- Department of Psychology, Oakland University, Rochester, MI, United States
Introduction: Although snakes are commonly housed in captivity, little research has investigated the impacts of common stimuli in their environments. Familiar scents, for example, may be indicative of threat or the lack thereof and may be associated with stress. Snakes have a highly developed sense of smell and can discriminate prey, mates, and kin by scent. Humans are regularly present in captive environments, but only one study has tested whether a single snake species discriminated scents of familiar and unfamiliar humans.
Methods: We investigated whether 19 snakes of nine species housed privately or at nature centers showed differential behavioral responses to the scents of familiar human handlers, unfamiliar humans, and control stimuli.
Results: There were no significant effects of condition, housing, or sociality on movement, investigation, or tongue-flicking rates.
Discussion: We did not replicate a previous finding with corn snakes, likely due to procedural or housing differences, which will be important for future explorations of this understudied topic.
1 Introduction
Although cognitive research with snakes has increased in recent decades, relatively less work has been done with snakes even compared to other reptiles, such as testudines (i.e., turtles, tortoises). Existing work has often focused on how snakes respond to chemosensory cues, especially with regard to prey stimuli (e.g., Burghardt, 1993; Burghardt et al., 2023; Burghardt and Schwartz, 1999; Greenbaum, 2004; Krause et al., 2025; Pernetta et al., 2009). Snakes use their vomeronasal organ to detect scents and pheromones (Halpern and Kubie, 1983) and there is good evidence that they can discriminate prey scents from control scents based on chemosensory information alone, with less clear evidence that they discriminate between different types of prey (e.g., Baeckens et al., 2017; Krause et al., 2025 in Pituophis catenifer). Some species also appear to discriminate predators from non-predators. For example, pine snakes (Pituophis melanoleucus) avoid chemical trails and odors of predators (e.g., Burger, 1989, 1990) and prefer the scent of other pine snakes to other sympatric species (Burger, 1990).
Several species of snake appear to recognize kin (e.g., Clark, 2004; Clark et al., 2012; Himes, 2002). This has been demonstrated through chemoreception indicated by differential tongue-flicking in neonate smooth snakes (Coronella austriaca, Pernetta et al., 2009). Chemosensory information has even been presented to test whether snakes can recognize themselves. Rattlesnakes (Crotalus horridus and Crotalus viridis) explored liners that they had soiled less than those soiled by a conspecific (Chiszar et al., 1991); however, this finding may indicate familiar recognition generally but not self-recognition specifically given that only the conspecific cues were novel. Male common garter snakes (Thamnophis sirtalis) discriminated between their own scent and those of their littermates, even when those littermates were fed the same diet (Burghardt et al., 2021). Implementing additional control conditions, Freiburger et al. (2024) found that garter snakes, but not ball pythons, appeared to pass an odor version of the mark test – the litmus test of self-recognition. These authors argued that the greater sociability of the garter snake may be responsible for these differences, pointing to the need for further comparisons of species that differ in sociability.
There is also ample evidence to suggest that snakes might recognize individual conspecifics based on their preferences to spend time with certain individuals over others (e.g., Amarello, 2012; Clark, 2004; Clark et al., 2012; Skinner et al., 2024a, b; Skinner and Miller, 2020, 2022). Yet, whether this recognition of familiar individuals is robust in various species and could extend to heterospecifics, such as humans, has not been investigated. In the only known study, corn snakes (Pantherophis guttatus) distinguished between scents of familiar and unfamiliar humans – showing a novelty response or preference for the unfamiliar scent - only if they received enriched environments (Nagabaskaran et al., 2021).
Unenriched captive environments may significantly impact sensory acuity, including odor discrimination. Alternatively, snakes raised with enrichment may be characterized by less anxiety (Hoehfurtner et al., 2021) or by distinct behavioral profiles, including increased efficiency in feeding indicative of superior motor skills. Enriched snakes habituate more quickly to novelty in an open field task, which indicates lower emotional reactivity and greater adaptivity to the environment. They may also be more successful in solving maze tasks, suggesting greater cognitive flexibility (Almi and Burghardt, 2006). Thus, there is reason to suspect that husbandry conditions may be associated with differential responses to chemosensory stimuli.
Given differences in the extent to which various snake species rely on chemosensory reception to detect prey (Baeckens et al., 2017), and the fact that only a single study with one species has tested responses to the scents of familiar humans, it is important to replicate and explore species differences. Snakes are quite diverse, even with regard to the sensory modality they prioritize during foraging (Ford and Burghardt, 1993; Tadić, 2023). We tested six corn snakes as well as 13 snakes of eight other species to determine if they differentiated the scents of familiar humans from those of unfamiliar humans. Most of our subjects belong to species that brumate communally, but five subjects were of species that do not engage in this behavior (four ball pythons and one Brazilian rainbow boa), allowing us to assess this aspect of sociability as a factor in expression of recognition.
Because snakes primarily use their tongue to investigate chemical stimuli via their vomeronasal organ (Daghfous et al., 2012), we examined tongue-flicking, as well as investigation of the scents through approach and orientation behavior and general activity/movement. Interactions with humans are inescapable in the lives of animals in managed care, which may impose stress. Potential stress may be partially mitigated by recognition of familiar humans that have proven to be harmless. If captive snakes respond differentially to cues of familiar humans compared to cues from unfamiliar humans, this would indicate recognition of familiar humans, which might mitigate stress at handling. We tested snakes that would encounter familiar handlers as well as less familiar humans by testing privately owned snakes in human homes and ambassador animals in nature centers. A better understanding of snake chemoreception may inform husbandry practices and benefit snake welfare.
2 Methods
2.1 Ethical statement
The study was approved by the IACUC of Oakland University (Protocol # 2022-1178).
2.2 Subjects
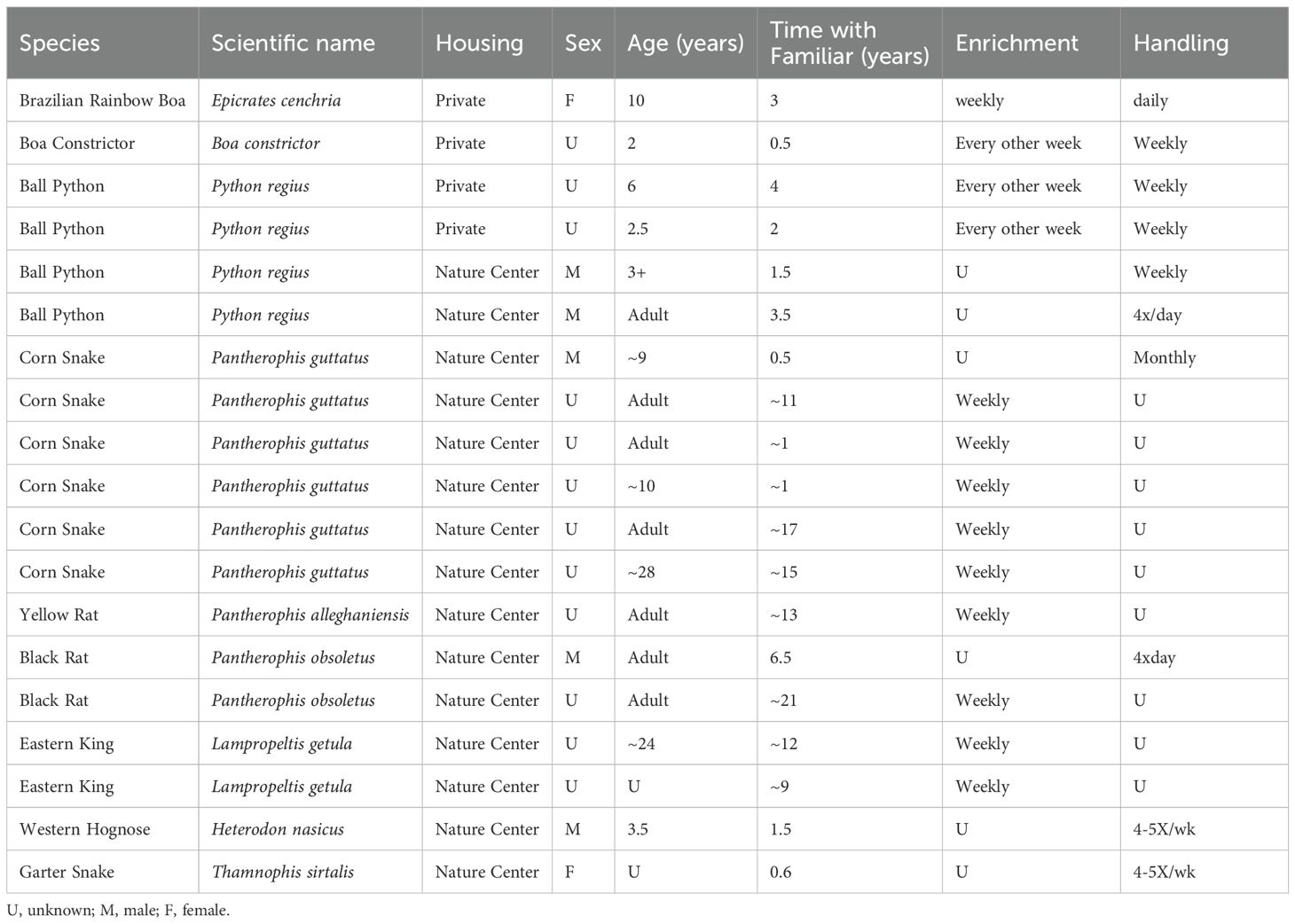
We tested 19 snakes (Table 1) that were housed at four different nature centers (n = 15) or privately owned (n = 4). All but two co-housed snakes (one corn snake and one yellow rat snake) were housed individually in terrariums furnished with foliage, hides, water dishes and other enrichment comparable to the enriched condition of Nagabaskaran et al. (2021). Unfortunately, we did not record the exact size of all terrariums but they were all approximately 32 gallons in size or larger.
2.3 Materials
Cotton rounds (Amazon Basics hypoallergenic 100% cotton) were used to swab the scent of a familiar handler and an unfamiliar person for each of the snakes. Two of the experimenters served as unfamiliar humans, gender matched for the familiar handler. Nitrile gloves were worn when collecting the swabbed cotton, which was then placed in Ziploc bags and sealed until testing. Cotton rounds were placed in plastic containers, which were placed in their home terrariums using tongs. All trials were filmed using a GoPro Hero 7. A stopwatch was used to time the trials.
2.4 Procedure
Each snake participated in three test trials on the same test day; one trial using a swab from a familiar handler, one trial using a swab from an unfamiliar human, and a control trial in which an un-swabbed cotton round was placed in their home terrarium. Trials were conducted in different random orders across test subjects. If there were multiple snakes at a location, each snake was tested once before any snake received a second or third trial, and the snakes were tested in the same order for each trial to maintain consistent inter-trial intervals (ITIs), which were at least 5 minutes except for the Rainbow boa whose ITIs were approximately 90 seconds. The neutral trail was inadvertently not filmed for one of the black rat snakes so no data for this trial are included in analyses.
Prior to testing, the handler and experimenters swabbed the cotton rounds against the inside of their arms to avoid hands or other areas that might have come in contact with soap or sanitizer and placed each round in a separate Ziploc bag, labeled appropriately. For each test trial, a researcher placed the assigned cotton round into a fresh plastic dish and the familiar handler placed the dish into the terrarium using tongs. A two-minute timer was begun as soon as the dish was placed on the floor of the terrarium. At the end of the trial, the handler removed the dish using tongs and the dish and cotton round were discarded. Each trial was video-taped for later coding by naïve research assistants.
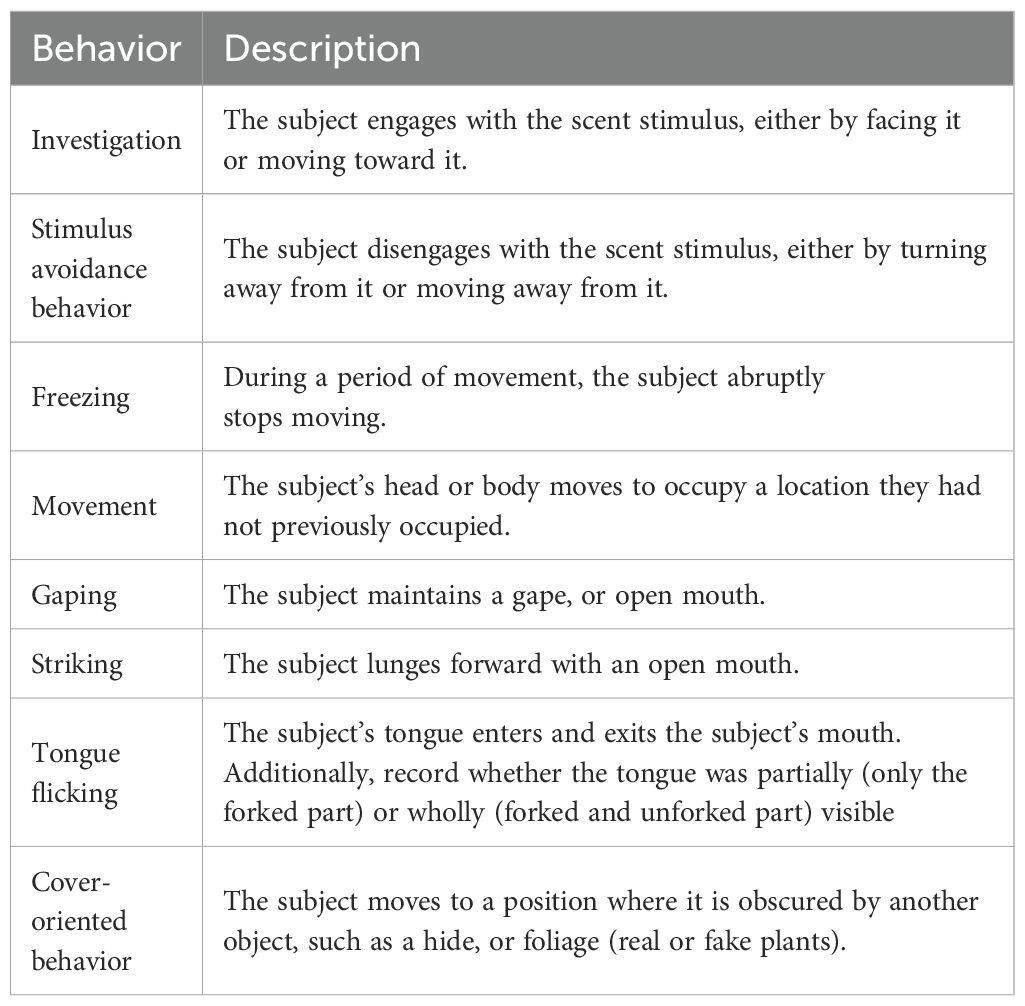
Coders used the behavioral coding software BORIS v.8.20. (Friard and Gamba, 2016) to code complete and partial tongue-flick counts, the duration of movement in seconds, and investigation of the scent during each trial in seconds. The behavioral ethogram provided to coders also included some other behaviors that were too infrequent to analyze, such as hiding, gaping, and striking (see Table 2).
2.5 Data analysis
Kolmogorov-Smirnov and Q-Q plots tests revealed that the data were not normally distributed; therefore, separate Friedman non-parametric tests were conducted to test for overall effects of condition (neutral, unfamiliar, familiar) for tongue-flicking (full and partial), movement duration, and investigation duration. To test the effects of the between subject factors of housing (home, nature center) and sociability (communal brumation or lack thereof) and their interactions with condition, the data were rank transformed and aligned rank transformation (ART) ANOVAs (Wobbrock et al., 2011) were conducted for each of the same four outcomes using SPSS version 29. Because sociability and housing overlapped significantly, we ran separate ANOVAs including only one of these two factors and its interactions with condition in each model. It should be noted that the data were zero inflated and there were multiple tied-ranks, which may impact the results.
3 Results
A secondary coder coded 21/56 (37.5%) of the trials. For movement duration, the Cronbach’s alpha was .96, for investigation duration, it was .95, for tongue-flicks full and partial, it was .91 and .79 respectively.
None of the Friedman tests were significant, all ps >.54. Furthermore, there were no significant main effects or interactions for any of the ART ANOVAs.
4 Discussion
We tested whether snakes of nine different species discriminated behaviorally when presented with familiar from unfamiliar human scents. Given that we tested only one or two snakes of each species except for the six corn snakes and four ball pythons, we could not determine species differences. Instead, we examined whether sociability (categorized on the basis of whether snakes brumated communally or not) or housing (nature center or private ownership) impacted responses to familiar and unfamiliar humans. We found no significant effects of these factors or condition and no interactions.
We caution against concluding that snakes do not recognize the scents of familiar humans because we presented snakes with only a single trial with each stimulus. We restricted presentations based on limited opportunities to test at the volunteer facilities and to prevent the unfamiliar scent from becoming familiar. Although we could have presented each snake with multiple unfamiliar scents, we would have had to repeat presentations of the familiar scent, creating a confound. The one other study to test responses to familiar human scents presented snakes with only two total trials in which both familiar and unfamiliar scents were presented simultaneously rather than including control trials (Nagabaskaran et al., 2021). However, these researchers also used longer trial durations, which may have led to better results. Future studies should use repeated trials, longer trial durations and longer ITIs (Burghardt, 1969).
Contrary to expectation, rates of tongue-flicking were relatively low. Baseline rates of tongue-flicking are typically higher in active predators compared to ambush predators (Baeckens et al., 2017) and two thirds of our subjects were active predators. However, even garter snake species may vary in activity levels, reflective of environmental factors such as predation risks. Species with faster life history strategies (i.e., shorter lifespans, earlier reproduction) tended to move and tongue-flick more often in an open field test (Gangloff et al., 2017). However, Freiburger et al. (2024) did not find significant differences in short tongue-flick frequency between garter snakes and ball pythons when investigating their own scents or those of conspecifics. Low rates of tongue flicking even in some of the active predators may be due to the lack of need to hunt for prey in captivity. It may also be an artifact of our procedure. Many prior studies of snake olfactory discrimination presented stimuli directly by the snout of the snakes (e.g., Burghardt, 1969; Cooper, 1998), and we did not do this here, as we wanted to explore spontaneous approach, avoidance and contact behavior. Because we did not present prey scents, we were not primarily interested in strikes, as in other related studies (e.g., Krause et al., 2025). Unfortunately, we did not collect information about when the snakes had last been fed, which may have also impacted their behavior during testing. We also lacked control over other aspects of their husbandry such as temperature.
Although we expected the ambush predators to be less responsive than the active predators, ambush predators do respond to pheromones (Duvall, 1979) and predator stimuli (Downes and Shine, 1998). Because humans could be considered predators for most species, all snakes might have been expected to display a stronger response to unfamiliar humans. We failed to replicate a previous finding that corn snakes provided with an enriched environment demonstrated a novelty response to an unfamiliar scent (Nagabaskaran et al., 2021). The difference in results cannot be attributed solely to species differences as six of our snakes were also corn snakes, or to a difference in enrichment, as all of our snakes received enrichment. Our companion and ambassador animals may have been exposed more often to unfamiliar humans compared to the laboratory snakes tested previously, which might reduce the likelihood of observing a novelty response.
Despite the strengths of comparing multiple species housed in different conditions, and a within-subject design that minimized extraneous factors, our study had limitations. Should tongue-flicking have been more prevalent and snakes easier to observe in their enriched enclosures, we could have further differentiated between oscillation number and duration as suggested by Gove and Burghardt (1983), as different forms of tongue-flicking may be context dependent and indicate different behavioral motivations. Even if the snakes discriminated the scents, they may have responded to familiarity in general rather than recognizing individuals. Freiburger et al.’s (2024) results suggested that garter snakes can distinguish between two familiar scents; themselves and a familiar conspecific, so future studies might present a test of discrimination of more than one familiar human. It is also possible that snakes use multimodal representations to recognize familiar individuals rather than relying on scent alone. Although our study suffers from the limitations of small sample sizes and lack of control over environmental factors, we hope to encourage others to attend to the impacts of human handling on species that are typically not considered social.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: OSF, https://osf.io/cxgb9/?view_only=285a2363b557451bb2343d9206c56c27.
Ethics statement
The animal studies were approved by Institutional Animal Care and Use Committee (IACUC) Oakland University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
JV: Formal Analysis, Writing – original draft, Methodology, Conceptualization, Project administration, Investigation. AJ: Writing – review & editing, Conceptualization, Investigation, Methodology, Data curation. JP: Investigation, Data curation, Methodology, Writing – review & editing, Conceptualization. KS: Writing – review & editing, Data curation. DL: Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are extremely grateful to Amanda Felk (Dinosaur Hill Nature Center), Jessica Fabian, Christopher Crame (Red Oaks Nature Center), Taylor Crews, Aware Wildlife Center and Chattachoochee Nature Center, who graciously provided access to their snakes for testing. We thank Gordon Burghardt for his willingness to lend his valuable expertise and feedback.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almi L. M. and Burghardt G. M. (2006). Environmental enrichment alters the behavioral profile of ratsnakes (Elaphe). J. Appl. Anim. Welfare Science. 9, 85–109. doi: 10.1207/s15327604jaws0902_1
Amarello M. (2012). Social Snakes? Non-random association patterns detected in a population of Arizona black rattlesnakes (Crotalus cerberus) (Tempe, Arizona, US: Unpublished Master’s thesis, Arizona State University).
Baeckens S., Van Damme R., and Cooper W. E. Jr. (2017). How phylogeny and foraging ecology drive the level of chemosensory exploration in lizards and snakes. J. Evolutionary Biol. 30, 627–640. doi: 10.1111/jeb.13032
Burger J. (1989). Following of conspecific and avoidance of predator chemical cues by pine snakes (Pituophis melanoleucus). J. Chem. Ecol. 15, 799–806. doi: 10.1007/BF01015178
Burger J. (1990). Response of hatchling pine snakes (Pituophis melanoleucus) to chemical cues of sympatric snakes. Copeia 1990, 1160–1163. doi: 10.2307/1446505
Burghardt G. M. (1969). Comparative prey-attack studies of newborn snakes of the genus Thamnophis. Behaviour 33, 77–113. doi: 10.1163/156853969X00332
Burghardt G. M. (1993). The comparative imperative: Genetics and ontogeny of chemoreceptive prey responses in natricine snakes. Brain Behav. Evol. 41, 138–146. doi: 10.1159/000113831
Burghardt G. M., Krause M. A., Placyk J. S., and Gillingham J. C. (2023). “Garter snakes of the Beaver archipelago: A story of plasticity and adaptation,” in Islands and snakes: Diversity and conservation. Eds. Lillywhite H. B. and Martins M. (Oxford, UK: Oxford University Press), 261–289.
Burghardt G. M., Partin A. M., Pepper H. E., Steele J. M., Liske S. M., Stokes A. E., et al. (2021). Chemically mediated self-recognition in sibling juvenile common garter snakes (Thamnophis sirtalis) reared on same or different diets: evidence for a chemical mirror? Behaviour 158, 1169–1191. doi: 10.1163/1568539X-bja10131
Burghardt G. M. and Schwartz J. M. (1999). “Geographic variations on methodological themes in comparative ethology: A natricine snake perspective,” in Geographic variation in behavior: Perspectives on evolutionary mechanisms. Eds. Foster S. A. and Endler J. A. (Oxford, UK: Oxford University Press), 69–94.
Chiszar D., Smith H. M., Bogert C. M., and Vidaurri J. (1991). A chemical sense of self in timber and prairie rattlesnakes. Bull. Psychonomic Soc. 29, 153–154. doi: 10.3758/BF03335221
Clark R. W. (2004). Kin recognition in rattlesnakes. Proc. R. Soc. London Ser. B: Biol. Sci. 271, S243–S245. doi: 10.1098/rsbl.2004.0162
Clark R. W., Brown W. S., Stechert R., and Greene H. W. (2012). Cryptic sociality in rattlesnakes (Crotalus horridus) detected by kinship analysis. Biol. Letters 8, 523–525. doi: 10.1098/rsbl.2011.1217
Cooper W. E. (1998). Evaluation of swab and related tests for responses by squamates to chemical stimuli. J. Chem. Ecol. 24, 841–866. doi: 10.1023/A:1022373517653
Daghfous G., Smargiassi M., Libourel P. A., Wattiez R., and Bels V. (2012). The function of oscillatory tongue-flicks in snakes: insights from kinematics of tongue-flicking in the banded water snake (Nerodia fasciata). Chem. Senses 37, 883–896. doi: 10.1093/chemse/bjs072
Downes S. and Shine R. (1998). Sedentary snakes and gullible geckos: Predator-prey coevolution in nocturnal rock-dwelling reptiles. Anim. Behav. 55, 1373–1385. doi: 10.1006/anbe.1997.0704
Duvall D. (1979). Western Fence lizard (Sceloporus occidentalis): Chemical signals, conspecific discriminations and release of a species-typical visual display. J. Exp. Zoology 210, 321–326. doi: 10.1002/jez.1402100215
Ford N. B. and Burghardt G. M. (1993). “Perceptual mechanisms and the behavioral ecology of snakes,” in Snakes: Ecology and behavior. Eds. Seigel R. A. and Collins J. T. (New York, NY, US: McGraw-Hill), 117–165.
Freiburger T., Miller N., and Skinner M. (2024). Olfactory self-recognition in two species of snake. Proc. R. Soc. B 291, 20240125. doi: 10.1098/rspb.2024.0125
Friard O. and Gamba M. (2016). BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. doi: 10.1111/2041-210X.12584
Gangloff E. J., Chow M., Leos-Barajas V., Hynes S., Hobbs B., and Sparkman A. M. (2017). Integrating behaviour into the pace-of-life continuum: Divergent levels of activity and information gathering in fast-and slow-living snakes. Behav. Processes 142, 156–163. doi: 10.1016/j.beproc.2017.06.006
Gove D. and Burghardt G. M. (1983). Context-correlated parameters of snake and lizard tongue-flicking. Anim. Behav. 31, 718–723. doi: 10.1016/S0003-3472(83)80227-9
Greenbaum E. (2004). The influence of prey-scent stimuli on predatory behavior of the North American copperhead Agkistrodon contortrix (Serpentes: Viperidae). Behav. Ecol. 15, 345–350. doi: 10.1093/beheco/arh011
Halpern M. and Kubie J. L. (1983). “Snake tongue flicking behavior: clues to vomeronasal system functions,” in Chemical Signals in Vertebrates 3. Eds. Müller-Schwarze D. and Silverstein R. M. (Springer, Boston, MA). doi: 10.1007/978-1-4757-9652-0_3
Himes J. (2002). The role of the midland water snake, Nerodia sipedon (Serpentes: Colubridae), as a predator: foraging behavior, kin recognition, and the response of prey. Amphibia-Reptilia 23, 333–342. doi: 10.1163/15685380260449216
Hoehfurtner T., Wilkinson A., Nagabaskaran G., and Burman O. H. (2021). Does the provision of environmental enrichment affect the behaviour and welfare of captive snakes? Appl. Anim. Behav. Sci. 239, 105324. doi: 10.1016/j.applanim.2021.105324
Krause M. A., Koharchik C., and Staples L. (2025). Responses to prey chemical cues in wild-caught, adult gopher snakes (Pituophis catenifer). J. Comp. Psychol. 139, 147–151. doi: 10.1037/com0000397
Nagabaskaran G., Burman O. H. P., Hoehfurtner T., and Wilkinson A. (2021). Environmental enrichment impacts discrimination between familiar and unfamiliar human odours in snakes (pantherophis guttata). Appl. Anim. Behav. Sci. 237, 105278. doi: 10.1016/j.applanim.2021.105278
Pernetta A. P., Reading C. J., and Allen J. A. (2009). Chemoreception and kin discrimination by neonate smooth snakes, Coronella Austriaca. Anim. Behav. 77, 363–368. doi: 10.1016/j.anbehav.2008.10.008
Skinner M., Hazell M., Jameson J., and Lougheed S. C. (2024a). Social networks reveal sex-and age-patterned social structure in Butler’s gartersnakes (Thamnophis butleri). Behav. Ecol. 35, 1–14. doi: 10.1093/beheco/arad095
Skinner M., Kumpan T., and Miller N. (2024b). Intense sociability in a “non-social” snake (Python regius). Behav. Ecol. Sociobiology 78, 113. doi: 10.1007/s00265-024-03535-7
Skinner M. and Miller N. (2020). Aggregation and social interaction in garter snakes (Thamnophis sirtalis sirtalis). Behav. Ecol. Sociobiology 74, 51. doi: 10.1007/s00265-020-2827-0
Skinner M. and Miller N. (2022). Stability and change in gartersnake social networks across ontogeny. Ethology 128, 257–267. doi: 10.1111/eth.13262
Tadić Z. (2023). Snakes: Slithering from sensory physiology to cognition. Comp. Cogn. Behav. Rev. 18, 95–120. doi: 10.3819/CCBR.2023.180006
Keywords: snakes, olfactory, human interactions, chemosensory perception, housing
Citation: Vonk J, Jordan A, Pappas J, Stellman K and Leibowitz D (2025) Scents for Serpentes: are familiar humans un-hiss-takable? Front. Ethol. 4:1634578. doi: 10.3389/fetho.2025.1634578
Received: 24 May 2025; Accepted: 29 July 2025;
Published: 15 August 2025.
Edited by:
Mystera M. Samuelson, University of Nebraska Medical Center, United StatesReviewed by:
Chelsea E. Martin, Loma Linda University, United StatesAndrew Heaton, Grand Bay National Estuarine Research Reserve, United States
Copyright © 2025 Vonk, Jordan, Pappas, Stellman and Leibowitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer Vonk, dm9ua0BvYWtsYW5kLmVkdQ==
 Jennifer Vonk
Jennifer Vonk Amity Jordan
Amity Jordan Jacob Pappas
Jacob Pappas