- 1Hamlyn Centre for Robotic Surgery, Imperial College London, London, United Kingdom
- 2Breast Unit, Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom
- 3Department of Histopathology, Imperial College Healthcare NHS Trust, London, United Kingdom
- 4Department of Surgery and Cancer, Imperial College, London, United Kingdom
Background: Confocal fluorescence microscopy (CFM) is a powerful optical biopsy technique which captures cellular resolution images of the tissue surface without the need for tissue fixation or sectioning. The evolution of CFM with miniaturization and fibre-based optics now allows rapid capture of wide field images with microscopic resolution. For in-situ diagnostics, there is growing evidence that CFM systems could rapidly and accurately identify breast cancer with clinically actionable results.
Review Focus: This comprehensive review discusses different technological advances in CFM systems and explores emerging trends in Artificial Intelligence (AI) and robotic integration in breast cancer imaging. The review further discusses the clinical implications of these technologies, including their potential to reduce re-excision rates following breast conserving surgery (BCS) and improve surgical workflow efficiency.
Methods: A comprehensive literature review using PubMed, Embase and Web of Science databases was conducted by three reviewers independently covering studies published from January 2013 to December 2024. We included studies that provided human tissue data (preclinical and clinical) relevant to breast cancer imaging, focusing on the technological features, intra-operative usability, and ease of use of different bench-top and fibre-based CFM systems. Research focusing on future trends and emerging challenges in standardizing imaging protocols for breast cancer CFM imaging and automating diagnostic workflows were also considered.
Results and conclusion: Of 1382 articles identified from database screening, 28 fulfilled the inclusion criteria. Only 10 clinical studies reported statistical differentiation among specimens. Bench-top CFM systems demonstrated high-resolution imaging with accuracy ranging 83%–99.6% making them effective for detailed tissue analysis. However, their size and operational complexity limit their use during live surgery. In contrast, fibre-based CFM systems offer miniaturized flexible micro-endoscopes that enable real-time, in-situ imaging with accuracy upto 94% demonstrating suitability for intra-operative diagnosis. Notably, fibre-bundle based Cellvizio® confocal laser endomicroscopy (CLE) system and line-scan CLE system can identify breast pathology but data is lacking on intra-operative diagnostic accuracy for margin assessment on wide local excision specimens. New developments like the commercial Histolog® Confocal Microscopy system (SamanTree Medical SA, Lausanne, Switzerland) has potential to identify missed tumour margins in up to 75% of cases, enhancing the accuracy of margin assessments.While these technologies are promising, several obstacles must be overcome before CFM can be widely adopted in routine surgical practice. Additionally, AI- powered automation in CFM, although promising, requires large-scale validation to ensure accurate real-time tissue classification. Integrating robotics and AI-enhanced CFM could greatly improve real-time surgical decision-making, minimizing interpretation errors and enhancing workflow efficiency.
1 Introduction
Breast cancer is second most common cancer diagnosed globally in 2020 with more than 55,000 new diagnoses each year in the United Kingdom (UK) alone Cancer Research UK (2023), Bray et al. (2024). The lifetime risk of breast cancer for women in the UK and United States of America (USA) is estimated at one in eight with a global estimate of over 2.3 million new breast cancer diagnoses resulting in nearly 685,000 deaths from the disease in 2020 Sung et al. (2021). Surgery remains the primary line of treatment, with breast conserving surgery (BCS) performed in 60%–70% of early-stage cases Macmillan et al. (2022). The adoption of oncoplastic BCS techniques has increased significantly in the past decade, emphasizing intra-operative breast margin assessment to preserve normal tissue without compromising oncological safety Fleming et al. (2020).
1.1 Current challenges in intra-operative margin assessment
A key determinant in local and distant recurrence after BCS is the histopathological status of the resection margins of wide local excisions (WLE) Moran et al. (2014), Chagpar et al. (2018). Despite advancements in localization techniques, positive or close margins persist in 15%–30% of WLE cases, often mandating a second operation to excise further tissue to clear margins Chagpar et al. (2018), Houssami et al. (2014). Surgical guidelines vary globally, with the Association of Breast Surgery (ABS) recommending a 1 mm margin for invasive cancer and a 2 mm margin for ductal carcinoma in-situ (DCIS), while American Society of Clinical Oncology (ASCO) accepts no tumour on ink as sufficient in preventing local recurrence Moran et al. (2014), American Society of Clinical Oncology (ASCO) (2020). Positive margins more than double the risk of ipsilateral breast tumour recurrence, leading to revision surgery, causing anxiety, inferior cosmesis and healthcare costs Lundgren et al. (2019). Due to the burden placed on patients and hospitals, it is of great importance to reduce the requirement for further surgery through obtaining negative margins intra-operatively.
The gold standard for determining margin status is post-operative histopathology. Tissue sections (5–20
1.2 Optical imaging for breast cancer margin assessment
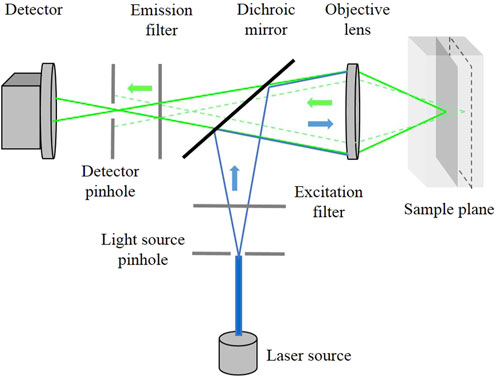
Optical fluorescence microscopy is one such promising intra-operative tool that enables real-time, cellular-level imaging, allowing surgeons to differentiate normal and malignant breast tissues. Fluorescence microscopic imaging of fresh breast specimens has been predominantly demonstrated using multiphoton microscopy (MPM) Wu et al. (2015), Yoshitake et al. (2016), Chen et al. (2020). Although the diagnostic accuracy of MPM is comparable to a histological assessment, the high cost of femtosecond lasers used for these systems creates a significant challenge to their clinical utility. Single photon fluorescence microscopy systems, such as CFM are less expensive allowing real-time, high-resolution visualization of tissues at cellular level. The working principle is shown in Figure 1.
By using laser light as a point-source and pin-hole aperture, the detector collects light only from the illuminated focused spot and rejects all the other out-of-focus light from the sample. This enables the CFM to image thin slices through a sample resulting in sharp focused cellular images without physically sectioning it. To create a 2D image, the laser spot is typically scanned point-by-point in raster-scan or spiral-scan patterns using a scan mirror. This makes CFM a promising tool for improving accuracy during surgery and reducing the need for additional procedures.
There have been significant developments in the use of CFM technique for the assessment of breast cancer tissues and resection margins in BCS Sandor et al. (2022), Gareau et al. (2012), Ragazzi et al. (2014), Krishnamurthy et al. (2019). Early studies demonstrated the feasibility of bench-top confocal strip mosaicking microscopes for ex-vivo breast tissue imaging, enabling large-area evaluation with microscopic resolution Larson et al. (2013), Abeytunge et al. (2013a). Parallelly, the introduction of fibre-based confocal laser endomicroscopy (CLE) has further expanded real-time intra-operative applications, with ex-vivo and in-vivo studies demonstrating its potential for rapid breast tissue diagnosis De Palma et al. (2015), Chang et al. (2015), Vyas et al. (2017). Both bench-top and fibre-based approaches offer diagnostic, cellular-level imaging in fresh tissues, supporting real-time intra-operative decision-making.
1.3 Recent advances and emerging frontiers
As the field of intra-operative imaging progresses, several new technologies have emerged that aim to address the limitations of conventional CFM approaches. Recently, Histolog® Confocal Microscopy and ex-vivo Fusion Confocal Microscopy (EVFCM) have demonstrated high levels of accuracy for IMA, offering an alternative to traditional histopathology Sandor et al. (2022), Togawa et al. (2023), Mathieu et al. (2024), Humaran et al. (2024). The Histolog® Scanner is a rapid confocal laser scanner for real-time imaging of excised tissue. Using a 488 nm laser and Histolog® Dip stain, it provides 2
Beyond hardware advancements, the integration of artificial intelligence (AI) in confocal imaging is transforming the way intra-operative diagnostics are performed. AI-powered machine learning algorithms are being developed to enhance real-time tissue classification, reducing human interpretation errors and variability Gu et al. (2017), Gu et al. (2018), Gu et al. (2020). AI-driven fluorescence analysis is also helping to standardize imaging protocols and improve automated tumour detection in surgical workflows Sung et al. (2021).
Additionally, robot-assisted confocal imaging is emerging as a way to enhance intra-operative precision. The integration of fibre-based CFM with surgical robotic platforms has demonstrated the potential to perform large area tissue imaging, improve motion stabilization, and optimize tissue penetration depth Zuo et al. (2016), Giataganas et al. (2018), Abdelaziz et al. (2024). The CRUK-EPSRC “MAMMOBOT” project has recently been presented by developing a millimeter scale steering soft robot integrated with flexible endomicroscope for real-time virtual histology of the ductal system and breast micro-architecture Berthet-Rayne et al. (2021). Further, a high-speed line-scan confocal laser endomicrosocpe (LS-CLE) system has been developed that can pass through the working channel of the MAMMOBOT platform well as soft polymer-based fiberbots and get real-time images of tissue micro-architecture at 120 fps, 10 times faster than conventional CLE systems Vyas et al. (2022), Abdelaziz et al. (2024). Feasibility studies have demonstrated effectiveness of LS-CLE to provide cellular resolution images of breast ducts, progressing from normal ducts to DCIS and invasive ductal carcinoma (IDC) in real-time by comparison to histopathology. AI-guided robotic-assisted CFM could play a pivotal role extending the use of fibre-based CFM systems in IMA and improving surgical outcomes.
1.4 Aims of this review
This review provides a comprehensive and critical update on novel CFM imaging techniques with potential for application in breast cancer surgery and with an emphasis on.
1. Comparing bench-top and fibre-based CFM systems for intra-operative diagnosis of breast cancer.
2. Assessing the effectiveness of CFM techniques in the detection of breast margin status against histopathology as the reference standard. Assessing imaging performance, diagnostic accuracy and clinical utility.
3. Identifying new frontiers such as integration with robotics and computer-aided diagnosis and discuss their role in improving the tumour margin assessment and clinical relevance.
2 Materials and methods
2.1 Study scope and definition of reference standard
The primary objective of this review is to evaluate the effectiveness of CFM techniques for intra-operative breast cancer margin assessment. Since this study did not involve direct patient data, ethics committee approval was not required. The reference standard for comparison was the routine hematoxylin and eosin (H
2.2 Literature search strategy
A comprehensive literature search was conducted following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The databases searched were PubMed, Embase, and Web of Science databases by three independent reviewers assessing articles published from January 2013 to December 2024. The study was conducted in August 2023 with a final revision on December 2024. The following search terms were selected in the title and/or abstract.
1. Confocal microscopy OR con-focal microscopy OR confocal adj3 mosaicking OR fluorescen* adj3 microscop* OR confocal adj3 microscop* OR con focal adj3 microscop* AND
2. Breast/OR breast. mp AND
3. breast* adj3 margin* OR breast adj5 tissue* OR biops* OR needle adj3 biops* OR fresh adj3 tissue* OR frozen adj3 tissue*
The literature search was restricted to English-language publications, covering all studies from January 2013 to December 2024. COVIDENCE software (Veritas Health Innovation, Melbourne, Australia) was used for literature management, and all retrieved records were screened by two independent reviewers (AE and NH), with a third expert adjudicator (KV) resolving any discrepancies.
Following screening of papers, all articles underwent full-text review by AE, NH and KV. The review included both technical and clinical studies. The focus was to identify studies evaluating fluorescence-based imaging in in-vivo and rapid ex-vivo imaging during breast cancer surgery. The following parameters were used to guide inclusion/exclusion of articles:
Inclusion criteria:
Exclusion criteria:
Our focus on human breast tissue imaging reflects the transition of CFM from technical validation to clinical translation. We prioritized studies that offer insights into real-world tissue acquisition, staining, and interpretation protocols, which are critical for clinical deployment in BCS. Animal and phantom models, while relevant for early-stage system development, were excluded to maintain clinical applicability.
2.3 Data extraction
All eligible studies included in this review were analyzed using a structured extraction table, developed by AE, NH, KV, and senior author DRL. The extraction framework was created in Microsoft Excel and all discrepancies were discussed until 100% agreement was achieved. The following parameters were extracted.
All selected studies involved human breast specimens, either preclinical or clinical in nature. No animal or phantom studies were included.
2.4 Statistical analysis
Due to the heterogeneity in study populations, imaging methodologies, and optical system specifications, a meta-analysis was not feasible. Instead, a qualitative synthesis of imaging performance was performed. Descriptive statistics were used to summarize diagnostic accuracy measures (sensitivity, specificity, accuracy) from individual studies.
3 Results
A total of 1382 references were imported to COVIDENCE following preliminary database search. 445 duplicate studies were removed, and 936 articles were screened for title and abstract assessment. 68 were selected for full-text review, with 28 studies meeting the final inclusion criteria. A Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) flow diagram summarizing the study selection process is illustrated in Figure 2.
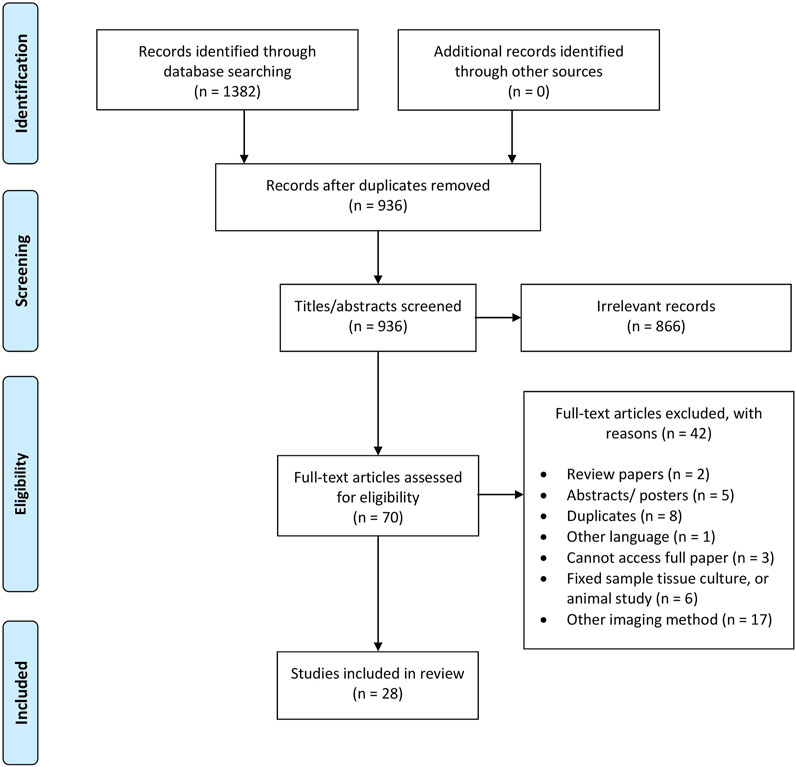
Figure 2. The flow diagram of identifying eligible studies and the different PRISMA-guideline selection phases, resulting in the total 28 included in this article.
The articles included in this review consisted of experimental studies (clinical and technical) assessing the utility and diagnostic accuracy of novel CFM modalities in breast cancer assessment. Available studies were categorised into bench-top CFM systems (Table 1, n = 21) and hand-held fibre-based systems (Table 2, n = 7). To aid visual interpretation of the technical and workflow differences between these imaging modalities, Figure 3 presents representative examples of the gold-standard H&E histopathology, alongside bench-top and fiber-based confocal fluorescence microscopy systems.
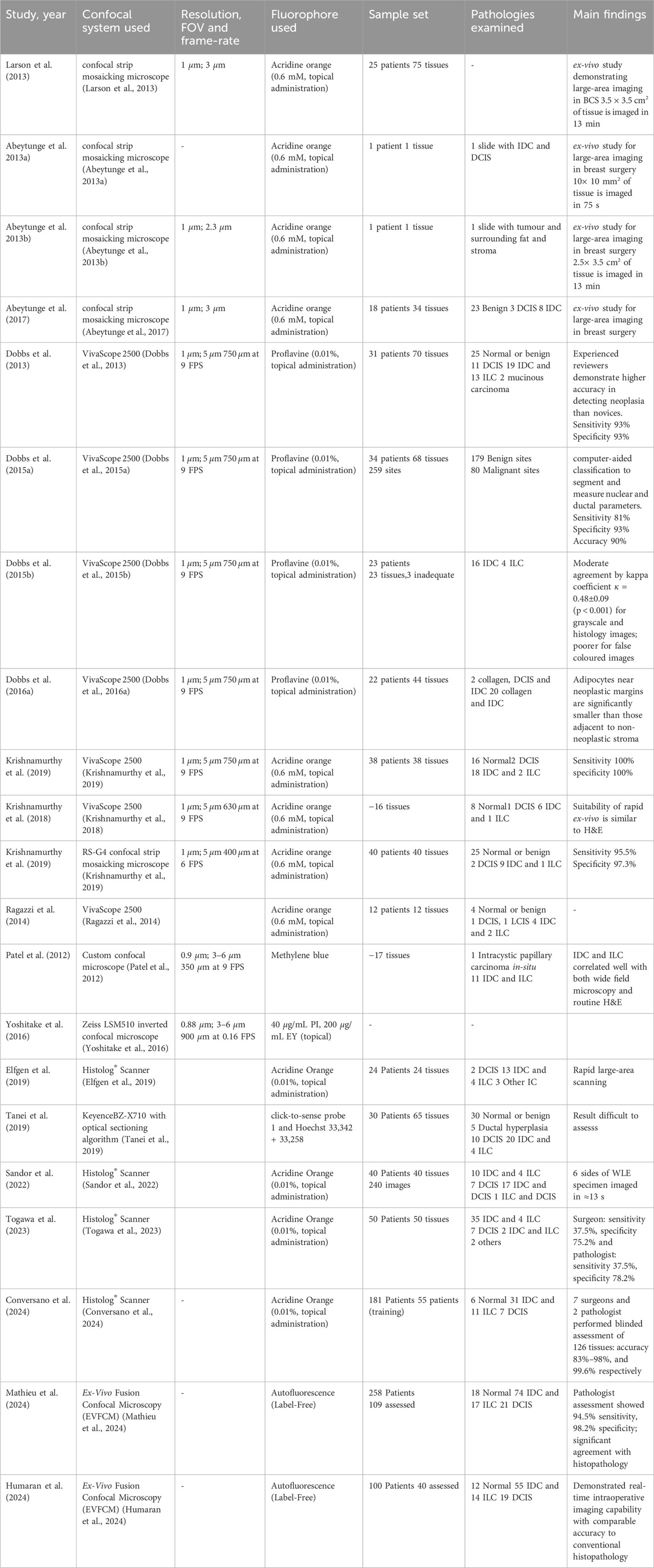
Table 1. Bench-top CFM (21 works). Legend to the table: FOV- Field of view, FPS- frames per second, DCIS- Ductal carcinoma in-situ, IDC- Invasive ductal carcinoma, ILC- Invasive lobular carcinoma, IC- Invasive carcinoma.
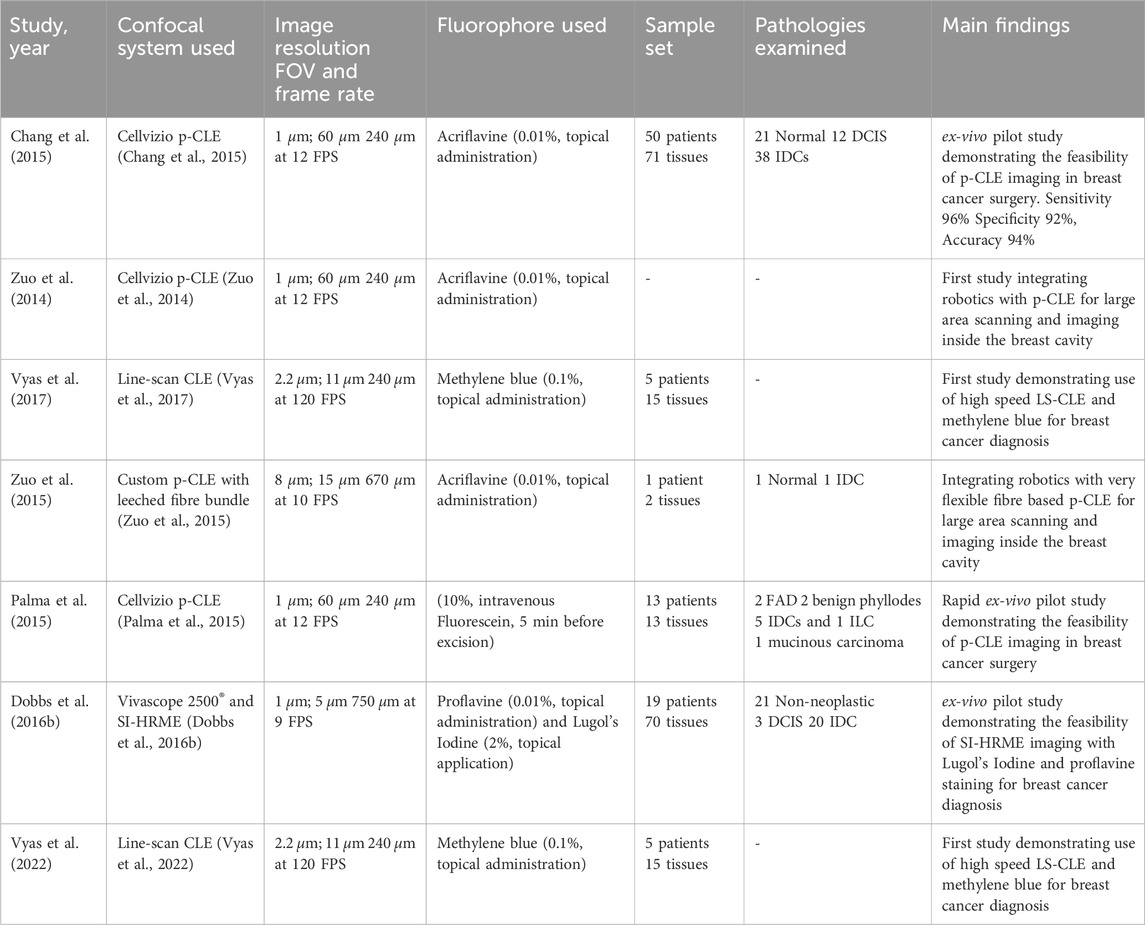
Table 2. Fibre based CLE (7 works). Legend to the table: p-CLE- Probe-based confocal laser endomicroscopy, LS-CLE- Line-scan confocal laser endomicroscopy, SI-HRME- Structured illumination based high resolution microendoscopy, FOV- Field of view, FPS- frames per second, DCIS- Ductal carcinoma in-situ, IDC- Invasive ductal carcinoma, ILC- Invasive lobular carcinoma.
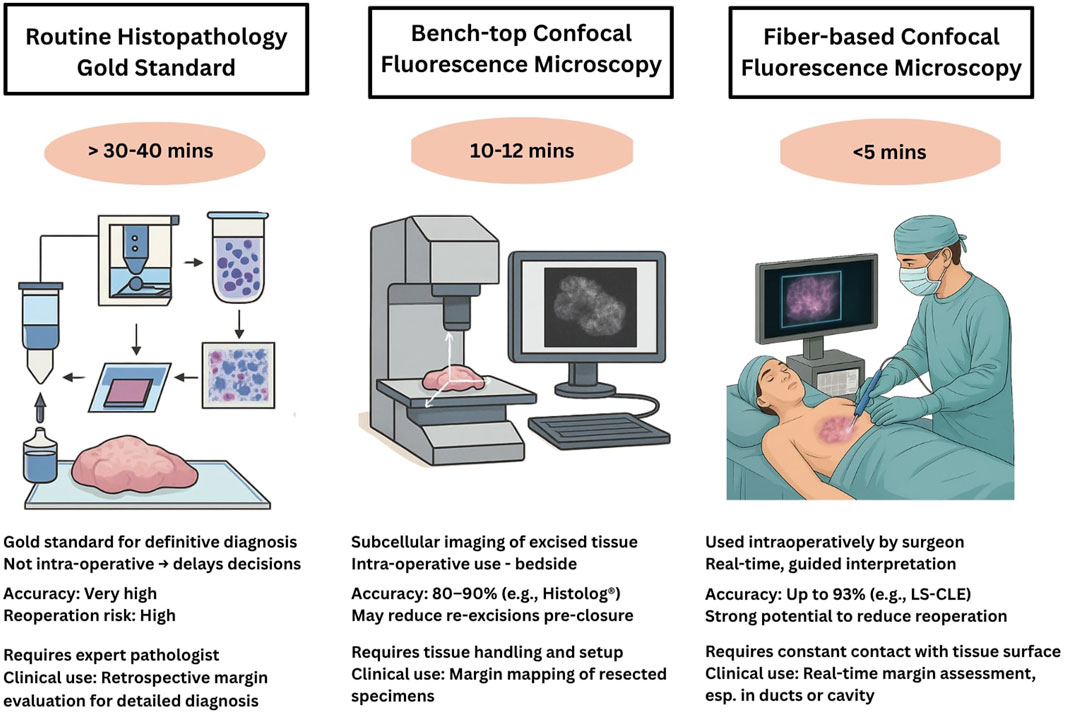
Figure 3. Comparative overview of tissue evaluation methodologies used during BCS. The left panel illustrates the gold standard of Hematoxylin and Eosin (H&E) stained histopathology, involving fixation, sectioning, and staining of resected tissue, typically requiring 30–40 min or longer. The center panel shows a bench-top CFM system, where excised tissue is placed on a motorized XYZ scanning stage for rapid ex-vivo 3D fluorescence imaging and digital histology review. The right panel depicts a fiber-based handheld CFM system, where the microscopic imaging probe is applied directly to the exposed surgical site intra-operatively, enabling real-time, sub-cellular resolution imaging. Together, these sub-figures compare sample processing, imaging workflows, and potential clinical application timelines across the three modalities.
3.1 Bench-top CFM systems for breast tissue imaging
The majority of the literature on benchtop CFM for breast tissue imaging has used the commercial CFM systems, the Vivascope® 2000 and Vivascope® 2500 (Caliber Imaging and Diagnostics, Rochester, NY, USA). These systems are designed for ex-vivo tissue and both provide microscopic resolution (
In 2013, confocal strip mosaicking microscope (CSMM) was developed as a modified version of Vivascope® 2000 to enable rapid scanning of large tissue areas while still maintaining microscopic resolution. With CSMM, a 10
The commercial Vivascope®2500 has shown promise in breast cancer imaging, particularly for core needle biopsies and small surgical excisions Dobbs et al. (2013), Dobbs J. et al. (2015), Krishnamurthy et al. (2018). Using 0.01% topical proflavine, an inexpensive topically applied dye, the researchers evaluated the ability of CFM to distinguish normal and neoplastic breast pathologies from gold standard histopathology. In this work, single 750
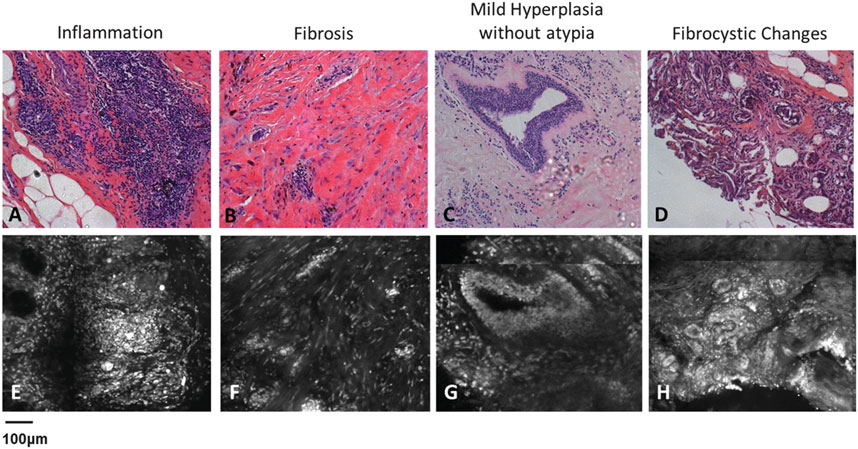
Figure 4. Demonstrates characteristic features of benign, non-neoplastic breast tissue. (A–D) represents H and E-stained images of inflammation, fibrosis, mild hyperplasia without atypia and fibrocystic changes while (E–H) represents features using CFM. Figure reproduced from Dobbs et al. (2013)).
To expand CFM’s clinical utility, researchers have explored techniques in computer-aided diagnosis (CAD) to automate tissue segmentation and improve diagnostic accuracy. One approach focused on automatically segmenting adipocytes to evaluate changes in adipocytes in the tumour microenvironment associated with IDC and DCIS in 22 cases Dobbs JL. et al. (2016). Dobbs et al. analyzed 179 benign and 80 malignant tissue sites from 34 patients. The study reported a sensitivity of 81%, specificity of 93%, and an overall diagnostic accuracy of 90% in distinguishing malignant from benign tissue using CAD-assisted CFM imaging. CAD analysis revealed that adipocytes near tumour margins were significantly smaller than those adjacent to non-neoplastic collagenous stroma, suggesting that changes in adipocyte morphology could serve as an indicator of tumour progression. These findings highlight the potential of CFM in assessing changes in the tumour micro-environment and understanding progression in malignancy with high accuracy. Additionally, a feasibility study involving 12 breast surgical specimens from 12 patients explored the use of Vivascope® 2500 with acridine orange, a rapid nuclear-staining agent, for intra-operative tissue evaluation Ragazzi et al. (2014). The study demonstrated that CFM could differentiate various breast tissue structures with sufficient contrast, reinforcing its potential as an intra-operative imaging tool. However, while these findings are promising, the study did not assess relevant clinical outcomes such as the accuracy of margin assessment, impact on re-excision rates, or long-term diagnostic reliability. Indeed whilst the studies highlighted above demonstrate the potential of CFM for breast cancer diagnostics, they have primarily been conducted on small tissue sections and biopsy samples, and further research is needed to assess its effectiveness in evaluating whole-margin status in larger WLE specimens.
The Histolog® Scanner (SamanTree Medical SA, Lausanne, Switzerland), is being explored as a tool for ex-vivo whole margin assessment in BCS. Sandor et al. conducted one of the earliest evaluations of Histolog® for WLE margin assessment Sandor et al. (2022). The authors analyzed 40 WLE specimens, comparing Histolog® imaging to intra-operative ultrasound and specimen radiography, rather than histopathology. Retrospective image review suggested that 30%–75% of patients requiring re-excision (n = 12) could have been identified earlier with confocal imaging. However, the wide range was attributed to tissue variability, differences in interpretation, and imaging method limitations. While the study demonstrated Histolog®‘s potential for intra-operative use, it also highlighted the need for direct histological validation and real-time clinical trials. While the study demonstrated Histolog®‘s potential for intraoperative use, it underscored the need for direct histological validation and standardized interpretation protocols.
Togawa et al. further investigated Histolog®‘s accuracy for breast WLE margin assessment using 50 tissue specimens Togawa et al. (2023). Surgeons had a sensitivity of 37.5% and specificity of 75.2%, whilst unsurprisingly pathologists achieved higher specificity of 78.2%. These results suggest that while Histolog® may support IMA, variability in interpretation indicates a need for additional standardization and training before it can be reliably implemented in routine surgical workflows. Conversano et al. as part of the HIBISCUSS project, evaluated 181 breast tissue samples, using 55 for training and the remainder for blinded tissue assessment Conversano et al. (2024). They found that pathologists demonstrated near-perfect diagnostic accuracy (99.6%), while surgeon interpretation ranged between 83% and 98% accuracy. These findings reinforce Histolog®‘s potential for high-resolution, ex-vivo imaging, but also emphasize that specialized training is necessary to optimize interpretation accuracy. Beyond WLE margins, recently Mathieu et al. and Mazzucchelli et al. explored Histolog®‘s applications in core needle biopsy assessment and patient-derived breast cancer organoid research, showing over 93% concordance with histopathology Mathieu et al. (2024), Mazzucchelli et al. (2024). Their findings suggest that Histolog® could be valuable in rapid breast tissue evaluation and personalized oncology research.
Collectively, these studies highlight the potential for wide-field confocal systems in intra-operative breast cancer diagnostics and precision BCS. However, despite the diagnostic accuracy shown in many studies conducted in academic research centres, up-front investment and the need for expert pathologists remain barriers to widespread adoption of existing bench-top CFM systems for breast cancer imaging. In addition, its low acquisition rate and small FOV can be prone to sampling errors, limiting its application for IMA of large specimens like whole breast margins in a timely manner.
3.2 Fibre-based CFM systems for breast tissue imaging
Fibre-based confocal laser endomicroscopy (CLE) is a popular optical biopsy technique that translates conventional CFM into a real-time in-vivo clinical modality De Palma et al. (2015), Chang et al. (2015). Flexible optical fibre bundles are used as small diameter imaging probes to acquire images at confined sites within the body by scanning the probe tip on the tissue surface. These probes can be inserted into the working channels of conventional endoscopes enabling rapid and non-invasive detection and classification of a tissue’s histopathological status Vyas et al. (2022).
Point-scanning probe-based confocal laser endomicroscopy (p-CLE) system and a range of imaging probes with different diameters and specifications are commercially available by Cellvizio® (Mauna Kea Technologies, Paris, France). For such systems, lateral and axial resolutions down to 0.5 and 3
Another pilot study reported the application of optical fibre bundle based structured illumination endomicroscopy (SI-HRME) using topical application of 0.01% proflavine and 2% Lugol’s iodine as contrast agents to detect breast cancer Dobbs J. et al. (2016). Fresh breast tissue specimens from 19 patients were stained with proflavine alone or Lugol’s Iodine and proflavine. Images of tissue specimens were acquired using a confocal microscope and an HRME system with and without structured illumination. Dobbs et al. reported that structured illumination along with proflavine staining could potentially be used to increase contrast in HRME images of breast tissue for rapid image acquisition. Further, the addition of Lugol’s Iodine did not increase mean contrast significantly for HRME or SI-HRME images. This study was also investigational as proflavine is currently not cleared to be routinely used for in-vivo human use.
For in-vivo imaging in routine clinical practice, it is desirable to use endomicroscopes with approved fluorescent dyes which are both safe for human use and provide sufficient contrast to distinguish between different tissue morphologies. Methylene Blue is one of the few FDA approved dyes for in-vivo human application and has been demonstrated previously for the treatment of methemoglobinemia and is an alternative to isosulphan blue for in-vivo localisation of non-palpable lesions and sentinel lymph node mapping during breast surgeries Zhang et al. (2019). In a 2017 study, a custom high-speed line-scan confocal laser endomicroscopy (LS-CLE) system operating at 660 nm was used in combination with topical 0.1% methylene blue to successfully demonstrate the first rapid morphological assessment of freshly excised breast cancer tissues Vyas et al. (2017). Images and mosaics of normal, benign and neoplastic breast tissue, acquired with a lateral resolution of 2.2
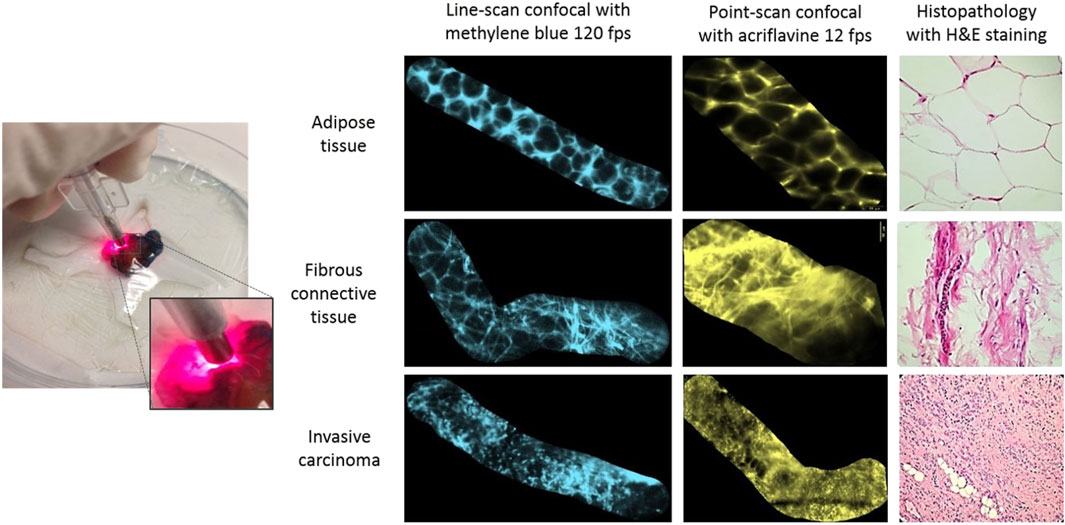
Figure 5. Comparison of acriflavine and methylene-blue aided rapid breast endomicroscopy imaging on normal, benign and neoplastic breast tissue specimens with routine H
While all the previous studies have been performed using non-specific dyes for imaging morphology of different breast pathologies, a recent study by Gao et al. demonstrated the molecular imaging capabilities of confocal endomicrosocpy Gao et al. (2017). A hand-held near-infrared dual axis confocal endomicroscope was developed to detect ErbB2 positive cells in breast tissue by using a specific targeting peptide labelled with IRDye800CW malemide (LiCor Biosciences) fluorophore. Images were acquired at five fps and in-vivo molecular imaging capabilities were demonstrated by assessing the uptake of specific peptide binding to human xenograft breast tumours expressing ErbB2.
3.3 New frontiers for p-CLE imaging
3.3.1 Computer-assisted image interpretation
Traditionally, histology images of biopsy samples are assessed by trained pathologists for disease diagnosis, classification, and treatment planning. While CFM images closely resemble histology, their interpretation remains a challenge for clinicians and surgeons with limited histopathology training. The high variability in p-CLE images and the presence of atypical conditions further complicate accurate manual diagnosis. As a result, CAD systems have been developed to improve diagnostic accuracy and reduce dependency on expert pathologists, enhancing the clinical utility of p-CLE imaging.
A component-based segmentation approach that utilizes internuclear distance, nuclear shape, ductal lumen diameter, and fluorescence intensity has demonstrated the potential of CAD for CFM imaging Dobbs JL. et al. (2015). Using Vivascope® 2500 with 0.01% proflavine staining, researchers evaluated freshly acquired breast tissues from 34 patients, including 22 malignant cases of IDC and DCIS. A decision tree model, validated against expert pathologist-confirmed histopathology, demonstrated high diagnostic accuracy in classifying IDC (92%) and DCIS (96%) Dobbs J. et al. (2015). The overall model sensitivity, specificity, and accuracy were 75%, 93%, and 88%, respectively.
The mosaicking feature adapted by most CLE systems enable to create a larger FOV by stitching adjacent image frames together. An Unsupervised Multimodal Graph Mining (UMGM) approach is reported to learn the discriminative features for p-CLE mosaics of breast tissue on a database of 700 p-CLE mosaics Gu et al. (2017). The mosaicking capability of p-CLE systems enables the reconstruction of large fields of view by stitching adjacent image frames together, improving the spatial coverage of imaging.
Since p-CLE is a relatively new imaging technique, the size of clinical dataset is also limited, making CAD analysis even more challenging. To address this, a Transfer Recurrent Feature Learning (TRFL) framework has been introduced for p-CLE video classification Gu et al. (2018). This two-stage method first learns discriminative features from individual p-CLE frames using generative adversarial networks (GANs) trained on both p-CLE and histology data. In the second stage, recurrent neural networks (RNNs) process frame-based features, handling variations in mosaic length and shape to achieve a classification accuracy of 84.1%.
3.3.2 Integration with robotics for large-area p-CLE imaging
One of the primary limitations of p-CLE is the restricted FOV, which is constrained by the fibre bundle probe size, typically ranging from 0.25 to 0.8 mm. This limitation poses significant challenges for surgeons in maintaining consistent probe-tissue contact, particularly when imaging large surgical areas such as breast WLE cavities. To address this, there has been increasing interest in robotic-assisted scanning systems to enhance p-CLE imaging capabilities, improve tissue coverage, and standardize image acquisition.
The first study to integrate robotics with p-CLE was conducted by Zuo et al., who demonstrated miniaturized and flexible robotic scanning systems designed for whole-breast cavity imaging Zuo et al. (2016). More recently, Giataganas et al. developed a portable robotic scanning probe capable of achieving micrometer-scale accuracy and generating mosaicked imaging fields of up to 14 mm2. Their system allows for stable, high-precision tissue scanning, reducing manual variability and improving intra-operative imaging reliability Giataganas et al. (2018).
Expanding on these advances, Abdelaziz et al. recently introduced a polymer-based flexible robotic system demonstrating high-precision large area CLE imaging applications on ex-vivo breast tissue Abdelaziz et al. (2024). p-CLE fibre was passed through the working channel of this flexible robot, enabling automated scanning across large tissue surfaces. This approach is designed to improve p-CLE probe positioning, reduce motion artifacts, and optimize tissue-contact consistency, thereby enhancing imaging accuracy and diagnostic confidence in intra-operative breast cancer assessment. In another study, Berthet et al. demonstrated the design and operation of Mammobot: A mm-scale steerable soft growing robot for precise fibre based ductoscopy for early breast cancer detection Berthet-Rayne et al. (2021). MAMMOBOT aims to access the breast through the nipple and navigate the mammary ducts to detect precursors of invasive breast cancers. Addressing limitations of the state-of-the-art, MAMMOBOT maintains a hollow inner lumen throughout its soft body, enabling the passing of instruments such as miniature endoscopes, biopsy needles, and optical probes for in-situ histopathology. Vyas et al. demonstrated the feasibility of LS-CLE as a flexible micro-ductoscope that could pass through the hollow lumen of MAMMOBOT and aid in identifying discernible features corresponding to normal ducts, DCIS and distinguishing them from IDC at sub-cellular scale. Such a miniaturized tool could be very useful as the next-generation mammary ductoscope for full duct outline mapping by detecting cancer or pre-cancerous lesions Vyas et al. (2022). Such robotic tools could be used hand-held or with an articulated or passive arm for large-area scanning applications. This could allow p-CLE systems to cover large areas of breast specimens or even for scanning breast resection cavities in-situ in a timely manner, increasing their clinical utility.
4 Discussion
4.1 Overview of findings and clinical implications
This review evaluated the role of confocal fluorescence microscopy (CFM) in breast cancer imaging, focusing on its technical features, clinical applications, and potential for IMA. Our analysis demonstrated that both bench-top and fibre-based CFM systems provide high diagnostic accuracy in distinguishing malignant from normal breast tissue, with reported sensitivities ranging from 81% to 96% and specificities from 85% to 99.6%.
Although all the reviewed studies focused on ex-vivo breast tissue imaging, the results highlight CFM’s potential for intra-operative integration in BCS. Accurate IMA remains a major challenge in breast cancer surgery, with re-operation rates ranging from 20% to 30% due to postoperative identification of positive margins. By enabling real-time, high-resolution imaging, CFM could improve intra-operative decision-making by enhancing intra-operative margin evaluation. However, further trials are required to demonstrate that CFM can reduce re-excision rates, improving oncological and cosmetic outcomes for patients undergoing BCS.
4.2 Diagnostic utility of CFM in breast cancer imaging
Our review found that CFM reliably provides sub-cellular resolution, enabling identification of nuclear and stromal features characteristic of malignant and benign breast tissue. Studies utilizing bench-top CFM systems (e.g., Histolog® Scanner, Vivascope® 2500) have demonstrated high diagnostic concordance with histopathology, with pathologists achieving accuracy rates as high as 99.6% in differentiating malignant from normal breast tissues. However, surgeon interpretation of confocal images was less consistent, with accuracy ranging from 83% to 98% Conversano et al. (2024).
Similarly, fibre-based p-CLE systems (e.g., Cellvizio®, LS-CLE) have been investigated for intra-operative applications, offering potential for real-time, in-vivo tissue imaging. Chang et al. demonstrated that topical acriflavine staining allowed p-CLE to distinguish neoplastic from normal breast tissue within 3 minutes, making it a potentially faster alternative to frozen section analysis. Palma et al. similarly showed that p-CLE with fluorescein was capable of accurately identifying IDC in breast quadrantectomy patients, reinforcing the potential of fibre-based CFM systems for rapid IMA during breast surgery.
Despite these promising results, CFM-based margin evaluation has been limited to retrospective and ex-vivo studies on small tissue cut-outs, with no current evidence supporting its real-time use in assessing breast cavity margins intra-operatively. This gap in research underscores the need for clinical trials evaluating CFM’s ability to guide surgical decision-making in real-time.
4.3 Margin assessment and implications for Re-operation reduction
One of the most critical applications of CFM in breast cancer surgery is its potential for IMA, allowing surgeons to determine tumour involvement at resection margins in near real-time. Several studies investigated CFM imaging for margin evaluation, particularly using the Histolog® Scanner for ex-vivo assessment of WLE specimens. Sandor et al. analyzed 40 WLE specimens, demonstrating that CFM-based retrospective review could have identified tumour-positive margins in up to 75% of cases Sandor et al. (2022). However, substantial variation in diagnostic performance was observed between surgeons (30% accuracy) and pathologists (58% accuracy), underscoring the need for improved standardization of image interpretation and AI-assisted classification tools. Further supporting these findings, the HIBISCUSS project demonstrated pathologist-led CFM image interpretation achieved near-perfect accuracy (99.6%), while surgeon interpretation ranged from 83% to 98% Conversano et al. (2024). While these results highlight the diagnostic capabilities of CFM, they also indicate that its optimal role may be as an adjunct to pathology workflows rather than a direct intra-operative imaging tool for surgical decision-making.
A major limitation in current CFM studies is the absence of data on its real-time application for in-vivo margin assessment. While CFM has demonstrated high diagnostic accuracy for localized areas of excised tissue, its feasibility for evaluating entire WLE specimens intra-operatively remains untested. Tissue heterogeneity, and variability in optical plane depth present additional challenges in obtaining high resolution imaging across large surgical specimens, necessitating further validation studies focusing on whole-margin assessment. Additionally, cancer-induced tissue stiffness variations may further hinder uniform imaging, and the high-resolution nature of CFM generates vast image datasets that could complicate real-time interpretation and clinical workflow integration. Combined with the issue of tissue stiffness, a single duct containing DCIS would need to be in the optical plane and detected correctly by the reader, which would pose further challenges to widespread use. Moreover, the shallow imaging depth (100
4.4 Fluorescent contrast agents in confocal imaging of breast tissue
CFM relies on exogenous dyes to generate high-contrast images that reveal critical cellular features—particularly nuclear and epithelial structures—which are essential for identifying malignancy. This is particularly relevant in BCS, where rapid assessment of surgical margins can influence real-time decision-making.
Since most breast cancers originate from the epithelial lining of ducts and lobules, accurate visualization of this surface layer becomes central to intra-operative imaging. Fluorescent contrast agents enable such visualization and are typically delivered via topical application or intravenous (IV) administration. Topical application allows for fast penetration (30–120 s), is safer for real-time surgery, and does not interfere with downstream histopathology. IV dyes circulate systemically and accumulate in tumor regions with leaky vasculature but require longer uptake times and raise safety/regulatory considerations. Several contrast agents have been evaluated in pre-clinical and early clinical studies.
While several fluorescent dyes show strong potential for margin assessment in BCS, most remain investigational or used off-label for intra-operative use. Clinical translation requires dyes that are both biocompatible and FDA-cleared, with rapid uptake and minimal impact on histology. Given the epithelial origin of most breast tumors, topical staining remains the most practical route for margin imaging during BCS.
4.5 Artificial intelligence – a critical frontier for clinical translation of confocal imaging
AI has emerged as a powerful enabler for the clinical translation of CFM, particularly in the context of BCS where real-time, high-resolution imaging of tumor margins is crucial. While the imaging capabilities of CFM are well established, its adoption in routine surgical workflows remains constrained by several key challenges—limited field of view, grayscale contrast interpretation, lack of standardization, and dependency on expert users. AI offers a compelling pathway to overcome these limitations and enhance the reliability, scalability, and usability of intraoperative confocal imaging platforms.
We identify the following four key application domains where AI can propel the clinical translation of CFM, enabling scalable, reliable, and surgeon-friendly intraoperative imaging platforms.
1. Automated Tissue Segmentation and Margin Identification. Deep learning models—particularly convolutional neural networks (CNNs) and recurrent neural networks (RNNs)—have shown success in segmenting epithelial structures, stromal boundaries, and tumor margins in grayscale confocal images with high precision. Accurate margin delineation is pivotal in BCS to reduce positive margins and avoid re-excision. AI-assisted segmentation reduces inter-observer variability and can offer real-time guidance during intraoperative imaging workflows Dobbs J. et al. (2015).
2. Large-Area Mosaicking and Margin Mapping. Due to the inherently small field-of-view of high-resolution CFM, real-time evaluation of surgical margins necessitates large-area mosaicking. AI-driven algorithms have demonstrated the capability to stitch thousands of confocal tiles into seamless wide-area maps (e.g.,
3. Tissue Classification and Decision Support. Supervised AI classifiers trained on annotated confocal datasets have achieved promising results in distinguishing between benign, malignant, and normal tissue types with diagnostic sensitivities exceeding 90% Gu et al. (2017), Gu et al. (2018), Gu et al. (2020). These tools hold significant potential to support intraoperative interpretation—especially in settings lacking on-site pathology—by offering rapid, standardized decision support to surgeons or radiologists.
4. Virtual Histopathology and Enhanced Visualization. To bridge the familiarity gap between grayscale fluorescence imaging and routine histopathology, researchers have applied generative adversarial networks (GANs) to generate virtual H&E-like images from confocal data ? Such AI-driven translation tools can improve interpretability for clinicians accustomed to color-stained histological images, easing the transition to digital and real-time modalities.
These innovations, while encouraging, are still at a developmental stage. Most AI models have been trained on small, single-center datasets, with limited validation across diverse histological subtypes or imaging devices. Interpretability remains a concern, particularly for high-stakes surgical decision-making. Regulatory frameworks are also evolving, and ethical concerns—such as bias, liability, and data privacy—must be addressed before clinical adoption can scale.
Nonetheless, the integration of AI into CFM represents a promising frontier in image-guided surgery. By enabling robust segmentation, classification, visualization, and surgeon-AI collaboration, these tools have the potential to transform intraoperative decision-making and reduce reoperation rates. As multi-center datasets become available and explainable AI methods mature, we anticipate these technologies to serve not only as interpretive aids, but as essential components of future digitally guided cancer surgery. A schematic of this evolving workflow is illustrated in Figures 6.
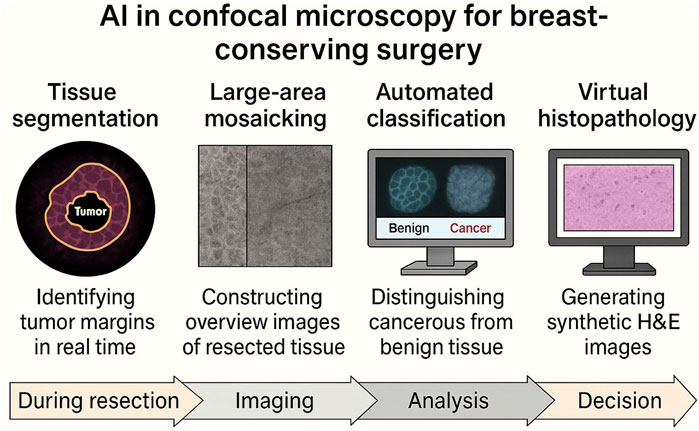
Figure 6. Integration of AI in the intraoperative confocal fluorescence microscopy (CFM) workflow for BCS. The schematic outlines the role of artificial intelligence across four key modules in the surgical imaging pipeline: 1) Tissue segmentation, assisting real-time tumor margin delineation at the point of resection; 2) Large-area mosaicking, enabling reconstruction of wide surgical margins from high-resolution confocal tiles; 3) Automated classification, supporting diagnostic interpretation of benign vs. malignant tissue patterns; and 4) Virtual histopathology, translating grayscale confocal images into H&E-like visual formats for enhanced interpretability. Each application aligns with a specific phase in the surgical workflow and addresses key limitations of conventional confocal imaging—positioning AI as a driver of real-time, scalable clinical adoption in BCS.
4.6 Challenges and future directions
Despite its promising applications, several challenges must be addressed before CFM can be routinely integrated into intra-operative breast cancer surgery.
Given that lobular carcinoma (6.6%) often presents with diffuse infiltration and DCIS (2.5%) lesions can be multi-focal, it is essential to examine and validate whether CFM exhibits the same level of diagnostic accuracy across different histological subtypes. Additionally, rarer breast pathologies, including mucinous carcinoma, fibroadenoma, phyllodes tumour, and lobular carcinoma in-situ collectively accounted for only 1.7% of analyzed specimens, highlighting a significant gap in confocal imaging validation.
For CFM to be effectively integrated into intra-operative surgical workflows, future research must go beyond validation in small tissue fragments and focus on real-time, in-vivo imaging of resection cavities and whole WLE specimens. This will provide direct evidence of its ability to assess margins intra-operatively and determine its clinical impact on reducing re-excision rates and optimizing surgical decision-making.
4.7 Limitations and barriers to clinical translation
While CFM offers promising capabilities for real-time margin assessment in breast conserving surgery, several barriers hinder its widespread clinical adoption. First, cost remains a significant constraint. Bench-top systems such as VivaScope or Histolog® can exceed USD 200,000, and even fiber-based systems—though relatively more affordable—require integration with surgical monitors, staff training, and dedicated imaging workflows. Unless clearly demonstrated to reduce re-excision rates or accelerate intra-operative decisions, their cost-effectiveness may be difficult to justify in resource-limited settings.
Second, training and usability pose practical challenges. Most confocal systems produce grayscale fluorescence images that differ significantly from conventional H&E slides. Interpretation requires familiarity with the imaging modality, and current systems rely heavily on expert visual assessment. This raises the need for structured training pathways, decision-support tools, and ideally, AI-assisted pattern recognition to aid non-pathologist users such as surgeons or radiologists. Some attempts to circumvent these challenges include depicting confocal images using post-processing techniques that provide a pink/purple contrast which is more akin to H&E (e.g., Histolog®).
Third, regulatory hurdles continue to limit routine use. Many of the fluorescent dyes used for nuclear contrast—such as acriflavine and proflavine—are not FDA-approved for clinical application. Devices like the Histolog® Scanner remain cleared only for research use in many jurisdictions, slowing adoption despite promising early results. Broader clinical integration will require not only regulatory approval of hardware, but also validation of contrast agents for safe and effective intra-operative use.
Finally, histological diversity presents interpretive challenges. While many studies have focused on invasive ductal carcinoma, breast cancer includes a spectrum of subtypes—such as lobular carcinoma, papillary lesions, and benign mimickers like sclerosing adenosis—that can appear deceptively similar on fluorescence imaging. Additionally, it is critical that work be done to better characterise cellular atypia that falls short of DCIS. These variations require further validation in large, histologically diverse datasets to ensure safe clinical deployment across tumor types.
5 Conclusion
Confocal fluorescence microscopy (CFM) holds significant potential as an intra-operative imaging tool for real-time tumor margin assessment in breast conserving surgery. By providing high-resolution, subcellular imaging, CFM addresses a key challenge in breast cancer surgery—reducing positive margin rates and re-operation rates (20%–30%). Both bench-top and fibre-based CFM systems offer high diagnostic accuracy, with bench-top systems like Histolog® excelling in resolution and wide-area imaging, while fiber-based systems like Cellvizio® p-CLE and LS-CLE allowing real-time intraoperative scanning.
The capacity to visualize cellular architecture within minutes offers a potential alternative to conventional frozen section analysis and may reduce re-excision rates. Yet, despite encouraging technical validation, clinical adoption remains nascent. Key barriers include the cost and complexity of implementation, training requirements for non-pathologist users, lack of standardized workflows, and the limited regulatory approval of contrast agents suited for intra-operative imaging.
Nonetheless, we view this as a moment of inflection and opportunity. CFM lies at the intersection of emerging technological trends: AI-driven diagnostic support, robotic-assisted imaging, and virtual histopathology. These developments are no longer distant ambitions—they are gradually entering clinical research pipelines. AI-enabled platforms that can segment, mosaic, and classify confocal images in real time are increasingly feasible. Robotic delivery systems offer the potential for precise probe control in anatomically constrained spaces, including intraductal regions. Together, these tools could shift intraoperative care from a reactive to a guided paradigm of real-time.
For the field to mature, the next steps must prioritize.
As engineers and clinicians work more closely at this frontier, we believe CFM offers more than just technical novelty - it signals a paradigm shift toward real-time digital pathology. With continued interdisciplinary effort, this technology may not only enhance the precision of cancer surgery but also redefine how we visualize and act on disease, in a timely manner.
Author contributions
KV: Investigation, Writing – review and editing, Formal Analysis, Writing – original draft, Methodology, Data curation, Validation, Visualization, Resources, Conceptualization. AE: Formal Analysis, Writing – original draft, Data curation, Conceptualization, Validation, Investigation, Writing – review and editing, Methodology, Software. NH: Writing – review and editing, Visualization, Investigation, Validation, Methodology. RR: Supervision, Writing – review and editing, Validation, Conceptualization. DL: Project administration, Supervision, Conceptualization, Writing – review and editing, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Engineering and Physical Science Research Council (EPSRC) UK grant EP/P012779. This work is also supported by Cancer Research UK (CRUK)/EPSRC funded project “MAMMOBOT”.
Acknowledgments
The authors also acknowledge the support of NIHR Imperial Biomedical Research Centre (BRC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelaziz, M. E., Zhao, J., Gil Rosa, B., Lee, H. T., Simon, D., Vyas, K., et al. (2024). Fiberbots: robotic fibers for high-precision minimally invasive surgery. Sci. Adv. 10, eadj1984. doi:10.1126/sciadv.adj1984
Abeytunge, S., Li, Y., Larson, B., Peterson, G., Seltzer, E., Toledo-Crow, R., et al. (2013a). Confocal microscopy with strip mosaicing for rapid imaging over large areas of excised tissue. J. Biomed. Opt. 18, 061227. doi:10.1117/1.jbo.18.6.061227
Abeytunge, S., Li, Y., Larson, B., Peterson, G., Toledo-Crow, R., and Rajadhyaksha, M. (2013b). Mobile large area confocal scanner for imaging tumor margins: initial testing in the pathology department. Adv. Biomed. Clin. Diagnostic Syst. XI (SPIE) 8572, 140–153. doi:10.1117/12.2005026
Abeytunge, S., Larson, B., Peterson, G., Morrow, M., Rajadhyaksha, M., and Murray, M. P. (2017). Evaluation of breast tissue with confocal strip-mosaicking microscopy: a test approach emulating pathology-like examination. J. Biomed. Opt. 22, 034002. doi:10.1117/1.jbo.22.3.034002
Berthet-Rayne, P., Sadati, S. M. H., Petrou, G., Patel, N., Giannarou, S., Leff, D. R., et al. (2021). Mammobot: a miniature steerable soft growing robot for early breast cancer detection. IEEE Robotics Automation Lett. 6, 5056–5063. doi:10.1109/lra.2021.3068676
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer J. Clin. 74, 229–263. doi:10.3322/caac.21834
Chagpar, A. B., Killelea, B. K., Tsangaris, T. N., Butler, M., Stavris, K., Li, F., et al. (2015). A randomized, controlled trial of cavity shave margins in breast cancer. N. Engl. J. Med. 373, 503–510. doi:10.1056/nejmoa1504473
Chang, T. P., Leff, D. R., Shousha, S., Hadjiminas, D. J., Ramakrishnan, R., Hughes, M. R., et al. (2015). Imaging breast cancer morphology using probe-based confocal laser endomicroscopy: towards a real-time intraoperative imaging tool for cavity scanning. Breast cancer Res. Treat. 153, 299–310. doi:10.1007/s10549-015-3543-8
Chen, Z., Guo, W., Kang, D., Wang, S., Zheng, L., Xi, G., et al. (2020). Label-free identification of early stages of breast ductal carcinoma via multiphoton microscopy. Scanning 2020, 1–8. doi:10.1155/2020/9670514
Chowdhry, V., Zeybek, B., Gupta, A., Kamran, S. C., Esserman, L. J., and Alvarado, M. D. (2022). Emerging technologies in intraoperative margin assessment during breast-conserving surgery: a comprehensive review. JAMA Surg. 157, 788–798. doi:10.1001/jamasurg.2022.1982
Conversano, A., Abbaci, M., van Diest, P., Roulot, A., Falco, G., Ferchiou, M., et al. (2023). Breast carcinoma detection in ex vivo fresh human breast surgical specimens using a fast slide-free confocal microscopy scanner: hibiscuss project. BJS Open 7, zrad046. doi:10.1093/bjsopen/zrad046
De Palma, G. D., Esposito, D., Luglio, G., Limite, G., Accurso, A., Sollazzo, V., et al. (2015). Confocal laser endomicroscopy in breast surgery: a pilot study. BMC cancer 15, 252–257. doi:10.1186/s12885-015-1245-6
Dobbs, J. L., Ding, H., Benveniste, A. P., Kuerer, H. M., Krishnamurthy, S., Yang, W., et al. (2013). Feasibility of confocal fluorescence microscopy for real-time evaluation of neoplasia in fresh human breast tissue. J. Biomed. Opt. 18, 106016. doi:10.1117/1.jbo.18.10.106016
Dobbs, J., Krishnamurthy, S., Kyrish, M., Benveniste, A. P., Yang, W., and Richards-Kortum, R. (2015a). Confocal fluorescence microscopy for rapid evaluation of invasive tumor cellularity of inflammatory breast carcinoma core needle biopsies. Breast cancer Res. Treat. 149, 303–310. doi:10.1007/s10549-014-3182-5
Dobbs, J. L., Mueller, J. L., Krishnamurthy, S., Shin, D., Kuerer, H., Yang, W., et al. (2015b). Micro-anatomical quantitative optical imaging: toward automated assessment of breast tissues. Breast Cancer Res. 17, 105–114. doi:10.1186/s13058-015-0617-9
Dobbs, J., Kyrish, M., Krishnamurthy, S., Grant, B., Kuerer, H., Yang, W., et al. (2016a). High resolution microendoscopy with structured illumination and lugol’s iodine staining for evaluation of breast cancer architecture. Opt. Biopsy XIV Toward Real-Time Spectrosc. Imaging Diagnosis (SPIE) 9703, 72–82.
Dobbs, J. L., Shin, D., Krishnamurthy, S., Kuerer, H., Yang, W., and Richards-Kortum, R. (2016b). Confocal fluorescence microscopy to evaluate changes in adipocytes in the tumor microenvironment associated with invasive ductal carcinoma and ductal carcinoma in situ. Int. J. cancer 139, 1140–1149. doi:10.1002/ijc.30160
Elfgen, C., Papassotiropoulos, B., Varga, Z., Moskovszky, L., Nap, M., Güth, U., et al. (2019). Comparative analysis of confocal microscopy on fresh breast core needle biopsies and conventional histology. Diagn. Pathol. 14, 58. doi:10.1186/.S13000-019-0835-Z
Fleming, F. J., Heneghan, H. M., Kelly, L., and McCartan, D. (2020). Intraoperative margin assessment in breast conserving surgery: a systematic review and meta-analysis of techniques and outcomes. Breast 53, 163–176. doi:10.1016/j.breast.2020.07.003
Gao, Z., Li, G., Li, X., Zhou, J., Duan, X., Chen, J., et al. (2017). In vivo near-infrared imaging of erbb2 expressing breast tumors with dual-axes confocal endomicroscopy using a targeted peptide. Sci. Rep. 7, 14404. doi:10.1038/s41598-017-13735-z
Gareau, D. S., Jeon, H., Nehal, K. S., and Rajadhyaksha, M. (2012). Rapid screening of cancer margins in tissue with multimodal confocal microscopy. J. Surg. Res. 178, 533–538. doi:10.1016/j.jss.2012.05.059
Giataganas, P., Hughes, M., Payne, C. J., Wisanuvej, P., Temelkuran, B., and Yang, G. Z. (2019). Intraoperative robotic-assisted large-area high-speed microscopic imaging and intervention. IEEE Trans. Biomed. Eng. 66, 208–216. doi:10.1109/tbme.2018.2837058
Gu, Y., Vyas, K., Yang, J., and Yang, G. Z. (2017). Medical image computing and computer assisted Intervention- MICCAI 2017: 20Th international conference, Quebec City, QC, Canada, September 11-13, 2017, proceedings, part III 20. Springer, 64–71.
Gu, Y., Vyas, K., Yang, J., and Yang, G. Z. (2019). Transfer recurrent feature learning for endomicroscopy image recognition. IEEE Trans. Med. imaging 38, 791–801. doi:10.1109/tmi.2018.2872473
Gu, Y., Vyas, K., Shen, M., Yang, J., and Yang, G. Z. (2021). Deep graph-based multimodal feature embedding for endomicroscopy image retrieval. IEEE Trans. Neural Netw. Learn. Syst. 32, 481–492. doi:10.1109/tnnls.2020.2980129
Houssami, N., Macaskill, P., Luke Marinovich, M., and Morrow, M. (2014). The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann. Surg. Oncol. 21, 717–730. doi:10.1245/s10434-014-3480-5
Humaran, D., Pérez-Anker, J., Fernández, P. L., Blay, L., Pascual, I., Castellà, E., et al. (2024). Unveiling a surgical revolution: the use of conventional histology versus ex vivo fusion confocal microscopy in breast cancer surgery. Cells 13, 1692. doi:10.3390/cells13201692
Jeevan, R., Cromwell, D., Trivella, M., Lawrence, G., Kearins, O., Pereira, J., et al. (2012). Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. Bmj 345, e4505. doi:10.1136/bmj.e4505
Kaczmarski, K., Wang, P., Gilmore, R., Overton, H. N., Euhus, D. M., Jacobs, L. K., et al. (2019). Surgeon re-excision rates after breast-conserving surgery: a measure of low-value care. J. Am. Coll. Surg. 228, 504–512e2. doi:10.1016/j.jamcollsurg.2018.12.043
Krishnamurthy, S., Meric-Bernstam, F., Lucci, A., Hwang, R. F., Kuerer, H. M., Babiera, G., et al. (2009). A prospective study comparing touch imprint cytology, frozen section analysis, and rapid cytokeratin immunostain for intraoperative evaluation of axillary sentinel lymph nodes in breast cancer. Cancer 115, 1555–1562. doi:10.1002/cncr.24182
Krishnamurthy, S., Cortes, A., Lopez, M., Wallace, M., Sabir, S., Shaw, K., et al. (2018). Ex vivo confocal fluorescence microscopy for rapid evaluation of tissues in surgical pathology practice. Archives Pathology and Laboratory Med. 142, 396–401. doi:10.5858/arpa.2017-0164-oa
Krishnamurthy, S., Ban, K., Shaw, K., Mills, G., Sheth, R., Tam, A., et al. (2019). Confocal fluorescence microscopy platform suitable for rapid evaluation of small fragments of tissue in surgical pathology practice. Archives Pathology and Laboratory Med. 143, 305–313. doi:10.5858/arpa.2018-0352-oa
Larson, B. A., Abeytunge, S., Murray, M., and Rajadhyaksha, M. (2013). Large area mapping of excised breast tissue by fluorescence confocal strip scanning: a preliminary feasibility study. Opt. Biopsy XI (SPIE) 8577, 17–22.
Lundgren, S., Garne, J. P., Emdin, S. O., Palm-Sjövall, M., Betheden, J., and Landberg, G. (2019). Increasing use of breast conservation in Sweden. Breast Cancer Res. Treat. 115, 831–837. doi:10.1007/.s10549-008-0131-2
Macmillan, R. D., McCulley, S. J., and Macmillan, M. T. (2022). Oncoplastic breast-conserving surgery: past, present and future. Ann. Surg. Oncol. 29, 4238–4252. doi:10.1245/s10434-022-11723-6
Mathieu, N., Pérez-Anker, J., and Blay, L. (2024). Ex-vivo fusion confocal microscopy for margin assessment in breast cancer surgery. Br. J. Surg. 111, 258–269. doi:10.1093/bjs/znad394
Mazzucchelli, S., Goldstein, E., and Sanchez-Herrero, E. (2024). Histolog® confocal microscopy for breast cancer research and patient-derived organoid imaging. Sci. Rep. 14, 992. doi:10.1038/s41598-024-39002-7
Moran, M. S., Schnitt, S. J., Giuliano, A. E., Harris, J. R., Khan, S. A., Horton, J., et al. (2014). Society of surgical Oncology–American Society for Radiation Oncology Consensus Guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann. Surg. Oncol. 21, 704–716. doi:10.1245/s10434-014-3481-4
Patel, R., Khan, A., Wirth, D., Kamionek, M., Kandil, D., Quinlan, R., et al. (2012). Multimodal optical imaging for detecting breast cancer. J. Biomed. Opt. 17, 066008. doi:10.1117/1.jbo.17.6.066008
Ragazzi, M., Piana, S., Longo, C., Castagnetti, F., Foroni, M., Ferrari, G., et al. (2014). Fluorescence confocal microscopy for pathologists. Mod. Pathol. 27, 460–471. doi:10.1038/modpathol.2013.158
Sandor, M. F., Schwalbach, B., Hofmann, V., Istrate, S. E., Schuller, Z., Ionescu, E., et al. (2022). Imaging of lumpectomy surface with large field-of-view confocal laser scanning microscope for intraoperative margin assessment-polarhis study. Breast 66, 118–125. doi:10.1016/j.breast.2022.10.003
St John, E. R., Al-Khudairi, R., Ashrafian, H., Athanasiou, T., Takats, Z., Hadjiminas, D. J., et al. (2017). Diagnostic accuracy of intraoperative techniques for margin assessment in breast cancer surgery: a meta-analysis. Ann. Surg. 265, 300–310. doi:10.1097/sla.0000000000001897
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Tanei, T., Pradipta, A. R., Morimoto, K., Fujii, M., Arata, M., Ito, A., et al. (2019). Cascade reaction in human live tissue allows clinically applicable diagnosis of breast cancer morphology. Adv. Sci. 6, 1801479. doi:10.1002/advs.201801479
Togawa, R., Hederer, J., Ragazzi, M., and Bruckner, T. (2023). Evaluation of lumpectomy surface with large field-of-view confocal laser scanning microscopy ’histolog® scanner’ for breast margin assessment. Breast 64, 33–41. doi:10.1016/j.breast.2023.03.001
Vyas, K., Hughes, M., Leff, D. R., and Yang, G. Z. (2017). Methylene-blue aided rapid confocal laser endomicroscopy of breast cancer. J. Biomed. Opt. 22, 020501. doi:10.1117/1.jbo.22.2.020501
Vyas, K., Ezzat, A., Asenov, M., Chauhan, M., Ramamoorthy, S., Jha, A., et al. (2022). 2022 conference on lasers and electro-optics. San Jose, CA: Optica Publishing Group, ATh4I–7.
Wu, Y., Fu, F., Lian, Y., Nie, Y., Zhuo, S., Wang, C., et al. (2015). Monitoring the progression from intraductal carcinoma to invasive ductal carcinoma based on multiphoton microscopy. J. Biomed. Opt. 20, 096007. doi:10.1117/1.jbo.20.9.096007
Yoshitake, T., Giacomelli, M. G., Cahill, L. C., Schmolze, D. B., Vardeh, H., Faulkner-Jones, B. E., et al. (2016). Direct comparison between confocal and multiphoton microscopy for rapid histopathological evaluation of unfixed human breast tissue. J. Biomed. Opt. 21, 126021. doi:10.1117/1.jbo.21.12.126021
Zhang, C., Jiang, D., Huang, B., Wang, C., Zhao, L., Xie, X., et al. (2019). Methylene blue–based near-infrared fluorescence imaging for breast cancer visualization in resected human tissues. Technol. cancer Res. and Treat. 18, 1533033819894331. doi:10.1177/1533033819894331
Zuo, S., Hughes, M., Giataganas, P., Seneci, C., Chang, T. P., and Yang, G. Z. (2014). in IEEE international conference on robotics and automation (ICRA) (IEEE), 3524–3530.
Zuo, S., Hughes, M., Seneci, C., Chang, T. P., and Yang, G. Z. (2015). Toward intraoperative breast endomicroscopy with a novel surface-scanning device. IEEE Trans. Biomed. Eng. 62, 2941–2952. doi:10.1109/tbme.2015.2455597
Keywords: confocal laser microscopy, confocal endomicroscopy, breast cancer, breast conserving surgery, fluorescence, resection margins, margin assessment technology, intra-operative imaging
Citation: Vyas K, Ezzat A, Holford N, Ramakrishnan R and Leff DR (2025) Confocal fluorescence microscopy for real-time breast cancer diagnosis: current advances and future perspectives. Front. Med. Eng. 3:1607453. doi: 10.3389/fmede.2025.1607453
Received: 07 April 2025; Accepted: 18 August 2025;
Published: 12 September 2025.
Edited by:
Lorenzo Vannozzi, Sant’Anna School of Advanced Studies, ItalyReviewed by:
Juliana Redondo, Sant’Anna School of Advanced Studies, ItalyBianca Cioni, Sant’Anna School of Advanced Studies, Italy
Copyright © 2025 Vyas, Ezzat, Holford, Ramakrishnan and Leff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel R. Leff, ZC5sZWZmQGltcGVyaWFsLmFjLnVr
†These authors share first authorship
 Khushi Vyas
Khushi Vyas Ahmed Ezzat
Ahmed Ezzat Nicholas Holford2
Nicholas Holford2 Daniel R. Leff
Daniel R. Leff