- 1Department of Zoology, Government General Degree College, Mohanpur, West Bengal, India
- 2Prafulla Chandra Sen Government Medical College and Hospital, Arambag, West Bengal, India
- 3Department of Zoology, The University of Burdwan, Rajbati, West Bengal, India
The Chilobrachys tarantula, a genus of Old-World tarantulas, is known for its unique behavior and venomous bites, which have varying effects on humans. This research provides a comprehensive overview of Chilobrachys species distribution, bite incidents, and medical treatment based on bioclimatic modeling and clinical case reports. Chilobrachys species thrive in regions with moderate daily temperature ranges (−7.9°C to 43.3°C), stable climates, and sufficient precipitation (0 mm to 2,325 mm), particularly in Southeast Asia. Key bioclimatic variables such as maximum temperature of the warmest month (BIO5) and precipitation during both warm and cold months (BIO18, BIO19) significantly influence species distribution. Field data from 24 confirmed Chilobrachys bites reveal that most incidents occur between April and July, with bite symptoms ranging from severe localized pain, swelling, and necrosis to systemic effects like fever and hemoglobinuria. Case studies demonstrate the cytotoxic effects of Chilobrachys venom, leading to complications such as cellulitis, necrosis, and compartment syndrome, particularly in untreated or delayed cases. Medical reports indicate that treatment involves immediate first aid (RICE protocol), pain management, antihistamines for allergic reactions, and antibiotics to prevent secondary infections. In severe cases, surgical intervention is required for necrosis and compartment syndrome management. Bioclimatic and clinical data emphasize the need for public awareness, healthcare training, and early intervention to mitigate the risks associated with Chilobrachys bites. This research provides crucial insights into the ecological and medical aspects of these tarantulas, aiding in the prevention and management of envenomation cases.
1 Introduction
Spiders are among the most diverse groups of arthropods, with over 50,000 known species globally (WSC, 2024). Unlike insects, spiders are not generally vectors for communicable diseases (Vetter and Isbister, 2008). Despite their abundance, most spiders pose little to no threat to humans due to their delicate mouthparts and venom that is either too weak or specifically adapted to incapacitate prey (Nentwig and Kuhn-Nentwig, 2012). However, a subset of species produces venom potent enough to cause localized skin reactions, systemic symptoms, or even neurotoxicity in humans (Isbister and White, 2004).
The epidemiology of spider bites is complex and often confounded by several factors, including recall bias, misdiagnosis of necrotic lesions resembling bites, and the frequent inability to accurately identify the species responsible (Isbister and Gray, 2002). Tarantulas, belonging to the family Theraphosidae, are among the most well-known spiders, both to the general public (Briggs and Hamilton, 2024) and to scientists who research toxins (Vetter and Isbister, 2008). The family Theraphosidae is taxonomically rich, comprising 168 genera and 1,117 species globally (WSC, 2024). Among these, the Asian genus Chilobrachys consists of 32 recognized species. These are large mygalomorph spiders, typically distinguished by their dark body coloration and unique morphological features such as a nearly straight arrangement of their anterior eyes and narrow scopulae at the tips of their metatarsi (Mondal et al., 2020a). These spiders are highly specialized for a burrowing lifestyle, constructing intricate shelters using environmental features such as mud walls, the bases of large trees, or cliff faces. Their burrows serve not only as shelter but also as strategic locations for hunting (Mondal et al., 2020a). Chilobrachys are nocturnal hunters, exhibiting unique predatory and reproductive behaviors that set them apart from many other tarantula species (Mondal et al., 2020a).
The venom of Chilobrachys spiders, particularly Chilobrachys jingzhao, is a sophisticated biochemical matrix with profound neurobiological and therapeutic relevance (Liao et al., 2007). Dominated by cystine-knot toxins (CKTs), known for their structural stability and specificity, the venom modulates voltage-gated ion channels, including sodium and potassium channels, by acting as pore blockers or gating modifiers (Chen et al., 2008). Toxins such as Jingzhaotoxin-V and Jingzhaotoxin-XIII selectively alter ion channel dynamics, shifting activation thresholds and trapping voltage sensors in resting states (Zeng et al., 2007). Structural features like conserved disulfide bonds enhance toxin stability and binding precision (Dresler et al., 2024). Transcriptomic studies (Chen et al., 2008) further reveal an array of novel peptides, highlighting the evolutionary adaptation of Chilobrachys venom for prey immobilization and defense, with significant implications for pharmacological applications (Liao et al., 2007). Research into tarantula venom has led to the discovery of novel toxins that target ion channels, including potassium and calcium channels, as well as recently identified toxins acting on acid-sensing ion channels (ASIC) (Escoubas et al., 2003; Wang et al., 2024).
The size and appearance of tarantulas may cause alarm, but their bites are generally considered harmless to humans (Lucas et al., 1994; Isbister et al., 2003). Most bites result in mild, localized symptoms, such as a brief stinging sensation and minimal inflammation. Serious systemic effects, such as dermonecrosis or other life-threatening conditions, are rare (Isbister et al., 2003). Nonetheless, tarantula venom can be lethal to domestic animals, especially dogs, with multiple reported fatalities (Isbister et al., 2003). While Chilobrachys spiders are generally not considered dangerous to humans, they have been implicated in more severe cases involving domestic animals. There have been multiple reports of fatal Chilobrachys bites in dogs (Robinson and Griffin, 1983). Additionally, two human fatalities have been attributed to Chilobrachys bites, although these cases involved complications such as gangrene and severe allergic reactions (Banerjee et al., 1997). Treatment for tarantula bites is largely supportive, focusing on wound cleaning, tetanus prophylaxis, elevation of the affected limb, immobilization, and the use of oral analgesics as necessary.
This research aims to provide a comprehensive analysis of the genus Chilobrachys, focusing on their behavior, geographic distribution, habitat preferences, and medical significance. The research also investigates bite cases, including clinical effects on both humans and domestic animals, and aims to establish a treatment protocol for Chilobrachys envenomation, particularly in regions where they are most commonly found. Through this work, we hope to improve our understanding of the medical importance of these spiders and enhance public health responses to Chilobrachys bites.
2 Materials and methods
2.1 Occurrence points data
The occurrence points for Chilobrachys species across Asia were sourced from the Global Biodiversity Information Facility (GBIF) database (GBIF, 2024), various social media platforms, and direct personal field observations. Initially, the dataset comprised 940 individual occurrence points. To minimize geographical sampling bias, a spatial thinning technique was applied, utilizing a 1.5-km × 1.5-km grid in accordance with the methodology outlined by Mukherjee and Mondal (2023). This process was conducted using the spThin package in R version 4.2.2. Additionally, points located over marine environments or those lacking essential environmental data were manually removed. Further exclusion of points with missing environmental variables was carried out using the “suppress similar visual warning” option during the model execution. After these refinements, a total of 921 valid occurrence points were retained for use in the final modeling process (Figure 1). The species’ country-level distribution was referenced from the WSC 2024 database (Figure 1).
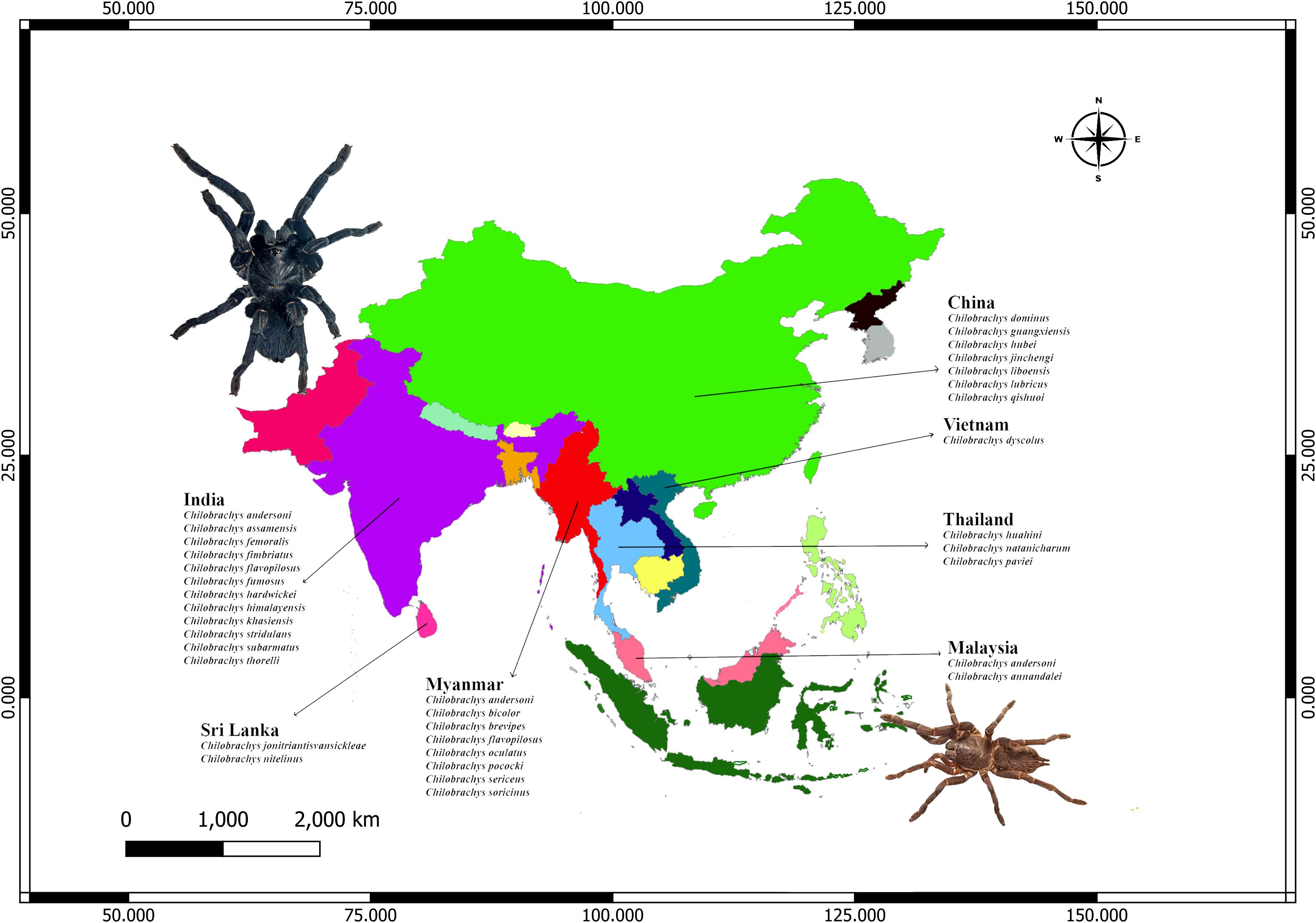
Figure 1. Geographic distribution of Chilobrachys species across South and Southeast Asia, based on compiled taxonomic literature and regional biodiversity records. Countries with confirmed Chilobrachys presence include India, Sri Lanka, Myanmar, China, Vietnam, Thailand, and Malaysia. India shows the highest diversity with 14 recorded species, followed by Myanmar and China.
2.2 Behavioral observations and natural history
For the detailed documentation of behavioral patterns and the natural history of the specimens, an opportunistic spot survey was conducted (Mirza and Mondal, 2018; Mondal et al., 2020b). Specimens were then identified in a controlled laboratory environment. The tools and equipment employed during this research included forceps, petri dishes, 70% ethanol solution, measuring scales, cameras, torches, rubber gloves, pens, notebooks, specimen containers, hygrometers, thermometers, and stereo zoom microscopes (Mirza and Mondal, 2018; Mukherjee and Mondal, 2020).
2.3 Environmental data
Environmental variables, including both bioclimatic and topographic factors, were obtained from the WorldClim v2.1 database (http://www.worldclim.org). All data were downloaded as GeoTiff files with a resolution of 30 arc-seconds. QGIS (http://qgis.osgeo.org/) was used to extract the relevant environmental variables specific to the Asian region. The bioclimatic variables represent long-term averages of monthly temperature and precipitation, capturing seasonal patterns and extremes (Kumar et al., 2021).
To prevent overfitting in the ecological model due to multicollinearity among the variables, the ENM Tools package (Warren et al., 2021) in R v4.2.2 was employed to calculate correlations between the raster layers. Environmental variables showing a correlation coefficient of ±0.8 or higher were removed from the analysis (Cao et al., 2021). The remaining uncorrelated variables were then selected for developing the final model.
2.4 Modeling overview
To predict the distribution of Chilobrachys species, maximum entropy modeling (MaxEnt) version 3.4.4 (https://www.cs.princeton.edu/schapire/maxent/) was employed. Out of the 19 initially considered bioclimatic variables, seven were ultimately retained to model the species’ distribution across Asia. The modeling parameters included a logistic output format, a regularization multiplier of 1, a maximum of 1,000 iterations, and a convergence threshold set at 0.00001 (Das et al., 2022). To control model complexity, the program’s auto features were utilized (Mukherjee et al., 2024). Model performance was assessed using a 10-fold cross-validation approach, dividing the occurrence data into 10 subsets. A 10-percentile training presence threshold was applied to enhance model accuracy.
The resulting habitat suitability map categorized the areas into four distinct distribution classes: NA (not suitable habitat: 0–0.2), Min (minimum suitable habitat: 0.2–0.4), Mod (moderate suitable habitat: 0.4–0.6), and Max (maximum suitable habitat: 0.6–1). The final distribution map was generated using QGIS version 3.28.
2.5 Bite documentation
Data on Chilobrachys spider bites were gathered over a 14-year span, from January 2010 to August 2024, using three different methods to document envenomations in humans.
2.5.1 Prospective data collection
Human bites by theraphosid spiders were systematically recorded as part of the broader research of Indian mygalomorph spiders (Mirza and Mondal, 2018).
2.5.2 Secondary data sources
Incidents were also compiled from published research papers and various news outlets.
2.5.3 Field surveys
Additional verified bite incidents were documented through spot surveys, where either photographs or detailed descriptions confirmed the occurrence of the bite.
Only verified cases were included in the research, adhering to established international protocols for confirmed spider bites (Isbister and Gray, 2002). To meet the criteria for verification, the following conditions were required:
Direct observation: The bite incident must have been directly witnessed.
Specimen identification: The offending spider needed to be captured during or immediately after the bite and submitted to an expert for taxonomic identification, or an adequate photographic record should be presented.
Symptom verification: The bite had to result in typical symptoms such as pain, discomfort, or other signs commonly associated with spider bites (Isbister and White, 2004; Vetter and Isbister, 2008).
For each verified bite incident, data were collected on demographics (age, gender, and location of the victim), details surrounding the bite (such as time, date, season, and exact location), and both local and systemic effects of the bite (onset, duration, and severity of symptoms). In certain instances, spiders were retrieved directly from the patient for identification purposes.
All specimens were identified according to the methodology outlined by Mondal et al. (2020a), and genus-level identification was consistently achieved, with species-level identification attempted wherever feasible, often in collaboration with expert arachnologists.
2.6 Treatment and management
First-aid treatment protocol has been developed in consultation with a trained, practicing physician for the Chilobrachys bite. Patients who will require transport to the hospital need intensive care in case of severe necrosis or secondary infection. We described our treatment procedure as successful (N = 3) for definitive bite cases.
3 Results
3.1 Distribution of Chilobrachys in Asia
Chilobrachys is widely distributed in the Indian subcontinent and Southeast Asia. India harbors 12 species, possibly due to its vast range of ecosystems making it a hotspot for this genus. Myanmar and China both have significant diversity (nine and seven species, respectively), playing major roles in the Chilobrachys genus’s Southeast Asian distribution. Thailand holds a middle ground with three species, while Sri Lanka and Malaysia have two species each. Vietnam has only one endemic species. Chilobrachys andersoni and Chilobrachys assamensis are common in northeastern regions like Assam and the Himalayan foothills. Chilobrachys fimbriatus, one of the better-known species, is native to Maharashtra, especially around forested areas like the Western Ghats. Despite being an island nation, Sri Lanka has two endemic species, Chilobrachys jonitriantisvansickeliae and Chilobrachys nitelinus. Myanmar is another region rich in Chilobrachys diversity, with nine recorded species, including C. andersoni (also found in India and Malaysia) and Chilobrachys bicolor. Its species also share similarities with those found in neighboring countries, reflecting some overlap in distribution. China has Chilobrachys dominus, Chilobrachys guangxiensis, Chilobrachys qishui, Chilobrachys jincheng, and Chilobrachys lubrica. Chilobrachys liboensis is particularly noted for its large size and aggressive temperament. Chilobrachys hubei is found in the central regions of China, showcasing a species that thrives in diverse environments from mountains to grasslands. While China’s species diversity is slightly less than that of India, its species are adapted to a wider range of ecological niches, from temperate forests to subtropical zones, distinguishing its Chilobrachys population. Vietnam hosts only one species Chilobrachys dyscolus, which is widespread and popular in the exotic pet trade due to its striking coloration and aggressive temperament. Thailand is the home of Chilobrachys huahini, Chilobrachys natanicharum, and Chilobrachys paviei. Malaysia hosts two species, Chilobrachys annandalei and Chilobrachys andersoni, with C. andersoni also found in India and Myanmar.
3.2 Natural history of Chilobrachys
Chilobrachys spiders prefer moist and humid environments, commonly found near water bodies such as rivers and streams. Their burrows are typically located in areas with semiarid, lateritic soil, where conditions are ideal for burrowing and creating silk-lined tunnels.
The burrows of Chilobrachys are often branched and lined with silk. These burrows serve as a home and a hunting ground. The spiders remain hidden within, waiting for prey to pass by. Juveniles of this genus differ slightly in their choice of habitat, often constructing their nests in elevated areas, such as tree trunks, and using disorganized zigzag webs at the entrance to block access. This protective web serves to shield the spider from potential predators and environmental threats.
Chilobrachys are primarily ambush predators, relying on the element of surprise to capture their prey. Both male and female spiders remain concealed within their burrows or crevices in tree trunks, waiting for unsuspecting prey to come within reach. This nocturnal hunting strategy is common across Theraphosidae spiders (Herberstein, 2011). However, males exhibit different behavior during the mating season, which is from March to July in the subcontinent. Driven by reproductive instincts, they leave their burrows and adopt a more active, wandering, or cursorial hunting style. The shift in predatory strategy during the mating season reflects the increased mobility of males as they search for potential mates.
Theraphosidae, including Chilobrachys, are opportunistic carnivores, preying on a wide range of organisms. Their diet includes a diverse array of insects, such as Orthopterans, Dipterans, Dictyopterans, Hymenopterans, and Lepidopterans (Mondal et al., 2020a; Mukherjee and Mondal, 2023). Additionally, they are known to prey on small vertebrates, including amphibians like frogs and toads, reptiles like small lizards, and even small mammals like rodents. Chilobrachys are strict carnivores, rejecting scavenging opportunities. Cannibalism is also observed in this genus, particularly among females, who may consume males following a failed mating attempt or when food resources are scarce (Herberstein, 2011). This wide dietary range highlights the spider’s adaptability and role as a versatile predator in its ecosystem.
The behavioral ecology and natural history of Chilobrachys reflect a well-adapted species that thrives in humid, moist environments but is flexible enough to occupy semiarid areas. Their burrowing, ambush predation, and mating rituals are finely tuned to their environment. However, the declining populations and the inherent risks in their reproductive behaviors pose significant challenges for the future survival of these spiders. A detailed understanding of their behavioral patterns, habitat preferences, and mating dynamics provides crucial insights into their ecological role and the factors affecting their conservation status.
3.3 Habitat suitability of Chilobrachys in Asia
The highest probability zones for Chilobrachys species occur in the northeastern parts of India, extending into the foothills of the Himalayas, and along the Western Ghats. This aligns with known Chilobrachys species like Chilobrachys assamensis, C. fimbriatus, and Chilobrachys hardwickei that are adapted to humid, tropical, and mountainous environments. Moderate occurrence is predicted in central and southern regions of India, indicating that Chilobrachys species thrive in less ideal but still favorable conditions. The northern plains and the drier regions of western India show lower likelihoods for Chilobrachys occurrence, correlating with the arid climate, where precipitation is limited (BIO14, BIO19). The central and southern parts of Sri Lanka show maximum probability. This corresponds well with C. jonitriantisvansickeliae and C. nitelinus, which prefer tropical climates with consistent rainfall and warm temperatures. Northern Sri Lanka shows a lesser probability, indicating that the Chilobrachys species are more concentrated in the wet, forested areas. Northern Myanmar, particularly in mountainous and forested regions, shows a high occurrence probability. This is expected given the presence of multiple species like C. andersoni and Chilobrachys pococki, which thrive in warm, moist environments with significant seasonal rainfall. The southern regions of China, bordering Myanmar, show moderate probability, reflecting the potential habitat for species like C. guangxiensis and Chilobrachys jinchengensis, which adapt to subtropical climates. However, the map (Figure 2) suggests that most of central and northern China is unsuitable for Chilobrachys species due to harsher climatic conditions, such as colder winters and lower precipitation (BIO5 and BIO19). Northern Vietnam and western Thailand are marked as high-probability areas. This makes sense for species like C. dyscolus in Vietnam and C. huahini in Thailand, which favor humid, tropical environments with consistent rainfall, as suggested by BIO18 and BIO14. Other areas, particularly central Vietnam and Thailand, still show moderate suitability, reflecting the potential spread of species to less humid areas, although with lower population densities. The central and northern parts of peninsular Malaysia show high probabilities, which would suit species like C. annandalei that prefers stable tropical environments. The southern regions show minimal likelihood for Chilobrachys presence, possibly due to less ideal climatic conditions, such as higher isothermality (BIO3) or lower precipitation during certain months (BIO14).
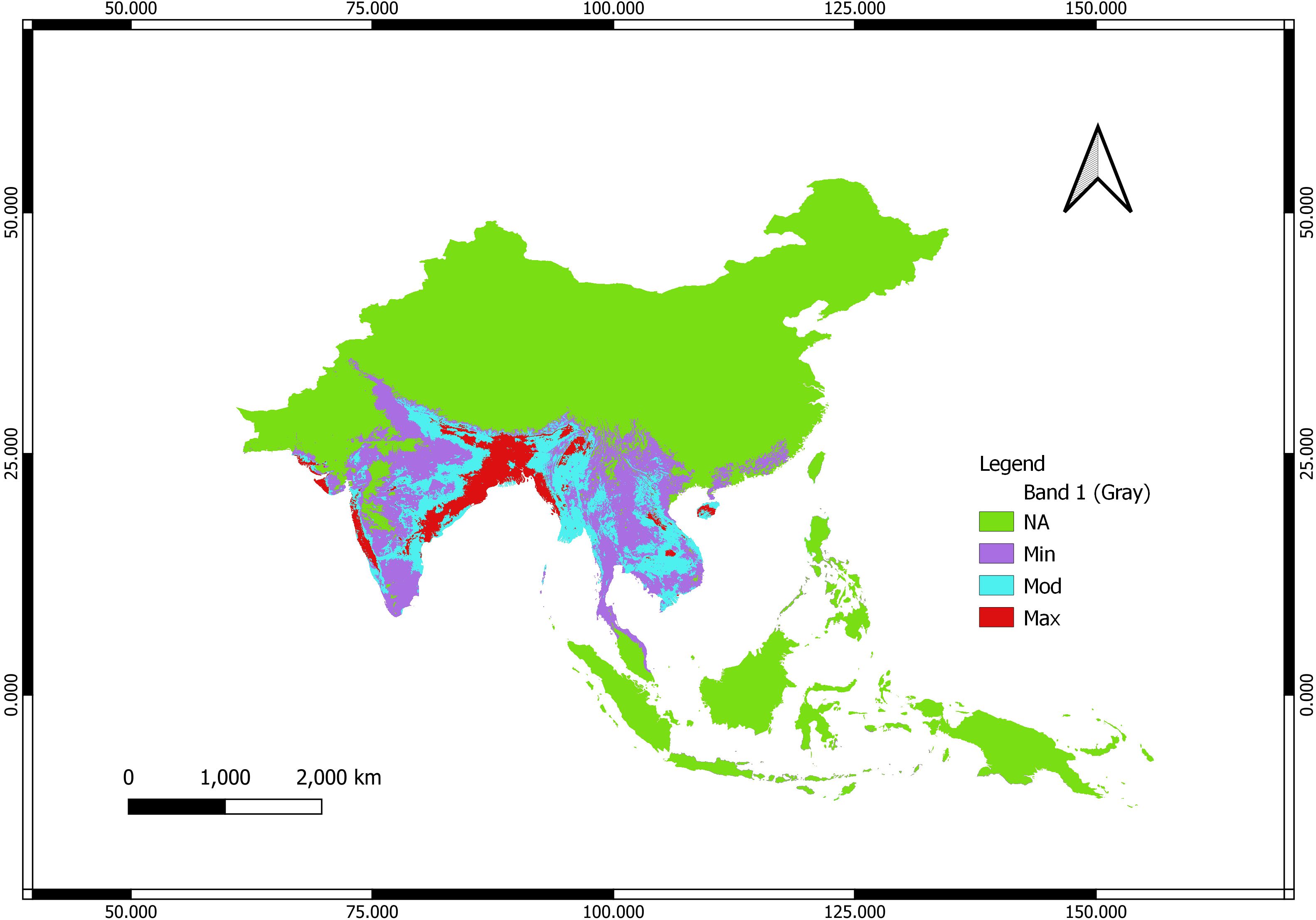
Figure 2. Habitat suitability of Chilobrachys color-coded according to the following legend: NA (green): no predicted occurrence of Chilobrachys species; Min (purple): minimum probability of occurrence; Mod (blue): moderate probability of occurrence; Max (red): maximum probability of occurrence.
The areas highlighted in red (734,216 km2) (Max) are regions with high precipitation (BIO18, BIO19), including tropical and subtropical zones, which support tarantula species that rely on consistent moisture. These areas also tend to have moderate (1,799,160 km2) to warm temperatures year-round (BIO5), another critical factor in Chilobrachys survival. Regions with moderate seasonality in temperature and precipitation (BIO15) appear to support moderate probabilities (blue) for Chilobrachys presence, suggesting that while these regions are suitable, the species might not thrive as abundantly. Areas with minimal precipitation, extreme seasonal variations, or extreme temperatures (such as central or northern China) show minimal probabilities, indicating that the species cannot adapt to these harsh environments.
3.4 Influence of bioclimatic variables on the distribution of Chilobrachys
The response curves (Figure 3) illustrate how Chilobrachys species respond to each bioclimatic variable, indicating the likelihood of the species’ presence based on the value of each variable. BIO2 represents the mean diurnal range (6.08°C to 14.85°C) (max temp − min temp), the curve shows a peak at approximately 10°C, after which suitability sharply decreases. Chilobrachys species seem to prefer regions with a moderate daily temperature range, where the difference between the hottest and coldest parts of the day is not too extreme. This suggests that regions with stable day–night temperatures are more suitable for Chilobrachys species, such as tropical or subtropical zones. BIO3 stands for isothermality response (24.23% to 84.92%), showing a slight peak at approximately 30%–50% isothermality, indicating that Chilobrachys species prefer areas where temperature variability between days and seasons is low. Stable climates, like those in Southeast Asia, are more conducive to species survival. BIO5 is the max temperature of the warmest month (17°C to 43.3°C), the response curve of which peaks at approximately 30°C–35°C but sharply declines at higher temperatures. This indicates that Chilobrachys species thrive in moderately warm regions but cannot tolerate extreme heat. BIO14 (0 mm to 157 mm) denotes precipitation of the driest month, which explains the sharp decline in species occurrence probability when precipitation falls below 50 mm, indicating a strong sensitivity to arid conditions during the driest month. Chilobrachys species depend heavily on moisture for survival, especially for burrow construction and maintaining suitable humidity levels. This makes regions with sufficient rainfall, even during the dry season, highly suitable. BIO15, pertaining to precipitation seasonality, directs a clear positive trend, with species occurrence increasing as precipitation seasonality rises. Chilobrachys species seem to prefer regions with distinct wet and dry seasons, as long as the overall precipitation levels are sufficient (BIO14). BIO18 (47 mm to 4,378 mm) denotes precipitation of the warmest quarter, which peaks at approximately 2,000–3,000 mm of rainfall during the warmest quarter, suggesting that Chilobrachys species favor regions with heavy rainfall during the warmest periods. This helps maintain adequate moisture in burrows and surrounding vegetation. BIO19 (2 mm to 1,887 mm), which is the precipitation of the coldest quarter, shows that Chilobrachys species can tolerate a wide range of cold-quarter precipitation, although higher precipitation (above 1,000 mm) is generally preferred. This variable is more relevant in regions with cooler winters, such as the foothills of the Himalayas, where species like C. assamensis and Chilobrachys fumosus are found.

Figure 3. The response curves of bioclimatic variables [BIO2 = mean diurnal range (mean of monthly (max temp − min temp)), BIO3 = isothermality (BIO2/BIO7) (×100), BIO5 = max temperature of the warmest month, BIO14 = precipitation of the driest month, BIO15 = precipitation seasonality (coefficient of variation), BIO18 = precipitation of the warmest quarter, BIO19 = precipitation of the coldest quarter] with distribution of Chilobrachys species.
The jackknife test of variable importance indicates that BIO5 (max temperature of the warmest month) consistently emerges as the most important variable across all analyses. This suggests that temperature extremes, particularly during the hottest periods of the year (17°C to 43.3°C), are a significant limiting factor for the distribution of Chilobrachys species. The areas where the maximum temperature exceeds their tolerance (above 35°C) will have lower habitat suitability. BIO19 (precipitation of the coldest quarter) and BIO18 (precipitation of the warmest quarter) are also important, indicating that precipitation during both the warmest and coldest months plays a crucial role in determining species presence. Regions with balanced rainfall throughout the year (moderate BIO18 and BIO19 values) are more likely to support Chilobrachys populations. BIO15 (precipitation seasonality), BIO2 (mean diurnal range), and BIO14 (precipitation of the driest month), though less significant than temperature and precipitation extremes, still contribute meaningfully to the model’s accuracy. They provide additional details on where Chilobrachys species can thrive, particularly in areas with less seasonal variability and more stable moisture conditions.
The ROC curve (Figure 4) demonstrates that the model’s accuracy is high, with a strong predictive power, and is effective in distinguishing between suitable and unsuitable habitats for Chilobrachys species with an approximate AUC value of 0.887. The curve shows that the model correctly predicts species presence in most cases, with relatively few false positives. This reinforces the idea that the bioclimatic variables selected are well-suited to predict Chilobrachys distributions.
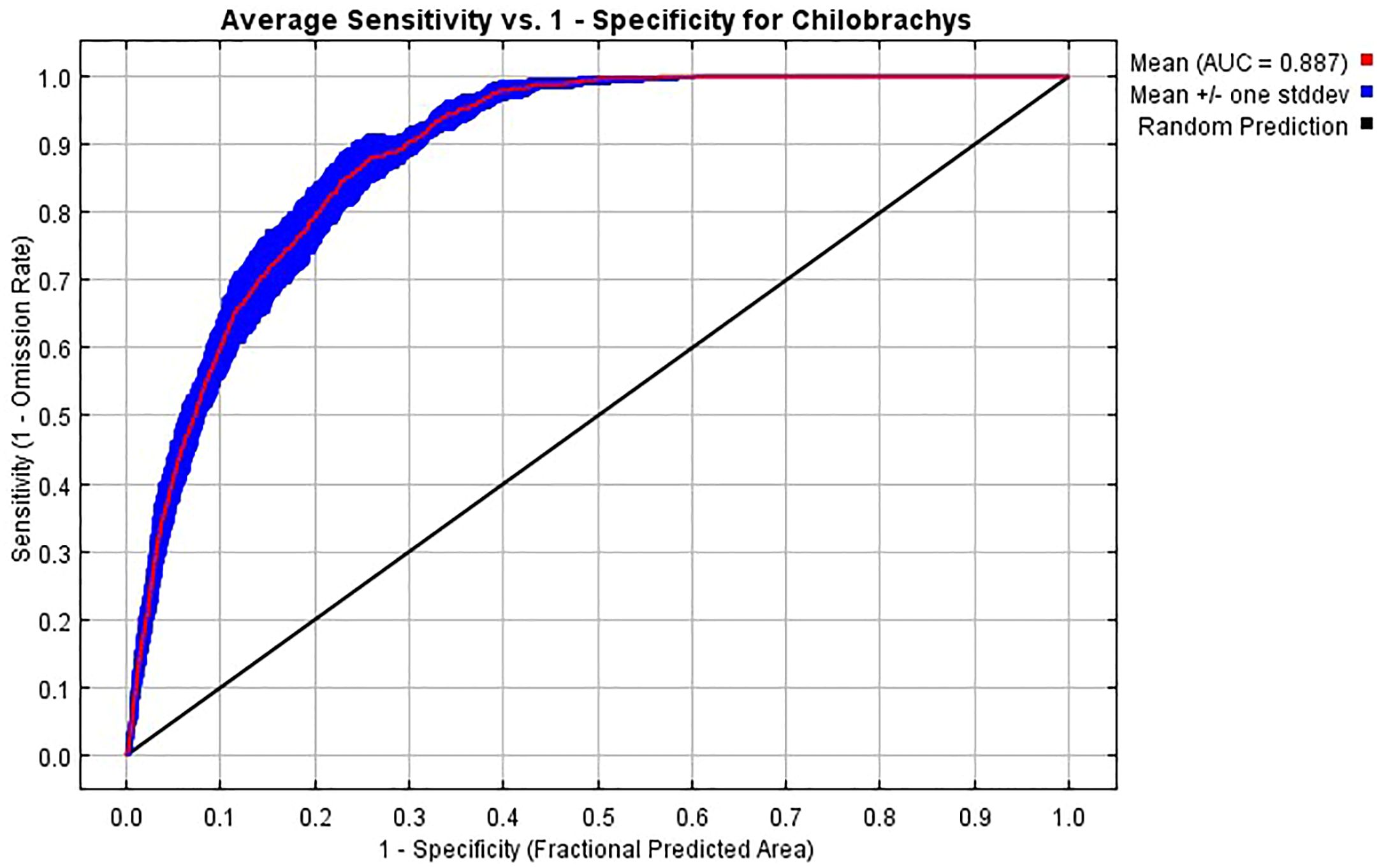
Figure 4. Receiver operating characteristic (ROC) curve showing the average model performance for predicting the distribution of Chilobrachys species based on environmental variables. The curve plots the average sensitivity (true positive rate) against 1−specificity (false positive rate) across multiple model replicates. The mean area under the curve (AUC) is 0.887, indicating a high predictive accuracy. The pink line represents the mean ROC curve, the blue shaded area indicates ±1 standard deviation, and the black diagonal line represents the random prediction threshold. The AUC value above 0.85 demonstrates a strong model performance in distinguishing suitable versus unsuitable habitats for Chilobrachys.
3.5 Chilobrachys bite
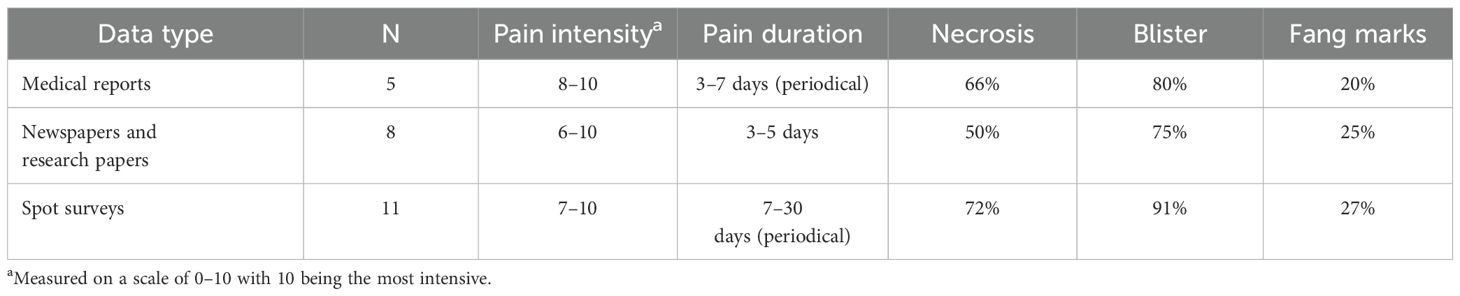
There were N = 24 confirmed bites by Chilobrachys spiders in humans, and five of these, which were followed up, were from medical reports. Among these five cases, three were directly observed by the authors. There were eight definite bites based on data from newspapers and research papers that provided a clear identifiable photograph of Chilobrachys (Table 1), and there were 11 more definitive bite cases according to spot surveys (Table 1). The most number of bites occurred in June (N = 7) followed by April (N = 5), May (N = 4), and July (N = 3) (Figure 5).
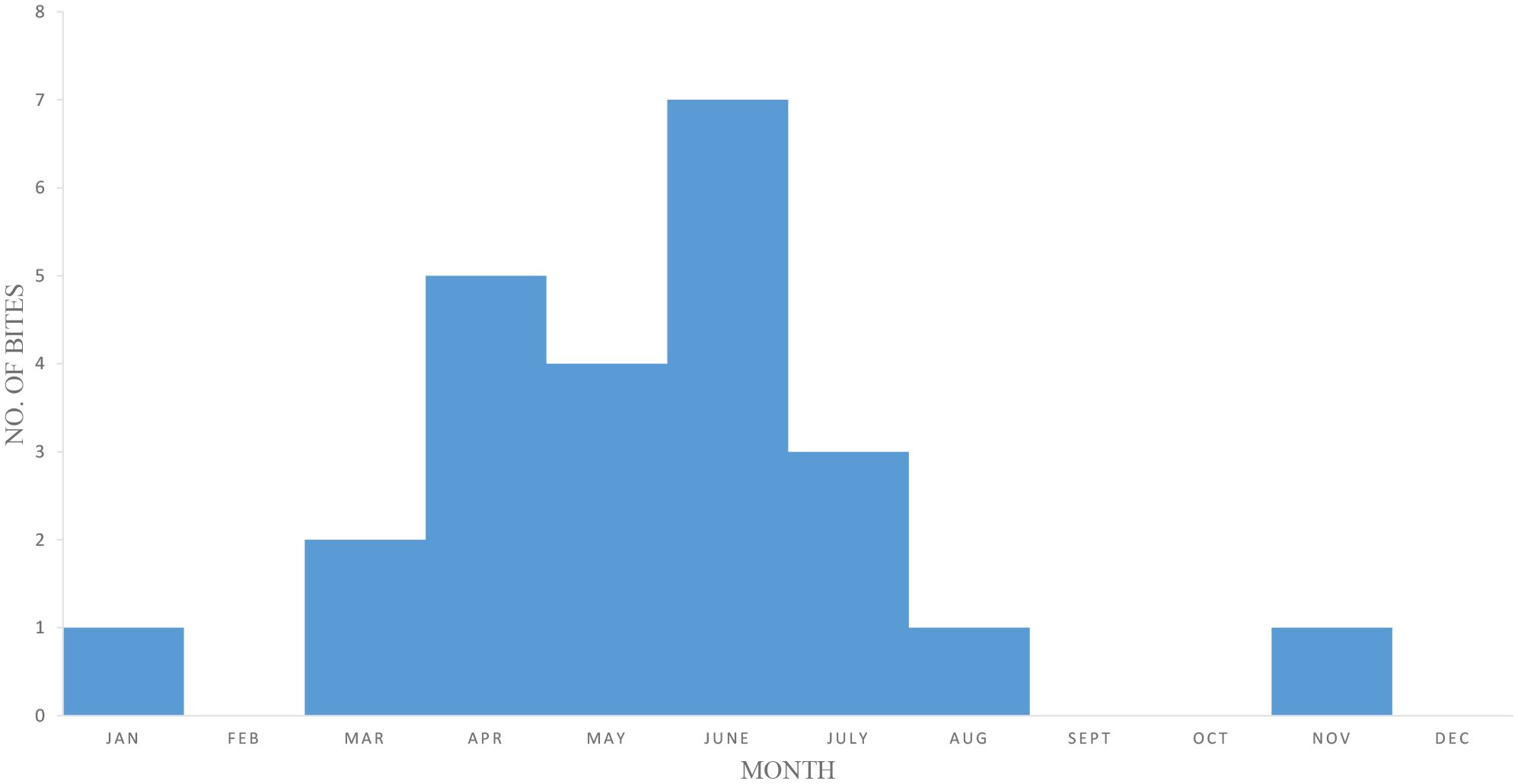
Figure 5. Temporal and seasonal distribution of Chilobrachys spider bite incidents from 2010 to 2024. A total of 24 confirmed envenomation cases were recorded, with the highest frequency observed during the pre-monsoon season (March to July), suggesting increased spider activity or human exposure during this period. Year-wise distribution of reported cases is as follows: 3 (2010), 1 (2011), 4 (2012), 0 (2013–2014), 2 (2015), 1 (2016), 0 (2017), 2 (2018), 2 (2019), 0 (2020–2021), 4 (2022), 3 (2023), and 2 (2024). The data indicate intermittent surges in bite reports, possibly linked to climatic variability and seasonal behavior of the genus Chilobrachys.
Medical reports (N = 5) indicated that pain intensity ranged from 8 to 10, suggesting high levels of pain experienced by individuals. Newspapers and research papers (N = 8) reported slightly broader variations, with pain intensity ranging from 6 to 10. Spot surveys (N = 11) revealed pain intensities between 7 and 10, also showing severe levels of pain, but the range is narrower compared to research paper reports. Overall, pain intensity appears consistently high across all sources, although newspapers and research papers indicate slightly more variation in pain levels. Medical reports noted that pain lasted between 3 and 7 days, with the duration being periodical, implying intermittent episodes of pain rather than continuous discomfort. Newspapers and research papers showed a slightly shorter pain duration of 3 to 5 days, but the periodicity of pain was not mentioned there. Spot surveys suggested a significantly longer period of pain, ranging from 7 to 30 days, again with periodical intervals of pain. Medical reports recorded necrosis in 66% of cases. Newspapers and research papers reported a lower frequency, with 50% of cases showing signs of necrosis. Spot surveys indicated the highest incidence, with 72% of cases showing necrosis. Medical reports showed that 80% of individuals experienced blister formation. Newspapers and research papers indicated that 75% of cases resulted in blisters. Spot surveys indicated the highest occurrence, with 91% of cases showing blisters. Medical reports showed that 20% of cases displayed fang marks, whereas newspapers and research papers reported fang marks in 25% of cases and spot surveys indicated fang marks in 27% of cases, slightly higher than the other two sources (Table 1).
3.5.1 Case report 1
A 65-year-old farmer from Mallarpur Birbhum, West Bengal presented to the emergency unit following a Chilobrachys bite on the left leg during work at a paddy field. When he presented to the local health center, he had swelling of the left leg with local erythema. He was given a tetanus toxoid injection and conservative treatment with a 20-min WBCT. There were no symptoms of neurotoxicity or envenomation, and the WBCT showed normal clotting. Local swelling and blister formation developed with blackening of the skin (Figure 6). Local ice application and elevation of the limbs relieved the pain momentarily. He did not have any disease like diabetes or chronic renal failure. Laboratory tests revealed blood urea 36 mg/dL with creatinine 1.8 mg/dL. Urine showed protein 1+ and hemoglobin 10.2 mg/dL. Blood LDH was 360 IU/L (N) and total leukocyte count was 18,700/cmm. Conservative management included intravenous fluid along with infusion of linezolid 600 mg BD and levofloxacin 500 mg. Local wound management involved cleansing with normal saline and superoxide solution and applying L-lysine hydrochloride and nadoxin ointment. Dead and necrotic tissue was removed regularly (Figure 6). Surgical consultation was conducted, and due to suspected compartment syndrome, debridement was done with regular dressing. After that, the patient started to recover. The case was thus finally clinically diagnosed as a Chilobrachys bite, resulting in cellulolytic change and the development of compartment syndrome. On follow-up (1 month), he had no more complications, and the wounds had almost healed (Figure 6).
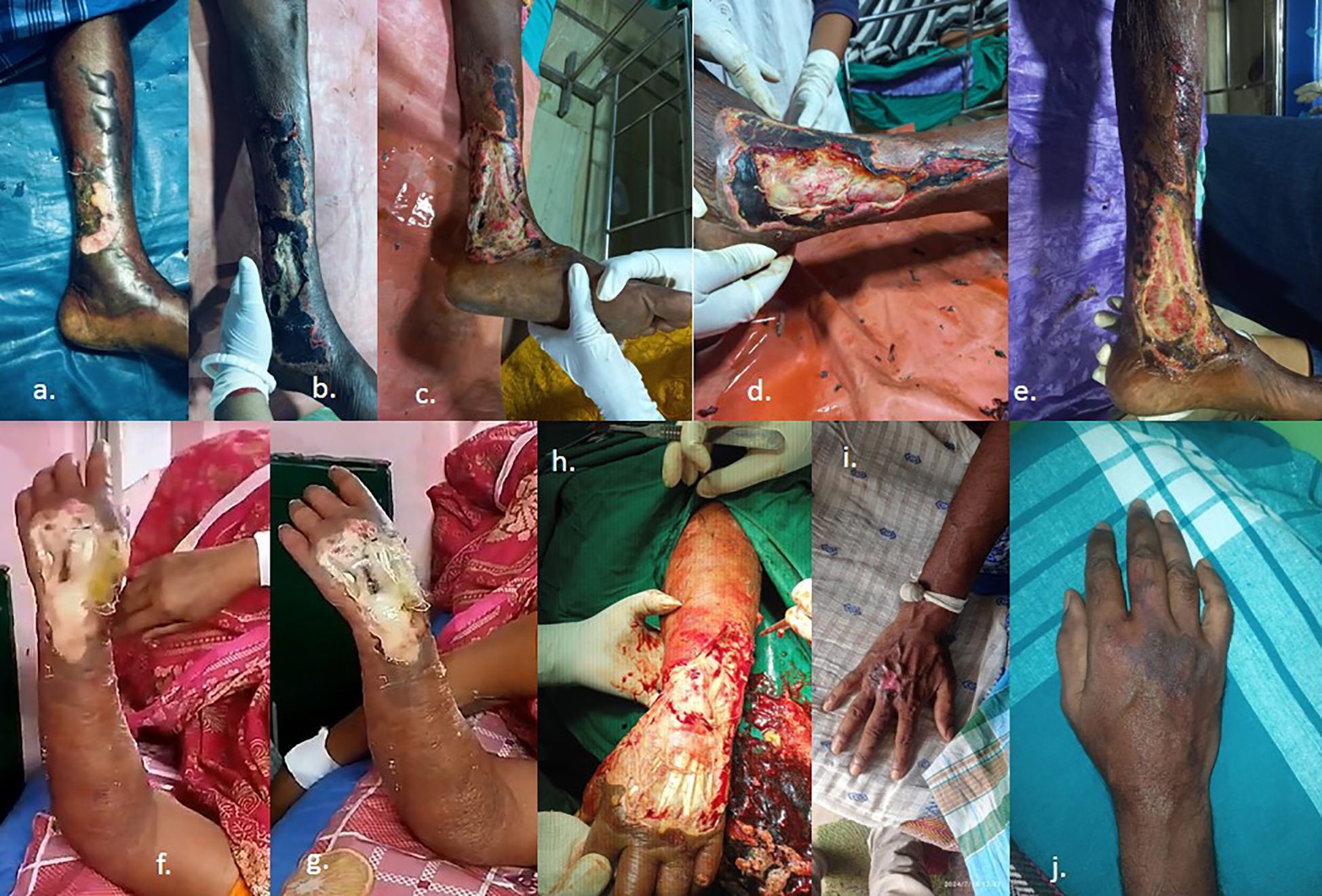
Figure 6. Clinical progression of envenomation of verified Chilobrachys bites. Case 1 (a–e): (a) Initial presentation with localized necrosis and skin discoloration on the lower leg. (b) Expansion of the necrotic area with blackened eschar formation. (c) Onset of tissue sloughing and deeper dermal involvement. (d) Advanced ulceration exposing underlying structures during debridement. (e) Chronic wound with extensive tissue loss and delayed healing. Case 2 (f–h): (f) Blistering and yellowish slough formation on the wrist. (g) Progression to full-thickness skin necrosis. (h) Surgical debridement showing exposed muscle and tendon structures. Case 3 (i–j): (i) Localized necrotic lesion on the dorsum of the hand with central eschar. (j) Marked vascular congestion and diffuse cyanosis extending to the fingers.
3.5.2 Case report 2
A 48-year-old female housewife from Tarapith, Birbhum, West Bengal presented to the emergency unit following a bite from a large hairy spider (identified as Chilobrachys sp. after physical verification of the spider specimen) on the left forearm and dorsal aspect during cooking. When she presented to the local health center, she had swelling of the left forearm and dorsal aspects of the hands with local erythema. She was given a tetanus toxoid injection and conservative treatment with a 20-min WBCT. There were no symptoms of neurotoxicity or envenomation, and the WBCT showed normal clotting. Local swelling and blister formation developed with blackening of the skin (Figure 6). Local ice application and elevation of the limbs relieved the pain momentarily. She did not have any disease like diabetes or chronic renal failure. Laboratory tests revealed blood urea 34 mg% with creatinine 1.4 mg% and hemoglobin 9.8 gm%, and the total leukocyte count was 16,500/cmm. Conservative management involved intravenous fluid along with infusion of linezolid 600 mg BD and levofloxacin 500 mg. Local wound management included cleansing with normal saline and superoxide solution and applying L-lysine hydrochloride and nadoxin ointment. Dead and necrotic tissue was removed regularly (Figure 6). Surgical consultation was made due to a cellulolytic change in the necrotic area of the left forearm of the dorsal aspect of the hand and compartment syndrome was suspected. Wound debridement and fasciotomy were performed under general anesthesia, and NPWT dressing was applied intermittently for 5 days, followed by regular dressing under proper aseptic maintenance. After that, the patient started to recover. The case was thus clinically diagnosed as a Chilobrachys spider bite. On follow-up (2 months), she has had no more complications. The wounds had almost healed, and proper functioning of the forearm and hands was observed (Figure 6).
3.5.3 Case report 3
A 57-year-old farmer from Baghmundi, Purulia, West Bengal presented to the emergency unit following a bite from a large hairy spider (identified as Chilobrachys sp. after physical verification of the spider specimen) on the right middle finger in the evening during work on a paddy field. When he presented to the local health center, he had swelling of the right hand with local erythema and a painful burning sensation radiating up to his shoulder. He was given a tetanus toxoid injection and conservative treatment with a 20-min WBCT. There were no symptoms of neurotoxicity or envenomation, and the WBCT showed normal clotting. Moreover, there was local swelling with blackening of the skin but no blisters (Figure 6). Local ice application and elevation of the limbs relieved the pain momentarily. He did not have any disease like diabetes or chronic renal failure. Laboratory tests revealed blood urea 38 mg/dL with creatinine 1.3 mg/dL and hemoglobin 10.8 mg/dL, and the total leukocyte count was 11,500/cmm. Conservative management involved intravenous fluid along with infusion of linezolid 600 mg BD and levofloxacin 500 mg and injection with promethazine and gabapentin 300 mg BD. Local wound management involved cleansing using normal saline and applying magnesium sulfate compression. After that, the patient started to recover. The case was thus clinically diagnosed as a spider bite and documented by a mobile photograph of a dead tarantula. On follow-up (10 days), he had no more complications. The wounds had almost healed, and there was proper functioning of the forearm and hands.
3.5.4 Medical symptoms of Chilobrachys bite
The bite of a Chilobrachys tarantula may present with varying degrees of local and systemic symptoms due to its cytotoxic and neurotoxic venom. Symptoms can develop immediately or be delayed up to 30 h post-bite. Here is a breakdown of the common symptoms:
3.5.4.1 Local symptoms
1. Pain: Immediate or delayed severe localized periodical pain at the bite site.
2. Pruritus (itching): Local itching around the bite area; may progress to generalized body pruritus.
3. Swelling: Progressive swelling at the bite site, which can extend to the surrounding limb (Ghosh et al., 2015).
4. Erythema: Redness and inflammation around the bite area form blisters.
5. Tissue necrosis: In severe cases, local tissue necrosis may develop (Banerjee et al., 1997).
6. Fang marks: Single or double fang marks may be visible at the bite site, but in most cases, fang marks are not visible.
7. Secondary infection: If not properly cleaned, there is a risk of bacterial infection at the bite site.
8. Abscess formation: In delayed cases, abscesses may develop.
3.5.4.2 Systemic symptoms
1. Dyspnea (breathing difficulty): This may result due to an allergic reaction.
2. Systemic pruritus: Itching may spread beyond the local bite area.
3. Fever: Mild to high fever may develop in some cases.
4. Anaphylaxis: In rare cases, severe allergic reactions may occur, causing anaphylaxis, which can be life-threatening.
5. Blackish urine (hemoglobinuria): Rarely, the bite may induce hemolysis and cause hemoglobin in the urine (Paul et al., 2012).
6. Secondary infection: Due to unhygienic treatment, severe secondary infection followed by gangrene may occur (Banerjee et al., 1997).
3.5.4.3 Primary treatment method for Chilobrachys bite
Initial treatment focuses on symptom management, preventing infection and addressing systemic reactions. The RICE protocol (rest, ice, compression, elevation) is essential in early care (Van Den Bekerom et al., 2012; Wang and Ni, 2021).
3.5.4.4 Immediate first aid
1. Rinse the bite area: Wash the bite area thoroughly with soap and water to reduce the risk of secondary infection and remove any venom.
2. Ice application: Apply ice packs to the bite site to reduce pain and swelling (15–20 min on, 15–20 min off).
3. Rest and elevation: Rest the affected limb and elevate it to decrease swelling.
4. Pain relief: Administer NSAIDs like naproxen or ibuprofen for pain control.
5. Tetanus prophylaxis: Administer a tetanus toxoid injection if the patient’s immunization status is unclear.
3.5.4.5 Allergic reaction management
1. Antihistamines: Administer antihistamines such as pheniramine or levocetirizine to control itching and mild allergic reactions.
2. Corticosteroid cream: Apply 1% hydrocortisone cream to reduce localized itching and inflammation.
3. Systemic allergic reaction: In case of severe allergic reactions or anaphylaxis, administer epinephrine and follow emergency protocols.
3.5.4.6 Clinical treatment protocol for Chilobrachys bite
Patients presenting with Chilobrachys bites should be monitored for a minimum of 24 h, especially if there is a history of systemic symptoms or severe local reactions. Follow-up should extend to a week, as symptoms may develop late.
3.5.4.7 Initial assessment
History: Gather detailed information regarding the time of bite, description of the spider, and the progression of symptoms.
Physical examination:
- Check vital signs (blood pressure, heart rate, temperature).
- Inspect and palpate the bite site for erythema, swelling and blister, necrosis, and fang marks.
- Conduct a neurological assessment to rule out systemic complications.
3.5.4.8 Monitoring and admission
Observation for 24 h: All patients should be monitored for at least 24 h, as symptoms may have a delayed onset. Follow-up is necessary for 7 days to check for late-developing symptoms.
Admission criteria:
- Severe swelling or necrosis.
- Signs of anaphylaxis.
- Evidence of compartment syndrome or systemic reactions such as hemolysis or renal failure.
3.5.4.9 Laboratory investigations
Complete blood count (CBC): To assess for leukocytosis, hemolysis, or infection.
Coagulation profile: Monitor for coagulopathy in severe cases.
Renal function tests: In cases of suspected hemolysis, check blood urea, creatinine, and urine analysis (for hemoglobinuria).
Liver function tests (LFTs): To check for systemic involvement.
Urine examination: Look for signs of hemoglobinuria, proteinuria, or infection.
3.5.4.10 Treatment protocols
3.5.4.10.1 Pain management
- NSAIDs: Oral diclofenac or aceclofenac for pain and inflammation.
- Local analgesics: Lidocaine or topical anesthetics if pain is severe.
3.5.4.10.2 Antihistamine therapy
- Oral pheniramine (three times a day) or levocetirizine (at bedtime) for pruritus.
- Consider adding ranitidine 150 mg twice daily as an H2 receptor blocker to reduce histamine-related effects.
3.5.4.10.3 Swelling and inflammation
- Ice and compression dressings: Apply MgSO4 and glycerin compression dressings to reduce swelling.
- Elevation: Elevate the limb to minimize edema.
3.5.4.10.4 Infection control
- Topical antibiotics: Apply mupirocin or other topical antibiotics to the bite area to prevent infection.
- Oral/IV antibiotics: If there are signs of secondary infection (fever, abscess, pus), prescribe antibiotics based on culture results.
3.5.4.10.5 Severe local necrosis
- Surgical debridement: In cases of significant necrosis, perform debridement to remove dead tissue.
- Consider fasciotomy if there is a risk of compartment syndrome due to swelling (Paul et al., 2012).
3.5.4.10.6 Systemic symptoms
- Dialysis: In cases of acute renal failure or hemolysis, initiate hemodialysis (Paul et al., 2012).
- Blood transfusion: If hemoglobin drops significantly (hemoglobinuria or hemolysis), consider blood transfusion.
3.5.4.10.7 Corticosteroids
- In severe inflammatory reactions unresponsive to NSAIDs, consider oral corticosteroids (e.g., prednisolone at 1 mg/kg).
3.5.4.11 Discharge and follow-up
- Patients without severe symptoms can be discharged after 24 h of observation if stable.
- Follow-up at 7 days to monitor for delayed symptoms, tissue necrosis, or secondary infections.
- Educate patients on signs of worsening conditions and the importance of early return to the hospital if symptoms progress.
3.5.4.12 Preventive measures and education
- Public awareness: Educate the public about washing bite areas with soap and water, seeking early medical attention for unknown bites, and avoiding handling tarantulas.
- Health worker training: Train healthcare workers to identify tarantula bites and administer proper first aid.
Chilobrachys tarantula bites, although rarely fatal, can cause significant local tissue damage and systemic reactions. Early identification, thorough cleaning of the bite area, symptomatic management, and timely intervention in severe cases are essential for preventing complications.
4 Discussion
This research reveals that Chilobrachys tarantulas have a clearly defined geographic distribution, primarily within tropical and subtropical regions. The results of this research provide valuable insights into the bioclimatic preferences of Chilobrachys species and the clinical implications of their bites on humans. The bioclimatic modeling results demonstrate that Chilobrachys species are highly sensitive to specific environmental variables, particularly temperature and precipitation (Gaspar et al., 2019), which directly affect their distribution. The peak suitability for Chilobrachys in areas with a moderate diurnal temperature range (BIO2) and maximum temperature of the warmest month (BIO5) between 30°C and 35°C highlights the species’ preference for relatively warm, stable climates, such as those found in tropical and subtropical regions. The sharp decline in habitat suitability beyond 35°C indicates that extreme temperatures act as a significant barrier to their distribution, potentially limiting their range expansion in the face of global warming (Branco and Cardoso, 2020). Additionally, the importance of stable precipitation patterns (BIO14, BIO18, BIO19) underscores the reliance of Chilobrachys on consistent moisture levels, which are necessary for burrow maintenance and overall survival (Gaspar et al., 2019; Mondal et al., 2020b). This is particularly relevant in regions experiencing shifts in rainfall patterns due to climate change (Mondal et al., 2022), which could either expand or contract the species’ suitable habitats depending on the nature of the changes.
The genus is particularly sensitive to extremes in temperature and precipitation (Gaspar et al., 2019). Precipitation stability, especially during critical growth periods, plays a crucial role in burrow maintenance, mating, and survival. The highest habitat suitability is found in regions where rainfall remains consistent throughout the year, which aligns with the natural history of Chilobrachys—burrowing spiders that rely on stable, humid environments (Nanayakkara and Vishwanath, 2013).
Additionally, the peak distribution in pre-monsoon and early monsoon periods, with most bites occurring from April to July, reflects both human activity and spider behavior. Agricultural practices during this period increase human–spider interactions, as Chilobrachys are more likely to be active due to mating season or displaced from their burrows due to soil disturbances (Roy et al., 2018).
The jackknife test of variable importance confirms that temperature extremes and precipitation during key periods of the year are the most critical factors influencing Chilobrachys distribution. These findings align with other arachnid distribution studies, further emphasizing that climatic stability is crucial for species survival (Branco and Cardoso, 2020). Given the relatively high AUC value of 0.887 in the model’s ROC curve, the predictive power of this analysis is robust, providing a reliable framework for predicting Chilobrachys presence in unstudied regions.
From a medical perspective, the research presents comprehensive data on Chilobrachys bite cases, with a focus on the clinical manifestation of their bites. The findings highlight significant local symptoms such as severe pain, erythema, and necrosis, which are consistent across case reports, spot surveys, and media sources (Ghosh et al., 2015). The prevalence of necrosis and blister formation in more than 70% of cases underscores the cytotoxic nature of Chilobrachys venom, which leads to localized tissue damage, requiring timely medical intervention (Guerrero-Vargas et al., 2015; Moreno-García et al., 2024). These clinical outcomes are corroborated by case reports of cellulitic changes and compartment syndrome, indicating the serious potential (Banerjee et al., 1997; Paul et al., 2012) of these bites if left untreated.
A notable observation from the data is the variation in pain intensity (Mortari et al., 2012) and duration reported across different sources. The spot surveys show significantly longer pain durations, possibly due to delays in treatment in remote areas or inadequate management of the bite. This suggests that timely medical intervention is crucial in minimizing the long-term effects of Chilobrachys envenomation (Paul et al., 2012). Moreover, the seasonal distribution of bite incidents, with a peak in June and the majority occurring during the pre-monsoon period (April to July), is likely related to increased human activity in agricultural fields during this time, which overlaps with the tarantulas’ active phase (Roy et al., 2018).
The three detailed case reports further illustrate the clinical diversity of Chilobrachys bites, ranging from mild swelling and erythema to more severe complications such as necrosis and compartment syndrome. The successful recovery of patients through a combination of conservative management, surgical debridement, and careful wound management highlights the effectiveness of the current treatment protocols when properly administered. However, the potential for severe complications in delayed or untreated cases (Banerjee et al., 1997) emphasizes the need for public awareness and early medical intervention, particularly in rural areas where tarantulas are more commonly encountered (Roy et al., 2018). Clinically, Chilobrachys bites can cause severe local and systemic symptoms, necessitating prompt treatment to avoid complications.
5 Conclusion
This research provides a comprehensive assessment of Chilobrachys tarantulas, encompassing their biogeographic distribution, habitat preferences, natural history, and medical impact on humans. Ecological modeling demonstrates that Chilobrachys thrive in warm, humid environments with moderate temperature ranges and consistent rainfall, which align with their burrowing behavior and natural habitat. These spiders are predominantly distributed in tropical and subtropical regions, with agricultural zones posing the highest risk for human–spider interactions due to soil disturbance and increased activity during the pre-monsoon and early monsoon seasons.
The medical data gathered from confirmed bite cases highlight the clinical importance of Chilobrachys tarantula envenomation. Although rarely fatal, bites can result in severe local symptoms such as intense pain, necrosis, blistering, and tissue damage. Systemic symptoms, while less frequent, include dyspnea, fever, and anaphylaxis in extreme cases. The research case reports emphasize the need for early medical intervention to prevent complications such as compartment syndrome and secondary infections.
The findings underline the significance of public awareness in endemic regions and the need for healthcare training to manage spider bites effectively (Guerrero-Vargas et al., 2021). Early identification of Chilobrachys bites, combined with appropriate wound care and surgical management in cases of necrosis, can mitigate the morbidity associated with these envenomations. Furthermore, the habitat suitability analysis suggests potential shifts in Chilobrachys distribution due to climate change, which could expose new populations to these spiders and their venom.
This research not only advances our understanding of Chilobrachys tarantulas from both ecological and medical perspectives but also provides essential guidance for public health strategies and clinical treatment protocols. By addressing gaps in knowledge about the species’ distribution and medical significance, this research lays the groundwork for future studies aimed at mitigating the health risks posed by these spiders, especially in the context of environmental change and increased human–spider interactions. The findings have important implications for both biodiversity conservation, particularly in the face of climate change, and public health, especially in regions where human–tarantula interactions are frequent. Future research should focus on refining bioclimatic models with finer-scale data and expanding medical documentation to improve both ecological predictions and clinical outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans because patients came to the hospital for their treatment. We used their personal and medical data here with their written consent. No experiments or trials have been done that is why ethical approval was not required. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AM: Methodology, Writing – review & editing, Investigation, Software, Writing – original draft, Conceptualization, Supervision, Visualization, Data curation, Formal analysis. SD: Methodology, Formal analysis, Data curation, Investigation, Writing – review & editing. SM: Methodology, Software, Writing – original draft, Formal analysis, Data curation, Visualization. SS: Visualization, Conceptualization, Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are thankful to the Department of Zoology, The University of Burdwan, West Bengal; Department of Zoology, Government General Degree College, Mohanpur, Paschim Medinipur; and Prafulla Chandra Sen Government Medical College and Hospital, Arambag for providing the infrastructural facilities. The authors are also thankful to Mr. Dhiman Bhattacharya, Mr. Debabrata Biswas, Mr. Debomay Chanda, and Mr. Akash Chatterjee during the field research and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Banerjee K., Banerjee R., Mukherjee A., and Ghosh D. (1997). Tarantula bite leads to death and gangrene. Indian J. Dermatology Venereology Leprology 63, 125–126.
Branco V. V. and Cardoso P. (2020). An expert-based assessment of global threats and conservation measures for spiders. Global Ecol. Conserv. 24, e01290. doi: 10.1016/j.gecco.2020.e01290
Briggs E. J. and Hamilton C. A. (2024). What does the history of Theraphosidae systematics tell us about the future of tarantula taxonomy? Front. Arachnid Sci. 3, 1445731. doi: 10.3389/frchs.2024.1445731
Cao Z., Zhang L., Zhang X., and Guo Z. (2021). Predicting the potential distribution of Hylomecon japonica in China under current and future climate change based on Maxent model. Sustainability 13, 11253. doi: 10.3390/su132011253
Chen J., Zhao L., Jiang L., Meng E., Zhang Y., Xiong X., et al. (2008). Transcriptome analysis revealed novel possible venom components and cellular processes of the tarantula Chilobrachys jingzhao venom gland. Toxicon 52, 794–806. doi: 10.1016/j.toxicon.2008.08.003
Das N., Mondal A., and Mandal S. (2022). Maximum entropy modelling for predicting the potential distribution of methanogens in Sundarban mangrove ecosystem, India. Theor. Appl. Ecol. 2, 42–47. doi: 10.25750/1995-4301-2022-2-042-047
Dresler J., Avella I., Damm M., Dersch L., Krämer J., Vilcinskas A., et al. (2024). A roadmap to the enzymes from spider venom: Biochemical ecology, molecular diversity, and value for the bioeconomy. Front. Arachnid Sci. 3, 1445500. doi: 10.3389/frchs.2024.1445500
Escoubas P., Bernard C., Lambeau G., Lazdunski M., and Darbon H. J. P. S. (2003). Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels, Protein Science Vol. 12. 1332–1343.
Gaspar C., Grămadă I., Oprea C.-I., and Ailincăi L.-I. (2019). Comparative observations on the hygiene conditions of the artificial spaces populated with tarantulas. Lucrari Stiintifice: Seria Medicina Veterinara 62, 344.
GBIF (2024). GBIF occurrence download. Available online at: https://doi.org/10.15468/dl.3mnm6n (Accessed 28th August 2024).
Ghosh M., Chakrabortty S., Mahanayak B., Ghosh K., and Talukdar S. (2015). Spider bite, a case from eastern India with review of literature. Toxicol. Int. 22 (3), 153–155. doi: 10.22506/ti/2015/v22/i3/137641
Guerrero-Vargas J. A., Buitrago J. R., Ayerbe S., Daza Flórez E., and Beltrán J. T. B. (2015). Scorpionism and dangerous species of Colombia. Scorpion Venom 4, 245–272. doi: 10.1007/978-94-007-6404-0_22
Guerrero-Vargas J. A., Ríos A. M. R., Ospina M. L. B., González S. A., and Ordóñez L. A. C. (2021). ICTs use since an interdisciplinary approach for support the training on ophidism a public health problem. Rev. Ingenierías Universidad Medellín 20, 13–34. doi: 10.22395/rium.v20n39a1
Herberstein M. E. (2011). Spider behaviour: flexibility and versatility (Cambridge University Press, Shaftesbury Road, Cambridge, CB2 8EA, England).
Isbister G. and Gray M. (2002). A prospective study of 750 definite spider bites, with expert spider identification. Qjm 95, 723–731. doi: 10.1093/qjmed/95.11.723
Isbister G. K., Seymour J. E., Gray M. R., and Raven R. J. (2003). Bites by spiders of the family Theraphosidae in humans and canines. Toxicon 41, 519–524. doi: 10.1016/S0041-0101(02)00395-1
Isbister G. K. and White J. (2004). Clinical consequences of spider bites: recent advances in our understanding. Toxicon 43, 477–492. doi: 10.1016/j.toxicon.2004.02.002
Kumar D., Rawat S., and Joshi R. (2021). Predicting the current and future suitable habitat distribution of the medicinal tree Oroxylum indicum (L.) Kurz in India. J. Appl. Res. Medicinal Aromatic Plants 23, 100309. doi: 10.1016/j.jarmap.2021.100309
Liao Z., Cao J., Li S., Yan X., Hu W., He Q., et al. (2007). Proteomic and peptidomic analysis of the venom from Chinese tarantula Chilobrachys jingzhao. Proteomics 7, 1892–1907. doi: 10.1002/pmic.200600785
Lucas S. M., Da Silva P. Jr., Bertani R., and Cardoso J. C. (1994). Mygalomorph spider bites: a report on 91 cases in the state of Sao Paulo, Brazil. Toxicon 32, 1211–1215. doi: 10.1016/0041-0101(94)90350-6
Mirza Z. A. and Mondal A. (2018). A new genus Gravelyia with two species of the family Nemesiidae (Araneae: Mygalomorphae) from India. Acta Arachnologica 67, 43–48. doi: 10.2476/asjaa.67.43
Mondal A., Chanda D., Patra S., and Barman M. (2020a). Phrynus whitei (Gervai) from the type locality Burdwan is actually Charinus bengalensis (Gravel). ZOO’S PRINT 35, 1–3.
Mondal A., Chanda D., Vartak A., and Kulkarni S. (2020b). A field guide to the spider genera of India. Ayan Mondal, 1–408.
Mondal A., Mondal S., and Mandal S. (2022). Empirical dynamic model deciphers more information on the nutrient (N)—phytoplankton (P)–zooplankton (Z) dynamics of Hooghly-Matla estuary, Sundarban, India. Estuarine Coast. Shelf Sci. 265, 107711. doi: 10.1016/j.ecss.2021.107711
Moreno-García Y., Ángel-Camilo K. L., Bueno-Ospina M. L., Ayerbe-González S., and Guerrero-Vargas J. A. (2024). Four physiopathological effects of the venom of Bothrocophias Colombianus (Rendahl & Vestergre) endemic to the Colombian Pacific: Toxinological profile of the venom of Bothrocophias Colombianus. Rev. Novedades Colombianas 19.
Mortari M. R., do Couto L. L., dos Anjos L. C., Mourão C. B. F., Camargos T. S., Guerrero-Vargas J. A. G., et al. (2012). Pharmacological characterization of Synoeca cyanea venom: An aggressive social wasp widely distributed in the Neotropical region. Toxicon 59, 163–170. doi: 10.1016/j.toxicon.2011.11.002
Mukherjee K. and Mondal A. (2020). Butterfly diversity in heterogeneous habitat of Bankura, West Bengal, India. J. Threatened Taxa 12, 15804–15816. doi: 10.11609/jott.5136.12.8.15804-15816
Mukherjee K. and Mondal A. (2023). A preliminary checklist of larval host plants of butterflies of Bankura and Purulia districts of West Bengal, India. J. Anim. Diversity 5, 1–18. doi: 10.61186/JAD.5.2.2
Mukherjee S. S., Patra D., and Hossain A. (2024). Range-wide prediction of habitat suitability for king cobras under current and future scenarios. Integr. Conserv 3 (2), 134–141. doi: 10.1002/inc3.v3.2
Nanayakkara R. P. and Vishwanath N. (2013). “Some notes on ground dwelling mygalomorph (ARANEAE) spiders of Sri Lanka,” in Proceedings of the national symposium on soil biodiversity. Proceedings of the National Symposium on Soil Biodiversity.
Nentwig W. and Kuhn-Nentwig L. (2012). Spider venoms potentially lethal to humans, Spider ecophysiology. Springer. p, 253–264. doi: 10.1007/978-3-642-33989-9_19
Paul R., Santra S., Banerjee A. K., and Mondal J. (2012). Spider bite presenting with acute renal failure from western part of West Bengal. Asian J. Pharm. Health Sci. 2, 445–447.
Robinson G. and Griffin G. (1983). Effects of a bite from a barking spider (Selenocosmia stirlingi Hoog). Victorian Nat 100, 116–117. doi: 10.5962/p.295624
Roy R., Khare R., Sarkar R., Sardar H., and Mukherjee S. (2018). “The four month hypothesis: an overview on the ecology and behaviour of chilobrachys sp spiders (Araneae: theraphosidae),” in International journal of innovative science and research technology International Journal of Innovative Science and Research Technology.
Van Den Bekerom M. P., Struijs P. A., Blankevoort L., Welling L., Van Dijk C. N., and Kerkhoffs G. M. (2012). What is the evidence for rest, ice, compression, and elevation therapy in the treatment of ankle sprains in adults? J. athletic Training 47, 435–443. doi: 10.4085/1062-6050-47.4.14
Vetter R. S. and Isbister G. K. (2008). Medical aspects of spider bites. Annu. Rev. Entomol. 53, 409–429. doi: 10.1146/annurev.ento.53.103106.093503
Wang Y., Guo S., Jee K. F., and Herzig V. (2024). Thou shalt not pass-arachnid venom peptides interacting with biological membranes. Front. Arachnid Sci. 3, 1490313. doi: 10.3389/frchs.2024.1490313
Wang Z.-R. and Ni G.-X. (2021). Is it time to put traditional cold therapy in rehabilitation of soft-tissue injuries out to pasture? World J. Clin. cases 9, 4116. doi: 10.12998/wjcc.v9.i17.4116
Warren D. L., Matzke N. J., Cardillo M., Baumgartner J. B., Beaumont L. J., Turelli M., et al. (2021). ENMTools 1.0: an R package for comparative ecological biogeography. Ecography 44, 504–511. doi: 10.1111/ecog.2021.v44.i4
World Spider Catalog (2024). “World spider catalog. Version 25.5,” in Natural history museum bern. Available at: http://wsc.nmbe.ch. doi: 10.24436/2
Keywords: bite symptom, treatment protocol, tarantula ecology, spider bite, tarantula habitat
Citation: Mondal A, Das S, Mukherjee SS and Saha S (2025) Distribution, habitat suitability, natural history, bite report, and medical importance of the Asian tarantula Chilobrachys spp. Front. Arachn. Sci. 4:1598438. doi: 10.3389/frchs.2025.1598438
Received: 23 March 2025; Accepted: 14 May 2025;
Published: 03 June 2025.
Edited by:
Gyorgy Panyi, University of Debrecen, HungaryReviewed by:
Shaodong Guo, University of the Sunshine Coast, AustraliaJimmy Alexander Guerrero Vargas, University of Cauca, Colombia
Copyright © 2025 Mondal, Das, Mukherjee and Saha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayan Mondal, bW9uZGFsYXlhbi56b29AZ21haWwuY29t
 Ayan Mondal
Ayan Mondal Shouvik Das2
Shouvik Das2