1
Department of Anesthesia, Carver College of Medicine, University of Iowa, Iowa City, IA, USA
2
Interdisciplinary Graduate Programs in Neuroscience and Genetics, University of Iowa, Iowa City, IA, USA
The fruit fly Drosophila melanogaster is an excellent model organism to identify genes and genetic pathways important for learning and memory. However, its small size makes surgical treatment and electrophysiological manipulation technically difficult, hampering the functional analysis of neuronal circuits that play critical roles in memory processing. To circumvent this problem, we developed a unique experimental strategy that uses the temperature-sensitive allele of the Drosophila dynamin gene, shibirets1 (shits1), in combination with the GAL4/UAS expression system. This strategy allows for rapid and reversible perturbation of synaptic neurotransmission in identifiable neurons, and analysis of the behavioral consequences of such manipulation in free-moving animals. Since its introduction in 2001, this GAL4/UAS-shits1 strategy has been widely used to study the neuronal basis of learning and memory. This review focuses on how this strategy has revitalized Drosophila memory research, and contributed to our understanding of dynamic neuronal processes that control various aspects of memory.
An exciting and challenging task in modern neuroscience is to gain a comprehensive understanding of how we learn and remember – at the molecular, cellular and systems levels. As a versatile model organism for behavioral genetics, the fruit fly Drosophila melanogaster has been successfully used to study individual genes and genetic pathways that are important for different aspects of memory (McGuire et al., 2005
). Notably, forward genetic screens for “memory mutants” in Drosophila have resulted in the identification of molecular cascades that are critical to learning and memory, and also remarkably well-conserved among evolutionarily diverse animal species. In order to fully elucidate how these genes and genetic pathways control memory processes, we must understand their roles in the context of the neurons and neuronal circuits responsible for the acquisition, consolidation and retrieval of memories. To link neuronal circuits to memory processes, it is necessary to manipulate the activity of identified neurons within intact animals, and to analyze the direct effects of this manipulation on memory-based behavioral modifications. Unfortunately, it is difficult to perform such experiments in Drosophila melanogaster through surgical or electrophysiological means, mainly because of the small size of this animal. To circumvent this problem, we have developed a strategy that couples the Drosophila dynamin mutant gene, shibire temperature-sensitive1 (shits1), with the GAL4/UAS binary expression system, to rapidly and reversibly suppress synaptic neurotransmission from targeted neurons in intact free-moving animals, through a mild temperature shift (Kitamoto, 2001
, 2002
). Here we explain the principles of this strategy and discuss how it has been utilized to gain a better understanding of neuronal mechanisms of Drosophila learning and memory. We focus in particular on the aversive associative olfactory learning paradigm.
Rapid and Reversible Suppression of Synaptic Neurotransmission by GAL4-Driven Expression of shibirets1
The Drosophila GAL4/UAS system (Brand and Perrimon, 1993
) has been widely used to drive the expression of UAS-linked transgenes in specific sets of identified neurons in order to study their roles in behavior. For example, particular set of neurons can be eliminated by introducing a cell death-inducing gene. Alternatively, functional properties of targeted neurons can be altered by expressing genetically engineered ion channels (to change neuronal excitability) or neurotoxins (to disrupt synaptic transmission). The relationships between particular neurons/neuronal functions and behavior can then be assessed using established assays. These non-conditional gene expression systems, however, have serious limitations with respect to studying the roles of neurons in behavior – due to the inherent plasticity of the nervous system. When a group of neurons is eliminated or their functions are continuously altered, the rest of the nervous system is likely to adjust (either structurally or functionally) to the perturbation. Such homeostatic responses from the nervous system may obscure the direct consequence of the targeted expression of UAS-linked transgenes. A second problem is that most, if not all, GAL4 driver lines exhibit substantial GAL4 activity during development. Thus, even if GAL4-driven expression of a transgene is restricted to a small number of neurons in the adult brain, the behavioral changes observed in adult animals could nevertheless arise as a consequence of undefined expression of the transgene during development. These considerations underscore the importance of the inducibility of transgene expression for analyzing the neuronal basis of behavior using the GAL4/UAS system. Only if a transgene exerts its expected function in the targeted neurons while the behavior is being examined, the observed behavioral changes can be directly link to the neurons expressing these genes.
UAS-linked transgenes can be conditionally expressed using the drug-inducible GeneSwitch GAL4 system (Osterwalder et al., 2001
) or the temperature-dependent TARGET system (McGuire et al., 2004
), and these conditional GAL4 systems are useful for eliminating any developmental effects of transgene expression. However, they are not suitable for analysis of the dynamic neuronal processes responsible for the temporal regulation of memory because of the lag time (several hours) between drug administration/temperature shift, respectively, and transgene expression. In addition, these conditional expression systems need several hours to terminate transgene activity once they are “turned off”. This kind of time resolution limits the usefulness of these systems for the functional study of neurons involved in temporally regulated memory processes.
The Drosophila gene shibire encodes dynamin (Chen et al., 1991
; van der Bliek and Meyerowitz, 1991
), a protein that plays a critical role in synaptic vesicle recycling in nerve terminals (Kosaka and Ikeda, 1983
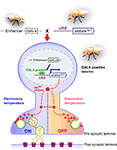
). The dynamin molecule encoded by the mutant gene shibire temperature-sensitive1 (shits1) has a single amino acid substitution in the GTPase domain, making it reversibly temperature-sensitive. shits1 mutants are paralyzed in a temperature-dependent manner, because the functions of shits1-encoded dynamin are impaired at restrictive temperature. This results in depletion of synaptic vesicles at the nerve terminals, and thus leads to suppression of neurotransmission in the neurons responsible for motor control (Figure 1
). The shits1 allele is semidominant regarding the paralytic phenotype (Kim and Wu, 1990
). Therefore, it was expected that would be possible to perturb neurotransmission in a temperature-dependent manner by overexpressing shits1 in the presence of its endogenous wild-type counterpart. We have demonstrated that this is indeed the case, using Cha-GAL4 to drive UAS-shits1 expression in the major excitatory (cholinergic) neurons in the Drosophila nervous system (Kitamoto, 2001
). Adult flies expressing shits1 in cholinergic neurons were apparently normal at permissive temperature, but became paralyzed within 2 min of being moved to the restrictive temperature; activity was immediately regained when the flies were returned to permissive temperature. A more specific behavioral defect was observed when shits1 expression was directed to the photoreceptor cells; both larvae and adults displayed a temperature-dependent defect in light-induced behavior, yet other behaviors were intact (Kitamoto, 2001
). These results demonstrated that UAS-shits1 can be used as a molecular switch for synaptic transmission in targeted neurons (Figure 1
).
Figure 1. Spatially and temporally restricted suppression of neurotransmission using the UAS-shits1 transgene. A GAL4 driver specific to neuronal subsets is crossed to the UAS-shits1 line. Progeny ectopically expressing shits1 in GAL4-positive neurons are raised at permissive temperature. When the temperature is shifted from permissive to restrictive, the shits1 product (temperature-sensitive dynamin) is rapidly inactivated and synaptic vesicle recycling is interrupted. As a result, the GAL4-positive neurons are depleted of synaptic vesicles and synaptic transmission is blocked. Behavioral consequences of spatial and temporal suppression of neurotransmission can be observed in free-moving animals. The shits1 product regains its activity and synaptic vesicles are restored immediately after the animals are returned to permissive temperature.
Drosophila has a remarkable ability to acquire, store and recall memory in various learning and memory paradigms. The most extensively studied of these paradigms is aversive olfactory conditioning (Davis, 2005
). Previous studies indicated that single odor-shock training (single training) induces protein synthesis independent memory that lasts for hours. The memory induced by single training is composed of at least three temporally distinct memory phases: short-term memory (STM), middle-term memory (MTM) and anesthesia-resistant memory (ARM). STM and MTM are labile memories and disrupted by anesthetic treatment such as a cold shock, whereas ARM, which develops during the first 30 min after training, is a form of consolidated memory and resistant to anesthetic treatment. Two protocols that are more intensive than single training, known as mass training and spaced training, are used to study the consolidation of aversive olfactory memory. In mass training, paired odor-shock stimuli are repeatedly presented without intervening rest periods. As in single training, mass training generates STM, MTM and ARM. Spaced training, in which repeated odor-shock stimuli are presented at intervals, is used to generate long-term memory (LTM), a protein synthesis-dependent form of consolidated memory that lasts for at least several days.
Roles of Neurons Intrinsic to the Mushroom Bodies
Which neurons are involved in the regulation of distinct memory phases? How does neuronal activity contribute to information processing relevant to the acquisition, consolidation and retrieval of memories? The UAS-shits1 transgene has been effectively used to gain important insights into these key questions. The primary brain region that has been studied in the context of olfactory memory is the mushroom body (MB) (Heisenberg, 2003
). Previous studies showed that aversive olfactory memory is disrupted in flies with genetically or chemically ablated MBs (Heisenberg et al., 1985
; de Belle and Heisenberg, 1994
), yet the MB-deficient flies are able to discriminate odors and respond to an electric shock. Although these results indicated that MBs play an essential role in olfactory memory, little was known about how MB-intrinsic neurons contribute to memory processing. The MB-intrinsic neurons known as Kenyon cells are broadly classified into three subtypes, the α/β, α′/β′ and γ neurons, according to the projection patterns of their axons (Lee et al., 1999
) (Figure 2
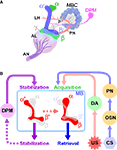
). The use of UAS-shits1 in combination with various GAL4 drivers that are specific to different subsets of Kenyon cells has revealed that these structurally distinct neurons are also functionally diverse in the temporal processing of olfactory memory.
Figure 2. Diverse roles of intrinsic and extrinsic mushroom body-associated neurons in the processing of aversive olfactory memory. (A) The mushroom body and associated neurons in one brain hemisphere are schematically represented (adapted from Armstrong et al., 1998
). The cell bodies of Kenyon cells (MBCs), the mushroom body-intrinsic neurons, are located in the dorsal and posterior cortices of the brain. They extend axons anteriorly through a structure called the peduncle (P). The axons of α/β and α′/β′ neurons bifurcate to form vertical (α and α′) and horizontal (β and β′) lobes. The axons of γ neurons do not bifurcate, and form only a horizontal lobe (γ). The primary olfactory information received by the olfactory neurons is transmitted through the antennal nerve (AN) to the first olfactory center antennal lobe (AL), where the information is processed and further transmitted to the mushroom bodies by the projection neurons (PN). The dorsal paired medial (DPM) neuron extends an axon that branches and terminates in all lobes of the mushroom body. (B) Conditioned stimuli (CS; e. g. odors) and unconditioned aversive stimuli (US; e.g. electric shock) are simultaneously presented to flies. The olfactory information received by the olfactory neurons (OSN) is conveyed to the mushroom bodies (MB) through the first-order interneuron, the projection neurons (PN). The neuronal circuits that transmit the aversive sensory information include dopaminergic neurons (DA). The information generated by CS and US converges at the MB, where aversive olfactory memory is formed. Neurotransmission from DPM neurons to the α′/β′ neurons, as well as that from the α′/β′ neurons, contributes to the stabilization of memory. Retrieval of both protein synthesis-independent, short-lasting memory and protein synthesis-dependent, long-term memory (LTM) require neurotransmission from the α/β neurons.
The α/β neurons, which constitute approximately 50% of the Kenyon cells, are labeled in the majority of the “MB-specific GAL4” lines (Aso et al., 2009
). When UAS-shits1 was preferentially expressed in the α/β neurons and neurotransmission from these neurons was temporally blocked by maintenance at the restrictive temperature only during single training, the flies avoided the shock-associated odor during the test period, showing that memory is formed, stored and retrieved under these conditions. Similarly, inhibiting α/β neuron output between the training and test periods did not significantly affect the memory-based avoidance behavior during testing. These results indicate that neurotransmission from the α/β neurons is dispensable for the acquisition and storage of olfactory memory. In marked contrast, blocking neurotransmission only during the test period (30 min to 3 h after training) eliminated the avoidance behavior, demonstrating that neurotransmission from the α/β neurons is required for the retrieval of aversive olfactory memory (Dubnau et al., 2001
; McGuire et al., 2001
). An important implication of this finding is that any aversive olfactory memory formation (presumably as changes in structures and/or functions of cellular components) occurs at or upstream of the α/β output synapses of the neuronal circuits involved in the processing memory. Memory is manifested as behavioral alterations in response to learned unconditioned stimuli (US) through neurotransmission from the α/β neurons in the MBs.
Either single or mass training can generate the consolidated form of memory known as ARM. Are the α/β neurons also required for ARM? ARM can be measured separately from STM and MTM by exposing animals to a cold shock 1 h after training, because labile STM and MTM are eliminated by this treatment. Blocking the output of the α/β neurons using UAS-shits1 has also been shown to impair ARM, indicating that neurotransmission from the α/β neurons is necessary for the retrieval of three types of short-lasting, protein synthesis-independent memory (Isabel et al., 2004
). In contrast to ARM, LTM is a protein synthesis-dependent, consolidated form of memory that is generated only after spaced training. Recent optical imaging studies demonstrated that spaced training, but not single or mass training, results in a robust increase in calcium influx into the α lobes, in a protein synthesis-dependent manner (Yu et al., 2006
). In addition, analysis of the alpha-lobes-absence (ala) mutants, which lack either the two vertical lobes (α and α′) or two of the three horizontal lobes (β and β′), suggested that LTM depends on the vertical lobes (Pascual and Preat, 2001
). These results suggested that neurons forming vertical lobes, in particular those of the α lobe, are important for LTM. Experiments with UAS-shits1 provided data in support of this notion. Indeed, the retrieval of memory 24 h after spaced training is significantly impaired when neurotransmission from the α/β neurons is blocked only during testing, indicating that the output of the α/β neurons is required for the retrieval of LTM. As in the case of STM, a memory trace for LTM is likely formed, at least partly, at or upstream of the α/β neuron output synapses (Isabel et al., 2004
).
The original model for aversive olfactory memory proposes that pairing conditioned stimuli (CS) with US leads to the sequential formation of STM and MTM, and that the latter is then processed into either ARM and/or LTM, depending on the training protocol (DeZazzo and Tully, 1995
). In this model, both ARM and LTM derive from MTM, and ARM and LTM coexist for 24 h after spaced training. Interestingly, however, it was shown that spaced training that produces LTM leads to disappearance of ARM in the ala mutants lacking vertical lobes (Isabel et al., 2004
). In addition, the experiments using UAS-shits1 indicate that ARM and LTM involve the same α/β subset of the MB neurons. These results suggest that ARM and LTM are mutually exclusive. Furthermore, normal ARM can be detected in mutants defective for STM (rut) or MTM (amn). Based on these collective results, it has been proposed that ARM is processed largely independently of the sequential STM-MTM-LTM pathway, but stored in the common group of neurons in the MBs (Isabel et al., 2004
).
With respect to aversive olfactory memory, the functional significance of the γ neurons, which account for approximately 30% of Kenyon cells (Aso et al., 2009
), has thus far not been shown. The α′/β′ neurons are relatively minor components of the MBs, comprising approximately 20% of the Kenyon cells (Aso et al., 2009
). Although they extend axons parallel to those of the α/β neurons, experiments using UAS-shits1 demonstrated that the roles of the α′/β′ neurons in olfactory memory processing are distinct from those of the α/β neurons. When neurotransmission from the α′/β′ neurons was blocked only during the test period by introducing UAS-shits1 in combination with GAL4 drivers that preferentially label the α′/β′ neurons, the memory-based avoidance behavior during the test period was not affected. This result shows that, unlike neurotransmission from the α/β neurons, that from α′/β′ neurons is dispensable for the retrieval of olfactory memory. Interestingly, however, when the α′/β′ neurons were blocked either during or after training, olfactory memory was severely impaired. Thus, output from the α′/β′ neurons appears to be required for the acquisition and stabilization of olfactory memory (Krashes et al., 2007
). An exciting line of future investigation will focus on how neurotransmission from the α′/β′ neurons contributes to the processing the memory that eventually depends on output of the α/β neurons for its retrieval.
Roles of Neurons Extrinsic to the Mushroom Bodies
The Dorsal Paired Medial (DPM) neurons are two large neurons that project extensively to all the lobes and the base of the peduncle of MB neurons. They were first identified as primary cells in the adult brain that express the putative neuropeptide encoded by the amnesiac gene (amn) (Waddell et al., 2000
), whose product plays an important role in stabilizing memory (Quinn et al., 1979
). amn mutants can learn and form STM, but their memory decays abnormally rapidly, within 30–60 min after training, and this results in severe defects in MTM. Because the amn memory defect is rescued by expressing the wild-type amn gene product preferentially in DPM neurons, these neurons are considered to be the site at which amn acts to stabilize olfactory memory. Results obtained from UAS-shits1 experiments revealed the significance of DPM neuron output in memory stabilization. When neurotransmission from DPM neurons was constantly blocked (during the training, storage and test periods), the flies displayed defects in memory that could be measured 1 h after training, as was the case in amn mutants. However, temporally blocking neurotransmission from DPM neurons only during the training or test period did not affect olfactory memory. Therefore, DPM neuron output is dispensable for memory acquisition and retrieval. In contrast, blocking DPM output for 30 min during the storage period (between training and testing) resulted in a significant impairment of 1-h memory, demonstrating that the DPM neuron is critical for stabilizing memory (Waddell et al., 2000
).
Considering that neurotransmission from the α′/β′ neurons is similarly required to stabilize memory during storage, and that DPM neurons heavily innervate the MB lobes – including the α′ and β′ lobes – it is possible that direct information flow from DPM neurons to the α′/β′ neurons is essential to stabilizing the newly formed memory. This possibility was tested by using flies that express an isoform of Drosophila Down syndrome cell adhesion molecule (Dscam17-2) in DPM neurons. The expression of Dscam17-2 in DPM neurons affected their development such that projections of DMP neurons to the α, β and γ lobes are significantly reduced, but those to the α′ and β′ lobes are relatively intact. In these genetically engineered flies with limited DPM projections to the MB α′/β′ neurons, aversive olfactory memory was not significantly affected. Importantly, a temporal block in DPM neuron output to the α′/β′ neurons, but not to most α/β and γ neurons, was enough to induce the amn-like memory phenotype (Krashes et al., 2007
). These results strongly suggest that stabilizing memory during the storage period mainly depends on neurotransmission from DPM neurons to a subset of MB neurons – the α′/β′ neurons.
It has been proposed that aversive olfactory memory is formed in the MBs, where sensory information concerning conditioned odor stimuli and unconditioned aversive stimuli converges, and that this establishes an association between CS and US (Davis, 2005
). Although significant progress has been made in understanding the molecular and neuronal mechanisms responsible for olfactory information processing (Hallem and Carlson, 2004
), little is known about how unconditioned electric shocks are sensed as aversive stimuli, and which neurons are involved in conveying US information to the MBs. In other organisms, monoaminergic interneurons are thought to transmit such information. In Drosophila, a study investigating mutants for dopa decarboxylase, which encodes an enzyme involved in the synthesis of both dopamine and serotonin, implicated these neurotransmitters in aversive olfactory learning (Tempel et al., 1984
). However, this result could not be reproduced by another study (Tully, 1987
). Given the controversial nature of these findings, UAS-shits1 was used to revisit this issue (Schwaerzel et al., 2003
). Tyrosine hydroxylase (TH) is the rate limiting enzyme for dopamine and expressed specifically in dopaminergic neurons. TH-GAL4, which was generated using regulatory DNA of the TH gene (Friggi-Grelin et al., 2003
), was used to direct UAS-shits1 to dopaminergic neurons. Although blocking neurotransmission from dopaminergic neurons before and during testing did not affect memory, blocking them only during the training period severely impaired aversive olfactory memory (Schwaerzel et al., 2003
). Interestingly, dopamine transmission was not required for appetitive olfactory learning using sugar as the US. These results provide strong support for the notion that dopaminergic neurons are part of the neuronal circuit for aversive US in Drosophila. There are approximately 120 dopaminergic neurons in the adult fly brain, and some of which extensively innervate the MBs (Riemensperger et al., 2005
). Future studies using GAL4 lines specific to a subset of brain dopaminergic neurons should reveal functional differences between dopaminergic neurons within the brain, and identify the particular neuronal circuit involved in aversive olfactory memory.
It is almost 30 years since the genetic analysis of learning and memory was initiated using Drosophila melanogaster as a model system. Since then, considerable progress has been made in identifying and characterizing genes and genetic interactions that are important for memory processes. Currently the Drosophila memory research field is aiming to attain a comprehensive understanding of the mechanisms that underlie learning and memory, by an approach that integrates molecular, cellular and systems analyses. Since its introduction to Drosophila behavioral research, the UAS-shits1 transgene has contributed significantly to the studies of temporal and spatial regulation of the neuronal activities responsible for learning and memory. Figure 2
summarizes the findings of the UAS-shits1 studies regarding the roles of intrinsic and extrinsic neurons of the MBs in different aspects of aversive olfactory memory. This knowledge provides a strong foundation for detailed functional and structural analyses in the future. Recently Pfeiffer et al. (2008)
have generated thousands of transgenic Drosophila lines in which GAL4 expression is directed to distinct (most of them small) subsets of cells in the adult brain (Pfeiffer et al., 2008
). In the case of the MBs in particular, an increasing number of GAL4 enhancer-trap strains have been identified that label specific subsets of its intrinsic and extrinsic neurons (Tanaka et al., 2008
). These new GAL4 lines will be used in combination with UAS-shits1, as well as other equally useful UAS-linked effector transgenes, to enable the conditional activation of identifiable neurons (Lima and Miesenbock, 2005
; Schroll et al., 2006
) and the optical imaging changes in the levels of important messengers including Ca2+, H+ and cAMP (Yu et al., 2004
). The same approach should be applied to other learning paradigms in Drosophila that involve sensory stimuli and brain neurons distinct from those involved in aversive olfactory learning. With a handful of valuable molecular and genetic tools, Drosophila research on memory and learning is poised to continue providing essential knowledge of the basic principles of learning and memory in the coming years.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the National Institute of Health grant MH62684.
Pfeiffer, B. D., Jenett, A., Hammonds, A. S., Ngo, T. T., Misra, S., Murphy, C., Scully, A., Carlson, J. W., Wan, K. H., Laverty, T. R., Mungall, C., Svirskas, R., Kadonaga, J. T., Doe, C. Q., Eisen, M. B., Celniker, S. E., and Rubin, G. M. (2008). Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 105, 9715–9720.