- 1Department of Otolaryngology-Head and Neck Surgery, University of Pittsburgh Medical Center, Pittsburg, PA, United States
- 2Cambridge University Hospitals NHS Foundation Trust, Otolaryngology, Addenbrooks Hospital, Cambridge, Cambridgeshire, United Kingdom
Autoimmune inner ear disease (AIED) is a rare cause of bilateral, progressive sensorineural hearing loss (SNHL) that often responds to immunosuppressive therapy. Once considered primarily a T cell–driven condition, recent evidence underscores the importance of innate immunity, with the NLRP3 inflammasome emerging as a central mediator of cochlear inflammation and tissue injury. Caspase 1 is triggered by NLRP3 inflammasome assembly, resulting in the production of the proinflammatory cytokines IL-1β and IL-18 and the induction of pyroptotic cell death. Its presence in cochlear macrophages and supporting cells points to a pivotal role in non-infectious inflammation and progressive sensorineural damage. Preclinical models and emerging clinical data implicate NLRP3 in AIED pathogenesis and support targeting this pathway therapeutically. This review examines the immunological mechanisms linking innate and adaptive responses in AIED, evaluates current and emerging therapies targeting NLRP3, and outlines future research directions. Targeting the NLRP3 inflammasome holds promise for improving diagnosis and developing precision treatments for autoimmune inner ear disease.
Introduction
Autoimmune inner ear disease (AIED) is a rare but significant cause of bilateral, progressive sensorineural hearing loss (SNHL) that responds, at least initially, to immunosuppressive therapy (Vambutas et al., 2014; Ciorba et al., 2018). First described in the early 1970s, AIED is estimated to account for less than 1% of all cases of SNHL, yet its elusive pathophysiology, lack of diagnostic biomarkers, and limited treatment options have rendered it a persistent clinical challenge (Vambutas and Pathak, 2016; Ribeiro et al., 2021). Patients typically present with rapidly progressive hearing loss, often accompanied by vestibular symptoms such as imbalance or vertigo, along with a history of corticosteroid responsiveness or a co-existing systemic autoimmune disease, suggesting an immune-mediated etiology (Girasoli et al., 2018; Ralli et al., 2018). Despite decades of clinical observation, the immunological mechanisms that drive AIED remain poorly defined.
Historically, AIED has been viewed through the lens of adaptive immunity, with autoantibodies, autoreactive T cells, and systemic autoimmune diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus) thought to contribute to the disease phenotype (Ciorba et al., 2018; Strum et al., 2020). However, this framework does not fully explain the clinical heterogeneity, episodic flares, and local tissue damage seen in AIED (Wang et al., 2025a). Recent advances in inner ear immunology have revealed the presence of resident immune cells, including macrophages and dendritic cells, that act as sentinels within the cochlea and vestibular organs (Miwa and Okano, 2022; Karayay et al., 2024; Keithley, 2022; Yuan et al., 2025a). These cells trigger and enhance immune activity through innate sensing mechanisms, with the NLRP3 inflammasome, a member of the NOD-like receptor family, now recognized as a key regulator of noninfectious inflammatory responses and autoimmune processes (Blevins et al., 2022; Akbal et al., 2022).
NLRP3 forms a protein complex within the cell's cytoplasm that, when triggered by cellular stress signals known as damage-associated molecular patterns (DAMPs), initiates the maturation of IL-1β and IL-18 and promotes a type of inflammatory cell death called pyroptosis (Blevins et al., 2022; Paik et al., 2021; Zheng et al., 2020; Yuan et al., 2025b). First identified in systemic autoinflammatory disorders, abnormal NLRP3 activation is increasingly recognized as a contributing factor in numerous autoimmune and neuroinflammatory conditions, such as multiple sclerosis, inflammatory bowel disease, and lupus nephritis (Corcoran et al., 2021; Ke et al., 2023). Importantly, components of the inflammasome machinery have been identified in the inner ear, and their dysregulation may contribute to cochlear inflammation, sensorineural degeneration, and irreversible hearing loss (Parekh and Kaur, 2023; Schiel et al., 2024; Nakanishi et al., 2022).
This review explores the emerging link between NLRP3 inflammasome activation and AIED, integrating insights from systemic autoimmune diseases, animal models of inner ear inflammation, and recent advances in innate immune signaling. We discuss the evidence supporting NLRP3 as a mechanistic driver of cochlear pathology, evaluate current and potential therapeutic strategies targeting this pathway, and highlight future research directions to advance diagnostic and treatment paradigms in AIED.
Immunopathogenesis of autoimmune inner ear disease (AIED)
AIED has traditionally been viewed as a T cell–mediated autoimmune disorder characterized by autoreactive CD4+ T cells and autoantibodies against inner ear antigens like cochlin and heat shock proteins (Ciorba et al., 2018; Wang et al., 2025a; Buniel et al., 2009). These adaptive immune responses, frequently involving Th1 and Th17 signaling molecules including IFN-γ and IL-17, play a significant role in driving cochlear inflammation and resulting tissue injury (Li et al., 2018; Kuwabara et al., 2017). However, the variability in autoantibody detection and the incomplete recapitulation of disease by passive transfer suggest additional immune mechanisms are involved (Wang et al., 2025a; Mendis et al., 2022).
AIED is likely multifactorial; triggers may include systemic autoimmunity, molecular mimicry after infection, local tissue stress, or breakdown of immune tolerance (Wang et al., 2025a). In this framework, NLRP3 inflammasome activation should be considered a critical amplifier of cochlear inflammation: it may act downstream of an initiating event, such as tissue injury or autoantibody deposition, and can propagate and sustain inflammation in concert with adaptive immune mechanisms (Murillo-Cuesta et al., 2025). Thus, NLRP3 is best conceptualized as a mechanistic node linking diverse triggers—including T cell–mediated signals—to common downstream injury pathways, rather than as the unique initiating antigenic stimulus (Arbore et al., 2016; Kelley et al., 2019). We emphasize that inflammasome activation does not replace adaptive immunity; rather, NLRP3 activity and T cell–mediated mechanisms likely act together: inflammasome-derived cytokines can amplify antigen presentation and T cell polarization, while adaptive responses can provide priming signals that facilitate inflammasome assembly (Zhang et al., 2021).
Multiple stimuli can induce NLRP3 activation in the cochlea, reflecting the diverse stressors and immune challenges present in the inner ear (Murillo-Cuesta et al., 2025). Sterile cellular stress, including oxidative damage, mitochondrial dysfunction, and ion flux alterations, can prime and activate the inflammasome (Murillo-Cuesta et al., 2025; Salminen et al., 2012). Injury to hair cells or supporting cells releases DAMPs, which serve as potent local danger signals. Microbial products or cochlear infection can also act as triggers, providing pathogen-associated molecular patterns (PAMPs) that engage NLRP3 (Murillo-Cuesta et al., 2025). Additionally, immune complexes, complement activation, and cytokines produced by adaptive immune cells, including IL-17 and TNFα, may provide secondary signals that amplify inflammasome activity (Evavold and Kagan, 2018). The relative contribution of each inducer likely varies across patients and clinical contexts, emphasizing the multifactorial and context-dependent nature of NLRP3 activation in autoimmune inner ear disease (Moltrasio et al., 2022).
Because NLRP3 responds to generic danger signals, establishing its disease-specific relevance requires direct spatial and temporal evidence linking inflammasome activity with adaptive immune responses in clinical AIED (Zhang et al., 2021). Key evidence would include elevated inflammasome components in perilymph or cochlear tissue from affected patients, correlation of these markers with disease activity or steroid responsiveness, identification of genetic or epigenetic variants that predispose to heightened NLRP3 responsiveness, and reproducible mitigation of cochlear injury by NLRP3 pathway blockade in models that recapitulate autoimmune features (Vambutas et al., 2014; Nakanishi et al., 2022; Murillo-Cuesta et al., 2025; Nakanishi et al., 2017). Future studies employing conditional genetic models and perilymph biomarker profiling will be critical to demonstrate specificity and strengthen the rationale for therapeutically targeting NLRP3 in AIED.
Recent research emphasizes the essential contribution of innate immune populations, especially cochlear macrophages and antigen-presenting cells, in the development of AIED (Figure 1; Miwa and Okano, 2022). These resident cells detect tissue stress through pattern recognition receptors such as NLRP3 and respond by adopting a proinflammatory phenotype that amplifies local immune responses (Chen et al., 2023; Wang et al., 2025b; Sebastian-Valverde and Pasinetti, 2020). Through cytokine secretion and antigen presentation, these cells play an essential role bridging innate detection mechanisms and adaptive immune responses within the specialized environment of the inner ear (Duan et al., 2022; Liu and Xu, 2023).
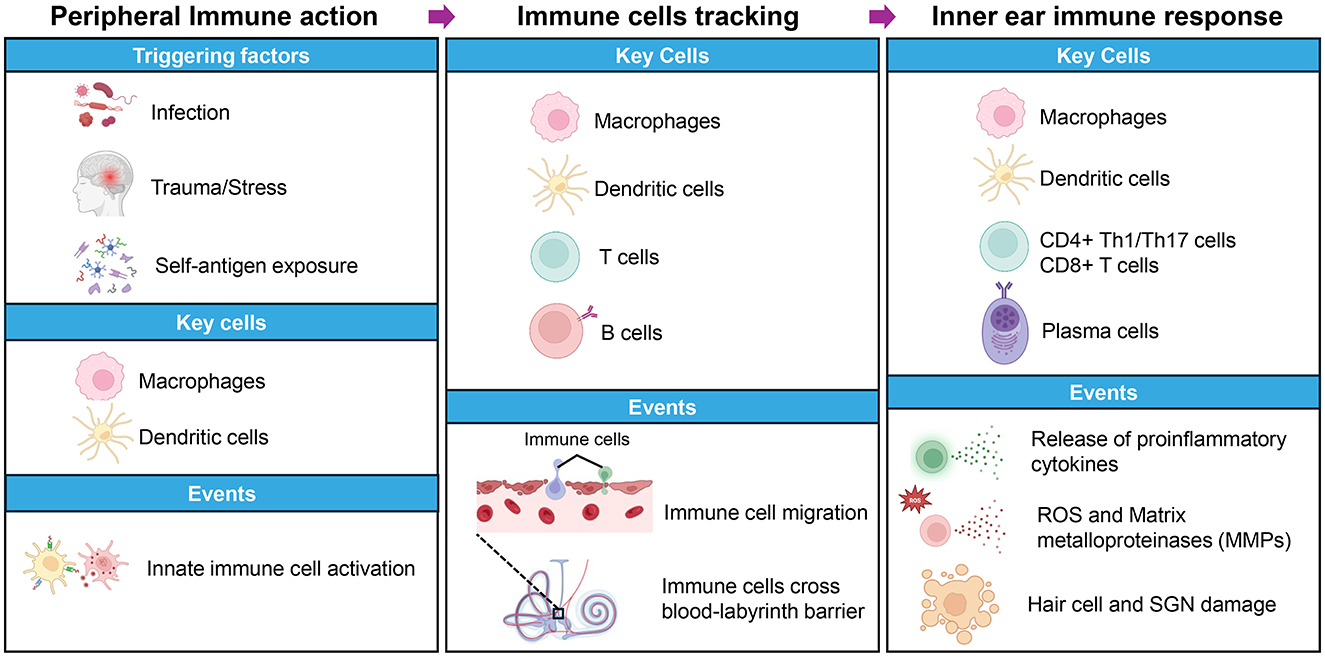
Figure 1. Overview of autoimmune inner ear disease (AIED) pathogenesis. This schematic depicts the major factors, immune cells, and molecular events driving the onset and progression of AIED. The left panel shows potential triggers such as infection, trauma or stress, and self-antigen exposure, along with early involvement of macrophages and dendritic cells that initiate innate immune responses. The middle panel highlights key immune players including macrophages, dendritic cells, T cells, and B cells and processes like immune cell migration and compromise of the blood labyrinth barrier. The right panel illustrates later disease stages, focusing on macrophages, dendritic cells, CD4+ Th1 and Th17 cells, CD8+ T cells, and plasma cells. These contribute to the release of proinflammatory cytokines, production of reactive oxygen species (ROS), and matrix metalloproteinases (MMPs), ultimately causing damage to hair cells and spiral ganglion neurons (SGN).
Proinflammatory cytokines such as IL-1β, TNFα, and IL-17 are key mediators of the inflammatory cascade in AIED (Miwa and Okano, 2022; Pathak et al., 2015). IL-1β, activated by the NLRP3 inflammasome, promotes leukocyte recruitment and tissue injury, while TNFα induces hair cell apoptosis and barrier disruption (Huang et al., 2021; Wu et al., 2022; Wood and Zuo, 2017). IL-17 further sustains inflammation by recruiting neutrophils and stimulating matrix degradation (Zenobia and Hajishengallis, 2015; Griffin et al., 2012). Together, these cytokines form a self-perpetuating loop of immune activation that underscores the potential of targeting innate immune pathways like NLRP3 for therapeutic intervention (Davis et al., 2011; Paik et al., 2025).
NLRP3 inflammasome: gatekeeper of innate immune activation and its role in autoimmune diseases
The NLRP3 inflammasome is a protein assembly located within the cytoplasm that plays a vital role as both sensor and effector in innate immune signaling (Kelley et al., 2019). Structurally, it consists of the receptor protein NLRP3, the adaptor ASC (apoptosis-associated speck-like protein containing a CARD), and the enzymatic effector caspase 1. When triggered, NLRP3 combines with ASC and pro-caspase-1 to create the inflammasome, which facilitates the conversion of pro-caspase-1 into its active enzyme form (Moretti and Blander, 2021; Que et al., 2024). This process leads to maturation and release of the highly inflammatory cytokines IL-1β and IL-18 and initiates a destructive form of programmed cell death known as pyroptosis (Wang et al., 2021).
NLRP3 activation takes place via two primary mechanisms: the classical pathway and an alternative pathway (Pellegrini et al., 2017). The classical pathway begins with a priming signal, typically mediated by Toll-like receptors or cytokine receptors, which upregulates NLRP3 and pro IL-1β production. This is followed by a secondary trigger such as cellular stress, ion imbalance, or mitochondrial injury, that initiates inflammasome formation (Kelley et al., 2019). The alternative pathway relies on intracellular detection of bacterial lipopolysaccharides by caspases 4 and 5 in humans (caspase 11 in mice), which subsequently triggers NLRP3 inflammasome assembly (Zheng et al., 2020; Yang et al., 2015). Both pathways culminate in robust inflammatory responses that eliminate pathogens but can also drive tissue damage if dysregulated.
While critical for pathogen defense, dysregulated NLRP3 activation is now understood to underlie chronic inflammation and autoimmunity across multiple organ systems (Ren et al., 2024). In conditions including lupus, rheumatoid arthritis, and multiple sclerosis, heightened NLRP3 activity has been associated with increased disease severity and advancement (Carnazzo et al., 2025). The inflammasome promotes tissue inflammation not only by releasing IL-1β and IL-18 but also by facilitating immune cell recruitment and activation, thereby bridging innate and adaptive immunity (Zheng et al., 2020). This bridge is critical, as NLRP3-mediated cytokines can act alongside T cell–mediated mechanisms to enhance antigen presentation, promote Th17 polarization, and support autoantibody production, collectively fueling autoimmune pathology (Zhang et al., 2021; Li et al., 2020; Accogli et al., 2025).
Insights from systemic autoimmune models highlight the NLRP3 inflammasome as a potential therapeutic target. Inhibiting NLRP3 or its downstream effectors reduces inflammatory damage and ameliorates clinical symptoms in animal models of lupus nephritis, arthritis, and neuroinflammation (Yu et al., 2021; Yin et al., 2022; Oliveira et al., 2021). These findings underscore the inflammasome's role as a nexus where innate immune sensing translates into chronic adaptive immune responses (Eisenbarth and Flavell, 2009). Given the shared immunopathogenic features, the NLRP3 inflammasome is increasingly viewed as a promising mechanistic link in autoimmune inner ear disease (Schiel et al., 2024), warranting further investigation into its specific contributions and therapeutic modulation in cochlear autoimmunity.
Evidence for NLRP3 in inner ear disease
Recent advances in inner ear immunology have identified the NLRP3 inflammasome as a critical driver of cochlear inflammation and a potential therapeutic target in inner ear disease (Schiel et al., 2024). This complex is predominantly found in resident macrophages, along with structurally supportive, non-immune cells including pillar and Deiters' cells (Figure 2; Hoa et al., 2020), indicating that both immune and structural cochlear elements are capable of mounting inflammasome-mediated responses (Pan et al., 2024).
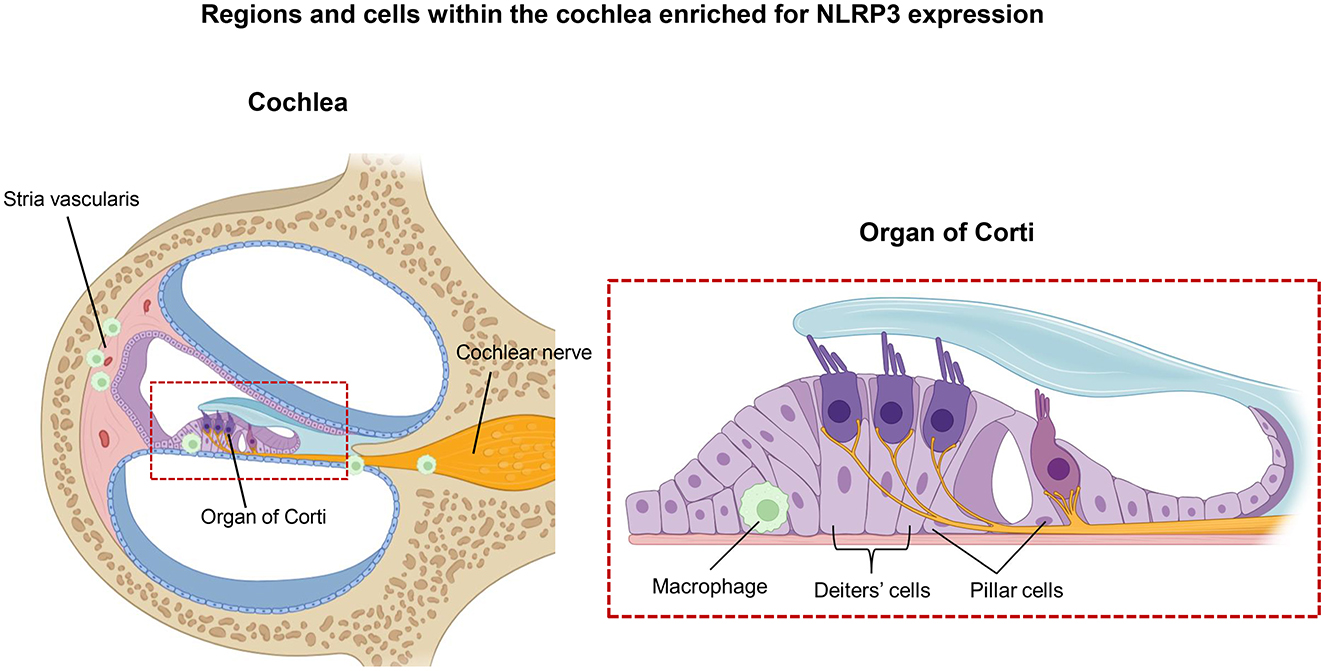
Figure 2. Regions and cells within the cochlea enriched for NLRP3 expression. Anatomical regions and specific cochlear cell types exhibiting elevated NLRP3 expression are shown, with emphasis on structural localization and cellular specificity. The left panel presents a cross-sectional view of the cochlea, highlighting key structures such as the stria vascularis and the cochlear nerve. A red box marks the location of the Organ of Corti, which is shown in greater detail in the right panel. In this magnified view, specific cell types, including macrophages, Deiters' cells, and pillar cells, are identified as sites of enriched NLRP3 expression.
Cochlear macrophages reside in the spiral ligament, basilar membrane, and stria vascularis (Ito et al., 2022). As tissue-resident sentinels, they constantly survey the cochlear microenvironment for pathogens, cell debris, and DAMPs, and upon activation, they can initiate robust proinflammatory cascades (Hough et al., 2022). These macrophages express canonical inflammasome components, including NLRP3, ASC, and caspase-1, and have been shown to respond dynamically to various insults, including infection, oxidative stress, and mechanical damage (Ma et al., 2022). The expression of NLRP3 in cochlear macrophages enables these cells to serve as primary effectors of inflammatory injury following acoustic trauma or infection (Schiel et al., 2024).
Notably, cochlear macrophages are heterogeneous in both location and function (Ye et al., 2025). Subsets in the stria vascularis, spiral ligament, and perivascular spaces differ in exposure to ions, metabolic demands, and local signals, suggesting that NLRP3 expression and activation thresholds may vary across these populations (Murillo-Cuesta et al., 2025; Gregory et al., 2023). Understanding these differences will require single-cell transcriptomics with spatial mapping, coupled with conditional NLRP3 ablation targeted to macrophage lineages, to determine which subsets are the key drivers of cochlear inflammasome activity.
Supporting cells of the organ of Corti, particularly pillar and Deiters' cells, are increasingly appreciated as immunocompetent (Wan et al., 2013; Hayashi et al., 2020). These cells not only express NLRP3 (Hoa et al., 2020) but also exhibit characteristics typically associated with microglia, including responsiveness to damage-associated cues. Pillar and Deiters' cells express receptors involved in innate sensing and can upregulate inflammasome components in response to local stress (Hayashi et al., 2020; Cai et al., 2014). While these supporting cells are not motile in the sense of cellular migration across the cochlea, they exhibit reactive morphological and transcriptional changes—including process extension, modifications of junctional proteins, and upregulation of inflammatory mediators—that position them to influence nearby hair cells and resident immune cells. These dynamic local responses suggest that supporting cells play an active role in shaping the cochlear immune environment and coordinating innate and adaptive responses during injury or inflammation (Hoa et al., 2020; Hu et al., 2018; Liu et al., 2018).
Animal models have been instrumental in delineating the pathophysiological role of the NLRP3 inflammasome in sensorineural hearing loss. In models of noise-induced hearing loss, acoustic overstimulation leads to a rapid upregulation of NLRP3 in cochlear tissues, accompanied by elevated expression of IL-1β and IL-18 (Sai et al., 2022). These cytokines contribute to blood-labyrinth barrier breakdown, recruitment of inflammatory cells, and apoptosis of hair cells, culminating in permanent threshold shifts. Therapeutic intervention with inflammasome inhibitors such as MCC950 (a selective NLRP3 inhibitor) or IL-1 receptor antagonist (IL-1Ra) has been shown to attenuate these pathological changes, leading to reduced hair cell death, diminished cytokine expression, and partial preservation of auditory function (Schiel et al., 2024; Ma et al., 2022). These findings not only support a mechanistic role for NLRP3 in cochlear pathology but also validate it as a therapeutic target in preclinical models.
Importantly, these preclinical findings are supported by emerging clinical evidence. Increased concentrations of IL-1β, ASC, and other inflammasome-related proteins have been identified in both the perilymph and bloodstream of patients with unexplained sensorineural impairment and immune-mediated inner ear disorders (Gregory et al., 2023; Rauch, 2014; Nakanishi et al., 2020). The presence of these markers correlates with disease severity and progression, suggesting that inflammasome activation may not only serve as a mechanistic link between inflammation and hearing loss but also hold promise as a diagnostic or prognostic biomarker (Nakanishi et al., 2022; Gregory et al., 2023). Furthermore, genetic studies are beginning to explore polymorphisms in NLRP3 and related genes that may predispose individuals to exaggerated inflammatory responses in the cochlea, although this remains an area for future investigation (Nakanishi et al., 2017).
Therapeutic targeting of NLRP3 in autoimmunity and inner ear diseases
The pivotal involvement of NLRP3 in sustaining chronic inflammation and contributing to tissue injury in autoimmune and inflammatory conditions has sparked considerable interest in designing therapies that block its activation (Figure 3; Li et al., 2020). Among the most promising approaches are small molecule inhibitors that directly target NLRP3 activation. One of the most studied compounds, MCC950, selectively blocks NLRP3 by preventing its ATPase activity, thereby inhibiting inflammasome assembly and subsequent IL-1β secretion (Li et al., 2022). MCC950 has advanced to clinical trials for disorders like cryopyrin-associated periodic syndromes and gout, while anakinra and canakinumab have been investigated in autoimmune conditions such as lupus and rheumatoid arthritis, as well as other long-standing inflammatory disorders, showing promising outcomes (Xu et al., 2019; Fu et al., 2017; Guo et al., 2018; Wu et al., 2024). Another emerging inhibitor, OLT1177 (dapansutrile), also selectively inhibits NLRP3 and has demonstrated anti-inflammatory effects in gout, heart failure, and neuroinflammation models with a favorable safety profile, advancing into early-phase clinical trials (Kluck et al., 2020; Sanchez-Fernandez et al., 2019).
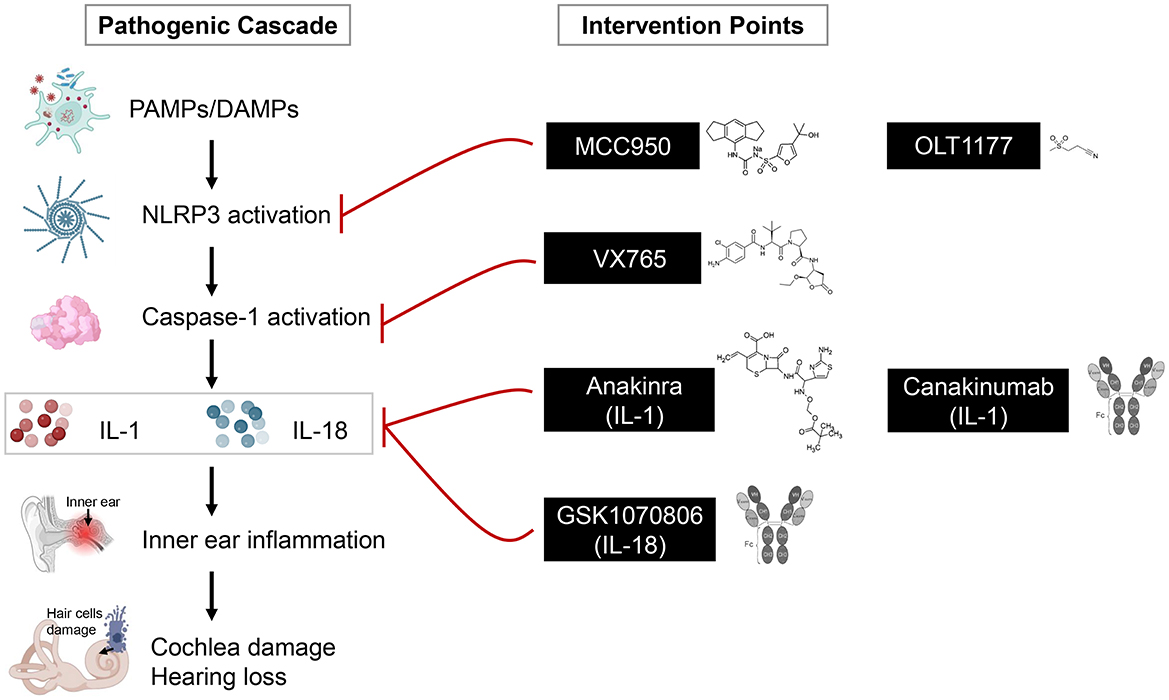
Figure 3. Pathogenic cascade and potential intervention points in inner ear inflammation. A simplified pathogenic cascade leading to inner ear damage is depicted, highlighting key stages of inflammation and corresponding therapeutic targets highlighted along the pathway. The left side illustrates the sequence of events beginning with initiating triggers including pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) such as viral infection, cellular stress, or inflammation. These triggers activate immune cells, which release inflammatory mediators that ultimately damage inner ear structures, particularly hair cells. The right side presents therapeutic compounds that intervene at specific stages of the cascade. NLRP3 inhibitors (MCC950, OLT1177), caspase-1 blockers (VX765), and cytokine-targeting agents such as IL-1 pathway inhibitors (anakinra, canakinumab) and an IL-18–neutralizing antibody (GSK1070806). MCC950, OLT1177, VX765, and anakinra are shown with their chemical structures, while canakinumab and GSK1070806 are represented with antibody structures.
Systemic corticosteroids produce broad anti-inflammatory effects and can reduce certain inflammasome-associated cytokines in multiple organs (Yang et al., 2020); however, corticosteroid responsiveness in AIED is heterogeneous and often incomplete (Breslin et al., 2020). Targeting NLRP3 directly offers a complementary strategy, as inhibitors or IL-1 pathway blockers can selectively block inflammasome assembly or IL-1β signaling (Sanchez-Fernandez et al., 2019). This approach may benefit patients who are corticosteroid-resistant or for whom chronic steroid exposure is undesirable (Vambutas et al., 2014). Accordingly, NLRP3 inhibitors should be positioned as precision agents for testing in steroid-refractory or inflammasome-high AIED phenotypes rather than as wholesale replacements for initial steroid therapy.
Complementing direct NLRP3 inhibitors, IL-1 blockers have been successfully employed to neutralize the key downstream effectors of inflammasome activation (Lara-Reyna et al., 2022). Anakinra, an IL-1 receptor blocker, and canakinumab, a monoclonal antibody targeting IL-1β, are approved by the FDA for treating autoinflammatory disorders and select autoimmune conditions (Dinarello et al., 2012). Preliminary evidence from case series suggests that anakinra may reduce cochlear inflammation and preserve hearing in patients with AIED, warranting further study in larger trials (Vambutas et al., 2014; Nakanishi et al., 2017). Canakinumab has been widely used in periodic fever syndromes and is being investigated for broader autoimmune indications, offering a translational opportunity for inner ear disorders characterized by IL-1β-mediated pathology (Dhimolea, 2010; De Benedetti et al., 2018). Together with NLRP3 inhibitors, these cytokine-targeting therapies provide a complementary or precision approach, particularly in patients who respond poorly to corticosteroids or exhibit high inflammasome activity (Vambutas et al., 2014). While these therapeutic approaches are promising, controlled clinical trials specifically targeting NLRP3 in inner ear disorders remain limited. Future research should focus on identifying the most effective dosing regimens, ideal timing for intervention, and sustained benefits for maintaining hearing function.
Recent animal models of noise-induced and autoimmune-mediated hearing loss show that selective blockade of NLRP3 with inhibitors such as MCC950 or IL-1 receptor antagonists can significantly attenuate cochlear inflammation, preserve hair cells, and prevent auditory threshold shifts (Ma et al., 2022). Therapeutic inhibition of the NLRP3 inflammasome with MCC950 or blockade of IL-1 signaling with Anakinra significantly preserves outer hair cells in chronic suppurative otitis media, demonstrating that targeting this pathway can effectively prevent inflammation-driven hearing loss (Schiel et al., 2024). Encouragingly, multiple clinical trials are currently underway to evaluate NLRP3 inflammasome inhibitors and IL-1 blockers in various autoimmune and inflammatory conditions. IL-1 receptor anakinra is being investigated in trials for corticosteroid-resistant AIED patients [ClinicalTrials.gov NCT01267994] (Vambutas et al., 2014). However, further clinical studies focusing specifically on inner ear diseases are needed to establish dosing, safety profiles, and therapeutic windows.
Knowledge gaps and future directions
Despite growing recognition of the NLRP3 inflammasome's role in inner ear inflammation and AIED, significant knowledge gaps remain, limiting our understanding and therapeutic progress. A major challenge is the lack of animal models that specifically recapitulate AIED with detailed analysis of the NLRP3 inflammasome pathway. Existing cochlear injury models primarily focus on noise-induced or ototoxic damage and do not fully mimic the autoimmune-mediated processes (Kurabi et al., 2017; Le et al., 2017; Steyger, 2021). Developing genetically engineered or inducible models that integrate adaptive autoimmunity with innate inflammasome activation will be critical to dissect the mechanistic contributions of NLRP3 in AIED pathogenesis and to evaluate targeted interventions in vivo.
Technological advances offer exciting opportunities to overcome current limitations in inner ear research. Notably, techniques like single-cell RNA sequencing and spatial transcriptomics offer exceptional detail in mapping cellular diversity and inflammasome-associated gene activity within the intricate environment of the cochlea (Molla Desta and Birhanu, 2025; Lee et al., 2021). These approaches would enable identification of specific immune and resident cell subsets expressing NLRP3 and related cytokines, and how these profiles differ between healthy and diseased states. Applying spatial transcriptomics is especially valuable in the cochlea, where precise anatomical localization of inflammasome activation relative to sensory hair cells and supporting structures can yield insights into disease mechanisms and targeted therapeutic windows (Cao et al., 2024).
Another key future direction is the discovery and validation of reliable biomarkers that reflect NLRP3 inflammasome activity in AIED. Peripheral blood markers such as serum IL-1β levels and caspase-1 enzymatic activity may serve as accessible surrogates of inner ear inflammation, facilitating diagnosis, disease monitoring, and assessment of treatment responses (Kim et al., 2022; Zhao et al., 2013). However, these systemic markers lack specificity and sensitivity for cochlear pathology. Therefore, exploring novel biomarker sources, including inner ear fluids like perilymph, or integrating multi-omics approaches, may improve disease stratification and therapeutic monitoring.
An emerging area warranting investigation is the epigenetic regulation of NLRP3 inflammasome components and related inflammatory pathways (Kaszycki and Kim, 2025; Raneros et al., 2021). Epigenetic processes including DNA methylation, histone alterations, and changes to chromatin structure can regulate the expression of inflammasome-related genes and modulate their activation sensitivity (Kaszycki and Kim, 2025; Handy et al., 2011; Poli et al., 2020). These regulatory layers are increasingly recognized as important modulators of immune cell function and may contribute to the chronicity and flare-ups observed in autoimmune inner ear disease. Understanding epigenetic control could reveal novel targets for therapeutic modulation, enabling more precise and sustained suppression of harmful inflammation without broadly impairing host defense.
Conclusion
The NLRP3 inflammasome emerges as a central integrator of innate immune pathology in AIED, linking cellular stress signals to proinflammatory cytokine release and subsequent adaptive immune activation. Its distinctive role bridging innate and adaptive immune responses highlights its essential contribution to cochlear inflammation and tissue injury that define AIED. Despite advances in understanding NLRP3′s function in systemic autoimmune diseases, its specific contributions within the specialized microenvironment of the inner ear remain underexplored.
Addressing these gaps requires comprehensive mechanistic studies utilizing advanced animal models and cutting-edge molecular techniques to delineate how NLRP3 inflammasome activation influences disease onset and progression in AIED. Concurrently, therapeutic exploration targeting NLRP3 and its downstream pathways holds great promise for developing precision treatments that can effectively mitigate inflammation, preserve hearing function, and improve patient quality of life. Ongoing collaborative research will be crucial to fully realize NLRP3′s promise as a diagnostic indicator and treatment focus for autoimmune inner ear disorders.
Author contributions
VY: Writing – original draft, Writing – review & editing. PK: Methodology, Writing – review & editing, Resources. AX: Resources, Methodology, Writing – review & editing. PS: Writing – original draft, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was funded by the National Institute of Health's National Institute for Deafness and Communication Disorders under award number R01DC019965.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Accogli, T., Hibos, C., Milian, L., Geindreau, M., Richard, C., Humblin, E., et al. (2025). The intrinsic expression of Nlrp3 in Th17 cells promotes their protumor activity and conversion into tregs. Cell. Mol. Immunol. 22, 541–556. doi: 10.1038/s41423-025-01281-y
Akbal, A., Dernst, A., Lovotti, M., Mangan, M. S. J., McManus, R. M., Latz, E., et al. (2022). How location and cellular signaling combine to activate the Nlrp3 inflammasome. Cell. Mol. Immunol. 19, 1201–1214. doi: 10.1038/s41423-022-00922-w
Arbore, G., West, E. E., Spolski, R., Robertson, A. A. B., Klos, A., Rheinheimer, C., et al. (2016). T Helper 1 immunity requires complement-driven Nlrp3 inflammasome activity in Cd4(+) T cells. Science 352:aad1210. doi: 10.1126/science.aad1210
Blevins, H. M., Xu, Y., Biby, S., and Zhang, S. (2022). The Nlrp3 inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front. Aging Neurosci. 14:879021. doi: 10.3389/fnagi.2022.879021
Breslin, N. K., Varadarajan, V. V., Sobel, E. S., and Haberman, R. S. (2020). Autoimmune inner ear disease: a systematic review of management. Laryngoscope Investig. Otolaryngol. 5, 1217–1226. doi: 10.1002/lio2.508
Buniel, M. C., Geelan-Hansen, K., Weber, P. C., and Tuohy, V. K. (2009). Immunosuppressive therapy for autoimmune inner ear disease. Immunotherapy 1, 425–434. doi: 10.2217/imt.09.12
Cai, Q., Vethanayagam, R. R., Yang, S., Bard, J., Jamison, J., Cartwright, D., et al. (2014). Molecular profile of cochlear immunity in the resident cells of the organ of corti. J. Neuroinflammation 11:173. doi: 10.1186/s12974-014-0173-8
Cao, J., Li, C., Cui, Z., Deng, S., Lei, T., Liu, W., et al. (2024). Spatial transcriptomics: a powerful tool in disease understanding and drug discovery. Theranostics 14, 2946–2968. doi: 10.7150/thno.95908
Carnazzo, V., Rigante, D., Restante, G., Basile, V., Pocino, K., Basile, U., et al. (2025). The entrenchment of Nlrp3 inflammasomes in autoimmune disease-related inflammation. Autoimmun. Rev. 24:103815. doi: 10.1016/j.autrev.2025.103815
Chen, S., Saeed, A., Liu, Q., Jiang, Q., Xu, H., Xiao, G. G., et al. (2023). Macrophages in immunoregulation and therapeutics. Signal. Transduct. Target Ther. 8:207. doi: 10.1038/s41392-023-01452-1
Ciorba, A., Corazzi, V., Bianchini, C., Aimoni, C., Pelucchi, S., Skarzynski, P. H., et al. (2018). Autoimmune inner ear disease (Aied): a diagnostic challenge. Int. J. Immunopathol. Pharmacol. 32:2058738418808680. doi: 10.1177/2058738418808680
Corcoran, S. E., Halai, R., and Cooper, M. A. (2021). Pharmacological inhibition of the Nod-like receptor family pyrin domain containing 3 inflammasome with Mcc950. Pharmacol. Rev. 73, 968–1000. doi: 10.1124/pharmrev.120.000171
Davis, B. K., Wen, H., and Ting, J. P. (2011). The inflammasome Nlrs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735. doi: 10.1146/annurev-immunol-031210-101405
De Benedetti, F., Gattorno, M., Anton, J., Ben-Chetrit, E., Frenkel, J., Hoffman, H. M., et al. (2018). Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N. Engl. J. Med. 378, 1908–1919. doi: 10.1056/NEJMoa1706314
Dinarello, C. A., Simon, A., and van der Meer, J. W. (2012). Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652. doi: 10.1038/nrd3800
Duan, T., Du, Y., Xing, C., Wang, H. Y., and Wang, R. F. (2022). Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 13:812774. doi: 10.3389/fimmu.2022.812774
Eisenbarth, S. C., and Flavell, R. A. (2009). Innate instruction of adaptive immunity revisited: the inflammasome. EMBO Mol. Med. 1, 92–98. doi: 10.1002/emmm.200900014
Evavold, C. L., and Kagan, J. C. (2018). How inflammasomes inform adaptive immunity. J. Mol. Biol. 430, 217–237. doi: 10.1016/j.jmb.2017.09.019
Fu, R., Guo, C., Wang, S., Huang, Y., Jin, O., Hu, H., et al. (2017). Podocyte activation of Nlrp3 inflammasomes contributes to the development of proteinuria in lupus nephritis. Arthritis Rheumatol. 69, 1636–1646. doi: 10.1002/art.40155
Girasoli, L., Cazzador, D., Padoan, R., Nardello, E., Felicetti, M., Zanoletti, E., et al. (2018). Update on vertigo in autoimmune disorders, from diagnosis to treatment. J. Immunol. Res. 2018:5072582. doi: 10.1155/2018/5072582
Gregory, G. E., Munro, K. J., Couper, K. N., Pathmanaban, O. N., and Brough, D. (2023). The Nlrp3 inflammasome as a target for sensorineural hearing loss. Clin. Immunol. 249:109287. doi: 10.1016/j.clim.2023.109287
Griffin, G. K., Newton, G., Tarrio, M. L., Bu, D. X., Maganto-Garcia, E., Azcutia, V., et al. (2012). Il-17 and Tnf-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 188, 6287–6299. doi: 10.4049/jimmunol.1200385
Guo, C., Fu, R., Wang, S., Huang, Y., Li, X., Zhou, M., et al. (2018). Nlrp3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin. Exp. Immunol. 194, 231–243. doi: 10.1111/cei.13167
Handy, D. E., Castro, R., and Loscalzo, J. (2011). Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation. 123, 2145–2156. doi: 10.1161/CIRCULATIONAHA.110.956839
Hayashi, Y., Suzuki, H., Nakajima, W., Uehara, I., Tanimura, A., Himeda, T., et al. (2020). Cochlear supporting cells function as macrophage-like cells and protect audiosensory receptor hair cells from pathogens. Sci. Rep. 10:6740. doi: 10.1038/s41598-020-63654-9
Hoa, M., Olszewski, R., Li, X., Taukulis, I., Gu, S., De Torres, A., et al. (2020). Characterizing adult cochlear supporting cell transcriptional diversity using single-cell Rna-Seq: validation in the adult mouse and translational implications for the adult human cochlea. Front. Mol. Neurosci. 13:13. doi: 10.3389/fnmol.2020.00013
Hough, K., Verschuur, C. A., Cunningham, C., and Newman, T. A. (2022). Macrophages in the cochlea; an immunological link between risk factors and progressive hearing loss. Glia 70, 219–238. doi: 10.1002/glia.24095
Hu, B. H., Zhang, C., and Frye, M. D. (2018). Immune cells and non-immune cells with immune function in mammalian cochleae. Hear. Res. 362, 14–24. doi: 10.1016/j.heares.2017.12.009
Huang, Y., Xu, W., and Zhou, R. (2021). Nlrp3 inflammasome activation and cell death. Cell. Mol. Immunol. 18, 2114–2127. doi: 10.1038/s41423-021-00740-6
Ito, T., Kurata, N., and Fukunaga, Y. (2022). Tissue-resident macrophages in the stria vascularis. Front. Neurol. 13:818395. doi: 10.3389/fneur.2022.818395
Karayay, B., Olze, H., and Szczepek, A. J. (2024). Mammalian inner ear-resident immune cells-a scoping review. Cells 13:1528. doi: 10.3390/cells13181528
Kaszycki, J., and Kim, M. (2025). Epigenetic regulation of transcription factors involved in Nlrp3 inflammasome and Nf-Kb signaling pathways. Front. Immunol. 16:1529756. doi: 10.3389/fimmu.2025.1529756
Ke, Q., Greenawalt, A. N., Manukonda, V., Ji, X., and Tisch, R. M. (2023). The regulation of self-tolerance and the role of inflammasome molecules. Front. Immunol. 14:1154552. doi: 10.3389/fimmu.2023.1154552
Keithley, E. M. (2022). Inner ear immunity. Hear. Res. 419:108518. doi: 10.1016/j.heares.2022.108518
Kelley, N., Jeltema, D., Duan, Y., and He, Y. The Nlrp3 inflammasome: an overview of mechanisms of activation regulation. Int. J. Mol. Sci. (2019) 20:3328. doi: 10.3390/ijms20133328
Kim, S. H., Lee, J. H., Jeong, H. J., Kim, J. M., Baek, W. K., Kim, T. H., et al. (2022). Clinical significance of elevated serum caspase-1 levels in patients with ankylosing spondylitis. Ann. Lab. Med. 42, 293–295. doi: 10.3343/alm.2022.42.2.293
Kluck, V., Jansen, T., Janssen, M., Comarniceanu, A., Efde, M., Tengesdal, I. W., et al. (2020). Dapansutrile, an oral selective Nlrp3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2, e270–e280. doi: 10.1016/S2665-9913(20)30065-5
Kurabi, A., Keithley, E. M., Housley, G. D., Ryan, A. F., and Wong, A. C. (2017). Cellular mechanisms of noise-induced hearing loss. Hear. Res. 349, 129–137. doi: 10.1016/j.heares.2016.11.013
Kuwabara, T., Ishikawa, F., Kondo, M., and Kakiuchi, T. (2017). The role of Il-17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm. 2017:3908061. doi: 10.1155/2017/3908061
Lara-Reyna, S., Caseley, E. A., Topping, J., Rodrigues, F., Jimenez Macias, J., Lawler, S. E., et al. (2022). Inflammasome activation: from molecular mechanisms to autoinflammation. Clin. Transl. Immunology 11:e1404. doi: 10.1002/cti2.1404
Le, T. N., Straatman, L. V., Lea, J., and Westerberg, B. (2017). Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol. Head Neck Surg. 46:41. doi: 10.1186/s40463-017-0219-x
Lee, Y., Bogdanoff, D., Wang, Y., Hartoularos, G. C., Woo, J. M., and Mowery, C. T. Xyzeq: spatially resolved single-cell rna sequencing reveals expression heterogeneity in the tumor microenvironment. Sci. Adv. (2021) 7:eabg4755. doi: 10.1126/sciadv.abg4755.
Li, G., You, D., Ma, J., Li, W., Li, H., Sun, S., et al. (2018). The role of autoimmunity in the pathogenesis of sudden sensorineural hearing loss. Neural Plast. 2018:7691473. doi: 10.1155/2018/7691473
Li, H., Guan, Y., Liang, B., Ding, P., Hou, X., Wei, W., et al. (2022). Therapeutic potential of Mcc950, a specific inhibitor of Nlrp3 inflammasome. Eur. J. Pharmacol. 928:175091. doi: 10.1016/j.ejphar.2022.175091
Li, Z., Guo, J., and Bi, L. (2020). Role of the Nlrp3 inflammasome in autoimmune diseases. Biomed. Pharmacother. 130:110542. doi: 10.1016/j.biopha.2020.110542
Liu, H., Chen, L., Giffen, K. P., Stringham, S. T., Li, Y., Judge, P. D., et al. (2018). Cell-specific transcriptome analysis shows that adult pillar and Deiters' cells express genes encoding machinery for specializations of cochlear hair cells. Front. Mol. Neurosci. 11:356. doi: 10.3389/fnmol.2018.00356
Liu, Y. C., and Xu, K. (2023). Macrophage-related immune responses in inner ear: a potential therapeutic target for sensorineural hearing loss. Front. Neurosci. 17:1339134. doi: 10.3389/fnins.2023.1339134
Ma, J. H., Lee, E., Yoon, S. H., Min, H., Oh, J. H., Hwang, I., et al. (2022). Therapeutic effect of Nlrp3 inhibition on hearing loss induced by systemic inflammation in a CAPS-associated mouse model. EBioMedicine 82:104184. doi: 10.1016/j.ebiom.2022.104184
Mendis, S., Longley, N., Morley, S., Korres, G., and Kaski, D. Autoimmune vestibulopathy-a case series. Brain Sci. (2022) 12:306. doi: 10.3390/brainsci12030306
Miwa, T., and Okano, T. (2022). Role of inner ear macrophages and autoimmune/autoinflammatory mechanisms in the pathophysiology of inner ear disease. Front. Neurol 13:861992. doi: 10.3389/fneur.2022.861992
Molla Desta, G., and Birhanu, A. G. (2025). Advancements in single-cell rna sequencing and spatial transcriptomics: transforming biomedical research. Acta Biochim. Pol. 72:13922. doi: 10.3389/abp.2025.13922
Moltrasio, C., Romagnuolo, M., and Marzano, A. V. (2022). Nlrp3 inflammasome and Nlrp3-related autoinflammatory diseases: from cryopyrin function to targeted therapies. Front. Immunol. 13:1007705. doi: 10.3389/fimmu.2022.1007705
Moretti, J., and Blander, J. M. (2021). Increasing complexity of Nlrp3 inflammasome regulation. J. Leukoc. Biol. 109, 561–571. doi: 10.1002/JLB.3MR0520-104RR
Murillo-Cuesta, S., Seoane, E., Cervantes, B., Zubeldia, J. M., and Varela-Nieto, I. (2025). Nlrp3 inflammasome and hearing loss: from mechanisms to therapies. J. Neuroinflammation. 22:225. doi: 10.1186/s12974-025-03561-w
Nakanishi, H., Kawashima, Y., Kurima, K., Chae, J. J., Ross, A. M., Pinto-Patarroyo, G., et al. (2017). Nlrp3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss Dfna34 responsive to anakinra therapy. Proc. Natl. Acad. Sci. USA. 114, E7766–E7775. doi: 10.1073/pnas.1702946114
Nakanishi, H., Prakash, P., Ito, T., Kim, H. J., Brewer, C. C., Harrow, D., et al. (2020). Genetic hearing loss associated with autoinflammation. Front. Neurol. 11:141. doi: 10.3389/fneur.2020.00141
Nakanishi, H., Yamada, S., Kita, J., Shinmura, D., Hosokawa, K., Sahara, S., et al. (2022). Auditory and vestibular characteristics of Nlrp3 inflammasome related autoinflammatory disorders: monogenic hearing loss can be improved by anti-interleukin-1 therapy. Front. Neurol. 13:865763. doi: 10.3389/fneur.2022.865763
Oliveira, C. B., Lima, C. A. D., Vajgel, G., and Sandrin-Garcia, P. The role of Nlrp3 inflammasome in lupus nephritis. Int. J. Mol. Sci. (2021) 22:12476. doi: 10.3390/ijms222212476
Paik, S., Kim, J. K., Shin, H. J., Park, E. J., Kim, I. S., Jo, E. K., et al. (2025). Updated insights into the molecular networks for Nlrp3 inflammasome activation. Cell. Mol. Immunol. 22, 563–596. doi: 10.1038/s41423-025-01284-9
Paik, S., Kim, J. K., Silwal, P., Sasakawa, C., and Jo, E. K. (2021). An update on the regulatory mechanisms of Nlrp3 inflammasome activation. Cell. Mol. Immunol. 18, 1141–1160. doi: 10.1038/s41423-021-00670-3
Pan, J., Wang, K., Qu, J., Chen, D., Chen, A., You, Y., et al. (2024). Activated tissue-resident macrophages contribute to hair cell insults in noise-induced hearing loss in mice. Commun. Biol. 7:1078. doi: 10.1038/s42003-024-06768-4
Parekh, S., and Kaur, T. (2023). Cochlear inflammaging: cellular and molecular players of the innate and adaptive immune system in age-related hearing loss. Front. Neurol. 14:1308823. doi: 10.3389/fneur.2023.1308823
Pathak, S., Stern, C., and Vambutas, A. (2015). N-acetylcysteine attenuates tumor necrosis factor alpha levels in autoimmune inner ear disease patients. Immunol. Res. 63, 236–245. doi: 10.1007/s12026-015-8696-3
Pellegrini, C., Antonioli, L., Lopez-Castejon, G., Blandizzi, C., and Fornai, M. (2017). Canonical and non-canonical activation of nlrp3 inflammasome at the crossroad between immune tolerance and intestinal inflammation. Front. Immunol. 8:36. doi: 10.3389/fimmu.2017.00036
Poli, G., Fabi, C., Bellet, M. M., Costantini, C., Nunziangeli, L., and Romani, L. Epigenetic mechanisms of inflammasome regulation. Int. J. Mol. Sci. (2020) 21:5758. doi: 10.3390/ijms21165758
Que, X., Zheng, S., Song, Q., Pei, H., and Zhang, P. (2024). Fantastic voyage: the journey of Nlrp3 inflammasome activation. Genes Dis. 11, 819–829. doi: 10.1016/j.gendis.2023.01.009
Ralli, M., D'Aguanno, V., Di Stadio, A., De Virgilio, A., Croce, A., Longo, L., et al. (2018). Audiovestibular symptoms in systemic autoimmune diseases. J. Immunol. Res. 2018:5798103. doi: 10.1155/2018/5798103
Raneros, A. B., Bernet, C. R., Florez, A. B., and Suarez-Alvarez, B. An epigenetic insight into nlrp3 inflammasome activation in inflammation-related processes. Biomedicines (2021) 9:1614. doi: 10.3390/biomedicines9111614
Rauch, S. D. (2014). Il-1beta Inhibition in autoimmune inner ear disease: can you hear me now? J. Clin. Invest. 124, 3685–3687. doi: 10.1172/JCI77197
Ren, W., Sun, Y., Zhao, L., and Shi, X. (2024). Nlrp3 inflammasome and its role in autoimmune diseases: a promising therapeutic target. Biomed. Pharmacother. 175:116679. doi: 10.1016/j.biopha.2024.116679
Ribeiro, R., Serodio, J. F., Amaral, M. C., Duarte, J. A., Durao, C., Mendes, N., et al. (2021). Sensorineural hearing loss and systemic autoimmune disease: the experience of a systemic immune-mediated diseases unit. Cureus 13:e14075. doi: 10.7759/cureus.14075
Sai, N., Yang, Y. Y., Ma, L., Liu, D., Jiang, Q. Q., Guo, W. W., et al. (2022). Involvement of Nlrp3-inflammasome pathway in noise-induced hearing loss. Neural Regen. Res. 17, 2750–2754. doi: 10.4103/1673-5374.339499
Salminen, A., Ojala, J., Kaarniranta, K., and Kauppinen, A. (2012). Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell. Mol. Life Sci. 69, 2999–3013. doi: 10.1007/s00018-012-0962-0
Sanchez-Fernandez, A., Skouras, D. B., Dinarello, C. A., and Lopez-Vales, R. (2019). Olt1177 (Dapansutrile), a selective Nlrp3 inflammasome inhibitor, ameliorates experimental autoimmune encephalomyelitis pathogenesis. Front. Immunol. 10:2578. doi: 10.3389/fimmu.2019.02578
Schiel, V., Bhattacharya, R., Gupta, A., Eftekharian, K., Xia, A., Santa Maria, P. L., et al. (2024). Targeting the Nlrp3 inflammasome in cochlear macrophages protects against hearing loss in chronic suppurative otitis media. J. Neuroinflammation. 21:223. doi: 10.1186/s12974-024-03212-6
Sebastian-Valverde, M., and Pasinetti, G. M. The Nlrp3 inflammasome as a critical actor in the inflammaging process. Cells (2020) 9:1552. doi: 10.3390/cells9061552
Steyger, P. S. (2021). Mechanisms of ototoxicity and otoprotection. Otolaryngol. Clin. North Am. 54, 1101–1115. doi: 10.1016/j.otc.2021.08.007
Strum, D., Kim, S., Shim, T., and Monfared, A. (2020). An update on autoimmune inner ear disease: a systematic review of pharmacotherapy. Am. J. Otolaryngol. 41:102310. doi: 10.1016/j.amjoto.2019.102310
Vambutas, A., Lesser, M., Mullooly, V., Pathak, S., Zahtz, G., Rosen, L., et al. (2014). Early efficacy trial of anakinra in corticosteroid-resistant autoimmune inner ear disease. J. Clin. Invest. 124, 4115–4122. doi: 10.1172/JCI76503
Vambutas, A., and Pathak, S. (2016). Aao: autoimmune and autoinflammatory (disease) in otology: what is new in immune-mediated hearing loss. Laryngoscope Investig. Otolaryngol. 1, 110–115. doi: 10.1002/lio2.28
Wan, G., Corfas, G., and Stone, J. S. (2013). Inner ear supporting cells: rethinking the silent majority. Semin. Cell Dev. Biol. 24, 448–459. doi: 10.1016/j.semcdb.2013.03.009
Wang, C., Yang, T., Xiao, J., Xu, C., Alippe, Y., Sun, K., et al Nlrp3 inflammasome activation triggers gasdermin d-independent inflammation. Sci. Immunol. (2021) 6:eabj3859. doi: 10.1126/sciimmunol.abj3859
Wang, M., Zhang, P., Li, Q., and Kong, C. (2025a). Investigating the process of autoimmune inner ear disease: unveiling the intricacies of pathogenesis and therapeutic strategies. Int. J. Med. Sci. 22, 179–187. doi: 10.7150/ijms.97831
Wang, X., Chen, L., Wei, J., Zheng, H., Zhou, N., Xu, X., et al. (2025b). The immune system in cardiovascular diseases: from basic mechanisms to therapeutic implications. Signal Transduct. Target Ther. 10:166. doi: 10.1038/s41392-025-02220-z
Wood, M. B., and Zuo, J. (2017). The contribution of immune infiltrates to ototoxicity and cochlear hair cell loss. Front. Cell. Neurosci. 11:106. doi: 10.3389/fncel.2017.00106
Wu, T., Zhou, J., Qiu, J., Song, Y., Guo, W., Cui, L., et al. (2022). Tumor necrosis factor-alpha mediated inflammation versus apoptosis in age-related hearing loss. Front. Aging Neurosci. 14:956503. doi: 10.3389/fnagi.2022.956503
Wu, X., Yang, J., Wu, J., and Yang, X. (2024). Therapeutic potential of Mcc950, a specific inhibitor of Nlrp3 inflammasome in systemic lupus erythematosus. Biomed. Pharmacother. 172:116261. doi: 10.1016/j.biopha.2024.116261
Xu, L., Zhang, C., Jiang, N., He, D., Bai, Y., Xin, Y., et al. (2019). Rapamycin combined with Mcc950 to treat multiple sclerosis in experimental autoimmune encephalomyelitis. J. Cell. Biochem. 120, 5160–5168. doi: 10.1002/jcb.27792
Yang, J., Zhao, Y., and Shao, F. (2015). Non-canonical activation of inflammatory caspases by cytosolic Lps in innate immunity. Curr. Opin. Immunol. 32, 78–83. doi: 10.1016/j.coi.2015.01.007
Yang, J. W., Mao, B., Tao, R. J., Fan, L. C., Lu, H. W., Ge, B. X., et al. (2020). Corticosteroids alleviate lipopolysaccharide-induced inflammation and lung injury via inhibiting Nlrp3-inflammasome activation. J. Cell. Mol. Med. 24, 12716–12725. doi: 10.1111/jcmm.15849
Ye, M., Zhang, C., Ding, D., Chen, G. D., Adler, H. J., Sharaf, R., et al. (2025). Organ of corti macrophages: a distinct group of cochlear macrophages with potential roles in supporting cell degeneration and survival. Front. Immunol. 16:1617146. doi: 10.3389/fimmu.2025.1617146
Yin, H., Liu, N., Sigdel, K. R., and Duan, L. (2022). Role of Nlrp3 inflammasome in rheumatoid arthritis. Front. Immunol. 13:931690. doi: 10.3389/fimmu.2022.931690
Yu, Q., Zhao, T., Liu, M., Cao, D., Li, J., Li, Y., et al. (2021). Targeting Nlrp3 inflammasome in translational treatment of nervous system diseases: an update. Front. Pharmacol. 12:707696. doi: 10.3389/fphar.2021.707696
Yuan, V. G., Xia, A., and Santa Maria, P. L. (2025a). Immunological mechanisms in Meniere's Disease. Front. Immunol. 16:1639916. doi: 10.3389/fimmu.2025.1639916
Yuan, V. G., Xia, A., and Santa Maria, P. L. (2025b). Chronic suppurative otitis media: disrupted host-microbial interactions and immune dysregulation. Front. Immunol. 16:1547206. doi: 10.3389/fimmu.2025.1547206
Zenobia, C., and Hajishengallis, G. (2015). Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol. 2000 69, 142–159. doi: 10.1111/prd.12083
Zhang, Y., Yang, W., Li, W., and Zhao, Y. (2021). Nlrp3 inflammasome: checkpoint connecting innate and adaptive immunity in autoimmune diseases. Front. Immunol. 12:732933. doi: 10.3389/fimmu.2021.732933
Zhao, R., Zhou, H., and Su, S. B. (2013). A critical role for interleukin-1beta in the progression of autoimmune diseases. Int. Immunopharmacol. 17, 658–669. doi: 10.1016/j.intimp.2013.08.012
Keywords: autoimmune inner ear disease (AIED), sensorineural hearing loss (SNHL), NLRP3 inflammasome, adaptive immunity, innate immunity, cochlear macrophages, immunopathology, precision medicine
Citation: Yuan VG, Kullar P, Xia A and Santa Maria PL (2025) NLRP3 inflammasome as a therapeutic target in autoimmune inner ear disease. Front. Audiol. Otol. 3:1673518. doi: 10.3389/fauot.2025.1673518
Received: 26 July 2025; Revised: 06 November 2025; Accepted: 14 November 2025;
Published: 26 November 2025.
Edited by:
Toru Miwa, Teikyo University Mizonokuchi Hospital, JapanReviewed by:
Mitsuo Paul Sato, Kindai University, JapanKazuaki Homma, Northwestern University, United States
Copyright © 2025 Yuan, Kullar, Xia and Santa Maria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincent G. Yuan, dmluY2VudHl1YW5AcGl0dC5lZHU=; Peter L. Santa Maria, c2FudGFtYXJpYXBAdXBtYy5lZHU=
 Vincent G. Yuan
Vincent G. Yuan Peter Kullar
Peter Kullar Anping Xia
Anping Xia Peter L. Santa Maria
Peter L. Santa Maria