- Zhejiang Cancer Hospital, Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences, Hangzhou, Zhejiang, China
Radiation-induced intestinal injury (RIII) poses a significant clinical challenge for patients undergoing pelvic or abdominal radiotherapy, characterized by dual features of acute symptoms (diarrhea, abdominal pain, rectal bleeding) and chronic complications (stricture, fistula, chronic pain), profoundly impacting quality of life. Despite high clinical prevalence, the molecular and cellular mechanisms underlying RIII remain poorly defined, hindering therapeutic development. Current diagnostic modalities (imaging, endoscopy) lack sensitivity and specificity for early detection or real-time monitoring. While biomarkers offer promise for non-invasive assessment and prognosis, existing candidates face limitations in reproducibility and clinical applicability. Therapeutic options, ranging from pharmaceuticals to surgery, show variable efficacy, underscoring the need for optimized strategies. This review systematically explores RIII pathogenesis, emphasizing radiation-induced immune dysregulation, epigenetic alterations, and gut microbiota dysbiosis. We discuss potential biomarkers, such as miRNA, fatty acid binding proteins (FABPs), etc. We categorize therapies into radioprotectors (pre-radiation use) and radiomitigators (post-radiation intervention), highlighting natural plant-derived compounds and traditional Chinese medicine (TCM) for their multi-target effects, alongside emerging approaches like stem cell and microbiota transplantation, with discussions on their therapeutic potential and clinical challenges. Crucially, we exclusively summarize recent clinical translation advances to accelerate drug development. Through critical evaluation of evidence, we propose future directions to refine risk stratification, enable timely intervention, and improve long-term outcomes for irradiated patients. This integrative analysis aims to bridge translational gaps and prioritize research avenues for RIII management.
1 Introduction
Radiation therapy stands as one of the most effective modalities for cancer treatment, applicable to over half of all cancer patients (1). However, ionizing radiation (IR) can induce varying degrees of damage to the human body. The small intestine, with its rapid cellular turnover, is highly sensitive to radiation. Gastrointestinal injury typically occurs when the entire intestinal tract is exposed to radiation doses exceeding 5 Gray (Gy) (2). Consequently, clinical radiotherapy targeting abdominal/pelvic tumors frequently leads to radiation-induced intestinal injury (RIII). Currently, over 75% of patients develop acute RIII within hours to weeks post-irradiation, manifesting as symptoms such as bleeding, pain, or diarrhea (3, 4). Furthermore, 5%–20% of patients progress to chronic RIII months or years later, characterized by irreversible tissue damage, intestinal fibrosis, or strictures (3, 4). In severe cases, RIII may result in intestinal necrosis, perforation, or even death (5, 6), underscoring its significant impact on long-term cancer survivors’ quality of life and the urgent need for effective management strategies.
While advanced imaging modalities such as abdominopelvic computed tomography (CT), magnetic resonance enterography (MRE), and confocal laser endomicroscopy (CLE) provide critical insights into morphological changes, their reliance on anatomical visualization restricts early functional assessment and prognostication (7, 8). With the in-depth study of the pathogenesis, more and more potential biomarkers have been discovered, which may become non-invasive assessment and prognosis prediction methods for RIII. The emergence of molecular diagnostics has renewed interest in identifying biomarkers capable of non-invasive detection and risk stratification. Preclinical and early clinical studies have identified promising candidates, including microRNAs, circulating exosomes (EVs), and gut microbiome diversity, which correlate with disease severity and treatment response.
Despite these advances, current management strategies remain predominantly palliative, relying on anti-diarrheal agents, corticosteroids, and surgical interventions that fail to address the underlying pathophysiology of RIII (7). This therapeutic gap highlights the urgent need for precision medicine frameworks that integrate biomarker-driven stratification and mechanism-targeted therapies (6).
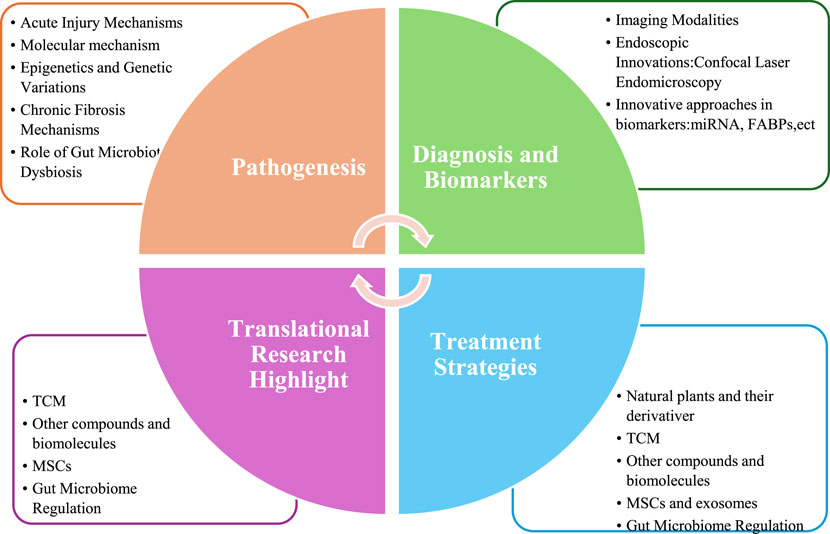
Recent preclinical and clinical studies have achieved significant progress, demonstrating that natural plants and their derivatives, traditional Chinese medicine (TCM), drug repurposing, mesenchymal stem cells (MSCs), and gut microbiome regulation can all serve as potential treatments for RIII. Some preclinical studies have laid the groundwork for translating experimental therapies into clinical practice (9–16). In this review, we synthesize the current understanding of RIII pathogenesis, evaluate emerging biomarkers with diagnostic and prognostic potential, and critically assess innovative treatment modalities. By integrating molecular insights with clinical translation, we aim to outline future directions for optimizing RIII management and improving long-term patient outcomes. The main content of the entire document consists of the following four parts (Pathogenesis, Diagnosis and Biomarkers, Treatment Strategies and Translational Research Highlight) as shown in Figure 1, and the last part is about the future exhibition booths (not included in Figure 1).
2 Pathogenesis
2.1 Acute injury mechanisms
RIII is a complex process that begins with intestinal cell damage and involves an interplay of direct DNA damage, oxidative stress, and dysregulated immune responses (Figure 2).
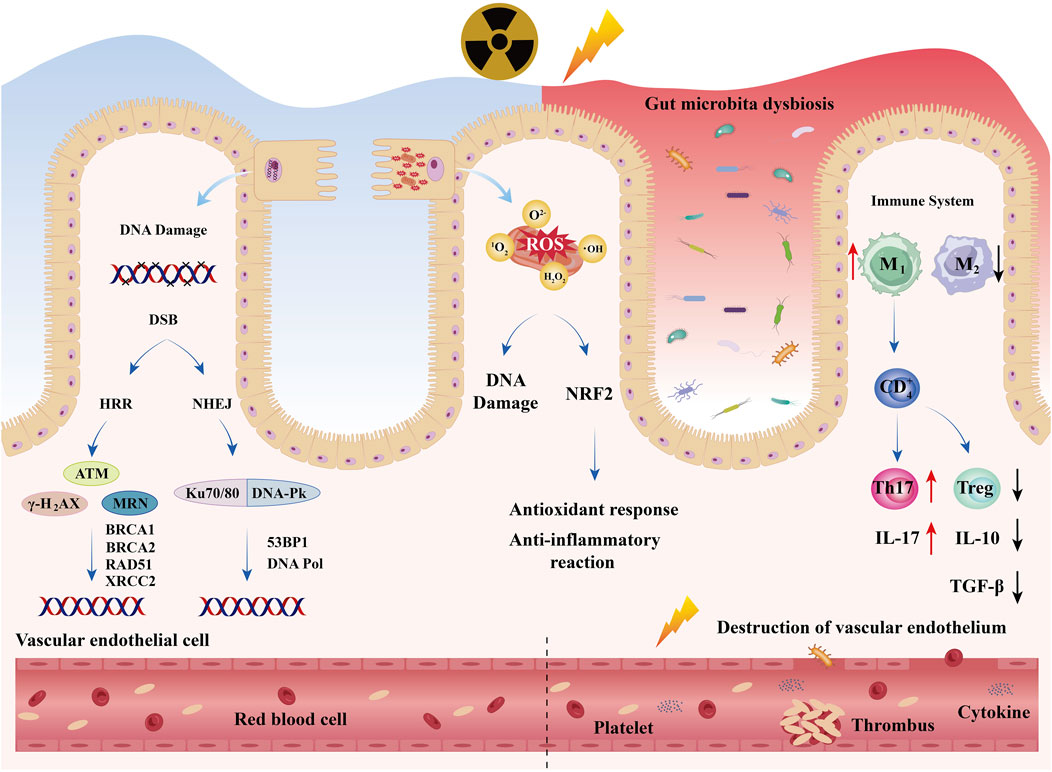
Figure 2. Acute Injury Mechanisms: RIII is a complex process that begins with intestinal cell damage and involves an interplay of direct DNA damage, oxidative stress, and dysregulated immune response. Meanwhile, radiation also causes dysbiosis of the intestinal microbiota and damage to vascular endothelium.
IR induces the formation of clustered DNA lesions, encompassing double-strand breaks (DSBs), single-strand breaks (SSBs), and base modifications (17). These lesions trigger the activation of the DNA damage response (DDR) pathway, which involves a cascade of molecular events including sensor proteins (MRN [MRE11-RAD50-NBS1] complex), signal transducers (ATM [Ataxia-Telangiectasia Mutated], ATR [ATM and Rad3-related], DNA-PKcs [DNA-dependent protein kinase catalytic subunit]), and downstream effectors (P53, CHK1/2 [Checkpoint kinase 1/2]). In the absence of proper repair mechanisms, these lesions may result in irreversible cellular damage or programmed cell death (18). The molecular mechanisms underlying these processes will be comprehensively elucidated in subsequent sections.
Concurrently, oxidative stress, mediated by reactive oxygen species (ROS), exacerbates DNA damage and disrupts cellular homeostasis (19). Ionizing radiation(IR) induces the radiolysis of water molecules, generating hydroxyl radicals (·OH), superoxide anions (O2−), and hydrogen peroxide (H2O2), which subsequently attack cellular components including lipids, proteins, and nucleic acids (20).
Dysfunction of the immune system is also a critical factor in the initiation and progression of RIII (21, 22). The intestinal mucosa and submucosa harbor numerous innate immune cells, including macrophages and natural killer cells (23). Macrophages bridge innate and adaptive immune responses and can undergo polarization in response to IR, differentiating into pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes (24). In murine models, IR promotes the activation of pro-inflammatory M1 macrophages while reducing anti-inflammatory M2 macrophages. Polarized macrophages secrete cytokines that activate T cells and induce adaptive immune responses. Immature cluster of differentiation four positive T lymphocyte (CD4+ T) cells can directly differentiate into helper T (Th) cell subsets (Th1, Th2, Th17) and regulatory T cells (Treg) (25).
The dysregulation of the Th17/Treg equilibrium represents a fundamental characteristic of inflammatory diseases (26). This pathological phenomenon has been extensively documented in total abdominal irradiation murine models, where advanced analytical techniques including flow cytometry and immunofluorescence staining have consistently demonstrated that ionizing radiation significantly upregulates intestinal Th17 cell populations while concurrently downregulating Treg cell populations (27). Th17 cells actively secrete pro-inflammatory cytokines, particularly interleukin (IL)-17, which subsequently induces the production of IL-6 and tumor necrosis factor (TNF)-α through positive feedback mechanisms. Conversely, Treg cells are responsible for the secretion of anti-inflammatory cytokines, specifically IL-10 and transforming growth factor (TGF)-β. These molecular events collectively contribute to the breakdown of the mucosal barrier integrity, enhancement of intestinal permeability, and facilitation of bacterial translocation, ultimately resulting in the amplification of inflammatory cascades and exacerbation of tissue damage.
2.2 Molecular mechanism
Serval study (28, 29) reveals that most IR-induced genes depend on a limited number of sensors and pathways, especially the DNA damage-activated kinase ATM, DNA-PK, the tumor suppressor P53, transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and the ROS-induced transcription factor nuclear factor erythroid 2-related factor 2 (NRF2). Furthermore, X-ray cross-complementing (XRCC) genes represent one of the most extensively investigated categories within the DNA repair gene family. The intricate mechanisms associated will be comprehensively elucidated in the subsequent sections.
Primarily, DSBs are primarily repaired through two distinct mechanisms: homologous recombination repair (HRR) and non-homologous end joining (NHEJ). DNA-PKcs functions as a crucial kinase in the NHEJ pathway, orchestrating both end processing and the assembly of the repair complex (30, 31). The DNA-PK complex is structurally composed of three key components: the catalytic subunit (DNA-PKcs) and two regulatory subunits (Ku70 and Ku80). Upon the occurrence of DSBs within a cell, the Ku70/Ku80 heterodimer exhibits rapid recognition capability and subsequently binds to the damaged DNA termini. This Ku complex then facilitates the recruitment of DNA-PKcs to the site of DNA damage, thereby assembling the complete and functional DNA-PK complex (32). Following its activation, DNA-PKcs initiates the DNA repair cascade through a series of molecular events, including autophosphorylation and the phosphorylation of various downstream protein substrates.
2.2.1 ATM
Recently, an investigation of RIII has revealed that the deubiquitinase USP15 serves as a pivotal regulator of ATM kinase stability. USP15 facilitates the stabilization of ATM through the deubiquitination of K48-linked polyubiquitin chains, thereby augmenting DNA damage repair (33). As we all know, ATM serves as a pivotal kinase in the DDR.
Upon DNA damage, ATM undergoes autophosphorylation and dissociates into active monomeric forms (31, 34, 35). It coordinates the DDR by phosphorylating diverse downstream targets, including:
a. DNA Repair: ATM kinase orchestrates the equilibrium between breast cancer type 1 susceptibility protein (BRCA1)-mediated HRR and TP53-binding protein 1(53BP1)-mediated NHEJ via the ATM/ATM and Rad3-related (ATR)- checkpoint kinase 1(Chk1) signaling axis (36–39).
b. Cell Cycle Regulation: ATM activates checkpoint kinases CHK2 and P53 to induce cell cycle arrest, allowing time for DNA repair. If damage is irreparable, ATM promotes apoptosis to prevent transmission of damaged DNA (40, 41).
c. Transcriptional Regulation: ATM kinase orchestrates the induction of interferon (IFN)-related gene expression through the activation of interferon regulatory factor 1 (IRF1) and concurrently stimulates the NF-κB signaling pathway, thereby modulating inflammatory and immune responses (42, 43). It also suppresses stimulator of interferon genes (STING)-mediated chronic IFN responses to balance immune homeostasis (29).
d. Antioxidant Response: ATM activates transcription factors such as NRF2 to counteract oxidative stress (44).
2.2.2 P53
The P53 protein, encoded by the TP53 gene, is one of the most critical tumor suppressor proteins within cells, renowned as the “Guardian of the Genome.”
DNA damage in intestinal epithelial cells can impede the ubiquitination process of P53, resulting in its activation and subsequent formation of P53 tetramers. These tetramers translocate to the nucleus, where they bind to specific DNA sequences and initiate the transcription of genes associated with apoptosis and cell cycle regulation (29). Notably, the radiation response of intestinal stem/progenitor cells exhibits differential regulation of both apoptosis-dependent and apoptosis-independent mechanisms mediated by P53 (45, 46). Specifically, the cell cycle inhibitory protein P21 plays a crucial role in preventing the proliferation of cells harboring mutated or damaged DNA by inducing G1 phase cell cycle arrest and initiating DNA repair processes (45). In contrast, P53 can also promote apoptosis through the transcriptional activation of pro-apoptotic genes, including P53 upregulated modulator of apoptosis (Puma), bcl-2-associated X protein (Bax), and oxidative stress-activated apoptosis (Oxa) (46).
The normal maintenance or post-injury regeneration of rapidly renewing tissues (including the gastrointestinal tract) relies on specific stem cell subpopulations (47). Studies demonstrate that PUMA gene-deficient mice exhibit greater protection of colon crypt base columnar cells (CBCs) against IR-induced apoptosis compared to P53-deficient mice (48, 49). Enhanced DNA repair and genomic stability mediated by P2 may further promote effective regeneration while preventing P53 activation-induced depletion of adult stem cells (50). Currently, no effective interventions exist against acute radiation-induced gastrointestinal injury. Small-molecule PUMA inhibitors may represent a novel therapeutic approach.
2.2.3 NF-κB
NF-κB, a family of highly conserved transcription factors, serves as a pivotal regulator in diverse biological processes, including immune responses, inflammatory reactions, cell survival, proliferation, and oxidative stress modulation (51). The activation mechanism of NF-κB is dependent on the phosphorylation of the IκB kinase (IKK) complex. Specifically, NF-κB facilitates the transcriptional activation of proinflammatory genes, such as TNF-α, IL-1β, IL-6, and cyclooxygenase (COX)-2, consequently orchestrating the intestinal inflammatory cascade triggered by IR (52).
Numerous investigations have systematically examined the impact of NF-κB inhibition on radiosensitivity across various experimental models (52–55). Particularly noteworthy are the findings from studies utilizing IKKβ-deficient intestinal epithelial cells, which demonstrated enhanced apoptotic activity associated with increased expression and activation of the tumor suppressor protein P53, concomitant with reduced levels of pro-survival Bcl-2 family members. These comprehensive results highlight the essential function of the NF-κB signaling pathway in maintaining intestinal epithelial integrity against radiation-induced cellular apoptosis in vivo, thereby establishing IKKβ as a crucial therapeutic target for the development of radioprotective interventions in the gastrointestinal tract (56).
Intriguingly, both ATM and DNA-PK exhibit dual regulatory capabilities, facilitating pro-apoptotic responses through P53 stabilization while simultaneously promoting anti-apoptotic responses via NF-κB activation (57). Furthermore, research has demonstrated that NF-κB can modulate the expression of the pro-apoptotic protein Bax, which is typically induced by P53 in colon cancer cells (58). These two transcription factors, NF-κB and P53, predominantly exhibit antagonistic functions (57), potentially competing for transcriptional co-activator interactions (57, 59). Notably, in certain cellular contexts, NF-κB appears to be essential for mediating the apoptotic effects initiated by P53 (60), highlighting the intricate and context-dependent nature of the interplay between these two signaling pathways.
2.2.4 STING
STING, a crucial intracellular signaling molecule, serves as a pivotal regulator in the innate immune system. IR induces DNA damage, which initiates the recognition of cytosolic DNA by cyclic GMP-AMP synthase (cGAS), thereby activating STING. Following this activation, TANK-binding kinase 1 (TBK1) phosphorylates IRF3, culminating in the expression of IFN-β. This cascade constitutes the cGAS-STING signaling pathway, which contributes to chronic inflammation and tissue damage, thereby exacerbating the pathological progression of RIII (61).
2.2.5 NRF2
NRF2, a pivotal transcription factor within the Cap’n’collar (CNC) family, serves as a master regulator of cellular antioxidant stress responses, detoxification metabolism, mitochondrial biogenesis, and autophagy, thereby playing an indispensable role in radiation protection (62). ROS initiate the dissociation of NRF2 from the Kelch-like ECH-associated protein 1 (KEAP1) complex, resulting in its activation (63). Upon activation, NRF2 orchestrates the transcriptional upregulation of downstream antioxidant genes, including NAD(P)H quinone oxidoreductase 1 (NQO1), superoxide dismutase 2 (SOD2), and glutathione (GSH), which collectively facilitate the repair of oxidatively damaged DNA, lipids, and proteins. Additionally, NRF2 directly modulates gene expression by binding to the antioxidant response element (ARE) within their promoter regions (64, 65).
In NRF2- deficient mice (61), intestinal tissues demonstrated markedly increased levels of cGAS, phosphorylated STING (pSTING), phosphorylated TBK1 (pTBK1), phosphorylated IRF3 (pIRF3), and IFN-β, while NRF2 overexpression downregulated these protein expressions. NRF2 knockdown in human intestinal epithelial cells (HIEC) led to activation of the cGAS-STING pathway and exacerbated DNA damage; in contrast, NRF2 overexpression or Pirin overexpression mitigated these effects. This study demonstrates that NRF2 directly upregulates Pirin gene expression through binding to ARE, consequently inhibiting the cGAS-STING pathway and attenuating RIII.
2.2.6 XRCC
Polymorphisms in XRCC genes may impair their ability to repair radiation-induced DNA damage, thereby contributing to the development of radiation enteropathy. Studies have demonstrated that specific XRCC variants are associated with reduced DNA repair efficiency and increased cellular susceptibility to radiation exposure.
Notably, the XRCC1 399 Arg/Gln polymorphism has been shown to possess significant predictive value for severe acute adverse effects induced by radiotherapy, particularly in cases of acute mucositis and gastrointestinal toxicity (66). Conversely, the XRCC1 194 Arg/Trp polymorphism exhibits a protective effect in women with cervical or endometrial cancer undergoing radiotherapy (67). Furthermore, gene silencing of XRCC2 leads to diminished repair of radiation-induced cellular damage, resulting in G2/M phase arrest and enhanced apoptosis (68). Additionally, the XRCC3 IVS5-14 polymorphic allele is significantly correlated with an elevated risk of late radiotherapy reactions, including fibrosis (67).
2.3 Epigenetics and genetic variations
Accumulating evidence highlights the pivotal roles of radiation-induced epigenetic regulation and biological processes in the pathogenesis and progression of RIII. The epigenetic system encompasses alterations in DNA methylation and histone modifications, enabling heritable gene silencing without modifying the underlying coding sequences. The development of “epigenetic therapies” shows considerable promise, particularly through the use of inhibitors targeting enzymes that regulate epigenetic modifications, such as DNA methyltransferases (DNMTs) and histone deacetylases (HDACs), which have exhibited promising radioprotective effects (69). Moreover, the reversibility of these processes and their involvement of multiple dysregulated enzymes suggest that inhibiting histone-modifying enzyme activity and modulating their levels represent viable therapeutic strategies for mitigating RIII.
IR exposure induces DNA methylation modifications, which in turn alter and regulate the expression of associated genes and proteins. This process runs concurrently with classical biological responses to radiation, ultimately resulting in tissue and organ damage (70). However, the expression of DNMTs in response to radiation exposure is influenced by various factors, including cell type, radiation dose, tissue type, and organism sex (71). To date, there is a lack of relevant research on the role of DNMTs in radiation-induced enteropathy; hence, this review does not address DNA methylation in this context. The subsequent sections elucidate the fundamental mechanisms of histone modifications and other critical protein post-translational modifications (PTMs), as well as their roles in RIII.
2.3.1 Histone modifications
Radiation acutely inhibits histone acetylation, leading to chromatin condensation and transcriptional repression. Multiple studies (72, 73) have demonstrated that histone deacetylase inhibitors (HDACi) exert mitigating effects on RIII.
In experimental studies (72) examining the protective efficacy of 7,8-diacetyloxy-4-methylthiocoumarin (DAMTC) against RIII in murine models subjected to total body irradiation (TBI), DAMTC administration at 24 h post-TBI activated anti-apoptotic signaling pathways mediated by the Bcl-2 protein family, consequently mitigating apoptosis in intestinal crypt progenitor/stem cells (ICPS) and villus stromal cells. This intervention facilitated both structural reconstruction and functional restoration of absorptive capacity. Furthermore, DAMTC attenuated TBI-induced DNA damage accumulation, suppressed cell cycle arrest, and conferred radioprotection to ICPS cells through the synergistic activation of anti-P53 and anti-P21 signaling pathways. Additionally, DAMTC enhanced crypt stem cell proliferation, upregulated antioxidant defense mechanisms, inhibited TBI-induced lipid peroxidation, and induced polarization of intestinal macrophages toward anti-inflammatory M2 phenotypes. These findings collectively demonstrate that DAMTC ameliorates RIII through epigenetic regulation via protein acetylation, initiating anti-apoptotic and pro-survival pathways that promote ICPS cell proliferation and maintenance.
In a distinct investigation (73) conducted on 10–12-week-old male C57BL/6 mice, 15 Gy gamma radiation was delivered to the whole abdomen, followed by intravenous trichostatin A (TSA) (150 ng/kg) administration after 1 h and 24 h. It found that the histone deacetylase inhibitor (HDACi) TSA significantly attenuated γ-histone 2AX(H2AX) expression and reduced poly ADP-ribose polymerase 1 (PARP1) and XRCC1 levels in the epithelial cells of the jejunum, suggesting its efficacy in mitigating radiation-induced DNA damage through the regulation of DNA repair mechanisms. Notably, through immunoblotting and qRT-PCR analysis, it was found that TSA administration restored β-catenin expression, decreased dickkopf-related protein 1(DKK1) and glycogen synthase kinase 3 β (GSK3β) levels, and substantially recovered Lgr5 and Bmi1 expression in the murine subjects. Additionally, intestinal morphology exhibited significant enhancement, with restoration of villus height and villus/crypt depth ratios. The research indicates that TSA ameliorates both acute and delayed radiation-induced intestinal damage by modulating the Wnt/β-catenin and TGFβ/Smad signaling pathways, thereby facilitating intestinal stem cell proliferation and regeneration.
What’s more, in another separate murine experiment involving 15Gy abdominal irradiation, TSA alleviated the oxidative-reductive imbalance induced by IR via the NRF2/GPX4/PINK1/PARKIN signaling pathway and reduced apoptosis in intestinal epithelial cells (74).Collectively, these findings (73, 74) underscore the therapeutic potential of HDACi-mediated histone acetylation activation as an innovative strategy for radiation protection. Considering the established clinical applications of HDACi in cancer therapy, the enhancement of protein acetylation may represent a promising avenue.
2.3.2 Non-histone protein modifications
Beyond histone modifications, other pivotal protein modifications, including phosphorylation, acetylation, and ubiquitination, play crucial roles in regulating cellular apoptosis, DNA repair, inflammatory responses, and intestinal barrier function to confer protective effects. The underlying mechanisms and recent research advancements are elaborated as follows (75, 76).
2.3.2.1 Phosphorylation
Following radiation exposure, key proteins such as ATM kinase, P53, and NRF2 undergo phosphorylation and subsequent activation, initiating downstream signaling pathways that promote DNA repair or induce cell cycle arrest, thereby mitigating oxidative stress-induced damage. The specific roles of these proteins have been extensively discussed in preceding section
2.3.2.2 Acetylation
P53 Lys382 acetylation: The acetylation of lysine 382 by CBP/p300 enhances P53’s DNA-binding affinity, thereby upregulating P21 expression to facilitate DNA repair (75). Future investigations may explore the application of acetylation mimetics to amplify P53 functionality and reduce apoptosis in intestinal cells.
2.3.2.3 Ubiquitination
The proteasome inhibitor bortezomib impedes the degradation of ubiquitinated P53 proteins, thereby stabilizing P53 and simulating a persistent stress state (76).
2.4 Chronic fibrosis mechanisms
Although acute radiation injury is generally reversible, chronic radiation-induced intestinal damage is characterized by progressive fibrosis, which can lead to long-term complications such as stricture formation, fistulae, and impaired intestinal function. In the context of chronic fibrosis, myofibroblasts act as the primary effector cells, driving structural remodeling through collagen secretion and extracellular matrix (ECM) deposition (6). Following radiation exposure, TGF-β1 activates connective tissue growth factor (CTGF) via the Smad3/4 signaling pathway, thereby promoting collagen synthesis and myofibroblast differentiation (77). As a downstream effector, CTGF is regulated by the Rho/ROCK pathway, which inhibits NF-κB activity (78). Platelet-derived growth factor-BB (PDGF-BB) enhances fibroblast proliferation and chemotaxis through tyrosine kinase receptors, synergizing with TGF-β1 to exacerbate fibrosis (79).
Reserve intestinal stem cells (rISCs) are activated in response to injury and contribute to tissue repair through the Wnt/β-catenin and Notch signaling pathways. Within 24 h post-radiation exposure, β-catenin undergoes nuclear translocation, thereby enhancing Lgr5+ cell proliferation (80) sustain stemness and facilitate tissue regeneration. Nevertheless, negative regulators of the Wnt-β-catenin pathway (DKK1, SFRP4) demonstrate significant upregulation by 72 h, leading to impaired crypt regeneration. BCN057, a canonical Wnt-β-catenin signaling agonist, enhances crypt formation efficiency threefold following radiation exposure (81). Under physiological conditions, BMP signaling counteracts Wnt activity to prevent crypt hyperproliferation and maintain villus-crypt gradients. BMPR1A-deficient murine models exhibit accelerated crypt regeneration post-radiation (82). Notch signaling synergizes with Wnt to promote secretory cell differentiation (e.g., goblet cells, Paneth cells), thereby preserving crypt architecture; however, excessive activation results in ISC depletion. γ-secretase inhibitors (e.g., DAPT) induce a 48-h delay in crypt regeneration (83).
Furthermore, epidermal growth factor (EGF) signaling plays a pivotal role in intestinal cell proliferation, tissue repair, and radioprotection. IR exposure stimulates IL-33 production by ISCs, thereby accelerating epithelial regeneration through EGFR-mediated upregulation of EGF in Paneth cells (78). IL-22 significantly enhances crypt cell survival following radiation exposure and augments organoid formation capacity in a dose-dependent manner, with its protective effects persisting for up to 7 days (84). Exogenous heparin-binding (HB)-EGF, a member of the EGF family, exhibits potent crypt-proliferative and radioprotective properties.
Gene expression profiling elucidates radiation-induced molecular alterations associated with macrophage polarization. Key mediators including TNFα, IL-6, IL-10, chemokine receptor 7 (Ccr7), heme oxygenase 1 (Hmox1), and IL-1β have been identified as crucial factors in M1/M2 polarization, with distinct expression patterns observed across macrophage subsets (85). Conventionally, M1 macrophages have been characterized as fibrinolytic cells due to their secretion of proteolytic enzymes, particularly matrix metalloproteinases (MMPs) and cathepsin K, which facilitate collagen degradation (86, 87). However, emerging evidence indicates that M1 macrophages also possess pro-fibrotic capabilities (88–90). The chronic activation of M1 pro-inflammatory signaling pathways, coupled with persistent and ineffective injury-repair cycles, ultimately results in tissue fibrosis and structural remodelin (78).
In addition, Bessout et al. (21) demonstrated that Th17 cells play a predominant role in radiation-induced colonic injury. Their experimental findings revealed that incubation of irradiated colonic smooth muscle cells with recombinant IL-17 protein significantly upregulates the expression of IL-6 and IL-1β, along with genes associated with extracellular matrix remodeling. Beyond its interaction with stromal cells through MAPK or NF-κB signaling pathways, IL-17 also contributes to fibrotic processes by facilitating neutrophil recruitment and augmenting neutrophil elastase activity (91).
2.5 Role of gut microbiota dysbiosis
Contemporary research demonstrates that IR instigates gut microbiota dysbiosis, fundamentally transforming its structural configuration, functional dynamics, and species diversity, while simultaneously modifying its metabolic profile (92, 93). The Firmicutes/Bacteroidetes (F/B) ratio is crucial for maintaining normal intestinal homeostasis. An increase or decrease in the F/B ratio is considered a sign of dysbiosis, with the former typically associated with obesity and the latter with inflammatory bowel disease (94). These alterations potentially exacerbate IR-induced tissue damage and amplify pro-inflammatory immune responses. In contrast, symbiotic microorganisms and their beneficial metabolic byproducts can reconstitute the disrupted intestinal microbial architecture during IR exposure, facilitate the restoration of homeostasis between anti-inflammatory and pro-inflammatory mechanisms, and consequently attenuate RIII (95).
In different mouse models that all received 18Gy radiation to the abdomen, the F/B ratio could increase or decrease (96, 97). Only the F/B ratio of the soluble gp130 transgenic mice exhibited a meaningful postirradiation decrease, whereas the F/B ratios of Galectin-1 KO mice and wild-type mice increased after irradiation (96). Notably, in radiation-resistant murine strains exhibiting prolonged survival post high-dose irradiation, the enhanced proliferation of Lachnospiraceae and Enterococcaceae families demonstrated a positive correlation with hematopoietic reconstitution and gastrointestinal mucosal repair, accompanied by elevated fecal concentrations of propionate and tryptophan-derived metabolites (98).
Following IR in non-human primates (NHPs; Macaca mulatta), the intestinal microbiota composition undergoes substantial modifications. The F/B ratio exhibited a marked decline from 1.2 to below 1.0 subsequent to 7.4 Gy radiation exposure. Of particular significance, the relative abundances of Actinobacteria, Fusobacterium, Prevotella, and Veillonella demonstrated a more than twofold increase, whereas Aerobacter and Acinetobacter populations experienced a remarkable reduction exceeding tenfold (99).
In clinical studies, radiotherapy targeting abdominal or pelvic tumors has been shown to significantly decrease the F/B ratio in 11 patients (100) and significantly reduce α-diversity of gut microbiota, while concurrently increasing the prevalence of pathogenic bacteria (e.g., Enterobacteriaceae, Escherichia coli) and decreasing beneficial microbial taxa (e.g., Bacteroides) (95). A longitudinal clinical cohort study has revealed a progressive decline in microbial diversity corresponding to the advancement of RIII (4). Furthermore, a robust correlation has been established between diminished bacterial diversity and the progression of RIII. Notably, elevated abundances of Clostridium cluster IV, Rothia, and Phascolarctobacterium demonstrate significant associations with the severity grading of RIII.
Thus, it can be seen that the changes in F/B in different models of RIII are not characteristic. The specific reasons need further research. However, in general, all studies have shown that exposure to IR leads to a decrease in the diversity and richness of the biological community, an increase in the abundance of pathogenic bacteria (Proteobacteria and Fusobacteria), and a reduction in beneficial bacteria (Faecalibacterium and Bifidobacterium) (92, 97). In the context of dynamic changes in the F/B ratio, the dynamic characteristics of the microbial composition of the two main bacterial groups and other components largely reflect the dysregulation of the intestinal microbiota during the occurrence and development of RIII.
Gut microbiota dysbiosis exacerbates RIII through the following mechanistic pathways (95):
a. Disruption of biological barriers: Pathogenic bacterial species (e.g., Escherichia coli) enzymatically degrade secretory immunoglobulin A (sIgA), thereby compromising the integrity of intestinal immune defenses.
b. Dysregulation of immune homeostasis: Lipopolysaccharide (LPS)-positive bacteria trigger the activation of proinflammatory cytokine cascades through Toll-like receptor 4 (TLR4)/NF-κB signaling pathways.
c. Suppression of anti-inflammatory mediators: The diminished production of probiotic-derived metabolites, particularly short-chain fatty acids (SCFAs) including butyrate, leads to reduced IL-10 activity.
3 Diagnosis and biomarkers
3.1 Imaging modalities
Currently, there are no clear diagnostic criteria for radiation-induced intestinal injury. In clinical practice, modern cross-sectional imaging techniques such as CT and MRE have become the preferred diagnostic tools due to their high resolution and non-invasive advantages (101, 102). CT provides high-resolution anatomical images through X-ray tomography, with radiation doses lower than traditional X-rays (total exposure <1 mSv) (103). It exhibits high sensitivity for detecting tumors, inflammation, and intestinal obstruction, particularly in emergency settings.
MRE, based on magnetic resonance imaging (MRI) principles, uses oral contrast agents to distend the intestines and generate high-resolution soft-tissue images via magnetic fields and radiofrequency pulses. This non-IR feature is a significant advantage for patients who have previously undergone radiotherapy. Additionally, MRE offers superior soft-tissue contrast resolution compared to CT, enabling clearer visualization of intestinal wall inflammation, fibrosis, and thickening (104, 105). Theoretically, T2-weighted imaging (T2WI) can clearly depict intestinal wall edema (high signal) and fibrosis (low signal), differentiating acute inflammation (predominantly edematous) from chronic injury (predominantly fibrotic). Diffusion-weighted imaging (DWI), on the other hand, quantifies inflammation activity by measuring the degree of water molecule diffusion restriction, aiding in the early detection of radiation-induced intestinal damage (106). This distinction is critical for guiding treatment decisions. Although we have not yet collected any real-world clinical data to validate this hypothesis, we believe that MRE could potentially emerge as one of the important modalities for diagnosing radiation-induced intestinal injury in the future. Nevertheless, the choice between MRE and CT often depends on institutional resources, patient tolerance, and specific clinical scenarios.
3.2 Endoscopic innovations: confocal laser endomicroscopy
The advancement of endoscopic technology has given rise to confocal laser endomicroscopy (CLE) as a promising tool for real-time, in vivo assessment of intestinal diseases. CLE is an endoscopic technique capable of obtaining ultra-high magnification images of the gastrointestinal mucosa (8), with the potential to enable real-time histological diagnosis (107). Its principle relies on low-power laser illumination of tissue and the subsequent detection of fluorescent light reflected back from the tissue through a pinhole (108). The term “confocal” refers to the alignment of both the illumination and collection systems within the same focal plane (109, 110). This design significantly enhances spatial resolution, permitting real-time cellular imaging and tissue architectural evaluation during endoscopy (8), thereby providing detailed insights into epithelial integrity, vascular changes, and inflammatory infiltration.
Currently, this technology has been extensively applied to characterize morphological features in inflammatory bowel disease (IBD) (111) and has also been utilized for microscopic mucosal descriptions in irritable bowel syndrome (IBS), infectious colitis (e.g., Clostridioides difficile infection) (112), and colonitis associated with graft-versus-host disease (GVHD) (111). Although the widespread adoption of CLE is limited by its high cost, steep learning curve, and requirement for specialized equipment, we propose that it could serve as a safe and feasible diagnostic tool for radiation-induced intestinal injury. Specifically, CLE may aid in assessing intestinal morphology, evaluating disease activity, and predicting treatment responses in this context.
3.3 Innovative approaches in biomarkers
MicroRNAs (miRNAs) are small non-coding RNAs involved in post-transcriptional regulation. Due to their stability in body fluids (e.g., serum, urine) and radiation sensitivity, they serve as promising candidate biomarkers for radiation exposure (19). Numerous studies have identified tissue-specific miRNA signatures associated with radiation exposure time and dose in animal models and patients. Rogers, C. J. et al. discovered modified miRNA profiles following radiation exposure and demonstrated that a miRNA panel could predict risks of neutropenia and pulmonary fibrosis in non-human primate models, with partial clinical validation in human samples (113). In intestinal tissues, radiation induces gene enrichment related to cell cycle regulation and metabolic processes. In irradiated mouse intestines, significant changes were observed in miR-34a and miR-215, which regulate cell cycle progression and DNA repair, thereby influencing intestinal mucosal repair post-radiation (19). These miRNAs may represent potential specific biomarkers for radioactive enteritis. However, future clinical translation must address challenges such as cross-species differences and dynamic complexity.
Fatty acid binding proteins (FABPs) are a class of low-molecular-weight cytosolic proteins widely expressed in intestinal epithelial cells. They are rapidly released into the bloodstream during tissue ischemia, oxidative stress, or inflammation, possessing significant potential as biomarkers for injury. Suchitra Sharma and colleagues (114) employed a data mining approach to retrieve six microarray datasets from the NCBI Gene Expression Omnibus (GEO) database and identified differentially expressed FABP genes across species. Subsequently, quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used to analyze the abundance of ten FABP-encoding genes in the intestinal tissues of male and female C57Bl/6 mice. The study revealed that FABP1 and FABP2 are highly expressed genes in the small intestine of both sexes. If elevated levels of FABP1/FABP2 are observed in the blood of humans exposed to radiation, rapid diagnostic assays (e.g., ELISA) could be developed to aid in early diagnosis.
Over time, circulating EVs have also been studied as biomarkers in liquid biopsies due to their high stability and presence in all body fluids. Exosomes are nanoscale vesicles secreted by cells, rich in proteins, lipids, and nucleic acids. By isolating exosomes from plasma and conducting metabolomic/lipidomic analyses, low-abundance biomarkers that are difficult to detect in traditional plasma analyses can be captured (115). Current research has focused on exosomes for studying radiation therapy complications. Institut de Radioprotection et de Sûreté Nucléaire (IRSN) identified protein components in EVs associated with coagulation and inflammatory processes in prostate cancer patients undergoing radiation therapy (116). A multicenter prospective study is exploring early detection of cardiac complications after breast cancer radiotherapy, with preliminary results showing dose-dependent increases in platelet- and endothelial-derived EVs (117). Further studies are needed to validate the role of exosomes in the early diagnosis of RIII.
The diversity of the gut microbiome is closely linked to radiation sensitivity, with shifts in microbial composition serving as early warning indicators of radiation-induced damage (100). Studies (19) have found that a sharp decline in Lactobacillus abundance in feces correlates negatively with the severity of RIII, while the proliferation of pathogenic bacteria (e.g., Clostridium perfringens) is associated with an increased risk of delayed intestinal fibrosis. Simply put, radiation exposure leads to a reduction in “beneficial bacteria” and an overgrowth of “pathogenic bacteria.” However, individual responses to radiation-induced microbiome changes vary significantly, potentially influenced by genetic factors, diet, baseline gut microbiota composition, and other variables. Future efforts should focus on developing machine learning-based predictive models that integrate multi-omics data (e.g., metagenomics, metabolomics) to enhance the precision of personalized interventions.
In conclusion, the diagnosis of RIII is evolving with the integration of advanced imaging techniques, innovative endoscopic tools, and novel biomarkers. These advancements are paving the way for more accurate and timely diagnosis, ultimately improving patient outcomes in this challenging condition.
4 Treatment strategies
The pathological process of RIII involves multi-layered molecular and cellular events. From acute-phase DNA damage and intestinal inflammation to chronic-phase fibrosis and microcirculation dysfunction, each stage presents potential therapeutic targets. Ongoing research into the mechanisms of RIII has revealed that certain novel drugs and treatment strategies exhibit significant therapeutic potential for RIII. Furthermore, current drug development efforts focus on creating Radioprotectors (for pre-radiation use) and Radiomitigators (for post-radiation use) (19). Radioprotectors are administered prior to radiation exposure as preventive agents to inhibit or minimize radiotherapy-induced adverse effects through mechanisms such as free radical scavenging and DNA damage prevention. In contrast, radiomitigators are employed post-radiation exposure as reactive agents to alleviate established radiation-related complications by modulating inflammatory cascades and promoting cellular repair (19). Therefore, the tables include corresponding categories. It should be noted that if RIII progresses to irreversible chronic ischemic necrosis and fibrosis of the intestinal wall, surgical intervention would be the most ideal treatment option (1, 118). The surgical treatment methods are not discussed in detail in this article. In this section, we review potential therapeutic strategies for RIII based on preclinical and clinical trials. But the content related to clinical trials will be uniformly presented in the next chapter.
4.1 Natural plants and their derivatives
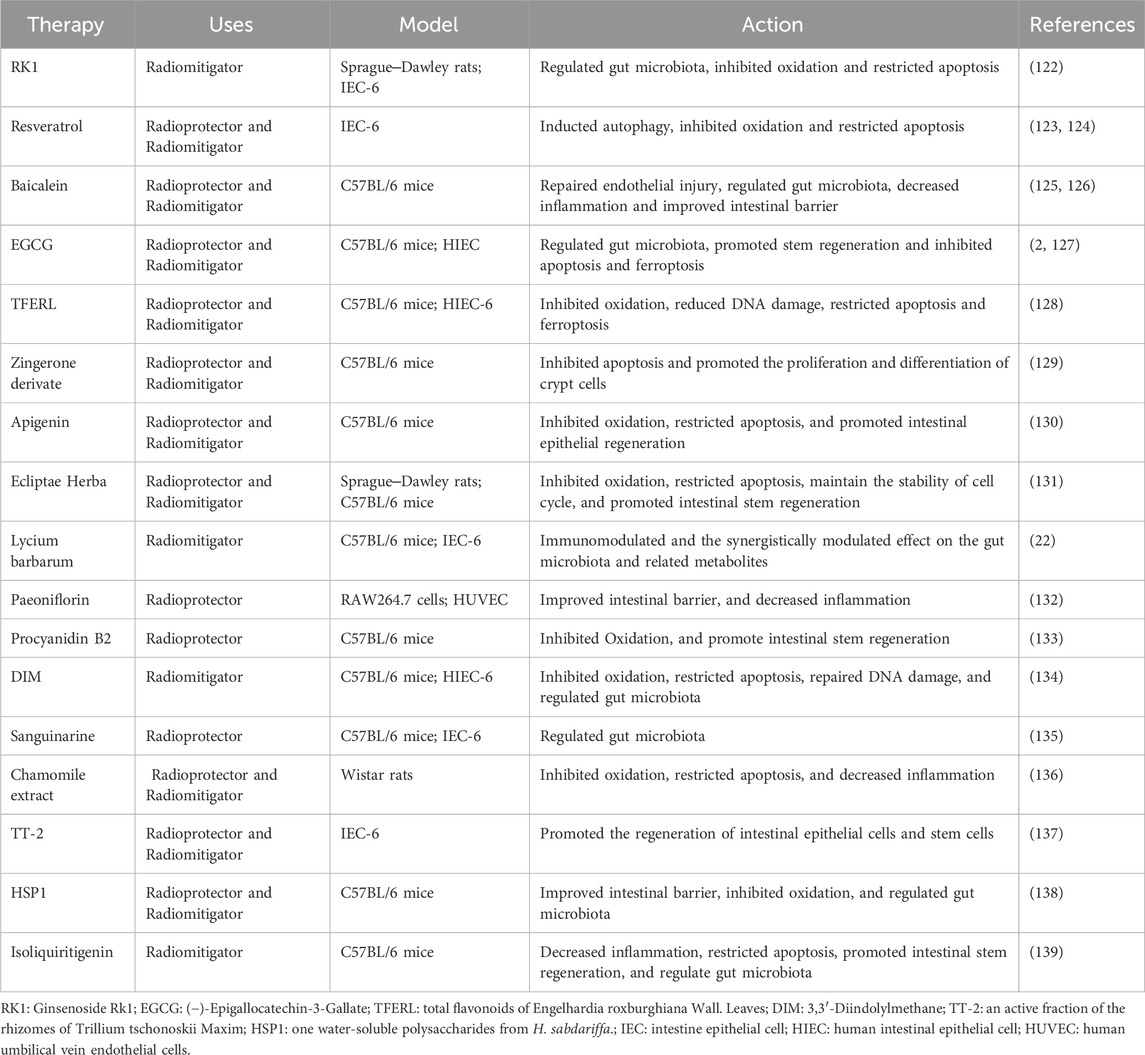
Studies have demonstrated that natural plants and their derivatives exhibit pharmacological activity and play a significant role in RIII (Table 1). Most of them mitigated RIII via improving intestinal barrier, inhibiting oxidation, regulating gut microbiota, decreasing inflammation, restricting apoptosis, promoting intestinal stem regeneration,etc.
Ginsenosides, extracted from Panax ginseng through thermal processing, are recognized as the primary bioactive components of ginseng. Ginsenoside Rk1 (RK1) (119), a rare ginsenoside, exhibits multiple bioactivities including anticancer effects, anti-apoptotic properties, anti-inflammatory responses, antioxidant capacity and antiplatelet aggregation effects. Accumulating evidence indicates that ginseng and its purified components containing RK1 demonstrate radioprotective potential by attenuating radiation-induced injury through oxidative stress reduction mechanisms (120, 121). Yilin Wang et al. (122) discovered that RK1 treatment significantly enhanced the survival rate of irradiated rats and significantly improved the intestinal mucosal structure damage observed through histological examination. RK1 treatment significantly alleviated the oxidative stress-induced apoptosis of intestinal epithelial cells induced by radiation. In addition, RNA-Seq identified 388 differentially expressed genes (DEGs), and indicated that the PI3K-AKT pathway might be the key signaling pathway for RK1 to exert therapeutic effects on RIII. Western blotting results showed that the expression levels of p-PI3K, p-AKT, and p-mTOR increased due to radiation were significantly inhibited by RK1.
Coincidentally, in another study on resveratrol (123), the PI3K/AKT/mTOR signaling pathway was also mentioned. Intestine epithelial cells (IEC)-6 were pretreated with resveratrol 2 h before radiation exposure. It was observed that resveratrol alleviated radiation-induced oxidative stress and cellular damage by activating autophagy through inhibition of the PI3K/AKT/mTOR signaling pathway, and upregulated SIRT1 expression post-irradiation, achieving an anti-apoptotic effect.
Secondly, for the treatment of RIII, protecting endothelial cells is of great significance. Baicalin is one of the natural plants and their derivatives which can affect endothelial cell function. Hyosun Jang et al. (125) observed that villi length was shortened, and intestinal crypt function was impaired in radiation-induced colitis mouse models. After treatment with baicalin, intestinal damage was alleviated, and intestinal barrier function was improved. Baicalin inhibited the expression of radiation-induced adhesion molecules in human umbilical vein endothelial cells (HUVECs) and in the intestines of irradiated mice, and reduced leukocyte infiltration in radiation-induced colitis.
Moreover, Meifang Wang et al. (126) also discovered that baicalein can re-adjust the composition pattern of intestinal microorganisms that has been disrupted by irradiation, increase the relative abundance ratio of Bacteroidetes and Firmicutes, and reduce the level of Proteobacteria. At the same time, baicalein inhibited the activation of P53 and mediated mitochondrial apoptosis and death receptor apoptosis in the intestine through P53. Eventually, it improved the intestinal structure, promoted the proliferation and regeneration ability of intestinal cells.
As for the regulation of gut microbiota, it is also a common mechanism of action for various drugs. Regulation of gut microbiota was also discovered in Lycium barbarum (22), Isoliquiritigenin (139), One water-soluble polysaccharides from H. sabdariffa (HSP1) (138), (−)-Epigallocatechin-3-Gallate (EGCG) (127), etc…
However, Li-Wei Xie et al. (2) conducted further research and discovered that in human intestinal epithelial HIEC cells, the same radiation protection effect was observed as in mice, manifested as a reduction in the number of γH2AX foci and a decrease in ferroptosis. In addition, EGCG lowered the level of ROS and activated transcription factor NRF2 and its downstream targets, including antioxidant proteins Slc7A11, HO-1, and GPX4. The use of NRF2 inhibitor ML385 eliminated the protective effect of EGCG, indicating that the activation of NRF2 is essential for the activity of EGCG. Therefore, EGCG can also counteract RIII by eliminating ROS and inhibiting apoptosis and ferroptosis.
Inhibition of ferroptosis can also be discovered in total flavonoids of Engelhardia roxburghiana Wall. leaves (TFERL). Seven days before and 7 days after the irradiation at 7.2 Gy, C57BL/6 mice were administered with different doses of TFERL (20, 40, and 80 mg/kg), and the researchers (128) found that TFERL alleviated RIII by reducing the damage of intestinal crypt/villus structure, increasing the number and proliferation of ISCs, and maintaining the integrity of intestinal epithelium after abdominal irradiation. Moreover, TFERL promoted the proliferation of irradiated HIEC-6 cells and reduced radiation-induced cell apoptosis and DNA damage. Mechanistic studies showed that TFERL promoted the expression of NRF2 and its downstream antioxidant proteins, and silencing NRF2 led to the loss of TFERL’s radiation protection effect, indicating that TFERL exerted radiation protection through activating the NRF2 pathway.
4.2 Traditional Chinese medicine (TCM)
TCM represents a comprehensive diagnostic, pathophysiological, and therapeutic system rooted in over two millennia of accumulated knowledge and clinical practice. This system encompasses herbal medicine, acupuncture, and various physical therapeutic modalities (140). Empirical studies have demonstrated that TCM exhibits significant protective effects against RIII. Chinese herbal decoction is a traditional form of herbal medicine in TCM, made by boiling a combination of dried herbs, roots, barks, seeds, or flowers in water. It is one of the most common and classic ways to administer herbal remedies, valued for its potency and ability to target specific health issues. In the following text, we list some traditional Chinese herbal decoction that have been studied for the treatment of RIII.
In accordance with the principles of TCM and through an integrated approach combining network pharmacology analysis with experimental validation, researchers (141) have identified that Liangxue Guyuan Yishen Decoction (LGYD) significantly enhances the survival rate o RIII model rats, mitigates radiation-induced intestinal pathological damage, and facilitates intestinal crypt regeneration. The investigation revealed that LGYD upregulates the expression of LGR5+ intestinal stem cell markers, stimulates the proliferation of crypt stem cells, augments tight junction protein expression, and restores intestinal epithelial barrier function. Through network pharmacology predictions and experimental validations, the study delineated the key signaling pathways underlying its mechanism: the Wnt signaling pathway was significantly activated (as evidenced by targets including β-catenin and c-Myc), while the MEK/ERK pathway was implicated in the regulation of cell proliferation and survival (demonstrated by alterations in ERK phosphorylation levels).
Liying Zhang et al. (142) demonstrated that Guiqi Baizhu Decoction (GQBZD) effectively mitigates immune impairment and intestinal microbiota dysbiosis induced by IR. In experimental studies utilizing rat models, the researchers observed that GQBZD significantly attenuated radiation-induced weight loss, ameliorated reductions in food and water intake, alleviated diarrhea, and improved quality of life scores following X-ray exposure. Furthermore, GQBZD exhibited notable anti-inflammatory properties and enhanced immune function. The formulation restored both
In parallel investigations, Yangyang L et al. (143) reported that Sprague-Dawley (SD) rats exposed to 6Gy X-ray irradiation exhibited marked colonic edema accompanied by ultrastructural damage to nuclear and mitochondrial components, increased expression of ROS, hypoxia-inducible factor 1-alpha (HIF-1α), and aquaporin-4 (AQP4), along with decreased expression and activity of Na+/K+-ATPase. However, GQBZD intervention effectively ameliorated colonic edema through the mitigation of hypoxia and oxidative stress, coupled with the regulation of AQP4 and Na+/K+-ATPase expression.
In the experimental investigation utilizing SD rats subjected to abdominal radiotherapy (144), compound kushen injection (CKI) was demonstrated to effectively mitigate radiation-induced intestinal inflammation, ulcerative lesions, and associated clinical manifestations while concurrently ameliorating intestinal tissue damage. Furthermore, CKI administration significantly reduced the concentrations of IL-1β, IL-6, and myeloperoxidase (MPO) within the intestinal mucosa, thereby exerting potent anti-inflammatory effects. Histopathological evaluations revealed that CKI treatment markedly decreased the apoptotic cell count in crypt regions, indicating its inhibitory capacity against epithelial cell apoptosis.
Shaoyao Decoction (SYD), a classical heat-clearing formula, is recognized for its efficacy in purging visceral real heat and is commonly employed in the treatment of various intestinal discomfort symptoms. Clinical evidence indicates its potential therapeutic effects on radiation enteritis. An X-ray-induced RIII model was established using C57BL/6 mice. Histopathological analysis revealed that SYD attenuated epithelial cell shedding and necrosis in the mucosal crypt layer while suppressing abnormal proliferation of damaged fibrous tissue. SYD significantly reduced serum levels of MDA, COX, LPS, and pro-inflammatory cytokines (IL-6, IL-1β, TNF-α), while enhancing the activity of the antioxidant enzyme SOD. TUNEL staining demonstrated that SYD decreased colon cell apoptosis; Western blot and qRT-PCR analyses confirmed that SYD upregulated the expression of Dclk-1, ATM, and MRE-11 genes and proteins, promoted the release of mitochondrial pro-apoptotic proteins Bax, Caspase-3, and Cyto-c, and concurrently increased the level of anti-apoptotic protein Bcl-2. Immunofluorescence analysis showed that SYD significantly reduced P53 protein expression and reversed the downregulation of Claudin-1 protein. These findings demonstrate that SYD effectively ameliorates X-ray-induced radiation enteritis in mice through multiple mechanisms, including tissue fibrosis reduction, inflammatory response inhibition, and regulation of the mitochondrial apoptotic pathway (pro-apoptotic and anti-apoptotic protein balance). The underlying mechanism may involve the regulation of DNA damage repair-related genes (Dclk-1, ATM, and MRE-11) and the restoration of intestinal barrier function mediated by P53 and Claudin-1 (145).
4.3 Other compounds and biomolecules
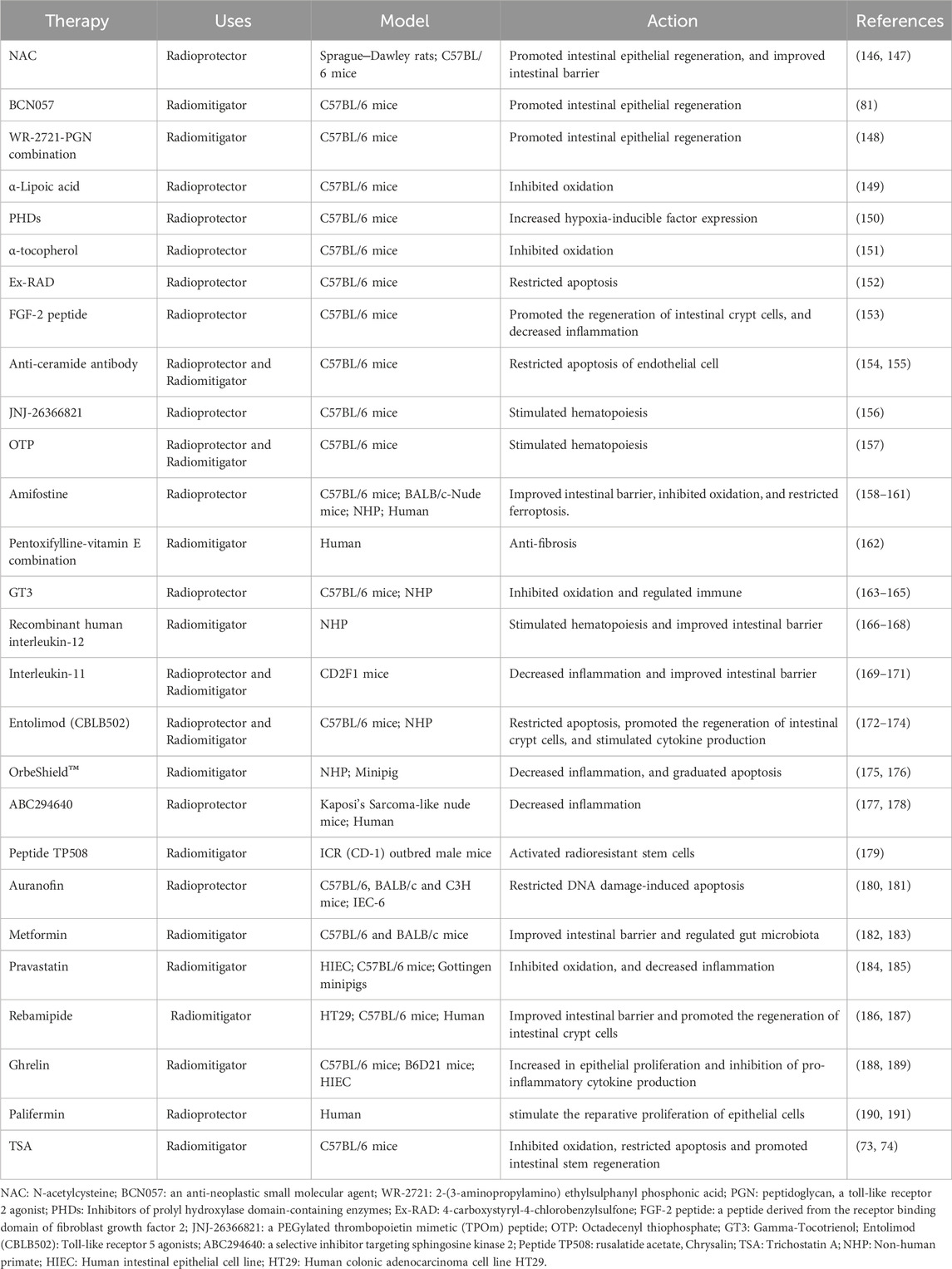
In addition to the aforementioned plant-derived drugs and traditional Chinese medicine formulations, numerous other significant compounds and biomolecules (Table 2) have been extensively validated for their efficacy in regulating RIII both prophylactically and therapeutically. Notably, this includes reusable therapeutic agents which have demonstrated substantial potential in clinical applications.
Firstly, emerging research has revealed that several established clinical agents demonstrate corresponding therapeutic efficacy in animal or cell models of RIII (Table 2), such as metformin, pravastatin, and auranofin. Metformin, the most extensively utilized hypoglycemic agent in clinical practice, exhibits multifaceted biological properties beyond its glucose-lowering effects, encompassing antioxidant, anti-inflammatory, and anti-apoptotic activities (192, 193). In an investigation into metformin’s impact on RIII, Hyosun Jang et al. conducted abdominal irradiation of mice with 13.5 Gy using X-rad-320, followed by metformin administration. The study (182) revealed that metformin treatment augmented the expression of tight junction proteins in epithelial cells and impeded bacterial translocation to mesenteric lymph nodes. Furthermore, metformin enhanced the population of ISCs by mitigating radiation-induced toxicity and facilitated epithelial cell regeneration through activation of the Wnt/β-catenin signaling pathway. Jing-Yu Yang et al. (183) demonstrated a significant increase in the abundance of lactic acid bacteria in the intestinal microbiota of patients receiving metformin during abdominal radiotherapy, with an inverse correlation observed between bacterial abundance and the duration of diarrhea. Additionally, the combination of metformin with Lactobacillus supplementation or FMT was found to activate the Farnesoid X Receptor (FXR) signaling pathway in intestinal epithelial cells, resulting in the upregulation of tight junction proteins and mucins, along with an increase in goblet cell numbers, thereby preserving the integrity of the intestinal epithelial barrier.
Pravastatin, a widely utilized therapeutic agent in clinical settings for the reduction of serum cholesterol levels, has been demonstrated to exhibit anti-inflammatory properties on endothelial cells. Hyosun Jang et al. (184) conducted a comprehensive investigation into the therapeutic efficacy of pravastatin on radiation-induced enteritis-damaged epithelial cells through both in vitro and in vivo experimental systems. The research findings revealed that pravastatin effectively suppressed radiation-induced intestinal inflammatory cytokines, specifically IL-6, IL-1β, and TNF-α, in irradiated InEpC cells. Furthermore, in murine models subjected to total abdominal irradiation and treated with pravastatin, histological impairments including villous shortening and intestinal crypt dysfunction were significantly ameliorated, with the differentiation of intestinal epithelial cells restored to normal physiological levels. In another investigation employing the minipig model by quantifying clinical symptoms, irradiated minipig demonstrated attenuated clinical manifestations, reduced intestinal inflammatory responses and endotoxin concentrations, and enhanced gastrointestinal mucosal integrity. Concurrently, through mRNA sequencing analysis, Kwak, Seo Young et al. proposed that pravastatin exerts its regulatory effects on epithelial integrity via modulation of MT2 expression in radiation-compromised epithelial cells (185).
Secondly, amifostine stands as the sole small-molecule radioprotective agent that has received approval from the U.S. Food and Drug Administration (FDA) in recent decades. And it has been extensively validated in murine radiation models to restore glutathione metabolism, neutralize free radicals, and exert potent antioxidant effects. This compound effectively suppresses arachidonic acid metabolism, thereby reducing pro-inflammatory mediators and modulating anti-inflammatory responses. Furthermore, amifostine demonstrates significant regulatory effects on bile acid, folate, vitamin A, and amino acid metabolism, facilitating the restoration of metabolic homeostasis. Through its dual mechanisms of supporting crypt stem cell regeneration and accelerating mucosal repair, it provides comprehensive protection for intestinal epithelial cells, ultimately delivering multi-faceted radioprotective effects on the jejunum (160).
Nevertheless, the severe adverse reactions associated with this compound have significantly constrained its clinical utility. To mitigate the toxicity concerns while preserving therapeutic efficacy, a series of cysteine-modified small peptides have been synthesized. Notably, compound 5 demonstrates radioprotective efficacy equivalent to that of amifostine, while exhibiting superior safety profiles. Intriguingly, mechanistic investigations have revealed that compound 5 exerts its cytoprotective effects through the inhibition of IR-induced ferroptosis, a distinct mechanism from that of amifostine (158).
Thirdly, previous studies (194–196) have revealed that the depletion of stem cell clonogens (SCCs) in the crypts of Lieberkühn is closely linked to ceramide-induced endothelial cell apoptosis within the mucosal microvascular network, which transmits apoptotic signals. A monoclonal antibody named 2A2, targeting ceramide, binds to ceramide and prevents the formation of “ceramide-rich platforms” in the endothelial cells of the gastrointestinal tract in irradiated mice (154). In addition, the successful generation of the single-chain variable fragment (scFv) derived from ceramide 6B5 antibody was accomplished, and subsequent experimental validation revealed that administration of 6B5 scFv significantly reduced mortality rates in murine models when administered subcutaneously 24 h post-exposure to a 90% lethal dose (15 Gy) of radiation in the context of RIII (155). This mechanism protects endothelial cells in the small intestinal lamina propria from apoptosis, promotes the recovery of crypt SCCs, and prevents murine mortality from RIII following high-dose radiation exposure.
The PEGylated thrombopoietin mimetic peptide (PEG-TPOm), which has demonstrated efficacy in treating murine hematopoietic acute radiation syndrome, has been unexpectedly found to exhibit vascular protective properties by mitigating hemorrhage and intestinal permeability. Additionally, it stimulates crypt cell proliferation and enhances survival rates when administered following radiation exposure (156).
Similarly, the small molecule octadecenyl thiophosphate (OTP), a synthetic analog of lysophosphatidic acid (LPA), exhibited significant radioprotective and radiomitigative effects in a murine model of total-body irradiation (TBI). A single dose of OTP administered within the time window of 12 h pre-irradiation to 26 h post-irradiation demonstrated a 50% reduction in mortality rate. Notably, even when administered during the 48–72 h post-irradiation period (corresponding to the late phase of acute radiation syndrome), OTP maintained a mortality reduction of ≥34%. Furthermore, OTP treatment enhanced jejunal crypt survival, improved intestinal glucose absorption capacity, reduced systemic endotoxin leakage, and mitigated radiation-induced intestinal injury (157).
Collectively, vascular-targeted therapeutic approaches for RIII, including anti-ceramide antibody administration and thrombopoietin mimetic application, effectively address the constraints inherent in conventional DNA repair-based therapeutic modalities through endothelial cell protection and regenerative promotion, thereby manifesting dual therapeutic efficacy encompassing both acute intervention and tissue restoration.
Lastly, antioxidants, particularly those compounds classified within the vitamin E category, have been extensively investigated and widely utilized as radioprotective agents (197). Gamma-Tocotrienol (GT3), a constituent of the vitamin E family distinguished by its potent antioxidant properties and inhibitory action on 3-hydroxy-3-methylglutaryl-coenzyme A reductase, has been demonstrated in numerous studies to markedly improve survival rates following radiation exposure in both murine and nonhuman primate models (163–165). In a landmark study (165), male CD2F1 mice administered with GT3 pretreatment exhibited 100% survival rate subsequent to exposure to 11 Gy of cobalt-60 gamma radiation—a dose that induces severe hematopoietic acute radiation syndrome accompanied by moderate gastrointestinal injury. Comprehensive proteomic analysis demonstrated that GT3 significantly modulated radiation-induced alterations in serum protein expression profiles, particularly affecting pathways associated with immune regulation, inflammatory responses, and cellular motility. The unprecedented achievement of complete survival in this animal model establishes GT3 as a prophylactic radioprotective agent, representing a paradigm shift in radiation countermeasure research.
4.4 Mesenchymal stem cells (MSCs) and exosomes
Since the first study (198) demonstrating the therapeutic value of MSCs in RIII by Sémont et al. in 2006, related explorations have continued to expand. Multiple studies have shown that MSCs prolong the survival of irradiated mice, with treated animals exhibiting hyperplastic intestinal villi characterized by increased proliferative cells and reduced apoptotic cells in crypts.
MSCs promote the growth of endogenous Lgr5+ ISCs by increasing the activation of the Wnt/β-catenin signaling pathway (199). And they could secrete IL-6 to promote epithelial regeneration (200). The research has found that MSCs increase the levels of serum R-spondin1, KGF, IL-10, and PGE2 to promote cell proliferation and inhibit apoptosis and inflammation (201). Moreover, MSC infusion can accelerate the formation of new blood vessels at radiation-damaged sites through the intrinsic repair effect triggered by the upregulation of expressed SDF-1, VEGF, basic fibroblast growth factor (bFGF), and Flk-1 (202). It is worth noting that MSC-conditioned medium also shows the potential to promote the regeneration of irradiated epithelial tissues (203). Additionally, Lindar et al. reported that repeated autologous transplantation of MSCs in a pig model of radiation-induced proctitis could counteract the inflammation of rectal mucosa by increasing the host’s IL-10 production and reduce collagen deposition by decreasing the expression of local Col1a2/Col3a1 and TGF-β/CTGF and improving the MMP/TIMP balance, protecting the rectum from radiation-induced fibrosis (204). These preclinical studies have demonstrated that MSC-based therapies have strong potential for repairing lesions associated with radiation-induced enteritis.
However, challenges such as tumor immune evasion risk due to MSC-mediated immunosuppression and allogeneic MSC intolerance have spurred interest in extracellular vesicles (EVs) as a safer alternative. Exosome is a type of EVs. Recent research (205, 206) confirms that mesenchymal stem cell-derived exosomes (MSC-exos) promote epithelial repair, maintain intestinal structural integrity, and mitigate radiation-induced gastrointestinal toxicity in mice, offering a promising cell-free therapeutic strategy. Leilei Yang et al. (207) discovered mesenchymal stem cell-derived exosomes (MSC-exos) injection alleviated intestinal inflammation, enhanced epithelial integrity, and upregulated stem cell markers (LGR5, OCT4) in a RIII mouse model. And in irradiated Lgr5+ intestinal epithelial stem cells (IESCs), MSC-exos promoted proliferation and suppressed apoptosis. MSC-exos reversed radiation-induced elevation of pro-inflammatory cytokines (TNF-α, IL-6) and miR-195 overexpression. Mechanistically, miR-195 inhibition by MSC-exos activated Akt and Wnt/β-catenin pathways, which are critical for IESC survival and regeneration. Nevertheless, the current body of research in this domain remains limited, and the therapeutic potential of exosomes warrants more extensive investigation through rigorous scientific studies.
4.5 Gut microbiome regulation
Probiotics, FMT, and microbial metabolites (e.g., SCFAs) restore intestinal health (95, 97). Probiotics like Saccharomyces boulardii, bifidobacteria, or synbiotics reduce radiocolitis incidence in mice and nonhuman primates. “Elite survivor” mice exposed to lethal radiation exhibit unique microbiota; transferring their gut microbiota to germ-free mice confers radiation resistance. SCFAs (acetate, propionate, butyrate) enhance radiation tolerance via DNA damage reduction and ROS suppression. Metabolomic studies identify tryptophan derivatives (1H-indole-3-carbaldehyde, kynurenine) as long-term Radioprotectors.
Prebiotics and probiotics exhibit synergistic effects in mitigating RIII. Inulin hydrogels (IGs) were employed as carriers for multi-strain probiotics (MSPs) comprising Clostridium butyricum, Bifidobacterium adolescentis, and Akkermansia muciniphila (9). Experimental findings demonstrated that the IG/MSP-based synbiotic formulation exhibited superior protective efficacy against ionizing RIII in comparison to individual probiotic strains and IGs alone. This synbiotic intervention effectively restored multiple physiological parameters to near-normal levels, including locomotor activity, intestinal barrier integrity (as evidenced by occludin and ZO-1 expression), histological damage, pro-inflammatory cytokine concentrations, gut microbiota composition, and short-chain fatty acid production. These collective data indicate that IGs significantly potentiate the therapeutic effects of probiotics in mitigating RIII.
Genetically Engineered Probiotics: With the progressive development of synthetic biology and the interdisciplinary integration of medical sciences, genetically engineered bacteria have exhibited remarkable potential in radiation-induced intestinal injury (RIII) precision therapy through the customization of microbial functionalities. D. F. Hamade et al. (10) engineered the second-generation probiotic Lactobacillus reuteri (LR-IL-22), which facilitates targeted delivery of interleukin-22 (IL-22) to mitigate intestinal damage, demonstrating significant enhancement of intestinal barrier integrity post-radiotherapy (P = 0.0167). This intervention effectively reduced concentrations of radiation-induced pro-inflammatory cytokines, specifically KC/CXCL1 (P = 0.002), IFN-γ (P = 0.0024), and plasma Eotaxin/CCL11 (P = 0.0088), while maintaining the population of Lgr5+GFP + intestinal stem cells (P = 0.0010) to facilitate intestinal regeneration. Moreover, in murine models with disseminated 2F8cis ovarian cancer, the combination of LR-IL-22 with fractionated radiotherapy (6.5 Gy × 4 fractions) resulted in substantial tumor burden reduction. When administered in conjunction with paclitaxel/carboplatin chemotherapy, this approach further improved survival outcomes. In addition to LR-IL-22, their research further validated that LR-IFN-β serves as an efficacious therapeutic agent for intestinal radioprotection and radiation damage mitigation (11). Their short half-life balances efficacy and safety, addressing bioagent retention challenges.
Overall, the engineered bacteria demonstrate dual therapeutic efficacy, functioning both as Radioprotectors to prevent radiation-induced damage and as mitigators to alleviate existing injuries, thereby transcending conventional single-mode protective strategies. This innovation establishes a novel “probiotics-cytokine synergistic radio-sensitization” paradigm that integrates microbial therapy with cytokine modulation to enhance radiation efficacy. Additionally, regarding the research on probiotics and FMT in clinical trials, it will be discussed in the “Translational Research Highlight” section.
5 Translational Research Highlight
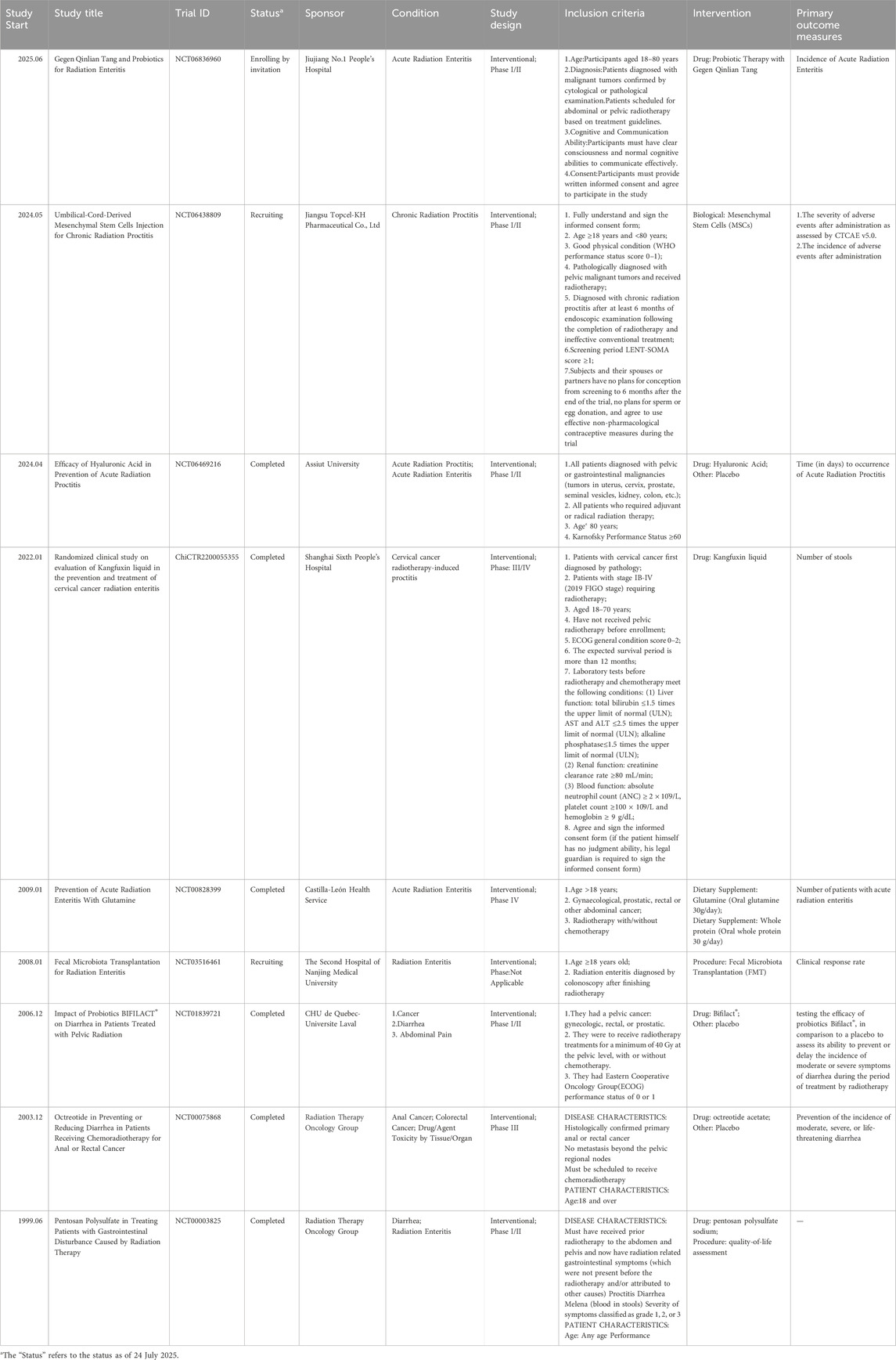
Since increased attention has been paid to gastrointestinal symptoms associated with radiotherapy, a growing number of researchers have initiated clinical trials in an effort to facilitate clinical translation. We have compiled all currently accessible relevant clinical trials into Table 3.
5.1 Traditional Chinese medicine (TCM)
The aforementioned therapeutic approach has exhibited substantial therapeutic potential in both cellular and animal models. To date, preliminary clinical translation efforts have yielded promising results, paving the way for further advancement.
In clinical practice, the herbal retention enema targeting RIII has achieved certain therapeutic effects. The base ingredients of the modified Baitouweng decoction (mBTWD) are mainly Baitouweng decoction, which consists of four herbs: Pulsatilla (Baitouweng), Coptis chinensis (Huanglian), tractat (Huangbo), and ash bark (Qinpi). Zihong Wu et al. (12) conducted a search for relevant randomized controlled trials (RCTs) in PubMed, Embase, Cochrane Library, Web of Science, CNKI, Wanfang Database, SionMed and China Science Journal Database. Eventually, 17 studies were included, involving a total of 1611 patients. mBTWD demonstrates significant efficacy in improving patients’ TCM syndrome scores (
A Phase I/II clinical trial (NCT06836960) titled “Gegen Qinlian Tang and Probiotics for Radiation Enteritis” is currently being conducted at Jiujiang No.1 People’s Hospital (China). This trial aims to recruit 60 patients with malignant tumors undergoing abdominal or pelvic radiotherapy. Participants will be randomly allocated into one of three groups: the control group (no intervention), the probiotics-only group (Bifidobacterium Tricell Capsules, administered twice daily at three capsules per dose), and the combination therapy group (probiotics combined with symptom-adapted Gegen Qinlian Tang). The primary outcome measure will focus on the incidence and severity of acute radiation enteritis, assessed using the RTOG/EORTC grading criteria (Grades 0–IV). Observations will be recorded daily during the radiotherapy period, with a follow-up duration of 3 months post-treatment. This study aims to provide evidence supporting the use of probiotics and herbal medicine as effective strategies to mitigate radiotherapy-induced side effects and enhance patient outcomes.
5.2 Other compounds and biomolecules
A randomized, double-blind, non-inferiority clinical trial (13) enrolled patients presenting with grade 2 or 3 diarrhea (as per the Common Terminology Criteria for Adverse Events version 4.0) following radical radiotherapy for pelvic malignancies. The study aimed to compare the therapeutic efficacy and safety profiles of the antisecretory agent Racecadotril versus the antispasmodic medication Loperamide in managing acute radiation enteritis. Between 2019 and 2022, a total of 162 patients were randomized into treatment groups. In the intention-to-treat analysis, 81 patients in the Racecadotril cohort demonstrated improvement from grade 2 or 3 diarrhea to grade 1 or 0 diarrhea (68 patients, 84%), while 70 patients (86.4%,) in the Loperamide group exhibited comparable clinical improvement (P = 0.66 > 0.05). The intergroup difference in improvement proportions was 2.4% (95% confidence interval [CI]: −8.5%–13.4%). As the upper limit of the 95% CI exceeded the predefined non-inferiority margin of 10% (13.4%), the non-inferiority of Racecadotril relative to Loperamide could not be established. Nevertheless, Racecadotril emerges as a preferred therapeutic option for acute radiation enteritis due to its preservation of intestinal motility, comparable clinical efficacy to Loperamide, and a more favorable adverse effect profile.
From June 2021 to January 2023, 86 patients with cervical cancer who received radiotherapy were included in a clinical trial (ChiCTR2200055355) (14) on the efficacy of kangfuxin liquid retention enema in alleviating RIII in cervical cancer patients. These patients were divided into two groups according to the treatment method: the kangfuxin liquid retention enema observation group and the control group, with 43 cases in each group. The results showed that the incidence of RIII in the observation group after radiotherapy was significantly lower, at 20.93%, while that in the control group was 41.86%. The average onset time of RIII in the observation group was significantly longer than that in the control group (P < 0.05). The incidence of moderate to severe radiation-induced enteritis in the observation group was significantly lower, at 2.33%, while that in the control group was 18.60%. In addition, after radiotherapy, the levels of CD3 and CD4 in the observation group increased, while the level of CD8 decreased, showing statistical differences compared with the control group (P < 0.05). The levels of neutrophils and C-reactive protein (CRP) in the observation group were lower than those in the control group, and the differences were statistically significant (P < 0.05). Thus, it can be concluded that the rehabilitative fluid retention enema therapy shows efficacy in delaying the onset of RIII and reducing its prevalence, thereby improving the clinical manifestations of cervical cancer patients after radiotherapy. This therapeutic effect can be attributed to the enhancement of immune function and the reduction of inflammatory mediators.
Similarly, a recent randomized controlled clinical trial evaluating the efficacy of hyaluronic acid enema in preventing acute radiation proctitis in cancer patients (especially those diagnosed with abdominal and pelvic tumors and subsequently requiring radiotherapy) has been completed (NCT06469216). However, no relevant data has been published so far. However, we are willing to believe that hyaluronic acid enema is as effective as kangfuxin liquid in treating acute radiation proctitis. A meta-analysis indicates that for patients with symptoms of vaginal and anorectal mucositis, local application of hyaluronic acid after radiotherapy and chemotherapy is an innovative approach for clinical treatment, which helps patients cope with adverse reactions (15).
A clinical trial (162) involving 10 patients with RIII treated with a combination of pentoxifylline and vitamin E found that the combined treatment could reduce symptoms by 41% and 80% respectively at 6 months and 18 months. In vitro, pentoxifylline and ascorbyl phenol synergistically inhibited the expression of TGF-β1 protein and mRNA. This inhibitory effect was achieved at the transcriptional level and led to subsequent inhibition of TGF-β1/Smad targets (Col Iα1, FN1, PAI-1, CTGF).
Notably, a substantial number of clinical trials investigating RIII failed to demonstrate anticipated outcomes. Alfonso Vidal-Casariego et al. (16) conducted a randomized, double-blind, Phase III clinical trial (NCT00828399) to evaluate the efficacy of glutamine in preventing acute radiation enteritis, which ultimately yielded negative findings. Similarly, Phase III randomized controlled trials investigating octreotide for diarrhea prevention during radiotherapy and chemotherapy for rectal cancer (NCT00075868) (208) and pentosanpolysulfate for managing radiation-induced diarrhea and hemorrhage (NCT00003825) (209) both produced unfavorable results.
5.3 Mesenchymal stem cells (MSCs)
MSC therapy has been applied to patients with severe gastrointestinal complications following radiotherapy (210). Surprisingly, in a case series by Voswinkel et al. (211), four patients achieved cessation of rectal bleeding, fistula healing, pain relief, and no tumor recurrence after MSC treatment.
A multicenter Phase I/II trial (NCT06438809) is currently evaluating this approach for chronic radiation-induced injuries refractory to conventional therapies. Zhaoshen Li et al. plan to enroll patients who fulfilled the inclusion criteria, had received radiotherapy followed by unsuccessful conventional treatment, and were diagnosed with chronic radiation proctitis through endoscopic examination conducted at least 6 months post-treatment. These patients will be administered umbilical cord-derived mesenchymal stem cells (120 million cells) via injection. The therapeutic response is monitored over a 24-month period following the administration. The primary endpoints include: the severity of adverse events assessed using CTCAE v5.0 within 28 days post-injection and the incidence of adverse events, with particular emphasis on evaluating the safety profile of MSC treatment for radiation proctitis. Additionally, on day 112 post-treatment, the improvement and recurrence rates of chronic radiation proctitis are evaluated to determine the efficacy of MSC therapy. This research outcome has the potential to substantially accelerate the clinical translation and application of MSC.
5.4 Gut microbiome regulation
A probiotic containing live lactobacillus acidophilus plus bifidobacterium bifidum was administered as an investigational agent in a double-blind clinical trial targeting RIII in patients diagnosed with locally advanced cervical carcinoma. In the placebo cohort (n = 31), 45% of participants manifested grade 2–3 diarrhea, whereas in the experimental group (n = 32), this incidence was reduced to 9% (P = 0.002). The utilization of anti-diarrheal medications in the placebo arm demonstrated a significant reduction(P = 0.003).
Furthermore, stool consistency in the treatment group exhibited marked improvement (P < 0.001). These findings substantiate that the probiotic containing live lactobacillus acidophilus plus bifidobacterium bifidum effectively mitigates the occurrence of diarrhea (212).
And another randomized, double-blind, placebo-controlled clinical trial (NCT01839721) was conducted to assess the efficacy of the probiotic formulation Bifilact® in managing moderate to severe therapeutic diarrhea during pelvic radiotherapy. A cohort of 246 patients was randomly allocated to receive either placebo or one of two dual-strain Bifilact® probiotic formulations (containing Lactobacillus LAC-361 and Bifidobacterium BB-536) at a standard dosage of twice daily (130 billion CFU). The findings indicated that the standard dosage of Bifilact® potentially reduces the incidence of grade 2-3-4 radiation-induced diarrhea by the conclusion of treatment in patients undergoing pelvic radiotherapy (213).
It is particularly noteworthy that Xiao Ding et al. conducted a pioneering clinical investigation (NCT03516461) into FMT as a therapeutic intervention for chronic radiation enteritis (CRE) (214). Between January and November 2018, five female patients (median age: 58 years; range: 45–81 years) with a median baseline RTOG/EORTC grade of 2 (range: 2–4) underwent FMT treatment. The therapeutic outcomes revealed that three patients exhibited significant clinical improvement, manifesting as reduced diarrhea, diminished rectal bleeding, alleviated abdominal/rectal pain, and improved fecal incontinence, accompanied by enhanced Karnofsky Performance Status (KPS) scores. Importantly, the procedure demonstrated an excellent safety profile, with no mortality or infectious complications associated with FMT observed throughout the study. During the extended follow-up period (8–18 months), only one mild FMT-related adverse event was documented. Furthermore, 16S rRNA sequencing analysis provided compelling evidence that FMT effectively modulated the intestinal microbiota composition in the treated patients. Currently, this clinical trial remains in the active recruitment phase (RECRUITING), and we anticipate the involvement of additional patients to strengthen the robustness of the research evidence.
6 Future perspectives
To address the unmet clinical needs of RIII, a precision medicine framework integratinggenomics-driven risk stratification, microbiome-based diagnostics, and AI-optimized radiotherapy planning represents a transformative paradigm. Below outlines actionable strategies for achieving this vision (Figure 3):
Figure 3. Future Perspectives The figure was created in https://BioRender.com.
6.1 Precision risk stratification via genomics and microbiome profiling
6.1.1 Genomic biomarkers for radiation sensitivity
Multi-gene panels for personalized risk assessment: Develop diagnostic panels incorporating radiation-responsive genes (e.g., ATM, TP53, XRCC1/2/3, NRF2) and single-nucleotide polymorphisms (SNPs) linked to DNA repair efficiency (e.g., XRCC1 399 Arg/Gln) and apoptosis regulation (e.g., P53 Pro/Arg72). These panels could predict individual susceptibility to acute mucositis, chronic fibrosis, or secondary malignancies.
Epigenetic biomarkers: Leverage DNA methylation signatures (e.g., NRF2-regulated genes) and histone modification patterns (e.g., H3K27ac in stem cell niches) to identify epigenetic dysregulation predisposing patients to RIII. Liquid biopsy-based methylation assays could enable non-invasive monitoring.
Pharmacogenomics for Radioprotectors: Identify genetic determinants of response to mitigators like PUMA inhibitors, HDAC inhibitors (e.g., TSA), or NRF2 activators (e.g., bardoxolone), optimizing drug selection for high-risk patients.
6.1.2 Gut microbiome as a predictive and therapeutic target
Microbiome-based risk prediction: Establish fecal microbiome signatures (e.g., decreased Lactobacillus/Akkermansia and increased Enterobacteriaceae) as early biomarkers for radiation enteropathy severity. Machine learning models integrating microbiome composition, metabolomics (e.g., SCFA levels), and host gene expression could improve prognostic accuracy.
Microbiome modulation for prevention: Engineer synbiotics or FMT protocols to enrich beneficial taxa (e.g., Clostridium XIVa clusters) and suppress pro-inflammatory/pathogenic species. Preclinical evidence suggests modulation of the gut microbiome can attenuate radiation-induced dysbiosis and fibrosis.
6.2 AI-driven optimization of radiotherapy planning
6.2.1 Anatomical and functional imaging-guided dose de-escalation
Organ-at-risk (OAR) segmentation: Utilize deep learning algorithms (e.g., convolutional neural networks) to automate segmentation of the small intestine and adjacent organs in CT/MRI scans, improving dose-sparing precision in intensity-modulated radiotherapy (IMRT) or proton therapy.
Predictive modeling of radiation toxicity: Train machine learning models on multi-parametric data (dose distribution, tissue oxygenation, microbiome profiles) to predict RIII incidence and severity. For example, a model integrating dose-volume histograms (DVHs) with gut microbiome alpha-diversity could identify patients at high risk for chronic fibrosis, prompting dose adjustments or adjuvant therapies.
6.2.2 Dynamic treatment adaptation
Real-time adaptive radiotherapy: Deploy AI-driven platforms to monitor anatomical changes (e.g., intestinal edema, fibrosis progression) during radiotherapy via weekly MRI or cone-beam CT. Adjust radiation beams dynamically to avoid high-dose exposure to injured regions.
Synthetic lethality strategies: Use AI to identify synthetic lethal interactions between radiation and specific gene mutations (e.g., ATM-deficient tumors) or microbiome profiles, enabling targeted combination therapies (e.g., PARP inhibitors for ATM-mutant cancers with concurrent gut radioprotection).
6.3 Translational platforms for therapeutic innovation
Organoid-based drug screening: Develop patient-derived intestinal organoids co-cultured with personalized gut microbiota to test radioprotective compounds (e.g., NRF2 agonists, exosome-based therapies) and identify responders/non-responders.
Nanotechnology-enabled therapeutics: Engineer nanoparticles targeting radiation-damaged crypts (e.g., Lgr5+ stem cells) to deliver NRF2 activators, DNA repair enzymes, or anti-inflammatory cytokines (e.g., IL-10) selectively to injured tissues.
6.4 Multidisciplinary collaboration and clinical validation
Clinical trials with precision endpoints: Design randomized trials stratifying patients by genomics/microbiome risk scores to evaluate the efficacy of tailored interventions (e.g., FMT plus IL-22 analogs vs standard care).
Global biobanking initiatives: Establish international consortia to collect longitudinal multi-omics data (genomics, transcriptomics, metabolomics, microbiome) from irradiated patients, enabling discovery of novel biomarkers and therapeutic targets.
Author contributions
WW: Writing – original draft, Writing – review and editing. YC: Software, Visualization, Writing – review and editing. ZY: Software, Visualization, Writing – review and editing. MC: Software, Visualization, Writing – review and editing. JH: Software, Visualization, Writing – review and editing. KQ: Software, Visualization, Writing – review and editing. JY: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express our gratitude to our colleagues at Zhejiang Cancer Hospital, Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences and to the associate editor and the reviewers for their useful feedback that improved this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hauer-Jensen, M, Denham, JW, and Andreyev, HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol (2014) 11(8):470–9. doi:10.1038/nrgastro.2014.46
2. Xie, LW, Cai, S, Zhao, TS, Li, M, and Tian, Y. Green tea derivative (-)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radic Biol Med (2020) 161:175–86. doi:10.1016/j.freeradbiomed.2020.10.012
3. Klopp, AH, Yeung, AR, Deshmukh, S, Gil, KM, Wenzel, L, Westin, SN, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology-RTOG 1203. J Clin Oncol (2018) 36(24):2538–44. doi:10.1200/JCO.2017.77.4273
4. Reis Ferreira, M, Andreyev, HJN, Mohammed, K, Truelove, L, Gowan, SM, Li, J, et al. Microbiota- and radiotherapy-induced gastrointestinal side-effects (mars) study: a large pilot study of the microbiome in acute and late-radiation enteropathy. Clin Cancer Res (2019) 25(21):6487–500. doi:10.1158/1078-0432.CCR-19-0960
5. Stacey, R, and Green, JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis (2014) 5(1):15–29. doi:10.1177/2040622313510730
6. Lu, L, Li, W, Chen, L, Su, Q, Wang, Y, Guo, Z, et al. Radiation-induced intestinal damage: latest molecular and clinical developments. Future Oncol (2019) 15(35):4105–18. doi:10.2217/fon-2019-0416
7. Loge, L, Florescu, C, Alves, A, and Menahem, B. Radiation enteritis: diagnostic and therapeutic issues. J Visc Surg (2020) 157(6):475–85. doi:10.1016/j.jviscsurg.2020.08.012
8. Pilonis, ND, Januszewicz, W, and di Pietro, M. Confocal laser endomicroscopy in gastro-intestinal endoscopy: technical aspects and clinical applications. Transl Gastroenterol Hepatol (2022) 7:7. doi:10.21037/tgh.2020.04.02
9. Du, S, Sun, R, Wang, M, Fang, Y, Wu, Y, Yuan, B, et al. Synergistic effect of inulin hydrogels on multi-strain probiotics for prevention of ionizing radiation-induced injury. Int J Biol Macromol (2025) 287:138497. doi:10.1016/j.ijbiomac.2024.138497
10. Hamade, DF, Espinal, A, Yu, J, Leibowitz, BJ, Fisher, R, Hou, W, et al. Lactobacillus reuteri releasing IL-22 (LR-IL-22) facilitates intestinal radioprotection for whole-abdomen irradiation (WAI) of ovarian cancer. Radiat Res (2022) 198(1):89–105. doi:10.1667/RADE-21-00224.1
11. Hamade, DF, Epperly, MW, Fisher, R, Hou, W, Shields, D, van Pijkeren, JP, et al. Release of Interferon-beta (IFN-beta) from Probiotic Limosilactobacillus reuteri-IFN-beta (LR-IFN-beta) mitigates gastrointestinal acute radiation syndrome (GI-ARS) following whole abdominal irradiation. Cancers (Basel) (2023) 15(6). doi:10.3390/cancers15061670
12. Wu, Z, Yin, B, Wang, Z, Song, E, You, F, and Wu, X. Clinical Evidence and potential mechanisms in Treating radiation enteritis with modified baitouweng decoction. Evidence-Based Complement Altern Med (2023) 2023(1). doi:10.1155/2023/9731315
13. P, BA, Sudha, SP, Mohan, P, Patil, N, Rahman, A, Gundapuneedi, BS, et al. Racecadotril versus loperamide in acute radiation enteritis: a randomized, double-masked, phase 3, noninferiority trial. Int J Radiat Oncol Biol Phys (2024) 118(3):616–25. doi:10.1016/j.ijrobp.2023.09.021
14. Yang, X, Gao, Z, and Fu, J. Efficacy of kangfuxin liquid retention enema in preventing and treating radiation-induced proctitis in cervical cancer patients: a randomized, open-label, phase III study. Int J Radiat Oncology*Biology*Physics (2024) 120(2). doi:10.1016/j.ijrobp.2024.07.030
15. Cosentino, D, and Piro, F. Hyaluronic acid for treatment of the radiation therapy side effects: a systematic review. Eur Rev Med Pharmacol Sci (2018) 22(21):7562–72. doi:10.26355/eurrev_201811_16298
16. Vidal-Casariego, A, Calleja-Fernández, A, Cano-Rodríguez, I, Cordido, F, and Ballesteros-Pomar, MD. Effects of oral glutamine during abdominal radiotherapy on chronic radiation enteritis: a randomized controlled trial. Nutrition (2015) 31(1):200–4. doi:10.1016/j.nut.2014.08.003
17. Santivasi, WL, and Xia, F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid Redox Signal (2014) 21(2):251–9. doi:10.1089/ars.2013.5668
18. Amundson, SA, Lee, RA, Koch-Paiz, CA, Bittner, ML, Meltzer, P, Trent, JM, et al. Differential responses of stress genes to low dose-rate gamma irradiation. Mol Cancer Res (2003) 1(6):445–52.
19. Shakyawar, SK, Mishra, NK, Vellichirammal, NN, Cary, L, Helikar, T, Powers, R, et al. A review of radiation-induced alterations of multi-omic profiles, radiation injury biomarkers, and countermeasures. Radiat Res (2023) 199(1):89–111. doi:10.1667/RADE-21-00187.1
20. Oh, YJ, Kwak, MS, and Sung, MH. Protection of radiation-induced DNA damage by functional cosmeceutical poly-gamma-glutamate. J Microbiol Biotechnol (2018) 28(4):527–33. doi:10.4014/jmb.1712.12016
21. Bessout, R, Demarquay, C, Moussa, L, Rene, A, Doix, B, Benderitter, M, et al. TH17 predominant T-cell responses in radiation-induced bowel disease are modulated by treatment with adipose-derived mesenchymal stromal cells. J Pathol (2015) 237(4):435–46. doi:10.1002/path.4590
22. Zheng, Y, Pang, X, Zhu, X, Meng, Z, Chen, X, Zhang, J, et al. Lycium barbarum mitigates radiation injury via regulation of the immune function, gut microbiota, and related metabolites. Biomed Pharmacother (2021) 139:111654. doi:10.1016/j.biopha.2021.111654
23. Choy, MC, Visvanathan, K, and De Cruz, P. An overview of the innate and adaptive immune system in inflammatory bowel disease. Inflamm Bowel Dis (2017) 23(1):2–13. doi:10.1097/MIB.0000000000000955
24. Franken, L, Schiwon, M, and Kurts, C. Macrophages: sentinels and regulators of the immune system. Cell Microbiol (2016) 18(4):475–87. doi:10.1111/cmi.12580
25. Tindemans, I, Joosse, ME, and Samsom, JN. Dissecting the heterogeneity in T-cell mediated inflammation in IBD. Cells (2020) 9(1). doi:10.3390/cells9010110
26. Clough, JN, Omer, OS, Tasker, S, Lord, GM, and Irving, PM. Regulatory T-cell therapy in Crohn's disease: challenges and advances. Gut (2020) 69(5):942–52. doi:10.1136/gutjnl-2019-319850
27. Zhang, LL, Xu, JY, Xing, Y, Wu, P, Jin, YW, Wei, W, et al. Lactobacillus rhamnosus GG alleviates radiation-induced intestinal injury by modulating intestinal immunity and remodeling gut microbiota. Microbiol Res (2024) 286:127821. doi:10.1016/j.micres.2024.127821
28. Watters, D. Molecular mechanisms of ionizing radiation-induced apoptosis. Immunol Cell Biol (1999) 77(3):263–71. doi:10.1046/j.1440-1711.1999.00824.x
29. Purbey, PK, Scumpia, PO, Kim, PJ, Tong, AJ, Iwamoto, KS, McBride, WH, et al. Defined sensing mechanisms and signaling pathways contribute to the global inflammatory gene expression output elicited by ionizing radiation. Immunity (2017) 47(3):421–34. doi:10.1016/j.immuni.2017.08.017
30. Burma, S, and Chen, DJ. Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA Repair (Amst) (2004) 3(8-9):909–18. doi:10.1016/j.dnarep.2004.03.021
31. Bensimon, A, Aebersold, R, and Shiloh, Y. Beyond ATM: the protein kinase landscape of the DNA damage response. FEBS Lett (2011) 585(11):1625–39. doi:10.1016/j.febslet.2011.05.013
32. Chaplin, AK, Hardwick, SW, Liang, S, Kefala Stavridi, A, Hnizda, A, Cooper, LR, et al. Dimers of DNA-PK create a stage for DNA double-strand break repair. Nat Struct Mol Biol (2020) 28(1):13–9. doi:10.1038/s41594-020-00517-x
33. Zhu, R, Li, M, Wang, D, Liu, C, Xie, L, Yang, Y, et al. USP15 regulates radiation-induced DNA damage and intestinal injury through K48-linked deubiquitination and stabilisation of ATM. Mol Med (2024) 30(1):205. doi:10.1186/s10020-024-00984-8
34. Lavin, MF, and Kozlov, S. ATM activation and DNA damage response. Cell Cycle (2007) 6(8):931–42. doi:10.4161/cc.6.8.4180
35. Derheimer, FA, and Kastan, MB. Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS Lett (2010) 584(17):3675–81. doi:10.1016/j.febslet.2010.05.031
36. Chen, CC, Kass, EM, Yen, WF, Ludwig, T, Moynahan, ME, Chaudhuri, J, et al. ATM loss leads to synthetic lethality in BRCA1 BRCT mutant mice associated with exacerbated defects in homology-directed repair. Proc Natl Acad Sci U S A (2017) 114(29):7665–70. doi:10.1073/pnas.1706392114
37. Rybanska-Spaeder, I, Reynolds, TL, Chou, J, Prakash, M, Jefferson, T, Huso, DL, et al. 53BP1 is limiting for NHEJ repair in ATM-deficient model systems that are subjected to oncogenic stress or radiation. Mol Cancer Res (2013) 11(10):1223–34. doi:10.1158/1541-7786.MCR-13-0252-T
38. Savic, V, Yin, B, Maas, NL, Bredemeyer, AL, Carpenter, AC, Helmink, BA, et al. Formation of dynamic gamma-H2AX domains along broken DNA strands is distinctly regulated by ATM and MDC1 and dependent upon H2AX densities in chromatin. Mol Cell (2009) 34(3):298–310. doi:10.1016/j.molcel.2009.04.012
39. Lei, T, Du, S, Peng, Z, and Chen, L. Multifaceted regulation and functions of 53BP1 in NHEJ-mediated DSB repair. Int J Mol Med (2022) 50(1). doi:10.3892/ijmm.2022.5145
40. Falck, J, Mailand, N, Syljuasen, RG, Bartek, J, and Lukas, J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature (2001) 410(6830):842–7. doi:10.1038/35071124
41. Powers, JT, Hong, S, Mayhew, CN, Rogers, PM, Knudsen, ES, and Johnson, DG. E2F1 uses the ATM signaling pathway to induce p53 and Chk2 phosphorylation and apoptosis. Mol Cancer Res (2004) 2(4):203–14.
42. Pamment, J, Ramsay, E, Kelleher, M, Dornan, D, and Ball, KL. Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signalling pathway. Oncogene (2002) 21(51):7776–85. doi:10.1038/sj.onc.1205981
43. Bournique, E, Sanchez, A, Oh, S, Ghazarian, D, Mahieu, AL, Manjunath, L, et al. ATM and IRAK1 orchestrate two distinct mechanisms of NF-kappaB activation in response to DNA damage. Nat Struct Mol Biol (2025). doi:10.1038/s41594-024-01417-0
44. Lee, JH. Oxidative stress and the multifaceted roles of ATM in maintaining cellular redox homeostasis. Redox Biol (2024) 75:103269. doi:10.1016/j.redox.2024.103269
45. Garner, E, and Raj, K. Protective mechanisms of p53-p21-pRb proteins against DNA damage-induced cell death. Cell Cycle (2008) 7(3):277–82. doi:10.4161/cc.7.3.5328
46. Leibowitz, BJ, Qiu, W, Liu, H, Cheng, T, Zhang, L, and Yu, J. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol Cancer Res (2011) 9(5):616–25. doi:10.1158/1541-7786.MCR-11-0052
47. Li, L, and Clevers, H. Coexistence of quiescent and active adult stem cells in mammals. Science (2010) 327(5965):542–5. doi:10.1126/science.1180794
48. Barker, N, van Es, JH, Kuipers, J, Kujala, P, van den Born, M, Cozijnsen, M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature (2007) 449(7165):1003–7. doi:10.1038/nature06196
49. Qiu, W, Leibowitz, B, Zhang, L, and Yu, J. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene (2010) 29(11):1622–32. doi:10.1038/onc.2009.451
50. Liu, D, Ou, L, Clemenson, GD, Gage, FH, Xu, Y, Chao, C, et al. Puma is required for p53-induced depletion of adult stem cells. Nat Cell Biol (2010) 12(10):993–8. doi:10.1038/ncb2100
51. Hayden, MS, and Ghosh, S. NF-κB in immunobiology. Cell Res (2011) 21(2):223–44. doi:10.1038/cr.2011.13
52. Linard, C, Ropenga, A, Vozenin-Brotons, MC, Chapel, A, and Mathe, D. Abdominal irradiation increases inflammatory cytokine expression and activates NF-kappaB in rat ileal muscularis layer. Am J Physiol Gastrointest Liver Physiol (2003) 285(3):G556–65. doi:10.1152/ajpgi.00094.2003
53. Pajonk, F, Pajonk, K, and McBride, WH. Apoptosis and radiosensitization of hodgkin cells by proteasome inhibition. Int J Radiat Oncol Biol Phys (2000) 47(4):1025–32. doi:10.1016/s0360-3016(00)00516-2
54. Yang, CR, Wilson-Van Patten, C, Planchon, SM, Wuerzberger-Davis, SM, Davis, TW, Cuthill, S, et al. Coordinate modulation of Sp1, NF-kappa B, and p53 in confluent human malignant melanoma cells after ionizing radiation. FASEB J (2000) 14(2):379–90. doi:10.1096/fasebj.14.2.379
55. Mangoni, M, Sottili, M, Gerini, C, Desideri, I, Bastida, C, Pallotta, S, et al. A PPAR-gamma agonist protects from radiation-induced intestinal toxicity. United Eur Gastroenterol J (2017) 5(2):218–26. doi:10.1177/2050640616640443
56. Egan, LJ, Eckmann, L, Greten, FR, Chae, S, Li, ZW, Myhre, GM, et al. IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci U S A (2004) 101(8):2452–7. doi:10.1073/pnas.0306734101
57. Magne, N, Toillon, RA, Bottero, V, Didelot, C, Houtte, PV, Gerard, JP, et al. NF-kappaB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett (2006) 231(2):158–68. doi:10.1016/j.canlet.2005.01.022
58. Bentires-Alj, M, Dejardin, E, Viatour, P, Van Lint, C, Froesch, B, Reed, JC, et al. Inhibition of the NF-kappa B transcription factor increases Bax expression in cancer cell lines. Oncogene (2001) 20(22):2805–13. doi:10.1038/sj.onc.1204343
59. Ravi, R, Mookerjee, B, van Hensbergen, Y, Bedi, GC, Giordano, A, El-Deiry, WS, et al. p53-mediated repression of nuclear factor-kappaB RelA via the transcriptional integrator p300. Cancer Res (1998) 58(20):4531–6.
60. Ryan, KM, Ernst, MK, Rice, NR, and Vousden, KH. Role of NF-kappaB in p53-mediated programmed cell death. Nature (2000) 404(6780):892–7. doi:10.1038/35009130
61. Xu, Y, Wang, L, Liao, H, Li, X, Zhang, Y, Chen, X, et al. Loss of Nrf2 aggravates ionizing radiation-induced intestinal injury by activating the cGAS/STING pathway via Pirin. Cancer Lett (2024) 604:217218. doi:10.1016/j.canlet.2024.217218
62. Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol (2013) 53:401–26. doi:10.1146/annurev-pharmtox-011112-140320
63. Panieri, E, Telkoparan-Akillilar, P, Suzen, S, and Saso, L. The NRF2/KEAP1 Axis in the regulation of tumor metabolism: mechanisms and therapeutic perspectives. Biomolecules (2020) 10(5). doi:10.3390/biom10050791
64. Reisman, SA, Yeager, RL, Yamamoto, M, and Klaassen, CD. Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol Sci (2009) 108(1):35–47. doi:10.1093/toxsci/kfn267
65. Ngo, V, and Duennwald, ML. Nrf2 and oxidative stress: a general overview of mechanisms and implications in human disease. Antioxidants (Basel) (2022) 11(12). doi:10.3390/antiox11122345
66. Zhao, J, Zhi, Z, Zhang, M, Li, Q, Li, J, Wang, X, et al. Predictive value of single nucleotide polymorphisms in XRCC1 for radiation-induced normal tissue toxicity. Onco Targets Ther (2018) 11:3901–18. doi:10.2147/OTT.S156175
67. De Ruyck, K, Van Eijkeren, M, Claes, K, Morthier, R, De Paepe, A, Vral, A, et al. Radiation-induced damage to normal tissues after radiotherapy in patients treated for gynecologic tumors: association with single nucleotide polymorphisms in XRCC1, XRCC3, and OGG1 genes and in vitro chromosomal radiosensitivity in lymphocytes. Int J Radiat Oncol Biol Phys (2005) 62(4):1140–9. doi:10.1016/j.ijrobp.2004.12.027
68. Liu, Q, Peng, Q, Zhang, B, and Tan, Y. X-ray cross-complementing family: the bridge linking DNA damage repair and cancer. J Transl Med (2023) 21(1):602. doi:10.1186/s12967-023-04447-2
69. Wei, J, Wang, B, Wang, H, Meng, L, Zhao, Q, Li, X, et al. Radiation-Induced normal tissue damage: oxidative stress and epigenetic mechanisms. Oxid Med Cell Longev (2019) 2019:3010342. doi:10.1155/2019/3010342
70. Antwih, DA, Gabbara, KM, Lancaster, WD, Ruden, DM, and Zielske, SP. Radiation-induced epigenetic DNA methylation modification of radiation-response pathways. Epigenetics (2013) 8(8):839–48. doi:10.4161/epi.25498
71. Raiche, J, Rodriguez-Juarez, R, Pogribny, I, and Kovalchuk, O. Sex- and tissue-specific expression of maintenance and de novo DNA methyltransferases upon low dose X-irradiation in mice. Biochem Biophys Res Commun (2004) 325(1):39–47. doi:10.1016/j.bbrc.2004.10.002
72. Venkateswaran, K, Shrivastava, A, Agrawala, PK, Prasad, AK, Devi, SC, Manda, K, et al. Mitigation of radiation-induced gastro-intestinal injury by the polyphenolic acetate 7, 8-diacetoxy-4-methylthiocoumarin in mice. Sci Rep (2019) 9(1):14134. doi:10.1038/s41598-019-50785-x
73. Dahiya, A, Rehan, A, P, KA, and Dutta, A. Trichostatin A mitigates acute and late effects of radiation in intestine by regulation of DNA damage repair and Wnt/TGFbeta/Smad signaling. Int J Radiat Biol (2025) 101(1):15–27. doi:10.1080/09553002.2024.2430250
74. Dahiya, A, Sharma, S, Agrawala, PK, and Dutta, A. Histone deacetylase inhibitor, Trichostatin A mitigates ionizing radiation induced redox imbalance by regulating NRF2/GPX4/PINK1/PARKIN signaling in mice intestine. Mol Biol Rep (2024) 51(1):943. doi:10.1007/s11033-024-09867-x
75. Zhang, J, Shen, L, and Sun, LQ. The regulation of radiosensitivity by p53 and its acetylation. Cancer Lett (2015) 363(2):108–18. doi:10.1016/j.canlet.2015.04.015
76. Xue, Y, Barker, N, Hoon, S, He, P, Thakur, T, Abdeen, SR, et al. Bortezomib stabilizes and activates p53 in proliferative compartments of both normal and tumor tissues in vivo. Cancer Res (2019) 79(14):3595–607. doi:10.1158/0008-5472.CAN-18-3744
77. Sun, YW, Zhang, YY, Ke, XJ, Wu, XJ, Chen, ZF, and Chi, P. Pirfenidone prevents radiation-induced intestinal fibrosis in rats by inhibiting fibroblast proliferation and differentiation and suppressing the TGF-β1/Smad/CTGF signaling pathway. Eur J Pharmacol (2018) 822:199–206. doi:10.1016/j.ejphar.2018.01.027
78. Zheng, M, Liu, Z, and He, Y. Radiation-induced fibrosis: mechanisms and therapeutic strategies from an immune microenvironment perspective. Immunology (2024) 172(4):533–46. doi:10.1111/imm.13788
79. Gervaz, P, Morel, P, and Vozenin-Brotons, MC. Molecular aspects of intestinal radiation-induced fibrosis. Curr Mol Med (2009) 9(3):273–80. doi:10.2174/156652409787847164
80. Jun, S, Jung, YS, Suh, HN, Wang, W, Kim, MJ, Oh, YS, et al. LIG4 mediates Wnt signalling-induced radioresistance. Nat Commun (2016) 7:10994. doi:10.1038/ncomms10994
81. Bhanja, P, Norris, A, Gupta-Saraf, P, Hoover, A, and Saha, S. BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury. Stem Cell Res Ther (2018) 9(1):26. doi:10.1186/s13287-017-0763-3
82. Qi, Z, Li, Y, Zhao, B, Xu, C, Liu, Y, Li, H, et al. BMP restricts stemness of intestinal Lgr5(+) stem cells by directly suppressing their signature genes. Nat Commun (2017) 8:13824. doi:10.1038/ncomms13824
83. van Es, JH, van Gijn, ME, Riccio, O, van den Born, M, Vooijs, M, Begthel, H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature (2005) 435(7044):959–63. doi:10.1038/nature03659
84. Chen, SM, Guo, BJ, Feng, AQ, Wang, XL, Zhang, SL, and Miao, CY. Pathways regulating intestinal stem cells and potential therapeutic targets for radiation enteropathy. Mol Biomed (2024) 5(1):46. doi:10.1186/s43556-024-00211-0
85. Chen, G, Xu, H, Yao, Y, Xu, T, Yuan, M, Zhang, X, et al. BMP signaling in the development and regeneration of cranium bones and maintenance of calvarial stem cells. Front Cell Dev Biol (2020) 8:135. doi:10.3389/fcell.2020.00135
86. Guo, X, Du, L, Ma, N, Zhang, P, Wang, Y, Han, Y, et al. Monophosphoryl lipid A ameliorates radiation-induced lung injury by promoting the polarization of macrophages to the M1 phenotype. J Transl Med (2022) 20(1):597. doi:10.1186/s12967-022-03804-x
87. Ma, PF, Gao, CC, Yi, J, Zhao, JL, Liang, SQ, Zhao, Y, et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J Hepatol (2017) 67(4):770–9. doi:10.1016/j.jhep.2017.05.022
88. Yeh, MH, Chang, YH, Tsai, YC, Chen, SL, Huang, TS, Chiu, JF, et al. Bone marrow derived macrophages fuse with intestine stromal cells and contribute to chronic fibrosis after radiation. Radiother Oncol (2016) 119(2):250–8. doi:10.1016/j.radonc.2016.01.025
89. Zhao, Z, Cheng, W, Qu, W, Shao, G, and Liu, S. Antibiotic alleviates radiation-induced intestinal injury by remodeling microbiota, reducing inflammation, and inhibiting fibrosis. ACS Omega (2020) 5(6):2967–77. doi:10.1021/acsomega.9b03906
90. Shen, S, Zhang, M, Wang, X, Liu, Q, Su, H, Sun, B, et al. Single-cell RNA sequencing reveals S100a9(hi) macrophages promote the transition from acute inflammation to fibrotic remodeling after myocardial ischemia‒reperfusion. Theranostics (2024) 14(3):1241–59. doi:10.7150/thno.91180
91. Wei, L, Liu, M, Xiong, H, and Peng, B. Up-regulation of IL-23 expression in human dental pulp fibroblasts by IL-17 via activation of the NF-κB and MAPK pathways. Int Endod J (2018) 51(6):622–31. doi:10.1111/iej.12871
92. Fernandes, A, Oliveira, A, Soares, R, and Barata, P. The effects of ionizing radiation on gut microbiota, a systematic review. Nutrients (2021) 13(9). doi:10.3390/nu13093025
93. Fernandes, A, Oliveira, A, Soares, R, and Barata, P. The effects of ionizing radiation on gut microbiota: what can animal models tell us? A systematic review. Curr Issues Mol Biol (2023) 45(5):3877–910. doi:10.3390/cimb45050249
94. Stojanov, S, Berlec, A, and Strukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms (2020) 8(11). doi:10.3390/microorganisms8111715
95. Yu, Y, Lin, X, Feng, F, Wei, Y, Wei, S, Gong, Y, et al. Gut microbiota and ionizing radiation-induced damage: is there a link? Environ Res (2023) 229:115947. doi:10.1016/j.envres.2023.115947
96. Chen, YF, Li, SC, and Huang, EY. Role of microbiota in radiation-induced small-bowel damage. J Radiat Res (2024) 65(1):55–62. doi:10.1093/jrr/rrad084
97. Li, Y, Yan, H, Zhang, Y, Li, Q, Yu, L, Li, Q, et al. Alterations of the gut microbiome composition and lipid metabolic profile in radiation enteritis. Front Cell Infect Microbiol (2020) 10:541178. doi:10.3389/fcimb.2020.541178
98. Guo, H, Chou, WC, Lai, Y, Liang, K, Tam, JW, Brickey, WJ, et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science (2020) 370(6516). doi:10.1126/science.aay9097
99. Kalkeri, R, Walters, K, Van Der Pol, W, McFarland, BC, Fisher, N, Koide, F, et al. Changes in the gut microbiome community of nonhuman primates following radiation injury. BMC Microbiol (2021) 21(1). doi:10.1186/s12866-021-02146-w
100. Wang, A, Ling, Z, Yang, Z, Kiela, PR, Wang, T, Wang, C, et al. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study. PLoS One (2015) 10(5):e0126312. doi:10.1371/journal.pone.0126312
101. Paulsen, SR, Huprich, JE, Fletcher, JG, Booya, F, Young, BM, Fidler, JL, et al. CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases. Radiographics (2006) 26(3):641–57. doi:10.1148/rg.263055162
102. Fisher, JG, Kalb, B, Martin, D, Dhere, T, Perez, SD, and Srinivasan, JK. Abdominal MRI without enteral contrast accurately detects intestinal fibrostenosis in patients with inflammatory bowel disease. Am Surg (2015) 81(11):1118–24.
103. Withers, PJ, Bouman, C, Carmignato, S, Cnudde, V, Grimaldi, D, Hagen, CK, et al. X-ray computed tomography. Nat Rev Methods Primers (2021) 1(1). doi:10.1038/s43586-021-00015-4
104. Minordi, LM, Bevere, A, Papa, A, Larosa, L, and Manfredi, R. CT and mri evaluations in crohn's complications: a guide for the radiologist. Acad Radiol (2022) 29(8):1206–27. doi:10.1016/j.acra.2021.07.025
105. Deepak, P, Axelrad, JE, and Ananthakrishnan, AN. The role of the radiologist in determining disease severity in inflammatory bowel diseases. Gastrointest Endosc Clin N Am (2019) 29(3):447–70. doi:10.1016/j.giec.2019.02.006
106. Sun, B, Liu, J, Li, S, Lovell, JF, and Zhang, Y. Imaging of gastrointestinal tract ailments. J Imaging (2023) 9(6). doi:10.3390/jimaging9060115
107. Al-Mansour, MR, Caycedo-Marulanda, A, Davis, BR, Alawashez, A, Docimo, S, Qureshi, A, et al. SAGES TAVAC safety and efficacy analysis confocal laser endomicroscopy. Surg Endosc (2020) 35(5):2091–103. doi:10.1007/s00464-020-07607-3
108. Fugazza, A, Gaiani, F, Carra, MC, Brunetti, F, Levy, M, Sobhani, I, et al. Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases: a systematic review and meta-analysis. Biomed Res Int (2016) 2016:4638683. doi:10.1155/2016/4638683
109. Al-Gubory, KH. Shedding light on fibered confocal fluorescence microscopy: applications in biomedical imaging and therapies. J Biophotonics (2019) 12(11):e201900146. doi:10.1002/jbio.201900146
110. Shao, Y. MOEMS 3-D scan mirror for single-point control of beam deflection and focus. J Micro/Nanolithography, MEMS, MOEMS (2005) 4(4). doi:10.1117/1.2107127
111. Bojarski, C, Gunther, U, Rieger, K, Heller, F, Loddenkemper, C, Grunbaum, M, et al. In vivo diagnosis of acute intestinal graft-versus-host disease by confocal endomicroscopy. Endoscopy (2009) 41(5):433–8. doi:10.1055/s-0029-1214604
112. Neumann, H, Gunther, C, Vieth, M, Grauer, M, Wittkopf, N, Mudter, J, et al. Confocal laser endomicroscopy for in vivo diagnosis of Clostridium difficile associated colitis - a pilot study. PLoS One (2013) 8(3):e58753. doi:10.1371/journal.pone.0058753
113. Rogers, CJ, Kyubwa, EM, Lukaszewicz, AI, Starbird, MA, Nguyen, M, Copeland, BT, et al. Observation of unique circulating miRNA signatures in non-human primates exposed to total-body vs. Whole thorax lung irradiation. Radiat Res (2021) 196(5):547–59. doi:10.1667/RADE-21-00043.1
114. Sharma, S, Rehan, A, Kumar, S, Verma, YK, and Dutta, A. Uncovering the potential of Fatty Acid Binding Proteins for predicting radiation-induced gastrointestinal injury. Chem Biol Lett (2025) 12(1). doi:10.62110/sciencein.cbl.2025.v12.1255
115. Cheema, AK, Hinzman, CP, Mehta, KY, Hanlon, BK, Garcia, M, Fatanmi, OO, et al. Plasma derived exosomal biomarkers of exposure to ionizing radiation in nonhuman primates. Int J Mol Sci (2018) 19(11). doi:10.3390/ijms19113427
116. Ribault, A, Benadjaoud, MA, Squiban, C, Arnaud, L, Judicone, C, Leroyer, AS, et al. Circulating microvesicles correlate with radiation proctitis complication after radiotherapy. Sci Rep (2023) 13(1):2033. doi:10.1038/s41598-022-21726-y
117. Walker, V, Crijns, A, Langendijk, J, Spoor, D, Vliegenthart, R, Combs, SE, et al. Early detection of cardiovascular changes after radiotherapy for breast cancer: protocol for a European multicenter prospective cohort study (MEDIRAD EARLY HEART study). JMIR Res Protoc (2018) 7(10):e178. doi:10.2196/resprot.9906
118. Lefevre, JH, Amiot, A, Joly, F, Bretagnol, F, and Panis, Y. Risk of recurrence after surgery for chronic radiation enteritis. Br J Surg (2011) 98(12):1792–7. doi:10.1002/bjs.7655
119. Hu, J-N, Xu, X-Y, Li, W, Wang, Y-M, Liu, Y, Wang, Z, et al. Ginsenoside Rk1 ameliorates paracetamol-induced hepatotoxicity in mice through inhibition of inflammation, oxidative stress, nitrative stress and apoptosis. J Ginseng Res (2019) 43(1):10–9. doi:10.1016/j.jgr.2017.07.003
120. Song, JY, Han, SK, Bae, KG, Lim, DS, Son, SJ, Jung, IS, et al. Radioprotective effects of ginsan, an immunomodulator. Radiat Res (2003) 159(6):768–74. doi:10.1667/0033-7587(2003)159[0768:reogai]2.0.co;2
121. Cho, HT, Kim, JH, Heo, W, Lee, HS, Lee, JJ, Park, TS, et al. Explosively puffed ginseng ameliorates ionizing radiation-induced injury of colon by decreasing oxidative stress-related apoptotic cell execution in mice. J Med Food (2019) 22(5):490–8. doi:10.1089/jmf.2018.4293
122. Wang, Y, Su, P, Zhuo, Z, Jin, Y, Zeng, R, Wu, H, et al. Ginsenoside Rk1 attenuates radiation-induced intestinal injury through the PI3K/AKT/mTOR pathway. Biochem Biophysical Res Commun (2023) 643:111–20. doi:10.1016/j.bbrc.2022.12.072
123. Qin, H, Zhang, H, Zhang, X, Zhang, S, Zhu, S, and Wang, H. Resveratrol protects intestinal epithelial cells against radiation-induced damage by promoting autophagy and inhibiting apoptosis through SIRT1 activation. J Radiat Res (2021) 62(4):574–81. doi:10.1093/jrr/rrab035
124. Truong, VL, Jun, M, and Jeong, WS. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors (2018) 44(1):36–49. doi:10.1002/biof.1399
125. Jang, H, Lee, J, Park, S, Kim, JS, Shim, S, Lee, SB, et al. Baicalein mitigates radiation-induced enteritis by improving endothelial dysfunction. Front Pharmacol (2019) 10:892. doi:10.3389/fphar.2019.00892
126. Wang, M, Dong, Y, Wu, J, Li, H, Zhang, Y, Fan, S, et al. Baicalein ameliorates ionizing radiation-induced injuries by rebalancing gut microbiota and inhibiting apoptosis. Life Sci (2020) 261:118463. doi:10.1016/j.lfs.2020.118463
127. Cai, S, Xie, L-W, Xu, J-Y, Zhou, H, Yang, C, Tang, L-F, et al. (-)-Epigallocatechin-3-Gallate (EGCG) modulates the composition of the gut microbiota to protect against radiation-induced intestinal injury in mice. Front Oncol (2022) 12. doi:10.3389/fonc.2022.848107
128. Wu, S, Tian, C, Tu, Z, Guo, J, Xu, F, Qin, W, et al. Protective effect of total flavonoids of Engelhardia roxburghiana Wall. leaves against radiation-induced intestinal injury in mice and its mechanism. J Ethnopharmacology (2023) 311. doi:10.1016/j.jep.2023.116428
129. Wu, J, Duan, Y, Cui, J, Dong, Y, Li, H, Wang, M, et al. Protective effects of zingerone derivate on ionizing radiation-induced intestinal injury. J Radiat Res (2019) 60(6):740–6. doi:10.1093/jrr/rrz065
130. Liu, D, Peng, R, Chen, Z, Yu, H, Wang, S, Dong, S, et al. The protective effects of apigenin against radiation-induced intestinal injury. Dose Response (2022) 20(3):15593258221113791. doi:10.1177/15593258221113791
131. Wu, J, Gou, W, Wang, Z, Chang, H, Li, D, Hou, W, et al. Discovery of the radio-protecting effect of Ecliptae Herba, its constituents and targeting p53-mediated apoptosis in vitro and in vivo. Acta Pharmaceutica Sinica B (2023) 13(3):1216–30. doi:10.1016/j.apsb.2022.09.003
132. Sheng, L, Hu, F, Yu, H, Tao, X, Jia, R, Gu, Y, et al. Paeoniflorin inhibits ASK1-TF Axis by up-regulating SOCS3 to alleviate radiation enteritis. Front Pharmacol (2022) 13:743708. doi:10.3389/fphar.2022.743708
133. Zhu, X, Tian, X, Yang, M, Yu, Y, Zhou, Y, Gao, Y, et al. Procyanidin B2 promotes intestinal injury repair and attenuates colitis-associated tumorigenesis via suppression of oxidative stress in mice. Antioxid Redox Signal (2020) 35(2):75–92. doi:10.1089/ars.2019.7911
134. Lu, L, Jiang, M, Zhu, C, He, J, and Fan, S. Amelioration of whole abdominal irradiation-induced intestinal injury in mice with 3,3'-Diindolylmethane (DIM). Free Radic Biol Med (2019) 130:244–55. doi:10.1016/j.freeradbiomed.2018.10.410
135. Gu, J, Zhao, L, Chen, YZ, Guo, YX, Sun, Y, Guo, Q, et al. Preventive effect of sanguinarine on intestinal injury in mice exposed to whole abdominal irradiation. Biomed Pharmacother (2022) 146:112496. doi:10.1016/j.biopha.2021.112496
136. Khayyal, MT, Kreuter, MH, Kemmler, M, Altmann, P, Abdel-Naby, DH, and El-Ghazaly, MA. Effect of a chamomile extract in protecting against radiation-induced intestinal mucositis. Phytother Res (2019) 33(3):728–36. doi:10.1002/ptr.6263
137. Song, F, Wang, S, Pang, X, Fan, Z, Zhang, J, Chen, X, et al. An active fraction of trillium tschonoskii promotes the regeneration of intestinal epithelial cells after irradiation. Front Cell Dev Biol (2021) 9:745412. doi:10.3389/fcell.2021.745412
138. Liu, L, Lu, X, Li, S, and Zhang, P. Polysaccharides isolated from Hibiscus sabdariffa L. mitigate intestinal radiation injury through relieving mucosal inflammation and reshaping gut microbiota in mice. J Funct Foods (2024) 121. doi:10.1016/j.jff.2024.106395
139. Chen, Y-p, Shi, L-l, Li, Y-y, Zhang, Y-M, Zhang, S-z, Hou, H-d, et al. Isoliquiritigenin alleviates radiation-induced intestinal injury in lung cancer by inhibiting TNF-α/caspase3 signaling pathway. Naunyn-Schmiedeberg's Arch Pharmacol (2025). doi:10.1007/s00210-025-04007-z
140. Wang, J, Wong, YK, and Liao, F. What has traditional Chinese medicine delivered for modern medicine? Expert Rev Mol Med (2018) 20:e4. doi:10.1017/erm.2018.3
141. Yan, Z, Yin, B, Wang, Y, Ni, Z, Feng, J, Yang, Q, et al. Therapeutic mechanism of Liangxue-Guyuan-Yishen decoction on intestinal stem cells and tight junction proteins in gastrointestinal acute radiation syndrome rats. J Radiat Res (2023) 64(6):880–92. doi:10.1093/jrr/rrad065
142. Zhang, LY, Zhou, T, Zhang, YM, Xu, XM, Li, YY, Wei, KX, et al. Guiqi baizhu decoction alleviates radiation inflammation in rats by modulating the composition of the gut microbiota. Evid Based Complement Alternat Med (2020) 2020:9017854. doi:10.1155/2020/9017854
143. Li, Y, Zhang, L, Zhang, Y, Miao, Z, Liu, Z, Zhou, G, et al. Potential molecular mechanism of Guiqi Baizhu Decoction in radiation-induced intestinal edema by regulating HIF-1a, AQP4 and Na(+)/K(+)-ATPase. Phytomedicine (2022) 107:154445. doi:10.1016/j.phymed.2022.154445
144. Harata-Lee, Y, Qu, Z, Bateman, E, Xiao, X, Keller, MD, Bowen, J, et al. Compound Kushen injection reduces severity of radiation-induced gastrointestinal mucositis in rats. Front Oncol (2022) 12:929735. doi:10.3389/fonc.2022.929735
145. Li, Z, Gao, Y, Du, L, Yuan, Y, Huang, W, Fu, X, et al. Anti-inflammatory and anti-apoptotic effects of Shaoyao decoction on X-ray radiation-induced enteritis of C57BL/6 mice. J Ethnopharmacol (2022) 292:115158. doi:10.1016/j.jep.2022.115158
146. Shukla, PK, Gangwar, R, Manda, B, Meena, AS, Yadav, N, Szabo, E, et al. Rapid disruption of intestinal epithelial tight junction and barrier dysfunction by ionizing radiation in mouse colon in vivo: protection by N-acetyl-l-cysteine. Am J Physiol Gastrointest Liver Physiol (2016) 310(9):G705–15. doi:10.1152/ajpgi.00314.2015
147. Mercantepe, F, Topcu, A, Rakici, S, Tumkaya, L, and Yilmaz, A. The effects of N-acetylcysteine on radiotherapy-induced small intestinal damage in rats. Exp Biol Med (Maywood) (2019) 244(5):372–9. doi:10.1177/1535370219831225
148. Liu, W, Chen, Q, Wu, S, Xia, X, Wu, A, Cui, F, et al. Radioprotector WR-2721 and mitigating peptidoglycan synergistically promote mouse survival through the amelioration of intestinal and bone marrow damage. J Radiat Res (2015) 56(2):278–86. doi:10.1093/jrr/rru100
149. Jeong, BK, Song, JH, Jeong, H, Choi, HS, Jung, JH, Hahm, JR, et al. Effect of alpha-lipoic acid on radiation-induced small intestine injury in mice. Oncotarget (2016) 7(12):15105–17. doi:10.18632/oncotarget.7874
150. Taniguchi, CM, Miao, YR, Diep, AN, Wu, C, Rankin, EB, Atwood, TF, et al. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci Transl Med (2014) 6(236):236ra64. doi:10.1126/scitranslmed.3008523
151. Singh, VK, Wise, SY, Singh, PK, Posarac, A, Fatanmi, OO, Ducey, EJ, et al. Alpha-tocopherol succinate-mobilized progenitors improve intestinal integrity after whole body irradiation. Int J Radiat Biol (2013) 89(5):334–45. doi:10.3109/09553002.2013.762137
152. Ghosh, SP, Kulkarni, S, Perkins, MW, Hieber, K, Pessu, RL, Gambles, K, et al. Amelioration of radiation-induced hematopoietic and gastrointestinal damage by Ex-RAD(R) in mice. J Radiat Res (2012) 53(4):526–36. doi:10.1093/jrr/rrs001
153. Zhang, L, Sun, W, Wang, J, Zhang, M, Yang, S, Tian, Y, et al. Mitigation effect of an FGF-2 peptide on acute gastrointestinal syndrome after high-dose ionizing radiation. Int J Radiat Oncol Biol Phys (2010) 77(1):261–8. doi:10.1016/j.ijrobp.2009.11.026
154. Rotolo, J, Stancevic, B, Zhang, J, Hua, G, Fuller, J, Yin, X, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest (2012) 122(5):1786–90. doi:10.1172/JCI59920
155. Rotolo, JA, Fong, CS, Bodo, S, Nagesh, PK, Fuller, J, Sharma, T, et al. Anti-ceramide single-chain variable fragment mitigates radiation GI syndrome mortality independent of DNA repair. JCI Insight (2021) 6(8). doi:10.1172/jci.insight.145380
156. Vercellino, J, Malachowska, B, Kulkarni, S, Bell, BI, Shajahan, S, Shinoda, K, et al. Thrombopoietin mimetic stimulates bone marrow vascular and stromal niches to mitigate acute radiation syndrome. Res Sq (2024). doi:10.21203/rs.3.rs-3946910/v1
157. Deng, W, Kimura, Y, Gududuru, V, Wu, W, Balogh, A, Szabo, E, et al. Mitigation of the hematopoietic and gastrointestinal acute radiation syndrome by octadecenyl thiophosphate, a small molecule mimic of lysophosphatidic acid. Radiat Res (2015) 183(4):465–75. doi:10.1667/RR13830.1
158. Zhang, J, Li, K, Zhang, Q, Zhu, Z, Huang, G, and Tian, H. Polycysteine as a new type of radio-protector ameliorated tissue injury through inhibiting ferroptosis in mice. Cell Death & Dis (2021) 12(2). doi:10.1038/s41419-021-03479-0
159. Andreassen, CN, Grau, C, and Lindegaard, JC. Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy. Semin Radiat Oncol (2003) 13(1):62–72. doi:10.1053/srao.2003.50006
160. Cheema, AK, Li, Y, Girgis, M, Jayatilake, M, Simas, M, Wise, SY, et al. Metabolomic studies in tissues of mice treated with amifostine and exposed to gamma-radiation. Sci Rep (2019) 9(1):15701. doi:10.1038/s41598-019-52120-w
161. Crook, A, De Lima, LA, Payne, T, Bhinderwala, F, Woods, J, Singh, VK, et al. Radiation exposure induces cross-species temporal metabolic changes that are mitigated in mice by amifostine. Sci Rep (2021) 11(1):14004. doi:10.1038/s41598-021-93401-7
162. Hamama, S, Gilbert-Sirieix, M, Vozenin, MC, and Delanian, S. Radiation-induced enteropathy: molecular basis of pentoxifylline-vitamin E anti-fibrotic effect involved TGF-β1 cascade inhibition. Radiother Oncol (2012) 105(3):305–12. doi:10.1016/j.radonc.2012.08.023
163. Singh, VK, Kulkarni, S, Fatanmi, OO, Wise, SY, Newman, VL, Romaine, PL, et al. Radioprotective efficacy of gamma-tocotrienol in nonhuman primates. Radiat Res (2016) 185(3):285–98. doi:10.1667/RR14127.1
164. Singh, VK, and Hauer-Jensen, M. γ-Tocotrienol as a promising countermeasure for acute radiation syndrome: current status. Int J Mol Sci (2016) 17(5). doi:10.3390/ijms17050663
165. Rosen, E, Fatanmi, OO, Wise, SY, Rao, VA, and Singh, VK. Gamma-tocotrienol, a radiation countermeasure, reverses proteomic changes in serum following total-body gamma irradiation in mice. Sci Rep (2022) 12(1):3387. doi:10.1038/s41598-022-07266-5
166. Gluzman-Poltorak, Z, Mendonca, SR, Vainstein, V, Kha, H, and Basile, LA. Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation. J Hematol Oncol (2014) 7:31. doi:10.1186/1756-8722-7-31
167. Singh, VK, and Seed, TM. An update on sargramostim for treatment of acute radiation syndrome. Drugs Today (Barc) (2018) 54(11):679–93. doi:10.1358/dot.2018.54.11.2899370
169. Burnett, AF, Biju, PG, Lui, H, and Hauer-Jensen, M. Oral interleukin 11 as a countermeasure to lethal total-body irradiation in a murine model. Radiat Res (2013) 180(6):595–602. doi:10.1667/rr13330.1
170. Hauer-Jensen, M. Toward development of interleukin-11 as a medical countermeasure for use in radiological/nuclear emergencies. Dig Dis Sci (2014) 59(7):1349–51. doi:10.1007/s10620-014-3074-x
171. Kumar, VP, Biswas, S, Sharma, NK, Stone, S, Fam, CM, Cox, GN, et al. PEGylated IL-11 (BBT-059): a novel radiation countermeasure for hematopoietic acute radiation syndrome. Health Phys (2018) 115(1):65–76. doi:10.1097/HP.0000000000000841
172. Krivokrysenko, VI, Toshkov, IA, Gleiberman, AS, Krasnov, P, Shyshynova, I, Bespalov, I, et al. The toll-like receptor 5 agonist entolimod mitigates lethal acute radiation syndrome in non-human primates. PLoS One (2015) 10(9):e0135388. doi:10.1371/journal.pone.0135388
173. Krivokrysenko, VI, Shakhov, AN, Singh, VK, Bone, F, Kononov, Y, Shyshynova, I, et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J Pharmacol Exp Ther (2012) 343(2):497–508. doi:10.1124/jpet.112.196071
174. Singh, VK, and Seed, TM. Entolimod as a radiation countermeasure for acute radiation syndrome. Drug Discov Today (2020) 26(1):17–30. doi:10.1016/j.drudis.2020.10.003
175. Legeza, VI, Grebenyuk, AN, and Drachev, IS. Radiomitigators: classification, pharmacological properties, and application prospects. Biol Bull (2020) 46(12):1625–32. doi:10.1134/s1062359019120045
176. Inc, S. OrbeShield™ for gastrointestinal acute radiation syndrome (GI ARS). Princeton, NJ: Soligenix Inc (2016). Available online at: http://www.soligenix.com/pipeline/vaccinesbiodefense/orbeshield-for-gastrointestinal-acute-radiation-syndrome-gi-ars/.
177. Dai, L, Bai, A, Smith, CD, Rodriguez, PC, Yu, F, and Qin, Z. ABC294640, A novel sphingosine kinase 2 inhibitor, induces oncogenic virus-infected cell autophagic death and represses tumor growth. Mol Cancer Ther (2017) 16(12):2724–34. doi:10.1158/1535-7163.MCT-17-0485
178. Britten, CD, Garrett-Mayer, E, Chin, SH, Shirai, K, Ogretmen, B, Bentz, TA, et al. A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin Cancer Res (2017) 23(16):4642–50. doi:10.1158/1078-0432.CCR-16-2363
179. Kantara, C, Moya, SM, Houchen, CW, Umar, S, Ullrich, RL, Singh, P, et al. Novel regenerative peptide TP508 mitigates radiation-induced gastrointestinal damage by activating stem cells and preserving crypt integrity. Lab Invest (2015) 95(11):1222–33. doi:10.1038/labinvest.2015.103
180. Nag, D, Bhanja, P, Riha, R, Sanchez-Guerrero, G, Kimler, BF, Tsue, TT, et al. Auranofin protects intestine against radiation injury by modulating p53/p21 pathway and radiosensitizes human colon tumor. Clin Cancer Res (2019) 25(15):4791–807. doi:10.1158/1078-0432.CCR-18-2751
181. Lee, ES, Kim, JS, Lee, H, Ryu, JY, Lee, HJ, Sonn, JK, et al. Auranofin, an anti-rheumatic gold Drug, aggravates the radiation-induced acute intestinal injury in mice. Front Pharmacol (2019) 10:417. doi:10.3389/fphar.2019.00417
182. Jang, H, Kim, S, Kim, H, Oh, SH, Kwak, SY, Joo, HW, et al. Metformin protects the intestinal barrier by activating goblet cell maturation and epithelial proliferation in radiation-induced enteropathy. Int J Mol Sci (2022) 23(11). doi:10.3390/ijms23115929
183. Yang, JY, Liu, MJ, Lv, L, Guo, JR, He, KY, Zhang, H, et al. Metformin alleviates irradiation-induced intestinal injury by activation of FXR in intestinal epithelia. Front Microbiol (2022) 13:932294. doi:10.3389/fmicb.2022.932294
184. Jang, H, Lee, J, Park, S, Myung, H, Kang, J, Kim, K, et al. Pravastatin attenuates acute radiation-induced enteropathy and improves epithelial cell function. Front Pharmacol (2018) 9:1215. doi:10.3389/fphar.2018.01215
185. Kwak, SY, Jang, WI, Park, S, Cho, SS, Lee, SB, Kim, MJ, et al. Metallothionein 2 activation by pravastatin reinforces epithelial integrity and ameliorates radiation-induced enteropathy. EBioMedicine (2021) 73:103641. doi:10.1016/j.ebiom.2021.103641
186. Lee, JS, Jeon, SW, Lee, HS, Kwon, YH, Nam, SY, Bae, HI, et al. Rebamipide for the improvement of gastric atrophy and intestinal metaplasia: a prospective, randomized, pilot study. Dig Dis Sci (2022) 67(6):2395–402. doi:10.1007/s10620-021-07038-7
187. Shim, S, Jang, HS, Myung, HW, Myung, JK, Kang, JK, Kim, MJ, et al. Rebamipide ameliorates radiation-induced intestinal injury in a mouse model. Toxicol Appl Pharmacol (2017) 329:40–7. doi:10.1016/j.taap.2017.05.012
188. Kwak, SY, Shim, S, Park, S, Kim, H, Lee, SJ, Kim, MJ, et al. Ghrelin reverts intestinal stem cell loss associated with radiation-induced enteropathy by activating Notch signaling. Phytomedicine (2020) 81:153424. doi:10.1016/j.phymed.2020.153424
189. Kiang, JG, Smith, JT, Cannon, G, Anderson, MN, Ho, C, Zhai, M, et al. Ghrelin, a novel therapy, corrects cytokine and NF-κB-AKT-MAPK network and mitigates intestinal injury induced by combined radiation and skin-wound trauma. Cell Biosci (2020) 10:63. doi:10.1186/s13578-020-00425-z
190. Finch, PW, Mark Cross, LJ, McAuley, DF, and Farrell, CL. Palifermin for the protection and regeneration of epithelial tissues following injury: new findings in basic research and pre-clinical models. J Cell Mol Med (2013) 17(9):1065–87. doi:10.1111/jcmm.12091
191. Lauritano, D, Petruzzi, M, Di Stasio, D, and Lucchese, A. Clinical effectiveness of palifermin in prevention and treatment of oral mucositis in children with acute lymphoblastic leukaemia: a case-control study. Int J Oral Sci (2014) 6(1):27–30. doi:10.1038/ijos.2013.93
192. Mortezaee, K, Shabeeb, D, Musa, AE, Najafi, M, and Farhood, B. Metformin as a radiation modifier; implications to normal tissue protection and tumor sensitization. Curr Clin Pharmacol (2019) 14(1):41–53. doi:10.2174/1574884713666181025141559
193. Palsson-McDermott, EM, and O'Neill, LAJ. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res (2020) 30(4):300–14. doi:10.1038/s41422-020-0291-z
194. Stancevic, B, and Kolesnick, R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett (2010) 584(9):1728–40. doi:10.1016/j.febslet.2010.02.026
195. Marathe, S, Schissel, SL, Yellin, MJ, Beatini, N, Mintzer, R, Williams, KJ, et al. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J Biol Chem (1998) 273(7):4081–8. doi:10.1074/jbc.273.7.4081
196. Santana, P, Pena, LA, Haimovitz-Friedman, A, Martin, S, Green, D, McLoughlin, M, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell (1996) 86(2):189–99. doi:10.1016/s0092-8674(00)80091-4
197. Cherdyntseva, N, Shishkina, A, Butorin, I, Murase, H, Gervas, P, and Kagiya, TV. Effect of tocopherol-monoglucoside (TMG), a water-soluble glycosylated derivate of vitamin E, on hematopoietic recovery in irradiated mice. J Radiat Res (2005) 46(1):37–41. doi:10.1269/jrr.46.37
198. Semont, A, Francois, S, Mouiseddine, M, Francois, A, Sache, A, Frick, J, et al. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol (2006) 585:19–30. doi:10.1007/978-0-387-34133-0_2
199. Gong, W, Guo, M, Han, Z, Wang, Y, Yang, P, Xu, C, et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis (2016) 7(9):e2387. doi:10.1038/cddis.2016.276
200. Francois, M, Birman, E, Forner, KA, Gaboury, L, and Galipeau, J. Adoptive transfer of mesenchymal stromal cells accelerates intestinal epithelium recovery of irradiated mice in an interleukin-6-dependent manner. Cytotherapy (2012) 14(10):1164–70. doi:10.3109/14653249.2012.684378
201. Saha, S, Bhanja, P, Kabarriti, R, Liu, L, Alfieri, AA, and Guha, C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One (2011) 6(9):e24072. doi:10.1371/journal.pone.0024072
202. Chang, P, Qu, Y, Liu, Y, Cui, S, Zhu, D, Wang, H, et al. Multi-therapeutic effects of human adipose-derived mesenchymal stem cells on radiation-induced intestinal injury. Cell Death Dis (2013) 4(6):e685. doi:10.1038/cddis.2013.178
203. Gao, Z, Zhang, Q, Han, Y, Cheng, X, Lu, Y, Fan, L, et al. Mesenchymal stromal cell-conditioned medium prevents radiation-induced small intestine injury in mice. Cytotherapy (2012) 14(3):267–73. doi:10.3109/14653249.2011.616194
204. Linard, C, Busson, E, Holler, V, Strup-Perrot, C, Lacave-Lapalun, JV, Lhomme, B, et al. Repeated autologous bone marrow-derived mesenchymal stem cell injections improve radiation-induced proctitis in pigs. Stem Cells Transl Med (2013) 2(11):916–27. doi:10.5966/sctm.2013-0030
205. Accarie, A, l'Homme, B, Benadjaoud, MA, Lim, SK, Guha, C, Benderitter, M, et al. Extracellular vesicles derived from mesenchymal stromal cells mitigate intestinal toxicity in a mouse model of acute radiation syndrome. Stem Cell Res Ther (2020) 11(1):371. doi:10.1186/s13287-020-01887-1
206. Li, H, Jiang, M, Zhao, SY, Zhang, SQ, Lu, L, He, X, et al. Exosomes are involved in total body irradiation-induced intestinal injury in mice. Acta Pharmacol Sin (2021) 42(7):1111–23. doi:10.1038/s41401-021-00615-6
207. Yang, L, Fang, C, Song, C, Zhang, Y, Zhang, R, and Zhou, S. Mesenchymal stem cell-derived exosomes are effective for radiation enteritis and essential for the proliferation and differentiation of Lgr5(+) intestinal epithelial stem cells by regulating mir-195/akt/beta-catenin pathway. Tissue Eng Regen Med (2023) 20(5):739–51. doi:10.1007/s13770-023-00541-0
208. Zachariah, B, Gwede, CK, James, J, Ajani, J, Chin, LJ, Donath, D, et al. Octreotide acetate in prevention of chemoradiation-induced diarrhea in anorectal cancer: randomized RTOG trial 0315. J Natl Cancer Inst (2010) 102(8):547–56. doi:10.1093/jnci/djq063
209. Pilepich, MV, Paulus, R, St Clair, W, Brasacchio, RA, Rostock, R, and Miller, RC. Phase III study of pentosanpolysulfate (PPS) in treatment of gastrointestinal tract sequelae of radiotherapy. Am J Clin Oncol (2006) 29(2):132–7. doi:10.1097/01.coc.0000203758.77490.fd
210. Tamarat, R, Satyamitra, MM, Benderitter, M, and DiCarlo, AL. Radiation-induced gastrointestinal and cutaneous injuries: understanding models, pathologies, assessments, and clinically accepted practices. Int J Radiat Biol (2024) 100(7):969–81. doi:10.1080/09553002.2024.2356544
211. Voswinkel, J, Francois, S, Gorin, NC, and Chapel, A. Gastro-intestinal autoimmunity: preclinical experiences and successful therapy of fistulizing bowel diseases and gut Graft versus host disease by mesenchymal stromal cells. Immunol Res (2013) 56(2-3):241–8. doi:10.1007/s12026-013-8397-8
212. Chitapanarux, I, Chitapanarux, T, Traisathit, P, Kudumpee, S, Tharavichitkul, E, and Lorvidhaya, V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol (2010) 5:31. doi:10.1186/1748-717X-5-31
213. Demers, M, Dagnault, A, and Desjardins, J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr (2014) 33(5):761–7. doi:10.1016/j.clnu.2013.10.015
Keywords: radiation-induced intestinal injury, pathogenesis, diagnosis, biomarkers, treatment strategies
Citation: Wu W, Cai Y, Yang Z, Chen M, Hu J, Qu K and Yang J (2025) Radiation-induced intestinal injury: from molecular mechanisms to clinical translation. Oncol. Rev. 19:1613704. doi: 10.3389/or.2025.1613704
Received: 17 April 2025; Accepted: 05 August 2025;
Published: 29 August 2025.
Edited by:
Deepa Kushwaha, Rare Genomics Institute, United StatesReviewed by:
Filippo Torrisi, University of Catania, ItalyWenbin Hou, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2025 Wu, Cai, Yang, Chen, Hu, Qu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Yang, eWFuZzk3MTI0QHNvaHUuY29t
†ORCID: Wen-Jue Wu, orcid.org/0000-0001-7996-7406; Jian Yang, orcid.org/0000-0002-3790-1545
 Wenjue Wu
Wenjue Wu Yubo Cai
Yubo Cai