- 1School of Life Sciences, University of Nottingham, Nottingham, United Kingdom
- 2Nanoscale and Microscale Research Centre, University of Nottingham, Nottingham, United Kingdom
Spiders have adapted to a wide range of ecological niches, accompanied by the diversification of their silk, which performs various ecological functions under different environmental conditions. This study investigates the physical properties of silk produced by highly mobile linyphiid spiders, whose airborne silken “sails” enable long-distance, high-altitude windborne dispersal. Environmental Scanning Electron Microscopy imaging demonstrated that linyphiid silk interacts with moisture in its surroundings, leading to changes in stiffness and increased torsion at relative humidities greater than 60%. The estimated tensile strength under low moisture conditions is estimated to be up to 1 GPa but drops by the order of a factor of 2 when exposed to moisture (>60% relative humidity) or to short (30 minute) bursts of short wavelength (UV-C) light. In contrast, storage at temperatures ranging from -18°C to 70°C had no significant impact on tensile strength. These findings demonstrate the resilience of linyphiid silk to low temperatures typical of high altitudes, with important implications for understanding wind-assisted dispersal, global spider distribution, and potential responses to climate change. We also show that the silk can withstand exposure to ultraviolet light at intensities that would induce damage or death to living tissue, albeit with an increased likelihood of fracture. Together these findings provide important insights needed to understand ecological processes and explore uses and limitations of synthetic analogues of these biological materials in medicine and engineering.
Introduction
Spiders play an important role in maintaining ecological balance in the numerous ecosystems in which they are a part (Nyffeler and Birkhofer 2017). The diversity of specialised silks that they use to fulfil a variety of ecological functions is central to their capacity to adapt to such a wide range of different ecological niches. Spiders use their silk to construct a diverse array of structures, each serving a specific function throughout their life history, from protective casings for developing embryos to elaborate snares to catch prey or delicate structures needed for successful mating (Blackledge, 2013).
The physical properties of silken structures are explained not only by the chemical composition of the silks themselves but by their interaction with the external environment. Some of the most well-characterised types of interactions between silks and their environment are those produced by orb-weaving spiders (Hopfe et al., 2024), but there remain substantial gaps in our knowledge of silks produced by other taxa. In this study, the physical properties of silk produced by a comparatively unexplored group, the linyphiids, are examined under common environmental pressures. The ways in which linyphiid spiders utilise their silk necessitates that the silk withstand a multitude of environmental extremes. Investigating these effects thus not only presents valuable insights into the ecology of linyphiid spiders and their responses to climate change but also offers potential to extend the range of possible templates for synthetic biomaterials in the fields of engineering and medicine.
Linyphiids are amongst the most numerous of spiders in the northern hemisphere (World Spider Catalogue, 2025). They predominantly inhabit damp leaf litter low to the ground but are also common in disturbed environments due to their tendency for long-distance aerial dispersal through a process known as ballooning (Schmidt and Tscharntke, 2005; Bell et al., 2005). While predicting the exact distance travelled during ballooning is challenging (Suter, 1999), it has been estimated that the distribution of dispersal distances is likely to be leptokurtic and that some linyphiids may travel 30 km or more per day in sustained optimal conditions (Thomas et al., 2003). Unlike orb-weavers, which construct vertical orb webs, linyphiids use their silk to produce horizontal sheet webs for prey capture (Benjamin and Zschokke, 2004). These webs comprise a dense matrix of horizontal sheets connected to vegetation, along with vertical capture lines (Cuff et al., 2022). The linyphiid webs that have been studied to date comprise fibres of different thicknesses, some of which may be coated in external, globular-like sticky substances (Benjamin et al., 2002; Eberhard, 2021). Unlike orb-weavers, these webs are not replaced daily with the spider primarily remaining on the underside of the sheet attacking prey from below once ensnared in the web. Although linyphiids appear to have the same types of silk storage gland and spinnerets as the orb weavers (Peters and Kovoor, 1991), the relative sizes of these appears to be different, with glands in linyphiids used to produce the most extensible strands (flagelliform silk), and sticky droplets (aggregate silk) being relatively reduced in size.
External factors, such as humidity, temperature and UV exposure are known to influence the physical properties of silk fibres. Low humidity typically renders silk more brittle and reduces its strain energy capacity (Blamires and Sellers, 2019). In contrast, under high humidity conditions, some silks increase in stiffness and can withstand a greater force than they would otherwise (Fazio et al., 2022; Cohen et al., 2021). This characteristic of ‘supercontraction’ is thought to have evolved 225 million years ago and is observed in many spider families, with the notable exception of the tarantulas (Agnarsson et al., 2009; Boutry and Blackledge, 2010). The phenomenon may have evolved to prevent webs from sagging under the weight of water, such as is observed for horizontal linyphiid webs, which may be more likely to naturally accumulate water than the vertical counterparts spun by orb-weavers (Brackenbury, 1997). Supercontraction is believed to be initiated when water molecules diffuse into the silk fibres, triggering the dissociation of intermolecular hydrogen bonds, which induces the swelling of the fibre and chain extension to accommodate the influx of water (Cohen et al., 2021). The rate of water uptake determines the extent of this change (Agnarsson et al., 2009). While this process enhances the strands stiffness to prevent immediate breakage, it also induces torsion within the fibre, generating significant stresses on the silk strand (Greco et al., 2021) that could ultimately adversely impact the webs capture efficiency. Recent theoretical work suggests that these environmental stressors, including moisture, may trigger bifurcation transitions in the silk’s mechanical response, shifting it between elongation and contraction under torsion, in a process known as the dual Poynting effect (Fraldi et al., 2025). Thus, through supercontraction, spider silk exploits bifurcation mechanics, allowing it to adapt to diverse environmental conditions. This adaptive behaviour may enhance the silk’s performance in processes such as wind-assisted dispersal and web construction, where it must withstand both high tensile forces and environmental stresses.
The breaking strain and stiffness of silk are also shown to be affected by temperature, decreasing as temperature increases (Maithani et al., 2022), although the silk itself can withstand temperatures far more extreme than those seen in nature, at the extremes of both cold (-60°C) and heat (300°C) (Gu et al., 2020; Yang et al., 2005). Ultraviolet rays (UV-A), to which there is exposure in nature, have been shown to exhibit a strengthening effect on spider silk, likely by fostering the formation of crosslinks between protein molecules within the silk fibre (Osaki and Osaki, 2011). Shorter wavelength UV-C rays, which are highly damaging to biological material and only expected to be encountered above the ozone layer appear to reduce tensile strength and strain at breakage (Perez-Rigueiro et al., 2007).
In this study we explore the effect of altered humidity and temperature, and exposure to biologically harmful UV-C rays, on the physical structure and properties of linyphiid silk. We use environmental Scanning Electron Microscopy (eSEM) and tests of tensile strength in order to fill a gap in our understanding of the properties of silk that is used by linyphiid spiders. These belong to the same spider clade, Araneoidea, which includes orb-weaving spiders such as nephilids and araneids, but linyphiid silk remains much less well-characterised than their orb-weaving counterparts. Our findings help shape our understanding of how these silks differ from those of the much better studied orb-weaver silks, and what this means for their ability to disperse under different environmental conditions.
Methods
Study site and sample
Linyphiid spiders (from the subfamily Linyphiinae) were collected by hand from parkland on the University of Nottingham campus. They were housed in transparent plastic bijou pots, containing a small wooden stick and wet tissue paper. The inclusion of these specific items aimed to create a suitable microenvironment for the spiders, offering a climbing structure and maintaining humidity to prevent desiccation. Spiders were stored in an opaque plastic tub in the laboratory, maintaining a consistent dark environment at room temperature (20°C) and watered regularly to keep moist.
Collection and treatment of silk
To extract silk, wooden sticks were used to gently lift the spider, which was then encouraged to descend, resulting in a strand of dragline silk forming between the wooden stick and the spider. This strand was then placed over the top of a plastic bijou pot. Tensile strength of silk strands was assessed by placing a small strip of tissue paper of known mass on the midpoint of the silk strand. 10 µl volumes of distilled water were added incrementally to the tissue paper using a P20 Gilson pipette. This process was repeated every minute until the silk strand snapped.
Treatment of silk samples with UV-C light was carried out by placing them in a sealed container with a UV-C light source (254 nm wavelength) for 30 minutes. This duration of exposure is used to sterilise surfaces because it induces damage or death to living tissue. Silks were exposed to a range of temperatures by being placed in a freezer (-18°C), fridge (5°C), or heat block (70°C) for 4 hours, respectively.
Ambient humidity in the outdoor environment where the silk would perform its natural function is approximately 80%. Samples were exposed to the following experimental conditions: (1) a laboratory setting (55% humidity at 20°C), (2) a dry room (30% humidity at 25°C), and (3) a sealed plastic box filled with water (100% humidity at 20°C).
Imaging using an environmental scanning electron microscope
To analyse the structural characteristics of silk, an environmental Scanning Electron Microscope FEI Qanta650 eSEM with a temperature-controlled Peltier stage was employed to capture high-resolution images. A sample of linyphiid web was carefully affixed to the stage and humidity reduced in the chamber to approximately 11%. Humidity was then increased incrementally over a period of approximately half an hour to a final value of 95% before being reduced back to 11%. The temperature of the stage was lowered to 2°C during this process of wetting in order to control the level of humidity by adjusting the chamber pressure, whilst maintaining constant temperature. Images of the samples were taken at multiple positions at each incremental change in humidity.
Analysis of data
Dimensions of silk strands were obtained from the eSEM images and used to estimate the tensile strength using the formula S=F/A where S =strength, F=ultimate force required to break the silk and A=cross sectional area. Comparisons between groups of silk treated in different ways were made using non-parametric Mann-Whitney U tests. The a priori expectation is that UV treatment reduces tensile strength thus tests were performed one-tailed. A conservative, two-tailed approach was taken when testing for significant effects of humidity and temperature.
Results
Silk structure
The eSEM imaging indicated a variety of strand thicknesses in the linyphiid web, notably the existence of larger silk strands with smaller strands attached to them, as well as multiple silk strands twisted together in a helical structure. (Figure 1). A range of thickness of strands was observed with an estimated range of 0.1- 2 μm in diameter. Some strands had observable thickened areas at regular intervals (Figure 2), consistent with some kind of external deposits. To account for this variability and to ensure consistency in our mechanical calculations, we adopted 2 μm, the upper end of the observed range, as a reference diameter for calculating cross-sectional area and tensile strength. This conservative choice guarantees that our reported tensile strength values represent minimum estimates, and we therefore acknowledge that the actual tensile strength of linyphiid silk is likely higher than reported.
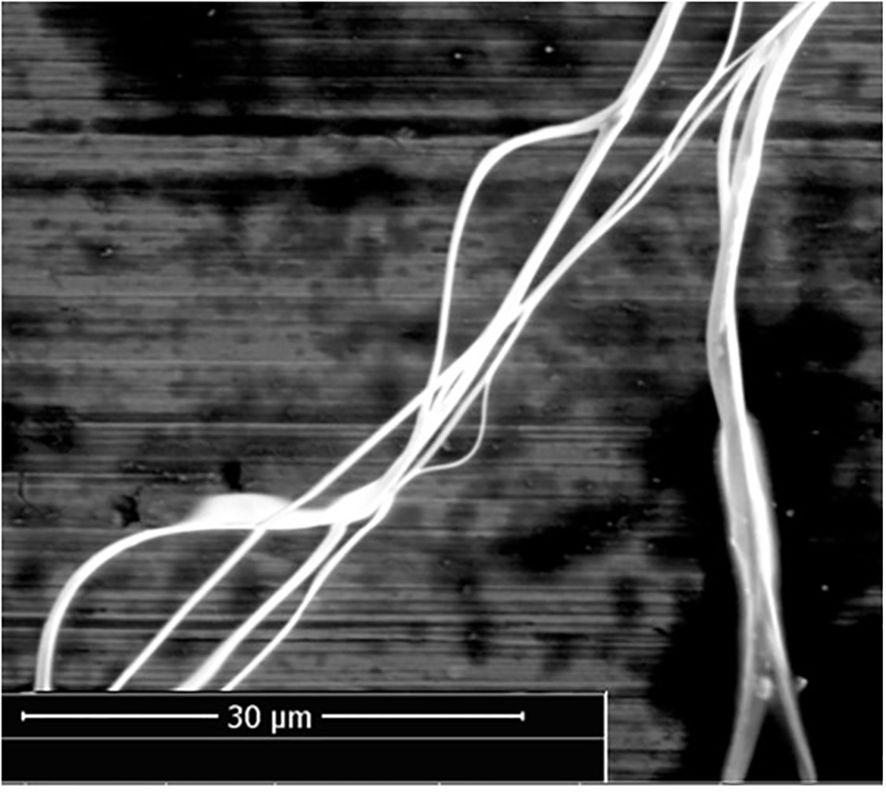
Figure 1. eSEM image taken at 20°C, 11.7% relative humidity, displaying the helical configuration of linyphiid silk strands, with multiple strands intricately entwined around each other. Strand diameter ranged between approximately 0.1- 2 μm.

Figure 2. eSEM image captured at 20 °C and 11.7% relative humidity. The image shows silk strands of varying diameter, with the larger strands exhibiting periodic thickened regions along their length. Arrow ‘A’ highlights the position of an external globular, adhesive-like coating, suggestive of a sticky surface deposit.
Humidity within the eSEM chamber was gradually increased from 11% to 95% and then reduced back to 11%. Five defined points on the sample were imaged at the initial humidity and at intervals throughout the increase. A representative series of images from one such point, illustrating the silk strands at various humidity levels, is shown in Figure 3. Observable changes in strand position occurred, consistent with fibre stiffening and contraction. Across all five points, these changes consistently appeared once the relative humidity exceeded 60%. In these images, the silk fibres appear to undergo not only contraction but also twisting or rotational movement, indicative of increased torsional stress within the fibres. This increased torsion is initiated by humidity-induced supercontraction and may contribute to the observed rearrangement and tightening of the silk strands.
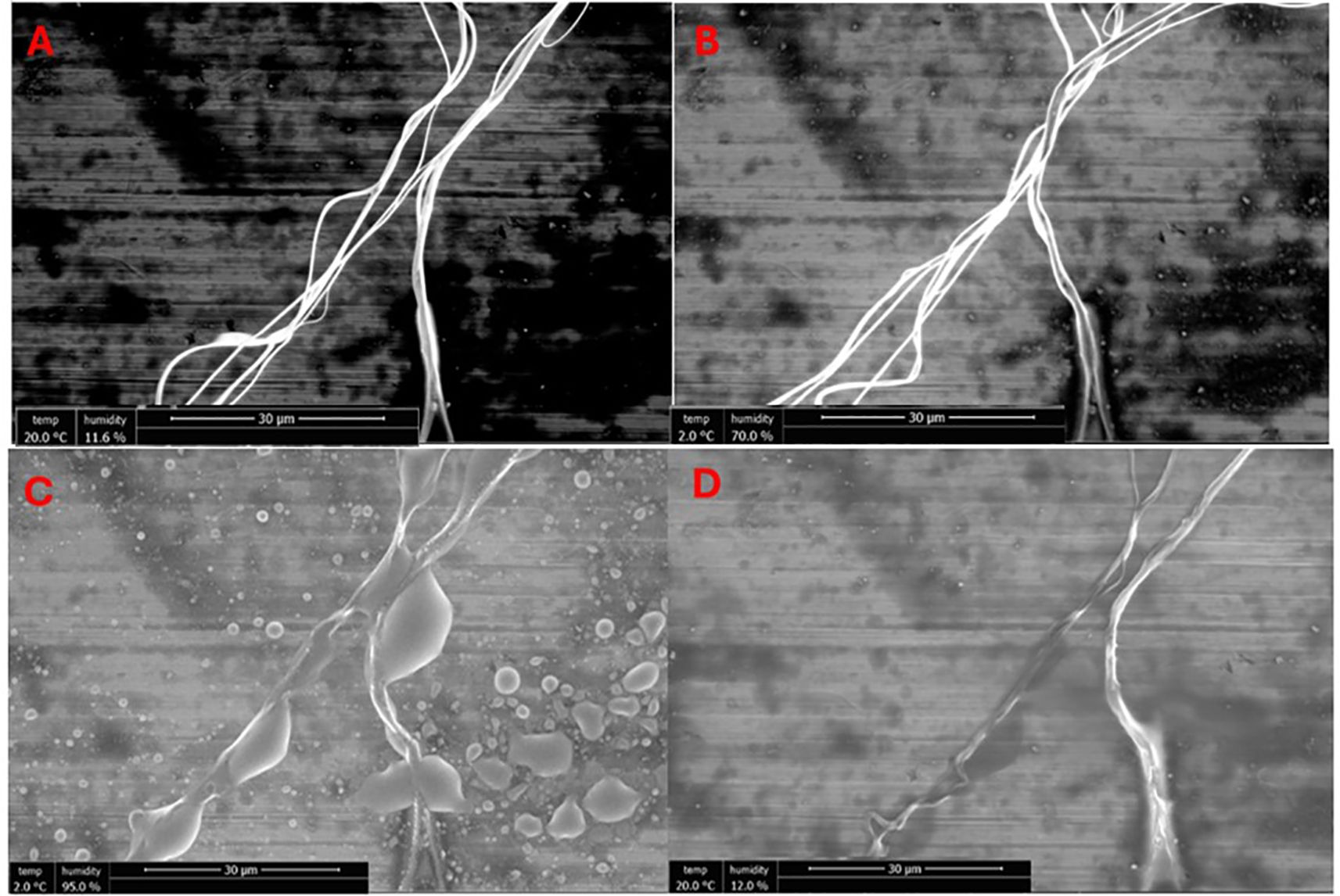
Figure 3. eSEM micrographs taken at the same location, and at the same magnification, showing linyphiid major ampullate at different levels of humidity, starting at 11.6% [image (A)], increasing to 70% [image (B)] and 95% [image (C)] and then subsequently reduced at the same rate to a final value of 12% [image (D)].
Estimates of tensile strength are reduced at high levels of humidity
Masses applied to the untreated (‘control’) silk strands under ambient laboratory conditions (55% humidity, 20°C) ranged from 0.0147 g to 0.0327 g with a mean mass of 0.0226 g, yielding an average tensile strength of 0.71 GPa and a maximum of 1.03 GPa (n=10 replicates) (Figure 4). A Mann-Whitney U test was used to determine the significance of the differences in strength between these samples and those exposed to different conditions. Samples stored in a dry room (30% humidity) had an average estimated tensile strength of 0.76 GPa (maximum estimated 1.46 GPa) but the difference was not significant compared with the values obtained under ambient conditions (U = 24, z = 0.06124, p > 0.05). In contrast, at maximum humidity (100%), the tensile strength was estimated to be significantly lower than at ambient room conditions (estimated mean tensile strength of 0.46 GPa, mean mass at breaking = 0.0147g. U = 4.5, z = 2.44949, p < 0.05).

Figure 4. This figure presents the tensile strength of spider silk across various environmental conditions. Crosses represent individual measurements, and red diamond markers indicate the mean tensile strength for each condition. The results highlight a significant decrease in tensile strength under UV exposure [p<0.00144] and 100% humidity [p<0. 01428]. In contrast, 30% humidity and temperature deviation, preserve or slightly enhance tensile strength performance.
Estimates of tensile strength are not significantly affected by temperature
The estimated tensile strength endured by silk strands treated at different temperatures exhibited no significant deviation from the ambient ‘control’ samples (-18°C sample: U = 9, z = -1.89835, p > 0.05; 5°C sample: U = 13, z = -1.40846, p > 0.05; 70°C sample: U = 15, z = -1.16351, p > 0.05). The samples treated at -18°C showed the highest deviation from the control group, with an average mass to breaking of 0.0294 g, corresponding to a tensile strength of 0.92 GPa. Estimates of tensile strength for the samples stored at 5°C sample were 0.88 GPa, and for 70°C samples were 0.82 GPa.
Ultraviolet-C irradiation reduces tensile strength
Results from the investigation of the UV-C treated silk samples were compared to those of the control group, with 0.0166 g (mean 0.0111 g) being the mass sufficient to break the untreated silk strands. The estimated mean tensile strength of the UV treated samples was 0.35 GPa with the estimated maximum being 0.52 GPa. These were significantly lower than those of the ambient ‘control’ samples (U = 2, z = 2.98279, p < 0.05).
Discussion
Linyphiids use their silk for long-distance dispersal as well as for creating silken structures for use in situ to catch prey. In this study we examine their silk and show that it likely alters its physical properties through interactions with the environment, specifically exposure to water. We find that silks become stiffer and more contracted as humidity increases but we do not find a similar response to lowered or raised temperatures, indicating that the silk is resistant to low temperatures that might be experienced during aerial dispersal at high altitude. We also identify areas where the individual silk fibres are covered with what may be external aggregations of proteins. Exposure to low-wavelength UV that is known to be harmful to living organisms also increases silk stiffness and the likelihood of fracture, but we show that the silk remains intact and retains approximately half its previous tensile strength.
Our eSEM imaging findings, which show that silk fibres appear to contract and shorten in the presence of moisture is consistent with the fibres going through the process of supercontraction, a phenomenon that has been observed in silk produced by other spiders. In addition to this contraction, fibres also exhibited visible twisting and rotation, suggesting the presence of torsional stress induced by humidity. This behaviour is likely driven by the disruption of hydrogen bonds within the silk’s amorphous regions, leading to realignment of molecular chains (Fraldi et al., 2025). Such torsional effects may facilitate the rearrangement and tightening of silk strands, potentially enhancing the structural cohesion of the web under changing environmental conditions. Interestingly we find evidence to suggest that in this case it occurs at humidity levels as low as 60% which is lower than what is seen in orb-weavers in which supercontraction usually starts at 75% humidity (Greco et al., 2021). This difference may reflect the evolutionary adaptation of linyphiid silk to the more temperate climates that they occupy, as opposed to the humid tropical climates of species that have previously been studied. The images that we obtained under different conditions illustrate the complex and diverse structure of the silk being studied. The different structural features observed may explain its ability to withstand particular environmental conditions and remain functional in its various ecological roles.
The supercontraction behaviour observed in this study, as well as in previous research, has been described and investigated through the development of advanced theoretical frameworks that capture the complex interplay between spider silk’s internal structure, environmental conditions, and mechanical response. For instance, Fazio et al. (2025a) present a model in which exposure to moisture causes silk to undergo a solid-solid phase transition. Within this model, external energy input provided by either increased humidity and water infiltration or temperature fluctuations is required to break the hydrogen bonds within the amorphous regions, causing the silk structure to transition from a glassy to a rubbery state (Fazio et al., 2025a). This transition is correlated to the silk’s hierarchical structure, comprising of a combination of highly ordered β-sheet nanocrystals and disordered amorphous regions (Fazio et al., 2025a). The major ampullate spidroins, MaSp1 and MaSp2, are fundamental to this structure with MaSp1 forming the stiff crystalline β-sheet domains that provide strength, while MaSp2, enriched in proline content, enhances the amorphous matrix’s flexibility and its affinity for water, making it particularly important for supercontraction (Fazio et al., 2025b). Recent advances using physically based machine learning approaches have further illuminated this behaviour. These models integrate physical principles such as elasticity, phase transitions, and hydration effects into the learning process, enabling machine learning algorithms to uncover mechanistic relationships across different scales rather than merely fitting data. Through this approach, it has been revealed that the onset of supercontraction is governed by specific structural reorganisations within the MaSp2-rich amorphous regions, where water acts as a plasticiser triggering large-scale fibre deformation (Fazio et al., 2024). Complementary studies on artificial spider silk fibres have demonstrated that temperature variations can amplify these hydration-driven transitions, highlighting the dynamic responsiveness of silk’s internal phases (Fazio et al., 2025a). Observations under eSEM closely align these theoretical predictions, capturing real-time morphological changes such as the fibre swelling and increased torsion that accompany supercontraction.
The tensile strength of orb-weaver silk has been regularly recorded at strengths around 1 GPa, although species differ considerably (Blackledge et al., 2013; Wen et al., 2023). There are far fewer data recorded for linyphiids, with only two published values of tensile strength, which are for a Neriene (1.13 GPa) and a Turinyphia (1.28 GPa) species respectively. Our values are similar (up to 1 GPa), an observation that further confirms the finding that whilst linyphiid silk is typically much thinner than that of orb-weaver silk – commonly 1-2 μm in diameter as compared with 3-6 μm and occasionally up to 10 μm in some orb-weavers (Andersson et al., 2016; IaChina et al., 2023) it retains a similar tensile strength. Orb-weaving spiders such as the Araneidae and the Linyphiidae are both part of the superfamily Araneoidea. Phylogenetically conserved features in their silks might explain the mechanism through which they both achieve these similar physical properties (Agnarsson, 2004), with natural selection responsible for maintaining and shaping them to fulfil their respective ecological functions (Tian et al., 2022).
Orb-weavers and their orb webs are required to absorb the impact of high-speed, often flying, prey without snapping and therefore, their hunting style necessitates a high tensile strength (Lefèvre and Auger, 2016). In contrast, linyphiid webs are positioned horizontally and are suited for catching prey that are proportionally smaller and moving at lower speeds (Benjamin and Zschokke, 2004). Our findings that the silk of linyphiids is of a similar strength despite the lower speed and smaller mass to catch is consistent with these prey items often encountering a single silk strand rather than their force being shared by the elements of an interconnected orb web. This may impose a selection pressure for increased silk strength and extensibility (Harmer et al., 2010). Linyphiids maintain their webs and cannot re-ingest them, unlike many orb-weavers, which may also create a need for them to withstand multiple encounters.
Conclusion
Our findings are also consistent with previous work on other spiders such as orb-weavers that has shown that exposure to water can increase stress and alter torsion within a fibre and decreases its overall tensile strength (Boutry and Blackledge, 2010). This suggests a fitness trade-off in spider silk supercontraction, wherein the benefits of supercontraction in high humidity conditions, such as preventing webs from sagging when wet, must outweigh the loss of ultimate tensile strength (Boutry and Blackledge, 2010). The performance that we observe of linyphiid silk in low levels of humidity may be attributed to its adaptation to the natural environments they inhabit. Linyphiid spiders are common in northern temperate environments characterised by lower average humidities when compared with tropical areas (Adams, 2014). Furthermore, the propensity of linyphiid spiders to engage in ballooning, which involves ascent into the higher atmosphere, an area that is typically marked by low humidity levels, may impose selection pressures for high-performance silk in low humidity environments. Our finding that linyphiid silk maintains its mechanical properties across a broad temperature range, is consistent with its suitability for operating at ground level and high altitude. This also points the way for using this silk as a template for suitable material for industrial applications that require materials capable of withstanding temperature fluctuations and that can also withstand exposure to UV wavelengths that are damaging to other biological materials.
Our findings show that there is a complex interplay between silks and the environment in which they operate. Studies of linyphiid silk will inform our understanding of how this particular group of spiders has diversified into such a range of ecological niches, and for our understanding of their likely response to changes in external climatic conditions. This understanding will help shape studies into the use of silk in industrial settings, providing new avenues for advancement in biomimetic spider silk studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All research conforms to UoN Code of Conduct and Research Ethics. The manuscript presents research on invertebrate animals, which do not require specific permission for their study.
Author contributions
DW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. NW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing, Funding acquisition. SG: Conceptualization, Formal Analysis, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the University of Nottingham.
Acknowledgments
This work was made possible by expertise provided by the Nanoscale and Microscale Research Centre (nmRC) at the University of Nottingham, which is an inter-disciplinary facility dedicated to supporting biomaterials characterisation. We would particularly like to thank Luke Norman, Mike Fay and Chris Parmenter in the nmRC for their unfailing enthusiasm and optimism, and for opening physical doors for us, as well as scientific ones. The eSEM facilities that we used were supported by the Engineering and Physical Sciences Research Council (EPSRC) under grant EP/L022494/1, and by the University of Nottingham. We would also like to acknowledge our gratitude to the School of Life Sciences at Nottingham for its continued encouragement and support for our work on 8-legged creatures, and to the community of Arachnologists distributed across the globe, who have guided, encouraged and provided valuable insights over many years.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams R. (2014). Field Guide to the Spiders of California and the Pacific Coast States. 1st ed. Berkeley: University of California Press.
Agnarsson I. (2004). Morphological phylogeny of cobweb spiders and their relatives (Araneae, Araneoidea, Theridiidae). Zool. J. Linn. Soc. 141, 447–626. doi: 10.1111/j.1096-3642.2004.00120.x
Agnarsson I., Boutry C., Wong S.-C., Baji A., Dhinojwala A., Sensenig A. T., et al. (2009). Supercontraction forces in spider dragline silk depend on hydration rate. Zoology 112, 325–331. doi: 10.1016/j.zool.2008.11.003
Andersson M., Johansson J., and Rising A. (2016). Silk spinning in silkworms and spiders. Int. J. Mol. Sci. 17, 1–14. doi: 10.3390/ijms17081290
Bell J. R., Bohan D. A., Shaw E. M., and Weyman G. S. (2005). Ballooning dispersal using silk: world fauna, phylogenies, genetics and models. Bull. Entomol. Res. 95, 69–114. doi: 10.1079/BER2004350
Benjamin S. P., Düggelin M., and Zschokke S. (2002). Fine structure of sheet-webs of Linyphia triangularis (Clerck) and Microlinyphia pusilla (Sundevall), with remarks on the presence of viscid silk. Acta Zool. 83, 49–59. doi: 10.1046/j.1463-6395.2002.00098.x
Benjamin S. P. and Zschokke S. (2004). Homology, behaviour and spider webs: web construction behaviour of Linyphia hortensis and L. triangularis (Araneae: Linyphiidae) and its evolutionary significance. J. Evol. Biol. 17, 120–130. doi: 10.1046/j.1420-9101.2004.00667.x
Blackledge T. A. (2013). “Spider silk: Molecular structure and function in webs,” in Spider Ecophysiology. Ed. Wolfgang N. (Springer, Berlin), 267–281. doi: 10.1038/srep00782
Blackledge T. A., Pérez-Rigueiro J., Plaza G. R., Perea B., Navarro A., Guinea G. V., et al. (2013). Sequential origin in the high-performance properties of orb spider dragline silk. Sci. Rep. 2, 1–5.
Blamires S. J. and Sellers W. I. (2019). Modelling temperature and humidity effects on web performance: implications for predicting orb-web spider (Argiope spp.) foraging under Australian climate change scenarios. Conserv. Physiol. 7, 1–12. doi: 10.1093/conphys/coz083
Boutry C. and Blackledge T. A. (2010). Evolution of supercontraction in spider silk: structure-function relationship from tarantulas to orb-weavers. J. Exp. Biol. 213, 3505–3514. doi: 10.1242/jeb.046110
Brackenbury J. H. (1997). Spider webs: Dew-loading of the linyphiid sheet-web. J. Zool. 242, 131–136. doi: 10.1111/j.1469-7998.1997.tb02934.x
Cohen N., Levin M., and Eisenbach C. H. (2021). On the origin of supercontraction in spider silk. Biomacromolecules 22, 993–1000. doi: 10.1021/acs.biomac.0c01747
Cuff J. P., Tercel M. P. T. G., Drake L. E., Vaughan I. P., Bell J. R., Orozco-ter Wengel P., et al. (2022). Density-independent prey choice, taxonomy, life history, and web characteristics determine the diet and biocontrol potential of spiders (Linyphiidae and Lycosidae) in cereal crops. Environ. DNA 4, 549–564. doi: 10.1002/edn3.272
Eberhard W. G. (2021). Possible self-assembly in linyphiid sheet webs. Arachnology 18, 882–892. doi: 10.13156/arac.2021.18.8.882
Fazio V., De Tommasi D., Pugno N. M., and Puglisi G. (2022). Spider silk mechanics: Predicting humidity and temperature effects. J. Mechan. Phys. Solids 164, 1–11. doi: 10.1016/j.jmps.2022.104857
Fazio V., Florio G., Pugno N. M., and Puglisi G. (2025a). Modeling spider silk supercontraction as a hydration-driven solid–solid phase transition. J. mechan. Phys. solids 195, 1–11. doi: 10.1016/j.jmps.2024.105959
Fazio V., Malay A. D., Numata K., Pugno N. M., and Puglisi G. (2025b). A physically-based machine learning approach inspires an analytical model for spider silk supercontraction. Adv. Funct. mater. 35, 1–17. doi: 10.1002/adfm.202420095
Fazio V., Pugno N. M., Giustolisi O., and Puglisi G. (2024). Physically based machine learning for hierarchical materials. Cell Rep. Phys. Sci. 5, 1–24. doi: 10.1016/j.xcrp.2024.101790
Fraldi M., Puglisi G., and Saccomandi G. (2025). Bifurcation-induced dual Poynting effect in transversely isotropic hyperelastic bodies. Proc. R. Soc. A Math. phys. Eng. Sci. 481, 1–17.10.1098/rspa.2024.0816
Greco G., Arndt T., Schmuck B., Francis J., Bäcklund F. G., Shilkova O., et al. (2021). Tyrosine residues mediate supercontraction in biomimetic spider silk. Commun. Mater. 2, 1–10. doi: 10.1038/s43246-021-00147-w
Gu Y., Yu L., Mou J., Wu D., Zhou P., and Xu M. (2020). Mechanical properties and application analysis of spider silk bionic material. e-Polymers 20, 443–457. doi: 10.1515/epoly-2020-0049
Harmer A. M. T., Blackledge T. A., Madin J. S., and Herberstein M. E. (2010). High-performance spider webs: integrating biomechanics, ecology and behaviour. J. R. Soc. Interface 8, 457–471. doi: 10.1098/rsif.2010.0454
Hopfe C., Ospina-Jara B., Schulze, Tischer M., Morales D., Reinhartz. V., et al. (2024). Impact of environmental factors on spider silk properties. Curr. Biol. 34, 56–67. doi: 10.1016/j.cub.2023.11.043
IaChina I., Fiutowski J., Rubahn H., Vollrath F., and Brewer J. R. (2023). Nanoscale imaging of major and minor ampullate silk from the orb web spider Nephila Madagascariensis. Sci. Rep. 13, 1–10. doi: 10.1038/s41598-023-33839-z
Lefèvre T. and Auger M. (2016). Spider silk as a blueprint for greener materials: A review. International Materials Reviews, 61(2), pp. 127–153.
Maithani A., Sahni I., and Joshi V. V. (2022). A review of thermal, physical, electrical properties of spider silk and their experimental setups. Mater. Today: Proc. 62, 3940–3949. doi: 10.1016/j.matpr.2022.04.565
Nyffeler M. and Birkhofer K. (2017). An estimated 400–800 million tons of prey are annually killed by the global spider community. Sci. Nat. 104, 30. doi: 10.1007/s00114-017-1440-1
Osaki S. and Osaki M. (2011). Evolution of spiders from nocturnal to diurnal gave spider silks mechanical resistance against UV irradiation. Polym. J. 43, 200–204. doi: 10.1038/pj.2010.119
Pérez-Rigueiro J., Elices M., Plaza G. R., Rueda J., and Guinea G. V. (2007). Fracture surfaces and tensile properties of UV-irradiated spider silk fibers. J. Polym. Sci. Part B Polym. Phys. 45, 786–793. doi: 10.1002/polb.21118
Peters H. M. and Kovoor J. (1991). The silk-producing system of Linyphia triangularis (Araneae, Linyphiidae) and some comparisons with Araneidae – Structure, histochemistry and function. Zoomorphology 111, 1–17. doi: 10.1007/BF01632706
Schmidt M. H. and Tscharntke T. (2005). Landscape context of sheetweb spider (Araneae: Linyphiidae) abundance in cereal fields. J. Biogeogr. 32, 467–473. doi: 10.1111/j.1365-2699.2004.01244.x
Suter R. B. (1999). An Aerial Lottery: The physics of ballooning in a chaotic Atmosphere. J. Arachnol. 27, 281–293.
Thomas C. F. G., Brain P., and Jepson P. C. (2003). Aerial activity of linyphiid spiders: modelling dispersal distances from meteorology and behaviour. J. Appl. Ecol. 40(5), 912–927.
Tian J., Zhan Y., Shi C., Ono H., and Tu L. (2022). Solenysa, a Cretaceous relict spider group in East Asia. Diversity 14, 1–16. doi: 10.3390/d14020120
Wen R., Wang S., Wang K., Yang D., Zan X, and Meng Q. (2023). Complete gene sequence and mechanical property of the fourth type of major ampullate silk protein. Acta Biomaterialia 155, 282–291.
Keywords: silk, super-contraction, torsion, tensile strength, spider
Citation: Wharton D, Weston N and Goodacre S (2025) Come rain or shine: effects of external conditions on the properties of linyphiid silk. Front. Arachn. Sci. 4:1580992. doi: 10.3389/frchs.2025.1580992
Received: 21 February 2025; Accepted: 05 May 2025;
Published: 02 June 2025.
Edited by:
Matjaž Gregorič, Research Centre of the Slovenian Academy of Sciences and Arts, SloveniaReviewed by:
Giuseppe Puglisi, Politecnico di Bari, ItalyMatjaz Kuntner, National Institute of Biology (NIB), Slovenia
Copyright © 2025 Wharton, Weston and Goodacre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Goodacre, c2FyYS5nb29kYWNyZUBub3R0aW5naGFtLmFjLnVr
 Daniel Wharton
Daniel Wharton Nicola Weston2
Nicola Weston2 Sara Goodacre
Sara Goodacre