector, indicating successful inoculation of the vaccines via the i.m. route. However, there was no substantial production of anti-ZIKV binding antibodies following immunization with the rVSVΔM-ZprME vaccine. Neutralizing antibody responses were detected after the third rVSVΔM-ZprME immunization in 1/4 animals (R10117). This animal had no detectable ZIKV plasma viremia post-challenge, indicating a potential role of neutralizing antibodies in protection by the vaccine. The absence of ZIKV envelope-specific T-cells after three immunizations and post-challenge further indicates that the rVSVΔM-ZprME vaccine did not elicit a cellular immune response. Due to the insufficient sample size of the rVSVΔM group in this pilot study, we did not have the power to evaluate statistical significance between the two vaccine groups. Nevertheless, these findings demonstrate rVSVΔM-ZprME elicits an ineffective humoral response, in contrast to the protective mechanism of rVSV-ZEBOV vaccine-induced antibody responses against Ebola virus (39, 40). The G glycoprotein is the main antigenic glycoprotein on the virion surface of VSV and may have immunodominant epitopes that would account for the antibody responses to the vector and inefficient production of ZIKV prME-specific antibodies. Future ZIKV vaccine studies evaluating a VSV vector in which the G gene has been removed from the vaccine construct would be valuable. However, elimination of the G gene would restrict the rVSV vector to a single round of replication, preventing its spread beyond the primary infected cells, also potentially influencing its immunogenicity.
While the rVSVΔM-ZprME vaccine was demonstrated to be highly effective in mice (29, 41), it was not fully protective in pigtail macaques in this pilot study that was limited by the number of animals. All animals were healthy adults and not considered to be of advanced age. However, we cannot exclude the possibility that the immunogenicity of a rVSV-based vaccine would be more robust in younger, juvenile macaques. Other possible reasons for reduced immunogenicity and efficacy include i) vaccine delivery route (e.g., aerosol or mucosal delivery) and ii) vaccine administration dose. Future studies would entail testing a greater vaccine dose and modification of immunogens expressed by the rVSV vaccine construct, such as VSVΔM expressing ZIKV Env (rVSVΔM-ZEnv) (29). Molecular approaches to the vaccine construct design that would enhance foreign antigen expression and reduce anti-vector immunity (i.e., elimination of the G gene), and consideration of adjuvant strategies could enhance overall vaccine immunogenicity. While Betancourt et al. showed higher anti-ZIKV Ab titers following i.v. injection of rVSVΔM-ZprME compared to i.m. route of delivery in mice, we elected to test the i.m. route of delivery in macaques. From a translational perspective, intramuscular injection is more practical and a single dose of rVSV vaccine delivered i.m. was previously shown to induce rapid antibody responses protecting macaques from Ebola and Marburg disease (6, 42). Here, the rVSVΔM-ZprME vaccine was administered as two intramuscular injections of 1×107 PFU spaced 4 weeks apart and then a higher boost immunization of 2×107 PFU was given at week 8, after which ZIKV NAb responses started to be detected. These administrations were clearly suboptimal to generate a robust immune response; thus, future work would entail testing a higher dose to ensure robust cellular and humoral immune responses are efficiently mounted, ideally achieved from a single dose administration.
Plasma viremia in animals that received the control rVSVΔM vector was higher compared to animals that received the rVSVΔM-ZprME vaccine, indicating the vaccine afforded some level of protection against ZIKV despite the low levels of immunogenicity. The Asian ZIKV FSS virus used for the heterologous challenge has a lower replicative fitness compared to America lineage isolates (34) and thus, causes a less robust infection in macaques; however, the ZIKV FSS strain was selected for this pilot study because it has been shown to cause severe fetal brain lesions in a pigtail macaque pregnancy model (31). Plasma viremia detected in the two control rVSVΔM animals was consistent with a historical study of pregnant pigtail macaques challenged with the same ZIKV FSS13025 (GenBank no. MH368551) strain and route of inoculation (31). De novo generation of ZIKV Env-specific T cells was also absent in the animals that received the control rVSVΔM vector 14 days post-challenge, indicating that low levels of virus replication was insufficient to produce enough antigen to stimulate a T cell response during this timeframe. Based on these findings and combined with inefficient ZIKV-specific immunity and low number of animals, we cannot conclude the rVSVΔM-ZprME vaccine was significantly protective in this pilot study. However, this study demonstrates that rVSVΔM-ZprME was well-tolerated in macaques and future vaccine studies are needed to further enhance the immunogenicity of the vaccine. Thus, the rVSVΔM-ZprME vaccine could provide highly relevant data to advance an rVSV-based ZIKV vaccine toward a clinical path. Toward this end, a successful ZIKV vaccine in non-pregnant adult female macaques, would be easily translatable into a maternal-fetal pigtail macaque model to assess vaccine protection against ZIKV vertical transmission and fetal injury following challenge (31, 43). This application could present major strides in developing a ZIKV vaccine that would not only protect adults against neurologic disease linked with ZIKV infection, but also pregnant women against the adverse effects of CZS (9).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The University of Washington’s Institutional Animal Care and Use Committee (IACUC) approved all experiments (IACUC Protocol Number 4158-10) and were in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and Animal Welfare.
Author contributions
JT-G and MO wrote the manuscript, led the study, generated data and performed data analysis. KV, TL, AM, LK, EF, and RG generated data and performed data analysis. DB and GB provided the vaccine constructs. SW, JA, and NI performed the experimental procedures, collected specimens, and provided care for the animals. RM performed necropsy, specimen collection, and gross and microscopic evaluation of tissues and organs. PE provided statistical guidance. GB, DF, and MG Jr. conceptualized the study and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was funded by the Washington National Primate Research Center and Institute of Translational Health Sciences Ignition award and by funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services P51-OD010425, U42-OD011123, AI143265 and AI145296. The laboratory work in this project was supported by funds from Florida Department of Health 7ZK21.
Acknowledgments
We want to thank InBios, Inc., Seattle, WA for allowing PDSL at WaNPRC to use their ZIKV IgM ELISA and associated spreadsheet for discovery and vaccine work in macaques.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2023.1108420/full#supplementary-material
References
1. Kuno G, Chang GJ. Full-length sequencing and genomic characterization of bagaza, kedougou, and zika viruses. Arch Virol (2007) 152(4):687–96. doi: 10.1007/s00705-006-0903-z
2. Dai L, Song J, Lu X, Deng Y-Q, Musyoki AM, Cheng H, et al. Structures of the zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe (2016) 19(5):696–704. doi: 10.1016/j.chom.2016.04.013
3. Grifoni A, Pham J, Sidney J, O'Rourke PH, Paul S, Peters B, et al. Prior dengue virus exposure shapes T cell immunity to zika virus in humans. J Virol (2017) 91(24). doi: 10.1128/JVI.01469-17
4. Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, et al. Specificity, cross-reactivity, and function of antibodies elicited by zika virus infection. Science (2016) 353(6301):823–6. doi: 10.1126/science.aaf8505
5. Wang L, Wang R, Wang L, Ben H, Yu L, Gao F, et al. Structural basis for neutralization and protection by a zika virus-specific human antibody. Cell Rep (2019) 26(12):3360–3368.e3365. doi: 10.1016/j.celrep.2019.02.062
6. Marzi A, Jankeel A, Menicucci AR, Callison J, O'Donnell KL, Feldmann F, et al. Single dose of a VSV-based vaccine rapidly protects macaques from marburg virus disease. Front Immunol (2021) 12:774026. doi: 10.3389/fimmu.2021.774026
7. Anaya JM, Rodriguez Y, Monsalve DM, Vega D, Ojeda E, Gonzalez-Bravo D, et al. A comprehensive analysis and immunobiology of autoimmune neurological syndromes during the zika virus outbreak in cucuta, Colombia. J Autoimmun (2017) 77:123–38. doi: 10.1016/j.jaut.2016.12.007
8. Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, et al. Guillain-Barre syndrome associated with zika virus infection. Lancet (2016) 387(10026):1482. doi: 10.1016/S0140-6736(16)30058-7
9. Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, et al. Characterizing the pattern of anomalies in congenital zika syndrome for pediatric clinicians. JAMA pediatrics. (2017) 171(3):288–95. doi: 10.1001/jamapediatrics.2016.3982
10. Styczynski AR, Malta J, Krow-Lucal ER, Percio J, Nobrega ME, Vargas A, et al. Increased rates of Guillain-barre syndrome associated with zika virus outbreak in the Salvador metropolitan area, Brazil. PloS Negl Trop Dis (2017) 11(8):e0005869. doi: 10.1371/journal.pntd.0005869
11. Gaudinski MR, Houser KV, Morabito KM, Hu Z, Yamshchikov G, Rothwell RS, et al. Safety, tolerability, and immunogenicity of two zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet (2018) 391(10120):552–62. doi: 10.1016/S0140-6736(17)33105-7
12. Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, et al. Rapid development of a DNA vaccine for zika virus. Science (2016) 354(6309):237–40. doi: 10.1126/science.aai9137
13. Modjarrad K, Lin L, George SL, Stephenson KE, Eckels KH, De La Barrera RA, et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated zika virus vaccine candidate: Phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet (2018) 391(10120):563–71. doi: 10.1016/S0140-6736(17)33106-9
14. Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3'-untranslated region. Am J Trop Med Hyg (2001) 65(5):405–13. doi: 10.4269/ajtmh.2001.65.405
15. Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature (2017) 543(7644):248–51. doi: 10.1038/nature21428
16. Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: Application for treatment of malignant disease. J Virol (2002) 76(2):895–904. doi: 10.1128/JVI.76.2.895-904.2002
17. Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life (2000) 50(2):135–8. doi: 10.1080/713803696
18. Melzer MK, Zeitlinger L, Mall S, Steiger K, Schmid RM, Ebert O, et al. Enhanced safety and efficacy of oncolytic VSV therapy by combination with T cell receptor transgenic T cells as carriers. Mol Ther Oncolytics. (2019) 12:26–40. doi: 10.1016/j.omto.2018.12.001
19. Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med (2016) 374(17):1647–60. doi: 10.1056/NEJMoa1502924
20. Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca suffit!). Lancet (2017) 389(10068):505–18. doi: 10.1016/S0140-6736(16)32621-6
21. Malenfant JH, Joyce A, Choi MJ, Cossaboom CM, Whitesell AN, Harcourt BH, et al. Use of Ebola vaccine: Expansion of recommendations of the advisory committee on immunization practices to include two additional populations - united states, 2021. MMWR Morb Mortal Wkly Rep (2022) 71(8):290–2. doi: 10.15585/mmwr.mm7108a2
22. Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Hu Z, et al. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med (2017) 376(4):330–41. doi: 10.1056/NEJMoa1414216
23. Ao Z, Ouyang MJ, Olukitibi TA, Warner B, Vendramelli R, Truong T, et al. A recombinant VSV-based bivalent vaccine effectively protects against both SARS-CoV-2 and influenza a virus infection. J Virol (2022) 96(18):e0133722. doi: 10.1128/jvi.01337-22
24. Furuyama W, Reynolds P, Haddock E, Meade-White K, Quynh Le M, Kawaoka Y, et al. A single dose of a vesicular stomatitis virus-based influenza vaccine confers rapid protection against H5 viruses from different clades. NPJ Vaccines (2020) 5(1):4. doi: 10.1038/s41541-019-0155-z
25. Furuyama W, Shifflett K, Pinski AN, Griffin AJ, Feldmann F, Okumura A, et al. Rapid protection from COVID-19 in nonhuman primates vaccinated intramuscularly but not intranasally with a single dose of a vesicular stomatitis virus-based vaccine. mBio (2022) 2022:e0337921. doi: 10.1101/2021.01.19.426885
26. O'Donnell KL, Gourdine T, Fletcher P, Shifflett K, Furuyama W, Clancy CS, et al. VSV-based vaccines reduce virus shedding and viral load in hamsters infected with SARS-CoV-2 variants of concern. Vaccines (Basel). (2022) 10(3). doi: 10.3390/vaccines10030435
27. Iyer AV, Pahar B, Boudreaux MJ, Wakamatsu N, Roy AF, Chouljenko VN, et al. Recombinant vesicular stomatitis virus-based west Nile vaccine elicits strong humoral and cellular immune responses and protects mice against lethal challenge with the virulent west Nile virus strain LSU-AR01. Vaccine (2009) 27(6):893–903. doi: 10.1016/j.vaccine.2008.11.087
28. Petersen JM, Her LS, Varvel V, Lund E, Dahlberg JE. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol Cell Biol (2000) 20(22):8590–601. doi: 10.1128/MCB.20.22.8590-8601.2000
29. Betancourt D, de Queiroz NM, Xia T, Ahn J, Barber GN. Cutting edge: Innate immune augmenting vesicular stomatitis virus expressing zika virus proteins confers protective immunity. J Immunol (2017) 198(8):3023–8. doi: 10.4049/jimmunol.1602180
30. O'Connor MA, Tisoncik-Go J, Lewis TB, Miller CJ, Bratt D, Moats CR, et al. Early cellular innate immune responses drive zika viral persistence and tissue tropism in pigtail macaques. Nat Commun (2018) 9(1):3371. doi: 10.1038/s41467-018-05826-w
31. Adams Waldorf KM, Nelson BR, Stencel-Baerenwald JE, Studholme C, Kapur RP, Armistead B, et al. Congenital zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat Med (2018) 24(3):368–74. doi: 10.1038/nm.4485
32. Basile AJ, Ao J, Horiuchi K, Semenova V, Steward-Clark E, Schiffer J. Performance of InBios ZIKV detect 2.0 IgM capture ELISA in two reference laboratories compared to the original ZIKV detect IgM capture ELISA. J Virol Methods (2019) 271:113671. doi: 10.1016/j.jviromet.2019.05.011
33. Robbiani DF, Bozzacco L, Keeffe JR, Khouri R, Olsen PC, Gazumyan A, et al. Recurrent potent human neutralizing antibodies to zika virus in Brazil and Mexico. Cell (2017) 169(4):597–609.e511. doi: 10.1016/j.cell.2017.04.024
34. Esser-Nobis K, Aarreberg LD, Roby JA, Fairgrieve MR, Green R, Gale M Jr. Comparative analysis of African and Asian lineage-derived zika virus strains reveals differences in activation of and sensitivity to antiviral innate immunity. J Virol (2019) 93(13). doi: 10.1128/JVI.00640-19
35. Clarke DK, Hendry RM, Singh V, Rose JK, Seligman SJ, Klug B, et al. Live virus vaccines based on a vesicular stomatitis virus (VSV) backbone: Standardized template with key considerations for a risk/benefit assessment. Vaccine (2016) 34(51):6597–609. doi: 10.1016/j.vaccine.2016.06.071
36. ElSherif MS, Brown C, MacKinnon-Cameron D, Li L, Racine T, Alimonti J, et al. Assessing the safety and immunogenicity of recombinant vesicular stomatitis virus Ebola vaccine in healthy adults: a randomized clinical trial. CMAJ (2017) 189(24):E819–27. doi: 10.1503/cmaj.170074
37. Jusu MO, Glauser G, Seward JF, Bawoh M, Tempel J, Friend M, et al. Rapid establishment of a cold chain capacity of -60 degrees c or colder for the STRIVE Ebola vaccine trial during the Ebola outbreak in Sierra Leone. J Infect Dis (2018) 217(suppl_1):S48–55. doi: 10.1093/infdis/jix336
38. Stein DR, Sroga P, Warner BM, Deschambault Y, Poliquin G, Safronetz D. Evaluating temperature sensitivity of vesicular stomatitis virus-based vaccines. Emerg Infect Dis (2019) 25(8):1563–6. doi: 10.3201/eid2508.190281
39. Marzi A, Engelmann F, Feldmann F, Haberthur K, Shupert WL, Brining D, et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci U S A. (2013) 110(5):1893–8. doi: 10.1073/pnas.1209591110
40. Menicucci AR, Jankeel A, Feldmann H, Marzi A, Messaoudi I. Antiviral innate responses induced by VSV-EBOV vaccination contribute to rapid protection. mBio (2019) 10(3). doi: 10.1128/mBio.00597-19
41. Emanuel J, Callison J, Dowd KA, Pierson TC, Feldmann H, Marzi A. A VSV-based zika virus vaccine protects mice from lethal challenge. Sci Rep (2018) 8(1):11043. doi: 10.1038/s41598-018-29401-x
42. Marzi A, Reynolds P, Mercado-Hernandez R, Callison J, Feldmann F, Rosenke R, et al. Single low-dose VSV-EBOV vaccination protects cynomolgus macaques from lethal Ebola challenge. EBioMedicine (2019) 49:223–31. doi: 10.1016/j.ebiom.2019.09.055
Keywords: Zika virus, nonhuman primate, vaccine, rVSV vector, immune response
Citation: Tisoncik-Go J, Voss KM, Lewis TB, Muruato AE, Kuller L, Finn EE, Betancourt D, Wangari S, Ahrens J, Iwayama N, Grant RF, Murnane RD, Edlefsen PT, Fuller DH, Barber GN, Gale M Jr and O’Connor MA (2023) Evaluation of the immunogenicity and efficacy of an rVSV vaccine against Zika virus infection in macaca nemestrina. Front. Virol. 3:1108420. doi: 10.3389/fviro.2023.1108420
Received: 26 November 2022; Accepted: 07 February 2023;
Published: 28 February 2023.
Edited by:
Matloob Husain, University of Otago, New ZealandReviewed by:
Albert Jonathan Auguste, Virginia Tech, United StatesLogan Banadyga, Public Health Agency of Canada (PHAC), Canada
Copyright © 2023 Tisoncik-Go, Voss, Lewis, Muruato, Kuller, Finn, Betancourt, Wangari, Ahrens, Iwayama, Grant, Murnane, Edlefsen, Fuller, Barber, Gale and O’Connor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer Tisoncik-Go, dGlzb25jaWtAdXcuZWR1; Megan A. O’Connor, bWVnYW5vY0B1dy5lZHU=
 Jennifer Tisoncik-Go
Jennifer Tisoncik-Go Kathleen M. Voss1,2,3
Kathleen M. Voss1,2,3 Thomas B. Lewis
Thomas B. Lewis LaRene Kuller
LaRene Kuller Richard F. Grant
Richard F. Grant Deborah H. Fuller
Deborah H. Fuller Glen N. Barber
Glen N. Barber Michael Gale Jr
Michael Gale Jr Megan A. O’Connor
Megan A. O’Connor