- 1Department of GI Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 2Department of Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 3Department of Interventional Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Radiation therapy (RT) and locoregional ablation are cornerstones of modern oncology, yet their therapeutic potential is frequently limited by the challenge of sparing healthy organs-at-risk (OARs) from treatment-related complications. Temporary organ displacement (TOD) techniques directly address this issue by creating a physical separation using ‘spacers’ during treatment, thereby minimizing collateral damage while enhancing therapeutic precision. The clinical benefits, including improved tumor control, reduced morbidity, and enhanced survival, are documented across malignancies of the head and neck, thorax, abdomen, and pelvis. To create a unified framework for this evolving field, this comprehensive review provides a systematic classification of TOD techniques based on invasiveness, administration, device technology and the accompanying treatment mo`dality. Furthermore, we synthesize key historical and recent innovations, from non-invasive maneuvers to advanced surgical spacers, to contextualize current practices. Finally, we address barriers to standardization and highlight emerging concepts such as meta-materials, computational modeling, and digital twins, which provide promising avenues for enhancing personalized cancer care and patient outcomes.
1 Introduction
Radiation toxicity and thermal injuries continue to pose significant clinical challenges in the management of cancer when using radiation therapy (RT) and locoregional ablation such as microwave ablation (MWA), radiofrequency ablation (RFA), and cryoablation (CA). Although these treatments are effective in targeting tumors, they can inadvertently damage adjacent organs-at-risk (OARs), resulting to acute and chronic complications that adversely affect the patient’s quality of life. Radiation toxicities arising from RT may present as mucositis, dermatitis, pneumonitis, and reduced blood cell counts (1). In contrast, locoregional ablations can cause pain, bleeding, abscess, and infection due to thermal damage or perforation of OARs involving the gastrointestinal (GI) tract, gallbladder, bile ducts, blood vessels, nerves, diaphragm, kidneys, or ureters (2–4). The severity of these toxicities is influenced by factors such as the total radiation dose or ablation power and duration, the extent of treatment margins, and the proximity of the tumor to OARs. To overcome these challenges, several strategies were established to enhance the safety and effectiveness of treatment. These approaches include the use of optimized fractionation schedules to minimize dose toxicities (5–7), the application of advanced stereotactic imaging and simulations for more precise tumor targeting and OAR delineation (6, 8–10), and the administration of protective agents to minimize toxicities (11–13).
As cancer treatment technologies became increasingly complex, driven by advanced imaging guidance and multi-modal therapies, temporary organ displacement (TOD) remains a simple yet often overlooked concept for protecting OARs. In the clinic, TOD techniques aim to physically separate adjacent OARs from treatment regions using a ‘spacer’ to avoid dose toxicities or injuries. Over time, a range of spacers were introduced to complement RT and locoregional ablation therapies, either by using the patient’s native tissues, repurposing existing implants or by developing new materials, ranging from liquid to solid forms, to improve local tumor control and overall survival (OS) (14–16). More specifically, advances in TOD techniques have led to enhanced effectiveness in maximizing treatment outcomes for numerous cancers involving the prostate (15, 17–19), pelvic (20), vagina (21), pancreas (22), liver (23–28), lung (29–31), peripheral nerves (32), and the head and neck (HNC) (33–37).
Early studies demonstrated the successful clinical translation of spacers, proving their safety, efficacy, and tolerability. These studies also showed that spacers effectively reduced acute side effects after prostate and cervical RT (38–40) and locoregional thermal ablation of the liver, kidney, lung, and bone (24, 28). Tang et al. (2018) provided a systematic review of spacers and their clinical implementation in RT (14), while Garnon et al. (2019) comprehensively review adjunctive thermoprotection strategies, specifically outlining techniques and materials designed to safeguard OARs during thermal ablation (24). Subsequently, reviews by Vaggers et al. (2021) (41) and Lapuz et al. (2024) (42) specifically addressed the use of hydrogel-based spacers in patients with prostate and gynecological cancers receiving brachytherapy (BT), respectively. Building on existing literature, our review provides a comprehensive and systematic classification of both historical and contemporary TOD techniques, detailing their invasiveness, modes of delivery, clinical applications, and device technologies. We synthesize key technical developments and critically assess the factors influencing their successful integration into radiotherapy and locoregional ablation workflows. In addition, we address current limitations in standardization and clinical adoption and explore promising opportunities for next-generation TOD solutions. Special attention is given to emerging trends such as personalized spacer design, advanced customization, and the integration of digital twin (DT) platforms, which hold the potential to further enhance precision and patient outcomes in cancer treatment.
2 A brief history of TOD in cancer therapy
In the late 1950s, awareness grew towards preserving ovarian function in patients with genitourinary (GU) cancers during RT (43–45). Notably, Batten and Brown’s clinical report in 1956 described an early form of organ displacement, using lead shells to shield ovaries from the radiation field (46). This principle was extended over the following decades; for instance, Steckel et al. demonstrated the use of catheter balloon occlusions in 1974 to protect against radiation-induced organ damage (47). Later, the introduction of adjunctive RT to treat colorectal adenocarcinomas led to complications such as radiation enteritis, prompting pre-operative RT involving abdominoperineal resection (48–50). In 1980, Gunderson et al. introduced the concept of ‘Operative displacement’ showcasing various techniques to protect OARs during post-operative RT, utilizing surgical techniques to displace and immobilize OARs (51). In 1985, surgical organ displacement methods such as ‘omental envelope’ (also known as ‘omental pedicle flap’ or ‘omental sling’) and retroverting the uterus were introduced, to protect the small intestines during pelvic radiation or to avoid potential risk of infertility, respectively (51–53).
At the same time, the proliferation of thermal ablation therapies in interventional radiology (IR) created the urgent need to protect OARs. Consequently, hydrodissection was widely adopted as a protective measure to prevent complications like necrosis or perforation by creating a fluid buffer that physically displaces healthy tissues from the ablation zone. This technique remains a cornerstone of safety in modern RFA, MWA, and CA procedures. Taking inspiration from lens-cortex separation (54) in the early 1980s, modern hydrodissection procedures in IR involves the injection of saline and takes advantage of spaces between the liver or kidney and diaphragm, body wall, bowel, pancreas or stomach. In the early 2000s, hydrodissection was shown to prevent nerve and bowel damage during the RFA of renal tumors (12, 55). When hydrodissection proves insufficient, other techniques are employed, including gas insufflation (56), balloon interposition (57) or probe manipulation (58). While both RT and thermal ablation use displacement techniques like balloons, their applications differ significantly. Organ displacement for image-guided thermal ablation is typically a one-time maneuver performed during the procedure, whereas in RT, the displacement must be reliably reproduced over several days for both treatment simulation and delivery (57, 59). Over time, technological innovations have enhanced material biocompatibility and patient safety, expanding the use of TOD techniques to various cancer types and the conception of more sophisticated spacers.
3 Classifications of TOD
We classify TODs primarily on invasiveness and mode of delivery followed by the specific methods and the necessary devices to achieve successful organ displacement. Figure 1 illustrates the nuances behind these classifications and show how they compare across different treatment modalities involving external beam RT (EBRT), BT, CA, or heat ablation (MWA, RFA, laser).
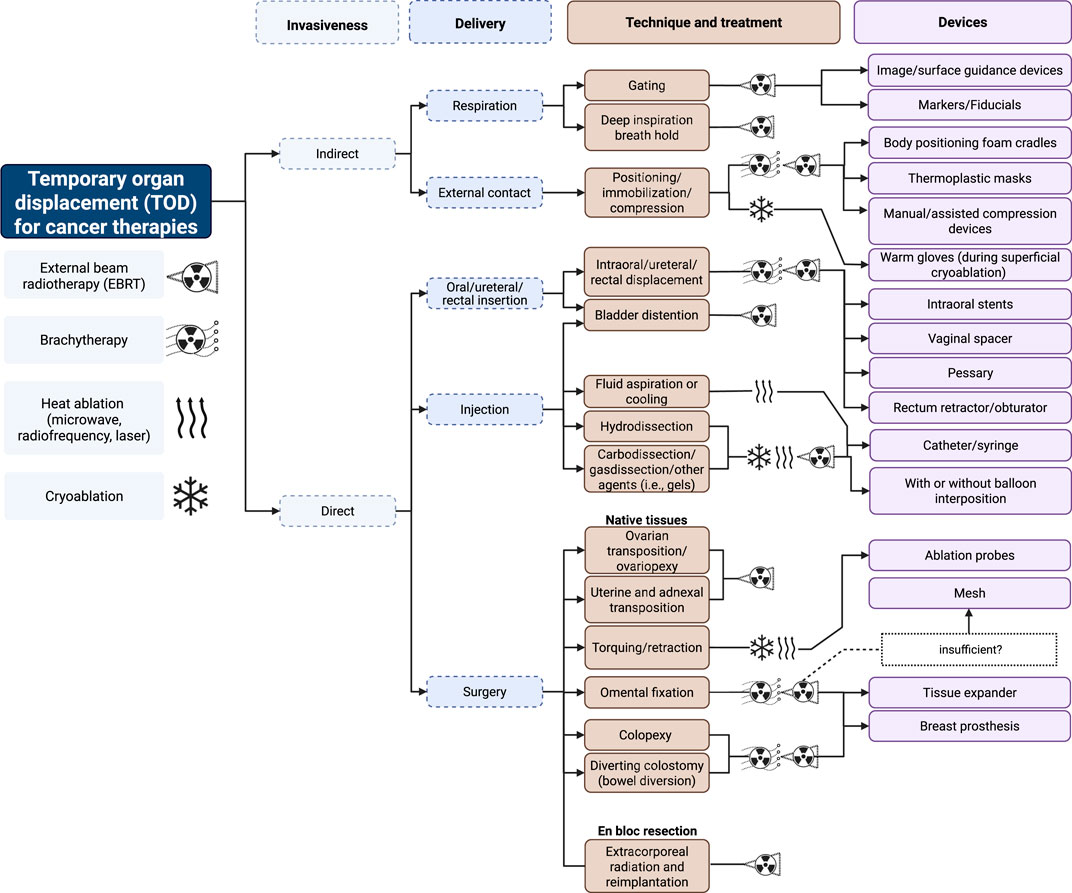
Figure 1. Classifications of TOD in cancer therapy. Created with BioRender.com.
3.1 Indirect TOD
RT commonly employs indirect TOD techniques that makes use of physiological actions or external/physical aids to displace OARs. These techniques include deep inspiration breath hold (DIBH) and various immobilization methods, such as thermoplastic molds and specialized cradles. More specifically, this section explores two primary indirect TOD techniques for organ displacement: physiological manipulation and external contact.
3.1.1 Physiological manipulation (swallowing and respiration)
Patients are often instructed to perform DIBH during RT to control their respiratory movements and lung-heart positions to minimize unwanted dose toxicities (see Figure 2) (60). Previous studies have demonstrated that DIBH can reduce non-target mean doses to the heart (15%–63%), medulla (15%–16%), and esophagus (6%–8%) without compromising target volumes during lung RT (61–64). Josipovic et al. (2019) ran a prospective trial assessing 69 patients with locally advanced non-small cell lung cancer (NSCLC) undergoing definitive RT, showing high compliance and target reproducibility in 50/69 patients (29). Another prospective trial also showed the efficacy of DIBH in patients with stage I/II breast cancer using an active breathing coordinator (ABC) device, showing a reduction in the affected heart volume during RT (with a mean absolute reduction of 1.5% in the heart expected tissue complication probability) (65). Similarly, the ABC device has been studied in patients with unresectable intrahepatic tumors undergoing RT, showing a reduction in normal liver irradiation while maximizing high-dose administration, and maintaining diaphragm and liver positions (66). While DIBH techniques and respiratory gating offer advantages in minimizing doses to OARs, it is important to note that the successful implementation of these methods depends on several factors guiding patient selection. These include the patient’s respiratory capabilities, the associated costs, convenience, and the potential benefits based on the tumor’s size, location, and type (67).

Figure 2. Comparison of Dose-Volume Histogram (DVH) during free breathing (FB) and deep inspiration breath hold (DIBH). (a, b) axial CT scan of the breast during FB (a) and DIBH (b). The red line shows the radiation field path used for RT. (c) the DVH plot illustrates dose reductions in the left anterior descending artery (LAD), left lung, and heart during DIBH (solid lines) vs. FB (dotted lines).
3.1.2 External contact
Prior literature demonstrated the feasibility and safety of using hand compressions and assisted compression devices to indirectly displace internal organ structures during before or during treatment. Here, Tuncali et al. (2006) demonstrated the effectiveness of manual hand compressions during magnetic resonance imaging (MRI) guided CA of renal tumors to displace the bowels (68). Other techniques showed the utility of external compression devices to achieve the optimal needle pathway and reduce the risk of injuring the bowel, bladder, and colon. Furthermore, these devices enable more accurate needle placement by providing stable compression and guidance, potentially increasing diagnostic yield and reducing the need for repeat biopsies (69, 70). More recently, 3D-printed compression paddles were developed to enhance needle access to difficult targets by allowing the displacement and immobilization of vital structures. These paddles safely guides the needles while ensuring the clinician’s hand is positioned away from the x-ray beam during computed tomography (CT) imaging to avoid unnecessary irradiation (71). In RT, non-invasive immobilization devices such as body foam cradles and thermoplastic masks have become essential components in treatment planning and delivery. For lung cancers, cervicothoracic immobilization devices have shown superior setup accuracy compared to traditional thoracoabdominal flat immobilization devices, particularly for patients requiring treatment of both primary lung lesions and supraclavicular lymph nodes (72). These devices significantly reduce organ motion and promote consistent patient positioning across multiple treatment sessions, with applications spanning various cancer types, including HNC (73–75), breast (76), lung (30, 31), prostate and bladder (77, 78), and paraspinal sarcomas (79) (see Figure 3).
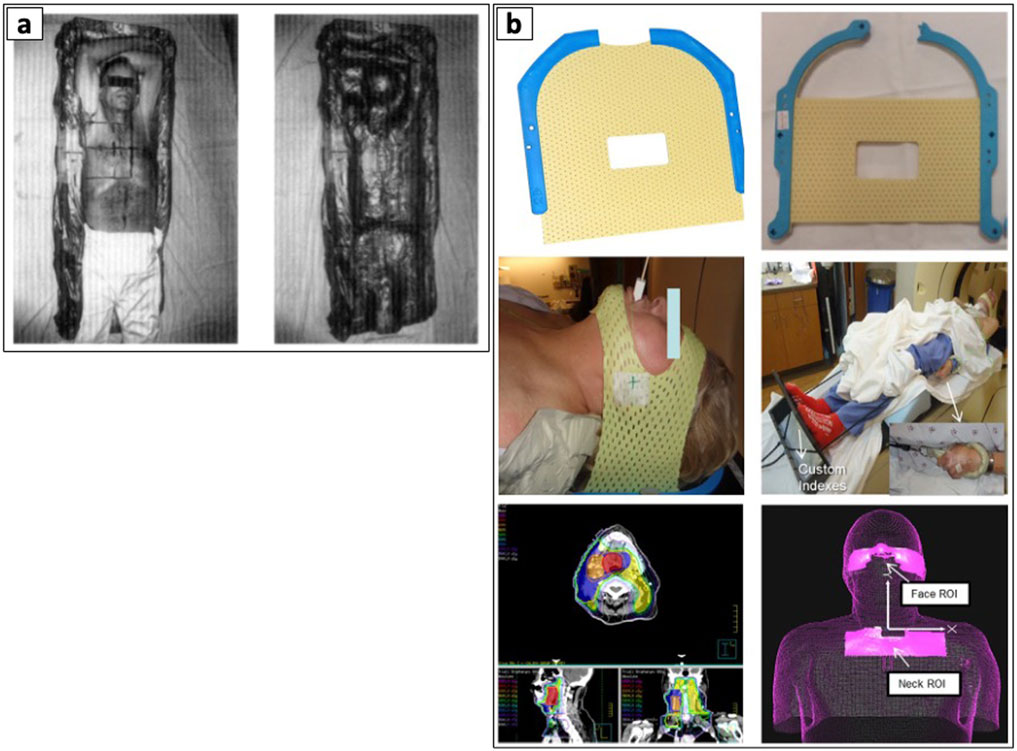
Figure 3. Examples of patient body immobilization devices. (a) Body foam. Reproduced with permission from “The prepared Styrofoam form (left), patient resting in the finished cradle (center), and the cradle without the patient demonstrating the close fit to the patient’s body (right)” by Gunilla C. Bentel, Lawrence B. Marks and Rupa Krishnamurthy licensed under CC-BY-NC-ND, and (b) Thermoplastic immobilization mask. Adapted with permission from “(a) Original short 3-point mask (Qfix, model RT-1876KSDGLF). (b) Mask modified with straight cuts at top and bottom. (c) Modified mask in place over only forehead and chin. (d) Overall patient setup. (e) Patient treatment plan with low neck coverage. (f) ROI selections on AlignRT relative to isocenter location. These two ROIs can be used to create a composite ROI for intrafractional tracking” by Bo Zhao, Genevieve Maquilan, Steve Jiang and David L. Schwartz, licensed under CC BY 4.0.
3.2 Direct TOD
Direct TOD techniques involve actions involving the direct physical manipulation of OARs using spacers, to achieve targeted organ displacement with or without immobilization. These techniques are categorized based on their delivery method, which includes oral/ureteral/rectal insertion, injection, or surgical implantation. In this section, we discuss each delivery methods, the associated technique(s) and their unique advantages in managing organ positioning and immobilization during RT and locoregional ablation therapies.
3.2.1 Oral, ureteral, and rectal insertion
3.2.1.1 Intraoral stents
The rising incidence of tongue cancer in the 1980s and 1990s sparked interest in interstitial BT applications. However, its use resulted in adverse complications due to radiation-induced oral mucositis (RIOM). Radium needles and iridium-192 hairpins emerged as a simple yet effective approach to mitigating RIOM, enhanced by precise displacement and positioning of intraoral tongue stents (33–35, 80–82). In 1984, Niwa et al. demonstrated one of the earliest clinical uses of a tongue stent to separate the lower gingiva from the tumor and prevent osteoradionecrosis during interstitial BT (83). Subsequently, Fujita et al. (1993–1994) demonstrated that silicone intraoral stents provided superior dose reduction and shorter fabrication times than acrylic stents (84). They later demonstrated an improved dose-reduction effect when combined with a Lipowitz metal (85). Tamamoto et al. (1996) further advanced this field by developing mouth guard-like oral spacers using acrylic resin to accommodate dentate and edentulous patients (86). Further advancements in digital dentistry have allowed for customization, more efficient fabrication and improved patient comfort during and after treatment (36, 37, 87, 88). Traditional and modern approaches in the clinic utilize several types of intraoral stents, such as shielding prostheses (82, 89), radiation carriers, positioning stents (36, 37, 87, 88, 90–95), and radiation mouthguards (96). These stents differ in their specific treatments, advantages, disadvantages, and materials used in their creation, offering a range of options to suit individual patient needs and treatment requirements (see Figure 4) (82).
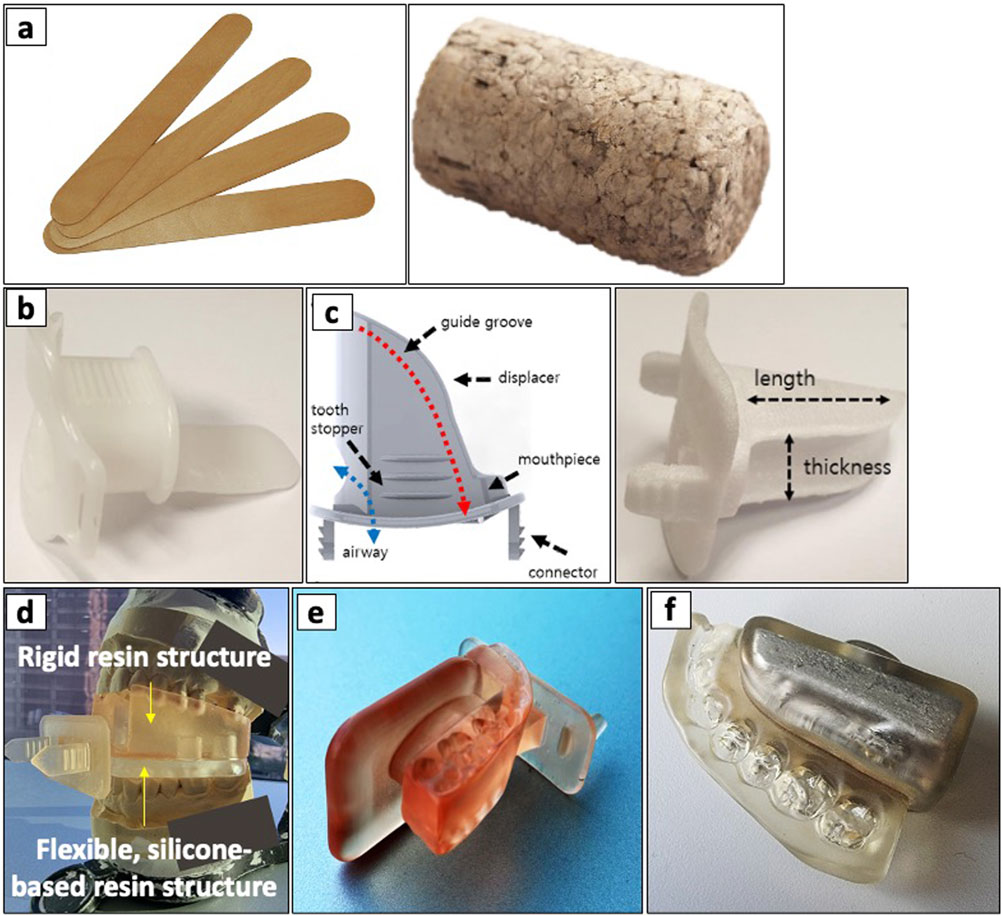
Figure 4. Types of intraoral stents ranging including non-customizable, semi-customizable, and customizable stents. (a) non-customizable stents including a stacked wooden tongue blade (left) and a cork (right). (b) a commercial tongue-depressing stent and (c) its semi-customized version (left) fabricated using 3D-printing (right). (d) a 3D-printed, customized tongue-depressing stent with flexible silicone-based resin structure, mounted on its dental impression. (e) a 3D-printed customized tongue-lateralizing intraoral stent. (f) a 3D-printed customized tongue-lateralizing stent filled with Lipowitz alloy. Adapted with permission from “a Top and b front views of the 3D model for the semi-customized tongue displacement device. It was printed using a 3D printer with a biocompatible material (c). d Commercially available standard mouthpiece, which has been the most commonly used device in H&N RT” by Chae-Seon Hong, Dongryul Oh, Sang Gyu Ju, Yong Chan Ahn, Cho Hee Na, Dong Yeol Kwon and Cheol Chong Kim, licensed under CC BY 4.0.
3.2.1.2 Intravaginal/rectal spacers
Few studies have shown that silicone-based materials in the form of repurposed breast implants and tissue expanders can effectively reduce radiation exposure to surrounding tissues, making them valuable components in RT (97–99). For patients with stage Ib-IIb cervical carcinoma, receiving high-dose-rate BT (HDR-BT), the intravaginal insertion of silicone-based Foley balloons filled with contrast medium were demonstrated to reduce rectal and bladder doses (100, 101). Recently, Ates et al. (2022) evaluated silicone-filled vaginal spacers in patients with rhabdomyosarcoma, receiving proton beam RT (PBRT), demonstrating effective vaginal wall displacement and reduced radiation exposure (21). Other areas involving the use of ureteral and rectal stent spacers, along with bladder distention devices, have shown promise in protecting patients with abdominal and pelvic malignancies receiving RT. Here, self-expandable ureteral stents were able to reduce radiation doses to pelvic structures in patients with cervical or rectal cancer, with significant improvements in stent patency and postoperative complications (102). Others including rectal retractors offer a less invasive alternative to surgery and injection, resulting in more successful sparing with lower complication rates (15, 16, 103, 104).
3.2.2 Injection-based TODs
Technological advancements in percutaneous abdominal ablation have led to the adoption of RFA and MWA for liver and kidney malignancies (105). While effective in achieving enhanced local tumor control, previous studies highlight potential risks to adjacent OARs, such as cholecystitis and gallbladder perforation (106). Liang et al. (2009) retrospectively analyzed 1,136 patients undergoing MWA for hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), and cholangio-HCC, reporting a 0.2% treatment-related mortality rate and a 2.6% major complication rate. Although complications were infrequent, their severity, ranging from liver abscess and bile duct injury to tumor seeding, hemorrhage, and skin burns, often resulted in significant morbidity and disability (107). These consequences have led to the development and adoption of injection techniques, such as hydrodissection, primarily used for lens-cortex separation and needle biopsy applications (108, 109). Other techniques involve the local administration of simple liquid solutions involving saline (110, 111) to more sophisticated hydrogel spacers (HS) (41, 112).
3.2.2.1 Hydrodissection
Hydrodissection utilizes fluid solutions to create a localized increase in pressure near the target organ, subsequently pushing OARs away from the treatment area. Standard hydrodissection procedures use local anesthetic, saline, 5% dextrose in water (D5W), or a dilute mixture of contrast agents for enhanced imaging visualization (see Figures 5a, b). Compared to other solutions, D5W is preferred due to its hypo-osmolar property, reducing risks of osmotic stress, and its slower absorption rate ideal for prolonged OAR separation. This is especially true for RFA procedures, where the use of D5W is the only fluid recommended due to its non-ionic composition, avoiding the spread of current and subsequent collateral damage to nearby tissues (113). Clinical studies have demonstrated the benefits of hydrodissection in various cancer treatments, showcasing its ability to protect OARs during biopsy procedures, RT, MWA, and RFA (109–111, 114–117). Studies have also demonstrated the safety and efficacy of hydrodissection in patients with papillary cancers receiving MWA and RFA (118–122). Furthermore, the infusion of saline into ureters, also known as ‘pyeloperfusion,’ has been shown to protect the urothelium, reducing the risks of urinary stricture and urinoma due to thermal damage from RWA, MWA, and CA (24). More recently, studies show that the injection of thermoprotective gels significantly reduce thermal damage in MWA, with similar benefits in image-guided biopsies, CA of the mediastinal, RFA of thyroids, and retroperitoneal tumor ablation (27, 118). As imaging techniques advanced, the need for visualization of the hydrodissection space developed, leading to using contrast agents. Using a 1:20 dilute mixture of iohexol-140 (Omnipaque; GE Healthcare, Princeton, New Jersey) in normal saline has been shown to improve the visibility of the fluid space under CT or MRI guidance, enabling controlled hydrodissection procedures (123). Advanced methods include the combination of balloon spacers with agents like collagen, hyaluronic acid (HA), saline with 5%–10% iohexol, fibrillar collagen, and thermoprotective gels (poloxamer 407) (124, 125). A multi-center study in prostate cancer confirmed that transperineal injection of a biodegradable balloon, ProSpace™ (BioProtect Ltd., Israel) between the prostate and rectum reduces rectal doses and improves immobilization during RT (126). Additionally, using multiple probes during MWA yields larger, symmetrical ablation zones and reduces treatment time (127). Andresciani et al. (2023) reported that dual-probe MWA with hydrodissection in 55 patients with HCC and unresectable liver metastases achieved safe ablation margins (2.5–166 cm3) for tumors (4.4–85 cm3) (128). Apart from liquid, air injection using a Chiba needle was employed to displace the small bowel during kidney RFA (2). However, this technique carries a higher risk of pain and potentially fatal air embolism if introduced intravenously. To address this limitation, Johnston et al. (2024) utilized a technique called ‘Carbodissection’, also known as ‘CO2 dissection’ or ‘gasdissection’, by injecting a highly soluble CO2 gas to displace the bowels during CA (56).

Figure 5. Examples of injectable spacers. (a) Baseline CT scan of a sacral chordoma, highlighting the proximity of the pelvic sacral tumor to the bowel at the S2-4 vertebrae. (b) Cone-beam CT image demonstrating successful temporary organ displacement (TOD) of the bowel, achieved through the injection of sterile saline via hydrodissection, creating a protective margin between the bowel and the tumor target. (a) and (b) Reproduced with permission from “Axial imaging of paraspinal non-seminomatous germ cell tumor treated to 2850 cGy” by Evangelia Katsoulakis, Stephen B Solomon, Majid Maybody, Douglas Housman, Greg Niyazov, Nadeem Riaz, Michael Lovelock, Daniel E Spratt, Joseph P Erinjeri, Raymond H Thornton and Yoshiya Yamada, licensed under CC BY 2.0. (c) Visualization of the TOD space between the prostate and rectum using SpaceOAR™ across varying timepoints. The SpacerOAR™ is a polyethylene glycol hydrogel material designed to spare the rectum during RT. Reproduced with permission from “T2-weighted magnetic resonance images of a spacer patient at baseline (a), post-application (b), and 12 months after spacer application (c)” by Mariados et al., licensed under CC-BY-NC-ND.
3.2.2.2 Hydrogel spacers (HS)
Transperineal injections of HA reduced rectal toxicities in prostate cancer RT (130, 131), but HA’s rapid degradation under radiation and its high viscosity limited its effectiveness (132, 133). Concurrently, bioabsorbable HS made from polyethylene glycol (PEG) emerged as a more reliable alternative, including SpacerOAR™ (Augmenix Inc., Waltham, MA, US) and DuraSeal® (Covidien, Mansfield, MA, US) (41, 134–137). Other bioresorbable hydrogel-based spacers made from hydroxypropyl methylcellulose (HPMC), such as Viscomet® (Sun Pharmaceutical Industries Ltd., Mumbai, India), have also demonstrated feasibility and safety primarily in patients with cervical cancers receiving BT (138). Moreover, Weber et al. (2012) found that transperineal injection of HS reduced rectal doses and improved target coverage in more advanced techniques involving intensity-modulated RT (IMRT), volumetric-modulated arc therapy (VMAT), and intensity-modulated proton therapy (IMPT) (126).
Compared to SpaceOAR™ and DuraSeal®’s ‘passive’ reversibility through natural biodegradation, non-animal, stabilized HA (NASHA®) formulations such as Barrigel™ (Palette Life Sciences, now acquired by Teleflex Inc., US) exhibit ‘active’ reversibility characteristics. The reversible nature of Barrigel™ provides additional safety advantages, as demonstrated by Hong et al. (2022), where rectal wall infiltration was successfully reversed with hyaluronidase administration without adverse effects (140). A multi-center randomized controlled trial conducted across the US, Australia, and Spain have further shown the effectiveness of Barrigel™ in 136 hypofractionated prostate cancer patients, with improved spacing maintenance and safety profile (141). The study demonstrated that 98.5% of patients treated with Barrigel™ achieved ≥25% reduction in radiation dose to the rectum, with patients averaging an 85% dose reduction. Importantly, this translated to significantly reduced acute grade 2 GI toxicity (2.9% vs. 13.8% for control group, p = 0.01). PEG-based spacers have similarly established strong evidence, with SpaceOAR™ studies demonstrating efficacy in reducing rectal ulcer incidence and increasing prostate-rectum distance in SBRT patients receiving 45 Gy in 5 fractions (142). A phase III trial with 222 patients confirmed significant reductions in late rectal toxicity with no device-related complications (see Figure 5c) (129), with 3-year follow-up data highlighting sustained benefits and reduced medical treatment requirements for bowel complications (143–145). Recent research further showed SpaceOAR™'s particular effectiveness in patients with larger prostates, significantly reducing high-dose rectal exposure and GI toxicities (146).
Given these benefits, post-prostatectomy RT (PPRT) represents an expanding application area requiring specialized investigation, requiring a well-designed study with clear criteria to assess the clinical effectiveness of HS (147). Hong et al. (2024) conducted a retrospective study evaluating 64 patients who received PPRT, showing the effective and safe use of SpaceOAR™ in at least 95% of patients (148). Furthermore, the ongoing landmark trial, the Barrigel® PPRT trial (NCT06496256, Teleflex Inc., US), aims to establish definitive efficacy in this patient population with the reduction in rectal volume receiving the prescribed dose as the primary endpoint. Beyond prostate applications, cervical cancer patients receiving HDR-BT were shown to benefit from other hydrogel-based spacers such as Suvenyl® (discontinued, Chugai Pharmaceutical Co., Tokyo, Japan) (149–155), MucoUp® (Seikagaku Co., Tokyo, Japan) (156, 157), and TraceIT® (Augmenix Inc., US) (158). More specifically, a study by Iijima et al. (2021) using Suvenyl® has shown that greater thickness and length of gel spacers lead to lower 2-cm3 covering doses to the rectum (152), though quality of evidence remains limited and requires further validation through larger prospective trials (42). In GI applications, Narang et al.’s (2024) study of six pancreatic adenocarcinoma patients injected with TraceIT® successfully separated the pancreas head and duodenum with a mean spacing of 0.77 cm without any device-related adverse events and showed no damage to the duodenum in patients undergoing Whipple resection (159). Additionally, studies have shown promising outcomes of Barrigel™ in retroperitoneal settings, with a recent report by Lee et al. (2025) demonstrating successful large bowel displacement during SBRT for adrenal and renal malignancies (160).
Current literature demonstrates excellent patient tolerance of HS implantation overall. However, documented complications include rectal discomfort, pain, bacterial prostatitis, and perineal abscess, though these occur rarely (134). Technical limitations require consideration, as imaging artifacts serve as contraindications for HS use (161). The reversibility advantage of NASHA® formulations over PEG-based alternatives provides enhanced safety profiles in cases of misplacement or complications, making stabilized HA an increasingly attractive option for complex anatomical applications where precision and safety are paramount.
3.2.3 Surgically placed TODs
Advanced interventions become necessary when high radiation doses increase the risk of nearby organ damage (162). Techniques like ovarian transposition to preserve fertility during pelvic irradiation for Hodgkin’s disease (163), colpopexy adapted from colorectal surgery for TOD (164), and extracorporeal procedures requiring reimplantation of resected bone after RT (165). This section focuses on surgical techniques for TOD during RT and discusses specific aspects of implantation, probe manipulation for organ positioning and immobilization in locoregional ablation therapies.
3.2.3.1 Surgery with implants
Synthetic implants are essential in surgical TOD, as they support and displace OARs to minimize radiation exposure. This is particularly important for GI cancer patients who have undergone prior resections, such as abdominoperineal resection and pelvic exenteration, where cavities must be filled to protect surrounding organs during RT (166). Sugarbaker et al. (1983) introduced a plastic mesh to fill pelvic cavities in post-operative EBRT patients, reducing radiation enteritis from 40% to less than 5% (167). Later studies show the utility of the Dexon mesh to displace and immobilize the small bowel during RT, with no cases of radiation enteritis or mesh-related complications in a study of 60 patients, 92% of whom reported improved QOL (168). Polyglycolic acid (PGA) meshes, widely used for TOD, dissolve over time to avoid surgical removal (169). Building on this experience, Sezeur et al. (2008) combined polyglactine 910 (Vicryl) mesh with a saline-filled silicone balloon, achieving a 95% success rate in preventing small bowel irradiation in 50 patients (170). Similarly, Jesseph et al. (2013) used tissue expanders and breast prostheses to protect the small bowel during PBRT with no complications (171), and Chan et al. (2019) demonstrated the effectiveness of a tissue expander combined with an absorbable pelvic mesh sling for protecting the small bowel during pelvic RT (172). Interestingly, it has been shown that silicone film drains folded in a fan-shaped configuration successfully separated the small intestine from the pelvic space in a porcine model, and were subsequently removed without invasive surgery (173) (see Figure 6a). Another study used a silicone vaginal insert, known as the ‘Gelhorn pessary’, to manage pelvic organ prolapse during chemo RT of the cervix, a strategy that may help minimize unnecessary irradiation to surrounding healthy tissues (174).

Figure 6. Examples of surgical TOD techniques. (a) a fan-shaped tube spacer inserted into the pelvic cavity of a porcine model, fixed to the abdominal walls using sutures. Adapted with permission from “Removal of the fan-shaped spacer in an animal model” by Norio Kubo, Takehiko Yokobori, Ryo Takahashi, Hiroomi Ogawa, Navchaa Gombodorj, Naoya Ohta, Tatsuya Ohno, Hiroshi Saeki, Ken Shirabe and Takayuki Asa, licensed under CC BY 4.0. (b) use of a Gore-Tex® Soft Tissue Patch between pancreas and GI tract. Adapted with permission from “Images of representative patients who underwent surgical spacer placement” by Dongha Lee, Shohei Komatsu, Kazuki Terashima, Hirochika Toyama, Yoshiro Matsuo, Daiki Takahashi, Masaki Suga, Naoko Nishimura, Kentaro Tai, Masahiro Kido, Yusuke Demizu, Sunao Tokumaru, Tomoaki Okimoto, Ryohei Sasaki and Takumi Fukumoto, licensed under CC BY 4.0. (c) setup of extracorporeal irradiation, with resected bone (left) wrapped for radiation (right). Reproduced with permission from “After resection of the tumor-bearing segment obvious tumor tissue as well as the biopsy tract are removed, whereas important functional structures such as the patellar tendon (⇨) are spared” by Andreas H. Krieg, Ulrich Lenze, Leandra Schultze, Markus W. Gross and Martin Haug, licensed under CC-BY-NC-ND.
Recent studies have demonstrated expanded polytetrafluoroethylene (ePTFE) Gore-Tex® sheets, a biocompatible, flexible, and impermeable material, for cosmetic means and TOD procedures (175). Gore-Tex® spacers can protect the GI tract during PBRT for locally advanced pancreatic cancer with improved tumor volume coverage while adhering to GI dose constraints (22) (see Figure 6b). Additionally, researchers have applied Gore-Tex® in image-guided IMRT for paraspinal tumors, PBRT for unresectable HCC, and other abdominal, pelvic, and retroperitoneal malignancies (176–178). More advanced materials, such as biologic devices made from natural tissues or biologically derived materials, offer superior biocompatibility compared to synthetic alternatives. A 2014 trial demonstrated that biologic meshes are more resistant to infection, resulting in fewer hernias and reinterventions (179). A biologic mesh derived from sterile cadaver skin, AlloDerm™ (LifeCell Corporation, acquired by Allergan Aesthetics, US) has been used as an implantable spacer for liver and pelvic tumors treated with RT (180, 181). In addition, Alloderm™ has also been demonstrated to displace OARs effectively, such as the small bowel, colon, and pancreas during PBRT and IMRT, minimizing GI toxicities (23, 182, 183).
3.2.3.2 Surgery with native tissues
While invasive, using the patient’s native tissues for surgical TOD techniques offers the most biocompatible method for displacing and securing organs, minimizing complications, and protecting healthy tissues during RT. Colopexy, a procedure that fixates the colon to the abdominal wall, was traditionally used for recurrent volvulus but has been adapted for TOD. The technique now includes using an omental ‘flap’, ‘sling’, or ‘envelope’ as a spacer to prevent dose toxicities to the bowels (184–186). Russ et al. (1984) demonstrated that omental transposition flaps achieved a 95% success rate in bowel displacement, reducing radiation enteritis from 30% to under 5% and improving QOL for 90% of patients (187). Another study demonstrated the feasibility of suturing the bladder to the abdominal wall and retroperitoneum, then distended with fluid to shield other peritoneal contents from radiation (52). Similarly, suturing the greater omentum to displace and immobilize the small intestines can effectively move them away from radiation targets (53). Uterine and adnexal transposition is a surgical technique used to protect reproductive organs from radiation damage, preserving fertility in women undergoing pelvic RT (189, 190). Ovarian transposition, also known as ovariopexy, has been used since the 1980s to protect the ovaries and fallopian tubes from radiation, reducing infertility during high-dose pelvic radiation for cancers such as Hodgkin’s disease, cervical and rectal cancers (191–193). More recently, Ribeiro et al. (2024) conducted a longitudinal study from 2017 to 2024 on laparoscopic transposition of the uterus and adnexa to the upper abdomen during pelvic RT, restoring them via rectosigmoidectomy post-RT. In a study of eight patients, six maintained uterine preservation with no significant complications. Follow-ups showed cervical ischemia in three patients, one death from carcinomatosis, and two successful pregnancies (194, 195).
3.2.3.3 Extracorporeal radiation and reimplantation
Extracorporeal irradiation and reimplantation is a surgical procedure for TOD in which surgeons remove a tumor-bearing bone segment, irradiate it with high-dose radiation outside the body, and then reimplant it free of tumor cells (196–198). First reported by Spira and Lubin in 1968, the technique aimed to preserve limb function and prevent amputation (165). A study by Davidson et al. (2005) involved 50 patients with bone malignancies who received 50 Gy of extracorporeal irradiation. At a mean follow-up of 38 months, 42 out of 50 patients were alive and disease-free. Functional outcomes were favorable, with a mean Musculoskeletal Tumor Society score of 77 and a Toronto Extremity Salvage score of 81 (199). A recent study by Krieg et al. (2019) on wide resection and reimplantation of irradiated autografts in patients with sarcomas showed good to excellent functional outcomes in 7 of 8 patients, demonstrating the potential of this technique for limb salvage in cases of tibial sarcomas (200) (see Figure 6c).
3.2.3.4 Probe manipulation
A simple approach for minimizing locoregional ablation damage to OARs involves probe manipulation, such as elevation or retraction. In CA procedures, clinicians carefully adjust a cryoprobe to target the tissue while avoiding adjacent structures (58). In renal tumors involving the chest wall, elevating the probe increases separation from the chest wall and pectoralis muscle while retracting protects the bowel. Aside from elevation and retraction, a similar probe manipulation technique involves applying torque or a rotational force to the ablation probe. Probe torquing can also temporarily displace organs to create space, as observed in cases of kidney tumors. Here, torquing the probe can shift the kidney position and increase the distance from the nearby bowel, preventing thermal injury to OARs during the subsequent freezing cycle. Adding gentle back-and-forth motion of the cryoprobe during the procedure prevents the probe from adhering to frozen tissue and allows minor adjustments in probe position to optimize ice ball formation and coverage (58, 201, 202). Clinicians often combine probe manipulation techniques with injections and surgical implantations. Through this, interventional radiologists can further reduce the risk of collateral damage during CA procedures near sensitive OARs (2, 55).
4 Spacers in the clinic
This section reviews key clinical factors and their role in promoting a safe and effective TOD. These include accurate patient selection, organ separation, immobilization, resource availability, spacer design, placement, and positioning verification. Figure 7 illustrates an overview of the current clinical landscape of TOD techniques in RT and locoregional ablation therapies. Although numerous studies have examined TOD, consensus regarding the indications and contraindications for TOD techniques remains unclear and current recommendations are based solely on the clinical factors outlined below.
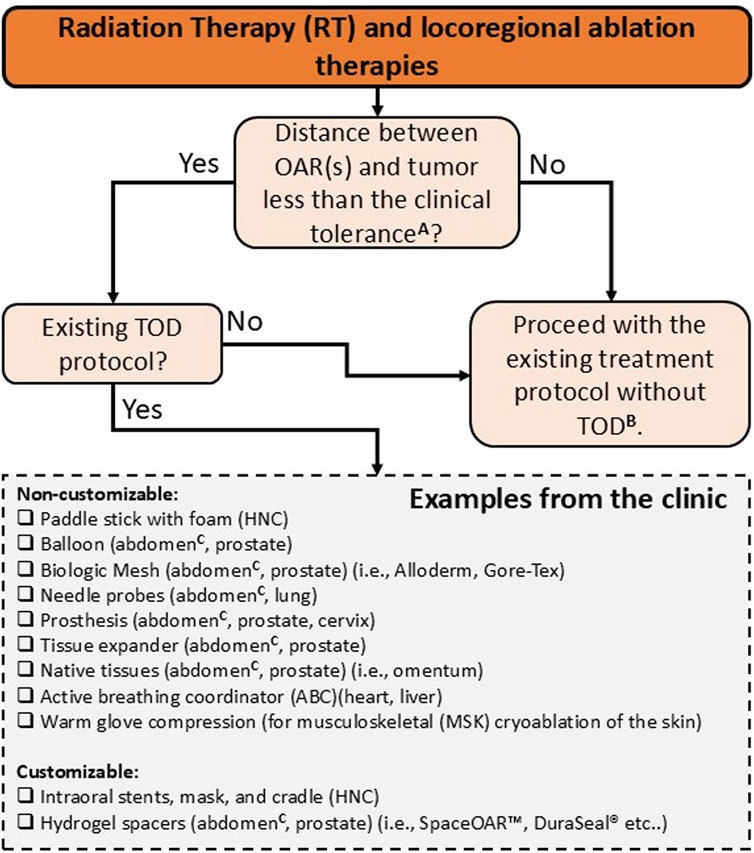
Figure 7. Indications and contra-indications for TOD use in RT and locoregional ablation therapies. Non-customizable devices, such as standard balloons and generic spacers, are designed for general use without the need for patient specificity. Customizable devices, including patient-specific spacers and immobilization systems, are tailored to fit unique anatomical features, potentially improving precision and comfort. A this clinical threshold is not universally defined and varies by modality, organ site, and image guidance capabilities. In RT, particularly with advanced modalities such as VMAT and IGRT, sub-centimeter threshold (<1 cm) between tumor and OARs are often feasible, but larger separations (>1 cm) may also require spacer use depending on individual anatomy, organ motion, and setup uncertainties. For percutaneous ablation, most studies use a <2 cm threshold to ensure complete tumor coverage and adequate OAR protection, but actual clinical tolerance should be tailored to patient-specific anatomy and organ-specific ablation protocols. B target structures are often underdosed to prioritize dose constraints to OARs in RT. In percutaneous ablation cases, target structures are treated at reduced power (watts) and ablation time to minimize collateral damage to adjacent healthy tissues. C abdomen includes liver, kidney, pancreas, and bowels.
4.1 Organ separation and immobilization
Intuitively, the tumor’s location and its distance from OARs provide crucial information for determining whether TOD is necessary and, if so, which specific TOD technique would be most appropriate. Tumor proximity to OARs indicates the need for intraoral stents, organ delineation, DIBH, or immobilization techniques in RT and hydrodissection or probe manipulation in locoregional ablation therapies. A standard method to measure these distances involves the manual measurement and annotation of CT slices, measuring the distance between boundary edges of 2D contours. More efficient and automated measurement methods utilize existing Euclidean pairwise distance or K-nearest neighbors (KNN) algorithms to measure the closest surface distance between 3D volumetric contours.
For RFA and MWA, tumors within 1 cm separation distance to OARs, such as the GI tract, diaphragm, gallbladder, major blood vessels, or bile ducts, indicate the necessity for hydrodissection (25, 203). Numerous studies have shown that maintaining a separation of at least 1 cm between the treatment margin and OAR(s) minimizes thermal damage (204–208), with hydrodissection achieving >1.5 cm separations (209). Ginat et al. (2010) recommend a safety margin of at least 2 cm for adjacent bowels to avoid complications (55). Although proximity to OARs presents challenges, successful ablations have been performed within 1 cm of these structures. Some patients achieved safe distances of 0.5–0.9 cm with hydrodissection (25, 119, 121, 203, 205). Moreover, researchers have demonstrated that minimally invasive manual hand compressions achieve a mean separation distance of 2.6 cm between the bowels and the renal tumor during CA (68).
RT centers use comparable tolerances, particularly for oropharyngeal, GI, and GU cancers. In HNC, past literature suggests intraoral stent thickness of >1 cm to prevent osteoradionecrosis during interstitial BT (84). Barcellini et al. (2022) highlighted the opportunity for omental flap spacers in patients with pelvic malignancies for carbon ion RT when tumor to OAR distance falls below 0.5 cm (184). In prostate cancers, HS injection has become increasingly prevalent, effectively reducing rectal toxicity by achieving >1 cm away from the prostate (129, 143, 210, 211). GI cancers have benefited from applying biologic mesh spacers, achieving immobilization of OARs and separation distances ranging from 0.5 to 4 cm in patients receiving PBRT, IMRT, and SBRT (23, 110). Tables 1, 2 present prospective clinical studies evaluating spacer techniques in patients receiving RT and locoregional ablation therapies, respectively. Studies were selected based on reporting both clinical treatment outcomes and quantitative measurements of achieved organ separation distances following spacer implementation.
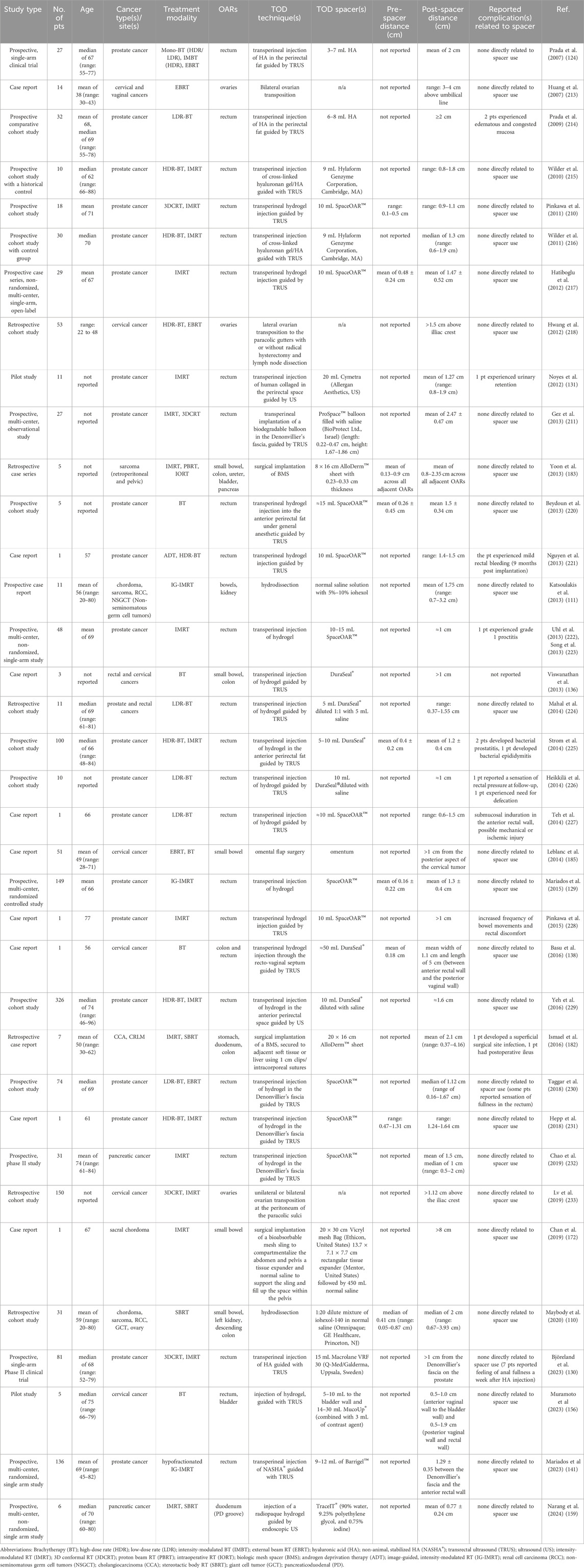
Table 1. Prospective clinical studies primarily reporting post-spacer distance measurements from patients (pts) receiving RT with TOD spacers.

Table 2. Prospective clinical studies primarily reporting post-spacer distance measurements from patients receiving locoregional ablation therapies with TOD spacers.
In conjunction with organ displacement, clinicians immobilize OARs to avoid further risks and uncertainties while ensuring treatment plans deploy as intended. Currently, a variety of spacers utilize immobilization during RT. These include intraoral stents to displace and immobilize the tongue and oral anatomy, along with immobilization masks (212); omental slings or pouches fixed to the abdominal wall via sutures while separating the stomach and bowels (187); multiple synthetic implants designed to fill empty cavities and ensure the bowels are immobilized (167); and immobilization cradles that further secure the patient on the treatment couch (31, 79). Standard immobilization techniques for ablation therapies like MWA, RFA, or CA involve using probe manipulation by directly inserting one or more probes to displace and stabilize OARs. Hydrodissection can immobilize organs by injecting fluid within potential spaces, however, gravity and movement often cause the fluid to shift, necessitating additional injections.
4.2 Resource availability
A clinic’s ability to offer options for TOD depends on essential resources, including spacer materials, tools, and protocols, as well as the presence of “champion” users willing to implement these procedures in their clinical practice. In locoregional ablation therapy, hydrodissection is a well-established technique for separating OARs during ablation therapies, valued for its simplicity and cost-effectiveness, despite its limited organ immobilization capabilities. RT presents a broader array of TOD techniques for HNC, GI, and GU cancers, including intraoral stents, hydrogels, balloons, and prostheses. This diversity, however, complicates consensus and reproducibility across clinical centers due to varying techniques, materials, protocols, and levels of expertise. Despite technological advancements, the widespread clinical adoption of TOD remains limited, particularly in RT settings, due to a lack of standardization and clinical evidence. While Tables 1, 2 highlight a greater number of clinical studies on TOD in RT compared to locoregional ablation therapies, the existing literature remains limited, especially for cancer types beyond prostate cancer.
4.3 Materials and design
Researchers have explored a wide range of materials and devices for TOD, frequently adapting tools from other medical fields like cosmetic surgery, patient positioning, and cataract procedures. Material density is a crucial yet often overlooked factor when considering TOD spacers for RT (177). The material density affects how materials attenuate photons, electrons, and other particles. Water-equivalent materials with densities near 0 Hounsfield units (HU) are ideal for accurate dose calculations (234, 235), while densities ranging from 0 to 500 HU can offer visibility in CT (‘radiopacity’), with minimal dose scattering and imaging artifacts (236).
Unlike RT, locoregional ablation therapies can use low- and high-density materials to reduce thermal conductivity, minimizing damage to surrounding tissues. For hydrodissection, fluids like 5% glucose (non-conductive and well-tolerated), saline (provides thermal protection but can spread heat during RFA, though studies suggest it can be protective), and sterile water (non-conductive but may cause fluid shifts) have been considered (25, 237, 238). The choice of fluid depends on the application and careful risk-benefit analysis. Furthermore, the atomic composition of spacer materials complicates CT imaging, as different materials with similar densities or X-ray attenuation may have distinct compositions. This variation in composition leads to ambiguity in material identification and affects the accuracy of TOD techniques and treatment planning.
Lastly, biocompatibility, tolerance, biodegradability, and stability are key concerns for TOD techniques, as materials can cause patient discomfort and pain (14). The use of biologic mesh in complex hernia repairs highlights this, with high morbidity and recurrence rates despite an initial promise (239–242). Additionally, bioprosthetic materials are significantly more expensive than synthetic alternatives, often lacking reimbursement and costing up to ten times more, with price variations among manufacturers (180). The ideal material for TOD should balance efficacy, safety, and cost-effectiveness. Ogino (2013) suggests that water-equivalent materials, which enable accurate dose distribution and function as spacers before resorption, would be optimal (177). However, further research is needed to develop materials that meet these criteria while addressing the challenges of artificial materials in clinical settings.
4.4 Placement, verification, and analysis
The success of a TOD technique relies not only on choosing appropriate spacer materials but also on the safe and precise placement of spacers. Following spacer placement, verification methods are crucial for confirming adequate organ separation, utilizing various imaging modalities such as CT/CBCT, MRI, and US. In addition to visual inspection, quantitative metrics are vital for assessing the effectiveness of spacers, including, but not limited to, surface distance measurements, geometrical analysis, and in silico simulations (i.e., dose-volume histograms (DVH) and thermal data generated from RT treatment plans and microwave ablation simulation models, respectively) (see Table 3).
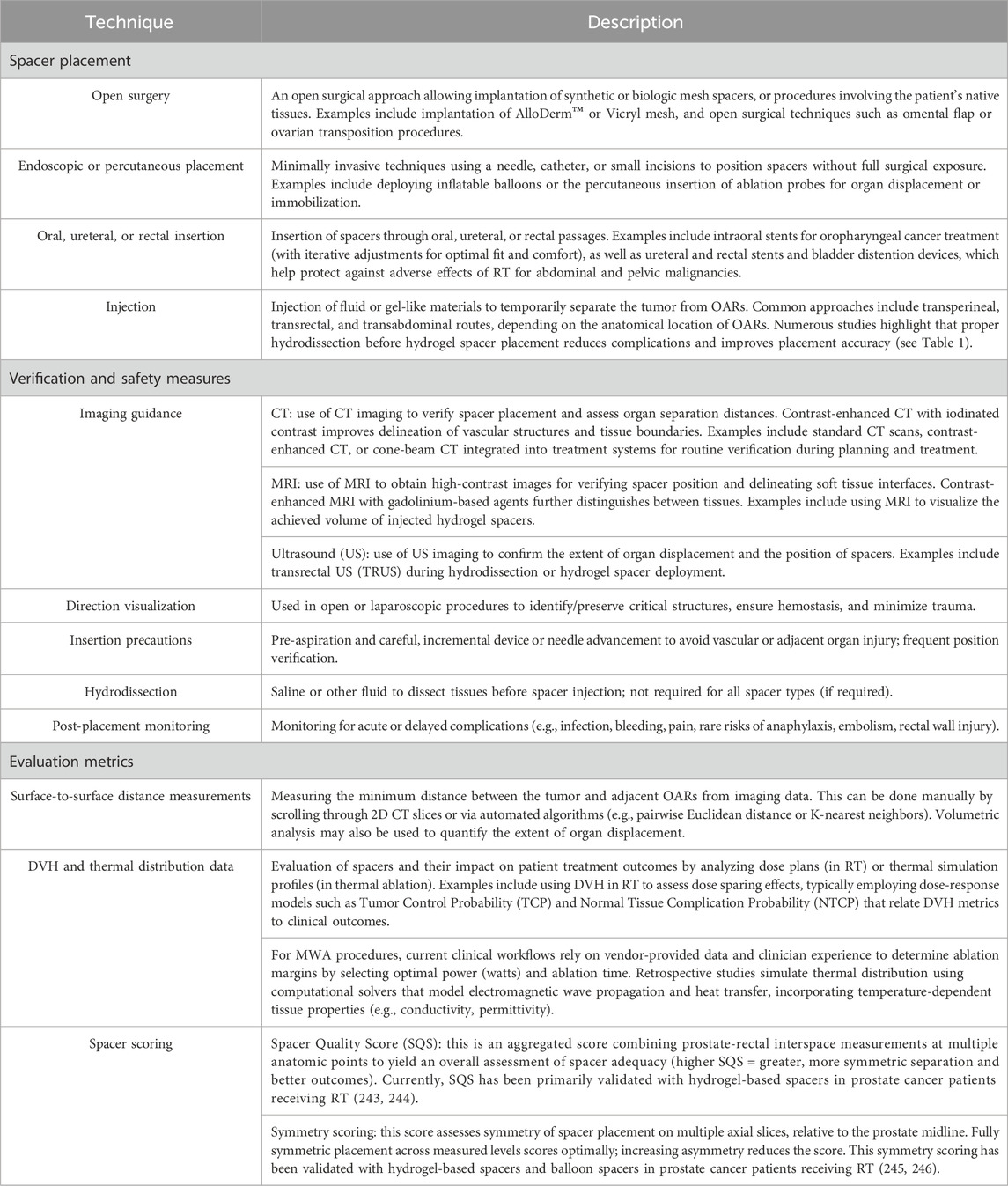
Table 3. Overview of supporting methods for TOD: spacer placement and immobilization, verification methods and safety measures, and evaluation metrics.
5 Opportunities and future directions
Based on the current literature, TOD technologies remain limited due to awareness of the benefits of using spacers in the clinic, their limited availability, differences in the users’ technical expertise, heterogeneous patient anatomy, and time-intensive workflows (i.e., simulation time and clinical implementation in patients). Haynes et al. (1997) state that the balance between patient treatment outcomes and QOL remains a therapeutic challenge for cancer treatment and, therefore, requires evidence-based treatment approaches (247). A review of the existing literature reveals a predominance of retrospective cohort studies and case reports, with relatively few randomized controlled trials and Phase II/III studies, limiting the strength of current evidence. Many studies rely on non-randomized, single-arm designs, which, while informative, may not provide definitive conclusions on treatment efficacy (see Tables 1, 2). Several key areas need more focus to enhance the role of TOD techniques in cancer management, which are discussed below (see Figure 8).
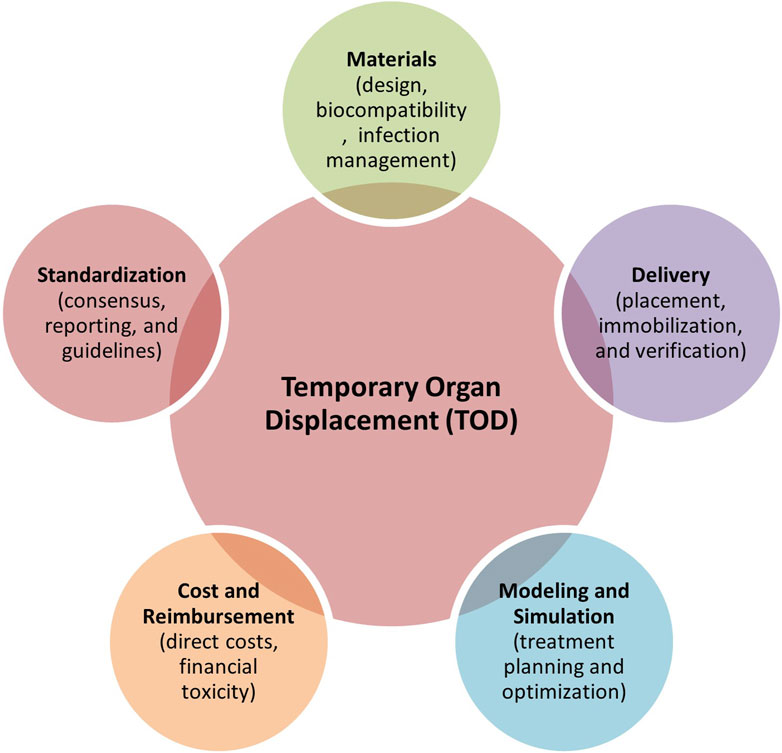
Figure 8. Five key areas requiring further development towards better clinical adoption of TOD techniques in cancer therapies.
5.1 Materials
Minimizing the risk of infection associated with introducing TOD spacers is crucial. This necessitates the development of improved sterile techniques, novel antimicrobial materials, and meticulous post-operative monitoring protocols. As highlighted earlier, the importance of material density and atomic composition in RT warrants further investigation to minimize dose perturbations and imaging artifacts when deploying spacers, necessitating quality control and assurance. Despite promising outcomes in using biologic spacers for RT, the ongoing risks associated with biologic mesh in hernia repair, as discussed by Brinas et al. (2018), must be carefully considered, and long-term follow-up studies are needed to assess the safety and efficacy of biologic spacers in oncologic applications (242).
5.2 Standardization
The lack of transparency and reproducibility issues alluded to by Ghaffari et al. (2020) regarding surgical and interventional procedures highlight the need for standardized reporting and rigorous quality control measures (161). Significant variability exists in the materials used for spacers, with ongoing debates regarding biocompatibility, density, contrast, cost, and manufacturing processes. There is no definitive consensus regarding which TOD technique is most appropriate for specific cancer therapies. Further research is needed to establish evidence-based guidelines for matching TOD techniques to specific tumor locations, sizes, and histological subtypes, considering RT and percutaneous ablation modalities. Standardized reporting guidelines are needed to address the correct and consistent reporting of variables and outcomes for reproducibility. This should include detailed descriptions of the spacer material (e.q., physical density, composition, manufacturer details), placement technique (e.q., open surgery, endoscopic/percutaneous, intraoral/ureteral/rectal insertion, or injection), imaging protocols (e.q., CT/CBCT, MRI, or US), metrics for analysis (e.q., measured separation distances between the tumor and the primary OAR, before and after spacer insertion), and any adverse events associated to spacer use. A thorough discussion of the limitations and potential biases of each study is also essential (161, 248).
5.3 Modeling and simulation
Current clinical trials rarely leverage biomechanical, radiation, or thermal modeling to optimize patient-specific treatment plans, particularly in spacer-mediated therapies, where variables like spacer material viscoelasticity, geometric conformity, and dynamic tissue interactions are critical for ensuring stable therapy delivery (161). Integrating these computational models into treatment workflows could significantly improve the safety and efficacy of targeted spacer delivery by validating spacer performance under thermal or mechanical stress (e.g., preventing displacement during RT) and simulating dose distribution to confirm spacer-mediated shielding of OARs. Future studies should prioritize multi-physics simulations that unify thermal diffusion, tissue biomechanics, and radiation dosimetry-for instance, finite element modeling to predict how hydrogel spacers alter dosimetry in prostate cancer or machine learning-enhanced thermal ablation simulations to optimize spacer conductivity for preventing bowel perforation. Combining machine learning with physics-based models could enable real-time adaptive planning, such as recalibrating radiation fields if intraoperative imaging detects spacer deformation or predicting spacer resorption rates to synchronize bioresorbable material degradation with fractionated treatment schedules. These advancements would not only maximize tumor control (e.g., enabling dose escalation) but also address risks, such as biomechanical displacement and unnecessary tissue damage.
5.4 Cost and reimbursement
As noted by Ogino (2013) the cost and reimbursement of procedures involving TOD vary widely across different countries, potentially limiting access to these interventions. Efforts are needed to advocate for equitable reimbursement policies that recognize the clinical value of TOD (177). Direct costs including specialized materials, operative time, and perioperative care must be weighed against indirect benefits such as reduced hospital stays, fewer complications, and improved QOL. Financial toxicity remains another significant concern in cancer therapies, as the inflated costs associated with the adoption of TOD may create barriers for patients and healthcare systems (249, 250). Addressing these economic challenges through policy reforms and cost-effectiveness studies is crucial to ensuring broader accessibility and equitable adoption of TOD techniques.
5.5 Delivery
Consensus towards the proper delivery method of TOD spacers remains unclear, with significant concerns regarding associated risks and complications for open surgical spacer placements as well as minimally invasive spacer injections. Image-guided percutaneous or endoscopic placements are increasingly preferred to enhance precision and minimize patient morbidity. While percutaneous approaches involving hydrogel spacers in RT are generally considered safer, they require precise injection within confined anatomical spaces to minimize fluid displacement (as observed in hydrodissection cases in IR), since inadequate placement can lead to ineffective organ separation and consequently result in dose toxicities (142). Although rare, they can also result in serious adverse events, including prostatitis and septic shock, underscoring the need for vigilant post-procedural monitoring, careful patient selection, and procedural expertise (251). The emergence of actively reversible NASHA® spacers such as Barrigel™ (Teleflex, US) represents a significant advancement in addressing these clinical concerns (141) and is currently undergoing a large-scale, multi-center trial (NCT06496256) across the US, Australia, and Spain for PPRT patients. Recent clinical experience has demonstrated that patients with rectal wall infiltration from Barrigel™ spacers, though are uncommon, were successfully managed and reversed using targeted hyaluronidase injection (140). This reversibility feature provides a crucial safety mechanism potentially transforming the risk-benefit profile of hydrogel spacer procedures in both RT and IR applications.
To further support the evaluation of spacers and their effectiveness, the scoring methodology introduced by Fischer-Valuck et al. (2017) utilizing symmetry-based measurements relative to the prostate midline has proven clinically useful towards characterizing the impact of hydrogel-based spacers on rectal doses (245). Charas et al. (2024 ASCO Annual Meeting, Chicago, US) expanded this scoring approach to include balloon spacers (BioProtect Balloon implant™ System, BioProtect, Israel), demonstrating improved symmetry and lower rectal doses compared to gel spacers (246). Other methods, such as the spacer quality score (SQS) developed by Grossman et al. (2023), emphasize the amount of separation and spatial relationship between the spacer and rectum (244). The SQS was externally validated in a cohort of prostate cancer patients receiving SBRT (n = 30), confirming clinical feasibility, while also highlighting the need for improvements to account for the disproportionate impact of a single poorly separated region on overall spacer adequacy (243).
Current practices are increasingly looking into implementing stereotactic guided procedures in IR, and we see this naturally translate into its use for TOD spacers since both utilize complementary image-guided methodologies and share common expertise within IR protocols. In RT, similar trends begin to form using existing treatment planning platforms and computational modeling to simulate and identify optimal TOD spacer placements (252–254). Emerging technologies, including robotic-assisted navigation, augmented reality platforms, and stereotactic guidance systems, provide opportunities to further refine the delivery of TOD spacers, maximizing therapeutic efficacy while reducing risks to OARs (255, 256). One advantage of these integrated approaches is the ability to potentially achieve real-time visualization and targeting precision that enables interventional radiologists to literally see through the patient during procedures, while maintaining the reproducible accuracy.
5.6 Smart spacers
The advent of advanced materials offers exciting possibilities for creating ‘smart’ spacers with enhanced functionality. Drawing upon the principles of 3D printing and computer-aided design (CAD), we can tailor the properties of meta-biomaterials to meet the specific needs of individual patients (257). For instance, spacers made from stimuli-responsive materials for contraction in response to external stimuli, such as US, could dynamically adjust organ separation during RT or locoregional ablation therapies (258). Similarly, closed-loop feedback balloon spacers, which integrate sensors for real-time monitoring of pressure or received dose, offer the potential for controlled dose perturbations (259). Additionally, engineered drug-eluting spacers that incorporate therapeutic agents can deliver more localized chemotherapy, anti-inflammatory agents, or other medications, reducing the risk of dose-related toxicities and thermal damage while protecting OARs (260, 261). In addition, 3D printed balloon spacers modified with high-atomic number (Z) materials have shown potential for intraoral and rectal organ displacement and protection in rats, though the adverse effects of radiation scattering from spacers with high atomic materials remain unexplored (262). Integrating these ‘smart’ materials into spacer workflows represents a step forward in personalized medicine, offering the potential to enhance the safety and efficacy of RT and locoregional ablation therapies.
5.7 Patient-centric design of spacers
Our review of spacer studies reveals a diverse range of techniques, devices, and clinical outcomes, highlighting that despite significant technological advances, a “one-size-fits-all” spacer for cancer patients remains elusive. The clinical implementation of spacers is determined on a case-by-case basis, with efforts directed toward addressing accuracy and reproducibility. Notably, in silico studies such as predicting the minimum spacer thickness required for definitive RT with carbon ions and photons for pelvic tumors (263) as well as analyzing the comparative dosimetric impacts of a thicker omentum spacer for abdominal and pelvic tumors in carbon-ion, proton, and photon RT (264), have provided valuable insights into optimizing spacer use. Additionally, the development of cooling devices aimed at minimizing oral mucositis (265–269) and preventing skin burns during RFA of superficial paraspinal masses underscores the innovative approaches being explored to mitigate treatment-related toxicities (2).
Despite these advances, several challenges remain. Maintaining long-term stability is critical, as some spacers may migrate or degrade over time, potentially compromising organ separation and the delivery of the planned therapy. Moreover, patient-specific anatomical variability necessitates individualized approaches to spacer placement, and further advancements in imaging modalities and processing techniques are required to enhance the visualization of both spacers and surrounding tissues. Future research should focus on developing novel spacer materials that offer improved biocompatibility, stability, and visibility on imaging. In parallel, integrating machine learning algorithms into treatment planning holds promise for automating spacer placement, predicting spacer behavior, and optimizing treatment outcomes. The overarching goal is to develop and implement TOD techniques that are safe and effective and tailored to each patient’s unique needs.
5.8 Digital twins
Recently, the National Academies of Sciences, Engineering, and Medicine published a white paper defining a DT as a collection of virtual information constructs replicating the structure, context, and behavior of a natural, engineered, or social system. The virtual model is continuously updated with data from its physical counterpart, can predict future states, and informs decisions that create value. The two-way interaction between the virtual model and the physical entity is central to the DT concept (270).
Within healthcare, DT is categorized into eight key areas: wellness, personalized medicine, clinical trials, biomarker and drug discovery, biomanufacturing, device design, surgical planning, and hospital management design and care coordination (see Figure 9). By integrating TOD into DT models that leverage real-time imaging data, virtual environments, and simulations, clinicians gain enhanced accuracy, guidance, and support to maximize patient treatment outcomes while minimizing complications. This integrated approach enables more precise and personalized cancer therapies, improving efficacy and safety. This section explores key concepts in RT and locoregional ablation therapies, focusing on the interface between TOD and DT.
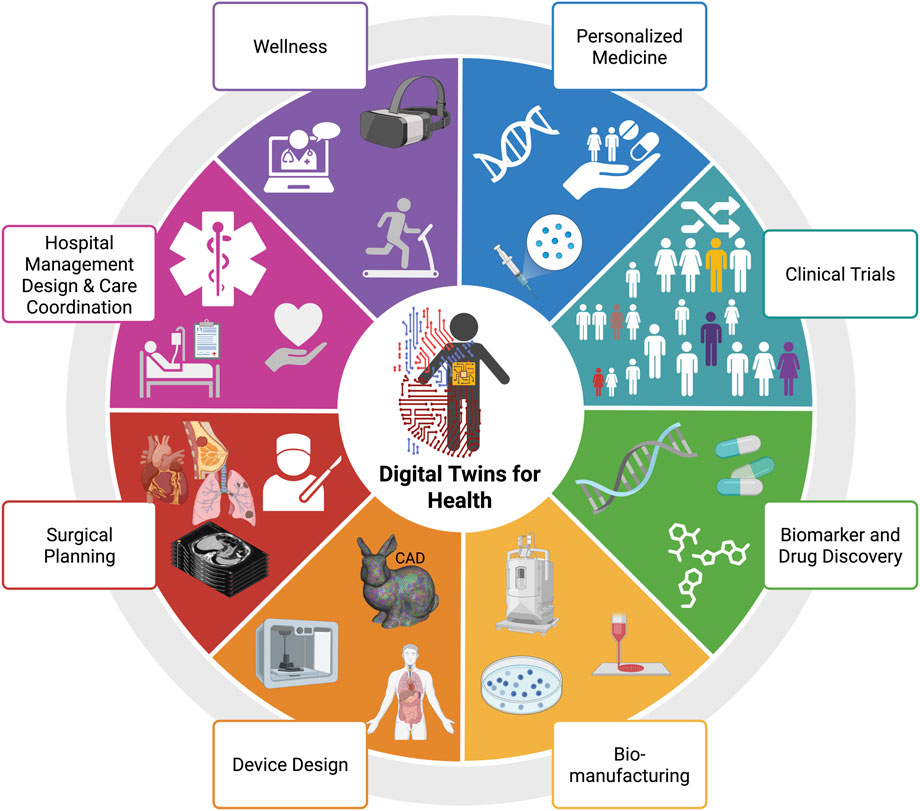
Figure 9. Primary applications of digital twins (DT) in healthcare. Adapted with permission from “Main applications of digital twins for health” by Evangelia Katsoulakis, Qi Wang, Huanmei Wu, Leili Shahriyari, Richard Fletcher, Jinwei Liu, Luke Achenie, Hongfang Liu, Pamela Jackson, Ying Xiao, Tanveer Syeda-Mahmood, Richard Tuli and Jun Deng, licensed under CC BY 4.0. Created with BioRender.com.
5.8.1 Planning, simulation, and clinical decision making in RT
Early concepts of DT towards minimizing RT damage have long been implemented in the areas of medical physics. Treatment planning systems (TPS) have utilized the synergy across 3D organ models and Monte Carlo models to maximize tumor dose prescription, accounting for predefined OAR constraints (6, 272). Later on, advanced image-guidance, tracking, and deformable image registration methods were introduced to accurately account for movements and breathing (10, 273–275). Other DT-based applications in oncology involve the integration of mechanistic modeling, which provides key insights into cancer dynamics, treatment, and patient outcomes (276–278).
Building on the importance of spacer placement and shape for improved RT dosimetry and planning (303, 304), recently developed DT-based spacer planning methods have emerged as an innovative solution, leveraging existing TPS and the finite element method (FEM) to understand spacer placement and its dosimetric impact better (see Figure 10). Kawaguchi et al. (2021) conducted a treatment planning study to investigate the dosimetric effects of a virtual spacer placed between the pancreas and surrounding OARs, highlighting the importance of accurate spacer placement to minimize non-target tissue coverage (279). Similarly, Yamada et al. (2019) compared the dosimetric impacts of a greater omentum spacer across carbon-ion, proton, and photon RT plans, showing notable dose-reduction effects of the virtual omental spacer on the GI tract (i.e., bowel, colon, rectum) (264). Furthermore, the same group developed a multi-regression model to predict the minimum spacer thickness required, that is, the minimum distance between the tumor and the GI tract, that allows for the delivery of definitive doses within the dose limits (D2% <77 Gy RBE) (263). Van Wijk et al. (2017) demonstrated the combination of a multi-regression model and an image deformation model using a unidirectional field not only to predict geometrical changes of the rectum and prostate in the presence of a virtual implantable rectum spacer but also to predict the resulting GI toxicity (280).
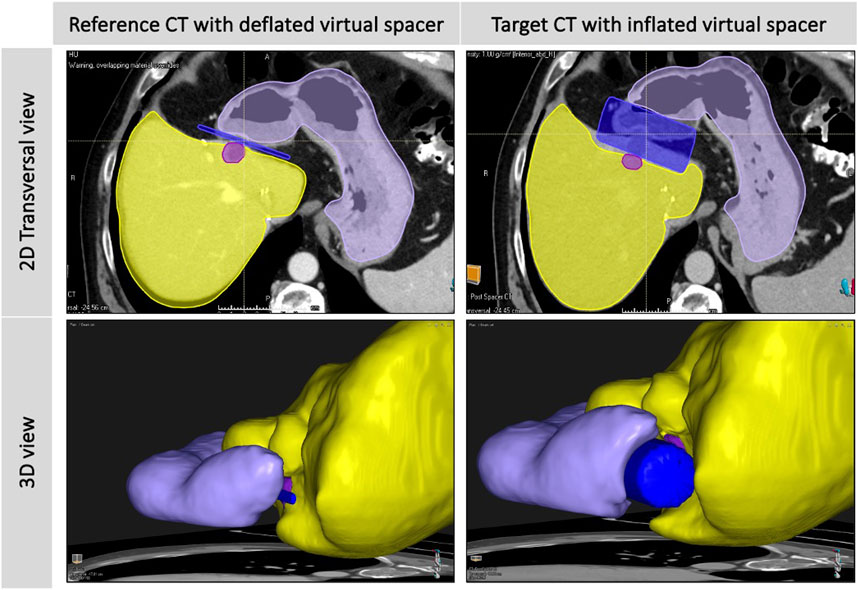
Figure 10. A diagram showing the in silico deformation of liver (yellow), stomach (purple), and tumor (light purple) structures, after simulating the inflation of a virtual cylindrical spacer (blue) from reference to target CT using a biomechanical deformable registration tool (Morfeus, RayStation V12.0, Raysearch Laboratories AB, Sweden).
More recently, Hooshangnejad et al. (2021) demonstrated a personalized approach by developing an FEM-based spacer simulation algorithm, FEMOSSA, to produce realistic deformations of the rectum and the prostate wall in the presence of a virtual HS (253). This algorithm, in combination with a Bayesian multi-regression model, has proven to be highly valuable as a potential clinical decision support tool for patients with pancreatic tumors receiving duodenal spacer placement procedures (252, 281). More recently, a robotic arm was developed to precisely control the placement of a ‘smart’ balloon applicator for brachytherapy and intraoperative RT. This innovation aims to enhance targeted tumor irradiation while effectively shielding OARs (SkinCure Oncology LLC, IL, United States) (259, 282, 283).
5.8.2 Planning, simulation, and clinical decision making in IR
Several simulation platforms have been proposed and adapted for planning thermal ablation procedures. More specifically, multi-physics solvers to compute electromagnetic power, temperature distribution, and specific absorption rates were investigated for MWA applications (284–287). Additionally, mathematical models involving isotherm contours, thermal effective doses, and the Arrhenius model have added value towards more accurate quantification of cell death during thermal ablation procedures (288, 289). Zhang et al. (2019) highlighted key computational approaches currently utilized for planning thermal ablation procedures encompassing the segmentation of anatomical structures from imaging scans, identifying the tumor’s centroid, and defining feasible skin entry zones through ‘hard’ or user-defined constraints. Subsequently, ‘Soft’ constraints are implemented to refine needle trajectory, and an optimization framework determines the ideal needle path. Despite significant algorithmic progress, clinical adoption remains limited primarily due to the lack of clinical evidence, highlighting a gap between research and practice (290).
A multi-modal approach utilizing a combination of DT-based technologies can possibly bridge this gap. Technologies such as deformable imaging methods, robotics, virtual reality, 3D printing, and stereotactic navigation systems have been integrated into IR planning protocols to enhance workflows and potentially improve treatment outcomes (8, 256, 291–296). More recently, Paolucci et al. (2025) highlight that automated, software-based workflows for ablation confirmation have transformed the assessment of technical success in percutaneous thermal ablation of malignant liver tumors. Conventional methods to measure minimal ablative margins (MAM) involves the use of anatomic landmarks, a method limited by operator bias and poor reproducibility. State-of-the-art software solutions now enable standardized, quantitative MAM measurement by integrating advanced imaging techniques, segmentation, and registration, many of which are enhanced by artificial intelligence. These digital tools provide rapid, accurate feedback during procedures, allowing for immediate re-ablation if margins are insufficient, and supporting improved local tumor control (255).
Commercial systems such as Ethicon’s NeuWave and Medtronic’s Emprint offer distinct MWA planning approaches (9). NeuWave integrates with picture archiving and communication systems (PACS) for lesion identification and antenna targeting but lacks a visual display of the predicted ablation zone, necessitating manual margin reference. In contrast, Emprint employs organ-specific workflows to automatically extract imaging data and calculate ablation times based on in vivo data. In addition, CAScination’s CAS-One IR integrates optical stereotactic navigation to enhance needle targeting (297–299). Innovations in stereotactic CT guidance with CAS-One IR have improved precision, reduced repositioning, and minimized complications such as bleeding and tumor dissemination (300, 301). Recent CT-based applications have successfully treated challenging lesions, as seen in a breast cancer liver metastasis in segment I that was precisely ablated with no recurrence after 18 months. While MWA offers advantages over RFA, such as resistance to the heat sink effect and the ability to create larger ablation zones, these benefits must be carefully managed to avoid damage to critical structures. Integrating advanced navigation systems like CAS-One IR in the clinic is a crucial step toward optimizing ablation outcomes and minimizing procedural risks (302).
6 Summary
In this review, we synthesized the considerable progress in the development of TOD techniques and devices utilized for RT and locoregional ablation therapies. Here, we provide a comprehensive classification of TOD based on key technical and clinical parameters. This structured approach is vital for addressing challenges such as the lack of standardized guidelines, variability in technical proficiency, and complex clinical workflows. Ultimately, this classification aims to strengthen clinical outcomes, foster broader adoption, and pave the way for the next-generation of more effective TOD techniques and better-tolerated cancer therapies. This review culminates in several key findings and future directions:
• From non-invasive and indirect TOD techniques such as deep inspiration breath-hold and advanced immobilization devices to more invasive and direct techniques like intraoral stents, injection, and surgical spacer placements, the field has experienced a remarkable diversification of organ displacement strategies. These innovations have enhanced the precision of radiation delivery and locoregional ablation therapies and significantly decreased treatment-related morbidities. The impact of these innovations extends beyond immediate clinical outcomes. By enabling more aggressive treatment regimens while reducing side effects, these techniques can improve long-term survival rates, enhance QOL for cancer survivors, and reduce the economic burden of managing treatment-related complications.
• Standardization of TOD techniques in clinical practice remains an evolving priority. While significant progress has been made in refining organ displacement strategies, universally accepted guidelines and protocols for TOD implementation remain limited. This absence of standardization risks compromising reproducibility, outcome comparability, and broader integration across cancer treatment centers. Addressing these challenges is critical to maximize clinical benefits, ensure high-quality care, enable informed treatment planning, as well as facilitate the development of robust clinical trials. Standardizing TOD techniques will empower clinicians to better interpret treatment effectiveness and safety, supporting evidence-based decisions and paving the way for broader adaptation in diverse healthcare settings.
• Integrating TOD techniques with emerging technologies promises to enhance and personalize cancer treatments further. Smart spacers utilizing meta-materials offer exciting possibilities for enhancing the precision of RT and thermal ablation therapies. These innovative devices can automatically tune to optimize treatment delivery while minimizing damage to healthy tissues. These advanced technologies can improve treatment outcomes, combined with artificial intelligence-driven treatment planning, real-time imaging, and adaptive therapies. The ongoing research in this field, including using sophisticated simulations to optimize spacer design and placement, underscores the medical community’s commitment to innovation.
Author contributions
RT: Methodology, Data curation, Visualization, Conceptualization, Project administration, Investigation, Validation, Supervision, Funding acquisition, Software, Formal Analysis, Writing – original draft, Resources, Writing – review and editing. ML: Data curation, Writing – original draft, Investigation, Writing – review and editing. AH: Writing – original draft, Investigation, Data curation, Writing – review and editing. AS: Investigation, Data curation, Writing – review and editing, Writing – original draft. AG: Formal Analysis, Data curation, Writing – original draft, Investigation, Writing – review and editing. KB: Writing – review and editing, Methodology, Supervision. BO: Supervision, Methodology, Writing – review and editing, Funding acquisition. EK: Validation, Supervision, Methodology, Writing – review and editing, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. R.B.T is supported by the Cancer Prevention and Research Institute of Texas (CPRIT) Training Program (award RP210028). E.J.K is supported by the Shirley Stein Research Award and MD Anderson Cancer Center Radiation Oncology Strategic initiatives.
Acknowledgments
We thank the CPRIT TRIUMPH Program for their support (award RP210028). Research reported in this publication was supported in part by resources of the Image Guided Cancer Therapy Research Program at The University of Texas MD Anderson Cancer Center, including a generous gift from the Apache Corporation and the Helen Black Image Guided Therapy Research Fund; and the National Cancer Institute of the National Institutes of Health (award R01CA235564).
Conflict of interest
RBT declares patent licensing agreement with Kallisio. KKB declares funding and royalties from RaySearch Laboratories; participation on the Clinical Advisory Board for RaySearch Laboratories. BCO declares research grants from Siemens Healthineers, Ethicon, Society of Interventional On-cology, and NIH; consulting fees from IO Life Sciences; co-development and license agreement between MD Anderson and an undisclosed party. EJK declares research grants from NIH, Philips Healthcare, GE Healthcare, Breakthrough Cancer, Stand up to Cancer, Project Purple, Elekta, Department of Defense, Nanobiotix, and EMD Serono; royalties from Taylor and Francis, LLC and licensing agreement with Kallisio regarding patent US11730561B2; consulting fees from IO Life Sciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. De Ruysscher, D, Niedermann, G, Burnet, NG, Siva, S, Lee, AWM, and Hegi-Johnson, F. Radiotherapy toxicity. Nat Rev Dis Primers (2019) 5:13. doi:10.1038/s41572-019-0064-5
2. Liddell, RP, and Solomon, SB. Thermal protection during radiofrequency ablation. Am J Roentgenology (2004) 182:1459–61. doi:10.2214/ajr.182.6.1821459
4. Chopra, S, Dodd, GD, Chanin, MP, and Chintapalli, KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. Am J Roentgenology (2003) 180:697–701. doi:10.2214/ajr.180.3.1800697
5. Thames, HD, Peters, LT, Withers, HR, and Fletcher, GH. Accelerated fractionation vs hyperfractionation: rationales for several treatments per day. Int J Radiat Oncology* Biology* Phys (1983) 9:127–38. doi:10.1016/0360-3016(83)90089-5
6. Bentel, GC, Nelson, CE, and Noell, KT. Treatment planning and dose calculation in radiation oncology. 3rd edn. Elsevier (1982).
7. Withers, HR. The four R’s of radiotherapy. Adv Radiat Biol (1975) 5:241–71. doi:10.1016/b978-0-12-035405-4.50012-8
8. Beyer, L, and Wiggermann, P. Planning and guidance: new tools to enhance the human skills in interventional oncology. Diagn Interv Imaging (2017) 98:583–8. doi:10.1016/j.diii.2017.07.004
9. Lyons, GR, and Pua, BB. Ablation planning software for optimizing treatment: challenges, techniques, and applications. Tech Vasc Interv Radiol (2019) 22:21–5. doi:10.1053/j.tvir.2018.10.005
10. Sotiras, A, Davatzikos, C, and Paragios, N. Deformable medical image registration: a survey. IEEE Trans Med Imaging (2013) 32:1153–90. doi:10.1109/tmi.2013.2265603
11. Smith, TA, Kirkpatrick, DR, Smith, S, Smith, TK, Pearson, T, Kailasam, A, et al. Radioprotective agents to prevent cellular damage due to ionizing radiation. J translational Med (2017) 15:232–18. doi:10.1186/s12967-017-1338-x
12. Lee, SJ, Choyke, LT, Locklin, JK, and Wood, BJ. Use of hydrodissection to prevent nerve and muscular damage during radiofrequency ablation of kidney tumors. J Vasc Interv Radiol (2006) 17:1967–9. doi:10.1097/01.RVI.0000248829.49442.0E
13. Liu, S, Zhao, Q, Zheng, Z, Liu, Z, Meng, L, Dong, L, et al. Status of treatment and prophylaxis for radiation-induced oral mucositis in patients with head and neck cancer. Front Oncol (2021) 11:642575–2021. doi:10.3389/fonc.2021.642575
14. Tang, Q, Zhao, F, Yu, X, Wu, L, Lu, Z, and Yan, S. The role of radioprotective spacers in clinical practice: a review. Quantitative Imaging Med Surg (2018) 8:514–24. doi:10.21037/qims.2018.06.06
15. Afkhami Ardekani, M, Ghaffari, H, Navaser, M, Zoljalali Moghaddam, SH, and Refahi, S. Effectiveness of rectal displacement devices in managing prostate motion: a systematic review. Strahlentherapie und Onkologie (2021) 197:97–115. doi:10.1007/s00066-020-01633-9
16. Sanei, M, Ghaffari, H, Ardekani, MA, Mahdavi, SR, Mofid, B, Abdollahi, H, et al. Effectiveness of rectal displacement devices during prostate external-beam radiation therapy: a review. J Cancer Res Ther (2021) 17:303–10. doi:10.4103/jcrt.jcrt_841_19
17. Karsh, LI, Gross, ET, Pieczonka, CM, Aliotta, PJ, Skomra, CJ, Ponsky, LE, et al. Absorbable hydrogel spacer use in prostate radiotherapy: a comprehensive review of phase 3 clinical trial published data. Urology (2018) 115:39–44. doi:10.1016/j.urology.2017.11.016
18. Repka, MC, Creswell, M, Lischalk, JW, Carrasquilla, M, Forsthoefel, M, Lee, J, et al. Rationale for utilization of hydrogel rectal spacers in dose escalated SBRT for the treatment of unfavorable risk prostate cancer. Front Oncol (2022) 12:860848. doi:10.3389/fonc.2022.860848
19. Forsthoefel, M, Hankins, R, Ballew, E, Frame, C, DeBlois, D, Pang, D, et al. Prostate cancer treatment with pencil beam proton therapy using rectal spacers sans endorectal balloons. Int J Part Ther (2022) 9:28–41. doi:10.14338/ijpt-21-00039
20. Dasmahapatra, KS, and Swaminathan, AP. The use of a biodegradable mesh to prevent radiation-associated small-bowel injury. Arch Surg (1991) 126:366–9. doi:10.1001/archsurg.1991.01410270114018
21. Ates, O, Zhao, L, Sobczak, D, Hua, CH, and Krasin, MJ. Dosimetric advantages of silicone-filled vaginal spacers in pediatric proton therapy. Int J Part Ther (2022) 9:64–70. doi:10.14338/ijpt-21-00044.1
22. Lee, D, Komatsu, S, Terashima, K, Toyama, H, Matsuo, Y, Takahashi, D, et al. Surgical spacer placement for proton radiotherapy in locally advanced pancreatic body and tail cancers: initial clinical results. Radiat Oncol (2021) 16:3. doi:10.1186/s13014-020-01731-z
23. Yoon, SS, Aloia, TA, Haynes, AB, Kambadakone, A, Kaur, H, Vauthey, JN, et al. Surgical placement of biologic mesh spacers to displace bowel away from unresectable liver tumors followed by delivery of dose-intense radiation therapy. Pract Radiat Oncol (2014) 4:167–73. doi:10.1016/j.prro.2013.07.007
24. Garnon, J, Cazzato, RL, Caudrelier, J, Nouri-Neuville, M, Rao, P, Boatta, E, et al. Adjunctive thermoprotection during percutaneous thermal ablation procedures: review of current techniques. Cardiovasc Intervent Radiol (2019) 42:344–57. doi:10.1007/s00270-018-2089-7
25. Levit, E, Bruners, P, Günther, RW, and Mahnken, AH. Bile aspiration and hydrodissection to prevent complications in hepatic RFA close to the gallbladder. Acta Radiologica (2012) 53:1045–8. doi:10.1258/ar.2012.120190
26. Zhang, LL, Xia, GM, Liu, YJ, Dou, R, Eisenbrey, J, Liu, JB, et al. Effect of a poloxamer 407-based thermosensitive gel on minimization of thermal injury to diaphragm during microwave ablation of the liver. World J Gastroenterol (2017) 23:2141–8. doi:10.3748/wjg.v23.i12.2141
27. Moreland, AJ, Lubner, MG, Ziemlewicz, TJ, Kitchin, DR, Hinshaw, JL, Johnson, AD, et al. Evaluation of a thermoprotective gel for hydrodissection during percutaneous microwave ablation: in vivo results. Cardiovasc Intervent Radiol (2015) 38:722–30. doi:10.1007/s00270-014-1008-9
28. Tsoumakidou, G, Buy, X, Garnon, J, Enescu, J, and Gangi, A. Percutaneous thermal ablation: how to protect the surrounding organs. Tech Vasc Interv Radiol (2011) 14:170–6. doi:10.1053/j.tvir.2011.02.009
29. Josipovic, M, Aznar, MC, Thomsen, JB, Scherman, J, Damkjaer, SM, Nygård, L, et al. Deep inspiration breath hold in locally advanced lung cancer radiotherapy: validation of intrafractional geometric uncertainties in the INHALE trial. The Br J Radiol (2019) 92:20190569. doi:10.1259/bjr.20190569
30. Negoro, Y, Nagata, Y, Aoki, T, Mizowaki, T, Araki, N, Takayama, K, et al. The effectiveness of an immobilization device in conformal radiotherapy for lung tumor: reduction of respiratory tumor movement and evaluation of the daily setup accuracy. Int J Radiat Oncology*Biology*Physics (2001) 50:889–98. doi:10.1016/S0360-3016(01)01516-4
31. Bentel, GC, Marks, LB, and Krishnamurthy, R. Impact of cradle immobilization of setup reproducibility during external beam radiation therapy for lung cancer. Int J Radiat Oncology*Biology*Physics (1997) 38:527–31. doi:10.1016/S0360-3016(97)00011-4
32. Endo, Y, Fukuzawa, T, Irie, M, Sasaki, H, Kudo, H, Ando, R, et al. Intraoperative placement of an absorbable spacer prior to radiation therapy for a malignant peripheral nerve sheath tumor. Case Rep Oncol (2022) 15:541–6. doi:10.1159/000524824
33. Brandão, TB, da Graça Pinto, H, Vechiato Filho, AJ, Faria, KM, de Oliveira, MCQ, Prado-Ribeiro, AC, et al. Are intraoral stents effective in reducing oral toxicities caused by radiotherapy? A systematic review and meta-analysis. J Prosthet Dent (2022) 128:1380–6. doi:10.1016/j.prosdent.2021.03.009
34. Appendino, P, Della Ferrera, F, Nassisi, D, Blandino, G, Gino, E, Solla, S, et al. Are intraoral customized stents still necessary in the era of Highly Conformal Radiotherapy for Head and Neck cancer? Case series and literature review. Rep Pract Oncol and Radiother (2019) 24:491–8. doi:10.1016/j.rpor.2019.07.012
35. Verrone, JR, Alves, Fd A, Prado, JD, Boccaletti, KW, Sereno, MP, Silva, MLG, et al. Impact of intraoral stent on the side effects of radiotherapy for oral cancer. Head and neck (2013) 35:E213–E217. doi:10.1002/hed.23028
36. Wilke, CT, Zaid, M, Chung, C, Fuller, CD, Mohamed, ASR, Skinner, H, et al. Design and fabrication of a 3D-printed oral stent for head and neck radiotherapy from routine diagnostic imaging. 3d Print Med (2017) 3:12. doi:10.1186/s41205-017-0021-4
37. Zaid, M, Koay, EJ, Bajaj, N, Mathew, R, Xiao, L, Agrawal, A, et al. A prospective parallel design study testing non-inferiority of customized oral stents made using 3D printing or manually fabricated methods. Oral Oncol (2020) 106:104665. doi:10.1016/j.oraloncology.2020.104665
38. Smeenk, RJ, Teh, BS, Butler, EB, van Lin, ENJT, and Kaanders, JHAM. Is there a role for endorectal balloons in prostate radiotherapy? A systematic review. Radiother Oncol (2010) 95:277–82. doi:10.1016/j.radonc.2010.04.016
39. Ghadjar, P, Budach, V, Köhler, C, Jantke, A, and Marnitz, S. Modern radiation therapy and potential fertility preservation strategies in patients with cervical cancer undergoing chemoradiation. Radiat Oncol (2015) 10:50. doi:10.1186/s13014-015-0353-4
40. Mok, G, Benz, E, Vallee, J-P, Miralbell, R, and Zilli, T. Optimization of radiation therapy techniques for prostate cancer with prostate-rectum spacers: a systematic review. Int J Radiat Oncology* Biology* Phys (2014) 90:278–88. doi:10.1016/j.ijrobp.2014.06.044
41. Vaggers, S, Rai, BP, Chedgy, ECP, de la Taille, A, and Somani, BK. Polyethylene glycol-based hydrogel rectal spacers for prostate brachytherapy: a systematic review with a focus on technique. World J Urol (2021) 39:1769–80. doi:10.1007/s00345-020-03414-6
42. Lapuz, C, Kain, M, Chao, M, Joon, DL, Dempsey, C, and Sim, J. Injectable bio-absorbable spacers in brachytherapy for gynecological cancers: a scoping review. J Contemp Brachytherapy (2024) 16:467–77. doi:10.5114/jcb.2024.146834
43. McCall, ML, Keaty, EC, and Thompson, JD. Conservation of ovarian tissue in the treatment of carcinoma of the cervix with radical surgery. Am J Obstet Gynecol (1958) 75:590–600. doi:10.1016/0002-9378(58)90614-8
44. Bieler, EU, Schnabel, T, and Knobel, J. Persisting cyclic ovarian activity in cervical cancer after surgical transposition of the ovaries and pelvic irradiation. The Br J Radiol (1976) 49:875–9. doi:10.1259/0007-1285-49-586-875
45. Krebs, C, Blixenkrone-møller, N, and Mosekilde, V. Preservation of ovarian function in early cervical cancer after surgical lifting of the ovaries and radiation therapy. Acta Radiologica: Ther Phys Biol (1963) 1:176–82. doi:10.3109/02841866309135076
46. Batten, R, and Brown, DE. Protection of ovaries from radiation. The Lancet (1956) 267:939–40. doi:10.1016/s0140-6736(56)91523-9
47. Steckel, RJ, Collins, JD, Snow, HD, Lagasse, LD, Barenfus, M, Anderson, DP, et al. Radiation protection of the normal kidney by selective arterial infusions. Cancer (1974) 34:1046–58. doi:10.1002/1097-0142(197410)34:4<1046::aid-cncr2820340414>3.0.co;2-l
48. Romsdahl, MM, and Withers, HR. Radiotherapy combined with curative surgery: its use as therapy for carcinoma of the sigmoid colon and rectum. Arch Surg (1978) 113:446–53. doi:10.1001/archsurg.1978.01370160104017
49. Roswit, B, Higgins, GA, and Keehn, RJ. Preoperative irradiation for carcinoma of the rectum and rectosigmoid colon: report of a National Veterans Administration randomized study. Cancer (1975) 35:1597–602. doi:10.1002/1097-0142(197506)35:6<1597::aid-cncr2820350618>3.0.co;2-s
50. Turner, SS, Vieira, EF, Ager, PJ, Alpert, S, Efron, G, Ragins, H, et al. Elective postoperative radiotherapy for locally advanced colorectal cancer. A preliminary report. Cancer (1977) 40:105–8. doi:10.1002/1097-0142(197707)40:1<105::aid-cncr2820400119>3.0.co;2-#
51. Gunderson, LL, Cohen, AM, and Welch, CE. Residual, inoperable or recurrent colorectal cancer. The Am J Surg (1980) 139:518–25. doi:10.1016/0002-9610(80)90330-x
52. Freund, H, Gunderson, L, Krause, R, and Fischer, JE. Prevention of radiation enteritis after abdominoperineal resection and radiotherapy. Surg Gynecol and Obstet (1979) 149:206–8.
53. DeLuca, FR, and Ragins, H. Construction of an omental envelope as a method of excluding the small intestine from the field of postoperative irradiation to the pelvis. Surg Gynecol Obstet (1985) 160:365–6.
54. Faust, KJ. Hydrodissection of soft nuclei. Am Intra-Ocular Implant Soc J (1984) 10:75–7. doi:10.1016/s0146-2776(84)80088-9
55. Ginat, DT, and Saad, WEA. Bowel displacement and protection techniques during percutaneous renal tumor thermal ablation. Tech Vasc Interv Radiol (2010) 13:66–74. doi:10.1053/j.tvir.2010.02.002
56. Johnston, EW, Basso, J, Mathiszig-Lee, J, Strauss, DC, and Fotiadis, N. Stable pneumoperitoneum using an automatic CO2 insufflation machine for safer cryoablation procedures. CardioVascular Interv Radiol (2024) 47:1417–9. doi:10.1007/s00270-024-03812-y
57. Yamakado, K, Nakatsuka, A, Akeboshi, M, and Takeda, K. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol (2003) 14:1183–6. doi:10.1097/01.rvi.0000086530.86489.05
59. Farrell, MA, Charboneau, JW, Callstrom, MR, Reading, CC, Engen, DE, and Blute, ML. Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. Am J Roentgenology (2003) 181:1315–7. doi:10.2214/ajr.181.5.1811315
60. Latty, D, Stuart, KE, Wang, W, and Ahern, V. Review of deep inspiration breath-hold techniques for the treatment of breast cancer. J Med Radiat Sci (2015) 62:74–81. doi:10.1002/jmrs.96
61. Giraud, P, Morvan, E, Claude, L, Mornex, F, Le Pechoux, C, Bachaud, JM, et al. Respiratory gating techniques for optimization of lung cancer radiotherapy. J Thorac Oncol (2011) 6:2058–68. doi:10.1097/JTO.0b013e3182307ec2
62. Marchand, VM, Zefkili, S, Desrousseaux, J, Simon, L, Dauphinot, C, and Giraud, P. Dosimetric comparison of free-breathing and deep inspiration breath-hold radiotherapy for lung cancer. Strahlentherapie und Onkologie (2012) 188:582–91. doi:10.1007/s00066-012-0129-9
63. Nissen, HD, and Appelt, AL. Improved heart, lung and target dose with deep inspiration breath hold in a large clinical series of breast cancer patients. Radiother Oncol (2013) 106:28–32. doi:10.1016/j.radonc.2012.10.016
64. Ottosson, W, Sibolt, P, Larsen, C, Lykkegaard Andersen, JA, Borissova, S, Mellemgaard, A, et al. Monte Carlo calculations support organ sparing in Deep-Inspiration Breath-Hold intensity-modulated radiotherapy for locally advanced lung cancer. Radiother Oncol (2015) 117:55–63. doi:10.1016/j.radonc.2015.08.032
65. Remouchamps, VM, Letts, N, Vicini, FA, Sharpe, MB, Kestin, LL, Chen, PY, et al. Initial clinical experience with moderate deep-inspiration breath hold using an active breathing control device in the treatment of patients with left-sided breast cancer using external beam radiation therapy. Int J Radiat Oncology*Biology*Physics (2003) 56:704–15. doi:10.1016/S0360-3016(03)00010-5
66. Dawson, LA, Brock, KK, Kazanjian, S, Fitch, D, McGinn, CJ, Lawrence, TS, et al. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncology*Biology*Physics (2001) 51:1410–21. doi:10.1016/s0360-3016(01)02653-0
67. Bergom, C, Currey, A, Desai, N, Tai, A, and Strauss, JB. Deep inspiration breath hold: techniques and advantages for cardiac sparing during breast cancer irradiation. Front Oncol (2018) 8:87. doi:10.3389/fonc.2018.00087
68. Tuncali, K, Morrison, PR, Tatli, S, and Silverman, SG. MRI-guided percutaneous cryoablation of renal tumors: use of external manual displacement of adjacent bowel loops. Eur J Radiol (2006) 59:198–202. doi:10.1016/j.ejrad.2006.04.013
69. De Kerviler, E, Guermazi, A, Cazals-Hatem, D, and Frija, J. Use of abdominal compression as an aid to CT-guided retroperitoneal biopsies. Am J Roentgenology (1996) 167:1346–7. doi:10.2214/ajr.167.5.8911219
70. Dachman, AH. A biopsy compression device for use in cross-sectional or fluoroscopic imaging. Am J Roentgenology (1998) 171:703–5. doi:10.2214/ajr.171.3.9725300
71. Epelboym, Y, Shyn, PB, Hosny, A, Kelil, T, Chick, JFB, Chauhan, NR, et al. Use of a 3D-printed abdominal compression device to facilitate CT fluoroscopy–guided percutaneous interventions. Am J Roentgenology (2017) 209:435–41. doi:10.2214/ajr.16.17188
72. Wan, B, Luo, S, Feng, X, Qin, W, Sun, H, Hou, L, et al. Superiority of integrated cervicothoracic immobilization in the setup of lung cancer patients treated with supraclavicular station irradiation. Front Oncol (2023) 13:1135879. doi:10.3389/fonc.2023.1135879
73. Bentel, GC, Marks, LB, Hendren, K, and Brizel, DM. Comparison of two head and neck immobilization systems. Int J Radiat Oncol Biol Phys (1997) 38:867–73. doi:10.1016/s0360-3016(97)00075-8
74. Hess, CF, Kortmann, R-D, Jany, R, Hamberger, A, and Bamberg, M. Accuracy of field alignment in radiotherapy of head and neck cancer utilizing individualized face mask immobilization: a retrospective analysis of clinical practice. Radiother Oncol (1995) 34:69–72. doi:10.1016/0167-8140(94)01497-Q
75. Zhao, B, Maquilan, G, Jiang, S, and Schwartz, DL. Minimal mask immobilization with optical surface guidance for head and neck radiotherapy. J Appl Clin Med Phys (2018) 19:17–24. doi:10.1002/acm2.12211
76. Lv, R, Yang, G, Huang, Y, and Wang, Y. Dosimetric effects of supine immobilization devices on the skin in intensity-modulated radiation therapy for breast cancer: a retrospective study. BMC Cancer (2021) 21:384. doi:10.1186/s12885-021-08119-6
77. Rosenthal, SA, Roach, M, Goldsmith, BJ, Catherine Doggett, E, Pickett, B, Yuo, HS, et al. Immobilization improves the reproducibility of patient positioning during six-field conformal radiation therapy for prostate carcinoma. Int J Radiat Oncology*Biology*Physics (1993) 27:921–6. doi:10.1016/0360-3016(93)90469-C
78. Kneebone, A, Gebski, V, Hogendoorn, N, and Turner, S. A randomized trial evaluating rigid immobilization for pelvic irradiation. Int J Radiat Oncology* Biology* Phys (2003) 56:1105–11. doi:10.1016/s0360-3016(03)00222-0
79. Lovelock, DM, Hua, C, Wang, P, Hunt, M, Fournier-Bidoz, N, Yenice, K, et al. Accurate setup of paraspinal patients using a noninvasive patient immobilization cradle and portal imaging. Med Phys (2005) 32:2606–14. doi:10.1118/1.1951042
80. Johnson, B, Sales, L, Winston, A, Liao, J, Laramore, G, and Parvathaneni, U. Fabrication of customized tongue-displacing stents: considerations for use in patients receiving head and neck radiotherapy. The J Am Dental Assoc (2013) 144:594–600. doi:10.14219/jada.archive.2013.0170
81. Kaanders, JH, Fleming, TJ, Ang, KK, Maor, MH, and Peters, LJ. Devices valuable in head and neck radiotherapy. Int J Radiat Oncology* Biology* Phys (1992) 23:639–45. doi:10.1016/0360-3016(92)90023-b
82. Singh, A, Rosen, EB, Randazzo, JD, Estilo, CL, Gelblum, DY, and Huryn, JM. Intraoral radiation stents—primer for clinical use in head and neck cancer therapy. Head and Neck (2021) 43:4010–7. doi:10.1002/hed.26848
83. Niwa, K, Morita, K, Kanazawa, H, and Yokoi, M. Usefulness of a radiolucent spacer in radiation therapy for cancer of the tongue. Gan No Rinsho (1984) 30:1861–5.
84. Fujita, M, Tamamoto, M, Hirokawa, Y, Kashiwado, K, Akagi, Y, Kashimoto, K, et al. Experimental and clinical studies on dose reduction effects of spacers in interstitial brachytherapy for carcinoma of the mobile tongue. Oral Surg Oral Med Oral Pathol (1993) 76:797–803. doi:10.1016/0030-4220(93)90054-8
85. Fujita, M, Hirokawa, Y, Tamamoto, M, Kashiwado, K, Akagi, Y, Kashimoto, K, et al. Dose-reducing effect of Lipowitz metal-embedded spacers in interstitial brachytherapy for carcinoma of the mobile tongue. Oral Surg Oral Med Oral Pathol (1994) 77:589–93. doi:10.1016/0030-4220(94)90316-6
86. Tamamoto, M, Fujita, M, Yamamoto, T, and Hamada, T. Techniques for making spacers in interstitial brachytherapy for tongue cancer. The Int J Prosthodont (1996) 9:95–8.
87. Tino, R, Roach, MA, Fuentes, GD, Agrawal, A, Zaid, M, Cooper, DJ, et al. Development and clinical implementation of a digital workflow utilizing 3D-printed oral stents for patients with head and neck cancer receiving radiotherapy. Oral Oncol (2024) 157:106944. doi:10.1016/j.oraloncology.2024.106944
88. Bruno, JS, Miranda-Silva, W, Guedes, VS, Parahyba, CJ, Moraes, FY, and Fregnani, ER. Digital workflow for producing oral positioning radiotherapy stents for head and neck cancer. J Prosthodont (2020) 29:448–52. doi:10.1111/jopr.13155
89. Wang, RR, and Olmsted, LW. A direct method for fabricating tongue-shielding stent. The J Prosthetic Dentistry (1995) 74:171–3. doi:10.1016/S0022-3913(05)80182-9
90. Ma, J, Chen, Z, Liu, S, Hu, W, Su, K, He, R, et al. The application of 3D-printed oral stents in intensity-modulated radiotherapy for oropharyngeal cancer and their dosimetric effect on organs at risk. Eur J Med Res (2023) 28:367. doi:10.1186/s40001-023-01333-x
91. Cleland, S, Crowe, SB, Chan, P, Chua, B, Dawes, J, Kenny, L, et al. Development of a customisable 3D-printed intra-oral stent for head-and-neck radiotherapy. Tech Innov and Patient Support Radiat Oncol (2022) 23:1–7. doi:10.1016/j.tipsro.2022.06.001
92. Mundee, T, Jongwannasiri, C, and Fuangrod, T. Design of 3D-printed universal oral stent for tongue immobilization in head and neck radiotherapy. Biomed Phys and Eng Express (2022) 9:015011. doi:10.1088/2057-1976/aca9d4
93. Prayongrat, A, Kitpanit, S, Lertbutsayanukul, C, Saikaew, P, Boonrueng, T, Mekayarajjananonth, T, et al. Digital fabrication of customized intraoral appliances for head and neck radiotherapy. Heliyon (2023) 9:e15374. doi:10.1016/j.heliyon.2023.e15374
94. Hong, C-S, Oh, D, Ju, SG, Ahn, YC, Na, CH, Kwon, DY, et al. Development of a semi-customized tongue displacement device using a 3D printer for head and neck IMRT. Radiat Oncol (2019) 14:79. doi:10.1186/s13014-019-1289-x
95. Ju, SG, Ahn, YC, Kim, Y, Park, SG, Choi, Y, Na, CH, et al. Development of a tongue immobilization device using a 3D printer for intensity modulated radiation therapy of nasopharyngeal cancer patients. Cancer Res Treat (2021) 53:45–54. doi:10.4143/crt.2020.572
96. Ahmed, ZU, Randazzo, JD, Huryn, JM, and Rosen, EB. Combination intraoral radiation mouthguard-positioning stent. J Cancer Res Ther (2022) 18:1162–4. doi:10.4103/jcrt.jcrt_825_19
97. Michalak, G, Taasti, V, Krauss, B, Deisher, A, Halaweish, A, and McCollough, C. A comparison of relative proton stopping power measurements across patient size using dual-and single-energy CT. Acta Oncologica (2017) 56:1465–71. doi:10.1080/0284186x.2017.1372625
98. Moyers, MF, Mah, D, Boyer, SP, Chang, C, and Pankuch, M. Use of proton beams with breast prostheses and tissue expanders. Med Dosimetry (2014) 39:98–101. doi:10.1016/j.meddos.2013.10.006
99. Reid, J, Smith, R, Borg, M, Dobbins, C, Gowda, R, Chryssidis, S, et al. Feasibility of spacers to facilitate postoperative radiotherapy for retroperitoneal sarcomas. J Med Imaging Radiat Oncol (2017) 61:812–8. doi:10.1111/1754-9485.12641
100. Eng, TY, Fuller, CD, Cavanaugh, SX, Blough, MM, Sadeghi, A, and Herman, T. Significant rectal and bladder dose reduction via utilization of Foley balloon catheters in high-dose-rate tandem and ovoid intracavitary brachytherapy of the uterine cervix. Int J Radiat Oncology*Biology*Physics (2004) 59:174–8. doi:10.1016/j.ijrobp.2003.09.090
101. Eng, TY, Patel, AJ, and Ha, CS. Rectal and bladder dose reduction with the addition of intravaginal balloons to vaginal packing in intracavitary brachytherapy for cervical cancer. Brachytherapy (2016) 15:312–8. doi:10.1016/j.brachy.2016.02.008
102. Wang, W, Gao, X, Chen, J, Liu, Z, Peng, L, and Wei, X. Metal stent for the ureteral stricture after surgery and/or radiation treatment for malignancy. BMC Urol (2021) 21:146. doi:10.1186/s12894-021-00912-6
103. Nilsson, K, Johansson, AK, Montelius, A, Turesson, I, Heikkinen, RO, Ljung, G, et al. Decreasing the dose to the rectal wall by using a rectal retractor during radiotherapy of prostate cancer: a comparative treatment planning study. J Radiother (2014) 2014:1–7. doi:10.1155/2014/680205
104. Ghaffari, H, Rostami, A, Ardekani, MA, Mofid, B, and Mahdavi, SR. Rectal wall sparing effect of a rectal retractor in prostate intensity-modulated radiotherapy. J Cancer Res Ther (2021) 17:383–8. doi:10.4103/jcrt.JCRT_701_19
105. Brace, CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol (2009) 38:135–43. doi:10.1067/j.cpradiol.2007.10.001
106. Gu, K, Kang, TW, Han, S, Cha, DI, Song, KD, Lee, MW, et al. Gastrointestinal tract perforation after radiofrequency ablation for hepatic tumor: incidence and risk factors. Eur J Radiol (2024) 177:111560. doi:10.1016/j.ejrad.2024.111560
107. Liang, P, Wang, Y, Yu, X, and Dong, B. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology (2009) 251:933–40. doi:10.1148/radiol.2513081740
108. Liu, Z, Chen, Z, and Liao, X. Modified hydrodissection as a safe and effective treatment modality for rotating lens in cataract surgery. Indian J Ophthalmol (2023) 71:1021–2. doi:10.4103/ijo.Ijo_2809_22
109. Asvadi, NH, and Arellano, RS. Hydrodissection-assisted image-guided percutaneous biopsy of abdominal and pelvic lesions: experience with seven patients. Am J Roentgenology (2015) 204:865–7. doi:10.2214/ajr.14.13040
110. Maybody, M, Soliman, MM, Yamada, Y, Tahour, D, Hsu, M, Moskowitz, CS, et al. Temporary organ displacement to escalate radiation dose to retroperitoneal tumors and decrease toxicity to organs at risk. J Vasc Interv Radiol (2020) 31:1578–86. doi:10.1016/j.jvir.2020.01.011
111. Katsoulakis, E, Solomon, SB, Maybody, M, Housman, D, Niyazov, G, Riaz, N, et al. Temporary organ displacement coupled with image-guided, intensity-modulated radiotherapy for paraspinal tumors. Radiat Oncol (2013) 8:150–8. doi:10.1186/1748-717x-8-150
112. Whalley, D, Hruby, G, Alfieri, F, Kneebone, A, and Eade, T. SpaceOAR hydrogel in dose-escalated prostate cancer radiotherapy: rectal dosimetry and late toxicity. Clin Oncol (2016) 28:e148–154. doi:10.1016/j.clon.2016.05.005
113. Campbell, C, Lubner, MG, Hinshaw, JL, del Rio, AM, and Brace, CL. Contrast media–doped hydrodissection during thermal ablation: optimizing contrast media concentration for improved visibility on CT images. Am J Roentgenology (2012) 199:677–82. doi:10.2214/ajr.11.7999
114. Bhatt, AA, Woodard, GA, and Lee, CU. Hydrodissection - practical applications in ultrasound-guided breast interventions. Clin Imaging (2021) 72:198–203. doi:10.1016/j.clinimag.2020.11.013
115. Ma, Y, Wu, T, Yao, Z, Zheng, B, Tan, L, Tong, G, et al. Continuous, large-volume hydrodissection to protect delicate structures around the thyroid throughout the radiofrequency ablation procedure. Eur Thyroid J (2021) 10:495–503. doi:10.1159/000519625
116. Moratti Gilberto, G, Mina Falsarella, P, Batalha Megale, A, Socolowski, LR, and Gobbo Garcia, R. Pressurized hydrodissection for CT-guided percutaneous peritoneal navigation: the hydro jet technique. Eur J Radiol (2021) 145:110042. doi:10.1016/j.ejrad.2021.110042
117. Lam, SKH, Reeves, KD, and Cheng, AL. Transition from deep regional blocks toward deep nerve hydrodissection in the upper body and torso: method description and results from a retrospective chart review of the analgesic effect of 5% dextrose water as the primary hydrodissection injectate to enhance safety. Biomed Res Int (2017) 2017:1–17. doi:10.1155/2017/7920438
118. Zheng, B, Zhang, P, Lv, Q, Wu, T, Liu, Y, Tang, J, et al. Development and preclinical evaluation of multifunctional hydrogel for precise thermal protection during thermal ablation. Bioactive Mater (2024) 31:119–35. doi:10.1016/j.bioactmat.2023.08.010
119. Zhao, Z-L, Wei, Y, Peng, LL, Li, Y, Lu, NC, Wu, J, et al. Upgraded hydrodissection and its safety enhancement in microwave ablation of papillary thyroid cancer: a comparative study. Int J Hyperthermia (2023) 40:2202373. doi:10.1080/02656736.2023.2202373
120. Yan, L, Li, Y, Li, XY, Xiao, J, Tang, J, and Luo, Y. Clinical outcomes of ultrasound-guided radiofrequency ablation for solitary T1N0M0 papillary thyroid carcinoma: a retrospective study with more than 5 years of follow-up. Cancer (2023) 129:2469–78. doi:10.1002/cncr.34802
121. Song, Y, Wu, M, Zhou, R, Zhao, P, and Mao, D. Application and evaluation of hydrodissection in microwave ablation of liver tumours in difficult locations. Front Oncol (2023) 13:1298757. doi:10.3389/fonc.2023.1298757
122. Li, N, Dong, Y, Ding, Y, Cui, G, Hua, Q, Xia, S, et al. Comparison of the efficacy and safety of ultrasound-guided radiofrequency ablation and microwave ablation for the treatment of unifocal papillary thyroid microcarcinoma: a retrospective study. Int J Hyperthermia (2024) 41:2287964. doi:10.1080/02656736.2023.2287964
123. Uman, S, Wang, LL, Thorn, SL, Liu, Z, Duncan, JS, Sinusas, AJ, et al. Imaging of injectable hydrogels delivered into myocardium with SPECT/CT. Adv Healthc Mater (2020) 9:e2000294. doi:10.1002/adhm.202000294
124. Prada, PJ, Fernández, J, Martinez, AA, de la Rúa, Á, Gonzalez, JM, Fernandez, JM, et al. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncology*Biology*Physics (2007) 69:95–102. doi:10.1016/j.ijrobp.2007.02.034
125. Majdalany, BS, Willatt, J, Beecham Chick, JF, Srinivasa, RN, and Saad, WA. Fibrillar collagen injection for organ protection during thermal ablation of hepatic malignancies. Diagn Interv Radiol (2017) 23:381–4. doi:10.5152/dir.2017.17120
126. Weber, DC, Zilli, T, Vallee, JP, Rouzaud, M, Miralbell, R, and Cozzi, L. Intensity modulated proton and photon therapy for early prostate cancer with or without transperineal injection of a polyethylen glycol spacer: a treatment planning comparison study. Int J Radiat Oncology*Biology*Physics (2012) 84:e311–e318. doi:10.1016/j.ijrobp.2012.03.028
127. Wright, AS, Lee, FT, and Mahvi, DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol (2003) 10:275–83. doi:10.1245/aso.2003.03.045
128. Andresciani, F, Pacella, G, Vertulli, D, Altomare, C, Bitonti, MT, Bruno, A, et al. Microwave ablation using two simultaneous antennas for the treatment of liver malignant lesions: a 3 year single-Centre experience. Int J Hyperthermia (2023) 40:2163309. doi:10.1080/02656736.2022.2163309
129. Mariados, N, Sylvester, J, Shah, D, Karsh, L, Hudes, R, Beyer, D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncology*Biology*Physics (2015) 92:971–7. doi:10.1016/j.ijrobp.2015.04.030
130. Björeland, U, Notstam, K, Fransson, P, Söderkvist, K, Beckman, L, Jonsson, J, et al. Hyaluronic acid spacer in prostate cancer radiotherapy: dosimetric effects, spacer stability and long-term toxicity and PRO in a phase II study. Radiat Oncol (2023) 18:1. doi:10.1186/s13014-022-02197-x
131. Noyes, WR, Hosford, CC, and Schultz, SE. Human collagen injections to reduce rectal dose during radiotherapy. Int J Radiat Oncology*Biology*Physics (2012) 82:1918–22. doi:10.1016/j.ijrobp.2011.02.034
132. Dong, J, Ren, B, Tian, Y, Peng, G, Zhai, H, Meng, Z, et al. Effects of radiation-induced skin injury on hyaluronan degradation and its underlying mechanisms. Molecules (2023) 28:7449. doi:10.3390/molecules28217449
133. Cowman, MK, Schmidt, TA, Raghavan, P, and Stecco, A. Viscoelastic properties of hyaluronan in physiological conditions. F1000Res (2015) 4:622. doi:10.12688/f1000research.6885.1
134. Afkhami Ardekani, M, and Ghaffari, H. Optimization of prostate brachytherapy techniques with polyethylene glycol–based hydrogel spacers: a systematic review. Brachytherapy (2020) 19:13–23. doi:10.1016/j.brachy.2019.08.009
135. Marnitz, S, Budach, V, Weißer, F, Burova, E, Gebauer, B, Vercellino, FG, et al. Rectum separation in patients with cervical cancer for treatment planning in primary chemo-radiation. Radiat Oncol (2012) 7:109. doi:10.1186/1748-717X-7-109
136. Viswanathan, AN, Damato, AL, and Nguyen, PL. Novel use of a hydrogel spacer permits reirradiation in otherwise incurable recurrent gynecologic cancers. J Clin Oncol (2013) 31:e446–447. doi:10.1200/jco.2012.47.9931
137. Takagawa, Y, and Itami, J. SpaceOAR hydrogel spacer in interstitial brachytherapy for intrapelvic recurrent endometrial cancer. BJR|caseReps (2022) 8:20210220. doi:10.1259/bjrcr.20210220
138. Basu, S, Manir, KS, Basu, A, and Ghosh, K. Rectal separation using hydroxypropyl methylcellulose in intracavitary brachytherapy of cervical cancer: an innovative approach. J Contemp Brachytherapy (2016) 5:399–403. doi:10.5114/jcb.2016.62951
140. Hong, A, Ischia, J, and Chao, M. Case report: reversal of hyaluronic acid rectal wall infiltration with hyaluronidase. Front Oncol (2022) 12:870388. doi:10.3389/fonc.2022.870388
141. Mariados, NF, Orio, PF, Schiffman, Z, Van, TJ, Engelman, A, Nurani, R, et al. Hyaluronic acid spacer for hypofractionated prostate radiation therapy: a randomized clinical trial. JAMA Oncol (2023) 9:511–8. doi:10.1001/jamaoncol.2022.7592
142. McLaughlin, MF, Folkert, MR, Timmerman, RD, Hannan, R, Garant, A, Hudak, SJ, et al. Hydrogel spacer rectal wall infiltration associated with severe rectal injury and related complications after dose intensified prostate cancer stereotactic ablative radiation therapy. Adv Radiat Oncol (2021) 6:100713. doi:10.1016/j.adro.2021.100713
143. Hamstra, DA, Mariados, N, Sylvester, J, Shah, D, Karsh, L, Hudes, R, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncology*Biology*Physics (2017) 97:976–85. doi:10.1016/j.ijrobp.2016.12.024
144. Hamstra, DA, Mariados, N, Sylvester, J, Shah, D, Gross, E, Hudes, R, et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: secondary analysis of a phase 3 trial. Pract Radiat Oncol (2018) 8:e7–e15. doi:10.1016/j.prro.2017.07.008
145. Pinkawa, M, Berneking, V, König, L, Frank, D, Bretgeld, M, and Eble, MJ. Hydrogel injection reduces rectal toxicity after radiotherapy for localized prostate cancer. Strahlentherapie und Onkologie (2017) 193:22–8. doi:10.1007/s00066-016-1040-6
146. Fagundes, M, Rodrigues, MA, Olszewski, S, Khan, F, McKenzie, C, Gutierrez, A, et al. Expanding the utilization of rectal spacer hydrogel for larger prostate glands (>80 cc): feasibility and dosimetric outcomes. Adv Radiat Oncol (2021) 6:100651. doi:10.1016/j.adro.2021.100651
147. Ghaffari, H. Is there a role for hydrogel spacer in post-prostatectomy radiotherapy setting? La radiologia Med (2019) 124:1062–3. doi:10.1007/s11547-019-01054-4
148. Hong, A, Bolton, D, Pham, T, Angus, D, Pan, D, Joon, DL, et al. Rectal spacer reduces gastrointestinal side effects of radiation post radical prostatectomy. Société Internationale d’Urologie J (2024) 5:111–21. doi:10.3390/siuj5020020
149. Kishi, K, Sonomura, T, Shirai, S, Sato, M, and Tanaka, K. Critical organ preservation in reirradiation brachytherapy by injectable spacer. Int J Radiat Oncology*Biology*Physics (2009) 75:587–94. doi:10.1016/j.ijrobp.2009.03.072
150. Kobayashi, R, Murakami, N, Chiba, T, Okuma, K, Inaba, K, Takahashi, K, et al. Effect of hyaluronate acid injection on dose-volume parameters in brachytherapy for cervical cancer. Adv Radiat Oncol (2022) 7:100918. doi:10.1016/j.adro.2022.100918
151. Murakami, N, Shima, S, Kashihara, T, Tselis, N, Kato, T, Takagawa, Y, et al. Hyaluronic gel injection into the vesicovaginal septum for high-dose-rate brachytherapy of uterine cervical cancer: an effective approach for bladder dose reduction. J Contemp Brachytherapy (2019) 11:1–7. doi:10.5114/jcb.2019.82612
152. Iijima, K, Murakami, N, Nakamura, S, Nishioka, S, Chiba, T, Kuwahara, J, et al. Configuration analysis of the injection position and shape of the gel spacer in gynecologic brachytherapy. Brachytherapy (2021) 20:95–103. doi:10.1016/j.brachy.2020.08.021
153. Kashihara, T, Murakami, N, Tselis, N, Kobayashi, K, Tsuchida, K, Shima, S, et al. Hyaluronate gel injection for rectum dose reduction in gynecologic high-dose-rate brachytherapy: initial Japanese experience. J Radiat Res (2019) 60:501–8. doi:10.1093/jrr/rrz016
154. Murakami, N, Nakamura, S, Kashihara, T, Kato, T, Shibata, Y, Takahashi, K, et al. Hyaluronic acid gel injection in rectovaginal septum reduced incidence of rectal bleeding in brachytherapy for gynecological malignancies. Brachytherapy (2020) 19:154–61. doi:10.1016/j.brachy.2019.11.004
155. Sakuramachi, M, Murakami, N, Nagao, A, Kojima, K, Miyata, Y, Kashihara, T, et al. Hydrogel spacer injection to the meso-sigmoid to protect the sigmoid colon in cervical cancer brachytherapy: a technical report. J Contemp Brachytherapy (2023) 15:465–9. doi:10.5114/jcb.2023.134174
156. Muramoto, Y, Murakami, N, Karino, T, Sugimoto, S, Takatsu, J, Oshima, M, et al. MucoUp® as a spacer in brachytherapy for uterine cervical cancer: a first-in-human experience. Clin Translational Radiat Oncol (2023) 42:100659. doi:10.1016/j.ctro.2023.100659
157. Takatsu, J, Murakami, N, Muramoto, Y, Karino, T, Oshima, M, Kosugi, Y, et al. Safe dose escalation and reduction of the fraction number of uterine cervical brachytherapy using a gel spacer in the rectovaginal and vesicouterine septum: a planning study. Brachytherapy (2024) 23:115–22. doi:10.1016/j.brachy.2023.10.003
158. Ahmed, O, Nguyen, VD, Ginsburg, M, and Barry, P. CT-guided placement of a polyethylene glycol hydrogel in brachytherapy for gynecologic malignancy to limit nontarget organ toxicity. J Vasc Interv Radiol (2019) 30:469–71. doi:10.1016/j.jvir.2018.12.002
159. Narang, AK, Hong, TS, Ding, K, Herman, J, Meyer, J, Thompson, E, et al. A multi-institutional safety and feasibility study exploring the use of hydrogel to create spatial separation between the pancreas and duodenum in patients with pancreatic cancer. Pract Radiat Oncol (2024) 14:e276–e282. doi:10.1016/j.prro.2023.11.011
160. Lee, SF, Harris, N, Yip, PL, Dean, J, Geary, B, Koufogiannis, G, et al. Utilization of a stabilized hyaluronic acid spacer in SBRT for retroperitoneal cancers: a case series and dosimetric analysis. Clin Translational Radiat Oncol (2025) 52:100943. doi:10.1016/j.ctro.2025.100943
161. Ghaffari, H, Afkhami Ardekani, M, and Refahi, S. In regard to ‘What is the quality of hydrogel spacer insertions? and which patients will benefit? A literature review. J Radiother Pract (2020) 19:403–4. doi:10.1017/S1460396920000035
162. Yavas, G., and Yazici, G. Toxicity management for central nervous system tumors in radiation oncology. In: Ozyigit, Selek, U, editors Prevention and management of acute and late toxicities in radiation oncology. Springer, Cham (2020). doi:10.1007/978-3-030-37798-4_1
163. Laios, A, Otify, M, Papadopoulou, A, Gallos, ID, and Ind, T. Outcomes of ovarian transposition in cervical cancer; an updated meta-analysis. BMC Womens Health (2022) 22:305. doi:10.1186/s12905-022-01887-8
164. Noé, GK. Genital prolapse surgery: what options do we have in the age of mesh issues? J Clin Med (2021) 10:267. doi:10.3390/jcm10020267
165. Spira, E, and Lubin, E. Extracorporeal irradiation of bone tumors. A preliminary report. Isr J Med Sci (1968) 4:1015–9.
166. Mathew, DAP, and Wagh, DMS. Abdominoperineal Excision in current era. Cancer Treat Res Commun (2022) 32:100580. doi:10.1016/j.ctarc.2022.100580
167. Sugarbaker, PH. Intrapelvic prosthesis to prevent injury of the small intestine with high dosage pelvic irradiation. Surg Gynecol Obstet (1983) 157:269–71.
168. Evans, DB, Shumate, CR, Ames, FC, and Rich, TA. Use of Dexon Mesh® for abdominal partitioning above the peritoneal reflection. Dis Colon and Rectum (1991) 34:833–5. doi:10.1007/bf02051081
169. Rodier, JF, Janser, JC, Rodier, D, Dauplat, J, Kauffmann, P, Bouedec, GL, et al. Prevention of radiation enteritis by an absorbable polyglycolic acid mesh sling. A 60-case multicentric study. Cancer (1991) 68:2545–9. doi:10.1002/1097-0142(19911215)68:12<2545::aid-cncr2820681202>3.0.co;2-f
170. Sezeur, A, Abbou, C, Chopin, D, Lottmann, H, Rey, P, and Leandri, J. Protection of the small intestine against irradiation by means of a removable adapted prosthesis: a preliminary study. Dig Surg (2008) 6:83–5. doi:10.1159/000171895
171. Jesseph, JM., Fitzek, MM., Shahnazi, K., Ko, SC., Howe, JR., Thornton, AF., and Change, AL. Surgical organ displacement for proton radiotherapy. Translational Cancer Res (2013) 1:247–54. doi:10.3978/j.issn.2218-676X.2012.12.05
172. Chan, DKH, Cheo, T, and Cheong, WK. Successful use of tissue expander and pelvic sling to exclude small bowel for high-dose pelvic irradiation. Int J Colorectal Dis (2019) 34:1043–6. doi:10.1007/s00384-019-03280-8
173. Kubo, N, Yokobori, T, Takahashi, R, Ogawa, H, Gombodorj, N, Ohta, N, et al. An abdominal spacer that does not require surgical removal and allows drainage of abdominal fluids in patients undergoing carbon ion radiotherapy. PLOS ONE (2020) 15:e0234471. doi:10.1371/journal.pone.0234471
174. Dawkins, JC, Lewis, GK, and Toy, EP. Cervical cancer complicating pelvic organ prolapse, and use of a pessary to restore anatomy for optimal radiation: a case report. Gynecol Oncol Rep (2018) 26:14–6. doi:10.1016/j.gore.2018.08.004
175. Godin, MS, Waldman, SR, and Johnson, CM. The use of expanded polytetrafluoroethylene (Gore-Tex) in rhinoplasty. A 6-year experience. Arch Otolaryngol - Head Neck Surg (1995) 121:1131–6. doi:10.1001/archotol.1995.01890100043007
176. Komatsu, S., Hori, Y., Fukumoto, T., Murakami, M., Hishikawa, Y., and Ku, Y. Surgical spacer placement and proton radiotherapy for unresectable hepatocellular carcinoma. World J Gastroenterol (2010) 16:1800–3. doi:10.3748/wjg.v16.i14.1800
177. Ogino, T. Surgical organ displacement: what is the best “materials and methods” for proton radiotherapy? Chin J Cancer Res (2013) 25:267–8. doi:10.3978/j.issn.1000-9604.2013.04.03
178. Fukumoto, T, Komatsu, S, Hori, Y, Murakami, M, Hishikawa, Y, and Ku, Y. Particle beam radiotherapy with a surgical spacer placement for advanced abdominal leiomyosarcoma results in a significant clinical benefit. J Surg Oncol (2010) 101:97–9. doi:10.1002/jso.21417
179. Musters, GD, Bemelman, WA, Bosker, RJ, Burger, JW, van Duijvendijk, P, van Etten, B, et al. Randomized controlled multicentre study comparing biological mesh closure of the pelvic floor with primary perineal wound closure after extralevator abdominoperineal resection for rectal cancer (BIOPEX-study). BMC Surg (2014) 14:58. doi:10.1186/1471-2482-14-58
180. Sainfort, A, Denis Hallouard, I, Hartmann, D, Aulagner, G, Francois, Y, Tiffet, O, et al. Xenograft biologic mesh in parietal and general surgery: technical assessment and review of clinical effectiveness and safety data. J Visc Surg (2016) 153:403–17. doi:10.1016/j.jviscsurg.2016.08.002
181. Köckerling, F, Alam, NN, Antoniou, SA, Daniels, IR, Famiglietti, F, Fortelny, RH, et al. What is the evidence for the use of biologic or biosynthetic meshes in abdominal wall reconstruction? Hernia (2018) 22:249–69. doi:10.1007/s10029-018-1735-y
182. Ismael, HN, Denbo, J, Cox, S, Crane, C, Das, P, Krishnan, S, et al. Biologic mesh spacer placement facilitates safe delivery of dose-intense radiation therapy: a novel treatment option for unresectable liver tumors. Eur J Surg Oncol (Ejso) (2016) 42:1591–6. doi:10.1016/j.ejso.2016.05.021
183. Yoon, SS, Chen, Y-L, Kambadakone, A, Schmidt, B, and DeLaney, TF. Surgical placement of biologic mesh spacers prior to external beam radiation for retroperitoneal and pelvic tumors. Pract Radiat Oncol (2013) 3:199–208. doi:10.1016/j.prro.2012.06.008
184. Barcellini, A, Mirandola, A, Fiore, MR, Orlandi, E, and Cobianchi, L. Omentum flap as a spacer before carbon ion radiotherapy for gynecological recurrences. A technical note. Cancer/Radiothérapie (2022) 26:599–603. doi:10.1016/j.canrad.2021.12.009
185. Leblanc, E, Narducci, F, Bresson, L, Durand-Labrunie, J, Taieb, S, Vanlerenberghe, E, et al. A new laparoscopic method of bowel radio-protection before pelvic chemoradiation of locally advanced cervix cancers. Surg Endosc (2014) 28:2713–8. doi:10.1007/s00464-014-3533-7
186. Jesseph, JM, Shahnazi, K, Fitzek, M, Chang, A, and Thornton, A. Organ displacement for proton radiotherapy. Int J Radiat Oncology*Biology*Physics (2009) 75:S730. doi:10.1016/j.ijrobp.2009.07.1663
187. Russ, JE, Smoron, GL, and Gagnon, JD. Omental transposition flap in colorectal carcinoma: adjunctive use in prevention and treatment of radiation complications. Int J Radiat Oncology*Biology*Physics (1984) 10:55–62. doi:10.1016/0360-3016(84)90412-7
189. Martin, JR, Kodaman, P, Oktay, K, and Taylor, HS. Ovarian cryopreservation with transposition of a contralateral ovary: a combined approach for fertility preservation in women receiving pelvic radiation. Fertil Sterility (2007) 87:189.e5–189.e7. doi:10.1016/j.fertnstert.2006.04.051
190. Buonomo, B, Multinu, F, Casarin, J, Betella, I, Zanagnolo, V, Aletti, G, et al. Ovarian transposition in patients with cervical cancer prior to pelvic radiotherapy: a systematic review. Int J Gynecol Cancer (2021) 31:360–70. doi:10.1136/ijgc-2020-001774
191. Turkgeldi, L, Cutner, A, Turkgeldi, E, Al Chami, A, Cassoni, A, Macdonald, N, et al. Laparoscopic ovarian transposition and ovariopexy for fertility preservation in patients treated with pelvic radiotherapy with or without chemotherapy. Facts Views Vis Obgyn (2019) 11:235–42.
192. Wo, JY, and Viswanathan, AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncology*Biology*Physics (2009) 73:1304–12. doi:10.1016/j.ijrobp.2008.12.016
193. Moawad, NS, Santamaria, E, Rhoton-Vlasak, A, and Lightsey, JL. Laparoscopic ovarian transposition before pelvic cancer treatment: ovarian function and fertility preservation. J Minimally Invasive Gynecol (2017) 24:28–35. doi:10.1016/j.jmig.2016.08.831
194. Ribeiro, R, Baiocchi, G, Obermair, A, Costa, CN, and Leitao, M. Uterine transposition for fertility preservation in pelvic cancers. Int J Gynecol Cancer (2024) 34:403–8. doi:10.1136/ijgc-2023-004992
195. Ribeiro, R, Rebolho, JC, Tsumanuma, FK, Brandalize, GG, Trippia, CH, and Saab, KA. Uterine transposition: technique and a case report. Fertil Sterility (2017) 108:320–4.e1. doi:10.1016/j.fertnstert.2017.06.016
196. Kancherla, NR, Paruchuri, S, Arvind, B, Peddamadyam, S, Eppakayala, S, and Cherukuri, N. Our experience with extracorporeal irradiation and reimplantation of the irradiated bone for the reconstruction of bone defects following tumor resection. Cureus (2024) 16:e52853. doi:10.7759/cureus.52853
197. Puri, A, Gulia, A, Agarwal, M, Jambhekar, N, and Laskay, S. Extracorporeal irradiated tumor bone: a reconstruction option in diaphyseal Ewing's sarcomas. Indian J Orthopaedics (2010) 44:390–6. doi:10.4103/0019-5413.69310
198. Takahashi, S, Okudaira, S, Sasai, K, and Kotoura, Y. En bloc resection, extracorporeal irradiation, and reimplantation of an entire tibia. J Orthopaedic Sci (2006) 11:298–302. doi:10.1007/s00776-006-1011-3
199. Davidson, AW, Hong, A, McCarthy, SW, and Stalley, PD. En-bloc resection, extracorporeal irradiation, and re-implantation in limb salvage for bony malignancies. The J Bone Joint Surg Br volume (2005) 87-B:851–7. doi:10.1302/0301-620x.87b6.15950
200. Krieg, AH, Lenze, U, Schultze, L, Gross, MW, and Haug, M. Extracorporeal irradiation and reimplantation of tumor-bearing bone segments following diaphyseal sarcoma resection at the tibia. Anticancer Res (2019) 39:2015–23. doi:10.21873/anticanres.13312
201. Kwak, K, Yu, B, Lewandowski, RJ, and Kim, DH. Recent progress in cryoablation cancer therapy and nanoparticles mediated cryoablation. Theranostics (2022) 12:2175–204. doi:10.7150/thno.67530
202. Hubbard, TJ, Aronson, SL, and Denegar, CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train (2004) 39:88–94.
203. Liu, C, He, J, Li, T, Hong, D, Su, H, and Shao, H. Evaluation of the efficacy and postoperative outcomes of hydrodissection-assisted microwave ablation for subcapsular hepatocellular carcinoma and colorectal liver metastases. Abdom Radiol (2021) 46:2161–72. doi:10.1007/s00261-020-02830-x
204. Lee, J, Rhim, H, Jeon, YH, Lim, HK, Lee, WJ, Choi, D, et al. Radiofrequency ablation of liver adjacent to body of gallbladder: histopathologic changes of gallbladder wall in a pig model. Am J Roentgenology (2008) 190:418–25. doi:10.2214/ajr.07.2526
205. Rhim, H, Dodd, GD, Chintapalli, KN, Wood, BJ, Dupuy, DE, Hvizda, JL, et al. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics (2004) 24:41–52. doi:10.1148/rg.241025144
206. Francica, G, and Marone, G. Ultrasound-guided percutaneous treatment of hepatocellular carcinoma by radiofrequency hyperthermia with a ‘cooled-tip needle’. A preliminary clinical experience. Eur J Ultrasound (1999) 9:145–53. doi:10.1016/s0929-8266(99)00022-1
207. Lewin, JS, Connell, CF, Duerk, JL, Chung, Y, Clampitt, ME, Spisak, J, et al. Invited. Interactive MRI-guided radiofrequency interstitial thermal ablation of abdominal tumors: clinical trial for evaluation of safety and feasibility. J Magn Reson Imaging (1998) 8:40–7. doi:10.1002/jmri.1880080112
208. Livraghi, T, Solbiati, L, Meloni, MF, Gazelle, GS, Halpern, EF, and Goldberg, SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology (2003) 226:441–51. doi:10.1148/radiol.2262012198
209. Arellano, RS, Garcia, RG, Gervais, DA, and Mueller, PR. Percutaneous CT-guided radiofrequency ablation of renal cell carcinoma: efficacy of organ displacement by injection of 5% dextrose in water into the retroperitoneum. Am J Roentgenology (2009) 193:1686–90. doi:10.2214/ajr.09.2904
210. Pinkawa, M, Escobar Corral, N, Caffaro, M, Piroth, MD, Holy, R, Djukic, V, et al. Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. Radiother Oncol (2011) 100:436–41. doi:10.1016/j.radonc.2011.09.005
211. Gez, E, Cytron, S, Yosef, RB, London, D, Corn, BW, Alani, S, et al. Application of an interstitial and biodegradable balloon system for prostate-rectum separation during prostate cancer radiotherapy: a prospective multi-center study. Radiat Oncol (2013) 8:96. doi:10.1186/1748-717X-8-96
212. Tryggestad, E, Christian, M, Ford, E, Kut, C, Le, Y, Sanguineti, G, et al. Inter- and intrafraction patient positioning uncertainties for intracranial radiotherapy: a study of four frameless, thermoplastic mask-based immobilization strategies using daily cone-beam CT. Int J Radiat Oncology*Biology*Physics (2011) 80:281–90. doi:10.1016/j.ijrobp.2010.06.022
213. Huang, K-G, Lee, C-L, Tsai, C-S, Han, C-M, and Hwang, L-L. A new approach for laparoscopic ovarian transposition before pelvic irradiation. Gynecol Oncol (2007) 105:234–7. doi:10.1016/j.ygyno.2006.12.001
214. Prada, PJ, Gonzalez, H, Menéndez, C, Llaneza, A, Fernández, J, Santamarta, E, et al. Transperineal injection of hyaluronic acid in the anterior perirectal fat to decrease rectal toxicity from radiation delivered with low-dose-rate brachytherapy for prostate cancer patients. Brachytherapy (2009) 8:210–7. doi:10.1016/j.brachy.2008.11.010
215. Wilder, RB, Barme, GA, Gilbert, RF, Holevas, RE, Kobashi, LI, Reed, RR, et al. Cross-Linked hyaluronan gel reduces the acute rectal toxicity of radiotherapy for prostate cancer. Int J Radiat Oncology*Biology*Physics (2010) 77:824–30. doi:10.1016/j.ijrobp.2009.05.069
216. Wilder, RB, Barme, GA, Gilbert, RF, Holevas, RE, Kobashi, LI, Reed, RR, et al. Cross-linked hyaluronan gel improves the quality of life of prostate cancer patients undergoing radiotherapy. Brachytherapy (2011) 10:44–50. doi:10.1016/j.brachy.2009.12.005
217. Hatiboglu, G, Pinkawa, M, Vallée, JP, Hadaschik, B, and Hohenfellner, M. Application technique: placement of a prostate–rectum spacer in men undergoing prostate radiation therapy. BJU Int (2012) 110:E647–E652. doi:10.1111/j.1464-410X.2012.11373.x
218. Hwang, JH, Yoo, HJ, Park, SH, Lim, MC, Seo, SS, Kang, S, et al. Association between the location of transposed ovary and ovarian function in patients with uterine cervical cancer treated with (postoperative or primary) pelvic radiotherapy. Fertil Sterility (2012) 97:1387–93.e2. doi:10.1016/j.fertnstert.2012.02.052
220. Beydoun, N, Bucci, JA, Chin, YS, Malouf, D, Enari, E, and Painter, SD. First report of transperineal polyethylene glycol hydrogel spacer use to curtail rectal radiation dose after permanent iodine-125 prostate brachytherapy. Brachytherapy (2013) 12:368–74. doi:10.1016/j.brachy.2013.01.164
221. Nguyen, PL, Devlin, PM, Beard, CJ, Orio, PF, O'Leary, MP, Wolfsberger, LD, et al. High-dose-rate brachytherapy for prostate cancer in a previously radiated patient with polyethylene glycol hydrogel spacing to reduce rectal dose: case report and review of the literature. Brachytherapy (2013) 12:77–83. doi:10.1016/j.brachy.2012.03.005
222. Uhl, M, van Triest, B, Eble, MJ, Weber, DC, Herfarth, K, and De Weese, TL. Low rectal toxicity after dose escalated IMRT treatment of prostate cancer using an absorbable hydrogel for increasing and maintaining space between the rectum and prostate: results of a multi-institutional phase II trial. Radiother Oncol (2013) 106:215–9. doi:10.1016/j.radonc.2012.11.009
223. Song, DY, Herfarth, KK, Uhl, M, Eble, MJ, Pinkawa, M, van Triest, B, et al. A multi-institutional clinical trial of rectal dose reduction via injected polyethylene-glycol hydrogel during intensity modulated radiation therapy for prostate cancer: analysis of dosimetric outcomes. Int J Radiat Oncology*Biology*Physics (2013) 87:81–7. doi:10.1016/j.ijrobp.2012.12.019
224. Mahal, BA, Ziehr, DR, Hyatt, AS, Neubauer-Sugar, EH, O'Farrell, DA, O'Leary, MP, et al. Use of a rectal spacer with low-dose-rate brachytherapy for treatment of prostate cancer in previously irradiated patients: initial experience and short-term results. Brachytherapy (2014) 13:442–9. doi:10.1016/j.brachy.2014.05.001
225. Strom, TJ, Wilder, RB, Fernandez, DC, Mellon, EA, Saini, AS, Hunt, DC, et al. A dosimetric study of polyethylene glycol hydrogel in 200 prostate cancer patients treated with high-dose rate brachytherapy±intensity modulated radiation therapy. Radiother Oncol (2014) 111:126–31. doi:10.1016/j.radonc.2014.02.011
226. Heikkilä, VP, Kärnä, A, and Vaarala, MH. DuraSeal® as a spacer to reduce rectal doses in low-dose rate brachytherapy for prostate cancer. Radiother Oncol (2014) 112:233–6. doi:10.1016/j.radonc.2014.08.012
227. Teh, AY, Ko, H-T, Barr, G, and Woo, HH. Rectal ulcer associated with SpaceOAR hydrogel insertion during prostate brachytherapy. BMJ case Rep (2014) 2014:bcr2014206931. doi:10.1136/bcr-2014-206931
228. Pinkawa, M, Schubert, C, Escobar-Corral, N, Holy, R, and Eble, MJ. Application of a hydrogel spacer for postoperative salvage radiotherapy of prostate cancer. Strahlentherapie und Onkologie (2015) 191:375–9. doi:10.1007/s00066-014-0769-z
229. Yeh, J, Lehrich, B, Tran, C, Mesa, A, Baghdassarian, R, Yoshida, J, et al. Polyethylene glycol hydrogel rectal spacer implantation in patients with prostate cancer undergoing combination high-dose-rate brachytherapy and external beam radiotherapy. Brachytherapy (2016) 15:283–7. doi:10.1016/j.brachy.2015.12.007
230. Taggar, AS, Charas, T, Cohen, GN, Boonyawan, K, Kollmeier, M, McBride, S, et al. Placement of an absorbable rectal hydrogel spacer in patients undergoing low-dose-rate brachytherapy with palladium-103. Brachytherapy (2018) 17:251–8. doi:10.1016/j.brachy.2017.11.006
231. Hepp, R, Eggert, T, Schabl, G, Herberholz, L, Petry, T, and Galalae, R. Salvage high-dose-rate brachytherapy for prostate cancer persistence after brachytherapy: repeated use of a polyethylene glycol hydrogel spacer. J Contemp Brachytherapy (2018) 10:169–73. doi:10.5114/jcb.2018.75602
232. Chao, M, Lim Joon, D, Khoo, V, Lawrentschuk, N, Ho, H, Spencer, S, et al. The use of hydrogel spacer in men undergoing high-dose prostate cancer radiotherapy: results of a prospective phase 2 clinical trial. World J Urol (2019) 37:1111–6. doi:10.1007/s00345-018-2502-5
233. Lv, X-j., Cheng, X-l., Tu, Y-q., Yan, D-d., and Tang, Q. Association between the location of transposed ovary and ovarian dose in patients with cervical cancer treated with postoperative pelvic radiotherapy. Radiat Oncol (2019) 14:230. doi:10.1186/s13014-019-1437-3
235. Gargett, MA, Briggs, AR, and Booth, JT. Water equivalence of a solid phantom material for radiation dosimetry applications. Phys Imaging Radiat Oncol (2020) 14:43–7. doi:10.1016/j.phro.2020.05.003
236. Neumann, W, Pusch, TP, Siegfarth, M, Schad, LR, and Stallkamp, JL. CT and MRI compatibility of flexible 3D-printed materials for soft actuators and robots used in image-guided interventions. Med Phys (2019) 46:5488–98. doi:10.1002/mp.13852
237. Mendiratta-Lala, M, Brook, OR, Midkiff, BD, Brennan, DD, Thornton, E, Faintuch, S, et al. Quality initiatives: strategies for anticipating and reducing complications and treatment failures in hepatic radiofrequency ablation. Radiographics (2010) 30:1107–22. doi:10.1148/rg.304095202
238. Laeseke, PF, Sampson, LA, Brace, CL, Winter, TC, Fine, JP, and Lee, FT. Unintended thermal injuries from radiofrequency ablation: protection with 5% dextrose in water. Am J roentgenology (2006) 186:S249–S254. doi:10.2214/ajr.04.1240
239. Morris-Stiff Mb Bch Frcs, GJ, and Hughes Ds Fracs Frcs, LE. The outcomes of nonabsorbable mesh placed within the abdominal cavity: literature review and clinical experience. J Am Coll Surgeons (1998) 186:352–67. doi:10.1016/s1072-7515(98)00002-7
240. Fansler, RF, Taheri, P, Cullinane, C, Sabates, B, and Flint, LM. Polypropylene mesh closure of the complicated abdominal wound. The Am J Surg (1995) 170:15–8. doi:10.1016/s0002-9610(99)80244-x
241. Karakousis, CP, Volpe, C, Tanski, J, Colby, ED, Winston, J, and Driscoll, DL. Use of a mesh for musculoaponeurotic defects of the abdominal wall in cancer surgery and the risk of bowel fistulas. J Am Coll Surgeons (1995) 181:11–6.
242. Brinas, P, Chalret du Rieu, M, Tuyeras, G, Julio, C, Kirzin, S, Ghouti, L, et al. Mid-term outcomes after biologic mesh use: does their performance meet our expectations? J Visc Surg (2018) 155:355–63. doi:10.1016/j.jviscsurg.2018.03.007
243. Giacometti, V, McLaughlin, O, Comiskey, P, Marshall, H, Houlihan, OA, Whitten, G, et al. Validation of a quality metric score to assess the placement of hydrogel rectal spacer in patients treated with prostate stereotactic radiation therapy. Adv Radiat Oncol (2024) 9:101396. doi:10.1016/j.adro.2023.101396
244. Grossman, CE, Folkert, MR, Lobaugh, S, Desai, NB, Kollmeier, MA, Gorovets, D, et al. Quality metric to assess adequacy of hydrogel rectal spacer placement for prostate radiation therapy and association of metric score with rectal toxicity outcomes. Adv Radiat Oncol (2023) 8:101070. doi:10.1016/j.adro.2022.101070
245. Fischer-Valuck, BW, Chundury, A, Gay, H, Bosch, W, and Michalski, J. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract Radiat Oncol (2017) 7:195–202. doi:10.1016/j.prro.2016.10.004
246. Charas, T, Ben Dor, Y, Bakouche, V, and Billan, S. Rectal spacer in radiotherapy for prostate cancer: correlation of spacer symmetry to rectal dosimetry. J Clin Oncol (2024) 42:e17102. doi:10.1200/JCO.2024.42.16_suppl.e17102
247. Haynes, RB, Sackett, DL, Richardson, WS, Rosenberg, W, and Langley, GR. Evidence-based medicine: how to practice and teach EBM. Can Med Assoc J (1997) 157:788.
248. Drabble, J, and Drury-Smith, H. What is the quality of hydrogel spacer insertions? and which patients will benefit? A literature review. J Radiother Pract (2020) 19:385–92. doi:10.1017/S1460396919000979
249. Smith, GL, Banegas, MP, Acquati, C, Chang, S, Chino, F, Conti, RM, et al. Navigating financial toxicity in patients with cancer: a multidisciplinary management approach. CA: a Cancer J clinicians (2022) 72:437–53. doi:10.3322/caac.21730
250. De Souza, JA, Yap, BJ, Wroblewski, K, Blinder, V, Araújo, FS, Hlubocky, FJ, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST). Cancer (2017) 123:476–84. doi:10.1002/cncr.30369
251. Markey, GE, Razdan, P, Jaipalli, S, and Rozzell, DM. Acute prostatitis and septic shock following rectal spacer placement: a case report of a pre-brachytherapy complication. Cureus (2025) 17:e85099. doi:10.7759/cureus.85099
252. Hooshangnejad, H, Youssefian, S, Narang, A, Shin, EJ, Rao, AD, Han-Oh, S, et al. Finite element-based personalized simulation of duodenal hydrogel spacer: spacer location dependent duodenal sparing and a decision support system for spacer-enabled pancreatic cancer radiation therapy. Front Oncol (2022) 12:833231. doi:10.3389/fonc.2022.833231
253. Hooshangnejad, H, Youssefian, S, Guest, JK, and Ding, K. FEMOSSA: patient-specific finite element simulation of the prostate–rectum spacer placement, a predictive model for prostate cancer radiotherapy. Med Phys (2021) 48:3438–52. doi:10.1002/mp.14990
254. Brenneman, RJ, Goddu, SM, Andruska, N, Roy, A, Bosch, WR, Fischer-Valuck, B, et al. Feasibility of same-day prostate fiducial markers, perirectal hydrogel spacer placement, and computed tomography and magnetic resonance imaging simulation for external beam radiation therapy for low-risk and intermediate-risk prostate cancer. Pract Radiat Oncol (2022) 12:e117–e122. doi:10.1016/j.prro.2021.09.015
255. Paolucci, I, Albuquerque Marques Silva, J, Lin, YM, Shieh, A, Ierardi, AM, Caraffiello, G, et al. Quantitative ablation confirmation methods in percutaneous thermal ablation of malignant liver tumors: technical insights, clinical evidence, and future outlook. Radiol Imaging Cancer (2025) 7:e240293. doi:10.1148/rycan.240293
256. Odisio, BC, Albuquerque, J, Lin, YM, Anderson, BM, O'Connor, CS, Rigaud, B, et al. Software-based versus visual assessment of the minimal ablative margin in patients with liver tumours undergoing percutaneous thermal ablation (COVER-ALL): a randomised phase 2 trial. The Lancet Gastroenterol and Hepatol (2025) 10:442–51. doi:10.1016/s2468-1253(25)00024-x
257. Zadpoor, AA. Mechanical performance of additively manufactured meta-biomaterials. Acta Biomater (2019) 85:41–59. doi:10.1016/j.actbio.2018.12.038
258. Balcerak-Woźniak, A, Dzwonkowska-Zarzycka, M, and Kabatc-Borcz, J. A comprehensive review of stimuli-responsive smart polymer materials—recent advances and future perspectives. Materials (2024) 17:4255. doi:10.3390/ma17174255
260. Fouladian, P, Kohlhagen, J, Arafat, M, Afinjuomo, F, Workman, N, Abuhelwa, AY, et al. Three-dimensional printed 5-fluorouracil eluting polyurethane stents for the treatment of oesophageal cancers. Biomater Sci (2020) 8:6625–36. doi:10.1039/d0bm01355b
261. Barmin, RA, Moosavifar, M, Dasgupta, A, Herrmann, A, Kiessling, F, Pallares, RM, et al. Polymeric materials for ultrasound imaging and therapy. Chem Sci (2023) 14:11941–54. doi:10.1039/d3sc04339h
262. Byrne, JD, Young, CC, Chu, JN, Pursley, J, Chen, MX, Wentworth, AJ, et al. Personalized radiation attenuating materials for gastrointestinal mucosal protection. Adv Sci (2021) 8:2100510. doi:10.1002/advs.202100510
263. Yamada, M, Miyasaka, Y, Kanai, T, Souda, H, Uematsu, K, Matsueda, R, et al. Prediction of the minimum spacer thickness required for definitive radiotherapy with carbon ions and photons for pelvic tumors: an in silico planning study using virtual spacers. J Radiat Res (2021) 62:699–706. doi:10.1093/jrr/rrab047
264. Yamada, M, Sato, H, Ieko, Y, Miyasaka, Y, Kanai, T, Yano, N, et al. In silico comparison of the dosimetric impacts of a greater omentum spacer for abdominal and pelvic tumors in carbon-ion, proton and photon radiotherapy. Radiat Oncol (2019) 14:207–10. doi:10.1186/s13014-019-1411-0
265. Walladbegi, J, Smith, SA, Grayson, AK, Murdoch, C, Jontell, M, and Colley, HE. Cooling of the oral mucosa to prevent adverse effects of chemotherapeutic agents: an in vitro study. J Oral Pathol and Med (2018) 47:477–83. doi:10.1111/jop.12696
266. Walladbegi, J, Henriksson, R, Tavelin, B, Svanberg, A, Larfors, G, Jädersten, M, et al. Efficacy of a novel device for cryoprevention of oral mucositis: a randomized, blinded, multicenter, parallel group, phase 3 trial. Bone Marrow Transpl (2022) 57:191–7. doi:10.1038/s41409-021-01512-6
267. Walladbegi, J, Gellerstedt, M, Svanberg, A, and Jontell, M. Innovative intraoral cooling device better tolerated and equally effective as ice cooling. Cancer Chemother Pharmacol (2017) 80:965–72. doi:10.1007/s00280-017-3434-2
268. Blacker, C, Kamsvåg, T, Bejhed, RS, and Ljungman, G. Primary evaluation of an air-cooling device to reduce oral mucositis: a pilot study in healthy volunteers. Med Oncol (2020) 37:110. doi:10.1007/s12032-020-01431-4
269. Blacker, C, Bejhed, RS, Frykholm, P, and Ljungman, G. Randomized cross-over study investigating the tolerability and side effects of an intra-oral air-cooling device compared to ice in healthy volunteers. Med Oncol (2022) 40:58. doi:10.1007/s12032-022-01932-4
270. National Academies of Sciences, E., and Medicine. Foundational research gaps and future directions for digital twins. Washington (2024).
271. Katsoulakis, E, Wang, Q, Wu, H, Shahriyari, L, Fletcher, R, Liu, J, et al. Digital twins for health: a scoping review. npj Digital Med (2024) 7:77. doi:10.1038/s41746-024-01073-0
272. Lyman, JT, and Wolbarst, AB. Optimization of radiation therapy, III: a method of assessing complication probabilities from dose-volume histograms. Int J Radiat Oncology* Biology* Phys (1987) 13:103–9. doi:10.1016/0360-3016(87)90266-5
273. Murphy, MJ, Balter, J, Balter, S, BenComo, JA, Das, IJ, Jiang, SB, et al. The management of imaging dose during image-guided radiotherapy: report of the AAPM Task Group 75. Med Phys (2007) 34:4041–63. doi:10.1118/1.2775667
274. Brock, K, Sharpe, M, Dawson, L, Kim, S, and Jaffray, D. Accuracy of finite element model-based multi-organ deformable image registration. Med Phys (2005) 32:1647–59. doi:10.1118/1.1915012
275. Hadjicharalambous, M, Roussakis, Y, Bourantas, G, Ioannou, E, Miller, K, Doolan, P, et al. Personalised in silico biomechanical modelling towards the optimisation of high dose-rate brachytherapy planning and treatment against prostate cancer. Front Physiol (2024) 15:1491144. doi:10.3389/fphys.2024.1491144
276. Wu, C, Lorenzo, G, Hormuth, DA, Lima, EABF, Slavkova, KP, DiCarlo, JC, et al. Integrating mechanism-based modeling with biomedical imaging to build practical digital twins for clinical oncology. Biophys Rev (2022) 3:021304. doi:10.1063/5.0086789
277. Stamatakos, GS, Kolokotroni, E, Panagiotidou, F, Tsampa, S, Kyroudis, C, Spohn, S, et al. In silico oncology: a mechanistic multiscale model of clinical prostate cancer response to external radiation therapy as the core of a digital (virtual) twin. Sensitivity analysis and a clinical adaptation approach. Front Physiol (2025) 16:1434739. doi:10.3389/fphys.2025.1434739
278. Pérez-Benito, Á, García-Aznar, JM, Gómez-Benito, MJ, and Pérez, MÁ. Patient-specific prostate tumour growth simulation: a first step towards the digital twin. Front Physiol (2024) 15:1421591. doi:10.3389/fphys.2024.1421591
279. Kawaguchi, H, Demizu, Y, Mukumoto, N, Ishihara, T, Miyawaki, D, Komatsu, S, et al. Efficacy of spacers in radiation therapy for locally advanced pancreatic cancer: a planning study. Anticancer Res (2021) 41:503–8. doi:10.21873/anticanres.14801
280. van Wijk, Y, Vanneste, BG, Walsh, S, van der Meer, S, Ramaekers, B, van Elmpt, W, et al. Development of a virtual spacer to support the decision for the placement of an implantable rectum spacer for prostate cancer radiotherapy: comparison of dose, toxicity and cost-effectiveness. Radiother Oncol (2017) 125:107–12. doi:10.1016/j.radonc.2017.07.026
281. Hooshangnejad, H, Han-Oh, S, Shin, EJ, Narang, A, Rao, AD, Lee, J, et al. Demonstrating the benefits of corrective intraoperative feedback in improving the quality of duodenal hydrogel spacer placement. Med Phys (2022) 49:4794–803. doi:10.1002/mp.15665
282. Fishman, K., and Vainer, Y. Systems and methods for real time beam sculpting intraoperative-radiation-therapy treatment planning (2019). USA patent US10940334B2. Available online at: https://patents.google.com/patent/US10940334B2/en.
283. Fishman, K., and Vainer, Y. Three-dimensional beam forming x-ray source (2024). US patent US12027341B2. Available online at: https://patents.google.com/patent/US12027341B2/e.
284. Yeniaras, E, Fuentes, DT, Fahrenholtz, SJ, Weinberg, JS, Maier, F, Hazle, JD, et al. Design and initial evaluation of a treatment planning software system for MRI-guided laser ablation in the brain. Int J Comput Assist Radiol Surg (2014) 9:659–67. doi:10.1007/s11548-013-0948-x
285. Schumann, C, Rieder, C, Haase, S, Teichert, K, Süss, P, Isfort, P, et al. Interactive multi-criteria planning for radiofrequency ablation. Int J Comput Assist Radiol Surg (2015) 10:879–89. doi:10.1007/s11548-015-1201-6
286. Fuentes, D, Cardan, R, Stafford, RJ, Yung, J, Dodd, GD, and Feng, Y. High-fidelity computer models for prospective treatment planning of radiofrequency ablation with in vitro experimental correlation. J Vasc Interv Radiol (2010) 21:1725–32. doi:10.1016/j.jvir.2010.07.022
287. Zhai, W, Xu, J, Zhao, Y, Song, Y, Sheng, L, and Jia, P. Preoperative surgery planning for percutaneous hepatic microwave ablation. In: Medical Image Computing And Computer-Assisted Intervention-MICCA 2008: 11th international conference, September 6–10, New York, NY. Springer (2008). p. 569–77. Part II 11.
288. Zhang, B, Moser, MA, Zhang, EM, Luo, Y, and Zhang, W. Numerical analysis of the relationship between the area of target tissue necrosis and the size of target tissue in liver tumours with pulsed radiofrequency ablation. Int J Hyperthermia (2015) 31:715–25. doi:10.3109/02656736.2015.1058429
289. Pearce, JA. Comparative analysis of mathematical models of cell death and thermal damage processes. Int J Hyperthermia (2013) 29:262–80. doi:10.3109/02656736.2013.786140
290. Zhang, R, Wu, S, Wu, W, Gao, H, and Zhou, Z. Computer-assisted needle trajectory planning and mathematical modeling for liver tumor thermal ablation: a review. Math biosciences Eng (2019) 16:4846–72. doi:10.3934/mbe.2019244
291. Lin, Y-M, Paolucci, I, O’Connor, CS, Anderson, BM, Rigaud, B, Fellman, BM, et al. Ablative margins of colorectal liver metastases using deformable CT image registration and autosegmentation. Radiology (2023) 307:e221373. doi:10.1148/radiol.221373
292. Heshmat, A, O’Connor, CS, Albuquerque Marques Silva, J, Paolucci, I, Jones, AK, Odisio, BC, et al. Using patient-specific 3D modeling and simulations to optimize microwave ablation therapy for liver cancer. Cancers (2024) 16:2095. doi:10.3390/cancers16112095
293. Guenette, JP, Himes, N, Giannopoulos, AA, Kelil, T, Mitsouras, D, and Lee, TC. Computer-based vertebral tumor cryoablation planning and procedure simulation involving two cases using MRI-visible 3D printing and advanced visualization. Am J Roentgenology (2016) 207:1128–31. doi:10.2214/ajr.16.16059
294. Koethe, Y, Xu, S, Velusamy, G, Wood, BJ, and Venkatesan, AM. Accuracy and efficacy of percutaneous biopsy and ablation using robotic assistance under computed tomography guidance: a phantom study. Eur Radiol (2014) 24:723–30. doi:10.1007/s00330-013-3056-y
295. Paolucci, I, Schwalbe, M, Prevost, GA, Lachenmayer, A, Candinas, D, Weber, S, et al. Design and implementation of an electromagnetic ultrasound-based navigation technique for laparoscopic ablation of liver tumors. Surg Endosc (2018) 32:3410–9. doi:10.1007/s00464-018-6088-1
296. Lin, Y-M, Paolucci, I, Anderson, BM, O’Connor, CS, Rigaud, B, Briones-Dimayuga, M, et al. Study protocol COVER-ALL: clinical impact of a volumetric image method for confirming tumour coverage with ablation on patients with malignant liver lesions. CardioVascular Interv Radiol (2022) 45:1860–7. doi:10.1007/s00270-022-03255-3
297. Widmann, G, Wallach, D, Toporek, G, Schullian, P, Weber, S, and Bale, R. Angiographic C-Arm CT–versus MDCT-Guided stereotactic punctures of liver lesions: nonrigid phantom study. Am J Roentgenology (2013) 201:1136–40. doi:10.2214/ajr.12.10405
298. Wallach, D, Toporek, G, Weber, S, Bale, R, and Widmann, G. Comparison of freehand-navigated and aiming device-navigated targeting of liver lesions. The Int J Med Robotics Computer Assisted Surg (2014) 10:35–43. doi:10.1002/rcs.1505
299. Toporek, G, Wallach, D, Weber, S, Bale, R, and Widmann, G. Cone-beam computed tomography-guided stereotactic liver punctures: a phantom study. Cardiovasc Interv Radiol (2013) 36:1629–37. doi:10.1007/s00270-013-0635-x
300. Lin, Y-M, Bale, R, Brock, KK, and Odisio, BC. Contemporary evidence on colorectal liver metastases ablation: toward a paradigm shift in locoregional treatment. Int J Hyperthermia (2022) 39:649–63. doi:10.1080/02656736.2021.1970245
301. Bale, R, Widmann, G, and Stoffner, DIR. Stereotaxy: breaking the limits of current radiofrequency ablation techniques. Eur J Radiol (2010) 75:32–6. doi:10.1016/j.ejrad.2010.04.013
302. Fischer, T, Lachenmayer, A, and Maurer, MH. CT-guided navigated microwave ablation (MWA) of an unfavorable located breast cancer metastasis in liver segment I. Radiol case Rep (2019) 14:146–50. doi:10.1016/j.radcr.2018.10.010
303. Svatos, M., Chell, E., Low, D. A., Pigrish, V., Orio, P. F., Miller, K., et al. (2024). Symmetry, separation, and stability: Physical properties for effective dosimetric space with a stabilized hyaluronic acid spacer. Med. Phys. 51 (9), 6231–6245. doi:10.1002/mp.17292
304. King, M. T., Svatos, M., Chell, E. W., Pigrish, V., Miller, K., Low, D. A., et al. (2024). Evaluating the quality-of-life effect of apical spacing with hyaluronic acid prior to hypofractionated prostate radiation therapy: a secondary analysis. Pract. Radiat. Oncol. 14 (3), e214–e219. doi:10.1016/j.prro.2023.11.010
Glossary
3D Three-dimensional
3DCRT 3D conformal radiation therapy
ABC Active breathing coordinator
ADT Androgen deprivation therapy
BMS Biologic mesh spacer
BT Brachytherapy
CA Cryoablation
CCA Cholangiocarcinoma
CAD Computer-aided design
CBCT Cone beam computed tomography
CRC Colorectal carcinoma
CRLM Colorectal liver metastasis
CT Computed tomography
D5W 5% dextrose in water
DIBH Deep inspiration breath hold
DT Digital twin
DVH Dose to volume histogram
EBRT External beam radiation therapy
ePTFE Expanded polytetrafluoroethylene
FB Free breathing
FEM Finite-element method
GCT Giant cell tumor
GI Gastrointestinal
GU Genitourinary
HA Hyaluronic acid
HCC Hepatocellular carcinoma
HDR High-dose rate
HNC Head and neck
HS Hydrogel spacer
HU Hounsfield units
ICRU International commission on radiation units and measurements
IG-IMRT Image-guided, intensity-modulated radiotherapy
IMBT Intensity-modulated brachytherapy
IMPT Intensity-modulated proton therapy
IMRT Intensity-modulated radiation therapy
IORT Intraoperative radiotherapy
IR Interventional radiology
LAD Left anterior descending artery
LDR Low-dose rate
MRI Magnetic resonance imaging
MWA Microwave ablation
NSCLC Non-small cell lung cancer
NSGCT Non-seminomatous germ cell tumors
NTCP Normal tissue complication probability
OAR Organs-at-risk
OS Overall survival
PACS Picture archiving and communication system
PBRT Proton beam radiation therapy
PD Pancreaticoduodenal
PEG Polyethylene glycol
PGA Polyglycolic acid
PTC Papillary thyroid carcinoma
PTMC Papillary thyroid microcarcinoma
QOL Quality of life
RCC Renal cell carcinoma
RFA Radiofrequency ablation
RIOM Radiation-induced oral mucositis
RT Radiation therapy
SBRT Stereotactic body radiation therapy
TCC Transitional cell carcinoma
TRUS Transrectal Ultrasound
TOD Temporary organ displacement
TPS Treatment planning system
TCP Tumor control probability
US Ultrasound
VMAT Volumetric arc radiation therapy
Keywords: organ sparing, radiation therapy, temporary organ displacement, organs-at-risk, dose toxicity, thermal ablation, non-target organ injury, spacers
Citation: Tino RB, Li M, Heshmat A, Suresh A, Guillaume A, Brock KK, Odisio BC and Koay EJ (2025) Protecting organs-at-risk in cancer therapies through temporary organ displacement: a comprehensive review. Oncol. Rev. 19:1655365. doi: 10.3389/or.2025.1655365
Received: 27 June 2025; Accepted: 30 July 2025;
Published: 05 September 2025.
Edited by:
Jeffrey Tuan, National Cancer Centre Singapore, SingaporeReviewed by:
Michael Chao, Olivia Newton-John Cancer Research Institute, AustraliaRaj Tiwari, Sengkang General Hospital, Singapore
Copyright © 2025 Tino, Li, Heshmat, Suresh, Guillaume, Brock, Odisio and Koay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rance B. Tino, cmJ0aW5vQG1kYW5kZXJzb24ub3Jn; Eugene J. Koay, ZWtvYXlAbWRhbmRlcnNvbi5vcmc=
 Rance B. Tino
Rance B. Tino Michael Li1
Michael Li1 Amirreza Heshmat
Amirreza Heshmat Kristy K. Brock
Kristy K. Brock Eugene J. Koay
Eugene J. Koay