- 1Department of Biology, The University of Western Ontario, London, ON, Canada
- 2Evidentia Institute of Knowledge Synthesis, London, ON, Canada
- 3Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, United Kingdom
- 4Department of Biology, CUNY Hunter College, New York, NY, United States
- 5Schulich School of Medicine and Dentistry, The University of Western Ontario, London, ON, Canada
- 6Department of Biomedical Science and Department of Human Health and Nutritional Science, University of Guelph, Guelph, ON, Canada
- 7School of Interdisciplinary Science, McMaster University, Hamilton, ON, Canada
- 8Department of Biochemistry and Biomedical Sciences, McMaster University, Hamilton, ON, Canada
- 9Department of Biology, York University, Toronto, ON, Canada
- 10Department of Psychology, University of British Columbia, Vancouver, BC, Canada
- 11School of Psychological and Social Sciences, University of Waikato, Hamilton, New Zealand
- 12Department of Psychology, The University of Western Ontario, London, ON, Canada
Vocal communication is widespread across animals, from mammals to amphibians. In recent years, rodents have become an increasingly valuable group in which to study vocal communication. Rodents offer rich opportunities to examine vocalizations from proximate and ultimate ethological perspectives. Here, we identify recent advances in ethological research on rodent vocal communication by synthesizing contemporary studies from the past decade. We carried out a scoping review of research published between 2014 and 2024. This review involved a broad search for peer-reviewed primary research studies in APA PsycINFO, Embase, MEDLINE, PubMed, Scopus, and Web of Science databases. The search yielded 403 eligible studies on rodent vocalizations. We extracted information about the ethological perspectives, species, research environment, and animal sex and age groups. We also identified studies that focused on method development. We found that rodent vocal communication studies varied across ethological perspectives, with more studies carried out on vocal mechanisms and adaptive functions than on development and evolution. These studies covered a broad range of 88 rodent species, with high species diversity in function and evolution studies and low species diversity in mechanism studies. Artificial environments were used more often than naturalistic environments, especially in mechanism and development studies. Naturalistic environments were common in function and evolution studies. Adult males were used more often than any other sex and age groups. The use of age groups, but not sexes, varied across ethological perspectives. Together, these findings highlight several advantages of contemporary rodent research, including opportunities to carry out in-depth studies of vocal mechanisms and to compare diverse species. Based on these findings, we also identify potential areas for future research. These research areas include non-mechanistic questions, as well as expanding species diversity, research environments, and animal sex and age groups. Rodent research from multiple ethological perspectives will be crucial for building a comprehensive understanding of animal acoustic communication.
1 Introduction
Vocalization is a fundamental behavior for communication in animals. As an ancestral form of acoustic signaling, vocalizations have a long phylogenetic history spanning tetrapod vertebrates from amphibians to mammals (Kelley, 2022). Individuals use vocalizations to convey information about their physical status and internal states, as well as information about the outside world like predators and food resources. Several decades of ethological research have been carried out on animal vocalizations from both proximate (mechanism and development) and ultimate (function and evolution) perspectives. This combination of ethological perspectives was initially described by Niko Tinbergen (e.g., Tinbergen’s four questions) (Tinbergen, 1963), and it has become a valuable integrative framework to study animal behavior (Krakauer et al., 2017). Most ethological research on animal communication, however, has been biased towards a handful of taxonomic groups, such as primates, birds, bats, and anurans (Nieder and Mooney, 2019; Mooney, 2020; Janik and Knörnschild, 2021; Kelley, 2022). To build a comprehensive understanding of the proximate and ultimate origins of acoustic communication, investigations are needed across a broader species range.
Rodents are another promising taxonomic group for exploring acoustic communication. With over 2,000 species, the order Rodentia makes up about 40% of known mammal species (Solari and Baker, 2007). Although traditionally, rodents were used as a model for olfactory communication (Eisenberg and Kleiman, 1972; Johnston, 2003), a complementary body of research also explored rodent communication in acoustic channels. Some of the first studies on rodent vocal communication were done in the mid-20th century, when researchers discovered that rats and mice can hear and vocalize in the ultrasonic frequency range (> 20 kHz), similar to bats (Gould and Morgan, 1941; Dice and Barto, 1952; Anderson, 1954; Rosenzweig et al., 1955; Zippelius and Schleidt, 1956; Ralls, 1967). Indeed, ‘bat detectors’ were some of the first types of equipment used in rodent studies (Brudzynski, 2009). Building on these initial discoveries, research shifted towards characterizing rodent vocalizations and their potential functions. One line of work showed that infant vocalizations may facilitate maternal care (Sewell, 1970; Noirot, 1972; Colvin, 1973). Another line of work showed that adult vocalizations may facilitate social interactions like mating and aggression (Barfield and Geyer, 1972; Sales, 1972a, 1972b; Mcintosh et al., 1978; Nyby and Whitney, 1978; Pomerantz et al., 1983). Together, these initial studies revealed that rodent species vocalize at a wide range of frequencies, from ultrasonic vocalizations to lower frequency calls (< 20 kHz) that are audible to humans (Roberts, 1975).
The turn of the century brought with it the widespread use of microphones and speakers integrated with computer software for acoustic recording, playback, and analysis (Stoddard, 1990; McGregor and Ranft, 1994). These technologies advanced the ethological study of rodent vocal communication from multiple perspectives (Branchi et al., 2001; Portfors, 2007; Scattoni et al., 2009; Wöhr and Schwarting, 2013; Brudzynski, 2014; Mooney, 2020; Kelley, 2022; Grijseels et al., 2024). Early studies revolved around characterizing communication at key life stages, primarily in laboratory rats (Rattus norvegicus) and mice (Mus musculus). Infant rats and mice readily vocalize when isolated from their mother and nest (Hofer, 1996; Wöhr and Schwarting, 2008), and these vocalizations evoke maternal care behaviors (Ehret, 1987; D’Amato et al., 2005; Okabe et al., 2010, 2013). An extensive line of early work examined the developmental trajectories, biomechanics, and neurobiological mechanisms that regulate infant vocalizations and maternal responses (Hofer, 1996). Ontogenetic processes like vocal tract maturation and control, or perhaps even learning, were thought to drive the acoustic changes from infantile to adult-like vocalizations (Grimsley et al., 2011). Studies showed that isolation-induced vocalizations involve biomechanical mechanisms related to cold exposure (Blumberg and Alberts, 1990), and vocalizations potentiated by maternal stimuli involve the parasympathetic nervous system (Shair et al., 2012). Infant vocalizations are regulated by opioid, oxytocin, and other neuromodulator systems (Hofer, 1996; Winslow et al., 2000; Moles et al., 2004). It is thought that isolation-induced vocalizations are behavioral markers of anxiety and negative affect (Wöhr and Schwarting, 2008; Wöhr et al., 2008a).
Juvenile communication during affiliative interaction is another research theme that has received much attention (Knutson et al., 1998; Panksepp et al., 2007). Young rats produce high-frequency 50-kHz vocalizations during, and in anticipation of, rough-and-tumble play with conspecifics (Knutson et al., 1998; Burgdorf et al., 2008). These high frequency vocalizations also occur alongside heterospecific play (Panksepp and Burgdorf, 2000; Schwarting et al., 2007), and the propensity to vocalize has a heritable component (Burgdorf et al., 2005). It is thought that high-frequency vocalizations are a behavioral marker of positive affect in juvenile and young adult rats (Panksepp and Burgdorf, 2000; Burgdorf et al., 2008). These vocalizations attract conspecifics (Wöhr and Schwarting, 2007) and involve dopaminergic and opioid neural systems that facilitate reward processing (Gordon et al., 2002; Wöhr and Schwarting, 2009).
In addition to infant and juvenile vocalizations, early studies characterized adult rodent vocalizations across many contexts. Adult rodents vocalize during, and anticipation of, aversive contexts like predator alarm (Blanchard et al., 1991), social defeat (Kroes et al., 2007), and fear conditioning (Wöhr et al., 2005; Borta et al., 2006; Kim et al., 2010). Adult rodents also produce a diverse vocal repertoire during, or in anticipation of, appetitive contexts like courtship (Holy and Guo, 2005; Yang et al., 2013) and interaction with familiar conspecifics (Wright et al., 2010). In rats, it is thought that low-frequency 22-kHz vocalizations communicate negative affect during aversive contexts, while higher-frequency 50-kHz vocalizations communicate positive affect during appetitive contexts (Schwarting et al., 2007; Wöhr et al., 2008b; Brudzynski, 2009). Supporting this idea, rats avoid playbacks of 22-kHz vocalizations but are attracted to 50-kHz vocalizations (Wöhr and Schwarting, 2007, 2012; Burgdorf et al., 2008). Adult rat and mouse vocalizations produced during appetitive and aversive contexts are associated with distinct hormonal and neural mechanisms (Depaulis et al., 1992; Goldstein et al., 1996). In mice, estrogen and androgen systems affect communication during appetitive contexts like mating (Ogawa et al., 2000). In rats, aversive 22-kHz vocalizations involve the mesolimbic cholinergic system, and brain areas like the amygdala, periaqueductal grey, and perirhinal cortex (Koo et al., 2004; Furtak et al., 2007; Kroes et al., 2007; Kholodar-Smith et al., 2008; Sadananda et al., 2008). Appetitive 50-kHz vocalizations involve the opioid and mesolimbic dopamine systems, and brain areas like the frontal cortex, nucleus accumbens, and paraventricular nuclei (Burgdorf et al., 2000; 2007; Thompson et al., 2006; Ciucci et al., 2007; Sadananda et al., 2008; Ahrens et al., 2009; Brudzynski, 2009; Wöhr and Schwarting, 2009; Wright et al., 2010).
Although laboratory rats (R. norvegicus) and mice (M. musculus) were the focus of early studies, several other rodents were also shown to communicate with acoustic signals. Other rodents include black rats (Rattus rattus) (Kaltwasser, 1990), deer mice (Peromyscus spp.) (Dice and Barto, 1952; Ralls, 1967; Wright and Brown, 2004; Kalcounis-Rueppell et al., 2006), degus (Octodon degus) (Nakano et al., 2013), gerbils (Meriones unguiculatus) (Hashimoto et al., 2004; Nishiyama et al., 2011), guinea pigs (Cavia porcellus) (Hennessy et al., 2006), hamsters (Allocricetulus spp., Cricetulus spp., Mesocricetus spp.) (Hashimoto et al., 2004; Kapusta et al., 2006; Simeonovska-Nikolova and Dekov, 2013), wild house mice (Mus musculus musculus) (Musolf et al., 2010; Hoffmann et al., 2012), naked-mole rats (Heterocephalus glaber) (Yosida et al., 2007; Yosida and Okanoya, 2009), voles (Myodes spp., Microtus spp.) (Colvin, 1973; Lepri et al., 1988; Kapusta et al., 2007; Szentgyorgyi et al., 2008), and wood mice (Sewell, 1970). In naturalistic settings, a common research theme was on the evolution of alarm calling and communicative complexity in rodents like yellow-bellied marmots (Marmota flaviventris) (Blumstein and Armitage, 1997; Blumstein and Munos, 2005; Shelley and Blumstein, 2005; Pollard and Blumstein, 2012), Richardson’s ground squirrels (Spermophilus richardsonii) (Sloan and Hare, 2008; Swan and Hare, 2008; Thompson and Hare, 2010), and other ground squirrel species (Eiler and Banack, 2004; Matrosova et al., 2007; Volodina et al., 2010; Schneiderova and Policht, 2012). These alarm calls have unique acoustic properties that communicate information about arousal and individual identity (Blumstein et al., 2004; Sloan and Hare, 2004, 2008; Blumstein and Recapet, 2009; Matrosova et al., 2009, 2010). Most naturalistic studies focused on vocalizations audible to humans, which are easier to record in the wild than more inconspicuous ultrasonic vocalizations. However, even ultrasonic vocalizations have been studied in some wild-living rodents, like deer mice (Peromyscus spp.) (Kalcounis-Rueppell et al., 2006, 2010; Briggs and Kalcounis-Rueppell, 2011; Petric and Kalcounis-Rueppell, 2013).
The widespread use of rodent models in ethological studies of vocal communication is relatively nascent compared to other tetrapod groups like birds, primates, bats, and anurans. And yet, several decades of work show that rodent vocalizations can be studied from different ethological perspectives across species, research environments, and demographic groups. During the past decade, in particular, a rapid evolution of technologies to detect and analyze rodent vocalizations is supporting a shift towards more rodent research (Neunuebel et al., 2015; Binder et al., 2021; Sterling et al., 2023). Here, we review contemporary research trends and advances in rodent vocal communication. Using a scoping review approach, we compile a list of ethological studies on rodent vocalizations from the past decade. We investigate how these studies fit into mechanism, development, function, and evolution perspectives. Then, we compare the rodent species used to examine vocal communication from these perspectives. Next, we examine research environments and demographic sex and age groups, across perspectives and species. We also identify potential research areas for future study. Finally, we summarize new technologies to record, detect, and analyze rodent vocalizations. Together, recent advancements in the integrative study of rodent vocalizations hold exciting opportunities for understanding how and why animals communicate.
2 Methods
2.1 Framework
Scoping reviews are conducted to provide the synthesis from existing literature on a broad topic, identify key concepts, evidence types, research gaps, and clarify definitions and terminology. This review followed the Arksey and O’Malley framework (Arksey and O’Malley, 2005), which consists of six steps: 1) identification of research questions; 2) identification of relevant studies in existing literature; 3) screening studies for eligibility; 4) data extraction; 5) data analysis; and 6) an optional stakeholder consultation. A stakeholder consultation was not performed as this review synthesizes existing knowledge from the field rather than exploring practical applications where stakeholder perspectives are relevant (Arksey and O’Malley, 2005).
2.2 Search strategy and study selection
A comprehensive search strategy was developed under two definitions: 1) Rodent species, such as “Mice”, “Rodent”, “Chinchilla”, and “Guinea pig”, and 2) Vocalization, such as “vocal repertoire”, “animal communication”, and “calling patterns”. The keywords were tailored to the interfaces of six databases: APA PsycINFO, Embase, MEDLINE, PubMed, Scopus, and Web of Science (Supplementary Data 1). The database search was conducted for primary research papers published between January 1, 2014 and December 31, 2024. A supplementary database search was conducted for primary research papers published between January 1, 2004 and December 31, 2013. Covidence (Veritas Health Innovation, Melbourne, Australia; available at www.covidence.org) was utilized for title and abstract screening, full-text screening, and extraction of publication metadata.
A duo-reviewer system was used to independently evaluate the records. Any conflicts between the two reviewers were resolved through consensus, with a third reviewer consulted when necessary to provide another perspective and facilitate the final decision. We included peer-reviewed primary research papers published in English. Studies were used regardless of geographic location and study setting. Non-research articles (e.g., errata, commentaries, book chapters, letters to the editor, protocols, editorials, perspectives and opinion pieces), thesis dissertations, conference proceedings, and reviews were excluded. Grey literature – research and information produced outside of traditional commercial publishing channels, including reports, theses, conference proceedings, and government documents, which may not undergo formal peer review – was not considered for inclusion in this study. Studies were excluded when they did not assess vocalization or when they used non-rodent species. Finally, we focused our search strategy on ethology-oriented research by excluding biomedical studies that used rodents as translational models for human disease (e.g., transgenic strains for a human disorder).
A streamlined version of this search strategy was carried out for research published between January 1, 2004 and December 31, 2013. Following the removal of duplicates, irrelevant studies were excluded at the title and abstract screening stage.
2.3 Data extraction
Data were extracted for eligible studies published between January 1, 2014 and December 31, 2024. The extraction form for each study was independently completed by two reviewers, detailing the methodological and study subject characteristics, as well as the principal findings. A third reviewer consolidated the extracted data through consensus. A fourth reviewer provided a final check of the extracted data for accuracy and formatted the data for statistical analyses. The Covidence metadata extraction template was adapted to include the following: first author’s last name, print publication year, title of study, study design, country where study was conducted, study objectives, and types of behaviors that were studied. Information collected for analysis purposes included the primary Tinbergen perspective(s) (i.e., mechanism, development, function, evolution) for vocalization-based study objectives, species, research environment (i.e., artificial, naturalistic, or both), animal sex (i.e., female, male, or both) and age groups (i.e., infant, juvenile, or adult).
The ‘mechanism’ Tinbergen perspective included studies on the physiological substrates that drive vocal production and perception. The mechanism perspective also was assigned to studies that characterized the spectral-temporal properties of vocalizations. The ‘development’ perspective included studies on how vocal behavior changes across time and in response to ontogenetic processes. The ‘function’ perspective included studies that focused on the meaning of vocalizations (e.g., information encoding), their effect on conspecifics, and their adaptive value for reproduction and survival. The ‘evolution’ perspective included phylogenetic studies that focused on describing inter-specific and inter-population variation in vocal behavior, as well as evolutionary processes like natural or artificial selection. Multiple perspectives were assigned if studies contained experiments from different perspectives. Studies were assigned to a ‘method’ perspective if their primary purpose was to design better hardware or software tools.
When assigning study species, multiple strains of laboratory-derived mice or rats were labeled as house mice (Mus musculus) or brown rats (Rattus norvegicus), respectively. When assigning the research environment, an ‘artificial’ category was used for studies that took place in experimental laboratory settings (e.g., home cage, testing arena). These were settings that were not designed to simulate an animal’s natural environment. The ‘naturalistic’ category was used for studies that took place in the wild or in semi-natural enclosures. The naturalistic category also was used when laboratory testing arenas were made to simulate aspects of an animal’s natural environment. Animal sex was assigned based on the reported sexes of the study subjects and stimuli. Studies that involved several animals that were not checked for sex, but came from a random population (e.g., infants, wild animals), were assigned as both sexes. When assigning age groups, ‘adult’ was used when authors noted that the animals were adults, young adults or subadults, or when an age group was not explicitly stated. Animals were assigned to the ‘juvenile’ group when authors identified them as adolescents or juveniles. The ‘infant’ group was used when animals were identified as infants, pups, and neonates.
2.4 Data analysis
Data were analyzed in R (v.3.5.3). Chi-square goodness of fit tests were used to examine whether the frequencies of studies assigned to distinct categories differed from expected proportions. These categories were not considered mutually exclusive, and so expected probabilities for chi-square tests were rescaled to a sum of 1. Spearman rank correlations were used to test whether study characteristics changed across publication years. Simpson Diversity Index (Keylock, 2005) was used to quantify species diversity across studies. This index (D) is a quantitative measure of biodiversity that takes species richness and evenness into account, with higher values indicating increased biodiversity. Phylogenetic trees of Rodentia species come from a published mammalian supertree (Fritz et al., 2009), and trees were plotted with R “ape” package (Paradis and Schliep, 2019).
3 Results
A total of 17,165 references were identified for screening between January 1, 2014 and December 31, 2024, (Figure 1), of which 9,439 duplicates were removed (133 manually and 9,306 via Covidence). 7,726 studies were used for title and abstract screening, and 6,073 of these studies were excluded as irrelevant. Then, 1,653 studies underwent full-text assessment. During this stage, 1,250 studies were excluded for various reasons: 760 were disease model studies, 248 had the wrong study design (e.g., review paper), 59 did not include a rodent species, and 183 did not assess vocalization. Metadata for disease model studies that were excluded at the full-text screening stage (n = 760) are provided in the Supplementary Materials (Supplementary Data 2). In total, 403 studies met the eligibility criteria and were used for data extraction and analyses (Supplementary Data 3). The publication dates of these studies included all years between 2014 to 2024. One of the included studies was published online in 2024 but was printed in a 2025 issue of the journal (Dymskaya et al., 2025). On average, there were 37 ± 5 (mean ± sd) studies published per year, ranging from 27 (in 2014) to 44 (in 2022).
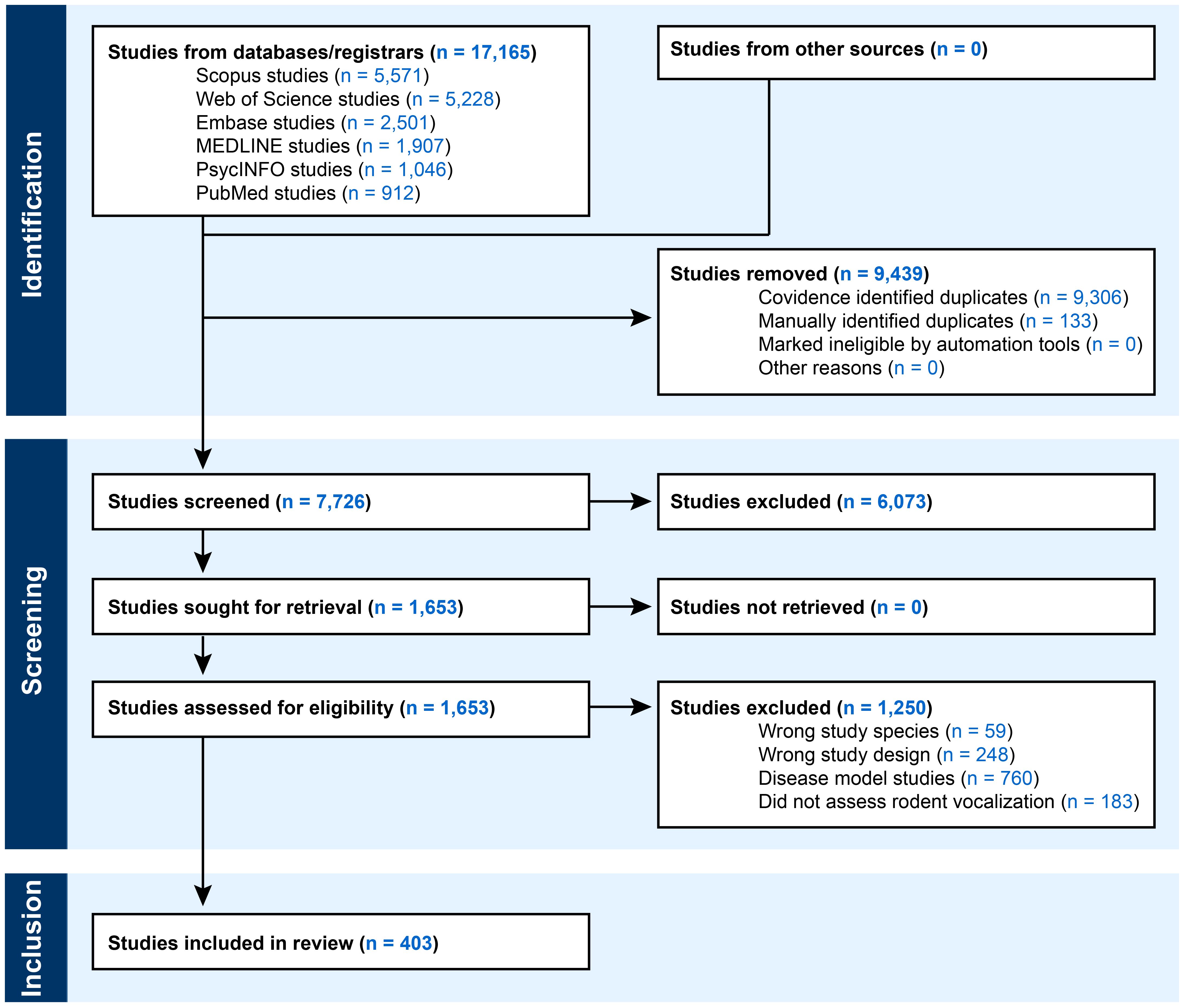
Figure 1. Study search and exclusion process. Studies were selected through a three stage process that involved study identification, screening, and inclusion. Identification involved the use of six databases to find potential studies published between January 1, 2014 and December 31, 2024. Duplicates and ineligible citations were removed. During the screening stage, studies that did not meet inclusion criteria were removed. During the inclusion stage, eligible studies were reviewed to extract information on research objectives, species, research environment, demographic group, and methods.
A supplementary search for eligible studies was carried out for research published between January 1, 2004 and December 31, 2013. A total of 10,374 references were identified for screening (Supplementary Figure 1), of which 5,780 duplicates were removed (9 manually and 5,771 via Covidence). 4,594 studies were used for title and abstract screening. Of these, 4,356 studies were excluded, which left 238 potentially eligible studies. These studies are listed in the supplement (Supplementary Data 4), and they were not used for data extraction and analyses.
3.1 Ethological perspectives
All ethological perspectives were represented in the 403 extracted studies (Figure 2). Of these extracted studies, 42% were assigned as mechanism (n = 171 studies), 17% as development (n = 70), 32% as function (n = 129), and 9% as evolution (n = 38). Most studies were focused on one of the four perspectives (n = 330), and a minority involved multiple perspectives (n = 38). The frequencies of studies categorized under the four perspectives deviated from the expected proportion of 0.25 (Chi-squared goodness of fit test: χ = 104.02, df = 3, p < 0.0001; Figure 2A). These frequencies were relatively stable across publication year. We found no support that yearly frequencies increased or decreased for mechanism (Spearman rank correlation: rho = 0.491, df = 9, p = 0.1292), development (rho = -0.155, df = 9, p = 0.6540), function (rho = -0.351, df = 9, p = 0.2902), or evolution (rho = -0.036, df = 9, p = 0.9244) studies (Figure 2B).
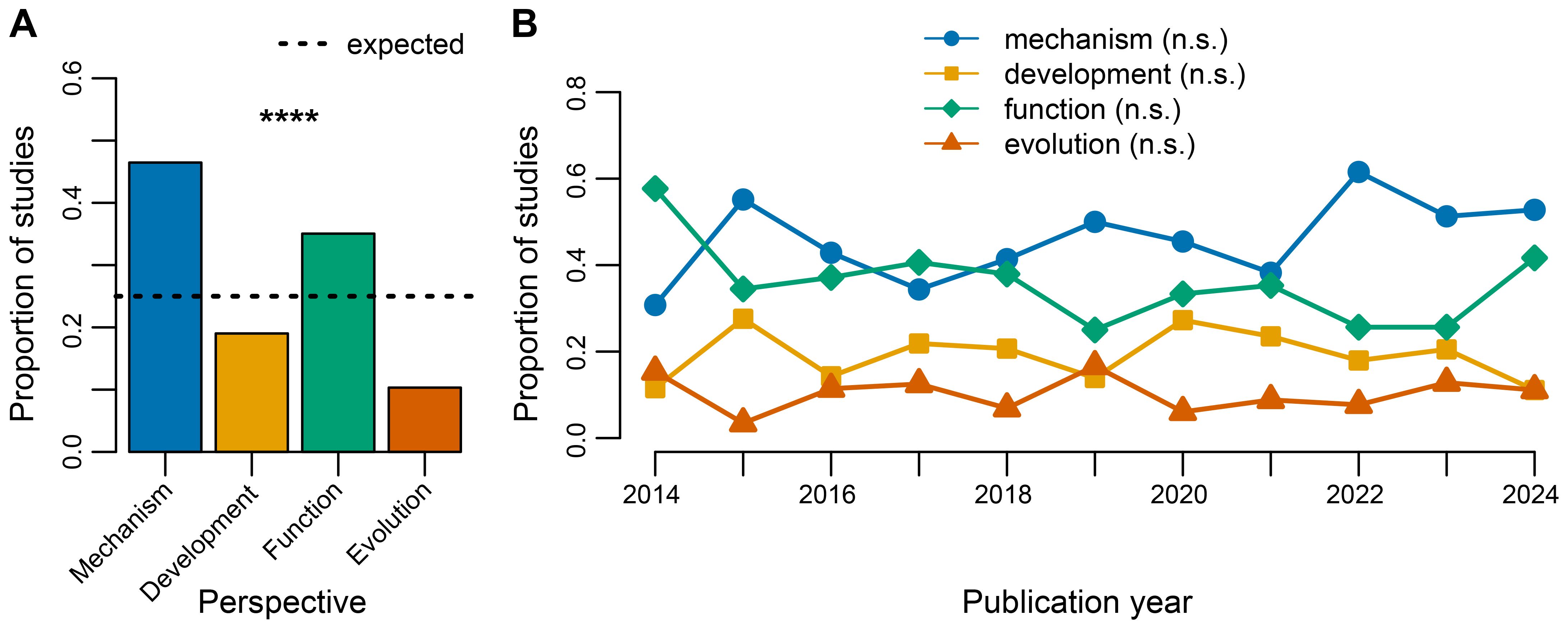
Figure 2. Proximate and ultimate approaches to rodent vocal communication studies. (A) Comparison of studies (n = 368) used to address research objectives related to mechanistic, developmental, functional, and evolutionary ethological perspectives. Frequencies of studies assigned to each of the perspectives (colored bars) are compared to the expected proportions (i.e., all perspectives have equal likelihood, shown with dotted line). (B) Associations between publication year and the frequencies of studies that focus on different ethological perspectives. Statistical significance is represented by n.s. p>0.05, ****p<0.0001.
3.2 Study species and species diversity
The extracted studies included 88 distinct rodent species across 43 Genus groups. Of these 88 species, 28% (n = 25 species) were used in mechanism studies, 18% (n = 16) in development studies, 45% (n = 40) in function studies, and 68% (n = 60) in evolution studies. The four most common species were brown rats (Rattus norvegicus, n = 170 studies), house mice (Mus musculus, n = 116 studies), California mice (Peromyscus californicus, n = 12), and Mongolian gerbils (Meriones unguiculatus, n = 10 studies). All other species were represented in seven or fewer studies. Most studies focused on single rodent species (n = 375 studies), and a minority included multiple species (n = 28 studies, 2–7 species per study). A variety of species were represented across all ethological perspectives (Figure 3). Rats (Rattus spp.) and mice (Mus spp.) were represented across all perspectives (Figure 3A). The only other Genus groups that were represented across all perspectives were deer mice (Peromyscus spp.), gerbils (Meriones spp.), grasshopper mice (Onychomys spp.), singing mice (Scotinomys spp.), and voles (Microtus spp.) (Figure 3A). Six distinct species were represented across all perspectives: Alston’s singing mice (S. teguina), brown rats (R. norvegicus), house mice (M. musculus), California mice (P. californicus), Mongolian gerbils (M. unguiculatus), and northern grasshopper mice (O. leucogaster).
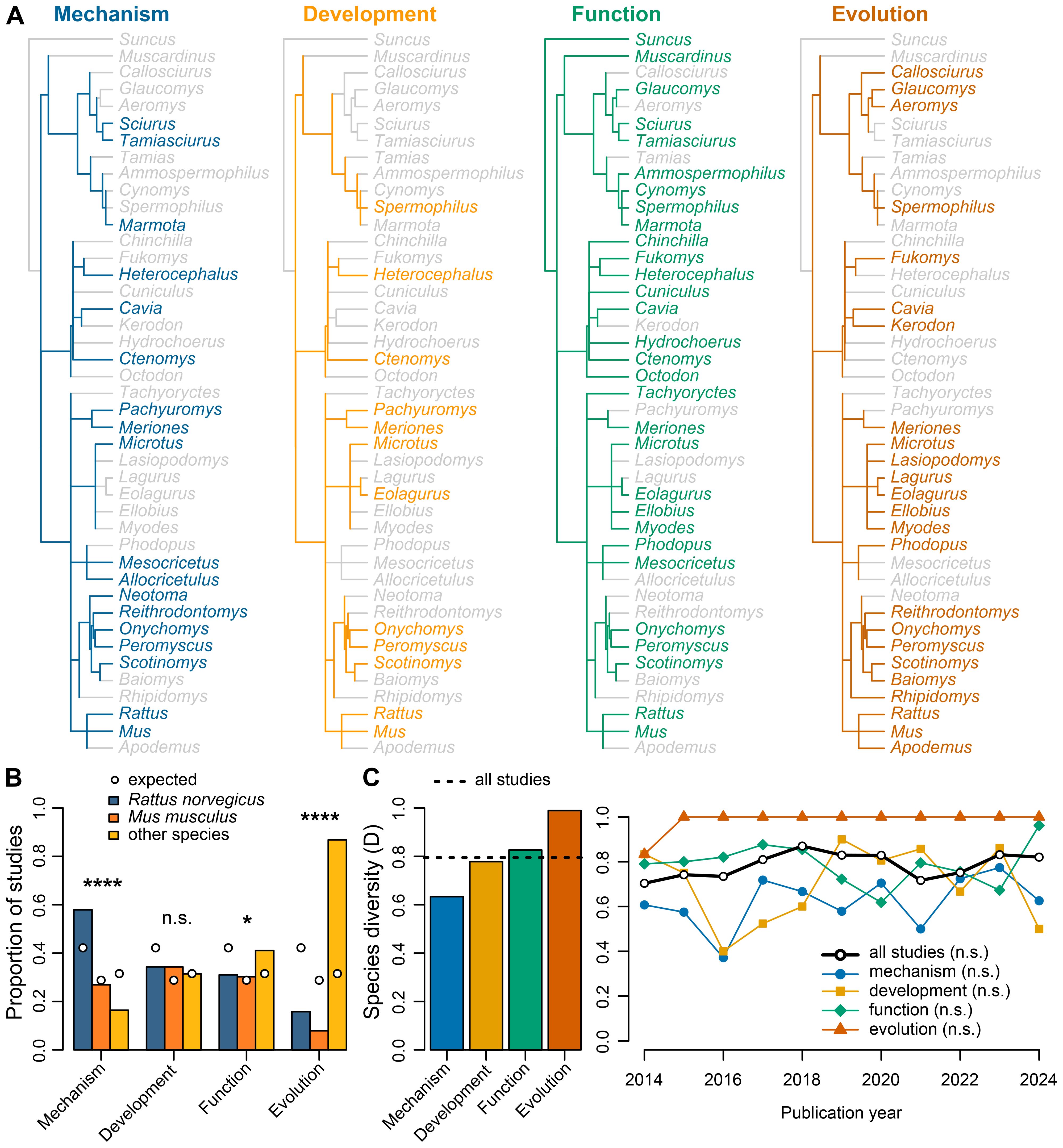
Figure 3. Rodentia species in vocal communication studies. (A) Phylogenetic trees for rodent species used to address research objectives related to mechanistic, developmental, functional, and evolutionary perspectives on vocal communication. Trees are mapped at the Genus level, with darker colored lines and text for groups represented in studies. Non-represented Genus groups are shown in light gray. (B) Comparison of rodent species groups used in studies for each ethological perspective. Species groups include brown rats (Rattus norvegicus, n = 170), house mice (Mus musculus, n = 116) and all other species combined (n = 127). Expected proportions (circle symbols) of each species group are calculated from the entire dataset (n = 403 studies). (C) Comparison of species diversity across studies that take different ethological perspectives (171 mechanism studies, 70 development, 129 function, and 38 evolution). Comparisons are shown for ethological perspectives (left) and across publication years (right). Species diversity is represented with the Simpson Diversity Index, with higher values representing increased diversity. Statistical significance is represented by n.s. p>0.05, *p<0.05, ****p<0.0001.
Ethological perspectives varied in their representation of three species groups – laboratory rats (R. norvegicus), laboratory mice (M. musculus), and non-traditional rodents (i.e., all other species combined) (Figures 3B, C). Frequencies of species groups deviated from expected for mechanism (Chi-squared goodness of fit test: χ = 22.92, df = 2, p < 0.0001), function (χ = 7.68, df = 2, p = 0.0215), and evolution (χ = 45.16, df = 2, p < 0.0001) studies, but not for development studies (χ = 1.77, df = 2, p = 0.4123). Expected proportions for each species group were based on the entire dataset. Overall, rats were used more than non-traditional rodents in mechanistic studies. Conversely, non-traditional rodents were used more than rats in functional studies, and non-traditional rodents were used more than rats and mice in evolution studies.
Species diversity also varied across ethological perspective (Figure 3C). The Simpson Diversity Index for the entire dataset was 0.79, which was consistently higher than mechanism studies (0.63) and lower than evolution studies (0.99) across publication year. Simpson Diversity Indices for development (0.78) and function (0.83) studies tended to be similar to the entire dataset. There was no evidence that Simpson Diversity Indices changed across publication year, either for the entire dataset (Spearman rank correlation: rho = 0.445, df = 9, p = 0.1728), or for mechanism (rho = 0.464, df = 9, p = 0.1543), development (rho = 0.118, df = 9, p = 0.7343), function (rho = -0.164, df = 9, p = 0.6339), and evolution (rho = 0.500, df = 9, p = 0.1173) studies.
3.3 Research environment
Of the 403 included studies, 91% (n = 367) were carried out in artificial environments, while 11% (n = 44) were carried out in naturalistic environments (artificial only = 359 studies, naturalistic only = 36, both artificial and naturalistic = 8). This bias towards artificial environments was consistent across years. The proportion of studies set in different environments did not correlate to publication year (Spearman rank correlations: artificial: rho = 0.227, df = 9, p = 0.5031; naturalistic: rho = -0.278, df = 9, p = 0.4080; Figure 4A).
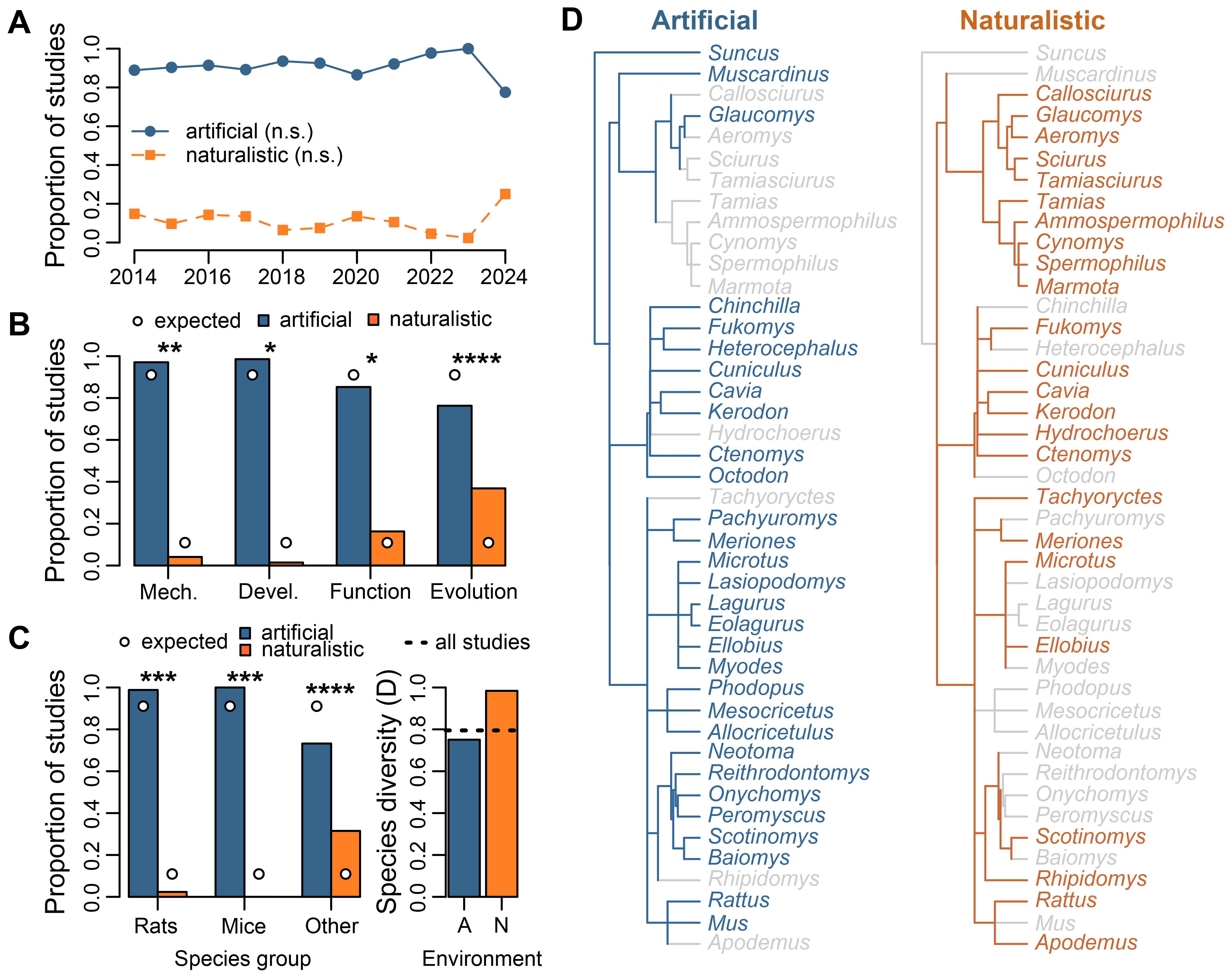
Figure 4. Research environments in rodent vocal communication studies. (A) Associations between publication year and the frequencies of studies carried out in artificial (n = 367 studies) and naturalistic (n = 44) environments. (B) Comparison of environments used in studies for each ethological perspective. Expected frequencies (circle symbols) of artificial and naturalistic environments are calculated from the entire dataset. (C) Comparison of environments used in studies for each rodent species group (left) and comparison of species diversity across artificial (‘A’) and naturalistic (‘N’) environments (right). Species groups include brown rats (Rattus norvegicus), house mice (Mus musculus) and all other species combined. Expected frequencies (circle symbols) of environments are calculated from the entire dataset. Species diversity is represented with the Simpson Diversity Index, with higher values representing increased diversity. (D) Phylogenetic trees for rodent species used in artificial and naturalistic environments. Trees are mapped at the Genus level, with darker colored lines and text for taxonomic groups represented in artificial and naturalistic studies. Non-represented Genus groups are shown in light gray. Statistical significance is represented by n.s. p>0.05, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Ethological perspectives varied in their representation of research environments (Figure 4B). Of the 367 artificial studies, 45% (n = 166 studies) were assigned to the mechanism perspective, 19% (n = 69) to development, 30% (n = 110) to function, and 8% (n = 29) to evolution. Of the 44 naturalistic studies, 16% (n = 7 studies) were assigned to the mechanism perspective, 3% (n = 1) to development, 48% (n = 21) to function, and 32% (n = 14) to evolution. Frequencies of each study environment deviated from expected for mechanism (Chi-squared goodness of fit test: χ = 8.03, df = 1, p = 0.0046), development (χ = 6.30, df = 1, p = 0.0121), function (χ = 3.89, df = 1, p = 0.0487), and evolution (χ = 21.48, df = 1, p < 0.0001) studies. Expected proportions for research environment were based on the entire dataset. Overall, artificial environments were represented more than expected in mechanism and development studies. Conversely, naturalistic environments were represented more than expected in function and evolution studies.
Species diversity also varied across research environments (Figures 4C, D). Artificial environments were used across the three main species groups (Rattus norvegicus: n = 168 studies; Mus musculus: n = 116; all others: n = 93). Naturalistic environments were only used for rats and non-traditional species (R. norvegicus: n = 4; M. musculus: n = 0; all others: n = 40). Frequencies of each environment deviated from expected for studies on laboratory rats (Chi-squared goodness of fit test: χ = 12.64, df = 1, p = 0.0004), laboratory mice (χ = 13.91, df = 1, p = 0.0002), and non-traditional species (χ = 52.20, df = 1, p < 0.0001). Expected proportions for each environment were based on the entire dataset. Overall, artificial environments were represented more than expected in laboratory rat and mice studies. Conversely, naturalistic environments were represented more than expected in non-traditional rodent studies. In total, artificial studies involved 62 rodent species across 30 Genus groups, while naturalistic studies involved 37 rodent species across 24 Genus groups (Figure 4D). Naturalistic studies had a higher Simpson Diversity index (D = 0.984; Figure 4D) than artificial studies (D = 0.751) and the entire dataset (D = 0.795).
3.4 Animal sex and age groups
Of the 403 extracted studies, 67% (n = 272) involved females, while 87% (n = 351) involved males (females only = 33 studies, males only = 112, both sexes = 239, unknown sex = 19). A bias towards male studies was consistent across years, and the proportions of studies involving either sex did not correlate with publication year (Spearman rank correlation: females: rho = 0.464, df = 9, p = 0.1543; males: rho = 0.347, df = 9, p = 0.2957; Figure 5A). In terms of age group, 81% of studies (n = 326) involved adults, while 16% (n = 65) involved juveniles and 19% (n = 78) involved infants. A bias towards adult studies was consistent across years, while juveniles and infants were used in a similar proportion of studies. The proportion of studies for age group did not correlate with publication year (Spearman rank correlation: adults: rho = -0.296, df = 9, p = 0.3766; juveniles: rho = -0.345, df = 9, p = 0.2994; infants: rho = 0.382, df = 9, p = 0.2484; Figure 5A).
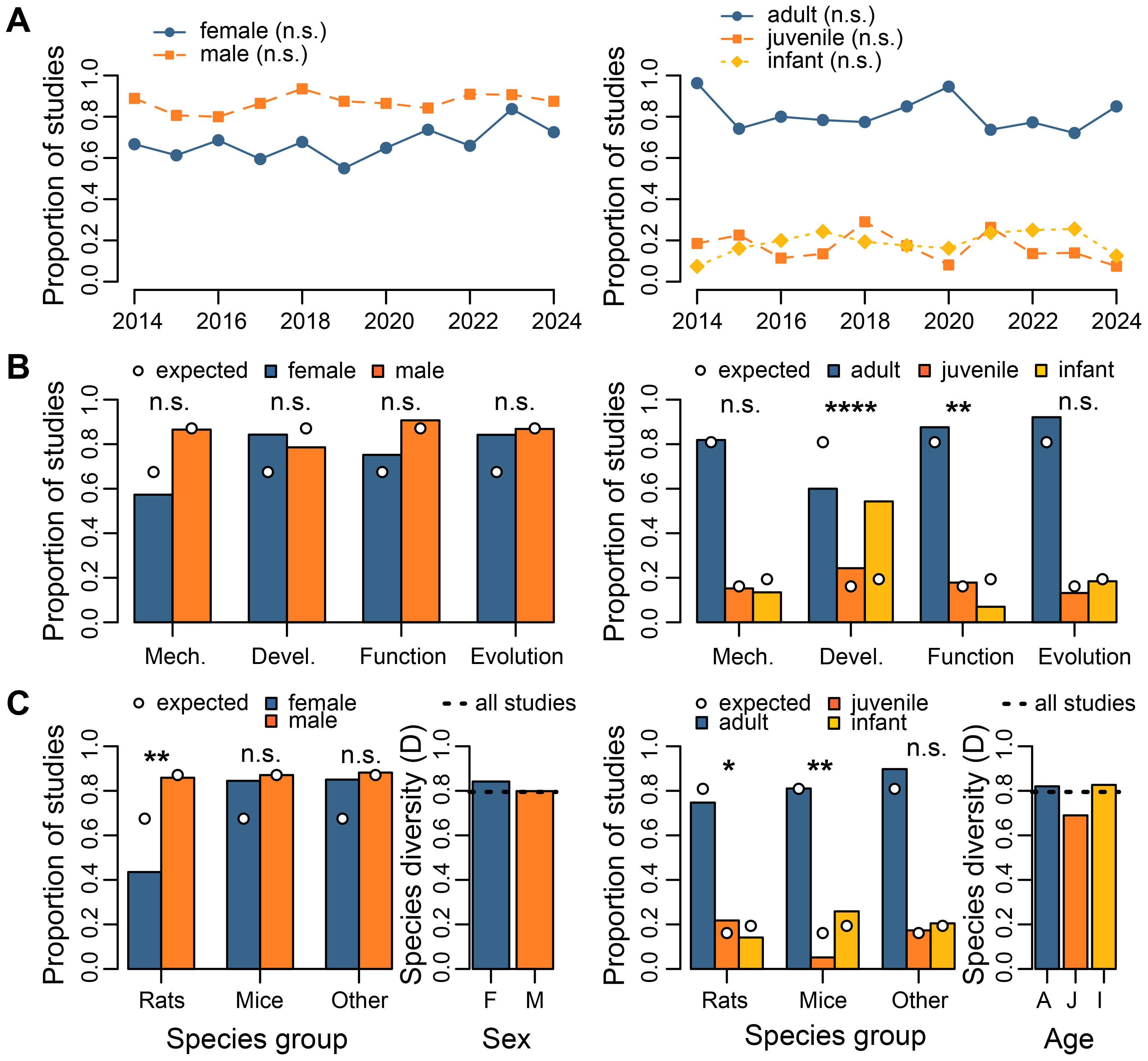
Figure 5. Sex and age of animals used in rodent vocal communication studies. (A) Associations between publication year and the frequencies of studies carried out with different sexes (left; 272 female studies, 351 male) and age groups (right; 326 adult studies, 65 juvenile, 78 infant). (B) Comparison of animal sex (left) and age groups (right) in studies for each ethological perspective. Expected frequencies (circle symbols) of sex and age groups are calculated from the entire dataset. (C) Comparison of animal sex (left) and age groups (right) in studies for each rodent species group and for species diversity. Species groups include brown rats (Rattus norvegicus), house mice (Mus musculus) and all other species combined. Sexes are females (‘F’) and males (‘M’), and age groups are adults (‘A’), juveniles (‘J’), and infants (‘I’). Expected frequencies (circle symbols) of sex and age groups are calculated from the entire dataset. Species diversity is assessed with the Simpson Diversity Index, with higher values representing increased diversity. Statistical significance is represented by n.s. p>0.05, *p<0.05, **p<0.01, ****p<0.0001.
Ethological perspectives were similar in their representation of animal sexes (Figure 5B). Of the 272 female studies, 36% (n = 98 studies) were assigned to the mechanism perspective, 22% (n = 59) to development, 36% (n = 97) to function, and 12% (n = 32) to evolution. Of the 351 male studies, 42% (n = 148 studies) were assigned to the mechanism perspective, 16% (n = 55) to development, 33% (n = 117) to function, and 9% (n = 33) to evolution. Frequencies of each animal sex did not deviate from expected for mechanism (Chi-squared goodness of fit test: χ = 1.46, df = 1, p = 0.2268), function (χ = 0.24, df = 1, p = 0.6229), and evolution (χ = 0.83, df = 1, p = 0.3651) studies. However, there was a tendency for the observed frequencies of animal sex to deviate from expected for development studies (χ = 3.04, df = 1, p = 0.0814), with females used more than expected and males used less. This was a marginal effect (p < 0.1) that did not pass the significance threshold (p < 0.05). Expected proportions for each sex were based on the entire dataset.
Ethological perspectives varied in their representation of animal ages (Figure 5B). Of the 326 adult studies, 43% (n = 140 studies) were assigned to the mechanism perspective, 13% (n = 42) to development, 35% (n = 113) to function, and 11% (n = 35) to evolution. Of the 65 juvenile studies, 40% (n = 26 studies) were assigned to the mechanism perspective, 26% (n = 17) to development, 35% (n = 23) to function, and 8% (n = 5) to evolution. Of the 78 infant studies, 29% (n = 23 studies) were assigned to the mechanism perspective, 49% (n = 38) to development, 12% (n = 9) to function, and 9% (n = 7) to evolution. Frequencies of the three age groups deviated from expected for development (Chi-squared goodness of fit test: χ = 40.17, df = 2, p < 0.0001) and function (χ = 11.37, df = 2, p = 0.0034) studies, but not for mechanism (χ = 2.83, df = 2, p = 0.2429) and evolution (χ = 0.60, df = 2, p = 0.7396) studies. Expected proportions for each age group were based on the entire dataset. Overall, adults were underrepresented, and infants overrepresented, in development studies. The opposite pattern was found in function studies.
Species diversity varied across animal sexes (Figure 5C). Females were used across the three main species groups (Rattus norvegicus: n = 74 studies; Mus musculus: n = 98; all others: n = 108), as were males (R. norvegicus: n = 146; M. musculus: n = 101; all others: n = 112). Frequencies of animal sexes deviated from expected for studies on laboratory rats (Chi-squared goodness of fit test: χ = 8.99, df = 1, p = 0.0027), but not for laboratory mice (χ = 2.53, df = 1, p = 0.1121) and non-traditional rodents (χ = 2.64, df = 1, p = 0.1043). Expected proportions for each sex were based on the entire dataset. Overall, females were represented less than expected in studies on laboratory rats. In total, studies that used female rodents involved 77 species across 40 Genus groups, while male studies involved 81 rodent species across 40 Genus groups (Supplementary Figure 2). Female studies showed a slightly higher Simpson Diversity Index (D = 0.842) than the entire dataset (D = 0.795; Figure 5C). Male studies had a similar diversity index (D = 0.799) to the entire dataset.
Species diversity also varied across animal age groups (Figure 5C). All age groups were used throughout the three main species groups (Rattus norvegicus: adults = 127 studies, juveniles = 37, infants = 24; Mus musculus: adults = 94, juveniles = 6, infants = 30; all others: adults = 114, juveniles = 22, infants = 26). Frequencies of animal age groups deviated from expected for studies on laboratory rats (chi-squared goodness of fit test: χ = 6.39, df = 2, p = 0.0410) and laboratory mice (χ = 11.41, df = 2, p = 0.0033), but not for non-traditional rodents (χ = 0.59, df = 2, p = 0.9708). Expected proportions for each age group were based on the entire dataset. Overall, adults and juveniles were overrepresented, and infants underrepresented, in rat studies. In mouse studies, juveniles were underrepresented and infants overrepresented. In total, studies that used adults rodents involved 85 species across 42 Genus groups, juvenile studies involved 22 rodent species across 18 Genus groups, and infant studies involved 29 rodent species across 15 Genus groups (Supplementary Figure 3). Studies carried out with adults showed a similar Simpson Diversity Index (D = 0.820) to the entire dataset (D = 0.795; Figure 5C). Juvenile studies had a lower diversity index (D = 0.690) than the entire dataset. Infant studies had a similar diversity index (D = 0.827) to the entire dataset.
3.5 Characteristics of method studies
Of the 403 extracted studies, (9%, n = 36) were identified as methods-focused studies. We found that the proportion of methods-focused studies tended to increase across publication year (Spearman rank correlations: rho = 0.584, df = 9, p = 0.0590). This correlation showed a marginal trend (p < 0.1) but did not pass the significance threshold (p < 0.05).
Method studies varied in the representation of different species groups – laboratory rats (Rattus norvegicus), laboratory mice (Mus musculus), and all other species combined. Frequencies of studies across these three species groups deviated from expected proportions based on the entire dataset (Chi-squared goodness of fit test: χ = 19.60, df = 2, p < 0.0001). Method studies only included 7% (n = 6 species) of the 88 rodent species: house mice (M. musculus, 23 studies), brown rats (R. norvegicus, 12 studies), northern and southern flying squirrels (Glaucomys sabrinus and G. volans, 2 studies), Mongolian gerbils (Meriones unguiculatus, 1 study), and Eastern chipmunks (Tamias striatus, 1 study). The Simpson Diversity Index across all method studies was 0.609, and there was no evidence that diversity indices were correlated with publication year (Spearman rank correlation: rho = -0.196, df = 9, p = 0.6137).
Method studies typically occurred in artificial settings with male study animals, but these observed patterns did not differ from expected patterns based on the entire dataset. Of the 36 method studies, 92% (n = 33) of them involved an artificial environment, and 8% (n = 3) involved naturalistic environments. However, the frequencies of these two study environments did not deviate from expected (Chi-squared goodness of fit test: χ = 0.21, df = 1, p = 0.6453). Methods studies tended to be male-biased, with 64% (n = 23) of them involving females and 81% (n = 29) involving males. However, frequencies of studies across the two sexes did not deviate from expected (Chi-squared goodness of fit test: χ = 0.01, df = 1, p = 0.9338).
Finally, method studies were biased towards adults and infants, with 78% (n = 28) of studies on adults, 3% (n = 1) on juveniles, and 31% (n = 11) on infants. The frequencies of the three age groups deviated from expected proportions based on the entire dataset (Chi-squared goodness of fit test: χ = 6.57, df = 2, p = 0.0375). Overall, infants were used more than expected, and juveniles were used less than expected.
4 Discussion
We present here an overview of contemporary trends and advancements in the ethological study of rodent vocal communication. We found that mechanism and function perspectives were more common than development and evolution perspectives. Also, studies from different perspectives varied in their use of species, research environments, and animal age groups. Overall, contemporary studies had higher species diversity when using an evolutionary perspective and lower species diversity when using a mechanistic perspective. Artificial environments were more common in mechanistic and developmental studies, while naturalistic environments were more common in functional and evolutionary studies. In addition, contemporary studies tended to use male adults more than other sex and age groups, but these demographics depended on the ethological perspective and taxonomic group. Species diversity also varied across research environments and demographic groups. Finally, methods-focused studies became more common over the past decade, and these studies introduced many new tools to better record, detect, and analyze vocalizations.
4.1 Ethological perspectives on rodent communication
Integration across ethological perspectives is important for developing a holistic understanding of behavior (Tinbergen, 1963; Krakauer et al., 2017). We found a strong bias towards mechanism studies on rodent vocalization, with relatively few studies focused on evolution and development. Function studies were relatively common, but not as common as mechanism studies. Such findings suggest that a current strength of rodent research is in characterizing the biological causes of vocal production and perception. This trend is expected, given that rodents are common in neuroscience research and method development (Bernstein and Boyden, 2011; Ellenbroek and Youn, 2016; Roth, 2016).
Contemporary mechanistic studies identified in this review make use of variety of neuroscience tools, such as pharmacological manipulations (Berz et al., 2021), optogenetics (Tschida et al., 2019), and brain-wide mapping of immediate early genes (Mai et al., 2023). This body of work shows that neural circuits for rodent vocalization span the entire brain, from regions of the hindbrain and midbrain, like retroambiguus and periaqueductal gray (Tschida et al., 2019), to regions of the forebrain, like nucleus accumbens and the motor cortex (Muller and Shair, 2016; Okobi et al., 2019). Several regions are also integral to vocal perception (e.g., auditory cortex and inferior colliculus) (Garcia-Lazaro et al., 2015; Agarwalla et al., 2023). Neural pathways that regulate vocal communication involve a variety of neuromodulator systems (e.g., dopamine, serotonin, androgens) (Furlanetti et al., 2016; Kikusui et al., 2021; Hood and Hurley, 2024). Outside of the brain, many morphological studies explore the biomechanics of rodent vocalization (Alves et al., 2016; Riede et al., 2020; Hakansson et al., 2022). This morphological work shows that rodent vocalizations involve the complex coordination of respiration and laryngeal structures. Indeed, some species emit vocalizations that are temporally strung together into song-like sequences with 'syntax' (Hertz et al., 2020), and others participate in speech-like dynamics of vocal ‘turn-taking’ (Okobi et al., 2019). Taken together, contemporary mechanism studies show that rodents are valuable animal models for investigations of vocal production and perception (Mooney, 2020; Kelley, 2022; Grijseels et al., 2024).
Complementary research from a function perspective provides insights into why rodents vocalize. Studies identified by this review show that vocalizations can facilitate advertisement displays (Burkhard et al., 2023a, 2023b; Siracusa et al., 2017), agonistic interactions (Keesom et al., 2015), juvenile play (Burke et al., 2018; Himmler et al., 2014), mating interactions (Finton et al., 2017; Neunuebel et al., 2015), pair bonding (Pultorak et al., 2018; Rieger et al., 2021), parental care behaviors (Harmon-Jones and Richardson 2021; Yu et al., 2020), and predator alarms (Loughry et al., 2019; McRae and Green, 2017; Wilson-Henjum et al., 2019). Although most of the functional studies identified in this review used observational methods (e.g., recording vocalizations in different contexts), it is important to recognize that playback experiments are equally important. Playback experiments allow researchers to determine the types of information encoded in vocalizations, as well as to categorize vocalizations into calls or songs. Playback studies identified in this review show that rodent vocalizations can encode identity, affective state, and referential information (Pultorak et al., 2017; Barker et al., 2021; Hammond et al., 2024). Playback studies further reinforce hypotheses that rodent vocalizations can facilitate advertisement displays, social interactions, and predator alarms (Seffer et al., 2014; Pultorak et al., 2017; Fendt et al., 2018; Okobi et al., 2019; Hood et al., 2023). These findings suggest that rodent vocalizations have adaptive value for parental care, social cohesion, courtship, territory defense, and predator avoidance (Grijseels et al., 2024).
Of the four perspectives, development and evolution were relatively uncommon in contemporary ethology studies on vocal communication. The development perspective offers insight into how rodent vocalizations change across the lifespan, and studies identified in this review show that rodent vocalizations gradually transition from infancy to adulthood (Campbell et al., 2014; Zaytseva et al., 2020; Warren et al., 2022). Moreover, rodent vocalizations are impacted by early-life experiences (Keesom et al., 2017), and they can be innate (Jefferson et al., 2023) or involve learning (Barker et al., 2021; Janik and Knörnschild, 2021; Volodin et al., 2023). Supporting earlier research, these contemporary studies show that several ontogenetic processes shape how communication behaviors emerge and change during development. This review also identified a small number of evolutionary studies that test phylogenetic hypotheses. For example, divergent evolution of vocal communication is thought to occur through allopatric or sympatric speciation (Von Merten et al., 2014). Acoustic variation in mating calls may support sympatric speciation by providing a means for reproductive isolation and species recognition (Campbell et al., 2019; Chen et al., 2017; Tamura et al., 2018; Wang et al., 2023). Vocal divergence may come about through inter-specific variation in temperament (Kalcounis-Rueppell et al., 2018). Inter and intra-specific comparisons provide insights into how morphological and neural structures evolved in ways that support or constrain vocalization (Fernández-Vargas et al., 2022; Kelley, 2022). Because of their expansive phylogeny, rodents are a great taxonomic group for testing hypotheses about how vocal communication changes across time. A valuable area for future rodent communication research would be to build upon the evolution perspective with more comparative studies.
4.2 Rodent communication across study taxa
Of the over 2,000 living species that make up Rodentia, 88 were identified in contemporary vocalization studies. On the one hand, these 88 species seem small when compared to the sheer number of potential rodent species. Many rodent taxa have yet to be used in communication studies. On the other hand, these study species cover a broad spectrum of 43 Genus groups. A strength of this phylogeny is that it covers species with diverse social systems, life history traits, habitats, and geographic distributions (Gliwicz and Taylor, 2002; Solari and Baker, 2007; Lukas and Clutton-Brock, 2013). This means that there are rich opportunities to explore variation in vocal behavior within and across species. Some studies identified by this review take advantage of novel model species to address unique study questions (Brito et al., 2017; Amaya and Areta, 2018). Other studies use comparative methods to examine variable and conserved traits amongst sister taxa (Kalcounis-Rueppell et al., 2018; Tamura et al., 2018; Jourjine et al., 2023). As a large taxonomic group, Rodentia is excellent for testing hypotheses about how vocal communication evolved in rodents and other mammals.
This review identified a couple potential limitations in how rodent species are used in contemporary studies. One potential limitation is that there are few species used in mechanistic research. Despite being the most common perspective, mechanistic studies had the least species diversity. The majority of mechanism studies used lab-derived strains of rats (R. norvegicus) and mice (M. musculus). This homogeneity means that there are missed opportunities to study behavioral phenomena that are not applicable to traditional laboratory rodents. For example, research on androgen hormones in California mice (P. Californicus) (Timonin et al., 2018) and heart rate in prairie voles (M. ochrogaster) (Stewart et al., 2015) has led to new insights into vocal mechanisms in socially monogamous species. Another potential limitation is that few species are studied from all four perspectives. Aside from laboratory rats and mice, we only found four species studied from all perspectives: Mongolian gerbils (M. unguiculatus) (Paraouty et al., 2021; Furuyama et al., 2022; Hardy et al., 2023; Volodin et al., 2024), California mice (P. californicus) (Johnson et al., 2017; Pultorak et al., 2017; Kalcounis-Rueppell et al., 2018; Malone et al., 2023), Northern grasshopper mice (O. leucogaster) (Pasch et al., 2016, 2017; Campbell et al., 2019; Kobrina et al., 2021), and singing mice (S. teguina) (Campbell et al., 2014; Giglio and Phelps, 2020; Burkhard et al., 2023a, 2023b). A potential direction for future work would be to integrate across perspectives in more species and Genus groups.
4.3 Rodent communication in artificial and naturalistic environments
Another advantage of rodent vocal communication research is the regular use of both artificial and naturalistic study environments. Within this spectrum, rodent studies identified in this review were carried out primarily in artificial environments. Common artificial environments included laboratory testing arenas like open field (Rojas-Carvajal et al., 2022) and the Y-maze (Asaba et al., 2014; Chabout et al., 2015). Artificial environments were often used in mechanism and development studies. This finding is consistent with the idea that controlled conditions are conducive to study designs that involve physiological manipulations (Furlanetti et al., 2016; Muller and Shair, 2016; Taylor et al., 2017) and sampling at precise ages (Schwarting, 2018; Kamitakahara et al., 2021). We also found that artificial environments were common in function studies. This finding is consistent with the idea that artificial environments are valuable for tracking communication in controlled contexts, such as food reward (Brenes and Schwarting, 2015; Wardak et al., 2024), aversive stimuli (Ayers et al., 2016; Burnett and Koprowski, 2020), social interaction (Warren et al., 2021; Chen et al., 2023), and social isolation (Schwarting and Wohr, 2018; Broadfoot et al., 2023; Piastolov et al., 2023). In contrast to proximate-level and function studies, artificial environments were underrepresented in evolution studies. Although rare, evolutionary studies in artificial environments provide unique insights into the phylogenetic history of rodent vocalizations. For example, research on pup isolation calls across Peromyscus species shows that vocalization types can be controlled by distinct genetic loci and evolve quickly among sister taxa (Jourjine et al., 2023).
One potential limitation of studies in artificial environments is low species diversity. We found that artificial studies largely focused on lab-derived strains of rats and mice. An area for future work would be to bring wild animal species into captivity for controlled studies. Of course, doing so involves unique ethical and logistical considerations. Animals may be stressed by being captured, brought into a new environment, and then handled by humans. Wild-caught animals are likely to carry pathogens, which may require quarantine, testing, and treatment. Also, wild-caught species may have unique housing requirements for space, temperature, food, enrichment, and social needs. Despite these challenges, this review identified several successful examples of wild-derived species studied in the lab. These species include California mice (P. californicus) (Pultorak et al., 2018), pinyon mice (Peromyscus truei) (Brzozowski et al., 2023), singing mice (S. teguina) (Campbell et al., 2014; Tripp and Phelps, 2024), and voles (Microtus spp.) (Stewart et al., 2015; Robison et al., 2016; Warren et al., 2022; Madrid et al., 2024). Singing mice, for instance, are insectivores from Central American cloud forests and grasslands. They thrive in captivity with extra humidity in their housing room and a carnivorous diet (Banerjee et al., 2019; Tripp and Phelps, 2024). North American voles vary in their social structure across species and seasons. Prairie voles (M. ochrogaster) are socially monogamous and thrive with stable female-male breeding pairs and social housing for weaned animals, while meadow voles (M. pennsylvanicus) show shifting levels of social affiliation with day length (Kenkel et al., 2021). Thus, although there are unique challenges, using wild-derived rodents in artificial environments is feasible.
We found a small collection of contemporary studies on rodent vocalizations in naturalistic environments. Naturalistic studies involved observations in wild habitats (e.g (Wilson et al., 2015; Brito et al., 2017; Loughry et al., 2019; Hrouzkova et al., 2020)), as well as in urban areas (McRae and Green, 2014, 2017) and semi-naturalistic enclosures in zoos (Schneiderova et al., 2017) or on farms (Lima et al., 2018, 2022). Naturalistic environments have even been set up in laboratories, where enclosures simulate burrow systems (Riede, 2014; Dvořáková et al., 2016; Heinla et al., 2021). We found that naturalistic studies tend to focus on ultimate rather than proximate perspectives. However, there is much to be gained by addressing proximate questions in naturalistic settings. Doing this will require researchers to consider unique ethical and logistical issues. Experimental manipulations in the wild could cause unnecessary stress or influence behavior in a way that affects reproductive success or survival. Also, strategies are needed to track individual animals as they move across their territories. Rodents are small and inconspicuous, and many of them produce ultrasonic vocalizations that are above the frequency range of human hearing (Wöhr and Schwarting, 2013; Brudzynski, 2014).
Despite ethical and logistical challenges, this review identified a handful of species where naturalistic studies were carried out from proximate perspectives. Model species include North American red squirrels (Tamiasciurus hudsonicus) and European ground squirrels (Spermophilus citellus). Long-term research on a wild population of North American red squirrels involves the continuous tracking of ear-tagged squirrels and routine trapping to collect biological samples (Sehrsweeney et al., 2019; Hare et al., 2024). Combined with behavioral observations and acoustic recordings, this long-term field research has supported studies on stress and reproductive condition. An alternative to studying rodents in the wild is to study them in semi-free ranging settings. The use of semi-natural outdoor enclosures has supported developmental research in European ground squirrels (Schneiderov et al., 2015). Taken together, valuable areas for future research would be to address proximate-level questions in naturalistic environments and to increase species diversity in artificial environments.
We also found that species diversity was higher in naturalistic over artificial studies, and these two environments tended to focus on different taxonomic groups. Some taxonomic groups have been studied in both environments (e.g., gerbils (Meriones), lemmings (Lagurus), mole voles (Ellobius), New World flying squirrels (Glaucomys), and rats (Rattus)). However, only a few species have been studied in both environments, such as the brown rat (R. norvegicus) (Takacs et al., 2016), guinea pig (Cavia intermedia) (Verzola-Olivio and Monticelli, 2017), and rock cavy (Kerodon rupestris) (Monticelli and Alencar-Jr, 2021). Artificial and naturalistic environments come with their own advantages and disadvantages. As such, future research that spans these environments in diverse species would be an important step for building a comprehensive understanding of rodent communication.
4.4 Rodent communication in females and males across the lifespan
Contemporary ethological research on rodent vocalization studies involved both sexes. A strength of these inclusive studies is that they occurred across both proximate and ultimate perspectives. On the other hand, we found some evidence for sex differences. Despite the use of females in over half of the studies, consistently more studies used males as subjects or stimuli. We found that this pattern is likely due to an underrepresentation of females in studies on laboratory rats. A potential area for future work would be to focus more on the female perspective. Indeed, female-oriented studies have led to findings that challenge existing assumptions about sex differences. Recent studies identified by this review show that female mice and rats vocalize with males during mating interactions (Neunuebel et al., 2015; Borner et al., 2016; Finton et al., 2017; Ronald et al., 2020). These findings challenge prior research that set the precedent that mating vocalizations were primarily from males (e.g (Sales, 1972b; Holy and Guo, 2005)).
Multiple age groups – from infancy to adulthood – were used in contemporary rodent vocalization studies. We found that both proximate and ultimate-level studies involved younger animals, which is crucial for a comprehensive understanding of vocal behavior across the lifespan. Despite the use of multiple age groups, however, infants were overrepresented in development studies. Infant studies that take a mechanistic, functional, or evolutionary perspective are an important complement to developmental work. For example, mechanistic studies describe unique neural circuits for infant vocalizations (Zimmer et al., 2019), and function studies describe unique roles for infant vocalizations in maternal and paternal care (Robison et al., 2016). We also found that contemporary studies on juvenile rodents showed low species diversity. This was likely due to an overrepresentation of juveniles in studies on laboratory rats (R. rattus) as opposed to mice (M. musculus) and other species. Rats have been an excellent model for juvenile studies because they engage in play behaviors that co-occur with vocalization (Himmler et al., 2014; Burke et al., 2017). However, studies in other species would be useful to characterize juvenile-specific behaviors and vocalizations across species.
4.5 Methodological innovations in rodent communication research
Methods-focused studies introduce new hardware and software tools that innovate the common versions. Types of common hardware used in contemporary rodent research include high-quality recording equipment like condenser microphones for ultrasonic vocalizations (e.g (Asaba et al., 2014; Sirotin et al., 2014; Binder et al., 2018; Cox et al., 2024)), directional shotgun microphones for audible vocalizations (e.g (McRae and Green, 2017; Lima et al., 2024), and their respective data acquisition systems. Other common hardware include high-quality electrostatic and dynamic speakers for acoustic playback experiments (Seffer et al., 2014; Schonfeld et al., 2020; Hood et al., 2023). Common software used in contemporary rodent studies allows researchers to record or play audio with recording and playback equipment. Common software also transforms audio recordings into spectrograms, detects vocalizations with user-defined thresholds or labels, and extracts an extensive array of spectro-temporal parameters. Example software packages include Avisoft SASLab Pro (Binder et al., 2018, 2020), Raven (Monticelli and Alencar-Jr, 2021; Fernández-Vargas et al., 2022; Eddington et al., 2024), and Metris Sonotrack (Riede, 2014; Jeon et al., 2019; Pupikina and Sitnikova, 2023).
This review identified a handful of methods-focused studies that innovated hardware tools. These innovations included novel equipment for passive wildlife recordings and sound localization. In the wild, ultrasonic microphones have been used to survey an area for various squirrel species (Diggins et al., 2020a, 2020b). Wearable microphones have been used to continuously record chipmunk vocalizations (Couchoux et al., 2015). In the lab, multiple condenser microphones around a testing arena (i.e., a microphone array) is an approach used to triangulate the locations of ultrasonic vocalizations (Neunuebel et al., 2015; Heckman et al., 2017; Warren et al., 2018; Oliveira-Stahl et al., 2023). Another novel method for sound localization is with an acoustic camera, which is a multi-microphone device that maps noise and vibration intensity (Matsumoto et al., 2022; Sterling et al., 2023). Combined with high-speed video to track animals, both of these non-invasive approaches enable researchers to assign caller identities in animal groups.
This review also identified several methods-focused studies that innovated software tools. A key innovation is new software for high-throughput processing of audio files. Example software packages include XBAT (Barker et al., 2014), MUPET (Van Segbroeck et al., 2017), Ultravox (Binder et al., 2018), DeepSqueak (Coffey et al., 2019), Mouse Song Analyzer (Binder et al., 2020), BootSnap (Abbasi et al., 2022), HybridMouse (Goussha et al., 2022), AMVOC (Stoumpou et al., 2023), and MoUSE (Kania et al., 2024). These software packages help to automate the detection, feature analysis, and classification of rodent vocalizations. Many of these software packages take advantage of computer vision and machine learning, including deep learning-based approaches like convolutional neural networks (CNNs). Other software innovations focus on computational pipelines and algorithms to improve downstream analyses of vocalization data. These innovations often improve the accuracy of caller identity assignments (Heckman et al., 2017; Oliveira-Stahl et al., 2023), linking vocalizations to other behaviors (Vendrig et al., 2019; John et al., 2023; Chen et al., 2024), and analysis of complex acoustic features (Barker and Johnson, 2017; Ivanenko et al., 2020).
Finally, we found that methods-focused studies tended to use a homogenous group of species, environments, and animal demographics. Method studies focused on rats and mice. They also were biased towards adult male study subjects in artificial environments, although these observed biases did not differ from expected patterns based on the entire dataset. Interestingly, method studies were more likely to include infants, and less likely to include juveniles. A potential area for future work would be to develop and validate tools for more species, environmental conditions, and demographic groups.
4.6 Limitations and future directions
A few limitations to this scoping review require consideration. First, it is unlikely that the search strategy could detect every relevant study. The search strategy included several databases for biological research, which should find most recent studies on rodent vocalization. However, these databases can still miss relevant studies from low-impact or obscure journals. In some cases, relevant studies may be missed due to vocalization terminology that was not included in the comprehensive list of search terms. In other cases, studies would be missed if vocalization terminology was not included in the abstract or title. For example, data has been published on vocal production during prairie vole (M. ochrogaster) pair bonding (Gustison et al., 2024), but this aspect of the research is not described in the title or abstract. Second, our search strategy focused on recent studies from the past decade, and therefore, older studies were not included in analyses. These older studies may focus on different perspectives, species, research environments, and demographic groups. Although outside the scope of this review, our database search strategy can be applied to alternative time periods (Supplementary Data 4).
Finally, our search strategy was focused on ethological studies and removed those that used rodents as ‘disease models’ to make translational inferences about human disease and disorders. Research from a translational perspective can provide valuable insights into rodent communication. Biomedical studies represent a major component of laboratory rodent research, and they often characterize mechanisms and developmental processes (Scattoni et al., 2009). Data from wildtype or untreated animals in biomedical studies can be comparable to data from animals in ethological studies. Moreover, experimental animals can be used to identify genetic and neural mechanisms. Although outside the scope of this review, our database search strategy can be used to synthesize rodent communication research from a translational perspective (Supplementary Data 2).
4.7 Conclusions
The proximate and ultimate origins of vocal communication are traditionally studied in anurans, birds, bats, and primates. This review synthesizes contemporary studies on vocal communication in Rodentia. These studies showcase rodents as a valuable taxonomic group for investigations across ethological perspectives, from mechanism and development to function and evolution. With several species, research environments, demographic groups, and novel technologies, rodents offer a plethora of opportunities for research at both proximate and ultimate levels. Integration across these ethological perspectives will help to build a comprehensive understanding of acoustic communication in rodents, mammals and vertebrates.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AA: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Data curation, Project administration. YW: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Data curation, Project administration. RH: Formal analysis, Investigation, Writing – review & editing, Data curation. ET: Formal analysis, Investigation, Writing – review & editing, Data curation. CW: Formal analysis, Investigation, Writing – review & editing, Data curation. SA: Data curation, Formal analysis, Investigation, Writing – review & editing. AR: Formal analysis, Investigation, Writing – review & editing, Data curation. MA: Formal analysis, Investigation, Writing – review & editing, Data curation. AA: Formal analysis, Investigation, Writing – review & editing, Data curation. HK: Formal analysis, Investigation, Writing – review & editing, Data curation. RKS: Formal analysis, Investigation, Writing – review & editing, Data curation. TS: Formal analysis, Investigation, Writing – review & editing, Data curation. AJ: Formal analysis, Investigation, Writing – review & editing, Data curation. SJ: Formal analysis, Investigation, Writing – review & editing, Data curation. MLG: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Open access funding for this work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN-2023-03603) to MLG.
Acknowledgments
We thank Ayesha Asif and Vandita Bhatnagar for their help with study screening. We also thank Jacqueline Kreller-Vanderkooy from the Information Literacy Team at the University of Guelph for assistance with refining the search strategy. Finally, we are grateful to three reviewers for their feedback on a previous version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fetho.2025.1563374/full#supplementary-material
References
Abbasi R., Balazs P., Marconi M. A., Nicolakis D., Zala S. M., and Penn D. J. (2022). Capturing the songs of mice with an improved detection and classification method for ultrasonic vocalizations (BootSnap). PLoS Comput. Biol. 18, e1010049. doi: 10.1371/journal.pcbi.1010049
Agarwalla S., De A., and Bandyopadhyay S. (2023). Predictive mouse ultrasonic vocalization sequences: Uncovering behavioral significance, auditory cortex neuronal preferences, and social-experience-driven plasticity. J. Neurosci. 43, 6141–6163. doi: 10.1523/JNEUROSCI.2353-22.2023
Ahrens A. M., Ma S. T., Maier E. Y., Duvauchelle C. L., and Schallert T. (2009). Repeated intravenous amphetamine exposure: rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behav. Brain Res. 197, 205–209. doi: 10.1016/j.bbr.2008.08.037
Alves J. A., Boerner B. C., and Laplagne D. A. (2016). Flexible coupling of respiration and vocalizations with locomotion and head movements in the freely behaving rat. Neural Plast. 2016, 4065073. doi: 10.1155/2016/4065073
Amaya J. and Areta J. (2018). Ontogeny of long-range vocalizations in a Neotropical fossorial rodent: the Anillaco Tuco-Tuco (Ctenomys sp.). PeerJ 6, e4334. doi: 10.7717/peerj.4334
Anderson J. W. (1954). The production of ultrasonic sounds by laboratory rats and other mammals. Science 119, 808–809. doi: 10.1126/science.119.3101.808
Arksey H. and O’Malley L. (2005). Scoping studies: towards a methodological framework. Int. J. Soc. Res. Method. 8, 19–32. doi: 10.1080/1364557032000119616
Asaba A., Okabe S., Nagasawa M., Kato M., Koshida N., Osakada T., et al. (2014). Developmental social environment imprints female preference for male song in mice. PLoS One 9, e87186. doi: 10.1371/journal.pone.0087186
Ayers L. W., Asok A., Blaze J., Roth T. L., and Rosen J. B. (2016). Changes in dam and pup behavior following repeated postnatal exposure to a predator odor (TMT): A preliminary investigation in Long-Evans rats. Dev. Psychobiology 58, 176–184. doi: 10.1002/dev.21362
Banerjee A., Phelps S. M., and Long M. A. (2019). Singing mice. Curr. Biol. 29, R190–R191. doi: 10.1016/j.cub.2018.11.048
Barfield R. J. and Geyer L. A. (1972). Sexual behavior: Ultrasonic postejaculatory song of the male rat. Science 176, 1349–1350. doi: 10.1126/science.176.4041.1349
Barker A. J., Veviurko G., Bennett N. C., Hart D. W., Mograby L., and Lewin G. R. (2021). Cultural transmission of vocal dialect in the naked mole-rat. Science 371, 503–507. doi: 10.1126/science.abc6588
Barker D. J., Herrera C., and West M. O. (2014). Automated detection of 50-kHz ultrasonic vocalizations using template matching in XBAT. J. Neurosci. Methods 236, 68–75. doi: 10.1016/j.jneumeth.2014.08.007
Barker D. J. and Johnson A. M. (2017). Automated acoustic analysis of 50-kHz ultrasonic vocalizations using template matching and contour analysis. J. Acoustical Soc. America 141, EL281. doi: 10.1121/1.4977990
Bernstein J. G. and Boyden E. S. (2011). Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn. Sci. 15, 592–600. doi: 10.1016/j.tics.2011.10.003
Berz A., Pasquini de Souza C., Wöhr M., and Schwarting R. K. W. (2021). Limited generalizability, pharmacological modulation, and state-dependency of habituation towards pro-social 50-kHz calls in rats. iScience. 24 (5), 102426. doi: 10.1016/j.isci.2021.102426
Binder M. S., Hernandez-Zegada C. J., Potter C. T., Nolan S. O., and Lugo J. N. (2018). A comparison of the Avisoft (5.2) and Ultravox (2.0) recording systems: Implications for early-life communication and vocalization research. J. Neurosci. Methods 309, 6–12. doi: 10.1016/j.jneumeth.2018.08.015
Binder M., Nolan S. O., and Lugo J. N. (2020). A comparison of the Avisoft (v.5.2) and MATLAB Mouse Song Analyzer (v.1.3) vocalization analysis systems in C57BL/6, Fmr1-FVB.129, NS-Pten-FVB, and 129 mice. J. Neurosci. Methods 346, 108913. doi: 10.1016/j.jneumeth.2020.108913
Binder M. S., Pranske Z. J., and Lugo J. N. (2021). Evaluating the DeepSqueak and Mouse Song Analyzer vocalization analysis systems in C57BL/6J, FVB.129, and FVB neonates. J. Neurosci. Methods 364, 109356. doi: 10.1016/j.jneumeth.2021.109356
Blanchard R. J., Blanchard D. C., Agullana R., and Weiss S. M. (1991). Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol. Behav. 50, 967–972. doi: 10.1016/0031-9384(91)90423-L
Blumberg M. S. and Alberts J. R. (1990). Ultrasonic vocalizations by rat pups in the cold: An acoustic by-product of laryngeal braking? Behav. Neurosci. 104, 808–817. doi: 10.1037/0735-7044.104.5.808
Blumstein D. T. and Armitage K. B. (1997). Alarm calling in yellow-bellied marmots: I. The meaning of situationally variable alarm calls. Anim. Behav. 53, 143–171. doi: 10.1006/anbe.1996.0285
Blumstein D. T. and Munos O. (2005). Individual, age and sex-specific information is contained in yellow-bellied marmot alarm calls. Anim. Behav. 69, 353–361. doi: 10.1016/j.anbehav.2004.10.001
Blumstein D. T. and Recapet C. (2009). The sound of arousal: The addition of novel non-linearities increases responsiveness in marmot alarm calls. Ethology 115, 1074–1081. doi: 10.1111/j.1439-0310.2009.01691.x
Blumstein D. T., Verneyre L., and Daniel J. C. (2004). Reliability and the adaptive utility of discrimination among alarm callers. Proc. R. Soc. B: Biol. Sci. 271, 1851–1857. doi: 10.1098/rspb.2004.2808
Borner A., Hjemdahl R., Gotz T., and Brown G. R. (2016). Ultrasonic vocalizations of female Norway rats (Rattus norvegicus) in response to social partners. J. Comp. Psychol. 130, 76–80. doi: 10.1037/com0000017
Borta A., Wöhr M., and Schwarting R. K. W. (2006). Rat ultrasonic vocalization in aversively motivated situations and the role of individual differences in anxiety-related behavior. Behav. Brain Res. 166, 271–280. doi: 10.1016/j.bbr.2005.08.009
Branchi I., Santucci D., and Alleva E. (2001). Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav. Brain Res. 125, 49–56. doi: 10.1016/s0166-4328(01)00277-7
Brenes J. C. and Schwarting R. K. W. (2015). Individual differences in anticipatory activity to food rewards predict cue-induced appetitive 50-kHz calls in rats. Physiol. Behav. 149, 107–118. doi: 10.1016/j.physbeh.2015.05.012
Briggs J. R. and Kalcounis-Rueppell M. C. (2011). Similar acoustic structure and behavioural context of vocalizations produced by male and female California mice in the wild. Anim. Behav. 82, 1263–1273. doi: 10.1016/j.anbehav.2011.09.003
Brito J. M., Tinoco N., Chávez D., Moreno-Cárdenas P., Batallas D., and Ojala-Barbour R. (2017). New species of arboreal rat of the genus Rhipidomys (Cricetidae, Sigmodontinae) from Sangay National Park, Ecuador. Neotropical Biodiversity 3, 65–79. doi: 10.1080/23766808.2017.1292755
Broadfoot C. K., Lenell C., Kelm-Nelson C. A., and Ciucci M. R. (2023). Effects of social isolation on 50-kHz ultrasonic vocalizations, affective state, cognition, and neurotransmitter concentrations in the ventral tegmental and locus coeruleus of adult rats. Behav. Brain Res. 437, 114157. doi: 10.1016/j.bbr.2022.114157
Brudzynski S. M. (2009). Communication of adult rats by ultrasonic vocalization: biological, sociobiological, and neuroscience approaches. ILAR J. 50, 43–50. doi: 10.1093/ilar.50.1.43
Brudzynski S. M. (2014). “Social origin of vocal communication in rodents,” in Biocommunication of Animals. Ed. Witzany G. (Springer Netherlands, Dordrecht), 63–79. doi: 10.1007/978-94-007-7414-8_5
Brzozowski R., Kobrina A., Mahoney S. M., and Pasch B. (2023). Advertising and receiving from heights increases transmission of vocalizations in semi-arboreal mice. Behav. Ecol. Sociobiology 77, 83. doi: 10.1007/s00265-023-03352-4
Burgdorf J., Knutson B., and Panksepp J. (2000). Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav. Neurosci. 114, 320–327. doi: 10.1037/0735-7044.114.2.320
Burgdorf J., Kroes R. A., Moskal J. R., Pfaus J. G., Brudzynski S. M., and Panksepp J. (2008). Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J. Comp. Psychol. 122, 357–367. doi: 10.1037/a0012889
Burgdorf J., Panksepp J., Brudzynski S. M., Kroes R., and Moskal J. R. (2005). Breeding for 50-kHz positive affective vocalization in rats. Behav. Genet. 35, 67–72. doi: 10.1007/s10519-004-0856-5
Burgdorf J., Wood P. L., Kroes R. A., Moskal J. R., and Panksepp J. (2007). Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav. Brain Res. 182, 274–283. doi: 10.1016/j.bbr.2007.03.010
Burke C. J., Kisko T. M., Pellis S. M., and Euston D. R. (2017). Avoiding escalation from play to aggression in adult male rats: The role of ultrasonic calls. Behav. Processes 144, 72–81. doi: 10.1016/j.beproc.2017.09.014
Burkhard T. T., Matz M., and Phelps S. M. (2023a). Patterns of repeatability and heritability in the songs of wild Alston’s singing mice, Scotinomys teguina. Anim. Behav. 200, 91–103. doi: 10.1016/j.anbehav.2023.03.012
Burkhard T. T., Sachs E. R., and Phelps S. M. (2023b). Female preferences for high vocal effort in singing mice. Behaviour 160, 275–297. doi: 10.1163/1568539X-bja10203
Burke C. J., Kisko T. M., Euston D. R., and Pellis S. M. (2018). Do juvenile rats use specific ultrasonic calls to coordinate their social play? Animal Behaviour, 140, 81–92. doi: 10.1016/j.anbehav.2018.03.019
Burnett A. D. and Koprowski J. L. (2020). Ultimate causes of antipredator vocalizations in a nonhibernating squirrel. Anim. Behav. 168, 225–230. doi: 10.1016/j.anbehav.2020.08.016
Campbell P., Arevalo L., Martin H., Chen C., Sun S., Rowe A. H., et al. (2019). Vocal divergence is concordant with genomic evidence for strong reproductive isolation in grasshopper mice (Onychomys). Ecol. Evol. 9, 12886–12896. doi: 10.1002/ece3.5770
Campbell P., Pasch B., Warren A. L., and Phelps S. M. (2014). Vocal ontogeny in neotropical singing mice (Scotinomys). PLoS One 9, e113628. doi: 10.1371/journal.pone.0113628
Chabout J., Sarkar A., Dunson D. B., and Jarvis E. D. (2015). Male mice song syntax depends on social contexts and influences female preferences. Front. Behav. Neurosci. 9. doi: 10.3389/fnbeh.2015.00076
Chen Z., Jia G., Zhou Q., Zhang Y., Quan Z., Chen X., et al. (2024). ARBUR, a machine learning-based analysis system for relating behaviors and ultrasonic vocalizations of rats. iScience 27, 109998. doi: 10.1016/j.isci.2024.109998
Chen Y., Xiang Z., Su Q., Qin J., and Liu Q. (2023). Vocal signals with different social or non-social contexts in two wild rodent species (Mus caroli and Rattus losea). Anim. Cogn. 26, 963–972. doi: 10.1007/s10071-023-01745-6
Chen Y., Su Q. Q., Qin J., and Liu Q. S. (2017). Call divergence in three sympatric Rattus species. J Acoust Soc Am. 142 (1), 29. doi: 10.1121/1.4990022
Ciucci M. R., Ma S. T., Fox C., Kane J. R., Ramig L. O., and Schallert T. (2007). Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: A preliminary study. Behav. Brain Res. 182, 284–289. doi: 10.1016/j.bbr.2007.02.020
Coffey K. R., Marx R. E., and Neumaier J. F. (2019). DeepSqueak: A deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology 44, 859–868. doi: 10.1038/s41386-018-0303-6
Colvin M. A. (1973). Analysis of acoustic structure and function in ultrasounds of neonatal Microtus. Behaviour 44, 234–263. doi: 10.1163/156853973X00418
Couchoux C., Aubert M., Garant D., and Reale D. (2015). Spying on small wildlife sounds using affordable collar-mounted miniature microphones: an innovative method to record individual daylong vocalisations in chipmunks. Sci. Rep. 5, 10118. doi: 10.1038/srep10118
Cox S. S., Brown B. J., Wood S. K., Brown S. J., Kearns A. M., and Reichel C. M. (2024). Neuronal, affective, and sensory correlates of targeted helping behavior in male and female Sprague Dawley rats. Front. Behav. Neurosci. 18. doi: 10.3389/fnbeh.2024.1384578
D’Amato F. R., Scalera E., Sarli C., and Moles A. (2005). Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav. Genet. 35, 103–112. doi: 10.1007/s10519-004-0860-9
Depaulis A., Keay K. A., and Bandler R. (1992). Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Exp. Brain Res. 90, 307–318. doi: 10.1007/BF00227243
Dice L. R. and Barto E. (1952). Ability of mice of the genus Peromyscus to hear ultrasonic sounds. Science 116, 110–111. doi: 10.1126/science.116.3005.110
Diggins C., Gilley L., Kelly C., and Ford W. (2020a). Using ultrasonic acoustics to detect cryptic flying squirrels: Effects of season and habitat quality. Wildlife Soc. Bull. 44, 300–308. doi: 10.1002/wsb.1083
Diggins C., Gilley L., Turner G., and Ford W. (2020b). Ultrasonic acoustic surveys of state endangered northern flying squirrels in the Pocono Mountains, Pennsylvania. J. Fish Wildlife Manage. 11, 644–653. doi: 10.3996/JFWM-20-020
Dvořáková V., Hrouzková E., and Šumbera R. (2016). Vocal repertoire of the social Mashona mole-rat (Fukomys darlingi) and how it compares with other mole-rats. Bioacoustics 25, 253–266. doi: 10.1080/09524622.2016.1141117
Dymskaya M. M., Volodin I. A., Smorkatcheva A. V., Rudyk A., and Volodina E. V. (2025). Field tests reveal acoustic variation of call types in a subterranean rodent, the Northern Mole Vole Ellobius talpinus. J. Mammalogy 106, 237–251. doi: 10.1093/jmammal/gyae123
Eddington V. M., Nichols H. K., Calistri-Yeh A., Young V. K. H., and Kloepper L. N. (2024). Graded alarm call behavior in wild fox squirrels (Sciurus Niger). J. Acoustical Soc. America 155, 1308–1314. doi: 10.1121/10.0024771
Ehret G. (1987). Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature 325, 249–251. doi: 10.1038/325249a0
Eiler K. and Banack S. (2004). Variability in the alarm call of golden-mantled ground squirrels (Spermophilus lateralis and S-saturatus). J. Mammalogy 85, 43–50. doi: 10.1644/1545-1542(2004)085<0043:VITACO>2.0.CO;2
Eisenberg J. F. and Kleiman D. G. (1972). Olfactory communication in mammals. Annu. Rev. Ecol. Systematics 3, 1–32. doi: 10.1146/annurev.es.03.110172.000245
Ellenbroek B. and Youn J. (2016). Rodent models in neuroscience research: is it a rat race? Dis. Models Mech. 9, 1079–1087. doi: 10.1242/dmm.026120
Fendt M., Brosch M., Wernecke K. E. A., Willadsen M., and Wöhr M. (2018). Predator odour but not TMT induces 22-kHz ultrasonic vocalizations in rats that lead to defensive behaviours in conspecifics upon replay. Sci. Rep. 8, 11041. doi: 10.1038/s41598-018-28927-4
Fernández-Vargas M., Riede T., and Pasch B. (2022). Mechanisms and constraints underlying acoustic variation in rodents. Anim. Behav. 184, 135–147. doi: 10.1016/j.anbehav.2021.07.011
Finton C. J., Keesom S. M., Hood K. E., and Hurley L. M. (2017). What’s in a squeak? Female vocal signals predict the sexual behaviour of male house mice during courtship. Anim. Behav. 126, 163–175. doi: 10.1016/j.anbehav.2017.01.021
Fritz S. A., Bininda-Emonds O. R. P., and Purvis A. (2009). Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol. Lett. 12, 538–549. doi: 10.1111/j.1461-0248.2009.01307.x
Furlanetti L. L., Coenen V. A., and Dobrossy M. D. (2016). Ventral tegmental area dopaminergic lesion-induced depressive phenotype in the rat is reversed by deep brain stimulation of the medial forebrain bundle. Behav. Brain Res. 299, 132–140. doi: 10.1016/j.bbr.2015.11.036
Furtak S. C., Allen T. A., and Brown T. H. (2007). Single-unit firing in rat perirhinal cortex caused by fear conditioning to arbitrary and ecological stimuli. J. Neurosci. 27, 12277–12291. doi: 10.1523/JNEUROSCI.1653-07.2007
Furuyama T., Shigeyama T., Ono M., Yamaki S., Kobayasi K. I., Kato N., et al. (2022). Vocalization during agonistic encounter in Mongolian gerbils: Impact of sexual experience. PLoS One 17, e0272402. doi: 10.1371/journal.pone.0272402
Garcia-Lazaro J. A., Shepard K. N., Miranda J. A., Liu R. C., and Lesica N. A. (2015). An overrepresentation of high frequencies in the mouse inferior colliculus supports the processing of ultrasonic vocalizations. PLoS One 10, e0133251. doi: 10.1371/journal.pone.0133251
Giglio E. M. and Phelps S. M. (2020). Leptin regulates song effort in Neotropical singing mice (Scotinomys teguina). Anim. Behav. 167, 209–219. doi: 10.1016/j.anbehav.2020.06.022
Gliwicz J. and Taylor J. R. E. (2002). Comparing life histories of shrews and rodents. Acta Theriologica 47, 185–208. doi: 10.1007/BF03192487
Goldstein L. E., Rasmusson A. M., Bunney B. S., and Roth R. H. (1996). Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J. Neurosci. 16, 4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996
Gordon N. S., Kollack-Walker S., Akil H., and Panksepp J. (2002). Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res. Bull. 57, 651–659. doi: 10.1016/s0361-9230(01)00762-6
Gould J. and Morgan C. (1941). Hearing in the rat at high frequencies. Science 94, 168. doi: 10.1126/science.94.2433.168
Goussha Y., Bar K., Netser S., Cohen L., Hel-Or Y., and Wagner S. (2022). HybridMouse: A hybrid convolutional-recurrent neural network-based model for identification of mouse ultrasonic vocalizations. Front. Behav. Neurosci. 15. doi: 10.3389/fnbeh.2021.810590
Grijseels D. M., Lemazina A., López-Jury L., and Barker A. J. (2024). Neural correlates of social signaling in rodents: An acoustic perspective. Curr. Opin. Neurobiol. 89, 102927. doi: 10.1016/j.conb.2024.102927
Grimsley J. M. S., Monaghan J. J. M., and Wenstrup J. J. (2011). Development of social vocalizations in mice. PLoS One 6, e17460. doi: 10.1371/journal.pone.0017460
Gustison M. L., Muñoz-Castañeda R., Osten P., and Phelps S. M. (2024). Sexual coordination in a whole-brain map of prairie vole pair bonding. eLife 12, RP87029. doi: 10.7554/eLife.87029
Håkansson J., Jiang W., Xue Q., Zheng X., Ding M., Agarwal A. A., et al. (2022). Aerodynamics and motor control of ultrasonic vocalizations for social communication in mice and rats. BMC Biol. 20 (1), 3. doi: 10.1186/s12915-021-01185-z
Hammond T., Brown S., Meddle S., Nielsen B., Lawrence A., and Bombail V. (2024). Feel-good songs: application of a novel playback paradigm to induce a positive affective state in juvenile male Wistar rats. Appl. Anim. Behav. Sci. 275, 106296. doi: 10.1016/j.applanim.2024.106296
Hardy K. A., Hart D. M., and Rosen M. J. (2023). Early-life stress affects Mongolian gerbil interactions with conspecific vocalizations in a sex-specific manner. Front. Behav. Neurosci. 17. doi: 10.3389/fnbeh.2023.1128586
Hare A., McAdam A., Dantzer B., Lane J., Boutin S., and Newman A. (2024). Reproductive state alters vocal characteristics of female North American red squirrels (Tamiasciurus hudsonicus). J. Mammalogy 105, 358–371. doi: 10.1093/jmammal/gyad128
Harmon-Jones S. K. and Richardson R. (2021). Maternal care, infant fear memory retention, and the moderating role of variations in separation-induced ultrasonic vocalizations. Dev Psychobiol. 63 (6), e22177. doi: 10.1002/dev.22177
Hashimoto H., Moritani N., Aoki-Komori S., Tanaka M., and Saito T. R. (2004). Comparison of ultrasonic vocalizations emitted by rodent pups. Exp. Anim. 53, 409–416. doi: 10.1538/expanim.53.409
Heckman J. J., Proville R., Heckman G. J., Azarfar A., Celikel T., and Englitz B. (2017). High-precision spatial localization of mouse vocalizations during social interaction. Sci. Rep. 7, 3017. doi: 10.1038/s41598-017-02954-z
Heinla I., Chu X., Agmo A., and Snoeren E. (2021). Rat ultrasonic vocalizations and novelty-induced social and non-social investigation behavior in a seminatural environment. Physiol. Behav. 237, 113450. doi: 10.1016/j.physbeh.2021.113450
Hennessy M. B., Miller E. E., and Shair H. N. (2006). Brief exposure to the biological mother “potentiates” the isolation behavior of precocial Guinea pig pups. Dev. Psychobiology 48, 653–659. doi: 10.1002/dev.20180
Hertz S., Weiner B., Perets N., and London M. (2020). Temporal structure of mouse courtship vocalizations facilitates syllable labeling. Commun. Biol. 3, 333. doi: 10.1038/s42003-020-1053-7
Himmler B. T., Kisko T. M., Euston D. R., Kolb B., and Pellis S. M. (2014). Are 50-kHz calls used as play signals in the playful interactions of rats? I. Evidence from the timing and context of their use. Behav. Processes 106, 60–66. doi: 10.1016/j.beproc.2014.04.014
Hofer M. A. (1996). Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology 21, 203–217. doi: 10.1016/0306-4530(95)00042-9
Hoffmann F., Musolf K., and Penn D. J. (2012). Spectrographic analyses reveal signals of individuality and kinship in the ultrasonic courtship vocalizations of wild house mice. Physiol. Behav. 105, 766–771. doi: 10.1016/j.physbeh.2011.10.011
Holy T. E. and Guo Z. (2005). Ultrasonic songs of male mice. PLoS Biol. 3, e386. doi: 10.1371/journal.pbio.0030386
Hood K. E. and Hurley L. M. (2024). Listening to your partner: Serotonin increases male responsiveness to female vocal signals in mice. Front. Hum. Neurosci. 17. doi: 10.3389/fnhum.2023.1304653
Hood K. E., Long E., Navarro E., and Hurley L. M. (2023). Playback of broadband vocalizations of female mice suppresses male ultrasonic calls. PLoS One 18, e0273742. doi: 10.1371/journal.pone.0273742
Hrouzkova E., Bernasova E., and Skliba J. (2020). Eavesdropping on a heterospecific alarm call in the giant root-rat (Tachyorytes macrocephalus), an important prey of the Ethiopian wolf (Canis simensis). J. Ethology 38, 121–124. doi: 10.1007/s10164-019-00618-1
Ivanenko A., Watkins P., van Gerven M. A. J., Hammerschmidt K., and Englitz B. (2020). Classifying sex and strain from mouse ultrasonic vocalizations using deep learning. PLoS Comput. Biol. 16, e1007918. doi: 10.1371/journal.pcbi.1007918
Janik V. M. and Knörnschild M. (2021). Vocal production learning in mammals revisited. Philos. Trans. R. Soc. B 376, 20200244. doi: 10.1098/rstb.2020.0244
Jefferson S. J., Gregg I., Dibbs M., Liao C., Wu H., Davoudian P. A., et al. (2023). 5-MeO-DMT modifies innate behaviors and promotes structural neural plasticity in mice. Neuropsychopharmacology 48, 1257–1266. doi: 10.1038/s41386-023-01572-w
Jeon S., Kim Y., Kim S., Suh S., and Cha H. (2019). Abuse potential of 2-(4-iodo-2, 5-dimethoxypheny1) N-(2-methoxybenzyl) ethanamine (25I-NBOMe); in vivo and ex vivo approaches. Neurochemistry Int. 125, 74–81. doi: 10.1016/j.neuint.2019.02.007
John S. R., Tiwari R., Goussha Y., Amar R., Bizer A., Netser S., et al. (2023). Simultaneous recording of ultrasonic vocalizations and sniffing from socially interacting individual rats using a miniature microphone. Cell Rep. Methods 3, 100638. doi: 10.1016/j.crmeth.2023.100638
Johnson S., Painter M., Javurek A., Murphy C., Howald E., Khan Z., et al. (2017). Characterization of vocalizations emitted in isolation by California mouse (Peromyscus californicus) pups throughout the postnatal period. J. Comp. Psychol. 131, 30–39. doi: 10.1037/com0000057
Johnston R. E. (2003). Chemical communication in rodents: from pheromones to individual recognition. J. Mammalogy 84, 1141–1162. doi: 10.1644/BLe-010
Jourjine N., Woolfolk M. L., Sanguinetti-Scheck J. I., Sabatini J. E., McFadden S., Lindholm A. K., et al. (2023). Two pup vocalization types are genetically and functionally separable in deer mice. Curr. Biol. 33, 1237–1248.e4. doi: 10.1016/j.cub.2023.02.045
Kalcounis-Rueppell M. C., Metheny J. D., and Vonhof M. J. (2006). Production of ultrasonic vocalizations by Peromyscus mice in the wild. Front. Zoology 3, 3. doi: 10.1186/1742-9994-3-3
Kalcounis-Rueppell M. C., Petric R., Briggs J. R., Carney C., Marshall M. M., Willse J. T., et al. (2010). Differences in ultrasonic vocalizations between wild and laboratory California mice (Peromyscus californicus). PLoS One 5, e9705. doi: 10.1371/journal.pone.0009705
Kalcounis-Rueppell M. C., Petric R., and Marler C. A. (2018). The bold, silent type: Predictors of ultrasonic vocalizations in the Genus Peromyscus. Front. Ecol. Evol. 6. doi: 10.3389/fevo.2018.00198
Kaltwasser M. T. (1990). Acoustic signaling in the black rat (Rattus rattus). J. Comp. Psychol. 104, 227–232. doi: 10.1037/0735-7036.104.3.227
Kamitakahara A. K., Ali Marandi Ghoddousi R., Lanjewar A. L., Magalong V. M., Wu H.-H., and Levitt P. (2021). MET receptor Tyrosine Kinase regulates lifespan ultrasonic vocalization and vagal motor neuron development. Front. Neurosci. 15. doi: 10.3389/fnins.2021.768577
Kania A., Ormaniec W., Zhylko D., Grzanka L., Piotrowska D., and Siódmok A. (2024). Joseph the MoUSE - mouse ultrasonic sound explorer. SoftwareX 25, 101606. doi: 10.1016/j.softx.2023.101606
Kapusta J., Sales G. D., and Czuchnowski R. (2007). Aggression and vocalization behaviour of three sympatric vole species during conspecific and heterospecific same-sex encounters. Behaviour 144, 283–305. doi: 10.1163/156853907780425730
Kapusta J., Szentgyorgyi H., Surov A., and Ryurikov G. (2006). Vocalization of two Palaearctic species of hamster: Eversmann hamster Allocricetulus eversmanni and grey hamster Cricetulus migratorius. Bioacoustics 15, 315–330. doi: 10.1080/09524622.2006.9753556
Keesom S. M., Rendon N. M., Demas G. E., and Hurley L. M. (2015). Vocal behaviour during aggressive encounters between Siberian hamsters, Phodopus sungorus. Anim. Behav. 102, 85–93. doi: 10.1016/j.anbehav.2015.01.014
Keesom S. M., Finton C. J., Sell G. L., and Hurley L. M. (2017). Early-life social isolation influences mouse ultrasonic vocalizations during male-male social encounters. PLoS One 12, e0169705. doi: 10.1371/journal.pone.0169705
Kelley D. B. (2022). Convergent and divergent neural circuit architectures that support acoustic communication. Front. Neural Circuits 16. doi: 10.3389/fncir.2022.976789
Kenkel W. M., Gustison M. L., and Beery A. K. (2021). A neuroscientist’s guide to the vole. Curr. Protoc. 1, e175. doi: 10.1002/cpz1.175
Keylock C. J. (2005). Simpson diversity and the Shannon–Wiener index as special cases of a generalized entropy. Oikos 109, 203–207. doi: 10.1111/j.0030-1299.2005.13735.x
Kholodar-Smith D. B., Allen T. A., and Brown T. H. (2008). Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behav. Neurosci. 122, 1178–1185. doi: 10.1037/a0012902
Kikusui T., Sonobe M., Yoshida Y., Nagasawa M., Ey E., de Chaumont F., et al. (2021). Testosterone increases the emission of ultrasonic vocalizations with different acoustic characteristics in mice. Front. Psychol. 12. doi: 10.3389/fpsyg.2021.680176
Kim E. J., Kim E. S., Covey E., and Kim J. J. (2010). Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One 5, e15077. doi: 10.1371/journal.pone.0015077
Knutson B., Burgdorf J., and Panksepp J. (1998). Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J. Comp. Psychol. 112, 65–73. doi: 10.1037/0735-7036.112.1.65
Kobrina A., Hidau M. K., Riede T., Guthrie O. W., and Pasch B. (2021). Age-related and noise-induced hearing loss alters grasshopper mouse (Onychomys) vocalizations. Hearing Res. 404, 108210. doi: 10.1016/j.heares.2021.108210
Koo J. W., Han J.-S., and Kim J. J. (2004). Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J. Neurosci. 24, 7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004
Krakauer J. W., Ghazanfar A. A., Gomez-Marin A., MacIver M. A., and Poeppel D. (2017). Neuroscience needs behavior: Correcting a reductionist bias. Neuron 93, 480–490. doi: 10.1016/j.neuron.2016.12.041
Kroes R. A., Burgdorf J., Otto N. J., Panksepp J., and Moskal J. R. (2007). Social defeat, a paradigm of depression in rats that elicits 22-kHz vocalizations, preferentially activates the cholinergic signaling pathway in the periaqueductal gray. Behav. Brain Res. 182, 290–300. doi: 10.1016/j.bbr.2007.03.022
Lepri J. J., Theodorides M., and Wysocki C. J. (1988). Ultrasonic vocalizations by adult prairie voles, Microtus ochrogaster. Experientia 44, 271–273. doi: 10.1007/BF01941736
Lima A., Lima S., Nogueira-Filho S., Held S., Mendl M., and Nogueira S. (2024). Object play as a positive emotional state indicator for farmed spotted paca (Cuniculus paca). Animals 14, 78. doi: 10.3390/ani14010078
Lima A. F., Lima S. G. C., Nogueira-Filho S. L. G., Held S., Paul E., Mendl M., et al. (2022). Vocal expression of emotions in farmed spotted paca (Cuniculus paca). Appl. Anim. Behav. Sci. 256, 105753. doi: 10.1016/j.applanim.2022.105753
Lima S., Sousa-Lima R., Tokumaru R., Nogueira S., and Nogueira S. (2018). Vocal complexity and sociality in spotted paca (Cuniculus paca). PLoS One 13, e0190961. doi: 10.1371/journal.pone.0190961
Loughry W. J., Oeser M., Anderson C. D., and Hoogland J. L. (2019). The importance of individual variation in the alarm calls of Gunnison’s prairie dogs. Anim. Behav. 150, 59–68. doi: 10.1016/j.anbehav.2019.01.019
Lukas D. and Clutton-Brock T. H. (2013). The evolution of social monogamy in mammals. Science 341, 526–530. doi: 10.1126/science.1238677
Madrid J. E., Pranic N. M., Chu S., Bergstrom J. J. D., Singh R., Rabinovich J., et al. (2024). Effects of short-term isolation on social behaviors in prairie voles. PLoS One 19, e0313172. doi: 10.1371/journal.pone.0313172
Mai L., Inada H., and Osumi N. (2023). Whole-brain mapping of neuronal activity evoked by maternal separation in neonatal mice: An association with ultrasound vocalization. Neuropsychopharmacol Rep. 43 (2), 239–248. doi: 10.1002/npr2.12337
Malone C. L., Rieger N. S., Spool J. A., Payette A., Riters L. V., and Marler C. A. (2023). Behavioral convergence in defense behaviors in pair bonded individuals correlates with neuroendocrine receptors in the medial amygdala. Behav. Brain Res. 452, 114556. doi: 10.1016/j.bbr.2023.114556
Matrosova V., Volodin I., and Volodina E. (2009). Short-term and long-term individuality in speckled ground squirrel alarm calls. J. Mammalogy 90, 158–166. doi: 10.1644/08-MAMM-A-032.1
Matrosova V. A., Volodin I. A., Volodina E. V., and Babitsky A. F. (2007). Pups crying bass: Vocal adaptation for avoidance of age-dependent predation risk in ground squirrels? Behav. Ecol. Sociobiology 62, 181–191. doi: 10.1007/s00265-007-0452-9
Matrosova V. A., Volodin I. A., Volodina E. V., and Vasilieva N. A. (2010). Stability of acoustic individuality in the alarm calls of wild yellow ground squirrels Spermophilus fulvus and contrasting calls from trapped and free-ranging callers. Die Naturwissenschaften 97, 707–715. doi: 10.1007/s00114-010-0686-7
Matsumoto J., Kanno K., Kato M., Nishimaru H., Setogawa T., Chinzorig C., et al. (2022). Acoustic camera system for measuring ultrasound communication in mice. iScience 25, 104812. doi: 10.1016/j.isci.2022.104812
McGregor P. K. and Ranft R. D. (1994). Equipment for sound analysis and playback: A survey. Bioacoustics 6, 83–86. doi: 10.1080/09524622.1994.9753273
Mcintosh T. K., Barfield R. J., and Geyer L. A. (1978). Ultrasonic vocalisations facilitate sexual behaviour of female rats. Nature 272, 163–164. doi: 10.1038/272163a0
McRae T. R. and Green S. M. (2014). Joint tail and vocal alarm signals of gray squirrels (Sciurus carolinensis). Behaviour 151, 1433–1452. doi: 10.1163/1568539X-00003194
McRae T. and Green S. (2017). Gray squirrel alarm call composition differs in response to simulated aerial versus terrestrial predator attacks. Ethology Ecol. Evol. 29, 54–63. doi: 10.1080/03949370.2015.1087433
Moles A., Kieffer B. L., and D’Amato F. R. (2004). Deficit in attachment behavior in mice lacking the µ-opioid receptor gene. Science 304, 1983–1986. doi: 10.1126/science.1095943
Monticelli P. F. and Alencar-Jr R. N. (2021). The acoustic behavior of the Brazilian caatinga big rodent is incongruent to its actual position in Hydrochoerinae. Behav. Processes 193, 104523. doi: 10.1016/j.beproc.2021.104523
Mooney R. (2020). The neurobiology of innate and learned vocalizations in rodents and songbirds. Curr. Opin. Neurobiol. 64, 24–31. doi: 10.1016/j.conb.2020.01.004
Muller J. M. and Shair H. N. (2016). Isolation-induced vocalization in the infant rat depends on the nucleus accumbens. Dev. Psychobiology 58, 1116–1123. doi: 10.1002/dev.21447
Musolf K., Hoffmann F., and Penn D. J. (2010). Ultrasonic courtship vocalizations in wild house mice, Mus musculus musculus. Anim. Behav. 79, 757–764. doi: 10.1016/j.anbehav.2009.12.034
Nakano R., Nakagawa R., Tokimoto N., and Okanoya K. (2013). Alarm call discrimination in a social rodent: adult but not juvenile degu calls induce high vigilance. J. Ethology 31, 115–121. doi: 10.1007/s10164-012-0355-8
Neunuebel J. P., Taylor A. L., Arthur B. J., and Egnor S. E. R. (2015). Female mice ultrasonically interact with males during courtship displays. eLife 4, e06203. doi: 10.7554/eLife.06203
Nieder A. and Mooney R. (2019). The neurobiology of innate, volitional and learned vocalizations in mammals and birds. Philos. Trans. R. Soc. B 375, 20190054. doi: 10.1098/rstb.2019.0054
Nishiyama K., Kobayasi K. I., and Riquimaroux H. (2011). Vocalization control in Mongolian gerbils (Meriones unguiculatus) during locomotion behavior. J. Acoustical Soc. America 130, 4148–4157. doi: 10.1121/1.3651815
Noirot E. (1972). Ultrasounds and maternal behavior in small rodents. Dev. Psychobiology 5, 371–387. doi: 10.1002/dev.420050410
Nyby J. and Whitney G. (1978). Ultrasonic communication of adult myomorph rodents. Neurosci. Biobehav. Rev. 2, 1–14. doi: 10.1016/0149-7634(78)90003-9
Ogawa S., Chester A. E., Hewitt S. C., Walker V. R., Gustafsson J.-Å., Smithies O., et al. (2000). Abolition of male sexual behaviors in mice lacking estrogen receptors α and β (αβERKO). Proc. Natl. Acad. Sci. 97, 14737–14741. doi: 10.1073/pnas.250473597
Okabe S., Nagasawa M., Kihara T., Kato M., Harada T., Koshida N., et al. (2010). The effects of social experience and gonadal hormones on retrieving behavior of mice and their responses to pup ultrasonic vocalizations. Zoological Sci. 27, 790–795. doi: 10.2108/zsj.27.790
Okabe S., Nagasawa M., Kihara T., Kato M., Harada T., Koshida N., et al. (2013). Pup odor and ultrasonic vocalizations synergistically stimulate maternal attention in mice. Behav. Neurosci. 127, 432–438. doi: 10.1037/a0032395
Okobi D. E., Banerjee A., Matheson A. M. M., Phelps S. M., and Long M. A. (2019). Motor cortical control of vocal interaction in neotropical singing mice. Science 363, 983–988. doi: 10.1126/science.aau9480
Oliveira-Stahl G., Farboud S., Sterling M. L., Heckman J. J., van Raalte B., Lenferink D., et al. (2023). High-precision spatial analysis of mouse courtship vocalization behavior reveals sex and strain differences. Sci. Rep. 13, 5219. doi: 10.1038/s41598-023-31554-3
Panksepp J. and Burgdorf J. (2000). 50-kHz chirping (laughter)? in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav. Brain Res. 115, 25–38. doi: 10.1016/S0166-4328(00)00238-2
Panksepp J. B., Jochman K. A., Kim J. U., Koy J. J., Wilson E. D., Chen Q., et al. (2007). Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One 2, e351. doi: 10.1371/journal.pone.0000351
Paradis E. and Schliep K. (2019). Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528. doi: 10.1093/bioinformatics/bty633
Paraouty N., Rizzuto C. R., and Sanes D. H. (2021). Dopaminergic signaling supports auditory social learning. Sci. Rep. 11, 13117. doi: 10.1038/s41598-021-92524-1
Pasch B., Abbasi M. Z., Wilson M., Zhao D., Searle J. B., Webster M. S., et al. (2016). Cross-fostering alters advertisement vocalizations of grasshopper mice (Onychomys): Evidence for the developmental stress hypothesis. Physiol. Behav. 157, 265–269. doi: 10.1016/j.physbeh.2016.02.012
Pasch B., Tokuda I. T., and Riede T. (2017). Grasshopper mice employ distinct vocal production mechanisms in different social contexts. Proc. R Soc. B 284, 20171158. doi: 10.1098/rspb.2017.1158
Petric R. and Kalcounis-Rueppell M. C. (2013). Female and male adult brush mice (Peromyscus boylii) use ultrasonic vocalizations in the wild. Behaviour 150, 1747–1766. doi: 10.1163/1568539X-00003118
Piastolov S. V., Volodin I. A., Vasilieva N. Y., Khrushchova A. M., Shekarova O. N., and Volodina E. V. (2023). Comparison of ultrasonic isolation calls of pure-breeding and interspecies hybrid Phodopus dwarf hamster pups. Behav. Processes 210, 104917. doi: 10.1016/j.beproc.2023.104917
Pollard K. A. and Blumstein D. T. (2012). Evolving communicative complexity: insights from rodents and beyond. Philos. Trans. R. Soc. B 367, 1869–1878. doi: 10.1098/rstb.2011.0221
Pomerantz S. M., Nunez A. A., and Jay Bean N. (1983). Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol. Behav. 31, 91–96. doi: 10.1016/0031-9384(83)90101-4
Portfors C. V. (2007). Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab. Anim. Sci. 46, 28–34.
Pultorak J., Alger S., Loria S., Johnson A., and Marler C. (2018). Changes in behavior and ultrasonic vocalizations during pair bonding and in response to an infidelity challenge in monogamous California mice. Front. Ecol. Evol. 6. doi: 10.3389/fevo.2018.00125
Pultorak J. D., Matusinec K. R., Miller Z. K., and Marler C. A. (2017). Ultrasonic vocalization production and playback predicts intrapair and extrapair social behaviour in a monogamous mouse. Anim. Behav. 125, 13–23. doi: 10.1016/j.anbehav.2016.12.023
Pupikina M. and Sitnikova E. (2023). Sex differences in behavior and learning abilities in adult rats. Life 13, 547. doi: 10.3390/life13020547
Ralls K. (1967). Auditory sensitivity in mice: Peromyscus and Mus musculus. Anim. Behav. 15, 123–128. doi: 10.1016/S0003-3472(67)80022-8
Riede T. (2014). Rat ultrasonic vocalization shows features of a modular behavior. J. Neurosci. 34, 6874–6878. doi: 10.1523/JNEUROSCI.0262-14.2014
Riede T., Schaefer C., and Stein A. (2020). Role of deep breaths in ultrasonic vocal production of Sprague-Dawley rats. J Neurophysiol. 123 (3), 966–979. doi: 10.1152/jn.00590.2019
Rieger N. S., Monari P. K., Hartfield K., Schefelker J., and Marler C. A. (2021). Pair-bonding leads to convergence in approach behavior to conspecific vocalizations in California mice (Peromyscus californicus). PLoS One 16 (8), e0255295. doi: 10.1371/journal.pone.0255295
Roberts L. H. (1975). The rodent ultrasound production mechanism. Ultrasonics 13, 83–88. doi: 10.1016/0041-624X(75)90052-9
Robison W. T., Myers M. M., Hofer M. A., Shair H. N., and Welch M. G. (2016). Prairie vole pups show potentiated isolation-induced vocalizations following isolation from their mother, but not their father. Dev. Psychobiology 58, 687–699. doi: 10.1002/dev.21408
Rojas-Carvajal M., Chinchilla-Alvarado J., and Brenes J. C. (2022). Muscarinic regulation of self-grooming behavior and ultrasonic vocalizations in the context of open-field habituation in rats. Behav. Brain Res. 418, 113641. doi: 10.1016/j.bbr.2021.113641
Ronald K. L., Zhang X., Morrison M. V., Miller R., and Hurley L. M. (2020). Male mice adjust courtship behavior in response to female multimodal signals. PLoS One 15, e0229302. doi: 10.1371/journal.pone.0229302
Rosenzweig M. R., Riley D. A., and Krech D. (1955). Evidence for echolocation in the rat. Science 121, 600–600. doi: 10.1126/science.121.3147.600
Roth B. L. (2016). DREADDs for neuroscientists. Neuron 89, 683–694. doi: 10.1016/j.neuron.2016.01.040
Sadananda M., Wohr M., and Schwarting R. K. W. (2008). Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci. Lett. 435, 17–23. doi: 10.1016/j.neulet.2008.02.002
Sales G. D. (1972a). Ultrasound and aggressive behaviour in rats and other small mammals. Anim. Behav. 20, 88–100. doi: 10.1016/s0003-3472(72)80177-5
Sales G. D. (1972b). Ultrasound and mating behaviour in rodents with some observations on other behavioural situations. J. Zoology 168, 149–164. doi: 10.1111/j.1469-7998.1972.tb01345.x
Scattoni M. L., Crawley J., and Ricceri L. (2009). Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 33, 508–515. doi: 10.1016/j.neubiorev.2008.08.003
Schneiderov I., Schnitzerov P., Uhlikov J., Brandl P., Zouhar J., and Mateju J. (2015). Differences in alarm calls of juvenile and adult European ground squirrels (Spermophilus citellus): Findings on permanently marked animals from a semi-natural enclosure. Zoo Biol. 34, 503–512. doi: 10.1002/zoo.21233
Schneiderova I. and Policht R. (2012). Acoustic analysis of the alarm call of the Anatolian ground squirrel Spermophilus xanthoprymnus: a description and comparison with alarm calls of the Taurus S. taurensis and European S. citellus ground squirrels. Die Naturwissenschaften 99, 55–64. doi: 10.1007/s00114-011-0870-4
Schneiderova I., Volodina E. V., Matrosova V. A., and Volodin I. A. (2017). One plus one: Binary alarm calls retain individual signature for longer periods than single-note alarms in the European ground squirrel (Spermophilus citellus). Behav. Processes 138, 73–81. doi: 10.1016/j.beproc.2017.02.014
Schonfeld L.-M., Zech M.-P., Schable S., Wohr M., and Kalenscher T. (2020). Lesions of the rat basolateral amygdala reduce the behavioral response to ultrasonic vocalizations. Behav. Brain Res. 378, 112274. doi: 10.1016/j.bbr.2019.112274
Schwarting R. K. W. (2018). Ultrasonic vocalization in female rats: A comparison among three outbred stocks from pups to adults. Physiol. Behav. 196, 59–66. doi: 10.1016/j.physbeh.2018.08.009
Schwarting R. K. W., Jegan N., and Wöhr M. (2007). Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav. Brain Res. 182, 208–222. doi: 10.1016/j.bbr.2007.01.029
Schwarting R. K. W. and Wohr M. (2018). Isolation-induced ultrasonic vocalizations in pups: A comparison between Long-Evans, Sprague-Dawley, and Wistar rats. Dev. Psychobiology 60, 534–543. doi: 10.1002/dev.21738
Seffer D., Schwarting R. K. W., and Wohr M. (2014). Pro-social ultrasonic communication in rats: insights from playback studies. J. Neurosci. Methods 234, 73–81. doi: 10.1016/j.jneumeth.2014.01.023
Sehrsweeney M., Wilson D. R., Bain M., Boutin S., Lane J. E., McAdam A. G., et al. (2019). The effects of stress and glucocorticoids on vocalizations: A test in North American red squirrels. Behav. Ecol. 30, 1030–1040. doi: 10.1093/beheco/arz044
Shair H. N., Smith J. A., and Welch M. G. (2012). Cutting the vagus nerve below the diaphragm prevents maternal potentiation of infant rat vocalization. Dev. Psychobiology 54, 70–76. doi: 10.1002/dev.20577
Shelley E. L. and Blumstein D. T. (2005). The evolution of vocal alarm communication in rodents. Behav. Ecol. 16, 169–177. doi: 10.1093/beheco/arh148
Simeonovska-Nikolova D. and Dekov O. (2013). Some aspects of the behavior and defensive vocalization of the Romanian hamster, mesocricetus newtoni. Acta Zoologica Bulgarica 65, 461–468. Available online at: https://acta-zoologica-bulgarica.eu/december-2013/.
Siracusa E., Morandini M., Boutin S., Humphries M., Dantzer B., Lane J., et al (2017). Red squirrel territorial vocalizations deter intrusions by conspecific rivals. Behaviour 154 (13-15), 1259–1273. doi: 10.1163/1568539X-00003467
Sirotin Y. B., Costa M. E., and Laplagne D. A. (2014). Rodent ultrasonic vocalizations are bound to active sniffing behavior. Front. Behav. Neurosci. 8. doi: 10.3389/fnbeh.2014.00399
Sloan J. L. and Hare J. F. (2004). Monotony and the information content of Richardson’s ground squirrel (Spermophilus richardsonii) repeated calls: Tonic communication or signal certainty? Ethology 110, 147–156. doi: 10.1111/j.1439-0310.2003.00955.x
Sloan J. L. and Hare J. F. (2008). The more the scarier: Adult Richardson’s ground squirrels (Spermophilus richardsonii) assess response urgency via the number of alarm signallers. Ethology 114, 436–443. doi: 10.1111/j.1439-0310.2008.01479.x
Solari S. and Baker R. J. (2007). Mammal species of the world: A taxonomic and geographic reference by D. E. Wilson; D. M. Reeder. J. Mammalogy 88, 824–830. doi: 10.1644/06-MAMM-R-422.1
Sterling M. L., Teunisse R., and Englitz B. (2023). Rodent ultrasonic vocal interaction resolved with millimeter precision using hybrid beamforming. eLife 12, e86126. doi: 10.7554/eLife.86126
Stewart A. M., Lewis G. F., Yee J. R., Kenkel W. M., Davila M. I., Sue Carter C., et al. (2015). Acoustic features of prairie vole (Microtus ochrogaster) ultrasonic vocalizations covary with heart rate. Physiol. Behav. 138, 94–100. doi: 10.1016/j.physbeh.2014.10.011
Stoddard P. K. (1990). Audio computers—Theory of operation and guidelines for selection of systems and components. Bioacoustics 2, 217–239. doi: 10.1080/09524622.1990.9753134
Stoumpou V., Vargas C., SChade P., Boyd J., Giannakopoulos T., and Jarvis E. (2023). Analysis of Mouse Vocal Communication (AMVOC): a deep, unsupervised method for rapid detection, analysis and classification of ultrasonic vocalisations. Bioacoustics 32, 199–229. doi: 10.1080/09524622.2022.2099973
Swan D. C. and Hare J. F. (2008). The first cut is the deepest: primary syllables of Richardson’s ground squirrel, Spermophilus richardsonii, repeated calls alert receivers. Anim. Behav. 76, 47–54. doi: 10.1016/j.anbehav.2007.11.008
Szentgyorgyi H., Kapusta J., and Marchlewska-Koj A. (2008). Ultrasonic calls of bank vole pups isolated and exposed to cold or to nest odor. Physiol. Behav. 93, 296–303. doi: 10.1016/j.physbeh.2007.09.015
Takacs S., Kowalski P., and Gries G. (2016). Natural and synthetic vocalizations of brown rat pups, Rattus norvegicus, enhance attractiveness of bait boxes in laboratory and field experiments. Pest Manage. Sci. 72, 1873–1882. doi: 10.1002/ps.4219
Tamura N., Boonkhaw P., Prayoon U., Kanchanasaka B., and Hayashi F. (2018). Mating calls are a sensitive indicator of phylogenetic relationships in tropical tree squirrels (Callosciurus spp.). Mamm. Biol. 93, 198–206. doi: 10.1016/j.mambio.2018.05.006
Taylor J. O., Urbano C. M., and Cooper B. G. (2017). Differential patterns of constant frequency 50 and 22 khz usv production are related to intensity of negative affective state. Behav. Neurosci. 131, 115–126. doi: 10.1037/bne0000184
Thompson A. B. and Hare J. F. (2010). Neighbourhood watch: Multiple alarm callers communicate directional predator movement in Richardson’s ground squirrels, Spermophilus richardsonii. Anim. Behav. 80, 269–275. doi: 10.1016/j.anbehav.2010.04.028
Thompson B., Leonard K. C., and Brudzynski S. M. (2006). Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav. Brain Res. 168, 64–73. doi: 10.1016/j.bbr.2005.10.012
Timonin M. E., Kalcounis-Rueppell M. C., and Marler C. A. (2018). Testosterone pulses at the nest site modify ultrasonic vocalization types in a monogamous and territorial mouse. Ethology 124, 804–815. doi: 10.1111/eth.12812
Tinbergen N. (1963). On aims and methods of Ethology. Z. für Tierpsychologie 20, 410–433. doi: 10.1111/j.1439-0310.1963.tb01161.x
Tripp J. A. and Phelps S. M. (2024). Females counter-sing, but response to male song differs by sex in Alston’s singing mouse. Biol. Lett. 20, 20230484. doi: 10.1098/rsbl.2023.0484
Tschida K., Michael V., Takatoh J., Han B.-X., Zhao S., Sakurai K., et al. (2019). A specialized neural circuit gates social vocalizations in the mouse. Neuron 103, 459–472.e4. doi: 10.1016/j.neuron.2019.05.025
Van Segbroeck M., Knoll A. T., Levitt P., and Narayanan S. (2017). MUPET-Mouse Ultrasonic Profile ExTraction: A signal processing tool for rapid and unsupervised analysis of ultrasonic vocalizations. Neuron 94, 465–485.e5. doi: 10.1016/j.neuron.2017.04.005
Vendrig N., Hemerik L., Pinter I., and ter Braak C. (2019). Relating ultrasonic vocalizations from a pair of rats to individual behavior: A composite link model approach. Statistica Neerlandica 73, 139–156. doi: 10.1111/stan.12144
Verzola-Olivio P. and Monticelli P. (2017). The acoustic repertoire of Cavia intermedia as a contribution to the understanding of the Caviidae communication system. Bioacoustics 26, 285–304. doi: 10.1080/09524622.2016.1278401
Volodin I. A., Klenova A. V., Kirilyuk V. E., Ilchenko O. G., and Volodina E. V. (2024). Ultrasonic alarm call of Mongolian gerbils (Meriones ungiuculatus) in the wild and in captivity: a potential tool for detecting inhabited colonies during population depression. Mamm. Biol. 104, 407–416. doi: 10.1007/s42991-024-00416-4
Volodin I., Kozhevnikova J., Ilchenko O., Sapozhnikova S., and Volodina E. (2023). Cross-fostering effects on ultrasonic calls in two gerbil species. Russian J. Theriology 22, 16–23. doi: 10.15298/rusjtheriol.22.1.02
Volodina E. V., Matrosova V. A., and Volodin I. A. (2010). An unusual effect of maturation on the alarm call fundamental frequency in two species of ground squirrels. Bioacoustics 20, 87–98. doi: 10.1080/09524622.2011.9753634
von Merten S., Hoier S., Pfeifle C., and Tautz D. (2014). A role for ultrasonic vocalisation in social communication and divergence of natural populations of the house mouse (Mus musculus domesticus). PLoS One 9 (5), e97244. doi: 10.1371/journal.pone.0097244. Erratum in: PLoS One. 2015 Jan 30;10(1):e0118130. doi: 10.1371/journal.pone.0118130
Wang W. C., Li Z. M., Chen Y., Zhang J. H., Zhang J. X., and Zhang Y. H. (2023). Species recognition and the divergences in the chemical and ultrasonic signals between two coexisting Rattus species. Curr Zool. 70 (4), 531–538. doi: 10.1093/cz/zoad035
Wardak A. D., Olszynski K. H., Polowy R., Matysiak J., and Filipkowski R. K. (2024). Rats that learn to vocalize for food reward emit longer and louder appetitive calls and fewer short aversive calls. PLoS One 19, e0297174. doi: 10.1371/journal.pone.0297174
Warren M. R., Campbell D., Borie A. M., Ford C. L. I., Dharani A. M., Young L. J., et al. (2022). Maturation of social-vocal communication in prairie vole (Microtus ochrogaster) pups. Front. Behav. Neurosci. 15. doi: 10.3389/fnbeh.2021.814200
Warren M. R., Sangiamo D. T., and Neunuebel J. P. (2018). High channel count microphone array accurately and precisely localizes ultrasonic signals from freely-moving mice. J. Neurosci. Methods 297, 44–60. doi: 10.1016/j.jneumeth.2017.12.013
Warren M. R., Spurrier M. S., Sangiamo D. T., Clein R. S., and Neunuebel J. P. (2021). Mouse vocal emission and acoustic complexity do not scale linearly with the size of a social group. J. Exp. Biol. 224, jeb239814. doi: 10.1242/jeb.239814
Wilson D. R., Goble A. R., Boutin S., Humphries M. M., Coltman D. W., Gorrell J. C., et al. (2015). Red squirrels use territorial vocalizations for kin discrimination. Anim. Behav. 107, 79–85. doi: 10.1016/j.anbehav.2015.06.011
Wilson-Henjum G. E., Job J. R., McKenna M. F., Shannon G., and Wittemyer G. (2019). Alarm call modification by prairie dogs in the presence of juveniles. J Ethol 37, 167–174. doi: 10.1007/s10164-018-0582-8
Winslow J. T., Hearn E. F., Ferguson J., Young L. J., Matzuk M. M., and Insel T. R. (2000). Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Hormones Behav. 37, 145–155. doi: 10.1006/hbeh.1999.1566
Wöhr M., Borta A., and Schwarting R. K. W. (2005). Overt behavior and ultrasonic vocalization in a fear conditioning paradigm: a dose-response study in the rat. Neurobiol. Learn. Memory 84, 228–240. doi: 10.1016/j.nlm.2005.07.004
Wöhr M., Dahlhoff M., Wolf E., Holsboer F., Schwarting R. K. W., and Wotjak C. T. (2008a). Effects of genetic background, gender, and early environmental factors on isolation-induced ultrasonic calling in mouse pups: an embryo-transfer study. Behav. Genet. 38, 579–595. doi: 10.1007/s10519-008-9221-4
Wöhr M., Houx B., Schwarting R. K. W., and Spruijt B. (2008b). Effects of experience and context on 50-kHz vocalizations in rats. Physiol. Behav. 93, 766–776. doi: 10.1016/j.physbeh.2007.11.031
Wöhr M. and Schwarting R. K. W. (2007). Ultrasonic communication in rats: can playback of 50-kHz calls induce approach behavior? PLoS One 2, e1365. doi: 10.1371/journal.pone.0001365
Wöhr M. and Schwarting R. K. W. (2008). Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav. Neurosci. 122, 310–330. doi: 10.1037/0735-7044.122.2.310
Wöhr M. and Schwarting R. K. W. (2009). Ultrasonic communication in rats: effects of morphine and naloxone on vocal and behavioral responses to playback of 50-kHz vocalizations. Pharmacology biochemistry Behav. 94, 285–295. doi: 10.1016/j.pbb.2009.09.008
Wöhr M. and Schwarting R. K. W. (2012). Testing social acoustic memory in rats: effects of stimulus configuration and long-term memory on the induction of social approach behavior by appetitive 50-kHz ultrasonic vocalizations. Neurobiol. Learn. Memory 98, 154–164. doi: 10.1016/j.nlm.2012.05.004
Wöhr M. and Schwarting R. K. W. (2013). Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 354, 81–97. doi: 10.1007/s00441-013-1607-9
Wright S. L. and Brown R. E. (2004). Sex differences in ultrasonic vocalizations and coordinated movement in the California mouse (Peromyscus californicus). Behav. Processes 65, 155–162. doi: 10.1016/j.beproc.2003.09.004
Wright J. M., Gourdon J. C., and Clarke P. B. S. (2010). Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacol. (Berl) 211, 1–13. doi: 10.1007/s00213-010-1859-y
Yang M., Loureiro D., Kalikhman D., and Crawley J. N. (2013). Male mice emit distinct ultrasonic vocalizations when the female leaves the social interaction arena. Front. Behav. Neurosci. 7. doi: 10.3389/fnbeh.2013.00159
Yosida S., Kobayasi K. I., Ikebuchi M., Ozaki R., and Okanoya K. (2007). Antiphonal vocalization of a subterranean rodent, the naked mole-rat (Heterocephalus glaber). Ethology 113, 703–710. doi: 10.1111/j.1439-0310.2007.01371.x
Yosida S. and Okanoya K. (2009). Naked mole-rat is sensitive to social hierarchy encoded in antiphonal vocalization. Ethology 115, 823–831. doi: 10.1111/j.1439-0310.2009.01677.x
Yu P., Yang M., Zhao H., Cao R., Chen Z., and Gong D. (2020). Characteristics of pup ultrasonic vocalizations and parental behavior responses in midday gerbils (Meriones meridianus). Physiol Behav. 224, 113075. doi: 10.1016/j.physbeh.2020.113075
Zaytseva A. S., Volodin I. A., Ilchenko O. G., and Volodina E. V. (2020). Audible calls and their ontogenetic relationship with ultrasonic vocalization in a rodent with a wide vocal range, the fat-tailed gerbil (Pachyuromys duprasi). Behav. Processes 180, 104241. doi: 10.1016/j.beproc.2020.104241
Zimmer M. R., Fonseca A. H. O., Iyilikci O., Pra R. D., and Dietrich M. O. (2019). Functional ontogeny of hypothalamic Agrp neurons in neonatal mouse behaviors. Cell 178, 44–59.e7. doi: 10.1016/j.cell.2019.04.026
Keywords: Rodentia, vocalization, acoustic signaling, evolution, function, development, mechanism, methods
Citation: Abud A, Wang Y, Hafter R, Tavakoli E, Wu C, Atapattu S, Raza A, Ali M, Al-Hadi A, Khalid H, Sukhija RK, Stoodley T, Joshi A, Joshi S and Gustison ML (2025) Advances in ethological approaches to explore rodent vocal communication. Front. Ethol. 4:1563374. doi: 10.3389/fetho.2025.1563374
Received: 19 January 2025; Accepted: 01 July 2025;
Published: 07 August 2025.
Edited by:
Giada Cordoni, University of Turin, ItalyReviewed by:
Theresa M. Kisko, Faculty of Psychology and Educational Sciences, BelgiumSusanna Pietropaolo, UMR5287 Institut de Neurosciences Cognitives et Intégratives d’Aquitaine (INCIA), France
Scott Nunes, University of San Francisco, United States
Copyright © 2025 Abud, Wang, Hafter, Tavakoli, Wu, Atapattu, Raza, Ali, Al-Hadi, Khalid, Sukhija, Stoodley, Joshi, Joshi and Gustison. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Morgan L. Gustison, bWd1c3Rpc29AdXdvLmNh
†These authors have contributed equally to this work and share first authorship
 Ali Abud1,2†
Ali Abud1,2† Yihan Wang
Yihan Wang Hamza Khalid
Hamza Khalid Rehmat K. Sukhija
Rehmat K. Sukhija Tobias Stoodley
Tobias Stoodley Morgan L. Gustison
Morgan L. Gustison