- 1Department of Otolaryngology – Head and Neck Surgery, The Ohio State University, Columbus, OH, United States
- 2Department of Biostatistics, The University of Iowa, Iowa City, IA, United States
This study aimed to determine whether the effect of changing stimulus polarity on the electrically evoked compound action potential (eCAP) threshold—referred to as the polarity effect—could be used to assess the degeneration of the peripheral axon of the cochlear nerve (CN) in human cochlear implant (CI) users. The polarity effect, measured using symmetric, biphasic pulses delivered in a monopolar-coupled stimulation mode, was systematically compared among four cochlear-implanted patient populations with differing CN statuses: children with cochlear nerve deficiency, children with biallelic Gap Junction Beta-2 gene mutations, children with idiopathic sensorineural hearing loss, and postlingually deafened adults with various etiologies. All patients used a Cochlear™ Nucleus® device in the test ear. The differences in the polarity effect across patient groups, as revealed by the results of Linear Mixed-Effect Models (LMMs), were not aligned with known anatomical differences in CN status for these patient groups regardless of quantification method. Therefore, peripheral axonal degeneration is unlikely to be the sole or primary biological contributor to the polarity effect measured using symmetric biphasic pulses in monopolar-coupled stimulation mode in human CI users.
1 Introduction
Given the emerging evidence highlighting the importance of the cochlear nerve (CN) function for CI clinical outcomes (e.g., Dong et al., 2023; Skidmore et al., 2023; Huy and Minh, 2024; Thompson et al., 2024), identifying biomarkers for CN health status has regained attention and interest in the field of cochlear implantation. Based on the results of computational simulation studies (Resnick et al., 2018; Rattay et al., 2001b,a; Rattay, 1999; Potrusil et al., 2020; Brochier et al., 2021; Kalkman et al., 2022), the polarity effect, which is defined as the difference in sensitivity of CN fibers to anodic vs. cathodic stimulation, has been suggested as a potential tool for assessing the degeneration of the peripheral axon of the CN in CI users. Specifically, these simulation results showed that cathodic stimuli presented at low stimulation levels activate the peripheral axons of healthy CN fibers to generate action potential. As stimulation level increases, the central axons become the action potential generation site. For CN fibers with degenerated peripheral axons, the cathodic stimulus needs to overcome the unmyelinated soma to activate the central axon. Compared with the central axon, the soma has high membrane capacitance. As a result, a 5- to 6-fold increase in stimulation strength is needed for the cathodic stimulus to initiate the action potential from the central axon (Rattay et al., 2001b). Therefore, the sensitivity to cathodic stimuli can be used as an indicator of peripheral axonal degeneration, with lower sensitivities indicating greater degeneration. One way to quantify this is to compare the sensitivity to cathodic stimuli with that to anodic stimuli. The rationale for this comparison is that results measured using anodic stimulation are not expected to be affected by peripheral axonal degeneration because it can directly stimulate the central axon of CN fibers in human CI users. Therefore, CN fibers with more extensive peripheral axonal degeneration are expected to show a larger decrease in sensitivity to cathodic stimuli as compared to anodic stimuli (i.e., larger polarity effects).
The polarity-dependent site of excitation is determined by the shape of the electrical field along CN fibers as well as by the trajectories of the neurons located in the electrical field (Rattay et al., 2001b,a). As a result, non-neural factors affecting the shape of electrical field, such as the relative position of the stimulating electrode to the CN, pulse configuration, and presentation mode of stimulation, also impact the polarity effect (Rattay et al., 2001b; Kalkman et al., 2022; Heshmat et al., 2021; Joshi et al., 2017). For example, Heshmat et al. (2021) reported a negligible difference in sensitivity to opposite stimulation polarities regardless of pulse configuration (i.e., monophasic, pseudomonophasic, triphasic, or biphasic pulse) or the degree of peripheral axonal degeneration when pulses were presented in the monopolar-coupled stimulation mode. Their simulation results showed that the action potential initiation sites for anodic-leading vs. cathodic-leading biphasic pulse were mostly the same when they were delivered in the monopolar-coupled stimulation mode by a peri-modiolar electrode. Based on their results, they concluded that this combination of pulse configuration, presentation mode and electrode type was not preferred to assess peripheral axonal degeneration of the CN. To distinguish the degeneration patterns of CN fibers across the cochlea based on the polarity effect, asymmetric pulse shapes presented in multipolar coupled stimulation modes with the consideration of the electrode-to-modiolus distance were recommended. However, Kalkman et al. (2022) recently demonstrated that the relative position of the stimulating electrode to the CN fibers significantly influenced the direction of change in the polarity effect elicited by monophasic pulses presented in the monopolar-coupled stimulation mode across neurons with varying degrees of peripheral axon integrity. Specifically, they found that neurons tended to have lower thresholds for cathodic, compared with anodic, monophasic pulses when stimulation was delivered via a peri-modiolar electrode. In contrast, when using a lateral wall electrode, the same neurons exhibited lower thresholds to anodic stimulation than to cathodic stimulation. This electrode array effect was more pronounced in neurons with intact or partially degenerated peripheral axons than in those with complete peripheral axon loss. As a result, Kalkman et al. (2022) questioned the reliability of the polarity effect as a consistent indicator of neural health.
While simulation results provide valuable insights into the potential effects of different factors on the polarity effect, all CN models developed to date utilize some amount of anatomical, morphometric data, biophysical properties and/or physiological data measured in non-human animal models (Skidmore et al., 2022). The applicability of these parameters to human listeners remains uncertain due to substantial differences in anatomical/morphometric and biophysical properties of CN fibers, cochlear volume, and etiology and duration of deafness between human listeners and all other mammalian species (Skidmore et al., 2022). Therefore, these simulation results need to be tested and validated in human listeners, rather than regarded as definitive.
To determine the applicability of these simulation results to human CI users, several studies have investigated the polarity effect using electrophysiological measures of the electrically evoked compound action potential (eCAP) or psychophysical measures in CI users (e.g., Goehring et al., 2019; Hughes, 2022, 2023; Hughes et al., 2018; Jahn and Arenberg, 2019a,b; Luo et al., 2020; Macherey et al., 2017; Mesnildrey et al., 2020; Undurraga et al., 2013; Xu et al., 2020; Carlyon et al., 2018; Herrmann et al., 2021; Macherey et al., 2008; Hughes et al., 2017). The eCAP is a near-field recording of neural responses generated by a group of electrically activated CN fibers (He et al., 2017). Overall, the results of these studies are inconsistent and do not fully align with the simulation findings. For example, Luo et al. (2020) and Hughes (2022) observed a significant polarity effect on the eCAP threshold (i.e., the lowest stimulation level to evoke an eCAP) measured using symmetric, biphasic pulses presented in the monopolar-coupled stimulation mode in pediatric and adult CI users, respectively, with lower eCAP thresholds measured for anodic-leading, biphasic pulses. Similarly, even though simulation results demonstrated a strong polarity effect on neural response threshold measured using asymmetric pulses (e.g., Rattay et al., 2001b; Rattay, 1999; Heshmat et al., 2021), this finding was not observed in human CI users at the auditory detection threshold level (Macherey et al., 2017; Undurraga et al., 2013; Macherey et al., 2006). Furthermore, the polarity effect on auditory detection threshold measured using multiphasic pulses presented in the monopolar- or partial tripolar-coupled stimulation mode in human CI users is not affected by the electrode-modiolus distance, electrode scalar location, or intracochlear resistance (Jahn and Arenberg, 2019a; Mesnildrey et al., 2020), which is not consistent with the strong impact of the relative position of the stimulating electrode to the CN on the polarity effect reported in several simulation studies (Rattay et al., 2001b; Rattay, 1999; Heshmat et al., 2021). Finally, regardless of differences in pulse configuration, presentation mode and evaluation methods, variations in polarity sensitivity at the threshold level across electrode locations within individual CI users are evident (e.g., Hughes, 2023; Jahn and Arenberg, 2019a,b). Factors accounting for these variations remain unknown. Overall, these results do not provide conclusive evidence supporting an association between the polarity effect and the degeneration of the peripheral axon of the CN in human CI users.
Histological results of human temporal bone studies revealed four patient populations with different statuses of the CN, providing an opportunity to better understand the polarity effect in human listeners. These populations include children with cochlear nerve deficiency (CND), children with biallelic Gap Junction Beta-2 (GJB2) gene mutations, children with idiopathic sensorineural hearing loss (SNHL) and normal-sized CNs, and postlingually deafened adult CI users. CND refers to a small (hypoplastic) or absent (aplastic) CN as revealed by high-resolution magnetic resonance imaging (MRI). The mean SGN count, calculated based on all histological results of human temporal bone studies for children with CND, is 5,739 (range: 0–15,714), representing approximately 16.4% of the SGN count expected for the corresponding age (i.e., 35,500; Wright et al., 1986; Glueckert et al., 2010; Nelson and Hinojosa, 2001; Haginomori et al., 2002; Chen et al., 2020; da Costa Monsanto et al., 2022). In comparison, the mean SGN count in children with SNHL and normal-sized CNs ranges from 14,302 to 20,523 (Miura et al., 2002). The axons of existing SGNs in children with CND are myelinated, with no apparent sign of degeneration (Wright et al., 1986; Glueckert et al., 2010). Additionally, the number of existing SGNs in children with CND follows a unique “base-to-apex” decreasing pattern (He et al., 2018, 2020), with more SGNs located at the more basal regions of the cochlea. GJB2 encodes the protein connexin 26, a gap junction protein expressed in the cochlea and epidermis. As a result, GJB2 mutation causes no noticeable neural damage in the CN (Jun et al., 2000). The results of human temporal bone studies demonstrated an age-related loss in SGN soma and an even greater loss in the peripheral axon (~50%) in adult listeners (Wu et al., 2019, 2023; Liu et al., 2015). Compared with the apical regions, where the electrode array of the Cochlear™ Nucleus® device ends (0.6–2 kHz; Dhanasingh and Jolly, 2017), the basal area of the cochlea shows the greatest loss in both SGN soma and peripheral axon (Wu et al., 2019, 2023; Liu et al., 2015). Noise exposure causes additional peripheral axon loss across different cochlear regions (Wu et al., 2021). A temporal bone study in one CI case showed total loss of peripheral axons for all surviving SGNs at the basal half of the cochlea. In contrast, peripheral axon counts in the apical cochlea of this case are similar to those of age-matched, non-implanted listeners (O'Malley et al., 2024). To summarize, children with CND represent a patient population with fewer but otherwise healthy SGNs with a known distribution pattern of existing SGNs, children with biallelic GJB2 gene mutations represent a patient population with minimal neural damage in the CN, postlingually deafened adult CI users represent a patient population with reduced SGN counts and substantial peripheral axon loss in the basal cochlea, and children with idiopathic SNHL and normal-sized CNs represent a patient population with less SGN soma and peripheral axon loss than adult CI users.
Currently, symmetric, biphasic pulses presented in a monopolar-coupled stimulation mode are standard programming settings for most CI users. As a result, this combination is still used to assess the polarity effect in recent studies aiming to develop clinical tools to assess local CN health or to identify biomarkers for CI clinical outcomes (e.g., Hughes, 2023; Marx et al., 2025; Konerding et al., 2022, 2025). As one step toward identifying the biological underpinnings of the polarity effect observed in human CI users, we leveraged datasets previously collected in children with CND, children with biallelic GJB2 gene mutations, and children with idiopathic SNHL using symmetric, biphasic pulses presented in a monopolar-coupled stimulation mode (Luo et al., 2020; Xu et al., 2020). Among these pediatric participants, all except one had a peri-modiolar electrode array in the test ear, with the remaining participant using a lateral wall array. These existing datasets were combined with results from four additional children with biallelic GJB2 gene mutations and systematically compared with those from postlingually deafened adult CI users to gain further insight into the polarity effect in human CI users.
In the current study, the polarity effect was defined as the difference in the eCAP threshold between the results measured using anodic-cathodic (AC) and cathodic-anodic (CA), biphasic pulses (i.e., thresholdAC – thresholdCA). Based on modeling data, lower anodic than cathodic thresholds (i.e., negative numbers) may indicate some degree of peripheral axonal degeneration. The polarity effect on the eCAP threshold was chosen because the literature suggested the polarity effect measured at the threshold level varies independently of electrode position and tissue impendence (Jahn and Arenberg, 2019a), and reflects the local CN health (Macherey et al., 2017). Therefore, eCAP parameters based on responses measured at high stimulation levels (e.g., slope of the eCAP amplitude growth function and the maximum eCAP amplitude) were not investigated in this study. Based on the literature, we hypothesized that the polarity effect on the eCAP threshold measured using symmetric, biphasic pulses presented in a monopolar-coupled stimulation mode would not be a reliable indicator of peripheral axonal degeneration in human CI users. Based on the proposed hypothesis, it was expected that the polarity effect on the eCAP threshold would not capture differences in peripheral axon loss either among the four patient groups or between basal and apical cochlear regions in adult CI users, as reported in the human temporal bone studies cited above.
2 Materials and methods
2.1 General methods
All participants were implanted with a Cochlear™ Nucleus® device (Cochlear Ltd., Macquarie, NSW, Australia) with a full electrode insertion in the test ear. Each participant had at least 6 months of listening experience with their CIs prior to testing. For pediatric participants, the anatomical status of the CN and the inner ear was determined based on the results of high-resolution MRI and Computed Tomography (CT) temporal bone scans, following the same protocol and criteria as described in our previous studies (Luo et al., 2020; He et al., 2018). The study was approved by the Biomedical Institutional Review Board of The Ohio State University. Written informed consents were obtained from participants and/or their legal guardians prior to participation.
For children with CND, three electrodes with measurable eCAPs were tested. These testing sites extended to the most apical electrode location where an eCAP could be recorded and had a relatively equal separation between testing electrodes. The electrodes were classified as the “basal,” the “middle,” and the “apical” electrode based on their relative locations among those with measurable eCAPs. For other pediatric participants, one basal electrode (electrode 3), one middle-array electrode (electrode 12), and one apical electrode (electrode 19 or 21) were tested. For adult participants, four electrodes across the array (electrodes 3, 9, 15, and 21) were tested. The electrode locations for testing were adjusted in cases of open or short circuits at the default electrode location and/or the absence of eCAPs at the maximum comfortable level. The CI electrodes tested in each participant for different experiments are listed in Table 1 and Supplementary Table 1.
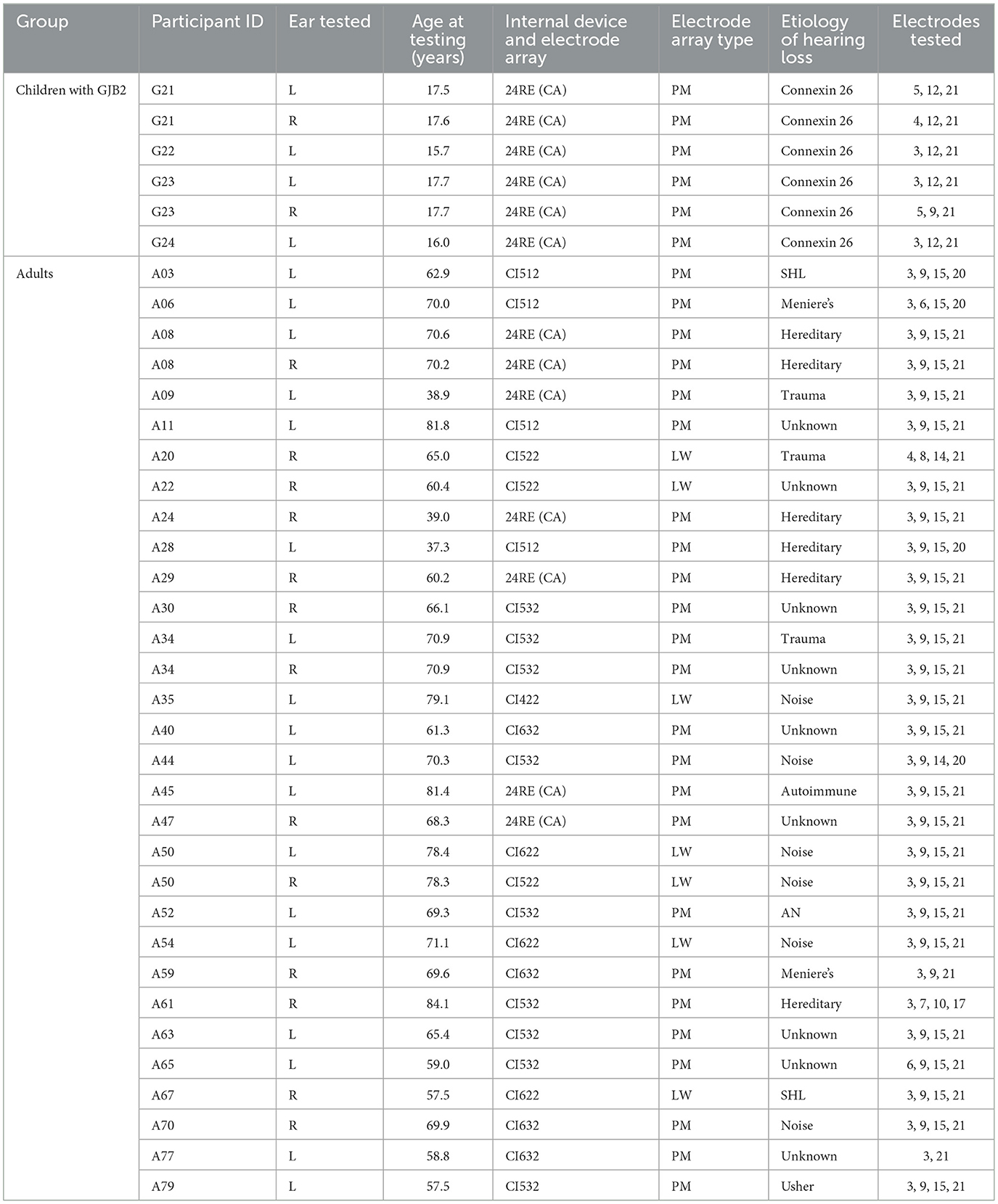
Table 1. Demographic information of the study participants whose data have not been reported in Luo et al. (2020) or Xu et al. (2020).
2.2 Participants
Data from 31 children with CND, 20 children with biallelic GJB2 gene mutations, and 34 children with idiopathic SNHL tested in our previous studies (Luo et al., 2020; Xu et al., 2020) were reanalyzed and included in this study. Supplementary Table 1 includes demographic information of these participants. Table 1 of these two studies (Luo et al., 2020; Xu et al., 2020) provide additional participant demographics. Four additional children with biallelic GJB2 gene mutations (one girl and three boys) ranging in age between 15.7 and 17.7 years (mean: 17.0 yrs, SD: 0.9 yrs) at the time of testing were recruited. Both ears were tested for two of these four children (G21 and G23). All except for one (G7) pediatric participant used a peri-modiolar electrode array in the test ear. In addition to these pediatric participants, 28 postlingually deafened adult CI users (14 females and 14 males), ranging in age between 37.3 and 84.1 years (mean: 65.9 yrs, SD: 11.7 yrs) at the time of testing, were recruited for this study. For three participants (A08, A34, and A50), both ears were tested. Only one ear was tested for all other adult participants. Detailed demographic information of these adult participants and the four newly tested children with biallelic GJB2 gene mutations is listed in Table 1. There is no overlap between the participants listed in Table 1 and Supplementary Table 1.
2.3 eCAP measurements procedures
The eCAP was measured using the Advanced Neural Response Telemetry (NRT) function that is implemented in the Custom Sound EP (v. 5.1 or v.6.2) software (Cochlear Ltd, Macquarie, NSW, Australia) and a two-pulse forward-masking-paradigm (Brown et al., 1990). The stimuli were charge-balanced, biphasic pulses with an interphase gap of 7 μs. In children with CND, pulse phase durations that were required to elicit a measurable eCAP ranged from 37 to 75 μs/phase. For one adult participant (A044), eCAPs were measured using a pulse phase duration of 37 μs/phase. For all other participants, a pulse phase duration of 25 μs/phase was used to measure eCAP responses. The stimulus was presented to individual CI electrodes in a monopolar-coupled stimulation mode via a Freedom or N5 sound processor interfaced with a programming pod. Parameters used for eCAP measures included a probe rate of 15 Hz, a masker-probe-interval of 400 μs, an effective sampling rate of 20,492 Hz, and sampling delays ranging between 98 and 122 μs.
For each of 31 ears of 31 children with CND, 27 ears of 24 children with biallelic GJB2 gene mutations, 35 ears of 34 children with idiopathic SNHL, and 31 ears of 28 postlingually deafened adult patients, eCAP input/output (I/O) functions were obtained for both anodic- and cathodic-leading, biphasic pulses at each CI electrode tested. The anodic-leading stimulus consisted of an anodic phase followed by a cathodic phase, and vice versa for the cathodic-leading stimulus. For each study participant, the C level for the anodic-leading stimulus (i.e., the anodic C level) was measured for each testing electrode using the same procedure as reported previously (He et al., 2018, 2024), and was used as the highest stimulation level tested in this study.
To obtain the eCAP I/O function, the masker pulse was presented at the anodic C level, the probe pulse was presented at 5–10 CLs below the anodic C level. The probe level was systematically decreased with steps of 1 CL for the first five steps and then in steps of 5 CLs until no eCAP response could be visually identified. The probe level was subsequently increased in steps of 1 CL until at least five eCAPs were recorded using this small step size.
2.4 Data analysis
Custom-designed scripts in MATLAB R2021b (MathWorks, MA) were used to measure the eCAP threshold which was defined as the lowest stimulation level that elicited an eCAP response with an amplitude of 5 μV or larger (Patrick et al., 2006). In some studies, the polarity effect was defined as the numerical difference in the lowest stimulation level quantified using a linear scale (Rattay et al., 2001b,a; Rattay, 1999; Heshmat et al., 2021). In other studies, it was defined as the ratio of current change (Luo et al., 2020; Xu et al., 2020) or the logarithm of this ratio (i.e., dB; e.g., Kalkman et al., 2022; Goehring et al., 2019; Hughes, 2022; Jahn and Arenberg, 2019b; Macherey et al., 2008). To better compare with the literature, the polarity effect was quantified using both a linear scale (in nC) and a logarithmic scale (in dB re: 1 nC) in this study. It was calculated by subtracting eCAP thresholds measured using cathodic-leading, biphasic pulses (CA) from those measured using anodic-leading, biphasic pulses (AC; i.e., thresholdAC – thresholdCA). Due to differences in pulse phase duration used in different participant groups, the eCAP thresholds shown in Figure 1 were quantified using a linear scale in nC.
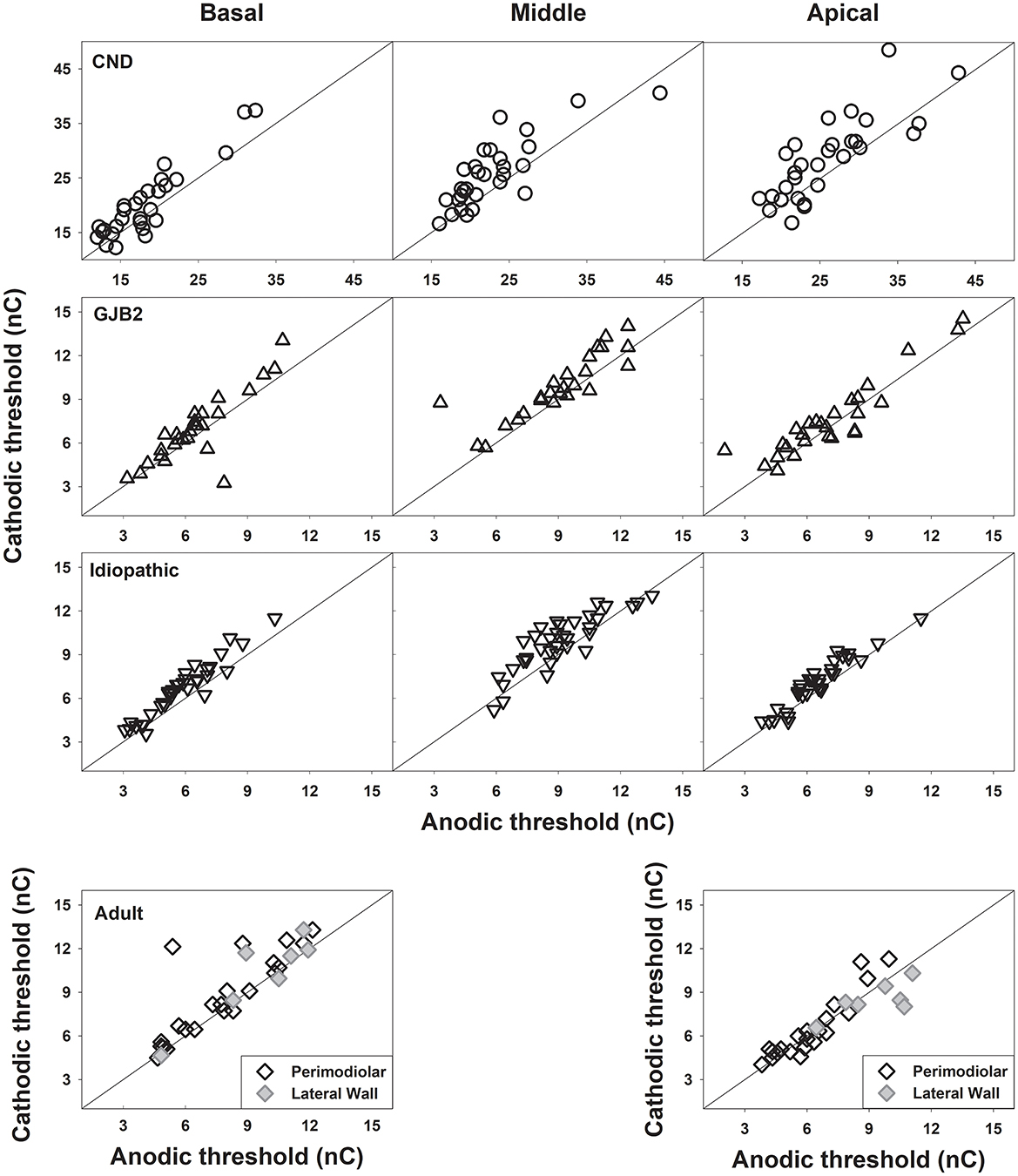
Figure 1. eCAP thresholds measured using cathodic-leading, biphasic pulses are plotted against those measured using anodic-leading, biphasic pulses for the pediatric participant group [top three rows] and the adult participant group [bottom row]. Each symbol represents the result from one participant, with different symbol types indicating different participant groups. The results are shown for each electrode location in each panel. In each panel, the diagonal line represents equal sensitivity to both polarities.
2.5 Statistical comparisons
Linear Mixed effects Models (LMMs) were used to: (1) compare the polarity effect at different electrode locations within each of four patient populations; (2) compare the polarity effect measured at different electrode locations among the three pediatric patient populations; and (3) compare the polarity effect measured at the most basal and the most apical electrode locations tested among four patient populations. All LMMs used a random intercept for subject and random slope for electrode to account for repeated measures by electrode, some individuals with measurements in both ears, and differing variances by electrode. Estimations were obtained using restricted maximum likelihood with Kenward-Roger degrees of freedom. Tukey's Honest Significant Difference (Tukey's HSD) method was applied to adjust for multiple comparisons. In adult CI users, two-tailed Pearson correlation analyses were used to assess the relationship between age at testing and the polarity effect, quantified using both scales. Separate analyses were performed for the most basal and apical electrode sites, with Bonferroni correction for multiple comparisons. All statistical analyses for this study were performed using R software v. 4.3.0 (R Core Team, 2023, https://www.R-project.org/) using the lme4 package (Bates et al., 2015). Statistical significance was determined at the 95% confidence level (i.e., p < 0.05).
3 Results
3.1 eCAP thresholds for different stimulation polarities
Figure 1 shows eCAP thresholds (in nC) measured for the cathodic-leading stimulus plotted against those measured for the anodic-leading stimulus at three electrode locations in the three pediatric patient groups, and at one basal and one apical electrode locations in adult patient group. These data were plotted using the same format as in Heshmat et al. (2021) to allow for direct comparison. While the results from children with biallelic GJB2 gene mutations and children with idiopathic SNHL cluster around the reference line, indicating equal sensitivity to both polarities, the results from children with CND exhibit a different pattern. Specifically, data from children with CND show clear deviations from the reference line, indicating differences in eCAP thresholds measured with different pulse polarities. Notably, these deviations tend to increase toward the more apical regions of the cochlea. The data shown for adult patients include results from 25 ears implanted with a peri-modiolar electrode array (black diamonds) and six ears implanted with a lateral-wall electrode array (gray diamonds). These results also exhibit deviations from the reference line; however, the deviations appear to be more pronounced at the basal electrode location, which contrasts with the pattern observed in children with CND.
3.2 Polarity effect results
Figure 2 illustrates the polarity effects on eCAP thresholds measured at three electrode locations in the pediatric patient groups and at four locations in adult patients. Figures 2a, b display the results using linear and logarithmic scales, respectively. These data clearly demonstrate differences in results across electrode locations within each participant group and among the groups. While different quantification scales affected the magnitude of these differences, they did not alter their direction. The statistical findings are summarized below.
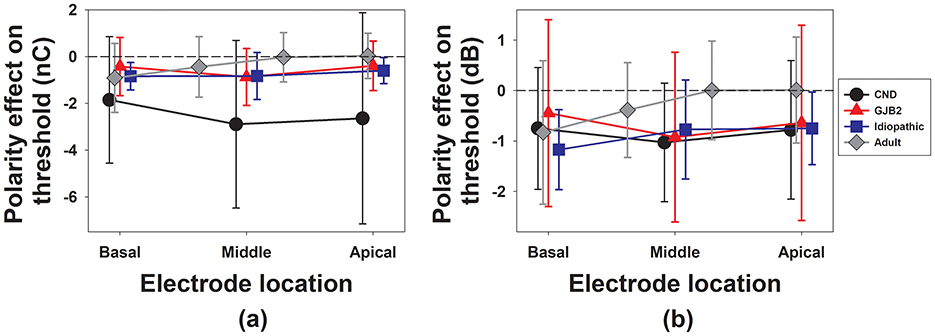
Figure 2. The means and standard deviations of the polarity effect on the eCAP threshold quantified using a linear scale (a) and a logarithmic scale (b) at each electrode location in different participant groups. Different symbol types represent different participant groups. The dashed line in each panel denotes the null polarity effect on the eCAP threshold.
3.2.1 Results quantified using a linear scale
For the three pediatric participant groups, the results of LMMs showed a significant effect of participant group [F(2, 84.53) = 4.55, p = 0.013]. The effect of electrode location [F(2, 79.43) = 2.39, p = 0.098] or the interaction between participant group and electrode location [F(4, 75.17) = 0.75, p = 0.456] did not reach statistical significance. The results of pairwise comparisons showed that children with CND had a significant larger polarity effect than both children with GJB2 (p = 0.001) and children with idiopathic SNHL (p < 0.001). It should be noted that the level of electric stimulation (in nC) required to evoke the eCAP in children with CND is much higher than that used for the other two groups of pediatric participants, which could have contributed to this significant group difference. There was no significant difference in the polarity effect between children with GJB2 and children with idiopathic SNHL (p = 0.952).
For the data measured in adult participants, the results of LMMs revealed a significant effect of electrode location [F(3, 25.68) = 9.83, p < 0.001]. Pairwise comparisons revealed that the polarity effect on the eCAP at the most basal electrode (electrode 3) was significantly larger than at the two apical electrodes: electrode 15 (p < 0.001) and electrode 21 (p = 0.024). No other pairwise differences were statistically significant (p > 0.05). No other pairwise comparison results were statistically significant (p > 0.05).
For the results measured at one basal and one apical electrode across four patient groups, the LMM showed a significant effect of participant group [F(3, 109.76) = 3.21, p = 0.026]. There was no significant effect of electrode location [F(1, 101.04) = 3.83, p = 0.053] or the interaction between participant group and electrode location [F(3, 103.45) = 2.31, p = 0.080]. Pairwise comparisons revealed that children with CND had a significantly larger polarity effect than all other participant groups. No other pairwise comparison results were statistically significant (p > 0.05). Table 2 includes details of these pairwise comparison results.
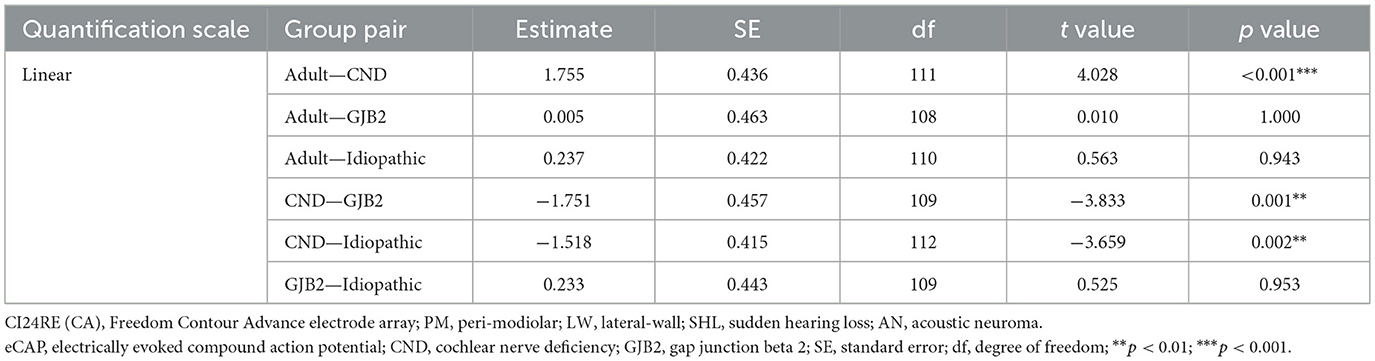
Table 2. Pairwise comparisons for the polarity effect quantified using a linear scale between adult participants and the three pediatric participant groups at two electrode locations.
3.2.2 Results quantified using a logarithmic scale
For the three pediatric participant groups, the LMM revealed no significant effect of participant group [F(2, 95.51) = 1.80, p = 0.171], electrode location [F(2, 96.00) = 0.85, p = 0.428] or their interaction [F(4, 95.09) = 0.83, p = 0.511].
Similar to the results quantified using a linear scale, the LMM showed a significant effect of electrode location [F(3, 25.72) = 9.03, p < 0.001] on the polarity effect in adult participants. Pairwise comparisons showed that the polarity effect on the eCAP measured at electrode 3 was significantly larger than that measured at electrode 15 (p < 0.001). No other pairwise comparison results were statistically significant (p > 0.05).
For the results measured at the two electrode locations across four patient groups, the LMM showed a significant effect of electrode location [F(1, 101.16) = 7.23, p = 0.008], with a larger polarity effect at the basal than the apical electrode, but there was no significant effect of participant group [F(3, 116.58) = 1.10, p = 0.353] or the interaction between these two factors [F(3, 105.63) = 1.94, p = 0.128].
3.2.3 Relationships with age at testing
Pearson correlation analyses showed no significant correlation between age at testing and the polarity effect, regardless of cochlear location or quantification scale. Detailed results are provided in Supplementary Table 2.
4 Discussion
4.1 Results discussion
One aim of this study was to confirm the simulation results reported in Heshmat et al. (2021) showing similar thresholds measured using AC or CA pulses when monopolar stimulation mode was used in patients with peri-modiolar electrodes. In contrast, our results in children with CND (Figure 1) clearly showed higher eCAP thresholds for the cathodic-leading pulse compared to those measured using the anodic-leading biphasic pulse. This pattern was particularly pronounced for the results measured at more apical electrode locations where fewer SGNs exist. We speculate that this may be due to differences in the relative position of the stimulating electrode to CN fibers in different intracochlear regions in children with CND. Specifically, the “effective” distance between the electrode and the remaining CN fibers may increase at more apical locations due to the reduced SGN population. As a result, a peri-modiolar electrode may function more like a lateral wall electrode in these regions, leading to lower thresholds for anodic stimulation in intact SGNs—consistent with findings reported by Kalkman et al. (2022). This speculation is supported by the diminished difference among the three pediatric patient populations when the polarity effect was quantified using a logarithmic scale to control for the group difference in the amount of “effective” current that stimulated existing SGNs (McKay, 2012). However, due to the limited literature on how substantial SGN loss affects the polarity effect, it remains unclear whether the observed pattern is directly attributable to the reduced SGN count in this unique patient population. Unfortunately, postoperative CT scans were not available in pediatric CI users because they are not part of standard clinical care. As a result, we were not able to conduct further data analysis to determine or disentangle the contributions of these two factors. It is worth noting that, although the threshold data measured in children with CND differed from those reported by Heshmat et al. (2021), our findings on the polarity effect—discussed below—are consistent with their recommendation against using symmetric biphasic pulses delivered in a monopolar coupled stimulation mode to assess peripheral axonal degeneration in human CI users.
The primary aim of this study was to determine whether the polarity effect on the eCAP threshold measured using symmetric biphasic pulse delivered in the monopolar-coupled stimulation mode could be used to assess the degeneration of the peripheral axon of the CN in human CI users. Regardless of the scale used to quantify this polarity effect, adult patients showed a larger polarity effect at the most basal electrode location tested in this study—the region where substantial peripheral axonal degeneration likely occurred (e.g., Wu et al., 2023; O'Malley et al., 2024)—than those measured at more apical regions of the cochlea. These results align with the proposed relationship between the polarity effect and peripheral axonal degeneration. However, the polarity effects measured in adult participants were comparable to those observed in children with GJB2—a patient population with no noticeable neural damage in the CN (Jun et al., 2000)—even at the most basal electrode locations, regardless of the quantification method. In other words, if the polarity effect measured using the stimulus and configuration employed in this study truly reflects peripheral axon degeneration, adult CI users with substantial peripheral axon loss, particularly in the basal region of the cochlea, would be expected to show greater polarity effects than children with GJB2. Contrary to these expectations, our data did not reveal significant group differences in polarity effects between these two patient populations. Therefore, these results clearly do not support the polarity effect observed in this study as a reliable indicator of peripheral axonal degeneration in CI users. The lack of group difference in the polarity effect between adult and pediatric CI users is also consistent with those reported in Jahn and Arenberg (2019b) in which the polarity effect on auditory detection threshold was evaluated using triphasic pulses. Overall, our findings do not support the use of polarity effect assessed using symmetric biphasic pulse delivered in the monopolar-coupled stimulation mode as an indicator of peripheral axonal degeneration in CI users, regardless of quantification method. The large polarity effect quantified on a linear scale in children with CND, along with the variation in polarity effects across electrode locations in adult CI users suggests that the measured polarity effect is associated with CN health. However, the specific aspects of CN health to which it relates remain unknown. Further studies are warranted to investigate the biological mechanisms underlying these polarity effects, utilizing the stimulus and presentation configurations employed in this study.
4.2 Study limitations
The first limitation is that the results and conclusions of this study are only applicable to those measured using symmetric, biphasic pulses presented in a monopolar-coupled stimulation mode. It remains unclear whether these results and explanations apply to studies using electrical pulses with other pulse shapes or presented in a non-monopolar-coupled stimulation mode. Further studies using asymmetric pulses presented in multipolar coupled stimulation modes in specific patient groups with well-characterized anatomical integrity of the CN are warranted to determine the biological underpinnings of this effect. Second, the limited number of adult participants implanted with a lateral-wall electrode array prevented us from evaluating the effect of electrode array type on the results. Third, due to the lack of postoperative CT scans for most participants, we could not assess the potential influence of electrode-to-modiolus distance, insertion angle, or insertion depth on the outcomes. These three factors should be considered in future studies.
5 Conclusions
Peripheral axonal degeneration is unlikely to be the sole or primary biological contributor to the polarity effect measured using symmetric biphasic pulses delivered in monopolar-coupled stimulation mode in human CI users. Further studies are warranted to determine the optimal combination of stimulation and recording parameters for assessing the polarity effect as a means of quantifying peripheral axonal degeneration of CN fibers in human CI users.
Data availability statement
The data that support the findings of this study are available from the authors upon reasonable request with permission from The Ohio State University.
Ethics statement
The data reported in this study were collected for projects that were approved by the biomedical Institutional Review Board (IRB) of the Ohio State University (IRB study #: 2017H0131, 2018H0344, and 2018N0005; PI: Shuman He), and the IRB of Shandong ENT Hospital (IRB study #: SENTP, 2016-2; PI: Lei Xu). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
SH: Formal analysis, Writing – original draft, Funding acquisition, Resources, Visualization, Project administration, Methodology, Supervision, Writing – review & editing, Conceptualization, Investigation, Validation. JO: Formal analysis, Writing – review & editing, Validation. ZG: Validation, Writing – review & editing, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was partially supported by grants from the National Institutes of Health awarded to SH [grant numbers: 1R01DC016038, 1R01DC017846, and R21DC019458].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fauot.2025.1693293/full#supplementary-material
References
R Core Team (2023). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Brochier, T., Guerit, F., Deeks, J. M., Garcia, C., Bance, M., Carlyon, R. P., et al. (2021). Evaluating and comparing behavioural and electrophysiological estimates of neural health in cochlear implant users. J. Assoc. Res. Otolaryngol. 22, 67–80. doi: 10.1007/s10162-020-00773-0
Brown, C. J., Abbas, P. J., and Gantz, B. (1990). Electrically evoked whole-nerve action potentials: data from human cochlear implant users. J. Acoust. Soc. Am. 88, 1385–1391. doi: 10.1121/1.399716
Carlyon, R. P., Cosentino, S., Deeks, J. M., Parkinson, W., and Arenberg, J. A. (2018). Effect of stimulus polarity on detection thresholds in cochlear implant users: relationships with average threshold, gap detection, and rate discrimination. J. Assoc. Res. Oto. 19, 559–567. doi: 10.1007/s10162-018-0677-5
Chen, J. X., Nourmahnad, A., O'Malley, J., Reinshagen, K., Nadol, J. B. Jr., and Quesnel, A. M. (2020). Otopathology in CHARGE syndrome. Laryngoscope Investig. Otolaryngol. 5, 157–162. doi: 10.1002/lio2.347
da Costa Monsanto, R., Knoll, R. M., de Oliveira Penido, N., Song, G., Santos, F., Paparella, M. M., et al. (2022). Otopathologic abnormalities in CHARGE syndrome. Otolaryngol. Head Neck Surg. 166, 363–372. doi: 10.1177/01945998211008911
Dhanasingh, A., and Jolly, C. (2017). An overview of cochlear implant electrode array designs. Hear. Res. 356, 93–103. doi: 10.1016/j.heares.2017.10.005
Dong, Y., Briaire, J. J., Stronks, H. C., and Frijns, J. H. M. (2023). Speech perception performance in cochlear implant recipients correlates to the number and synchrony of excited auditory nerve fibers derived from electrically evoked compound action potentials. Ear Hear. 44, 276–286. doi: 10.1097/AUD.0000000000001279
Glueckert, R., Rask-Andersen, H., Sergi, C., Schmutzhard, J., Mueller, B., Beckmann, F., et al. (2010). Histology and synchrotron radiation-based microtomography of the inner ear in a molecularly confirmed case of CHARGE syndrome. Am. J. Med. Genet. A. 152A, 665–673. doi: 10.1002/ajmg.a.33321
Goehring, T., Archer-Boyd, A., Deeks, J. M., Arenberg, J. G., and Carlyon, R. P. A. (2019). Site-selection strategy based on polarity sensitivity for cochlear implants: effects on spectro-temporal resolution and speech perception. J. Assoc. Res. Otolaryngol. 20, 431–448. doi: 10.1007/s10162-019-00724-4
Haginomori, S., Sando, I., Miura, M., and Casselbrant, M. L. (2002). Temporal bone histopathology in CHARGE association. Ann. Otol. Rhinol. Laryngol. 111, 397–401. doi: 10.1177/000348940211100503
He, S., Shahsavarani, B. S., McFayden, T. C., Wang, H., Gill, K. E., Xu, L., et al. (2018). Responsiveness of the electrically stimulated cochlear nerve in children with cochlear nerve deficiency. Ear Hear. 39, 238–250. doi: 10.1097/AUD.0000000000000467
He, S., Skidmore, J., Bruce, I. C., Oleson, J. J., and Yuan, Y. (2024). Peripheral neural synchrony in postlingually deafened adult cochlear implant users. Ear Hear. 45, 1125–1137. doi: 10.1097/AUD.0000000000001502
He, S., Teagle, H. F. B., and Buchman, C. A. (2017). The electrically evoked compound action potential: from laboratory to clinic. Front. Neurosci. 11:339. doi: 10.3389/fnins.2017.00339
He, S., Xu, L., Skidmore, J., Chao, X., Jeng, F. C., Wang, R., et al. (2020). The effect of interphase gap on neural response of the electrically stimulated cochlear nerve in children with cochlear nerve deficiency and children with normal-sized cochlear nerves. Ear Hear. 41, 918–934. doi: 10.1097/AUD.0000000000000815
Herrmann, D. P., Kretzer, K. V. A., Pieper, S. H., and Bahmer, A. (2021). Effects of electrical pulse polarity shape on intra cochlear neural responses in humans: triphasic pulses with anodic and cathodic second phase. Hear. Res. 412:108375. doi: 10.1016/j.heares.2021.108375
Heshmat, A., Sajedi, S., Schrott-Fischer, A., and Rattay, F. (2021). Polarity sensitivity of human auditory nerve fibers based on pulse shape, cochlear implant stimulation strategy and array. Front. Neurosci. 15:751599. doi: 10.3389/fnins.2021.751599
Hughes, M. L. (2022). Characterizing polarity sensitivity in cochlear implant recipients: demographic effects and potential implications for estimating neural health. J. Assoc. Res. Otolaryngol. 23, 301–318. doi: 10.1007/s10162-021-00824-0
Hughes, M. L. (2023). Electrically evoked compound action potential polarity sensitivity, refractory-recovery, and behavioral multi-pulse integration as potential indices of neural health in cochlear-implant recipients. Hear. Res. 433. doi: 10.1016/j.heares.2023.108764
Hughes, M. L., Choi, S., and Glickman, E. (2018). What can stimulus polarity and interphase gap tell us about auditory nerve function in cochlear-implant recipients? Hear. Res. 359, 50–63. doi: 10.1016/j.heares.2017.12.015
Hughes, M. L., Goehring, J. L., and Baudhuin, J. L. (2017). Effects of stimulus polarity and artifact reduction method on the electrically evoked compound action potential. Ear Hear. 38, 332–343. doi: 10.1097/AUD.0000000000000392
Huy, P. T., and Minh, L. T. Q. (2024). Efficacy of cochlear implantation in cochlear nerve deficiency children - a single center study. Am. J. Otolaryngol. 45. doi: 10.1016/j.amjoto.2024.104428
Jahn, K. N., and Arenberg, J. G. (2019a). Evaluating psychophysical polarity sensitivity as an indirect estimate of neural status in cochlear implant listeners. J. Assoc. Res. Otolaryngol. 20, 415–430. doi: 10.1007/s10162-019-00718-2
Jahn, K. N., and Arenberg, J. G. (2019b). Polarity sensitivity in pediatric and adult cochlear implant listeners. Trends Hear. 23, 1–22. doi: 10.1177/2331216519862987
Joshi, S. N., Dau, T., and Epp, B. A. (2017). Model of electrically stimulated auditory nerve fiber responses with peripheral and central sites of spike generation. J. Assoc. Res. Otolaryngol. 18, 323–342. doi: 10.1007/s10162-016-0608-2
Jun, A. I., McGuirt, W. T., Hinojosa, R., Green, G. E., Fischel-Ghodsian, N., Smith, R. J., et al. (2000). Temporal bone histopathology in connexin 26-related hearing loss. Laryngoscope 110, 269–275. doi: 10.1097/00005537-200002010-00016
Kalkman, R. K., Briaire, J. J., Dekker, D. M. T., and Frijns, J. H. M. (2022). The relation between polarity sensitivity and neural degeneration in a computational model of cochlear implant stimulation. Hear. Res. 415. doi: 10.1016/j.heares.2021.108413
Konerding, W., Arenberg, J., Kral, A., and Baumhoff, P. (2025). Cochlear health alters the polarity effect and spike-initiation sites in guinea pigs. Hear. Res. 465:109341. doi: 10.1016/j.heares.2025.109341
Konerding, W., Arenberg, J. G., Kral, A., and Baumhoff, P. (2022). Late electrically-evoked compound action potentials as markers for acute micro-lesions of spiral ganglion neurons. Hear. Res. 413. doi: 10.1016/j.heares.2020.108057
Liu, W., Edin, F., Atturo, F., Rieger, G., Lowenheim, H., Senn, P., et al. (2015). The pre- and post-somatic segments of the human type I spiral ganglion neurons–structural and functional considerations related to cochlear implantation. Neuroscience 284, 470–482. doi: 10.1016/j.neuroscience.2014.09.059
Luo, J., Xu, L., Chao, X., Wang, R., Pellittieri, A., Bai, X., et al. (2020). The effects of GJB2 or SLC26A4 gene mutations on neural response of the electrically stimulated auditory nerve in children. Ear Hear. 41, 194–207. doi: 10.1097/AUD.0000000000000744
Macherey, O., Carlyon, R. P., Chatron, J., and Roman, S. (2017). Effect of pulse polarity on thresholds and on non-monotonic loudness growth in cochlear implant users. J. Assoc. Res. Otolaryngol. 18, 513–527. doi: 10.1007/s10162-016-0614-4
Macherey, O., Carlyon, R. P., van Wieringen, A., Deeks, J. M., and Wouters, J. (2008). Higher sensitivity of human auditory nerve fibers to positive electrical currents. J. Assoc. Res. Otolaryngol. 9, 241–251. doi: 10.1007/s10162-008-0112-4
Macherey, O., van Wieringen, A., Carlyon, R. P., Deeks, J. M., and Wouters, J. (2006). Asymmetric pulses in cochlear implants: effects of pulse shape, polarity, and rate. J. Assoc. Res. Otolaryngol. 7, 253–266. doi: 10.1007/s10162-006-0040-0
Marx, M., Laborde, M. L., Algans, C., Tartayre, M., and James, C. J. (2025). Barriers to early progress in adult cochlear implant outcomes. Ear Hear. 46, 98–110. doi: 10.1097/AUD.0000000000001559
McKay, C. M. (2012). Forward masking as a method of measuring place specificity of neural excitation in cochlear implants: a review of methods and interpretation. J. Acoust. Soc. Am. 131, 2209–2224. doi: 10.1121/1.3683248
Mesnildrey, Q., Venail, F., Carlyon, R. P., and Macherey, O. (2020). Polarity sensitivity as a potential correlate of neural degeneration in cochlear implant users. J. Assoc. Res. Otolaryngol. 21, 89–104. doi: 10.1007/s10162-020-00742-7
Miura, M., Sando, I., Hirsch, B. E., and Orita, Y. (2002). Analysis of spiral ganglion cell populations in children with normal and pathological ears. Ann. Otol. Rhinol. Laryngol. 111, 1059–1065. doi: 10.1177/000348940211101201
Nelson, E. G., and Hinojosa, R. (2001). Aplasia of the cochlear nerve: a temporal bone study. Otol. Neurotol. 22, 790–795. doi: 10.1097/00129492-200111000-00013
O'Malley, J. T., Wu, P. Z., Kaur, C., Gantz, B. J., Hansen, M. R., Quesnel, A. M., et al. (2024). Delayed hearing loss after cochlear implantation: re-evaluating the role of hair cell degeneration. Hear. Res. 447:109024. doi: 10.1016/j.heares.2024.109024
Patrick, J. F., Busby, P. A., and Gibson, P. J. (2006). The development of the nucleus freedom cochlear implant system. Trends Amplif. 10, 175–200. doi: 10.1177/1084713806296386
Potrusil, T., Heshmat, A., Sajedi, S., Wenger, C., Johnson Chacko, L., Glueckert, R., et al. (2020). Finite element analysis and three-dimensional reconstruction of tonotopically aligned human auditory fiber pathways: a computational environment for modeling electrical stimulation by a cochlear implant based on micro-CT. Hear. Res. 393:108001. doi: 10.1016/j.heares.2020.108001
Rattay, F. (1999). The basic mechanism for the electrical stimulation of the nervous system. Neuroscience 89, 335–346. doi: 10.1016/S0306-4522(98)00330-3
Rattay, F., Leao, R. N., and Felix, H. (2001a). A model of the electrically excited human cochlear neuron. II. Influence of the three-dimensional cochlear structure on neural excitability. Hear. Res. 153, 64–79. doi: 10.1016/S0378-5955(00)00257-4
Rattay, F., Lutter, P., and Felix, H. (2001b). A model of the electrically excited human cochlear neuron. I. Contribution of neural substructures to the generation and propagation of spikes. Hear. Res. 153, 43–63. doi: 10.1016/S0378-5955(00)00256-2
Resnick, J. M., O'Brien, G. E., and Rubinstein, J. T. (2018). Simulated auditory nerve axon demyelination alters sensitivity and response timing to extracellular stimulation. Hear. Res. 361, 121–137. doi: 10.1016/j.heares.2018.01.014
Skidmore, J., Oleson, J. J., Yuan, Y., and He, S. (2023). The relationship between cochlear implant speech perception outcomes and electrophysiological measures of the electrically evoked compound action potential. Ear Hear. 44, 1485–1497. doi: 10.1097/AUD.0000000000001389
Skidmore, J., Ramekers, D., Bruce, I. C., and He, S. (2022). Comparison of response properties of the electrically stimulated auditory nerve reported in human listeners and in animal models. Hear. Res. 426:108643. doi: 10.1016/j.heares.2022.108643
Thompson, N. J., Park, L. R., O'Connell, B. P., Zdanski, C. J., Brown, K. D., Anderson, M. R., et al. (2024). Factors that influence performance in pediatric cochlear implant recipients with cochlear nerve deficiency. Cochlear Implants Int. 25, 191–196. doi: 10.1080/14670100.2024.2316457
Undurraga, J. A., Carlyon, R. P., Wouters, J., and van Wieringen, A. (2013). The polarity sensitivity of the electrically stimulated human auditory nerve measured at the level of the brainstem. J. Assoc. Res. Otolaryngol. 14, 359–377. doi: 10.1007/s10162-013-0377-0
Wright, C. G., Brown, O. E., Meyerhoff, W. L., and Rutledge, J. C. (1986). Auditory and temporal bone abnormalities in CHARGE association. Ann. Otol. Rhinol. Laryngol. 95, 480–486. doi: 10.1177/000348948609500509
Wu, P. Z., Liberman, L. D., Bennett, K., de Gruttola, V., O'Malley, J. T., Liberman, M. C., et al. (2019). Primary neural degeneration in the human cochlea: evidence for hidden hearing loss in the aging ear. Neuroscience 407, 8–20. doi: 10.1016/j.neuroscience.2018.07.053
Wu, P. Z., O'Malley, J. T., de Gruttola, V., and Liberman, M. C. (2021). Primary neural degeneration in noise-exposed human cochleas: correlations with outer hair cell loss and word-discrimination scores. J. Neurosci. 41, 4439–4447. doi: 10.1523/JNEUROSCI.3238-20.2021
Wu, P. Z., O'Malley, J. T., and Liberman, M. C. (2023). Neural degeneration in normal-aging human cochleas: machine-learning counts and 3D mapping in archival sections. J. Assoc. Res. Otolaryngol. 24, 499–511. doi: 10.1007/s10162-023-00909-y
Xu, L., Skidmore, J., Luo, J., Chao, X., Wang, R., Wang, H., et al. (2020). The effect of pulse polarity on neural response of the electrically stimulated cochlear nerve in children with cochlear nerve deficiency and children with normal-sized cochlear nerves. Ear Hear. 41, 1306–1319. doi: 10.1097/AUD.0000000000000854
Keywords: cochlear nerve, neural health, polarity effect, peripheral axon degeneration, cochlear implant
Citation: He S, Oleson JJ and Gao Z (2025) Polarity sensitivity to symmetric, biphasic monopolar pulses is not a reliable indicator of peripheral axonal degeneration in human cochlear implant users. Front. Audiol. Otol. 3:1693293. doi: 10.3389/fauot.2025.1693293
Received: 26 August 2025; Accepted: 10 October 2025;
Published: 31 October 2025.
Edited by:
Arianna Di Stadio, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Wiebke Konerding, Hannover Medical School, GermanyVictor Adenis, Harvard Medical School, United States
Copyright © 2025 He, Oleson and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuman He, c2h1bWFuLmhlQG9zdW1jLmVkdQ==
†ORCID: Shuman He orcid.org/0000-0002-6409-7388
Jacob J. Oleson orcid.org/0000-0001-6343-3274
Zi Gao orcid.org/0009-0000-2508-4448
 Shuman He
Shuman He Jacob J. Oleson
Jacob J. Oleson Zi Gao
Zi Gao